ABSTRACT
Aim
To evaluate the effect of different add-ons on the flexural strength (FS) of glass ionomer cement (GIC).
Materials and methods
Around 72 samples were fabricated and divided among the following six different groups: group I—control (conventional GIC—nonmodified), group II—GIC powder modified with 3% titanium dioxide (TiO2) and liquid is unmodified, group III—powder modified with 10% nanohydroxyapatite (nHA) and liquid is unmodified, group IV—powder is unmodified and Liquid is modified with 10% chitosan (CH), group V—powder is modified with 3% TiO2 and liquid is modified with 10% CH, and group VI—powder is modified with 10% nHA and liquid is modified with 10% CH. The samples were then subjected to a three-point bending test on a universal testing machine for the evaluation of FS. The results obtained were analyzed statistically using the analysis of variance (ANOVA) test.
Result
The mean FS value of group V depicts significantly high FS among all groups (29.42 ± 3.35). A significant difference was present in FS amongst all the groups that is groups V>II>IV>VI>III>I.
Conclusion
Glass ionomer cement (GIC) powder can be modified with nHA, nanotitanium, and GIC liquid can be modified with CH to improve its FS.
Clinical significance
Glass ionomer cement (GIC) supplemented with additives like nanoparticles (NPs) and CH can be used as an enhanced filling material due to its potential antibacterial properties and in areas with a high masticatory load.
How to cite this article
Showkat I, Chaudhary S, Sinha AA, et al. Comparative Evaluation of Flexural Strength of Conventional Glass Ionomer Cement and Glass Ionomer Cement Modified with Chitosan, Titanium Dioxide Nanopowder and Nanohydroxyapatite: An In Vitro Study. Int J Clin Pediatr Dent 2023;16(S-1):S72–S76.
Keywords: Chitosan, Flexural strength, Glass ionomer cement, In vitro study, 10% nanohydroxyapatite
Introduction
A chemical reaction between calcium fluoro-aluminosilicate glass and polyacrylic acid, itaconic acid, and maleic acid forms the basis for glass ionomer cement (GIC), which was developed by Wilson and Kent, for dental restorative purposes.1 Its use as luting cement, fissure sealant, core build, bases, and restorative materials has been due to its advantageous chemical adhesive and fluoride charging properties.2 But certain disadvantages like low wear resistance and low flexural strength (FS) have made its use unfavorable in many cases3 and restricted its use in certain regions.4 Nanoparticle (NP) technology has been introduced which has been found to enhance the physical properties of GIC.
Chitosan (CH) is a linear polysaccharide obtained from the deacetylation of chitin. CH has reactive hydroxyl groups at C2, C3, and C6 positions, respectively and has a rigid crystalline structure through intramolecular hydrogen bonding.5 Interfacial surface tension developed between various components present in the matrix of GI has been found to reduce because of CH and also forms good interaction between them. A favorable outcome of the rise in surface area and density of charge leads to interactive surrounding medium as well. The addition of nanohydroxyapatite (nHA) into GICs may enhance the mechanical properties as well as improve the biocompatibility of GIC. The composition is the same as of enamel and dentin and can improve the strength of cement with respect to the tooth.6
Significant enhancement of wear resistance, compressive and FS, and hardness has been found with the addition of TiO2 NPs to GIC at 3 and 5% (w/w)3 which maybe because of the NPs addition in GIC.7
Hence, this study was done to evaluate the FS of conventional GIC and modified GIC with TiO2 nanopowder and hydroxyapatite.
Materials and Methods
This study was conducted in the Department of Pediatrics and Preventive Dentistry, in Kothiwal Dental College and Research Centre, Moradabad, and associated with ITS, Ghaziabad, Uttar Pradesh, India.
The study evaluated the FS of conventional GIC and modified GIC with CH, nanotitanium, and nHA.
Armamentarium profile:
- Instruments
- Gloves.
- Mask.
- Agate spatula.
- Paper pad.
- Glass slab.
- Explorer.
- Brass mold.
- Abrasive paper (600, 1000 grit).
- Materials
- Conventional GIC (type IX).
- Around 3% NPs of TiO2 (w/w).
- Around 10% nHA (w/w).
- Around 10% CH solution (v/v).
- Putty material.
- Equipment
- Instron 10 kN Taiwan.
- Micromotor with the handpiece.
Modification of GI with CH
For the preparation of 0.3 N acetic acid, 1.8 mL of anhydrous acetic acid was prepared by the addition of 0.1 l distilled water in a standard flask. Around 20 mg of CH were weighed and dissolved in the similar solution prepared above to get 0.2 mg/mL solution of CH. Also, 1/10 mL of the prepared CH solution was dissolved in 0.9 mL of GIC liquid to prepare a 10% v/v CH-modified GIC solution.6
Modified GI with Nanotitanium
Around 0.3 mg TiO2 powder with 9 mg of GIC to prepare modified GI with 3% TiO2 nanopowder.
Modified GI with nHA
Around 1 mg of nHA was added to 9 mg of GI to get a modified GI with 10% TiO2 nanopowder.
Methodology
In the present study for fabrication of appropriate shape and size, a rectangular brass die shape with dimensions of 25 mm length × 2 mm width × 2 mm thickness was used. Then a putty index of the die was made to form the mold. The index was then packed with GIC and distributed among the following six groups:
| Groups | Powder | Liquid |
|---|---|---|
| Group I—control (conventional GIC—nonmodified) |
GIC powder | Polyacrylic acid |
| Group II (powder modified) |
GIC powder + 3% TiO2 | Polyacrylic acid |
| Group III (powder modified) |
GIC powder + 10% nHA | Polyacrylic acid |
| Group IV (liquid modified) | GIC powder | Polyacrylic acid + CH |
| Group V (both powder and liquid modified) | GIC powder + 3% TiO2 | Polyacrylic acid + CH |
| Group VI (Both powder and liquid modified) | GIC powder + 10% nHA | Polyacrylic acid + CH |
The GIC in the index was covered with a glass slide until the initial setting.
The material was moisture proofed with Vaseline. The material after the setting was retrieved from the index.
Flexural strength (FS) values were measured using a universal testing machine (Instron 10 kN Taiwan). Each sample was evaluated using an Instron universal testing machine, and the load to fracture was recorded on a blended indenter in a universal load testing machine.
The results when statistically analyzed depicted a rise in FS in all groups than conventional GIC, but modified GIC with 3% TiO2 nanopowder and 10% CH solution depicted high FS (29.42 mean FS).
This shows that modification of GIC with nHA, nanotitanium, and CH improves FS.
Further, in vivo, studies are still required to explore the improvement in FS of GIC with these particles.
Results
The recorded data was evaluated using Statistical Package for the Social Sciences software. Data was depicted as mean ± standard deviation (SD). Analysis of variance (ANOVA) was used. The student's unpaired t-test was done. A p-value of <0.05 was considered significant.
Group V shows significantly high FS among all the groups (29.42 ± 3.35). A statistically significant difference in FS was found amongst all groups that is groups V>II >IV > VI>III >I.
Table 1 shows the descriptive stats of FS in different groups. It shows that among all the groups, group I has the least FS which is 5.26 MPa and group V has the mean highest strength which is 29.42 MPa.
Table 1.
Descriptive statistics of FS among various groups
| N | Mean | SD | 95% confidence interval for mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Group I | 12 | 5.26 | 1.03 | 4.61 | 5.91 | 3.21 | 6.83 |
| Group II | 12 | 27.81 | 3.50 | 25.59 | 30.04 | 21.06 | 32.37 |
| Group III | 12 | 10.97 | 1.96 | 9.72 | 12.21 | 5.88 | 13.01 |
| Group IV | 12 | 24.33 | 4.23 | 21.64 | 27.01 | 17.74 | 30.95 |
| Group V | 12 | 29.42 | 3.35 | 27.29 | 31.55 | 23.55 | 35.31 |
| Group VI | 12 | 16.52 | 1.81 | 15.37 | 17.67 | 14.07 | 20.58 |
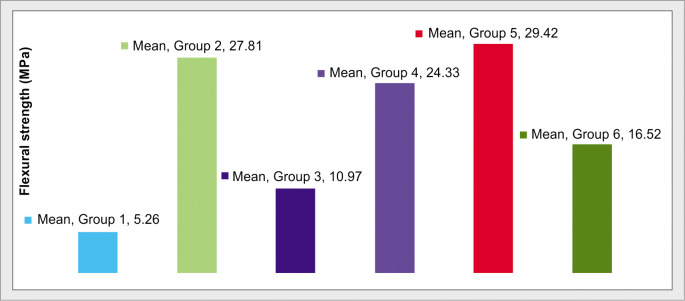
Figure 1 bar chart showing FS in groups. This bar chart depicts a higher mean strength of group V (29.42 MPa) followed by group II (27.81 MPa), group IV (24.33 MPa), group VI (16.52 MPa), group III (10.97 MPa). Group I showed the lowest FS which is 5.26 MPa compared to all other groups.
Fig. 1.
Comparison of FS of all groups
Table 2 depicts ANOVA for FS values depicts an F-value of 137.741 and a p-value < 0.001.
Table 2.
ANOVA for comparing mean transverse strength among the groups
| Sum of squares | Degree of freedom | Mean square | F-value | p-value | |
|---|---|---|---|---|---|
| Between groups | 5689.274 | 5 | 1137.855 | 137.741 | <0.001* |
| Within groups | 545.214 | 66 | 8.261 | ||
| Total | 6234.488 | 71 |
Table 3 shows a comparative intergroup of FS among various groups. The intergroup comparison shows a statistically significant difference among all the groups except groups II vs V.
Table 3.
Least significance difference test for intergroup comparison based on FS among various groups
| Group comparison | Mean difference | p-value | 95% confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| I vs II | −22.55 | <0.001* | −24.90 | −20.21 |
| I vs III | −5.71 | <0.001* | −8.05 | −3.36 |
| I vs IV | −19.06 | <0.001* | −21.41 | −16.72 |
| I vs V | −24.16 | <0.001* | −26.50 | −21.82 |
| I vs VI | −11.26 | <0.001* | −13.60 | −8.92 |
| II vs III | 16.85 | <0.001* | 14.50 | 19.19 |
| II vs IV | 3.49 | 0.004* | 1.15 | 5.83 |
| II vs V | −1.61 | 0.175 | −3.95 | 0.73 |
| II vs VI | 11.29 | <0.001* | 8.95 | 13.64 |
| III vs IV | −13.36 | <0.001* | −15.70 | −11.02 |
| III vs V | −18.46 | <0.001* | −20.80 | −16.11 |
| III vs VI | −5.55 | <0.001* | −7.90 | −3.21 |
| IV vs V | −5.10 | <0.001* | −7.44 | −2.75 |
| IV vs VI | 7.81 | <0.001* | 5.46 | 10.15 |
| V vs VI | 12.90 | <0.001* | 10.56 | 15.25 |
*Statistically significant difference (p-value < 0.05); *p<0.05, significant difference; **p< 0.001, highly significant difference
Table S1 shows the composition of materials used in this study.
Table S1.
Composition of materials used in this study
| Restorative material | Composition | Manufacturer |
|---|---|---|
| Conventional GIC type IX (powder and liquid) | Powder Silicon dioxide—24.9% Aluminum oxide—14.2% Aluminum fluoride—4.6% Calcium fluoride—12.8% Sodium hexafluoroaluminate—19.2% Aluminum phosphate—24.2% Strontium Liquid—polyacrylic acid Itaconic acid Maleic acid Tricarballylic Tartaric acid Water |
GC Corporation, Tokyo, Japan |
| 10% CH liquid | CH powder Glacial acetic acid Water |
Everest, Bengaluru, India |
| 3% Nanotitanium powder | NPs/nanopowder (TiO2, anatase, 10–20 nm, and 99.9%) | Nano Research Lab, Jharkhand, India |
| 10% nHA powder | Nanoparticles/nanopowder (calcium hydroxide phosphate, 20–80 nm, and 99.5%) | Nano Research Lab, Jharkhand, India |
Discussion
Glass ionomer cement (GIC) possesses certain advantages of chemical adherence to the tooth structure and acts as a fluoride reserve, which has led to its use as a restorative material. However, the major issue faced with this material has been low flexural strength.3 The need to improve these cement has led to strengthening concepts. Earlier approaches dealt with the amalgamation of ceramics, glass, or metal particles into GIC. Today's GIC concept is well understood, which in turn has paved the way for new formulations.5
The integration of nanosized particles can ameliorate the mechanical properties of GIC used in restoration.8
Hydroxyapatite (HA) has an effect on both the degree of polysalt bridge and reaction integration of the GI, which improves the FS of the final set cement. It is soluble in acids and results in the availability of calcium ions from the surface of HA.7
The integration of HA granules could increase the FS of GIC without compromising its inherent favorable characteristics.9
Titanium dioxide (TiO2) nanoparticles have been suggested for use as strengthening fillers to dental resin restoration materials. TiO2 is biocompatible.1
Chitosan (CH) is a natural product with replenishing antibacterial properties and enhancing FS.7 It has hydroxyl and acetamide groups, these bind to the hydroxyl group of powder, and with the help of hydrogen bonding, the carboxyl group binds with polyacrylic acid. This bonding reduces interfacial tension among GIC components and hence leads to improvement of FS.5
The FS test determines a clinical situation of the forces exerted by the opposing cusp.10 It is also considered to be the most appropriate test of the strength of GICs.11
Group I
In the present study, the control group depicts the lowest FS among all groups which is 5.26 ± 1.03 MPa. On applying ANOVA for intergroup comparison, it showed a significant difference (significance at p < 0.001) between groups II (significance at p < 0.001), III (significance at p < 0.001), IV (significance at p < 0.001), and V (significance at p < 0.001).
The interfacial tension between each component is more leading to poor mechanical performance. Therefore, materials used in reducing the interfacial tension or to increase the adhesion among the components results in good mechanical outcomes.12
Group II
The FS of group II as modified GIC powder with 3% TiO2 nanopowder and liquid unmodified has higher values, that is, 27.81 ± 3.50 MPa than groups I, III, IV, and VI. On applying ANOVA for intergroup comparison, it showed a significant difference (significance at p < 0.001) between groups I (significance at p < 0.001), III (significance at p < 0.001), IV (significance at p < 0.004), and VI (significance at p < 0.001). It has lower FS than group V which showed a nonsignificant difference (p = 0.175).
Elaska et al. found that improved FS of the GI was significant at 3% and 5% (w/w) TiO2 nanoparticles.1
Group III
The FS of group III has low values that are 10.97 ± 1.96 MPa than groups V, II, IV, and V. On applying ANOVA for intergroup comparison, it showed a significant difference (significance at p < 0.001) between groups V (significance at p < 0.001), II (p < 0.001), IV (significance at p < 0.001), and VI (significance at p < 0.001).
Nicholson et al. depicted the incorporation of HA into GIC in the literature. In this study, an increase in HA nanopowder has led to a decrease in FS. The incorporation of excess NPs lowers the association of the particles and ionomer. Hence, HA does not react with the ionomer to form the cross-linking.11
Basir et al. reported that the nanoparticles increased the FS more than the microparticles of HA but also the best values found were for the addition of 5 wt% as compared to each particle's size separately. On the contrary, NPs gave higher results than micro.13
Group IV
The FS of group IV has higher values that are 24.33 ± 4.23 MPa than groups I, III, and VI and lower values than groups II and V. On applying ANOVA for intergroup comparison, it showed a significant difference (significance at p < 0.001) between groups II (significance at p < 0.001), III (significance at p < 0.001), IV (significance at p < 0.001), and V (significance at p < 0.001).
Petri et al. investigated the addition of 0.0044 wt% of CH led to a rise in the FS. High CH content than 0.022 wt% indicates poor outcomes. The number of fluoride ions released from CH-modified GIC was much high than commercial GIC.12
Group V
The FS of group V has the highest value which is 29.42 ± 3.35 MPa among all groups. On applying ANOVA for intergroup comparison, it showed a significant difference (significance at p < 0.001) between groups I (significance at p < 0.001), II (p < 0.001), III (significance at p < 0.001), IV (significance at p < 0.001) and VI (significance at p < 0.001). The present study depicted that with modified GIC liquid with CH and powder also with TiO2 NP, improved mechanical outcomes in terms of FS can be seen as compared to modifying GIC polyacrylic acid with CH and GIC powder with HA NPs.
Ibrahim et al. modified GIC with three percent (w/w) TiO2/NP, 10% (v/v) CH solution, or dually modified with TiO2/CH. The control group was nonmodified GIC. The synergistic effect of the modifications was indicated by an increase in flexural and compressive strength.7
The results of the above-stated studies are similar to the present study.
Group VI
The FS of group VI has a lower value that is 16.52 ± 1.81 MPa than groups V, II, and IV and higher than groups I and III. On applying ANOVA for intergroup comparison, it showed a significant difference (significance at p < 0.001) between groups I (significance at p < 0.001), II (p < 0.001), III (significance at p < 0.001), IV (significance at p < 0.001), and V (significance at p < 0.001).
Barandehfard et al. inferred that the addition of HA resulted in an increase in the FS of the GIC.9
Basir et al. conducted an experimental study, in a total of 252 light cure-improved GI samples were divided into six groups as a control group (0%) and the nHA groups (1, 2, 5, 7, and 10%) on the basis of mass percent. Around 108 samples were used for the testing of FS. It was concluded that 5% nHA results in a rise in FS.13
The results show that all groups have improved FS than conventional GIC, but modified GIC with 3% TiO2 nanopowder and 10% CH solution showed the highest FS.
Inference from this Study
Flexural strength (FS) of groups V>II>IV>VI>III>I.
Conclusion
Within the limitations, the following conclusions were:
A significant difference was seen in FS in all groups.
Groups V>II>IV>VI>III>I.
Group V showed high FS.
The results of the present study depict that powdered GIC can be modified with nHA and nanotitanium to improve MPA while CH can be added to liquid which alone as well as synergistically has improved the FS of GIC.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Wilson AD, Kent BE. The glass-ionomer cement, a new translucent dental filling material. J Appl Chem. 1971;21:313–313. [Google Scholar]
- 2.Elaska SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: influence on physical and antibacterial properties. J Dent. 2011;39(9):589–598. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, et al. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl Oral Sci. 2015;23(3):321–328. doi: 10.1590/1678-775720140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khurshid Z, Zafar M, Qasim S, et al. Advances in nanotechnology for restorative dentistry. Materials (Basel) 2015;8(2):717–731. doi: 10.3390/ma8020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyapina MG, Tzekova M, Dencheva M, et al. Nanoglass-ionomer cements in modern restorative dentistry. J of IMAB. 2016;22(2):1160–1165. doi: 10.5272/jimab.2016222.1160. [DOI] [Google Scholar]
- 6.Moshaverinia A, Ansari S, Moshaverinia M, et al. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008;4(2):432–440. doi: 10.1016/j.actbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim MA, Meera Priyadarshini B, Neo J, et al. Characterization of chitosan/TiO2 nano-powder modified glass-ionomer cement for restorative dental applications. J Esthet Restor Dent. 2017;29(2):146–156. doi: 10.1111/jerd.12282. [DOI] [PubMed] [Google Scholar]
- 8.Najeeb S, Khurshid Z, Zafar MS, et al. Modifications in glass ionomer cements: nano-sized fillers and bioactive nanoceramics. Int J Mol Sci. 2016;17(7):1134. doi: 10.3390/ijms17071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barandehfard F, Rad MS, Hosseinnia A, et al. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceramics International. 2016;42(15):1–41. doi: 10.1016/j.ceramint.2016.08.122. [DOI] [Google Scholar]
- 10.Azlisham NAF, Abdul Rahman FS, Mohamad D. Flexural and morphological properties of Newly developed glass ionomer cement (GIC) with the incorporation of 3-acetylcoumarin. Malays J Micros. 2015;11:11–15. [Google Scholar]
- 11.Nicholson JW, Hawkins SJ, Smith JE. The incorporation of hydroxyapatite into glass polyalkenoate (“glass ionomer”) cements: a preliminary study. J Mater Sci Mater Med. 1993;(04):418–421. [Google Scholar]
- 12.Petri DF, Donegá J, Benassi AM, et al. Preliminary study on chitosan modified glass ionomer restoratives 2007. Dent Mater. 2007;23(8):1004–1010. doi: 10.1016/j.dental.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Basir MM, Ataei M, Rezvani MB, et al. Effect of incorporation of various amounts of nano-sized hydroxyapatite on the mechanical properties of a resin modified glass ionomer. J Dent Sch. 2012;30(4):216–223. [Google Scholar]