Summary
Background
Previous studies have reported that tafamidis treatment was associated with better outcomes in patients with transthyretin amyloid cardiomyopathy (ATTR-CM) compared with those without tafamidis treatment. Therefore, we aimed to systematically assess the association of tafamidis treatment with outcomes in patients with ATTR-CM.
Methods
The protocol for this systematic review and meta-analysis was registered in the PROSPERO (CRD42022381985). Pubmed, Ovid Embase, Scopus, Cochrane Library, and Web of Science were interrogated to identify studies that evaluated the impact of tafamidis on prognosis in ATTR-CM, from January 1, 2000 to June 1, 2023. A random-effects model was used to determine the pooled risk ratio (RR) for the adverse endpoints. In addition, the main outcomes included all-cause death or heart transplantation, the composite endpoints included all-cause death, heart transplantation, cardiac-assist device implantation, heart failure exacerbations, and hospitalization.
Findings
Fifteen studies comprising 2765 patients (mean age 75.9 ± 9.3 years; 83.7% male) with a mean follow-up duration of 18.7 ± 17.1 months were included in the meta-analysis. There was a decrease in left ventricular ejection fraction (LVEF) (standard mean differences (SMD: −0.17; 95% confidence interval (CI), −0.31 to −0.03; P = 0.02) but were no significant differences in intraventricular septum (IVS) thickness or global longitudinal strain (GLS) after tafamidis treatment. However, subgroup analysis showed no significant deterioration in LVEF in the patients with wild-type ATTR after tafamidis treatment (SMD: −0.11; 95% CI, −0.34 to 0.12, P = 0.34). In addition, the group with tafamidis treatment had a decreased risk for all-cause death or heart transplantation compared to patients without treatment (the pooled RR, 0.44; 95% CI, 0.31–0.65; P < 0.01). Subgroup analysis showed that there was no significant difference of tafamidis on the outcomes in patients with wild-type or hereditary ATTR (RR, 0.44; 95% CI, 0.27–0.73 versus 0.21, 95% CI, 0.11–0.40, P = 0.08). Furthermore, tafamidis treatment was associated with a lower risk of the composite endpoint (RR, 0.57; 95% CI, 0.42–0.77; P < 0.01).
Interpretation
Our findings suggested that there was no significant deterioration in LVEF in the patients with wild-type ATTR after tafamidis treatment. In addition, tafamidis treatment was associated with a low risk of all-cause death and adverse cardiovascular events.
Funding
This work was supported by grants from the Natural Science Foundation of Sichuan Province [Grant Number: 23NSFSC4589] and the National Natural Science Foundation of China [Grant Number: 82202248].
Keywords: Tafamidis, Transthyretin amyloid cardiomyopathy, ATTR, Prognosis
Research in context.
Evidence before this study
We sought studies across Pubmed, Ovid Embase, Scopus, Cochrane Library, and Web of Science between January 1, 2000 and June 1, 2023. We included observational cohort studies, randomized controlled trials, and early-phase clinical trials published in peer-reviewed journals accompanied by a complete analysis of the impact of tafamidis on prognosis in patients with ATTR-CM. Duplicated studies were excluded using Endnote version X9 (Clarivate Analytics). The studies with a mean/median follow-up time greater than 6 months and published in English were considered. The editorials, reviews, commentaries, conference abstracts, and case reports were excluded. The quality of included studies was independently assessed using the Cochrane risk of bias tool for randomized controlled trials and the Newcastle-Ottawa Scale for observational studies.
Added value of this study
There was no significant deterioration in LVEF in the patients with wild-type ATTR after tafamidis treatment and tafamidis treatment is associated with a low risk of all-cause death, heart transplant, heart assist device implantation, heart failure exacerbations and hospitalization in patients with ATTR-CM.
Implications of all the available evidence
Our systematic meta-analysis highlights the survival benefit of tafamidis treatment, which strengthens the evidence for recommendations for the treatment of patients with ATTR-CM. Further research, encompassing larger sample sizes and long-term follow-up, is warranted to evaluate the efficacy of tafamidis in the treatment of ATTR.
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a severe disease characterized by an accumulation of amyloid fibrils composed of misfolded transthyretin protein in the heart.1 The prevalence of ATTR-CM was varied in certain patient cohorts, such as 16% in patients with severe calcific aortic stenosis undergoing transcatheter aortic valve implantation (TAVI),2 13% in patients with heart failure (HF) with preserved ejection fraction (HFpEF) and with left ventricular hypertrophy (LVH).3 ATTR-CM is a rapidly progressive disorder with a median survival of 2–6 years.4 Early diagnosis of patients with ATTR-CM and the timely initiation of therapy is important.
Traditional therapies, such as beta-blockers or angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB) for symptomatic relief, cannot reverse the underlying cause of ATTR-CM. Recent evidence suggests that the treatment with tafamidis is beneficial in patients with ATTR-CM.1,4 Tafamidis is a transthyretin stabilizer that can potently occupy thyroxine-binding sites and keep the tetrameric structure of the transthyretin protein from dissociating into intermediates transthyretin protein with high specificity.5 In the ATTR-ACT trial, tafamidis was reported to be associated with improved survival in patients with ATTR-CM,6 similar to other observational studies.7, 8, 9, 10, 11, 12, 13, 14 Moreover, other studies have reported that tafamidis delays structural and functional changes in the left ventricle.15,16 Nevertheless, changes in the left ventricle, following tafamidis treatment, vary within these studies and its efficacy is limited by small sample sizes, low number of events, and short follow-up periods. Up to date, there were systematic reviews on the efficacy of tafamidis in patients with ATTR-CM but no meta-analysis had been conducted.
Thus, we conducted a systematic review and random-effects meta-analysis for estimating the efficacy of tafamidis in patients with ATTR-CM.
Methods
Search strategy and selection criteria
A systematic interrogation across Pubmed, Ovid Embase, Scopus, Cochrane Library and Web of Science was conducted by two independent reviewers (HYC and ZHT) to identify publications of studies that assessed the efficacy of tafamidis on outcomes in patients with ATTR-CM between January 1, 2000 and June 1, 2023. The detailed search protocol is provided in the Supplemental Material.
A systematic review and meta-analysis of studies relating to the treatment of ATTR-CM patients with tafamidis was performed in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and was previously registered with PROSPERO (CRD42022381985).17 The ethical approval and informed consent of participants were waived for this study type. The inclusion criteria were as follows: 1) observational cohort studies (both retrospective and prospective) or randomized controlled trials; 2) early-phase clinical trials published in peer-reviewed journals accompanied by complete analysis; 3) studies examining the impact of tafamidis on prognosis in patients with ATTR-CM; 4) studies with a mean/median follow-up time greater than 6 months; and 5) studies published in English. The exclusion criteria included editorials, reviews, commentaries, conference abstracts, and case reports.
Data analysis
Two independent reviewers (HYC and ZHT) screened publication titles and abstracts, based on the inclusion and exclusion criteria, and then further confirmed whether the full text met the inclusion criteria. Duplicated studies were excluded using Endnote version X9 (Clarivate Analytics). Any discrepancy was resolved by a third reviewer (JW). Eligible study data were extracted by the two reviewers (HYC and ZHT) using a predetermined data collection table. Study characteristics including the first author, year of publication, study design, sample size, patient population, and study duration, as well as patient characteristics including age, sex, NYHA class, ATTR type, race, baseline and outcome measures, were extracted. The primary endpoint was defined as all-cause death or heart transplantation, while the composite endpoints included all-cause death, heart transplantation, cardiac-assist device implantation, heart failure exacerbations and hospitalization. The differences in left ventricular ejection fractions (LVEF), intraventricular septum (IVS) thickness, and global longitudinal strain (GLS) on echocardiograms or cardiovascular magnetic resonance (CMR) imaging between the before and after tafamidis treatment were extracted. For the study from Bezard et al.,14 the numbers of endpoints were extracted by survival curves using IPDfromKM.18 In addition, we conducted propensity score matching (PSM) in two studies7,13 that underwent PSM for inclusion to control baseline differences. Furthermore, four studies,19, 20, 21, 22 provided as median (range), were converted into mean ± SD for pooled SMDs calculation.
The quality of included studies was independently assessed by two reviewers (HYC and ZHT) using the Cochrane risk of bias tool23 for randomized controlled trials and the Newcastle-Ottawa Scale24 for observational studies. Using the Cochrane risk of bias tool, the following 7 domains were evaluated: random-sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of data, selective reporting, and other biases. Each domain was assessed based on low, unclear, or high risk of bias. Using the Newcastle-Ottawa Scale, a total of 8 domains, namely representativeness, selection, exposure ascertainment, the outcome at baseline, comparability, outcome assessment, follow-up duration, and adequacy of follow-up, were used for scoring the study quality.
All statistical analyses were performed using R, version 4.2.1 (Rstudio). Dichotomous variables were reported as numbers (proportions), and continuous variables were shown as mean ± SD or median (IQR). Risk ratios (RRs) with 95% confidence intervals (CI) were calculated for a dichotomous outcome according to the random-effects model, and standard mean differences (SMDs) were presented for the comparisons of the changes of continuous variables before and after tafamidis treatment. The heterogeneity within the included studies was assessed based on Cochran's Q test and Higgins I2 values. I2 values of 25%, 50%, and 75% showed mild, moderate, and high heterogeneity, respectively. Subgroup analysis was performed for exploring heterogeneity. We performed sensitivity analysis and Begg’s test which was used to test publication bias. The analysis was considered significant when P < 0.05.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data used in the study and accept the final responsibility for the decision to submit to the publication.
Results
Study selection and patients’ characteristics
The systematic literature search identified 3437 related studies, including 415 from PubMed, 1320 from Ovid Embase, 128 from Cochrane Library, 855 from Scopus and 719 from Web of Science. 1728 articles were further screened based on title and abstract after the removal of duplicates. The full texts of 37 articles were reviewed. Ten conference abstracts, 8 articles25, 26, 27, 28, 29, 30, 31, 32 with overlapping data from the ATTR-ACT study,6 and 4 studies33, 34, 35, 36 without available data for analysis were excluded. Finally, fifteen articles,6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16,19, 20, 21, 22 involving a total of 2765 patients, were included in the meta-analysis. The study and patient characteristics are summarized in Table 1 and Table 2. 1251 (45.2%) of 2765 patients with ATTR-CM received tafamidis treatment. The weighted mean age of patients was 75.9 ± 9.3 years, and 2316 (83.7%) patients were male. The average follow-up duration was 18.7 ± 17.1 months. 678 (28.0%) patients died or received heart transplantation in nine studies.6, 7, 8, 9, 10, 11, 12, 13, 14 In addition, a total of 1060 patients with wild-type ATTR (wtATTR) in 4 articles7,10,11,13 and 78 patients with hereditary ATTR (hATTR) in 1 article12 were included for subgroup analysis. A flowchart of the study is shown in Fig. 1. A detailed risk of bias analysis for the included studies is presented in Supplementary Table S1 and Supplementary Table S2.
Table 1.
Baseline characteristics of the included studies.
| First author | Published year | Study type | Patients, no. | The median follow-up duration, months | Treatment | Patient populationa | Endpoint |
|---|---|---|---|---|---|---|---|
| Merlini et al.21 | 2013 | Phase II | 21 | 12 | Tafamidis 20 mg/d | Cardiomyopathic; cardio-neuropathic; neuropathic | Only include for the analysis of changes in LVEF and IVS |
| Maurer et al.20 | 2015 | Phase II | 35 | 12 | Tafamidis 20 mg/d | Cardiomyopathic | Only include for the analysis of changes in LVEF and IVS |
| Sultan et al.8 | 2017 | Retrospective | 64 | 13.6 ± 5.6b | Tafamidis 20 mg/d | Cardiomyopathic | All-cause death |
| Rosenblum et al.9 | 2018 | Retrospective | 120 | 22.8 (12–33.6) | Tafamidis, diflunisalc | Cardiomyopathic | All-cause death and OHT |
| Maurer et al.6 | 2018 | Phase III | 441 | 30 | Tafamidis 80 mg/d or 20 mg/d | Cardiomyopathic | All-cause death, heart transplant, and cardiac mechanical assist devices |
| Gospodinova et al.12 | 2020 | Prospective | 78 | 30 (15–48) | Tafamidis 20 mg/d | Cardiomyopathic; cardio-neuropathic | All-cause death |
| Bézard et al.14 | 2020 | Prospective | 646 | 21.0 (6.2–45.2) | Tafamidis 80 mg/d or 20 mg/d | Cardiomyopathic; cardio-neuropathic; neuropathic | All-cause death, heart transplant, and cardiac decompensation |
| Ochi et al.10 | 2022 | Retrospective | 82 | 16.7 (8.8–23.8) | Tafamidis 80 mg/d | Cardiomyopathic | All-cause death, HF hospitalizations, and pacemaker implantation |
| Hussain et al.11 | 2022 | Prospective | 107 | 13.5 | Tafamidis | Cardiomyopathic | Death, hospitalization for heart failure |
| Giblin et al.22 | 2022 | Retrospective | 45 | 12 | Tafamidis 61 mg/d | Cardiomyopathic | Only include for the analysis of changes in LVEF, IVS, and GLS |
| Ichikawa et al.16 | 2023 | Retrospective | 41 | 16 ± 8 | Tafamidis | Cardiomyopathic | Only include for the analysis of changes in LVEF, IVS, and GLS |
| Ghoneem et al.13 | 2023 | Retrospective | 842d | 12 | Tafamidis | Cardiomyopathic | All-cause death, heart failure exacerbations |
| Takashio et al.7 | 2023 | Retrospective | 66d | 37.5 ± 18.1 | Tafamidis 80 mg/d | Cardiomyopathic | All-cause death, heart failure hospitalization |
| Rettl et al.15 | 2023 | Prospective | 137 | 9.5 ± 5.1b | Tafamidis 61 mg/d or 20 mg/d | Cardiomyopathic | Only include for the analysis of changes in LVEF, IVS, and GLS |
| Chamling et al.19 | 2023 | Retrospective | 40 | 12 ± 3b | Tafamidis 61 mg/d | Cardiomyopathic | Only include for the analysis of changes in LVEF and GLS |
NI = not included; ATTR = transthyretin amyloidosis; OHT = orthotopic heart transplant; HF = heart failure; LVEF = left ventricular ejection fraction; IVS = intraventricular septum; GLS = global longitudinal strain.
The 15 patients with pure neuropathy were excluded from the meta-analysis.
The average follow-up time of the included patients.
13 patients receiving diflunisal in this study were excluded from the meta-analysis.
The numbers of patients were from cohorts after propensity score matched.
Table 2.
Patients' demographic and clinical characteristics.
| First author | Published year | Patients, no. | Treatment group | Age, years | Male, no. (%) | NYHA class ≤ Ⅱ, no. (%) | hATTR, no. (%) | White, no. (%) |
|---|---|---|---|---|---|---|---|---|
| Merlini et al.21 | 2013 | 21 | Tafamidis | 63.1 ± 9.9 | 13 (62) | NR | 21 (100) | NR |
| Maurer et al.20 | 2015 | 35 | Tafamidis | 76.7 (68.7–86.5)b | 29 (83) | 30 (86) | 4 (11) | 35 (100) |
| Sultan et al.8 | 2017 | 29 | TRACS (without tafamidis) | 73.8 ± 5.7 | 27 (93) | 22 (75.6) | 11 (38) | 18 (62) |
| 35 | Fx1B-201 (On tafamidis) | 76.4 ± 4.6 | 32 (91) | 33 (94) | 4 (11) | 32 (91) | ||
| Rosenblum et al.9 | 2018 | 29 | On stabilizera | 75 ± 9 | 28 (97) | 23 (79) | 3 (10) | 27 (93) |
| 91 | Not on stabilizer | 75 ± 8 | 77 (85) | 36 (40) | 33 (36) | 50 (55) | ||
| Maurer et al.6 | 2018 | 264 | Tafamidis | 74.5 ± 7.2 | 241 (91.3) | 186 (70.5) | 63 (23.9) | 211 (79.9) |
| 177 | Placebo | 74.1 ± 6.7 | 157 (88.7) | 114 (64.4) | 43 (24.3) | 146 (82.5) | ||
| Gospodinova et al.12 | 2020 | 60 | Tafamidis | 56 (54–61)b | 39 (50) | 49 (63) | 78 (100) | 78 (100) |
| 18 | Without tafamidis | |||||||
| Bézard et al.14 | 2021 | 98 | Tafamidis | 69 ± 10 | 73 (74) | 52 (57) | 81 (83) | NR |
| 533 | Without tafamidis | 78 ± 9 | 443 (83.1) | 271 (55.8) | 127 (23.8) | NR | ||
| Ochi et al.10 | 2022 | 38 | Tafamidis | 78.4 ± 5.9 | 33 (87) | 33 (92) | 0 (0) | NR |
| 44 | Without tafamidis | 84.3 ± 4.6 | 38 (86) | 13 (30) | 0 (0) | NR | ||
| Hussain et al.11 | 2022 | 63 | Tafamidis | 83.6 (80.5–88.5)b | 53 (84.1) | 22 (41.5) | 2 (3.2) | 56 (88.9) |
| 44 | Without tafamidis | 84.2 (79.7–90.3)b | 32 (72.7) | 7 (20.6) | 2 (4.5) | 33 (75) | ||
| Giblin et al.22 | 2022 | 23 | Tafamidis | 79.1 ± 6.0 | 23 (100) | NR | 2 (9) | NR |
| 22 | Without tafamidis | 78.2 ± 6.7 | 22 (91) | NR | 1 (5) | NR | ||
| Ichikawa et al.16 | 2023 | 41 | Tafamidis | 75.6 ± 6.9 | 37 (90.2) | 33 (80.5) | 7 (17.1) | NR |
| Ghoneem et al.13 | 2023 | 421c | Tafamidis | 76.8 ± 8.6 | 361 (85.7) | NR | 0 (0) | 268 (63.7) |
| 421c | Without tafamidis | 76.2 ± 9.0 | 365 (86.7) | NR | 0 (0) | 270 (64.1) | ||
| Takashio et al.7 | 2023 | 33c | Tafamidis | 77.3 ± 6.8 | 30 (91) | 21 (64) | 0 (0) | NR |
| 33c | Without tafamidis | 77.0 ± 5.6 | 27 (82) | 18 (55) | 0 (0) | NR | ||
| Rettl et al.15 | 2023 | 62 | Tafamidis 61 mg | 78.5 (6.6)b | 50 (81) | 29 (47) | NR | NR |
| 21 | Tafamidis 20 mg | 74.1 (5.9)b | 19 (91) | 13 (62) | NR | NR | ||
| 54 | Without tafamidis | 78.0 (8.0)b | 44 (82) | 29 (54) | NR | NR | ||
| Chamling et al.19 | 2023 | 20 | Tafamidis | 76 (73–81)b | 18 (90) | NR | 0 (0) | NR |
| 20 | Without tafamidis | 80 (75–82)b | 15 (75) | NR | 0 (0) | NR |
The values are mean ± standard deviation, unless otherwise indicated.
NR = not reported; NYHA = New York Heart Association; hATTR = hereditary transthyretin amyloidosis.
Stabilizer includes diflunisal and tafamidis; 13 patients receiving diflunisal in this study were excluded from the meta-analysis.
The value was presented as median (IQR).
All data of patients were from cohorts after propensity score matched.
Fig. 1.
Flowchart for included studies for themeta-analysis.
Outcomes
The primary endpoint: all-cause death or heart transplantation
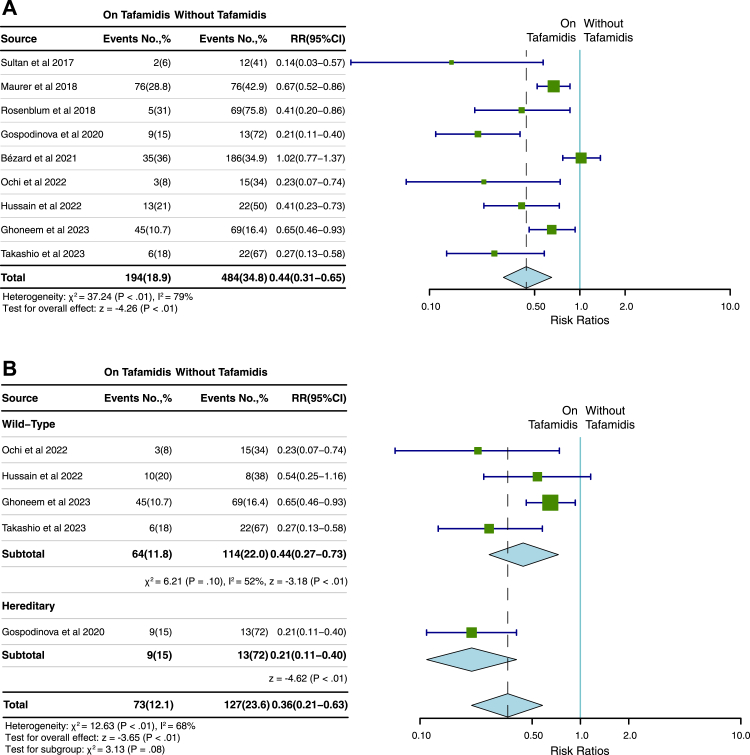
Nine studies,6, 7, 8, 9, 10, 11, 12, 13, 14 involving a total of 2418 patients, were included to analyse the relationship between tafamidis and the primary endpoint of all-cause death or heart transplantation. 194 (18.9%) of 1028 patients that received tafamidis treatment reached the primary endpoint while 484 (34.8%) of 1390 patients without tafamidis treatment reached the primary endpoint. Patients who received tafamidis treatment were associated with a significantly lower RR (RR, 0.44; 95% CI, 0.31–0.65; P < 0.01; Fig. 2A) compared to the patients who did not receive tafamidis with a significant heterogeneity (I2 = 79%, P < 0.01). A subgroup analysis according to ATTR type showed that no significant difference of tafamidis on primary endpoint was identified between wtATTR and hATTR (RR, 0.44; 95% CI, 0.27–0.73 versus 0.21; 95% CI, 0.11–0.40, P = 0.08; I2 = 68%) (Fig. 2B). The results remained valid after the removal of the study from Bezard et al.14 (Supplementary Figure S1A). There was significant publication bias (P = 0.04) while the sensitivity analysis revealed that the overall effect size remained robust when each study was removed in turn (Supplementary Figure S2A). In addition, subgroup analysis showed there was no significant publication bias by Begg’s test (P = 0.5).
Fig. 2.
Pooled RR of tafamidis treatment for death or heart transplantation. The pooled RR for the all-cause death or heart transplantation (A) in patients with ATTR-CM and the subgroup analysis by types of ATTR (B). ATTR-CM = transthyretin amyloid cardiomyopathy; No. = number; RR = risk ratio.
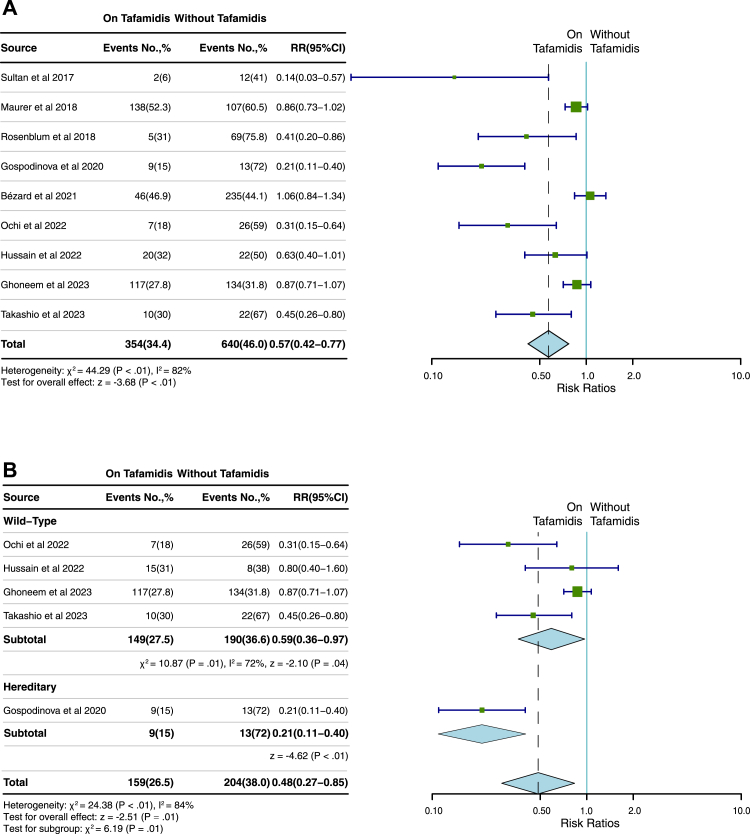
The composite endpoints
The same nine studies6, 7, 8, 9, 10, 11, 12, 13, 14 were used to analyse the impact of tafamidis on the composite endpoint of all-cause death, heart transplant, heart assist device implantation, heart failure exacerbations and hospitalizations. During the follow-up period, 354 (34.4%) of 1028 patients reached the composite endpoint in the group with tafamidis treatment, while 640 (46.0%) of 1390 patients reached the composite endpoint in patients without tafamidis treatment. Patients that received tafamidis treatment were associated with a significantly lower RR (RR, 0.57; 95% CI, 0.42–0.77; P < 0.01; Fig. 3A) compared to the patients that did not with a significant heterogeneity (I2 = 82%, P < 0.01). Subgroup analysis according to ATTR type showed that tafamidis treatment decreased the risk of composite endpoint in both wtATTR and hATTR, respectively (wtATTR: RR, 0.59; 95% CI, 0.36–0.97, P = 0.04; hATTR: 0.21; 95% CI, 0.11–0.40, P < 0.01) (Fig. 3B). The results remained valid after the removal of the study from Bezard et al.14 (Supplementary Figure S1B). There was significant publication bias (P = 0.04) while the sensitivity analysis revealed that the overall effect size remained robust when each study was removed in turn (Supplementary Figure S2B). In addition, subgroup analysis showed there was no significant publication bias by Begg’s test (P = 0.17).
Fig. 3.
Pooled RR of tafamidis treatment for the composite endpoint. The pooled RR for the composite endpoint (A) in patients with ATTR-CM and the subgroup analysis by types of ATTR (B). ATTR-CM = transthyretin amyloid cardiomyopathy; No. = number; RR = risk ratio.
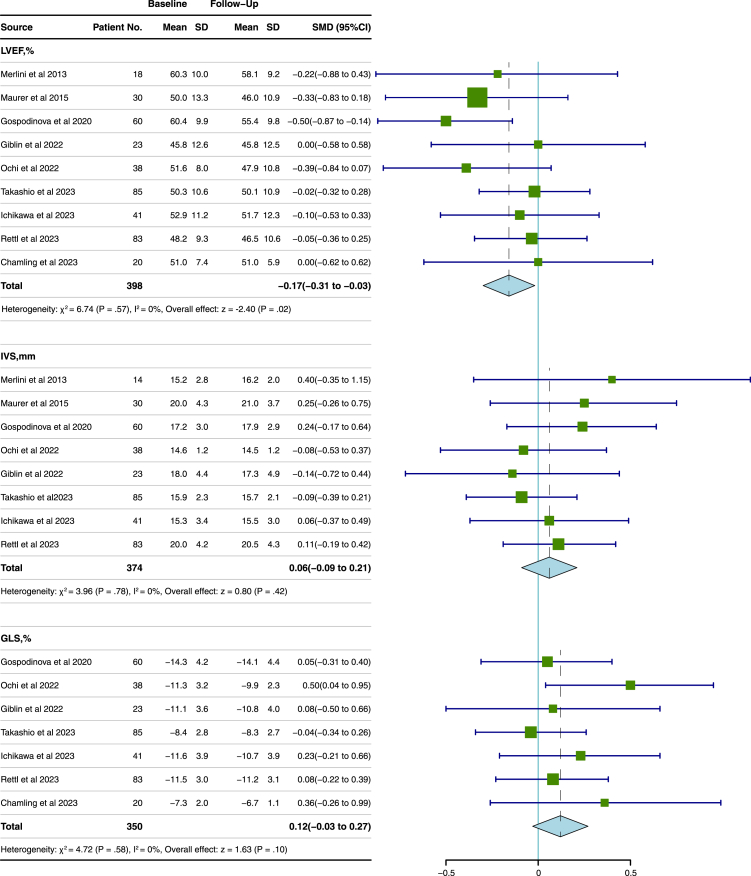
Cardiac remodeling in patients with and without tafamidis treatment
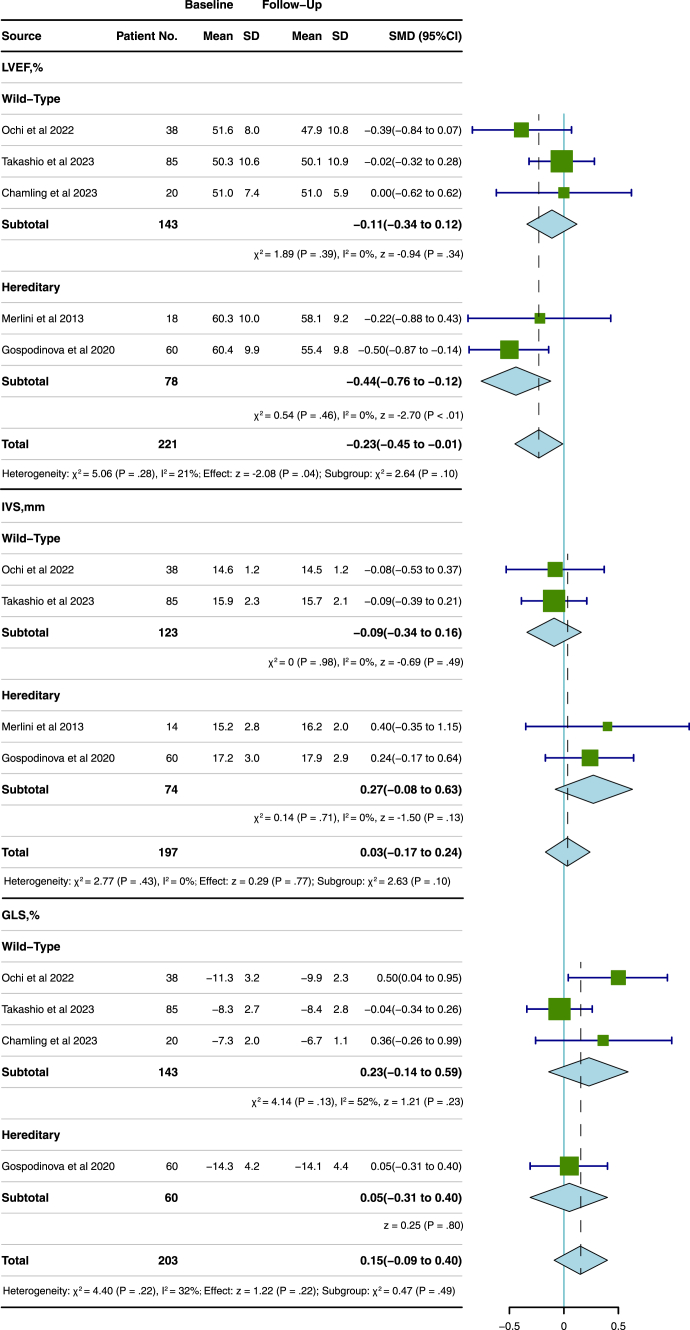
Nine studies,7,10,12,15,16,19, 20, 21, 22 involving a total of 398 patients, were analysed to compare the differences of LVEF, IVS, and GLS on echocardiograms or CMR after receiving tafamidis in patients with ATTR-CM. There was a significant decrease in LVEF (SMD, −0.17; 95% CI, −0.31 to −0.03; P = 0.02; Fig. 4) after tafamidis treatment. However, subgroup analysis by types of ATTR showed no significant deterioration in LVEF in the patients with wtATTR after tafamidis treatment (SMD, −0.11; 95% CI, −0.34 to 0.12, P = 0.34). In addition, there was no significant change in IVS (entire group: SMD, 0.06, 95% CI, −0.09 to 0.21, P = 0.42; Fig. 4; wtATTR: SMD, −0.09; 95% CI, −0.34 to 0.16, P = 0.49; hATTR: SMD, 0.27; 95% CI, −0.08 to 0.63, P = 0.13, Fig. 5) and GLS (SMD, 0.12, 95% CI, −0.03 to 0.27, P = 0.11; Fig. 4, wtATTR: SMD, 0.23, 95% CI, −0.14 to 0.59, P = 0.23; hATTR: SMD, 0.05, 95% CI, −0.31 to 0.40, P = 0.8, Fig. 5) in the entire ATTR-CM or the subgroups with wtATTR or hATTR after tafamidis treatment. No significant heterogeneity was identified across changes of LVEF (I2 = 0%, P = 0.57) and IVS (I2 = 0%, P = 0.78), and GLS (I2 = 0%, P = 0.58). Begg’s test suggested that there was no significant publication bias within included studies (P = 0.83 for LVEF changes; P = 0.62 for IVS changes; P = 0.051 for GLS changes).
Fig. 4.
Pooled SMD of tafamidis treatment for changes in LVEF, IVS, and GLS in patients with ATTR-CM. The pooled SMD represented the changes from baseline to follow-up after tafamidis treatment. ATTR-CM = transthyretin amyloid cardiomyopathy; SMD = standard mean difference; LVEF = left ventricular ejection fraction; IVS = intraventricular septum; GLS = global longitudinal strain.
Fig. 5.
Subgroup analysis of tafamidis treatment for changes in LVEF, IVS, and GLS in patients with wtATTR and hATTR. ATTR-CM = transthyretin amyloid cardiomyopathy; wtATTR = wild-type ATTR; hATTR = hereditary ATTR; SMD = standard mean difference; LVEF = left ventricular ejection fraction; IVS = intraventricular septum; GLS = global longitudinal strain.
Discussion
This meta-analysis summarized fifteen different published studies and evaluated the impact of tafamidis on prognosis in patients with ATTR. Our findings suggested that tafamidis treatment was statistically significantly associated with reductions in all-cause death or heart transplantation and composite endpoints in patients with the entire ATTR or the subgroups with wtATTR or hATTR. In addition, our study revealed no significant deterioration in LVEF in the patients with wtATTR after tafamidis treatment, which indicated that the responsiveness to tafamidis treatment may be better in the patients with wtATTR than hATTR.
Previous studies demonstrated that LVEF decreased with disease progression in ATTR. In our meta-analysis, we also found that there was a significant decrease in LVEF despite tafamidis treatment, which is consistent with the study by Maurer et al.6 Maurer et al. demonstrated that, although LVEF decreased in patients with tafamidis treatment, there was a smaller decrease in LVEF in 264 patients who received tafamidis compared to 177 patients who received placebo from baseline at 30 months (tafamidis group: denotes least square (LS) mean LVEF: −2.82% (standard error (SE) = 0.85); placebo group: LS mean LVEF: −4.34% (SE = 1.10).6 This relatively lower reduction in LVEF in patients receiving tafamidis suggests that tafamidis halts disease progression in ATTR.
A previous meta-analysis37 reported that the patients with wtATTR were associated with higher survival compared with hATTR. In addition, within the cohort of patients with hATTR, the patients with the Val30Met variant had a more benign course and better survival than the patients with Val122Ile or Thr60Ala variant, which highlighted the possibility that the natural course or responsiveness to tafamidis treatment may be different between the genetic variants. Our study reported no significant deterioration in LVEF in the patients with wtATTR rather than hATTR after tafamidis treatment by the subgroup analysis, reflecting that the responsiveness to tafamidis treatment may be better in the patients with wtATTR than hATTR. These results need to be further validated within a larger sample and the impact of tafamidis on functional changes by echocardiography or CMR in hATTR with different variants needs to be further explored.
Additionally, previous studies reported that patients that received tafamidis had an improvement in physical performance, 6-min walk distance, N-terminal prohormone of brain natriuretic peptide, and the Kansas City Cardiomyopathy Questionnaire–Overall Summary score.6,20,38 These results supported that tafamidis treatment helps improve quality of life by halting disease progression.
Several previous studies reported patients with ATTR-CM that received tafamidis treatment were associated with a lower risk of a heart transplant, all-cause death, or cardiovascular-related hospitalizations. The primary composite endpoint was evaluated in 1 RCT trial and 8 non-RCT studies. The RCT study of the placebo-controlled phase 3 study showed that all-cause mortality and cardiovascular-related hospitalizations were both lower in patients with the treatment of tafamidis compared with the placebo over the 30-month follow-up (all-cause mortality: HR, 0.70; 95% CI, 0.51–0.96; cardiovascular-related hospitalizations: relative risk ratio, 0.68; 95% CI, 0.56–0.81).6 Another retrospective study, including 122 patients with ATTR-CM, also showed a similar reduction in death or heart transplant under the treatment of stabilizers based on multivariable Cox analysis (hazard ratio, 0.37; 95% confidence interval, 0.19–0.75; P = 0.003).9 In addition, 3 real-world studies reported that patients treated with tafamidis had reduced risk of all-cause mortality or cardiovascular-related hospitalization compared with untreated patients.7,11,13 Our systematic meta-analysis is in agreement with prior studies, highlighting the survival benefit of tafamidis treatment, which strengthens the evidence for recommendations for the treatment of patients with ATTR-CM. In addition, our subgroup analysis of ATTR types affirms that tafamidis treatment could reduce risk in both wtATTR and hATTR.
Our meta-analysis has several limitations. First, only 1 RCT was included in the analysis. Second, the included studies had a mean follow-up duration of approximately 20 months, thereby necessitating further research to determine the longer-term efficacy of tafamidis. Third, the sample size of hATTR was relatively small. Fourth, the heterogeneity remained moderate even after subgroup analyses by types of ATTR. Nonetheless, this meta-analysis of available data provides evidence supporting favorable outcomes of tafamidis in patients with ATTR-CM.
According to our findings, in patients with ATTR-CM, tafamidis treatment was associated with a low risk of all-cause death, heart transplant, heart assist device implantation, heart failure exacerbations or hospitalizations. In addition, tafamidis treatment may decrease deterioration in LVEF in patients with wtATTR. Further research, encompassing larger sample sizes and long-term follow-up, is warranted to evaluate the efficacy of tafamidis in the treatment of ATTR.
Contributors
JW, HYC, and ZHT participated in the design of the study, literature search, data extraction, data analysis, and interpretation, performed the statistical analysis, and wrote the original draft. Thus, these three authors contributed equally to this paper. YCC and GVG conceived, designed, and coordinated the study. YCC, YH, GVG, and HK edited and reviewed the manuscript. JQZ, YWX, KW, and HK carried out data acquisition. JQZ, YWX, and KW conducted the data analysis. JW, HYC, and ZHT registered the study in PROSPERO. JW and YCC verified the underlying data. All the authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Data sharing statement
Once the data datasets have been de-identified and all the main findings have been published, all data will be shared upon request by the corresponding author (YCC) for research purposes.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
The authors thank Dr. Mariana Gospodinova from the Clinic of Cardiology, Medical Institute, Ministry of Interior Sofia for helping us to confirm the information used in this study. This work was supported by grants from the Natural Science Foundation of Sichuan Province [Grant Number: 23NSFSC4589] and the National Natural Science Foundation of China [Grant Number: 82202248].
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102172.
Appendix A. Supplementary data
References
- 1.Lane T., Fontana M., Martinez-Naharro A., et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. [DOI] [PubMed] [Google Scholar]
- 2.Castaño A., Narotsky D.L., Hamid N., et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González-López E., Gallego-Delgado M., Guzzo-Merello G., et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 4.Marques N., Azevedo O., Almeida A.R., et al. Specific therapy for transthyretin cardiac amyloidosis: a systematic literature review and evidence-based recommendations. J Am Heart Assoc. 2020;9(19) doi: 10.1161/JAHA.120.016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulawa C.E., Connelly S., Devit M., et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 7.Takashio S., Morioka M., Ishii M., et al. Clinical characteristics, outcome, and therapeutic effect of tafamidis in wild-type transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2023;10(4):2319–2329. doi: 10.1002/ehf2.14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan M.B., Gundapaneni B., Schumacher J., Schwartz J.H. Treatment with tafamidis slows disease progression in early-stage transthyretin cardiomyopathy. Clin Med Insights Cardiol. 2017;11 doi: 10.1177/1179546817730322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenblum H., Castano A., Alvarez J., Goldsmith J., Helmke S., Maurer M.S. TTR (Transthyretin) stabilizers are associated with improved survival in patients with TTR cardiac amyloidosis. Circ Heart Fail. 2018;11(4) doi: 10.1161/CIRCHEARTFAILURE.117.004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochi Y., Kubo T., Baba Y., et al. Early experience of tafamidis treatment in Japanese patients with wild-type transthyretin cardiac amyloidosis from the Kochi amyloidosis cohort. Circ J. 2022;86(7):1121–1128. doi: 10.1253/circj.CJ-21-0965. [DOI] [PubMed] [Google Scholar]
- 11.Hussain K., Macrinici V., Wathen L., et al. Impact of tafamidis on survival in a real-world community-based cohort. Curr Probl Cardiol. 2022;47(12) doi: 10.1016/j.cpcardiol.2022.101358. [DOI] [PubMed] [Google Scholar]
- 12.Gospodinova M., Sarafov S., Chamova T., et al. Cardiac involvement, morbidity and mortality in hereditary transthyretin amyloidosis because of p.Glu89Gln mutation. J Cardiovasc Med. 2020;21(9):688–695. doi: 10.2459/JCM.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 13.Ghoneem A., Bhatti A.W., Khadke S., et al. Real-world efficacy of tafamidis in patients with transthyretin amyloidosis and heart failure. Curr Probl Cardiol. 2023;48(6) doi: 10.1016/j.cpcardiol.2023.101667. [DOI] [PubMed] [Google Scholar]
- 14.Bezard M., Kharoubi M., Galat A., et al. Natural history and impact of treatment with tafamidis on major cardiovascular outcome-free survival time in a cohort of patients with transthyretin amyloidosis. Arch Cardiovasc Dis Suppl. 2021;13(1):27–28. doi: 10.1002/ejhf.2028. [DOI] [PubMed] [Google Scholar]
- 15.Rettl R., Duca F., Binder C., et al. Impact of tafamidis on myocardial strain in transthyretin amyloid cardiomyopathy. Amyloid. 2023;30(1):127–137. doi: 10.1080/13506129.2022.2131385. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa Y., Oota E., Odajima S., et al. Impact of tafamidis on echocardiographic cardiac function of patients with transthyretin cardiac amyloidosis. Circ J. 2023;87(4):508–516. doi: 10.1253/circj.CJ-22-0683. [DOI] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N., Zhou Y., Lee J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21(1):111. doi: 10.1186/s12874-021-01308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamling B., Bietenbeck M., Korthals D., et al. Therapeutic value of tafamidis in patients with wild-type transthyretin amyloidosis (ATTRwt) with cardiomyopathy based on cardiovascular magnetic resonance (CMR) imaging. Clin Res Cardiol. 2023;112(3):353–362. doi: 10.1007/s00392-022-02035-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer M.S., Grogan D.R., Judge D.P., et al. Tafamidis in transthyretin amyloid cardiomyopathy: effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail. 2015;8(3):519–526. doi: 10.1161/CIRCHEARTFAILURE.113.000890. [DOI] [PubMed] [Google Scholar]
- 21.Merlini G., Planté-Bordeneuve V., Judge D.P., et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013;6(6):1011–1020. doi: 10.1007/s12265-013-9512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giblin G.T., Cuddy S.A.M., González-López E., et al. Effect of tafamidis on global longitudinal strain and myocardial work in transthyretin cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2022;23(8):1029–1039. doi: 10.1093/ehjci/jeac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Wells G.A., Shea B., O'Connell D., et al. 2015. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 25.Li B., Alvir J., Stewart M. Extrapolation of survival benefits in patients with transthyretin amyloid cardiomyopathy receiving tafamidis: analysis of the tafamidis in transthyretin cardiomyopathy clinical trial. Cardiol Ther. 2020;9(2):535–540. doi: 10.1007/s40119-020-00179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Rozenbaum M., Casey M., Sultan M.B. Estimating the effect of tafamidis on cardiovascular-related hospitalization in NYHA class III patients with transthyretin amyloid cardiomyopathy in the presence of death. Cardiology. 2022;147(4):398–405. doi: 10.1159/000525883. [DOI] [PubMed] [Google Scholar]
- 27.Miller A.B., Januzzi J.L., O'Neill B.J., et al. Causes of cardiovascular hospitalization and death in patients with transthyretin amyloid cardiomyopathy (from the tafamidis in transthyretin cardiomyopathy clinical trial [ATTR-ACT]) Am J Cardiol. 2021;148:146–150. doi: 10.1016/j.amjcard.2021.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Nativi-Nicolau J., Judge D.P., Hoffman J.E., et al. Natural history and progression of transthyretin amyloid cardiomyopathy: insights from ATTR-ACT. ESC Heart Fail. 2021;8(5):3875–3884. doi: 10.1002/ehf2.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sperry B.W., Hanna M., Maurer M.S., et al. Association of tafamidis with health status in patients with ATTR cardiac amyloidosis: a post hoc analysis of the ATTR-ACT randomized clinical trial. JAMA Cardiol. 2023;8(3):275–280. doi: 10.1001/jamacardio.2022.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damy T., Garcia-Pavia P., Hanna M., et al. Efficacy and safety of tafamidis doses in the tafamidis in transthyretin cardiomyopathy clinical trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2021;23(2):277–285. doi: 10.1002/ejhf.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozenbaum M.H., Garcia A., Grima D., et al. Health impact of tafamidis in transthyretin amyloid cardiomyopathy patients: an analysis from the tafamidis in transthyretin cardiomyopathy clinical trial (ATTR-ACT) and the open-label long-term extension studies. Eur Heart J Qual Care Clin Outcomes. 2022;8(5):529–538. doi: 10.1093/ehjqcco/qcab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vong C., Boucher M., Riley S., Harnisch L.O. Modeling of survival and frequency of cardiovascular-related hospitalization in patients with transthyretin amyloid cardiomyopathy treated with tafamidis. Am J Cardiovasc Drugs. 2021;21(5):535–543. doi: 10.1007/s40256-021-00464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Socie P., Benmalek A., Cauquil C., et al. Comparison between tafamidis and liver transplantation as first-line therapy for hereditary transthyretin amyloidosis. Amyloid. 2023:1–10. doi: 10.1080/13506129.2023.2177986. [DOI] [PubMed] [Google Scholar]
- 34.Bhambri R., Colavecchia A.C., Bruno M., et al. Real-world characteristics of patients with wild-type transthyretin amyloid cardiomyopathy: an analysis of electronic healthcare records in the United States. Am J Cardiovasc Drugs. 2023;23(2):197–206. doi: 10.1007/s40256-022-00563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razvi Y., Porcari A., Di Nora C., et al. Cardiac transplantation in transthyretin amyloid cardiomyopathy: outcomes from three decades of tertiary center experience. Front Cardiovasc Med. 2023;9 doi: 10.3389/fcvm.2022.1075806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A., Peterson A., Marchant N., et al. Baseline characteristics and secondary medication adherence among Medicare patients diagnosed with transthyretin amyloid cardiomyopathy and/or receiving tafamidis prescriptions: a retrospective analysis of a Medicare cohort. J Manag Care Spec Pharm. 2022;28(7):766–777. doi: 10.18553/jmcp.2022.28.7.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonopoulos A.S., Panagiotopoulos I., Kouroutzoglou A., et al. Prevalence and clinical outcomes of transthyretin amyloidosis: a systematic review and meta-analysis. Eur J Heart Fail. 2022;24(9):1677–1696. doi: 10.1002/ejhf.2589. [DOI] [PubMed] [Google Scholar]
- 38.Rettl R., Mann C., Duca F., et al. Tafamidis treatment delays structural and functional changes of the left ventricle in patients with transthyretin amyloid cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2022;23(6):767–780. doi: 10.1093/ehjci/jeab226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.