Highlights
-
•
Mutant histone H3 drives radioresistance in DMG.
-
•
Regulation of ALDH1A3 expression in DMG is Wnt/β-Catenin-dependent.
-
•
EYA4 acts as a bona fide tumor suppressor in DMG.
-
•
Intrinsic vulnerabilities of DMG may be targeted with PI3K/mTOR and ALDH inhibitors.
Keywords: Diffuse midline glioma (DMG), Cancer stem cells, H3K27M, Resistance, Radiosensitization
Abstract
Therapeutic resistance remains a major obstacle to preventing progression of H3K27M-altered Diffuse Midline Glioma (DMG). Resistance is driven in part by ALDH-positive cancer stem cells (CSC), with high ALDH1A3 expression observed in H3K27M-mutant DMG biopsies. We hypothesized that ALDH-mediated stemness and resistance may in part be driven by the oncohistone itself. Upon deletion of H3K27M, ALDH1A3 expression decreased dramatically and was accompanied by a gain in astrocytic marker expression and a loss of neurosphere forming potential, indicative of differentiation. Here we show that the oncohistone regulates histone acetylation through ALDH1A3 in a Wnt-dependent manner and that loss of H3K27M expression results in sensitization of DMGs to radiotherapy. The observed elevated Wnt signaling in H3K27M-altered DMG likely stems from a dramatic suppression of mRNA and protein expression of the Wnt inhibitor EYA4 driven by the oncohistone. Thus, our findings identify EYA4 as a bona fide tumor suppressor in DMG that upon suppression, results in aberrant Wnt signaling to orchestrate stemness and differentiation. Future studies will explore whether overexpression of EYA4 in DMG can impede growth and invasion. In summary, we have gained mechanistic insight into H3K27M-mediated regulation of cancer stemness and differentiation, which provides rationale for exploring new therapeutic targets for DMG.
Introduction
Diffuse midline glioma (DMG) is a highly aggressive high-grade glioma (HGG) that arises in the brain stem. Survival rates remain dismal and so do therapeutic options. Radiotherapy has remained the standard of care owing to the difficulty of drugs permeating the blood-brain barrier (BBB). New drugs and delivery techniques like convection enhanced delivery (CED) are being developed and provide hope for future therapeutic paradigms for DMG [1].
Seminal studies have identified DMG's driver mutation in Histone H3 and expanded our understanding of this epigenetically regulated disease. H3K27M controls the methylation of target genes by inhibiting the PRC2 complex and inducing a defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 [2]. At the same time, the acetylation of target genes increases, with both mechanisms regulating an aberrant transcriptional program that results in gliomagenesis.
Acquired and intrinsic resistance mechanisms to radiotherapy likely stem from this aberrant transcriptional program, co-occurring mutations, and the emergence of cancer stem cells (CSCs) [3], [4], [5]. These mechanisms ultimately contribute to tumor progression in 100% of cases. A recent study provided evidence that chemo- and radiosensitivity is regulated in part by the H3K27M mutation [6]. This study showed that low- and high-grade gliomas that express the H3.3K27M mutation exhibited increased resistance to chemotherapeutics and radiotherapy compared to H3.3 wildtype cells, providing strong evidence of a dominant negative role of the oncohistone [6].
CRISPR gene editing strategies are successfully used as tools to study cancer and adaptations of these technologies have emerged to improve diagnosis and treatment [7]. However, many limitations, including off-target effects, delivery, and immunogenicity will have to be resolved before CRIPSR strategies become a reality in cancer therapy. For DMG, the use of CRISPR has contributed to our understanding of the role the histone H3 oncogene plays. Importantly, DMGs in which the mutant copy of histone H3 was removed failed to form tumors when implanted into the brain of mice [2]. This indicates the promise of targeting the oncohistone for curative treatments. Moreover, it underscores the need to develop new therapeutic strategies to target the oncohistone directly or identify and target downstream signaling pathways/nodes until CRISPR technologies become a reality for the curative treatment of DMG.
To gain a better understanding of the role of H3K27M in tumorigenesis, treatment response and tumor progression and identify the oncohistone's downstream targets, we identified and explored H3K27M effectors as regulators of DMG stemness and differentiation. Furthermore, we investigated therapeutic options, specifically those that target the stemness program induced by H3K27M, that may be used as radiosensitizers and inhibitors of tumor progression.
Materials and methods
Cell lines
Isogenic pair for H3K27M: Human SU-DIPG XIII parental (H3K27M), CRISPR edited SU-DIPG XIII K27M-KO isogenic pair, and BT245 H3K27M parental and CRISPR edited BT245 K27M-KO isogenic pair was kindly provided by Dr. Jabado (Pediatrics, McGill University) [2]. The murine intrauterine electroporation (IUE) model was generated as previously described using the following 4 plasmids together: (a) PBase, (b) PB-CAG-DNp53-Ires-luciferase (dominant-negative TP53, referred to herein as TP53), (c) PB-CAG-PdgfraD824V-Ires-EGFP (PDGFRA D842V), and (d) PB-CAG-H3.3 K27M-Ires-EGFP (H3K27M), referred to herein as the PPK model [8]. For the PPW model, mutant TP53 and PDGFRA as described for the PPK model was used with H3.3 wildtype expressing plasmid instead of the mutant H3.3. Neurospheres, generated from the PPW (H3.3WT) and PPK (H3.3K27M), were provided by Dr. Koschmann (Pediatrics, University of Michigan) [8]. Murine neuronal stem cells (mNSCs) H3 wildtype (wt) and overexpressing H3K27M (K27M) cells were provided by Dr. Venneti (Neuropathology, University of Michigan) [9]. Cells were maintained in Tumor Stem Neurobasal-A medium mixed 1:1 with DMEM F-12 supplemented with 10 mM HEPES, 100 mm Sodium Pyruvate, non-essential Amino Acids, GlutaMAX-I supplement, 100X Antibiotic-Antimycotic, B27(-A) (all from Invitrogen), 20 ng/ml of human-FGF and human-EGF, 10 ng/ml of human PDGF-AA and BB (all from Shenandoah Biotech) and 10 ng/ml heparin (StemCell Technologies). Cells obtained from other institutions were not re-authenticated in our laboratory. Human neurospheres were dissociated in TrypLE Express (Thermo Fisher) and 2D cultures with Trypsin-EDTA (Gibco). Human SF8628 cell lines were obtained from Millipore and were cultured according to manufacturer instructions. Each lot of Millipore's cells was genotyped by STR analysis to verify the unique identity of the cell line. All cell lines were regularly checked for mycoplasma contamination using MycoAlert Mycoplasma Detection Kit (Lonza). Cell lines were cultured for a period of ∼ 2 months, after which they were replaced by new thaws.
Lentiviral infection
Lentiviral plasmid expressing either ALDH1A3 (ALDH1A3- pLentil-GIII-CMV-RFP-2A-Puro, cat no 117250610495) or control plasmid (pLentil-GIII-CMV-RFP-2A-Puro, cat no LV591) were purchased from ABM. Lentiviral particles were generated by co-transfection of ALDH1A3 or empty plasmids with packaging plasmid into 293 T cells as previously described [10]. Media was replaced 4 h post-transfection. For lentiviral infection, supernatant collected after 48 h from 293T transfected was filtered (0.45 µm) and polybrene (10 µg/mL) was added before infecting the target cells. SU-DIPG XIII K27M-KO and BT245 K27M-KO cells were incubated with virus particles generated from ALDH1A3 or control plasmid for 6 h, before replacing them with fresh media. Cells were then allowed to grow for 5 days and collected for flow cytometry analysis.
Flow cytometry
SU-DIPG XIII K27M-KO and BT245 K27M-KO cells were harvested and single cell suspensions were prepared. ALDH1A3 overexpressing cells were identified based on RFP positivity on Synergy Head cell sorter and analyzed in FCS Express 7 cytometry software at the University of Michigan Flow Cytometry Core.
Bru sequencing
Bru sequencing (Bru-seq) was performed as described previously [5]. For irradiation experiments, SU-DIPG XIII cells were irradiated with 5 Gy using a C-320 Biological Irradiator (Kimtron Inc., Oxford, CT) and incubated for 1 h at 37 °C before being labeled with 2 mM bromouridine for 30 min. Bru-labeled RNA was then captured and cDNA libraries were prepared and sequenced at the University of Michigan Sequencing Core using an Illumina NovaSeq 6000 sequencer as described previously [11,12]. The sequencing reads were first aligned to the ribosomal RNA repeating unit (GenBank U13369.1), the mitochondrial genome, and the EBV genome using Bowtie2 2.3.3 [13]. The unaligned reads were then mapped to the human genome build hg38 using STAR 2.5.3a [14]. Gene annotations were from GENCODE version 27 [15]. Differential gene expression was performed using DESeq2 1.18.1 [16]. Log2 fold-changes were modified using the apeglm shrinkage estimator [17] and genes with adjusted p-value < 0.05 and fold-change > 1.5 up or down were considered significant. Gene set/pathway enrichment (over-representation analysis) was performed using WebGestalt (both via the web interface at http://webgestalt.org and the R package WebGestaltR 0.4.4). FDR < 0.05 was required for enriched gene sets.
ChIP sequencing
Ten million SU-DIPG XIII cells were seeded 24 h prior to treatment and incubated with GSK458 for 2 h at 10 µM. Cells were washed with PBS and lysed with RIPA lysis buffer (Thermo Fisher) supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail, Roche) and phosphatase inhibitors (PhosSTOP, Roche). Following 30-minute incubation with RIPA, the cell lysate was sonicated. Protein concentrations of whole-cell lysates were determined using Lowry assays (Bio-Rad). Samples containing 750 µg of protein were used per immunoprecipitation (IP) reaction. Protein of interest was immunoprecipitated using Thermo Fisher Dynabead A kit (cat no 10006D) following the manufacturer's instructions. For immunoprecipitation the H3K27ac antibody cat no ab4729 from Abcam and IgG included in the Diagenode kit was used. Following immunoprecipitation, half of the precipitate was analyzed via western blot or submitted to the University of Michigan Epigenomics Core for sequencing and analysis.
Reads were trimmed using TrimGalore 0.4.5 and CutAdapt 1.15 with the following parameters: –adapter AGATCGGAAGAGC -e 0.1 –stringency 6 –length 20 –nextseq 20.
Trimmed reads were aligned to hg19 using Bowtie2 2.3.4.1 [13] with the following parameters: -X 2000, and defaults multi-seed length of 20 bp with 0 mismatches. Duplicate reads were marked and removed with Picard 2.20.2 (https://broadinstitute.github.io/picard/). Alignments below a MAPQ threshold of 10 were removed using SAMtools 1.2 (http://www.htslib.org/) [18] and the parameters -q 10 -F 1024. Reads completely overlapping the ENCODE Problematic Regions [19] were removed with bedtools2 2.28.0 [20]. DeepTools 3.3.0 [21] was used to calculate coverage (bigWig format) and IP efficiency plots.
Sample-wise peaks were called using MACS2 2.1.2 [22] in its default (narrow peak) mode using treatment-matched input as control. Parameters used: –format BAM –gsize hs. Peaks were merged over all samples using bedops 2.4.26 [23] and differential peak analysis was performed using the R package edgeR 3.28.1. In the absence of biological replicates, the BCV (biological coefficient of variance) parameter was manually tuned. Models were then fit (glmFit function) and tested (likelihood ratio test) for the contrast representing GSK458 vs control (DMSO). A p-value < 0.05 and log2 fold-change > 1 or < -1 were used to determine differential binding. Peaks were annotated using the R package annotatr 1.12.1 [24].
Western blot
Cells were seeded 24 h prior to treatment and incubated with the respective inhibitors for 2 hours or as otherwise indicated. Cells were lysed with RIPA lysis buffer (Thermo Fisher Scientific) supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail, Roche) and phosphatase inhibitors (PhosSTOP, Roche). Histone protein was extracted and purified by acid extraction as described previously [25]. Western blotting was performed as described previously [5]. Primary antibodies were obtained from Cell Signaling Technology against GFAP, H3K27ac, and β-catenin; from Abcam against ALDH1A3, EYA4 (ab251675) and β Actin; and from MilliporeSigma against H3K27M. Secondary HRP-conjugated antibodies were purchased from Jackson ImmunoResearch. ECL-Plus substrate (Bio-Rad) and Bio-Rad ChemiDoc MP imager were used according to the manufacturer's recommendations.
Histone extraction
Histone extraction was performed as previously described [9,25]. Cells were seeded at a density of 5 × 106 cells. After 24 hours cells were pelleted, washed with PBS and resuspended in 1 ml hypotonic lysis buffer (10 mM Tris-Cl pH 8.0, 1 mM KCl, 1.5 mM MgCl2, 1 mM DTT, protease and phosphatase inhibitors) and incubated for 30 min at 4 °C while rotating. The sample was centrifuged at 10,000 g, at 4 °C for 10 min and pellet resuspended in 400 µl of 0.4 N H2SO4 prior to overnight incubation at 4 °C while rotating. Next day, the sample was centrifuged at 16,000 g, and supernatant transferred to a fresh tube and mixed with 132 μl TCA added dropwise. After incubation on ice for 30 min, the histones were pelleted and washed with ice-cold acetone two times. Supernatant was discarded and the histone pellet air-dried at room temperature for 20 min and resuspended in ddH2O water prior to storage or use at -20 °C.
Acetyl coenzyme A assay
Acetyl-CoA level was determined with the PicoProbe acetyl-CoA assay kit (ab87546; Abcam). Briefly, 5 × 106 cells were harvested and homogenized in an assay buffer using a hand homogenizer on ice until effective lysis was achieved. The homogenate was centrifuged at 10,000 g for 10 min at 4 C. The supernatant was collected and after deproteinization, intracellular levels of acetyl-CoA were measured in accordance with the manufacturer's instructions. In brief, the supernatant was collected and deproteinized with 1 M of perchloric acid and 2 M of potassium hydroxide. The intracellular levels of acetyl-CoA in the supernatant were measured with PicoProbe by fluorescence (Ex = 535\ EM = 587 nm).
Cell viability assay
SU-DIPG XIII and BT245 isogenic cells (3 × 106) were cultured and 24 h later radiated at indicated doses (2, 4 and 6 Gy). Immediately after irradiation, cells were harvested and seeded at a density of 2500 cells/well in 96 well plates and incubated for 10 days. Titer-Glo cell viability assay (Promega, Madison, WI) was performed to assess cell viability using Envision multi-label plate reader (PerkinElmer).
Irradiation
Irradiation was carried out using Philips RT250 (Kimtron Medical) at indicated doses at the University of Michigan Comprehensive Cancer Center Experimental Irradiation Core.
Conditioned media experiment
SU-DIPG XIII parental and H3K27M KO cells were grown in T75 flask at a density of 8 × 106 cells/25 ml. Conditioned media (CM) was collected after 24 or 48 h, centrifuged at 1800 rpm for 5 min, and the supernatant aliquoted and stored at -80 °C for later experiments. On the day of the experiment CM was thawed and 20 ml was heat inactivated at 95 °C for 30 min. DIPG XIII H3K27M KO cells were seeded at a density of 3 × 106 on T25 flask in 10 ml of CM, which was either heat-inactivated or left untreated, from H3 K27M or H3K27M KO cells. Cells were grown in CM for either 24 or 48 h prior to lysis and RNA isolation using Qiagen Kit and qRT-PCR.
RNA isolation and quantitative PCR
RNA was extracted using QIAshredder and RNeasy Mini Kit (Qiagen) and reverse transcription was performed with QuantiTect Reverse Transcription Kit (Qiagen) using 1.5 µg RNA. qRT-PCR was performed with QuantiTect SYBR Green PCR Kit (Qiagen) on Eppendorf Realplex 2 Master cycler. All samples were run in technical replicates. qRT-PCR primers are listed in Supplemental Table 1.
Clonogenic survival assay
SF8628 cells were seeded at a density of 500 cells/well in 6 well plates. Cells were treated either alone or in combination therapy with varying doses of GDC-0084 or Disulfiram (DSF). On day 14, media was removed, and colonies were stained using crystal violet (0.1% in 20% methanol: Sigma-Aldrich). Colonies were manually counted if they contained > 50 cells as assessed by microscopy. Surviving fraction (SF) was calculated as the number of colonies divided by the number of plated cells (corrected for plating efficiency). IC50 values were calculated using nonlinear regression with variable slope by GraphPad Prism software.
Therapeutics
GSK458, GDC-0084 and DSF were purchased from Cayman Chemicals (Ann Arbor, MI). Stock solutions of 10 mM were prepared in 100% DMSO.
Neurosphere assay
2500 cells were seeded per well in 96 well plates after being treated or left untreated. Neurosphere size and formation was evaluated at indicated days post-seeding by microscopic imaging of three different fields of view per timepoint and condition. Neurosphere size and number was quantified on microscopic images with ImageJ software using the average over the three fields of view for four independent experiments as performed by three different raters.
Statistics
Statistical analysis not described above was performed using GraphPad Prism v7 (GraphPad Software, La Jolla, CA, USA). Statistical analysis is presented from three independent experiments unless otherwise indicated and data represents mean ± SEM. p-values were calculated using an unpaired t-test or one-way ANOVA. In all cases, p ≤ 0.05 were considered significant. In addition, qRT-PCRs and alamarBlue analysis was performed in technical triplicates. P-values were calculated using unpaired t-test unless otherwise indicated.
Results
Regulation of ALDH1A3 isoform by mutant histone H3
To gain mechanistic insight into the role of histone H3 mutations in the pathogenesis of DMG we performed nascent RNA Bru sequencing (Bru-seq) in patient-derived DMG neurospheres with the H3.3 K27M mutation and compared it to CRISPR edited cells (H3K27M-KO), where editing resulted in a frameshift deletion of the mutant allele as previously described [2]. A total of 4162 genes were found to be upregulated and 3971 downregulated upon oncohistone deletion (Fig. 1A). Stemness genes were upregulated in H3K27M cells, whereas genes important for differentiation were upregulated in H3K27M-KO cells (Fig. 1B). Not surprisingly, signaling pathways pertaining to oncogenesis, stemness and differentiation were found to be distinct between parental (H3K27M) and H3K27M-KO cells (Fig. 1C), which was further supported by morphological changes upon H3K27M deletion (Fig. 1D). The latter finding was confirmed in murine neuronal stem cells (mNSCs), where mutant or wildtype H3 was overexpressed (mNSC K27M or wt) [9] (Fig. 1D), and in murine neurospheres isolated from tumors of an intrauterine electroporation (IUE) mouse model [8] (Fig. 1D). In the latter model, cells stemming from tumors developed by injection of mutant TP53 (dominant negative TP53), PDGFRA (D842V) and wildtype histone (PPW) adhered to the plastic whereas cells stemming from tumors developed by injection of the same plasmids, but mutant (H3.3K27M) histone 3 (PPK) grew as 3D neurospheres (Fig. 1D). Bru-seq data analysis of ALDH1A3 and GFAP, selected stemness and differentiation genes, respectively, showed a dramatic elevated level of transcription of ALDH1A3 (Fig. 1E) and a decrease of GFAP (Fig. 1F) in DIPG XIII H3K27M mutant cells, which was further validated by qRT-PCR (Fig. 1G and H) and western blotting (Fig. 1I). To characterize downstream targets of ALDH and understand the consequences of targeting it, we analyzed the mRNA expression of ALDH target genes in Bru-seq data sets and found that genes previously described as ALDH targets [26] were downregulated in KO cells expressing low levels of ALDH1A3 when compared to parental cells (Fig. 1J and K). We further validated these findings by qRT-PCR of selected ALDH downstream targets (Fig. 1L and M). In summary, our findings implicate a role for histone 3 in affecting differentiation potential of neuronal precursor cells.
Fig. 1.
Gene regulation of stemness and differentiation by mutant H3. Parental (H3K27M) and CRISPR edited (H3K27M-KO) SU-DIPG XIII cells were utilized for Bru sequencing (Bru-seq) analysis. (A) Volcano plot comparing H3K27M-KO to H3K27M expressing cells. Red points are genes meeting the significance thresholds: adjusted p-value < 0.05 and log2 fold change > 1.5. (B) Heatmap of Bru-seq expression of select genes from parental (H3K27M) and CRISPR edited (H3K27M-KO) SU-DIPG XIII cells and (C) KEGG pathways enriched in differentially expressed genes. Depicted pathways are top 10 upregulated in H3K27M cells (FDR < 0.05). (D) Representative microscope images of human and murine isogenic pairs of DIPG cells. (E) and (F) Bru-seq coverage of ALDH1A3 and GFAP expression in isogenic SU-DIPG XIII cells, respectively. (G) and (H) qRT-PCR analysis of ALDH1A3 and GFAP, respectively, in isogenic DIPG XIII cells, normalized to GAPDH and represented as fold change ± SEM from three independent experiments. Statistically, significance was determined using t-test and indicated (*p < 0.05, ****p < 0.00005). (I) Representative western blot analysis in isogenic DIPG XIII cells, for ALDH1A3, GFAP and H3K27M. Western blots were performed three times. (J-K) Venn diagrams identifying overlapping ALDH1A3 up- and downregulated downstream target genes, respectively, in Bru-seq data from parental (H3K27M) compared to CRISPR edited (H3K27M-KO) SU-DIPG XIII cells and compared to a publicly available data set [26] where ALDH overexpression or ALDH siRNA mediated knock-down was compared to controls in MDA-MB-231 or MDA-MB-468 cells, respectively. Tables depict overlapping up- and downregulated downstream targets. (L-M) qRT-PCR of selected up- and downregulated ALDH downstream targets in parental (H3K27M) when compared to CRISPR edited (H3K27M-KO) SU-DIPG XIII cells. Expression of selected genes was normalized to GAPDH and represented as fold change ± SEM from three independent experiments. Statistically, significance was determined using t-test and indicated (*p < 0.05, **p < 0.005).
H3K27M-KO cells display differentiated glioma cell (DGC)-like transcriptional signature
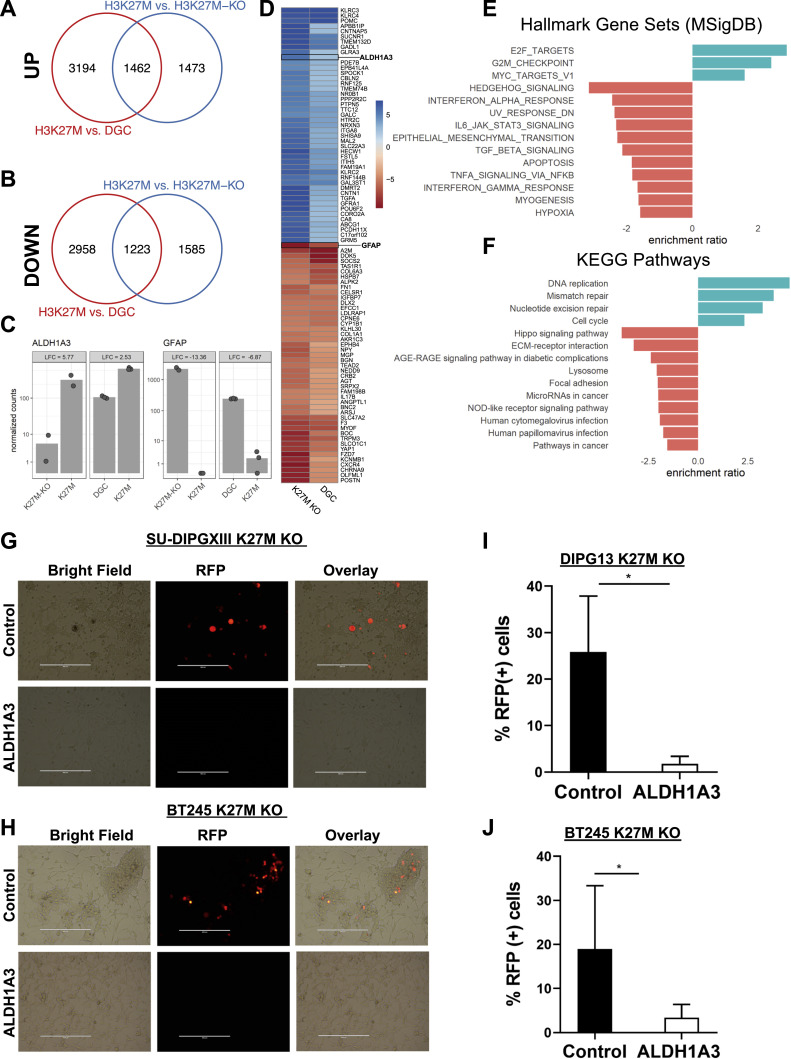
To gain insight into gene signature changes pertaining to the differentiation potential of DMGs upon deletion of mutant histone H3 (H3K27M-KO), we compared Bru-seq data from the DIPG XIII isogenic pair with publicly available RNA sequencing data from differentiated glioma cells (DGC) [27]. Differentiation of the DMG gliomaspheres was induced by fetal bovine serum (FBS)-containing media. Transcriptome analysis of DGCs indicated that they resembled differentiated astrocyte-like cells [27], thus providing insight into the gene signature of differentiated cells. Comparison of these two data sets found a significant overlap of 1462 upregulated (Fig. 2A) and 1223 downregulated (Fig. 2B) genes and distinct gene signatures in H3 mutant DMGs when compared to DGC and H3K27M-KO, including the stemness gene ALDH1A3 and the differentiation gene GFAP (Fig. 2C and D). Pathway enrichment analyses of Hallmark and KEGG gene sets indicated upregulation of E2F signaling, G2M checkpoint, MYC, DNA replication and repair and cell cycle pathways in H3 mutant DMGs, indicative of a stemlike-program (Fig. 2E and F). To test the extent of differentiation in mutant H3 deleted DMGs and determine whether rescue of a stemness program was possible, we transduced DIPG XIII- and BT245-H3K27M-KO cells with lentivirus expressing ALDH1A3 and RFP (ALDH1A3- pLentil-GIII-CMV-RFP-2A-Puro) or RFP (pLentil-GIII-CMV-RFP-2A-Puro) alone. Using fluorescence microscopy and flow cytometry to assess RFP expression, we found that cells were expressing RFP, but not ALDH1A3-RFP. The failure to express ALDH1A3-RFP, but not RFP alone, suggests a suppression of ALDH1A3 in KO cells and potentially indicates non-reversible cell differentiation (Fig. 2G–J). In summary, our results indicate that deletion of mutant H3 results in a gene expression program like that of differentiated cells that is not ‘rescued’ upon expression of ALDH1A3.
Fig. 2.
H3K27M removal results in cell differentiation. RNA expression from DIPG XIII cells (H3K27M) was compared to CRISPR-edited (H3K27M-KO) cells and differentiated (DGCs) DIPG XIII (H3K27M) cells using two different data sets (Bru-seq/Galban Lab, n = 2 and RNA-seq/Mbah et al [27], n = 3). (A-B) Venn diagrams of significantly upregulated genes (A) and downregulated genes (B) in parental (H3K27M) compared to CRISPR edited (H3K27M-KO) or differentiated SU-DIPG XIII cells (DGC). (C) Gene expression normalized counts for ALDHIA3 and GFAP in K27M, K27M-KO and DGC cells. The bars represent the mean of normalized counts. (D) Heatmap of the top differentially regulated genes in differentiated glioma cells (DGC) [27] and K27M-KO cells each compared to DIPG XIII K27M. The scale is log2 fold-change. (E) and (F) Significantly enriched Hallmark gene sets and KEGG pathways for up- (blue) and downregulated (red) genes in DIPG XIII K27M each compared to DGC [27] and K27M KO cells. (G-H) DIPG XIII K27M KO and BT245 K27M KO cells were infected with lentiviral constructs ALDH1A3- pLentil-GIII-CMV-RFP-2A-Puro to express ALDH1A3 and cherry fluorescent protein, and with pLentil-GIII-CMV-RFP-2A-Puro to express cherry fluorescent protein only. Five days post infection fluorescence was evaluated by microscopy (G and H) and flow cytometry (I and J). Representative bright field and fluorescence microscopy images are shown in (G) and (H). (I) and (J) % RFP positive cells measured at 587 nm excitation and 610 nm emission wavelength by flow cytometry are depicted from three independent experiments with SEM and statistical significance assessed using unpaired t-test. *p < 0.05.
Mutant histone H3 drives radioresistance in DMG
Radiotherapy remains the standard of care for the treatment of DMG, yet tumors almost always recur due to radioresistance. To study the impact of mutant H3 deletion and the potential to radiosensitize DMGs, DIPG XIII isogenic cells were irradiated, and changes in transcription were assessed using Bru-seq. Short-term response to radiotherapy (90 min) resulted in almost no upregulation of gene expression in either the mutant (H3K27M) or CRISPR-edited (H3K27M-KO) DMGs, likely due to the p53 mutational status of DIPG XIII cells (Fig. 3A and B). In fact, in both the mutant and CRISPR-edited cells, more genes were down- than upregulated upon radiation and 64 were found to be downregulated especially in the DIPG XIII parental cells (Fig. 3C and D). The two genes upregulated in the parental included STARD5 and DENND3, which are involved in cholesterol biosynthesis and autophagy. STARD5’s upregulation was validated by qRT-PCR (Fig. 3E). Notably, regulation of intracellular cholesterol may represent a targetable mechanism of radioresistance that has been described for other malignancies [28,29]. 53 downregulated genes were found in both the mutant and edited cells, and 8 were found to be exclusively downregulated in the H3 deleted cells. Surprisingly, genes downregulated in the DIPG XIII parental cells (H3K27M) upon radiation included WEE1, AURKA and BUB1B, which are all known to promote radioresistance in glioblastoma multiforme and other malignancies; thus, we expected them to be upregulated as mechanisms of radioresistance in DMG. These findings are surprising and will be compared in future studies to long-term radiation responses. The 8 genes found to be downregulated in the KO cells were CA10, CNTNAP5, HIST1H3J, KNL1, MKI67, NMU, PIWIL3, and TMEM132D. To explore the consequences of radiotherapy in H3K27M-KO cells in vitro, DIPG XIII and BT 245 isogenic cell pairs were exposed to various doses of radiation. Neurosphere formation (Fig. 3F and G) and cell viability (Fig. 3H and I) were found to be diminished in the cells wherein H3 was deleted, indicating radiosensitivity when compared to H3 mutant cells. These results indicate that H3 deletion changes the response to and outcome of radiotherapy.
Fig. 3.
H3K27M mutation leads to radioresistance in isogenic DMG cell lines. SU-DIPG XIII isogenic cells were irradiated at 5 Gy or left untreated and after 60 min incubated with 2 mM bromouridine for 30 min. 90 min post IR cells were collected in Trizol and RNA isolated. Bromouridine labeled RNA was then pulled down using anti-bromouridine antibody and library preps performed and sequenced as previously described [11]. (A) and (B). Volcano plots depicting up- and downregulated genes in H3K27M (top) and KO (bottom) cells. Red points are genes meeting the significance thresholds: adjusted p-value < 0.05 and log2 fold change > 1.5. (C) Venn diagram showing genes upregulated in DIPG isogenics depict genes identified in H3K27M or KO only and overlapping genes. (D) Venn diagram showing downregulated genes in same cells. (E) qRT-PCR of a selected upregulated gene in H3K27M cells compared to CRISPR edited (H3K27M-KO) SU-DIPG XIII cells. Expression of selected gene was normalized to GAPDH and represented as fold change ± SEM from three independent experiments. Statistically, significance was determined using t-test and indicated (**p < 0.005). (F) and (G) Isogenic DIPG XIII and BT245 cells were set up for neurosphere assays immediately after irradiation at doses ranging from 2–6 Gy (F) or 2–4 Gy (G) and microscopic images at 10x magnification taken 10 days post IR. (H) and (I) Proliferation assays using TiterGlo 10 days post IR in isogenic DIPG or BT 245, respectively. *p < 0.05, **p < 0.005 and ***p < 0.0005.
Regulation of ALDH1A3 expression in DMG depends on Wnt/β-catenin signaling
To glean insight into the transcriptional regulation of the stemness program in DMG, particularly ALDH1A3, we interrogated gene expression changes of known ALDH1A3 regulators, which include the Wnt signaling pathway. Wnt itself is inversely regulated by the tumor suppressor EYA4, which interestingly, we found to be highly upregulated in H3K27M-KO cells in our Bru-seq data obtained from isogenic DIPG XIII (Fig. 4A). Increased expression of EYA4 in the H3K27M-KO cells was also validated by qRT-PCR and western blotting (Fig. 4B and C). Next, we evaluated whether the reduced expression of EYA4 in parental cells resulted in an elevated expression of Wnt genes in DMGs. Wnt genes 7B, 11, 5B, 4, 9A and 2B were found to be upregulated in parental cells when compared to H3K27M-KO (Fig. 4D) in Bru-seq data, which was further validated for selected Wnt genes by qRT-PCR (Fig. 4E). To prove that cell-specific, secreted factors are responsible for the regulation of ALDH1A3 gene expression, we performed conditioned media (CM) experiments in the DIPG XIII isogenic pair. As demonstrated in Fig. 4F ALDH1A3 mRNA expression was dramatically induced in DIPG XIII K27M-KO cells when incubated with CM stemming from parental cells. Furthermore, heat inactivation abrogated this observed effect, indicating its mediation by secreted factors in the media that were inactivated when degraded by heat (Fig. 4F). Inhibition of Wnt using the pan-Wnt inhibitor XAV-939 in DIPG XIII parental cells abrogated ALDH1A3 expression, further supporting a Wnt-dependent mechanism of regulating ALDH1A3 expression (Fig. 4G). The canonical β-catenin signaling axis depends on Wnt binding to β-catenin, resulting in protein stabilization and translocation of β-catenin to the nucleus to activate transcription of target genes like ALDH1A3 [26]. We interrogated β-catenin expression in isogenic DIPG XIII and as expected, β-catenin protein expression was found to be elevated in DIPG XIII parental cells (Fig. 4H), supporting our hypothesis of Wnt/β-catenin dependent ALDH1A3 regulation in DMG.
Fig. 4.
WNT/β-catenin pathway dependent regulation of ALDH1A3. (A) Bru-seq coverage for EYA4 in isogenic SU-DIPG XIII cells. (B) qRT-PCR of EYA4 in isogenic SU-DIPG XIII. Data were normalized to GAPDH. Values were represented as mean ± SEM from three independent experiments. Statistically, significance was determined using unpaired t-test (P = 0.000034). (C) Representative western blot analysis of EYA4 expression in isogenic SU-DIPG XIII (n = 3). (D) Bru-seq analysis of Wnt genes in DIPG XIII K27M and KO cells. Bru-seq was performed in replicates of two. (E) qRT-PCR of Wnt genes in isogenic SU-DIPG XIII. Data were normalized to GAPDH and fold change was calculated and compared with DIPG XIII K27M KO cells. Values were represented as mean ± SEM from three independent experiments. Statistically, significance was determined using t-test and indicated (*p < 0.05, **p < 0.005, ***p < 0.0005). (F) Conditioned media was obtained from isogenic cells grown in TSM complete media for 24 or 48 h. Media was then collected, and heat inactivated at 95 °C for 30 min or left untreated. Isogenic cells were then treated for 24 or 48 h with indicated media prior to RNA isolation followed by qRT-PCR for ALDH1A3. Data was normalized to GAPDH and represented as fold change ± SEM from three independent experiments. Statistical significance was determined using t-test and indicated (***p < 0.0005). (G) qRT-PCR of ALDH1A3 in isogenic SU-DIPG XIII cells treated with 10 µM pan-Wnt inhibitor XAV-939 for 24 hrs. Statistically, significance was determined using unpaired t-test and indicated (***p < 0.0005, n.s. = no statistical significance). (H) Representative western blot analysis of β-catenin expression in isogenic SU-DIPG XIII (n = 3).
Oncohistone H3K27M increases acetylation through ALDH1A3
Most gene expression changes observed during DMG tumorigenesis are thought to be dependent on alterations of epigenetic modifications. While changes in methylation are likely a direct consequence of the H3 mutation, modifications in acetylation often co-occur and result in an aberrant transcriptional program [30], [31], [32]. ALDH1A3 has previously been reported to increase acetyl coenzyme A, resulting in acetylation of lysine 27 on Histone 3 at enhancer sites and increased gene expression [33] (Fig. 5A). Thus, we tested whether acetyl coenzyme A production is elevated in ALDH1A3 high, H3K27M mutant cells. As seen in Fig. 5B, production of acetyl coenzyme A significantly decreased upon deletion of the H3K27M mutation, indicating regulation by the oncohistone. Furthermore, this decrease in acetyl coenzyme A production affected the acetylation of H3K27 itself (Fig. 5C). Next, we tested whether inhibition of ALDH1A3 with the pan-ALDH inhibitor Disulfiram (DSF) in H3K27M mutant cells affected acetylation of H3 at lysine 27. As depicted in Fig. 5D H3K27M cells treated with DSF showed decreased acetyl-H3K27 by western blotting. To glean insight into gene expression changes caused by H3K27 acetylation, we compared gene expression profiles obtained from our Bru-seq analysis in isogenic DIPG XIII to publicly available ChIP Sequencing (ChIP-Seq) data, where acetylated DNA was pulled down using acetyl H3K27 antibody [34]. The top 10 genes, which were downregulated upon H3 deletion and where expression changes were likely caused by modifications in acetylation, are depicted in Fig. 5E. Next, we validated that observed changes in acetylation of selected genes including ALDH1A3 caused a change in gene expression (Fig. 5F). Previous reports have described a regulatory role for the ALDH1A3 enzyme in the acetylation of H3K27 through its conversion of acetaldehyde to acetate and production of acetyl coenzyme to mark active enhancers [33]. Based on our findings thus far, we propose a model by which mutant H3 reduces the expression of the tumor suppressor EYA4, thereby inducing the expression of Wnt/β-catenin signaling. This in turn activates the expression of ALDH1A3 to regulate the pool of acetate and Co-A enzyme, and thereby acetylation, to induce an altered transcriptional program (Fig. 5G, left). Deletion of H3 results in a reversal of this transcriptional program and induces cell differentiation (Fig. 5G, right).
Fig. 5.
ALDH1A3 regulates acetylation of H3K27. (A) Schematic of ALDH1A3 dependent regulation of acetyl coenzyme A. (B) Percent change in acetyl coenzyme A production in isogenic DIPG XIII cells. Experiments were performed three times. Statistical significance was determined using unpaired t-test with **p < 0.005. (C) Western blot detecting acetylated H3K27 and mutant H3K27M in isogenic SU-DIPG XIII cells. (D) Representative western blot of acetyl-H3K27 in DIPG XIII parental cells treated with 100 µM Disulfiram (DSF) or equimolar concentration of DMSO for 24 h. (E) Heat map of top 10 transcriptionally upregulated genes in SU-DIPG XIII parental (K27M) cells that were also acetylated at H3K27M. Heat maps were generated by comparing Bru-seq data [5] to ChIP-Seq data [34] in isogenic SU-DIPG XIII cells. (F) qRT-PCR of selected genes and ALDH1A3 identified in (E). Values were represented as mean ± SEM from three independent experiments. Statistically, significance was determined using t-test and indicated (*p < 0.05, **p < 0.005). (G) Proposed model of Wnt/β-catenin dependent regulation in H3K27M altered DMG.
PI3K/mTOR inhibition effectively targets stemness program in DMG
In previous studies of DMG, we have described PI3K/mTOR signaling to be elevated specifically in ALDH positive CSCs, which were successfully targeted using a clinically relevant pan-PI3K/mTOR inhibitor, GDC-0084 [5]. We now ask the question whether PI3K/mTOR inhibition reverses stemness by regulating Wnt signaling and stemness gene programs like ALDH1A3. Analysis of Bru-seq data obtained from ALDH positive DMGs treated with GSK458, a PI3K/mTOR inhibitor, showed a dramatic reduction of Wnt7B in treated cells (Supplemental Fig. 1A). We also assessed expression of stemness genes in the same data set, which were mostly found to be downregulated in parental cells upon GSK458 treatment (Supplemental Fig. 1B). Next, we asked whether the acetylation, and thus, transcription program of PI3K/mTOR treated cells resembles that of mutant H3 deleted cells, which would indicate a viable treatment option until targeted H3K27M inhibitors become available. ChIP-Seq data of GSK458-treated DIPG XIII cells using acetyl-H3K27 antibody were compared to publicly available ChIP-Seq data performed in isogenic DIPG XIII cells [34]. A significant overlap in acetylation of H3K27 was detected between GSK458-treated cells and H3K27M-KO cells (Supplemental Fig. 1C and D). In both cell types 99 genes were found to gain acetylation (Supplemental Fig. 1C) while 170 genes lost H3K27 acetylation (Supplemental Fig. 1D). Furthermore, when we compared the expression with acetylation profiles in GSK458-treated or H3K27M-KO-treated cells each normalized to H3K27M, we identified acetylated genes with increased expression in GSK458-treated cells and H3K27M-KO cells (Supplemental Fig. 1E) and genes that lost acetylation and showed decreased expression (Supplemental Fig. 1F). Interestingly, stemness (PROM1) and differentiation (POU3F1) genes were among the genes modulated by GSK458 or oncohistone deletion (Supplemental Fig. 1E and F).
Combination of PI3K/mTOR and ALDH inhibition to eradicate cancer stem cells in DMG
Our finding that PI3K/mTOR inhibition epigenetically modifies a stemness and differentiation program (see Supplemental Fig. 1) provides sufficient rationale to test PI3K/mTOR inhibitors in combination with ALDH inhibition to target the mutant H3 dependent stemness program in DMG. Several DMG cell lines (SU-DIPG XIII, DIPG-007, SF8628) were used to test combination therapies of two clinically relevant, BBB-penetrant inhibitors of PI3K/mTOR (GDC-0084) and ALDH (DSF). Cells were treated with various concentrations of GDC-0084 and DSF alone and in combination to identify maximal inhibition and synergy. Viability of DIPG XIII and DIPG-007 neurospheres was significantly decreased when cells were treated in combination compared to single agents alone or control treated cells (Fig. 6A and C). High synergy with the two inhibitors was observed using Combenefit software (Fig. 6B and D). Likewise, a significant growth reduction and colony formation capacity was observed when GDC-0084 was combined with DSF in adherent SF8628 cells (Fig. 6E and F), which also translated into statistical reduction of IC50 of each drug when used in combination (Fig. 6G and H). This data was further supported by Combenefit software analysis, which confirmed synergy when both drugs were combined (Fig. 6I). In conclusion, these data suggest that intrinsic vulnerabilities of DMG may be targeted with PI3K/mTOR and ALDH inhibitors.
Fig. 6.
Combination therapy in DMG to target CSCs. (A and C) Viability of SU-DIPG XIII and DIPG-007 neurospheres, treated with various concentrations of GDC-0084 and DSF alone or in combination for 72 h using TiterGlo. Experiments were performed three times each in triplicate. Statistical significance was determined using unpaired t-test. *p < 0.05, n.s. = no statistical significance. (B and D) Synergy of the drug treatment determined in SU-DIPG XIII and DIPG-007 cells, respectively, with Loewe model in Combenefit software. (E and F) Colony formation assays of adherent SF8628 cells treated with various concentrations of GDC-0084 and DSF alone or in combination for 13 days. (E) Number of colonies were counted for GDC or DSF treatment either alone or in combination of GDC (0.5 µM) with DSF (0.25 µM) and (F) % survival calculated at indicated concentration. Experiments were performed a minimum of three times in duplicate wells each. Statistical significance was determined using unpaired t-test. **p < 0.005, n.s. = no statistical significance. (G-H) IC50 from experiment performed in (E) was calculated for GDC or DSF alone or DSF (0.25 µM) titrated into GDC (G), or GDC (0.5µM) titrated into DSF (H). Statistical significance was determined using unpaired t-test. *p < 0.05, **p < 0.005. (I) Synergy of drug treatment with HSA model in Combenefit software.
Discussion
DMG remains an incurable disease and treatment options are desperately needed. In a previous study, we identified aldehyde dehydrogenase expressing cancer stem cells (ALDH+ CSC) in DMG as contributors to tumor progression [5]. Our transcriptomic studies identified PI3K/mTOR signaling as an intrinsic vulnerability of ALDH+ CSCs and thus, a means by which to target them. In this study, we investigated the regulation of a specific ALDH isoform, ALDH1A3, in DMG. We uncovered a mechanism by which the disease-causing histone H3K27M mutation transcriptionally decreases the expression of the Wnt inhibitor EYA4, thereby inducing Wnt signaling in DMG. In turn, Wnt/β-catenin signaling was found to induce ALDH1A3 expression, which prevented differentiation, likely by increasing acetylation of H3K27 at gene promoters of stemness and differentiation genes. Previous reports have described EYA4 as a bona fide tumor suppressor [35,36], and here, we have identified EYA4 as a bona fide tumor suppressor in DMG that upon suppression, results in aberrant Wnt signaling to orchestrate stemness and differentiation. In addition, previous studies have shown a regulatory role for the ALDH1A3 enzyme in the acetylation of H3K27 through the conversion of acetaldehyde to acetate and production of acetyl coenzyme to mark active enhancers [33]. Removal of the mutant histone by CRISPR editing resulted in morphological changes, a gene profile that was found to resemble astrocyte-like cells, and a dramatic increase in the expression of two astrocytic markers, GFAP and ALDH1L1. Interestingly, this differentiated phenotype could not be rescued by overexpressing the stem cell gene ALDH1A3. In fact, expression of RFP alone, but not ALDH1A3-RFP, was achieved in both H3K27M KO lines. This suggests that ALDH1A3 expression is actively suppressed in these cells and may indicate terminal differentiation of the CRISPR edited H3K27M-KO cells. Consequences of histone H3K27M deletion included radiosensitization of DMGs, a finding that encourages the development of future therapeutics targeting the mutant histone directly or via downstream signaling pathways such as those described here.
Using technology that allows for identification of newly synthesized RNA, we identified a transcriptional program that depends on the mutational status of H3 and explains observed morphological changes in human and murine DMG cell lines. Our transcriptomic analysis identified numerous stem cell genes, including ALDH1A3, to be highly upregulated in DMG cells when compared to H3K27M-KO cells. Furthermore, the astrocyte markers GFAP and ALDH1L1, among others, were downregulated in parental cells, but highly upregulated upon correction of the mutation, indicating differentiation to astrocytes. This is an interesting finding supported by recent studies that have provided insight into the cell of origin in DMG [37]. Upon expression of the mutant histone H3.3, neuronal stem cells give rise to gliomas when co-occurring with p53 mutations [37]. Interestingly, oncohistone expression induced a transcriptional program, which resembled that of undifferentiated oligodendrocyte precursor cells (OPCs), providing evidence of a stem-like phenotype previously described for DMG [38]. One recent study provides insight into pathological underpinnings of the oncohistone and a mechanism by which the mutation alters stem cell growth, epigenetic regulation, and differentiation potential [39]. Oncohistone expression results in a gene program that supports tissue properties that enable acquisition of additional mutations that cooperate with H3K27M mutation in genesis of DMG [39]. Our transcriptome comparisons to differentiated astrocyte-like cells [27] demonstrated a significant overlap with genes regulated in KO, further supporting a role for mutant H3 in preventing differentiation. Although the role of H3K27M in radiosensitization has been explored and found to be dominant negative [6], we showed for the first time that correction of the mutation impacts the cell's therapeutic sensitivity, a finding that supports the development of therapeutics that directly or indirectly affect the oncohistone or its downstream signaling pathways. Interestingly, radiation induced changes were mostly found to be downregulating gene expression in our model. This may be explained by the mutational status of p53. Early radiation induced transcriptome changes in other malignancies have been shown to be dependent on wildtype p53 expression [40], and since DIPG XIII cells carry a mutation in p53, radiation-induced upregulation of gene expression may not occur at early timepoints. Future studies will be designed to compare long- to short-term radiation responses in DMG. Our study accords with the published findings we discuss here and contributes additional mechanistic insight. Further, we provide new therapeutic targets, like ALDH1A3, to disrupt the stemness program observed in DMG.
Until brain penetrant small molecule inhibitors targeting the mutant histone are available or CRISPR editing delivery to the brain becomes feasible, the identification of alternative downstream pathways to treat radioresistant DMGs remains a critical avenue of investigation. Interestingly, our transcriptomic findings of irradiated DMGs indicate that targeting cholesterol biosynthesis may increase the efficacy of radiotherapy. This is supported by a study where the Menin inhibitor MI-2 was used to target cholesterol biosynthesis, particularly lanosterol synthase, to restore sensitivity to radiotherapy in DMG [29]. Importantly, clinically approved and frequently used inhibitors of cholesterol like Simvastatin, which was found to sensitize radioresistant prostate cancer [28], could be repurposed in DMG for enhancing the efficacy of radiotherapy. Our study also provides rationale for exploring EYA4 overexpression strategies or Wnt/β-catenin pathway inhibitors for the treatment of DMG. We found that Wnt/β-catenin regulates the expression of ALDH1A3 and showed that blockade of Wnt by XAV-939 affects ALDH1A3 expression. A new Wnt/tankyrase inhibitor, NVP-TNKS656, was recently tested in colorectal cancer patient-derived sphere cultures and mouse models to combat therapeutic resistance observed in a clinical trial of colorectal cancer patients treated with PI3K or AKT inhibitors [41]. We have shown that ALDH+ CSCs in DMG exhibit elevated PI3K/mTOR signaling and that use of the brain penetrant PI3K/mTOR inhibitor GDC-0084 reduces tumor growth in an orthotopic patient-derived DMG model [5]. Future therapeutic avenues to treat DMG may therefore include targeting CSCs and stemness with a combination of PI3K/mTOR inhibitors such as GDC-0084 and Wnt/β-catenin inhibitors. Its regulation by the mutant histone through Wnt/β-catenin prompted us to explore the possibility of targeting DMG with the combination of pan-ALDH inhibitors and GDC-0084 that we used in previous studies [5]. We tested DSF, a pan-ALDH inhibitor, for proof-of-principle, as DSF has been shown to reverse resistance to chemotherapeutic drugs by inhibiting the ALDH isoforms [42] and the P-glycoprotein (Pgp) multidrug efflux pump [43]. Our study indicates exquisite synergy between these two drugs and suggests that resistance caused by CSCs may be prevented by treating DMG post-radiotherapy with DSF and GDC-0084 to prevent emergence and abundance of ALDH positive DMGs that promote tumor progression [5]. We are diligently working on developing mouse models for DMGs that recapitulate the disease and allow for therapeutic testing. However, currently available models provide limited windows for testing therapeutics and thus in vivo testing of the above-described drug combination will be the goal of future studies.
We acknowledge additional limitations of this study. First, although we have utilized human and murine DMG lines, these lines have distinct mutations and the mechanism of ALDH1A3 regulation may not apply to all H3K27M mutant DMG if other co-occurring mutations are present. Secondly, the cell lines we have used to mimic H3K27M correction were generated using CRISPR editing [2], which resulted in a frame shift mutation and complete deletion of H3K27M expression, which may be different than a correction of the mutation and a full recovery of histone H3 expression from both alleles. Future studies will focus on developing optimal pre-clinical mouse models for therapeutic testing.
In summary, our study provides new insight into the pathobiology of mutant histone H3 in regulating a stemness program that prevents differentiation and leads to resistance to radiotherapy. Furthermore, it presents viable new therapeutic avenues to radiosensitize DMGs and target tumor progression until mutant H3 inhibitors become available.
CRediT authorship contribution statement
Monika Sharma: Investigation, Methodology, Formal analysis, Data curation. Ivana Barravecchia: Investigation, Formal analysis, Data curation. Brian Magnuson: Formal analysis, Methodology. Sarah F. Ferris: Investigation, Data curation. April Apfelbaum: Investigation, Data curation. Nneka E. Mbah: Data curation. Jeanette Cruz: Investigation, Data curation. Varunkumar Krishnamoorthy: Investigation, Data curation. Robert Teis: Investigation, Data curation. McKenzie Kauss: Investigation, Data curation. Carl Koschmann: Writing – review & editing. Costas A. Lyssiotis: Writing – review & editing. Mats Ljungman: Writing – review & editing, Formal analysis. Stefanie Galban: Conceptualization, Methodology, Writing – original draft, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
CAL has received consulting fees from Astellas Pharmaceuticals, Odyssey Therapeutics, and T-Knife Therapeutics, and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting the GOT1-pathway as a therapeutic approach (US Patent No: 2015126580-A1, 05/07/2015; US Patent No: 20190136238, 05/09/2019; International Patent No: WO2013177426-A2, 04/23/2015). All other authors declare that no conflicts of interest exist.
Acknowledgments
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NIH) Grant 1R01NS13151501 (SG), the ChadTough Defeat DIPG Foundation (SG), the University of Michigan Rogel Cancer Center “First and Goal” funding (SG), the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) Grants R01-NS124607 and R01-NS119231 (CK), the Department of Defense Grant CA201129P1 (CK), the University of Michigan Pediatric Brain Tumor Initiative (CAL), the National Cancer Institute of NIH Grants 1 R01 CA213214 and P30CA046592 (ML), and the National Human Genome Research Institute of NIH Grant UM1 HG009382 (ML).
Acknowledgments
The authors wish to thank Michelle Paulsen in assisting with Bru Sequencing under grant support from 1 R01 CA213214 and UM1 HG009382 (ML). Flow cytometry work was performed at the University of Michigan Flow Cytometry Core, which is supported by NCI under award numbers P30CA046592 and P30 CA04659229. The authors wish to thank Drs. Uday B. Maachani, Mark M. Souweidane, Sriram Venneti and Michelle Monje-Deisseroth for providing the DMG cell lines SU-DIPG IV, SU-DIPG XIII, HSJD-DIPG 007 and SU-DIPG 29, respectively. The isogenic DIPG XIII and BT245 cells were a kind gift from the Jabado Laboratory [2]. We would like to thank the Koschmann Laboratory for providing us with the murine isogenic neurospheres (PPK and PPW) obtained from the intrauterine electroporation model [8] and the Venneti Laboratory for providing us with the isogenic murine neuronal stem cell pair [9]. We would also like to acknowledge Dr. Jabado's group, who made the RNA and ChIP Sequencing data publicly available [34] and Dr. Lyssiotis's group, who made the RNA sequencing data from gliomaspheres and differentiated glioma cells publicly available [27].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2023.100931.
Appendix. Supplementary materials
References
- 1.Souweidane M.M., et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19(8):1040–1050. doi: 10.1016/S1470-2045(18)30322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harutyunyan A.S., et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat. Commun. 2019;10(1):1262. doi: 10.1038/s41467-019-09140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackay A., et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537. doi: 10.1016/j.ccell.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu G., et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014;46(5):444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surowiec R.K., et al. Transcriptomic analysis of diffuse intrinsic pontine glioma (DIPG) identifies a targetable ALDH-positive subset of highly tumorigenic cancer stem-like cells. Mol. Cancer Res. 2021;19(2):223–239. doi: 10.1158/1541-7786.MCR-20-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakotomalala A., et al. H3.3K27M mutation controls cell growth and resistance to therapies in pediatric glioma cell lines. Cancers. 2021;13(21) doi: 10.3390/cancers13215551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katti A., et al. CRISPR in cancer biology and therapy. Nat. Rev. Cancer. 2022;22(5):259–279. doi: 10.1038/s41568-022-00441-w. [DOI] [PubMed] [Google Scholar]
- 8.Miklja Z., et al. Everolimus improves the efficacy of dasatinib in PDGFRalpha-driven glioma. J. Clin. Invest. 2020;130(10):5313–5325. doi: 10.1172/JCI133310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung C., et al. Integrated metabolic and epigenomic reprograming by H3K27M mutations in diffuse intrinsic pontine gliomas. Cancer Cell. 2020;38(3):334–349. doi: 10.1016/j.ccell.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galban S., et al. Cytoprotective effects of IAPs revealed by a small molecule antagonist. Biochem. J. 2009;417(3):765–771. doi: 10.1042/BJ20081677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulsen M.T., et al. Use of Bru-seq and BruChase-seq for genome-wide assessment of the synthesis and stability of RNA. Methods. 2014;67(1):45–54. doi: 10.1016/j.ymeth.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulsen M.T., et al. Coordinated regulation of synthesis and stability of RNA during the acute TNF-induced proinflammatory response. Proc. Natl. Acad. Sci. U. S. A. 2013;110(6):2240–2245. doi: 10.1073/pnas.1219192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langmead B., Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat. Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobin A., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankish A., et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47(D1):D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Love M.I., et al. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu A., et al. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35(12):2084–2092. doi: 10.1093/bioinformatics/bty895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H., et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amemiya H.M., et al. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 2019;9(1):9354. doi: 10.1038/s41598-019-45839-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinlan A.R., Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez F., et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44(W1):W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neph S., et al. BEDOPS: high-performance genomic feature operations. Bioinformatics. 2012;28(14):1919–1920. doi: 10.1093/bioinformatics/bts277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavalcante R.G., Sartor MA. Annotatr: genomic regions in context. Bioinformatics. 2017;33(15):2381–2383. doi: 10.1093/bioinformatics/btx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shechter D., et al. Extraction, purification and analysis of histones. Nat. Protoc. 2007;2(6):1445–1457. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- 26.Marcato P., et al. Aldehyde dehydrogenase 1A3 influences breast cancer progression via differential retinoic acid signaling. Mol. Oncol. 2015;9(1):17–31. doi: 10.1016/j.molonc.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbah N.E., et al. Therapeutic targeting of differentiation state-dependent metabolic vulnerabilities in DIPG. bioRxiv. 2022;2022 doi: 10.1101/2022.03.01.482555. [DOI] [Google Scholar]
- 28.Chen Y.A., et al. Simvastatin sensitizes radioresistant prostate cancer cells by compromising DNA double-strand break repair. Front. Pharmacol. 2018;9:600. doi: 10.3389/fphar.2018.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips R.E., et al. Target identification reveals lanosterol synthase as a vulnerability in glioma. Proc. Natl. Acad. Sci. U. S. A. 2019;116(16):7957–7962. doi: 10.1073/pnas.1820989116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johung T.B., Monje M. Diffuse intrinsic pontine glioma: new pathophysiological insights and emerging therapeutic targets. Curr. Neuropharmacol. 2017;15(1):88–97. doi: 10.2174/1570159x14666160509123229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammad F., et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat. Med. 2017;23(4):483–492. doi: 10.1038/nm.4293. [DOI] [PubMed] [Google Scholar]
- 32.Ehteda A., et al. Dual targeting of the epigenome via FACT complex and histone deacetylase is a potent treatment strategy for DIPG. Cell Rep. 2021;35(2) doi: 10.1016/j.celrep.2021.108994. [DOI] [PubMed] [Google Scholar]
- 33.Li D., et al. ALDH1A3 coordinates metabolism with gene regulation in pulmonary arterial hypertension. Circulation. 2021;143(21):2074–2090. doi: 10.1161/CIRCULATIONAHA.120.048845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krug B., et al. Pervasive H3K27 acetylation leads to ERV expression and a therapeutic vulnerability in H3K27M gliomas. Cancer Cell. 2019;35(5):782–797. doi: 10.1016/j.ccell.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo S.J., et al. EYA4 inhibits hepatocellular carcinoma growth and invasion by suppressing NF-kappaB-dependent RAP1 transactivation. Cancer Commun. 2018;38(1):9. doi: 10.1186/s40880-018-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S.J., et al. EYA4 acts as a new tumor suppressor gene in colorectal cancer. Mol. Carcinog. 2015;54(12):1748–1757. doi: 10.1002/mc.22247. [DOI] [PubMed] [Google Scholar]
- 37.Haag D., et al. H3.3-K27M drives neural stem cell-specific gliomagenesis in a human iPSC-derived model. Cancer Cell. 2021;39(3):407–422. doi: 10.1016/j.ccell.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Filbin M.G., et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science. 2018;360(6386):331–335. doi: 10.1126/science.aao4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kfoury-Beaumont N., et al. The H3K27M mutation alters stem cell growth, epigenetic regulation, and differentiation potential. BMC Biol. 2022;20(1):124. doi: 10.1186/s12915-022-01324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkata Narayanan I., et al. Transcriptional and post-transcriptional regulation of the ionizing radiation response by ATM and p53. Sci. Rep. 2017;7:43598. doi: 10.1038/srep43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arques O., et al. Tankyrase inhibition blocks Wnt/beta-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin. Cancer Res. 2016;22(3):644–656. doi: 10.1158/1078-0432.CCR-14-3081. [DOI] [PubMed] [Google Scholar]
- 42.Koppaka V., et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012;64(3):520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cvek B. Targeting malignancies with disulfiram (Antabuse): multidrug resistance, angiogenesis, and proteasome. Curr. Cancer Drug Targets. 2011;11(3):332–337. doi: 10.2174/156800911794519806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.