Abstract
The return of individual genomic results (ROR) to research participants is still in its early phase, and insight on how individuals respond to ROR is scarce. Studies contributing to the evidence base for best practices are crucial before these can be established. Here, we describe a ROR procedure conducted at a population-based biobank, followed by surveying the responses of almost 3000 participants to a range of results, and discuss lessons learned from the process, with the aim of facilitating large-scale expansion. Overall, participants perceived the information that they received with counseling as valuable, even when the reporting of high risks initially caused worry. The face-to-face delivery of results limited the number of participants who received results. Although the participants highly valued this type of communication, additional means of communication need to be considered to improve the feasibility of large-scale ROR. The feedback collected sheds light on the value judgements of the participants and on potential responses to the receipt of genetic risk information. Biobanks in other countries are planning or conducting similar projects, and the sharing of lessons learned may provide valuable insight and aid such endeavors.
Subject terms: Outcomes research, Human behaviour, Personalized medicine
Introduction
Recent advances in human genomics and genetics, reduced costs of DNA sequencing and genotyping, new statistical methods for the calculation of personal disease risks, and the growing number of large, longitudinal, population-based biobanks have created an environment suitable for the piloting of opportunistic genomic screening and personalized medicine approaches using existing genomic data. Broadly agreed-upon recommendations and policies regarding the return of individual results (ROR) to research participants have been established [1, 2]. Medical actionability is the key criterion for ROR; other important factors that should be considered include the clinical significance, the analytical validity and feasibility of responsible and effective ROR, and the option to opt out of ROR [2–4]. Legal, practical, and societal factors have been identified as challenges that impact the feasibility of large-scale, long-term ROR [4, 5]. Recommendations have been made to address these challenges prospectively; they include the addressing of ROR during the initial consent procedure and the allocation of specific funds for ROR [2, 4].
Attitudes toward the return of research findings to participants are generally supportive across different stakeholders. This support persists even for conditions that are low risk or of no serious health importance, and it is not limited to conditions that can be treated or prevented [6, 7]. Participants prefer or even expect to receive results and report ROR as a factor affecting their trust in researchers and influencing their likelihood of participating in research [7–9].
Recently, support has increased for the consideration of the value judgements of participants with regard to ROR. More active engagement of participants could potentially improve trust and ensure biobank sustainability [8, 10, 11]. The extent to which ROR motivates participants varies between countries [12]. In Estonia, surveys have shown that the majority of the public would be interested in receiving genetic information [13]. Over the years, thousands of Estonian Biobank (EstBB) participants have expressed their interest in ROR. In previous ROR projects, biobank staff have offered the disclosure of individual genetic results with relatively large genetic effect sizes to a limited numbers of participants [14–16]. One argument against disclosure is the potential harm caused by the receipt of risk-related information. Previous reports on ROR to biobank participants, to our knowledge, have not supported this fear [17, 18]. Only few EstBB participants have reported feeling uncomfortable, but the majority have appreciated the information received and report no regrets [14, 15].
Although a consensus favors ROR, the number of ongoing initiatives is limited, and optimal procedures remain undefined and likely context specific. In this paper, we share an approach to offering ROR to biobank participants who expressed interest. We discuss lessons learned from this initiative and describe how the risk information was perceived based on feedback from almost 3000 participants.
Methods
The EstBB cohort
The EstBB is a population-based biobank established in 2000 [19]. The Estonian Human Genes Research Act regulates biobank maintenance, participants’ rights, and the use of their samples and data [20]. By 2011, the biobank had collected biological samples and health information from 51,000 adult participants. This information has been updated regularly from nationwide databases [21]. By 2017, all 51,000 participants had been genotyped using the Global Screening Array version 1 (Illumina, Inc.).
The EstBB has grown substantially since 2017, and had samples and information from more than 207,000 adult participants at the end of 2021. The cohort described here was enrolled before 2017; thus, genotype data were available for all individuals. On average, ROR occurred about 9 years after biobank enrollment.
ROR workflow
The ROR initiative for biobank participants was introduced to the public in the spring of 2017, at a press conference that was covered in the national news. Further information on how to sign up was available on the biobank’s website. The first visits for ROR were registered in the fall of 2017. No individual invitations were sent to biobank participants.
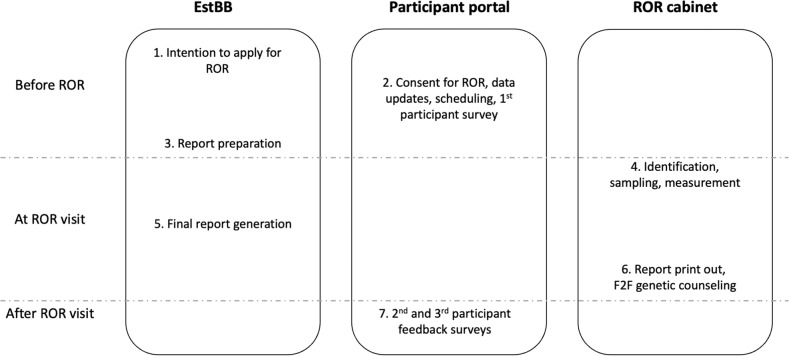
The ROR procedure, from registration to feedback collection, was approved by the ethics committee of the University of Tartu (no. 271/T-22) and is summarized in seven steps (Fig. 1):
Fig. 1. The EstBB ROR workflow.
EstBB, Estonian Biobank; ROR, return of individual genomic results; F2F, face-to-face.
Expression of interest and biobank participant identification
Information about the initiative is provided and online registration is performed on the biobank’s website. After providing proof of identification, individuals can express interest in ROR by completing a short application. Project participation status is confirmed within 2 weeks if the individual is confirmed to be an EstBB participant. When confirmed, a link to the participant portal is e-mailed to the participant.
Consent for ROR, data updates, and visit registration
The participant portal can be accessed by providing proof of identification (ID card or Mobile ID). A short description of different categories of results is provided (Textbox S1). Participants can opt in separately for ROR, the provision of a new blood sample, and/or the updating of their information by electronically signing a consent form. Reflective of their preferences, they then update their personal health information by filling out a short questionnaire, and survey on expectations of ROR. Finally, a participant can select a suitable venue, date, and time for ROR from a web-based calendar. Each participant receives two reminders via e-mail prior to the visit.
Risk calculation and report preparation
The calendar offers dates for ROR starting from 2 weeks after registration, to provide time for generating reports. Different sections of the report are gathered from the respective workgroups. Personalized reports can be viewed and printed only by previously appointed staff members after log-in to ROR portal using their ID cards.
ROR visit: identification, sampling, and measurement
The participant meets with a recruitment assistant and provides proof of identification. Their blood pressure, weight, height, hip and waist measurements are specified and entered into the participant portal, and a new blood sample is collected.
Final report generation
PRS are calculated and included in the report together with newly updated information about measurements and diagnoses. Consultants print the report.
ROR visit continues: face-to-face (F2F) counseling
The participant meets with a consultant who introduces the report, explains the meaning of the results, and answers any questions. Up to this point the participant can opt out from ROR. A printout of the report is given to the participant. In the presence of clinically actionable high-impact genetic variants, a second visit is scheduled to follow up after an independent validation of the finding using the newly acquired blood sample.
The consultants include a total of 8 different individuals - medical doctors, a genetic counselor, and two genetic counseling trainees. The F2F consultations are semi-structured and last 30 min on average.
Participant feedback surveys
After the visit, participants receive an e-mail reminder to provide feedback on the visit and information received. For long-term follow-up, the participants are asked to fill out a survey 6 months after ROR.
Sections of the report
PRSs for common complex traits
The joint effect of SNPs describing genetic predisposition for a disease is modelled using polygenic risk scores (PRS) which are in essence weighted sum of allele dosages of a selected subset of SNPs. PRS were calculated for type 2 diabetes (T2D), coronary heart disease (CHD) and early (before 45 years of age) menopause. The PRS for T2D included 7502 independent SNPs and was calculated using double weighting [22]. Weights were taken from a meta-analysis of T2D studies [23]. PRSs for CHD were calculated using ~46,000 non-palindromic SNPs, with weights taken from stage 2 of the CARDIoGRAMplusC4D Consortium meta-analysis [24]. The PRS for early menopause was based on 740 independent SNPs taken from a meta-analysis of studies of the age at menopause [25]; a threshold of p < 5 * 10−4 and regression coefficients from the same study were used as weights. The risk scores’ predictive characteristics in the EstBB database were checked before returning results to participants.
All PRSs were presented as in the sample report (Fig. S1), where the top quintile of the PRS distribution was considered as high risk. Additionally, for T2D, the report includes a graph illustrating the absolute disease risk as a function of age and provides an overall disease risk calculation that includes the weight, waist-to-height ratio, existing hypertension, and current smoking as modifiable risk factors.
Moderate risk factors and carrier screening
Report sections on adult-type hypolactasia and exfoliative glaucoma were based on the genotypes of the rs4988235 and rs2165241 variants, respectively. Individuals carrying two risk alleles were deemed to be at increased risk for these traits. A section on thrombophilia was based on common moderate risk factors in F5 (p.R534Q, rs6025) and F2 (c.*97 G > A, rs1799963). Report sections on carrier screening included the most frequent pathogenic allele for Wilson disease [p.H1069Q (rs76151636) in ATP7B] and for cystic fibrosis [p.F508del (rs113993960) in CFTR]. As only a limited number of findings were validated, the reports included comments that participants’ carrier status should be re-confirmed before the implementation of reproductive planning.
High-impact variants
We extracted all coding variants for genes specified in the gene list of the American College of Medical Genetics and Genomics Secondary Findings v2.0 [26]. These variants were cross-referenced with the ClinVar dataset [27] for restriction to those with likely or known pathogenic classifications and minor allele frequencies <0.5% in the gnomAD database [28]. For additional estimation of the pathogenicity of expected pathogenic variants, up to 12 different in silico prediction algorithms were used, as described previously [15]. All high-risk variants considered for ROR were validated with Sanger sequencing. The ROR procedure of high-impact variants involved an additional F2F visit and referral to a medical geneticist, as described previously [15].
Pharmacogenomics
Details of the translation of genotype data into pharmacogenomic recommendations are provided elsewhere [29]. The developed pipeline involves the creation of pharmacogenetic reports for biobank contributors using whole-genome sequencing, exome sequencing, and/or genotyped and imputed microarray data as input. Genotype data for 11 clinically important pharmacogenes (CYP2C19, CYP2C9, CYP2D6, CYP3A5, CYP4F2, DPYD, IFNL3, SLCO1B1, TPMT, UGT1A1, and VKORC1) are phased, and for the translation to relevant pharmacogenetic alleles, we used allele definition tables from the Pharmacogenomics Knowledge base [30] and the prescription guidelines of the Clinical Pharmacogenetics Implementation Consortium [31]. The pipeline first defines the nonfunctional star alleles that override other alleles and then tests for the remaining pharmacogenetic alleles [29]. We provided a summary report of recommendations for up to 32 medications for each participant, and longer drug-specific guidelines that participants could share with their physicians (Fig. S2).
Participant feedback surveys
Participants’ feedback was collected using three surveys that were developed based on findings from analogous previous studies [15, 16, 32–34]. The first survey was provided to the participants during registration, it included questions about the participant’s emotional state and expectations. After the ROR visit, each participant received an e-mail with a link to the participant portal, where the second survey was available for them to fill out within 4 weeks. The survey included questions about the participant’s satisfaction with and psychological responses to the information received. The third survey was provided with a reminder e-mail 6 months after ROR visitation, included questions about ROR-related decision regret, perceived personal control and coping, psychological adjustment, communication, and support, as well as reported health behavior and healthcare utilization. Participants who had not returned previous surveys could respond to current surveys.
Statistical analyses of the survey results were conducted using SAS software version 9.4. (SAS Institute Inc.). The chi-square test with Bonferroni correction was used to examine the effect of ROR with counseling. An ordinary logistic regression model was used to estimate the effect of receiving risk information on the emotional response of participants. In the analysis of a subset of the participants reporting uncomfortable feelings, participants who responded “agree” or “somewhat agree” to feeling worried, tense and upset were defined as “worriers”. The binary logistic regression was used to estimate the risk of “worriers” vs “non-worriers”.
Results
Cohort
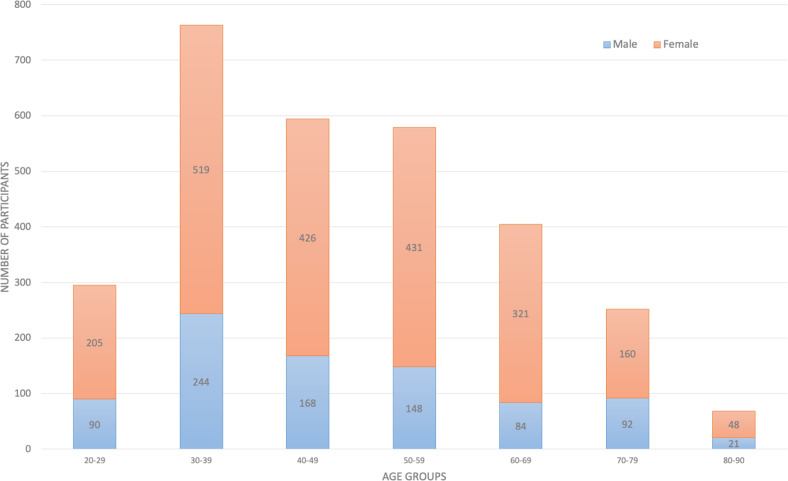
Over the 30-month project period, 9325 i.e. approximately 18% of the eligible 51,000 individuals had entered the portal and 2957 (6% of 51,000) individuals received results with F2F counseling at two locations: Tartu (n = 2163) and Tallinn (n = 794). The age and gender distributions of these participants were similar to those for the entire biobank cohort, described previously [19]. All age groups were represented, and 71% of the participants were female (Fig. 2). As of 2020, 13 of the 2957 participants had died.
Fig. 2. Age and gender distribution of participants receiving ROR.
ROR return of individual genomic results.
Findings reported
The reports varied depending on the genotype data available for given participants; not all participants received results from all sections (Table 1). The majority of participants received PRSs for T2D and CHD, as well as data on pharmacogenomics and moderate risk factors such as lactose intolerance. Information on high-impact variants and carrier status required NGS data and was thus available for a minority (22%) of the participants.
Table 1.
Findings reported and proportions of participants receiving high-risk information.
| Section of the report | How many received this info | High risk reported | ||
|---|---|---|---|---|
| N | % | N | % | |
| PRS and overall risk for T2D | 2916 | 98.6 | 569** | 19.5 |
| PRS for CHD and MI | 2916 | 98.6 | 559 | 19.2 |
| Early onset menopause PRS* | 2080 | 70.3 | 443 | 21.3 |
| Thrombophilia (F5, F2) | 2532 | 85.6 | 125 | 4.9 |
| α1-antitrypsin deficiency | 2531 | 85.6 | 92 | 3.6 |
| Carrier (cystic fibrosis) | 653 | 22.1 | 11 | 1.7 |
| Carrier (Wilson disease) | 2279 | 77.1 | 25 | 1.1 |
| Lactose intolerance associated genotype | 2956 | 100 | 786 | 26.6 |
| Exfoliation glaucoma risk | 2956 | 100 | 821 | 27.8 |
| ACMG findings (HBOC, CRC) | 666 | 22.5 | 22 | 3.3 |
| Pharmacogenomics | 2894 | 97.9 | 2834 | 97.9 |
*Women only; **T2D PRS only
PRS polygenic risk score, T2D type 2 diabetes, CHD coronary heart disease, MI myocardial infarction, F5 factor V, F2 factor II, ACMG American College of Medical Genetics and Genomics, HBOC hereditary breast and ovarian cancer, CRC colorectal cancer.
Survey results
Responders
Overall, 2905 (98%) participants completed the first survey, 41% responded to the post-visit survey, and 31% responded to the survey distributed at 6 months. Similar to the overall ROR cohort, 70% of responders were female. Response rates did not differ between genders. Although participants in all 10-year age groups responded, response rates were highest for those aged 40–79 years (42–51% after ROR visits and 33–36% after 6 months) and lower for those aged <40 and >79 years (28–36% after ROR visits and 22–27% after 6 months).
General assessments of reports
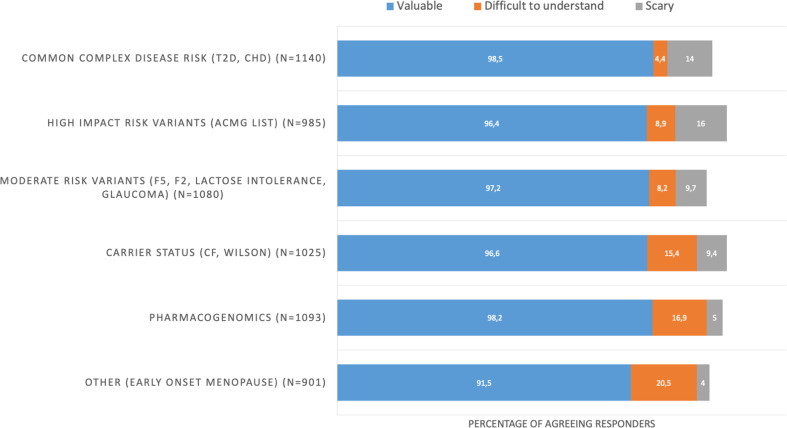
Overall, over 91% of responders valued all report sections, with small differences between sections (Fig. 3). The early-onset menopause and common complex trait PRSs were considered to be difficult to understand by 20.5% and 4.4% of responders, respectively. Some sections of the report were considered to be scary by 5–16% of responders (common complex traits, 14%; high-impact variants, 16%; pharmacogenomics, 5%).
Fig. 3. Report sections considered to be valuable, difficult to understand, and scary as reported by participants shortly after ROR.
ROR return of individual genomic results.
Emotional responses to ROR
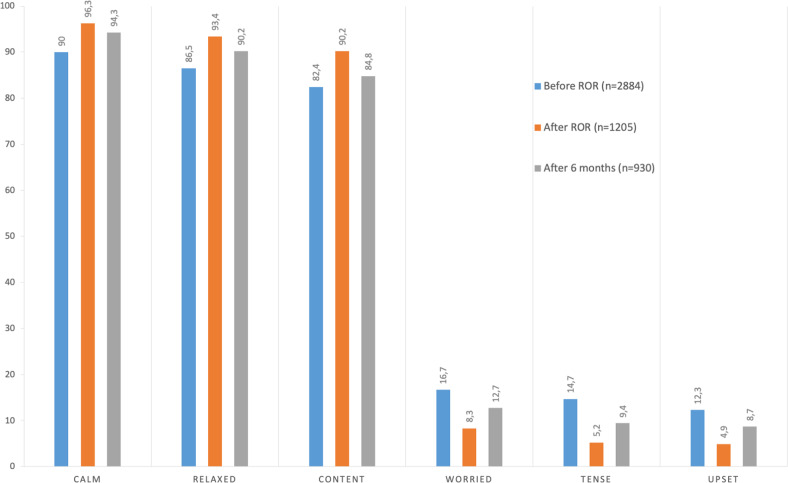
Responders tended to feel calm, relaxed, and content before, immediately after, and 6 months after ROR (Fig. 4). They tended to respond in the negative to questions about whether they were feeling worried, tense, and upset. The proportions of participants feeling calm, relaxed, and content increased significantly immediately after ROR (p < 0.05). After 6 months, responses were more mixed, but the proportions of responders feeling calm and relaxed remained significantly larger than before ROR (p < 0.05). Conversely, the proportions of participants feeling worried, tense, and upset decreased significantly immediately after ROR (p < 0.05); although these differences were somewhat smaller after 6 months, they remained significant compared with before ROR (p < 0.05).
Fig. 4. Proportions of participants reporting positive (calm, relaxed, content) and uncomfortable feelings (worried, tense, upset) before and immediately after ROR and 6 months later.
ROR return of individual genomic results.
One-third of the participants that commented on most appreciated aspect of ROR mentioned enjoyable and understandable communication as a positive experience (Table S3). Common themes on the least enjoyable aspects involved receiving high-risk information, the reports not being as extensive as expected, or not containing information in specific areas of interest.
Responses to specific report content
Overall, 559 and 569 participants received results that contained high PRSs for CHD or T2D, respectively. The latter had no effect on feelings reported immediately or 6 months after ROR. High PRSs for CHD were associated with feeling more worried (odds ratio (OR) = 1.47), tense (OR = 1.75), and upset (OR = 1.53), and less calm (OR = 0.65), relaxed (OR = 0.68), and content (OR = 0.76) immediately after ROR (Table S1). These effects were no longer significant after 6 months.
In total, 125 participants received information about moderate risk variants, such as thrombophilia. Risk reporting was associated with feelings of worry (p = 0.02) and upset (p = 0.04) immediately after ROR. After 6 months, these effects were no longer observed. Twenty-two participants received information about carrier status for high-impact variants. This risk information was not associated with any difference in response immediately after ROR compared with participants who did not receive high-impact risk variant information.
A total of 666 participants had a section on high-impact risk variants in their reports. This was associated with a content feeling in the long term (OR = 1.49; 95% confidence interval, 1.13–2.00). This effect was not seen for other feelings or immediately after ROR. Similarly for moderate risk factors such as thrombophilia, participants reported feeling more content after 6 months than did participants who did not receive such information in their report (n = 342, p = 0.04). Participants appreciated ROR and viewed it as valuable, although it could be worrying and upsetting.
Worries and regrets
The proportions of “worriers” was greatest before ROR [n = 197 (6.83%)] and smaller immediately after ROR [n = 30 (2.49%)] and after 6 months [n = 44 (4.73%)]. Being a worrier before ROR was associated with hypertension and age (Table S2), and was the only significant risk factor for being a worrier immediately and 6 months after ROR.
Six months after ROR, the majority of responders considered their decision to receive ROR to be right (92% of 930 individuals) or wise (86%), and 92% reported that they would make the same decision again. The majority of responders did not think that the decision caused them harm (96%) and had no regrets (95%). Only 10 individuals reported feeling regret, and 8 individuals felt that the ROR decision caused them harm (Fig. S3).
Discussion
The EstBB offered a range of individual genetic results to biobank contributors expressing interest with the goal of learning from the process and assessing the possibility of offering ROR on large-scale i.e. to all interested participants. Over a 30-month period, 2957 participants received results. Although the receipt of information on high-risk conditions was sometimes associated with uncomfortable feelings, these were not long-lasting and the participants generally considered ROR to be valuable, understandable, and not scary. They expressed that they would have liked to receive more information. The greatest bottleneck to providing ROR to all interested biobank contributors was the limited resources for providing F2F counseling. Besides lessons learned during the process of ROR to interested biobank participants, we identified multiple areas that need further exploration (Box 1).
Box 1 Summary of lessons learned in setting up and carrying out broad ROR at EstBB.
| Would recommend | Would do differently | Needs exploration | |
|---|---|---|---|
| Regulations and consent | Right for ROR was included in EstBB’s initial recruitment consent and in the biobank legislation before recruitment began in 2002. Separate consent was created for ROR, specifying the approach and types of results offered. | As the biobank was established decades ago, the initial consent form was missing specific plans regarding ROR. Ideally, a description of the approach and types of results potentially offered would be provided at the time of recruitment. | Participants were unable to select from ROR categories. What would their preferences be if this were an option? What would the long-term consequences be? |
| Modes of communication | Use of an online interface (participant portal) for consent provision, data collection, and scheduling. Great means for participant engagement and bidirectional information flow. | Exclusively digital signing of the consent form could limit ROR access in a population-based cohort with a wide range of demographic characteristics. Ideally, other options should be available. | If future communication occurred primarily through the participant portal, how would this approach impact ROR participation or risk perception? |
| Report and results offered | A wide range of results offered. In addition to rare clinically significant findings, PRSs for common complex diseases and pharmacogenomic information were included to make results available to the majority of interested participants. A printed copy of the report was provided to each participant. |
The reporting of different sets of results depending on the datasets available created confusion and frustration among participants. Participants expressed interest in receiving electronic copies of the report as well. |
If the report evolves over time, should new amendments be added to it and be available to participants directly through the participant portal? Should alerts about updates be sent? |
| Counseling |
Participants valued F2F counseling sessions highly. Semi-structured genetic counseling was offered by a variety of specialists, likely reflective of the mainstreaming of genetic counseling. |
F2F counseling created a significant bottleneck due to the lack of specialists available. EstBB has a nationwide cohort, but ROR was offered in only two locations because of the use of F2F counseling. |
If tele-genetic counseling were offered as an alternative to F2F counseling, what proportion of participants would be interested? |
| Participant feedback and follow-up | Collection of participant feedback for the examination of ROR responses and consideration of participant preferences in the future. | The offering of a wide range of results simultaneously complicated the analysis of responses. | The impact of and response to different forms of results presentation needs to be investigated further. |
| Translation | Pre-established collaboration with clinical partners enabled the smooth transfer of participants with high-impact clinically significant findings from the research to the healthcare setting. | Health-care workers should be engaged early, as they might be approached with the report, and unclear roles and responsibilities can create fear and tension. | How can therapeutic misconceptions best be avoided or corrected, when EstBB is involved with several personalized medicine feasibility studies in clinical settings? |
| Educational needs | ROR can be seen as an opportunity to improve participants’ overall genetic literacy. | Transparency is needed to assure realistic expectations; information about the logic underlying ROR projects and what types of results to expect is particularly necessary when ROR-related consent and information are vague at the time of recruitment. | The extent to which ROR increases overall genetic literacy needs to be explored further. Could additional online educational efforts related to selected basic genetic knowledge aid the understanding and practical usability of ROR? |
Responses to ROR
Concern has been raised about the potential harmful effect of ROR for biobank populations, as genetic information can be difficult to understand or scary. The majority of responders in our cohort considered all sections of the report to be valuable, and only a minority considered some report sections to be difficult to understand or scary. On average, participants tended to feel calm, relaxed, and content, not worried, tense, or upset; most participants stated that they would opt for ROR again, with only 1% expressing regret or considering the process to be harmful. A minority of responders reported feeling uncomfortable, primarily before ROR. Individuals with health concerns and younger individuals in our cohort tended to be more nervous before ROR, and pre-ROR anxiety increased the likelihood of uncomfortable feelings after ROR. This could reflect fear of the unknown or the expectation of receiving uncomfortable risk information. More detailed information on the ROR process and potential results would help to alleviate such anxiety, highlighting the importance of having an option for genetic counseling during ROR, as some participants may need more support than others.
ROR with counseling had a significant positive effect on the feelings that responders reported, most strongly directly thereafter. The same effect was reflected in their feedback comments praising the F2F counselling. Other comments of the participants suggest that uncomfortable feelings before ROR are attributable to high expectations about the receipt of genetic risk information or answers to specific concerns based on family histories. These unmet expectations highlight the need for more transparency and education about the main objective of a biobank, the type of results that most research projects yield, and the ethical and legal considerations addressed before ROR. All of these factors influence the ROR approach and the number and type of results that can be offered.
Whereas our previous projects involved ROR for single risk-related genetic findings, the reports provided in the current project contained a range of results. Considering that risk information can be perceived differently depending on the severity of the condition, level of risk, or actionability, the most responsible and effective communication approach may vary among contexts [35]. Indeed, the response involving uncomfortable feelings seems to be related to specific findings, communication or the presentation of results, and the personalities of the participants. The presentation of results provided to our cohort varied, depending on the report section and specific finding. In most cases, we provided recommendations on how to reduce the overall risk. For T2D, however, the impact of the participant’s weight and hypertension on their overall risk was also illustrated graphically. This difference from the CHD reporting may explain the significant impact that high CHD PRSs had on the feelings of the participants after ROR. Communication about preventive measures and actionability are known to influence the attitudes of participants toward the receipt of risk information [6]. Participants appreciated recommendations on how to reduce risks, which give them a sense of control and feeling of contentment. This information is valuable for planning risk communication in future ROR projects and consideration of alternative modes of ROR. These findings are in agreement with the results of previous EstBB and other ROR projects, in which participants valued the information that they received, even though some reported uncomfortable feelings [14, 15, 17].
Consideration of the participant-initiated approach to ROR
There is widespread public interest in the receipt of personal genetic information [8, 13]. Although this interest may be related mainly to potential health benefits, the public is interested in receiving results beyond those with clinical utility [8], and this expectation is also increasing among biobank participants. Previous ROR projects have focused on clinically significant findings [14–16]. As high-impact clinical findings are relatively rare, this approach means that genetic reporting is not conducted for the majority of a given cohort. The goal of offering findings to anyone expressing interest requires the inclusion of a wider variety of results. We witnessed that the interest in receiving results was greater than our capabilities, and when receiving ROR, the majority of participants expressed interest in receiving even more results.
The EstBB and the legislation governing it have several advantages in terms of broad-scale ROR feasibility. For example, some biobanks work with anonymized data and are not able or allowed to recontact study participants. Nevertheless, this approach will be challenging in the long term. This pilot study was conducted with the first 51,000 contributors, who were enrolled in the biobank before 2017. Although all participants were familiarized with the project, consented to participation, updated their phenotypic data, and gave feedback to the biobank through the participant portal, ROR with F2F counseling was offered in only two locations, which created a major bottleneck. The biobank cohort has since grown by another 150,000 individuals, increasing the demand for ROR. Ongoing research and the constantly growing knowledge base are expanding the list of results that can be offered to participants beyond clinically relevant, actionable findings. These factors necessitate the use of alternative means of communication (i.e., digital) to improve access, reduce waiting times, and enable the continuity of ROR [36]. A recent Finnish study explored the response of participants to the provision of CHD risk information through an interactive web tool [37]. Although one-third of responders reported receiving concerning information, almost 90% thought that the information was understandable and useful, and motivated them to engage in positive health behaviors. Offering ROR through a participant portal is one alternative that could increase availability and access. New results could be added continuously, and participants could update their phenotypic data and provide feedback about their experience on the platform. Ideally, different options would be available to accommodate individuals who lack technological skills or would benefit from a conversation with a counselor.
Conclusions and future perspectives
Here, we describe an approach for offering ROR to a population of interested biobank contributors, and their responses to a variety of results. The overall response to ROR was positive, and participants commonly expressed interest in receiving more results. Due to the timeframe and funding available for this study, access to ROR was limited to 3000 participants. To better balance participants’ interest in and the feasibility of ROR [11], alternative modes of communication should be considered. As ROR is likely to become an integral activity for population-based biobanks, ROR initiatives with different approaches need to be shared, including the experiences of participants and research staff, to facilitate learning and define best practices.
Biobanks and the healthcare sector face challenges related to the communication of genetic risk information. Responses from participants who receive results inform future plans for the provision of genomic risk information in a range of healthcare settings, and decision making about when F2F counseling is required and when other modes of communication should be considered. The best means of communication will vary depending on the type of results provided and the traits addressed. Furthermore, ROR to biobank participants offers a testbed for piloting opportunistic screening and for cost-benefit analysis of such an approach. Ultimately, ROR of clinically relevant findings should be fully incorporated in healthcare settings, thus allowing biobanks to remain strictly as research facilities and discovery platforms. Additionally, biobanks could offer participants information of other value (e.g., personal utility, guiding better life-style choices), thereby engaging them in research, improving collaborative efforts, and increasing trust in biobanks and science in general [10].
Supplementary information
Author contributions
(CRediT statements). LL: Conceptualization, Formal analysis, Investigation, Writing - Original Draft, Visualization. AR: Methodology, Formal analysis, Investigation, Writing - Review & Editing, Visualization, Project administration. MP: Methodology, Investigation. TN: Methodology, Validation, Writing - Review & Editing. KL: Methodology. KK: Methodology. SR: Methodology. RM: Methodology. MK: Methodology. HA: Methodology, Investigation, Project administration. MN: Investigation, Writing - Review & Editing. AK: Investigation. IN: Investigation. MLT: Data Curation. EK: Formal analysis. MP: Methodology, Software. KM: Methodology, Software. AA: Methodology, Project administration. LM: Methodology, Writing - Review & Editing, Supervision, Funding acquisition. KF: Methodology, Formal analysis, Visualization, Writing - Review & Editing, Supervision, Funding acquisition. NT: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing - Review & Editing, Supervision, Funding acquisition. AM: Conceptualization, Resources, Writing - Review & Editing, Supervision, Funding acquisition.
Funding
This research was supported by the European Union through the European Regional Development Fund (project no. 2014-2020.4.01.15-0012 and SLTAT16148T/TK148) and by Personal Research Funding grants from the Estonian Research Council (PRG555, PRG1095, PRG184, PRG1197).
Data availability
Data mentioned in the paper can be found within the published article and its supplementary files, and additional data generated or analysed during this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The ROR procedure, from registration to feedback collection, including the informed consent procedure, was approved by the ethics committee of the University of Tartu (no. 271/T-22). Informed consent was obtained from all participants as required by the REC. The biobank has been described previously [19] and more details about the biobank can be found at the biobank’s website https://genomics.ut.ee/en/content/estonian-biobank.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Neeme Tõnisson, Andres Metspalu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-022-01196-6.
References
- 1.de Wert G, Dondorp W, Clarke A, Dequeker EMC, Cordier C, Deans Z, et al. Opportunistic genomic screening. Recommendations of the European Society of Human Genetics. Eur J Hum Genet. 2021;29:365–77. doi: 10.1038/s41431-020-00758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis ACF, Knoppers BM, Green RC. An international policy on returning genomic research results. Genome Med. 2021;13:115. doi: 10.1186/s13073-021-00928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics. 2008;36:219–48. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budin-ljøsne I, Mascalzoni D, Soini S, Machado H, Kaye J, Bentzen HB, et al. Feedback of individual genetic results to research participants: is it feasible in Europe? Biopreserv Biobank. 2016;14:241–8. doi: 10.1089/bio.2015.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West KM, Blacksher E, Cavanaugh KL, Fullerton SM, Umeukeje EM, Young BA, et al. At the research-clinical interface: returning individual genetic results to research participants. Clin J Am Soc Nephrol. 2020;15:1181–9. doi: 10.2215/CJN.09670819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton A, Morley KI, Bragin E, Firth HV, Hurles ME, Wright CF, et al. Attitudes of nearly 7000 health professionals, genomic researchers and publics toward the return of incidental results from sequencing research. Eur J Hum Genet. 2015;24:1–9. doi: 10.1038/ejhg.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vears DF, Minion JT, Roberts SJ, Cummings J, Machirori M, Blell M, et al. Return of individual research results from genomic research: a systematic review of stakeholder perspectives. Plos One. 2021;16:e0258646. doi: 10.1371/journal.pone.0258646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins CH, Mapes BM, Jerome RN, Villalta-Gil V, Pulley JM, Harris PA. Understanding what information is valued by research participants, and why. Health Aff. 2019;38:399–407. doi: 10.1377/hlthaff.2018.05046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facio FM, Brooks S, Loewenstein J, Green S, Biesecker LG, Biesecker BB. Motivators for participation in a whole-genome sequencing study: implications for translational genomics research. Eur J Hum Genet. 2011;19:1213–7. doi: 10.1038/ejhg.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcguire AL, Majumder MA, Villanueva AG, Bardill J, Juli M, Boerwinkle E, et al. Importance of participant-centricity and trust for a sustainable medical information commons. J Law Med Ethics. 2019;47:12–20. doi: 10.1177/1073110519840480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botkin JR, Mancher M, Busta ER, Downey AS. Returning individual research results to participants: guidance for a new research paradigm. Washington, DC: The National Academies Press; 2018. [PubMed]
- 12.Milne R, Morley KI, Almarri MA, Atutornu J, Baranova EE, Bevan P, et al. Return of genomic results does not motivate intent to participate in research for all: perspectives across 22 countries. Genet Med. 2022;24:1120–9. doi: 10.1016/j.gim.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Sotsiaalministeerium. Eesti elanike teadmised, hoiakud, kartused ja ootused personaalmeditsiini osas Uuringuaruanne Kevad 2015 [Internet]. Sotsiaalministeerium. 2015. Available from: http://sm.ee/et/personaalmeditsiini-juhtprojekti-eeluuring.
- 14.Alver M, Palover M, Saar A, Läll K, Zekavat SM, Tõnisson N, et al. Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia. Genet Med. 2018;21:1173–80. [DOI] [PMC free article] [PubMed]
- 15.Leitsalu L, Palover M, Sikka TT, Reigo A, Kals M, Parn K, et al. Genotype-first approach to the detection of hereditary breast and ovarian cancer risk, and effects of risk disclosure to biobank participants. Eur J Hum Genet. 2021;29:471–81. [DOI] [PMC free article] [PubMed]
- 16.Leitsalu L, Alavere H, Jacquemont S, Kolk A, Maillard AM, Reigo A, et al. Reporting incidental findings of genomic disorder-associated copy number variants to unselected biobank participants. Per Med. 2016;13:303–14. [DOI] [PubMed]
- 17.Haukkala A, Kujala E, Alha P, Salomaa V, Koskinen S, Swan H, et al. The return of unexpected research results in a biobank study and referral to health care for heritable long QT syndrome. Public Health Genomics. 2013;16:241–50. doi: 10.1159/000354105. [DOI] [PubMed] [Google Scholar]
- 18.Blout Zawatsky CL, Shah N, Machini K, Perez E, Christensen KD, Zouk H, et al. Returning actionable genomic results in a research biobank: analytic validity, clinical implementation, and resource utilization. Am J Hum Genet. 2021;12:2224–37. [DOI] [PMC free article] [PubMed]
- 19.Leitsalu L, Haller T, Esko T, Tammesoo M-L, Alavere H, Snieder H, et al. Cohort profile: Estonian biobank of the Estonian genome center, university of Tartu. Int J Epidemiol. 2015;44:1137–47. [DOI] [PubMed]
- 20.Riigikogu. Human Genes Research Act [Internet]. 2000 [cited 2014 Jun 27]. Available from: https://www.riigiteataja.ee/en/eli/531102013003/consolide.
- 21.Leitsalu L, Alavere H, Tammesoo M, Leego E, Metspalu A. Linking a population biobank with national health registries — The Estonian experience. J Pers Med. 2015;5:96–106. doi: 10.3390/jpm5020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Läll K, Mägi R, Morris A, Metspalu A, Fischer K. Personalized risk prediction for type 2 diabetes: the potential of genetic risk scores. Genet Med. 2017;19:322–9. doi: 10.1038/gim.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–90. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham G, Havulinna AS, Bhalala OG, Byars SG, De Livera AM, Yetukuri L, et al. Genomic prediction of coronary heart disease. Eur Heart J. 2016;37:3267–78. doi: 10.1093/eurheartj/ehw450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47:1294–303. doi: 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. ACMG Statement Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2. 0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017;19:249–55. [DOI] [PubMed]
- 27.Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–7. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisberg S, Krebs K, Lepamets M, Kals M, Mägi R, Metsalu K, et al. Translating genotype data of 44,000 biobank participants into clinical pharmacogenetic recommendations: challenges and solutions. Genet Med. 2019;21:1245–54. [DOI] [PMC free article] [PubMed]
- 30.Barbarino JM, Whirl-Carrillo M, Altman RB, Klein TE. PharmGKB: a worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018;10:e1417. doi: 10.1002/wsbm.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharm Ther. 2011;89:464–7. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray SW, Martins Y, Feuerman LZ, Bernhardt BA, Biesecker BB, Christensen KD, et al. Social and behavioral research in genomic sequencing: approaches from the Clinical Sequencing Exploratory Research Consortium Outcomes and Measures Working Group. Genet Med. 2014;16:727–35. doi: 10.1038/gim.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31:301–6. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 34.Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Med Decis Mak. 2003;23:281–92. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 35.Ormond KE, Hallquist MLG, Buchanan AH, Dondanville D, Cho MK, Smith M. Developing a conceptual, reproducible, Rubric-based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background. Genet Med. 2019;21:727–35. doi: 10.1038/s41436-018-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bombard Y, Hayeems RZ. How digital tools can advance quality and equity in genomic medicine. Nat Rev Genet. 2020;21:505–6. doi: 10.1038/s41576-020-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widén E, Junna N, Ruotsalainen S, Surakka I, Mars N, Ripatti P, et al. How Communicating Polygenic and Clinical Risk for Atherosclerotic Cardiovascular Disease Impacts Health Behavior: an Observational Follow-up Study. Circ Genom Precis Med. 2022;15:e003459. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data mentioned in the paper can be found within the published article and its supplementary files, and additional data generated or analysed during this study are available from the corresponding author upon reasonable request.