Abstract
The timely administration of antivenom is the most effective method currently available to reduce the burden of snakebite envenoming (SBE), a neglected tropical disease that most often affects rural agricultural global populations. There is increasing interest in the development of adjunctive small molecule and biologic therapeutics that target the most problematic venom components to bridge the time-gap between initial SBE and the administration antivenom. Unique combinations of these therapeutics could provide relief from the toxic effects of regional groupings of medically relevant snake species. The application a PRISMA/PICO literature search methodology demonstrated an increasing interest in the rapid administration of therapies to improve patient symptoms and outcomes after SBE. Advice from expert interviews and considerations regarding the potential routes of therapy administration, anatomical bite location, and species-specific venom delivery have provided a framework to identify ideal metrics and potential hurdles for the development of a field-based medical device that could be used immediately after SBE to deliver adjunctive therapies. The use of subcutaneous (SC) or intramuscular (IM) injection were identified as potential routes of administration of both small molecule and biologic therapies. The development of a field-based medical device for the delivery of adjunctive SBE therapies presents unique challenges that will require a collaborative and transdisciplinary approach to be successful.
Keywords: Snakebite, Adjunctive, Pre-hospital, Field-based, Medical device, Autoinjector
Graphical abstract
Highlights
-
•
Treatment of snakebite envenoming with antivenom is time sensitive.
-
•
Adjunctive small molecule and biologic therapies could also be used to treat snakebite.
-
•
Considerations for the delivery of therapies with a medical device are presented.
-
•
Subcutaneous or intramuscular injection of therapies is feasible.
-
•
Transdisciplinary collaboration is required to develop a medical device for snakebite.
1. Introduction
The idea of developing novel therapies for the treatment of snakebite envenoming (SBE) has existed ever since humans have recognized that a bite from a venomous snake causes a host of negative physiological effects that may ultimately lead to a painful and rapid death. The ancient Roman physician Galen who may have attended to Cleopatra's death wrote, “human and prompt executions were made in Alexandria with the intervention of cobras” and “there is nothing more dangerous in life than poisons, and the bites of noxious animals … one may avoid most dangers by fleeing from them, but poisons and venomous animals are treacherous enemies, since, if one is careless, he can take poison by mistake, or he falls victim to a quick bite without warning.“(Kuhn, 2011) The symbol of the snake has impacted medical symbology from Greek and Roman times becoming first the entwined snake around the Rod of Asclepius, the Greek God of healing and medicine, and later the a ubiquitous medical symbol known as the caduceus(Tsoucalas and Androutsos, 2019), and many ancient cultures detailed the specific effects of bites from particular snake species as well as potential remedies to prevent the negative outcomes from such bites(Sanchez and Harer, 2019; Scarborough, 1977; Slouber, 2012, 2016; Walker-Meikle, 2014). Once such ancient remedy, Theriac, was touted as a “universal antidote” for a variety of diseases, toxins, and snakebites and included ingredients such as opium and snake flesh(Karaberopoulos et al., 2012). Ancient remedies often attempted to reduce the negative effects of snake venom or to draw venom out of the local wound site, and in some cases amputation of an affected extremity was used.
It was not until the simultaneous development of species specific antivenoms in 1894 by Calmette, Phisalix and Bertrand, that a highly effective remedy for SBE became available(Calmette, 1894; Phisalix and Bertrand, 1894). The history of antivenom development has been recently reviewed(Pucca et al., 2019). Antivenom research over the last 130 years has revealed how to produce these immunologic derived antivenoms on a large scale at a reasonable economic cost, that intravenous (IV) administration of species-specific antivenom can be highly effective by providing rapid systemic distribution resulting in significant reductions in morbidity and mortality, and that despite their advantages, antivenoms still possess limitations as a treatment for SBE.
SBE primarily affects poor rural communities of Southeast Asia, sub-Saharan Africa, and South America where agricultural activities and low socioeconomic conditions contribute to increases in snake–human interaction(Harrison et al., 2009). For millions of people around the world, the risk of SBE is a daily concern. Global estimates reveal that approximately 500,000 individuals either die or are disfigured on an annual basis as the result of SBE(Gutiérrez et al., 2017). When one considers the effects of SBE on family units and communities, the number of affected individuals increases into the millions. Studies from India(Bawaskar et al., 2008; Bawaskar and Bawaskar, 2002; Chaaithanya et al., 2021; Darshani et al., 2021; Kadam et al., 2021; Suchithra et al., 2008), Southeast Asia(Patra and Mukherjee, 2021; Schioldann et al., 2018), Africa(Benjamin et al., 2020a; Blaylock, 2005; Chippaux, 2011; Chippaux et al., 2019; Godpower et al., 2019; Iliyasu et al., 2015; Chuat et al., 2021; Moos et al., 2021; Steinhorst et al., 2021), and South America(Cristino et al., 2021; Gutiérrez, 2014; Hansson et al., 2013; Magalhães et al., 2019; Ochoa-Avilés et al., 2020) reveal that rural agricultural communities bear the largest burden of SBE. These regions possess unique factors that lead to increased vulnerability to the consequences of SBE including a high density and variety of venomous snakes coupled to with inequalities in health care and a lack of access to effective therapy(Longbottom et al., 2018).
In 2017, the World Health Organization (WHO) classified SBE as a neglected tropical disease and subsequently in 2019, they presented an integrated strategy with the overarching aim of reducing the global burden of SBE 50% by the year 2030(Lancet, T., 2017; Williams et al., 2019; World Health Organization, 2019). The major focus of the WHO strategy to address the global burden of SBE, is to foster increases in global production and testing of polyvalent antivenom on a regional basis and to improve access to quality health care and facilities that stock appropriate regional antivenoms. However, the WHO strategy also recognizes that antivenom has limitations as a treatment for SBE(World Health Organization, 2019). Antivenoms are typically produced from horses, donkeys, goats, or sheep, and thus can cause life-threatening adverse reactions in humans. Additionally, antivenom does not reverse some of the negative impacts of venom components such as local tissue necrosis as well as kidney and organ damage, particularly if administered many hours after the SBE has occurred. All antivenoms must be administered via intravenous (IV) injection as “serotherpy” because they are rendered inactive by the acidic and enzymatic conditions found in the gut. Antivenoms may have issues related to folding instabilities, aggregation or solubility when produced in lyophilized form. When in liquid form, antivenoms require cold chain storage, a luxury often not available in many tropical or sub-tropical regions with high SBE burdens.
As a result of these limitations and the fact that many SBE victims lack access to timely antivenom therapy, the WHO recommends that a strategy to “accelerate the development of pre-hospital treatments” should be pursued to help reduce the global burden of SBE(World Health Organization, 2019). The WHO's strategy recognizes that there is an urgent need to bridge the time-gap between an SBE event in the field and subsequent administration of IV antivenom. It is well established that delays in the administration of antivenom lead to increases in mortality and morbidity related to SBE(da Silva Souza et al., 2018; Gutiérrez et al., 2021b; Habib and Abubakar, 2011; Iliyasu et al., 2015; Longbottom et al., 2018; Michael et al., 2017; Pintor et al., 2021). Thus, the successful treatment of SBE with antivenom becomes a race against time that is guided by the mantra, “Time is Tissue”, as the severity of venom effects are proportional to the elapsed time between envenoming and when antivenom can be administered(Boyer et al., 2015). This paper focuses on a strategy to bridge this time-gap by examining considerations for the development of a field-based medical device that could be used to deliver adjunctive SBE therapies immediately after a bite has occurred.
2. Envenoming therapy timeline (ETT)
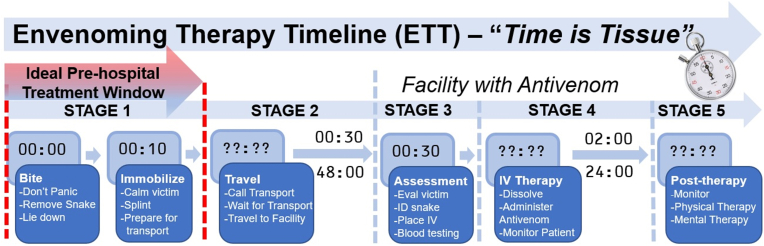
When an individual is bitten by a venomous snake, a time-sensitive cascade of events rapidly ensues(Cristino et al., 2021). The longer it takes for an SBE victim to receive IV antivenom the more likely that disfigurement or death will result(Boyer et al., 2015; Iliyasu et al., 2015). The only therapy that has scientifically validated efficacy for treating SBE in humans is the administration of either species-specific or polyvalent antivenom (see section 4.4.2 describing the differences between these two types of antivenom). Administration of IV antivenom thus represents the “gold-standard” for SBE treatment(Bénard-Valle et al., 2015; Gutiérrez et al., 2017). The post-SBE journey to treatment can be described as the envenoming therapy timeline (ETT) that can be divided into five stages (Fig. 1). Mortality and morbidity related to SBE could be reduced by bridging the time gap between initial SBE and the administration of IV antivenom with the field-based administration of adjunctive therapies during Stage 1 (and possibly Stage 2) of the ETT.
Fig. 1.
Envenoming therapy timeline (ETT), representing the five stages along the timeline required for post-SBE treatment. Stage 1: victim and wound stabilization during the first 10 min after SBE represent an ideal pre-hospital treatment window, Stage 2: the variable time required for victim transport to reach a medical facility that stocks regional snake antivenom, Stage 3: the time required for victim assessment by trained medical personnel at a hospital facility, Stage 4: the variable time for the administration of IV antivenom, and Stage 5: the variable time required for post-therapy assessment and follow-up.
The first stage of the ETT represents the short time interval (∼10 min) immediately following SBE. Approaches such as the application of a tourniquet, excision and vacuum extraction, cryotherapy, the application of herbal remedies or snakestones by traditional healers, and even amputation have been utilized during this stage (see section 3.2 for further discussion). These approaches ultimately result in negative outcomes and delay of IV administration of IV antivenom(Bénard-Valle et al., 2015). Best practices for the proper administration of first aid to an SBE victim during this stage are presented in Section 3.1. It has been proposed that Stage 1 of the ETT would be an ideal time to administer adjunctive therapeutic agents to inactivate, bind or destroy the harmful components of the venom thus reducing their negative effects while the victim is prepared to travel to a medical facility (Gutiérrez et al., 2007). Much of this paper will focus on providing evidence for this approach and considerations for the development of methods to deliver such therapies in a field-based setting. The administration of pre-hospital therapies should not significantly delay when the victim begins their journey to a medical facility with appropriate regional antivenom and should not be viewed as a “cure” for snakebite or discourage a victim's journey to a medical facility for appropriate care.
The second stage of the ETT is transporting the SBE victim to an appropriate medical facility for IV antivenom therapy. As encounters with venomous snakes are typically in rural environments, SBE victims seeking medical care must then begin a lengthy, stressful, and arduous journey typically by foot, boat, motorcycle, or other vehicle(Hansson et al., 2013; Longbottom et al., 2018; Potet et al., 2021). On a global perspective, it is estimated that approximately 95% of those in areas with endemic medically relevant snakes reside more than 1 h travel time to a medical facility with the ability to administer antivenom(Longbottom et al., 2018). For example, studies performed by the Asclepius Snakebite Foundation, an organization that treats SBE victims in West Africa, show that SBE victims most often travel by motorcycle on a journey taking up to several days to receive medical care, with an average transit time of 8 h(Brandehoff, 2022). This example illustrates that the method of transport may not allow for a victim to be immobilized properly for the duration of travel (see section 3.1). Ideally, adjunctive therapies administered during Stage 1 would reduce the destructive effects and systemic spread of venom toxins during travel. Depending on the method of transport, one could also envision the administration of additional adjunctive therapies during this travel phase, recognizing that logistical complications may make this impossible.
The third stage of the ETT begins with the arrival of the SBE victim at a medical facility with appropriate regional antivenom in stock where initial patient assessment by qualified medical staff can begin. Arrival at such a facility does not represent the end of the ETT as delays are often encountered while a patient is assessed for signs of envenoming including pain, swelling, and ptosis(Benjamin et al., 2020b; Gutiérrez et al., 2017). The use of ELISA-based venom detection kits could be used to confirm envenoming, but these are only widely available in Australia(Theakston and Laing, 2014). In addition, preliminary blood coagulopathy tests may be performed and attempts may be made to identify the species of snake to allow for the appropriate choice of antivenom(Benjamin et al., 2020b). During this time, an IV line should be established to obtain blood samples, measure baseline indicators, and to determine if blood coagulability issues exist. The IV line may also be used to provide supportive therapies or pain management as well as for the administration of antivenom if indicated(Gutiérrez et al., 2017). Adjunctive therapies to antivenom could be administered during this stage, but if the time duration of Stages 1 and 2 is significant, their effect may be attenuated (see section 4 for further discussion).
The fourth stage of the ETT involves the preparation and administration of antivenom to the SBE patient. In cases where lyophilized antivenom must be reconstituted under sterile conditions, additional delays of between 30 and 60 min may occur(Gerardo, 2021). Administration of IV antivenom can be a slow process often taking many hours depending on the severity of symptoms and the required dosage.(Brandehoff et al., 2023) Patients must be monitored for adverse reactions over the course of antivenom treatment. Such reactions can be reduced by the administration of adrenaline(de Silva et al., 2011; Gutiérrez et al., 2017). Organ and system failures must be detected and treated early in during Stage 4 of the ETT.
The fifth and final stage of the ETT involves continued patient assessment, often for several days or even weeks to monitor potential antivenom related serum sickness or persistent blood coagulopathy issues and to assess wound healing(Boyer et al., 1999). Longer term rehabilitation may be needed in cases of amputation or debilitation, and many patients experience significant mental health effects including post-traumatic stress disorder that may require additional counseling and therapy(Bhaumik et al., 2020; Williams et al., 2011b).
3. Treatment strategies for SBE
3.1. Best practices for first aid management of SBE
First aid intervention strategies for SBE have a complex history that has evolved over the last century, and bites are rare enough that few have sufficient training regarding the most effective ways to render first aid for SBE. Outcomes are improved with knowledge of basic SBE first aid, awareness of resources for obtaining help, and avoidance of practices that cause more harm than good. The most important consideration is that first aid should stabilize the victim for ultimate transport to the closest medical facility with regional antivenom as quickly as possible. Fundamental actions that improve victim health and survival immediately after SBE include calming the victim to reduce heart rate and venom spread, splinting affected extremities to further limit the spread of venom through hemodynamic stabilization, maintaining the airway, and in the case of elapid envenoming(Sutherland et al., 1979), the proper application of pressure-immobilization bandages/pads(Rogers and Winkel, 2005). It should be noted that pressure immobilization may not be recommended for crotalid (viper) bites(Bush et al., 2004; Seifert et al., 2011). Here we present currently recommended best practices as summarized in several recent reviews and publicly available resources(Avau et al., 2016; Benjamin et al., 2020b; Hamza et al., 2021; Parker-Cote and Meggs, 2018; World Health Organization, 2016; Warrell and Williams, 2023).
Guidelines for first aid administered to an SBE victim (adapted from WHO recommendations) (World Health Organization, 2016).
-
1)
Move victim away from the snake that caused the bite.
-
2)
If possible, carefully determine species of snake (take photos if possible) but do not risk harm or waste time doing so.
-
3)
Reassure and calm the victim by stating that dry bites are common and most venomous snakebites do not cause immediate death.
-
4)
Remove tight objects from the body to avoid harm if swelling occurs.
-
5)
If supplies and training permit, apply a pressure immobilization pad/bandage if an elapid bite is confirmed or cannot be excluded.
-
6)
Immobilize the victim completely on their left side in the recovery position in case of vomiting. Minimize movement of affected limb with splint or sling.
-
7)
Where available, contact emergency services, poison control centers or make inquiries on internet or with local residents for advice about nearby health facilities most capable of administering palliative care and antivenom.
-
8)
Carefully transport victim to closest appropriate medical facility as soon as possible.
-
9)
Monitor airway and breathing and resuscitate if necessary.
-
10)
If available, paracetamol (acetaminophen) may be given for severe pain.
3.2. Ineffective pre-hospital intervention strategies for SBE
Recommendations for treatment of SBE during the mid-1900s were generally directed towards inactivating, removing or slowing the action of venom near the local site of envenoming, and preventing systemic spread of toxins using tourniquets (Blackman and Dillon, 1992; Pope and Peterson, 1946; Russell, 1967). First aid techniques that are at best questionably effective or, at worst, severely detrimental, have been reviewed(Bénard-Valle et al., 2015; Fry, 2018; Parker-Cote and Meggs, 2018). These discouraged methods include the use of immediate autotomy (self-amputation)(Bénard-Valle et al., 2015), tourniquets(Amaral et al., 1998; Bush and Kinlaw, 2015), suction devices(Alberts et al., 2004; Bush, 2004; Bush et al., 2000; Holstege, 2006), incisions(Hall, 2001), cryotherapy(Canul-Caamal et al., 2020; Frank, 1971), electric shock(Howe and Meisenheimer, 1988; Johnson et al., 1987), “medicinal” stones(Baldwin, 1995), and herbal or traditional remedies(Puzari et al., 2022; Gomes et al., 2010; Martz, 1992). Unfortunately, the use of many of these ineffective techniques persist not only in rural areas with high burdens of SBE(Ameade et al., 2021; Chaaithanya et al., 2021; Chuat et al., 2021; Mahmood et al., 2019; Michael et al., 2011; Singaravelu et al., 2021), but also in clinical, government, and military settings(Afroz et al., 2023; Bhargava et al., 2020; Chen et al., 2017; Johnson et al., 2013; Michael et al., 2017, 2018; Wilkins et al., 2018). The use of unproven and unregulated commercial snakebite kits over the past one hundred years has continued to the present day. These “first-aid kits” often contain antiseptic wipes, blades to make incisions, restriction bands or chords for tourniquets, and a suction device. These commercial kits are typically not approved by regulatory authorities and their use may provide the victim with a false sense of security and lead to further harm(Fry, 2018; Gellert, 1992).
4. Adjunctive field-based therapies for pre-hospital management of SBE
4.1. Benefits of early intervention after SBE
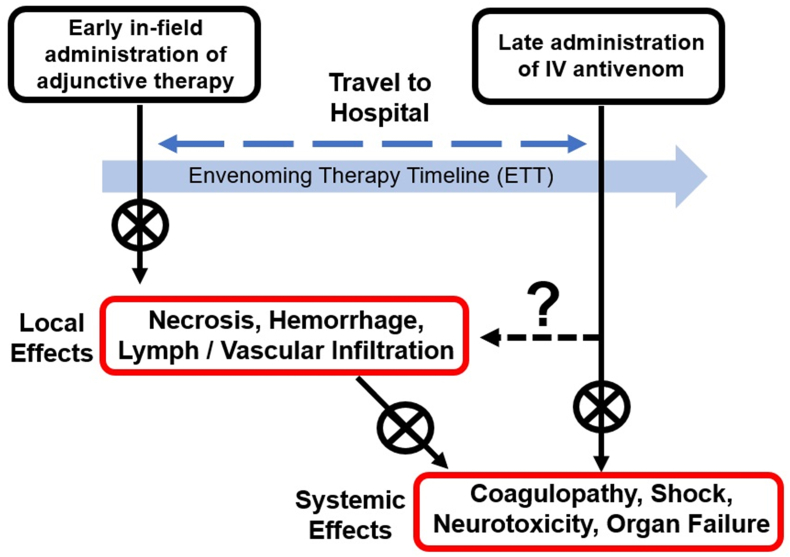
The current mechanism to provide life-saving therapies to an SBE victim involves waiting until Stage 4 of the ETT to administer IV antivenom (Fig. 1). This approach could be considered a late intervention strategy. In 2007, Gutierrez et al. outlined a future approach to treat viperid SBE that might first involve intervention with an immediate administration of venom toxin inhibitors post-SBE aimed at preventing local tissue damage and potentially the systemic effects of venom toxins (Fig. 2).(Gutiérrez et al., 2007) While this field-based administration of therapeutics was suggested for viperid SBE, the general concepts could be applied for any SBE. A proposed advantage to this early intervention strategy was to mitigate the harmful local effects of venom components, effects that generally cannot be fully reversed with IV antivenom, with the local in situ administration of therapies. It was proposed that such an early intervention would delay the onset of both local and systemic effects, thus providing additional time for an SBE victim to travel to a medical facility. The time for an SBE victim to travel to a medical facility with regional antivenom ranges between hours to days, with long travel times leading to increases in mortality and morbidity.
Fig. 2.
Potential benefits of early field-based administration of adjunctive SBE therapy along the envenoming therapy timeline (ETT). The immediate administration of adjunctive therapeutics after SBE may halt local effects such as necrosis, hemorrhage, and infiltration of venom into the vascular and lymphatic system. This early intervention strategy may also help reduce the systemic effects of venom toxins such as altered coagulopathy, neurotoxicity, and organ failure. While administration of IV antivenom is often highly effective at preventing systemic effects, these agents typically do not reverse the immediate effects of venom on local tissues. Mortality and morbidity due to SBE increases the longer it takes for the victim to travel to a medical facility with regional antivenom (adapted from Gutiérrez et al.) (Gutiérrez et al., 2007).
4.2. Tracing the “idea” of pre-hospital adjunctive SBE therapy
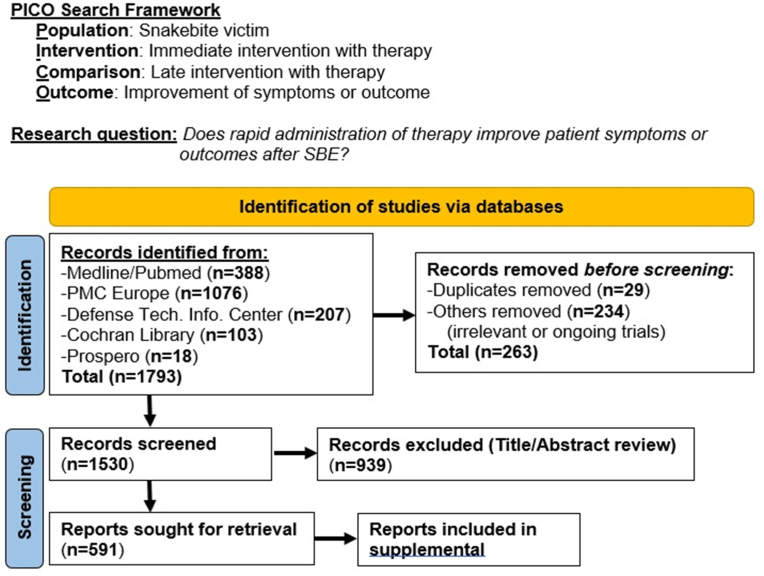
Where did the idea of administering a therapy immediately after SBE begin and how has it evolved? To address this question, we have applied a PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) that incorporates a PICO (Population, Intervention, Comparison, Outcome) framework to search the relevant literature from the late 1800s to 2022 (Fig. 3).(Eriksen and Frandsen, 2018; Page et al., 2021) Our search question was defined as, “Does rapid administration of therapy improve patient symptoms or outcomes after SBE?” A total of 1793 records were identified from Medline/Pubmed, PMC Europe, Defense Technology Information Center, Cochran Library, and Prospero. After initial screening for duplicates, ongoing trials, and removal based on title or abstract irrelevance, 591 records were identified for retrieval and further evaluation. In addition to citations identified using peer-reviewed article databases, a number of popular literature references have also been identified in our analysis and several are mentioned below. While a full review of both peer-reviewed and popular literature references is beyond the scope of this article, these records, search methodology, and associated search terms are provided as supplemental material.
Fig. 3.
Application of PRISMA/PICO search methodology to identify research articles related to the question, “Does rapid administration of therapy improve patient symptoms or outcomes after SBE?” A total of 1793 records were identified from a variety of databases, and after screening, the number sought for retrieval was reduced to 591 records. See supplemental material for a full listing of records and search methodology.
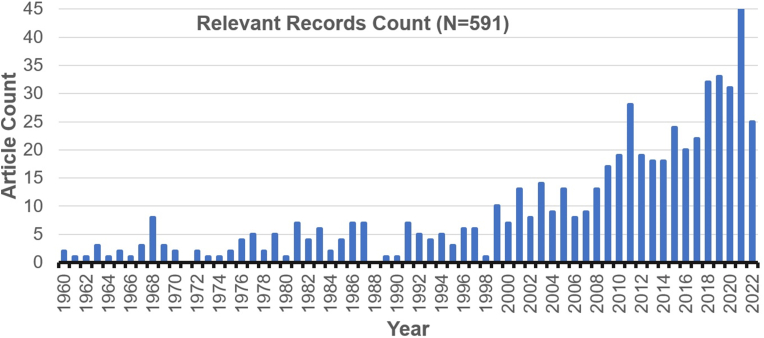
The last 25 years has seen a dramatic increase in articles dealing with rapid delivery and evaluation of therapies for SBE in a first aid or field-based setting (Fig. 4). Using the PICO framework outlined above, we found that between 1889 and 1959 there were a total of ten relevant articles and between 1960 and 1998 there were an average of 3–4 articles per year. However, after 1999 the number of relevant articles per year steadily increased to 45 in 2021 (note: this review only covered thru July 2022).
Fig. 4.
Number of articles published by year with relevance to the search question, “Does rapid administration of therapy improve patient symptoms or outcomes after SBE?” The last 25 years has seen a dramatic increase in articles dealing with rapid delivery and evaluation of therapies for SBE in a first aid or field-based setting. Details of search terms, search methodology, and a full listing of records are provided in the supplemental material.
In the late 1950s and early 1960s, the United States Army funded a significant research program investigating the effects of and treatments for SBE. Flowers and Goucher at the U.S. Army Medical Research and Development Command in Fort Knox, KY investigated the effect of immediate subcutaneous injection of the metal chelating agent ethylene-diaminetetraacetic acid (EDTA) at the site of envenoming by either Bothrops asper (fer-de-lance) or Agkistrodon piscivorus (cottonmouth moccasin) in both rabbits and chimpanzee(Flowers and Goucher, 1963, 1965). Two major conclusions from the study were: 1) “The prompt local use of EDTA is helpful in diminishing local edema, hemorrhage, and necrosis following cottonmouth and fer-de-lance envenomation”, and 2) “EDTA affects the local activity of venom and appears to be a useful adjunct to specific antivenin therapy.” The results of this study suggest that the prompt local use of EDTA is helpful in diminishing local edema, hemorrhage, and necrosis following cottonmouth and fer-de-lance envenoming.
In 1975, researchers investigating the use of local trypsin injection after SBE stated, “Trypsin can be put into pocketable first-aid auto-injector and can be used after snakebite at the possible earlier time.“(Yü-liang et al., 1975) This represents one of the earliest statements clearly identifying how an adjunctive therapy for SBE might be delivered in the field. The idea of using an autoinjector for in-field administration of therapies immediately after SBE picked up momentum in the mid-2010s when an opinion piece in the New York Times written by Matthew Lewin (California Academy of Sciences) entitled, “The Killers Underfoot” stated that, “The world needs the snakebite equivalent of EpiPens — epinephrine autoinjectors used to stop allergic reactions. The ideal field treatment would have fewer side effects than antivenin and could be administered easily and inexpensively on site before the venom has spread. Nearly every study of snakebite has shown that survival depends most on shortening the time between bite and treatment. Reducing the lag time from hours to minutes is crucial to saving lives.“(Lewin, 2014) Since that time, an ever increasing number of research groups have been working on developing novel adjunctive therapies for SBE, and often mention the use of rapid field-based delivery of therapy. In 2021, a review of inhibitors that would complement antivenom as therapies for SBE states that, “The possibility of introducing therapies that could be administered in the field rapidly after the snakebite, with a good safety profile and without requiring specialized medical personnel, is being actively considered as a step forward in the management of envenomings."(Gutiérrez et al., 2021a). Only very recently has there been discussion of designing delivery methods for adjunctive therapies for SBE in the form of microneedles(Tiwari et al., 2022). This article seeks to expand this discussion.
Our initial review of the literature has revealed that since the 1950s, there has been an ever-growing effort to discover and develop adjunctive therapeutic compounds that could be administered immediately after SBE in a field-based setting to reduce negative outcomes and bridge the time-gap until IV antivenom can be administered. However, little attention has been devoted to developing the methods by which these potential therapies could be effectively delivered in the field immediately following SBE. Potential reasons for this deficiency in research on delivery methods for SBE therapies include challenges related to.
-
1)
Administration of therapies in austere rural environments
-
2)
Stability of therapies and compatibility of delivery methods particularly in tropical and sub-tropical environments
-
3)
Training requirements to effectively administer therapies
-
4)
Development of therapy formulations acting as regional or universal therapies
-
5)
Cost-effective production of therapies and associated delivery methods
-
6)
Regulatory limitations on development and implementation
-
7)
Procuring funding as SBE burdens are highest in economically challenged regions
-
8)
False perceptions that effective treatment is widely available in regions with high SBE burden (Asia, sub-Saharan Africa, South America)
-
9)
False perceptions that IV antivenom can reverse and treat all effects of SBE
4.3. Which venom components should adjunctive therapies target?
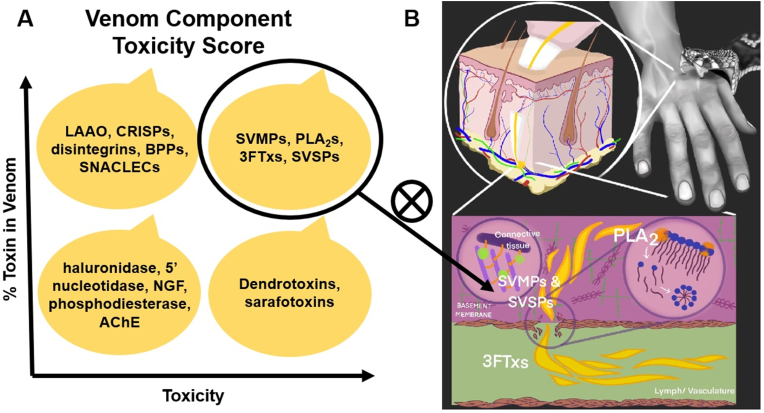
Most medically relevant snakes (those capable of causing harm to humans via envenoming) are members of one of only three clades: the families Elapidae (cobras, mambas, sea snakes, taipans, and their relatives) and Viperidae (vipers and pit vipers, including adders, rattlesnakes, and their relatives) and the subfamily Atractaspidinae (mole vipers/stiletto snakes)(Casewell et al., 2020). Through a combination of toxicological and proteomic analyses, toxicity scores have been developed to help identify the most relevant venom toxins that adjunctive SBE therapies should address (Laustsen et al., 2015). These scores are based on evaluations of specific activities (myotoxic, hemorrhagic, etc.) and lethality as well as relative abundances in the major medically relevant snake species. Based on this scoring regime, the four main categories of potential venom targets are those with: (a) high toxicity and high abundance, (b) high toxicity and lower abundance, (c) high abundance and lower toxicity, and (d) low abundance and low toxicity (Fig. 5A). It has been proposed that the first of these groupings, venom targets with both high abundance and high toxicity including snake venom metalloproteinases (SVMPs), phospholipase A2s (PLA2s), three-finger toxins (3FTs), and to a somewhat lesser extent, snake venom serine proteases (SVSPs), should receive the most attention in terms of developing potential adjunctive therapies for SBE (Fig. 5B).(Gutiérrez et al., 2021a; Laustsen et al., 2015) While there is significant inter-species variation in venom components, this approach seeks to maximize the likelihood that therapies administered would provide an SBE victim with broad protection by focusing on the most harmful and universally “common” venom targets. Fortunately, most medically relevant snakes are either elapids or vipers, with elapid venom consisting of mainly PLA2s and 3FTxs with lower amounts of SVMPs and SVSPs, and viper venom consisting mainly of PLA2s, SVMPs, and SVSPs with virtually no 3FTxs(Sanhajariya et al., 2018).
Fig. 5.
A: representation of toxicity scores where the major medically relevant snake venom toxins are distributed into four groups according to their percent abundance in venom and their toxicity (Abbreviations: LAAO: L-amino acid oxidases; CRISPs: cysteine-rich secretory proteins; BPPs: bradykininpotentiating peptides; SNACLECs: C-type lectin-like proteins; SVMPs: snake venom metalloproteinases; PLA2s: phospholipases A2s; 3FTxs: three-finger toxins; SVSPs: snake venom serine proteases; NGF: nerve growth factor; AChE: acetylcholinesterase) (adapted from Gutiérrez et al.)(Gutiérrez et al., 2021a). B: representation of SBE showing the initial bite followed by injection of venom into subcutaneous tissue and the generalized actions of PLA2s, SVMPs, SVSPs, and 3FTXs. Venom components with both high abundance and high toxicity (circled) should be prioritized for the development of novel inhibitors (X) that could be administered in the field immediately after SBE.
In addition to targeting venom toxins, there is growing interest in the potential to disrupt venom absorption by limiting intrinsic lymphatic pumping, the dominant propulsive mechanism in humans under resting conditions(Aukland, 2005; Olszewski and Engeset, 1980; Schmid-Schonbein, 1990), by paralyzing smooth muscle contraction involved with lymphatic flow(Saul et al., 2011). The reason for this interest is that these toxins are thought to enter systemic circulation mainly by lymphatic absorption or direct vascular entry. High molecular weight toxins such as SVMPs and SVSPs enter the bloodstream through the lymphatic system, while lower molecular weight toxins such as PLA2s and 3FTXs may enter the vasculature directly(Neri-Castro et al., 2020; Paniagua et al., 2012, 2017, 2019; Sanhajariya et al., 2018; Supersaxo et al., 1990; van Helden et al., 2014, 2019; Vergara et al., 2016).
4.4. Potential adjunctive field-based SBE therapies
4.4.1. Small molecule adjunctive therapies for SBE
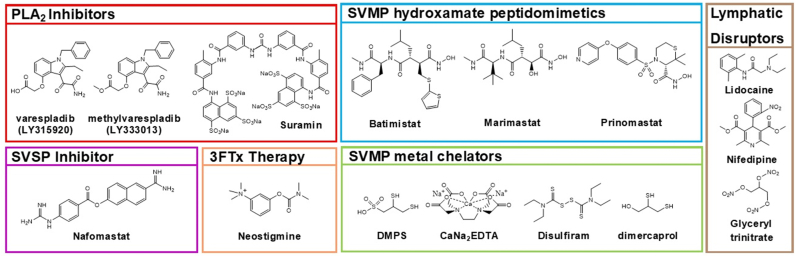
There are a number of promising small molecule therapeutics that could be used to target PLA2s, SVMPs, and SVSPs whereas drugs targeting non-enzymatic neurotoxins such as the 3FTxs represent a more challenging target that existing small molecule drugs may not be able to address(Gutiérrez et al., 2021a). In addition, there are several small molecules that reduce venom effects by the disrupting lymphatic absorption. An increasingly popular approach to the development of drugs for SBE therapy is to utilize “repurposed” molecules that were developed to address other diseases and often have significant clinical efficacy and toxicological data(Bulfone et al., 2018; Puzari et al., 2021). Ethno-pharmaceuticals for use as SBE therapy also show promise and reviews are provided elsewhere(Puzari et al., 2022). The leading candidates for small molecule adjunctive therapies for SBE have been reviewed and are presented here (Fig. 6) (Bulfone et al., 2018; Clare et al., 2021; Gutiérrez et al., 2021a; Hamza et al., 2021; Laustsen et al., 2016; Puzari et al., 2021).
Fig. 6.
Structures of small molecule therapeutics with potential utility as field-administered adjunctive therapies against SBE. Molecules that inhibit PLA2s include, varespladib, methyl varespladib, and suramin. Molecules that inhibit SVMPs include the hydroxamate peptidomimetics batimistat, marimastat, and prinomastat. Metal chelators DMPS, CaNa2EDTA, disulfram, and dimercaprol can inhibit SVMPs. The molecule nafomastat shows promise as a SVSP inhibitor. Neostigmine can be used to reverse the neurotoxic effects of elapid bite, while lidocaine, nifedipine, and glyceryltrinitrate can disrupt lymphatic flow. Many of these molecules are repurposed drugs including both methyl varespladib and DMPS which are currently in human clinical trials as potential treatments for SBE.
The PLA2 inhibitor varespladib (LY315920) was originally developed for treatment of pancreatitis and sepsis and, later, entered phase III trials for the treatment of coronary heart disease(Nicholls et al., 2014). More recently, varespladib and its orally bioavailable pro-drug methyl varespladib (LY333013) have been repurposed by Ophirex(Bulfone et al., 2018), and have been shown to reduce mortality in a variety of in vivo models by protecting against neurotoxic, myotoxic, and coagulopathic effects brought about by PLA2 toxins found in a variety of elapid and viper venoms from different geographic regions(Bittenbinder et al., 2018; Bryan-Quirós et al., 2019; Chowdhury et al., 2021a; Dashevsky et al., 2021; Gutiérrez et al., 2020; Lewin et al., 2016, 2018a, 2018b; Salvador et al., 2021; Tan et al., 2022; Wang et al., 2018; Youngman et al., 2020; Zdenek et al., 2020; Zinenko et al., 2020). Orally administered methyl varespladib represents a likely candidate for wide-spread use as an adjunctive treatment of SBE and has recently entered a multicenter, randomized, double-blind, placebo-controlled, phase 2 study in the United States and India(Carter et al., 2022). The anti-trypanosomal drug suramin, inhibits the myotoxic and cytotoxic activities of a variety of geographically distinct presynaptic PLA2 neurotoxins including β-bungarotoxin, taipoxin, ammodytoxin, bothropstoxin-I, and provides protection against the myotoxic effects in venom from a number of North and South American crotalids as well as the Asian viper Echis carinatus(Arruda et al., 2002; de Oliveira et al., 2003; Fathi et al., 2011; Hajari et al., 2019; Murakami et al., 2005; Salvador et al., 2018).
The peptidomimetic hydroxamate containing SVMP inhibitors batimastat, and its more bioavailable cousin marimastat(Fischer et al., 2019), were first generation matrix metalloproteinase (MMP) inhibitors originally developed as anti-cancer therapies(Li et al., 2020; Rasmussen and McCann, 1997; Winer et al., 2018), but have been repurposed as potential SBE therapies(Preciado and Pereañez, 2018). Both are potent in vitro inhibitors of a variety of isolated SVMPs, and in several animal models injected with whole venoms, have provided protection against hemorrhage, skin necrosis, and mortality(Arias et al., 2017; Escalante et al., 2000; Howes et al., 2007; Layfield et al., 2020; Menzies et al., 2022; Rucavado et al., 2000). The MMP inhibitor prinomastat (AG-3340) has also been reinvestigated and found to inhibit SVMPs and whole venom from E. ocellatus(Howes et al., 2007). In addition, prinomastat has displayed dual-inhibitory properties as it inhibits both SVMPs responsible for factor Xa activation in procoagulant venoms from a variety of viper species(Chowdhury et al., 2021b), while at the same time inhibiting PLA2 toxins of all the African spitting cobras(Chowdhury et al., 2021a).
Because SVMPs require a divalent cation such as zinc for activity, the use of metal chelating agents such as CaNa2EDTA(Flowers and Goucher, 1963, 1965; Gowda et al., 2011; Howes et al., 2007; León et al., 1998; Rucavado et al., 2000; Silva-Neto et al., 2018), tetraethyl thiuram disulfide (Disulfiram/TTD)(Nanjaraj Urs et al., 2015; Rudresha et al., 2021), 2,3-dimercapto-1-propanesulfonic acid (DMPS/Unithiol)(Albulescu et al., 2020a; Menzies et al., 2022; Xie et al., 2020a), and dimercaprol (British anti-Lewisite) (Albulescu et al., 2020b; Xie et al., 2020b) for SBE therapy has been demonstrated in a variety of animal models. These agents have traditionally been used to treat metal intoxication(Flora and Pachauri, 2010), but have found a use as repurposed drugs for SBE therapy. Despite the fact that these molecules typically exhibit non-specific zinc binding, the oral administration of the chelator DMPS provides in vivo neutralization of local and systemic effects of Echis ocellatus venom(Albulescu et al., 2020a, 2020b). Orally administered DMPS has recently entered phase I clinical trials in Kenya as a potential SBE therapy(Abouyannis et al., 2022).
Inhibitors specifically targeting SVSPs are challenging due to off target effects on serine proteases found in the blood clotting cascade(Gutiérrez et al., 2021a). While SVSPs can have significant effects upon envenoming, they have received less attention as potential targets because they are typically found in lower quantities in most snake venoms. However, the serine protease inhibitor nafamostat was recently shown to broadly neutralize the SVSP activity found in South American, African, and Asian viper venoms in a dose-dependent manner(Albulescu et al., 2020b).
The α-neurotoxins are non-enzymatic peptide toxins present in the venom of elapid snakes and belong to the superfamily of three finger fold proteins (3FPs) that competitively inhibit a variety of molecular targets, most notably the nicotinic acetylcholine receptor (nAChR). Upon binding, these molecules abolish normal neurotransmission leading to paralysis and subsequent respiratory failure(Nirthanan et al., 2017). The most well characterized of this superfamily are the three-finger toxins (3FTxs) that exhibit a wide range of biological activities, including cytotoxicity, proteinase activity, and neurotoxicity(Kessler et al., 2017; Utkin, 2019). The small molecule neostigmine is an acetylcholinesterase (AChE) inhibitor that prevents the breakdown of acetylcholine (ACh) thus prolonging neurotransmission. Neostigmine has been used either IM or IP in mouse models to reverse the neurotoxic effects of 3FTxs found in elapid venom(Banerjee et al., 1976; Irshad et al., 2021). In addition, internasal administration of neostigmine had similar effects on a single human patient in a clinically controlled setting at very high concentration (8.4% w/v)(Lewin et al., 2013, 2014). While controversial, neostigmine is commonly used in small doses as part of India's national snakebite management protocol despite potentially significant side effects(Mahadevan and Jacobsen, 2008; Naguib and Kopman, 2018; Karthika and Satapathy, 2021). Neostigmine has recently been suggested as a transdermal therapy for elapid SBE, but this approach has yet to be validated(Tiwari et al., 2022). Discovering drugs that counteract non-enzymatic toxins such as 3FTxs remains challenging, and is more likely to be addressed with larger biologic molecules such as phage display peptides(Lynagh et al., 2020), receptor decoys(Albulescu et al., 2019), DNA aptamers(Alomran et al., 2022; Chen et al., 2016), and recombinant antibodies(Laustsen et al., 2015). With the recent structural determination of the molecular mechanism of α-neutrotoxin binding to muscle-type nAChRs(Nys et al., 2022), it is likely that increased research on the use of small molecules to inhibit these toxins will increase.
Drugs that disrupt the lymphatic absorption of venom components were demonstrated first using a topical application of glyceryl trinitrate, a nitric oxide donor that reduced lymphatic transit in the legs of human subjects approximately three-fold, and increased time to respiratory arrest by approximately 50% in rats injected with Pseudonaja textilis venom(Saul et al., 2011). However, the use of glyceryl trinitrate to treat SBE in humans should be carefully considered as its use could invoke a rapid decline in blood pressure resulting in cardiovascular collapse for patients also utilizing contraindicated vasodilating drugs (e.g. phosphodiesterase 5 (PDE-5) inhibitors such as sildinafil/Viagra)(Webb et al., 1999). Fortunately, similar results were found for both lymphatic transit time and time to respiratory arrest using topical applications of the Ca2+ channel antagonist nifedipine and the local anesthetic lidocaine (lignocaine) in the same model system(van Helden et al., 2014). Importantly, lidocaine can reduce lymphatic transit times up to 60 min when administered SC. Conversely, lymphatic flow is increased when both lidocaine and epinephrine were administered in combination(Kwon and Sevick-Muraca, 2016). Because epinephrine is often given to help reduce anaphylaxis when SBE patients are administered antivenom, the use of lidocaine in combination with epinephrine should be carefully considered(de Silva et al., 2011). The recent finding that the crotamine-like protein from Crotalus durissus terrificus activates calcium influx, decreases nitric oxide release, and increases rates of lymphatic transport of large molecular weight molecules, provides additional evidence that therapeutics targeting lymphatic flow shows promise(Si et al., 2023).
The ability of small molecules to inhibit or alter the action of multiple toxin isoforms from the major venom toxin families (PLA2s, SVMPs, SVSPs, 3FTxs) or that disrupt lymphatic absorption of venom components provides the opportunity to develop drug combinations that one day may be used as a pan-specific therapy(Clare et al., 2021; Hall et al., 2022). This approach has been demonstrated when a combination of marimastat and varespladib showed synergistic efficacy over the individual drugs and prevented mortality in mice when exposed to venom from the most medically important vipers of South Asia, Africa, and Central America(Albulescu et al., 2020b; Xie et al., 2020a). There are also a growing number of examples demonstrating the synergistic effects of small molecule drugs when used in combination with traditional biologic antivenoms (see section 4.5) (de Souza et al., 2022; Lay et al., 2023; Silva-Carvalho et al., 2022; Silva et al., 2021).
While small molecules hold tantalizing potential for the treatment of SBE in humans, their use remains experimental, and none have been clinically validated. However, the use of small molecule drugs as therapies for SBE are envisioned as adjunctive to conventional polyvalent antivenom, and their use represents a much different approach than current practice(Bulfone et al., 2018; Clare et al., 2021; Gutiérrez et al., 2021a; Hamza et al., 2021; Laustsen et al., 2016; Puzari et al., 2021). The advantages that small molecules drugs possess when compared to conventional biologic antivenom therapy have been summarized and include cross-species inhibition, stability, timeliness and ease of administration, lower immunogenicity, and affordability(Clare et al., 2021). Small molecule drugs can be administered by a variety of convenient and safe routes and generally have relatively short half-lives coupled with rapid systemic and local tissue penetration due to their large volumes of distribution. In contrast, biologic antivenoms must be administered IV, and because of their small volume of distribution, are characterized by rapid systemic distribution with little extravascular local penetration. Thus, antivenoms are often not effective in addressing local SBE effects, particularly if treatment is given well after envenoming has occurred. In contrast, the small molecule therapeutics mentioned above have the potential to reduce the local tissue damage inflicted by many venoms, particularly if applied near the site of envenoming. It is possible that combinations of these repurposed pharmaceuticals may have enhanced synergistic effects towards a variety of venom toxins, and if administered immediately after envenoming, could provide additional time for an SBE victim to be administered IV antivenom.
4.4.2. Biologic adjunctive therapies for SBE
Biologic therapies are generally classified as substances derived from living organisms that either occur naturally or can be synthesized in the laboratory and are used to treat disease. Biologic therapies such as the antibodies found in antivenom are effective due to their inherent specificity resulting from the in vivo selection and maturation process involved in their production. This specificity also presents a significant limitation with respect to their use for treatment of SBE. It is well established that inter- and intra-specific variations in snake venom composition render the specific antibodies found in existing animal-derived antivenoms ineffective against heterologous toxins found in different venoms(Casewell et al., 2014). As a result, monovalent antivenoms raised against a particular species of snake are often ineffective in treating snakebite by different, even closely related species, and thus, an SBE victim must be given antivenom that is specific to the offending snake species.
Unfortunately, most regions with high SBE burden (Southeast Asia, sub-Saharan Africa, Central and South America) have diverse populations of venomous snakes with overlapping geographic ranges often making it difficult for clinicians to determine exactly which species caused an envenoming, thus, polyvalent antivenom is often employed. Polyvalent antivenom is produced when pooled venom from a variety of regionally important medically relevant snake species is used to inoculate production animals (i.e. horse, sheep, goat, donkey), and the resulting antibodies are then isolated(Gutiérrez et al., 2017). In order to reduce the risk of adverse reactions or to improve bioavailability, some antivenom manufacturers will further process the whole IgG antibodies extracted from animal plasma by enzymatic digestion with either pepsin or papain. Pepsin digestion removes the Fc moiety from the antigen-binding (Fab) fragment producing a F(abʹ)2 fragment, while papain digestion produces smaller Fab fragments(Gutiérrez et al., 2017; World Health Organization, 2017). While there are examples of successful production and application of both mono- and polyvalent antivenoms, they still possess the limitations discussed previously (Section 1) and the number of global manufacturers, and thus supplies, have decreased in the last decade(Alirol et al., 2015; Potet et al., 2021; Williams et al., 2019).
It is helpful to classify biologic therapies by their biophysical properties and molecular size, typically expressed as a molecular weight in kiloDaltons (kDa). For the purposes of this discussion, we also characterize larger sized nanoparticle and polymer derived therapies as biologic therapies despite their “unnatural” nature. The biophysical properties and molecular size affect both the pharmacodynamics (PD) and pharmacokinetics (PK) of therapeutic molecules. Pharmacodynamics describe a molecule's in vivo ability to neutralize specific venom toxins, whereas pharmacokinetics describe the rates of absorption, distribution, metabolism, and excretion which are generally characterized by parameters such as volume of distribution (Vd), bioavailability (F), clearance (CL), maximum concentration in plasma (Cmax), and elimination half-life (t1/2)(Laustsen et al., 2018). The PK properties of biologic therapies such as IgG, F(abʹ)2, and Fab antivenom used for SBE, play a critical role in their effectiveness. In general, as the molecular weight of the biologic therapy increases, therapy half-life tends to increase. Conversely, as molecular weight increases, volume of distribution and thus peripheral tissue distribution and penetration tends to decrease(Gutiérrez et al., 2003). Thus, smaller Fab fragments are more rapidly cleared via renal filtration resulting in a shortened half-life, but can penetrate extravascular tissues more easily, due to their larger volume of distribution. Conversely, larger IgGs do not penetrate extravascular tissues readily due to a small volume of distribution and have much longer half-lives because they are not filtered renally and are recycled by the neonatal Fc receptor (FcRn) of cells in the mononuclear phagocytic system(Tabrizi et al., 2006). The F(abʹ)2 fragments appear to have PK profiles intermediate to those of Fab and IgGs.
There are several potential formats of biologic therapy that could be considered as field-administered adjunctive therapies for SBE. These include antibody formats(Hamza et al., 2021; Laustsen et al., 2018), antibody-like protein scaffold formats(Jenkins et al., 2019), peptide formats(Komives et al., 2017; Laustsen et al., 2022; Lipps, 1996; Lynagh et al., 2020; Silva et al., 2021; Titus et al., 2017), DNA/RNA aptamer formats(Alomran et al., 2022; Ascoët and De Waard, 2020; Chen et al., 2016), and nanoparticle polymer formats(Karain et al., 2016; Nakamoto et al., 2021; Nakamoto et al., 2020; O'Brien et al., 2016; O'Brien et al., 2018). Each format has its own advantages and disadvantages centered around attributes such as safety, immunogenicity, serum half-life, rates of systemic or peripheral distribution and penetration, method of selection (in vivo vs. in vitro), cost to manufacture, stability, and solubility. The major biologic therapy formats that show potential as adjunctive therapies for SBE are summarized in Table 1.
Table 1.
The attributes considered for potential biologic and large molecule (polymer) formats for use as SBE therapies include molecular weight (kDa), serum half-life, human immunogenicity, tissue distribution and penetration inferred from volume of distribution, selection technique, and cost to manufacture. Scores for these attributes were adapted from recent reviews(Laustsen et al., 2018; Ascoët and De Waard, 2020; Hamza et al., 2021; Knudsen et al., 2019; Laustsen, 2019), or inferred based on general trends of pharmacokinetics or published reports. The major antibody formats used in either existing animal-derived antivenoms (where indicated) or experimental recombinant/monoclonal antivenom include IgG: whole IgG antibody (∼150 kDa), F(ab’)2: pepsin-digested IgG antigen-specific region (∼110 kDa), Fab: papain-digested antigen-specific region (∼50 kDa), scFv: single-chain variable fragments (∼25 kDa), and Camelid VHH (nanobodies): single-domain antigen-specific fragments (∼15 kDa). Additional formats with promising potential as a therapy for SBE include antibody-like protein scaffold formats (∼1.5–20 kDa), peptide formats (∼1–10 kDa), DNA/RNA aptamer formats (∼3–30 kDa), and nanoparticle polymer formats (variable kDa).
| Therapy Format | Mol. Wt. |
Serum Half-Life |
Immunogenicity |
Tissue Dist./Penetration |
In vivo maturation |
Cost to Manufacture |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (kDa) | Long | Medium | Short | High | Low | High | Medium | Low | Yes | No | Low | Medium | High | |
| Antibody Format | ||||||||||||||
| Animal-derived polyclonal IgG | ∼150 | X | X | X | X | X | ||||||||
| Animal-derived polyclonal F(ab′)2 | ∼110 | X | X | X | X | X | ||||||||
| Animal-derived polyclonal Fab | ∼50 | X | X | X | X | X | ||||||||
| Human IgG | ∼150 | X | X | X | X | X | ||||||||
| Human F(ab′)2 | ∼110 | X | X | X | X | X | ||||||||
| Human Fab | ∼50 | X | X | X | X | X | ||||||||
| Human scFv | ∼25 | X | X | X | X | X | ||||||||
| Camelid VHH (Nanobodies) | ∼15 | X | X | X | X | X | ||||||||
| Bispecific IgG | ∼150 | X | X | X | X | X | ||||||||
| Bi/multispecific VH and VHH | ∼10–15 | X | X | X | X | X | ||||||||
| Antibody-like scaffold proteins | ∼1.5–20 | X | X | X | X | X | ||||||||
| Peptide Format | ||||||||||||||
| Phage display peptides | ∼1–10 | X | X | X | X | X | ||||||||
| pepB/pepC | ∼0.7 | X | X | X | X | X | ||||||||
| LTNF Peptides | ∼1.5 | X | X | X | X | X | ||||||||
| DNA/RNA aptamers | ∼3–30 | X | X | X | X | X | ||||||||
| Nanoparticles | Variable | ? | ? | ? | X | ? | ? | ? | X | X | ||||
Given the complexities of the biologic formats presented in Table 1, the question arises, “Is there a role for biologic therapies for the pre-hospital treatment of SBE”? In a comparison of different biologic therapy formats, Lausten et al. argues that it is unlikely that the animal-derived IgG antibody format will be used in a field setting for several reasons(Laustsen et al., 2018). First, these preparations can cause life-threatening adverse reactions, a situation that would only exacerbate the precarious situation of SBE. Second, without IV administration, IgGs are slow to absorb or can be degraded via other potential routes of administration (see section 5.1 below for additional discussion). Third, the distribution of IgGs are mostly restricted to the intravascular compartment, where they are effective in neutralizing systemically acting toxins for many days due to their long elimination half-life, but they have limited ability to act on extravascular tissues near the site of envenoming. However, because smaller antibody fragments, peptides, or aptamers have larger volumes of distribution allowing them to penetrate tissue compartments more effectively, they have the potential to neutralize both toxins present in or around the bite wound, and toxins that have reached the systemic circulation or target organs. Most of these smaller formats have lower human immunogenicity, particularly those that involve the use of recombinant, monoclonal, or “humanized” technologies(Laustsen et al., 2018). Unfortunately, these smaller biologic formats have a shorter elimination half-life, and may require increased or prolonged dosing for maximal effect. The use of abiotic toxin binding nanoparticles administered subcutaneously at the site of SBE to reduce the extent of local tissue damage and mitigate the systemic distribution of toxins is an exciting prospect(Nakamoto et al., 2020; O'Brien et al., 2018), but issues related to safety and toxicity still need to be investigated.
4.5. Regional therapies required
Venomous snakes are widely distributed, especially in tropical countries, from sea level to altitudes of up to 4900 m (Gloydius himalayanus) and occur in ranges of latitude from 47 °S in Argentina (Bothrops ammodytoides) to 72 °N into the Arctic circle (Vipera berus)(World Health Organization, 2017). In addition, tremendous venom component diversity exists across phylogenetic levels of venomous snakes(Casewell et al., 2014). As a result of these geographic and biological variations, it is unlikely that a universal antidote or therapy could be developed for SBE covering all venomous snakes. Fortunately, the WHO has classified the venomous snake species representing the greatest threat to public health and divided them into two categories(World Health Organization, 2017). Category 1 (CAT 1) are species with the highest medical importance, having widespread distribution, causing numerous SBEs, and resulting in high levels of morbidity, disability, or mortality. Category 2 (CAT 2) are species of secondary medical importance capable of causing morbidity, disability, or mortality, but lack solid epidemiological or clinical data, and are less frequently implicated owing to their activity cycles, behavior, habitat preferences or occurrence in areas remote from large human populations. The WHO considers snakes in both CAT 1 and CAT 2 as species for which antivenom production is important, however CAT 1 species should be given the highest priority for antivenom production as they are responsible for greater SBE burdens. In addition, based on current herpetological, epidemiological, and medical literature, the WHO has divided the medically relevant snake species into four main regions: Europe, the Americas, Africa, and Australasia. Each of these main regions is further divided into sub-regions with the aim of helping antivenom producers identify cohorts of “regional” snake species for the production of polyvalent antivenom.
The use of these regional groupings of medically relevant snakes, and considerations about the commonalities between their venom components, could be used to guide the types of adjunctive therapies that might be combined to provide broad cross-species protection to SBE victims.(Bulfone et al., 2018; Clare et al., 2021; Gutiérrez et al., 2021a; Laustsen, 2018). For example, medically relevant snakes in the Americas or Europe are dominated by viperids while those in Australia are elapids. Thus, a combination of adjunctive therapies for SBE victims in the Americas or Europe might include small molecule inhibitors of PLA2s, SVMPs, SVSPs, and metal chelators, while SBE victims in Australia might benefit more from a combination of small molecule PLA2 inhibitors and elapid specific 3FTx inhibitors. Several recent studies demonstrate the synergy provided when a combination of therapies was utilized to address SBE. The first study examined the synergistic use of a SVMP inhibitor (marimastat) and a PLA2 inhibitor (varespladib) with an in vivo mouse model demonstrating increased protection against medically relevant vipers of Africa, South Asia and Central America when the therapies were used in combination(Albulescu et al., 2020b). A second study examined the use of varespladib and antivenom to the South American rattlesnake Crotalus durissus terrificus, a venom that contains both the presynaptically-acting heterodimeric PLA2 crotoxin and the cell penetrating myotoxin crotamine(de Souza et al., 2022). This study demonstrated that varespladib was effective in reducing the neuromuscular blocking activity of C. d. terrificus venom and acted synergistically with antivenom. A third study demonstrated in vivo synergistic effects in a mouse model when the SVSP inhibitor peptides pepB and pepC were used in combination with bothropic antivenom. This approach significantly reduced coagulopathy and hemorrhage induced by B. jararaca venom(Silva et al., 2021). A fourth in vitro study examined a combination of varespladib and Chinese D. siamensis (Russell's viper) antivenom finding that when administered 60 min after envenoming, the combination failed to produce better results than varespladib alone, demonstrating that the window of time in which antivenom remains effective is relatively short compared to the small molecule inhibitor varespladib(Lay et al., 2023).
While the last three studies mentioned above used small molecules in combination with animal-derived antivenom, the future use of the smaller biologic formats mentioned above, including recombinant or monoclonal antivenom fragments, would eliminate concerns related to adverse reactions. A recent review of next-generation biologic antivenoms summarized views concerning future SBE treatments as follows: “Due to their complexity, snake venoms are unlikely to be neutralized by a single molecule, and this makes ‘one hit’ drugs therapeutically unfeasible. Furthermore, as snake venoms are highly diverse and different across continents, developing a ‘universal antivenom’ that theoretically would be able to neutralize all key toxins from all snakes across the world seems highly infeasible and irrelevant. However, future antivenoms will most likely be quite complex therapeutic products with mixed compositions of toxin-neutralizing modalities.” (Knudsen et al., 2019) Combining different biologic therapy formats with small molecule drugs is an exciting possibility for SBE therapy, and such an approach will require significant research on therapy formulations and effective routes of administration.
5. Considerations for delivery of field-administered therapies for SBE
5.1. Route of SBE therapy administration
Since its inception, the United States Food and Drug Administration (USFDA) has approved a total of approximately 35,000 drugs for human use. The routes of administration of these drugs can be broken down into six major categories with the following distributions: oral (62.02%), injection (22.5%), cutaneous (8.70%), mucosal (5.22%), inhalation (1.24%), and a remaining category defined as “other” (0.34%)(Zhong et al., 2018). Clearly, oral (PO) drug delivery remains the most appealing delivery route due to the ease of administration coupled with high patient compliance. As a result, generic drug companies favor the development of oral medications with approximately 70% of their formulations being delivered via the oral route. The PLA2 inhibitor methyl varespladib entered clinical trials in 2022 as an oral medication for the treatment of SBE in both the United States and India(Carter et al., 2022). In addition, a smaller trial involving both oral and IV administration of the metal chelator (DMPS) as a potential treatment of SBE has recently started in Kenya where the pharmacokinetics and tolerability of DMPS will be determined(Abouyannis et al., 2022).
Although at first glance oral delivery of SBE therapeutics appears ideal, there are some significant disadvantages to this approach. The low pH and presence of digestive enzymes in the gastrointestinal tract can degrade some therapeutics, particularly biologic therapeutics, well before absorption into the bloodstream. Additionally, many drugs become insoluble at low pH significantly reducing their bioavailability, and drugs taken orally can have variable absorption rates due to an inability to effectively cross the epithelial lining in the gut resulting in variable serum concentrations. Furthermore, drugs ingested orally can be inactivated by hepatic enzymes before entering systemic circulation, and many medications may cause gastrointestinal side effects. Finally, oral administration can only be applied to conscious patients capable of swallowing(Alqahtani et al., 2021; Jain, 2020).
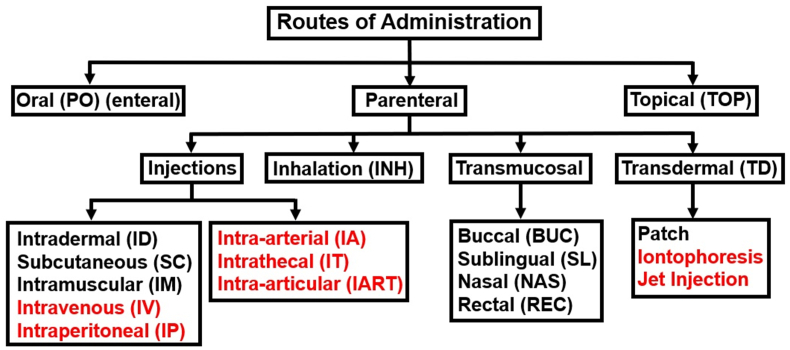
Many researchers have reported on the potential of administration of therapies for SBE in the field immediately after a bite. However, few have discussed a route or method to deliver these agents with most authors simply stating that therapies could be “administered” or “given” in the field immediately after envenoming. The nasal (NAS) administration of neostigmine to counteract the neurotoxic effects of elapid envenoming shows promise, but this approach has only been confirmed in a hospital setting for a single human patient (Lewin et al., 2013). An ideal route of delivery for SBE therapies in the field would be compatible with both small molecule drugs and larger biologic formats discussed above (see section 4, 4.4.1.4.2), and should maximize their bioavailability, systemic and local distribution, and metabolic stability. In addition, therapies given in the field should be easily self-administered or given by individuals without specialized training and account for the possibility of victim incapacitation or unconsciousness. The route of administration should not cause significant pain or fear as these can reduce patient willingness to receive therapy(Berteau et al., 2010; Gupta et al., 2011). The major routes of administration that might be considered for administration of adjunctive therapies for the field-administration of SBE therapies are shown in Fig. 7.
Fig. 7.
The major routes of administration (and related abbreviations) that might be considered for administration of adjunctive therapies for the field-based treatment of SBE. Routes that would involve specialized medical equipment or trained personnel are highlighted in red. Adapted from Udaykumar et al.(Udaykumar, 2017).
To evaluate convenient potential routes of delivery for their ability to deliver adjunctive therapeutics for SBE in the field, we have performed a viability analysis by examining a variety of parameters that are relevant for SBE therapies (Table 2). To each parameter we assigned scores of zero, one, two, or three representing a particular route's effectiveness as being none, low, moderate, or high, respectively. Tallying up individual parameter scores resulted in a cumulative “viability score” for each of the routes of delivery. This approach is meant to highlight the advantages and disadvantages each delivery route may have and is not meant to exclude any particular route from consideration. A combination of either small molecule or biologic therapies tailored to treat regional snake species of medical importance might also require several routes of delivery.
Table 2.
Viability analysis of convenient SBE therapy routes of delivery listed in descending order of cumulative viability score. Individual scores for each parameter were assigned as 0 = none, 1 = low, 2 = moderate, and 3 = high. The anatomical routes of therapy administration considered in this analysis are oral (OP), buccal (BUC), sublingual (SL), rectal (REC), nasal (NAS), intradermal (ID), subcutaneous (SC), intramuscular (IM), intravenous (IV), transdermal (TD), topical (TOP), and pulmonary inhalation (INH). IV administration is shown for comparison but would require specialized training. Parameters for evaluation were selected as relevant for adjunctive SBE therapies given in the field immediately after envenoming and scores were assigned based adaptations from previous work(Jain, 2020; Jin et al., 2015; Jonaitis et al., 2021).
| Route of Delivery | Ease of Self-Admin. | Dose Control | Volumn greater 1 mL/1g | Bioavail. | Avoid Deg. (Stomach/Liver) | Rapid Systemic Dist. | Onset of Action | Protection from infection | Tolerate Biologics | Rapid Dist. Locally | Uncon. Admin. | Pain Free | Viabililty Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intramuscular (IM) | 2 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 30 |
| Subcutaneous (SC) | 2 | 3 | 2 | 3 | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 29 |
| Nasal (NAS) | 3 | 1 | 1 | 2 | 3 | 2 | 3 | 3 | 2 | 2 | 3 | 3 | 28 |
| Intravenous (IV) | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 2 | 3 | 1 | 28 |
| Buccal (BUC) | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | 1 | 1 | 3 | 3 | 27 |
| Sublingual (SL) | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 26 |
| Resp. Inhilation (INH) | 3 | 2 | 1 | 2 | 3 | 2 | 3 | 3 | 3 | 2 | 0 | 2 | 26 |
| Rectal (REC) | 2 | 1 | 3 | 2 | 2 | 2 | 2 | 3 | 1 | 2 | 3 | 2 | 25 |
| Intradermal (ID) | 3 | 2 | 0 | 2 | 3 | 1 | 1 | 2 | 3 | 1 | 3 | 2 | 23 |
| Oral (PO) | 3 | 3 | 3 | 2 | 0 | 2 | 2 | 3 | 0 | 1 | 0 | 3 | 22 |
| Topical (TOP) | 3 | 1 | 1 | 1 | 3 | 0 | 1 | 3 | 1 | 1 | 3 | 3 | 21 |
| Transdermal (TD) | 3 | 1 | 0 | 1 | 3 | 1 | 1 | 3 | 0 | 2 | 3 | 3 | 21 |
The viability analysis presented is meant to illustrate that there are multiple viable routes of therapy delivery for adjunctive treatment of SBE. The cumulative viability scores are composite estimates and will be different for individual therapeutics due to differences in chemical and physical properties as well drug formulation. What becomes apparent is that oral (PO) delivery is a viable option but is hampered by its inability to be used with an unconscious victim, and its inability to tolerate biologic therapies due to chemical modification by the liver and degradation primarily in the stomach. On the other hand, routes of delivery that do not involve injection of therapies such as nasal (NAS), sublingual (SL), buccal (BUC), respiratory inhalation (INH), and even rectal (REC) administration are viable options for the delivery of a wide variety of therapeutics. These routes avoid potential complications from infection or hematoma and will cause little to no pain when administered, however, they suffer from limited bioavailability for polar or biologic therapeutics and their reduced ability to cross mucosal membranes. Administration by TD, TOP, and ID methods are convenient but are limited with respect to large dosing, bioavailability, and ability of polar or biologic therapies to infiltrate below the dermal layer to provide significant local or systemic distribution(Benson et al., 2019).
Intramuscular (IM) and subcutaneous (SC) injection of therapeutics appear to have the most cumulative advantages for SBE therapy administration. These advantages include avoidance of gastrointestinal or hepatic modification or degradation well as excellent dose control and absorption characteristics providing relatively rapid local and systemic distribution. While IM and SC routes are not without pain or risk of infection, their ability to be delivered to an unconscious victim or to be self-administered are clear advantages. It should be noted that the possibility of local hematoma formation may be a disadvantage of SC or IM delivery, particularly if the therapy is injected local to an envenoming site that is already severely swollen or developing necrosis. This situation may be avoided if SC or IM injection is provided immediately after envenoming before these symptoms appear or by injection at a site other than the envenoming site.
The use of multiple routes of administration for SBE therapeutics may be required for the effective use of small molecule drugs, biologic therapies, or combinations of both. Different administration routes may be required due to the specific mechanisms of absorption and distribution for individual therapies. In addition, as discussed in section 4.5, the treatment of SBEs appropriate therapies may require different routes of administration based on regional differences in venomous snake populations.
5.2. SBE location
The location of envenoming could affect the success of pre-hospital treatment of SBE due to differences in tissue composition and vascularization. Reviews of North American and Indian SBE incidents reveal strikingly consistent locations of SBE with approximately 80% are to the lower extremities (foot, ankle, calf), 18% to the upper extremities (hand, wrist, forearm), 2% (thigh, torso, shoulder, back) (Kulkarni and Anees, 1994; Russell et al., 1975; Willson, 1908) Similar findings are presented in a review of SBE incidents in sub-Saharan Africa between 1970 and 2010 with approximately 76 percent of SBE occurring on the lower extremities(Chippaux, 2011). In Europe, SBE locations are divided more equally between lower and upper extremities(Chippaux, 2012). Interestingly, there is growing evidence that trends in the anatomical location of SBE may be changing with respect to socioeconomic conditions and gender with females and people with lower socioeconomic conditions differing from males and those with higher socioeconomic conditions(Albuquerque et al., 2013; Blackman and Dillon, 1992; O'Neil et al., 2007). It is unclear how epidemiological differences may affect the pre-hospital delivery of SBE therapies.
The location of SBE is important when it comes to the use of adjunctive field-based therapies and their ability to rapidly distribute either systemically or locally. When SBE occurs on an extremity, the likelihood of amputation or surgical fasciotomy is increased, particularly if antivenom is given late during the ETT(Bennett et al., 1961; Hall, 2001; Russell et al., 2021; Stewart et al., 1989). The absorption kinetics of both venom components and potential therapies will undoubtedly vary depending on the location and vascularization of the tissue surrounding the site of SBE. The rapid use of adjunctive therapies at or near the site of envenoming may slow the progression of venom toxicity and absorption and decrease the need for surgical intervention.
5.3. Species-specific venom delivery apparatus
Snakes that utilize venom for the capture of prey are often classified by the position of their fangs as either rear- or front-fanged snakes, with the majority of medically relevant snakes being front-fanged (Viperidae and Elapidae)(Vonk et al., 2008). The venom glands in viperids and elapids are enclosed in a muscularized fibrous sheath that allows the venom glands to inject venom by contraction of the compressor muscle(Young and Kardong, 2007). In elapids, the venom fang is attached to a rigid maxillary bone, and thus is always erect. However, viperid snakes have a maxilla capable of rotating, enabling the fang to be erected or laid parallel to the jaw when in the relaxed state. The ability to rotate the maxilla is why viperids are said to possess the most efficient venom delivery systems of all venomous snakes(Pucca et al., 2020).
The venom delivery apparatus can affect the volume of venom injected, fang penetration width and depth, and prevalence of dry bites. The fang length of venomous snakes ranges between 5.0 cm in certain vipers (Bitis gabonica, Bitis rhinoceros) to as little as 0.2 cm in some elapids (Pseudonaja guttata)(Mirtschin et al., 2017). There are studies related to fang length, inter-fang distance, and venom yield for some medically relevant snakes available, but additional measurements would be useful to compare these attributes for regional groupings of medically relevant snakes(Braga et al., 2022; de Roodt et al., 2016; de Roodt et al., 1998; Gao et al., 2019; Hayes et al., 2017; Mirtschin et al., 2017; Mirtschin et al., 2002; Morrison et al., 1983; Nascimento da Costa et al., 2020). The amount of venom delivered as well as the depth of tissue penetration may affect the pre-hospital treatment of SBE in a variety of ways. Large venom volumes and deeper injection depths would require larger dosing to counteract venom absorption and distribution at both a local and systemic level.
Therapies used for field-based treatment of SBE must be safe when administered even when a dry bite occurs. Dry bites occur when venom is not injected when a snakebite occurs. A review of sub-Saharan SBE incidents revealed that dry bites occurred in approximately 23% of reported cases(Chippaux, 2011). while a review of 3025 global SBE incidents, revealed a similar proportion of dry bites for viperids (14.7%) and elapids (14.5%) providing evidence that there is little difference in the proportion of dry bites between these two snake families(Pucca et al., 2020). To avoid the complications due to adverse reactions, the administration of animal-derived antivenom is only recommended when a bite and the resulting symptoms are confirmed. In contrast, the administration of small molecule or non-immunogenic biologic therapies with good safety profiles would avoid this complication.
6. IM or SC administration of SBE therapies using a medical device
6.1. Identification of ideal attributes for a medical device
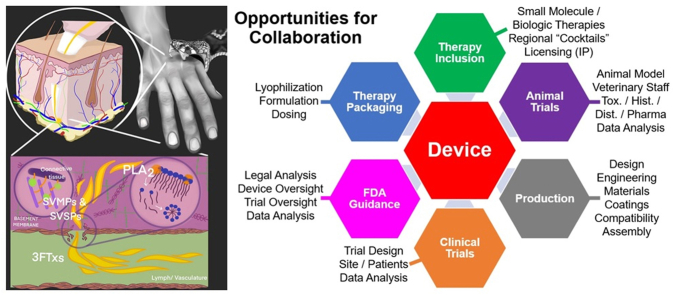
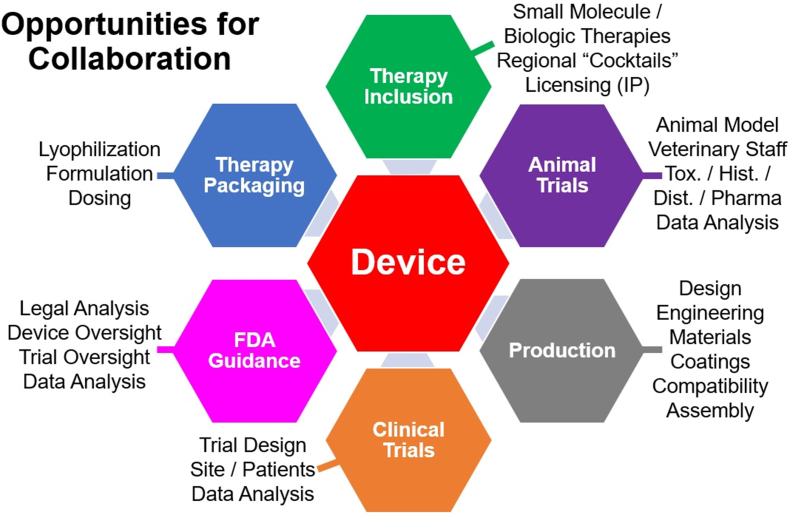
Given the above considerations related to route of administration and variabilities in bite location and venom delivery, our group has recently focused our efforts on the development of a field-based medical device for the subcutaneous (SC) and/or intramuscular (IM) administration of adjunctive therapies for SBE. As previously mentioned, the idea of using a device such as an autoinjector immediately after envenoming, was originally proposed in 1975 and has seen increasing interest in the last decade (Section 4.2). While there has been both medical and industrial interest in the development of such an approach, there is currently no commercially available device for the treatment of SBE.
Through a series of in-depth interviews with physicians and veterinarians that treat SBE, as well as industrial and academic groups involved in the development of therapies for SBE (see supplemental material), we have identified several “ideal” attributes that a device for in-field delivery of either small molecule or biologic adjunctive SBE therapies should possess.
The device should:
-
1.
Be compatible and complementary with existing first aid and health care regimes.
-
2.
Not cause a delay in patient travel to a medical facility with antivenom.
-
3.
Be compatible with self-administration using one hand.
-
4.
Induce little pain when administered.
-
5.
Be compatible with a variety of bite locations.
-
6.
Be small enough to be carried by an individual in the field.
-
7.
Have a rugged design to withstand demanding conditions.
-
8.
Have a long shelf-life when stored in tropical and sub-tropical environments.
-
9.
Require no external power source to function.
-
10.
Be inexpensive to manufacture and have a low cost to the end user.
-
11.
Focus on delivery of rapidly distributing small molecule and/or non-immunogenic biologic therapy formats.
We envision that such a device could deliver therapies immediately after SBE via SC or IM injection at or near the local site of envenoming, or at a remote location. Depending on the therapy format (small molecule or non-immunogenic biologic formats), SC or IM delivery of therapeutics at the local site would provide direct inhibition of venom components and potentially reduce their systemic absorption and distribution (Table 2). Delivery of therapies to a site distant from the envenoming site would most likely involve IM delivery as SC therapy delivery would take longer to reach systemic circulation. IM delivery may avoid further damaging the envenoming site, however this approach would require therapeutics that could reach local tissues via rapid absorption and extravascular distribution. Such a device could deliver therapy rapidly, like an Epipen autoinjector (>1 s), or more slowly, like an insulin infusion pump (minutes to hours). A successful device would need flexibility to accommodate variables discussed previously (see sections 5, 5.2.3) such as bite location, volume of venom injected, and the spacing and depth of venom injection. In addition, the device should be compatible with dry bites and not further damage the already vulnerable tissue where envenoming occurs. Fortunately, there is a good track record for the successful development and widespread use of such devices for the delivery of either small molecule or biologic therapeutics(Collins et al., 2020).
6.2. Human use-case and engineering metrics
Identifying and evaluating human use-case scenarios and patient risks that can be mitigated, controlled, and managed throughout a product's lifecycle is critical for the development of combination products that combine both therapy and a device to administer that therapy(DeGrazio and Paskiet, 2020). Thus, we have identified potential use-related issues at the onset of the design process to enhance the safety and improve the usability of the product. This approach is recommended by regulatory bodies such as FDA who suggest that manufacturers investigate use-related problems that have occurred in the past with devices that are similar to the one under development and apply lessons learned to the development of the new product(Gupta and Pidgeon, 2016). Most of the safety and use-case metrics for our proposed device mimic those found for the use of mechanical autoinjectors or infusion pumps. However, additional factors related to the painful, stressful, emotional, and emergent nature of SBE, both for the victim and bystanders, were identified. In addition to use-case scenarios and safety considerations, expert opinions gleaned from personal interviews (see supplemental material) helped us develop the ideal engineering metrics that our proposed device should possess (Table 3).
Table 3.
Engineering metrics for a field-based device for the administration of adjunctive therapies for SBE. The identified units and the corresponding reasons for the choice of those units are provided. The (*) indicates that SC administration is ideal, but may involve IM administration depending on bite location.
| Metric | Ideal Units | Reason |
|---|---|---|
| Volume Injected | 2–5 mL | Encapsulate venom 0.1–2.0 mL |
| Dia. Injection Bolus | 3–5 cm | Majority of inter-fang distance |
| Depth Injection Bolus | 0.5–2 cm | Majority of fang length |
| Needle Depth | 0.5–1 cm | Subcutaneous* injection |
| Time to Inject/Deliver | 10–300 s | Patient compliance |
| Shelflife | 1–5 yr | Stable to heat/moisture |
| Device Width | <8 cm | Hold in adult hand |
| Device Length | <30 cm | Easily Packable |
| Device Weight | <300 g | Ease of transport, use |
| # steps to use | 2-5 steps | Simplicity |
| Power | Mech. | No external power required |
A complicating factor related to SBE is the anatomical location of envenoming, particularly if therapies are to be administered near the bite location. As discussed in section 5.2, the majority of SBEs occur on the lower and upper extremities, however, bites can take place at any location. Individual differences in skin thickness and subcutaneous tissue depth as measured by ultrasonography are related not only to the anatomical location in question, but also to factors such as body mass index (BMI), hydration, age, and gender(Alexander and Miller, 1979; Seidenari et al., 1994). A representative study evaluating the subcutaneous administration of insulin in adults (N = 101) found that the average skin thickness of adult males ranges from 0.6 mm to 2.60 mm and in adult females from 1.55 mm to 3.00 mm, giving an average skin thickness in adults of 1.92 mm(Jain et al., 2013). Likewise, the subcutaneous tissue thickness in the arms of adult males ranged from 1.65 mm to 14.65 mm, whereas it ranged from 3.30 mm to 18.20 mm in females, giving an average subcutaneous tissue thickness in adults of 9.45 mm. Since these average thicknesses have such large variations, it is difficult to predict the exact route of administration if therapies are meant to be administered close to the site of SBE. Thus, we have identified between 5 mm and 10 mm as an ideal needle length for delivery of SC therapies, however, depending on the location of therapy administration, we recognize that IM delivery is a possibility (Table 3). Ideal therapies included in such a device would be equally effective if administered by either a SC or IM route.
Factors such as average venomous snake fang length, inter-fang distance, and venom amount (bolus) helped us arrive at the identified units related to therapy volume, needle depth and potential area of coverage. The use of single needle or multi-needle injection designs are possible approaches to meet these metrics. Opinions of medical experts and experimental studies indicate that administration of SC or IM therapies exceeding volumes of 1–2 mL can cause significant pain that leads to reduced patient compliance(Berteau et al., 2010, 2015; Gupta et al., 2011; Hill et al., 2016; Kim et al., 2012). Thus, the larger volume of injection described necessitates the use of either lower injection/infusion rates or the distribution of the injection volume over a larger surface are. Again, the use of a multi-needle approach to address these issues may be considered. To maximize the shelf-life of the therapies contained in the device, considerations regarding the use of lyophilized components that are solubilized when needed could be considered. The self-life is a parameter that is particularly relevant for biologic therapies due to the increased likelihood of degradation given the high temperatures and humidities that most regions with high burdens of SBE experience. Expert interviews helped us identify metrics related to size and simplicity of use in an austere environment (see supplemental material).
6.3. Development hurdles
The development of medical devices, pharmaceuticals, or combination products that involve both have an increasingly difficult and expensive path to successful implementation and ultimate commercialization(DeGrazio and Paskiet, 2020; Gopalaswamy and Gopalaswamy, 2008). This presents a difficult challenge with respect to the development of such a device for the treatment of SBE because the populations most affected typically have the least financial resources and medical infrastructure available to them (see section 1). Therefore, therapies, or the devices used to administer them, must have a minimal cost to the end user if they are to be widely adopted in the regions of the world that would benefit most from their use. To achieve this goal, production costs must be kept to a minimum, and the development of effective “low-tech” devices over expensive multi-component “high-tech” or “smart” devices appears warranted.
Given the magnitude of SBE on a global scale, there is clearly a need for improved therapies for SBE and the accompanying ways to deliver them. However, defining a need is not the same as defining a market for a product. A market for a product is defined as the number and types of entities (consumers) paying for an item, and thus can be used to calculate the potential revenue generated by sales of the product. In the case of a device to deliver therapies for SBE in the field, potential consumers could be government entities (military, national health systems, etc.), local hospital and EMS systems, or potentially small employers, villages, or even individuals. Lessons can be gleaned from experiences with antivenom funding and distribution that could be applied to market issues for such a device. Studies of the global antivenom markets(Gutiérrez, 2012, 2019; Habib and Brown, 2018; Williams et al., 2011a), and markets in South East Asia,(Patikorn et al., 2022), Africa(Brown, 2012; Habib et al., 2020; Potet et al., 2019), Australia(Isbister, 2023), and South America(Fan and Monteiro, 2018), reveal that significant markets exist in these regions, but supply chain, quality control, and distribution issues prevent supplies from adequately reaching these markets.
The markets for small molecule and non-immunogenic biologic adjunctive therapies have the potential for global expansion if they are shown to be effective, can be widely distributed, and have a low cost. Lessons can be learned from the recent pathway of development for the repurposed PLA2 inhibitor (varespladib) by Ophirex(Lewin, 2022). The development pathway for varspladib started with small initial investments used to gather results demonstrating in vitro followed by in vivo efficacy, and these results were then used to leverage much larger private and defense related investments to help support the advancement of varespladib into human clinical trials as an adjunctive therapy for SBE. By analogy, if such adjunctive therapies can follow similar pathways and become widely used, we anticipate that the market for a device to deliver these therapies may also expand.
The process of regulatory approval for pharmaceuticals, medical devices and combination products for use in humans will be different depending on the country or region for which approval is sought (Lamph, 2012; Sweet et al., 2011; Van Norman, 2016; Darrow et al., 2021; Drashti et al., 2019). An approach called “bridging” that may speed the regulatory approval of a device for the treatment of SBE could be used. Bridging occurs when a new medical device delivers a therapy that has already received regulatory approval. This approach focuses the approval process on the device rather than the therapy and can often expedite the approval of the device(USFDA, 2019). Despite the advantages of bridging, combination products are still subject to often difficult early-stage hurdles related to material selection, drug-device interactions, and biocompatibility testing(DeGrazio and Paskiet, 2020; Gopalaswamy and Gopalaswamy, 2008). Regulatory approval by the FDA is often considered the gold standard, but since the burden of SBE is very low in the United States, regulatory approvals from other regional agencies may be more appropriate. Fortunately, there is increasing awareness of the unique challenges that therapies for addressing neglected diseases such as SBE possess, and some regulatory agencies are attempting to facilitate more streamlined approval processes(Mukherjee, 2023).
7. Opportunities for transdisciplinary collaboration
There has long been a recognition that collaborative transdisciplinary approaches can be highly successful when addressing the challenges presented by SBE(Gutiérrez, 2016; Gutiérrez et al., 2006, 2022). Such a collaborative spirit would be necessary for the successful development of a device for the field-based administration of adjunctive SBE therapies, and a summary of potential collaborative entities is provided in Fig. 8. In addition to small molecule and biologic therapy developers, we envision collaborative efforts involving medical device engineers and manufacturers, therapy formulation and packaging experts, animal testing and toxicology partners, legal and regulatory entities, as well as government and industrial partners that can assist with ultimate clinical testing, validation, and implementation of the device.
Fig. 8.
Opportunities for collaboration in the development of a field-based device for the administration of adjunctive therapies for SBE.
8. Conclusion
In this review, we have presented a summary of practices for treatment of SBE and highlighted the crucial role that the timely delivery of antivenom plays in SBE therapy. The time to travel to a medical facility for the administration of antivenom is situationally dependent given the geographically remote nature of most SBE incidents. In the last three decades, there has been increasing interest in the development of adjunctive therapeutics to bridge this time-gap between initial SBE and the administration antivenom, and a summary of relevant literature is provided as supplemental material. We presented potential points of intervention along the envenoming therapy timeline (ETT) and discussed the possibility of delivering adjunctive therapies during the initial minutes or hours following SBE with the aim of reducing overall SBE mortality and morbidity. Small molecule and biologic therapies with the highest potential for use as adjunctive SBE therapies targeting the most relevant venom components (PLA2s, SVMPs, SVSPs, and 3FTxs) were presented, as was the possibility of combining therapeutics to provide protection against regional groupings of medically relevant snake species. Considerations for the development of a field-based medical device that could deliver adjunctive SBE therapies including the route of therapy administration, anatomical bite location, and species-specific venom delivery were presented. The advice gleaned from expert interviews is provided as supplemental material and was used in conjunction with the above considerations to develop ideal metrics and identify potential development hurdles for a medical device that could administer adjunctive SBE therapies via SC or IM injection in a field-based setting. Future publications will detail the specific designs, functionality, and testing of the device our group is developing for these purposes. We look forward to seeing what the future holds for the development of adjunctive SBE therapies and the devices that will be needed to deliver them.
Funding
This work was supported by the Office of Undergraduate Research, the Technology and Commercialization Center, and the College of Arts & Sciences at Grand Valley State University. Additional funding was provided by a Michigan Economic Development Corporation University Early-Stage Proof-of-Concept Fund (ADVANCE) grant (grant number MEDC:12,202,021).
Ethical statement
No animals or human subjects were used for experiments related to the preparation of this manuscript.
Data and analysis is freely available upon request.
Literature sources for adapted material have been appropriately acknowledged in the manuscript.
This manuscript has not been submitted for review with any other entity.
The use of expert interviews is presented in the supplemental material. Interviewees were aware that they were providing responses for academic use. Sensitive or confidential disclosures are not included in these summaries.
A Credit of Authorship has been included in the manuscript.
No generative AI was used in the preparation of the manuscript. All writing was performed by the corresponding author.
A declaration of competing interests was submitted and included as part of the manuscript. The status of intellectual property associated with this project can be classified as “Patent Pending”. The corresponding author (RMW) is associated with this intellectual property.
If errors are discovered, the corresponding author is responsible to notify the journal editor or publisher and cooperate with the editor to retract or correct the paper if deemed necessary by the editor.
The manuscript contains images that comply with the specific policy for graphical images applied by Toxicon X.
No clinical trials are reported in the manuscript.
CRediT authorship contribution statement
R. Marshall Werner: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Funding acquisition, Formal analysis, Data curation, Conceptualization. Allison N. Soffa: Writing – review & editing, Visualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:R. Marshall Werner reports financial support and equipment, drugs, or supplies were provided by Michigan Economic Development Corporation. RMW is associated with intellectual property (patent-pending) related to the commercial development of a medical device for the field-based administration of adjunctive snakebite envenoming therapies.
Acknowledgements
The authors would like to acknowledge the assistance of Kelly Martin (Tekna, Kalamazoo, MI) for help with identification of ideal device metrics and use-case scenarios. We would also like to acknowledge Anna White at the Grand Valley State University Library for assistance with search methods and terms. We would like to acknowledge Dr. Rebecca Werner and Dr. Robert M. Werner (Sr.) for their assistance with manuscript proofing and editing.
Handling Editor: Ray Norton
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2023.100169.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abouyannis M., FitzGerald R., Ngama M., Mwangudzah H., Nyambura Y.K., Ngome S., Riako D., Babu L., Lewa F., Else L., Dily Penchala S., Orindi B., Mumba N., Kalama B., Ndungu F.M., Adetifa I., Khoo S., Lalloo D.G., Casewell N.R., Hamaluba M. TRUE-1: trial of repurposed unithiol for snakebite envenoming phase 1 (safety, tolerability, pharmacokinetics and pharmacodynamics in healthy Kenyan adults) Wellcome open research. 2022;7:90. doi: 10.12688/wellcomeopenres.17682.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afroz A., Siddiquea B.N., Shetty A.N., Jackson T.N.W., Watt A.D. Assessing knowledge and awareness regarding snakebite and management of snakebite envenoming in healthcare workers and the general population: a systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2023;17 doi: 10.1371/journal.pntd.0011048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts M.B., Shalit M., LoGalbo F. Suction for venomous snakebite: a study of mock venom extraction in a human model. Ann. Emerg. Med. 2004;43:181–186. doi: 10.1016/S0196-0644(03)00813-8. [DOI] [PubMed] [Google Scholar]
- Albulescu L.-O., Hale M.S., Ainsworth S., Alsolaiss J., Crittenden E., Calvete J.J., Evans C., Wilkinson M.C., Harrison R.A., Kool J., Casewell N.R. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci. Transl. Med. 2020;12:542. doi: 10.1126/scitranslmed.aay8314. eaay8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu L.-O., Kazandjian T., Slagboom J., Bruyneel B., Ainsworth S., Alsolaiss J., Wagstaff S.C., Whiteley G., Harrison R.A., Ulens C., Kool J., Casewell N.R. A decoy-receptor approach using nicotinic acetylcholine receptor mimics reveals their potential as novel therapeutics against neurotoxic snakebite. Front. Pharmacol. 2019;10:848. doi: 10.3389/fphar.2019.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu L.-O., Xie C., Ainsworth S., Alsolaiss J., Crittenden E., Dawson C.A., Softley R., Bartlett K.E., Harrison R.A., Kool J., Casewell N.R. A therapeutic combination of two small molecule toxin inhibitors provides broad preclinical efficacy against viper snakebite. Nat. Commun. 2020;11:6094. doi: 10.1038/s41467-020-19981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque P.L., Silva Junior G.B., Jacinto C.N., Lima C.B., Lima J.B., Veras M.d.S.B., Daher E.F. Brazil. Vol. 55. Revista do Instituto de Medicina Tropical de São; Paulo: 2013. In: Epidemiological Profile of Snakebite Accidents in a Metropolitan Area of Northeast; pp. 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander H., Miller A.D. Determining skin thickness with pulsed ultra sound. J. Invest. Dermatol. 1979;72:17–19. doi: 10.1111/1523-1747.ep12530104. [DOI] [PubMed] [Google Scholar]
- Alirol E., Lechevalier P., Zamatto F., Chappuis F., Alcoba G., Potet J. Antivenoms for snakebite envenoming: what is in the research pipeline? PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomran N., Chinnappan R., Alsolaiss J., Casewell N.R., Zourob M. Exploring the utility of ssDNA aptamers directed against snake venom toxins as new therapeutics for snakebite envenoming. Toxins. 2022;14:469. doi: 10.3390/toxins14070469. https://www.mdpi.com/2072-6651/14/7/469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqahtani M.S., Kazi M., Alsenaidy M.A., Ahmad M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.618411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral C.F.S., Campolina D., Dias M.B., Bueno C.M., Rezende N.A. Tourniquet ineffectiveness to reduce the severity of envenoming after Crotalus durissus snake bite in Belo Horizonte, Minas Gerais, Brazil. Toxicon. 1998;36:805–808. doi: 10.1016/S0041-0101(97)00132-3. [DOI] [PubMed] [Google Scholar]
- Ameade E.P.K., Bonney I., Boateng E.T. Health professionals' overestimation of knowledge on snakebite management, a threat to the survival of snakebite victims—a cross-sectional study in Ghana. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0008756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A.S., Rucavado A., Gutiérrez J.M. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon. 2017;132:40–49. doi: 10.1016/j.toxicon.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Arruda E., Silva N., Moraes R., Melo P. Effect of suramin on myotoxicity of some crotalid snake venoms. Braz. J. Med. Biol. Res. 2002;35:723–726. doi: 10.1590/S0100-879X2002000600013. [DOI] [PubMed] [Google Scholar]
- Ascoët S., De Waard M. Diagnostic and therapeutic value of aptamers in envenomation cases. Int. J. Mol. Sci. 2020;21:3565. doi: 10.3390/ijms21103565. https://www.mdpi.com/1422-0067/21/10/3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukland K. Arnold Heller and the lymph pump. Acta Physiol. Scand. 2005;185:171–180. doi: 10.1111/j.1365-201X.2005.01470.x. [DOI] [PubMed] [Google Scholar]
- Avau B., Borra V., Vandekerckhove P., De Buck E. The treatment of snake bites in a first aid setting: a systematic review. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin M. The snakestone experiments: an early modern medical debate. Isis. 1995;86:394–418. doi: 10.1086/357237. [DOI] [PubMed] [Google Scholar]
- Banerjee R.N., Sahni A.L., Chacko K.A. In: Animal, Plant, and Microbial Toxins: Volume 2 Chemistry, Pharmacology, and Immunology. Ohsaka A., Hayashi K., Sawai Y., Murata R., Funatsu M., Tamiya N., editors. Springer New York; Boston, MA: 1976. Neostigmine in the treatment of elapidae bites; pp. 475–481. [Google Scholar]
- Bawaskar H., Bawaskar P., Punde D., Inamdar M., Dongare R., Bhoite R. Profile of snakebite envenoming in rural Maharashtra, India. J. Ass. Phys. India. 2008;56:88–95. [PubMed] [Google Scholar]
- Bawaskar H.S., Bawaskar P.H. Profile of snakebite envenoming in Western Maharashtra, India. Trans. R. Soc. Trop. Med. Hyg. 2002;96:79–84. doi: 10.1016/S0035-9203(02)90250-6. [DOI] [PubMed] [Google Scholar]
- Bénard-Valle M., Neri-Castro E.E., Boyer L., Jackson T.N.W., Sunagar K., Clarkson M., Fry B.G. In: Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery. Fry B.G.), editor. Oxford University Press; New York, NY: 2015. Ineffective traditional and modern techniques for the treatment of snakebite. [Google Scholar]
- Benjamin J.M., Abo B.N., Brandehoff N. Review article: snake envenomation in Africa. Current Tropical Medicine Reports. 2020;7:1–10. doi: 10.1007/s40475-020-00198-y. [DOI] [Google Scholar]
- Benjamin J.M., Brandehoff N.N., Wilson C.B., Hall M.A., Greene S., Maddry M.J., Collazo M., Grisham N.C.J., Dye S.C., Lee A.S.J. 2020. Global Snake Envenomation Management.https://jts.amedd.army.mil/assets/docs/cpgs/Global_Snake_Envenomation_Management_30_Jun_2020_ID81.pdf (cpg id: 81). URL: [Google Scholar]
- Bennett J.E., Brelsford H.G., Lewis S.R., Blocker T.G. Distal extremity necrosis after snakebite. Plast. Reconstr. Surg. 1961;28:385–393. doi: 10.1097/00006534-196110000-00005. https://journals.lww.com/plasreconsurg/Fulltext/1961/10000/DISTAL_EXTREMITY_NECROSIS_AFTER_SNAKE_BITE.5.aspx [DOI] [PubMed] [Google Scholar]
- Benson A.E.H., Grice E.J., Mohammed Y., Namjoshi S., Roberts S.M. Topical and transdermal drug delivery: from simple potions to smart technologies. Curr. Drug Deliv. 2019;16:444–460. doi: 10.2174/1567201816666190201143457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteau C., Filipe-Santos O., Wang T., Rojas H.E., Granger C., Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med. Dev. Evid. Res. 2015;8:473–484. doi: 10.2147/MDER.S91019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteau C., Schwarzenbach F., Donazzolo Y., Latreille M., Berube J., Abry H., Cotten J., Feger C., Laurent P.E. Evaluation of performance, safety, subject acceptance, and compliance of a disposable autoinjector for subcutaneous injections in healthy volunteers. Patient Prefer. Adherence. 2010;4:379–388. doi: 10.2147/ppa.s13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava S., Kumari K., Sarin R.K., Singh R. First-hand knowledge about snakes and snake-bite management: an urgent need. Nagoya J. Med. Sci. 2020;82:763–774. doi: 10.18999/nagjms.82.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S., Kallakuri S., Kaur A., Devarapalli S., Daniel M. Mental health conditions after snakebite: a scoping review. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittenbinder M.A., Zdenek C.N., Op den Brouw B., Youngman N.J., Dobson J.S., Naude A., Vonk F.J., Fry B.G. Coagulotoxic cobras: clinical implications of strong anticoagulant actions of African spitting naja venoms that are not neutralised by antivenom but are by LY315920 (varespladib) Toxins. 2018;10:516. doi: 10.3390/toxins10120516. http://www.mdpi.com/2072-6651/10/12/516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J.R., Dillon S. Venomous snakebite: past, present, and future treatment options. J. Am. Board Fam. Pract. 1992;5:399–405. doi: 10.3122/jabfm.5.4.399. [DOI] [PubMed] [Google Scholar]
- Blaylock R. The identification and syndromic management of snakebite in South Africa. S. Afr. Fam. Pract. 2005;47:46–53. [Google Scholar]
- Boyer L., Alagón A., Fry B.G., Jackson T.N.W., Sunagar K., Chippaux J.-P. Oxford University Press; New York: 2015. Signs, Symptoms, and Treatment of Envenomation; pp. 32–60. [Google Scholar]
- Boyer L.V., Seifert S.A., Clark R.F., McNally J.T., Williams S.R., Nordt S.P., Walter F.G., Dart R.C. Recurrent and persistent coagulopathy following pit viper envenomation. Arch. Intern. Med. 1999;159:706–710. doi: 10.1001/archinte.159.7.706. [DOI] [PubMed] [Google Scholar]
- Braga J.R.M., Oitaven L.P.C., da Rocha M.M.T., Vieira S.E.M., Rúbio D.T., Sant’anna S.S., Grego K.F. Influence of size, sex and age on venom yield of Bothrops leucurus (serpentes, viperidae) in captivity conditions. Basic Appl. Herpetol. 2022;36:31–45. doi: 10.11160/bah.233. [DOI] [Google Scholar]
- Brandehoff N., Dalton A., Daugherty C., Dart R.C., Monte A.A. Total CroFab and Anavip antivenom vial administration in US rattlesnake envenomations: 2019–2021. J. Med. Toxicol. 2023;19:248–254. doi: 10.1007/s13181-023-00941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandehoff N., Personal Communication . 2022. Asclepius Snakebite Foundation, Medical Director. [Google Scholar]
- Brown N.I. Consequences of neglect: analysis of the sub-Saharan African snake antivenom market and the global context. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan-Quirós W., Fernández J., Gutiérrez J.M., Lewin M.R., Lomonte B. Neutralizing properties of LY315920 toward snake venom group I and II myotoxic phospholipases A2. Toxicon. 2019;157:1–7. doi: 10.1016/j.toxicon.2018.11.292. [DOI] [PubMed] [Google Scholar]
- Bulfone T.C., Samuel S.P., Bickler P.E., Lewin M.R. Developing small molecule therapeutics for the initial and adjunctive treatment of snakebite. J. Trop. Med. 2018;2018, 1-10. doi: doi: 10.1155/2018/4320175. 10.10.1155/2018/4320175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush S.P. Snakebite suction devices don’t remove venom: they just suck. Ann. Emerg. Med. 2004;43:187–188. doi: 10.1016/j.annemergmed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Bush S.P., Green S.M., Laack T.A., Hayes W.K., Cardwell M.D., Tanen D.A. Pressure immobilization delays mortality and increases intracompartmental pressure after artificial intramuscular rattlesnake envenomation in a porcine model. Ann. Emerg. Med. 2004;44:599–604. doi: 10.1016/j.annemergmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bush S.P., Hegewald K.G., Green S.M., Cardwell M.D., Hayes W.K. Effects of a negative pressure venom extraction device (extractor) on local tissue injury after artificial rattlesnake envenomation in a porcine model. Wilderness Environ. Med. 2000;11:180–188. doi: 10.1580/1080-6032(2000)011[0180:EOANPV]2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Bush S.P., Kinlaw S.B. Management of a pediatric snake envenomation after presentation with a tight tourniquet. Wilderness Environ. Med. 2015;26:355–358. doi: 10.1016/j.wem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Calmette A. Vol. 8. Annales de l'Institut Pasteur.; 1894. pp. 275–291. (Contribution à l'étude du venin des serpents. Immunisation des animaux et traitement de l'envenimation). [Google Scholar]
- Canul-Caamal M.A., Madrigal-Anaya J.D.C., Pastelin-Palacios R., Escalante-Galindo P., Moreno-Eutimio M.A. Cryotherapy as a coadjuvant in crotaline snakebite management with F(ab')(2) antivenom: a randomized pilot study. Compl. Ther. Med. 2020;54 doi: 10.1016/j.ctim.2020.102569. [DOI] [PubMed] [Google Scholar]
- Carter R.W., Gerardo C.J., Samuel S.P., Kumar S., Kotehal S.D., Mukherjee P.P., Shirazi F.M., Akpunonu P.D., Bammigatti C., Bhalla A., Manikath N., Platts-Mills T.F., Lewin M.R. The BRAVO clinical study protocol: oral varespladib for inhibition of secretory phospholipase A2 in the treatment of snakebite envenoming. Toxins. 2022;15:1–14. doi: 10.3390/toxins15010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell N.R., Jackson T.N.W., Laustsen A.H., Sunagar K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020;41:570–581. doi: 10.1016/j.tips.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell N.R., Wagstaff S.C., Wuster W., Cook D.A.N., Bolton F.M.S., King S.I., Pla D., Sanz L., Calvete J.J., Harrison R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA. 2014;111:9205–9210. doi: 10.1073/pnas.1405484111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaithanya I.K., Abnave D., Bawaskar H., Pachalkar U., Tarukar S., Salvi N., Bhoye P., Yadav A., Mahale S.D., Gajbhiye R.K. Perceptions, awareness on snakebite envenoming among the tribal community and health care providers of Dahanu block, Palghar district in Maharashtra, India. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Gui L., Kan T., Li S., Qiu C. A survey of snakebite knowledge among field forces in China. Int. J. Environ. Res. Publ. Health. 2017;14:15. doi: 10.3390/ijerph14010015. https://www.mdpi.com/1660-4601/14/1/15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-J., Tsai C.-Y., Hu W.-P., Chang L.-S. DNA aptamers against Taiwan banded krait α-bungarotoxin recognize Taiwan cobra cardiotoxins. Toxins. 2016;8:66. doi: 10.3390/toxins8030066. https://www.mdpi.com/2072-6651/8/3/66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.-P. Estimate of the burden of snakebites in sub-Saharan Africa: a meta-analytic approach. Toxicon. 2011;57:586–599. doi: 10.1016/j.toxicon.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Chippaux J.-P. Epidemiology of snakebites in Europe: a systematic review of the literature. Toxicon. 2012;59:86–99. doi: 10.1016/j.toxicon.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Chippaux J.-P., Massougbodji A., Habib A.G. 2019. The WHO Strategy for Prevention and Control of Snakebite Envenoming: A sub-Saharan Africa Plan (SciELO Brasil) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A., Lewin M.R., Zdenek C.N., Carter R., Fry B.G. The relative efficacy of chemically diverse small-molecule enzyme-inhibitors against anticoagulant activities of African spitting cobra (Naja species) venoms. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.752442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A., Zdenek C.N., Lewin M.R., Carter R., Jagar T., Ostanek E., Harjen H., Aldridge M., Soria R., Haw G., Fry B.G. Venom-induced blood disturbances by palearctic viperid snakes, and their relative neutralization by antivenoms and enzyme-inhibitors. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.688802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuat M., Alcoba G., Eyong J., Wanda F., Comte E., Nkwescheu A., Chappuis F., Hudelson P. Dealing with snakebite in rural Cameroon: a qualitative investigation among victims and traditional healers. Toxicon X. 2021 doi: 10.1016/j.toxcx.2021.100072. 9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare R.H., Hall S.R., Patel R.N., Casewell N.R. Small molecule drug discovery for neglected tropical snakebite. Trends Pharmacol. Sci. 2021;42:340–353. doi: 10.1016/j.tips.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Collins D.S., Sánchez-Félix M., Badkar A.V., Mrsny R. Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics. J. Contr. Release. 2020;321:475–482. doi: 10.1016/j.jconrel.2020.02.036. [DOI] [PubMed] [Google Scholar]
- Cristino J.S., Salazar G.M., Machado V.A., Honorato E., Farias A.S., Vissoci J.R.N., Silva Neto A.V., Lacerda M., Wen F.H., Monteiro W.M., Sachett J.A.G. A painful journey to antivenom: the therapeutic itinerary of snakebite patients in the Brazilian amazon (the qualisnake study) PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Souza A., de Almeida Gonçalves Sachett J., Alcântara J.A., Freire M., Alecrim M.d.G.C., Lacerda M., de Lima Ferreira L.C., Fan H.W., de Souza Sampaio V., Monteiro W.M. Snakebites as cause of deaths in the Western Brazilian amazon: why and who dies? Deaths from snakebites in the amazon. Toxicon. 2018;145:15–24. doi: 10.1016/j.toxicon.2018.02.041. [DOI] [PubMed] [Google Scholar]
- Darrow J.J., Avorn J., Kesselheim A.S. FDA regulation and approval of medical devices: 1976-2020. JAMA. 2021;326:420–432. doi: 10.1001/jama.2021.11171. [DOI] [PubMed] [Google Scholar]
- Darshani S., Gnanathasan A., Arambepola C., Chang T. Knowledge on prevention, diagnosis and treatment of snakebite envenoming among doctors in snakebite-dense regions in Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2021;115:984–991. doi: 10.1093/trstmh/trab112. [DOI] [PubMed] [Google Scholar]
- Dashevsky D., Bénard-Valle M., Neri-Castro E., Youngman N.J., Zdenek C.N., Alagón A., Portes-Junior J.A., Frank N., Fry B.G. Anticoagulant micrurus venoms: targets and neutralization. Toxicol. Lett. 2021;337:91–97. doi: 10.1016/j.toxlet.2020.11.010. [DOI] [PubMed] [Google Scholar]
- de Oliveira M., Cavalcante W.L.G., Arruda E.Z., Melo P.A., Dal-Pai Silva M., Gallacci M. Antagonism of myotoxic and paralyzing activities of bothropstoxin-I by suramin. Toxicon. 2003;42:373–379. doi: 10.1016/S0041-0101(03)00166-1. [DOI] [PubMed] [Google Scholar]
- de Roodt A.R., Boyer L.V., Lanari L.C., Irazu L., Laskowicz R.D., Sabattini P.L., Damin C.F. Venom yield and its relationship with body size and fang separation of pit vipers from Argentina. Toxicon. 2016;121:22–29. doi: 10.1016/j.toxicon.2016.08.013. [DOI] [PubMed] [Google Scholar]
- de Roodt A.R., Dolab J.A., Galarce P.P., Gould E., Litwin S., Dokmetjian J.C., Segre L., Vidal J.C. A study on the venom yield of venomous snake species from Argentina. Toxicon. 1998;36:1949–1957. doi: 10.1016/S0041-0101(98)00119-6. [DOI] [PubMed] [Google Scholar]
- de Silva H.A., Pathmeswaran A., Ranasinha C.D., Jayamanne S., Samarakoon S.B., Hittharage A., Kalupahana R., Ratnatilaka G.A., Uluwatthage W., Aronson J.K., Armitage J.M., Lalloo D.G., de Silva H.J. Low-dose adrenaline, promethazine, and hydrocortisone in the prevention of acute adverse reactions to antivenom following snakebite: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza J., Oliveira I.C.F., Yoshida E.H., Cantuaria N.M., Cogo J.C., Torres-Bonilla K.A., Hyslop S., Silva Junior N.J., Floriano R.S., Gutiérrez J.M., Oshima-Franco Y. Effect of the phospholipase A2 inhibitor varespladib, and its synergism with crotalic antivenom, on the neuromuscular blockade induced by Crotalus durissus terrificus venom (with and without crotamine) in mouse neuromuscular preparations. Toxicon. 2022;214:54–61. doi: 10.1016/j.toxicon.2022.05.001. [DOI] [PubMed] [Google Scholar]
- DeGrazio F., Paskiet D. Injectable combination product development: facilitating risk-based assessments for efficiency and patient centric outcomes. J. Pharmaceut. Sci. 2020;109:2101–2115. doi: 10.1016/j.xphs.2020.03.020. [DOI] [PubMed] [Google Scholar]
- Drashti P., Kothari C.S., Shantanu S., Manan S. In-depth review on ‘innovation and regulatory challenges of the drug delivering medical devices. J. Gene Med. 2019;15:18–28. doi: 10.1177/1741134318821143. [DOI] [Google Scholar]
- Eriksen M.B., Frandsen T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J. Med. Libr. Assoc. : JMLA. 2018;106:420–431. doi: 10.5195/jmla.2018.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante T., Franceschi A., Rucavado A., Gutierrez J.M. Effectiveness of batimastat, a synthetic inhibitor of matrix metalloproteinases, in neutralizing local tissue damage induced by bap1, a hemorrhagic metalloproteinase from the venom of the snake Bothrops asper. Biochem. Pharmacol. 2000;60:269–274. doi: 10.1016/s0006-2952(00)00302-6. [DOI] [PubMed] [Google Scholar]
- Fan H.W., Monteiro W.M. History and perspectives on how to ensure antivenom accessibility in the most remote areas in Brazil. Toxicon. 2018;151:15–23. doi: 10.1016/j.toxicon.2018.06.070. [DOI] [PubMed] [Google Scholar]
- Fathi B., Harvey A.L., Rowan E.G. Suramin inhibits the early effects of PLA(2) neurotoxins at mouse neuromuscular junctions: a twitch tension study. J. Venom Res. 2011;2:6–10. [PMC free article] [PubMed] [Google Scholar]
- Fischer T., Senn N., Riedl R. Design and structural evolution of matrix metalloproteinase inhibitors. Chem. Eur J. 2019;25:7960–7980. doi: 10.1002/chem.201805361. [DOI] [PubMed] [Google Scholar]
- Flora S.J.S., Pachauri V. Chelation in metal intoxication. Int. J. Environ. Res. Publ. Health. 2010;7:2745–2788. doi: 10.3390/ijerph7072745. https://www.mdpi.com/1660-4601/7/7/2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers H.H., Goucher C.R. Vol. 21. Rep US Army Med Res Lab; 1963. https://apps.dtic.mil/dtic/tr/fulltext/u2/432899.pdf (The Effect of EDTA on the Extent of Tissue Damage Caused by the Venoms of Bothrops atrox (Fer-de-lance) and Agkistrodon piscivorus (Cottonmouth Moccasin)). URL: [DOI] [PubMed] [Google Scholar]
- Flowers H.H., Goucher C.R. The effect of EDTA on the extent of tissue damage caused by the venoms of Bothrops atrox and Agkistrodon piscivorus. Toxicon. 1965;2:221–224. doi: 10.1016/0041-0101(65)90020-6. [DOI] [PubMed] [Google Scholar]
- Frank H.A. Snakebite or frostbite: what are we doing? An evaluation of cryotherapy for envenomation. Calif. Med. 1971;114:25–27. [PMC free article] [PubMed] [Google Scholar]
- Fry B.G. Snakebite: when the human touch becomes a bad touch. Toxins. 2018;10:170. doi: 10.3390/toxins10040170. https://www.mdpi.com/2072-6651/10/4/170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Yin Y., Qu Y., Wang J., Lin L., Lu H., Ji X. Identifying intraspecific variation in venom yield of Chinese cobra (Naja atra) from ten populations in mainland China. Asian Herpetol. Res. 2019;10:32–40. doi: 10.16373/j.cnki.ahr.180041. [DOI] [Google Scholar]
- Gellert G.A. Snake-venom and insect-venom extractors: an unproved therapy. N. Engl. J. Med. 1992;327 doi: 10.1056/nejm199210293271820. 1322-1322. [DOI] [PubMed] [Google Scholar]
- Gerardo C.J. Duke University hospital; 2021. (Personal Communicaton) Professor and Chief, Division of Emergency Medicine at. [Google Scholar]
- Godpower M., Ibrahim A., Bukar G. Primary prevention of snakebite envenoming in resource-limited settings: a narrative review. Environmental Disease. 2019;4:37–44. doi: 10.4103/ed.ed_11_19. [DOI] [Google Scholar]
- Gomes A., Das R., Sarkhel S., Mishra R., Mukherjee S., Bhattacharya S., Gomes A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010;48:865–878. [PubMed] [Google Scholar]
- Gopalaswamy S., Gopalaswamy V. CRC press, Taylor & Francis Group; Boca Raton: 2008. Combination Products: Regulatory Challenges and Successful Product Development; p. 264. [Google Scholar]
- Gowda C.D.R., Shivaprasad H.V., Kumar R.V., Rajesh R., Saikumari Y.K., Frey B.M., Frey F.J., Sharath B.K., Vishwanath B.S. Characterization of major zinc containing myonecrotic and procoagulant metalloprotease malabarin from non lethal Trimeresurus malabaricus snake venom with thrombin like activity: its neutralization by chelating agents. Curr. Top. Med. Chem. 2011;11:2578–2588. doi: 10.2174/156802611797633375. [DOI] [PubMed] [Google Scholar]
- Gupta J., Park S.S., Bondy B., Felner E.I., Prausnitz M.R. Infusion pressure and pain during microneedle injection into skin of human subjects. Biomaterials. 2011;32:6823–6831. doi: 10.1016/j.biomaterials.2011.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.P., Pidgeon A. An analytical approach to identifying potential use-related issues concerning a medical device under development. J. Med. Eng. Technol. 2016;40:61–71. doi: 10.3109/03091902.2015.1132785. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M. Improving antivenom availability and accessibility: science, technology, and beyond. Toxicon. 2012;60:676–687. doi: 10.1016/j.toxicon.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M. Reducing the impact of snakebite envenoming in Latin America and the Caribbean: achievements and challenges ahead. Trans. R. Soc. Trop. Med. Hyg. 2014;108:530–537. doi: 10.1093/trstmh/tru102. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M. Understanding and confronting snakebite envenoming: the harvest of cooperation. Toxicon. 2016;109:51–62. doi: 10.1016/j.toxicon.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M. Global availability of antivenoms: the relevance of public manufacturing laboratories. Toxins. 2019;11:5. doi: 10.3390/toxins11010005. https://www.mdpi.com/2072-6651/11/1/5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Albulescu L.-O., Clare R.H., Casewell N.R., Abd El-Aziz T.M., Escalante T., Rucavado A. The search for natural and synthetic inhibitors that would complement antivenoms as therapeutics for snakebite envenoming. Toxins. 2021;13:451. doi: 10.3390/toxins13070451. https://www.mdpi.com/2072-6651/13/7/451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Borri J., Giles-Vernick T., Duda R., Habib A.G., Malhotra A., Martín G., Pintor A.F.V., Potet J., Scott T., Bolon I., de Castañeda R.R. Understanding and tackling snakebite envenoming with transdisciplinary research. PLoS Neglected Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017;3:1–20. doi: 10.1038/nrdp.2017.63. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., León G., Lomonte B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin. Pharmacokinet. 2003;42:721–741. doi: 10.2165/00003088-200342080-00002. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Lewin M.R., Williams D.J., Lomonte B. Varespladib (LY315920) and methyl varespladib (LY333013) abrogate or delay lethality induced by presynaptically acting neurotoxic snake venoms. Toxins. 2020;12:131. doi: 10.3390/toxins12020131. https://www.mdpi.com/2072-6651/12/2/131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Lomonte B., Leon G., Rucavado A., Chaves F., Angulo Y. Trends in snakebite envenomation therapy: scientific, technological and public health considerations. Curr. Pharmaceut. Des. 2007;13:2935–2950. doi: 10.2174/138161207782023784. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Maduwage K., Iliyasu G., Habib A. Snakebite envenoming in different national contexts: Costa Rica, Sri Lanka, and Nigeria. Toxicon X. 2021;9–10 doi: 10.1016/j.toxcx.2021.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Theakston R.D.G., Warrell D.A. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.G., Abubakar S.B. Factors affecting snakebite mortality in North-Eastern Nigeria. International Health. 2011;3:50–55. doi: 10.1016/j.inhe.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Habib A.G., Brown N.I. The snakebite problem and antivenom crisis from a health-economic perspective. Toxicon. 2018;150:115–123. doi: 10.1016/j.toxicon.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Habib A.G., Musa B.M., Iliyasu G., Hamza M., Kuznik A., Chippaux J.-P. Challenges and prospects of snake antivenom supply in sub-Saharan Africa. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajari Z., Fathi B., Saadatfar Z., Zare A. Antagonistic effects of suramin against the venom of snake, Echis carinatus, on the circulatory system of developing chicken embryos. Iranian Journal of Toxicology. 2019;13:13–18. doi: 10.32598/ijt.13.1.569.1. [DOI] [Google Scholar]
- Hall E.L. Role of surgical intervention in the management of crotaline snake envenomation. Ann. Emerg. Med. 2001;37:175–180. doi: 10.1067/mem.2001.113373. [DOI] [PubMed] [Google Scholar]
- Hall S.R., Rasmussen S.A., Crittenden E., Dawson C.A., Bartlett K.E., Westhorpe A.P., Albulescu L.-O., Kool J., Gutiérrez J.M., Casewell N.R. Repurposed drugs and their combinations prevent morbidity-inducing dermonecrosis caused by diverse cytotoxic snake venoms. bioRxiv. 2022 doi: 10.1101/2022.05.20.492855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza M., Knudsen C., Gnanathasan C.A., Monteiro W., Lewin M.R., Laustsen A.H., Habib A.G. Clinical management of snakebite envenoming: future perspectives. Toxicon X. 2021;11 doi: 10.1016/j.toxcx.2021.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E., Sasa M., Mattisson K., Robles A., Gutiérrez J.M. Using geographical information systems to identify populations in need of improved accessibility to antivenom treatment for snakebite envenoming in Costa Rica. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.A., Hargreaves A., Wagstaff S.C., Faragher B., Lalloo D.G. Snake envenoming: a disease of poverty. PLoS Neglected Trop. Dis. 2009;3:e569. doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes W.K., Corbit A.G., Cardwell M.D., Herbert S.S. Interfang distances of rattlesnakes: sexual, interspecific, and body size-related variation, and implications for snakebite research and management. Wilderness Environ. Med. 2017;28:101–107. doi: 10.1016/j.wem.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Hill R.L., Wilmot J.G., Belluscio B.A., Cleary K., Lindisch D., Tucker R., Wilson E., Shukla R.B. Comparison of drug delivery with autoinjector versus manual prefilled syringe and between three different autoinjector devices administered in pig thigh. Medical devices (Auckland, N.Z.). 2016;9:257–266. doi: 10.2147/mder.s83406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege C.P. Skin damage following application of suction device for snakebite. Ann. Emerg. Med. 2006;48:113. doi: 10.1016/j.annemergmed.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Howe N.R., Meisenheimer J.L. Electric shock does not save snakebitten rats. Ann. Emerg. Med. 1988;17:254–256. doi: 10.1016/S0196-0644(88)80118-5. [DOI] [PubMed] [Google Scholar]
- Howes J.M., Theakston R.D., Laing G.D. Neutralization of the haemorrhagic activities of viperine snake venoms and venom metalloproteinases using synthetic peptide inhibitors and chelators. Toxicon. 2007;49:734–739. doi: 10.1016/j.toxicon.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Iliyasu G., Tiamiyu A.B., Daiyab F.M., Tambuwal S.H., Habib Z.G., Habib A.G. Effect of distance and delay in access to care on outcome of snakebite in rural North-Eastern Nigeria. Rural Rem. Health. 2015;15:76–81. https://search.informit.org/doi/10.3316/informit.223800636408692 [PubMed] [Google Scholar]
- Irshad V.S., Godhiwala P., Kumar S., Bagga C., Gupta A. Role of neostigmine in neurotoxic snake bite. J. Evol. Med. Dent. Sci. 2021;10:1095–1097. doi: 10.14260/jemds/2021/234. [DOI] [Google Scholar]
- Isbister G.K. Antivenom availability, delays and use in Australia. Toxicon X. 2023;17 doi: 10.1016/j.toxcx.2022.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S.M., Pandey K., Lahoti A., Rao P.K. Evaluation of skin and subcutaneous tissue thickness at insulin injection sites in Indian, insulin naïve, type-2 diabetic adult population. Indian J. Endocrinol. Metab. 2013;17:864–870. doi: 10.4103/2230-8210.117249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K.K. In: Drug Delivery Systems. Jain K.K., editor. Springer New York; New York, NY: 2020. An overview of drug delivery systems; pp. 1–54. [Google Scholar]
- Jenkins T.P., Fryer T., Dehli R.I., Jürgensen J.A., Fuglsang-Madsen A., Føns S., Laustsen A.H. Toxin neutralization using alternative binding proteins. Toxins. 2019;11:53. doi: 10.3390/toxins11010053. https://www.mdpi.com/2072-6651/11/1/53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.-F., Zhu L.-L., Chen M., Xu H.-M., Wang H.-F., Feng X.-Q., Zhu X.-P., Zhou Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence. 2015;9:923–942. doi: 10.2147/PPA.S87271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Rimmer J., Mount G., Gurney I., Nicol E.D. Challenges of managing snakebite envenomation in a deployed setting. J. Roy. Army Med. Corps. 2013;159:307–311. doi: 10.1136/jramc-2013-000047. [DOI] [PubMed] [Google Scholar]
- Johnson E.K., Kardong K.V., Mackessy S.P. Electric shocks are ineffective in treatment of lethal effects of rattlesnake envenomation in mice. Toxicon. 1987;25:1347–1349. doi: 10.1016/0041-0101(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Jonaitis L., Marković S., Farkas K., Gheorghe L., Krznarić Ž., Salupere R., Mokricka V., Spassova Z., Gatev D., Grosu I., Lijović A., Mitrović O., Saje M., Schafer E., Uršič V., Roblek T., Drobne D. Intravenous versus subcutaneous delivery of biotherapeutics in IBD: an expert's and patient's perspective. BMC Proc. 2021;15:25. doi: 10.1186/s12919-021-00230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam P., Ainsworth S., Sirur F.M., Patel D.C., Kuruvilla J.J., Majumdar D.B. Approaches for implementing society-led community interventions to mitigate snakebite envenoming burden: the she-India experience. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaberopoulos D., Karamanou M., Androutsos G. The theriac in antiquity. Lancet. 2012;379:1942–1943. doi: 10.1016/S0140-6736(12)60846-0. [DOI] [PubMed] [Google Scholar]
- Karain B.D., Lee M.K.H., Hayes W.K. C60 fullerenes as a novel treatment for poisoning and envenomation: a proof-of-concept study for snakebite. J. Nanosci. Nanotechnol. 2016;16:7764–7771. doi: 10.1166/jnn.2016.12851. [DOI] [Google Scholar]
- Karthika I.K., Satapathy A.K. Neurotoxic snake envenomation: neostigmine-induced paradoxical weakness. Indian J. Pediatr. 2021;88 doi: 10.1007/s12098-020-03602-7. 406-406. [DOI] [PubMed] [Google Scholar]
- Kessler P., Marchot P., Silva M., Servent D. The three-finger toxin fold: a multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 2017;142:7–18. doi: 10.1111/jnc.13975. [DOI] [PubMed] [Google Scholar]
- Kim Y.-C., Park J.-H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen C., Ledsgaard L., Dehli R.I., Ahmadi S., Sørensen C.V., Laustsen A.H. Engineering and design considerations for next-generation snakebite antivenoms. Toxicon. 2019;167:67–75. doi: 10.1016/j.toxicon.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Komives C.F., Balderrama M., Cifelli A., Sanchez E.E., Suntravat M., Rathore A.S., Joshi V., White B. Opossum peptide that can neutralize rattlesnake venom is expressed in Escherichia coli. Biotechnol. Prog. 2017;33:81–86. doi: 10.1002/btpr.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C.G. vol. 14. Cambridge University Press), De Theriaca ad Pisonem; Cambridge, MA: 2011. p. 237. (The Complete Works of Galen (Claudii Galeni Opera Omnia) Kuhn). 1964-65. [Google Scholar]
- Kulkarni M., Anees S. Snake venom poisoning: experience with 633 cases. Indian Pediatr. 1994;31 https://europepmc.org/article/med/7875785 1239-1239. [PubMed] [Google Scholar]
- Kwon S., Sevick-Muraca E.M. Effect of lidocaine with and without epinephrine on lymphatic contractile activity in mice in vivo. J. Anesth. 2016;30:1091–1094. doi: 10.1007/s00540-016-2260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamph S. Regulation of medical devices outside the European Union. J. R. Soc. Med. 2012;105:12–21. doi: 10.1258/jrsm.2012.120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet The. Snake-bite envenoming: a priority neglected tropical disease (Editorial) Lancet. 2017;390:2. doi: 10.1016/S0140-6736(17)31751-8. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H. Toxin-centric development approach for next-generation antivenoms. Toxicon. 2018;150:195–197. doi: 10.1016/j.toxicon.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H. How can monoclonal antibodies be harnessed against neglected tropical diseases and other infectious diseases? Expet Opin. Drug Discov. 2019;14:1103–1112. doi: 10.1080/17460441.2019.1646723. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., Engmark M., Milbo C., Johannesen J., Lomonte B., Gutiérrez J.M., Lohse B. From fangs to pharmacology: the future of snakebite envenoming therapy. Curr. Pharmaceut. Des. 2016;22:5270–5293. doi: 10.2174/1381612822666160623073438. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., Gless B.H., Jenkins T.P., Meyhoff-Madsen M., Bjärtun J., Munk A.S., Oscoz S., Fernández J., Gutiérrez J.M., Lomonte B., Lohse B. In vivo neutralization of myotoxin II, a phospholipase A2 homologue from Bothrops asper venom, using peptides discovered via phage display technology. ACS Omega. 2022;7:15561–15569. doi: 10.1021/acsomega.2c00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen A.H., Lohse B., Lomonte B., Engmark M., Gutiérrez J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a toxicity score. Toxicon. 2015;104:43–45. doi: 10.1016/j.toxicon.2015.07.334. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., María Gutiérrez J., Knudsen C., Johansen K.H., Bermúdez-Méndez E., Cerni F.A., Jürgensen J.A., Ledsgaard L., Martos-Esteban A., Øhlenschlæger M., Pus U., Andersen M.R., Lomonte B., Engmark M., Pucca M.B. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon. 2018;146:151–175. doi: 10.1016/j.toxicon.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Lay M., Liang Q., Isbister G.K., Hodgson W.C. In vitro efficacy of antivenom and varespladib in neutralising Chinese Russells viper (Daboia siamensis) venom toxicity. Toxins. 2023;15:62. doi: 10.3390/toxins15010062. https://www.mdpi.com/2072-6651/15/1/62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layfield H.J., Williams H.F., Ravishankar D., Mehmi A., Sonavane M., Salim A., Vaiyapuri R., Lakshminarayanan K., Vallance T.M., Bicknell A.B., Trim S.A., Patel K., Vaiyapuri S. Repurposing cancer drugs batimastat and marimastat to inhibit the activity of a group I metalloprotease from the venom of the Western diamondback rattlesnake, Crotalus atrox. Toxins. 2020;12:309. doi: 10.3390/toxins12050309. https://www.mdpi.com/2072-6651/12/5/309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León G., Estrada R., Chaves F., Rojas G., Ovadia M., Gutiérrez J.M.a. Inhibition by CaNa2EDTA of local tissue damage induced by Bothrops asper (terciopelo) venom: application in horse immunization for antivenom production. Toxicon. 1998;36:321–331. doi: 10.1016/S0041-0101(97)00114-1. [DOI] [PubMed] [Google Scholar]
- Lewin M. 2022. (Personal Communication), Founder of the Center for Exploration and Travel Health and Snakebite Project at the California Academy of Sciences in San Francisco, and Founder of Ophirex. [Google Scholar]
- Lewin M., Samuel S., Merkel J., Bickler P. Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins. 2016;8:248. doi: 10.3390/toxins8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R. New York Times; 2014. The Killers Underfoot; p. 6.https://www.nytimes.com/2014/04/13/opinion/sunday/the-killers-underfoot.html April 13, 2014. [Google Scholar]
- Lewin M.R., Bickler P., Heier T., Feiner J., Montauk L., Mensh B. Reversal of experimental paralysis in a human by intranasal neostigmine aerosol suggests a novel approach to the early treatment of neurotoxic envenomation. Clinical Case Reports. 2013;1:7–15. doi: 10.1002/ccr3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R., Gilliam L.L., Gilliam J., Samuel S.P., Bulfone T.C., Bickler P.E., Gutiérrez J.M. Delayed LY333013 (oral) and LY315920 (intravenous) reverse severe neurotoxicity and rescue juvenile pigs from lethal doses of Micrurus fulvius (Eastern coral snake) venom. Toxins. 2018;10:479. doi: 10.3390/toxins10110479. https://www.mdpi.com/2072-6651/10/11/479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R., Gutiérrez J.M., Samuel S.P., Herrera M., Bryan-Quirós W., Lomonte B., Bickler P.E., Bulfone T.C., Williams D.J. Delayed oral LY333013 rescues mice from highly neurotoxic, lethal doses of Papuan taipan (Oxyuranus scutellatus) venom. Toxins. 2018;10:380. doi: 10.3390/toxins10100380. https://www.mdpi.com/2072-6651/10/10/380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R., Samuel S.P., Wexler D.S., Bickler P., Vaiyapuri S., Mensh B.D. Early treatment with intranasal neostigmine reduces mortality in a mouse model of Naja naja (Indian cobra) envenomation. J. Trop. Med. 2014;6 doi: 10.1155/2014/131835. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Tay F.R., Yiu C.K.Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020;207 doi: 10.1016/j.pharmthera.2019.107465. [DOI] [PubMed] [Google Scholar]
- Lipps B.V. Lethal toxin neutralizing factor from opossum serum as a treatment for snakebites and various types of allergies. Toxicon. 1996;34:165. doi: 10.1016/0041-0101(96)83728-7. [DOI] [Google Scholar]
- Longbottom J., Shearer F.M., Devine M., Alcoba G., Chappuis F., Weiss D.J., Ray S.E., Ray N., Warrell D.A., Ruiz de Castañeda R., Williams D.J., Hay S.I., Pigott D.M. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392:673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynagh T., Kiontke S., Meyhoff-Madsen M., Gless B.H., Johannesen J., Kattelmann S., Christiansen A., Dufva M., Laustsen A.H., Devkota K., Olsen C.A., Kümmel D., Pless S.A., Lohse B. Peptide inhibitors of the α-cobratoxin–nicotinic acetylcholine receptor interaction. J. Med. Chem. 2020;63:13709–13718. doi: 10.1021/acs.jmedchem.0c01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães S.F.V., Peixoto H.M., Moura N., Monteiro W.M., de Oliveira M.R.F. Snakebite envenomation in the Brazilian amazon: a descriptive study. Trans. R. Soc. Trop. Med. Hyg. 2019;113:143–151. doi: 10.1093/trstmh/try121. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Jacobsen I. National snakebite management protocol (India), 2008 (shortened version) Indian J Emerg Pediatr. 2008;1:63–81. [Google Scholar]
- Mahmood M.A., Halliday D., Cumming R., Thwin K.T., Myitzu M., White J., Alfred S., Warrell D.A., Bacon D., Naing W., Aung H., Thein M.M., Chit N.N., Serhal S., Nwe M.T., Aung P.P., Peh C.A. Inadequate knowledge about snakebite envenoming symptoms and application of harmful first aid methods in the community in high snakebite incidence areas of Myanmar. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz W. Plants with a reputation against snakebite. Toxicon. 1992;30:1131–1142. doi: 10.1016/0041-0101(92)90429-9. [DOI] [PubMed] [Google Scholar]
- Menzies S.K., Clare R.H., Xie C., Westhorpe A., Hall S.R., Edge R.J., Alsolaiss J., Crittenden E., Marriott A.E., Harrison R.A., Kool J., Casewell N.R. In vitro and in vivo preclinical venom inhibition assays identify metalloproteinase inhibiting drugs as potential future treatments for snakebite envenoming by Dispholidus typus. Toxicon. 2022;X doi: 10.1016/j.toxcx.2022.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G.C., Aliyu I., Grema B.A., De-Kaa N.L.P. Prehospital care practices for venomous snakebites in resource-limited settings: a narrative review. Arch. Med. Health Sci. 2017;5:237. doi: 10.4103/amhs.amhs_93_17. [DOI] [Google Scholar]
- Michael G.C., Grema B.A., Aliyu I., Alhaji M.A., Lawal T.O., Ibrahim H., Fikin A.G., Gyaran F.S., Kane K.N., Thacher T.D., Badamasi A.K., Ogwuche E. Knowledge of venomous snakes, snakebite first aid, treatment, and prevention among clinicians in Northern Nigeria: a cross-sectional multicentre study. Trans. R. Soc. Trop. Med. Hyg. 2018;112:47–56. doi: 10.1093/trstmh/try028. [DOI] [PubMed] [Google Scholar]
- Michael G.C., Thacher T.D., Shehu M.I.L. The effect of pre-hospital care for venomous snake bite on outcome in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2011;105:95–101. doi: 10.1016/j.trstmh.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Mirtschin P., Rasmussen A., Weinstein S. CSIRO Publishing; Clayton South, Australia: 2017. Australia's Dangerous Snakes: Identification, Biology and Envenoming; p. 432. [Google Scholar]
- Mirtschin P.J., Shine R., Nias T.J., Dunstan N.L., Hough B.J., Mirtschin M. Influences on venom yield in Australian tigersnakes (Notechis scutatus) and brownsnakes (Pseudonaja textilis: elapidae, serpentes) Toxicon. 2002;40:1581–1592. doi: 10.1016/S0041-0101(02)00175-7. [DOI] [PubMed] [Google Scholar]
- Moos B., Williams D., Bolon I., Mupfasoni D., Abela-Ridder B., Ruiz de Castaneda R. A scoping review of current practices on community engagement in rural East Africa: recommendations for snakebite envenoming. Toxicon X. 2021;11 doi: 10.1016/j.toxcx.2021.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J.J., Pearn J.H., Coulter A.R., Tanner C., Charles N.T. The quantity of venom injected by elapid snakes. Toxicon. 1983;21:309–312. doi: 10.1016/0041-0101(83)90217-9. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. The United States Food and Drug Administration (FDA) regulatory response to combat neglected tropical diseases (NTDS): a review. PLoS Neglected Trop. Dis. 2023;17 doi: 10.1371/journal.pntd.0011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M.T., Arruda E.Z., Melo P.A., Martinez A.B., Calil-Eliás S., Tomaz M.A., Lomonte B., Gutiérrez J.M., Arni R.K. Inhibition of myotoxic activity of Bothrops asper myotoxin II by the anti-trypanosomal drug suramin. J. Mol. Biol. 2005;350:416–426. doi: 10.1016/j.jmb.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Naguib M., Kopman A.F. Neostigmine-induced weakness: what are the facts? Anaesthesia. 2018;73:1055–1057. doi: 10.1111/anae.14322. [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Escalante T., Gutiérrez J.M., Shea K.J. A biomimetic of endogenous tissue inhibitors of metalloproteinases: inhibition mechanism and contribution of composition, polymer size, and shape to the inhibitory effect. Nano Lett. 2021;21:5663–5670. doi: 10.1021/acs.nanolett.1c01357. [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Zhao D., Benice O.R., Lee S.-H., Shea K.J. Abiotic mimic of endogenous tissue inhibitors of metalloproteinases: engineering synthetic polymer nanoparticles for use as a broad-spectrum metalloproteinase inhibitor. J. Am. Chem. Soc. 2020;142:2338–2345. doi: 10.1021/jacs.9b11481. [DOI] [PubMed] [Google Scholar]
- Nanjaraj Urs A.N., Yariswamy M., Ramakrishnan C., Joshi V., Suvilesh K.N., Savitha M.N., Velmurugan D., Vishwanath B.S. Inhibitory potential of three zinc chelating agents against the proteolytic, hemorrhagic, and myotoxic activities of Echis carinatus venom. Toxicon. 2015;93:68–78. doi: 10.1016/j.toxicon.2014.11.224. [DOI] [PubMed] [Google Scholar]
- Nascimento da Costa T., Mota-da-Silva A., Colombini M., Moura-da-Silva A.M., Medeiros de Souza R., Monteiro W.M., Bernarde P.S. Relationship between snake size and clinical, epidemiological and laboratory aspects of Bothrops atrox snakebites in the Western Brazilian amazon. Toxicon. 2020;186:160–167. doi: 10.1016/j.toxicon.2020.08.010. [DOI] [PubMed] [Google Scholar]
- Neri-Castro E., Bénard-Valle M., Paniagua D., Boyer V.L.D., Possani L., López-Casillas F., Olvera A., Romero C., Zamudio F., Alagón A. Neotropical rattlesnake (Crotalus simus) venom pharmacokinetics in lymph and blood using an ovine model. Toxins. 2020;12:455. doi: 10.3390/toxins12070455. https://www.mdpi.com/2072-6651/12/7/455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls S.J., Kastelein J.J.P., Schwartz G.G., Bash D., Rosenson R.S., Cavender M.A., Brennan D.M., Koenig W., Jukema J.W., Nambi V., Wright R.S., Menon V., Lincoff A.M., Nissen S.E. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- Nirthanan S., Awal W., Niranjan N.R. In: Snake Venoms. Inagaki H., Vogel C.-W., Mukherjee A.K., Rahmy T.R., Gopalakrishnakone P., editors. Springer Netherlands; Dordrecht: 2017. Snake a-neurotoxins and the nicotinic acetylcholine receptor; pp. 215–252. [Google Scholar]
- Nys M., Zarkadas E., Brams M., Mehregan A., Kambara K., Kool J., Casewell N.R., Bertrand D., Baenziger J.E., Nury H., Ulens C. The molecular mechanism of snake short-chain α-neurotoxin binding to muscle-type nicotinic acetylcholine receptors. Nat. Commun. 2022;13:4543. doi: 10.1038/s41467-022-32174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J., Lee S.-H., Onogi S., Shea K.J. Engineering the protein corona of a synthetic polymer nanoparticle for broad-spectrum sequestration and neutralization of venomous biomacromolecules. J. Am. Chem. Soc. 2016;138:16604–16607. doi: 10.1021/jacs.6b10950. [DOI] [PubMed] [Google Scholar]
- O'Brien J., Lee S.H., Gutiérrez J.M., Shea K.J. Engineered nanoparticles bind elapid snake venom toxins and inhibit venom-induced dermonecrosis. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil M.E., Mack K.A., Gilchrist J., Wozniak E.J. Snakebite injuries treated in United States emergency departments, 2001–2004. Wilderness Environ. Med. 2007;18:281–287. doi: 10.1580/06-WEME-OR-080R1.1. [DOI] [PubMed] [Google Scholar]
- Ochoa-Avilés A., Heredia-Andino O.S., Escandón S.A., Celorio-Carvajal C.A., Arias-Peláez M.C., Zaruma-Torres F., Caldeira C.A.d.S., Soares A.M., Da Silva S.L. Viperidae snakebites in Ecuador: a review of epidemiological and ecological aspects. Toxicon X. 2020;7 doi: 10.1016/j.toxcx.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski W.L., Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am. J. Physiol. Heart Circ. Physiol. 1980;239:H775–H783. doi: 10.1152/ajpheart.1980.239.6.H775. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- Paniagua D., Jiménez L., Romero C., Vergara I., Calderón A., Benard M., Bernas M., Rilo H., De Roodt A., D'Suze G. Lymphatic route of transport and pharmacokinetics of Micrurus fulvius (coral snake) venom in sheep. Lymphology. 2012;45:144–153. [PubMed] [Google Scholar]
- Paniagua D., Vergara I., Boyer L., Alagón A. In: Snake Venoms. Inagaki H., Vogel C.-W., Mukherjee A.K., Rahmy T.R., Gopalakrishnakone P., editors. Springer Netherlands; Dordrecht: 2017. Role of lymphatic system on snake venom absorption; pp. 453–474. [Google Scholar]
- Paniagua D., Vergara I., Román R., Romero C., Benard-Valle M., Calderón A., Jiménez L., Bernas M.J., Witte M.H., Boyer L.V., Alagón A. Antivenom effect on lymphatic absorption and pharmacokinetics of coral snake venom using a large animal model. Clin. Toxicol. 2019;57:727–734. doi: 10.1080/15563650.2018.1550199. [DOI] [PubMed] [Google Scholar]
- Parker-Cote J., Meggs W.J. First aid and pre-hospital management of venomous snakebites. Trav. Med. Infect. Dis. 2018;3:45. doi: 10.3390/tropicalmed3020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patikorn C., Ismail A.K., Abidin S.A.Z., Blanco F.B., Blessmann J., Choumlivong K., Comandante J.D., Doan U.V., Ismail Z.M., Khine Y.Y., Maharani T., Nwe M.T., Qamruddin R.M., Safferi R.S., Santamaria E., Tiglao P.J.G., Trakulsrichai S., Vasaruchapong T., Chaiyakunapruk N., Taychakhoonavudh S., Othman I. Situation of snakebite, antivenom market and access to antivenoms in ASEAN countries. BMJ Glob. Health. 2022;7 doi: 10.1136/bmjgh-2021-007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra A., Mukherjee A.K. Assessment of snakebite burdens, clinical features of envenomation, and strategies to improve snakebite management in Vietnam. Acta Trop. 2021;216 doi: 10.1016/j.actatropica.2021.105833. [DOI] [PubMed] [Google Scholar]
- Phisalix C., Bertrand G. Sur la propriété antitoxique du sang des animaux vaccinés contre le venin de vipère. CR Soc. Biol. 1894;46:111–113. [Google Scholar]
- Pintor A.F.V., Ray N., Longbottom J., Bravo-Vega C.A., Yousefi M., Murray K.A., Ediriweera D.S., Diggle P.J. Addressing the global snakebite crisis with geo-spatial analyses – recent advances and future direction. Toxicon X. 2021;11 doi: 10.1016/j.toxcx.2021.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C.H., Peterson L.W. Treatment of poisoning with rattlesnake venom: experiments with negative pressure, tourniquet and bulb suction. Arch. Surg. 1946;53:564–569. doi: 10.1001/archsurg.1946.01230060574004. [DOI] [PubMed] [Google Scholar]
- Potet J., Beran D., Ray N., Alcoba G., Habib A.G., Iliyasu G., Waldmann B., Ralph R., Faiz M.A., Monteiro W.M., de Almeida Gonçalves Sachett J., di Fabio J.L., Cortés M.d.l.Á., Brown N.I., Williams D.J. Access to antivenoms in the developing world: a multidisciplinary analysis. Toxicon X. 2021;12 doi: 10.1016/j.toxcx.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potet J., Smith J., McIver L. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preciado L.M., Pereañez J.A. Low molecular mass natural and synthetic inhibitors of snake venom metalloproteinases. Toxin Rev. 2018;37:19–26. doi: 10.1080/15569543.2017.1309550. [DOI] [Google Scholar]
- Pucca M.B., Cerni F.A., Janke R., Bermudez-Mendez E., Ledsgaard L., Barbosa J.E., Laustsen A.H. History of envenoming therapy and current perspectives. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.01598. doi:10.10.3389/fimmu.2019.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucca M.B., Knudsen C., Oliveira I.S., Rimbault C., Cerni F.A., Wen F.H., Sachett J., Sartim M.A., Laustsen A.H., Monteiro W.M. Current knowledge on snake dry bites. Toxins. 2020;12:668. doi: 10.3390/toxins12110668. https://www.mdpi.com/2072-6651/12/11/668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzari U., Fernandes P.A., Mukherjee A.K. Advances in the therapeutic application of small-molecule inhibitors and repurposed drugs against snakebite. J. Med. Chem. 2021;64:13938–13979. doi: 10.1021/acs.jmedchem.1c00266. [DOI] [PubMed] [Google Scholar]
- Puzari U., Fernandes P.A., Mukherjee A.K. Pharmacological re-assessment of traditional medicinal plants-derived inhibitors as antidotes against snakebite envenoming: a critical review. J. Ethnopharmacol. 2022;292 doi: 10.1016/j.jep.2022.115208. [DOI] [PubMed] [Google Scholar]
- Rasmussen H.S., McCann P.P. Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol. Ther. 1997;75:69–75. doi: 10.1016/S0163-7258(97)00023-5. [DOI] [PubMed] [Google Scholar]
- Rogers, I.R., Winkel, K.D., 2005. Struan Sutherland’s “rationalisation of first-aid measures for elapid snakebite”—a commentary. Wilderness Environ. Med. 16, 160–163. doi: 10.1580/ER20-04.1. [DOI] [PubMed]
- Rucavado A., Escalante T., Franceschi A., Chaves F., León G., Cury Y., Ovadia M., Gutiérrez J.M. Inhibition of local hemorrhage and dermonecrosis induced by Bothrops asper snake venom: effectiveness of early in situ administration of the peptidomimetic metalloproteinase inhibitor batimastat and the chelating agent CaNa2EDTA. Am. J. Trop. Med. Hyg. 2000;63:313–319. doi: 10.4269/ajtmh.2000.63.313. [DOI] [PubMed] [Google Scholar]
- Rudresha G.V., Urs A.P., Manjuprasanna V.N., Milan Gowda M.D., Jayachandra K., Rajaiah R., Vishwanath B.S. Echis carinatus snake venom metalloprotease-induced toxicities in mice: therapeutic intervention by a repurposed drug, tetraethyl thiuram disulfide (disulfiram) PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F.E. First-aid for snake venom poisoning. Toxicon. 1967;4:285–289. doi: 10.1016/0041-0101(67)90057-8. [DOI] [PubMed] [Google Scholar]
- Russell F.E., Carlson R.W., Wainschel J., Osborne A.H. Snake venom poisoning in the United States: experiences with 550 cases. JAMA. 1975;233:341–344. doi: 10.1001/jama.1975.03260040035020. [DOI] [PubMed] [Google Scholar]
- Russell J.J., Schoenbrunner A., Janis J.E. Snake bite management: a scoping review of the literature. Plast Reconstr Surg Glob Open. 2021;9 doi: 10.1097/GOX.0000000000003506. e3506-e3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador G.H.M., Borges R.J., Lomonte B., Lewin M.R., Fontes M.R.M. The synthetic varespladib molecule is a multi-functional inhibitor for PLA2 and PLA2-like ophidic toxins. Biochim. Biophys. Acta Gen. Subj. 2021;1865 doi: 10.1016/j.bbagen.2021.129913. [DOI] [PubMed] [Google Scholar]
- Salvador G.H.M., Dreyer T.R., Gomes A.A.S., Cavalcante W.L.G., dos Santos J.I., Gandin C.A., de Oliveira Neto M., Gallacci M., Fontes M.R.M. Structural and functional characterization of suramin-bound mjtx-I from Bothrops moojeni suggests a particular myotoxic mechanism. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-28584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez G., Harer W.B. In: Toxicology in Antiquity. second ed. Wexler P., editor. Academic Press; 2019. Chapter 3 - toxicology in ancient Egypt; pp. 73–82. [Google Scholar]
- Sanhajariya S., Duffull S.B., Isbister G.K. Pharmacokinetics of snake venom. Toxins. 2018;10:73. doi: 10.3390/toxins10020073. https://www.mdpi.com/2072-6651/10/2/73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul M.E., Thomas P.A., Dosen P.J., Isbister G.K., O'Leary M.A., Whyte I.M., McFadden S.A., van Helden D.F. A pharmacological approach to first aid treatment for snakebite. Nat. Med. 2011;17:809–811. doi: 10.1038/nm.2382. [DOI] [PubMed] [Google Scholar]
- Scarborough J. vol. 19. American Institute of the History of Pharmacy; University of Wisconsin Press; 1977. pp. 3–23.http://www.jstor.org/stable/41110050 (Nicander's Toxicology I: Snakes. Pharmacy in History). [PubMed] [Google Scholar]
- Schioldann E., Mahmood M.A., Kyaw M.M., Halliday D., Thwin K.T., Chit N.N., Cumming R., Bacon D., Alfred S., White J., Warrell D., Peh C.A. Why snakebite patients in Myanmar seek traditional healers despite availability of biomedical care at hospitals? Community perspectives on reasons. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schonbein G.W. Microlymphatics and lymph flow. Physiol. Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Seidenari S., Pagnoni A., Di Nardo A.D., Giannetti A. Echographic evaluation with image analysis of normal skin: variations according to age and sex. Skin Pharmacol. Physiol. 1994;7:201–209. doi: 10.1159/000211295. [DOI] [PubMed] [Google Scholar]
- Seifert S., White J., Currie B.J. Pressure bandaging for North American snake bite? No. Clin. Toxicol. 2011;49:883–885. doi: 10.3109/15563650.2011.626424. [DOI] [PubMed] [Google Scholar]
- Si H., Yin C., Wang W., Davies P., Sanchez E., Suntravat M., Zawieja D., Cromer W. Effect of the snake venom component crotamine on lymphatic endothelial cell responses and lymph transport. Microcirculation. 2023;30 doi: 10.1111/micc.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Carvalho R., Gaspar M.Z., Quadros L.H.B., Lobo L.G.G., Giuffrida R., Santarém C.L., Silva E.O., Gerez J.R., Silva N.J., Hyslop S., Lomonte B., Floriano R.S. Partial efficacy of a Brazilian coralsnake antivenom and varespladib in neutralizing distinct toxic effects induced by sublethal Micrurus dumerilii carinicauda envenoming in rats. Toxicon. 2022;213:99–104. doi: 10.1016/j.toxicon.2022.04.014. [DOI] [PubMed] [Google Scholar]
- Silva-Neto A.V., Santos W.G., Botelho A.F.M., Diamantino G.M.L., Soto-Blanco B., Melo M.M. Use of EDTA in the treatment of local tissue damage caused by the Bothrops alternatus venom. Arq. Bras. Med. Vet. Zootec. 2018;70:1529–1538. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-09352018000501529&nrm=iso [Google Scholar]
- Silva G.M., Berto D.H., Lima C.A., Waitman K.B., Lima C.F.G., Prezoto B.C., Vieira M.L., Rocha M.M.T., Gonçalves L.R.C., Andrade S.A. Synergistic effect of serine protease inhibitors and a bothropic antivenom in reducing local hemorrhage and coagulopathy caused by Bothrops jararaca venom. Toxicon. 2021;199:87–93. doi: 10.1016/j.toxicon.2021.06.009. [DOI] [PubMed] [Google Scholar]
- Singaravelu K.P., Pandit V.R., Chinnakali P., Bammigatti C. Pre-hospital care and its association with clinical outcome of snakebite victims presenting at a tertiary care referral hospital in South India. Trop. Doct. 2021;51:77–80. doi: 10.1177/0049475520966958. [DOI] [PubMed] [Google Scholar]
- Slouber M. Oxford University Press; New York: 2016. Early Tantric Medicine: Snakebite, Mantras, and Healing in the Garuda Tantras; p. 386. [Google Scholar]
- Slouber M.J. University of California; Berkeley: 2012. Gāruda Medicine: A History of Snakebite and Religious Healing in South Asia; p. 340. Dissertation Archive. [Google Scholar]
- Steinhorst J., Aglanu L.M., Ravensbergen S.J., Dari C.D., Abass K.M., Mireku S.O., Adu Poku J.K., Enuameh Y.A.K., Blessmann J., Harrison R.A., Amuasi J.H., Stienstra Y. ‘The medicine is not for sale’: practices of traditional healers in snakebite envenoming in Ghana. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R.M., Page C.P., Schwesinger W.H., McCarter R., Martinez J., Aust J.B. Antivenin and fasciotomy/debridement in the treatment of the severe rattlesnake bite. Am. J. Surg. 1989;158:543–547. doi: 10.1016/0002-9610(89)90188-8. [DOI] [PubMed] [Google Scholar]
- Suchithra N., Pappachan J.M., Sujathan P. Snakebite envenoming in Kerala, South India: clinical profile and factors involved in adverse outcomes. Emerg. Med. J. 2008;25:200–204. doi: 10.1136/emj.2007.051136. [DOI] [PubMed] [Google Scholar]
- Supersaxo A., Hein W.R., Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharmaceut. Res. 1990;7:167–169. doi: 10.1023/A:1015880819328. [DOI] [PubMed] [Google Scholar]
- Sutherland S.K., Coulter A.R., Harris R.D. Rationalisation of first-aid measures for elapid snakebite. Lancet. 1979;313:183–186. doi: 10.1016/S0140-6736(79)90580-4. [DOI] [PubMed] [Google Scholar]
- Sweet B.V., Schwemm A.K., Parsons D.M. Review of the processes for FDA oversight of drugs, medical devices, and combination products. J. Manag. Care Pharm. 2011;17:40–50. doi: 10.18553/jmcp.2011.17.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi M.A., Tseng C.-M.L., Roskos L.K. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov. Today. 2006;11:81–88. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- Tan C.H., Lingam T.M.C., Tan K.Y. Varespladib (LY315920) rescued mice from fatal neurotoxicity caused by venoms of five major Asiatic kraits (Bungarus spp.) in an experimental envenoming and rescue model. Acta Trop. 2022;227 doi: 10.1016/j.actatropica.2021.106289. [DOI] [PubMed] [Google Scholar]
- Theakston R.D.G., Laing G.D. Diagnosis of snakebite and the importance of immunological tests in venom research. Toxins. 2014;6:1667–1695. doi: 10.3390/toxins6051667. https://www.mdpi.com/2072-6651/6/5/1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus J.K., Kay M.K., Glaser C.J.J. Application of phage display for the development of a novel inhibitor of PLA(2) activity in western cottonmouth venom. J. Venom Res. 2017;8:19–24. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5735792/ [PMC free article] [PubMed] [Google Scholar]
- Tiwari N., Aggarwal G., Jain G.K., Mittal G. Multi-drug loaded microneedles for emergency treatment of snakebite envenomation. Med. Hypotheses. 2022;165 doi: 10.1016/j.mehy.2022.110908. [DOI] [Google Scholar]
- Tsoucalas G., Androutsos G. In: Toxicology in Antiquity. second ed. Wexler P., editor. Academic Press; 2019. Chapter 17 - Asclepius and the snake as toxicological symbols in ancient Greece and Rome; pp. 257–265. [Google Scholar]
- Udaykumar P. third ed. Edition. Jaypee Brothers Medical Publishers; New Delhi: 2017. Pharmacology for Physiotherapy Students; p. 298. [Google Scholar]
- USFDA . 2019. Bridging for Drug-Device and Biologic-Device Combination Products (Draft Guidance for Industry)https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bridging-drug-device-and-biologic-device-combination-products URL: [Google Scholar]
- Utkin Y.N. Last decade update for three-finger toxins: newly emerging structures and biological activities. World J. Biol. Chem. 2019;10:17–27. doi: 10.4331/wjbc.v10.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden D.F., Dosen P.J., O'Leary M.A., Isbister G.K. Two pathways for venom toxin entry consequent to injection of an Australian elapid snake venom. Sci. Rep. 2019;9:8595. doi: 10.1038/s41598-019-45022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden D.F., Thomas P.A., Dosen P.J., Imtiaz M.S., Laver D.R., Isbister G.K. Pharmacological approaches that slow lymphatic flow as a snakebite first aid. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman G.A. Drugs, devices, and the FDA: Part 2: an overview of approval processes: FDA approval of medical devices. JACC (J. Am. Coll. Cardiol.): Basic to Translational Science. 2016;1:277–287. doi: 10.1016/j.jacbts.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara I., Castillo E.Y., Romero-Piña M.E., Torres-Viquez I., Paniagua D., Boyer L.V., Alagón A., Medina L.A. Biodistribution and lymphatic tracking of the main neurotoxin of Micrurus fulvius venom by molecular imaging. Toxins. 2016;8:85. doi: 10.3390/toxins8040085. https://www.mdpi.com/2072-6651/8/4/85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk F.J., Admiraal J.F., Jackson K., Reshef R., de Bakker M.A.G., Vanderschoot K., van den Berge I., van Atten M., Burgerhout E., Beck A., Mirtschin P.J., Kochva E., Witte F., Fry B.G., Woods A.E., Richardson M.K. Evolutionary origin and development of snake fangs. Nature. 2008;454:630–633. doi: 10.1038/nature07178. [DOI] [PubMed] [Google Scholar]
- Walker-Meikle K. Toxicology and treatment: medical authorities and snake-bite in the middle ages. Korot. 2014;22:85–104. [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang J., Zhang D., Xiao H., Xiong S., Huang C. Exploration of the inhibitory potential of varespladib for snakebite envenomation. Molecules. 2018;23:391. doi: 10.3390/molecules23020391. http://www.mdpi.com/1420-3049/23/2/391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell D.A., Williams D.J. Clinical aspects of snakebite envenoming and its treatment in low-resource settings. Lancet. 2023;401:1382–1398. doi: 10.1016/S0140-6736(23)00002-8. [DOI] [PubMed] [Google Scholar]
- Webb D.J., Freestone S., Allen M.J., Muirhead G.J. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am. J. Cardiol. 1999;83:21–28. doi: 10.1016/S0002-9149(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Wilkins D., Burns D.S., Wilson D., Warrell D.A., Lamb L.E.M. Snakebites in Africa and Europe: a military perspective and update for contemporary operations. BMJ Military Health. 2018;164:370. doi: 10.1136/jramc-2017-000883. [DOI] [PubMed] [Google Scholar]
- Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D., Habib A.G., Junghanss T., Fan H.W., Turner M., Harrison R.A., Warrell D.A. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.J., Gutiérrez J.-M., Calvete J.J., Wüster W., Ratanabanangkoon K., Paiva O., Brown N.I., Casewell N.R., Harrison R.A., Rowley P.D., O'Shea M., Jensen S.D., Winkel K.D., Warrell D.A. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteonomics. 2011;74:1735–1767. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Williams S.S., Wijesinghe C.A., Jayamanne S.F., Buckley N.A., Dawson A.H., Lalloo D.G., de Silva H.J. Delayed psychological morbidity associated with snakebite envenoming. PLoS Neglected Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson P. Snake poisining in the United States: a study based on the analysis of 740 cases. Arch. Intern. Med. 1908;I(4):516–570. doi: 10.1001/archinte.1908.00050040075004. [DOI] [Google Scholar]
- Winer A., Adams S., Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol. Cancer Therapeut. 2018;17:1147–1155. doi: 10.1158/1535-7163.Mct-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . second ed. Edition. WHO Regional Office for South-East Asia; New Delhi: 2016. Guidelines for the Management of Snakebites.https://apps.who.int/iris/handle/10665/249547 URL: [Google Scholar]
- World Health Organization . 2017. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins (Replacement of Annex 2 of Who Technical Report Series, No. 964) (Geneva, Switzerland) p. 192.https://www.who.int/publications/m/item/snake-antivenom-immunoglobulins-annex-5-trs-no-1004 URL: [Google Scholar]
- World Health Organization . In: Abela-Ridder B., editor. vol. 70. 2019. Snakebite envenoming: a strategy for prevention and control.https://www.who.int/publications/i/item/9789241515641 (Control of Neglected Tropical Diseases). Geneva, Switzerland. [Google Scholar]
- Xie C., Albulescu L., Bittenbinder M.A., Somsen G., Vonk F., Casewell N.R., Kool J. Neutralizing effects of small molecule inhibitors and metal chelators on coagulopathic viperinae snake venom toxins. Biomedicines. 2020;8:297. doi: 10.3390/biomedicines8090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Slagboom J., Albulescu L.-O., Somsen G.W., Vonk F.J., Casewell N.R., Kool J. Neutralising effects of small molecule toxin inhibitors on nanofractionated coagulopathic crotalinae snake venoms. Acta Pharm. Sin. B. 2020;10:1835–1845. doi: 10.1016/j.apsb.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.A., Kardong K.V. Mechanisms controlling venom expulsion in the Western diamondback rattlesnake, Crotalus atrox. J. Exp. Zool. Part A: Ecological Genetics and Physiology. 2007;307A:18–27. doi: 10.1002/jez.a.341. [DOI] [PubMed] [Google Scholar]
- Youngman N.J., Walker A., Naude A., Coster K., Sundman E., Fry B.G. Varespladib (LY315920) neutralises phospholipase A2 mediated prothrombinase-inhibition induced by Bitis snake venoms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;236 doi: 10.1016/j.cbpc.2020.108818. [DOI] [PubMed] [Google Scholar]
- Yü-liang H., Ju-chin T., Yi-ti H., Tz'u-chüan L., Hsing-liang C., Ching-yen L. Experimental studies on curing elapid bite with trypsin. Sci. Sin. 1975;18:396–405. http://europepmc.org/abstract/MED/1198093 PMID: 1198093. [PubMed] [Google Scholar]
- Zdenek C.N., Youngman N.J., Hay C., Dobson J., Dunstan N., Allen L., Milanovic L., Fry B.G. Anticoagulant activity of black snake (Elapidae: pseudechis) venoms: mechanisms, potency, and antivenom efficacy. Toxicol. Lett. 2020;330:176–184. doi: 10.1016/j.toxlet.2020.05.014. [DOI] [PubMed] [Google Scholar]
- Zhong H., Chan G., Hu Y., Hu H., Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10:263. doi: 10.3390/pharmaceutics10040263. https://www.mdpi.com/1999-4923/10/4/263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinenko O., Tovstukha I., Korniyenko Y. PLA2 inhibitor varespladib as an alternative to the antivenom treatment for bites from Nikolsky’s viper Vipera berus nikolskii. Toxins. 2020;12(356):1–7. doi: 10.3390/toxins12060356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.