Highlights
-
•
Maternal body mass index (BMI) could influence stillbirth risk.
-
•
The risk of stillbirth is significantly higher in overweight/obese individuals.
-
•
Appropriate pre-pregnancy BMI can reduce the risk of stillbirth.
-
•
Counselling women on the importance of pre-pregnancy BMI is crucial.
Keywords: Japanese, Maternal body mass index, Obesity, Overweight, Stillbirth, Women
Abstract
The relationship between high body mass index (BMI) >25 kg/m2 and risk for stillbirth in the Japanese population remains unclear. This study aimed to estimate the impact of maternal obesity on the risk of stillbirth in a Japanese population. This prospective cohort study used data from the Japan Environment and Children’s Study, which recruited pregnant individuals between 2011 and 2014. A total of 93,772 fetuses were considered eligible for inclusion in this study. Stillbirth (fetal death before or during labor at ≥22 completed weeks of gestation) rates were compared among four pre-pregnancy BMI groups: underweight (<18.5 kg/m2), reference (18.5 to <25.0 kg/m2), overweight (25.0 to <30.0 kg/m2), and obese (≥30.0 kg/m2). The association between pre-pregnancy BMI and the risk of stillbirth was estimated using multiple logistic regression analyses. The overall stillbirth incidence was 0.33% (305/93,722). Compared with the reference group, the risk of stillbirth was significantly higher in the overweight group (adjusted odds ratio [aOR]: 1.55; 95% confidence interval [CI]: 1.08–2.23) and the obese group (aOR: 2.60; 95% CI: 1.59–4.24). The overall incidence of early stillbirth (i.e., <28 weeks) was 0.17% (155/93,722). Similarly, after adjusting for potential confounding factors, the risk of early stillbirth was significantly higher in the obese group (aOR: 4.33; 95% CI: 2.44–7.70). Increased maternal BMI was associated with an increased risk of stillbirth in the Japanese population. Therefore, counselling women planning for pregnancy on the importance of an appropriate pre-pregnancy BMI to minimize the risk of stillbirth is important.
1. Introduction
Stillbirth, defined as fetal death before or during labor at ≥22 completed weeks of gestation, affects 3.0 per 1,000 deliveries in Japan (Haruyama et al., 2018, Sugai et al., 2017); its prevalence is much higher in low- and middle-income countries (Blencowe et al., 2016, Carmichael et al., 2015). The loss of an unborn baby is a tragic event for a family; it substantially impacts parental mental health and leads to high rates of depression and anxiety (Carmichael et al., 2015). The overall prevalence of stillbirths has declined in recent decades; however, this decrease is largely limited to late stillbirth (i.e., ≥28 weeks) and can be attributed to improved obstetric care and prevention of intrapartum complications (Haruyama et al., 2018, Maeda, 2014).
In Japan, the perinatal mortality rate has declined significantly over the past 30 years and is among the lowest in the world (Haruyama et al., 2018). However, the prevalence of early stillbirth (i.e., <28 weeks), which comprises almost half the total stillbirths, has not declined (Carmichael et al., 2015). Therefore, it is important to identify the risk factors of stillbirth and establish effective and appropriate prevention strategies to improve perinatal outcomes.
To date, several risk factors for stillbirth have been identified, including obesity (Aune et al., 2014, Flenady et al., 2011), older maternal age, nulliparity (Gardosi et al., 2013), history of previous stillbirth (Lamont et al., 2015), diabetes mellitus (DM) (Management of stillbirth: obstetric care consensus, 2020), hypertensive disorders (Management of stillbirth: obstetric care consensus, 2020), smoking (Pineles et al., 2016), systemic lupus erythematosus (SLE) (Buyon et al. 2015), and abnormal thyroid function (Nijkamp et al., 2016). A meta-analysis from 13 high-income Western countries showed that being overweight and obese (body mass index [BMI] >25 kg/m2) were the highest-ranking modifiable risk factors for stillbirth among mothers (Flenady et al., 2011). However, it remains unclear whether this relationship applies to the Japanese population, considering that the maternal physique differs among individuals of different races and ethnicities (Deurenberg et al., 2002, NCD Risk Factor Collaboration (NCD-RisC), 2016). It should be acknowledged that, on average, pregnant women in Japan are physically shorter and thinner than those in Western countries (Deurenberg et al., 2002, NCD Risk Factor Collaboration (NCD-RisC), 2016). Interestingly, a retrospective cross-sectional study using the Japan Society of Obstetrics and Gynecology Perinatal Database reported no significant association between being overweight/obese and stillbirth rates (Haruyama et al., 2018). However, to the best of our knowledge, this is the only study to date to address the risk factors for and the causes of stillbirth in Japan. Among the risk factors related to stillbirth, being overweight/obese is a potentially modifiable factor. Evidence relating maternal physique to stillbirth is still insufficient in Japan, and Haruyama et al.'s findings concerning the association between obesity and stillbirth differ from those reported in studies conducted in other countries. To further reduce the incidence of this tragic pregnancy complication, the relationship between being overweight/obese and the incidence of stillbirth in the Japanese population should be extensively examined. Examining the association between obesity and stillbirth in different countries and ethnic groups may provide a new opportunity to elucidate the pathways leading to stillbirth. Therefore, studies on obesity and stillbirth in Japanese population are also important for the medical/obstetric community outside of Japan.
The primary purpose of this study was to determine whether obesity increases the risk of stillbirth, for which data from the Japan Environment and Children’s Study (JECS) was used. Moreover, our secondary purpose was to analyze the association between obesity and early stillbirth (i.e., <28 weeks).
2. Materials and methods
2.1. Study population
The JECS is an ongoing nationwide prospective birth cohort study. The study was conducted at 15 Regional Centers in Japan (including Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyusyu/Okinawa). Details of the JECS have been previously described (Ishitsuka et al., 2017, Kawamoto et al., 2014, Suzumori et al., 2020). Previous JECS’s studies revealed the representativeness of the study population to the general population of Japan (Michikawa et al., 2015, Michikawa et al., 2018).
This study was conducted in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology Statement guidelines for observational studies. In this study, pregnant women were recruited between January 2011 and March 2014. The eligibility criteria for participation included residence in the study areas at the time of recruitment, expected delivery date after August 2011, comprehension of the Japanese language, and completion of a self-administered questionnaire.
A total of 104,062 fetal records were included in this cohort. This study used the jecs-ta-201901930-qsn dataset, released in October 2019 and revised in February 2020. We included only those mothers for whom complete obstetric and demographic data were available.
2.2. Ethics statement
The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions. Written informed consent was obtained from all participants. The JECS was conducted in accordance with the Declaration of Helsinki and other national regulations and guidelines.
2.3. Data collection
The study participants completed questionnaires throughout the pregnancy (i.e., during the first and second/third trimesters) and postpartum periods (1 month after delivery) (Ishitsuka et al., 2017, Kawamoto et al., 2014, Suzumori et al., 2020). The medical records at the time of registration and immediately after vaginal delivery or cesarean section were transcribed by physicians, midwives/nurses, and/or Research Co-ordinators. Information regarding maternal or paternal demographic factors was transcribed from the medical records during pregnancy.
2.4. Exposure definitions
The pre-pregnancy height and weight of the participants were transcribed from their medical records or the questionnaire. Pre-pregnancy BMI was calculated as weight in kilograms divided by height in meters squared according to the World Health Organization standard. Participants were categorized into four groups for analysis, according to their pre-pregnancy BMI: underweight group (<18.5 kg/m2), reference group (18.5–<25.0 kg/m2), overweight group (25.0–<30.0 kg/m2), and obese group (≥30.0 kg/m2) (EURO-PERISTAT, the Japanese Society of Obstetrics and Gynecology, 2021). Moreover, participants were categorized into four groups according to the appropriate BMI categories for Asian populations, recommended by the World Health Organization, as follows: underweight (<18.5 kg/m2), normal (18.5–<23.0 kg/m2), overweight (23.0 to <27.5 kg/m2), and obese (≥27.5 kg/m2) (WHO Expert Consultation, 2004).
2.5. Covariate definitions
The patients’ obstetric records transcriptions included maternal age at delivery, parity (Gardosi et al., 2013), pre-pregnancy BMI (Aune et al., 2014, Flenady et al., 2011), parental smoking status, exposure to secondhand smoking (Pineles et al., 2016), use of assisted reproductive technology (ART) (Sarmon et al., 2021), and infants born of multiple births (Hayata et al., 2022). In addition, using the questionnaires completed during early pregnancy, data were collected regarding the presence of hypertension (HT), DM, and thyroid disease, which are known to be associated with stillbirth (Management of stillbirth: obstetric care consensus, 2020, Nijkamp et al., 2016).
ART was defined as in vitro fertilization or intracytoplasmic sperm injection. Further, maternal age was divided into three groups (i.e., <19, 20–35, and ≥35 years). Thyroid disease was defined as hyperthyroidism or hypothyroidism. The information on parental smoking status and exposure to secondhand smoking was collected using the self-administered questionnaires in the 1st trimester. In this regard, the expectant mothers were asked to reveal whether they or their partners had “Never smoked,” “Previously did, but quit before realizing current pregnancy,” “Previously did, but quit after realizing current pregnancy,” or were “Currently smoking.” Accordingly, participants and their partners were categorized into three groups for analysis: “never smoked or quit smoking before pregnancy” group, “quit smoking after pregnancy” group, and “continued smoking” group.
Regarding secondhand smoking, the expectant mothers answered how often they were exposed to tobacco smoke at home, workplace, or any other indoor places before and during pregnancy, respectively, by choosing frequency options from either “Almost never/ Never,” “1 day a week,” “2–3 days a week,” “4–6 days a week,” or “Every day.” For analysis, “1 day per week” to “4–6 days per week” were re-categorized as “several times per week,” and secondhand smoking was categorized into three groups for analysis: “seldom,” “several times per week,” and “every day.”
2.6. Outcomes
Stillbirth was defined as fetal death before or during labor at ≥22 completed weeks of gestation. Moreover, stillbirth was further classified into early stillbirth (i.e., <28 weeks) and late stillbirth (i.e., ≥28 weeks) (Carmichael et al., 2015). The World Health Organization-recommended definition of fetal death as that occurring after 22 weeks of gestation for the purposes of general statistics and for the registration of stillbirths is used for Japanese demographics and was adopted for the cohort of this study. All births, including stillbirths, were confirmed by a physician, and data on the fetus were obtained by the physician who examined the patient.
2.7. Statistical analysis
One-way analysis of variance and χ2 tests were used to evaluate the association between stillbirth and potential confounding factors. Further, one-way analysis of variance was used to analyze continuous variables such as maternal age, and the χ2 test was used for categorical variables, such as the incidence of obstetric complications.
Multiple logistic regression analyses were conducted to estimate the association between pre-pregnancy BMI and the risk of stillbirth. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated after adjusting for potential confounders. All analyses were performed using IBM SPSS Statistics for Windows (version 25.0; IBM Corp., Armonk, NY, USA). The significance level was set at P <0.05.
3. Results
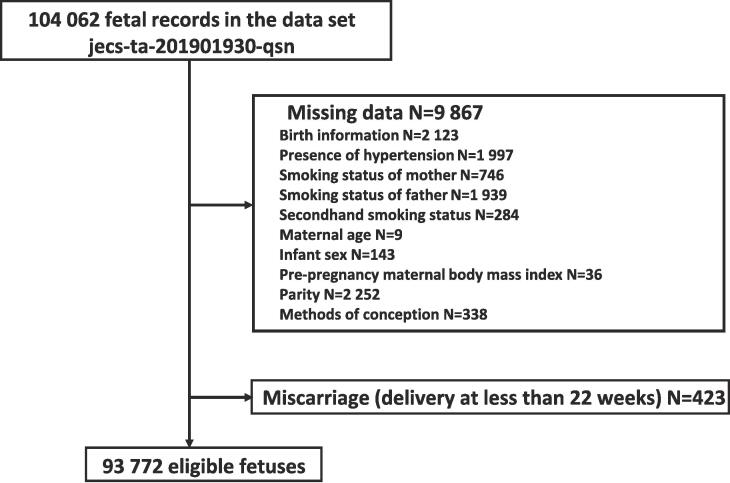
A total of 104,062 fetal records were included in the cohort. We excluded 2,123 fetuses because they lacked information regarding the birth. We also excluded women with missing data (presence of HT, n = 1,997; smoking status of mother, n = 746; smoking status of father, n = 1,939; secondhand smoking status, n = 284; maternal age, n = 9; infant sex, n = 143; pre-pregnancy BMI, n = 36; parity, n = 2,252; and methods of conception, n = 338). Finally, 423 cases were excluded because they were considered miscarriages that resulted in delivery at less than 22 weeks (Fig. 1).
Fig. 1.
Flow diagram showing the recruitment and exclusion of fetus recruited to the JECS study, 2011–2014 in Japan.
A total of 93,772 fetuses were considered eligible for inclusion in the study. Of these, 305 (0.33%) were stillbirths and 155 (0.17%) were early stillbirths. The mean maternal age was 31.2 ± 5.0 years, and the mean maternal pre-pregnancy BMI was 21.2 ± 3.3 kg/m2, with 37,767 nulliparous women (40.3%), 48,043 male infants (51.2%), 438 women with HT before pregnancy (0.47%), and 835 women with DM before pregnancy (0.89%).
Table 1 summarizes the clinical characteristics of the women enrolled in this study.
Table 1.
Baseline characteristics of women in the study population, the Japan Environment and Children’s Study: Japan, 2011–2014.
| Pre-pregnancy body mass index | <18.5 kg/m2 | 18.5–<25.0 kg/m2 | 25.0–<30.0 kg/m2 | ≥30.0 kg/m2 |
|---|---|---|---|---|
| n = 15,037 | n = 68,641 | n = 7,704 | n = 2,390 | |
| Maternal age, years | 30.4 ± 5.0 | 31.4 ± 5.0 | 31.8 ± 5.0 | 31.2 ± 5.0 |
| <19, years | 189 (1.3) | 433 (0.6) | 49 (0.6) | 6 (0.3) |
| 20–34, years | 11,601 (77.1) | 49,019 (71.4) | 5,210 (67.6) | 1,610 (67.4) |
| ≥35, years | 3,247 (21.6) | 19,189 (28.0) | 2,445 (31.7) | 774 (32.3) |
| Hypertension | 30 (0.20) | 234 (0.34) | 94 (1.2) | 80 (3.3) |
| Diabetes mellitus | 66 (0.44) | 436 (0.64) | 176 (2.3) | 157 (6.6) |
| Thyroid disease | 245 (1.6) | 1,376 (2.0) | 185 (2.4) | 42 (1.8) |
| Assisted reproductive technology | 408 (2.7) | 2,215 (3.2) | 255 (3.3) | 68 (2.8) |
| Male infant | 7,772 (51.7) | 35,079 (51.1) | 3,988 (51.8) | 1,204 (50.4) |
| Nulliparity | 8,480 (56.4) | 40,889 (59.6) | 5,053 (65.6) | 1,583 (66.2) |
| Stillbirth | 36 (0.24) | 213 (0.31) | 38 (0.49) | 18 (0.75) |
| Earlier stillbirth | 19 (0.13) | 106 (0.15) | 16 (0.21) | 14 (0.59) |
| Later stillbirth | 17 (0.11) | 107 (0.16) | 22 (0.29) | 4 (0.17) |
| Infant born of multiple births | 276 (1.8) | 1 334 (2.0) | 154 (2.0) | 61 (2.6) |
| Maternal smoking status | ||||
| Never or quit smoking before pregnancy | 12,073 (80.3) | 57,246 (83.4) | 6,031 (78.3) |
1,785 (74.7) |
| Quit smoking after pregnancy | 2,147 (14.3) | 8,504 (12.4) | 1,163 (15.1) | 402 (16.8) |
| Continued smoking | 817 (5.4) | 2,891 (4.2) | 510 (6.6) | 203 (8.5) |
| Paternal smoking status | ||||
| Never or quit smoking before pregnancy | 7,366 (49.0) | 35,225 (51.3) |
3,764 (48.8) | 1,113 (46.6) |
| Quit smoking after pregnancy | 383 (2.5) | 1,677 (2.4) | 166 (2.2) | 46 (1.9) |
| Continued smoking | 7,288 (48.5) | 31,739 (46.2) | 3,774 (49.0) | 1,231 (51.5) |
| Secondhand smoking status | ||||
| Seldom | 7,594 (50.5) | 35,264 (51.4) | 3,432 (44.5) | 958 (40.0) |
| Several times per week | 4,627 (30.8) | 21,641 (31.5) | 2,635 (34.2) | 812 (34.0) |
| Every day | 2,816 (18.7) | 11,736 (17.1) | 1,637 (21.2) | 620 (25.9) |
Data are presented as means ± standard deviations or as numbers (%).
There was a statistically significant difference among the four groups according to pre-pregnancy BMI in terms of maternal age (<19, 20–34, and ≥35 years), HT before pregnancy, DM before pregnancy, thyroid disease before pregnancy, ART, nulliparity, stillbirth, early stillbirth, late stillbirth, maternal smoking status (never smoked or quit smoking before pregnancy, quit smoking after pregnancy, and continued smoking), paternal smoking status (never smoked or quit smoking before pregnancy and continued smoking), and secondhand smoking status (seldom, several times per week, or every day). After adjusting for potential confounding factors, the risk of stillbirth was significantly higher in the overweight (adjusted OR [aOR]: 1.55; 95% CI: 1.08–2.23) and obese groups (aOR: 2.60; 95% CI: 1.59–4.24) (Table 2). Further, we did not find a significant association between being underweight and the incidence of stillbirth (aOR: 0.71; 95% CI: 0.49–1.04). Similarly, after adjusting for potential confounding factors, the risk of early stillbirth was significantly higher in the obese group (aOR: 4.33; 95% CI: 2.44–7.70) (Table 3). Finally, when analyzed using the Asian BMI criterion, the risk of stillbirth did not increase significantly in the overweight group (aOR: 0.89; 95% CI: 0.54–1.46); however, it increased significantly in the obese group (aOR: 3.21; 95% CI: 1.97–5.23). Moreover, we did not find a significant association between being underweight and the incidence of stillbirth (aOR: 0.75; 95% CI: 0.43–1.28).
Table 2.
Association between pre-pregnancy body mass index and stillbirth in the Japan Environment and Children’s Study; Japan, 2011–2014.
| Pre-pregnancy BMI | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| <18.5 kg/m2 | 0.77 (0.54–1.10) | 0.71 (0.49–1.04) |
| 18.5–<25.0 kg/m2 | 1.00 | 1.00 |
| 25.0–<30.0 kg/m2 | 1.59 (1.13–2.25) | 1.55 (1.08–2.23) |
| ≥30.0 kg/m2 | 2.43 (1.50–3.95) | 2.60 (1.59–4.24) |
*Regression analysis was performed after adjusting for pre-pregnancy BMI, maternal age at delivery, parity, parental smoking status, exposure to secondhand smoking, ART, infant sex, infants born of multiple birth, HT, DM, and thyroid disease.
ART, assisted reproductive technology; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HT, hypertension; OR, odds ratio.
Table 3.
Association between pre-pregnancy body mass index and stillbirth under 28 weeks of gestation in the Japan Environment and Children’s Study; Japan, 2011–2014.
| Pre-pregnancy BMI | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| <18.5 kg/m2 | 0.82 (0.50–1.33) | 0.75 (0.44–1.28) |
| 18.5–<25.0 kg/m2 | 1.00 | 1.00 |
| 25.0–<30.0 kg/m2 | 1.35 (0.80–2.28) | 1.22 (0.68–2.18) |
| ≥30.0 kg/m2 | 3.81 (2.79–6.66) | 4.33 (2.44–7.70) |
*Regression analysis was performed after adjusting for pre-pregnancy BMI, maternal age at delivery, parity, parental smoking status, exposure to secondhand smoking, ART, infant sex, infants born of multiple birth, HT, DM, and thyroid disease.
ART, assisted reproductive technology; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HT, hypertension; OR, odds ratio.
4. Discussion
The main results of this nationwide prospective cohort study are as follows: first, after adjusting for potential confounding factors, the risk of stillbirth was significantly higher in the maternal overweight (pre-pregnancy BMI: 25.0–<30.0 kg/m2) and obese (pre-pregnancy BMI >30 kg/m2) groups, but not in the underweight (pre-pregnancy BMI: <18.5 kg/m2) group; second, the risk of early stillbirth was significantly higher in the obese group after adjusting for potential confounding factors. To the best of our knowledge, this investigation is the first detailed evaluation of the association between pre-pregnancy BMI and the incidence of stillbirth in the Japanese general population.
The positive dose–response relationship between increased maternal BMI before conception and stillbirth risk can be explained by several biological mechanisms. First, being overweight or obese is associated with an increased risk of DM (Kodama et al., 2012), gestational HT (Bodnar et al., 2007), preeclampsia (O’Brien et al., 2003), gestational diabetes (Chu et al., 2007), and congenital anomalies (Stothard et al., 2009). All of these conditions have been strongly associated with the risk of stillbirth, according to previous reports. In this study, among the cases that resulted in stillbirth, the questionnaires administered in the 2nd/3rd trimesters and at 1 month after delivery elicited insufficient responses and could not be used for analysis. Therefore, it was not possible to fully examine the confounding effect of preeclampsia, gestational HT, gestational diabetes, and congenital anomalies. However, DM and HT were significantly more common in the overweight and obese groups, which is consistent with previous reports (Bodnar et al., 2007, Kodama et al., 2012;). Second, hyperlipidemia with decreased prostacyclin production increases thromboxane production, increases the risk of placental thrombosis, decreases placental perfusion, and may even cause both placental infarction and abruption in late pregnancy (Craven and Ward, 2002, Eldor, 2001, Moldenhauer et al., 2003, Stone et al., 1994). However, it should be noted that the aforementioned mechanisms, through which obesity causes stillbirths, cannot be directly proven from the present data.
Despite slight differences in the definitions of obesity and study population (Aune et al., 2014, Flenady et al., 2011), several studies have reported an association between increased maternal BMI and stillbirth (Aune et al., 2014, Flenady et al., 2011). The current study’s findings are consistent with these previous reports (Aune et al., 2014, Flenady et al., 2011). In contrast, in a retrospective cross-sectional study using the nationwide perinatal database in Japan, Haruyama et al. (2018) reported that being overweight and obese did not have a significant association with stillbirth; however, being underweight showed a protective effect. Unlike our study and other previous reports (Aune et al., 2014, Flenady et al., 2011), women in Haruyama’s study were categorized into four groups for analysis, according to their pre-pregnancy BMI values: underweight (<18.5 kg/m2), normal (18.5–< 23.0 kg/m2), overweight (23.0–<30.0 kg/m2), and obese (≥30.0 kg/m2) (Haruyama et al., 2018). When we re-analyzed our data according to this pre-pregnancy BMI classification, we found that the risk of stillbirth did not increase significantly in the overweight group (aOR: 1.18; 95% CI: 0.88–1.58), whereas it increased significantly in the obese group (aOR: 2.56; 95% CI: 1.56–4.21). Moreover, we did not find a significant association between being underweight and the incidence of stillbirth (aOR: 0.70; 95% CI: 0.48–1.03). The difference between the results of our study and those of Haruyama’s study can be attributed to the study population.
In Japan, approximately 50% of the deliveries are managed in small birth centers and level I hospitals (Hirata et al., 2021, Hosono et al., 2019). However, unlike our study of the general Japanese population, the cases registered in Haruyama’s study were mostly from secondary and tertiary facilities, and the stillbirth rate (0.56%) was much higher than that based on the vital statistics (Haruyama et al., 2018).
We examined the relationship between pre-pregnancy BMI and stillbirth according to this Asian BMI criterion for our study population. Considering our results for the general Japanese population and previous studies (Aune et al., 2014, Flenady et al., 2011), we believe that, at minimum, the obese individuals should be recognized as being at a high risk for stillbirth.
Unlike late stillbirth (i.e., ≥28 weeks), which can be reduced by improvements in obstetric care and prevention of intrapartum complications (Carmichael et al., 2015), early stillbirth (i.e., <28 weeks) is one of the major concerns of perinatal management. In this study, we found a significant association between obesity and early stillbirth. Considering the biological mechanisms of stillbirth, it is possible that avoiding obesity will lead to fewer early stillbirths. However, we were not able to adequately perform multiple logistic regression analyses for the association between the incidence of late stillbirth and obesity. This limitation was attributed to the fact that none of the participants in the group under 19 years of age had late stillbirths; further, maternal age, which is an important risk factor for stillbirth, could not be incorporated in the analysis of late stillbirths.
The association between maternal preconception low BMI and adverse perinatal outcomes has been extensively reviewed in the literature. A lower maternal pre-pregnancy BMI increases the risk of preterm birth, low birth weight, and small-for-gestational age fetuses (Enomoto et al., 2016, Lynch et al., 2014, Tang et al., 2021). Low maternal weight is a common health problem, especially in Japan, considering that there is a strong tendency among reproductive-age individuals to have a lower body weight (Nakanishi et al., 2022). Among Japanese women aged ≥20 years, the prevalence of underweight (<18.5 kg/m2) was 11.5% according to the 2019 National Health and Nutrition Survey of Japan (Nakanishi et al., 2022); the corresponding prevalence was 15.9% in our study. Women in Japan are typically thinner than those in Western countries (Deurenberg et al., 2002, NCD Risk Factor Collaboration (NCD-RisC), 2016).
According to our results, it can be stated that being underweight is not associated with adverse perinatal outcomes, such as stillbirth; however, the possibility that being underweight may have a marginally protective effect on stillbirth cannot be completely ruled out. Therefore, the association between being underweight and the incidence of stillbirth derived from this study should be interpreted with caution.
In summary, the association of stillbirth with BMI is similar to that noted in other populations. This is important, as it implies that the pathways leading to stillbirth are common across different countries and ethnic groups, suggesting a biologic construct to stillbirth and not purely a socioeconomic construct. In the future, it may be possible to reduce stillbirth not only in Japan but also worldwide by conducting detailed studies and experiments on the mechanism by which obesity causes stillbirth.
4.1. Study limitations
This study had several limitations. We did not evaluate all potential risk factors for stillbirth, including SLE (Buyon et al., 2015) and chronic kidney disease (CKD) (Zhang et al., 2015). This is because no women with SLE or CKD in the current study had stillbirths. However, this does not indicate that SLE or CKD are not risk factors for stillbirth. It should be noted that it is difficult to accurately identify all mild diseases (diagnosed or undiagnosed) in a nationwide cohort study. Moreover, it was not possible to fully assess the role of the smoking status in the 2nd trimester (Pineles et al., 2016), which is shown to be associated with the risk of stillbirth. As aforementioned, in cases that resulted in stillbirth, the responses to the questionnaires at the time of 2nd/3rd trimesters were insufficient in this study. Further, confounders that were not measured in this study may be associated with stillbirth. Additionally, the generalizability of our results may be limited because of the homogeneity of this cohort, which included almost exclusively Japanese women. Finally, although the study results may not change, detailed information on how to confirm stillbirth (e.g., assessment of vital signs and use of the Apgar score) is not available in the JECS data set.
5. Conclusions
In conclusion, increased maternal BMI is associated with an increased risk of stillbirth in the Japanese population. To reduce the risk of stillbirth, it is essential to counsel women planning for pregnancy on the importance of an appropriate pre-pregnancy BMI and weight management guidelines.
CRediT authorship contribution statement
Satoshi Shinohara: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration. Ryoji Shinohara: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Reiji Kojima: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Sayaka Horiuchi: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Sanae Otawa: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Megumi Kushima: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Kunio Miyake: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Hideki Yui: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Tadao Ooka: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Yuka Akiyama: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Hiroshi Yokomichi: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Zentaro Yamagata: Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the participants of the JECS. The findings and conclusions of this study are solely the responsibility of the authors and do not represent the official views of the above-mentioned government agency. Members of the JECS Group as of 2022 include: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Author contributions
Satoshi Shinohara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Satoshi Shinohara: Concept and design. All authors: Acquisition, analysis, or interpretation of data. All authors: Writing – review & editing, critical revision of the manuscript for important intellectual content. Satoshi Shinohara: Statistical analysis. Ryoji Shinohara: Administrative, technical, or material support. Satoshi Shinohara and Zentaro Yamagata: Supervision.
Funding/financial disclosures
The Japan Environment and Children's Study (JECS) is funded by the Ministry of the Environment, Japan. However, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the government of Japan.
Data availability statement
Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of May 30, 2003 amendment on September 9, 2015) to deposit the data containing personal information publicly. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiologic data. All inquiries regarding access to the data should be sent to the following email address: jecs-en@nies.go.jp.
Data availability
The authors do not have permission to share data.
References
- Aune D., Saugstad O.D., Henriksen T., Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311:1536–1546. doi: 10.1001/jama.2014.2269. [DOI] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Jassir F.B., Say L., Chou D., Mathers M., Hogan D., Shiekh S., Qureshi Z.U., You D., Lawn J.E. (2016) National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob. Health. 2016;4:e98–e108. doi: 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- Bodnar L.M., Catov J.M., Klebanoff M.A., Ness R.B., Roberts J.M. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18:234–239. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- Buyon J.P., Kim M.Y., Guerra M.M., Laskin C.A., Petri M., Lockshin M.D., Sammaritano L., Branch D.W., Porter T.F., Sawitzke A., Merrill J.T., Stephenson M.D., Cohn E., Garabet L., Salmon J.E. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann. Intern. Med. 2015;163:153–163. doi: 10.7326/M14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael, S.L., Blumenfeld, Y.J., Mayo, J., Wei, E., Gould, J.B., Stevenson, D.K., Shaw, G.M., 2015. Prepregnancy obesity and risks of stillbirth. PLoS One 10, e0138549. https://doi.org/10.1371/journal.pone.0138549. [DOI] [PMC free article] [PubMed]
- Chu S.Y., Callaghan W.M., Kim S.Y., Schmid C.H., Lau J., England L.J., Dietz P.M. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- Craven C., Ward K. Stillbirth: tissue findings with environmental and genetic links. Semin. Perinatol. 2002;26:36–41. doi: 10.1053/sper.2002.29836. [DOI] [PubMed] [Google Scholar]
- Deurenberg P., Deurenberg-Yap M., Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes. Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- Eldor A. Thrombophilia and its treatment in pregnancy. J. Thromb. Thrombolysis. 2001;12:23–30. doi: 10.1023/a:1012730325902. [DOI] [PubMed] [Google Scholar]
- Enomoto, K., Aoki, S., Toma, R., Fujiwara, K., Sakamaki, K., Hirahara, F., 2016. Pregnancy outcomes based on pre-pregnancy body mass index in japanese women. PLoS One 11, e0157081. https://doi.org/10.1371/journal.pone.0157081. [DOI] [PMC free article] [PubMed]
- EURO-PERISTAT, the Japanese Society of Obstetrics and Gynecology (JSOG) Perinatal Committee Report 2021. (2021) https://www.jsog.or.jp/news/pdf/20210616_shuuchi.pdf (accessed 1 Jun 2021).
- Flenady V., Koopmans L., Middleton P., Frøen J.F., Smith G.C., Gibbons K., Coory M., Gordon A., Ellwood D., McIntyre H.D., Fretts R., Ezzati M. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- Gardosi, J., Madurasinghe, V., Williams, M., Mali,k A., Francis, A., 2013. Maternal and fetal risk factors for stillbirth: population based study. BMJ 346, f108. https://doi.org/10.1136/bmj.f108. [DOI] [PMC free article] [PubMed]
- Haruyama R., Gilmour S., Ota E., Abe S.K., Rahman M.M., Nomura S., Miyasaka N., Shibuya K. Causes and risk factors for singleton stillbirth in Japan: Analysis of a nationwide perinatal database, 2013–2014. Sci. Rep. 2018;8:4117. doi: 10.1038/s41598-018-22546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayata, E., Nakata, M., Morita, M., 2022. Time trend analysis of perinatal mortality, stillbirth, and early neonatal mortality of multiple pregnancies for each gestational week from the year 2000 to 2019: A population-based study in Japan. PLoS One 17, e0272075. https://doi.org/10.1371/journal.pone.0272075. [DOI] [PMC free article] [PubMed]
- Hirata K., Kimura T., Hirano S., Wada K., Kusuda S., Fujimura M. Outcomes of outborn very-low-birth-weight infants in Japan. Arch. Dis. Child Fetal Neonatal Ed. 2021;106:131–136. doi: 10.1136/archdischild-2019-318594. [DOI] [PubMed] [Google Scholar]
- Hosono S., Tamura M., Isayama T., Sugiura T., Kusakawa I., Ibara S., Ishikawa G., Okuda M., Sekizawa A., Tanaka H., Masaoka N., Morizane M., Arahori H., Kabe K., Kubo M., Wada M. Neonatal cardiopulmonary resuscitation project in Japan. Pediatr. Int. 2019;61(7):634–640. doi: 10.1111/ped.13897. [DOI] [PubMed] [Google Scholar]
- Ishitsuka K., Nakayama S.F., Kishi R., Mori C., Yamagata Z., Ohya Y., Kawamoto T., Kamijima M. Japan Environment and Children's Study: backgrounds, activities, and future directions in global perspectives. Environ. Health Prev. Med. 2017;22:61. doi: 10.1186/s12199-017-0667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Nitta H., Murata K., Toda E., Tsukamoto N., Hasegawa M., Yamagata Z., Kayama F., Kishi R., Ohya Y., Saito H., Sago H., Okuyama M., Ogata T., Yokoya S., Koresawa Y., Shibata Y., Nakayama S., Michikawa T., Takeuchi A., Satoh H. Rationale and study design of the Japan environment and children's study (JECS) BMC Public Health. 2014;14:25. doi: 10.1186/1471-2458-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S., Horikawa C., Fujihara K., Heianza Y., Hirasawa R., Yachi Y., Sugawara A., Tanaka S., Shimano H., Iida K.T., Saito K., Sone H. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am. J. Epidemiol. 2012;176:959–969. doi: 10.1093/aje/kws172. [DOI] [PubMed] [Google Scholar]
- Lamont, K., Scott, N.W., Jones, G.T., Bhattacharya, S., 2015. Risk of recurrent stillbirth: systematic review and meta-analysis. BMJ 350, h3080. https://doi.org/10.1136/bmj.h3080. [DOI] [PubMed]
- Lynch, A.M., Hart, J.E., Agwu, O.C., Fisher, B.M., West, N.A., Gibbs, R.S., 2014. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am. J. Obstet. Gynecol. 210, 428.e1–9. https://doi.org/10.1016/j.ajog.2013.12.011. [DOI] [PubMed]
- Maeda K. Highly improved perinatal states in Japan. J. Obstet. Gynaecol. Res. 2014;40:1968–1977. doi: 10.1111/jog.12485. [DOI] [PubMed] [Google Scholar]
- Management of stillbirth: obstetric care consensus no, 10. 2020. Management of Stillbirth: Obstetric Care Consensus No, 10. Obstet Gynecol 135, e110–e132. https://doi.org/10.1097/AOG.0000000000003719. [DOI] [PubMed]
- Michikawa T., Nitta H., Nakayama S.F., Ono M., Yonemoto J., Tamura K., Suda E., Ito H., Takeuchi A., Kawamoto T., Kawamoto T., Saito H., Kishi R., Yaegashi N., Hashimoto K., Yasumura S., Mori C., Hirahara F., Yamagata Z., Inadera H., Kamijima M., Konishi I., Iso H., Shima M., Fukumoto M., Suganuma N., Hara T., Katoh T. The Japan Environment and Children's Study (JECS): A preliminary report on selected characteristics of approximately 10 000 pregnant women recruited during the first year of the study. J. Epidemiol. 2015;25:452–458. doi: 10.2188/jea.JE20140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa T., Nitta H., Nakayama S.F., Yamazaki S., Isobe T., Tamura K., Suda E., Ono M., Yonemoto J., Iwai-Shimada M., Kobayashi Y., Suzuki G.o., Kawamoto T. Baseline profile of participants in the Japan Environment and Children's Study (JECS) J. Epidemiol. 2018;28(2):99–104. doi: 10.2188/jea.JE20170018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldenhauer J.S., Stanek J., Warshak C., Khoury J., Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am. J. Obstet. Gynecol. 2003;189:1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Saijo Y., Yoshioka E., Sato Y., Kato Y., Nagaya K., Takahashi S., Ito Y., Kobayashi S., Miyashita C., Ikeda-Araki A., Kishi R., Kamijima M., Yamazaki S., Ohya Y., Yaegashi N., Hashimoto K., Mori C., Ito S., Yamagata Z., Inadera H., Nakayama T., Iso H., Shima M., Kurozawa Y., Suganuma N., Kusuhara K., Katoh T. Severity of low pre-pregnancy body mass index and perinatal outcomes: the Japan Environment and Children's Study. BMC Pregnancy Childbirth. 2022;22:121. doi: 10.1186/s12884-022-04418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijkamp J.W., Korteweg F.J., Groen H., Timmer A., Van Den Berg G., Bossuyt P.M., Mol B.W., Erwich J.J. Thyroid function testing in women who had a stillbirth. Clin. Endocrinol. (Oxf) 2016;85:291–298. doi: 10.1111/cen.13002. [DOI] [PubMed] [Google Scholar]
- O’Brien T.E., Ray J.G., Chan W.S. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Pineles B.L., Hsu S., Park E., Samet J.M. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am. J. Epidemiol. 2016;184:87–97. doi: 10.1093/aje/kwv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmon K.G., Eliasen T., Knudsen U.B., Bay B. Assisted reproductive technologies and the risk of stillbirth in singleton pregnancies: a systematic review and meta-analysis. Fertil. Steril. 2021;116:784–792. doi: 10.1016/j.fertnstert.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Stone J.L., Lockwood C.J., Berkowitz G.S., Alvarez M., Lapinski R., Berkowitz R.L. Risk factors for severe preeclampsia. Obstet. Gynecol. 1994;83:357–361. [PubMed] [Google Scholar]
- Stothard K.J., Tennant P.W., Bell R., Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Sugai M.K., Gilmour S., Ota E., Shibuya K. Trends in perinatal mortality and its risk factors in Japan: Analysis of vital registration data, 1979–2010. Sci. Rep. 2017;7:46681. doi: 10.1038/srep46681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumori N., Ebara T., Matsuki T., Yamada Y., Kato S., Omori T., Saitoh S., Kamijima M., Sugiura‐Ogasawara M. Effects of long working hours and shift work during pregnancy on obstetric and perinatal outcomes: A large prospective cohort study-Japan Environment and Children's Study. Birth. 2020;47(1):67–79. doi: 10.1111/birt.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Zhu X., Chen Y., Huang D., Tiemeier H., Chen R., Bao W., Zhao Q. Association of maternal pre-pregnancy low or increased body mass index with adverse pregnancy outcomes. Sci. Rep. 2021;11:3831. doi: 10.1038/s41598-021-82064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Ma X.X., Hao L., Liu L.J., Lv J.C., Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin. J. Am. Soc. Nephrol. 2015;10:1964–1978. doi: 10.2215/CJN.09250914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.
Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of May 30, 2003 amendment on September 9, 2015) to deposit the data containing personal information publicly. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiologic data. All inquiries regarding access to the data should be sent to the following email address: jecs-en@nies.go.jp.