Abstract
Phytoliths are opal silica particles formed within plant tissues. Diatoms are aquatic, single-celled photosynthetic algae with silica skeletons. Phytolith and diatom morphotypes vary depending on local environmental and climatic conditions and because their silicate structures preserve well, the study of phytolith and diatom morphotypes can be used to better understand paleoclimatic and paleoenvironmental dynamics and changes. This article presents original data from an 820cm-deep stratigraphy excavated at the Hazen diatomite deposits, a high-elevation desert paleolake in the Fernley District, Northern Nevada, USA. The site has been studied for an assemblage of fossilized threespine stickleback, Gasterosteus doryssus, that reveal adaptive evolution. For this study, a total of 157 samples were extracted at 20 cm intervals covering approximately 24,500 years. After extraction, the samples were mounted on slides and viewed under 400-1000x light microscopy, enabling classification of 14 phytolith and 45 diatom morphotypes. Our data support paleoenvironmental reconstructions of the Hazen Miocene paleolake.
Keywords: Palaeoecology, Botany, Phytoliths, Diatoms, Paleontology
Specifications Table
| Subject | Ecology |
| Specific subject area | Palaeoecological reconstruction of Miocene paleolake local environment |
| Data format | Raw |
| Type of data | Table, Figure |
| Data collection | Sediment samples were extracted from Miocene paleolake diatomite deposits using metal chisels. The sediment was then ground with a needle tool in an 1.5ml eppendorf tube and mounted onto glass slides using a medium viscosity oil-based mounting agent and sealed with polymer nitrocellulose. Data was collected by brightfield optical microscopy using a Meiji MT4300L at 40x-100x magnification. Morphotypes were photographed using a Meiji Techno HD1500T camera. Morphotypes were identified via comparison to published literature. |
| Data source location | Institution: Loyola University Chicago • City/Town/Region: Chicago • Country: USA • Latitude and longitude for collected samples/data: -119.18379, 39.496 (WGS84) |
| Data accessibility | Repository name: Figshare Repository DOI: 10.6084/m9.figshare.22715866 Direct URL to data: https://figshare.com/articles/figure/Botanical_Microfossil_Morphotypes_-_Hazen_Diatomite_Formation/22715866 |
1. Value of the Data
-
•
The fossil phytolith and diatom data can be used to reconstruct palaeoecological histories of local and regional vegetation, volcanic and fire activity, and other environmental variables.
-
•
Explainable changes in abundance and composition of ancient microfossil communities may help predict how modern life might respond to similar environmental change.
-
•
The paleoenvironment reconstruction may help explain observed adaptive evolution by the threespine stickleback fish (Gasterosteus doryssus) collected from the same stratigraphic sections.
2. Objective
This article presents original phytolith and diatom data from a currently high-elevation desert paleolake in Northern Nevada (Fernley District, USA) comprised of Miocene diatomite [1,2]. The samples were originally collected to study the fossilized threespine stickleback, Gasterosteus doryssus. 157 samples spanning approximately 24,500 years of stratigraphical deposition were extracted following a published protocol [3] to identify diatom and phytolith morphotypes. The objective of this study was to offer a new dataset for future study of paleoenvironmental and paleoclimatic contexts of paleolakes from the Hazen Miocene. Micrographs and morphological and identification details of phytolith and diatom morphotypes can be found in Fig. 1 and Table 1.
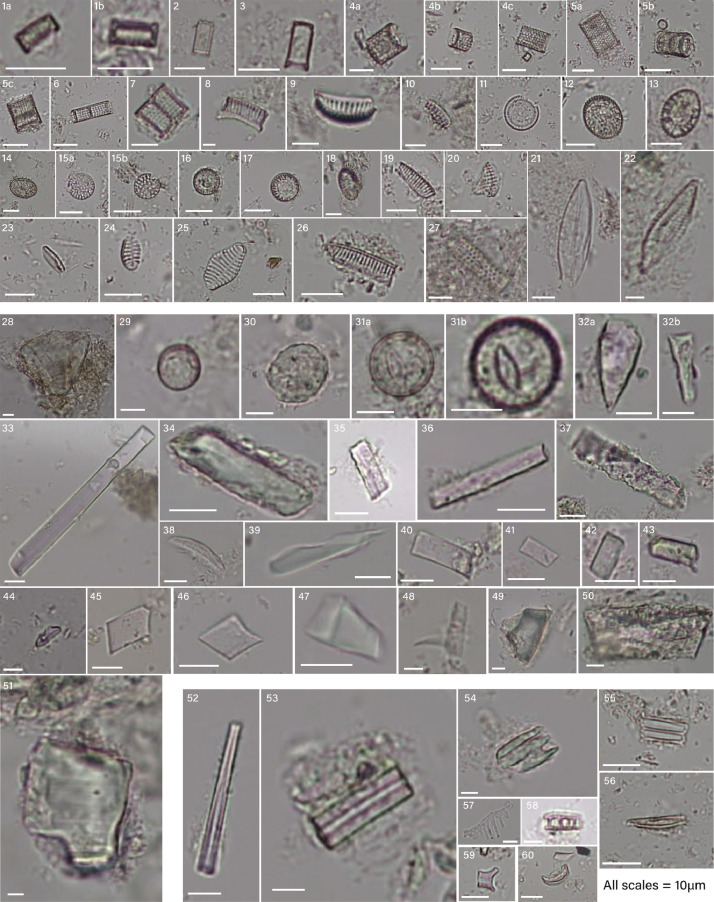
Fig. 1.
Paleontological microfossils, including diatoms, phytoliths, sponge spicules, and unidentified morphotypes. Diatoms: 1a-1b,2,3, unidentified centric; 4a-4c,6,7, Aulacoseira spp.; 5a-5c, Aulacoseira tenella; 8,10, Cymbella spp.; 9, Cymbella cymbiformis; 11,16, Lindavia rossii; 12,13,14,18, Pliocaenicus spp.; 15a-15b, Stephanodiscus spp.; 17, Cyclostephanos tholiformis; 19,20, Semiorbis spp.; 21,22, Craticula spp.; 23, Fragilariales spp.; 24, Geissleria spp.(?); 25, Punctastriata mimetica; 26, unidentified; 27, Aulacoseira subarctica. Phytoliths: 28, Poacae, Bulliform flabellate; 29,31a-31b, Broad-leaf/Conifer, Spheroid psilate; 30, Broad-leaf/Conifer, Spheroid ornate; 32a-32b, Panicoideae (?), Bilobate (fragmented); 33,34,35,36,39,43, unidentified, Elongate entire (Type 1); 37, unidentified, Elongate entire (Type 2); 38, unidentified, Elongate arcuate; 40,41,42,45,46, unidentified, Polygonal tabular; 44, Poacae, Rondel; 47,49, Broad-leaf/Conifer, Polyhedral (slereid); 48, Poacae, Acicular (hair-like); 49,50,51, Broad-leaf/Conifer, Blocky. Other: 52-53, sponge spicule fragments; 54-60, unidentified.
Table 1.
Phytolith, diatom and other microfossil morphotype descriptions and identifications.
| Microfossil type | Morphology | Identification | Fig. 1 Panel | References |
|---|---|---|---|---|
| Diatom | Araphid | Fragilaria vaucheriae | [5] | |
| Fragilariales spp. | 23 | [5] | ||
| Odontidium spp. | [5] | |||
| Pseudostaurosira brevistriata | [5] | |||
| Pseudostaurosira trainorii | [5] | |||
| Punctastriata mimetica | 25 | [15] | ||
| Staurosira construens binodis | [5] | |||
| Staurosira construens venter | [5] | |||
| Staurosirella leptostauron dubia | [5] | |||
| Staurosirella leptostauron | [5] | |||
| Staurosirella pinnata | [5] | |||
| Staurosirella spp. | [5] | |||
| Tetracyclus spp. | [5] | |||
| Asymmetric Biraphid | Amphora coffeaeformis | [5] | ||
| Amphora spp. | [5] | |||
| Cymbella cymbiformis | 9 | [12] | ||
| Cymbella spp. | 8,10 | [5] | ||
| Encyonema spp. | [5] | |||
| Gomphoneis spp. | [5] | |||
| Centric | unidentified | 1a-1b,2,3 | [5] | |
| Actinocyclus spp. | [5] | |||
| Alveophora americana | [18] | |||
| Aulacoseira spp. | 4a-4c,6,7 | [5] | ||
| Aulacoseira subarctica | 27 | [16] | ||
| Aulacoseira tenella | 5a-5c | [11] | ||
| Aulacoseira ambigua | [5] | |||
| Aulacoseira canadensis | [5] | |||
| Aulacoseira pusilla | [5] | |||
| Lindavia rossii | 11,16 | [13] | ||
| Pliocaenicus spp. | 12,13,14,18 | [5] | ||
| Stephanodiscus spp. | 15a-15b | [5] | ||
| Chaetoceros spp. | [5] | |||
| Semiorbis spp. | 19,20 | [5] | ||
| unidentified | 26 | [5] | ||
| Epithemioid | Epithemia musculus | [5] | ||
| Eunotioid | Geissleria spp. (?) | 24 | [5] | |
| Craticula spp. | 21,22 | [5] | ||
| Eunotia spp. | [5] | |||
| Semiorbis spp. | [5] | |||
| Monoraphid | Cocconeis spp. | [5] | ||
| Planothidium apiculatum | [5] | |||
| Planothidium delicatulum | [5] | |||
| Nitzschioid | Nitzschia fonticola | [5] | ||
| Surirelloid | Surirella amphioxys | [5] | ||
| Symmetric Biraphid | Anomoeneis spp. | [5] | ||
| Anomoeneis sculpta | [5] | |||
| Navicula spp. | [5] | |||
| Phytolith | Bulliform flabellate | Poaceae | 28 | [4] |
| Spheroid psilate | Broadleaf/Conifer | 29, 31a-31b | [4] | |
| Spheroid ornate | Broadleaf/Conifer | 30 | [4] | |
| Spheroid granulate | Broadleaf/Conifer | [4] | ||
| spheroid plicate | Broadleaf/Conifer | [4] | ||
| Bilobate | Panicoideae/Poaceae | 32a-32b | [4] | |
| Cross (polylobate) | Panicoideae/Poaceae | [4] | ||
| Elongate entire (Type 1) | unidentified | 33,34,35,36,39,43 | [17] | |
| Elongate entire (Type 2) | unidentified | 37 | [17] | |
| Elongate arcuate | unidentified | 38 | [4] | |
| Polygonal tabular | unidentified | 40,41,42,45,46 | [4] | |
| Rondel | Poaceae | 44 | [4] | |
| Polyhedral (sclereid) | Broadleaf/Conifer | 47,49 | [4] | |
| Acicular (hair-like) | Poaceae | 48 | [4] | |
| Blocky | Broadleaf/Conifer | 49,50,51 | [4] | |
| Tracheary annulate | unidentified | [4] | ||
| Other | Sponge spicule | unidentified | 52,53 | [4] |
| Undetermined | unidentified | 54,55,56,57,58,59, 60 |
N/A |
3. Data Description
The dataset includes 14 phytolith morphotypes and 45 identifiable diatom morphotypes >3µm. Phytolith morphotypes were described according to ICPN2.0 [4]. Phytoliths originated from both arboreal and grassland sources. Grassland morphotypes included bulliform flabellate (Fig. 1; 28), rondel (Fig. 1; 44), bilobates (Fig, 1; 32a-32b), and acicular (Fig. 1; 48) phytolith. Arboreal phytoliths included spheroids, both psilate (Fig. 1; 29, 31a-31b) and ornate (Fig. 1; 30), polyhedral sclereids (Fig. 1; 47,49), and blockies (Fig. 1; 49,50,51). Other phytolith morphotypes included elongate entire (Fig. 1; Type 1, 33,34,35,36,39,43; Type 2, 37), elongate arcuate (Fig. 1; 38), and polygonal tabulars (Fig. 1; 40,41,42,45,46).
Diatom morphotypes were evaluated based on the Database Diatoms of North America [5]. Diatoms were mostly centric and biraphid with some eunotioid and araphids present. Some centric diatoms were unidentifiable (Fig. 1; 1a-1b,2,3). Some were identifiable to the class or family level, including Aulacoseira spp. (Fig. 1; 4a-4c) and Stephanodiscus spp. (Fig. 1; 15a-15b), and some to the species level: A. tenella (Fig. 1; 5a-5c), L. rossii (Fig. 1; 11,16), C. tholiformis (Fig. 1; 17), and A. subarctica (Fig. 1; 27). Asymmetric Biraphid diatoms included Cymbella spp. (Fig. 1; 8,10) and C. cymbiformis (Fig. 1; 9). Symmetric Biraphid diatoms included Craticula spp. (Fig. 1; 21,22) and Geissleria spp. (Fig. 1; 24). Araphid diatoms were particularly small and were identified as Fragilariales spp. (Fig. 1; 24), with one species-level identification of P. mimetica (Fig. 1; 25). Finally, two Eunotioid diatoms were isolated, one non-identifiable (Fig. 1; 26) and the other identified as Semiorbis spp. (Fig. 1; 16,20). There were seven unidentified diatom morphotypes.
Other microremains included fragmented sponge spicules (Fig. 1; 52,53) and indeterminate microfossils (Fig. 1; 54,55,56,57,58,59,60).
4. Experimental Design, Materials and Methods
4.1. Experimental design
The ‘Bot-Meps’ Protocol [3] was followed for the sampling and slide preparation processes used to develop the presented dataset. Here is a brief summary of the major steps:
-
(1)
A 5mm wide chisel was used to outline a 1 cm × 1 cm section as a sampling region. This was done in a fume hood onto a protective surface.
-
(2)
The same chisel was used to separate the 1 × 1 cm sample from the rock matrix, at depths ranging from 2-4 cm, depending on the thickness of the specimen.
-
(3)
The sample was placed in 1.5 mL Eppendorf tubes and ground to a fine powder using a needle tool. Between each sample the tools used were cleaned to prevent cross-contamination.
-
(4)
Resulting powder was mounted onto glass slides with medium viscosity mounting oil and sealed.
-
(5)
Slides were analyzed using a brightfield optical microscope with 40 and 100 x objective le5ses and 10 x eye lenses. Microphotographs were achieved using a Meiji Techno HD1500T microscope camera.
4.2. Materials
Diatomite samples came from Pit L, Quarry D, of the Hazen Diatomite Deposits, a 10.3 Myo Miocene paleolake (Fig. 2) from Northern Nevada [6,7]. Phytoliths and diatoms were extracted from diatomite samples at 20 cm intervals over 820 cm of section that captured adaptive evolution by G. doryssus [6], [8], [9], [10].
Fig. 2.
Location of the study area (c) within the state of Nevada (b) in the United States of America (a).
4.3. Methods
The samples were extracted and prepared following the ‘Bot-MEPS’ Protocol [3], though because our samples were free of carbonates and organic residues (Cerasoni, unpublished data) we did not follow the steps to remove those residues, nor did we need the heavy liquid flotation separation technique. The resulting ground samples were analyzed by brightfield optical microscopy at 40x and 100x magnification. The identification of each morphotype was carried out by matching with a high degree of confidence size, shape, surface texture and unique features to previously published databases and standards [11], [12], [13], [14], [15], [16], [17], [18]. All microfossils that did not match any known published diatom or phytolith morphotype were recorded as unidentified, but still presented here.
Ethics Statement
All authors have read and follow the ethical requirements for publication in Data in Brief and confirming that the current work does not involve human subjects, animal experiments, or any data collected from social media platforms.
CRediT authorship contribution statement
Jacopo Niccolò Cerasoni: Conceptualization, Investigation, Methodology, Software, Data curation, Visualization, Writing – original draft, Writing – review & editing. Megan C. O'Toole: Data curation, Methodology, Software, Writing – original draft. Richa Patel: Data curation, Methodology, Software. Yoel E. Stuart: Conceptualization, Investigation, Writing – review & editing.
Acknowledgments
Limitations
Not applicable.
Acknowledgements
We thank Dr. Michael A. Bell, Dr. Patricia Holroyd, and Joseph Schluep for their expertise and assistance throughout all aspects of our study and for their help in accessing, understanding and having the proper equipment to work on the studied assemblage. This project was funded in part by a National Science Foundation CAREER award to Y.E.S. (EAR 2145830).
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jacopo Niccolò Cerasoni, Email: jcerasoni@luc.edu.
Yoel E. Stuart, Email: ystuart@luc.edu.
Data Availability
References
- 1.Bell M.A., Sadagursky M.S., Baumgartner J.V. Utility of lacustrine deposits for the study of variation within fossil samples. Palaios. 1987:455–466. [Google Scholar]
- 2.Houseman M.D. Proceedings of the 39th Forum on the Geology of Industrial Minerals: Nevada Bureau of Mines and Geology Special Publication. Vol. 33. 2004. Late Miocene diatomite formation near Fernley, Nevada, Betting on industrial minerals; pp. 142–152. [Google Scholar]
- 3.O’Toole M.C., Stuart Y.E., Cerasoni J.N. Botanical Microfossil Extraction from Paleontological Sediments - Bot-MEPS’ Protocol, protocols.io. 2022 doi: 10.17504/protocols.io.bp2l69kz5lqe/v2. [DOI] [Google Scholar]
- 4.International Committee for Phytolith Taxonomy (ICPT) International code for phytolith nomenclature (ICPN) 2.0. Ann. Botany. 2019;124(2):189–199. doi: 10.1093/aob/mcz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaulding, et al. Diatoms.org: supporting taxonomists, connecting communities. Diatom Research. 2021;36(4):291–304. doi: 10.1080/0269249X.2021.2006790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell M.A., Travis M.P., Blouw D.M. Inferring natural selection in a fossil threespine stickleback. Paleobiology. 2006;32(4):562–577. doi: 10.1666/05026.1. [DOI] [Google Scholar]
- 7.J.N. Cerasoni, M.A. Bell, Y.E. Stuart, Geomorphology of Miocene diatomite deposits from Hazen, Nevada, with stratigraphy of a stickleback (Gasterosteus doryssus) fossil fish sequence. PaleoBios (in review).
- 8.Gene H., Bell M.A., Travis M.P. Evolution toward a new adaptive optimum: phenotypic evolution in a fossil stickleback lineage. Evolution. 2008;62(3):700–710. doi: 10.1111/j.1558-5646.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 9.Stuart Y.E., Travis M.P., Bell M.A. Inferred genetic architecture underlying evolution in a fossil stickleback lineage. Nature Ecol. Evol. 2020;4(11):1549–1557. doi: 10.1038/s41559-020-01287-x. [DOI] [PubMed] [Google Scholar]
- 10.Voje K.L., Bell M.A., Stuart Y.E. Evolution of static allometry and constraint on evolutionary allometry in a fossil stickleback. J. Evol. Biol. 2022;35(3):423–438. doi: 10.1111/jeb.13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siver P.A., Kling H. Morphological observations of Aulacoseirausing scanning electron microscopy. Can. J. Bot. 1998;75:1807–1835. [Google Scholar]
- 12.Patrick R.M., Reimer C.W., W C. The Diatoms of the United States, exclusive of Alaska and Hawaii. V. 2 Monogr. Acad. Natural Sci. Philadelphia. 1975;13 [Google Scholar]
- 13.Nakov T., Guillory W., Julius M., Theriot E., Alverson A. Towards a phylogenetic classification of species belonging to the diatom genus Cyclotella (Bacillariophyceae): transfer of species formerly placed in Puncticulata, Handmannia, Pliocaenicus and Cyclotella to the genus Lindavia. Phytotaxa. 2015;217(3):249–264. [Google Scholar]
- 14.Håkansson H., Kling H. The current status of some very small freshwater diatoms of the genera Stephanodiscus and Cyclostephanos. Diatom Res. 1990;5(2):273–287. [Google Scholar]
- 15.Morales E.A. Observations of the morphology of some known and new fragilarioid diatoms (Bacillariophyceae) from rivers in the USA. Phycol. Res. 2005;53:113–133. doi: 10.1111/j.1440-183.2005.00378.x. [DOI] [Google Scholar]
- 16.Gibson C.E., Anderson N.J., Haworth E.Y. Aulacoseira subarctica: taxonomy, physiology, ecology and palaeoecology. Eur. J. Phycol. 2003;38:83–101. doi: 10.1080/0967026031000094102. [DOI] [Google Scholar]
- 17.Runge F. The opal phytolith inventory of soils in central Africa—quantities, shapes, classification, and spectra. Rev. Palaeobotany Palynol. 1999;107(1-2):23–53. [Google Scholar]
- 18.Usoltseva M., Kociolek J.P., Khursevich G. Three new species of Alveolophora (Aulacoseiraceae, Bacillariophyceae) from Miocene deposits in western North America. Phycologia. 2013;52(1):109–117. doi: 10.2216/12-022.1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.