Abstract
Purpose
Skeletal dysplasias are rare genetic disorders that are characterized by abnormal development of bone and cartilage. There are multiple medical and non-medical treatments for specific symptoms of skeletal dysplasias e.g. pain, as well as corrective surgical procedures to improve physical functioning. The aim of this paper was to develop an evidence-gap map of treatment options for skeletal dysplasias, and their impact on patient outcomes.
Methods
We conducted an evidence-gap map to identify the available evidence on the impact of treatment options on people with skeletal dysplasias on clinical outcomes (such as increase in height), and dimensions of health-related quality of life. A structured search strategy was applied to five databases. Two reviewers independently assessed articles for inclusion in two stages: titles and abstracts (stage 1), and full text of studies retained at stage 2.
Results
58 studies fulfilled our inclusion criteria. The included studies covered 12 types of skeletal dysplasia that are non-lethal with severe limb deformities that could result in significant pain and numerous orthopaedic interventions. Most studies reported on the effect of surgical interventions (n = 40, 69%), followed by the effect of treatments on dimensions of health quality-of-life (n = 4, 6.8%) and psychosocial functioning (n = 8, 13.8%).
Conclusion
Most studies reported on clinical outcomes from surgery for people living with Achondroplasia. Consequently, there are gaps in the literature on the full range of treatment options (including no active treatment), outcomes and the lived experience of people living with other skeletal dysplasias. More research is warranted to examine the impact of treatments on health-related quality-of-life of people living with skeletal dysplasias, including their relatives to enable them to make preference- and valued based decisions about treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11136-023-03431-z.
Keywords: Skeletal dysplasia, Evidence gap map, Clinical outcomes, Quality-of-life, Psychosocial functioning
Background
Skeletal dysplasia is an overarching term for rare genetic disorders that are characterized by the abnormal development of bone and cartilage, which can also affect muscle, tendons, and ligaments. There are over 461 heterogeneous skeletal dysplasias [1] with an estimated overall prevalence of 2.3 per 10,000 live births [2]. The majority of skeletal dysplasias first present at a young age [3, 4].
There are numerous sub-types of skeletal dysplasias that are non-lethal, but can result in severe limb deformities and significant pain and discomfort that could lead to loss of mobility and require numerous orthopaedic interventions. One such sub-type are metaphyseal chondrodysplasias, which are a diverse group of genetic disorders characterized by flaring and irregularity of various metaphyses (long bones) and vertebrae (spinal bones) [3]. The most common metaphyseal chondrodysplasia is Metaphyseal Chondrodysplasia type Schmid (MCDS, ORPHA174). MCDS is an ultra-rare genetic condition, with an estimated prevalence of three to six individuals per million of the population [5]. It is caused by a mutation in the X-type collagen, with signal characteristics of short stature with abnormally short arms and legs (short-limbed dwarfism) and bowed legs (genu varum) [6]. A secondary sub-type are epiphyseal dysplasias which are grouped by the condition’s spinal involvement [7].
Understanding the lived experience of those with a skeletal dysplasia is important to providing optimal care throughout their healthcare journey [8]. Due to the nature of the conditions, those with skeletal dysplasia have increased levels of pain, and in adults with skeletal dysplasia those with higher levels of pain are more likely to report mental health concerns [9]. In addition to higher levels of pain, those with skeletal dysplasia have poorer health-related quality of life (HRQoL) outcomes and experience increased limitations to their physical functioning [10, 11].
The treatment and management of symptoms of those with a skeletal dysplasia is complex and unique to the individual, and is focused on improving symptoms, physical function and prevention of complications in later life. Treatments include non-medical and medical treatments, as well as corrective surgeries to improve deformities, physical function and reduce pain [6]. These interventions often commence from an early age, with surgical interventions occurring at different stages in an individual’s growth development and remaining growth potential [4]. Surgical treatment for symptoms of skeletal dysplasia continues to develop in an effort to reduce the number of repeated corrective surgical procedures [4]. Non-medical treatment includes orthoses and physical therapy, which can help correct particular deformities of the hip and knee, and posture [12]. Physical therapy may involve strengthening exercises for the lower extremities which, in part, may help with pain management. Further pain management options include the long-term use of analgesic medication and/or hot, moist packs [13].
With advances in genetic medicine, new treatments are emerging in the form of targeted drug therapies. These treatments have the potential to decelerate or prevent the development of skeletal dysplasias [14]. The efficacy of drug treatments varies by genetic condition: for example, growth hormone therapy is indicated as a treatment for short stature for people with achondroplasia or hypochondroplasia, but not MCDS [15]. In the case of MCDS, carbamazepine, a drug treatment for epilepsy and other seizure disorders, trigeminal neuralgia, and bipolar disorder is undergoing a clinical trial to establish its effectiveness for reducing endoplasmic reticulum stress and so improve bone growth and patient outcomes [6, 16].
Despite the treatment options available and the potential for emerging therapies, outcomes vary considerably between individuals affected by skeletal dysplasia. There are currently no systematic reviews or evidence gap maps of treatment options for skeletal dysplasias and their impact on clinical outcomes and HRQoL.
We formulated the following review question to produce an evidence gap map:
For children/adults diagnosed with non-lethal skeletal dysplasias with severe limb deformities and significant pain and discomfort that could lead to loss of mobility and require numerous orthopaedic interventions, what is the evidence for the impact of available treatment options on clinical outcomes and health-related quality of life?
Methods
We adhered to the Preferred Reporting Items for Systematic Review and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines [17], and guidance for producing evidence gap maps [18].
Eligibility criteria
Study designs
All study designs (qualitative and quantitative) with a sample size of ≥ 10 participants were eligible for inclusion in the review.
Population
Children or adults with a diagnosis of one of 30 skeletal dysplasias that shared similarities in terms of being non-lethal with severe limb deformities and significant pain and discomfort that could lead to loss of mobility and require numerous orthopaedic interventions. The list of conditions eligible for inclusion was developed by consulting with a geneticist with specialist expertise in skeletal dysplasias, specifically MCDS (see online resource 1). Studies were excluded if most participants (≥ 50%) did not have a diagnosis of these skeletal dysplasias.
Interventions
Any type of surgery, medical (pharmacological) or non-medical treatment: physical therapy, orthoses, supported self-management for health and lifestyle change, counselling, or specialist services for mental health.
Outcomes
Clinical outcomes (e.g., changes in height or skeletal measurements, correction of deformities, complication rate), and dimensions of HRQoL (physical functioning, pain, mental health/well-being, psychosocial functioning and satisfaction).
Search strategy
The search strings to identify relevant studies were applied to the following bibliographic databases: MedLine, PsychInfo, CINHAL, EMBASE, and SCOPUS. The structured search strategy was devised using a combination of subject indexing terms and free text search terms covering the period up to March 2021. The search terms were identified through background literature screening. In addition, experts in the field of skeletal dysplasia were contacted to request papers that may not yet be available in the public domain. There was no restriction placed on publication date, but studies were restricted to English, Spanish or Italian publications. An example of the search strategy applied to EMBASE can be found in online resource – online resource 2.
Selection of studies
Three reviewers (NM, JH, DF) independently screened the titles and abstracts identified by the search strategy. Studies retained at this stage were read in full-text, and their relevance for inclusion was independently determined by two reviewers (NM, JH) with reference to a study selection form (online resource 1). A third reviewer (DF) adjudicated on any disagreements between these two reviewers that could not be resolved via discussion.
Risk of bias
Due to the extreme rarity of the genetic conditions in the general population, study designs were expected to be predominantly observational, with small sample sizes. For this reason, we did not assess methodological quality, as the primary aim was to develop an evidence-gap map.
Data synthesis
A systematic mapping exercise was conducted to produce an evidence gap map of research across a range of treatment options and outcomes for people living with non-lethal skeletal dysplasias that are non-lethal with severe limb deformities and significant pain and discomfort that could lead to loss of mobility and require numerous orthopaedic interventions. A structured data extraction form was used to extract data to produce an evidence gap-map of: type of skeletal dysplasia; treatment type (surgical, pharmacological, physical therapy, supported self-management for health and lifestyle change, orthoses); outcome category (clinical, HRQoL [scales that report across dimensions of HRQoL—psychosocial functioning, mental health/wellbeing, pain, physical functioning, and satisfaction], or report on dimensions of HRQoL as single measure derived from HRQoL measurement scales, or single item measure scales (e.g. VAS for pain severity); and direction of treatment effect (whether the treatment had no effect, a positive, or negative impact on outcomes). Clustered bar graphs were produced to convey frequency of studies for: type of skeletal dysplasia and type of treatment; and type of treatment and type of outcomes. An evidence gap map was produced to convey the effects of treatments, by type, on outcomes for each type of skeletal dysplasia.
Results
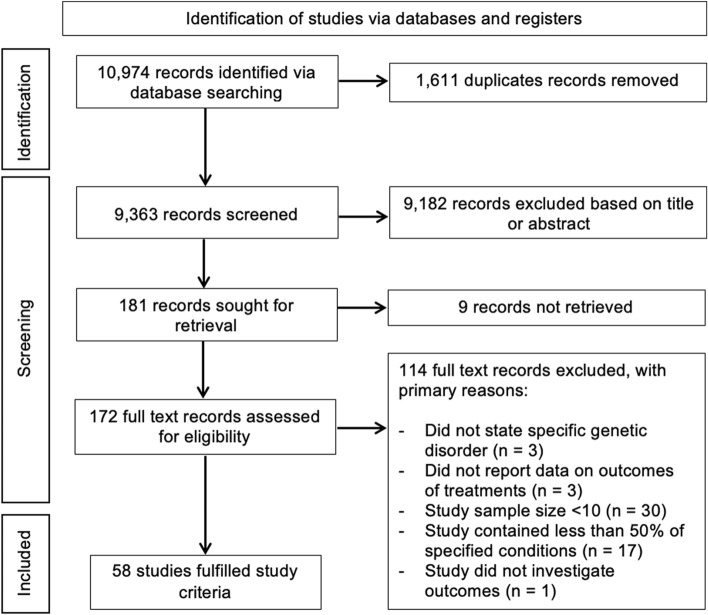
A total of 10,974 records (9,363 after de-duplication) were identified by the search strategy, with 181 full text studies evaluated for inclusion in the evidence-gap map (Fig. 1). From these 181 studies, a total of 58 studies met the eligibility criteria [5, 15, 19–74].
Fig. 1.
Flow diagram summarising the process used to identify the included studies. An overview of the characteristics of each included study is provided in online resource 3
Out of the 58 included studies, four were randomised control trials [22, 32, 53, 54], 25 were retrospective case studies [5, 23–25, 27, 28, 30, 34, 35, 39, 41, 42, 45, 46, 48, 49, 57, 59, 65–69, 71, 73], 15 were retrospective cohort studies [20, 21, 26, 29, 31, 36, 38, 40, 47, 55, 58, 60, 61, 70, 74], eight were controlled before and after studies [15, 51, 52, 56, 62–64, 72], two were prognostic studies [19, 37], and one study was a non-randomised control trial [50]. There were three cross-sectional studies that used patient focused surveys [33, 43, 44].
Included studies were conducted across 23 countries: Japan (n = 13) [15, 31, 34, 38–40, 43, 46, 53, 54, 63, 64, 72] United States of America (USA; n = 12) [19, 23, 25, 26, 28, 44, 55–58, 70, 71]; South Korea (n = 10) [35–37, 42, 48, 49, 59–61, 68]; United Kingdom (UK; n = 5) [22, 27, 30, 45, 51]; Italy (n = 4) [20, 21, 67, 74]; France (n = 2) [50, 66]; China (n = 2) [29, 41]; the Netherlands (n = 2) [65, 69]. One study was conducted each in Greece (62), Spain (47), and Turkey (24). Five of the studies utilised multi-national cohorts: Australia, Canada and UK (33); Australia, France, UK and USA (52); Croatia and USA (73); Austria, Tunisia and Russia (5); and Sweden, Norway, Finland, Denmark and Germany (32).
This evidence gap map is comprised of data from 3,136 participants. Sample sizes ranged from 10 to 567 with a mean age of participants ranging from 2.25 to 51.2 years. The ages reported in the studies ranged from 0.4 to 79 years. Out of the 58 studies, 35 of the studies focused on children and adolescents, with 23 reporting on interventions and outcomes across children, adolescents and adult age groups (see Appendix C). Seven studies did not report on the sex of participants [27, 30, 33, 38, 40, 48, 57] and in a further study [59] the sex of the participants was unclear. Out of the 51 studies that reported sex of the participants, there were 1,070 females and 1,036 males. The included studies were published between 1990 [22] and 2019 [43].
Types of treatment and skeletal dysplasia
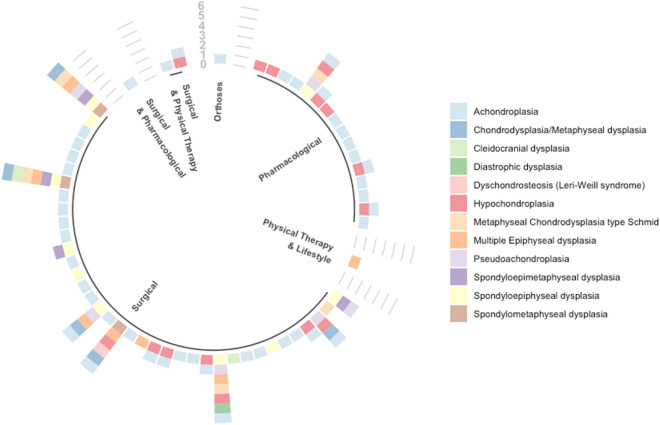
Included studies covered 12 types of skeletal dysplasia (see Fig. 2). 17 were multi-condition studies (including two or more skeletal dysplasias) [15, 19–21, 33, 34, 38–40, 45, 47, 56, 58, 64, 66, 73, 74]. Most studies focused on, or included people with Achondroplasia (ACH; n = 44), followed by Hypochondroplasia (HCH; n = 15), spondyloepiphyseal dysplasia (SED; n = 10), multiple epiphyseal dysplasia (MED; n = 7), pseudoachondroplasia (PAch; n = 6), chondrodysplasia/metaphyseal dysplasia (MD; n = 4), spondyloepimetaphyseal dysplasia (SEMD; n = 4), spondylometaphyseal dysplasia (SMD; n = 3), cleidocranial dysplasia (CCD; n = 2) and one study each for diastropic dysplasia and Leri Weill syndrome. Only five studies reported on treatments for individuals with MCDS [5, 15, 33, 66, 73].
Fig. 2.
Evidence gap map—studies by type of skeletal dysplasia and treatment type
The treatment focus of included studies across all skeletal dysplasias was primarily surgical (n = 40, 69% of studies), followed by pharmacology (n = 16, 28%), physical therapy and lifestyle behavioural change interventions (n = 1, 2%), and orthotic treatment (n = 1, 2%). Three of the surgical focused studies included additional treatments—one with pharmacological [43] and two with physical therapy [30, 74].
Treatments and outcomes
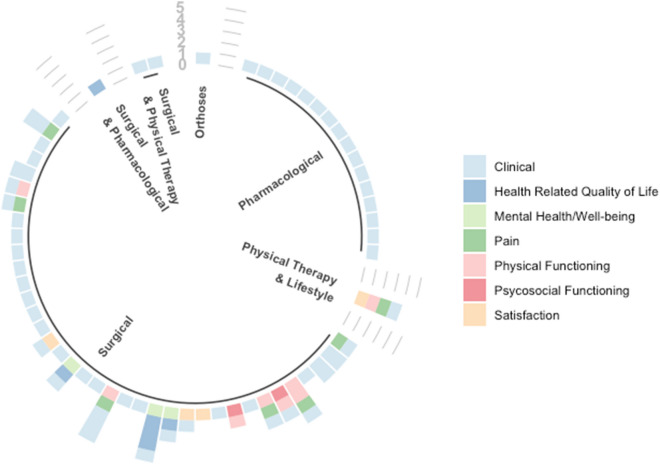
Of the 40 studies of surgical treatment, 93% reported on clinical related outcomes such as increased length or height (see Fig. 3). Overall, out of the 58 included studies (n = 11, 19% reported on non-clinical outcomes (dimensions of HRQoL) such as their physical functioning and satisfaction with surgery. Two studies reported on use of physical therapy after surgical treatments for people with skeletal dysplasias [30, 45]. Despite this, physical functioning was assessed in only 10 studies, and was done primarily after surgery nine studies. One study that focused on individuals with MCDS reported on the effects of a pharmacological treatment on growth [15]. One study examined the effect of physical therapy after a lifestyle behaviour change intervention on physical functioning for individuals with MED, which was assessed using the Harris Hip Score (HHS) and a visual analogue scale (VAS) for pain and stiffness [37]. Only one study reported on use of orthoses that aimed to improve the extent of thoracolumbar kyphosis for children with achondroplasia [41]. Seven studies reporting on pain outcomes [23, 25, 28, 42, 70] after surgery assessed with symptom scores (HHS or VAS). One study reported on pain after physical therapy [45] and another after a lifestyle change intervention [37].
Fig. 3.
Evidence gap map—type of treatment and number of outcomes reported by study
Dimensions of HRQoL was reported in four studies (6.8%) [35, 36, 43, 46]. Only one study reported on psychosocial functioning after surgery, while another reported on patient satisfaction with surgical treatment [24, 33]. Mental health/well-being outcomes were measured in three studies involving people with achondroplasia, which reported on changes in body image and self-esteem after surgery [35, 36, 46].
Of the three studies that reported physical therapy, none commented on specific outcomes linked to this treatment. Instead, these studies indicated that physical therapy was used as part of the treatment as a rehabilitative or clinical therapy combined with surgery [30, 45], or used in combination with other forms of conservative treatment [37].
Surgery
Most of the evidence examining surgical treatments demonstrates potential improvement on clinical outcomes (e.g. growth—limb length and height, bone angle, hip alignment, dental outcomes) after surgical interventions; however, due to the variability of the conditions and the treatments it is not possible to compare these meaningfully. The effect of surgical interventions on HRQoL or dimensions of HRQoL were reported in studies involving Achondroplasia patients (see Table 1) [19, 23, 24, 28, 35, 36, 43, 46]. There seems to be no evidence of a difference on the HRQoL (assessed with SF-36) of surgical interventions of limb lengthening [35, 36, 46]. Additionally, there is no evidence of improvement in HRQOL (assessed with SF-36) for spinal surgery in people with achondroplasia [43]. There were no further studies reporting on the effect of surgical interventions using validated HRQoL instruments for any other type of skeletal dysplasia.
Table 1.
Health-related quality of life measures and additional measures
| Treatment type and condition | Health-related quality of life scale | Single item or other measures | Key finding |
|---|---|---|---|
| Humeral lengthening (monolateral external fixators): Achondroplasia [35] | SF-36 [75]: reports physical component summary (PCS), mental component summary (MCS), & total component summary (TCS) | Rosenberg self-esteem score [76] | Significantly improved the SF-36 score and Rosenberg self-esteem score |
| Tibial and femoral lengthening: Ilizarov ring fixator (Tibia), monolateral external fixators (Femoral): Achondroplasia [36] |
SF-36 [75]: reports PCS, MCS & TCS PedsQL(for children between 8 and 12 years of age)[18] |
Rosenberg self-esteem score [76]; AAOS lower limb outcomes [77] | Significantly improved the Rosenberg self-esteem score but no difference in other measures |
| Humeral Lengthening (external fixation with Ilizarov method): Achondroplasia [46] | SF-36 [75]: reports PCS & MCS | Rosenberg self-esteem score [76] | PCS, MCS and Rosenberg self-esteem score are only reported post-surgery. Scores are considered higher than those without lengthening surgery reported in the literature |
| Multi-study examining historical treatment for height, humeral lengthening, orthodontics, andenotonsillectomy, ear ventilation tubing, foramen magnum surgery, spinal surgery: Achondroplasia [43] | SF-36 [75]: reports PCS, MCS & role/social component summary | Self-reporting of getting in and out of vehicles, stepping up stairs, nursing care service use and wheelchair use after surgery |
Treatment improved outcomes (single item and other measures) SF-36 scores significantly deteriorated after spinal surgery and showed no difference for the other treatments |
| Spinal surgery (lumbar decompression): Achondroplasia [23] | – | Symptom score: leg weakness, numbness or pain; incontinence; abnormal reflexes; walking tolerance | Treatment significantly improved outcomes |
| Humeral lengthening (monorail external fixators): Achondroplasia [24] | – | DASH score: disabilities to the arm, shoulder and hand | Treatment significantly improved outcomes |
| Hip surgery (osteotomy): SED Congenita [25] | – | Gait analysis and presence of hip pain | No difference in gait outcomes, some reduction in pain |
| Spinal surgery (historical laminectomy): Achondroplasia [28] | – | Independence indicated by RANKIN level [78] | No difference in independence level, however walking distance increased |
| Hip conservative treatment (limited weight bearing, control body eight, physical therapy and intermittent pain medications): MED [37] | – | Harris Hip Score [79]; visual analogue scale for stiffness [80] | Significantly improved outcomes in the Harris Hip Score and the visual analogue scale for stiffness |
| Hip surgery (total hip arthroplasty): MED [42] | – | Harris Hip Score | Significantly improved Harris Hip Score |
| Hip surgery (total hip arthroplasty): SED [71] | – | Harris Hip Score; Pain symptom score | Significantly improved Harris Hip Score and pain symptom score |
In the study, examining historical interventions on HRQoL of people with Achondroplasia, the impact of foramen magnum surgery reported a mixed effect on HRQoL assessed with the SF-36 [43]. The findings were represented as a combination of the physical component summary (PCS), mental component summary (MCS), role/social component summary (RCS). Foramen magnum surgery significantly increased the MCS score, yet significantly reduced the RCS score; increased height also significantly increased the PCS score on the SF-36 [43].
Kim et al. (2012) reported no evidence of a difference in the HRQoL measures assessed with AAOS [77], total SF-36 [75] and PedsQl [81] after the intervention; however, a significant positive impact was observed in the Rosenberg self-esteem score with an absolute difference of 3.1 (p < 0.001) between the surgical and non-surgical group. Additionally, for the PCS of the SF-36, it provides weak evidence for lower HRQoL of patients who had surgery, with an absolute difference of − 10.59 (surgical score: 45.04; non-surgical score: 55.63; p = 0.0508).
A further study did not link HRQoL to surgical outcomes but did comment on the mental and psychological well-being of patients before surgery and the effect of the interventions on satisfaction after surgery [21]. In their study, Aldegheri and Dall’Oca [21] indicated that short stature had psychosocial implications that negatively impacted on their social and emotional relationships, although after surgery 95% would repeat the surgical procedure.
Pain appeared to improve with spinal surgery and hip alignment for those with PAch and SED, respectively [19, 25, 71]. For people with SEMD, hip alignment was not associated with any differences in pain, whereas an improvement in pain after surgery was not observed for people with MED [42, 66].
Physical functioning was improved in people with achondroplasia and MED after limb lengthening and hip alignment, respectively [24, 37, 42, 43]. There was an overall deteriorating effect of reducing independence after spinal surgery, as measured by a Rankin score in people with achondroplasia; improvements in independence were recorded by a point improvement of the Rankin score for those who receive surgery quickly (within 6 months of occurrence) [28].
One study examined individuals’ satisfaction after surgical intervention and observed that satisfaction is improved in hip alignment surgery and limb lengthening for SED, DD, PAch and MED, and in spinal surgery for DD [33]. However, lengthening deteriorated satisfaction for those with PAch [33]. A study on MED indicated no clinical complaints from patients were observed after surgery, supporting the findings for improvements for MED [37].
There was no evidence linking pharmacological treatments with HRQoL outcomes.
Physical therapy
There were gaps in the evidence for the impact of physical therapy on clinical outcomes apart from weight bearing as measured via the HHS for people with MED [37]. Additionally there was no evidence for the impact of physical therapy on HRQoL or mental health/well-being.
Lifestyle change and orthotic treatment
Gaps in the evidence were reported for the impact of health and lifestyle change interventions and orthoses on any outcomes, including HRQoL, with exception of spinal orthosis for improving height for people with achondroplasia [41] and the use of conservative treatment improving the HHS in people with MED [37].
Discussion
This evidence gap map summarises the available treatment options and their impact on individual’s symptoms for individuals with non-lethal skeletal dysplasia with severe limb deformities and significant pain and discomfort that could lead to loss of mobility and require numerous orthopaedic interventions. Despite there being a number of studies available for the treatment of skeletal dysplasias, only five examine outcomes for people living with MCDS, with most articles focusing on Achondroplasia. Many of the treatment options available focus on clinical outcomes, with minimal investigation of their impact on HRQoL. Across all studies there is a focus on surgical and pharmacological interventions, with relatively few focussed on health and lifestyle modification, physical therapy, and orthotic treatment. Within the identified studies, the evidence suggested, overall, that surgical interventions may be likely to improve clinical outcomes, except for surgery for SMD and SED (hip alignment), SEMD (limb lengthening), and achondroplasia (spinal surgery). In studies of achondroplasia, the impact of treatment on HRQoL-related outcomes such as physical and psychosocial functioning, and mental health/well-being were prominent.
The inclusion of HRQoL outcomes after surgery was limited. Seven out of 58 publications discussed or briefly noted, the psychosocial and physical outcomes, and all were linked to surgical interventions, with the majority suggesting an improvement on activities of daily living, physical functioning, and presence of pain. However, some evidence indicates that certain surgical treatments do not lead to an improvement of psychosocial functioning. No evidence of a difference was found, however, for increased independence (psychosocial functioning) [28] after a laminectomy, the presence of stiffness did not show an improvement after conservative treatment [37] and gait showed no evidence of a difference post-operatively after a hip osteotomy [25]. A study reported reduced waddle after surgery in those with achondroplasia and HCH [74]. Patient satisfaction and, in some cases, parental satisfaction, was reported to be higher after some surgical procedures [33, 34, 37, 48].
The evidence gap map demonstrates that there are several gaps in evidence on non-surgical treatment and assessment of HRQoL for people living with skeletal dysplasias that were the focus of this study. We identified only four studies that explicitly examined the effects of treatments using validated HRQoL instruments with people living with skeletal dysplasia, and all used the SF-36 [75, 82] measure, further only one of these studies used the AAOS and PedsQl in conjunction with SF-36 [43]. Only one publication reported a significant improvement in HRQoL assessed with the SF-36 measure after surgical treatment with monolateral external fixators [35]. In a different study, spinal surgery significantly deteriorated SF-36 score [43]. It is evident that a gap exists on the effects of interventions on HRQoL in relation to SF-36 measurements, however this gap could be the result of the SF-36 score being validated for adults and not younger age groups. In addition, a further study [33] reported an apparently positive effect on satisfaction with surgery; however, there was no evidence that surgery for MCDS and PAch improved satisfaction with outcomes.
Despite identifying evidence that pharmacological and surgical treatments had positive effects on clinical outcomes for the dysplasias studied, these findings may not be generalised to types of skeletal dysplasia that were not eligible for inclusion in our study. For instance, the height results from growth hormone used for people with Achondroplasia may not be the same for people with CCD. In addition, due to the small number of publications on the use of physical therapy, health and lifestyle changes and orthoses [37, 41], more evidence is required. Further research should examine the preferences and experiences of individuals with skeletal dysplasias for treatments of symptoms and whether surgical and non-surgical treatments provide evidence of improved HRQoL. This research should also determine if there are age-specific differences linked to outcomes of HRQoL, as existing literature on HRQoL of demonstrates age and gender related differences [83].
To the best of our knowledge, this is the first evidence map on the outcomes of the treatments of non-lethal skeletal dysplasias. The strengths of this study is the methodological rigour with which it was performed, which included an extensive search strategy, a comprehensive summary of the results and an independent assessment on each stage of the study selection process. However, several articles were excluded for having fewer than 10 participants, which may have resulted in studies exploring the impact of treatment on a broader range of outcomes being missed. Additionally, due to the small sample sizes, the rarity of skeletal dysplasias in the general population, and the methodological variation amongst studies, the methodological quality of the studies included in the evidence-gap map could not be adequately assessed.
To conclude, there is a dearth of studies linking treatment of skeletal dysplasias with HRQoL outcomes. More research is needed to understand the lived experiences of those living with skeletal dysplasia, and how different treatment options impact on their HRQoL to inform decision-making about optimal treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We wish to thank Dr Marta Bertoli from The Newcastle Upon Tyne Hospitals NHS Foundation Trust and Dr Michael Wright from Newcastle University and Institute of Human Genetics International Centre for Life, and Professor Michael Briggs from Newcastle University, for their guidance on inclusion criteria. We also acknowledge Dr Nawaraj Bhattarai and Eoin Moloney from the Newcastle University for useful discussions about this work.
Abbreviations
- MCDS
Metaphyseal chondrodysplasia type Schmid
- USA
United States of America
- UK
United Kingdom
- HRQOL
Health Related Quality-of-Life
- ACH
Achondroplasia
- CCD
Cleidocranial dysplasia
- DD
Diastropic dysplasia
- HCH
Hypochondroplasia
- LWS
Leri Weillis syndrome
- MD
Metaphyseal dysplasia
- MED
Multiple epiphyseal dysplasia
- PAch
Pseudoachondroplasia
- SED
Spondyloepiphyseal dysplasia
- SEMD
Spondyloepimetaphyseal dysplasia
- SMD
Spondylometaphyseal dysplasia
Author contributions
NM, JF, DF, LC, LV and FP: conceived the study. NM, JF, DF: searched and assessed the studies, while NM: synthesised the relevant included papers. NM and DF, collated, analysed and interpreted the studies identified. All authors provided intellectual input, contributed to the drafting process, and approved the final manuscript.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. This project has received funding support from the European Union’s Horizon 2020 research and innovation programme under agreement No 754825.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This is an evidence gap map, that utilises existing literature. No ethics approval is required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mortier GR, Cohn DH, Cormier-Daire V, Hall C, Krakow D, Mundlos S, Nishimura G, Robertson S, Sangiorgi L, Savarirayan R, Sillence D, Superti-Furga A, Unger S, Warman ML. Nosology and classification of genetic skeletal disorders: 2019 revision. American Journal of Medical Genetics Part A. 2019;179(12):2393–2419. doi: 10.1002/ajmg.a.61366. [DOI] [PubMed] [Google Scholar]
- 2.Orioli I, Castilla E, Barbosa-Neto J. The birth prevalence rates for the skeletal dysplasias. Journal of Medical Genetics. 1986;23(4):328–332. doi: 10.1136/jmg.23.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lachman R, Rimoin D, Spranger J. Metaphyseal chondrodysplasia, Schmid type clinical and radiographic deliniation with a review of the literature. Pediatric Radiology. 1988;18(2):93–102. doi: 10.1007/BF02387549. [DOI] [PubMed] [Google Scholar]
- 4.Stevens PM, Novais EN. Multilevel guided growth for hip and knee varus secondary to chondrodysplasia. Journal of Pediatric Orthopaedics. 2012;32(6):626–630. doi: 10.1097/BPO.0b013e3182567a79. [DOI] [PubMed] [Google Scholar]
- 5.Al Kaissi A, Ghachem MB, Nabil NM, Kenis V, Melchenko E, Morenko E, Grill F, Ganger R, Kircher SG. Schmid’s type of metaphyseal chondrodysplasia: Diagnosis and management. Orthopaedic Surgery. 2018;10(3):241–246. doi: 10.1111/os.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabir AH, Cole T. The evolving therapeutic landscape of genetic skeletal disorders. Orphanet Journal of Rare Diseases. 2019;14(1):300. doi: 10.1186/s13023-019-1222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panda A, Gamanagatti S, Jana M, Gupta AK. Skeletal dysplasias: A radiographic approach and review of common non-lethal skeletal dysplasias. World journal of radiology. 2014;6(10):808. doi: 10.4329/wjr.v6.i10.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apajasalo M, Sintonen H, Rautonen J, Kaitila I. Health-related quality of life of patients with genetic skeletal dysplasias. European Journal of Pediatrics. 1998;157(2):114–121. doi: 10.1007/s004310050781. [DOI] [PubMed] [Google Scholar]
- 9.Jennings SE, Ditro CP, Bober MB, Mackenzie WG, Rogers KJ, Conway L, Duker AL. Prevalence of mental health conditions and pain in adults with skeletal dysplasia. Quality of Life Research. 2019;28(6):1457–1464. doi: 10.1007/s11136-019-02102-2. [DOI] [PubMed] [Google Scholar]
- 10.Constantinides C, Landis SH, Jarrett J, Quinn J, Ireland PJ. Quality of life, physical functioning, and psychosocial function among patients with achondroplasia: A targeted literature review. Disability and Rehabilitation. 2022;44(21):6166–6178. doi: 10.1080/09638288.2021.1963853. [DOI] [PubMed] [Google Scholar]
- 11.Dhiman N, Albaghdadi A, Zogg CK, Sharma M, Hoover-Fong JE, Ain MC, Haider AH. Factors associated with health-related quality of life (HRQOL) in adults with short stature skeletal dysplasias. Quality of Life Research. 2017;26(5):1337–1348. doi: 10.1007/s11136-016-1455-7. [DOI] [PubMed] [Google Scholar]
- 12.Skworc A, Osiadło G, Sławska H, Jezela-Stanek A, Marciniak S. The influence of rehabilitation treatment on the patient's motor development with Metaphyseal Chondrodysplasia, Schmid type – case study. Pediatria Polska. 2017;92(2):214–217. doi: 10.1016/j.pepo.2016.10.006. [DOI] [Google Scholar]
- 13.Khanal D. Metaphyseal dysplasia: A rare case report. J Clin Case Rep. 2016;6(726):2. [Google Scholar]
- 14.Briggs MD, Bell PA, Wright MJ, Pirog KA. New therapeutic targets in rare genetic skeletal diseases. Expert opinion on orphan drugs. 2015;3(10):1137–1154. doi: 10.1517/21678707.2015.1083853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanazawa H, Tanaka H, Inoue M, Yamanaka Y, Namba N, Seino Y. Efficacy of growth hormone therapy for patients with skeletal dysplasia. Journal of Bone & Mineral Metabolism. 2003;21(5):307–310. doi: 10.1007/s00774-003-0425-7. [DOI] [PubMed] [Google Scholar]
- 16.Forouhan M, Sonntag S, Boot-Handford RP. Carbamazepine reduces disease severity in a mouse model of metaphyseal chondrodysplasia type Schmid caused by a premature stop codon (Y632X) in the Col10a1 gene. Human Molecular Genetics. 2018;27(22):3840–3853. doi: 10.1093/hmg/ddy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.White H, Albers B, Gaarder M, Kornør H, Littell J, Marshall Z, Matthew C, Pigott T, Snilstveit B, Waddington H, Welch V. Guidance for producing a Campbell evidence and gap map. Campbell systematic review. 2020 doi: 10.1002/cl2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ain MC, Chaichana KL, Schkrohowsky JG. Retrospective study of cervical arthrodesis in patients with various types of skeletal dysplasia. Spine. 2006;31(6):E169–174. doi: 10.1097/01.brs.0000202758.61848.61. [DOI] [PubMed] [Google Scholar]
- 20.Aldegheri R. Distraction osteogenesis for lengthening of the tibia in patients who have limb-length discrepancy or short stature. Journal of Bone & Joint Surgery - American. 1999;81(5):624–634. doi: 10.2106/00004623-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Aldegheri R, Dall'Oca C. Limb lengthening in short stature patients. Journal of Pediatric Orthopaedics, Part B. 2001;10(3):238–247. [PubMed] [Google Scholar]
- 22.Appan S, Laurent S, Chapman M, Hindmarsh PC, Brook CG. Growth and growth hormone therapy in hypochondroplasia. Acta Paediatrica Scandinavica. 1990;79(8–9):796–803. doi: 10.1111/j.1651-2227.1990.tb11557.x. [DOI] [PubMed] [Google Scholar]
- 23.Baca KE, Abdullah A, Ting BL, Schkrohowsky JG, Hoernschemeyer DG, Carson BS, Ain MC. Surgical decompression for lumbar stenosis in pediatric achondroplasia. Journal of Pediatric Orthopaedics. 2010;30(5):449–454. doi: 10.1097/BPO.0b013e3181e00c66. [DOI] [PubMed] [Google Scholar]
- 24.Balci HI, Kocaoglu M, Sen C, Eralp L, Batibay SG, Bilsel K. Bilateral humeral lengthening in achondroplasia with unilateral external fixators: Is it safe and does it improve daily life? Bone and Joint Journal. 2015;97B(11):1577–1581. doi: 10.1302/0301-620x.97b11.36037. [DOI] [PubMed] [Google Scholar]
- 25.Bayhan IA, Abousamra O, Rogers KJ, Bober MB, Miller F, Mackenzie WG. Valgus hip osteotomy in children with spondyloepiphyseal dysplasia congenita: Midterm results. Journal of Pediatric Orthopedics. 2017;30:30. doi: 10.1097/BPO.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 26.Beals RK, Stanley G. Surgical correction of bowlegs in achondroplasia. Journal of Pediatric Orthopaedics, Part B. 2005;14(4):245–249. doi: 10.1097/01202412-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Bridges NA, Hindmarsh PC, Brook CG. Growth of children with hypochondroplasia treated with growth hormone for up to 3 years. Hormone Research. 1991;36(Suppl 1):56–60. doi: 10.1159/000182190. [DOI] [PubMed] [Google Scholar]
- 28.Carlisle ES, Ting BL, Abdullah MA, Skolasky RL, Schkrohowsky JG, Yost MT, Rigamonti D, Ain MC. Laminectomy in patients with achondroplasia: the impact of time to surgery on long-term function. Spine. 2011;36(11):886–892. doi: 10.1097/BRS.0b013e3181e7cb2a. [DOI] [PubMed] [Google Scholar]
- 29.Chang H, Wei J, Wang Y, Jia J, Gao X, Li X, Feng H. Restorative treatment strategies for patients with cleidocranial dysplasia. Acta odontologica Scandinavica. 2015;73(6):447–453. doi: 10.3109/00016357.2014.983541. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson J, Aftab S, Bradish C. Achondroplasia and limb lengthening: Results in a UK cohort and review of the literature. Journal of Orthopaedics. 2015;12(1):31–34. doi: 10.1016/j.jor.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada D, Namba N, Hanioka Y, Ueyama K, Sakamoto N, Nakano Y, Izui M, Nagamatsu Y, Kashiwagi H, Yamamuro M, Ishiura Y, Ogitani A, Seino Y. Final adult height in long-term growth hormone-treated achondroplasia patients. European Journal of Pediatrics. 2017;176(7):873–879. doi: 10.1007/s00431-017-2923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertel NT, Eklof O, Ivarsson S, Aronson S, Westphal O, Sipila I, Kaitila I, Bland J, Veimo D, Muller J, Mohnike K, Neumeyer L, Ritzen M, Hagenas L. Growth hormone treatment in 35 prepubertal children with achondroplasia: A five-year dose-response trial. Acta Paediatrica. 2005;94(10):1402–1410. doi: 10.1111/j.1651-2227.2005.tb01811.x. [DOI] [PubMed] [Google Scholar]
- 33.Hunter AG. Perceptions of the outcome of orthopedic surgery in patients with chondrodysplasias. Clinical Genetics. 1999;56(6):434–440. doi: 10.1034/j.1399-0004.1999.560605.x. [DOI] [PubMed] [Google Scholar]
- 34.Kashiwagi N, Suzuki S, Seto Y, Futami T, Kashiwagi N, Suzuki S, Seto Y, Futami T. Bilateral humeral lengthening in achondroplasia. Clinical Orthopaedics & Related Research. 2001;391:251–257. doi: 10.1097/00003086-200110000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Agashe MV, Song SH, Choi HJ, Lee H, Song HR. Comparison between upper and lower limb lengthening in patients with achondroplasia: a retrospective study. Journal of Bone & Joint Surgery - British. 2012;94(1):128–133. doi: 10.1302/0301-620X.94B1.27567. [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ, Balce GC, Agashe MV, Song SH, Song HR. Is bilateral lower limb lengthening appropriate for achondroplasia?: midterm analysis of the complications and quality of life. Clinical Orthopaedics & Related Research. 2012;470(2):616–621. doi: 10.1007/s11999-011-1983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SJ, Ramanathan AK, Jeon YS, Song HR. The fate of hips that are conservatively treated in multiple epiphyseal dysplasia. Journal of Pediatric Orthopaedics, Part B. 2017;26(6):526–531. doi: 10.1097/BPB.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 38.Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. Journal of Pediatric Orthopaedics. 2007;27(6):629–634. doi: 10.1097/BPO.0b013e318093f523. [DOI] [PubMed] [Google Scholar]
- 39.Kitoh H, Mishima K, Matsushita M, Nishida Y, Ishiguro N. Early and late fracture following extensive limb lengthening in patients with achondroplasia and hypochondroplasia. Bone & Joint Journal. 2014;96(9):1269–1273. doi: 10.1302/0301-620X.96B9.33840. [DOI] [PubMed] [Google Scholar]
- 40.Kubota T, Wang W, Miura K, Nakayama H, Yamamoto K, Fujiwara M, Ohata Y, Tachibana M, Kitaoka T, Takakuwa S, Miyoshi Y, Namba N, Ozono K. Serum NT-proCNP levels increased after initiation of GH treatment in patients with achondroplasia/hypochondroplasia. Clinical Endocrinology. 2016;84(6):845–850. doi: 10.1111/cen.13025. [DOI] [PubMed] [Google Scholar]
- 41.Leilei X, Yetian L, Fei S, Chao X, Yong Q, Zezhang Z, Xu L, Li Y, Sheng F, Xia C, Qiu Y, Zhu Z. The efficacy of brace treatment for thoracolumbar kyphosis in patients with achondroplasia. Spine. 2018;43(16):1133–1138. doi: 10.1097/brs.0000000000002586. [DOI] [PubMed] [Google Scholar]
- 42.Lim SJ, Park YS, Moon YW, Jung SM, Ha HC, Seo JG, Lim S-J, Park Y-S, Moon Y-W, Jung S-M, Ha H-C, Seo J-G. Modular cementless total hip arthroplasty for multiple epiphyseal dysplasia. Journal of Arthroplasty. 2009;24(1):77–82. doi: 10.1016/j.arth.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Matsushita M, Kitoh H, Mishima K, Yamashita S, Haga N, Fujiwara S, Ozono K, Kubota T, Kitaoka T, Ishiguro N. Physical, mental, and social problems of adolescent and adult patients with achondroplasia. Calcified Tissue International. 2019;104(4):364–372. doi: 10.1007/s00223-019-00518-z. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee D, Pressman BD, Krakow D, Rimoin DL, Danielpour M. Dynamic cervicomedullary cord compression and alterations in cerebrospinal fluid dynamics in children with achondroplasia: Review of an 11-year surgical case series. Journal of Neurosurgery: Pediatrics. 2014;14(3):238–244. doi: 10.3171/2014.5.Peds12614. [DOI] [PubMed] [Google Scholar]
- 45.Myers GJC, Bache CE, Bradish CF. Use of distraction osteogenesis techniques in skeletal dysplasias. Journal of Pediatric Orthopaedics. 2003;23(1):41–45. doi: 10.1097/01241398-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Nakano-Matsuoka N, Fukiage K, Harada Y, Kashiwagi N, Futami T. The prevalence of the complications and their associated factors in humeral lengthening for achondroplasia: Retrospective study of 54 cases. Journal of Pediatric Orthopaedics Part B. 2017;26(6):519–525. doi: 10.1097/bpb.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 47.Noonan KJ, Leyes M, Forriol F, Canadell J. Distraction osteogenesis of the lower extremity with use of monolateral external fixation. A study of two hundred and sixty-one femora and tibiae. Journal of Bone & Joint Surgery - American Volume. 1998;80(6):793–806. doi: 10.2106/00004623-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Park HW, Kim HS, Hahn SB, Yang KH, Choi CH, Park JO, Jung SH. Correction of lumbosacral hyperlordosis in achondroplasia. Clinical Orthopaedics and Related Research. 2003;414:242–249. doi: 10.1097/01.blo.0000081936.75404.a4. [DOI] [PubMed] [Google Scholar]
- 49.Park KW, Garcia RAN, Rejuso CA, Choi JW, Song HR. Limb lengthening in patients with achondroplasia. Yonsei Medical Journal. 2015;56(6):1656–1662. doi: 10.3349/ymj.2015.56.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto G, Cormier-Daire V, Le Merrer M, Samara-Boustani D, Baujat G, Fresneau L, Viaud M, Souberbielle JC, Pineau JC, Polak M. Efficacy and safety of growth hormone treatment in children with hypochondroplasia: comparison with an historical cohort. Hormone Research in Paediatrics. 2014;82(6):355–363. doi: 10.1159/000364807. [DOI] [PubMed] [Google Scholar]
- 51.Ramaswami U, Rumsby G, Spoudeas HA, Hindmarsh PC, Brook CG. Treatment of achondroplasia with growth hormone: Six years of experience. Pediatric Research. 1999;46(4):435–439. doi: 10.1203/00006450-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Savarirayan R, Irving M, Bacino CA, Bostwick B, Charrow J, Cormier-Daire V, Kim-Hanh Le Quan S, Dickson P, Harmatz P, Phillips J, Owen N, Cherukuri A, Jayaram K, Jeha GS, Larimore K, Ming-Liang C, Labed AH, Day J, Julie H-F, Le Quan Sang K-H. C-Type natriuretic peptide analogue therapy in children with achondroplasia. New England Journal of Medicine. 2019;381(1):25–35. doi: 10.1056/NEJMoa1813446. [DOI] [PubMed] [Google Scholar]
- 53.Seino Y, Moriwake T, Tanaka H, Inoue M, Kanzaki S, Tanaka T, Matsuo N, Niimi H. Molecular defects in achondroplasia and the effects of growth hormone treatment. Acta Paediatrica Supplement. 1999;88(428):118–120. doi: 10.1111/j.1651-2227.1999.tb14369.x. [DOI] [PubMed] [Google Scholar]
- 54.Seino Y, Yamanaka Y, Shinohara M, Ikegami S, Koike M, Miyazawa M, Inoue M, Moriwake T, Tanaka H. Growth hormone therapy in achondroplasia. Hormone Research. 2000;53(Suppl 3):53–56. doi: 10.1159/000023534. [DOI] [PubMed] [Google Scholar]
- 55.Serhan Er M, Abousamra O, Rogers K, Palocaren T, Takemitsu M, Mackenzie WG, Akyol Y, Campbell JW. Upper cervical fusion in children with spondyloepiphyseal dysplasia congenita. Journal of Pediatric Orthopedics. 2017;37(7):466–472. doi: 10.1097/bpo.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 56.Shohat M, Tick D, Barakat S, Bu X, Melmed S, Rimoin DL. Short-term recombinant human growth hormone treatment increases growth rate in achondroplasia. Journal of Clinical Endocrinology & Metabolism. 1996;81(11):4033–4037. doi: 10.1210/jcem.81.11.8923856. [DOI] [PubMed] [Google Scholar]
- 57.Sisk EA, Heatley DG, Borowski BJ, Leverson GE, Pauli RM, Sisk EA, Heatley DG, Borowski BJ, Leverson GE, Pauli RM. Obstructive sleep apnea in children with achondroplasia: Surgical and anesthetic considerations. Otolaryngology-Head & Neck Surgery. 1999;120(2):248–254. doi: 10.1016/S0194-5998(99)70414-6. [DOI] [PubMed] [Google Scholar]
- 58.Sitoula P, Mackenzie WG, Shah SA, Thacker M, Ditro C, Holmes L, Jr, Campbell JW, Rogers KJ. Occipitocervical fusion in skeletal dysplasia: A new surgical technique. Spine. 2014;39(15):E912–918. doi: 10.1097/brs.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 59.Song MH, Lee T-J, Song JH, Song H-R. Sustained hip flexion contracture after femoral lengthening in patients with achondroplasia. BMC Musculoskeletal Disorders. 2018 doi: 10.1186/s12891-018-2344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song SH, Agashe MV, Huh Y-J, Hwang S-Y, Song H-R. Physeal growth arrest after tibial lengthening in achondroplasia 23 children followed to skeletal maturity. Acta Orthopaedica. 2012;83(3):282–287. doi: 10.3109/17453674.2012.678802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song SH, Kim SE, Agashe MV, Lee H, Refai MA, Park YE, Choi HJ, Park JH, Song HR. Growth disturbance after lengthening of the lower limb and quantitative assessment of physeal closure in skeletally immature patients with achondroplasia. Journal of Bone and Joint Surgery - Series B. 2012;94B(4):556–563. doi: 10.1302/0301-620x.94b4.28375. [DOI] [PubMed] [Google Scholar]
- 62.Stamoyannou L, Karachaliou F, Neou P, Papataxiarchou K, Pistevos G, Bartsocas CS. Growth and growth hormone therapy in children with achondroplasia: A 2 year experience. American Journal of Medical Genetics. 1997;72(1):71–76. doi: 10.1002/(SICI)1096-8628(19971003)72:1<71::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka H, Kubo T, Yamate T, Ono T, Kanzaki S, Seino Y. Effect of growth hormone therapy in children with achondroplasia: Growth pattern, hypothalamic-pituitary function, and genotype. European Journal of Endocrinology. 1998;138(3):275–280. doi: 10.1530/eje.0.1380275. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka N, Katsumata N, Horikawa R, Tanaka T. The comparison of the effects of short-term growth hormone treatment in patients with achondroplasia and with hypochondroplasia. Endocrine Journal. 2003;50(1):69–75. doi: 10.1507/endocrj.50.69. [DOI] [PubMed] [Google Scholar]
- 65.Thomeer RT, van Dijk JM. Surgical treatment of lumbar stenosis in achondroplasia. Journal of Neurosurgery. 2002;96(3 Suppl):292–297. doi: 10.3171/spi.2002.96.3.0292. [DOI] [PubMed] [Google Scholar]
- 66.Trigui M, Pannier S, Finidori G, Padovani JP, Glorion C. Coxa vara in chondrodysplasia: Prognosis study of 35 hips in 19 children. Journal of Pediatric Orthopaedics. 2008;28(6):599–606. doi: 10.1097/BPO.0b013e3181831ec8. [DOI] [PubMed] [Google Scholar]
- 67.Trivella G, Zambito A, Aldegheri R, Leso P, Burton M. Functional and aesthetic results of leg lengthening in Achondroplastic patients. Physiotherapy (United Kingdom) 1991;77(11):724–726. doi: 10.1016/s0031-9406(10)62040-8. [DOI] [Google Scholar]
- 68.Vaidya SV, Song HR, Lee SH, Suh SW, Keny SM, Telang SS. Bifocal tibial corrective osteotomy with lengthening in achondroplasia: An analysis of results and complications. Journal of Pediatric Orthopedics. 2006;26(6):788–793. doi: 10.1097/01.bpo.0000242429.83866.97. [DOI] [PubMed] [Google Scholar]
- 69.Vleggeert-Lankamp C, Peul W. Surgical decompression of thoracic spinal stenosis in achondroplasia: Indication and outcome. Journal of Neurosurgery: Spine. 2012;17(2):164–172. doi: 10.3171/2012.4.Spine1220. [DOI] [PubMed] [Google Scholar]
- 70.Weiner DS, Mirhaidari GJM, Morscher MA, Gothard MD, Adamczyk MJ. Results through skeletal maturity of planned fibular nonunion for the treatment of genu varum in achondroplasia: An observational retrospective study. Medicine. 2019;98(44):e17723. doi: 10.1097/MD.0000000000017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyles CC, Panos JA, Houdek MT, Trousdale RT, Berry DJ, Taunton MJ. Total hip arthroplasty reduces pain and improves function in patients with spondyloepiphyseal dysplasia: A long-term outcome study of 50 cases. Journal of Arthroplasty. 2019;34(3):517–521. doi: 10.1016/j.arth.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamate T, Kanzaki S, Tanaka H, Kubo T, Moriwake T, Inoue M, Seino Y. Growth hormone (GH) treatment in achondroplasia. The Journal of Pediatric Endocrinology. 1993;6(1):45–52. doi: 10.1515/JPEM.1993.6.1.45. [DOI] [PubMed] [Google Scholar]
- 73.Yilmaz G, Oto M, Thabet AM, Rogers KJ, Anticevic D, Thacker MM, Mackenzie WG. Correction of lower extremity angular deformities in skeletal dysplasia with hemiepiphysiodesis: a preliminary report. Journal of Pediatric Orthopedics. 2014;34(3):336–345. doi: 10.1097/BPO.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 74.Zambito A, Polo A, Agostini S, Aldegheri R. Functional outcome after lower-limb lengthening in short stature using the callotasis method. Europa Medicophysica. 2000;36(4):197–204. [Google Scholar]
- 75.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Quality of Life Research. 1994;3(1):7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg M. Society and the adolescent self-image. Princeton University Press; 2015. [Google Scholar]
- 77.Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments: Reliability, validity, and sensitivity to change. JBJS. 2004;86(5):902–909. doi: 10.2106/00004623-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Sulter G, Steen C, De Keyser J. Use of the barthel index and modified rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. doi: 10.1161/01.STR.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 79.Harris W. Traumatic arthritis of the hip after dislocation and acetabular fractures: An end-result study using a new method of result evaluation. J Bone Joint Surg [Am] 1969;51(4):737–755. doi: 10.2106/00004623-196951040-00012. [DOI] [PubMed] [Google Scholar]
- 80.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. The Journal of pain. 2003;4(7):407–414. doi: 10.1016/S1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 81.Davis S, Hynan L, Limbers C, Andersen C, Greene M, Varni J, Iannaccone S. The PedsQL in pediatric patients with Duchenne muscular dystrophy: Feasibility, reliability, and validity of the pediatric quality of life inventory neuromuscular module and generic core scales. Journal of Clinical Neuromuscular Disease. 2010;11(3):97–109. doi: 10.1097/CND.0b013e3181c5053b. [DOI] [PubMed] [Google Scholar]
- 82.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Michel G, Bisegger C, Fuhr DC, Abel T, The KG. Age and gender differences in health-related quality of life of children and adolescents in Europe: A multilevel analysis. Quality of Life Research. 2009;18(9):1147. doi: 10.1007/s11136-009-9538-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.