Abstract
Fresh produce continues to be the main source of foodborne illness outbreaks, particularly in developing countries where water stress results in the use of surface wastewater all year round for irrigation of vegetables. The objective of the current study was to evaluate the microbial quality of lettuce irrigated with wastewater from Onyasia river. Lettuce and soil were sampled from selected vegetable farms on the Eastern gate of the Ghana Atomic Energy Commission land alongside surface wastewater from the Onyasia river, which is used as the main source for irrigation. Samples were analyzed for aerobic mesophilic plate counts, total coliforms count, fecal coliforms count, Salmonella counts and intestinal parasites using standard methods. Surface wastewater was found to be contaminated with mean fecal coliform counts of log 3.50 cfu/100 mL. Enterobacter cloacae, Acinetobacter baumannii, and Klebsiella pneumonia were also isolated from the wastewater samples. No intestinal parasite egg was detected in wastewater samples. While fecal coliforms and Salmonella spp were not detected, mean aerobic mesophilic plate counts (log 4.82 cfu/g) and total coliforms count (log 3.50 cfu/g) were recorded in the lettuce samples. Enterobacter asburiae, Acinetobacter baumannii, Klebsiella variicola and Citrobacter freundii were isolated from lettuce. Infective larvae of helminths were observed on lettuce samples at a density of 36/g-648/g with a mean of 342/g. Soil samples recorded a mean aerobic mesophilic plate counts of log 6.14 cfu/g, total coliforms count of log 4.90 cfu/g while fecal coliforms and Salmonella spp were not detected (<1 cfu/g) Soil samples yielded a mean infective larval count of 1941.5 larvae/g and a Strongyle count of 12 eggs/g. Even though less than 1 cfu/g of Salmonella spp were found, the study found lettuce to be contaminated with other foodborne bacteria pathogens, opportunistic bacteria pathogens, eggs and infective larvae of intestinal parasites of health importance. As a consequence, the microbial food safety risk associated with wastewater irrigated vegetables was observed to be high with possible public health implications. It is recommended that wastewater from the Onyasia River should be treated before use for irrigation of lettuce.
Keywords: Microbial quality, Wastewater, Lettuce, Onyasia river, Parasites
1. Background
Consumption of fresh fruits and vegetables has increased globally due to urbanization, with more consciousness of the need for a healthy diet, and year-round availability as a result of global trade [1,2]. Unfortunately, fresh produce including vegetables remains one of the main causes of foodborne illnesses globally [3,4]. Even though developing countries bear most of the burden of foodborne disease [5], local outbreaks in the 21st century can turn into international emergencies due to the speed and range of product distribution [6] Foodborne pathogens associated with fruits and vegetables are known to cause sicknesses such as Hepatitis A, Norovirus, Cyclospora spp, Aeromonas spp., Bacillus cereus, Campylobacter spp., Clostridium botulinum, E. coli, L. monocytogenes, S. enterica, Shigella spp., Staphylococcus spp., Yersinia enterocolitica and human intestinal parasites in humans [2,7,8]. Foodborne pathogens contaminate vegetables along the food production chain and can result in food borne diseases or outbreaks with the consequence of economic losses, increased healthcare costs, loss of productivity, reduction in quality of life, and mortality [9] if appropriate decontamination methods are not applied before consumption. Unfortunately, emerging food safety issues such as increased antimicrobial resistance in bacteria, changes in virulence due to biofilm formation [10], the phenomenon of internalization of enteric pathogens in various vegetables [11], climate change [12], the growing immune-compromised population [10] and minimal processing of vegetables exacerbates the food safety risk associated with vegetables particularly, those eaten raw.
Treatment of soil with organic fertilizers and/or sewage sludge and the use of untreated wastewater for irrigation are some of the major factors that result in contamination of vegetables with pathogens [13]. Additionally, the application of technologies such as cutting, slicing, peeling, and shredding will remove the natural protective barriers of the intact plant and open the possibility for providing a suitable medium for the growth of contaminating microorganisms [14]. It is clear from the foregoing that the production of fresh vegetables embraces different activities such as growing, harvesting, post-harvest treatment, and processing. Within all these activities, specific hazards exist that affect product safety and quality and might therefore pose a health risk for the consumer.
Water stress particularly in the urban and peri-urban areas in developing countries where increased demand of fresh produce coupled with the lack of clean water resources drives a lot of farmers into the use of polluted surface water for vegetable growing [15,16] In the urban and peri-urban areas of Ghana, untreated wastewater from streams, rivers and gutters are used for vegetable production [17] Notable vegetables produced are lettuce, cabbage, carrots, spinach, beet root and other vegetables, however lettuce is mostly cultivated due to its high demand for salads. Wastewater has been increasingly recognized as a reliable and cost-effective source of water, particularly for agricultural or industrial applications if appropriately treated [18]. For instance, less than 5% of all households in Ghana are connected to piped sewerage systems that are linked to sewage treatment plants [19]. In the two major cities (Accra and Kumasi), an estimated 72% and 43%, respectively, of the sludge produced ends up untreated in the environment or in the sea [20,21].
To reduce microbial contamination of vegetables, the [22] guidelines recommended a multiple barrier approach which is intended to protect livelihood and safeguard public health [15]. The multi-barrier approach follows the Hazard Analysis and Critical Control Points (HACCP) concept [23]. This implies that the health risks associated with low quality water used in agriculture can be safely managed. if the stakeholders along the contamination pathway comply with the multiple-barrier approach.
Ghana serves as a reference may be of the only African country, with the [22] guidelines in its irrigation policy in support of safe wastewater irrigation [15]. Yet, evidence after more than a decade of introduction of these guidelines in irrigation policy in Ghana, does not indicate any reduced risks, changed risk perception, or behavior [[17], [24], [25], [26], [27],]. This indicates that, the related legislation (like municipal by-laws) and regulations that empower institutions to implement them are lacking [15]. To safeguard public health and sustain livelihoods, there is the need to evaluate the microbial safety of wastewater irrigated lettuce along the Onyasia River in Ga East Municipal Assembly in Greater Accra Region of Ghana.
2. Materials and methods
2.1. Sampling
2.1.1. The study area
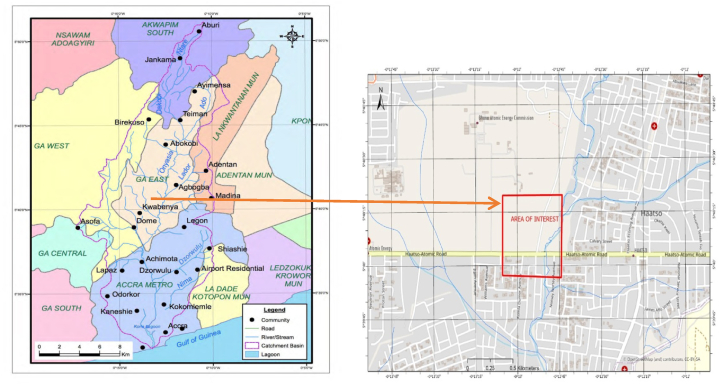
The Onyasia river runs within the Odaw river catchment, stretching from Akuapem Mountains to the sea at Korle Gonno (Fig. 1), all within several Metropolitan, Municipal and District Assemblies (MMDA) This river passes through communities such as Abokobi, Kwabenya, Haatso, and Dome, all in the Ga East District of the Greater Accra region of Ghana. The Onyasia river serves as a source of irrigation water for nearby vegetable farms [28].
Fig. 1.
Odaw River basin showing the MMDA and some major townships within its Catchment [29].
The study area in the rectangle.
This river receives effluents from Legon and Achimota Hospital, Legon and other academic institutions in the catchment area from the waste stabilization ponds at the University of Ghana [30].
2.1.2. Sampling site
The study was conducted on vegetable farms located in the rectangle labeled ‘Area of Interest’ in Fig. 1. Forty (40) lettuce farmers out of the two hundred (200) farmers of the GAEC Farmers' Association who grow vegetables along the Onyasia River use its water for their farming activities [28]. To our knowledge, reports of microbiological assessment of lettuce specifically from this site are scanty.
2.1.3. Sampling of lettuce
Convenient sampling of some members of the GAEC Farmers’ Association [28] was done from January to September 2021, and was carried out when the lettuce samples were ready for harvest. Thirty (30) samples were collected under normal purchase conditions for each month. A minimum of 3 composite samples-each containing 2 whole lettuces were collected from farmers, randomly selected and put in sterile polythene bags and then transported on ice to the laboratory where they were analyzed immediately or stored at 4 °C and analyzed within 24 h.
2.1.4. Irrigation wastewater sampling for bacteriological analysis
The procedure of [31] was used for the sampling of irrigation water with some modification. Twenty-seven water samples were collected from the Onyasia river. Sampling of the irrigation water sources was carried out between the hours of 8 and 10 in the morning at the time farmers are irrigating. At each site (upstream, midstream and downstream which were about 500 m apart), sterilized 200 mL glass bottles were used to collect water from three different points along the river. One composite sample was pooled and then used in the laboratory for different analysis.
2.1.5. Irrigation wastewater sampling for helminths egg quantification
Two-liter samples of irrigation water were collected after deliberately disturbing the water to stimulate the agitation that might occur when farmers are filling their watering cans. A total of 27 samples were collected from the upstream, midstream and downstream. This is intended to bring out the eggs, as they usually settle out under their own weight [30]. Samples were then collected at three different locations as above and a composite sample pooled for analysis.
2.1.6. Soil sampling for microbiological analysis
Thirty (30) soil samples for each month were collected from each vegetable farm as follows; samples were collected from the top 10 cm layer, where most bacteria are concentrated [29], using sterile spatulas and a 60 mm diameter soil auger. Soil samples were combined into one composite sample for the initial estimates of fecal coliform and helminth egg populations. The soil samples (approximately 500 g) were then placed in sterile polythene bags and immediately transported to the laboratory for analysis. Samples were placed in another polythene bag to retain humidity and kept refrigerated at (4 °C) until used within 24 h. The analysis of each composite sample was carried out in duplicates.
2.2. Laboratory analysis
2.2.1. Quantification of indicator bacteria populations in soil, lettuce, and irrigation wastewater
Soil, lettuce and irrigation wastewater samples were prepared for coliform population estimates as follows: soil samples were thoroughly mixed and two subsamples analyzed. A subsample of 20 g (dry weight) of each was weighed into 180 mL of sterile phosphate-buffered saline and shaken vigorously for about 2 min. Lettuce samples were aseptically cut into small pieces and mixed thoroughly. A subsample of 20 g (fresh weight) of each was weighed into 180 mL of phosphate-buffered saline and shaken vigorously for about 2 min. The membrane filtration method as described by [,[31], [32], [33], [34], [35]] was adopted for the quantification of indicator organisms in wastewater and standard laboratory methods employing the pour plate technique were used for lettuce and soil samples. Xylose lysine deoxycholate (XLD) agar (Oxoid) was used as selective media for Salmonella spp. and Shigella spp and Eosin Methylene Blue (EMB) (Oxoid) as selective media for both general coliforms and fecal coliforms. General coliforms were incubated at 37 °C and fecal coliforms at 44 °C.
2.2.2. Confirmation of bacteria isolates
Confirmation and identification of isolates were done using the Matrix-Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectrometry (Daltonics GmbH, Leipzig, Germany) technique. Pure cultures were overlaid on the MALDI-TOF matrix and spectrum for each microorganism, also called the peptide mass fingerprint (PMF), was automatically generated by the software. The PFM spectrum of unknown organisms was then matched with the proteome database reference to provide microbial identification.
2.2.3. Quantification and identification of helminth eggs in irrigation wastewater, soil, and lettuce
Helminth eggs were enumerated following the concentration method [29]. This is a modified US-EPA method using ZnSO4 solution (specific gravity, 1.2). About 100 g of lettuce and soil samples were each separately washed in about 1 L of sterile distilled water. The washed water from each sample and irrigation wastewater were then poured into separate containers and allowed to stand overnight to enable the helminth eggs to settle completely. About 90% of the supernatant was removed using a suction pump and transferred into 50 mL centrifugation tubes. The container was rinsed with distilled water and the rinsed water added into the centrifuge tubes. The tubes were then centrifuged at 1500 rpm for 3 min. The supernatant was poured off and the deposit re-suspended in 40 mL ZnSO4 solution. The mixture was homogenized with a sterile spatula and centrifuged at 1500 rpm for 3 min. At a specific gravity of 1.2 (ZnSO4), helminth eggs float leaving other sediments at the bottom of the centrifuge tube. The ZnSO4 supernatant was poured into a 2 L flask and distilled water added to the 1 L mark. This was allowed to stand overnight at room temperature for the eggs to resettle. As much supernatant as possible was sucked up and the deposit re-suspended by shaking. This was then transferred into a centrifuge tube. The deposit was re-suspended in 15 mL acid/alcohol buffer solution (H2SO4 at 0.1 N at 35% ethanol, i.e., 350 mL ethanol and 5.16 mL H2SO4) and about 5 mL ethyl acetate was added. The mixture was shaken and the centrifuge tube occasionally opened to let gas out before centrifuging at 2200 rpm for 3 min. After centrifugation, a diphasic solution (aqueous and lipophilic phase representing the acid/alcohol and ethyl acetate, respectively) was formed. With the aid of a micropipette, large volumes of the supernatant (starting from the lipophilic and then the aqueous phase) were sucked up leaving about 1 mL of the deposit. The deposit was then transferred onto a Sedgwick-Rafter cell, observed under a light microscope and the eggs counted. Eggs were identified by shape, size and color. The identities of specific helminth eggs were established using color charts for the diagnosis of intestinal parasites [35] and were identified to the genus level. Microbial food safety assessed under the current study were selected based on availability of resources and were analyzed and reported as described by [36].
2.3. Data analysis
The microbial counts (colony forming units, cfu/g) were transformed into logarithms (log) and means were determined.
3. Results
Results of the levels of bacterial contamination in irrigation wastewater, lettuce, and soil are presented in Table 1.
Table 1.
Bacterial contamination in counts (log cfu/g) in soil/lettuce samples and counts in (log cfu/100 mL) wastewater samples.
| Microbial (Bacterial) Count (log CFU/g) | Sample |
|||
|---|---|---|---|---|
| Wastewater | Lettuce | Soil | ||
| Aerobic Plate Count | Recorded Count | 5.80 ± 0.13 | 4.82 ± 2.55 | 6.14 ± 0.38 |
| Standard | NA | ≤6.69* | NA | |
| Total Coliform | Recorded Count | 4.77 ± 0.45 | 3.50 ± 1.00 | 4.90 ± 0.15 |
| Standard | NA | <4* | NA | |
| Faecal Coliform | Recorded Count | 3.5 ± 1.00 | <1 | <1 |
| Standard | <3/100 mLa | <2* | NA | |
| Salmonella | Count | <1 | <1 | NA |
| Standard | <1 | <1 | <1 | |
The results indicate that all samples were contaminated with aerobic mesophilic bacteria (APC) and total coliforms (TCC) Faecal coliforms (FCC) were however found in only surface wastewater samples while Salmonella was not detected in any of the samples.
Counts in surface wastewater samples were APC; log 5.70 cfu/100 mL - log 5.89 cfu/100 mL (log 5.80 cfu/100 mL), TCC; log 4.34 cfu/100 mL - log 4.98 cfu/100 mL (log 4.77 cfu/100 mL) and FCC; 0-log 3.80 cfu/100 mL (log 3.50 cfu/100 mL) (Table 1). Lettuce samples recorded APC of log 2.71 cfu/g - log 6.31 cfu/g (log 4.82 cfu/g) and TCC {log 2.79 cfu/g - log 4.21 cfu/g (log 3.5 cfu/g)}, while pooled soil samples yielded APC{log 5.77 cfu/g - log 6.31 cfu/g (log 6.14 cfu/g)} and TCC of log 4.88 cfu/g-log 5.09 cfu/g (log 4.90 cfu/g) (Table 1). Whereas the mean FCC of surface wastewater of log 3.50 cfu/100 mL was above permissible level (<log 3 cfu/100 mL), the average APC (log 4.82 cfu/g) and TCC (log 3.5 cfu/g) of lettuce were within the ICMSF permissible limits of ≤ log 6.69 cfu/g and < log 4.0 cfu/g, respectively (Table 1).
The distribution of bacteria, genera, and species among wastewater, lettuce, and soil samples is shown in Table 2.
Table 2.
Bacterial occurrence and diversity in surface wastewater, lettuce, and soil samples.
| Isolate | Source (sample type) |
||
|---|---|---|---|
| Wastewater | Lettuce | Soil | |
| Acinetobacter baumannii | + | + | – |
| Citrobacter freundii | – | + | + |
| Enterobacter aerogenes | – | – | + |
| Enterobacter asburiae | – | + | + |
| Enterobacter cloacae | + | – | – |
| Klebsiella pneumonia | + | - | – |
| Klebsiella variicola | – | + | – |
| Pseudomonas otitidis | – | – | + |
+ indicates the presence of bacteria species and − indicates absence of bacteria species.
Table 2 shows the distribution of bacterial isolates identified in the different samples. Of all 8 bacteria species identified, surface wastewater recorded three, lettuce four, and soil four (Table 2). Enterobacter spp was the only genus that occurred in the samples (Enterobacter cloacae in water, Enterobacter asburiae in lettuce and soil, and Enterobacter aerogenes in soil) (Table 2). Three species (Enterobacter cloacae, Acinetobacter baumannii, and Klebsiella pneumonia) were identified in wastewater, four species (Enterobacter asburiae, Acinetobacter baumannii, Klebsiella variicola, and Citrobacter freundii) in lettuce and four species (Enterobacter asburiae, Enterobacter aerogenes, Citrobacter freundii, and Pseudomonas otitidis) from soil samples (Table 2).
Results of parasitological analysis for counts of different stages (eggs, larvae, and cyst) of parasite contamination of irrigation wastewater, lettuce and soil are summarized in Table 3.
Table 3.
Intestinal parasite contamination levels and diversity of samples.
| Parameter | Sample |
|||
|---|---|---|---|---|
| Wastewater | Lettuce | Pooled soil sample | ||
| Parasite density | Infective larvae/g | ND | 342 ± 432.75 | 1941.5 ± 2444.47 |
| Strongyle ova/g | ND | ND | 12 | |
| Type of parasite | Type of parasite | ND | Nematode | Nematode |
| Standard [22] | Helminth | ≤ 1 egg/L | ||
Reported values are means ± standard deviation. ND-not detected.
Parasites were recovered from soil and lettuce samples but not from the surface wastewater samples, and they were mainly in the eggs and larvae forms (Table 3). Infective larval counts recorded in lettuce samples ranged between 36 and 648 eggs/g with a mean of 342 eggs/g, while pooled soil samples yielded an infective larval counts of 213–3670 larvae/g with a mean of 1941.5 and Strongyle eggs count of 12 eggs/g. Intestinal parasite eggs were not found in the surface wastewater used for irrigation and this is within the WHO accepted contamination level of ≤1 egg/L (Table 3).
4. Discussion
This study found surface wastewater used for irrigation contaminated with a mean fecal coliform count of log 3.50 cfu/100 mL, above the WHO permissible level of <3/100 mL [39] (Table 1). This observation is consistent with the findings of [25,40,41] who reported irrigation water used in the Accra metropolis to be contaminated with mean total and faecal coliform counts above the WHO recommended limit for irrigation water. The presence of thermotolerant coliforms or E. coli in water always indicates recent faecal contamination and the likely contamination with pathogenic human and/or animal gut microbes [39], unlike general coliforms which are found ubiquitously in nature, including in plants, soil, animals, and humans [39]. The growth of E. coli even at 44 °C has been reported to confirm E. coli as an indicator of recent faecal contamination [42]. The poor quality of surface wastewater observed in the current study could generally be due to anthropogenic activities, such as the disposal of human and animal waste into drains, open defecation around farms, and of fresh poultry manure used to amend soils in the vegetable farms which are all washed as run-off into the irrigation water source.
Specific bacteria isolates identified in irrigation wastewater were Enterobacter cloacae, Acinetobacter baumannii, and Klebsiella pneumonia (Table 2). Both Enterobacter cloacae and Klebsiella pneumonia are general coliforms and may have contaminated water from plants, soil, or animals/humans. Acinetobacter spp are typically free-living saprophytes, found almost everywhere, commonly distributed in the environment [41]. It is not surprising that they were isolated in the current study as they may have contaminated irrigation wastewater from the environment.
Helminth eggs count as a water quality indicator were observed in this study to be within WHO accepted contamination level of ≤1 egg/L [22]. [43] reported a helminth eggs concentration ranging from 5 to 10 eggs per liter of water used for irrigation by farmers. Evidence indicates that the presence and number of helminth eggs in water and soil vary with the infection rate prevailing in the community or natural factors, such as rainfall [[44], [45]]. Low prevalence of helminth infection in the community could have accounted for the acceptable levels recorded in the irrigation wastewater in the current study.
The current study found mean counts of aerobic mesophilic bacteria (APC, 4.82) and total coliforms (TCC, 3.5) in the lettuce samples to be within ICMSF permissible levels of ≤6.69 and <4.0 respectively [38]. Fecal coliforms and Salmonella spp were however below the detection limit of <1 cfu/g (Table 1). Notwithstanding the acceptable levels of APC and TCC observed, some foodborne pathogens and opportunistic pathogens were identified in the lettuce. Four bacteria species (Enterobacter asburiae, Acinetobacter baumannii, Klebsiella variicola, and Citrobacter freundii) were identified from lettuce (Table 2). Enterobacter asburiae is a general coliform opportunistic pathogen associated with different human diseases such as community-acquired pneumonia, soft tissue infections, and wound infections [7]. Results of the present study suggest that it can also be a potential pathogen in marketed fresh produce like lettuce in agreement with [[46], [45]]. Acinetobacter baumannii is associated with bacteraemia, pulmonary infections, meningitis, diarrhea, and notorious nosocomial infections with mortality rates from 8% to 35% [47]. Acinetobacter baumannii is also known for its rapid development of resistance to a wide range of antimicrobials and its ability to survive and persist under dry conditions in the environment for a very long time [41]. The isolation of Acinetobacter spp from lettuce in the current study agrees with [45]. Klebsiella variicola can be found in several associations with its environment, such as contaminated soil, rivers, and wastewater [48] and may have contaminated lettuce from the afore mentioned sources. Klebsiella variicola as well as K. pneumoniae, are opportunistic pathogens responsible for infections such as bloodstream infections (BSIs), respiratory tract infections, and urinary tract infections (UTIs) and a potential reservoir of multidrug-resistant genes [48]. Citrobacter freundii has been associated with foodborne outbreaks as a contaminant from both the guts of animals/man and the environment [49]. Gastrointestinal diseases linked to fresh produce has been associated with members of the family Enterobacteriaceae, most especially Citrobacter species [50,51]. The findings from the current study agree with the findings by [47,52] who reported that fresh produce has increasingly become recognized not only as significant reservoirs of foodborne pathogens but also for opportunistic pathogens. Opportunistic pathogens are mainly associated with nosocomial infections in immune-compromised and multi antibiotic-resistance [53,54]. Unfortunately, the size of the immunocompromised population outside the hospital setting is growing and antibiotic resistance has also become a global public health issue [10]. The isolation of these bacteria from lettuce in the current study therefore raises some serious concerns, particularly because lettuce is mostly eaten raw.
Lettuce samples were observed to be contaminated with larval forms of intestinal parasites with a mean larval count of 342 larvae/g is in agreement with [55] who also recovered parasite larvae from lettuce. These free-living nematodes feed on bacteria, algae or small organic particles. Free-living forms of parasitic nematodes, in particular, the filariform stages of Ancylostoma duodenale, Necator americanus or Strongyloides stercoralis are considered as a subject of workers and public health concern [56].
According to [57], transmission of intestinal helminth infection is through ingestion of eggs or larval forms. The high mean of larval count recorded in lettuce in the current study is therefore worrying. Particularly, evidence indicates that in most developing countries, the poverty of farmers and retailers is a major issue contributing to less or no disinfection of fresh produce before marketing [58]. Larvae recovered in the current study on lettuce may end up on the table of the consumers leading to infection [59]. It is not surprising that helminth infections are among the major public health problems in developing countries with significant morbidity and mortality in endemic countries [60]. In Ghana, worm infestations were seventh of the top twenty causes of morbidity at the outpatients’ department with a steady increase from 2.1% in 2011 to 3.6% in 2016 [61].
The current study observed a mean aerobic bacterial counts of log 6.14 cfu/g in the soil samples in contrast with [62] who reported that 1 g of soil may contain up to 1.5 × 1010 CFU/g of bacteria. According to [31], microbial contamination of soil varies markedly among different soil types and that may have accounted for the low counts observed in the current study. Four bacteria species (Enterobacter asburiae, Enterobacter aerogenes, Citrobacter freundii and Pseudomonas otitidis) were identified in the soil samples (Table 2). It is known that soil is one of the major reservoirs of microbial biodiversity (i.e., soil microbiota, which includes bacteria, archaea, fungi, protists, and viruses), playing a crucial role in agricultural ecosystems [62]. Isolation of bacteria from soil in the current study is therefore not surprising. Pseudomonas spp are known to form communities with other microbes in the soil and play a great influence on plant growth and vitality [63]. Evidence, however, indicates that bacterial pathogens of animal and human origin also survive easily in soil [63] and persist in the plant spermosphere-rhizosphere or phyllosphere, due to the formation of bacterial biofilms facilitating their adherence [64]. The phenomenon of internalization of enteric pathogens in various vegetables has been reported [11].
According to [65], eggs or larvae of all major helminths require a period of development in the soil to become infective before transmission. It is not surprising therefore, that the highest mean larval counts (1941.5 larvae/g) and mean egg counts (12 eggs/g) were observed in soil samples in the current study (Table 3). The recovery of Strongyle eggs from soil (Table 3) is in agreement with report by [57] who reported that it is one of the most prevalent intestinal parasites in Ghana. According to [66], eggs are persistent in the environment and have low infective dose; this makes their recovery in soil a matter of concern.
5. Conclusion
The current study found lettuce irrigated with wastewater and on the soil, it was farmed to be contaminated with foodborne bacteria pathogens, opportunistic bacteria pathogens, eggs and infective larvae of intestinal parasites, even though Salmonella spp were below detection limit of 1 cfu/g. These contaminants may end up contaminating the produce and this is very worrying. Consequentially, the microbial food safety risk associated with wastewater irrigated lettuce was observed to be high with possible public health implications. Isolation of foodborne pathogens and opportunistic pathogens in the current study is therefore worrying, since they may end up contaminating lettuce. There is a need for more management control measures to be put in place to mitigate this risk.
6. Recommendations
We recommend based on our findings that lettuce farmers need to be educated on the WHO guidelines on safe irrigation practices, that safe water is provided for the farmers, that irrigation water quality should be monitored, that wastewater should not be used to irrigate vegetables eaten raw without being treated, that only properly composted manures be used, that steps should be put in place to minimize the presence or activities of animals on farms, that all surfaces and implements that touch fresh produce must be treated as food contact surfaces, that quality of postharvest water that contacts fresh produce during cleaning, grading, cooling, and application of surface treatments is of good quality.
7. Declaration
Author contribution statement
1) conceived and designed the experiments: TM, BTO.
2) performed the experiments; TM, IA, BTO, AM, EAA.
3) analyzed and interpreted the data; TM, BTO, FCKO, SNAN, AA,
4) contributed reagents, materials, analysis tools or data; TM, BTO, FCKO, SNAN, AA.
5) wrote the paper; TM, BTO, FCKO, SNAN, AA.
7.1. Data availability statement
Data will be made available on request.
Declaration of competing interest
We declare that there is no conflict of interest between us as authors of the submitted manuscript.
Acknowledgments
The authors acknowledge the vegetable farmers association within the sampling site for their cooperation. The authors gratefully acknowledge the unknown referees for constructive suggestions, which were helpful in substantial improvement of the article.
References
- 1.Global Panel on Agriculture and Food Systems for Nutrition (GPAFSN) Urban Diets and Nutrition: Trends, Challenges and Opportunities for Policy Action. 2017. https://www.glopan.org/sites/default/files/Downloads/GlobalPanelUrbanizationPolicyBrief.pdf Accessed. [Google Scholar]
- 2.Carstens C.K., Salazar J.K., Darkoh C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019;10:2667. doi: 10.3389/fmicb.2019.02667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Truchado P., Allende A. La implicación de las frutas y hortalizas en las toxiinfecciones alimentarias y la relevancia del estado fisiológico de las bacterias. vol. 196. Arbor; 2020. Relevance of fresh fruits and vegetables in foodborne outbreaks and the significance of the physiological state of bacteria; pp. 1–9. [Google Scholar]
- 4.López-Gálvez F., Gómez P.A., Artés F., Artés-Hernández F., Aguayo E. Interactions between microbial food safety and environmental Sustainability in the fresh produce supply chain. Foods. 2021;10:1655. doi: 10.3390/foods10071655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delia G. Hemel Hempstead, UK: Evidence on Demand. 2015. Food safety in developing countries: an overview.https://hdl.handle.net/10568/68720 [Google Scholar]
- 6.Fung F., Huei-Shyong W., Suresh M. Food safety in the 21st century. Biomed. J. 2018;41:887–895. doi: 10.1016/j.bj.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC national outbreak reporting system (NORS) available from. 2017. https://wwwn.cdc.gov/norsdashboard/ accessed.
- 8.Osaili T.M., Hasan F., Al-Nabulsi A.A., Olaimat A.N., Ayyash M., Obaid R.S., Holley R. A worldwide review of illness outbreaks involving mixed salads/dressings and factors influencing product safety and shelf life. Food Microbiol. 2023 doi: 10.1016/j.fm.2023.104238. [DOI] [PubMed] [Google Scholar]
- 9.Focker M., van der Fels-Klerx H.J. Economics applied to food safety. Curr. Opin. Food Sci. 2020;36:18–23. [Google Scholar]
- 10.Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/ [Google Scholar]
- 11.Ge C., Lee C., Nangle E., Li J., Gardner D., Kleinhenz M., Lee J. Impact of phytopathogen infection and extreme weather stress on internalization of Salmonella Typhimurium in lettuce. Int. J. Food Microbiol. 2014;168:24–31. doi: 10.1016/j.ijfoodmicro.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Ramona Y., Darmayasa I.B.G., Kusuma A.A.N.N., Line M. Diversity of biocontrol agents, isolated from several sources, inhibitory to several fungal plant pathogens. BiodiverJ Biol Diversity. 2021;22(1) [Google Scholar]
- 13.Allende A., Monaghan J. Irrigation water quality for leafy crops: a perspective of risks and potential solutions. Int. J. Environ. Res. Publ. Health. 2015;12:7457–7477. doi: 10.3390/ijerph120707457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivek K., Suranjoy S.S., Ritesh W., Soberly M., Baby Z., Baite H., Mishra S., Pradhan R.C. A review on postharvest management and advances in the minimal processing of fresh-cut fruits and vegetables. J. Microbiol. Biotechnol. Food Sci. 2019;8(5):1178–1187. [Google Scholar]
- 15.Amponsah O., Vigre H., Schou T.W., Boateng E.S., Braimah I., Abaidoo R.C. Assessing low quality water use policy framework: case study from Ghana. Resour. Conserv. Recycl. 2015;97:1–15. [Google Scholar]
- 16.Drechsel P., Qadir M., Galibourg D. The WHO guidelines for safe wastewater use in agriculture: a review of implementation challenges and possible solutions in the global South. Water. 2022;14:864. [Google Scholar]
- 17.Quansah J.K., Escalante C.L., Kunadu A.P.H., Saalia F.K., Chen J. Pre- and post-harvest practices of urban leafy green vegetable farmers in Accra, Ghana and their association with microbial quality of vegetables produced. Agriculture. 2020;10:18. [Google Scholar]
- 18.UNESCO . United Nations Educational, Scientific and Cultural Organisation (UNESCO); Paris, France: 2017. WWAP the United Nations World Water Development Report 2017. Wastewater: the Untapped Resource; p. 198. [Google Scholar]
- 19.Monney I., Antwi-Agyei P. Journal of Water Sanitation and Hygiene for Development; 2018. Beyond the MDG Water Target to Universal Water Coverage in Ghana: the Key Transformative Shifts Required; pp. 127–141. [Google Scholar]
- 20.Amoah P. Thesis (PhD) Ghana. Kwame Nkrumah University of Science and Technology; 2008. Wastewater irrigated vegetable production: contamination pathway for health risk reduction in Accra, Kumasi and Tamale - Ghana. [Google Scholar]
- 21.Mansour G., Esseku H. Urban Sanitation Research Initiative Ghana: Accra; Ghana: 2017. Situation Analysis of the Urban Sanitation Sector in Ghana. [Google Scholar]
- 22.Who . ume 2. WHO; Geneva: 2006. p. 219pp. (Guidelines for the Safe Use of Wastewater, Excreta and Grey Water: Wastewater Use in Agriculture). [Google Scholar]
- 23.Keraita B., Drechsel P., Konradsen F. Up and down the sanitation ladder: harmonizing the treatment and multiple-barrier perspectives on risk reduction in wastewater irrigated agriculture. Irrigat. Drain. Syst. 2010;24:23–35. [Google Scholar]
- 24.Fianko J.R., Korankye M.B. Quality characteristics of water used for irrigation in urban and peri-urban agriculture in greater Accra region of Ghana: health and environmental risk. West Afr. J. Appl. Ecol. 2020;28:131–143. [Google Scholar]
- 25.Abass K., Ganle J.K., Afriyie K. ‘The germs are not harmful’: health risk perceptions among consumers of peri-urban grown vegetables in Kumasi, Ghana. Geojournal. 2017;82:1213–1227. [Google Scholar]
- 26.Antwi-Agyei P., Peasey A., Biran A., Bruce J., Ensink J. Risk perceptions of wastewater use for urban agriculture in Accra,Ghana. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry of Works, Housing (Mwh) MWH; Accra: 2018. Greater Accra Resilient and Integrated Development Project, Ibrd/ida Credit (P164330) Environmental and Social Management Framework (Esmf) Draft Esmf for the Garid. [Google Scholar]
- 28.Banson K.E., Asare D., Heng L., Cobbinah J.F., Adu-Sarkodieh A. Impact of small-scale irrigation technologies on poverty alleviation among peri-urban and urban farmers. J. Life Sci. 2014;8(2) [Google Scholar]
- 29.Orlofsky E., Bernstein N., Sacks M., Vanshika A., Benami M., Kundu A., Maki M., Woutrina S., Wuertz S., Shapiro K., Gillor O. Comparable levels of microbial contamination in soil and on tomato crops after drip irrigation with treated wastewater or potable water. Agric. Ecosyst. Environ. 2016;215:140–150. [Google Scholar]
- 30.Cornish G.A., Mensah E., Ghesquière P. HR Wallingford Ltd; Wallingford, UK: 1999. Water Quality and Peri-Urban Irrigation. An Assessment of Surface Water Quality for Irrigation and its Implications for Human Health in the Peri-Urban Zone of Kumasi, Ghana. Report OD/TN 95. [Google Scholar]
- 31.Adomako L.A., Yirenya-Tawiah D., Nukpezah D., Abrahamya A., Labi A.K., Grigoryan R., Zachariah .R. Reduced bacterial counts from a sewage treatment plant but increased counts and antibiotic resistance in the recipient stream in Accra, Ghana—a cross-Sectional study. Trop med inf disease. 2021;6(2):79. doi: 10.3390/tropicalmed6020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . World Health Organization; Geneva: 1996. Analysis of Wastewater for Use in Agriculture -A Laboratory Manual of Parasitological and Bacteriological Techniques. [Google Scholar]
- 33.Schwartzbrod J. France: University of Nancy; 2001. A Collection of Methods of Analysis of Helminth Eggs and Cysts in Wastewater, Sludge, Soils and Crops. [Google Scholar]
- 34.Yang X., Wu Q., Huang J., Wu S., Zhang J., Chen L., Zhang Y. Prevalence and characterization of Salmonella isolated from raw vegetables in China. Food Control. 2020;109 [Google Scholar]
- 35.WHO, Bench Aids for the Diagnosis of Intestinal Parasites. World Health Organization; Geneva: 1994. [Google Scholar]
- 36.FAO . WHO; Rome: 2021. Microbiological Risk Assessment - Guidance for Food. Microbiological Risk Assessment Series No. 36. [DOI] [Google Scholar]
- 37.Who . Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture. World Health Organization; Geneva: 1989. Report of WHO Scientific group. WHO) Technical Report Series No. 778. 1989. [PubMed] [Google Scholar]
- 38.vol. 6. Microbial Ecology of food commodities; New York: 1998. ICMSF (international commission on microbiological Specification for foods. (Microorganisms in Foods). Blackie. [Google Scholar]
- 39.Niyoyitungiye L., Giri A., Ndayisenga M. Assessment of coliforms bacteria contamination in lake tanganyika as bioindicators of recreational and drinking water quality South. Asian J Res Microbiol. 2020;6(3):9–16. [Google Scholar]
- 40.Akrong M.O., Ampofo J.A., Danso S.K.A. The quality and health implications of urban irrigation water used for vegetable production in the Accra metropolis. J. Environ. Protect. 2012;3:1509–1518. [Google Scholar]
- 41.Almasaudi S.B. Acinetobacter spp. as nosocomial pathogens: epidemiology and resistance features. Saudi J. Biol. Sci. 2018;25(3):586–596. doi: 10.1016/j.sjbs.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odonkor S.T., Mahami T. Escherichia coli as a tool for disease risk assessment of drinking water sources. Hindawi, Int J Microbiol. 2020 doi: 10.1155/2020/2534130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amoah P., Keraita B., Akple M., Drechsel P., Abaidoo R.C., Konradsen F. vol. 141. International Water Management Institute. Research Report; 2011. Low-cost Options for Reducing Consumer Health Risks from Farm to Fork where Crops Are Irrigated with Polluted Water in West Africa; p. 4. [Google Scholar]
- 44.Amoah I.D., Kumari S., Poovendhree R., Stenström T.A., Bux F. Impact of informal settlements and wastewater treatment plants on helminth egg contamination of urban rivers and risks associated with exposure. Environ. Monit. Assess. 2020;192:713. doi: 10.1007/s10661-020-08660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Kharousi Z.S., Guizani N., Al-Sadi M.A., Al-Bulushi I.M., Shaharoona B. Hiding in fresh fruits and vegetables: opportunistic pathogens may cross geographical barriers. Hindawi Int J Microbiol. 2016:1–15. doi: 10.1155/2016/429241. Article ID 4292417 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizi K., Ghazvini K., Farsiani H. Clinical and pathogenesis overview of Enterobacter infections. Rev. Clin. Med. 2020;6(4):146–154. [Google Scholar]
- 47.Cornejo-Jua ´rez P., Cevallos M.A., Castro-Jaimes S., Castillo-Ramı ´rez S., Vela ´zquez-Acosta C., Martı ´nez-Oliva D., et al. High mortality in an outbreak of multidrug resistant Acinetobacter baumannii infection introduced to an oncological hospital by a patient transferred from a general hospital. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez-Medina N., Barrios-Camacho H., Duran-Bedolla J., Garza-Ramos U. Emerging Microbes & Infections; 2019. Klebsiella Variicola: an Emerging Pathogen in Humans; p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aminharati F., Ehrampoush M.H., Soltan Dallal M.M., Yaser M., Dehghani T.A.A., Z Rajab R., freundii Citrobacter. Foodborne disease outbreaks related to environmental conditions in yazd province, Iran. Iran. J. Public Health. 2019;48(6):1099–1105. [PMC free article] [PubMed] [Google Scholar]
- 50.Kemajou T.S., Awemu G.A., Digban K.A., Oshoman C.E., Ekundayo O.L., Ajugwo A.O. Microbiological studies of vegetable leaves sold in elele market, rivers-state, Nigeria. J. Transm. Dis. Immun. 2017;1:1–4. [Google Scholar]
- 51.Aminu F., Ali M. Isolation and identification of microorganisms associated with spoilage of cabbage (Brassica oleracea) in Sabon-gari market Kano, Nigeria. Int. J. Adv. Acad. Res. | Sci., Technol. Eng. 2017;3:2. [Google Scholar]
- 52.Adegun B.R., Oluduro O.A., Aregbesola O.A. Isolation and molecular characterization of citrobacter species in fruits and vegetables sold for consumption in ILE-IFE, Nigeria. Scientific African. 2019;6 [Google Scholar]
- 53.Martin R.M., Bachman M.A. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018;8:4. doi: 10.3389/fcimb.2018.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Annavajhala M.K., Gomez-Simmonds A., Uhlemann A.C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front. Microbiol. 2019;10:44. doi: 10.3389/fmicb.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eraky M.A., Rashed S.M., Nasr M.E., El-Hamshary A.M.S., El-Ghannam A.S. Parasitic contamination of commonly consumed fresh leafy vegetables in benha, Egypt. J Parasitol Res. 2014;2014:1–7. doi: 10.1155/2014/613960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization . World Health Organization Regional Office for the Eastern Mediterranean Regional Centre for Environmental Health Activities Amman – Jordan; 2004. Integrated Guide to Sanitary Parasitology. [Google Scholar]
- 57.Ahiadorme M., Morhe E. Soil transmitted helminthes infections in Ghana: a ten-year review. Pan Afr Med J. 2020;35:131. doi: 10.11604/pamj.2020.35.131.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridolfi C., Hoffman V., Baral S. International Food Policy Research Institute; Washington, DC, USA: 2018. Post-Harvest Losses: Global Scale, Solutions, and Relevance to Ghana. [Google Scholar]
- 59.Shumbej T., Girum T. Helminthes infections in light of an ongoing intervention in endemic areas of Guragae zone, Southern Ethiopia: an implication for neglected tropical diseases elimination in Ethiopia by 2020. Tropical Diseases, Travel Medicine and Vaccines. 2019;5:8. doi: 10.1186/s40794-019-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amissah-Reynolds P.K., Dekugmen D.Y., Aboagye V., Monney I., Nuamah F., Awimbe-Ndego E. Parasitic contamination in ready-to-eat salads in the Accra metropolis, Ghana. South Asian Journal of Parasitology. 2019;3(4):1–11. [Google Scholar]
- 61.Torsvik V., Goksøyr J., Daae F.L. High diversity in DNA of soil bacteria. Appl. Environ. Microbiol. 1990;56:782–787. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Z., He J., Quan Z., Wu C., Sheng R., Zhang L., Geisen S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020;148 [Google Scholar]
- 63.Cruz D., Cisneros R., Benítez A., Zúñiga-Sarango W., Peña J., Fernández H., Jaramillo A. Gram-negative bacteria from organic and conventional agriculture in the hydrographic basin of loja: quality or pathogen reservoir? Agronomy. 2021;11:2362. [Google Scholar]
- 64.Szczech M., Kowalska B., Smoli′ nska U., Maciorowski R., Oskiera M., Michalska A. Microbial quality of organic and conventional vegetables from polish farms. Int. J. Food Microbiol. 2018;286:155–161. doi: 10.1016/j.ijfoodmicro.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Oyewole E.O., Simon-Oke I.A. Ecological risk factors of soil-transmitted helminths infections in Ifedore district, Southwest Nigeria. Bull. Natl. Res. Cent. 2022;46(1):13. [Google Scholar]
- 66.Amoah I.D., Abubakari A., Stenström T.A., Abaidoo R.C., Seidu R. Contribution of wastewater irrigation to soil transmitted helminths infection among vegetable farmers in Kumasi, Ghana. PLoS Neglected Trop. Dis. 2016;10(12) doi: 10.1371/journal.pntd.0005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.