Abstract
Chylothorax caused by superior vena cava (SVC) syndrome is a rare but potentially life-threatening complication requiring a multidisciplinary diagnosis and management approach. We present a case of a 27-year-old female with end-stage renal disease who developed chylothorax secondary to SVC syndrome caused by venous stenosis from a tunneled hemodialysis (HD) catheter. The patient had a history of ongoing hemodialysis through a tunneled catheter placed in the right internal jugular vein approximately seven months before presentation. She presented with dyspnea, chest pain, and a large left-sided pleural effusion. A multidisciplinary diagnostic workup and management included 2 thoracentesis, pleural fluid studies, serial radiological tests, right and left heart catheterizations, and blood serum studies with flow cytometry. They revealed that SVC stenosis around the hemodialysis catheter was causing the patient's pathology. The patient underwent veno-plasty of the right SVC and brachiocephalic veins and replacement of HD catheter leading to the resolution of the chylothorax and significant improvement in respiratory symptoms. This report will highlight the approach to diagnosing and managing chylothorax and a review of existing medical literature.
Keywords: Chylothorax, Superior vena cava syndrome
Introduction
Chylothorax is a significant clinical phenomenon defined by the accumulation of lymphatic fluid in the pleural cavity [1]. Although rare, its etiology can be categorized into traumatic causes, most commonly due to surgery, and nontraumatic reasons, which are mainly attributed to malignancy [2]. Superior vena cava (SVC) syndrome is a term to describe a group of symptoms caused by the compression or obstruction of the SVC. Although these are distinct entities, SVC syndrome has been postulated to produce chylothorax in relatively few cases in the literature [3,4]. We herein present a case of a young female with a history significant for end-stage renal disease (ESRD) with a hemodialysis catheter in place who developed a chylothorax due to SVC syndrome. We discuss our approach to diagnosis and management and review the existing literature.
Case presentation
Our patient is a 27-year-old female with a past medical history significant for end-stage renal disease secondary to focal segmental glomerulosclerosis and hypertension. She presented with progressive dyspnea, dry cough, and left-sided pleuritic chest pain. She was diagnosed and treated for pneumonia 2 weeks before presentation with a 5-day course of amoxicillin/clavulanic acid and doxycycline without clinical improvement.
On examination, she was afebrile, saturating well on room air, and had a high blood pressure of 148/89 and eupneic. Expansion of the lungs was symmetric, with decreased air entry on the left compared to the right and mild crackles. Right internal jugular hemodialysis (HD) catheter and left upper extremity arteriovenous fistula were present.
Computed tomography (CT) of the chest revealed a large left pleural effusion associated with the complete collapse of the left lower lobe and mild left upper lobe atelectasis. And small pericardial effusion (Fig. 1). Echocardiogram confirmed a small pericardial effusion.
Fig. 1.
CT of the chest (blue arrow) large left pleural effusion. (Red arrow) Dialysis catheter.
The patient underwent left-sided ultrasound-guided thoracentesis. About 1600 cc of turbid yellow fluid was aspirated. Pleural fluid analysis revealed exudative fluid and an elevated triglyceride level of 361 mg/dL without any microbiological growth or evidence of malignancy (Table 1). Follow-up chylomicrons test and flow cytometry confirmed diagnosis of chylothorax without evidence of lymphoma or acute leukemia.
Table 1.
Pleural fluid analysis at day 2 from ultrasound-guided left thoracocentesis.
| Tests on pleural fluid | D 2 results | Units |
|---|---|---|
| Color | Yellow | Subjective |
| Appearance | Turbid | Subjective |
| Fluid WBC | 3657 | mm3 |
| Fluid RBC | 3000 | mm3 |
| Fluid Neutrophils | 3 | % |
| Fluid Lymphocytes | 97 | % |
| Fluid Glucose | 93 | mg/dL |
| Fluid Protein | 3.3 | mg/dL |
| Fluid LDH | 120 | International Units/L |
| Fluid Cholesterol | <50 | mg/dL |
| Fluid Triglycerides | 361 | mg/dL |
| Serum Total Protein | 6.9 | g/dL |
| Chylomicrons | Positive | N/A |
LDH, lactate dehydrogenase; RBC, red blood cells; WBC, white blood cells.
The nephrology specialist continued intermittent dialysis. Cardiology was consulted for left and right heart catheterization to evaluate the cardiac chamber pressures, given that the echocardiography findings were concerning for pericardial effusion, which could be a potential cause for the now re-accumulating chylothorax. The patient required a second left-sided thoracocentesis due to worsening chest pain and pressure on day 6 of admission with aspirating 1250 cc of milky white pleural fluid. A heart catheterization revealed normal left and right heart chamber pressures and no evidence of constrictive pericarditis. Interventional pulmonology was consulted for further workup of the etiology of the chylothorax and possible long-term management. Interventional radiology specialists were consulted for advanced imaging, given a clinical suspicion of SVC obstruction being the culprit for the patient's presentation and etiology for chylothorax.
On admission day 10, the central HD catheter was removed, and a venogram study revealed 50% moderate stenosis of the distal SVC and more severe local stenosis of the proximal SVC entering the right atrium (Fig. 2). Veno-plasty of the right SVC and brachiocephalic veins was performed, and the HD catheter was replaced. The day following the procedure, the patient noted improved shortness of breath and chest pain. Follow-up serial chest X-rays showed continued improvement and resolution of pleural pathology.
Fig. 2.
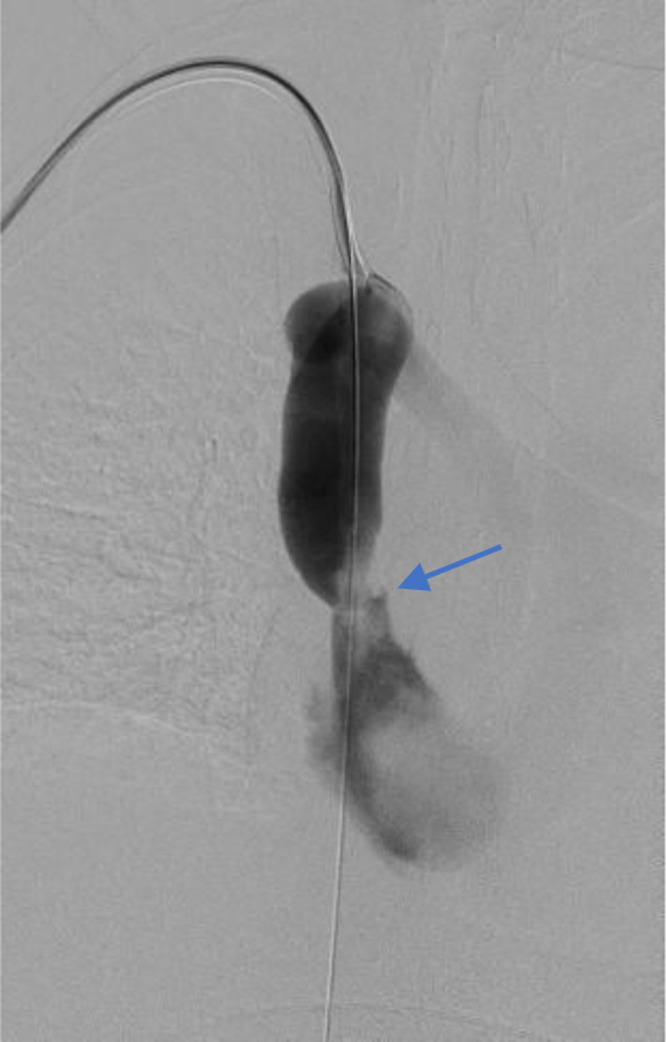
Venogram displaying enlarged superior vena cava and site of stenosis (Blue arrow).
After being admitted to the hospital for 13 days, the patient was discharged in stable condition. She was instructed to follow up with interventional pulmonology for additional imaging to assess for complete resolution of chylothorax. On follow-up, her pulmonary issues had resolved.
Discussion
Chylothorax is an uncommon cause of pleural effusion and typically occurs secondary to extravasation of the chyle into the pleural space [5]. In those cases, the pleural fluid has a milky appearance and is characterized by elevated triglyceride levels (>110 mg/dL) and the presence of chylomicrons [6].
While the differential for chylothorax is extensive, this pathology is typically classified into traumatic or nontraumatic chylothorax [7]. Traumatic chylothorax can be iatrogenic, secondary to damage to lymphatics during thoracic surgeries and procedures, such as esophageal surgery, subclavian vein catheterization, or central venous catheterization [7]. Traumatic chylothorax can also be noniatrogenic and caused by direct trauma to the thoracic duct from penetrating blunt trauma, childbirth, or spine dislocation [7].
Nontraumatic chylothorax also has a broad differential. Primary disease of the lymph vessels is uncommon, with only a few cases reported in the literature [8]. Common causes of nontraumatic chylothorax include malignant and benign tumors, sarcoidosis, amyloidosis, SVC thrombosis, and congenital duct anomalies [7].
Regardless of the underlying cause and type of chylothorax, diagnosis is always made through thoracentesis and laboratory analysis of pleural fluid. In particular, lipoprotein analysis identifying chylomicrons in pleural fluid is the gold standard for confirming the diagnosis [7]. Another strong predictor of chylothorax diagnosis is the presence of elevated triglycerides on fluids analysis (>110 mg/dL) [9]. Furthermore, patients with high suspicion of traumatic chylothorax should be monitored closely, especially those with persistent drainage from a chest drain. In nontraumatic cases, a CT chest, abdomen, and pelvis should be considered to assess for lymphadenopathy and masses because of the strong association between malignancies and chylothorax. Finally, lymphangiography can be considered in patients where the leakage site is not evident [10].
The management of chylothorax should be centered around identifying and treating the underlying cause and implementing noninvasive measures to decrease chyle production. If noninvasive measures are ineffective, interventions such as pleurodesis, thoracic duct ligation, or embolization may be necessary [5,11]. Noninvasive measures such as dietary modifications, octreotide therapy, or thoracentesis should be attempted to reduce chyle production during diagnosis and as a bridge to long-term management. Somatostatin analogs and octreotide reduce intestinal chyle production, thereby decreasing the volume of chyle traveling through the thoracic duct. If the pathology is caused by malignancy, such as obstructing mass, or hematological/oncological conditions, such as lymphoma, then a combination of chemotherapy, radiation, and or surgery should be performed and evaluated on a case-by-case basis [11,12].
SVC syndrome is an infrequent cause of chylothorax, with only a few cases reported in the literature. While the pathophysiology of this entity is not fully understood, it is hypothesized that the increased pressure from the SVC obstruction leads to increased pressure in the thoracic duct [1]. This eventually leads to chyle leakage in the pleural space and the development of a chylothorax. In those cases, treating the underlying cause of SVC syndrome is crucial for the chylothorax to resolve [1].
Conclusion
In conclusion, this case report highlights the rare occurrence of SVC syndrome causing chylothorax in a young female with ESRD. SVC syndrome should be considered a possible cause of chylothorax in ESRD patients, particularly those with central venous catheterization or pathologies that compromise SVC integrity. Although the diagnostic process might be challenging, a high index of clinical suspicion paired with imaging examinations, pleural fluid analysis, and a multidisciplinary approach can aid in making a timely diagnosis. In our case, we outlined the complete diagnostic process and the stepwise approach to managing the pathology presented.
Patient consent
Informed consent was obtained from the patient for the publication of this case report, ensuring confidentiality and voluntary participation.
Footnotes
Competing Interests: We, the undersigned authors, collectively declare that we have no conflicts of interest to report related to this research project.
Disclaimer: This research was supported in whole or in part by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
- 1.Austin A, Al-Faris F, Modi A, Chopra A. A transudative chylothorax associated with superior vena cava syndrome. Respirat Med Case Rep. 2019;28 doi: 10.1016/j.rmcr.2019.100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kho SS, Tie ST, Chan SK, Yong MC, Chai SL, Voon PJ. Chylothorax and central vein thrombosis, an under-recognized association: a case series. Respirol Case Rep. 2017;5(3):e00221. doi: 10.1002/rcr2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes AO, Ribeiro S, Neves J, Mendonça T. Uncommon etiologies of chylothorax: superior vena cava syndrome and thoracic aortic aneurysm. Clin Respirat J. 2015;9(2):185–188. doi: 10.1111/crj.12122. [DOI] [PubMed] [Google Scholar]
- 4.Barracano R, Scognamiglio G, Palma M, Sica G, Merola A, Borrelli N, et al. Chylothorax due to superior vena cava obstruction in a patient with complex congenital heart disease. JACC Case Rep. 2021;3(5):736–739. doi: 10.1016/j.jaccas.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley LE, Ataya A. Clinical approach and review of causes of a chylothorax. Respirat Med. 2019;157:7–13. doi: 10.1016/j.rmed.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal V, Doelken P, Sahn SA. Pleural fluid analysis in chylous pleural effusion. Chest. 2008;133(6):1436–1441. doi: 10.1378/chest.07-2232. [DOI] [PubMed] [Google Scholar]
- 7.McGrath EE, Blades Z, Anderson PB. Chylothorax: etiology, diagnosis, and therapeutic options. Respirat Med. 2010;104(1):1–8. doi: 10.1016/j.rmed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P) Medicine. 1999;78(5):321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Staats BA, Ellefson RD, Budahn LL, Dines DE, Prakash UB, Offord K. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc. 1980;55(11):700–704. [PubMed] [Google Scholar]
- 10.Ngan H, Fok M, Wong J. The role of lymphography in chylothorax following thoracic surgery. Br J Radiol. 1988;61(731):1032–1036. doi: 10.1259/0007-1285-61-731-1032. [DOI] [PubMed] [Google Scholar]
- 11.McGrath EE, Blades Z, Anderson PB. Chylothorax: etiology, diagnosis and therapeutic options. Respir Med. 2010;104:1–8. doi: 10.1016/j.rmed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Doerr CH, Allen MS, Nichols FC, Ryu JH. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80(7):867–870. doi: 10.4065/80.7.867. [DOI] [PubMed] [Google Scholar]



