Abstract
Badgers (Meles meles) are a major tuberculosis (TB) reservoir in Europe, with the potential to transmit infection to cattle. Here we assessed whether a recently described oral tuberculosis vaccine based on heat-inactivated Mycobacterium bovis (HIMB), delivered as edible baits, can protect badgers from infection. Eight badgers were given individually five baits, each one consisting of a ball of peanut butter, natural peanut and oat flakes including a dose of the vaccine containing 5 × 107 colony-forming units. In parallel, a control group of seven badgers did not receive the vaccine. One month and a half later a second dose of the vaccine was offered to the vaccinated group. Ninety-four days after the second dose, all badgers were challenged with M. bovis (103 colony-forming units per animal) delivered endobronchially to the right middle lung lobe. Clinical, immunological, pathological and bacteriological variables were measured throughout the whole study to assess the efficacy of the vaccine. Two vaccinated animals showed high bacterial load of M. bovis and worsening of pathological lesions of TB. Conversely, the other six vaccinated animals showed slight improvement in bacterial load and pathology with respect to the control group. These results suggest that delivering the TB vaccine via food bait can partially protect wild badger populations, although vaccination can lead to either protection or tolerization, likely depending on the animal's immune status and general condition at the time of vaccination. Further optimization of the vaccination trial/strategy is needed to reduce the rate of tolerization, such as altering vaccine dose, number of doses, type of bait, use of adjuvants or route of administration.
Keywords: Tuberculosis, Badger, Mycobacterium bovis heat-inactivated bait oral vaccine, HIMB, Efficacy
1. Introduction
Tuberculosis (TB) is a contagious, chronic infectious disease caused by Mycobacterium tuberculosis complex (MTC) bacteria. Its severe social, economic and public health effects have made TB a major challenge over the course of human and animal history. The disease affects both domestic and wild animals worldwide [1]. European badgers (Meles meles) serve as TB reservoirs in the UK and Ireland [2,3], and potentially also in Spain and France, especially in areas where MTC bacteria are prevalent in cattle [4,5].
In addition to culling badgers, vaccinating them has been proposed as a long-term control strategy in the UK and Ireland [6]. The only vaccine currently licensed for use in badgers is intramuscularly injected Bacillus Calmette-Guérin (BCG) vaccine in the UK [7], and this vaccine contains live attenuated bacilli. This vaccine may not be sufficiently robust for widespread administration to wildlife via food bait, and the fact that it contains live bacteria may lead to adverse environmental effects and sensitization of cattle.
Inactivated vaccines such as this based on heat-inactivated Mycobacterium bovis (HIMB) may be more robust than the BCG vaccine under field conditions [8]. The fact that the vaccine contains only dead bacteria may make it safer for widespread release into the environment. Manual, oral vaccination with vaccines based on HIMB has been shown to protect several animal species against experimentally induced progressive disease: captive wild boar (Sus scrofa) [9], wild Molokai pig [10], red deer (Cervus elephus) [11] and badger [12]. HIMB in edible baits has also been shown to protect free-ranging piglets of wild boar in a TB-endemic area [8].
As a further step toward widespread oral vaccination based on HIMB, we assessed whether delivering the vaccine in edible bait could protect captive European badgers from experimental challenge.
2. Materials and methods
2.1. Badgers, baits and experimental design
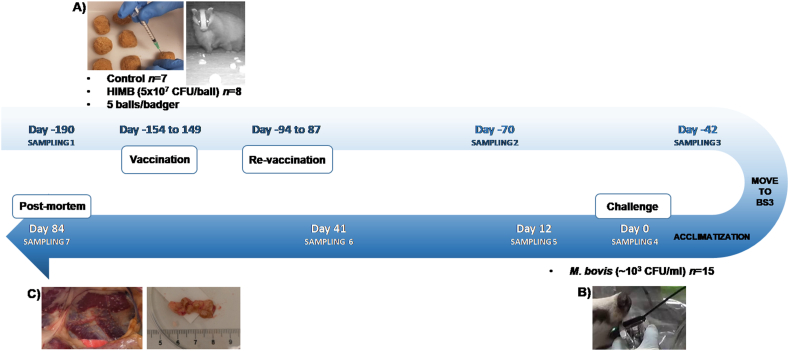
Fifteen young adult badgers, seven of which were male and eight females, were trapped between March and September 2018 in Nava, Asturias (43°21′30ℙN, 5°30′20ℙW), where cattle are known to be free of TB [13]. Before inclusion in the study, badgers were confirmed to be negative for avian or bovine TB using Interferon-Gamma (IFN-γ) Release Assay (IGRA) enzyme linked immunosorbent assay (ELISA), IGRA ELISPOT and an ELISA against M. bovis P22 (P22 ELISA) (see section 2.2). Given the difficulties to trap wild animals, obtaining 15 animals represented a substantial challenge that imposed a lack of homogeneity in their genetic and environmental background that must be considered in the interpretation of results. All badgers were housed in stable social groups, i.e., badgers coming from the same field sett or coming from different setts but cohabiting for at least one month without fights, in an outdoor purpose-built facility at the Servicio Regional de Investigación y Desarrollo Agroalimentario (SERIDA, Gijón, Spain). The floor of pens contained a wire-based liner with digging soil above it. Animals had permanent access to a large wooden sett with internal straw bedding, water for drinking and bathing, and various enrichment features such as wooden structures and branches. Food was provided and animal welfare monitored daily. All animals remained in good general clinical condition throughout the study.
Badgers were anesthetized using a cocktail of butorphanol, medetomidine and ketamine as described [12] and were weighed and tagged with a subcutaneous microchip in the left side of the neck. Then, eight animals (5 male, 3 female) were randomly allocated and vaccinated via edible baits with HIMB [5 × 107 heat-inactivated colony-forming units (CFU)/ml], while the remaining seven animals (2 male, 5 female) were vaccinated with baits not containing HIMB and keep as the control group. The time course of the experiment is shown in Fig. 1. The vaccinated group received a first dose of the vaccine at days −154 to −148 followed by a second dose at days −94 to −87 at SERIDA facilities. The vaccine was delivered orally in balls (diameter, 3 cm) containing manually mixed peanut butter, natural peanuts and oat flakes. Balls were injected with 200 μl of HIMB vaccine, corresponding to 107 inactivated CFU per bait. To verify bait palatability, bait without vaccine was placed in setts around days −170 to −160, and all baits were eaten. Each badger in the vaccinated group was individually given five baits in its own sett and allowed to eat them for two days. The duration of vaccination was seven days for vaccinating all animals. Control badgers had ad libitum access to baits without vaccine. During re-vaccination, the same protocol than in the first vaccination was followed (five baits per animal). Fifty-two days after re-vaccination all badgers were moved within their wooden setts to the NEIKER- Basque Institute for Agricultural Research and Development, where they were housed in the biosafety level 3 facilities. Vaccinated and control animals were kept in separate rooms. Ninety-four days after re-vaccination, each badger was experimentally challenged with M. bovis (103 CFU) delivered endobronchially as previously described [12,14,15]. This day was considered day 0. At three times before challenge (days −190, −70, and −42), at challenge (day 0), and at three times after challenge (days 12, 41 and 84), blood was sampled from the animals after anesthetizing them using the cocktail described above. Blood samples from the jugular vein were collected into serum tubes and heparinized blood tubes (Vacutainer®, BD Diagnostics, Plymouth, UK). Rectal temperature and body weight were measured at every blood sampling. At the three samplings after challenge, urine was obtained by bladder massage, and tonsillar, nasal and faecal swabs were collected. On day 84, all badgers were euthanized by intravenously injection of a lethal dose of sodium pentobarbital (Dolethal®, Vetoquinol, Madrid, Spain). Tissues, tracheal swabs and feces were also collected during post-mortem examination.
Fig. 1.
Experimental design. European badgers (Meles meles) were given food bait prepared with or without heat-inactivated Mycobacterium bovis (HIMB), then challenged with M. bovis infection. Day 0 corresponds to the day of experimental challenge. “Sampling” refers to when animals were anesthetized, and blood was sampled. At some samplings, urine as well as tonsillar, nasal and faecal swabs were collected. A) Bait used for delivering the vaccine. B) Inoculation of M. bovis using an endoscope. C)Post-mortem examination of tuberculosis lesions. CFU, colony-forming units; BS3: biosecurity level 3 facilities.
2.2. Immunology on blood samples
2.2.1. Cellular assays
2.2.1.1. Whole blood IGRA ELISA
Interferon gamma release assay (IGRA) was performed as previously described [7,12,16]. Briefly, 0.75 ml of heparinized blood diluted 1:1 in RPMI medium (Fisher, Hampton, USA) supplemented with 1% sodium heparin (Roche, Basilea, Switzerland) and 1% penicillin/streptomycin (pen/strep) (Fisher, Hampton, USA) was stimulated with antigens: bovine or avian tuberculins (PPD-B and PPD-A, respectively, CZ Vaccines, Porriño, Spain) at a final concentration of 30 μg/ml; pokeweed mitogen (PWM) at a final concentration of 5 μg/ml (Sigma-Aldrich, St. Louis, USA); CFP-10/ESAT-6 (CE) (Lionex, Braunschweig, Germany) cocktail at a final concentration of 5 μg/ml protein; M. bovis P22 complex at a concentration of 10 μg/ml or without antigen (nil negative control) (RPMI + Pen/strep), and kept at 37 °C with 5% CO2 for 16–20 h. Post-incubation, supernatants were collected in duplicate aliquots (250 μl) and stored at −80 °C. IFN-γ levels were assessed by sandwich ELISA using anti-badger IFN-γ capture monoclonal antibody 10H6–C1 and biotinylated monoclonal antibody 11B9 (Animal and Plant Health Agency, UK). Optical density (OD) was measured with 450 nm filters by ELISA reader. The cut-off point of 0.044 (using combined PPD-B and PPD-A values) was used to identify positivity to M. bovis infection [16].
2.2.1.2. IGRA ELISPOT
A direct IGRA ELISPOT assay was also conducted as previously described [12,17]. Freshly isolated peripheral blood mononuclear cells (PBMC) (2 × 105 cells in 100 μl per duplicated wells) diluted in RPMI complete medium made up of 5% fetal calf serum (Sigma-Aldrich, St. Louis, USA), 1% pen/strep, 1% non-essential amino acid (Gibco, Hampton, USA), and 0.1% mercaptoethanol (Fisher, Hampton, USA) were used. PBMC were stimulated with PPD-B and PPD-A at a final concentration of 30 μg/ml, mitogen Concanavalin A (ConA) (Sigma-Aldrich, St. Louis, USA), antigen 85A (a secretory protein of M. tuberculosis and BCG), antigens CFP-10/ESAT-6 at final concentrations of 5 μg/ml each and M. bovis P22 complex at a concentration of 10 μg/ml or no antigen (nil) (RPMI complete) for 16–20 h in wells pre-coated with monoclonal antibody 10H6–C1(10 mg/ml) in carbonate buffer. The IFN-γ producing cells were detected using biotinylated monoclonal antibody 11B9. The ELISPOT results were expressed as number of spot forming units per million cells.
2.2.1.3. PBMC IGRA ELISA
Isolated PBMC were also used for IGRA ELISA. A volume of 700 μl PBMC were stimulated with 700 μl of PPD-B and PPD-A at a final concentration of 30 μg/ml, mitogen ConA, antigens CFP-10/ESAT-6 at final concentrations of 5 μg/ml and M. bovis P22 complex at a concentration of 10 μg/ml or nil (RPMI complete). Plates (48 wells) were kept at 37 °C with 5% CO2 for 20–24 h and centrifuged at 800g for 10 min. Supernatants (250 μL) were taken in duplicate and frozen at −80 °C. An IGRA previously described for stimulated whole blood (see above) was carried out and analyzed using PPD-B and PPD-A values with a cut-off of 0.044 nm [16].
2.2.2. Humoral (serological) assay
2.2.2.1. P22 ELISA
Serum samples were tested by an in-house indirect ELISA to detect antibodies against the M. bovis P22 complex in duplicate, following the protocol previously described [18]. Briefly, plates were coated with P22 at 10 μg/ml overnight in phosphate buffer saline (PBS) and then blocked with 5% skimmed milk powder solution (prepared in PBS). After three washes with PBS containing 0.05% Tween-20 (PBST), sera were added to duplicate wells as a 1:100 dilution in PBS-skim milk and incubated for 60 min. Horseradish peroxidase-conjugated CF2/HRPo anti-badger IgG (100 μl) (Animal and Plant Health Agency, UK) was diluted to 1.5 μg/ml in PBS and added to the plates and incubated for 30 min. Plates were incubated with 3,3′,5,5′-tetramethylbenzidine substrate (Perbio, Skane Lan, Sweden) for 15 min in the dark at room temperature. The reaction was stopped by adding 100 μl of 2 M H2SO4. Optical density (OD) was measured at 450 nm using an ELISA reader (Varioskan Lux, Thermo Scientific, Waltham, USA). Negative control serum samples from UK tuberculosis-free captive badgers were included in every plate in quadruplicate. Positive controls were obtained from UK badgers experimentally infected with M. bovis. Sample results were expressed as an ELISA percentage E%, calculated using the following formula:
E% = mean sample OD/(2 x mean of negative control OD) x 100%. The cut-off point was set up in previous work [18]; serum samples with E% values greater than 120 were considered positive.
2.3. Heat-inactivated Mycobacterium bovis vaccine (HIMB)
Heat-inactivated Mycobacterium bovis vaccine (HIMB) was produced by NEIKER. The strain, first isolated from a naturally infected wild boar on Coletsos medium, was propagated in Middlebrook 7H9 broth enriched with oleic acid-albumin-dextrose-catalase (OADC Enrichment; Difco, Tucker, USA) for 2–3 weeks [9]. This vaccine was prepared following the protocol previously described [9], with an inactivation step at 84–85 °C for 45 min.
2.4. Mycobacterium bovis challenge
An M. bovis field strain (SB0339) isolated from a tuberculous wild boar was diluted to ∼103 CFU/ml in PBS and loaded in 3-ml luer-lock syringes. Syringes were vortexed and the challenge inoculum (1 ml) was immediately instilled into anesthetized badgers via a catheter through a fibroscope (channel, 1.8 mm; dimensions, 3.6 mm × 70 cm; Olympus URF P2, Bolton, UK). The inoculum was administered into the bronchial entrance of the right middle lung lobe while animals lay ventrally recumbent as described [12,14,15]. In order to prevent inoculum drainage into the left lung, badgers were placed on their right side until they recovered from general anesthesia.
2.5. Post-mortem examination
On day 84, 28 tissues freshly removed from all badgers were examined post-mortem as described [12,14,15]. The following tissues were collected: tonsils, salivary glands, left and right parotid lymph nodes (LN), left and right mandibular LN, left and right retropharyngeal LN, anterior and posterior mediastinal LN, left and right bronchial LN, lung lobes, mediastinum, left and right axillary LN, heart, left and right inguinal LN chains, left and right popliteal LN, spleen, hepatic LN, mesenteric LN, liver, and left and right kidneys. Sterile instruments were changed between tissues to avoid bacterial cross-contamination. The severity of gross lesions was assessed as described [12] by veterinary pathologists blinded to badger group allocation. Some tissue samples were frozen at −20 °C for later culture tests or fixed in 10% buffered formalin for later histology (Table 1). Fixed lungs were inflated with 10% buffered formalin for subsequent magnetic resonance imaging (MRI), then examined directly for visible lesions.
Table 1.
Analysis of badger tissues.
| Tissue | Site | MRI | Microbiology | Histology |
|---|---|---|---|---|
| Lung region | Left Cranial Lung Lobe | Y | N | N |
| Left Caudal Lung Lobe | Y | N | N | |
| Right Cranial Lung Lobe | Y | N | N | |
| Right Middle Lung Lobe | Y | N | N | |
| Right Caudal Lung Lobe | Y | N | N | |
| Accessory Lung Lobe | Y | N | N | |
| Pleura Mediastinum | N | Y | Y | |
| Trachea | N | Y | Y | |
| Lymphoid | Right Parotid LN | N | Y | Y |
| Left Parotid LN | N | Y | Y | |
| Right Mandibular LN | N | Y | Y | |
| Left Mandibular LN | N | Y | Y | |
| Right Retropharyngeal LN | N | Y | Y | |
| Left Retropharyngeal LN | N | Y | Y | |
| Tonsils | N | Y | Y | |
| Right Bronchial LN | N | Y | Y | |
| Left Bronchial LN | N | Y | Y | |
| Posterior Mediastinal LN | N | Y | Y | |
| Land Axillary LN Chains | N | Y | Y | |
| Land Inguinal LN Chains | N | Y | Y | |
| Land Popliteal LN | N | Y | Y | |
| Hepatic LN | N | Y | Y | |
| Mesenteric LN | N | Y | Y | |
| Other | Salivary Gland | N | Y | Y |
| Spleen | N | Y | Y | |
| Liver | N | Y | Y | |
| Kidneys | N | Y | Y | |
| Heart | N | Y | Y |
MRI: Magnetic resonance imaging; Y: Yes; N: No; LN: lymph node.
2.6. Magnetic resonance imaging (MRI) acquisition and analysis
The formalin fixed lungs were scanned with a 3T Magnetom® Verio MRI scanner (Siemens, Erlangen, Germany) available at the Phénotypage par Imagerie in/ex vivo de l’Animal à la Molécule (PIXANIM) platform. This device, endoted with a strong magnetic field, ensured high quality images of 15 ex-vivo badgers' lungs. For this purpose, a 32 phased array torso coil (split into 2, 16 anterior and 16 posterior) was positioned either side of the lung under investigation [12]. Two separate MRI acquisition images were performed individually on each lung to obtain two different and complementary image contrasts: one image set had a T1 contrast (spin-lattice or longitudinal relaxation time) and the other had a T2 contrast (spin-spin or transverse relaxation time). The T1 MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) and the T2 SPACE (Sampling Perfection with Application-optimized Contrasts) were both acquired in 3D mode. In addition, the TB granulomas/lesions differ from the healthy parenchyma of the lung because they differ in intensity level on the T1 and T2 images. The MRI acquisition parameters for these two distinct anatomical images were set as follows: (i) T1 3D: Repetition Time (TR) = 1970 ms, Echo Time (TE) = 3.01 ms, Inversion Time (TI) = 900 ms, flip angle = 9°. Field-of-View (FOV): 180 × 180 mm2, matrix: 448 × 4482 and a slice thickness of 0.4 mm (with 192 slices) resulting in a voxel size of 0.4x0.4x0.4 mm3. The inter-echo spacing was 9.7 ms. A bandwidth of 190 Hz/Px was used, 4 Number of Excitations (NEX) and an integrated Parallel Acquisition Technique (iPAT) of 2 was used producing an acquisition time of 30 min 53 s; (ii) T2 3D: TR = 2270 ms, TE = 138 ms, flip angle = 140°, Echo Train Length (ETL): 682 and Turbo Factor: 165. The FOV was 180 × 180 mm2, matrix: 320 × 3202 and slice thickness: 0.5 mm (with 144 slices), making a voxel size: 0.57x0.57x0.50 mm3. The inter-echo spacing was 6.56 ms. A bandwidth of 295 Hz/Px was used, 2 NEX were set generating an acquisition time of 21 min 49 s.
The analysis of the MRI was done into two steps; they were first converted and afterwards underwent segmentation. In step 1, the Digital Imaging and Communication in Medicine (DICOM) images were exported from the acquisition machine onto a PC analysis workstation. The DICOM images were then converted to the Neuroimaging Informatics Technology Initiative (NIfTI). In step 2, the NIfTI images were read with the ITK Snap software, which is generally used to segment 3D medical image structures. The segmentation was then done automatically with a Human-Machine Interface (HMI) created in-situ. This HMI could generate a bit mask by assigning 1 to voxels thresholded between a minimum and a maximum value and by assigning 0 to the remaining part of the image. These minimum and maximum were discriminated on the NIfTI images. The HMI directly superimposed the mask on the T1 NIfTI images with the ITK Snap software. The voxels under investigation were then colorized in red (or in any other color). After that, it was necessary to correct the automatic segmentation by a manual segmentation and consequently eliminate the voxels that did not correspond to lesions. To do this the T2 images were loaded simultaneously with the T1 images which allowed confirmation of the belonging of the spots to a specific class. Indeed, T2 offered a different contrast after T1 and hence confirmed TB lesion on the images. At the end, once the segmentation process was satisfactory, the final bit mask was superimposed with the T1 NIfTI images to directly locate the TB granulomas on them. It was then possible to edit the volumetry panel that corresponded to the segmentation and thus to the lesions. All segmentations were afterwards blindly re-read through a third party for validation.
2.7. Bacterial isolation, real time-polymerase chain reaction (RT-PCR) and spoligotyping
Tissues were weighed and individually homogenized in 5–15 ml saline solution (0.85%) with a GentleMacs dissociator (Miltenyi Biotec, Madrid, Spain). After that 100 μl of each homogenate was inoculated in one BD BBL™ mycobacteria growth indicator tube (MGIT™) (Becton Dickinson, Franklin Lakes, USA) supplemented with BACTEC™ MGIT™ growth supplement and PANTA™ antibiotic mixture according to manufacturer's instructions (Becton Dickinson, Franklin Lakes, USA), and onto three modified 7H11 agar plates using BBL™ 7H11 agar (Becton Dickinson, Franklin Lakes, USA) as previously reported [19]. The inoculated plates were incubated at 37 °C for 12 weeks and tubes introduced in a BACTEC™ MGIT™ 960 System for an incubation protocol of 42 days. Afterwards, CFU were counted, and the total bacterial load was calculated, as the mean number of colonies per gram of tissue on the 7H11 plates. All the colonies growing on plates/tubes were confirmed as members of the MTC by multiplex PCR [20]. Additionally, MTC colonies from three positive cultures from each badger, obtained from LN from the head, the thorax and any other location outside the thorax were spoligotyped [21].
Clinical samples including tonsillar and nasal swabs from samplings at days 12, 41 and 84 post-infection (PI), and tracheal swab obtained at necropsy, were individually homogenized in 1 ml saline solution (0.85%). 100 μl of the resulting suspensions were plated onto three solid medium plates and inoculated in one MGIT tube. The same amount of urine (100 μl) was cultured in the same way as swab homogenates. Two grammes of feces obtained at necropsy and faecal swabs collected from days 41 and 84 PI, were homogenized in 38 ml or 15 ml saline solution (0.85%), respectively, and decontaminated with oxalic acid (5% final volume) before being inoculated in the same media. All the positive plates/tubes were confirmed as MTC by multiplex PCR [20]. A 100-base logarithm transformation was applied to the bacterial load to normalize data and to reduce the bacterial quantification to a 0-to-4 scale similar to that of the histopathological score. The scale of positive results ranging from 1 to 4 was complemented with the results of MGIT culture; when plating was negative and MGIT positive a value of 0.1 was assigned. The mean value of each one of the three tissue types (lung [2 sites], lymphoid [15 sites] and other [5 sites]) was calculated for each individual in order to assess any differential effects of vaccination according to tissue that could be of epidemiological/microbiological significance.
2.8. Histopathology
Samples were processed using standard protocols and stained with hematoxylin-eosin or with Ziehl-Neelsen staining specific for acid-fast bacilli. Histological scores were calculated for each tissue. Granulomas were assigned a score from 1 to 4 as previously described [12,14]: 1, presence of lymphocytes, epithelioid cells and plasma cells; 2, presence of the same cell types as 1 as well as the presence of a coagulative necrotic center; 3, presence of a caseous necrotic center; or 4, presence of a caseous and mineralized necrotic center. Tissue sections showing only active lymphoid follicles were scored as 0. Tissues were scored for abundance of acid-fast bacilli from 0 (none) to 2 (abundant). The histopathology score was based on the most severe lesion observed on the section. Individual histopathology scores for granuloma and acid-fast bacilli for each of the three tissue categories were averaged to yield a final score for each animal (see Supplementary Material _Raw data. Histology, culture and MRI). Tissues that were suspected of containing tuberculous lesions but were negative by culture or Ziehl-Neelsen staining were not included in the calculation of the final score.
2.9. Statistical analysis
Results were analyzed attending to in vivo and post-mortem variables. The in vivo variables were weight loss ([maximum weight between challenge and final -final weight]/maximum weight between challenge and final), rectal temperature, serology (P22 ELISA), cellular immune response (whole blood IGRA ELISA and IGRA ELISPOT) and isolation from clinical samples (swabs, feces and urine). Regarding the post-mortem, the variables were gross and microscopic lesion scores, lesion volume with MRI, plate or tube isolation (bacteriological) score, combination of both culture types, and a geometric combination of both histopathological (microscopic) lesions and isolation (Combined score). The descriptive analysis clearly showed that there were two vaccinated animals with much higher scores than the rest and, as a consequence, were considered as potentially representing a sub-group resulting from the effect of unknown factors given the lack of homogeneity linked to use of randomly captured wild animals as the experimental subjects (see Results section). Since all the response dependent variables were quantitative (continuous or ordinal), the statistical approach was the linear model, either analysis of variance for treatment/response type classes, correlation for quantitative variables or analysis of covariance for both types of independent variables. All these analyses were carried out using the jamovi application for descriptive, ANOVA, ANCOVA, linear regression and correlation analysis (Jamovi, Version 2.2 computer software). In order to test the two suspected sub-groups, goodness of fit (see Table 2 in Results section) was compared for a model comparing the pre-planned treatment groups and another one splitting the vaccinated animals into two sub-groups according to their type of response (see below). For treatment effect comparison, no correction was applied for pre-planned marginal means comparison (vaccination versus control groups), but the Tukey correction was applied to any other comparison. The Cohen's d was shown as a measure of size of effect of the treatment with respect to the control. MRI volumes were natural logarithm transformed and submitted to ANOVA.
Table 2.
Goodness of fit of the two linear models for the effect of treatment on clinical, microbiological, pathological and combined variables.
| Variable | Model | Adjusted R2 | AIC |
|---|---|---|---|
| Weight gain | Treatment | 0.032098 | −17.819 |
| Treatment response type | 0.39787 | −24.139 | |
| Increase | 1139.5% | −35.5% | |
| Isolation | Treatment | 0.057009 | 125.45 |
| Treatment response type | 0.89492 | 93.333 | |
| Increase | 1469.8% | −25.6% | |
| Lesion volume | Treatment | 0.051694 | 378.06 |
| Treatment response type | 0.89214 | 346.25 | |
| Increase | 1625.8% | −8.4% | |
| Combined score | Treatment | −0.053005 | 141.18 |
| Treatment response type | 0.85842 | 111.88 | |
| Increase | 1719.5% | −20.8% |
AIC: Akaike information criterion.
2.9.1. Post-hoc analysis
According to the hypothesis of two types of response a new categorical variable was created meant to represent a different response to vaccination and challenge without making any assumption on its cause. It separated sub-group of badgers identified as 3919 and 3920 from the HIMB vaccinated group that showed larger weight loss, more advanced granulomas, numerous colonies of M. bovis on culture plates, and a very high lesion volume in lungs (see Results section). Those animals seemed to respond in a different way to vaccination and they were defined as “divergent” for a post-hoc analysis in which a model was built for one control and two vaccine response types (standard and divergent).
Standard statistical significance was declared at p = 0.05; however, most actual values are shown and, if lower than 0.15, discussed as potentially affected by the low number of animals and their individual heterogeneity.
3. Results
3.1. Overall model fitting
Model fit for the two models is shown in Table 2. It shows that the treatment response type substantially improved the pre-planned two levels treatment model. However, to better understand a general or a more detailed view on badger vaccination, both models are presented throughout the results section.
3.2. Clinical signs
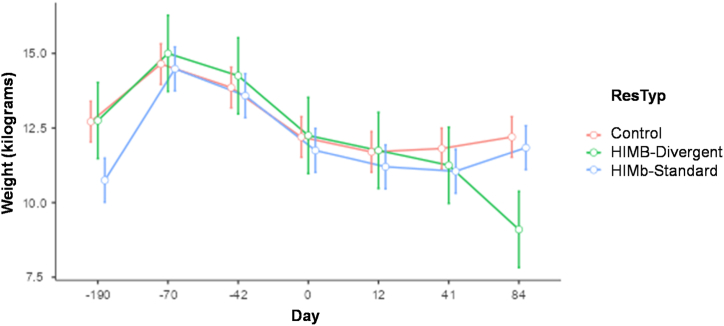
All animals remained in good condition throughout the study. Mean body weight was 11.93 kg (SD 2.45 kg) before vaccination, 12.03 kg (SD 1.65 kg) at challenge, and 11.64 kg (SD 2.07 kg) at post-mortem examination. Body weight increased just after vaccination (Fig. 2), but steadily decreased from −70 day, though it showed signs of an increase by the last sampling on day 84 for vaccinated group and on day 41 for control group. Body weight of the two divergent vaccinated animals that showed substantially worse response than the other vaccinated animals evolved similarly as the body weight in the control group and among the other six vaccinated animals, except that their post-mortem weight was lower than that in controls (p = 0.0354) or the other six vaccinated animals (p = 0.0668). This was best summarized by the weight loss at the end of the experiment that was non-significant when treatment groups were compared (p = 0.2478). However, it showed a highly significant difference when post-hoc response type groups were considered (p = 0.0189). According to this analysis, the divergent group weight loss was significantly lower than the control (p = 0.0067) and the standard (p = 0.0114) that did not differ between them (p = 0.7450). At all sampling times, mean rectal temperature was similar across all groups (HIMB/control and divergent/standard/control).
Fig. 2.
Mean body weight in control and vaccinated badgers. ResTyp: Treatment and type of response. Weight: Mean group weight at sampling time. Standard error bars are included for each sampling date.
3.3. Cellular and humoral immune response
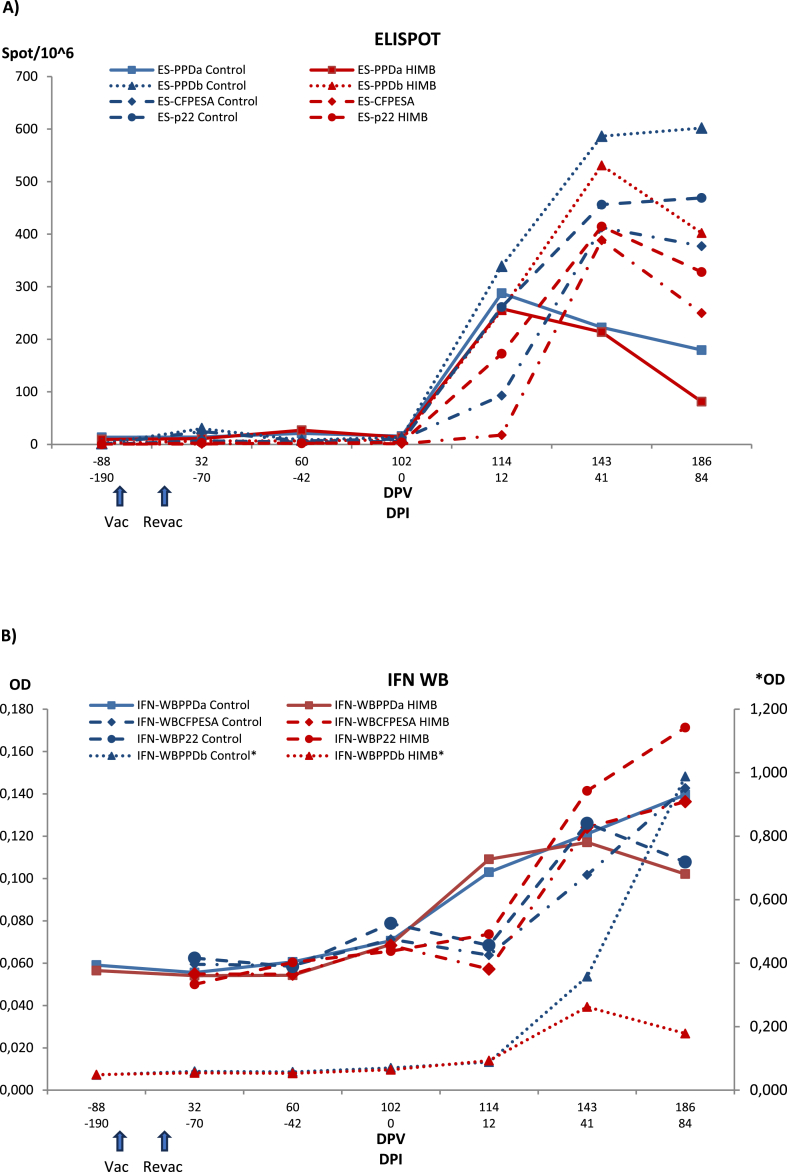
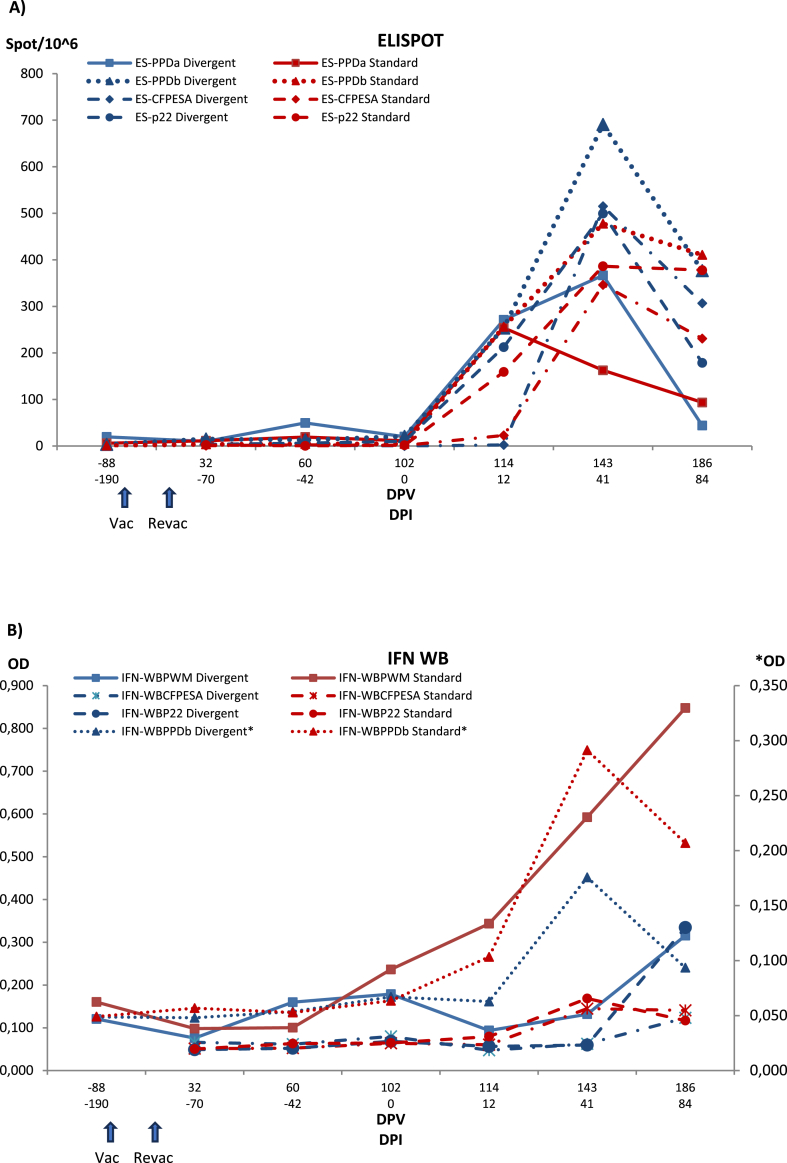
Fig. 3, Fig. 4 show the treatment group evolution of the cellular immune response in blood determined by ELISPOT, whole blood IGRA and PBMC IGRA ELISA with different antigens (PPD-B, PPD-A, CFP-10/ESAT-6 and P22 and nil negative control) as well the specific anti-P22 antibodies response.
Fig. 3.
Evolution of IGRA ELISPOT (A) and whole blood IGRA ELISA (IFN WB) (B) immune response in control and vaccinated badgers. Each panel shows results with the different antigens in each method and matrix for each treatment group. Control: Control group. HIMB: HIMB vaccinated group. PPDa: Avian tuberculosis PPD. PPDb: Bovine tuberculosis PPD. CFPESA: CFP-10 and ESAT-6 MTC-specific antigen cocktail. Series with an asterisk are scaled to the right vertical axis. Vac: vaccination. Revac: Re-vaccination. DPV: Days post-vaccination. DPI: Days post-infection. OD: Optical density.
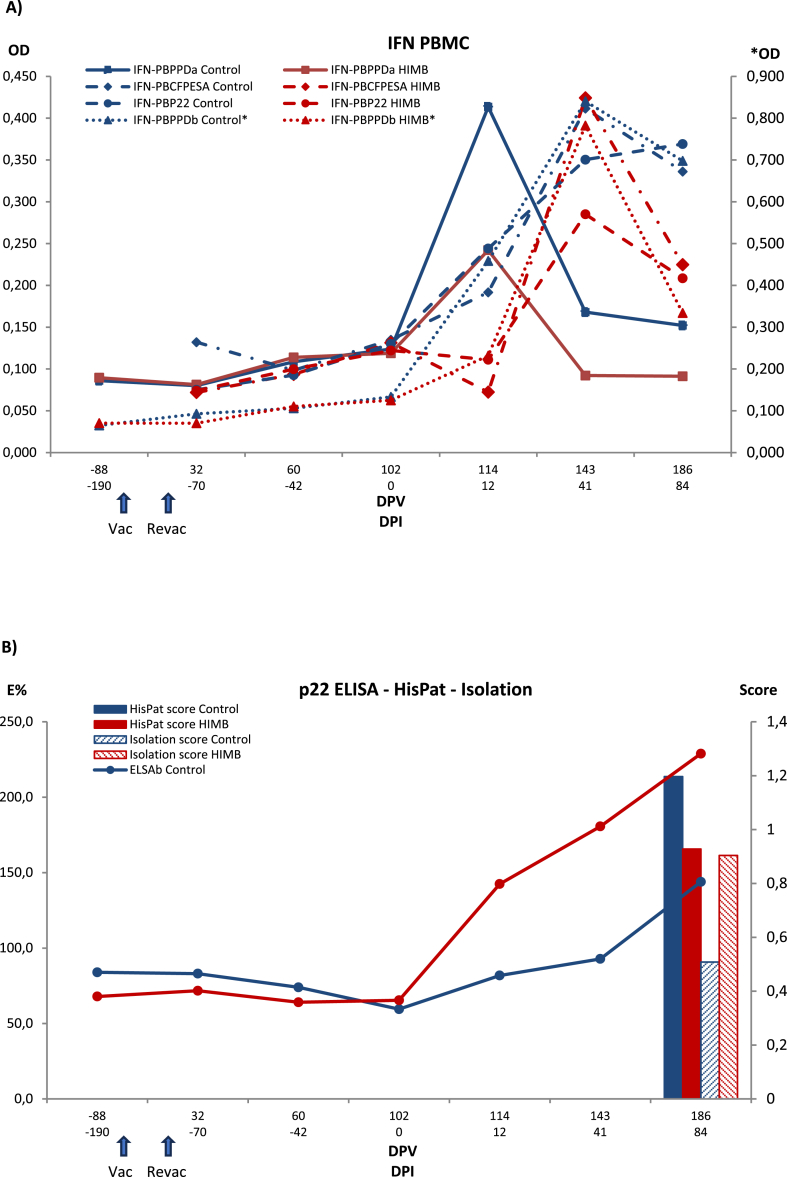
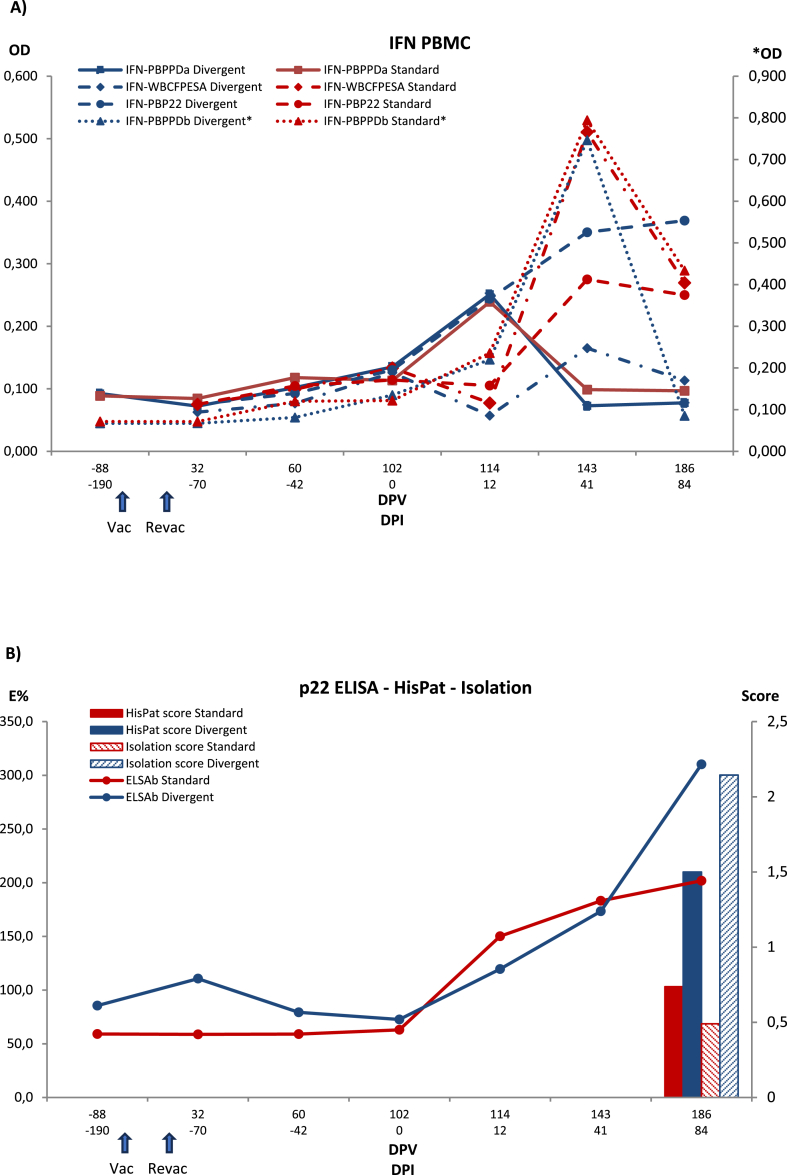
Fig. 4.
Evolution of PBMC IGRA ELISA (IFN PBMC) (A) and P22 ELISA immune response and bacteriological and histopathological outcomes (B) in control and vaccinated badgers. Each panel shows results with the different antigens in each method and matrix for each treatment group. Control: Control group. HIMB: HIMB vaccinated group. PPDa: Avian tuberculosis PPD. PPDb: Bovine tuberculosis PPD. CFPESA: CFP-10 and ESAT-6 MTC-specific antigen cocktail. Series with an asterisk are scaled to the right vertical axis. Vac: vaccination. Revac: Re-vaccination. DPV: Days post-vaccination. DPI: Days post-infection. OD: Optical density.
3.3.1. IGRA ELISPOT
ELISPOT response remained essentially stable up to challenge. All groups response started increasing by 12 days PI, significantly for all antigens but CFP-10/ESAT-6 and P22 (PPD-A, ptukey = 0.0003; PPD-B, pt < 0.0001; CFP-10/ESAT-6, pt = 0.9261; P22, pt = 0.0158 for control group and PPD-A, pt = 0.0005; PPD-B, pt = 0.0046; CFP-10/ESAT-6, pt = 1.0000; P22, pt = 0.2948 for HIMB group). By 41 days PI all but PPD-A (pt = 0.0003 and pt = 0.0005) responses clearly increased (pt < 0.0001 for all groups and antigens); then they stabilized or slightly decreased, with a slight lower responsiveness in the vaccinated group by day 84 that became statistically significant relative to control group for PPD-A (pt = 0.0214), PPD-B (pt = 0.0019), CFP-10/ESAT-6 (pt = 0.0214), and P22 (pt = 0.0356).
3.3.2. Whole blood IGRA ELISA
The whole blood IGRA reactivity to PPD-A remained at very low levels until the first sampling PI when a slight non-significant increase was observed relative to the first sampling at vaccination. Only by the last sampling a small difference was observed between control and HIMB group (p = 0.0936). The more specific response to PPD-B stayed at minimal and very homogeneous values until the sixth sampling at 41 days PI when a small non-significant increase was observed especially in control group (pt = 0.9961), that kept increasing until the last sampling when it became statistically significant for control group (pt = 0.0383), but not for HIMB group (pt = 1.0000). From −70 days PI to 41 days PI, P22 and CFP-10/ESAT-6 values remained low and not different for both treatment groups. At day 41 PI, both groups showed a rise in the CFP-10/ESAT-6 antigen response (control p = 0.9014, HIMB, p = 01455) with respect to the first sampling values. This increase continued until the end of the experiment when differences became more significant (control, p = 0.0562, HIMB, p = 0.0358) with no difference between them (p = 0.7873). For P22, the HIMB group still slightly increased, while the control group showed a decrease making both groups difference nearly significant (p = 0.0720).
3.3.3. PBMC IGRA ELISA
PBMC IGRA group means were nearly identical between control and HIMB groups until challenge. Afterwards, the HIMB group consistently had lower PBMC IGRA means with all antigens, and the differences kept below significance except for control and HIMB group means for PPD-A at the first follow-up after challenge (p = 0.0393) and the last for PPD-B (p = 0.0160). CFP10/ESAT6 cocktail and P22 IGRA means showed a rising pattern right after the first PI sampling (p = 0.0496 and p = 0.0173 relative to −190 days PI at 41 days PI for control and HIMB groups, respectively), but differences between the two remained below statistical significance (p > 0.3000 and p > 0.1500, respectively, at 12-, 41- and 84-days PI).
3.3.4. Cellular immune response in standard and divergent vaccinated badgers
Splitting the HIMB vaccinated group into standard and divergent response individuals (Fig. 5, Fig. 6), some variables had a different behavior in the divergent badgers. The ELISPOT readings of the divergent animals at 41 days PI showed a peak in PPD-A, PPD-B and CFP-10/ESAT-6 cocktail relative to both control (PPD-A, p = 0.0866; PPD-B, p = 0.2912; CFP-10/ESAT-6, p = 0.2337; p22, p = 0.1863) and standard response (PPD-A, p = 0.0181; PPD-B, p = 0.0361; CFP-10/ESAT-6, p = 0.0554; P22, p = 0.6775). In the whole blood IGRA, the only difference between standard and divergent for PPD-A antigen occurred at 12 days PI (p = 0.0275) when standard behaved similar to control. By 41 days PI all groups had equal means (p > 0.8660) while, at 84 days PI standard and divergent were similar (p = 0.5946), but nearly different to control (p > 0.1390). No differences were observed between standard and divergent for PPD-B at any time, except at 84 days PI (p = 0.7887), when both differed from control (p = 0.0080 and p = 0.0337). For CFP-10/ESAT-6 cocktail, the single difference between groups was observed at 41 days PI, when the standard was higher than divergent (p = 0.0320) and control (p = 0.0966). Regarding P22 antigen, differences were observed between standard and divergent at 41 days PI and at 84 days PI. At 41 days PI, standard was higher than divergent (p = 0.0346), which did not differ from control (p = 0.1863). At 84 days PI, divergent was higher than control (p < 0.0001) and standard (p < 0.0001).
Fig. 5.
Evolution of IGRA ELISPOT (A) and whole blood IGRA ELISA (IFN WB) (B) immune response, with post-hoc groups according to the type of response at vaccination (divergent or standard). Each panel shows results with the different antigens in each method and matrix for each type of response to HIMB vaccination. PPDa: Avian tuberculosis PPD. PPDb: Bovine tuberculosis PPD. CFPESA: CFP-10 and ESAT-6 MTC-specific antigen cocktail. Divergent: Divergent response animals. Standard: Standard response group. PPDa: Avian tuberculosis PPD. PPDb: Bovine tuberculosis PPD. CFPESA: CFP-10 and ESAT-6 MTC-specific antigen cocktail. Series with an asterisk are scaled to the right vertical axis.
Vac: vaccination. Revac: Re-vaccination. DPV: Days post-vaccination. DPI: Days post-infection. OD: Optical density.
Fig. 6.
Evolution of PBMC IGRA ELISA (IFN PBMC) (A) and P22 ELISA immune response and bacteriological and histopathological outcomes (B), with post-hoc groups according to the type of response at vaccination (divergent or standard). Each panel shows results with the different antigens in each method and matrix for each type of response to HIMB vaccination. PPDa: Avian tuberculosis PPD. PPDb: Bovine tuberculosis PPD. CFPESA: CFP-10 and ESAT-6 MTC-specific antigen cocktail. Divergent: Divergent response animals. Standard: Standard response group. PPDa: Avian tuberculosis PPD. PPDb: Bovine tuberculosis PPD. CFPESA: CFP-10 and ESAT-6 MTC-specific antigen cocktail. Series with an asterisk are scaled to the right vertical axis. Vac: vaccination. Revac: Re-vaccination. DPV: Days post-vaccination. DPI: Days post-infection. OD: Optical density.
In the PBMC IGRA ELISA matrix, no differences were found between standard and divergent at any sampling (p > 0.8470), although the control was nearly significantly different from standard (p = 0.0606), but not from divergent (p = 0.2226) regarding PPD-A antigen. For PPD-B, no differences were observed between standard and divergent (p > 0.8370) although by the last sampling the divergent had a lower mean than the standard (p = 0.1478) and the control (p = 0.0088). Regarding the CFP-10/ESAT-6 cocktail, standard and divergent maintained similar means until the second sampling after challenge at 41 days PI, when the standard had a mean similar to the control (p = 0.4348), but marginally different from the divergent (p = 0.0655). By the last sampling (84 days PI) all groups had similar means (p > 0.2230). No significant differences were observed for P22, although the divergent group had lower mean than control (p = 0.1337), but not than standard (p = 0.4281).
3.3.5. P22 ELISA
No animals were positive by the P22 ELISA prior to vaccination or challenge. However, the day of challenge (day 0), one animal was positive to the test in vaccinated group. The number of positive animals rose 12 days PI in HIMB vaccinated group [2/8; 25% (CI 95% 7.15–59.09)] while no reactive animals were observed in control group. The levels of antibodies peaked at 41 days PI in vaccinated group [4/8; 50% (CI95% 21.52–78.48)], and 5 animals out of 8 [62.5% (CI95% 30.57–86.32)] were positive to ELISA at the end of the study. Regarding control group, 2/7 [28.57% (CI95% 8.22–64.11)] and 3/7 [42.86% (CI 95% 15.82–74.95) were positive at 41 days PI and at the end of the study. In addition, significant differences were observed in the antibodies’ level at 41 and 84 days PI in the vaccinated group compared to control group at the end of the study (p < 0.001) (Fig. 4). When vaccinated group was analyzed by dividing into standard and divergent, no significant differences were observed (p = 0.3096), although by the last sampling day the divergent had a higher mean than the standard (Fig. 6).
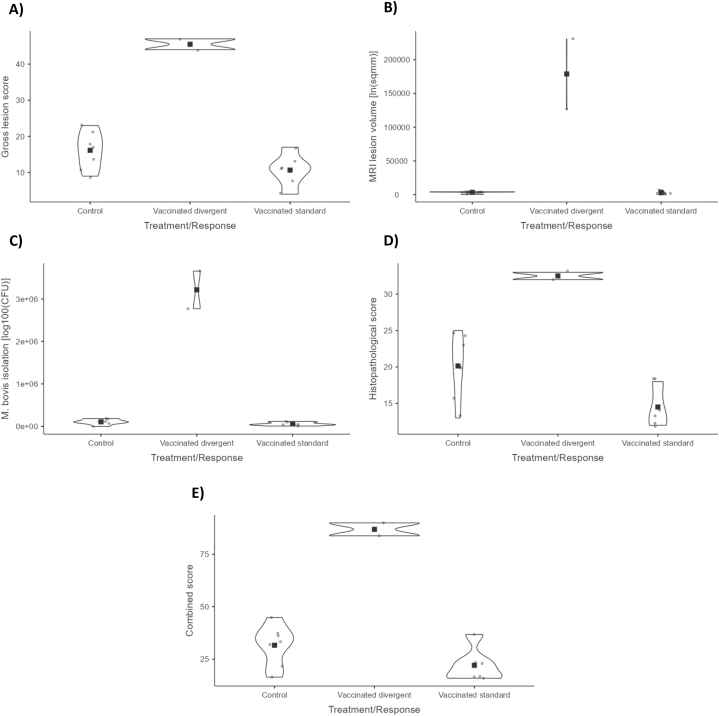
3.4. Post-mortem gross tuberculous lesions
All badgers presented gross tuberculous lesions at necropsy (Fig. 7 and Supplementary Material _Raw data. Gross TB lesions). No significant difference between HIMB (19.38) and control (16.14) group mean score was observed (p = 0.6296). However, lesion severity score for the two divergent vaccinated badgers (45.50) was significantly higher than the score for the other six standard vaccinated animals (10.67, p < 0.0001) and the score for control animals (16.14, p < 0.0001; 182%; d = −6.32). The six standard vaccinated animals’ comparison with the control group did not reach the conventional p-value threshold (p = 0.0555; 34%; d = 1.18), but when the body condition at capture variable was added to the overall model, the difference became significant (p = 0.0486).
Fig. 7.
Lesion assessment, isolation scores, and histopathology scores for control and vaccinated animals (divergent or standard). A) Gross lesion scores. B) Magnetic resonance imaging (MRI) lesion volume, after natural log transformation. C)M. bovis colony-forming units (CFU) per gram, after log100 transformation. D) Histopathological score. E) A score combining the results from panels (A)–(D).
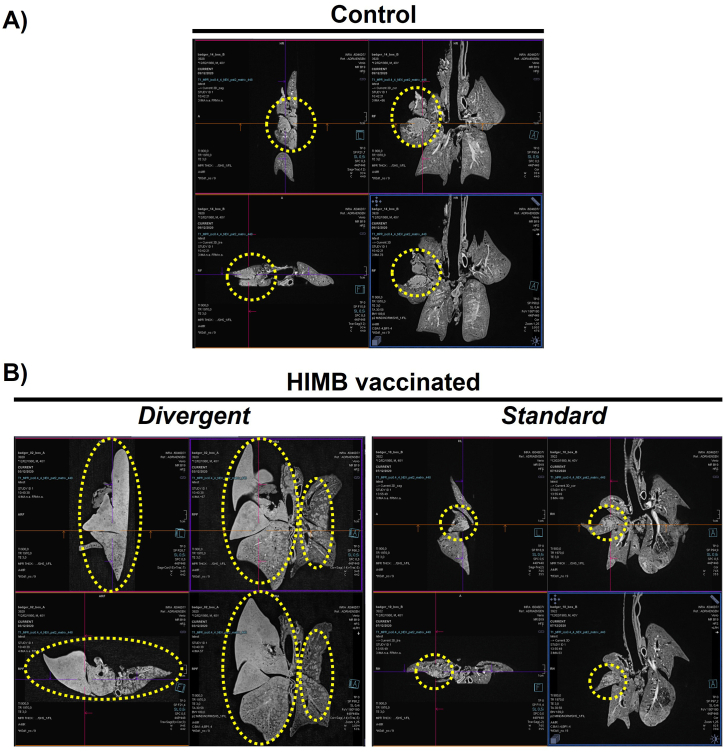
3.5. Magnetic resonance imaging (MRI) analysis
The MRI analysis showed a broad range of lesion extension in badgers - ‘severely damaged’, ‘noticeably damaged’, and ‘not damaged or with low damage’ - (Fig. 8), whereby TB lesion volume could be precisely calculated in mm3 for each animal. The right middle lung lobe, where the inoculum was placed, consistently presented the most severe lesions of the whole lung. The overall quantitative comparison with the general linear model showed no significant differences between HIMB vaccinated and control group (control: 3424 mm3 versus (vs.) HIMB: 46876 mm3; p = 0.2071). However, highly significant differences were observed between divergent (178950 mm3) and control (3424 mm3) or standard (2852 mm3) (p < 0.0001 divergent vs. control or standard and p = 0.9623 between the latter). That is, on average, the treated animals had more severe lesions than the controls (−1269% volume lesion reduction; d = 0.69). However, that could be split into a strong increase comparing control with divergent (−5127% volume lesion reduction; d = −8.23) and a slight decrease comparing control with standard (17% volume lesion reduction; d = 0.03).
Fig. 8.
Magnetic resonance imaging of lungs in control (A) and vaccinated (B) badgers. Lesions are demarcated in dotted yellow circles. Lesions in the control group were classified as “noticeably damaged”, while those in the two vaccinated animals (“divergent”) that showed much higher scores on post-mortem, histopathology, culture and MRI tests than the other six vaccinated animals (“standard”) were classified as “severely damaged”. The lesions in the vaccinated standard animals were classified as “low damage”.
3.6. Microbiology isolation, real time-polymerase chain reaction (RT-PCR) and spoligotyping
A total of 329 tissues were individually cultured (see Supplementary_Raw data. Histology, culture and MRI). All the spoligotyped isolates corresponded with the challenge strain SB0339. Mycobacterium avium complex (MAC) mycobacteria were isolated from the left bronchial LN of one vaccinated badger (3921) and Mycobacterium spp. from the popliteal LN of other vaccinated badger (3923). Additionally, a total of 135 clinical samples (30 tonsillar swabs, 30 nasal swabs, 30 faecal swabs, 15 urine samples, 15 tracheal swabs, and 13 faecal samples) were also individually tested (see Supplementary Material_Raw data. Culture clinical samples). Mycobacterium bovis was isolated in 7 tonsillar swabs (5 from day 41 PI and 2 from day 84 PI), 4 faecal samples (2 from day 41 PI and 2 from day 84 PI), 3 tracheal swabs from day 84 PI, 2 nasal swabs (1 from day 41 PI and 1 from day 84 PI), and 2 urine samples from day 84 PI. Those positive clinical samples were constantly found in divergent animals from day 42 PI to post-mortem. The remaining positive clinical samples were from one control and two HIMB vaccinated badgers in tonsillar swabs at day 41 PI, and from a control badger in a tracheal swab and a HIMB-vaccinated badger in faecal samples at day 84 PI. The overall isolation rate from all samples was much higher (FET p = 0.0016) in HIMB group (22.54%) than in the control group (3.23%) (Fig. 7). However, this was due to the high frequency of isolations in the divergent group (76.47%) compared to both control and standard (FET p < 0.0001), while the standard response group frequency (5.56%) did not differ from that of controls (FET p = 0.6626). The frequency of M. bovis isolation in combined plate and tube cultures was significantly higher in HIMB animals than in the control ones (HIMB: 46.3% vs. control: 22.7%; p < 0.0001). When divergent animals 3919 and 3920 were excluded, the percentage of positive samples in vaccinated animals decreased to 28.8% (FET p = 0.2770). Regarding tissues, in lung region, standard response type group yielded the same proportion of positive samples than the control group (50%; FET p = 1.000), slightly higher in lymphoid tissue (34.4% vs. 23.8%; FET p = 0.1141) and slightly lower in other tissues (3.3% vs 8.6%; FET p = 0.2770).
Considering the total amount of bacteria isolated in tissues, controls had lower total counts (10729 CFU/g) than HIMB vaccinated animals (847293 CFU/g), although the difference was not statistically significant (p = 0.2110). Splitting the vaccinated into standard and divergent showed that most of the bacterial load in the vaccinated group were isolated in the two divergent animals (3213875 CFU/g; p < 0.0001 compared to both control –d = −16.51- and standard –d = 16.77- groups). On the other hand, the standard vaccinated badgers had a slightly lower load (58560 CFU/g; p = 0.8888) than the controls (45% reduction; d = 0.26). Comparison of bacterial loads by type of tissue showed no significant differences of vaccinated (11571 CFU/g) to control (2430 CFU/g) in lung region (p = 0.3211), lymphoid tissue (772237 CFU/g; p = 0.2135) and other tissues (63581 CFU/g vs. 218 CFU/g; p = 0.2140. When focusing on the type of response groups, again the main differences were observed between divergent (37970 CFU/g in lung region; 2921889 CFU/g in lymphoid tissue; and 254016 CFU/g in other tissues) and both control (2430 CFU/g, p = 0.0046 in lung region, 104561 CFU/g, p < 0.0001 in lymphoid tissue, and 218 CFU/g, p > 0.0001 in other tissues) and standard response (2772 CFU/g, p = 0.0055 in lung region; 55686 CFU/g, p < 0.0001 in lymphoid tissue; and 102 CFU/g, p < 0.0001 in other tissues). Results did not differ among them in any tissue (p > 0.5997) although the bacterial burdens were 14% higher and 47% and 53% lower in the standard vs. the control group in each tissue, respectively.
3.7. Histopathology
All badgers showed microscopic lesions compatible with TB (see Fig. 7 and Supplementary material _Raw data. Histology, culture and MRI). Consistent with the results for visible lesion scores, HIMB vaccination reduced the severity of granulomas compared with controls. Type 4 granulomas (see Methods section) were observed in 3/7 (42.86%) control animals vs. none of the HIMB vaccinated badgers. The mediastinum showed TB microscopic lesions in all badgers except for one HIMB badger. The right bronchial, posterior mediastinal and hepatic LN sustained the most severe damage. Indeed, the most frequently affected LN outside of the thoracic cavity was the hepatic LN (15/15, 100%). Tonsils were affected in three (37.5%) HIMB badgers and in salivary glands of two (28.58%) control badgers. Mesenteric LN was affected in 3/7 (42.86%) control and 2/8 (25%) HIMB badgers. Spleen showed TB granulomas in two control and two HIMB badgers. Two control badgers and one HIMB badger presented TB granulomas in liver. Kidneys were affected in one control and two HIMB badgers.
The vaccinated group showed a lower mean lesion severity score (19.00) but the difference with the control group (20.14; 6% reduction; d = 0.1628) was not significant (p = 0.7581). However, the two divergent vaccinated badgers (32.50) showed a significant difference from controls (20.14; 61% increase; p = 0.0010; d = −3.44) and standard (14.50; p < 0.0001; d = 5.01). This represented a reduction of lesions in the standard response group of 28% (p = 0.0154; d = 1.57) with respect to the control. According to tissues, the vaccinated group showed a slightly decreased histopathological mean score in lung and other tissues compared to control (1.50 vs. 2.14, d = 0.88; p = 0.1110 and 0.88 vs. 1.86, d = 0.50, p = 0.3496; respectively) and slightly increased in lymphoid tissue (16.63 vs. 16.14; p = 0.8660; d = −0.0891). The response type analysis showed a reduction of the scores in lung region (2.00 vs. 2.14, 7% reduction, d = 0.20, p = 0.8083), but an increase in lymphoid (27.00 vs. 16.14, 67% increase, d = −3.89, p = 0.0004) and other tissues (3.5 vs. 1.86, 88% increase, p = 0.2290) for the divergent animals. In the standard response group, however, a reduction of lesions was a trend in all tissues (1.33, 38% reduction, d = 1.13, p = 0.0656 for lung region; 13.17, d = 107, 18% reduction, p = 0.0797 for lymphoid tissue, and 0; 100% reduction, d = 1.15, p = 0.0612 for other tissues).
3.8. Summary of main variables
A summary of main variables group values is shown in Table 3 and raw individual data for all variables can be found in Supplementary_Raw data. All data.
Table 3.
Summary of main variables group mean values.
| Treatment/Response | Control | Vaccinated divergent | Vaccinated standard | ||
|---|---|---|---|---|---|
| Pathology/Microbiology | Sex | Female | 5 | 0 | 3 |
| Male | 2 | 2 | 3 | ||
| Gross lesion score | Mean | 16.14 | 45.50 | 10.67 | |
| SEM | 1.93 | 1.50 | 1.80 | ||
| p (control) | – | 0.0067 | 0.7450 | ||
| MRI lesion volume | Mean | 3423.86 | 178950.00 | 2851.67 | |
| SEM | 458.89 | 52050.00 | 991.99 | ||
| p (control) | – | 0.0001 | 0.9623 | ||
| M. bovis isolation | Mean | 107209.08 | 3213874.67 | 58560.30 | |
| SEM | 23995.60 | 442813.11 | 16824.53 | ||
| p (control) | – | <0.0001 | 0.6504 | ||
| Histopathological score | Mean | 20.14 | 32.50 | 14.50 | |
| SEM | 1.65 | 0.50 | 1.15 | ||
| p (control) | – | 0.0010 | 0.0154 | ||
| Combined score | Mean | 31.64 | 86.90 | 22.05 | |
| SEM | 3.65 | 3.10 | 3.23 | ||
| p (control) | – | <0.0001 | 0.0689 | ||
| ELISPOT | PPD-B | Mean | 3005 | 1875 | 2030.42 |
| SEM | 372.89 | 897.5 | 213.64 | ||
| p (control) | – | 0.1261 | 0.0636 | ||
| CFP-10/ESAT-6 | Mean | 1881.79 | 1525 | 1131.67 | |
| SEM | 349.49 | 422.5 | 236.64 | ||
| p (control) | – | 0.5754 | 0.12066 | ||
| P22 | Mean | 2340.36 | 885 | 1865.83 | |
| SEM | 449.65 | 227.5 | 338.66 | ||
| p (control) | – | 0.85 | 0.4111 | ||
| Whole blood IGRA ELISA | PPD-B | Mean | 0.99 | 0.09 | 0.21 |
| SEM | 0.71 | 0.03 | 0.07 | ||
| p (control) | – | 0.4173 | 0.3113 | ||
| CFP-10/ESAT-6 | Mean | 0.14 | 0.12 | 0.14 | |
| SEM | 0.02 | 0.07 | 0.05 | ||
| p (control) | – | 0.8155 | 0.9718 | ||
| P22 | Mean | 0.11 | 0.33 | 0.12 | |
| SEM | 0.01 | 0.28 | 0.03 | ||
| p (control) | – | 0.0445 | 0.8985 | ||
| PBMC IGRA ELISA | PPD-B | Mean | 0.7 | 0.09 | 0.43 |
| SEM | 0.17 | 0.01 | 0.14 | ||
| p (control) | – | 0.0755 | 0.2708 | ||
| CFP-10/ESAT-6 | Mean | 0.34 | 0.11 | 0.27 | |
| SEM | 0.09 | 0.05 | 0.09 | ||
| p (control) | – | 0.2352 | 0.4167 | ||
| P22 | Mean | 0.37 | 0.11 | 0.25 | |
| SEM | 0.2 | 0.05 | 0.09 | ||
| p (control) | – | 0.4486 | 0.6368 | ||
| P22 ELISA | P22 | Mean | 143.9 | 310.22 | 201.79 |
| SEM | 55.38 | 158.22 | 85.3 | ||
| p (control) | – | 0.2765 | 0.4794 | ||
SEM: Standard error of mean. PPD-B: Bovine tuberculin. MRI: Magnetic resonance imaging.
3.9. Combined score
The pathological and microbiological combined score represents an overall simplified criterion for vaccine protection evaluation (Fig. 7). When comparing both groups of animals, the HIMB vaccinated group presented a slightly higher combined score (38.26) than the control (31.64, 21% increase, p = 0.5960; d = −0.28). However, excluding the divergent animals, the standard group score showed a 30% reduction over the control group (d = 1.1, p = 0.0689).
Regarding tissues, no treatment effect (p = 0.3232) was observed in lung, lymphoid tissue (p = 0.5033) or other tissues (p = 0.2490). When the divergent group was considered apart, in lung there was a marginal effect of treatment (p = 0.0948), but this effect was very strong in lymphoid tissue (0 < 0.0001) and other tissues (p < 0.0001). In lung the standard response group had a mean index of 2.32 that was 43% lower than that of the control (4.07, d = 1.03, p = 0.0893), while the divergent had a 29% increase that was not significant (p = 0.4058, d = −0.69). In lymphoid tissue the index was 22.23 for the control group, while the divergent had an index of 70.05, higher (p < 0.0001) than both control and standard (19.05, 24% lower than control, p = 0.1175, d = 0.94). In other tissues, the divergent group had a mean index of 11.6, higher than control (p < 0.0001, d = −4.82, 395% increase) and standard (0.68, 71% reduction, d = 5.69, p < 0.0001).
3.10. Correlations
No consistent correlations between in vivo and post-mortem variables were found, although at a few time points a correlation between the two variables was found. The only highly significant correlations for the whole data set were between IGRA on whole blood with P22 antigen and lung colony number and whole blood P22 (r = 0.8681, p < 0.0001), but it was due to a single divergent animal (badger 3919) with an extremely high value both in lung colony numbers and IGRA. Excluding this animal, it changed the correlation to negative and a non-significant value (r = 0.5423, p = 0.0451).
Regarding the control group, several correlations were observed relating ELISPOT PPD-B (r = −0.9638, p = 0.0005), CFP-10/ESAT-6 (−0.9710, p = 0.0003), P22 (−0.9683, p = 0.0003) at −70 days PI, PPD-B (r = −0.8835, p = 0.0083), CFP-10/ESAT-6 (−0.9778, p = 0.0001) and P22 (−0.9692, p = 0–0003) at −42 days PI and CFP-10/ESAT-6 (r = −0.9706, p = 0.0003) at 12 days PI, whole blood IGRA with PPD-B (0.9243, p = 0.0029) and CFP-10/ESAT-6 (−0.9535, p = 0.0009) at 12 days PI and PBMC IFN-ɣ with PPD-B (r = −0.9666, p = 0.0004), CFP-10/ESAT-6 (r = −0.9689, p = 0.0003), P22 (−0.9697, p = 0.0003) and Nil (−0.8794, p = 0.0091) at −70 days PI and CFP-10/ESAT-6 (r = −9693, p = 0.0003) at 12 days PI with MRI lung lesion volume. Also, whole blood IGRA negatively correlated with colony number (r = −0.9528, p = 0.0009) and lymphoid colony number (r = −0.9590, p = 0.0006) at last control.
Regarding vaccinated animals, ELISPOT readings at −42 days PI with P22 also showed strong correlations with several post-mortem variables like overall animal lesion volume (r = 0.9843, p < 0.0001), histopathological score (r = 0.8654, p = 0.0055) and number of colonies (r = 0.9468, p = 0.0004), isolation score (p = 0.8695, p = 0.0050) and combined index (r = 0.8860, p = 0.0034), as well as with lymphoid histopathological score (r = 0.08769, p = 0.0042), number of colonies (r = 0.9441, p = 0.0004), isolation score (r = 0.8609, p = 0.0060) and combined index (r = 0.8860, p = 0.0034). P22 ELISA at −70 days PI correlated well with most variables: gross lesions (r = 0.9156, p = 0.0014), lesion volume (r = 0.9391, p = 0.0005), overall histopathological score (0.8465, p = 0.0080), overall colony number (r = 0.9226, p = 0.0011), isolation score (r = 0.8707, p = 0.0049), overall combined index (r = 0.8769, p = 0.0042), as well as with lymphoid tissue histopathology (r = 0.8547, p = 0.0069), number of colonies (r = 0.9219, p = 0.0011), isolation score (r = 0.8524, p = 0.0072) and combined index (r = 0.8712, p = 0.0048). Restricting vaccination analysis to the standard response group, the −70 days PI whole blood IGRA kept as the only time point related to the post-mortem outcome. In this case, strong correlations were found for P22 and no antigen with overall isolation (r = 0.9558, p = 0.0029 and r = 0.9230, p = 0.0087, respectively) and combined (r = 0.9436, p = 0.0047 and r = 0.9585, p = 0.0028, respectively) scores, as well as with lymphoid isolation (r = 0.9708, p = 0.0013 and r = 0.9381, p = 0.0056, respectively) and combined (r = 0.9265, p = 0.0079 and r = 0.9704, p = 0.0013, respectively) scores.
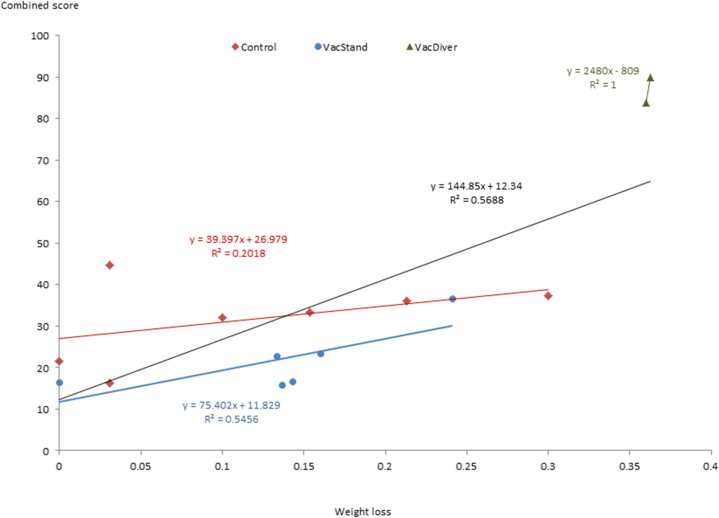
Clinical, microbiological and pathological variables at post-mortem control correlated well. The weight loss correlated best with gross lesion score (r = 0.8333, p = 0.0001), overall isolation score (r = −0.7652, p = 0.0009) and lymphoid tissue isolation score (r = −0.7606, p = 0.0010). Combined score very strongly correlated with the microbiological (r = 0.9715, p < 0.0001), gross (r = 0.9556, p < 0.0001) and histopathological (r = 0.9402, p < 0.0001) scores from which it originated and also had a strong correlation with weight loss (r = −0.7542, p = 0.0012) (Fig. 9) confirming its value as a summary synthetic variable. If sex was considered, weight loss (p = 0.0387) was larger in males. Regarding immune variables, only ELISPOT at the end of the follow-up showed a clear trend (p = 0.0613) for males having a higher cellular response to PPD-B.
Fig. 9.
Correlation between weight loss and combined score in vaccinated standard (VacStand) or divergent (VacDiver) badgers. Black line represents all data.
4. Discussion
Our study examined the efficacy of HIMB vaccine orally administered via edible baits on badgers after challenge with 103 CFU of M. bovis delivered endobronchially. This strategy led to successful infection of all badgers, leading to the formation of tuberculous lesions in the respiratory system and other tissues. These results are similar to those reported in previous studies from the UK, Ireland and Spain [7,12,14,15].
Overall, HIMB vaccinated animals had higher clinical, microbiological and pathological scores than the controls, although these differences were not statistically significant due to the high variability observed within the HIMB group.
4.1. Standard versus divergent vaccinated badgers
4.1.1. Clinical, bacteriological and pathological outcomes
Taking into account the type of response to vaccination within the vaccinated group, two different groups of animals were observed - standard and divergent -. In that regard, the six standard response vaccinated animals showed some reduction in the bacterial load at post-mortem, which in some cases achieved statistical significance and correlated with a reduction in the severity and volume of gross and microscopic lesions. In sharp contrast, the two divergent response animals suffered a significant increase in the average number of lesions and bacterial load after infection, compared to the remaining vaccinated badgers. As already pointed out, the animals used in the experiment were trapped in an area without evidence of recent M. bovis infection and were negative to all tests for TB. However, it cannot be ruled out sensitization to other mycobacteria or even immune training by other pathogens or any other unknown predisposing condition [12]. Therefore, our experiment might have been conditioned by the different immune or physiological backgrounds of our subjects, as expected in field trials, with a result of 75% standard and 25% divergent response in vaccinated badgers. Interestingly, the observation that from at least day 41 the two divergent animals continuously excreted mycobacteria via nasal and tracheal discharge, as well as via urine and feces (continuously exposing the other six vaccinated animals to the pathogen), supports the partial protective efficacy of HIMB vaccine and bait vaccination strategy on 75% of the vaccinated animals. For example, in humans the constant reinfection can result in the presence of bacilli in the upper lung lobes, where they can grow faster and present a delayed immune response [22].
4.1.2. Hypothesis for divergent response
Vaccination can, in some cases, exacerbate manifestations of subsequent disease instead of mitigating it [23]. This has been observed in previous studies, under similar or different conditions (e.g., heterologous vaccination), with vaccination based on mycobacterial antigens in mice [24,25], high-dose BCG vaccination in badgers [14] and vaccination with inactivated M. paratuberculosis in pigs [26]. One possible explanation for this is a differed immune response in some individuals by shifting towards a humoral type 2 immune response, or a lymphatic dysfunction, such as lymphoma as reported in badgers after oral BCG vaccination [27]. We did not, however, observe signs of such dysfunction in our study. A third potential explanation is T cell exhaustion, when T cells are unable to clear chronic infection due to persistent antigen and chronic T cell stimulation [28]. This hypothesis requires explanation, and it is further discussed. Finally, a fourth explanation could be related to the endobronchial challenge not being solely distributed to the right middle lung lobe, i.e., an overspill during the procedure. In that regard, we did not observe any difference in divergent animals in comparison with those with a standard response or the control group, in MRI or pathological studies. However, endobronchial route is per se an aggressive route for lung tissue, and further studies should consider either the oral or aerosol routes for challenge, more similar to the natural route of infection based on previous studies [4].
4.1.3. Innate, adaptative or trained immune response
Classically, host immunity is divided into innate and adaptive immune responses [29]. The former reacts rapidly and non-specifically to pathogens, whereas the latter responds in a slower but specific manner, with the generation of long-lived immunological memory. During an infection, innate immunity is the first to react (the inflammatory reaction), taking no longer than minutes to hours to be fully activated [30]. This is crucial for the host defense in the early phases of a new infection. Innate immunity is generally able to eliminate the pathogens efficiently; however, initial clearance of infection can fail due to the high number or virulence of invading pathogens. In these situations, lymphocytes and adaptive immune mechanisms are activated, which allow specific recognition and elimination of the pathogen. Establishment of adaptive immunity needs 1–2 weeks and is important for host defense during the latter phases of an infection and during secondary infections due to its capacity to “remember” and respond more effectively to re-stimulation [31]. Therefore, innate immunity may mediate at least some of the protective effects of vaccination, as it has been shown for other TB vaccines such as BCG, which induce T cell-independent protection against both target (MTC) and non-target pathogens such as Candida albicans, Schistosoma mansoni, or influenza virus in animals or yellow fever in humans [[32], [33], [34], [35], [36]]. The memory associated to this type of protection has been defined as trained immunity [30] and is responsible of a stronger and more efficient response to secondary challenges by agents sharing the so-called pathogen associated molecular patterns (PAMP) with the primary agent. These PAMP usually are highly repetitive small molecule polymers (polysaccharides, fatty acids and peptides and its combinations) present as structural elements of living organisms.
4.1.4. Vaccine immunogenicity: exhaustion hypothesis
In the present study, based on low adaptive cellular and humoral immune response in vaccinated animals, HIMB vaccine protective responses might have provided an increased but non-specific protective response against M. bovis infection. Responses to specific antigens in the cellular assays indicate a low-level of this response after vaccination, but not in the humoral assays, except for divergent animals. Standard vaccinated group seemed to react in a delayed and less intense manner than controls although non-significantly. Those results suggest that vaccination acted according to the expected trained immunity model causing either protection or tolerization, this likely depending on the individual immune status at vaccination. That is why T cell exhaustion must be considered in the divergent animals. When an infection cannot be cleared by the host, and the pathogenic antigens persist in time, pathogen-specific T cells curtail or reduce their anti-pathogen function to avoid causing excessive damage to normal tissues, with a result of an expansion of the inflamed areas and pathogen burden at an overall more severe intensity [37]. Exhausted T cells express inhibitory receptors but can retain some anti-pathogen effector function, ultimately resulting in a pathogen-host stalemate. On those circumstances, exhausted T cells result of an early divergence between effective memory T cell precursors and precursors of the exhausted T cell lineage [37], which would correspond to the ”standard” and “divergent” response in vaccinated badgers in our study, respectively. This could be further extended to explain the TB lesion location in lymphoid tissues as a result of M. bovis tropism for LN resident central memory T lymphocytes (Tcm) rather than for tissue effector T lymphocytes (Tem) [38]. We speculate that body condition, may influence whether such exhaustion occurs. Six of the vaccinated animals lost substantial weight soon after challenge, yet their condition improved; in contrast, the two divergent vaccinated animals began to lose weight after challenge, even though their body weight initially was comparable to that of control animals. This could be compatible with the exhaustion hypothesis in the sense that animals with a good nutritional status would be able to efficiently react to the infectious challenge, while those with a negative balance will mount an inefficient but strong response. According to this, the timing and variables measured in this experiment might have failed to identify key factors that determine the standard/divergent type response pathways. Alternatively, the variables used in this experiment might not properly represent the epidemiological and clinical consequences of infection and vaccine protection. Which trajectory/response a given host follows likely depends on immune status and perhaps physiological balance at vaccination. These hypotheses should be explored in further studies with larger samples, which may need to measure a more comprehensive array of variables to take into account body condition and immune status before and after vaccination, as well as after challenge. Differences in environment, exercise space or access to water/food in BSL3 facilities compared to vaccination facilities were excluded, since same welfare conditions were maintained along the study.
4.2. Comparison with other models
Our results here contrast with those of our previous study, in which the same HIMB vaccine was applied directly to tonsils and found to partially protect the vaccinated badgers against progressive disease [12]. In the present work, using edible bait to vaccinate animals meant that we could not ensure what dose reached the oral mucous membranes. Vaccination with HIMB via edible bait has already been shown to be effective for protecting wild boar [8,39] and wild Molokai pig [10]. Therefore, further work should explore how to optimize variables such as vaccine dose, number of doses, route of administration and potential use of adjuvants [40] to ensure efficient protection of badgers.
5. Conclusions
In summary, our study highlights the need to consider the initial general condition of animals in studies of field vaccination. Inadequate condition may increase the risk that orally administered vaccines will tolerize, rather than protect animals. If body condition is good, it could be expected a substantial protective effect in the order of 60% reduction in all variables. If the body condition is low, any outcome can be expected. This strongly supports the need of homogenizing experimental groups related to body condition in those wild randomly trapped badgers for further studies focused on the vaccine protection assessment. If that is not considered, assuming our sample is representative of Asturian conditions, it can be expected a 75% favorable or neutral effect and a 25% pathogenic effect of oral vaccination. Further studies should explore how such tolerization (divergence) occurs with oral vaccines based on HIMB, which may guide efforts to minimize it.
Funding
This work is result of the I + D + i research project RTI2018-096010-B-C21, funded by the Spanish MCIN/AEI/10.13039/501100011033/Ministry of Science, Innovation and the European Regional Development Funds (FEDER Una manera de hacer Europa). This work was also partially funded by the Principality of Asturias, PCTI 2021–2023 (GRUPIN: IDI-2021-000102) and European Regional Development Fund. The cost of the running MRI was financed by the European Commission in the context of Horizon 2020 – Vetbionet Transnational Access Activities (TNA) call. DEFRA also funded APHA staff for their participation in the study. Dr. Cristina Blanco Vázquez was granted with a predoctoral fellowship CPD2016-0142 funded by MCIN/AEI/10.13039/501100011033 and FSE “El FSE invierte en tu futuro”. Gloria Herrero-García was granted with a predoctoral fellowship by Junta de Castilla y León and FSE (LE036-20).
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Animal Ethics Committees of the Government of the Principality of Asturias (license PROAE 47/2018) and the Diputación Foral de Bizkaia (license 201944).
CRediT authorship contributions
AB and JMP conceived and designed the experiments. RAJ, CBV, MB, JMP, LVC, SL, DD, IAS, ABME, HA, GHG, JMG, RC and AB performed the experiments. RAJ, MB, SL, DD, HA and AB analyzed and interpreted the data. All authors wrote the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank their colleagues from SERIDA, NEIKER, APHA, INRAE and ULE for helping with sample processing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19349.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Barberis I., Bragazzi N.L., Galluzzo L., Martini M. The history of tuberculosis: from the first historical records to the isolation of Koch's bacillus. J Prev Med Hyg. 2017;58 E9–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly C.A., Woodroffe R., Cox D.R., Bourne F.J., Cheeseman C.L., Clifton-Hadley R.S., Wei G., Gettinby G., Gilks P., Jenkins H., Johnston W.T., Le Fevre A.M., McInerney J.P., Morrison W.I. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature. 2006;439:843–846. doi: 10.1038/nature04454. [DOI] [PubMed] [Google Scholar]
- 3.Murphy D., Gormley E., Costello E., O'Meara D., Corner L.A. The prevalence and distribution of Mycobacterium bovis infection in European badgers (Meles meles) as determined by enhanced post mortem examination and bacteriological culture. Res. Vet. Sci. 2010;88:1–5. doi: 10.1016/j.rvsc.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Blanco Vázquez C., Barral T.D., Romero B., Queipo M., Merediz I., Quirós P., Armenteros J.Á., Juste R., Domínguez L., Domínguez M., Casais R., Balseiro A. Spatial and temporal distribution of Mycobacterium tuberculosis complex infection in Eurasian badger (Meles meles) and cattle in Asturias. Animals (Basel) 2021;11:1294. doi: 10.3390/ani11051294. Spain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne A., Boschiroli M.L., Gueneau E., Moyen J.L., Rambaud T., Dufour B., Gilot-Fromont E., Hars J. Bovine tuberculosis in “Eurasian” badgers (Meles meles) in France. Eur. J. Wildl. Res. 2012;59:331–339. doi: 10.1007/s10344-012-0678-3. [DOI] [Google Scholar]
- 6.Gormley E., Costello E. Tuberculosis and badgers: new approaches to diagnosis and control. J. Appl. Microbiol. 2003;94 doi: 10.1046/j.1365-2672.94.s1.9.x. Suppl:80–6S. [DOI] [PubMed] [Google Scholar]
- 7.Chambers M.A., Rogers F., Delahay R.J., Lesellier S., Ashford R., Dalley D., Cheeseman C., Hanks C., Murray A., Palphramand K., Pietravalle S., Smith G.C., Tomlinson A., Walker N.J., Wilson G.J., Corner L.A., Rushton S.P., Shirley M.D., Gettinby G., McDonald R.A., Hewinson R.G. Bacillus Calmette-Guérin vaccination reduces the severity and progression of tuberculosis in badgers. Proc. Biol. Sci. 2011;278 doi: 10.1098/rspb.2010.1953. 1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díez-Delgado I., Sevilla I.A., Romero B., Tanner E., Barasona J.A., White A.R., Lurz P.W.W., Boots M., de la Fuente J., Domínguez L., Vicente J., Garrido J.M., Juste R.A., Aranaz A., Gortázar C. Impact of piglet oral vaccination against tuberculosis in endemic free-ranging wild boar populations. Prev. Vet. Med. 2018;155:11–20. doi: 10.1016/j.prevetmed.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Garrido J.M., Sevilla I.A., Beltrán-Beck B., Minguijón E., Ballesteros C., Galindo R.C., Boadella M., Lyashchenko K.P., Romero B., Geijo M.V., Ruiz-Fons F., Aranaz A., Juste R.A., Vicente J., de la Fuente J., Gortázar C. Protection against tuberculosis in Eurasian wild boar vaccinated with heat-inactivated Mycobacterium bovis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nol P., Wehtje M.E., Bowen R.A., Robbe-Austerman S., Thacker T.C., Lantz K., Rhyan J.C., Baeten L.A., Juste R.A., Sevilla I.A., Gortázar C., Vicente J. Effects of inactivated Mycobacterium bovis vaccination on Molokai-origin wild pigs experimentally infected with virulent M. bovis. Pathogens. 2020;9:E199. doi: 10.3390/pathogens9030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas J., Risalde M.Á., Serrano M., Sevilla I.A., Geijo M., Ortíz J.A., Fuertes M., Ruíz-Fons J.F., de la Fuente J., Domínguez L., Juste R.A., Garrido J., Gortázar C. The response of red deer to oral administration of heat-inactivated Mycobacterium bovis and challenge with a field strain. Vet. Microbiol. 2017;208:195–202. doi: 10.1016/j.vetmic.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Balseiro A., Prieto J.M., Álvarez V., Lesellier S., Davé D., Salguero F.J., Sevilla I.A., Infantes-Lorenzo J.A., Garrido J.M., Adriaensen H., Juste R.A., Barral M. Protective effect of oral BCG and inactivated Mycobacterium bovis vaccines in European badgers (Meles meles) experimentally infected with M. bovis. Front. Vet. Sci. 2020;7:41. doi: 10.3389/fvets.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MAPA 2022. https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/programatb2022_tcm30-583922.pdf
- 14.Lesellier S., Palmer S., Gowtage-Sequiera S., Ashford R., Dalley D., Davé D., Weyer U., Salguero F.J., Nunez A., Crawshaw T., Corner L.A.L., Hewinson R.G., Chambers M.A. Protection of Eurasian badgers (Meles meles) from tuberculosis after intra-muscular vaccination with different doses of BCG. Vaccine. 2011;29 doi: 10.1016/j.vaccine.2011.03.028. 3782–90. [DOI] [PubMed] [Google Scholar]
- 15.Murphy D., Costello E., Aldwell F.E., Lesellier S., Chambers M.A., Fitzsimons T. Corner LAL, Gormley E. Oral vaccination of badgers (Meles meles) against tuberculosis: comparison of the protection generated by BCG vaccine strains Pasteur and Danish. Vet. J. 2014;200 doi: 10.1016/j.tvjl.2014.02.031. 362–7. [DOI] [PubMed] [Google Scholar]
- 16.Dalley D., Davé D., Lesellier S., Palmer S., Crawshaw T., Hewinson R.G., Chambers M. Development and evaluation of a gamma-interferon assay for tuberculosis in badgers (Meles meles). Tuberculosis. Edinb) 2008;88:235–243. doi: 10.1016/j.tube.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Lesellier S., Boschiroli M.L., Barrat J., Wanke C., Salguero F.J., García-Jiménez W.L., Nunez A., Godinho A., Spiropoulos J., Palmer S., Dave D., Anderson P., Boucher J.M., de Cruz K., Henault S., Michelet L., Gowtage S., Williams G.A., Nadian A.K., Monchâtre-Leroy E., Boué F., Chambers M.A., Richomme C. Detection of live M. bovis BCG in tissues and IFN-γ responses in European badgers (Meles meles) vaccinated by oropharyngeal instillation or directly in the ileum. BMC Vet. Res. 2019;15:445. doi: 10.1186/s12917-019-2166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Infantes-Lorenzo J.A., Dave D., Moreno I., Anderson P., Lesellier S., Gormley E., Domínguez L., Balseiro A., Gortázar C., Domínguez M., Salguero F.J. New serological platform for detecting antibodies against Mycobacterium tuberculosis complex in European badgers. Vet. Med. Sci. 2019;5 doi: 10.1002/vms3.134. 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers M.A., Aldwell F., Williams G.A., Palmer S., Gowtage S., Ashford R., Dalley D.J., Davé D., Weyer U., Salguero F.J., Nunez A., Nadian A.K., Crawshaw T., Corner L.A., Lesellier S. The effect of oral vaccination with Mycobacterium bovis BCG on the development of tuberculosis in captive European badgers (Meles Meles) Front. Cell. Infect. Microbiol. 2017;7:6. doi: 10.3389/fcimb.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevilla I.A., Molina E., Tello M., Elguezabal N., Juste R.A., Garrido J.M. Detection of mycobacteria by culture and DNA-based methods in animal-derived food products purchased at Spanish supermarkets. Front. Microbiol. 2017;8:1030. doi: 10.3389/fmicb.2017.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamerbeek J., Schouls L., Kolk A., van Agterveld M., van Soolingen D., Kuijper S., Bunschoten A., Molhuizen H., Shaw R., Goyal M., Embden van. J. Simultaneous detection and strain differentiation of Mycobacterium bovis for diagnosis and epidemiology. J. Clin. Microbiol. 1997;35:907–914. doi: 10.1128/JCM.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardona P.J. Revisiting the natural history of tuberculosis. The inclusion of constant reinfection, host tolerance, and damage-response frameworks leads to a better understanding of latent infection and its evolution to active disease. Arch. Immunol. Ther. Exp. 2010;58:7–14. doi: 10.1007/s00005-009-0062-5. [DOI] [PubMed] [Google Scholar]
- 23.Tizard I.R. Tizard IR Editor. Vaccines for Veterinarians. Elsevier; Amsterdan: 2021. Adverse consequences of vaccination; pp. 115–130. [DOI] [Google Scholar]
- 24.Moreira A.L., Tsenova L., Haile Aman M., Bekker L.G., Freeman S., Mangaliso B., Schröder U., Jagirdar J., Rom W.N., Tovey M.G., Freedman V.H., Kaplan G. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect. Immun. 2002;70:2100–2107. doi: 10.1128/IAI.70.4.2100-2107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner J., Rhoades E.R., Keen M., Belisle J.T., Frank A.A., Orme I.M. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect. Immun. 2000;68:1706–1709. doi: 10.1128/IAI.68.3.1706-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen K.J., Hansen M.S., Heegaard P.M.H., Benn C.S., Jungersen G. The effect of inactivated Mycobacterium paratuberculosis vaccine on the response to a heterologous bacterial challenge in pigs. Front. Immunol. 2019;10:1557. doi: 10.3389/fimmu.2019.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianco C., Lesellier S., Barrat J., Richomme C., Boschiroli M.L., Núñez A. Subclinical BCG-osis in a captive badger (Meles meles) with lymphoma. J. Comp. Pathol. 2020;176:76–80. doi: 10.1016/j.jcpa.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Blank C.U., Haining W.N., Held W., Hogan P.G., Kallies A., Lugli E., Lynn R.C., Philip M., Rao A., Restifo N.P., Schietinger A., Schumacher T.N., Schwartzberg P.L., Sharpe A.H., Speiser D.E., Wherry E.J., Youngblood B.A., Zehn D. Defining 'T cell exhaustion'. Nat. Rev. Immunol. 2019;19 doi: 10.1038/s41577-019-0221-9. 665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Netea M.G., van der Meer J.W. Trained immunity: an ancient way of remembering. Cell Host Microbe. 2017;21:297–300. doi: 10.1016/j.chom.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Netea M.G., Balkwill F., Chonchol M., Cominelli F., Donath M.Y., Giamarellos-Bourboulis E.J., Golenbock D., Gresnigt M.S., Heneka M.T., Hoffman H.M., Hotchkiss R., Joosten L.A.B., Kastner D.L., Korte M., Latz E., Libby P., Mandrup-Poulsen T., Mantovani A., Mills K.H.G., Nowak K.L., O'Neill L.A., Pickkers P., van der Poll T., Ridker P.M., Schalkwijk J., Schwartz D.A., Siegmund B., Steer C.J., Tilg H., van der Meer J.W.M., van de Veerdonk F.L., Dinarello C.A. A guiding map for inflammation. Nat. Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farber D.L., Netea M.G., Radbruch A., Rajewsky K., Zinkernagel R.M. Immunological memory: lessons from the past and a look to the future. Nat. Rev. Immunol. 2016;16:124–128. doi: 10.1038/nri.2016.13. [DOI] [PubMed] [Google Scholar]
- 32.Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., Reusken C.B.E.M., Benn C.S., Aaby P., Koopmans M.P., Stunnenberg H.G., van Crevel R., Netea M.G. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. e5. [DOI] [PubMed] [Google Scholar]
- 33.Goodridge H.S., Ahmed S.S., Curtis N., Kollmann T.R., Levy O., Netea M.G., Pollard A.J., van Crevel R., Wilson C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016;16:392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer J.C., Ganguly R., Waldman R.H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J. Infect. Dis. 1977;136:171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- 35.Tribouley J., Tribouley-Duret J., AppriouM Effect of Bacillus Callmette Guerin (BCG) on the receptivity of nude mice to Schistosoma mansoni. C. R. Seances Soc. Biol. Fil. 1978;172:902–904. [PubMed] [Google Scholar]
- 36.van 't Wout JW., Poell R., van Furth R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992;36:713–719. doi: 10.1111/j.1365-3083.1992.tb03132.x. [DOI] [PubMed] [Google Scholar]
- 37.Yao C., Sun H.W., Lacey N.E., Ji Y., Moseman E.A., Shih H.Y., Heuston E.F., Kirby M., Anderson S., Cheng J., Khan O., Handon R., Reilley J., Fioravanti J., Hu J., Gossa S., Wherry E.J., Gattinoni L., McGavern D.B., O'Shea J.J., Schwartzberg P.L., Wu T. Single-cell RNA-seq reveals TOX as a key regulator of CD8(+) T cell persistence in chronic infection. Nat. Immunol. 2019;20:890–901. doi: 10.1038/s41590-019-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 39.Beltrán-Beck B., Romero B., Sevilla I.A., Barasona J.A., Garrido J.M., González-Barrio D., Díez-Delgado I., Minguijón E., Casal C., Vicente J., Gortázar C., Aranaz A. Assessment of an oral Mycobacterium bovis BCG vaccine and an inactivated M. bovis preparation for wild boar in terms of adverse reactions, vaccine strain survival, and uptake by nontarget species. Clin. Vaccine Immunol. 2014;21:12–20. doi: 10.1128/CVI.00488-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balseiro A., Thomas J., Gortázar C., Risalde M.A. Development and challenges in animal tuberculosis vaccination. Pathogens. 2020;9:472. doi: 10.3390/pathogens9060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.