Abstract
The human brain choroid plexus (ChP) is a highly organized secretory tissue with a complex vascular system and epithelial layers in the ventricles of the brain. The ChP is the body's principal source of cerebrospinal fluid (CSF); it also functions as a barrier to separate the blood from CSF, because the movement of CSF through the body is pulsatile in nature. Thus far, it has been challenging to recreate the specialized features and dynamics of the ChP in a physiologically relevant microenvironment. In this study, we recapitulated the ChP structure by developing a microfluidic chip in accordance with established design rules. Furthermore, we used image processing and analysis to mimic CSF flow dynamics within a rlcking system; we also used a hydrogel containing laminin to mimic brain extracellular matrix (ECM). Human ChP cells were cultured in the ChP-on-a-chip with in vivo-like CSF dynamic flow and an engineered ECM. The key ChP characteristics of capillaries, the epithelial layer, and secreted components were recreated in the adjusted microenvironment of our human ChP-on-a-chip. The drug screening capabilities of the device were observed through physiologically relevant drug responses from breast cancer cells that had spread in the ChP. ChP immune responses were also recapitulated in this device, as demonstrated by the motility and cytotoxic effects of macrophages, which are the most prevalent immune cells in the ChP. Our human ChP-on-a-chip will facilitate the elucidation of ChP pathophysiology and support the development of therapeutics to treat cancers that have metastasized into the ChP.
Keywords: Choroid plexus, Organ-on-a-chip, Cerebrospinal fluid dynamics, Brain metastasis, Tumor-immune microenvironment
Graphical abstract
Highlights
-
•
Microfluidic platform to model the human brain choroid plexus.
-
•
Key choroid plexus feature recapitulation such as capillaries and epithelial layers.
-
•
Recreation of cerebrospinal fluid flow and brain extracellular matrix.
-
•
Brain metastasis modeling using choroid plexus-on-a-chip with immune system.
-
•
Recreation of anti-cancer drug response and immune reaction in in vivo-like state.
1. Introduction
The human brain choroid plexus (ChP) is a highly organized secretory tissue with a complex vascular system and epithelial layers in the ventricles of the brain [1,2]. The ChP is the body's principal source of cerebrospinal fluid (CSF); it separates blood from CSF, through a function known as the blood-CSF barrier (BCSFB) [3]. The composition of CSF differs from the composition of plasma in that CSF contains lower levels of calcium, glucose, and proteins [4]. CSF also plays key roles in providing buoyant physical protection for the brain and facilitating the development of an epithelial–endothelial convolute by circulation through the cerebral aqueduct [5,6]. The movement of CSF through the body is pulsatile in nature, driven primarily by the cardiac cycle [[7], [8], [9]]. Under this unique dynamic stimulation, the ChP exhibits a specialized structure in which epithelial cells joined at tight junctions form an outer epithelial layer that surrounds fenestrated capillaries with low expression levels of tight junction proteins [1,10]. The vasculature of the ChP differs from the vasculature of the blood-brain barrier (BBB), where high expression of tight junctions is elicited by critical extracellular matrix (ECM) factors such as laminin and other stromal cells (e.g., pericytes) [[11], [12], [13]]. Importantly, laminin and brain stromal cells help to enhance the expression of occludin in the BBB, where ChP capillaries exhibits low occludin expression [10,[14], [15], [16], [17]]. Metastasis (i.e., leptomeningeal metastasis [LM]) can occur within this specialized contruct. Breast cancer cells are among the most common tumors that metastasize to the central nervous system; the reported incidence of LM occurring in breast cancer is reported to be approximately 5% [18]. Because tumor cells require an adequate blood supply to grow and develop a lesion after brain metastasis, they often spread into ChP capillaries [19,20]. Macrophages, the most prevalent population of immune cells in the ChP, support cancer growth by promoting invasion and immunosuppression rather than anti-tumor responses [21,22]. For example, the expression of human epidermal growth factor receptor 2 (HER2) in cancer cells can allow upregulation of macrophage motility while promoting tumor proliferation [[23], [24], [25]], such that cancer cellular phenotypes (e.g., HER2 expression) can be tuned via dynamic flow [26]. The HER2-targeting drug trastuzumab is typically used as a form of intrathecal therapy in the treatment of disseminated breast cancer cells [27,28]. Its anti-cancer efficacy can be enhanced through the synergetic effect obtained by introducing both trastuzumab and macrophages into the system [29].
The development of the ChP progresses under dynamic CSF flow, such that dynamic conditions affect its physiology [1,30]. Moreover, dynamic ECM factors (e.g., laminin) play key roles in determining the characteristics of the BCSFB and capillaries in the ChP [31]. In addition to ChP physiology, the dynamic conditions can significantly affect the tumor microenvironment (TME), including its immune system [[32], [33], [34], [35], [36]]. The effects of CSF flow dynamics on drug responses in the brain are also regarded as critical factors, but they remain unexplored [37]. Notably, a clinical study demonstrated that dynamic CSF flow can affect patient responses to intrathecal chemotherapy for tumors [38]. Therefore, analyses of the physiology and pathophysiology of the human ChP, as well as drug tests for the treatment of disorders in the ChP, require recreation of this dynamic microenvironment in preclinical models.
A lack of disease models for these features has hindered drug testing in the ChP very difficult. Drugs targeting human meningeal diseases have failed in animal models because of disparities between humans and animals [[39], [40], [41], [42]]. Two-dimensional cell culture of the ChP has limited translatability because it lacks the complexity of three-dimensional (3D) tissue structures [43]. Similarly, the specialized features of the ChP have hindered studies of its physiology. Further understanding of central nervous system barriers, including the ChP, is necessary for therapeutic development [44]. However, it remains challenging to recapitulate the specialized architecture and dynamics of the ChP with a physiologically relevant microenvironment. To address the limitations of conventional preclinical models, several in vitro models have been developed to recapitulate the complexity and specialized structure of the ChP. Recently, Pellegrini et al. developed a ChP organoid that recapitulated the BCSFB properties and successfully produced CSF; they also reproduced an in vivo-like drug response in ChP organoids that was similar to the response observed in clinical trials [45]. Although the organoid model was able to recreate the critical characteristics of the ChP barrier, it lacked a vascular system and immune microenvironment, which are critical components of its physiology. Moreover, this organoid model did not exhibit in vivo-like CSF dynamic stimulation, which may have a major role in ChP physiology. Another group reconstructed a neurovascular unit under the influence of CSF using organ-on-a-chip technology, which yielded the complex 3D structure [46]. This neurovascular-unit-on-a-chip represented BBB contact with brain parenchyma and CSF channels; it was used to investigate metastatic behavior in breast cancer cells. However, despite the inclusion of a CSF channel, this microfluidic model was not able to recreate a capillary–epithelium structure in the ChP with brain-specific ECM and CSF dynamics. Moreover, no immune system was constructed, and anti-cancer drug testing was not conducted in this model. Few in vitro models have successfully recreated ChP structure with critical ECM factors and CSF flow dynamics. A better understanding of ChP physiology and pathophysiology (e.g., cancer metastasis and immune responses) requires recapitulation of these critical components in preclinical models of the ChP [43].
Here, we developed a human ChP-on-a-chip with recreated ChP architecture and CSF dynamics, which can be used as a model of brain metastasis for drug screening and immune response analysis. This platform utilizes open microfluidic patterning to enable the simple construction of a complex multilayer consisting of a ChP vascular network and epithelial layer, in accordance with established design rules. The ECM was engineered using laminin, which is a critical ECM component for the construction of endothelial and epithelial barriers in the brain. The ChP reconstructed in this platform was subjected to dynamic flow that physiologically resembles in vivo CSF flow. This pulsatile CSF flow was reproduced using a rocking system; its fluid dynamics were analyzed by image processing and computer simulation to facilitate the recreation of human CSF flow dynamics. Our analysis confirmed that the unique oscillatory motion of the device was similar to the motion of CSF, including an in vivo-like stroke volume, flow rate, and frequency. In our platform, we demonstrated high expression of tight junctions in neurovasculature when vessels were co-cultured with pericytes in the engineered ECM, as expected in brain ECM. However, the introduction of dynamic CSF fluid flow led to significant downregulation of tight junction expression in the neurovasculature, consistent with ChP capillaries. Moreover, epithelial cells lining the vasculature exhibited ciliogenesis, an increased cellular coverage area, and increased tight junction expression upon exposure to recapitulated CSF flow. Enzymatic analysis of the established co-culture was conducted, after exposure to dynamic flow; the levels of calcium, glucose, and protein were consistent with previous findings in CSF. To validate the use of our human ChP-on-a-chip as a drug screening platform, we reconstructed the TME in the ChP by spreading HER2-overexpressing breast cancer cells (i.e., SKBR3 cells) in the platform, followed by treatment with a typical intrathecal anti-HER2 therapy [[47], [48], [49]]. Under dynamic flow, HER-2 overexpression in cancer cells was maintained; the anti-cancer effect of anti-HER2 therapy was greater than the effect under static conditions. To recreate a TME with an immune system, macrophages were introduced into the platform; their motility was upregulated and their cytotoxic effects on cancer cells were significantly reduced under dynamic flow, compared with static conditions. When the TME with macrophages was exposed to trastuzumab under dynamic conditions, the anti-cancer effect was much greater than the effects of those under static conditions.
Overall, our microfluidic chip with a reconstituted ChP under in vivo-like CSF flow exhibited a physiologically relevant tumor-immune microenvironment, as well as characteristics of the ChP. Our human ChP-on-a-chip with recapitulated CSF dynamics can serve as a powerful tool for better understanding of human ChP pathophysiology (including the role of the immune system), and it will contribute to the development of therapies for cancers related to LM.
2. Materials and methods
2.1. Device design and fabrication
In this research, we designed prototype designs using SolidWorks (Dassault Systèmes), a solid modeling computer-aided design (CAD) program. The prototyped device was then fabricated using a high-resolution DLP 3D printer (Fig. 3, Fig. 4D Systems). The resin used was MED-AMB 10 (3D Systems), a rigid bio-compatible resin capable of meeting ISO 10993-5 and −10 standards for biocompatibility (cytotoxicity, sensitization and irritation). The fabricated device was washed with isopropanol for 10 min and cured in a UV chamber for 60 min. Single-sided PSA (Pressure Sensitive Adhesive) film (IS-00820, IS Solution, Korea) was bonded to the bottom of the device to enclose the device.
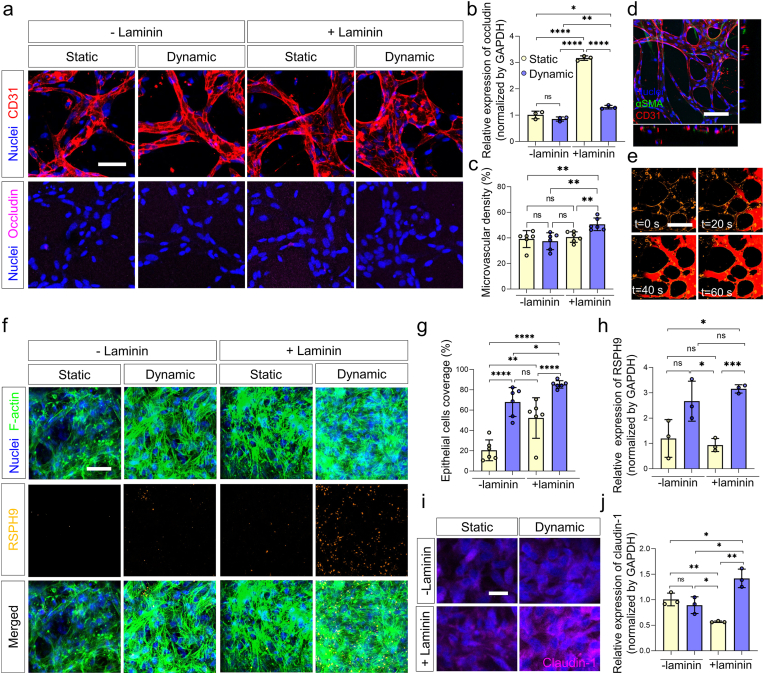
Fig. 3.
In vivo-like choroid plexus capillaries and epithelium as a blood-CSF barrier (BCSFB) in the engineered microenvironment to reconstruct the human ChP. a Confocal images of immunostainned nuclei (blue), CD31 (red), and occludin (magenta) expressed on the microvessels cultured in each condition (scale bar: 50 μm). b RT-qPCR results of occludin expressed in 3D cell cultured HBMECs and HBVPs in each cell culture condition (-laminin, +laminin, static, and dynamic) (n = 3 for each condition, *p < 0.05, **p < 0.01, and ****p < 0.0001 by student t-test). c Microvascular density of microvessels cultured in each culture condition (n = 6 for each condition, **p < 0.01 by student t-test). d Representative confocal image of nuclei (blue), αSMA (green), CD31 (red) expressed on the in vivo-like ChP capillaries (scale bar: 100 μm). e Beads flow in the perfusable microvessels cultured in the engineered microenvironment (blood vessels, yellow; 0.2 μm microbeads, red). f Immunostaining of f-actin (green), FoxJ1 (red), RSPH9 (yellow) expressed on HCPEpiCs cultured in the ChP epithelium-on-a-chip in each culture condition. (scale bar: 50 μm) g Quantification of the coverage of the HCPEpiCs (n = 6 for each condition, *p < 0.05, **p < 0.01, and ****p < 0.0001 by student t-test). h qRT-PCR results RSPH9 expressed on the epithelium by varying ECM and flow conditions (n = 3 for each condition, *p < 0.05 and ***p < 0.001 by student t-test). i-j (i) qRT-PCR results (n = 3 for each condition, *p < 0.05, and **p < 0.01 by student t-test) and (j) confocal images of nuclei (blue) and claudin-1 (magenta) expressed on the epithelium by varying ECM and flow conditions (lam: laminin) (scale bar: 20 μm). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
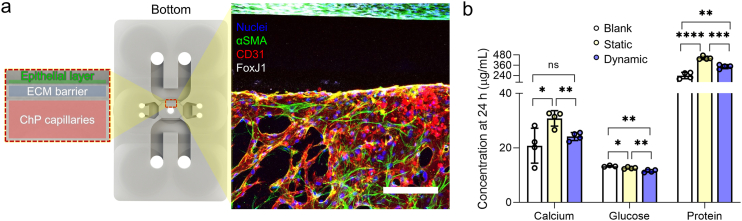
Fig. 4.
The ChP capillaries-epithelium complex cultured and the level of factors from the complex in each condition. a Confocal microscopy image (right, scale bar: 200 μm) of nuclei (blue), αSMA (green), CD31 (red), and FoxJ1 (grey) when the ChP capillaries-epithelium complex was cultured in the engineered ECM under the dynamic flow with the described assembly in the ChP-on-a-chip (left). b Quantification of calcium, glucose, and protein concentrations in the serum-free media extracted in static and dynamic culture conditions, respectively (n = 3 for control in glucose and n = 4 for other conditions, respectively, *p < 0.05, **p < 0.01, ***p < 0.001and ****p < 0.0001 by student t-test). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Design rules of open microfluidic patterning
Prior to liquid patterning, the 3D printed chips were irradiated with oxygen plasma using a plasma treatment system (Femto Science, Korea) at 70 W for 1 min to create a hydrophilic surface. 2 μL of bovine fibrinogen solution (Sigma, USA; 2.5 mg mL−1) mixed with bovine thrombin (Sigma, USA; 1 U mL−1) was injected into the central channel through the patterning hole. The gel was allowed to crosslink for 10 min, and then the diameter of the gel surface was measured after contraction.
2.3. Cell preparation
Primary human brain microvascular endothelial cells (HBMEC; Cell systems; # ACBRI 376) at passage 4–5 were cultured in flasks coated with 50 μgmL−1 fibronectin (Sigma-Aldrich) using endothelial cell medium (Sciencell; #1001). Human brain vascular pericytes (HBVP; Sciencell; #1200) at passage 3–4 were maintained in 1 mg mL−1 poly-l-lysine (PLL, Sigma-Aldrich) coated flasks using pericyte medium (Sciencell; #1201). Human choroid plexus epithelial cells (HCPEpiC; Sciencell; #1310) at passage 3 were cultured in flasks coated with 1 mg mL−1 poly-l-lysine (PLL, Sigma-Aldrich) using epithelial cell medium (Sciencell; #4101). Fibronectin and PLL coating procedures were achieved following the manufacturer's instruction. The cells were maintained in an incubator at 37 °C in 5% CO2 for 3 days. SKBR3 and MCF7 cells were obtained from Korean cell line bank (KCLB, Korea) and cultured in Dulbecco's Modified Eagle's Medium (DMEM) including 10% fetal bovine serum (HyClone, USA) and 1% penicillin–streptomycin (Gibco, USA). THP-1 cells (a human leukemia monocytic cell line) were cultured in Roswell Park Memorial Institute Medium-1640 (RPMI-1640) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 0.05 nM 2-Mercaptoethanol. The culture medium was changed every 2–3 days, and the cells were resuspended at a density of 0.3 × 106 cells mL−1 for every media change. THP-1 cells were then seeded in 6-well plates at a density of 1 × 106 cells/well and treated with 100 nM phorbol 12-myristate 13-acetate (PMA). After 48 h, only adherent cells were collected using 0.25% Trypsin-EDTA to be used for the tumor cell killing assay.
2.4. Construction of the human ChP chip system
Prior to liquid patterning, the 3D printed chips were irradiated with oxygen plasma using a plasma treatment system (Femto Science, Korea) at 70 W for 1 min to create a hydrophilic surface. A 50 μL mixture of suspension with 8 × 106 cells mL−1 of HBMECs and HBVPs (HBMECs: HBVPs = 4:1, volume ratio), bovine fibrinogen solution (Sigma, USA; 2.5 mg mL−1), and laminin from human placenta (Sigma, USA; 0.1 mg mL−1) with 1 μL of bovine thrombin (Sigma, USA; 1 U mL−1) was prepared. The 1.8 μL of mixture was used to fill each central microchannel by injecting it through the central hole. After allowing 10 min for the patterned mixture to cross-link at room temperature, the ECM with high concentration bovine fibrinogen solution (10 mg mL−1) was injected into the flow channels through their respective entry holes and aspirated to leave a small gel barrier in the lower channels next to the central channel, where the fibrin gel serves as a barrier between capillaries and epithelium cells. Endothelial cell medium supplemented with vascular endothelial growth factor (VEGF; Peprotech; 50 ng mL−1), fibroblast growth factor (FGF; Gibco; 20 ng mL−1), epidermal growth factor (EGF; Peprotech; 20 ng mL−1) was prepared, and 200 μL of the medium was loaded in the chip reservoirs after 10 min of cross-linking at room temperature. The endothelial cells were patterned to form perfusable blood vessels by injecting 10 μL of suspension with 5 × 106 cells mL−1 HBMECs and HBVPs (HBMECs:HBVPs = 4:1, volume ratio) in the side channels and tilting the device by 90° for 30 min in an incubator at 37 °C in 5% CO2 to allow the cells to adhere on the gel surface in the central channels. After 6 days culturing the HBMECs and HBVPs in an incubator at 37 °C in 5% CO2, 20 μL of cell suspension with 5 × 106 cells mL−1 HCPEpiCs was loaded in each flow channel and the chip was tilted by 90° for 30 min in an incubator at 37 °C in 5% CO2 to allow the cells to adhere on the gel surface in the lower channels of the flow channels to construct the ChP epithelium. The cocktail medium was changed daily. After 24 h to stabilize the epithelium, each reservoir of the flow channels was filled with 150 μL of the serum-free medium and the in vivo-like CSF flow was applied to the microfluidic chip by loading the platform on the rocker and culturing the system for 24 h in the incubator (Supplementary video S1).
2.5. Construction of the ChP epithelium-on-a-chip system
Prior to filling the microchannels, the adhesive films were irradiated with oxygen plasma using a plasma treatment system (Femto Science, Korea) at 70 W for 20 s to convert the hydrophobic film surface to hydrophilic, which were then adhered to the 3D printed chips. A 50 μL mixture of bovine fibrinogen solution (Sigma, USA; 2.5 mg mL−1) and laminin from human placenta (Sigma, USA; 0.1 mg mL−1) with 1 μL of bovine thrombin (Sigma, USA; 1 U mL−1) was prepared. The 15 μL mixture was injected into the bottom microchannels through the injection holes. After allowing 10 min for the patterned mixture to cross-link at room temperature, a 20 μL suspension with 5 × 106 cells mL−1 HCPEpiCs was loaded in each top channel and the chip was filled with media after 30 min in an incubator at 37 °C in 5% CO2 to allow the cells to adhere on the gel surface in the bottom channels to construct the ChP epithelium. The medium was changed daily. After 24 h to stabilize the epithelium, each reservoir was filled with 150 μL of the serum-free medium and the in vivo-like CSF flow was applied to the microfluidic chip by loading the platform on the rocker and culturing the system for 24 h in an incubator at 37 °C in 5% CO2 (Supplementary video S1).
2.6. Construction of the TME in human ChP chip system
Prior to filling the microchannels, the 3D printed chips were irradiated with oxygen plasma using a plasma treatment system (Femto Science, Korea) at 70 W for 1 min to convert the hydrophobic surface to hydrophilic. Prior to loading cells in the microfluidic chip, SKBR3 and MCF7 cells were labeled with CellTraceTM CFSE (1:1000; Invitrogen, USA). 50 μL mixture of suspension with 8 × 106 cells mL−1 of HBMECs, HBVPs, cancer cells (HBMECs:HBVPs:cancer cells = 8:2:1 vol ratio), bovine fibrinogen solution (Sigma, USA; 2.5 mg mL−1), and laminin from human placenta (Sigma, USA; 0.1 mg mL−1) with 1 μL of bovine thrombin (Sigma, USA; 1 U mL−1) was prepared. The 1.8 μL of mixture was introduced into each central microchannel by injecting it through the central hole. After allowing 10 min for the patterned mixture to cross-link at room temperature, the ECM with high concentration bovine fibrinogen solution (10 mg mL−1) was injected into the flow channels through their respective entry holes and aspirated to leave a small gel barrier in the lower channels next to the central channel, where the fibrin gel serves as a barrier between capillaries and epithelium cells. 200 μL of the medium was loaded in reservoirs after 10 min for cross-linking at room temperature. The endothelial cells were patterned to form perfusable blood vessels by injecting 10 μL suspension with 5 × 106 cells mL−1 HBMECs and HBVPs (HBMECs:HBVPs = 4:1, volume ratio) in the side channels and tilting the device by 90° for 30 min in an incubator at 37 °C in 5% CO2 to allow the cells to adhere on the gel surface in the central channels. After 4 days culturing the cells in an incubator at 37 °C in 5% CO2, 20 μL suspension with 5 × 106 cells mL−1 HCPEpiCs was loaded in each flow channel and the chip was tilted by 90° for 30min in an incubator at 37 °C in 5% CO2 to allow the cells to adhere on the gel surface in the lower channels of the flow channels to construct the ChP epithelium. After 24 h to stabilize the epithelium, each reservoir of the flow channels was filled with 150 μL of the serum-free medium and the in vivo-like CSF flow was applied to the microfluidic chip by loading the platform on the rocker and culturing the system for 24 h in an incubator at 37 °C in 5% CO2 (Supplementary video S1).
2.7. Image processing
An image pre-processing and an analyzing algorithm was developed using Python (3.7.0) and the OpenCV library. A live video was taken of the rocking motion of the microfluidic platform using a 60 frame rate camera and each video was taken for 20–25 s. The video was taken from the ground level for each shot, meaning the camera was fixed parallel to the bottom of the rocking panel in order to take videos with the platform and reservoirs in a stationary position. This was necessary in order to outline each reservoir and isolate the media movement in the area calculation process (Supplementary video S2). Around 1200–1500 images were obtained from each video. The platform was filled with magenta-colored media (DMEM) which has a similar viscosity as cell media to allow for identifying and marking the media in the images to create a mask (Supplementary video S3). A color mask (HSV threshold (56,52,0)) was implemented as an image pre-processing filter for all frames. The images were filtered using the mask, and the areas in the two media reservoirs filled with media were isolated. In order to quantify the area filled with media, we developed an image analysis algorithm for real-time volume tracking and OpenCV-based contour detecting. Continuous measurements from multiple frames were used to measure flow rate by calculating the difference in area between the two reservoirs. Total volume error was also measured and calculated to verify that the measurements were reliable. Finally, we plotted a time-flow rate graph for each condition.
2.8. Computational fluid dynamics
COMSOL software (COMSOL Multiphysics 5.5) was used to evaluate the channel wall and interior shear stress under a varying flowrate condition. A rectangular channel with dimensions of 2.3 mm length × 1 mm width × 2 mm height was modeled with a fluid with the properties of water flowing inside the inlet. The simulation estimated the velocity fields U inside the mesh by solving the Navier-Stokes equation with continuity, assuming no-slip boundary conditions in stationary mode. Finally, shear stress was obtained considering the shear rate calculated as dU/dz multiplied by the dynamic viscosity of the fluid μ. The flowrate data was imported into COMSOL from a spreadsheet using piecewise cubic interpolation. The varying flowrate condition was fixed at the inlet and the other port was set as an open boundary without any normal stress. We assumed laminar flow considering the Reynolds number calculated to be less than 100 as a reasonable assumption. Other assumptions considered included Newtonian behavior of the fluid, water dynamic viscosity, and the presence of gravity.
2.9. Real-time qRT-PCR
The samples were treated with TRIzol reagent (Invitrogen, USA) to extract total RNA. The isolated RNA, after quantification in Epoch Microplate Spectrometer (Agilent Technologies, USA), was reverse transcribed with TOPscript™ cDNA Synthesis Kit (Enzynomics, Republic of Korea). Later, qPCR was conducted using primers obtained from prior publications (Table 1) and SYBR Green TOPreal™ qPCR 2X PreMIX (Enzynomics, Republic of Korea) in Quantstudio 3 Real-Time PCR instrument (Applied Biosystems, USA). Both cDNA synthesis and qPCR were conducted according to the manufacturer's instructions. The relative expression level of each target gene was calculated using the comparative 2−ΔΔCT method. GAPDH was taken as the reference gene.
Table 1.
Primers used for qRT-PCR.
| Genes | Forward sequence | Reverse sequence |
|---|---|---|
| GAPDH | CGCTGAGTACGTCGTGGAGT | AGAGGGGGCAGAGATGATG |
| Occludin | CGGCAATGAAACAAAAGGCAG | GGCTATGGTTATGGCTATGGCTAC |
| Claudin-1 | GTCTTTGACTCCTTGCTGAATCTG | CACCTCATCGTCTTCCAAGCAC |
| RSPH9 | ATGCTGGTTAAGCGCGACTAC | TTCACCACAGACGACTGCG |
2.10. Media components quantification
The supernatants were collected and frozen at −80 °C until used. Glucose and calcium concentrations in the medium were measured using enzymatic assay kits EIAGLUC from ThermoFisher Scientific for glucose, and MAK022 from Sigma for calcium detection. The assays were conducted following the manufacturer's instructions.
2.11. Preparation for drug treatment
Trastuzumab (Selleckchem, USA) was diluted into 10 μg mL−1 with the serum-free endothelial cell medium and applied to the flow channel reservoirs. The concentration was determined by referring to the reported in vitro inhibitory concentration of trastuzumab [50].
2.12. Live imaging and live/dead assay
Macrophages were labeled with Far Red (1:1000; Invitrogen, USA), prior to live-cell imaging. Blood vessels were stained with fluorescein-conjugated Ulex Europaeus Agglutinin 1 (1:2000; Vector, USA), and dead cells were labeled by adding SYTOXTM Blue (1:1000; Invitrogen, USA) to the culture medium immediately before imaging. Macrophages at a density of 4 × 106 cells mL−1 were attached to both sides of the central channel by tilting the device and incubated for at least 30 min. The motility and cytotoxic activities of macrophages were then monitored for 16 h with a Nikon Eclipse Ti2-E inverted microscope under 5% CO2 at 37 °C.
2.13. Immunocytochemistry
Immunofluorescence imaging was performed to visualize cell-specific marker expression. The samples were fixed with 4% (w/v) PFA (Santa Cruz Biotechnology, San Diego, CA, USA) in PBS (Gibco, USA) for 15 min at RT, followed by permeabilization in 0.1% Triton X-100 (Sigma-Aldrich) for 15 min. The samples were then treated with 3% bovine serum albumin (BSA; Sigma-Aldrich) for 40 min. The following antibodies were used for immunocytochemistry and were incubated: AlexaFluor 594 conjugated mouse anti-CD31 (1:200; BioLegend), AlexaFluor 647 conjugated mouse anti-CD31 (1:200; BioLegend), AlexaFluor 488 conjugated mouse anti-α-SMA (1:200; R&D systems), AlexaFluor 594 conjugated mouse anti-occludin (1:200; Invitrogen), mouse anti-FoxJ1 (1:200, Invitrogen), rabbit anti-RSPH9 (1:200, Sigma), goat anti-CD163 (1:200, R&D systems), and Hoechst 33342 (1:1000; Invitrogen). Also, Alexa Fluor 488 and 594 Phalloidin (1:200, Invitrogen) was used to stain f-actin. They were all treated for 2 days at 4 °C. The samples were then incubated with fluorescence conjugated secondary antibodies: goat anti-rabbit AlexaFluor 647 (1:200; Invitrogen), goat anti-mouse AlexaFluor 594 (1:200; Invitrogen), and donkey anti-goat AlexaFluor 633 (1:200; Invitrogen) for overnight at 4 °C for the target visualization. Fluorescently visualized samples were examined using a Ti2-Eclipse inverted microscope with NIS elements software (Nikon, Japan).
2.14. Statistical image analysis
Fiji (http://fiji.sc.), open access software, was used to analyze confocal images of each target visualization. Confocal 3D images were converted to 2D image by z-projection. The images for the area measurement were then converted into binary masked images by applying identical threshold conditions. Finally, the area of the visualized targets including microvascular density, epithelial cells coverage, and cilia formation was measured using Fiji directly. To quantify cell viability based on the z-projection confocal images, the number of cancer cells and dead cells were counted using the Fiji Cell Counter plugin. Macrophage movement was tracked using the Chemotaxis and Migration tools (https://ibidi.com/). All statistical analyses included unpaired student's t-tests to obtain statistical comparisons of analyzed values. The p value thresholds for statistical significance were set and represented in the graph as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
3. Results and discussion
3.1. Human ChP model with in vivo-like physical traits
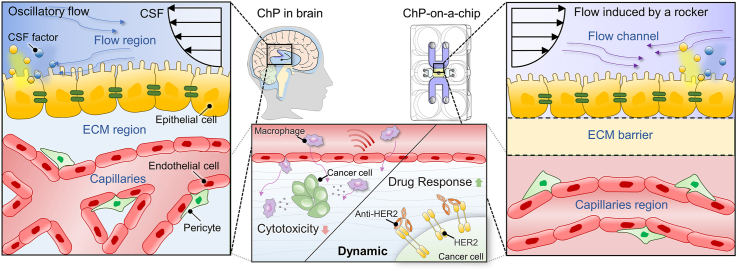
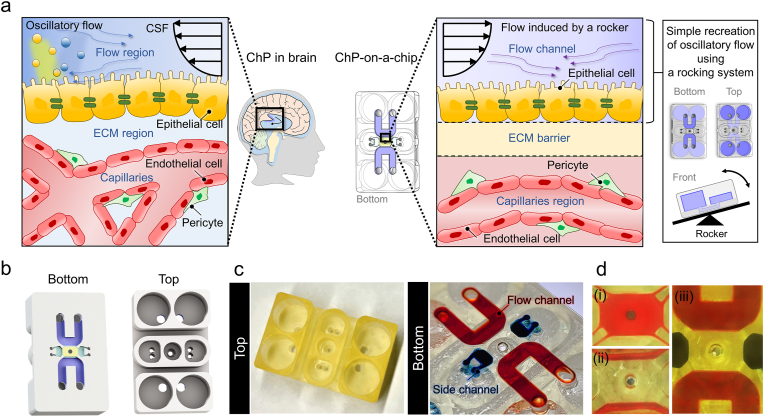
Our microengineered human ChP model reconstitutes a complex multilayer structure with ChP capillaries and epithelium, which enables drug testing and immune reaction analysis of breast cancer cells spread into the ChP system (Fig. 1a). An in vivo-like CSF dynamic flow and engineered brain-specific ECM was integrated to recapitulate ChP physiology (Fig. 1a). The microfluidic platform consists of one central channel, two microchannels for gel loading, two side channels, and four media reservoirs (Fig. 1b–d). The central channel of the platform mimics the ChP vascular system, and the microchannels for gel loading function as an ECM barrier to separate the capillaries and epithelial cell layer. Two flow channels provide routes to inject ChP epithelial cells for the formation of an epithelium layer, and the channels also function as pathways for in vivo-like CSF flow. The side channels serve as a lane for patterning cells such as endothelial cells to create a perfusable vascular network or macrophages for other assays. The sequence of microchannel patterning is indicated in Fig. 1d.
Fig. 1.
Engineered human choroid plexus (ChP)-on-a-chip to reconstitute ChP physiology under CSF flow. a Schematic description of the 3D microfluidic chip to recapitulate the human ChP in the brain and TME in the ChP by engineering in vivo-like cerebrospinal fluid (CSF) flow dynamics. b, c (b) Schematic and (c) photograph of the top and bottom of the human ChP-on-a-chip. d Sequential steps for the microfluidic patterning; (i) Fill the central channel with a hydrogel mixed with the cell suspension by capillary-guided fluid patterning and wait for polymerization. (ii) Fill and withdraw a blank hydrogel through an injection hole of the flow channel to make the hydrogel remain only underneath the sides of the central channel as a blank ECM zone to separate the flow channels and central channels. And wait for polymerization. (iii) Fill the flow channels and the side channels with cell suspension or media.
The dimensions of the central channel and the geometry of the microposts were established by design rules meant to avoid hydrogel constriction, which can lead to bubble formation on the sides of the central channel (Fig. S1). These design rules allowed successful micropatterning of multi-microchannels without any bubble traps. We confirm that our device with ChP-relevant architecture allowed the successful recapitulation of human in vivo ChP and its physiological features.
3.2. CSF fluid flow analysis
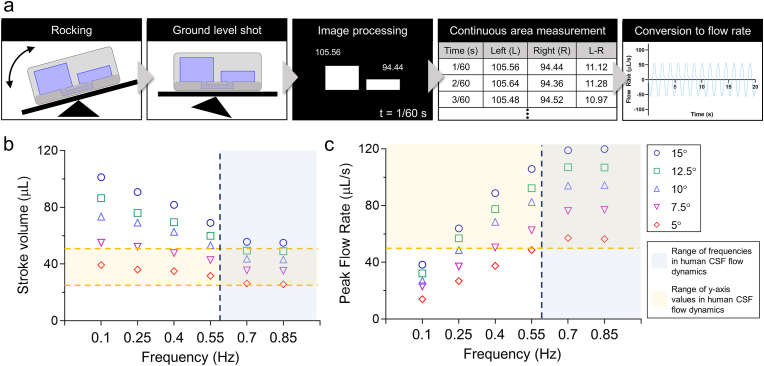
The pulsatile flow of the CSF in the ventricles of the brain is predominantly induced by the cardiac cycle [[7], [8], [9]]. The stroke volume of this distinctive CSF movement in the cerebral aqueduct ranged from 30 to 50 μL [[51], [52], [53], [54]], where stroke volume is the net volume of fluid pulsating back and forth [55,56]. Peak flow rate, another typical MRI-derived amplitude measure, ranged from 50 to 150 μL s−1 [57]. Another key value measured in CSF circulation is frequency, where the range varied from 0.008 Hz to the frequency of respiration which can be as high as 0.7 Hz [58,59]. To measure the parameters of CSF flow, we developed a real-time flow measuring system where the dynamic flow within a microfluidic platform was analyzed using image processing technology (Fig. 2a). Our image processing system utilized color masking and contour detecting steps for real-time volume tracking while calculating total volume error for reliable data extraction (Fig. S2, Supplementary video S1 – S3). We extracted 30 time - flow rate plots with stroke volume and peak flow rate while varying the frequency and tilting angle of the rocking system (frequency: 0.1–0.85 Hz, angle: 5–15°) (Fig. 2b, c, S3, and S4). To verify the accuracy of the extracted values from image processing, we compared the extracted values with actual liquid measurements from inside the media reservoir. The actual measurements were done by collecting and measuring the liquid in the device's reservoir when the platform was in the most tilted state under the lowest frequency setting, 0.1 Hz. These values were compared with the calculated values from image processing with which there was little disparity (Fig. S5). Once the flow frequencies were obtained, they were compared to in vivo CSF flow frequencies in order to choose an appropriate value. In vivo CSF flow is mainly affected by the cardiac cycle, and the frequency of normal brain CSF flow is within the 0.6–1.2 Hz frequency range [[60], [61], [62]]. Among the rocking settings that can represent CSF flow dynamics, the setting with the lowest flow values (0.7 Hz, 26.7 μl of stroke volume, 57 μl/s of peak flow rate) was used to investigate the physiological changes in the ChP microenvironment with minimal dynamic effects. Computational simulations of fluid dynamics within the microfluidic channel revealed how the shear stress was applied to the center channel (Supplementary video S4). Our image processing system for analyzing dynamic fluid flow served as a strong tool to recapitulate in vivo CSF dynamics in the microfluidic platform.
Fig. 2.
Design of the ChP-on-a-chip and fluid dynamics analysis via image processing to recapitulate CSF circulation in the microfluidic platform. a Flow dynamics analysis process using image processing. b, c Plots of (b) frequency – stroke volume and (c) frequency – peak flow rate extracted through the image processing in each angle and frequency of rocking system with the indicated range of the values in human CSF flow dynamics.
3.3. Recapitulation of the ChP capillary system
ChP capillaries are highly vascularized and fenestrated in nature with low tight junction expression [1,10,63]. Capillaries in other neurovascular units show high tight junction expression, which results from brain-specific ECM components including laminin and interaction with surrounding stromal cells such as pericytes [[11], [12], [13], [14], [15], [16]]. On the other hand, ChP capillaries exhibit low tight junction expression even though they reside in a similar ECM and cellular microenvironment [10,17]. Such vascular features have been known to be affected by mechanical cues (i.e., fluid flow) [64,65]. Due to these factors, we hypothesized that the CSF's dynamic flow was responsible for these unique characteristics of the ChP capillaries. Tight junction expression was compared using occludin, one of the typical tight junction proteins, in a microvascular network modified with laminin for when it was subjected to dynamic in vivo-like flow and when it was not (Fig. 3a and b). RT-qPCR data of occludin quantification showed significantly upregulated occludin expression in vasculature cultured in the modified ECM without in vivo-like CSF flow, while the expression of occludin was significantly decreased when cultured in the modified ECM with in vivo-like CSF flow. Additionally, the microvascular density of the vascular network cultured in each condition was quantified (Fig. 3c). The microvasculature cultured in the engineered ECM with dynamic flow exhibited the greatest microvascular density compared to microvessels cultured in other conditions. When the microvasculature was cultured with the culture condition deriving ChP capillaries-like characteristics, it showed a lumenized structure and formed a perfusable network (Fig. 3d and e, Supplementary video S5). These results suggest that our microvasculature cultured in brain-specific ECM with in vivo-like CSF dynamic flow is physiologically relevant to ChP capillaries.
3.4. Reconstruction of the ChP epithelium
The ChP consists of a lining of epithelial tissue known as BCSFB, which separates the CSF from the vascular system and restricts the molecular exchange by complex architectural features including high tight junction expression [66,67]. Particularly, claudin-1 is one of the typical tight junction proteins which are highly expressed on the BCSFB [[68], [69], [70]]. This specialized epithelial barrier plays a critical role in the secretion of the CSF, where the cilia built on the epithelium is related to the regulation of the CSF production [71,72]. The cilia formation can be affected by the CSF dynamic flow and indicated by several markers including FoxJ1 and RSPH9 [5,73].
We presented the characteristics of the ChP epithelial layers and their function can be recreated in our microengineered ChP culture condition. To scrutinize the response of epithelial layers in each culture condition, we developed the ChP epithelium-on-a-chip, which reconstructs the flow channels and reservoirs of the human ChP-on-a-chip, while facilitating the investigation of the ChP epithelium in a horizontal direction (Fig. S6). The epithelial cells in the ChP epithelium-on-a-chip are applied to the same fluid dynamic condition with the ones in the human ChP epithelium-on-a-chip according to the computational simulation (Supplementary video S4). When the HCPEpiCs patterned in the channels were cultured in the device for 48 h in each condition, the epithelial cell layer exhibited the highest coverage and expression of ciliogenesis markers, FoxJ1 and RSPH9, expressing portions when cultured in the adjusted ECM under in vivo-like CSF flow relative to other culture conditions (Fig. 3f–h, Fig. S7). Moreover, the ChP epithelium cultured in engineered ECM under dynamic flow showed the highest claudin-1 expression compared to those in other microenvironments (Fig. 3i and j). Our results demonstrated that the adjusted microenvironment with dynamic flow and specialized ECM can provide a ChP epithelium-like culture condition with consistent epithelial layers and ciliogenesis.
3.5. Function of the ChP capillaries-epithelium complex
The ChP consists of both a vascular network and epithelial layer, which are known to produce CSF [71,72,74]. This fluidic production is related to the epithelial regulation of transporting ions and water between the blood and the ventricles, which indicates the process is associated with capillaries-epithelium complex [4]. Through the interactive procedure, CSF with distinctive molecular components such as lower calcium and glucose levels relative to the plasma is generated [75].
The ChP capillaries were co-cultured with epithelium in the adjusted microenvironment within the device (Fig. 4a). To examine whether the ChP capillaries-epithelium complex functions as the CSF source under dynamic flow, we conducted enzymatic assays to measure the concentrations of calcium, glucose, and protein in the serum-free media extracted from the device when exposed to each flow condition. The enzymatic assays were conducted 24 h after the beginning of each flow condition as the secretion of CSF is known to vary over the duration of a day, and therefore quantified CSF secretion values are taken every 24 h [76,77]. In vivo CSF has lower values of calcium, glucose, and protein compared to human serum, indicating a lower production of these substances in the ChP. Likewise, in our device, the dynamic condition had significantly lower levels of calcium, glucose, and protein compared to the same values in the static condition, displaying the same trend as in in vivo CSF (Fig. 4b). We concluded that the ChP epithelium as well as the ChP capillaries were recapitulated in the engineered ECM under in vivo-like CSF dynamic flow, where the released molecules in the ChP capillaries-epithelium complex cultured in the microengineered condition presented physiologically relevant levels with CSF.
3.6. Drug response and immune reaction in ChP TME
Breast cancer is one of the most common types of cancer to spread to the leptomeninges with 12–35% of breast cancer resulting in LM [78,79]. This metastasis is related to the cancer cells spread in the ChP, since cancer cells pass through the ChP to infiltrate the CSF, where the metastatic cells can circulate freely and lead to LM [80]. In the ChP, the most prevalent immune cells are macrophages, which also include tumor-associated macrophages (TAMs). TAMs are generally described as M2-like macrophages and play a significant role in tumor progression and invasion [21,22]. M2-like TAMs are highly correlated with poor prognosis of breast cancer because they can promote tumor cell growth, allowing tumors to escape phagocytosis [81]. These aggressive features have mostly been observed in HER2-positive breast cancer cells, which are more likely to progress metastasis [[82], [83], [84]]. In the tumor-immune microenvironment, immunosuppressive molecules are highly expressed on the surface of tumor cells under fluid shear stress, allowing tumor cells to escape immunosurveillance and phagocytosis by macrophages [85,86]. The immunosuppressive expression upregulated due to fluidic shear stress could lead to the low percentage of macrophage-mediated killing of tumor cells in the in vivo dynamic condition.
One of the typical therapeutic methods to treat breast cancer cells metastasized to the brain is an anti-HER2 drug, called trastuzumab, which is intrathecally administered to target HER2-positive breast cancer cells disseminated in the brain [47,87,88]. The key phenotypes for therapeutic targets such as HER2 can be modulated in a physically in vivo-like microenvironment [89]. On the other hand, the anti-cancer efficacy can be further improved through the synergetic effect of introducing both trastuzumab and macrophages into the system [29].
Though the probability of this type of tumor metastasis through or into the ChP is relatively rare compared to other brain metastases, a better understanding of the pathophysiology is required for its treatment. For a better understanding of oncology, anti-cancer drug response as well as tumor-immune reaction needs to be unveiled. To verify the utility of the human ChP-on-a-chip as a drug testing and immuno-oncology screening platform, we reconstructed breast cancer spreading in the ChP, where typical intrathecal therapy was tested and macrophages were applied to investigate tumor-immune reaction. HER2-overexpressing breast cancer cells, SKBR3 cells, were dispersed in the ChP capillaries and THP-1 cell-derived macrophages were disseminated through the capillaries, which would be differentiated into M2-like TAM under breast TME [90]. Trastuzumab was delivered through the flow channels, which reflects the intrathecal therapeutic method of injecting the drug via CSF.
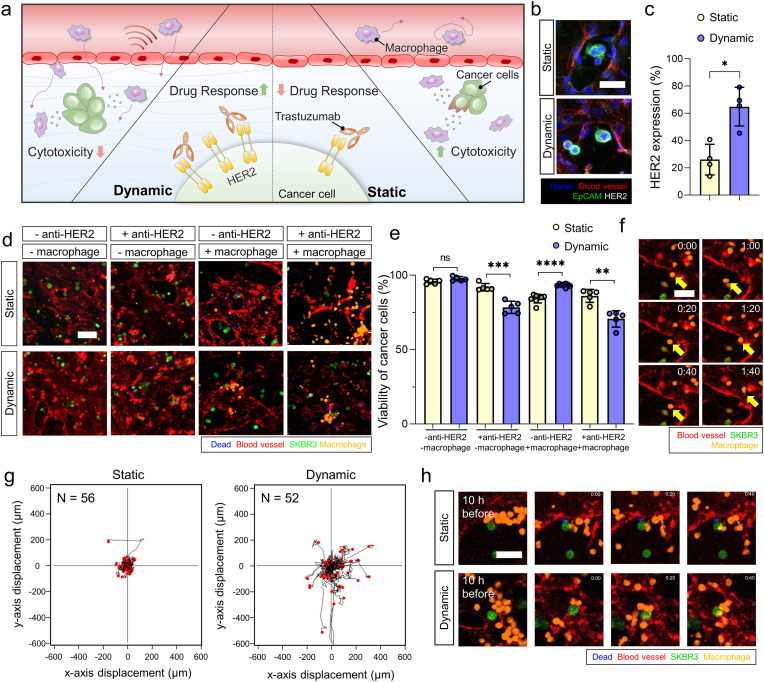
Likewise, the ChP model for the dispersed HER2-overexpressing breast cancer cells was constructed to compare HER2 expression and the response to trastuzumab or macrophages in static and dynamic conditions. We expected that HER2 overexpression would be maintained, and the cytotoxic effects would be valid in the in vivo-like dynamic condition, while the cytotoxic response would be mild when exposed to macrophages in the dynamic condition (Fig. 5a). When both anti-HER2 therapy and macrophages are applied to the ChP model, the cytotoxic effects would be the highest under physiologically relevant conditions [29]. We quantified HER2 expression levels from SKBR3 cells cultured in the ChP model in each condition. HER2 expression was significantly higher in the dynamic condition compared to the static condition (Fig. 5b and c). When SKBR3 cells were exposed to anti-HER2 therapy, their viability significantly decreased in the dynamic condition compared to the static condition (Fig. 5d, e, and S9). When macrophages were applied to our model in each condition, we observed the distinctive antitumor activity of polarized macrophages under the dynamic flow condition, where macrophages often failed to eliminate tumor cells in close contact despite their high motility (Fig. 5d–h, and S9). Interestingly, it was observed that only under the dynamic condition, macrophages showed increased CD163 expression and an elongated shape (Fig. S8), which is a morphological characteristic of M2 macrophages [91]. This distinct morphology was easily distinguishable from the round and flattened macrophages typically observed under the static condition. These findings confirm that dynamic flow-induced polarization specifically drives macrophage differentiation into the M2 subset. Moreover, when anti-HER2 and macrophages were applied to SKBR3 cells in the ChP chip, cytotoxic effects were highest under the dynamic condition compared to those in the static condition (Fig. 5d, e, 5h and S9). To compare anti-HER2 responses of HER2-negative cells, MCF7, to the SKBR3 cells under each condition, a model for the dispersed MCF7 cells was also constructed. We expected that the cytotoxic effect would be higher in SKBR3 than MCF7 when the ChP with the breast cancer cells was treated with trastuzumab in a physiologically relevant microenvironment (Fig. S10a). When the anti-HER2 drug was applied to each model under in vivo-like CSF flow, the death rate was significantly upregulated when SKBR3 cells were exposed to the anti-HER2 drug whereas there was no significant change in viability of MCF7 cells under the drug treatment (Figs. S10b and S10c). We suggest that low HER2 expression in MCF7 cells, maintained in an in vivo-like condition, might result in no significant change in cell viability when exposed to the anti-HER2 drug [50]. On the other hand, no significant change in cytotoxic effects of both MCF7 and SKBR3 cells was observed when trastuzumab was applied to the models under static condition (Figs. S10b and S10c).
Fig. 5.
Drug responses to the intrathecal therapy and immune reaction of breast cancer cells in the human ChP-on-a-chip. a Illustration of drug response when applied to an anti-HER2 drug, trastuzumab, tumor-immune reaction, and motility of macrophages of a breast cancer cell line, SKBR3 under each flow condition. b-c (b) Confocal images and (c) quantification of HER2 expression (nuclei, blue; blood vessels, red; EpCAM, green; HER2, grey) (scale bar: 30 μm) (n = 4 for each condition, *p < 0.05 by student t-test). d-e (d) Confocal images and (e) quantification of viability of SKBR3 cells (green), when applied to anti-HER2, trastuzumab, or macrophages in each flow condition (blood vessels, red; dead signals, blue; macrophages, orange) (scale bar: 30 μm) (n = 8 for macrophage without anti-HER2 therapy and n = 5 for other conditions, respectively, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by student t-test). f Confocal images of intra and extravasating macrophages under dynamic flow in the human ChP-on-a-chip. The yellow arrow indicates the intra and extravasating macrophage (scale bar: 50 μm). g Trajectory plot of the macrophage migration under static and dynamic conditions, respectively (n = 56 for static condition and n = 52 for dynamic condition). h Confocal images of macrophage – breast cancer cytotoxic response in static and dynamic condition, respectively (SKBR3 cells, green; blood vessels, red; dead signals, blue; macrophages, orange) (scale bar: 50 μm). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Along with investigating the cytotoxic response by immune reaction, we also examined the motility of macrophages in the TME of the ChP model in each condition. Adhesion molecules in human endothelial cells that are involved in transmigration could also be selectively regulated by a physiologically relevant range of biomechanical forces [92,93]. This could lead to higher chances of monocyte/macrophage transendothelial migration and infiltration with its high motility in our model under dynamic relative to static condition (Fig. 5f–h, Supplementary video S6 – S11).
In summary, we recapitulated the physiological relevance of the unique tumor-immune system of the ChP. The ChP model with dynamic CSF flow closely mimics highly motile macrophages with little cytotoxic ability and also recapitulates the anti-cancer drug response with its synergetic effects with macrophages. It offers a more in vivo-like environment to understand immune-tumor cell interactions in the TME and represent human drug responses.
4. Conclusion
In this research, we developed a human ChP-on-a-chip, which recapitulates the human capillaries-epithelium complex in the ChP system through several processes. The design of the microfluidic device allowed for the construction of a multi-layered capillaries – epithelium complex of the ChP, and the engineered ECM with brain-specific components recreated a more accurate brain microenvironment. Furthermore, in vivo-like CSF flow was recreated using dynamic fluid flow and was applied to the microfluidic platform to recapitulate the ChP dynamic microenvironment. After successfully recapitulating the human ChP, breast cancer cells were spread in the ChP and drugs targeting the cancer cells were tested. Moreover, the immune reaction between macrophages and breast cancer cells in the ChP was reconstituted and investigated using the platform. In conclusion, this human ChP model to recapitulate the human brain microenvironment can provide a powerful tool in which pathophysiology and drug response can be reliably studied.
Credit author statement
Jungeun Lim: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Stephen Rhee: Methodology, Validation, Investigation, Writing - Review & Editing. Hyeri Choi: Validation, Investigation, Writing - Review & Editing, Visualization. Jungseub Lee: Software, Visualization. Shruthy Kuttappan: Validation, Formal analysis. Tri Tho Yves Nguyen: Formal analysis. Sunbeen Choi: Validation, Formal analysis. YongTae Kim: Writing – review & editing. Noo Li Jeon: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) [2021R1A3B1077481]; the Ministry of Health and Welfare, South Korea [HP20C0146010020]; the Ministry of Education [NRF-2022R1I1A1A01072579]; and the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) [P0011266].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2023.100773.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Lun M.P., Monuki E.S., Lehtinen M.K. Nat. Rev. Neurosci. 2015;16:445–457. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rua R., McGavern D.B. Trends Mol. Med. 2018;24:542–559. doi: 10.1016/j.molmed.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javed K., Reddy V., Lui F. StatPearls Publishing; 2021. Neuroanatomy, Choroid Plexus, StatPearls. [Internet] [PubMed] [Google Scholar]
- 4.Brown P.D., Davies S.L., Speake T., Millar I.D. Neuroscience. 2004;129:955–968. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagenlocher C., Walentek P., Müller C., Thumberger T., Feistel K. Cilia. 2013;2:12. doi: 10.1186/2046-2530-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felten D.L., O'Banion M.K., Maida M.S. In: Netter's Atlas of Neuroscience. third ed. Felten D.L., O'Banion M.K., Maida M.S., editors. Elsevier; Philadelphia: 2016. 6 - ventricles and the cerebrospinal fluid; pp. 85–91. [Google Scholar]
- 7.Brinker T., Stopa E., Morrison J., Klinge P. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bapuraj J.R., Londy F.J., Delavari N., Maher C.O., Garton H.J.L., Martin B.A., Muraszko K.M., Ibrahim E.-S.H., Quint D.J. J. Magn. Reson. Imag. 2016;44:463–470. doi: 10.1002/jmri.25160. [DOI] [PubMed] [Google Scholar]
- 9.Bedussi B., Almasian M., de Vos J., VanBavel E., Bakker E.N. J. Cerebr. Blood Flow Metabol. 2018;38:719–726. doi: 10.1177/0271678X17737984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bock M., Vandenbroucke R.E., Decrock E., Culot M., Cecchelli R., Leybaert L. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2014;588:1259–1270. doi: 10.1016/j.febslet.2014.02.060. [DOI] [PubMed] [Google Scholar]
- 11.Wu C., Ivars F., Anderson P., Hallmann R., Vestweber D., Nilsson P., Robenek H., Tryggvason K., Song J., Korpos E., Loser K., Beissert S., Georges-Labouesse E., Sorokin L.M. Nat. Med. 2009;15:519–527. doi: 10.1038/nm.1957. [DOI] [PubMed] [Google Scholar]
- 12.Luissint A.-C., Artus C., Glacial F., Ganeshamoorthy K., Couraud P.-O. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautam J., Zhang X., Yao Y. Sci. Rep. 2016;6 doi: 10.1038/srep36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menezes M.J., McClenahan F.K., Leiton C.V., Aranmolate A., Shan X., Colognato H. J. Neurosci. 2014;34:15260–15280. doi: 10.1523/JNEUROSCI.3678-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen M.S., Routhe L.J., Moos T. J. Cerebr. Blood Flow Metabol. 2017;37:3300–3317. doi: 10.1177/0271678X17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson N., Campbell M. Front. Mol. Neurosci. 2021;14 doi: 10.3389/fnmol.2021.752781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitanihirwe B.K.Y., Lizano P., Woo T.-U.W. Mol. Psychiatr. 2022;27:3573–3582. doi: 10.1038/s41380-022-01623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman S.A., Krabak M.J. Cancer Treat Rev. 1999;25:103–119. doi: 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]
- 19.Yano S., Shinohara H., Herbst R.S., Kuniyasu H., Bucana C.D., Ellis L.M., Davis D.W., McConkey D.J., Fidler I.J. Cancer Res. 2000;60:4959–4967. [PubMed] [Google Scholar]
- 20.Tabouret E., Chinot O., Metellus P., Tallet A., Viens P., Goncalves A. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 21.You H., Baluszek S., Kaminska B. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You H., Baluszek S., Kaminska B. Theranostics. 2020;10:2949–2964. doi: 10.7150/thno.40783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W.-C., Zhang X.-F., Peng J., Li X.-F., Wang A.-L., Bie Y.-Q., Shi L.-H., Lin M.-B., Zhang X.-F. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/6304701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linde N., Casanova-Acebes M., Sosa M.S., Mortha A., Rahman A., Farias E., Harper K., Tardio E., Reyes Torres I., Jones J., Condeelis J., Merad M., Aguirre-Ghiso J.A. Nat. Commun. 2018;9:21. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang S.U., Cho S.Y., Jeong H., Han J., Chae H.Y., Yang H., Sung C.O., Choi Y.-L., Shin Y.K., Kwon M.J. Lab. Invest. 2022;102:376–390. doi: 10.1038/s41374-021-00699-y. [DOI] [PubMed] [Google Scholar]
- 26.Gensbittel V., Kräter M., Harlepp S., Busnelli I., Guck J., Goetz J.G. Dev. Cell. 2021;56:164–179. doi: 10.1016/j.devcel.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Romond E.H., Perez E.A., Bryant J., Suman V.J., Geyer C.E., Davidson N.E., Tan-Chiu E., Martino S., Paik S., Kaufman P.A., Swain S.M., Pisansky T.M., Fehrenbacher L., Kutteh L.A., Vogel V.G., Visscher D.W., Yothers G., Jenkins R.B., Brown A.M., Dakhil S.R., Mamounas E.P., Lingle W.L., Klein P.M., Ingle J.N., Wolmark N. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 28.Tabouret E., Chinot O., Metellus P., Tallet A., Viens P., Gonçalves A. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 29.Shi Y., Fan X., Deng H., Brezski R.J., Rycyzyn M., Jordan R.E., Strohl W.R., Zou Q., Zhang N., An Z. J. Immunol. 2015;194:4379–4386. doi: 10.4049/jimmunol.1402891. [DOI] [PubMed] [Google Scholar]
- 30.Kompaníková P., Bryja V. Cell. Mol. Life Sci. 2022;79:304. doi: 10.1007/s00018-022-04314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilling T., Engelbertz C., Decker S., Korte D., Hüwel S., Galla H.-J. Cell Tissue Res. 2002;310:19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 32.Suresh S. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Follain G., Herrmann D., Harlepp S., Hyenne V., Osmani N., Warren S.C., Timpson P., Goetz J.G. Nat. Rev. Cancer. 2020;20:107–124. doi: 10.1038/s41568-019-0221-x. [DOI] [PubMed] [Google Scholar]
- 34.Nia H.T., Munn L.L., Jain R.K. Science. 2020:370. doi: 10.1126/science.aaz0868. eaaz0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasudevan J., Jiang K., Fernandez J.G., Lim C.T. Acta Biomater. 2022;163:351–364. doi: 10.1016/j.actbio.2022.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Wang M., Jiang H., Liu X., Wang X. iScience. 2022;25 doi: 10.1016/j.isci.2022.104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naseri Kouzehgarani G., Feldsien T., Engelhard H.H., Mirakhur K.K., Phipps C., Nimmrich V., Clausznitzer D., Lefebvre D.R. Adv. Drug Deliv. Rev. 2021;173:20–59. doi: 10.1016/j.addr.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Eltobgy M., Huntoon K., Musgrave N., Shaikhouni A., Hardesty D.A., Giglio P., Elder J.B. J. Neuro Oncol. 2021;153:161–167. doi: 10.1007/s11060-021-03756-0. [DOI] [PubMed] [Google Scholar]
- 39.Callaway E., Butler D. Nature. 2016;529:263–264. doi: 10.1038/nature.2016.19189. [DOI] [PubMed] [Google Scholar]
- 40.Kaur R., Sidhu P., Singh S. J. Pharmacol. Pharmacother. 2016;7:120–126. doi: 10.4103/0976-500X.189661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callaway E., Butler D. 2016. Nature. [DOI] [PubMed] [Google Scholar]
- 42.Bird S.M., Bailey R.A., Grieve A.P., Senn S. Pharmaceut. Stat. 2017;16:100–106. doi: 10.1002/pst.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellegrini L., Lancaster M.A. Curr. Opin. Cell Biol. 2021;73:41–49. doi: 10.1016/j.ceb.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay M. Nat. Methods. 2020;17:875. doi: 10.1038/s41592-020-0950-5. 875. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrini L., Bonfio C., Chadwick J., Begum F., Skehel M., Lancaster M.A. Science. 2020;369 doi: 10.1126/science.aaz5626. eaaz5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyu Z., Park J., Kim K.-M., Jin H.-J., Wu H., Rajadas J., Kim D.-H., Steinberg G.K., Lee W. Nat. Biomed. Eng. 2021;5:847–863. doi: 10.1038/s41551-021-00744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zagouri F., Sergentanis T.N., Bartsch R., Berghoff A.S., Chrysikos D., de Azambuja E., Dimopoulos M.-A., Preusser M. Breast Cancer Res. Treat. 2013;139:13–22. doi: 10.1007/s10549-013-2525-y. [DOI] [PubMed] [Google Scholar]
- 48.Maximiano S., Magalhães P., Guerreiro M.P., Morgado M. BioDrugs. 2016;30:75–86. doi: 10.1007/s40259-016-0162-9. [DOI] [PubMed] [Google Scholar]
- 49.Mack F., Baumert B.G., Schäfer N., Hattingen E., Scheffler B., Herrlinger U., Glas M. Cancer Treat Rev. 2016;43:83–91. doi: 10.1016/j.ctrv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Barok M., Tanner M., Köninki K., Isola J. Breast Cancer Res. 2011;13:R46. doi: 10.1186/bcr2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greitz D. Acta Radiol. Suppl. 1993;386:1–23. [PubMed] [Google Scholar]
- 52.Bateman G.A., Levi C.R., Schofield P., Wang Y., Lovett E.C. Neuroradiology. 2005;47:741–748. doi: 10.1007/s00234-005-1418-0. [DOI] [PubMed] [Google Scholar]
- 53.Wagshul M.E., Chen J.J., Egnor M.R., McCormack E.J., Roche P.E. Journal of Neurosurgery JNS. 2006;104:810–819. doi: 10.3171/jns.2006.104.5.810. [DOI] [PubMed] [Google Scholar]
- 54.Stoquart-ElSankari S., Balédent O., Gondry-Jouet C., Makki M., Godefroy O., Meyer M.-E. J. Cerebr. Blood Flow Metabol. 2007;27:1563–1572. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- 55.Bradley J.W.G., Scalzo D., Queralt J., Nitz W.N., Atkinson D.J., Wong P. Radiology. 1996;198:523–529. doi: 10.1148/radiology.198.2.8596861. [DOI] [PubMed] [Google Scholar]
- 56.Balédent O., Henry-Feugeas M.-C., a. C, Idy-Peretti I. Invest. Radiol. 2001;36:368–377. doi: 10.1097/00004424-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Wagshul M.E., Eide P.K., Madsen J.R. Fluids Barriers CNS. 2011;8:5. doi: 10.1186/2045-8118-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strik C., Klose U., Erb M., Strik H., Grodd W. J. Magn. Reson. Imag. 2002;15:251–258. doi: 10.1002/jmri.10084. [DOI] [PubMed] [Google Scholar]
- 59.Bailon R., Laguna P., Mainardi L., Sornmo L. 2007. 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society2007; pp. 6674–6677. [DOI] [PubMed] [Google Scholar]
- 60.Cordes D., Haughton V.M., Arfanakis K., Carew J.D., Turski P.A., Moritz C.H., Quigley M.A., Meyerand M.E. Am. J. Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 61.Daouk J., Bouzerar R., Baledent O. Acta Radiol. 2016;58:977–982. doi: 10.1177/0284185116676655. [DOI] [PubMed] [Google Scholar]
- 62.Klostranec J.M., Vucevic D., Bhatia K.D., Kortman H.G.J., Krings T., Murphy K.P., terBrugge K.G., Mikulis D.J. Radiology. 2021;301:502–514. doi: 10.1148/radiol.2021202043. [DOI] [PubMed] [Google Scholar]
- 63.Anrather J. In: Primer on Cerebrovascular Diseases. second ed. Caplan L.R., Biller J., Leary M.C., Lo E.H., Thomas A.J., Yenari M., Zhang J.H., editors. Academic Press; San Diego: 2017. Chapter 28 - pathophysiology of the peripheral immune response in acute ischemic stroke; pp. 139–145. [Google Scholar]
- 64.Komarova Y.A., Kruse K., Mehta D., Malik A.B. Circ. Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng Z., Shu B., Zhang Y., Wang M. Arterioscler. Thromb. Vasc. Biol. 2019;39:e233–e243. doi: 10.1161/ATVBAHA.119.312580. [DOI] [PubMed] [Google Scholar]
- 66.Redzic Z. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Javed K., Reddy V., Lui F. StatPearls Publishing; Treasure Island (FL: 2021. Neuroanatomy, Choroid Plexus. [PubMed] [Google Scholar]
- 68.Szmydynger-Chodobska J., Pascale C.L., Pfeffer A.N., Coulter C., Chodobski A. Cerebrospinal Fluid Res. 2007;4:11. doi: 10.1186/1743-8454-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kratzer I., Vasiljevic A., Rey C., Fevre-Montange M., Saunders N., Strazielle N., Ghersi-Egea J.-F. Histochem. Cell Biol. 2012;138:861–879. doi: 10.1007/s00418-012-1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinemann A., Galm I., Chip S., Nitsch C., Maly I.P. Front. Neuroanat. 2016;10 doi: 10.3389/fnana.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Narita K., Takeda S. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Telano L.N., Baker S. StatPearls Publishing; Treasure Island (FL: 2021. Physiology, Cerebral Spinal Fluid. [PubMed] [Google Scholar]
- 73.Narita K., Kozuka-Hata H., Nonami Y., Ao-Kondo H., Suzuki T., Nakamura H., Yamakawa K., Oyama M., Inoue T., Takeda S. Biology Open. 2012;1:815–825. doi: 10.1242/bio.20121081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva-Vargas V., Doetsch F. Science. 2020;369:143–144. doi: 10.1126/science.abd0269. [DOI] [PubMed] [Google Scholar]
- 75.Artru A.A. In: Cottrell and Young's Neuroanesthesia. fifth ed. Cottrell J.E., Young W.L., editors. Mosby; Philadelphia: 2010. Chapter 3 - cerebrospinal fluid; pp. 60–74. [Google Scholar]
- 76.Nilsson C., Stahlberg F., Thomsen C., Henriksen O., Herning M., Owman C. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992;262:R20–R24. doi: 10.1152/ajpregu.1992.262.1.R20. [DOI] [PubMed] [Google Scholar]
- 77.Wichmann T.O., Damkier H.H., Pedersen M. Front. Hum. Neurosci. 2022;15 doi: 10.3389/fnhum.2021.737217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarke J.L., Perez H.R., Jacks L.M., Panageas K.S., DeAngelis L.M. Neurology. 2010;74:1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Rhun E., Taillibert S., Chamberlain M.C. Surg. Neurol. Int. 2013;4:S265–S288. doi: 10.4103/2152-7806.111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kokkoris C.P. Cancer. 1983;51:154–160. doi: 10.1002/1097-0142(19830101)51:1<154::aid-cncr2820510130>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 81.Qiu S.-Q., Waaijer S.J.H., Zwager M.C., de Vries E.G.E., van der Vegt B., Schröder C.P. Cancer Treat Rev. 2018;70:178–189. doi: 10.1016/j.ctrv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Witzel I., Oliveira-Ferrer L., Pantel K., Müller V., Wikman H. Breast Cancer Res. 2016;18:8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bailleux C., Eberst L., Bachelot T. Br. J. Cancer. 2021;124:142–155. doi: 10.1038/s41416-020-01175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klingen T.A., Chen Y., Aas H., Wik E., Akslen L.A. Hum. Pathol. 2017;69:72–80. doi: 10.1016/j.humpath.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Huang Q., Hu X., He W., Zhao Y., Hao S., Wu Q., Li S., Zhang S., Shi M. Am. J. Cancer Res. 2018;8:763–777. [PMC free article] [PubMed] [Google Scholar]
- 86.Takimoto C.H., Chao M.P., Gibbs C., McCamish M.A., Liu J., Chen J.Y., Majeti R., Weissman I.L. Ann. Oncol. 2019;30:486–489. doi: 10.1093/annonc/mdz006. [DOI] [PubMed] [Google Scholar]
- 87.Franzoi M.A., Hortobagyi G.N. Crit. Rev. Oncol. Hematol. 2019;135:85–94. doi: 10.1016/j.critrevonc.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 88.Zagouri F., Zoumpourlis P., Le Rhun E., Bartsch R., Zografos E., Apostolidou K., Dimopoulos M.-A., Preusser M. Cancer Treat Rev. 2020;88 doi: 10.1016/j.ctrv.2020.102046. [DOI] [PubMed] [Google Scholar]
- 89.Jiang K., Liang L., Lim C.T. iScience. 2021;24 doi: 10.1016/j.isci.2021.102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li D., Ji H., Niu X., Yin L., Wang Y., Gu Y., Wang J., Zhou X., Zhang H., Zhang Q. Cancer Sci. 2020;111:47–58. doi: 10.1111/cas.14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McWhorter F.Y., Wang T., Nguyen P., Chung T., Liu W.F. Proc. Natl. Acad. Sci. USA. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagel T., Resnick N., Atkinson W.J., Dewey C.F., Jr., Gimbrone M.A., Jr. J. Clin. Investig. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morigi M., Zoja C., Figliuzzi M., Foppolo M., Micheletti G., Bontempelli M., Saronni M., Remuzzi G., Remuzzi A. Blood. 1995;85:1696–1703. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.