Abstract
Purpose
An increasing amount of evidence suggests that psoriasis and nonalcoholic steatohepatitis (NASH) may occur simultaneously, whereas the underlying mechanisms remain unclear. Our research aims to explore the potential comorbidity mechanism in psoriasis and nonalcoholic steatohepatitis.
Materials and Methods
The expression profiles of psoriasis (GSE30999, GSE13355) and NASH (GSE24807, GSE17470) were downloaded from GEO datasets. Next, common differently expressed genes (DEGs) of psoriasis and NASH were investigated. Then, GO and KEGG enrichment, protein interaction network (PPI) construction, and hub gene identification for DEGs were performed. Finally, immune cells expression, target genes predicted by common miRNAs, and transcription factors interaction analysis for hub genes were carried out.
Results
Twenty DEGs were identified in totally. GO analysis revealed response to the virus was the most enriched term, and hepatitis C and coronavirus disease‐COVID‐19 infection‐associated pathways were mainly enriched in KEGG. A total of eight hub genes were collected, including IFIT1, IFIT3, OAS1, HPGDS, IFI27, IFI44, CXCL10, IRF9, and 11 TFs were predicted. Then, neutrophils and monocytes were identified as immune cells that express the most hub genes. Moreover, five common miRNAs for psoriasis and NASH and one common miRNAs (hsa‐miR‐1305)‐mRNAs (CHL1, MBNL2) network were presented.
Conclusion
CHL1 and MBNL2 may participate in the process of psoriasis and NASH via regulating hsa‐miR‐1305, and together with eight hub genes may be potential therapeutic targets for future treatment for the co‐occurrence of these two diseases. This comprehensive bioinformatic analysis provides new insights on molecular pathogenesis and identification of potential therapeutic targets for the co‐occurrence of them.
Keywords: bioinformatics, differentially expressed genes, microRNAs (miRNAs), nonalcoholic steatohepatitis, psoriasis
1. INTRODUCTION
Psoriasis is a common, chronic papulosquamous skin disease occurring worldwide with an incidence of about 2%, presenting at any age, and leading to both physical and psychological substantial burden for individuals and society. 1 Typical histologic findings of psoriasis contain spinous hypertrophy with elongation of the epidermal process, granular thinning, hyperkeratosis and dyskeratosis, dermal vasodilation, and neutrophil infiltration. There are two main causes of psoriasis: innate genetic susceptibility as well as acquired environmental triggers, which include infections, HIV, hypocalcemia, stress, alcohol consumption, obesity, smoking, and drugs. 1 So far, it has been broadly acknowledged that psoriasis is triggered by T cell activation associated with proinflammatory cytokines secretion, including interleukin (IL)−17A, IL‐22, IL‐23, tumor necrosis factor‐α (TNF‐α), and interferon‐γ (IFN‐γ). 2 , 3 Meanwhile, psoriasis is associated with an increased incidence of many comorbidities, which comprise cardiovascular disease, inflammatory bowel disease, nonalcoholic fatty liver disease, and various metabolic diseases including diabetes mellitus, obesity, hypertension, and dyslipidemia compared with the general population. 1 , 4 , 5
Nonalcoholic steatohepatitis (NASH), a type of liver damage that is strongly associated with visceral adiposity and metabolic syndrome, is considered as the progressive form of nonalcoholic fatty liver disease (NAFLD), and has become a major cause of cirrhosis and liver cancer. 6 , 7 , 8 Although many individuals with simple steatosis develop no more severe disease, NASH can progress to both cirrhosis and even end‐stage liver disease. 9 So far, it has become one of the leading causes of liver‐related morbidity and mortality worldwide and a primary indication for liver transplantation. 7 , 10 , 11 , 12 , 13 A number of inherited and environmental factors increase the risk of NASH and influence its progression. Existing studies have been revealing its possible pathogenesis including metabolic stress, inflammation, and fibrosis. 8 Nevertheless, the exact pathophysiology of NASH has not been completely understood yet. Moreover, some research has identified that once hereditary conditions are excluded, nearly all patients diagnosed with NAFLD share the characteristics associated with metabolic risks, such as obesity, type 2 diabetes mellitus, hypertension, and dyslipidemia, sharing the same pathophysiologic mechanism of insulin resistance. 7 , 14 , 15
The above contents suggest that both psoriasis and NASH are related to some systematic metabolic diseases and inflammatory responses. What's more, the identical pathogenic link between psoriasis and NAFLD is chronic inflammation and peripheral insulin resistance, and many metabolic diseases have been shown to cause both psoriasis and NAFLD, where obesity comes firstly. 16 Also, more evidence indicates that there is a bidirectional association between psoriasis and NASH from the perspective of clinical research. So far, it has been identified that patients with psoriasis were at significantly increased risk for NAFLD compared with the general population. 14 Other teams reported that patients with psoriasis and NAFLD at the same time had more severe psoriasis in comparison with patients who had psoriasis alone, and NAFLD was a significant predictor of higher Psoriasis Area and Severity Index (PASI) scores. 14 Contrarily, PASI was a remarkable and independent predictor of NAFLD grade. 17
From the clinical level, despite there is an obvious clinical relationship between psoriasis and NASH, the etiology of the co‐occurrence of the two diseases is unknown. In terms of mechanism, although there are many researches on differentially expressed genes (DEGs) in psoriasis 18 , 19 , 20 , 21 and NAFLD 22 , 23 , 24 , 25 separately, which revealed possible mechanisms in each disease, the common DEGs in both of them have not been investigated yet. So far, on the basis of previous research, we inferred that there are similar mechanisms in the pathogenesis of psoriasis and NASH, for example, some common DEGs and pathways. Therefore, we use bioinformatics approaches here to verify the hypothesis. As a result, the purpose of this study is to explore the relationship between psoriasis and NASH, discover the underlying comorbidity mechanism by bioinformatics analysis, and provide certain data support as well as innovative perspectives for the joint prevention and treatment of psoriasis and NASH.
2. MATERIAL AND METHODS
2.1. Data source
The GEO dataset (https://www.ncbi.nlm.nih.gov/geo/) contains millions of microarray datasets and high‐throughput sequences submitted by researchers worldwide. We used the key word “Psoriasis” or “NASH” to search gene expression datasets. Inclusion and exclusion criteria: (1) the test specimens should be Homo sapiens. (2) The gene expression profiles should include cases as well as controls. (3) The samples of both diseases should be local pathological tissues, which means skin tissues for psoriasis and liver tissues for NASH. (4) The sequencing platform should be consistent. (5) The patients receiving clinical intervention are excluded. As a result, GEO datasets: GSE30999 & GSE13355 for psoriasis and GSE24807 & GSE17470 for NASH were selected. For these datasets, we used GEO2R online for further analysis. The information of these datasets is displayed in Table 1, including disease types, platforms, sample types, quantities, and experiment types.
TABLE 1.
Details of the GEO datasets.
| Disease | Series | Platform | Case | Control | Total | Experiment type |
|---|---|---|---|---|---|---|
| Psoriasis | GSE30999 | GPL570 | 85 | 85 | 170 | Array |
| GSE13355 | GPL570 | 58 | 64 | 122 | Array | |
| GSE31037 | GPL10999 | 47 | 20 | 67 | High throughput sequencing | |
| NASH | GSE24807 | GPL570 | 12 | 5 | 17 | Array |
| GSE17470 | GPL570 | 7 | 4 | 11 | Array | |
| GSE33857 | GPL10656 | 7 | 12 | 19 | Array |
Abbreviations: NASH, nonalcoholic steatohepatitis; NAFLD, nonalcoholic fatty liver disease; DEGs, differently expressed genes; PPI, protein protein network; TFs, transcription factors; IL, interleukin; TNF‐α, tumor necrosis factor‐α; IFN‐γ, interferon‐γ; PASI, psoriasis area and severity index; BP, biological process; CC, cellular component; MF, molecular function; HGNC, human gene nomenclature committee; FC, fold change; ROS, reactive oxygen species; JNK, Jun N‐terminal kinase; IFIT, IFN‐γ stimulated genes; OASs, oligoadenylate synthetases; ISGs, interferon‐stimulated genes; CXCL10, interferon gamma‐induced protein 10; RA, rheumatoid arthritis; SLE, systemic lupus rythematosus; COVID‐19, coronavirus disease 2019; miRNAs, MicroRNAs; CHL1, cell adhesion molecule L1 like.
2.2. Identification of DEGs
GEO2R online was used to identify DEGs. When multiple probes corresponded to a single gene symbol, the first probe was selected. When some probes did not correspond to any gene symbol, they were removed. The criteria of DEGs are |logFC (fold change)|≥1 and adjusted p‐value < 0.05. Their common DEGs were obtained by drawing Venn diagram via http://bioinformatics.psb.ugent.be/webtools/Venn/.
2.3. Enrichment analysis of common DEGs
R Package “clusterProfiler” was used to perform GO and KEGG pathway enrichment analysis. The GO analysis contains three terms: biological process (BP), cellular component (CC), and molecular function (MF). R Package “ggplot2” was used for visualization. R version is 4.2.1.
2.4. PPI network construction and hub genes selection
The complex regulatory relationships among interested proteins were explored via String (https://cn.string‐db.org/). The combined score over 0.4 was set as statistically significant. The Degree algorithm of Cytohubba in Cytoscape was used to explore hub genes. HUGO Gene Nomenclature Committee (HGNC) database (https://www.genenames.org/) is a standard website for providing a unique symbol for all genes on the human genome, which was used to search details of each hub gene.
2.5. Enrichment analysis and expression in immune cells of hub genes
GeneMANIA (http://www.genemania.org) is an effective tool to identify the interconnectedness of gene sets, where we established the co‐expression network of the hub genes. Human Protein Atlas Database (https://www.proteinatlas.org/) is a useful immunohistochemical database of many genes, through which we explored the expression of each hub gene in various immune cells.
2.6. Prediction of transcription factors (TFs)
TRRUST (https://www.grnpedia.org/trrust) is a database for identifying target genes of common TFs and regulatory relationship between TFs, which is used to predict transcriptional regulatory networks. TFs that regulate the hub genes were explored in TRRUST where p‐value < 0.05 was set in the analysis.
2.7. Identification of common miRNAs in psoriasis and NASH
MicroRNAs carry out gene regulation by degrading mRNAs or inhibiting their function. R Package “edgeR” and GEO2R online were used to identify the miRNAs associated with two diseases via GEO datasets (GSE31037 for psoriasis, GSE33857 for NASH), and the information of these datasets was displayed in Table 1, including disease types, platform, sample types, quantities, and experiment type. The miRNAs associated with psoriasis and NASH were obtained and intersected, in addition, miRDB database (http://mirdb.org) was used to search for the mature miRNA for further analysis. TAM 2.0 (http://www.lirmed.com/tam2) was used to perform miRNA function analysis. The terms ranked by p‐values and p‐value < 0.05 were identified as significant.
2.8. The common miRNAs‐mRNAs network
MiRTarbase is a miRNA target interactions database (https://mirtarbase.cuhk.edu.cn) validated experimentally, which included plentiful miRNAs and target genes verified by experiments. The miRNAs–mRNAs regulated network was established after taking the intersection of common DEGs and predicted consensus miRNAs in psoriasis and NASH.
2.9. Ethics exemption statement
According to the latest governmental legal ethical regulation titled “Ethical Review Measures for Life Science and Medical Research Involving Human Beings,” issued and approved by the National Science and Technology Ethics Committee and State Council of PR China on the Feb 18th, 2023, a study utilizing public database data is exempt from ethical review.
3. RESULTS
3.1. Identification of common DEGs
The data analysis process is shown in the flowchart (Figure 1). There were 3002 DEGs obtained in GSE30999, 936 in GSE13355, 2679 in GSE24807 and 1436 in GSE17470 (Figure 2A–D). A Venn diagram intersection revealed 16 common upregulated DEGs (IFI27, OAS1, CWH43, CXCL10, IRF9, IFIT3, DNASE1L3, CD36, VSNL1, FAM26F, SLC16A6, IFIT1, UNC93A, LYNX1, IFI44, CTSC) and four common downregulated DEGs (HPGDS, MBNL2, CHL1, IGFL2) (Figure 2E,F).
FIGURE 1.
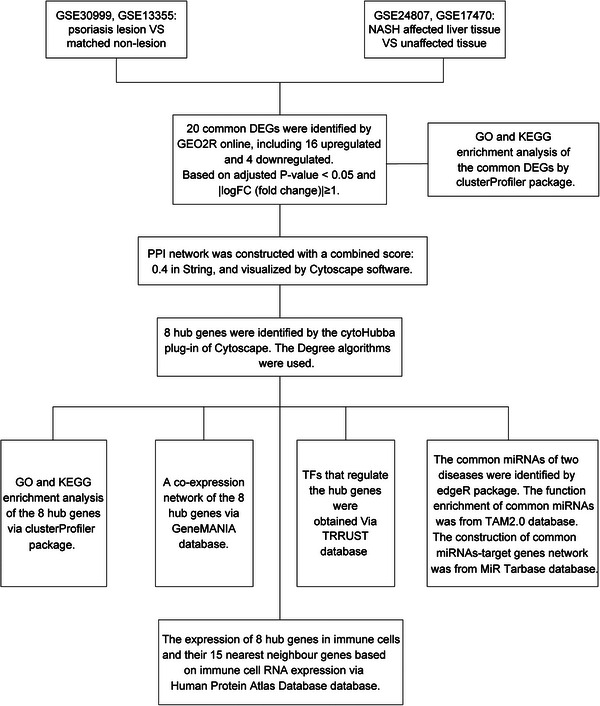
The flowchart of this study.
FIGURE 2.
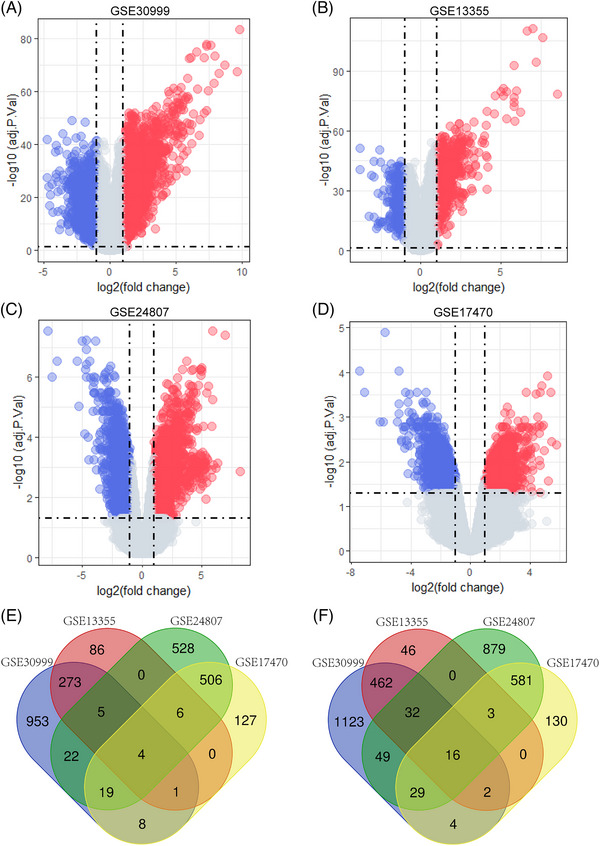
Volcano map of GSE30999, GSE13355, GSE24807, and GSE17470 and Venn diagram of common DEGs. (A) The volcano map of GSE30999. (B) The volcano map of GSE13355. (C) The volcano map of GSE24807. (D) The volcano map of GSE17470. Red represents upregulated genes, and blue represents downregulated genes. (E) The four datasets have an overlap of four common downregulated genes. (F) The four datasets have an overlap of 16 common upregulated genes.
3.2. Enrichment analysis of common DEGs
Analysis of GO and KEGG pathway enrichment were performed to explore the functions and pathways of the 20 common DEGs. From GO analysis, major biological processes (BPs) and molecular functions (MFs) were shown in Table S1, including each function and their p‐values. According to the KEGG pathway, major enrichment pathways were shown in Table S1 as well, including each pathway and their p‐values. Then, bar charts (Figure 3A), bubble charts (Figure 3B), and network plots (Figure 3C) were presented respectively to show the enrichment results. These results expressed strongly that viral infection, immune response, and lipid metabolism are primarily responsible for the comorbidity of psoriasis and NASH.
FIGURE 3.
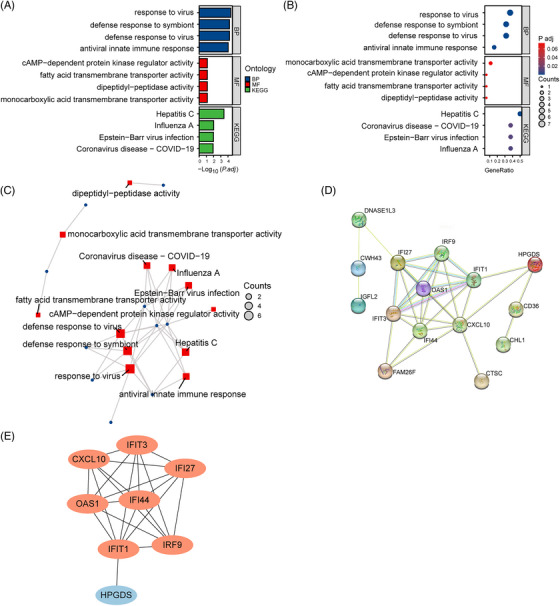
Enrichment analysis and PPI network of common DEGs. (A) Enrichment analysis of common DEGs is shown with bar chart in BP, MF, and KEGG. (B) Enrichment analysis of common DEGs is shown with bubble diagram. (C) Enrichment analysis of common DEGs is shown with network planning. (D) PPI network of common DEGs contains 15 nodes and 31 interactions. (E) Eight hub genes are selected from PPI network. Orange represents upregulated genes, and blue represents downregulated genes.
3.3. PPI network construction and identification of hub genes
Cytoscape was used to build PPI networks containing 15 nodes and 31 interactions for the common DEGs (Figure 3D). Eight hub genes were evaluated and selected by the Degree algorithm of Cytohubba in Cytoscape, containing IFIT1, OAS1, HPGDS, IFI27, IFI44, CXCL10, IFIT3, and IRF9 (Figure 3E), which were acquired at the intersection of the Upset diagram. Orange represents upregulated genes, and blue represents downregulated genes. The details of their roles were searched in the HGNC database (Table S2).
3.4. Co‐expression and enrichment analysis of hub genes
According to the co‐expression network of hub genes by using GeneMANIA, we identified top five related functions and their different weight interaction, including co‐expression of 57.24%, physical interactions of 31.80%, pathway 8.40%, co‐localization of 2.34%, and predicted functional relationships of 0.22% (Figure 4A). Analysis of GO and KEGG pathway enrichment was performed to explore the functions and pathways of the eight hub genes. From GO analysis, major biological processes (BPs), cellular components (CCs), and molecular functions (MFs) were shown in Table S3, including each function and their p‐values. According to the KEGG pathway, major enrichment pathways were shown in Table S3 as well, including each pathway and their p‐values. The results emphasized the important role of various viral infections, chemokines, lipid metabolism, and inflammatory factors in these two diseases, and bar charts (Figure 4B), bubble charts (Figure 4C), and network plots (Figure 4D) were presented respectively to show the enrichment results. Moreover, the expression of hub genes in various immune cells was shown by the chart, and neutrophils and monocytes have been identified as cells that express the most hub genes (Figure 5A–H). What's more, the network of eight hub genes and their nearest neighbor genes based on immune cell RNA expression was shown (Figure 6A).
FIGURE 4.
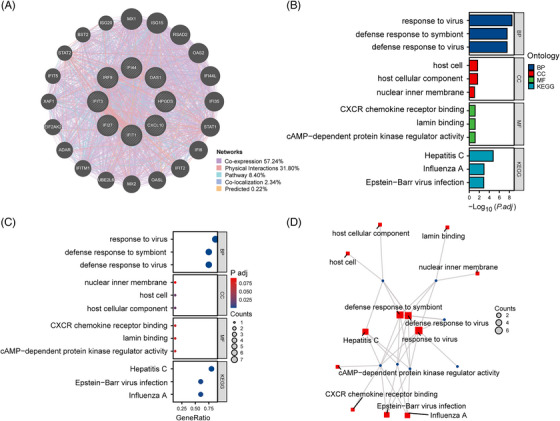
Identification and analysis of hub genes. (A) Hub genes and their co‐expression genes network. (B) Enrichment analysis of hub genes is shown with bar chart in BP, CC, MF, and KEGG. (C) Enrichment analysis of hub genes is shown with bubble diagram. (D) Enrichment analysis of hub genes is shown with network planning.
FIGURE 5.
Exploration of hub genes in immune cells. (A–H) Eight bar charts showing expression of each hub gene in immune cells in decreasing order.
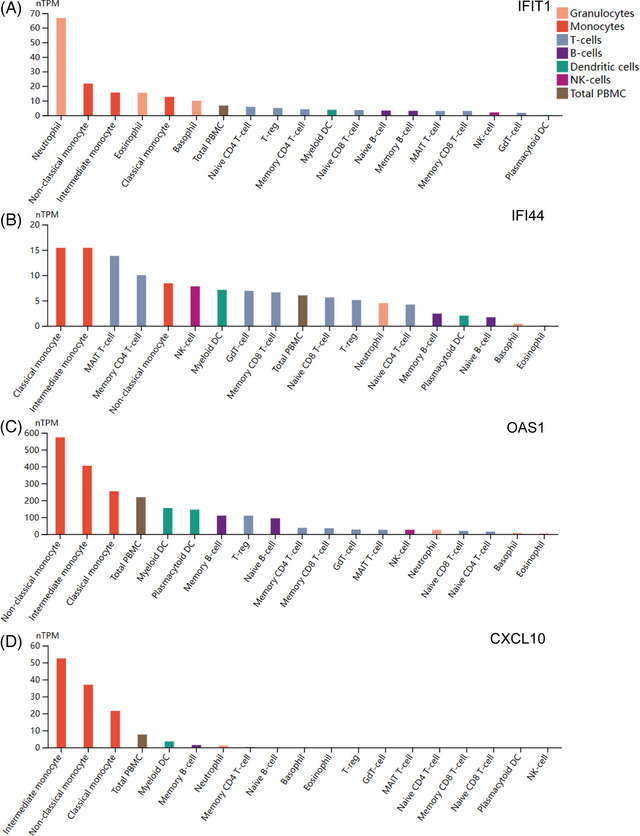
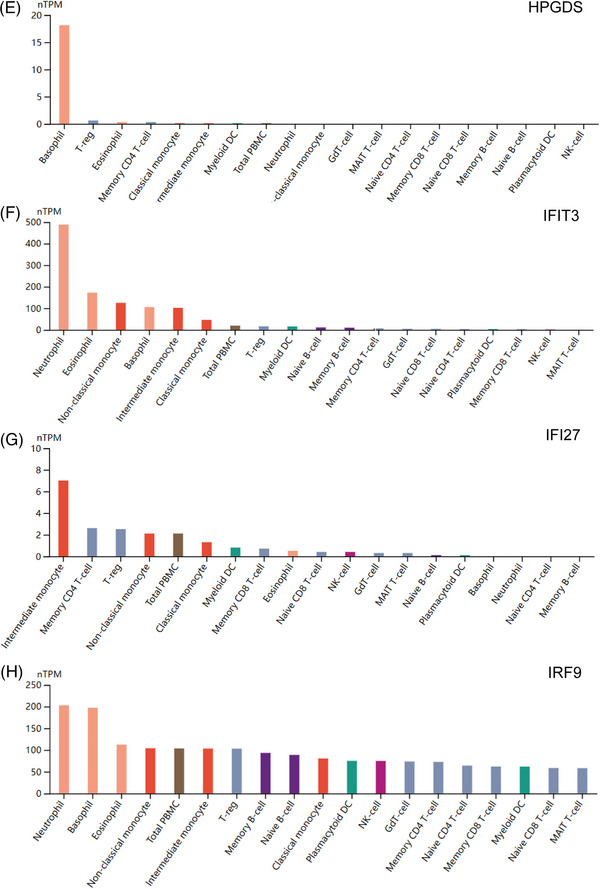
FIGURE 6.
Exploration of hub genes with their neighbor genes and their transcription factors. (A) The network of hub genes and their nearest neighbors. Orange represents hub genes and yellow represents their neighbors based on immune cell RNA expression. (B) Regulatory network of TFs. Orange represents related hub genes, and blue represents TFs.
3.5. TFs prediction of hub genes
On the basis of the TTRUST database, 11 TFs were obtained that regulate the hub genes, including STAT3, POU2F1, IRF1, IRF3, IRF7, NFKB1, RELA, SPI1, STAT1, STAT2, and BRCA1. Details such as descriptions, corresponding target genes, mode of regulation, and references (PMID) have been recorded (Table S4). The TFs–Hub genes network was constructed, including 16 nodes and 13 edges (Figure 6B).
3.6. Exploration of common miRNAs in psoriasis and NASH
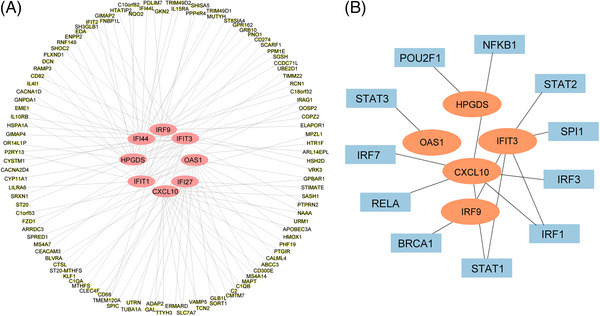
Totally, 96 miRNAs related to psoriasis and 84 miRNAs related to NASH were identified via the GEO datasets (GSE31037, GSE33857) and Linux operating system. There were five common miRNAs between psoriasis and NASH shown in the Veen diagram, including hsa‐miR‐1305, hsa‐miR‐483‐3p, hsa‐miR‐139‐3p, hsa‐miR‐142‐5p, hsa‐miR‐142‐3p (Figure 7A).
FIGURE 7.
Exploration of miRNAs. (A) Five overlapping miRNA in psoriasis and psoriasis. (B) Cell specificity of common miRNAs, top seven are shown. (C) Function of common miRNAs via heat map. (D) Function of common miRNAs via bar plot, top nine are shown. (E) Two overlapping genes between target genes of miRNAs and common DEGs. (F) The miRNAs‐mRNAs network. Blue represents miRNAs and yellow represents common DEGs.
As a result, these miRNAs are cell‐specific to COBL‐a rinderpest (‐C) infection, hepatic sinusoidal endothelial cells, amniotic epithelial cells, CD19+B cells, CD14+ monocytes, pineal gland, and chorionic membrane cells, which were shown as a bar chart (Figure 7B). Also, these miRNAs were involved in the top nine functions: regulation of Wnt signaling pathway, erythrocyte differentiation, cell migration, T‐cell differentiation, wound healing, innate immunity, hematopoiesis, regulation of stem cell and inflammation, presented by cell chart (Figure 7C) and bar chart (Figure 7D).
3.7. The common miRNAs‐mRNAs network
A total of 2394 target genes were obtained via miRTarbase. Two important genes were found in both 2394 target genes and 20 common DEGs, including CHL1 and MBNL2 (Figure 7E). Then, the miRNAs‐mRNAs network was established, presenting the relationship between one miRNA and two mRNAs (Figure 7F).
4. DISCUSSION
In our research, we obtained 20 DEGs, eight hub genes and five common miRNAs via bioinformatic methods and further investigated 11 TFs based on experimental databases in psoriasis and NASH. Meanwhile, the enrichment of biological processes, molecular functions, and signaling pathways most relevant to these genes as well as miRNAs has been identified.
The primary pathogenesis of psoriasis is: (1) Genetic contributions: The major genetic risk factor for early‐stage psoriasis is HLA‐C*06:02. 1 (2) Environmental triggers: Psoriasis requires various environmental triggers, including stress, infection, alcohol consumption, smoking, drugs, sunlight, weight gain, and obesity. 1 (3) Crosstalk between innate and adaptive immune systems and feedforward amplification of inflammation: IL‐23 produced by dendritic cells stimulates Th17 cells to release IL‐17/22, which leads to hyperplasia of keratinocytes and inflammation of skin by activating JAK‐STAT and NF‐κB signaling pathways. 1 The primary pathogenesis of NASH is: (1) Injured hepatocytes release factors promoting accumulation of immune cells which produce hepatotoxic substances and irritate further injury and inflammation. 26 , 27 (2) Excessive lipid accumulation in liver causes hepatocellular lipotoxicity via cellular and organelle oxidative stresses, increases the production of reactive oxygen species (ROS), and eventually leads to hepatic inflammation by activating NF‐κB or c‐Jun N‐terminal kinase (JNK) pathways where insulin resistance plays a large role. 14 , 15 , 28 (3) A proinflammatory cytokine cascade with recruitment of amounts of immune cells contributes to NASH. 26 , 27 Meanwhile, there are also researches that specifically summarize the mechanisms of comorbidities of PSO and NSAH from perspectives of epidemiology, genetics, microbiology, and immunology. They put forward that IL‐17 signaling pathway, which plays a crucial role in psoriasis, is also especially significant to facilitate the progression in NASH by regulating adipogenesis and glucose metabolism. 14 , 15
According to our findings, IFIT1, IFIT3, OAS1, HPGDS, IFI27, IFI44, CXCL10, and IRF9 are eight hub genes for both psoriasis and NASH. As members of IFIT (IFN‐γ stimulated genes) family, IFIT1 and IFIT3 may act as a regulator of anti‐inflammatory and immune responses. 29 , 30 In some researches, IFIT1 was identified low expression when interventions were used to suppress nonalcoholic hepatitis, 29 , 31 and the expression of IFIT1 and IFIT3 was validated as upregulated in psoriasis, 21 , 32 , 33 , 34 which was consistent with the high expression in both psoriasis and NASH we found. OAS1 is a member of OASs (2′−5′‐oligoadenylate synthetases) family, which also contains OAS2, OAS3, and OASL, being IFN‐induced enzymes with multiple antiviral activities. The overexpression of OAS1 in psoriasis and low expression after biologics were revealed in some researches, 34 , 35 accordant with ours. Although OAS1 has not been studied in NASH so far, OAS2, which belongs to the same family as OAS1, related to cell growth, inflammation, and the immune response, has been shown to be overexpressed in cirrhosis when NASH subsequently progresses to the terminal stage, 36 accordant with ours as well. As the only downregulated hub gene in our study, HPGDS is a cytosolic protein, mainly located in mast cells and antigen‐presenting cells, which converts prostaglandin H2 into anti‐inflammatory factors prostaglandin D2. 37 , 38 It has been reported that enhanced inflammatory response in oviduct is due to the deficiency of HPGDS. 38 Another research confirmed that HPGDS was downregulated in diabetic wounds, and its deficiency delayed normal mice wound healing. 37 Despite there have been no research on the relationship between HPGDS and psoriasis or NASH, we hypothesize that due to its low expression in both diseases, the ability to suppress inflammation is weakened, which contributes to the development of psoriasis and NASH. IFI27 and IFI44 are some of few ISGs (Interferon‐stimulated genes) with antibacterial activity which are cellular products that mediate the type I interferon response against a wide range of invading viruses. 39 Same as the results of this study yield, IFI27 is highly expressed in psoriasis, and has been identified the involvement in proliferation of skin keratinocytes in imiquimod‐induced psoriasis‐like skin. 40 IRF9 is a member of the interferon regulatory transcription factor family, which function in the regulation of immune cells, cell cycles, and apoptosis in response to various stimulation. 41 A gene cluster comprising PI3, IRF9, IFIT1, and NMI were found as positively correlated and differentially co‐expressed for women. 41 From another aspect, IRF9 knockout increased inflammation insulin resistance, hepatic steatosis, in mice on high‐fat diet. 42 CXCL10 (interferon gamma‐induced protein 10) is classified as an inflammatory chemokine functionally, and is identified to combine with receptor CXCR3 and regulate immune responses via the activation and recruitment of T cells, eosinophils, and monocytes, which often amplifies effects of other cytokines. 43 , 44 , 45 Increases of serum and/or tissue expressions of CXCL10 in various autoimmune diseases like rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) have been reported. 44 Other researchers identified that CXCL10 expressed highly in many Th1‐type human inflammatory diseases, including psoriasis, which is an organ‐specific autoimmune disease as well. 20 , 46 Moreover, CXCL10 correlates positively with the incidence of obesity and type 2 diabetes and is upregulated in NASH because of the activation of NF‐κB signal pathway and oxidative stress through CYP2E1 and C/EBPβ. 43
Among our enrichment results, multiple types of viral infections, metabolic processes, and pathways were speculated to be associated with these two diseases mostly, including hepatitis C, influenza A, Epstein‐Barr virus infection, coronavirus disease‐COVID‐19, measles, herpes simplex virus 1 infection, glutathione metabolism, arachidonic acid metabolism, NOD‐like receptor signaling pathway, RIG‐I‐like receptor signaling pathway, IL‐17 signaling pathway, toll‐like receptor signaling pathway, C‐type lectin receptor signaling pathway, and TNF signaling pathway. From our data, it seems that viral infection draws close ties with psoriasis and NASH. Meanwhile, HCV and HPV infection were reported as an inducing factor for psoriasis. 47 , 48 , 49 In hepatitis C patients, elevated tumor necrosis factor‐α may cause progression of hepatic disease including NASH and trigger psoriasis in patients with a certain predisposition. 47 , 50 , 51 Other researches have uncovered a critical innate immune pathway where RIG‐I‐mediated antiviral response is critical to trigger IL‐23 expression and the development of psoriatic pathology in psoriasis. 52 IRF‐3/7, as the predicted TFs for our hub genes, together with NF‐κB being the main downstream signaling pathways of RIG‐I, may promote the production of type I interferon and IFN‐α via pDCs, contributing to initiate psoriasis. 52 Also, hepatic steatosis can result from direct viral cytopathic effect in genotype 3 HCV infection. 51 In an observational study, researchers found that HPV infection triggered plaque‐type psoriasis by promoting lesion to an inflammatory state, upregulating of nerve growth factor, and finally leading to classical pathological features of psoriasis, including keratinocyte proliferation, angiogenesis, and T cell activation. 53 , 54 There was a novel coronavirus named as SARS‐CoV‐2 triggering an outbreak of coronavirus disease 2019 (COVID‐19) at the end of 2019. A few new‐onset psoriasis were reported in the context of COVID‐19 infection. 55 , 56 Stimulation of toll‐like receptor 3 by viral RNA, causing dysregulation of innate immune response and production of IL‐36‐γ and CXCL8 may be the possible pathogenesis of COVID‐19 infection leading to psoriasis, 49 , 57 , 58 , 59 and the hyperinflammatory state of COVID‐19 may exacerbate psoriasis. 49 , 60 Moreover, COVID‐19 infection can trigger inflammatory cytokine storms involving both the innate and the cellular adaptive immunity, which play a critical role in the pathogenesis of liver disease and exacerbate steatohepatitis. 61
MicroRNAs (miRNAs) are short (20–24 nt) non‐coding RNAs that are involved in post‐transcriptional regulation of gene expression in multicellular organisms by affecting both the stability and translation of mRNAs. Our findings identified five common miRNAs for both psoriasis and NASH, including hsa‐miR‐1305, hsa‐miR‐483‐3p, hsa‐miR‐139‐3p, hsa‐miR‐142‐5p, and hsa‐miR‐142‐3p. So far, hsa‐miR‐1305 has been found to participate in the regulation of TGF‐β pathway members, which were related to lymphocytes T activation, virus infections, ventricular remodeling, and HF progression, and also has been identified to be associated with type 2 diabetes mellitus, which is well known to be a usual metabolic disease. 62 , 63 Later, its relationship with cell cycles and differentiation was revealed. Low expression of hsa‐miR‐1305 facilitated the maintenance of pluripotency and increased cell survival, while its upregulation increased cell apoptosis and sped up G1/S transition. 64 As so much abnormal proliferation of keratinocytes in psoriasis, it may be a potential therapeutic method by upregulating hsa‐miR‐1305. In common metabolic diseases, hsa‐miR‐483‐3p was proved to inhibit the repair of endothelial cells in type 2 diabetes and contribute to high risk of cardiovascular diseases. 65 Moreover, as a terminal disease stage of NASH, liver cirrhosis could cause cirrhotic cardiomyopathy, where hsa‐miR‐483‐3p was found to be a key miRNA to promote its occurrence and progression. 66 However, hsa‐miR‐139‐3p has only been proved to participate in various cancers, 67 , 68 and there has been no research about its relationship with psoriasis, NASH, or other metabolic diseases, which suggests more further studies. As for hsa‐miR‐142‐3p and hsa‐miR‐142‐5p, some researchers identified their participation in metabolic diseases, including insulin resistance and obesity in preadolescent children 69 , 70 as well as metabolic syndrome‐associated cardiovascular disease by regulating metabolic and inflammatory signals. 71 So, we suppose hsa‐miR‐142‐3p and hsa‐miR‐142‐5p may take part in psoriasis and NASH as well, and even be possible therapies for them, which need more experiments.
By taking the intersection of 20 DEGs and 2394 target genes predicted by the common miRNAs, two DEGS (CHL1, MBNL2) and one miRNA (hsa‐miR‐1305) were obtained at the end of our findings. CHL1 (cell adhesion molecule L1 like) was discovered as promotion of neurite growth and a coreceptor with integrins to enhance cell migration, and it has been linked to metabolic diseases. In type 2 diabetes, CHL1 was proved to reduce insulin resistance by activating AKT pathway and reducing inflammatory factor expression levels including IL‐6 and TNF‐α, and be downregulated in type 2 diabetes. 72 , 73 , 74 As a member of RNA binding protein MBNL family, MBNL2 was identified to be low expression in cancers and myotonic dystrophy type 1. 14 , 15 , 16 Despite no researches have investigated its expression in metabolic diseases related with psoriasis or NASH, an enrichment of PI3K/AKT pathway in MBNL2‐depleted cells was identified. 75 So we assume that upregulating the expression of MBNL2 may induce less inflammatory factors by inhibiting PI3K/AKT pathway, and be a potential therapeutic target for the co‐occurrence of psoriasis and NASH. Although there has been no research about the relationship between CHL1, MBNL2, and hsa‐miR‐1305, we infer that CHL1 and MBNL2 may participate in the process of psoriasis and NASH via regulating hsa‐miR‐1305 based on our results, and may be a potential therapeutic target for future treatment for the co‐occurrence of psoriasis and NASH.
However, there are still some limitations in our study: (1) We did not verify further by experiments even if we try to choose high‐throughput sequencing data and experimentally‐based databases during our research. (2) Due to the limitations of public dataset sample size and sequencing platform, the datasets we chose are not all blood samples, and supplemental blood samples will be more comprehensive. (3) The sample size of NASH is not large enough.
5. CONCLUSION
IFIT1, IFIT3, OAS1, HPGDS, IFI27, IFI44, CXCL10, and IRF9 are eight hub genes we got for both psoriasis and NASH, and this provides new approaches and possible targets for the treatment of the co‐occurrence of these two diseases in the future. Moreover, a potential relationship between the common miRNA (hsa‐miR‐1305) and DEGs (CHL1, MBNL2) were shown at last. Further experiments will be investigated to verify how hsa‐miR‐1305 plays a significant role in the pathogenesis of both psoriasis and NASH by regulating CHL1 and MBNL2.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank the researchers who are generous and have uploaded their experimental data onto online databases. We thank Professor Zheng (The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China) for her methodological assistances.
Xian N, Bai R, Guo J, et al. Bioinformatics analysis to reveal the potential comorbidity mechanism in psoriasis and nonalcoholic steatohepatitis. Skin Res Technol. 2023;29:e13457. 10.1111/srt.13457
Ningyi Xian and Ruimin Bai contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article.
REFERENCES
- 1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301‐1315. doi: 10.1016/S0140-6736(20)32549-6 [DOI] [PubMed] [Google Scholar]
- 2. Qu Y, Li D, Xiong H, Shi D. Transcriptional regulation on effector T cells in the pathogenesis of psoriasis. Eur J Med Res. 2023;28(1):182. doi: 10.1186/s40001-023-01144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnekoh H, Erpenbeck L. Neutrophilic dermatoses—pathomechanistic concepts and therapeutic developments. J Dtsch Dermatol Ges. 2023;21(4):374‐380. doi: 10.1111/ddg.15055 [DOI] [PubMed] [Google Scholar]
- 4. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840‐848. doi: 10.1111/bjd.18245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo L, Guo Y, Chen L, Zhu J, Li C. Crosstalk between cholesterol metabolism and psoriatic inflammation. Front Immunol. 2023;14:1124786. doi: 10.3389/fimmu.2023.1124786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powell EE, Wong VWS, Rinella M. Non‐alcoholic fatty liver disease. Lancet. 2021;397(10290):2212‐2224. doi: 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 7. Musso G, Saba F, Cassader M, Gambino R. Lipidomics in pathogenesis, progression and treatment of nonalcoholic steatohepatitis (NASH): recent advances. Prog Lipid Res. 2023;91:101238. doi: 10.1016/j.plipres.2023.101238 [DOI] [PubMed] [Google Scholar]
- 8. Brennan PN, Elsharkawy AM, Kendall TJ, Loomba R, Mann DA, Fallowfield JA. Antifibrotic therapy in nonalcoholic steatohepatitis: time for a human‐centric approach. Nat Rev Gastroenterol Hepatol. 2023;2:1‐10. doi: 10.1038/s41575-023-00796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee WH, Najjar SM, Kahn CR, Hinds TD. Hepatic insulin receptor: new views on the mechanisms of liver disease. Metabolism. 2023;145:155607. doi: 10.1016/j.metabol.2023.155607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kazankov K, Jørgensen SMD, Thomsen KL, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):145‐159. doi: 10.1038/s41575-018-0082-x [DOI] [PubMed] [Google Scholar]
- 11. Haldar D, Kern B, Hodson J, et al. Outcomes of liver transplantation for non‐alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71(2):313‐322. doi: 10.1016/j.jhep.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsochatzis E, Coilly A, Nadalin S, et al. International liver transplantation consensus statement on end‐stage liver disease due to nonalcoholic steatohepatitis and liver transplantation. Transplantation. 2019;103(1):45. doi: 10.1097/TP.0000000000002433 [DOI] [PubMed] [Google Scholar]
- 13. Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non‐alcoholic‐steatohepatitis–related liver transplantation waitlist additions in the United States. Hepatology. 2019;70(2):487‐495. doi: 10.1002/hep.29473 [DOI] [PubMed] [Google Scholar]
- 14. Bellinato F, Gisondi P, Mantovani A, Girolomoni G, Targher G. Risk of non‐alcoholic fatty liver disease in patients with chronic plaque psoriasis: an updated systematic review and meta‐analysis of observational studies. J Endocrinol Invest. 2022;45(7):1277‐1288. doi: 10.1007/s40618-022-01755-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ortolan A, Lorenzin M, Tadiotto G, et al. Metabolic syndrome, non‐alcoholic fatty liver disease and liver stiffness in psoriatic arthritis and psoriasis patients. Clin Rheumatol. 2019;38(10):2843‐2850. doi: 10.1007/s10067-019-04646-7 [DOI] [PubMed] [Google Scholar]
- 16. Carrascosa JM, Bonanad C, Dauden E, Botella R, Olveira‐Martín A. Psoriasis and nonalcoholic fatty liver disease. Actas Dermo‐Sifiliográficas (English Edition). 2017;108(6):506‐514. doi: 10.1016/j.adengl.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 17. Abedini R, Salehi M, Lajevardi V, Beygi S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. Clin Exp Dermatol. 2015;40(7):722‐727. doi: 10.1111/ced.12672 [DOI] [PubMed] [Google Scholar]
- 18. Dorai S, Alex Anand D. Differentially expressed cell cycle genes and STAT1/3‐driven multiple cancer entanglement in psoriasis, coupled with other comorbidities. Cells. 2022;11(23):3867. doi: 10.3390/cells11233867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He H, Bissonnette R, Wu J, et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J Allergy Clin Immunol. 2021;147(1):199‐212. doi: 10.1016/j.jaci.2020.05.048 [DOI] [PubMed] [Google Scholar]
- 20. Luo Y, Luo Y, Chang J, Xiao Z, Zhou B. Identification of candidate biomarkers and pathways associated with psoriasis using bioinformatics analysis. Hereditas. 2020;157(1):30. doi: 10.1186/s41065-020-00141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang YJ, Sun YZ, Gao XH, Qi RQ. Integrated bioinformatic analysis of differentially expressed genes and signaling pathways in plaque psoriasis. Mol Med Rep. 2019;20(1):225‐235. doi: 10.3892/mmr.2019.10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Ma L, Ge Z, Pan Y, Xie L. Key genes associated with non‐alcoholic fatty liver disease and polycystic ovary syndrome. Front Mol Biosci. 2022;9:888194. doi: 10.3389/fmolb.2022.888194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu Y, Yu F, Wu Y, et al. Identification of genes and key pathways underlying the pathophysiological association between nonalcoholic fatty liver disease and atrial fibrillation. BMC Med Genomics. 2022;15(1):150. doi: 10.1186/s12920-022-01300-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Govaere O, Cockell S, Tiniakos D, et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med. 2020;12(572):eaba4448. doi: 10.1126/scitranslmed.aba4448 [DOI] [PubMed] [Google Scholar]
- 25. Jiang ZY, Zhou Y, Zhou L, Li SW, Wang BM. Identification of key genes and immune infiltrate in nonalcoholic steatohepatitis: a bioinformatic analysis. Biomed Res Int. 2021;2021:1. doi: 10.1155/2021/7561645 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Park SJ, Garcia Diaz J, Um E, Hahn YS. Major roles of kupffer cells and macrophages in NAFLD development. Front Endocrinol (Lausanne). 2023;14:1150118. doi: 10.3389/fendo.2023.1150118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huby T, Gautier EL. Immune cell‐mediated features of non‐alcoholic steatohepatitis. Nat Rev Immunol. 2022;22(7):429‐443. doi: 10.1038/s41577-021-00639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim KH, Lee MS. Pathogenesis of nonalcoholic steatohepatitis and hormone‐based therapeutic approaches. Front Immunol. 2018;9:485. Accessed May 6, 2023. https://www.frontiersin.org/articles/10.3389/fendo.2018.00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang R, Yao L, Lin X, Hu X, Wang L. Exploring the potential mechanism of Rhodomyrtus tomentosa (Ait.) Hassk fruit phenolic rich extract on ameliorating nonalcoholic fatty liver disease by integration of transcriptomics and metabolomics profiling. Food Res Int. 2022;151:110824. doi: 10.1016/j.foodres.2021.110824 [DOI] [PubMed] [Google Scholar]
- 30. Hwang JW, Lee KJ, Choi IH, Han HM, Kim TH, Lee SH. Decreased expression of type I (IFN‐β) and type III (IFN‐λ) interferons and interferon‐stimulated genes in patients with chronic rhinosinusitis with and without nasal polyps. J Allergy Clin Immunol. 2019;144(6):1551‐1565. e2. doi: 10.1016/j.jaci.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janssen AWF, Betzel B, Stoopen G, et al. The impact of PPARα activation on whole genome gene expression in human precision cut liver slices. BMC Genomics. 2015;16:760. doi: 10.1186/s12864-015-1969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zou A, Jian Q. CXCL10 and its related key genes as potential biomarkers for psoriasis: evidence from bioinformatics and real‐time quantitative polymerase chain reaction. Medicine (Baltimore). 2021;100(38):e27365. doi: 10.1097/MD.0000000000027365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li X, Zhou W, Wang D. Integrative bioinformatic analysis identified IFIT3 as a novel regulatory factor in psoriasis. J Cell Biochem. 2022;123(12):2066‐2078. doi: 10.1002/jcb.30332 [DOI] [PubMed] [Google Scholar]
- 34. Tang S, Jiang W, Xu P, et al. Integrated bioinformatic analysis of key biomarkers and signalling pathways in psoriasis. Scott Med J. 2022;67(1):7‐17. doi: 10.1177/00369330221078993 [DOI] [PubMed] [Google Scholar]
- 35. Huang YZ, Zheng YX, Zhou Y, et al. OAS1, OAS2, and OAS3 contribute to epidermal keratinocyte proliferation by regulating cell cycle and augmenting IFN‐1‒induced Jak1‒signal transducer and activator of transcription 1 phosphorylation in psoriasis. J Invest Dermatol. 2022;142(10):2635‐2645. e9. doi: 10.1016/j.jid.2022.02.018 [DOI] [PubMed] [Google Scholar]
- 36. Blencowe M, Karunanayake T, Wier J, Hsu N, Yang X. Network modeling approaches and applications to unravelling non‐alcoholic fatty liver disease. Genes. 2019;10(12):966. doi: 10.3390/genes10120966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ouyang L, Qiu D, Fu X, et al. Overexpressing HPGDS in adipose‐derived mesenchymal stem cells reduces inflammatory state and improves wound healing in type 2 diabetic mice. Stem Cell Res Ther. 2022;13:395. doi: 10.1186/s13287-022-03082-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bridges PJ, Jeoung M, Shim S, et al. Hematopoetic prostaglandin D synthase: an ESR1‐Dependent oviductal epithelial cell synthase. Endocrinology. 2012;153(4):1925‐1935. doi: 10.1210/en.2011-1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu YW, Chen YJ, Shi N, et al. IL36G is associated with cutaneous antiviral competence in psoriasis. Front Immunol. 2022;13:971071. doi: 10.3389/fimmu.2022.971071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsieh W‐L, Huang Y‐H, Wang T‐M, Ming Y‐C, Tsai C‐N, Pang J‐HS. IFI27, a novel epidermal growth factor‐stabilized protein, is functionally involved in proliferation and cell cycling of human epidermal keratinocytes. Cell Prolif. 2015;48(2):187‐197. doi: 10.1111/cpr.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sevimoglu T, Turanli B, Bereketoglu C, Arga KY, Karadag AS. Systems biomarkers in psoriasis: integrative evaluation of computational and experimental data at transcript and protein levels. Gene. 2018;647:157‐163. doi: 10.1016/j.gene.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 42. Zhang C, Liu S, Yang M. The role of interferon regulatory factors in non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Gastroenterol Insights. 2022;13(2):148‐161. doi: 10.3390/gastroent13020016 [DOI] [Google Scholar]
- 43. Zhang X, Shen J, Man K, et al. CXCL10 plays a key role as an inflammatory mediator and a non‐invasive biomarker of non‐alcoholic steatohepatitis. J Hepatol. 2014;61(6):1365‐1375. doi: 10.1016/j.jhep.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 44. Lee EY, Lee ZH, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev. 2009;8(5):379‐383. doi: 10.1016/j.autrev.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 45. Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN‐gamma‐inducible protein 10 (IP‐10; CXCL10)‐deficient mice reveal a role for IP‐10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195‐3204. doi: 10.4049/jimmunol.168.7.3195 [DOI] [PubMed] [Google Scholar]
- 46. Ramessur R, Corbett M, Marshall D, et al. Biomarkers of disease progression in people with psoriasis: a scoping review. Br J Dermatol. 2022;187(4):481‐493. doi: 10.1111/bjd.21627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Imafuku S, Nakayama J. Profile of patients with psoriasis associated with hepatitis C virus infection. J Dermatol. 2013;40(6):428‐433. doi: 10.1111/1346-8138.12112 [DOI] [PubMed] [Google Scholar]
- 48. Jain SP, Gulhane S, Pandey N, Bisne E. Human papilloma virus infection and psoriasis: did human papilloma virus infection trigger psoriasis? Indian J Sex Transm Dis AIDS. 2015;36(2):201‐203. doi: 10.4103/0253-7184.167178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou S, Yao Z. Roles of infection in psoriasis. Int J Mol Sci. 2022;23(13):6955. doi: 10.3390/ijms23136955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian Z, Xu C, Yang P, et al. Molecular pathogenesis: connections between viral hepatitis‐induced and non‐alcoholic steatohepatitis‐induced hepatocellular carcinoma. Front Immunol. 2022;13:984728. doi: 10.3389/fimmu.2022.984728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hwang SJ, Lee SD. Hepatic steatosis and hepatitis C: still unhappy bedfellows? J Gastroenterol Hepatol. 2011;26(Suppl 1):96‐101. doi: 10.1111/j.1440-1746.2010.06542.x [DOI] [PubMed] [Google Scholar]
- 52. Zhu H, Lou F, Yin Q, et al. RIG‐I antiviral signaling drives interleukin‐23 production and psoriasis‐like skin disease. EMBO Mol Med. 2017;9(5):589‐604. doi: 10.15252/emmm.201607027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen ML, Kao WM, Huang JY, Hung YM, Wei JCC. Human papillomavirus infection associated with increased risk of new‐onset psoriasis: a nationwide population‐based cohort study. Int J Epidemiol. 2020;49(3):786‐797. doi: 10.1093/ije/dyaa027 [DOI] [PubMed] [Google Scholar]
- 54. Simeone P, Teson M, Latini A, Carducci M, Venuti A. Human papillomavirus type 5 in primary keratinocytes from psoriatic skin. Exp Dermatol. 2005;14(11):824‐829. doi: 10.1111/j.1600-0625.2005.00358.x [DOI] [PubMed] [Google Scholar]
- 55. Rouai M, Rabhi F, Mansouri N, Jaber K, Dhaoui R. New‐onset guttate psoriasis secondary to COVID‐19. Clin Case Rep. 2021;9(7):e04542. doi: 10.1002/ccr3.4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mathieu RJ, Cobb CBC, Telang GH, Firoz EF. New‐onset pustular psoriasis in the setting of severe acute respiratory syndrome coronavirus 2 infection causing coronavirus disease 2019. JAAD Case Rep. 2020;6(12):1360‐1362. doi: 10.1016/j.jdcr.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181(6):1304‐1306. doi: 10.1111/bjd.18203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin‐36‐receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365(7):620‐628. doi: 10.1056/NEJMoa1013068 [DOI] [PubMed] [Google Scholar]
- 59. Hewson CA, Jardine A, Edwards MR, Laza‐Stanca V, Johnston SL. Toll‐like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J Virol. 2005;79(19):12273‐12279. doi: 10.1128/JVI.79.19.12273-12279.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ozaras R, Berk A, Ucar DH, Duman H, Kaya F, Mutlu H. Covid‐19 and exacerbation of psoriasis. Dermatol Ther. 2020;33(4):e13632. doi: 10.1111/dth.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID‐19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18‐24. doi: 10.1016/j.ejim.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wicik Z, Eyileten C, Jakubik D, et al. ACE2 interaction networks in COVID‐19: a physiological framework for prediction of outcome in patients with cardiovascular risk factors. J Clin Med. 2020;9(11):3743. doi: 10.3390/jcm9113743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lan X, Han J, Wang B, Sun M. Integrated analysis of transcriptome profiling of lncRNAs and mRNAs in livers of type 2 diabetes mellitus. Physiol Genomics. 2022;54(2):86‐97. doi: 10.1152/physiolgenomics.00105.2021 [DOI] [PubMed] [Google Scholar]
- 64. Jin S, Collin J, Zhu L, et al. A Novel Role for miR‐1305 in regulation of pluripotency‐differentiation balance, cell cycle, and apoptosis in human pluripotent stem cells. Stem Cells. 2016;34(9):2306‐2317. doi: 10.1002/stem.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuschnerus K, Straessler ET, Müller MF, Lüscher TF, Landmesser U, Kränkel N. Increased expression of miR‐483‐3p impairs the vascular response to injury in type 2 diabetes. Diabetes. 2019;68(2):349‐360. doi: 10.2337/db18-0084 [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y, Wang Z, Zhao L, et al. Comprehensive evaluation of circRNAs in cirrhotic cardiomyopathy before and after liver transplantation. Int Immunopharmacol. 2023;114:109495. doi: 10.1016/j.intimp.2022.109495 [DOI] [PubMed] [Google Scholar]
- 67. Xia G, Wang A, Li L. hsa_circ_0000218/hsa‐miR‐139‐3p/SOX4 regulatory feedback circuit influences the proliferation and apoptosis of gastric cancer cells. Cytotechnology. 2022;74(1):89‐98. doi: 10.1007/s10616-021-00509-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu Z, Chen S, Zuo T, et al. miR‐139‐3p/Wnt5A Axis inhibits metastasis in hepatoblastoma. Mol Biotechnol. 2023. doi: 10.1007/s12033-023-00714-1 [DOI] [PubMed] [Google Scholar]
- 69. Oses M, Medrano M, Margareto Sanchez J, et al. Peripheral blood mononuclear cells‐expressed miRNA profiles derived from children with metabolic‐associated fatty liver disease and insulin resistance. Pediatr Obes. 2022;17(12):e12966. doi: 10.1111/ijpo.12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oses M, Margareto Sanchez J, Portillo MP, Aguilera CM, Labayen I. Circulating miRNAs as biomarkers of obesity and obesity‐associated comorbidities in children and adolescents: a systematic review. Nutrients. 2019;11(12):2890. doi: 10.3390/nu11122890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu L, Zhou X, Chen J, Li X. Potential of ATP5MG to treat metabolic syndrome‐associated cardiovascular diseases. Front Cardiovasc Med. 2022;9:921778. doi: 10.3389/fcvm.2022.921778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Taneera J, Dhaiban S, Hachim M, et al. Reduced expression of Chl1 gene impairs insulin secretion by down‐regulating the expression of key molecules of β‐cell function. Exp Clin Endocrinol Diabetes. 2021;129(12):864‐872. doi: 10.1055/a-1014-2544 [DOI] [PubMed] [Google Scholar]
- 73. Jiang H, Liu Y, Qian Y, et al. CHL1 promotes insulin secretion and negatively regulates the proliferation of pancreatic β cells. Biochem Biophys Res Commun. 2020;525(4):1095‐1102. doi: 10.1016/j.bbrc.2020.03.040 [DOI] [PubMed] [Google Scholar]
- 74. Bacos K, Perfilyev A, Karagiannopoulos A, et al. Type 2 diabetes candidate genes, including PAX5, cause impaired insulin secretion in human pancreatic islets. J Clin Invest. 2023;133(4):e163612. doi: 10.1172/JCI163612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai J, Wang N, Lin G, et al. MBNL2 Regulates DNA Damage Response via Stabilizing p21. International Journal of Molecular Sciences. 2021;22(2):783. doi: 10.3390/ijms22020783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
Data sharing is not applicable to this article.


