Abstract
HNRNPU encodes a multifunctional RNA-binding protein that plays critical roles in regulating pre-mRNA splicing, mRNA stability, and translation. Aberrant expression and dysregulation of HNRNPU have been implicated in various human diseases, including cancers and neurological disorders. We applied a next generation sequencing based assay (EPIC-NGS) to investigate genome-wide methylation profiling for >2 M CpGs for 7 individuals with a neurodevelopmental disorder associated with HNRNPU germline pathogenic loss-of-function variants. Compared to healthy individuals, 227 HNRNPU-associated differentially methylated positions were detected. Both hyper- and hypomethylation alterations were identified but the former predominated. The identification of a methylation episignature for HNRNPU-associated neurodevelopmental disorder (NDD) implicates HNPRNPU-related chromatin alterations in the aetiopathogenesis of this disorder and suggests that episignature profiling should have clinical utility as a predictor for the pathogenicity of HNRNPU variants of uncertain significance. The detection of a methylation episignaure for HNRNPU-associated NDD is consistent with a recent report of a methylation episignature for HNRNPK-associated NDD.
Subject terms: DNA methylation, Metabolic disorders, Genetics research
Introduction
Advances in genomics have resulted in increasingly large numbers of genes being identified as causing neurodevelopmental disorders (NDDs) [1, 2]. HNRNPU encodes a component of a multiprotein complex that binds heterogeneous nuclear RNA and scaffold-attached DNA [3, 4]. Other members (n = 32) of the large heterogeneous nuclear ribonucleoprotein family that have been implicated in human disease include HNRNPH1, HNRNPH2, HNRNPK, HNRNPR and SYNCRIP [5]. Following suggests that inactivation of HNRPNU might contribute to the neurological and neurodevelopmental phenotype of 1q34q44 microdeletion syndrome [6, 7] as de novo mutations in HNRNPU were reported in rare cases of epileptic encephalopathy [8, 9]. Subsequently, the phenotype associated with pathogenic variants in HNRNPU was extended to include early-onset seizures, severe intellectual disability, speech impairment, hypotonia, microcephaly and ventriculomegaly [10–13]. Dysmorphic features (high arched eyebrows, long palpebral fissures, overhanging columella, widely spaced teeth and thin upper lip) have also been described [11, 12].
Interpreting the potential pathogenicity of variants of uncertain significance (VUSs) remains a major challenge in many areas clinical genetics, including the diagnosis of NDDs [14–16]. A major cause of NDDs are variants in chromatin modifying genes (CMGs) (e.g., histone lysine methyltransferases or histone acetylases etc.) and for many of these disorders, evidence of disordered epigenetic regulation can be detected through alterations of DNA methylation patterns (episignatures) in peripheral blood [2, 17]. The identification of CMG-associated NDD specific episignatures can be used to aid variant interpretation and suggest candidate CMGs in unsolved NDDs [15, 17–22]. In addition to a role in posttranscriptional RNA processing, HNRNPU (also known as scaffold attachment factor A (SAF-A)) is also reported to have roles in gene transcription, maintenance of higher-order chromatin structure and X-inactivation via Xist [23–25]. Recently, a methylation episignature was described for Au-Kline syndrome, a NDD associated with germline mutations in HNRNPK [16]. In the light of this finding, we investigated whether HNRNPU-related NDD was associated with a methylation episignature.
Subjects and methods
We performed genome-wide methylation profiling of >2 M CpGs with a targeted next generation sequencing assay (Illumina TruSeq® Methyl Capture EPIC NGS) as described previously [17]. Written informed consent was obtained for all participants and the study was approved by South Birmingham Research Ethics Committee.
Genomic DNA with HNRNPU pathogenic mutations (n = 7) were extracted from whole blood by standard methods. Bisulfite conversion, library preparation, target enrichment and sequencing (Illumina NextSeq 2000) were performed at the Cambridge University Department of Medical Genetics Stratified Medicine Core Laboratory (SMCL) as described previously [17]. Raw methylation beta-values were extracted by RnBeads R package (https://rnbeads.org). Data pre-processing and bioinformatics analysis, and detection and visualisation of methylation episignatures were performed according to our standard procedure (see Lee et al. [17, 26]). If a significant batch effect (age, gender, batch-based) was detected, the target variables were adjusted by surrogate variable analysis (SVA) using the sva package. The p-value of differentially methylated sites was determined either by a two-sided Welch test or by a linear model employed in the limma package, and the combined p-values (for CpG islands) were determined by Fisher’s method. During the process, neighbouring CpGs combined together and assigned as ‘DMB (differentially methylated blocks).’ DMBs were combined based on their functional similarity. Only DMBs (including CpG Islands) with a p-value lower than 0.01 and a methylation difference between controls and diseases group of more than 20% were considered significant for genome-wide CpG site methylation analysis. A summary of the sequencing coverage and sequencing reads is included in Supplementary Table 1.
Results
Clinical and genetic features of HNRNPU patient cohort
The seven individuals studied had been diagnosed with a HNRNPU-NDD after the identification of a germline HNRNPU variant (see Table 1). All HNRNPU variants were assessed as likely pathogenic or pathogenic and were predicted to have a loss-of-function effect (6 were predicted, in the absence of nonsense-mediated mRNA decay, to result in a truncated gene product and one patient was predicted to have PBRM1 haploinsufficiency as a result of a de novo deletion of exons 1–11) (Table 1). The positions of the truncating variants are plotted on the HNRNPU protein in Fig. 1.
Table 1.
Genetic characteristics of patients with HNRNPU variants.
| Patient ID | Age at DNA sampling | Sex | Variant Details (HNRNPU NM_004501.3) | Protein effect | Reported previously |
|---|---|---|---|---|---|
| Patient 1 | 2 | Male | de novo deletion of exons 1–11 | Haploinsufficiency | Patient-14 (Taylor et al., 2022 [27]) |
| Patient 2 | 23 | Female | de novo c.847_857del, p.(Phe283Serfs*5) | Truncating | Patient-13 (Durkin et al., 2021 [12]) |
| Patient 3 | 12 | Female | de novo c.23_24delTAinsA, p.(Val8Glufs*4) | Truncating | Patient-4 (Yates et al., 2017 [11]) |
| Patient 4 | 24 | Male | de novo c.1450 C > T, p.(Arg484*) | Truncating | Patient-3 (Durkin et al., 2021 [12]) |
| Patient 5 | 3 | Male | de novo c.1624dup p.(Gln542Pro*8) | Truncating | Not published |
| Patient 6 | 22 | Female | de novo c.706_707del, p.(Glu236Thrfs*6) | Truncating | Patient-20 (Durkin et al., 2021 [12]) |
| Patient 7 | 2 | Male | de novo c.2365 C > T, p.(Arg789*) | Truncating | Not published |
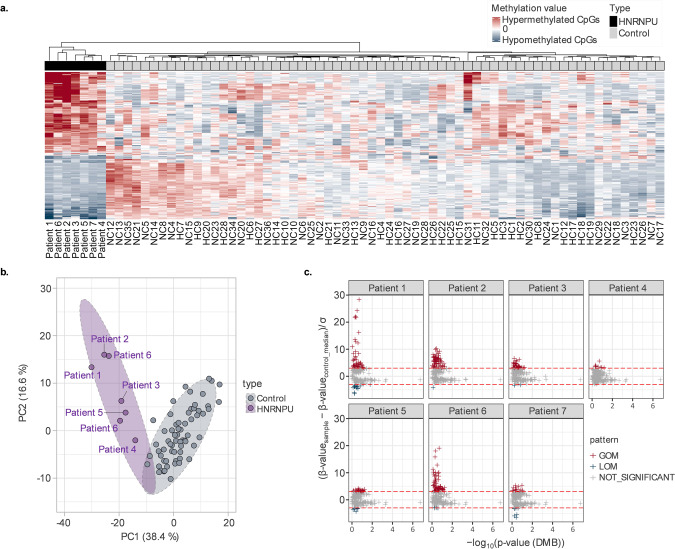
Fig. 1. HNRNPU-specific methylation episignature.
a The genome-wide methylation episignatures of HNRNPU samples were determined by calculating the mean normalised methylation beta-value relative to the control group. Unsupervised clustering analysis revealed a clear separation between the 7 patients and the control group, with approximately 55% of differentially methylated positions (DMPs) exhibiting hypermethylated profiles and approximately 44% showing hypomethylation. b To eliminate potential biases introduced by normalised data, a PCA clustering analysis was performed based on preprocessed beta-values. The results demonstrated that 227 DMPs were able to effectively differentiate the HNRNPU group from the control group. c Scatter plots were generated by comparing the methylation beta-values of individuals with the mean values of the healthy control group, using a confidence interval of ±3 standard deviations (3 SD). A significant pattern of the number of DMPs with gain of methylation (GOM) compared to loss of methylation (LOM) DMPs was detected (17.6% hypermethylated, 2.08% hypomethylated).
The frequency of the clinical features displayed by the 7 individuals with a HNRNPU variant is summarised in Table 1. The overall frequency of clinical features such as seizures, developmental delay, intellectual disability, and hypotonia (Table 2) in the study cohort was similar to that in a previously reported series [27] of 17 patients with HNRNPU-NDD (1 patient from the current cohort were also represented in this previous series). Table 3 provides detailed overview of clinical characteristics of current (n = 7) cohort with an update on those patients previously published in Taylor et al. and Durkin et al. [12, 27].
Table 2.
Frequencies of clinical features in HNRNPU-associated neurodevelopmental disorder.
| Clinical Features | Frequency (%) in current series (n = 7) | Frequency (%) in Taylor et al. (n = 17) |
|---|---|---|
| Seizures | 100 | 100 |
| Global developmental delay | 100 | 100 |
| Intellectual disability | 100 | 94 |
| Dysmorphism | 100 | 94 |
| Hypotonia | 100 | 88 |
| Neonatal hypotonia | 28 | 65 |
| Neonatal feeding difficulties | 14 | 59 |
| Autistic features | 57 | 50 |
| Cardiac abnormality | 14 | 44 |
| Abnormality on brain MRI | 28 | 38 |
Table 3.
Clinical characteristics of patients in current cohort with HNRNPU variants.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Decipher ID (in DDD cohort) | DDD-270453 | DDD-268390 | DDD-305034 | DDD-279875 | |||
| Sex | Male | Female | Female | Male | Male | Female | Male |
| Age of DNA sampling (decimal age in years) | 2 | 23 | 7 | 24 | 3 | 22 | 2 |
| HNRNPU Variant | |||||||
| Heterozygous cDNA change | Deletion exons 1–11 | c.847_857del | c.23_24del | c.1450 C > T | c.1624dup | c.706_707del | c.2365 C > T |
| Amino acid change | Not applicable | p.Phe283SerfsTer5 | p.Val8Glufs*4 | p.Arg484Ter | p.Gln542ProfsTer8 | p.Glu236Thrfs*6 | p.Arg789* |
| Inheritance | De novo | De novo | De novo | De novo | De novo | De novo | De novo |
| Additional genetic defect | No | No | No | No | No | No | No |
| Pregnancy/delivery | |||||||
| Gestational age at birth (weeks) | 40 + 2 | 37 | 38 | 40 | 39 | 41 + 2 | 40 |
| Birth weight (kilograms) | 3.7 | 2.85 | 2.41 | 2.63 | 2.76 | 2.68 | 3.7 |
| Neonatal concerns | |||||||
| Hypotonia | No | Yes | No | No | No | Yes | No |
| Feeding difficulties | No | Yes | No | No | No | No | No |
| Other | No | No | No | No | Hypoglycaemia, jaundice, concerns with thermoregulation | No | No |
| Development | |||||||
| Intellectual disability | Yes | Yes | Yes | Yes | Yes | Yes | Too young |
| Mild/Moderate/Severe | Moderate | Severe | Severe | Moderate | Mild | Moderate-severe | |
| Global dev. delay | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Language delay | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Age of first words | 23 months | 36 months | 5 years | 18 months | 24 months | 24 months | absent |
| Motor delay | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Age of sitting unsupported | 14 months | 15 months | 2–2.5 years | 10 months | 9 months | NK | |
| Age of first steps | 3 years | 3 years | 5 years | 20 months | 30 months | 24 months | 22 months |
| Behavioural Features | |||||||
| Any psychiatric diagnosis | No | Yes | Very sociable | No | Autism spectrum disorder | Difficult behaviour | Autism spectrum disorder |
| Other psychopathology | No | Episodic hyperventilation with apnoea, cyanotic episodes in between | hand flapping | No | No formal diagnosis | ||
| Neurological | |||||||
| Hypotonia | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Epilepsy | Yes | Yes | No | Yes | Yes | Yes | Yes |
| EEG abnormality | Yes | Yes | No | Yes | No | Yes | No |
| MRI-brain | Normal | Abnormal | Normal | Not performed | Abnormal | Normal | Normal |
| Delayed myelinisation | No | No | No | No | No | ||
| Corpus callosum | No | No | No | No | No | ||
| Colpocephaly | No | No | No | No | No | ||
| Ventriculomegaly | No | No | No | Yes | No | ||
| Other | Normal MRI | Minor frontal atrophy | Optic nerve hypoplasia | Periventricular gliosis foci | Normal | ||
| Seizures | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Age at first seizure | 11 months, febrile | 12 months | One seizure only | 16 months | 24 months | ||
| Seizure type | Tonic-clonic | Tonic, tonic-clonic | febrile | Febrile seizures | Atypical febrile, Generalised tonic clonic, absences | ||
| Refractory seizures | Yes | Yes | No | No | No | No | |
| Cardiac abnormalities | No | No | none | No | No | Atrial septal defect | No |
| Renal abnormalities | No | No | none | No | No | No | Vesicoureteral reflux grade 4, surgery |
| Dysmorphism | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Eyes | No | Strabismus | Epicanthic folds | Elongated palpebral fissures | Normal | Upslanting palpebral fissures | Normal, arched eyebrows |
| Nose | No | Prominent nasal bridge | Upturned nose | Flat nasal bridge | Depressed nasal base | Flat nasal bridge | Normal |
| Mouth | Thin upper vermilion | Smooth philtrum, think upper vermilion | Thin upper vermilion | Downturned corners of mouth | Thin upper vermilion | Large | |
| Ear | No | No | Low-set, posteriorly rotated ears | No | Low-set ears with fistula | No | Pretragal tag (right) |
| Forehead | No | No | No | No | No | No | Frontal bossing |
| Other | Single palmar crease, sacral dimple | Drooling, short 4/5 metacarpals, rhizomelic shortening | Bilateral 2/3 syndactyly | Hirsutism, broad thumb | Valgus knees and feet | Broad thumbs | |
| Other clinical features | Hypermetropia, VSDbilateral undescended testes | Hand wringing, bruxism | Drooling, recurrent urinary tract infections, short stature, cyclical vomiting, squint | Type 1 diabetes mellitus, Barrett’s oesophagus, bilateral undescended testes | Stereotypical hand movements and poor eye contact | Scoliosis, pes planus, cold feet | Partial growth hormone deficiency. Short stature. Respond to treatment with growth hormone |
| Tests performed in past | Epilepsy targeted panel | DDD Trio WES | DDD Trio WES | DDD Trio WES | |||
| Karyotyping | No | Yes | Yes | Yes | Yes | Yes | No |
| Array | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Single gene tests | No | Yes | Yes - UBE3A & Angelman testing | Yes | No | Yes | No (had fragile X) |
| Metabolic testing | No | No | No | No | No | No | Yes |
| Muscle biopsy | No | No | No | No | No | No | No |
| Other | SHOX MLPA | ||||||
| Current medical treatment | Levetiracetam | Sodium Valproate, Lamotrigine | Growth hormone injections | Valproate | No treatment at present | Valproate/ Clobazam | |
CpG methylation profile
We identified 227 HNRNPU specific methylation episignature with an adjusted p-value (p < 0.01) and a methylation difference between controls (n = 64) and HNRNPU group (n = 7) of more than 20% (Fig. 1). The principal component analysis (PCA) of unsupervised clustering revealed that HNRNPU group samples are distinguished from healthy controls based on their methylation episignature (Fig. 1b). More specifically, by analysing the methylation beta-values of patients with the mean values of ±3 standard deviation (3 SD) confidence interval from healthy individuals, a significant gain or loss of methylation was observed (see Fig. 1c) with a slightly higher level of hypermethylation patterns across DMRs (227 DMRs of 7 samples (nDMP=1,589)) which showed 17.6% hypermethylated, 2.08% hypomethylated. Moreover, patient 1 (de novo del in exon 1–11), patient 2 (de novo c.847_857del, p.(Phe283Serfs*5)), and patient 6 (c.706_707del, p.(Glu236Thrfs*6)) exhibited similar hypermethylated patterns in 2 CpG islands (Grch37:chr8:145749856-145750410 and Grch37:chr16:89632593-89632799) while milder hypermethylated signatures observed in other patients; patient 3 (de novo c.23_24delTAinsA, p.(Val8Glufs*4)), patient 4 (de novo c.1450 C > T, p.(Arg484*)), patient 5 (de novo c.1624dup p.(Gln542Pro*8)), and patient 7 (c.2365 C > T p.(Arg789*)) based on hierarchical clustering in Fig. 1a.
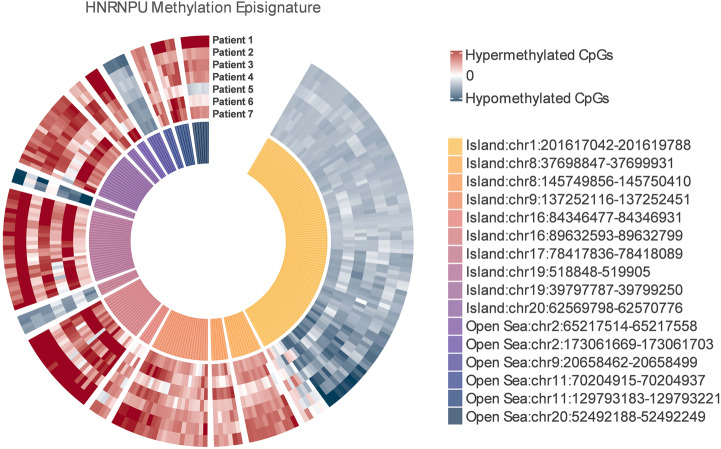
Out of 227 DMPs, 16 DMBs including 10 CpG islands and 6 Open Sea regions were identified as significant (Fig. 2). Based on normalised beta-values (normalised value by mean control value [(β-value sample − β-valuecontrol_mean)/σ]), 12 DMBs were found to be hypermethylated and 4 DMBs were hypomethylated. Genes associated with hypomethylated CpGs are NAV1, LRFN1, FOCAD and those related to hypermethylated DMBs are ADGRA2, LRRC14, LRRC24, RXRA, WFDC1, TPGS1, LINC02245, SLC1A4, PPFIA1, PRDM10. Two of these genes have been linked with human disease previously, biallelic germline pathogenic variants in SLC1A4 were reported to cause an autosomal recessively inherited disorder characterised by spastic tetraplegia, thin corpus callosum and progressive microcephaly (MIM 616657) [28–30] and compound heterozygous or homozygous mutations in FOCAD were associated with severe congenital liver disease (MIM 619991) [31].
Fig. 2. Genomic location and methylation pattern for significantly altered CpGs in HNRNPU group.
A total of 16 differentially methylated blocks (DMBs) including 10 CpG islands and 6 Open Sea regions were identified as significant among 7 HNRNPU patients. Based on methylation values, 12 DMBs were found to be hypermethylated (Island 2, 3, 4, 5, 6, 8, 10 and Open Sea 1, 2, 4, 5, 6) and 4 DMBs (Island 1, 7, 9 and Open Sea 3) were hypomethylated.
Inspection of hypermethylation/hypomethylation profiles in individual cases (see Fig. 1) showed some variability in the extent of methylation alterations, but there was no obvious relationship apparent between this variability and the type of variant or, for truncating variants, the position of the predicted effect on the HNRNPU gene product.
Discussion
We found evidence of methylation alterations in blood DNA from patients with HNRNPU-associated NDD and this, to our knowledge, is the first report of a methylation episignature for HNRNPU inactivation by a NGS-based assay. Recently, Rooney et al. [32] published DNA methylation signatures for 9 pathogenic/likely pathogenic and 1 VUS HNRNPU variants using data from Illumina EPIC methylation array assay which interrogates fewer CpGs.
The role of members of the heterogeneous nuclear ribonucleoproteins in NDDs (HNRNPH1, HNRNPH2, HNRNPK, HNRNPR and HNRNPU) and cancer (HNRNPA1, HNRNPA2B1, HNRNPC, HNRNPD, HNRNPF, HNRNPK, HNRNPR, and HNRNPU) has been the subject of recent investigations but the relationships between the individual function of disease-associated HNRNPs and the mechanisms of relevant disorders is not well defined [5, 33]. Our finding of a disordered epigenetic state in peripheral blood DNA from patients with pathogenic variants in HNRNPU is consistent with the report of Choufani et al. [16] who described a methylation episignature for HNRNPK-associated NDD in 9 individuals. Whereas Choufani et al. [16] used a methylation bead array platform targeting ~850,000 CpGs for methylation profiling, we used a NGS-based assay targeting >2 M CpGs. The difference in methodology and analytical approaches used by Choufani et al. [16] and us limits the detailed comparison of the methylation episignatures from the two conditions. Whereas Choufani et al. [16] identified 429 statistically significant CpG DMPs in their AKS discovery cohort (n = 6) using a false discovery rate adjusted p-value of 0.05 and a minimum methylation difference of 10%, we employed a p-value of less than 0.01 and a more stringent minimum methylation difference of 20% and identified 227 DMPs in our HNRNPU-NDD cohort (n = 7). However, no overlapping DMBs were found between these 227 DMPs and the 429 CpGs identified from the EPIC array in the HNRNPK-NDD cohort (though in the HNRNPK-associated episignature of the 429 CpGs identified using the EPIC array, only 178 of these CpGs were present within the target regions of the EPIC-NGS analysis). We note that both in our cohort and in the findings from the HNRNPK-AKS cohort studied by Choufani et al. [16], significantly altered DMP events comprised both hypermethylation and hypomethylation alterations (see Fig. 1). However, whereas in our HNRNPU cohort, DMPs showed predominantly hypermethylated DMPs (12/16 DMBs), the episignatures from HNRNPK-AKS from Choufani et. al. indicated more hypomethylated DMPs than hypermethylated DMPs [16].
The differences in methylation profiling platforms between our investigations and those of Rooney et al. [32] limit a direct detailed comparison of the respective results but there are similarities in the overall episignature patterns with both hyper and hypomethylation alterations. For example, whilst we identified 16 differentially methylated blocks (DMBs) (including 10 CpG islands) among 7 HNRNPU patients (12 of which were hypermethylated and 4 were hypomethylated), Rooney et al. [32] identified 18 differentially methylated regions (including 12 CpG islands) with 12 being hypermethylated and 4 hypomethylated. We have previously used EPIC-NGS methodology to identify methylation episignatures in a range of chromatin disorders (e.g., Kabuki syndrome Type 1, KMT2B-DYS28, Luscan-Lumish syndrome (SETD2) and Rabin–Pappas syndrome (SETD2) from healthy controls [17, 26], however a much wider range of NDDs have been studied by methylation array profiling and Rooney et al. [32] compared DNAm patterns in their HNRNPU cohort to 56 other NDDs and identified most overlap between the differentially methylated positions within the episignatures for HNPNPU with those for velocardiofacial syndrome and BAFopathy cohorts.
The analysis of methylation episignatures in chromatin disorders can often inform likely pathogenicity of variants of uncertain significance (VUSs). Candidate pathogenic HNRNPU missense variants are rare [27] and in our cohort all of the patients had a pathogenic loss of function HNRNPU variants, so we were not able to formally confirm the utility of DNAm testing for variant interpretation [1]. Nevertheless, the extent of the significant DMPs for our HNRNPU cohort suggest that episignature profiling will have clinical utility as a predictor for clarifying pathogenicity of HNRNPU VUSs (as described previously for HNRNPK variants [16]). In cases of a suspected chromatin disorder in which a VUS is predicted to be non-pathogenic episignature analysis may suggest another diagnosis or suggest the presence of an undetected pathogenic variant [1]. Thus the differential diagnosis of HNRNPK-NDD includes Kabuki syndrome and comparison of the methylation signatures for these two disorders would enable them to be distinguished by methylome analysis [16]. Indeed we note that in their recent paper Rooney et al. [32] reported (a) a HNRNPU in frame deletion (p.(Glu279del)) which did not demonstrate the HNRNPU episignature and (b) an undiagnosed patient who did demonstrate a HNRNPU DNAm profile suggestive of HNRNPU NDD and was then subsequently found to harbour a candidate pathogenic variant in HNRNPU (c.1720_1722delAAG p.(Lys574del)).
The main differential diagnosis of HNRNPU-related NDD can be wide due to a number of causes associated with developmental impairment- epileptic encephalopathy (DEE). However, the common differentials include Rett and Angelman syndromes [12] Although Rett syndrome is caused by mutations in the methyl-CpG-binding protein 2 (MECP2) it is not associated with a DNA methylation signature and though methylation changes occur in a subset of Angelman syndrome patients, these are generally limited to the imprinted SNURF:TSS-DMR at chromosome 15q11q13 [33, 34]. Therefore, the presence of the relevant DNAm episignature in a child with a clinical suspicion of HNRNPU-NDD would be consistent with this diagnosis rather than any other conditions causing DEE including Rett or Angelman syndrome.
HNRNPU is abundantly expressed in the developing mouse brain and biallelic loss of HNRNPU function was associated with cortical cell death in a genetically-engineered mouse model [35]. Prominent features of HNRNPU-NDD include developmental delay, epileptiform seizures, speech and language impairment and behavioural alterations (e.g., autistic features or aggressiveness). Abnormal brain imaging is common (but the range of anomalies is variable) and cardiac and renal structural defects also occur. Transcriptomic studies in the brains of homozygous and heterozygous HNRNPU-deficient mouse models demonstrated widespread effects on gene expression, particularly in the homozygote mice affecting multiple signalling pathways including synaptogenesis, neuroinflammation and (cell cycle control [36]. Evidence for disordered RNA splicing (a known role of HNRNPU) was detected in HNRNPU mutant mice brain cortex [31]. Though RNA splicing is critical for brain development, our findings suggest that the pathogenesis of HNRNPU-NDD might also be related to disordered epigenetic regulation of gene expression. Epigenomic and transcriptomic analysis of HNRNPU mutant mice might provide further insights into potential disease mechanisms.
Finally, it has been noted previously that rare, apparently healthy, individuals with HNRNPU truncating variants may be found in the gnomAD data set (https://gnomad.broadinstitute.org) [12]. This might reflect a lack of detailed phenotypic information or variability of phenotypic expression. However, methylation episignature analysis of such individuals might provide novel insights into genotype-epigenotype-phenotype relationships.
Supplementary information
Acknowledgements
We thank the patients and families for their participation in this study. The views expressed are those of the authors and not necessarily those of the NHS or Department of Health. We acknowledge support from the NIHR UK Rare Genetic Disease Research Consortium.
Author contributions
MB: conceived study, collated patient samples, clinical data; ERM, SL and MB: wrote manuscript. Methylome analyses and investigation were performed by SL, EO, FR, FD, EM and ERM. EO and ERM provided supervision; All authors contributed to data collection and all authors critically reviewed and edited the manuscript.
Funding
MB is funded by Medical Research Council (MR/V037307/1) academic salary support. The HNRNPU research is funded by the Sheffield Children’s Hospital Charity (CA21001). This research was co-funded by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014 and NIHR203312) and Rosetrees Trust (to EO, SL and ERM). The University of Cambridge has received salary support (ERM) from the NHS in the East of England through the Clinical Academic Reserve.
Data availability
Data available on request from the authors (subject to patient consent).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
HNRNPU-related neurodevelopmental disorder: creating an international registry and natural history study. REC reference: 22/NE/0125 IRAS project ID: 314583 (North East - Newcastle & North Tyneside 2 Research Ethics Committee). Molecular Pathology of Human Genetic Disease, IRAS project ID: 50895 (South Birmingham REC).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eamonn R. Maher, Email: erm1000@medschl.cam.ac.uk
Meena Balasubramanian, Email: m.balasubramanian@sheffield.ac.uk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-023-01422-9.
References
- 1.Sadikovic B, Levy MA, Kerkhof J, Aref-Eshghi E, Schenkel L, Stuart A, et al. Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet Med. 2021;23:1065–74. doi: 10.1038/s41436-020-01096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy MA, McConkey H, Kerkhof J, Barat-Houari M, Bargiacchi S, Biamino E, et al. Novel diagnostic DNA methylation episignatures expand and refine the epigenetic landscapes of Mendelian disorders. HGG Adv. 2022;3:100075. doi: 10.1016/j.xhgg.2021.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romig H, Fackelmayer FO, Renz A, Ramsperger U, Richter A. Characterization of SAF-A, a novel nuclear DNA binding protein from HeLa cells with high affinity for nuclear matrix/scaffold attachment DNA elements. EMBO J. 1992;11:3431–40. doi: 10.1002/j.1460-2075.1992.tb05422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu B, Su S, Patil DP, Liu H, Gan J, Jaffrey SR, et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun. 2018;9:420. doi: 10.1038/s41467-017-02770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillentine MA, Wang T, Hoekzema K, Rosenfeld J, Liu P, Guo H, et al. Rare deleterious mutations of HNRNP genes result in shared neurodevelopmental disorders. Genome Med. 2021;13:63. doi: 10.1186/s13073-021-00870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballif BC, Rosenfeld JA, Traylor R, Theisen A, Bader PI, Ladda RL, et al. High-resolution array CGH defines critical regions and candidate genes for microcephaly, abnormalities of the corpus callosum, and seizure phenotypes in patients with microdeletions of 1q43q44. Hum Genet. 2012;131:145–56. doi: 10.1007/s00439-011-1073-y. [DOI] [PubMed] [Google Scholar]
- 7.Thierry G, Bénéteau C, Pichon O, Flori E, Isidor B, Popelard F, et al. Molecular characterization of 1q44 microdeletion in 11 patients reveals three candidate genes for intellectual disability and seizures. Am J Med Genet A. 2012;158A:1633–40. doi: 10.1002/ajmg.a.35423. [DOI] [PubMed] [Google Scholar]
- 8.Epi4K Consortium; Epilepsy Phenome/Genome Project. De novo mutations in the classic epileptic encephalopathies. Nature. 2013;501:217–21. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Kovel CGF, Brilstra EH, Van Kempen MJA, Van‘t Slot R, Nijman IJ, Afawi Z, et al. Targeted sequencing of 351 candidate genes for epileptic encephalopathy in a large cohort of patients. Mol Genet Genom Med. 2016;4:568–80. doi: 10.1002/mgg3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramswig NC, Lüdecke HJ, Hamdan FF, Altmüller J, Beleggia F, Elcioglu NH, et al. Heterozygous HNRNPU variants cause early onset epilepsy and severe intellectual disability. Hum Genet. 2017;136:821–34. doi: 10.1007/s00439-017-1795-6. [DOI] [PubMed] [Google Scholar]
- 11.Yates TM, Vasudevan PC, Chandler KE, Donnelly DE, Stark Z, Sadedin S, et al. De novo mutations in HNRNPU result in a neurodevelopmental syndrome. Am J Med Genet A. 2017;173:3003–12. doi: 10.1002/ajmg.a.38492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durkin A, Albaba S, Fry AE, Morton JE, Douglas A, Beleza A, et al. Clinical findings of 21 previously unreported probands with HNRNPU-related syndrome and comprehensive literature review. Am J Med Genet A. 2020;182:1637–54. doi: 10.1002/ajmg.a.61599. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Hoekzema K, Vecchio D, Wu H, Sulovari A, Coe BP, et al. Large-scale targeted sequencing identifies risk genes for neurodevelopmental disorders. Nat Commun. 2020;11:4932. doi: 10.1038/s41467-020-18723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choufani S, Cytrynbaum C, Chung BHY, Turinsky AL, Grafodatskaya D, Chen YA, et al. NSD1 mutations generate a genome-wide DNA methylation signature. Nat Commun. 2015;6:10207. doi: 10.1038/ncomms10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher DT, Cytrynbaum C, Turinsky AL, Siu MT, Inbar-Feigenberg M, Mendoza-Londono R, et al. CHARGE and Kabuki syndromes: gene-specific DNA methylation signatures identify epigenetic mechanisms linking these clinically overlapping conditions. Am J Hum Genet. 2017;100:773–88. doi: 10.1016/j.ajhg.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choufani S, McNiven V, Cytrynbaum C, Jangjoo M, Adam MP, Bjornsson HT, et al. An HNRNPK-specific DNA methylation signature makes sense of missense variants and expands the phenotypic spectrum of Au-Kline syndrome. Am J Hum Genet. 2022;109:1867–84. doi: 10.1016/j.ajhg.2022.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Ochoa E, Barwick K, Cif L, Rodger F, Docquier F, et al. Comparison of methylation episignatures in KMT2B- and KMT2D-related human disorders. Epigenomics. 2022;14:537–47. doi: 10.2217/epi-2021-0521. [DOI] [PubMed] [Google Scholar]
- 18.Aref-Eshghi E, Schenkel LC, Lin H, Skinner C, Ainsworth P, Paré G, et al. The defining DNA methylation signature of Kabuki syndrome enables functional assessment of genetic variants of unknown clinical significance. Epigenetics. 2017;12:923–33. doi: 10.1080/15592294.2017.1381807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aref-Eshghi E, Rodenhiser DI, Schenkel LC, Lin H, Skinner C, Ainsworth P, et al. Genomic DNA methylation signatures enable concurrent diagnosis and clinical genetic variant classification in neurodevelopmental syndromes. Am J Hum Genet. 2018;102:156–74. doi: 10.1016/j.ajhg.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aref-Eshghi E, Bourque DK, Kerkhof J, Carere DA, Ainsworth P, Sadikovic B, et al. Genome-wide DNA methylation and RNA analyses enable reclassification of two variants of uncertain significance in a patient with clinical Kabuki syndrome. Hum Mutat. 2019;40:1684–9. doi: 10.1002/humu.23833. [DOI] [PubMed] [Google Scholar]
- 21.Aref-Eshghi E, Kerkhof J, Pedro VP, Barat-Houari M, Ruiz-Pallares N, Andrau JC, et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 Mendelian neurodevelopmental disorders. Am J Hum Genet. 2020;106:356–70. doi: 10.1016/j.ajhg.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciolfi A, Foroutan A, Capuano A, Pedace L, Travaglini L, Pizzi S, et al. Childhood-onset dystonia-causing KMT2B variants result in a distinctive genomic hypermethylation profile. Clin Epigenet. 2021;13:157. doi: 10.1186/s13148-021-01145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada N, Hasegawa Y, Yue M, Hamada T, Nakagawa S, Ogawa Y. Xist Exon 7 contributes to the stable localization of Xist RNA on the inactive X-chromosome. PLoS Genet. 2015;11:e1005430. doi: 10.1371/journal.pgen.1005430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan H, Lv P, Huo X, Wu J, Wang Q, Cheng L, et al. The nuclear matrix protein HNRNPU maintains 3D genome architecture globally in mouse hepatocytes. Genome Res. 2018;28:192–202. doi: 10.1101/gr.224576.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Song D, Zhu B, Wang X. The role of nuclear matrix protein HNRNPU in maintaining the architecture of 3D genome. Semin Cell Dev Biol. 2019;90:161–7. doi: 10.1016/j.semcdb.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Menzies L, Hay E, Ochoa E, Docquier F, Rodger F, et al. Epigenotype-genotype-phenotype correlations in SETD1A and SETD2 chromatin disorders. Hum Mol Genet. 2023;11:ddad079. doi: 10.1093/hmg/ddad079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor J, Spiller M, Ranguin K, Vitobello A, Philippe C, Bruel A, et al. Expanding the phenotype of HNRNPU-related neurodevelopmental disorder with emphasis on seizure phenotype and review of literature. Am J Med Genet A. 2022;188:1497–514. doi: 10.1002/ajmg.a.62677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srour M, Hamdan FF, Gan-Or Z, Labuda D, Nassif C, Oskoui M, et al. A homozygous mutation in SLC1A4 in siblings with severe intellectual disability and microcephaly. Clin Genet. 2015;88:e1–4. doi: 10.1111/cge.12605. [DOI] [PubMed] [Google Scholar]
- 29.Damseh N, Simonin A, Jalas C, Picoraro JA, Shaag A, Cho MT, et al. Mutations in SLC1A4, encoding the brain serine transporter, are associated with developmental delay, microcephaly and hypomyelination. J Med Genet. 2015;52:541–7. doi: 10.1136/jmedgenet-2015-103104. [DOI] [PubMed] [Google Scholar]
- 30.Heimer G, Marek-Yagel D, Eyal E, Barel O, Levi DO, Hoffmann C, et al. SLC1A4 mutations cause a novel disorder of intellectual disability, progressive microcephaly, spasticity and thin corpus callosum. Clin Genet. 2015;88:327–35. doi: 10.1111/cge.12637. [DOI] [PubMed] [Google Scholar]
- 31.Moreno Traspas R, Teoh TS, Wong PM, Maier M, Chia CY, Lay K, et al. Loss of FOCAD, operating via the SKI messenger RNA surveillance pathway, causes a pediatric syndrome with liver cirrhosis. Nat Genet, 2022;54:1214–26. doi: 10.1038/s41588-022-01120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rooney K, van der Laan L, Trajkova S, Haghshenas S, Relator R, Lauffer P, et al. DNA methylation episignature and comparative epigenomic profiling of HNRNPU-related neurodevelopmental disorder. Genet Med. 2023;27:100871. doi: 10.1016/j.gim.2023.100871. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Yang X, Zhang G, Wang L, Zhu Z, Zhang W, et al. Heterogeneous nuclear ribonucleoprotein K promotes the progression of lung cancer by inhibiting the p53-dependent signaling pathway. Thorac Cancer. 2022;13:1311–21. doi: 10.1111/1759-7714.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buiting K, Groß S, Lich C, Gillessen-Kaesbach G, El-Maarri O, Horsthemke B. Epimutations in Prader-Willi and Angelman syndromes: A molecular study of 136 patients with an imprinting defect. Am J Hum Genet. 2003;72:571–7. doi: 10.1086/367926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eggermann T, Perez de Nanclares G, Maher ER, Temple IK, Tümer Z, Monk D, et al. Imprinting disorders: a group of congenital disorders with overlapping patterns of molecular changes affecting imprinted loci. Clin Epigenet. 2015;7:123. doi: 10.1186/s13148-015-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sapir T, Kshirsagar A, Gorelik A, Olender T, Porat Z, Scheffer IE, et al. Heterogeneous nuclear ribonucleoprotein U (HNRNPU) safeguards the developing mouse cortex. Nat Commun. 2022;13:4209. doi: 10.1038/s41467-022-31752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors (subject to patient consent).