Abstract
Vanillin, a plant-derived antimicrobial volatile substance, has potential microbial control applications in the food industry. However, the effect of vanillin on the food-borne pathogen Escherichia coli (E. coli) O157:H7 has not been well studied. This study aims to explore the antibacterial mechanism of vanillin against E. coli O157:H7. The minimum inhibitory concentration (MIC) and antibacterial effect of vanillin were determined by microdilution. Scanning electron microscopy (SEM) was used to observe the damage of vanillin to the cell membrane, while cell membrane potential and the leakage of nucleic acid protein were measured to explore the effect of vanillin on the membrane system. Confocal laser scanning and intracellular adenosine triphosphate (ATP) concentration determination were utilized to investigate the effects of vanillin on the energy, life, and death of E. coli. Finally, transcriptome sequencing was conducted to investigate the gene expression differences induced by vanillin treatment. The results showed that vanillin treatment effectively controlled E. coli O157:H7 with an MIC of 2 mg/mL. After treatment, damage to the membrane system, depolarization of the membrane, and leakage of nucleic acid and protein were observed. Meanwhile, vanillin treatment caused decreased ATP content and cell death. Transcriptome analysis showed that vanillin treatment significantly affected the expression of genes involved in cell membrane formation, tricarboxylic acid (TCA) cycling pathway, and oxidative phosphorylation pathway in E. coli O157:H7. In conclusion, membrane damage and energy metabolism disruption are important mechanisms of vanillin's inhibitory effect on E. coli O157:H7. This study provides new insights into the molecular reaction mechanism of vanillin against E. coli O157:H7, highlighting its potential as an antibacterial substance for preventing E. coli contamination in the food industry.
Keywords: Vanillin, E. coli O157:H7, Antibacterial mechanism, Transcriptome
Highlights
-
•
Vanillin can effectively inhibit Escherichia coli O157: H7.
-
•
Vanillin damages the membrane system of Escherichia coli O157: H7.
-
•
Vanillin affected the energy balance of Escherichia coli O157: H7.
-
•
The transcriptome of Escherichia coli O157: H7 treated with vanillin was confirmed.
1. Introduction
Escherichia coli (E. coli) is a diverse group of Gram-negative bacteria, with some strains being non-pathogenic [1]. However, certain strains of E. coli are capable of producing toxins and have a low infectious dose, making them highly transmissible through various media. This bacterium can persist in the environment for extended periods, proliferate within food sources, and ultimately pose significant threats to human health and safety. Notably, E. coli O157:H7 is an important food-borne pathogen associated with severe health risks. This strain is classified as an enterohaemorrhagic E. coli and is capable of producing the Shiga toxin, which can cause hemorrhagic colitis, hemolytic uremic syndrome, acute renal failure, and other severe diseases [[2], [3], [4]].
In current food production and research, various methods have been proven effective in controlling E. coli O157:H7, including physical and chemical treatments. Physical treatments, such as pasteurization [5] and new methods such as irradiation, plasma treatment, and pulsed electric field [[6], [7], [8], [9]], have shown promise in controlling E. coli O157:H7. However, these methods have limitations in their applicability to different food types and the conditions of treatment facilities and equipment [10]. Chemical treatments, including organic acids, sodium hypochlorite, and ozone aqueous solutions, have been widely used in the food industry due to their low cost [[11], [12], [13]]. Different types of chemical disinfectants have specific applications. For instance, organic acids are effective in controlling E. coli O157:H7 on meat products, whereas sodium hypochlorite is commonly used to control E. coli O157:H7 on fruit and vegetable products [14,15]. Despite the low cost, the potential toxicity, impact on food safety, and potential drug resistance of these chemical disinfectants cannot be ignored [16,17]. Therefore, the development of safer, harmless, and environmentally friendly control agents such as plant-derived natural compounds, probiotics, and antagonistic bacteria to control food-borne bacteria is of great significance to promote the healthy and sustainable development of the food industry.
Vanillin (4-hydroxy-3-methoxybenzaldehyde), a phenolic formaldehyde compound derived from plants, has gained significant attention in food processing due to its wide range of applications [18]. Natural vanillin is mainly extracted from vanilla orchid pods and is also found in essential oil extracts from various plants [19], including mulberry seed essential oil [20]. Previous studies have reported the antibacterial properties of vanillin, which exhibits similar biological properties to common phenolic compounds in plant essential oils such as carvacrol, thymol, and eugenol [21]. In vitro experiments have demonstrated the antibacterial effect of vanillin on different food-related bacteria, yeast, and molds [22,23]. Moreover, the lower cytotoxicity of vanillin as compared to other phenolic compounds has been reported, indicating its potential as a safe antimicrobial agent [24]. Given its natural origin and effective antibacterial properties, vanillin holds significant promise for the control of E. coli O157:H7 in the food industry.
It is generally believed that vanillin is a membrane pore-forming compound, which can inhibit the growth of E. coli by destroying its cell membrane [25]. In the study of the mechanism of vanillin inhibiting E. coli, it has been confirmed that vanillin destroys the integrity of E. coli cell membrane by staining method, and through the detection of the ion concentration of E. coli cells, the damage of cell homeostasis and the inhibition of energy metabolism caused by vanillin damaging E. coli cell membrane are identified from the perspective of potassium ion balance and pH homeostasis [22]. On the basis of the above research, vanillin is often used as an effective antibacterial substance in the development of composite antibacterial materials to inhibit E. coli [26]. However, up to now, there have been few studies on how vanillin destroys the membrane system of E. coli, and the target and mechanism of vanillin's action on E. coli cell membrane cannot be explained clearly. There is also a lack of systematic studies to explore the effects of vanillin on E. coli.
As a new type of omics [27], transcriptomics is different from most traditional techniques (dye penetration, electron microscopy imaging and in vitro DNA binding assay) that cannot elucidate the mechanism of antibacterial molecules of natural antibacterial substances. Transcriptomics can generate large amounts of data through high-throughput sequencing technology, which can analyze extremely complex features [28]. The molecular mechanism was also elucidated. Existing studies have explored the molecular mechanism of cold stress and essential oil substances inducing bacterial inactivation through transcriptomic techniques [29,30]. Therefore, the aims of this study were to (i) confirm the inhibitory effect of vanillin on the growth of E. coli O157:H7, (ii) investigate the impact of vanillin treatment on the membrane and energy systems of E. coli O157:H7, and (iii) elucidate the mode of action of vanillin against E. coli O157:H7 through transcriptome analysis.
2. Materials and methods
2.1. Reagents
Vanillin [HPLC≥98%, CAS 121-33-5] was obtained from Shanghai Yuanye Bio & Technology Co., Ltd., (Shanghai, China). Vanillin was then dissolved in water containing 2% anhydrous ethanol (v/v) and sterilized using 0.22-μm Acrodisc filters. The solutions were then stored at 4 °C until analysis. All other chemicals used in this study were of analytical grade.
2.2. Bacteria culture
E. coli O157:H7 ATCC 35150 strain was obtained from the Mulberry Laboratory at Zhejiang University (Zhejiang, China) and stored accordingly. The first activated strain was cultured in LB medium at 37 °C for 18 h until it reached the logarithmic growth phase or remained stable in the stationary phase.
2.3. Determination of the antibacterial effect
2.3.1. Determination of minimum inhibitory concentration (MIC)
The MIC of vanillin against E. coli O157:H7 was determined using the modified agar dilution method [31]. Briefly, different volumes of vanillin solution were added to 150 mL of sterilized LB agar medium at a temperature of 55 °C to achieve concentrations of 0.1 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 1.5 mg/mL, and 2.0 mg/mL. A blank control containing 2% ethanol solution was also prepared. The LB culture solution containing vanillin solution was mixed and poured into Petri plates, and after drying, bacterial suspensions of E. coli O157:H7 at a concentration of 1.0 × 106 CFU/mL were inoculated onto the plates. The Petri plates were inverted and incubated at 37 °C for 24 h, and the colony-forming units were counted. The concentration at which no colony growth was observed was recorded as the MIC. Each treatment was performed in triplicate as biological replicates.
2.3.2. Determination of growth curve
Growth curves were constructed to evaluate the effect of different concentrations of vanillin on the growth of E. coli O157:H7 according to a modified method primarily described by Qian [32]. The E. coli O157:H7 culture was first diluted to a concentration of 1.0 × 106 CFU/mL in the logarithmic growth phase. Then, 100 μL of the bacterial culture was added to each well of a 96-well plate, followed by the addition of an equal volume (100 μL) of vanillin solution to each well to obtain final concentrations of 1/4 MIC, 1/2 MIC, and 3/4 MIC. A negative control was prepared using 2% ethanol solution. The plates were incubated at 37 °C in a humidified incubator, and cell growth was monitored every 2 h by measuring the optical density at 600 nm using a full-wavelength scanning multifunctional reader (Synergy H1, Biotek, USA).
2.4. Detection of effects on the bacterial plasma membrane
2.4.1. Scanning electron microscopy (SEM) analysis
E. coli O157:H7 cells were treated with 0, 1/2 MIC, 1 MIC, and 2 MIC vanillin solution, incubated at 37 °C for 4 h, washed three times with phosphate buffer saline (PBS) (pH 7.0) and resuspended with 2.5% glutaraldehyde PBS. The bacterial pellets were then fixed at 4 °C for 12 h. The fixed samples were rinsed three times with 0.1 M, pH 7.0 PBS for 15 min each time. Subsequently, the samples were dehydrated with a gradient concentration of ethanol solution (including six concentrations of 30%, 50%, 70%, 80%, 90%, and 95%) for 15 min each and then treated with 100% ethanol for 20 min. Finally, the samples were dried using a fully automatic critical point drier (Hitachi HCP-2, Japan), coated with a thin layer of gold, and observed using a scanning electron microscope (Hitachi SU-8010, Japan) to evaluate the effects of vanillin treatment on the ultrastructure of E. coli O157:H7 cells.
2.4.2. Determination of the membrane potential
In this study, the lipophilic anionic fluorescent dye DiBAC4(3) was used to detect the membrane potential of E. coli O157:H7 cells. DiBAC4(3) is a highly sensitive and specific probe for detecting cell membrane potential changes. It is non-fluorescent by itself and only emits fluorescence when it enters the cell and binds to intracellular proteins [33]. When the membrane potential changes, DiBAC4(3) fluorescence intensity also changes, indicating cell depolarization or hyperpolarization. Therefore, DiBAC4(3) was employed to investigate the effects of vanillin treatment on the membrane potential of E. coli cells.
E. coli O157:H7 cells were suspended in PBS to a concentration of 1.0 × 106 CFU/mL in the logarithmic growth phase. Different concentrations of vanillin solution (0, 1/2 MIC, 1 MIC, and 2 MIC) were added to the bacterial suspension, followed by incubation at 37 °C for 4 h. Next, a 200 μL of cell suspension was mixed with 1 μM of fluorescent probe DiBAC4(3) (Shanghai Maokang Biotechnology Co., Ltd., China) in a black 96-well microtiter plate. After incubation at 37 °C for 15 min in the dark, a fully functional enzyme marker (Synergy H1, Biotek, Vermont, USA) was used with excitation and emission wavelengths set at 492 nm and 515 nm, respectively, to measure the fluorescence intensity.
2.4.3. Determination of nucleic acid and protein leakage
E. coli O157:H7 cells were exposed to different concentrations of vanillin solution (0, 1/2 MIC, 1 MIC, and 2 MIC) for 4 h. After treatment, the bacterial suspension was centrifuged at 6000 rpm for 5 min, and the supernatant was collected and allowed to stand overnight. The following day, the supernatant samples were analyzed for UV absorption intensity at 260 nm and 280 nm by adding 1 μL of the supernatant dropwise to the ultramicroscopic spectrophotometer.
2.5. Determination of intracellular adenosine triphosphate (ATP)
The quantification of intracellular ATP was based on the ATP bioluminescence method and an ATP assay kit (A095-1-1, Nanjing, China) to detect the intracellular ATP content of E. coli O157:H7 cells following treatment with vanillin solution.
E. coli O157:H7 cells in the logarithmic growth stage were centrifuged at 6000 rmp for 2 min and resuspended in PBS and treated with vanillin solution of varying concentrations (0, 1/2 MIC, 1 MIC, and 2 MIC) at 37 °C for 4 h. After incubation, 1 mL of bacterial suspension was transferred to 1.5 mL Eppendorf tubes and incubated for 30 min at 37 °C. Cell lysate buffer (100 μL) was added to each tube, and the mixture was centrifuged at 4000 rpm for 5 min to collect the supernatant. A total of 20 μL of supernatant was added to a black 96-well microtiter plate, followed by the addition of 100 μL of ATP analysis mixture to each well. The absorbance of each well was measured using a full-function enzyme standard (Synergy H1, Biotek, USA), and three biological replicates were conducted for each group.
2.6. Confocal laser scanning microscopy (CLSM) observation
The SYTO 9/PI Live/Dead Bacteria Dual Staining Kit (Shanghai Maokang Biotechnology Co., Ltd., China) was used to detect E. coli O157:H7 necrosis. SYTO 9 is a green-fluorescent nucleic acid stain that can penetrate cell membranes of all cells. PI is a red fluorescent and normally impermeable to the cell membrane of live cells, but enters the membrane of dead or damaged cells and stains the nucleic acid with red fluorescence [34]. E. coli O157:H7 in the logarithmic growth phase was selected and suspended in sterile PBS at a concentration of 1.0 × 106 CFU/mL. The bacteria were then treated with different concentrations of vanillin solution (0, 1/2 MIC, 1 MIC, and 2 MIC) at 37 °C for 4 h. The treated E. coli O157:H7 was centrifuged at 6000 rpm for 5 min, and the supernatant was discarded. The centrifuged bacteria were washed with sterile PBS three times and resuspended in sterile PBS. In the single-staining group, 10 μL of PI or SYTO 9 was added to a 1.5 mL centrifuge tube. In the double-staining group, 5 μL of PI and 5 μL of SYTO 9 were added. The samples were mixed well and incubated at 25 °C in the dark for 30 min. The stained suspension was centrifuged at 6000 rpm for 5 min, the supernatant was discarded, and the cells were resuspended in sterile PBS. The samples were observed with a confocal laser scanning microscope (TCS SP5, Germany) with excitation and emission wavelengths set to 490 nm and 635 nm for PI and 480 nm and 500 nm for SYTO 9.
2.7. Transcriptome analysis
2.7.1. RNA extraction, library construction, and sequencing
Total RNA was extracted from E. coli O157:H7 cells using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. The RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Subsequently, mRNA was isolated by removing ribosomal RNA (rRNA) from the total RNA. The isolated mRNA was then fragmented into short fragments using a fragmentation buffer, followed by the construction of a chain-specific library [35]. After the library was validated, the different libraries were pooled according to the requirements of the effective concentration and target off-machine data volume, and subjected to Illumina sequencing.
2.7.2. Transcriptome data analysis
After filtering out low-quality reads, Bowtie2 was used to align the clean reads to the reference genome. The expression levels of each gene were calculated based on the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) method, and differential expression analysis was conducted between the treated and control groups using the edgeR R package (v3.18.1). Gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed on the differentially expressed genes (DEGs) using the cluster Profiler R package.
2.8. Statistical analysis
All experiments were performed in triplicate and repeated at least twice to ensure the reliability of data. Statistical analysis was conducted using the statistical product and service solutions (SPSS) statistical package 23.0 (SPSS Inc. Chicago, USA). The effects of the treatments were determined by analysis of variance (ANOVA), and significant differences (P < 0.05) were separated using the Waller–Duncan multiple range test.
3. Results and discussion
3.1. Antibacterial effect of vanillin
3.1.1. The MIC of vanillin
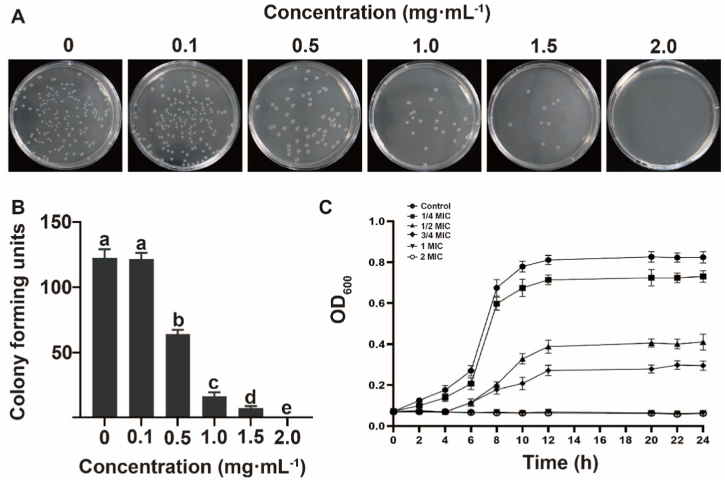
Vanillin has been demonstrated to have effective antibacterial properties against E. coli O157:H7, with an MIC of 2 mg/mL (Fig. 1 A, B). This finding is consistent with previous research on the antibacterial effect of compound essential oils containing vanillin as the main component, which reported MICs of 0.63–2.5 mg/mL against E. coli O157:H7 [36]. When compared to other natural compounds of plant origin with antibacterial activity against E. coli O157:H7, such as active compounds from Punica granatum pericarp (0.05 mg/mL) [37], Litsea cubeba essential oil (0.5 mg/mL) [38], Cymbopogon citratus extract (0.63 mg/mL) [39], Quercus infectoria nut galls (1.56 mg/mL) [40], Paeonia suffruticosa and buds extract (6.25 mg/mL) [41], Wasabi (10 mg/mL) [42], and pecan shell extracts (15 mg/mL) [43], vanillin has a moderate antibacterial effect. Based on the research on oregano essential oil [36] and oregano essential oil pectin edible film [44], it is possible that the use of natural compounds derived from plants to construct new antibacterial composite materials may have better antibacterial effects, and the antibacterial effect of vanillin also has the potential to be further improved. In conclusion, vanillin is a promising natural compound for controlling E. coli O157:H7 in food and related industries.
Fig. 1.
Antibacterial effect of vanillin on E. coli O157:H7 and growth curve of E. coli O157:H7. (A) The growth of E. coli O157:H7 treated with vanillin after one day of culture. (B) Growth statistics of E. coli O157:H7 treated with vanillin after one day of culture. Each value represents the mean of independent triplicate measurements. Different lowercase letters (a, b, and c) represent significant differences (p < 0.05), and vertical bars represent the mean ± standard error (mean ± SEM). (C) Growth curves for E. coli O157:H7. Each value represents the average of three independent measurements.
3.1.2. The growth curve of E. coli O157:H7
To further investigate the inhibitory effect of vanillin on the growth of E. coli O157:H7, we conducted a growth curve determination using concentrations ranging from 1/4 MIC to 2 MIC. Under normal conditions, the growth curve of the control group followed the S-shaped model, with the logarithmic phase reached after 6 h and stabilization phase within 12 h (Fig. 1C). Compared to the control OD values, the 1/4 MIC group showed growth inhibition while the 1/2 MIC and 3/4 MIC groups exhibited significant inhibition of bacterial growth. Furthermore, the bacterial OD600 values of the 1 MIC and 2 MIC groups remained almost unchanged, indicating that vanillin derived from mulberry seeds can effectively slow down or even completely inhibit the growth of E. coli O157:H7.
3.2. Vanillin affects the plasma membrane of E. coli O157:H7
3.2.1. SEM
SEM was used to observe the antibacterial effect of vanillin on E. coli O157:H7 (Fig. 2A–D). The membrane morphology of E. coli O157:H7 was normal in the control group, and the cells were plump with clear rod-shaped borders. Under the condition of 1/2 MIC treatment, the morphology of E. coli O157:H7 basically failed to change significantly, most of which could present a round rod-shaped border. However, some cells began to shrink on the surface, further indicating that vanillin has a certain inhibitory effect on E. coli O157:H7 under 1/2 MIC treatment while failing to change the cell morphology of E. coli O157:H7 significantly. Treatment with MIC destroyed the cell structure of E. coli O157:H7 and collapsed the cell surface in a large area, thereby resulting in significant changes in the cell morphology of E. coli O157:H7 different from that of the control group and the 1/2 MIC group. When the dose reached 2 MIC, the damage to the membrane of E. coli O157:H7 was more severe. This phenomenon was similar to the SEM observation results of E. coli O157:H7 treated with monocaprin and carvacrol [45], Cudrania tricuspidata fruit essential oil [46]. Therefore, it can be speculated that the direct damage of vanillin to the cell membrane of E. coli O157:H7, resulting in significant changes in the cell morphology, is one of its antibacterial mechanisms.
Fig. 2.
Scanning electron micrographs of E. coli O157:H7 under different treatments. (A) Untreated E. coli O157:H7. (B) E. coli O157:H7 treated with vanillin at 1/2MIC. (C) E. coli O157:H7 treated with vanillin at MIC. (D) E. coli O157:H7 treated with vanillin at 2MIC.
3.2.2. Membrane potential
A stable cell membrane potential is one of the most important symbols of the integrity of the cell membrane. Cell membrane damage and loss of membrane integrity often coincide with depolarization of the membrane. After vanillin was treated with E. coli O157:H7, the fluorescence intensity detected was significantly enhanced (Fig. 3A). The fluorescence intensity of E. coli O157:H7 was significantly higher than that of the control group under the condition of 1/2 MIC. The fluorescence intensity continued to increase significantly with the increase in treatment intensity, indicating that vanillin treatment will destroy the membrane structure of E. coli O157:H7. Consequently, the cell membrane was depolarized, and the ion balance inside and outside the cell was broken down. Previous studies have shown that various treatments, including 460–470 nm light-emitting diodes [47], quercetin [48], and single card protein combined with carvall treatment [45], have presented a similar antibacterial mechanism to E. coli O157:H7, causing depolarization of E. coli O157:H7 cell membrane similar to vanillin treatment.
Fig. 3.
Effects of vanillin on membrane potentials and nucleic acid and protein leakage in E. coli O157:H7. (A) Membrane potentials. (B) Nucleic acid and protein leakage. Each value represents the mean of independent triplicate measurements. Different lowercase letters (a, b, and c) represent significant differences (p < 0.05), and vertical bars represent the mean ± standard error (mean ± SEM).
3.2.3. Leakage of nucleic acids and proteins
Vanillin has been shown to cause damage to the membrane system of E. coli O157:H7, resulting in the release of important cellular contents such as nucleic acids and proteins that are necessary for maintaining normal physiological activities. Nucleic acids carry unique genetic information, and proteins play a structural and catalytic role in maintaining the structure and vitality of cells [49,50]. The extent of nucleic acid leakage from E. coli O157:H7 can be evaluated by measuring absorbance at 260 nm, and vanillin treatment has been shown to cause this leakage. Under MIC treatment, the A260 value was significantly higher than that in the control group and 1/2 MIC treatment. Moreover, as the treatment intensity increased, the leakage of nucleic acid also increased, indicating that vanillin causes severe damage to E. coli O157:H7. The protein leakage from E. coli O157:H7 can be assessed by measuring absorbance at 280 nm (Fig. 3B). It was found that protein leakage and nucleic acid leakage shared similar trends, suggesting that vanillin treatment at MIC and above concentrations may cause irreversible damage to the plasma membrane of E. coli O157:H7, leading to the release of a large amount of intracellular substances, including nucleic acids and proteins with important biological functions. Similar to vanillin, nanoparticles containing phenolic compounds [51] and zinc oxide nanoparticles [52] have also been shown to disrupt the membrane structure of E. coli O157:H7, leading to leakage of nucleic acids and proteins.
3.3. Intracellular ATP concentrations
Vanillin has been shown to damage the membrane integrity of E. coli O157:H7, disrupting the intracellular ionic balance and affecting energy production [22]. Intracellular ATP is a critical molecule for storage and function, with important roles in signal transduction and enzymatic reactions [53]. Treatment with vanillin at 1/2 MIC significantly decreased the intracellular ATP content compared to the control group (Fig. 4). As the concentration of vanillin increased, the intracellular ATP content continued to decrease, with only 20.67 μmol/L and 15.33 μmol/L at 1 MIC and 2 MIC, respectively. The reduction in ATP content in E. coli O157:H7 cells may be due to two reasons: the direct leakage of ATP caused by the destruction of cell membrane structure and the disruption of ATP hydrolysis reaction balance caused by ion balance breakdown [54,55]. Similar to vanillin, other antibacterial treatments have been shown to cause a loss of intracellular ATP in E. coli O157:H7, such as Spanish oregano, Chinese cinnamon, and savory essential oils [56], mustard essential oil [57], and Ib-AMP1 treatment [58]. These results suggest that the reduction of ATP content in E. coli O157:H7 is one of the antibacterial mechanisms of vanillin treatment.
Fig. 4.
Effects of vanillin on intracellular ATP levels in E. coli O157:H7. Each value represents the mean of independent triplicate measurements. Different lowercase letters (a, b, and c) represent significant differences (p < 0.05), and vertical bars represent the mean ± standard error (mean ± SEM).
3.4. CLSM observation
As shown in Fig. 5, untreated control cells exhibit strong green fluorescence, indicating intact cell membranes. After treatment with 1/2 MIC of vanillin, the green fluorescence significantly decreased while the red fluorescence significantly increased, indicating vanillin-induced cell membrane damage and cell death of E. coli O157:H7. As the vanillin treatment concentration increased, there were fewer fluorescent green cells observed in the visual field. Under MIC vanillin treatment, only a few green cells can be observed in the visual field, indicating that cell growth and differentiation are strongly inhibited due to severe membrane damage, ultimately leading to cell death. Similar studies involving silver colloid nanoparticles [59], zinc oxide [60] and bacteriophage PP01 [61] have shown that these antibacterial treatments also kill E. coli O157:H7 cells, achieving the desired antibacterial effect.
Fig. 5.
Effects of vanillin on cell Live/Dead of E. coli O157:H7 by CLSM.
3.5. Transcriptome analysis
3.5.1. Global analysis of the transcriptome data
Early on in the experiment, it was found that the treatment of E. coli with MIC vanillin would give rise to cell death and cell content leaking. Therefore, it was not suitable for transcriptome analysis, as the majority of them would come from dead cells, failing to objectively reflect the influence of vanillin treatment on the physiological response of E. coli [62]. As a result, the transcriptome of the blank control group and 1/2 MIC vanillin treated E. coli were examined.
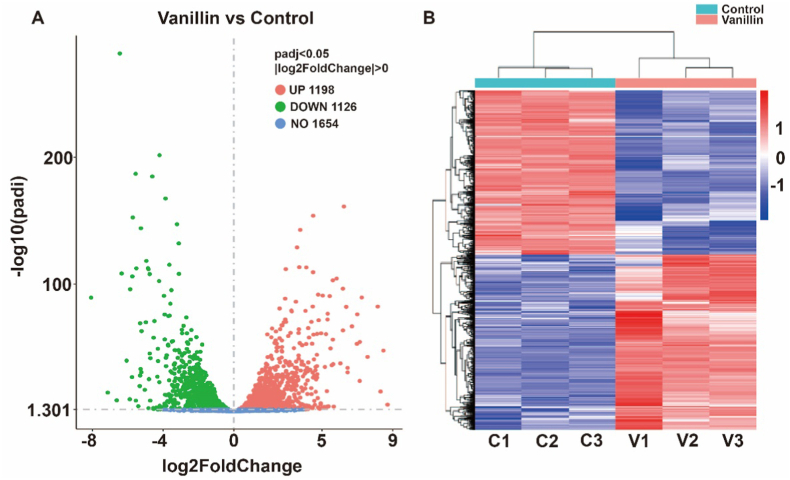
The experiment, through comparing the transcriptome data between vanillin-treated group and control group, screened a total of 2324 DEGs, including 1198 up-regulated genes and 1126 down-regulated genes. The results were presented in the form of volcano map (Fig. 6A) and heat map (Fig. 6B) to visualize the differential gene expression between the two groups, demonstrating the difference of gene expression in E. coli induced by vanillin treatment.
Fig. 6.
Heat and volcano maps of differential genes in E. coli induced by vanillin treatment. (A) Volcano Plots of DEGs. The red dots represent up-regulated DEGs, the green dots represent down-regulated DEGs and the blue dots represent genes with no significant changes in expression. (B) Heatmap of DEGs. Each row represents the expression pattern of a single gene and each column corresponds to a single sample. The expression levels are represented by colored tags, with red representing the highest levels of expression and blue representing the lowest levels of expression.
3.5.2. GO and KEGG analysis of DEGs
GO enrichment analysis was performed, and the function of DEGs was annotated (Fig. 7A) to understand the function of DEGs between vanillin treatment group and control group. The results of GO enrichment analysis can be classified into three categories: biological processes, cell components, and molecular functions. Under biological processes, the following terms were mainly identified: small molecule metabolic process (GO:0044281), organic acid metabolic process (GO:0006082), oxoacid metabolic process (GO:0043436), biosynthetic process (GO:0009058), organic substance biosynthetic process (GO:1901576), organic cyclic compound biosynthetic process (GO:1901362), and carboxylic acid metabolic process (GO:0019752). Under cell components, the following terms were mainly identified: outer membrane (GO:0019867), cell periphery (GO:0071944), plasma membrane (GO:0005886), membrane protein complex (GO:0098796), intracellular (GO:0005622), and cytoplasm (GO:0005737). Under molecular functions, the following terms were mainly identified: oxidoreductase activity (GO:0016491), transcription factor binding (GO:0008134), and ion binding (GO:0043167).
Fig. 7.
Enrichment of DEGs in GO terms and KEGG pathways. (A) Most enriched GO terms (vanillin versus control). (B) Statistical enrichment of differential expression genes in KEGG pathways. Important pathways were outlined in red bubbles.
KEGG enrichment analysis was utilized to explore the biological functional pathways of DEGs. The top 20 pathways were selected from all the differential KEGG enrichment pathways as shown in the figure, mainly including the citrate cycle (TCA cycle) and oxidative phosphorylation (Fig. 7B). These results provide a basis for further screening and analysis of key DEGs.
3.5.3. DEGs associated with cell membrane components
The cell membrane serves as a protective barrier for bacteria and plays a crucial role in maintaining cell integrity and shape. The cell membrane is composed of complex components of peptidoglycan, lipopolysaccharide, lipid bilayer, and lipoprotein, which are of great importance to the biological function of the cell membrane [63]. The analysis of DEGs related to cell membrane components showed that vanillin treatment significantly affects the components of the cell membrane, including the plasma membrane, membrane protein complex, and outer membrane (Fig. 8A–C). Compared with the control group, the expression of ATP synthase (atpA, atpC, and atpD) involved in ATP activity and proton transmembrane transport was up-regulated after vanillin treatment. This protein on the cell membrane of E. coli can convert the energy inherent in the transmembrane electrochemical gradient into the energy of covalent phosphate anhydride bond, which is an important mechanism for E. coli to directly convert the membrane potential into useful chemical energy [64]. Vanillin treatment can damage the cell membrane, potentially destroying the function of ATP synthase, or breaking down the ion balance inside and outside the cell, and inducing the cell to produce more ATP synthase to maintain the normal function of the cell [51]. Furthermore, vanillin treatment caused up-regulation of potA, potG, malK, ugpC, and other genes. malK and ugpC are involved in the formation of E. coli extracellular binding protein-dependent transport systems Ugp and Mal, transporting Sn-glycerol-3-phosphate and maltose, respectively [65]. potA and potG participate in the spermidine-putrescine transport system [66]. The up-regulated expression of these outer membrane proteins, which are essential to cell membrane function, further indicates the damage effect of vanillin on the cell membrane, resulting in the need for the synthesis of more outer membrane proteins to maintain the normal function of cells.At the same time, after vanillin treatment, the expression of permease-related genes in E. coli was down-regulated, such as lldP encoding lactic acid permease, mlaE encoding permease, and osmE encoding osmotic-induced lipoprotein. This type of enzyme can assist substances to enter the cell along the concentration gradient [67]. The damage of vanillin to the cell membrane may have an impact on its permeability, and the substance can enter the cell more easily along the concentration gradient. Therefore, the gene encoding this type of enzyme will A decrease in expression. Interestingly, vanillin treatment led to down-regulation of ompA, a typical outer membrane protein that is crucial for the stability and integrity of cell membranes [68]. The same phenomenon has been observed in studies of ultra-small gold nanoclusters against Gram-negative bacteria, which may be related to vanillin disrupting cell membrane integrity [69]. These findings further indicate that the inhibitory mechanism of vanillin against E. coli involves the destruction of cell membrane integrity, stability, and biological functions.
Fig. 8.
Heat map of typical DEGs associated with cell membrane components in E. coli, including (A)Plasma Membrane, (B)Membrane Protein Complex and (C) Outer Membrane. Red indicates up-regulated genes while blue indicates down-regulated genes.
3.5.4. DEGs related to energy metabolism
Energy metabolism plays a vital role in molecular function, biological process, physiological behavior, growth and even survival of organisms [70]. Oxidative phosphorylation and tricarboxylic acid (TCA) metabolism are two critical pathways for energy production. In the oxidative phosphorylation pathway of E. coli, vanillin treatment gave rise to the up-regulation of numerous genes (Fig. 9A and B). Among these genes, NuoL, NuoM, and NuoN participated in the formation of complex I/NDH-the prop-translocating NADH-quinone oxidoreductase [71], while sdhA, sdhB, and sdhD participated in forming complex II (succinate-ubiquinone oxidoreductase) [72]. The up-regulated expression of these critical components of the respiratory chain indicated that vanillin treatment would activate the respiratory chain of E. coli, and substances were consumed for ATP synthesis. However, measuring the intracellular ATP content revealed that the ATP content of E. coli treated with vanillin demonstrated a significant decrease. This may be attributed to the fact that the stress-induced response failed to produce enough ATP to balance the shortage of ATP [73], further indicating that vanillin causes ATP loss or consumption in E. coli as one of its inhibitory mechanisms. In addition, vanillin treatment gave rise to up-regulation and down-regulation of the TCA cycle pathway genes in E. coli, possibly due to the disruption of energy metabolic balance induced by vanillin treatment. Hence, differences in up-regulation and down-regulation of different metabolic steps occurred in the TCA cycle. In general, the effect of vanillin on the energy metabolism of E. coli is one of its antibacterial mechanisms.
Fig. 9.
Heat map of typical DEGs associated with energy metabolism in E. coli, including (A) Oxidative Phosphorylation and (B) Citrate Cycle (TCA cycle). Red indicates up-regulated genes while blue indicates down-regulated genes.
Among these DEGs, genes associated with energy metabolism, genes associated with cell membrane components, were significant differences in the expression of vanillin in E. coli cells. In Fig. 10, the effect of vanillin on E. coli is graphically summarized. Vanillin has been shown to inhibit the growth of E. coli through the destruction of cell membranes, and also affect the energy metabolism of E. coli [22]. By comparative transcriptomic analysis, we further found that vanillin treatment may affect the cell membrane components of E. coli, mainly including some proteins that control the ion balance inside and outside the cell, which will have a certain degree of impact on the maintenance of the ion balance of E. coli cells. The destruction of cellular ion balance further affects the energy metabolism in the cell, resulting in the disturbance of energy metabolism. However, some studies have pointed out that plant essential oils can inhibit the synthesis of bacterial cell membranes by inhibiting energy metabolism, so as to achieve the effect of inhibiting bacteria. The specific mechanism remains to be further explored. In general, through transcriptomic analysis, we further clarified the effects of vanillin on the cell membrane and energy metabolism of E. coli, and also screened and analyzed the genes mainly affected by vanillin, which helped to further clarify the molecular mechanism of vanillin inhibition of E. coli.
Fig. 10.
Diagram of key genes of E. coli affected by vanillin treatment.
4. Conclusions
In conclusion, vanillin was shown to be effective in controlling the growth of E. coli O157:H7 with a MIC of 2 mg/mL. Vanillin treatment damages the membrane structure of E. coli O157:H7, resulting in membrane depolarization and leakage of nucleic acids and proteins in the cell. Additionally, vanillin treatment decreases the ATP content in E. coli O157:H7 cells, leading to cell death. Transcriptome analysis confirmed these findings at the gene level. After sublethal concentrations of vanillin was compressed into E. coli O157:H7, the expression of genes related to the plasma membrane, membrane protein complex, and outer membrane of E. coli O157:H7 revealed significant differences, indicating the damage of vanillin to the membrane of E. coli O157:H7. Cells require more synthetic-related substances to maintain the normal function of cell membrane structure. Furthermore, the expression levels of genes related to oxidative phosphorylation and the TCA cycling pathways in E. coli O157:H7 showed significant differences, indicating that vanillin damages the energy balance of E. coli O157:H7. E. coli O157:H7 is required to synthesize more ATP through TCA pathway and oxidative phosphorylation pathway to sustain normal life activities. These results provide new insights into the antimicrobial mechanism of vanillin and suggest its potential as a plant-derived antimicrobial substance in the food industry to control E. coli O157:H7 contamination.
Author contribution statement
Peiyao Chen: Conceived and designed the experiments; Performed the experiments.
Yinxin Liu: Analyzed and interpreted the data; Wrote the paper.
Cheng Li: Performed the experiments.
Shuhao Hua: Contributed reagents, materials, analysis tools or data.
Cui Sun: Conceived and designed the experiments.
Lingxia Huang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
4.1. Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Zhejiang Provincial Agricultural Major Technology Cooperative Promotion Program [2022XTTGCS03], the Key laboratory of silkworm and bee resource utilization and innovation of Zhejiang Province [2020E10025], the Science and Technology Plan Project in Zhejiang Province [LGN21C200016], the Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties [2021C02072-5],the Agricultural Major Technology Collaborative Promotion Project of Zhejiang Province [2022XTTGCS03]. We thank the Bio-ultrastructure analysis Lab of Analysis center of Agrobiology and environmental sciences, Zhejiang University for SEM support.
References
- 1.Rohatgi A., Gupta P. Natural and synthetic plant compounds as anti-biofilm agents against Escherichia coli O157:H7 biofilm. Infect. Genet. Evol. 2021;95 doi: 10.1016/j.meegid.2021.105055. [DOI] [PubMed] [Google Scholar]
- 2.Li Y.K., Chen H., Shu M., Zhong C., Bi Y., Yang H.H., Wu G.P. Isolation, characterization and application of an alkaline resistant virulent bacteriophage JN01 against Escherichia coli O157:H7 in milk and beef. LWT--Food Sci. Technol. 2021;144 doi: 10.1016/j.lwt.2021.111266. [DOI] [Google Scholar]
- 3.Lim J.Y., Yoon J.W., Hovde C.J. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J. Microbiol. Biotechnol. 2010;20(1):5–14. doi: 10.4014/jmb.0908.08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rybarczyk J., Kieckens E., Vanrompay D., Cox E. In vitro and in vivo studies on the antimicrobial effect of lactoferrin against Escherichia coli O157:H7. Vet. Microbiol. 2017;202:23–28. doi: 10.1016/j.vetmic.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Chauret C. Survival and control of Escherichia coli O157:H7 in foods, beverages, soil and water. Virulence. 2011;2(6):593–601. doi: 10.4161/viru.2.6.18423. [DOI] [PubMed] [Google Scholar]
- 6.Cui H.Y., Ma C.X., Lin L. Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control. 2016;66:8–16. doi: 10.1016/j.foodcont.2016.01.035. [DOI] [Google Scholar]
- 7.Jeong S., Marks B.P., Ryser E.T., Moosekian S.R. Inactivation of Escherichia coli O157:H7 on lettuce, using low-energy X-ray irradiation. J. Food Protect. 2010;73(3):547–551. doi: 10.4315/0362-028X-73.3.547. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Gonzalez O., Walkling-Ribeiro M., Jayaram S., Griffiths M.W. Cross-protective effects of temperature, pH, and osmotic and starvation stresses in Escherichia coli O157:H7 subjected to pulsed electric fields in milk. Int. Dairy J. 2011;21(12):953–962. doi: 10.1016/j.idairyj.2011.07.003. [DOI] [Google Scholar]
- 9.Schilling M.W., Yoon Y., Tokarskyy O., Pham A.J., Williams R.C., Marshall D.L. Effects of ionizing irradiation and hydrostatic pressure on Escherichia coli O157:H7 inactivation, chemical composition, and sensory acceptability of ground beef patties. Meat Sci. 2009;81(4):705–710. doi: 10.1016/j.meatsci.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Puligundla P., Lim S. Biocontrol approaches against Escherichia coli O157:H7 in foods. Foods. 2022;11(5) doi: 10.3390/foods11050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman S., Ding T., Oh D.H. Inactivation effect of newly developed low concentration electrolyzed water and other sanitizers against microorganisms on spinach. Food Control. 2010;21(10):1383–1387. doi: 10.1016/j.foodcont.2010.03.011. [DOI] [Google Scholar]
- 12.Fu Y.Z., Deering A.J., Bhunia A.K., Yao Y. Biofilm of Escherichia coli O157:H7 on cantaloupe surface is resistant to lauroyl arginate ethyl and sodium hypochlorite. Int. J. Food Microbiol. 2017;260:11–16. doi: 10.1016/j.ijfoodmicro.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Mohan A., Pohlman F.W. Role of organic acids and peroxyacetic acid as antimicrobial intervention for controlling Escherichia coli O157:H7 on beef trimmings. LWT--Food Sci. Technol. 2016;65:868–873. doi: 10.1016/j.lwt.2015.08.077. [DOI] [Google Scholar]
- 14.Gomez-Aldapa C.A., Torres-Viela M.R., Amaya-Acosta M.A., Rangel-Vargas E., Villaruel-Lopez A., Castro-Rosas J. Behavior of thirteen foodborne bacteria on whole Hass avocado and potential of roselle calyx extracts as alternative disinfectant agents of avocado. J. Food Saf. 2017;37(4) doi: 10.1111/jfs.12351. [DOI] [Google Scholar]
- 15.Koo O.K., Kim S.M., Kang S.H. Antimicrobial potential of Leuconostoc species against E-coli O157:H7 in ground meat. Journal of the Korean Society for Applied Biological Chemistry. 2015;58(6):831–838. doi: 10.1007/s13765-015-0112-0. [DOI] [Google Scholar]
- 16.Chauhan R., Singh N., Pal G.K., Goel G. Trending biocontrol strategies against Cronobacter sakazakii: a recent updated review. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109385. [DOI] [PubMed] [Google Scholar]
- 17.Piper J.D., Piper P.W. Benzoate and sorbate salts: a systematic review of the potential hazards of these invaluable preservatives and the expanding spectrum of clinical uses for sodium benzoate. Compr. Rev. Food Sci. Food Saf. 2017;16(5):868–880. doi: 10.1111/1541-4337.12284. [DOI] [PubMed] [Google Scholar]
- 18.Al-Naqeb G., Ismail M., Bagalkotkar G., Adamu H.A. Vanillin rich fraction regulates LDLR and HMGCR gene expression in HepG2 cells. Food Res. Int. 2010;43(10):2437–2443. doi: 10.1016/j.foodres.2010.09.015. [DOI] [Google Scholar]
- 19.Hyldgaard M., Mygind T., Meyer R.L. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao H.Q., Guo M.M., Wang L.Q., Sun C., Huang L.X. Identification and antioxidant capacity of free and bound phenolics in six Varieties of mulberry seeds using UPLC-ESI-QTOF-MS/MS. Antioxidants. 2022;11(9) doi: 10.3390/antiox11091764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuentes C., Fuentes A., Barat J.M., Ruiz M.J. Relevant essential oil components: a minireview on increasing applications and potential toxicity. Toxicol. Mech. Methods. 2021;31(8):559–565. doi: 10.1080/15376516.2021.1940408. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald D.J., Stratford M., Gasson M.J., Ueckert J., Bos A., Narbad A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004;97(1):104–113. doi: 10.1111/j.1365-2672.2004.02275.x. [DOI] [PubMed] [Google Scholar]
- 23.Maisch N.A., Bereswill S., Heimesaat M.M. Antibacterial effects of vanilla ingredients provide novel treatment options for infections with multidrug-resistant bacteria - a recent literature review. Eur J Microbiol Immunol. 2022;12(3):53–62. doi: 10.1556/1886.2022.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuentes C., Ruiz-Rico M., Fuentes A., Barat J.M., Ruiz M.J. Comparative cytotoxic study of silica materials functionalised with essential oil components in HepG2 cells. Food Chem. Toxicol. 2021;147 doi: 10.1016/j.fct.2020.111858. [DOI] [PubMed] [Google Scholar]
- 25.Lander B.A., Checchi K.D., Koplin S.A., Smith V.F., Domanski T.L., Isaac D.D., Lin S. Extracytoplasmic stress responses induced by antimicrobial cationic polyethylenimines. Curr. Microbiol. 2012;65(5):488–492. doi: 10.1007/s00284-012-0182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campardelli R., Pettinato M., Drago E., Perego P. Production of vanillin-loaded zein sub-micron electrospun fibers for food packaging applications. Chem. Eng. Technol. 2021;44(8):1390–1396. doi: 10.1002/ceat.202100044. [DOI] [Google Scholar]
- 27.Lamas A., Regal P., Vazquez B., Miranda J.M., Franco C.M., Cepeda A. Transcriptomics: a powerful tool to evaluate the behavior of foodborne pathogens in the food production chain. Food Res. Int. 2019;125 doi: 10.1016/j.foodres.2019.108543. [DOI] [PubMed] [Google Scholar]
- 28.Cunha B.R.D., Zoio P., Fonseca L.P., Calado C.R.C. Technologies for high-throughput identification of antibiotic mechanism of action. Antibiotics-Basel. 2021;10(5) doi: 10.3390/antibiotics10050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Z., Yuan K., Zhou Q., Lu C., Du L., Liu F. Mechanism of antifungal activity of Perilla frutescens essential oil against Aspergillus flavus by transcriptomic analysis. Food Control. 2021;123 doi: 10.1016/j.foodcont.2020.107703. [DOI] [Google Scholar]
- 30.Li Y., Zhou D., Hu S., Xiao X., Yu Y., Li X. Transcriptomic analysis by RNA-seq of Escherichia coli O157:H7 response to prolonged cold stress. LWT--Food Sci. Technol. 2018;97:17–24. doi: 10.1016/j.lwt.2018.06.025. [DOI] [Google Scholar]
- 31.Ballestero-Tellez M., Docobo-Perez F., Rodriguez-Martinez J.M., Conejo M.C., Ramos-Guelfo M.S., Blazquez J., Rodriguez-Bano J., Pascual A. Role of inoculum and mutant frequency on fosfomycin MIC discrepancies by agar dilution and broth microdilution methods in Enterobacteriaceae. Clin. Microbiol. Infection. 2017;23(5):325–331. doi: 10.1016/j.cmi.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Qian W.D., Fu Y.T., Liu M., Wang T., Zhang J.N., Yang M., Sun Z.H., Li X., Li Y.D. In vitro antibacterial activity and mechanism of vanillic acid against carbapenem-resistant Enterobacter cloacae. Antibiotics-Basel. 2019;8(4) doi: 10.3390/antibiotics8040220. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Sanchez E., Garcia S., Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2010;76(20):6888–6894. doi: 10.1128/AEM.03052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallouk Y., Vayssier-Taussat M., Bonventre J.V., Polla B.S. Heat shock protein 70 and ATP as partners in cell homeostasis. Int. J. Mol. Med. 1999;4(5):463–474. doi: 10.3892/ijmm.4.5.463. (Review) [DOI] [PubMed] [Google Scholar]
- 35.Parkhomchuk D., Borodina T., Amstislavskiy V., Banaru M., Hallen L., Krobitsch S., Lehrach H., Soldatov A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009;37(18) doi: 10.1093/nar/gkp596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan W.Q., Teo C., Yuk H.G. Combined antibacterial activities of essential oil compounds against Escherichia coli O157:H7 and their application potential on fresh-cut lettuce. Food Control. 2019;96:112–118. doi: 10.1016/j.foodcont.2018.09.005. [DOI] [Google Scholar]
- 37.Voravuthikunchai S.P., Sririrak T., Limsuwan S., Supawita T., Iida T., Honda T. Inhibitory effects of active compounds from Punica granatum pericarp on verocytotoxin production by enterohemorrhagic Escherichia coli O157 : H7. J. Health Sci. 2005;51(5):590–596. doi: 10.1248/jhs.51.590. [DOI] [Google Scholar]
- 38.Dai J.M., Li C.Z., Cui H.Y., Lin L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157:H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021;338 doi: 10.1016/j.ijfoodmicro.2020.108989. [DOI] [PubMed] [Google Scholar]
- 39.Zulfa Z., Chia C.T., Rukayadi Y. In vitro antimicrobial activity of Cymbopogon citratus (lemongrass) extracts against selected foodborne pathogens. Int. Food Res. J. 2016;23(3):1262–1267. [Google Scholar]
- 40.Suwalak S., Voravuthikunchai S.P. Morphological and ultrastructural changes in the cell structure of enterohaemorrhagic Escherichia coli O157:H7 following treatment with Quercus infectoria nut galls. J. Electron. Microsc. 2009;58(5):315–320. doi: 10.1093/jmicro/dfp024. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y.D., Zhang Y.L., Zong H., Lu X.Y., Shen W., Bin Z.G. Chemical constituents, antibacterial activity and mechanism of Paeonia suffruticosa Andr. buds extract against Staphylococcus aureus and Escherichia coli O157:H7. Nat. Prod. Res. 2021;35(6):1005–1009. doi: 10.1080/14786419.2019.1610961. [DOI] [PubMed] [Google Scholar]
- 42.Lu Z.J., Dockery C.R., Crosby M., Chavarria K., Patterson B., Giedd M. Antibacterial activities of Wasabi against Escherichia coli O157:H7 and Staphylococcus aureus. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yemmireddy V.K., Cason C., Moreira J., Adhikari A. Effect of pecan variety and the method of extraction on the antimicrobial activity of pecan shell extracts against different foodborne pathogens and their efficacy on food matrices. Food Control. 2020;112 doi: 10.1016/j.foodcont.2020.107098. [DOI] [Google Scholar]
- 44.Alvarez M.V., Ortega-Ramirez L.A., Gutierrez-Pacheco M.M., Bernal-Mercado A.T., Rodriguez-Garcia I., Gonzalez-Aguilar G.A., Ponce A., Moreira M.D., Roura S.I., Ayala-Zavala J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma M.M., Zhao J.X., Yan X.H., Zeng Z.L., Wan D.M., Yu P., Xia J.H., Zhang G.H., Gong D.M. Synergistic effects of monocaprin and carvacrol against Escherichia coli O157:H7 and Salmonella Typhimurium in chicken meat preservation. Food Control. 2022;132 doi: 10.1016/j.foodcont.2021.108480. [DOI] [Google Scholar]
- 46.Bajpai V.K., Sharma A., Baek K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32(2):582–590. doi: 10.1016/j.foodcont.2013.01.032. [DOI] [Google Scholar]
- 47.Hyun J.E., Moon S.K., Lee S.Y. Antibacterial activity and mechanism of 460-470 nm light-emitting diodes against pathogenic bacteria and spoilage bacteria at different temperatures. Food Control. 2021;123 doi: 10.1016/j.foodcont.2020.107721. [DOI] [Google Scholar]
- 48.Lee I.H., Cho E.R., Kang D.H. The effect of quercetin mediated photodynamic inactivation on apple juice properties at different temperature and its bactericidal mechanism. Food Control. 2023;144 doi: 10.1016/j.foodcont.2022.109362. [DOI] [Google Scholar]
- 49.Hu W., Li C.Z., Dai J.M., Cui H.Y., Lin L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA) Ind. Crop. Prod. 2019;130:34–41. doi: 10.1016/j.indcrop.2018.12.078. [DOI] [Google Scholar]
- 50.Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azi F., Li Z.Y., Xu P., Dong M.S. Transcriptomic analysis reveals the antibacterial mechanism of phenolic compounds from kefir fermented soy whey against Escherichia coli 0157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 2022;383 doi: 10.1016/j.ijfoodmicro.2022.109953. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y., He L., Mustapha A., Li H., Hu Z.Q., Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009;107(4):1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x. [DOI] [PubMed] [Google Scholar]
- 53.Mempin R., Tran H., Chen C.N., Gong H., Ho K.K., Lu S.W. Release of extracellular ATP by bacteria during growth. BMC Microbiol. 2013;13 doi: 10.1186/1471-2180-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ultee A., Kets E., Smid E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999;65(10):4606–4610. doi: 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez E., Garcia S., Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2010;76(20):6888–6894. doi: 10.1128/AEM.03052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oussalah M., Caillet S., Lacroix M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157 : H7 and Listeria monocytogenes. J. Food Protect. 2006;69(5):1046–1055. doi: 10.4315/0362-028X-69.5.1046. [DOI] [PubMed] [Google Scholar]
- 57.Turgis M., Han J., Caillet S., Lacroix M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control. 2009;20(12):1073–1079. doi: 10.1016/j.foodcont.2009.02.001. [DOI] [Google Scholar]
- 58.Wu W.H., Di R., Matthews K.R. Antibacterial mode of action of ib-AMP1 against Escherichia coli O157:H7. Probiotics and Antimicrobial Proteins. 2013;5(2):131–141. doi: 10.1007/s12602-013-9127-1. [DOI] [PubMed] [Google Scholar]
- 59.Rastogi S.K., Rutledge V.J., Gibson C., Newcombe D.A., Branen J.R., Branen A.L. Ag colloids and Ag clusters over EDAPTMS-coated silica nanoparticles: synthesis, characterization, and antibacterial activity against Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2011;7(3):305–314. doi: 10.1016/j.nano.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahin E., Musevi S.J., Aslani A. Antibacterial activity against Escherichia coli and characterization of ZnO and ZnO-Al2O3 mixed oxide nanoparticles. Arab. J. Chem. 2017;10:S230–S235. doi: 10.1016/j.arabjc.2012.07.027. [DOI] [Google Scholar]
- 61.Akusobi C., Chan B.K., Williams E., Wertz J.E., Turner P.E. Parallel evolution of host-attachment proteins in phage PP01 populations adapting to Escherichia coli O157:H7. Pharmaceuticals. 2018;11(2) doi: 10.3390/ph11020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang A.G., Ren Q.W., Wu Y.L., Wu C., Cheng Y.Y. Investigation into the antibacterial mechanism of biogenic tellurium nanoparticles and precursor tellurite. Int. J. Mol. Sci. 2022;23(19) doi: 10.3390/ijms231911697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown L., Wolf J.M., Prados-Rosales R., Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015;13(10):620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker J.E. The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans. 2013;41:1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- 65.Hekstra d., Tommassen J. Functional exchangeability of the abc proteins of the periplasmic binding protein-dependent transport-systems ugp and mal of Escherichia-coli. J. Bacteriol. 1993;175(20):6546–6552. doi: 10.1128/jb.175.20.6546-6552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pistocchi r., Kashiwagi k., miyamoto S., nukui e., Sadakata y., Kobayashi h., Igarashi k. Characteristics of the operon for a putrescine transport-system that maps at 19 minutes on the Escherichia-coli chromosome. J. Biol. Chem. 1993;268(1):146–152. [PubMed] [Google Scholar]
- 67.Abramson J., Wright E.M. Function trumps form in two sugar symporters, LacY and vSGLT. Int. J. Mol. Sci. 2021;22(7) doi: 10.3390/ijms22073572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guan G.L., Zhang L.N., Zhu J.X., Wu H., Li W.X., Sun Q.J. Antibacterial properties and mechanism of biopolymer-based films functionalized by CuO/ZnO nanoparticles against Escherichia coli and Staphylococcus aureus. J. Hazard Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123542. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y.X., Malkmes M.J., Jiang C., Wang P., Zhu L.Y., Zhang H.M., Zhang Y.H., Huang H., Jiang L. Antibacterial mechanism and transcriptome analysis of ultra-small gold nanoclusters as an alternative of harmful antibiotics against Gram-negative bacteria. J. Hazard Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.126236. [DOI] [PubMed] [Google Scholar]
- 70.Wang N., Qian Z.X., Luo M.W., Fan S.J., Zhang X.J., Zhang L.Y. Identification of salt stress responding genes using transcriptome analysis in green alga chlamydomonas reinhardtii. Int. J. Mol. Sci. 2018;19(11) doi: 10.3390/ijms19113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torres-Bacete J., Sinha P.K., Matsuno-Yagi A., Yagi T. Structural contribution of C-terminal segments of NuoL (ND5) and NuoM (ND4) subunits of complex I from Escherichia coli. J. Biol. Chem. 2011;286(39):34007–34014. doi: 10.1074/jbc.M111.260968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ackrell B. Cytopathies involving mitochondrial complex II. Mol. Aspect. Med. 2002;23(5):369–384. doi: 10.1016/S0098-2997(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 73.Tian X.J., Yu Q.Q., Shao L.L., Silva-Vera W., Li X.M., Dai R.T. Comparative transcriptomic study of Escherichia coli O157:H7 in response to ohmic heating and conventional heating. Food Res. Int. 2021;140 doi: 10.1016/j.foodres.2020.109989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.