Abstract
Rainfed agriculture which is the mainstay of the Rwandan economy has been severely affected by prolonged droughts and climate change impacts, resulting in severe food insecurity. In the Eastern Province, the adoption of monocropping (MnC) systems at dissent driven by the CIP may critically worsen the rain-fed agricultural gains against mixed cropping (MxC) systems in drought conditions. Therefore, this study aimed to analyze and compare soil organic carbon (SOC) stocks and simulated maize biomass and grain yields under MnC and MxC systems in Kayonza District, Rwanda. Soil samples (n = 96) were collected in 0–30 and 30–60 cm depths following the stratified simple random sampling technique. The SOC stocks were determined following the guidelines of the FAO of 2018. The biomass and grain yield for the past 20 years (2001–2021) was simulated using a calibrated and validated AquaCrop model (version 6.1) using daily climate data obtained from RMA, and maize crop, raw soil, and land management features collected at the field. The data were analyzed using IBM SPSS software (version 25). The results show that the SOC stocks of MxC soils were significantly (p < 0.001) higher (67.4 ± 1.8 tC ha−1) than that of the MnC soils (52.0 ± 3.8 tC ha−1). The depths avowed more highly significant (p < 0.001) SOC stocks in topsoils (0–30 cm depth) than that of the subsoils (30–60 cm depth) in the two cropping systems. This indicates that MxC sequesters more carbon and revamps soil C pools than the MnC system. The results also indicate that the simulated biomass and grain yields were highly significantly (p < 0.001) higher more and stable in MxC than in MnC fields for the entire past 20 years. Harnessing these findings, as C pools were monitored and analyzed in this study, N-bio-chemistry dynamics should also be conducted thereby comparing its environmental pools and impacts to both below and above-ground ecotones.
Keywords: AquaCrop model, Carbon sequestration, Climate change, Yields
1. Introduction
Currently, climate change is the major global environmental threat that jeopardizes humans and their socio-economic activities, biodiversity, and different ecosystems [1,2]. It poses major threats to food and nutrition security systems [3]. Herewith, agriculture is the most vulnerable and sensitive sector due to its dependency on climate factors such as rainfall, temperature, and extreme weather events [4,5]. Both developed and developing countries have experienced the impacts of climate change [6]. But developing countries, particularly Sub-Saharan Africa (SSA), have been severely affected due to their dependencies on small-scale (accounting for 96% of overall crop production) and rain-fed agriculture, with limited adaptive capacities and living in warmer regions of the globe, whereto the warming undermines their farming practices [7,8]. In East Africa (EAC), subsistent and rainfed small-scale farmers have been identified as the most vulnerable groups, and the extent of vulnerability depends on exposure, sensitivity, and adaptive capacity of the household [[9], [10], [11]]. Since 1930, Rwanda experienced the onset of climate change, herewith extreme weather variables (like temperature) started to steadily increase up-to-date [12]. The agriculture sector has been alarmingly experiencing impacts of climate change harnesses the abrupt-intensive rainfall and temperature patterns [8,13]; driven by unstainable practices [14], which channel it to failure. This climate-based calamity has intensified food insecurity and economic losses in many parts of Rwanda, particularly in drought-hot spot areas of Eastern Province, including Kayonza District, as well as in flooded parts of the country. Drought, floods, and landslides have been causing land degradation, death of livestock, crop failure, and yield decline (particularly maize and beans) [13,15,16]. This yield decline is harnessing land decline and fragmentation due to growing population, limited financial capital, and climate hazards on slope lands (including soil erosion, floods, landslides, and droughts) [17]. In the study conducted by Rwanda Environment Management Authority (REMA) in three zones of Rwanda on the effects of floods and landslides, economic losses (i.e., agricultural, livestock, infrastructural losses) counted as 43% of losses are from the middle zone, 24% in the upstream and 33% in the downstream zones, respectively. This dramatic loss in the middle zone has harnessed unsustainable cultivation practices compared to the upstream and downstream ones. This loss was more than 58 million USD (accounting for 1.4% of the overall GDP of 2011/2012), to date, this loss still occurs at each year [15]. The Rwandan soil loss rate is expected to be at least 1.4 million tons each year, resulting in economic expenses of US$320.000 to the national budget per year [18,19]. In the Eastern province (including Kayonza District), about 19% of households are food insecure, often related to the instability of rural incomes, access to land, ownership of animals, and events such as crop failures and seasonal scarcities, which lead to poor accessibility and availability to food commodities [20]. Food-insecure people are among the poorest and small-scale farmers. About 50,000 poor and food-insecure rural households representing 225,000 individuals and more have been classified in Ubudehe (socio-economic category 1 and 2), particularly women and youth, accounting for 51.5% and 69.8% respectively [16,21]. For instance, through food relief, MINAGRI has provided 6469.631 MT of maize and 3135.376 MT of beans to feed those starved families [21]. The total economic cost of vulnerable crops in the drought-prone areas have estimated at approximately 8.8 billion Rwandan francs (∼US$ 8,800,000) in seasons 2016A (i.e., long-term rainfall season) and B (short-term rainfall season), and hitherto needs more budget [16]. In addition, climate-related hazards have been accelerating the enormous depletion of SOC stocks―as much as 421 Mg ha−1 yr−1, with mean national rates of 250 Mg ha−1 yr−1 in croplands (i.e., occupy 56% of the national area and contribute to about 95% of the national soil loss) [13]. Yet, Rwanda's economy stands in the agriculture sector. It employs 72% of the total active population, and provides more than 90% of the national food supply, and 70% of export revenues, accounting for 33% of the Gross Domestic Product (GDP) [18,[22], [23], [24], [25]]. About 96% of rural households rely on agriculture for their livelihoods―wherein 80% of them are subsistence farmers who use mostly rain-fed production systems [19,20]. To achieve national food security and Sustainable Development Goals (SDGs), transformative strategies (e.g., Strategic Plan for the Transformation of Agriculture, PSTA), agricultural policies and programs (e.g., CIP and Land Use Consolidation, LUC) as well as approaches that integrate climate change into their heart, have been recommended [4]. Climate-Smart Agriculture (CSA) and sustainable agricultural intensification (SAI) are the current approaches [4], that embrace the sustainable agricultural intensified and agroecological systems that improve soil health [26]. These systems positively influence ecological benefits, conserve natural resources and ecosystem services, increase productivity and income via low-input use and efficiency, build resilience to climate change, and mitigate GHG emissions [27]. The integration of mixed cropping systems is the backbone for small-scale farmers for sustainably stabilizing crop production systems and increasing C sinks under climate shocks [[28], [29], [30]]. Mixed cropping systems provide more diversified yields for farmers, improve soil properties, increase C sequestration potential, and control pest infestation [2,31]. However, there is still a misunderstanding and scant information on maize-monocropping versus mixed cropping systems harnessing the pledges of land use consolidation under CIP and smallholder farming systems. Consequently, sometimes these mismatches appeal to the local leaders to take serious decisions for uprooting those mixed crops. This resulted in confrontations between farmers and leaders; and significantly declined yields of staple crops like maize, banana, bush bean, and Irish potato for small-scale farmers [24,32], which are worsened by climate change. Hence, understanding the relationship between cropping systems and carbon stock and biomass is crucial and keen, whilst a cropping system has either a positive or negative influence on soil properties, carbon stock, and yield imbalance. In Rwanda, many researches focused on the impacts of land uses (e.g., forest, cropland, rangeland, and grassland) on carbon stock with poor emphasis on individual components of land uses. For instance, monocropping and mixed cropping are among the elements of cropland (as land use), they act differently on carbon stock in the soils and crop biomass yields. Moreover, the impact of subsistence agriculture on carbon stock is characterized by the low level of management skills where low or no external inputs are applied, which may, in turn, have lessened carbon stock, consequently this was not studied. Under the imminent climate change, such information on carbon emission and stock is very crucial for forming strategies that increase productivity via increased carbon stock and decrease emissions via revamped carbon sinks into the soil-plant system. Following this push-and-pull scenario, this study compared monocropping and mixed cropping systems based on selected soil properties, SOC stocks, simulated maize biomass, and grain yields under a changing climate in Kayonza District, Eastern Province of Rwanda.
2. Materials and methods
2.1. Study area
This study was conducted in the Rukara and Gahini sectors of Kayonza District in the Eastern Province of Rwanda. It is located at 30°28′0″E − 30°40′0″E and 1°38′0″S ‒ 1°54′0″S (Fig. 1). The total area of the study area was 9173 ha. It has an altitude ranging between 1000 m and 1200 m above sea level. It is located 114 km distance eastward away from Kigali. This study area is inherently classified and located into eastern agropastoral and semi-arid pastoral livelihood zones [32]. The landscape is dominated by hills, plains, and plateaus. Soils are infertile, dominated by Ferralsols and Leptosols, with Histosols in lowland areas [33]. They are generally composed of high clay, silt contents, and a low proportion of sand in top-soils; while sub-soils are dominated by hard soils laying on bed-rocks from 1 m deep accoutered on North and East of the district in the layout of Rukara and Gahini sectors [34].
Fig. 1.
The map of the study area: A is Rwanda, B is Eastern Province and C denotes Gahini and Rukara sectors (Source: own map generated by using QGIS 3.18).
Agriculture is almost done in lowlands, plateaus, and plains. Crops grown in the District are maize (Zea mays L.), cassava (Manihot esculenta), paddy rice (Oryza sativa), sorghum (Sorghum vulgare), bananas (Musa sapientum and Musa paradisiaca), sweet potatoes (Ipomoea batatas) and Irish potatoes (Solanum tuberosum), yams (Dioscorea alata) and taro (Colocasia esculenta), common beans (Phaseolus vulgaris), peas (Pisum sativum), groundnuts (Arachis hypogaea), soya beans (Soja hispida and Glycine max), vegetables and fruits [35]. Unfortunately, the District agrarian is seriously affected by drought with low precipitations mainly in season B [agricultural season with low rainfall amount] and C [agricultural season with zero rainfall amount], which hinder agricultural production, food security, and economy of the district, the communities and the country at all. The rainfall is a bi-modal pattern. Yearly, the climate of the study area is characterized by two distinct rainy seasons: from March to May and from October to December. The area is also characterized by two dry seasons: a long-dry season from June to September, and a short-dry season from January–February. In the District, the average annual rainfall is 898 mm; and the average annual temperature is 19.7 °C. Maximum temperatures range between 19.7 and 31 °C, while the minimum ranges between 14.9 and 19.7 °C. The temperature data were collected from the Kwangire station in Kayonza District.
2.2. Contextualized meaning: monocropping versus mixed cropping systems
Monocropping which is known as sole cropping, or monoculture, is a farming system of growing a single crop on the same field, year after year, with defined row patterns [36]. On the other hand, “mixed cropping, also known as intercropping, polyculture, or co-cultivation, is a form of a crop production system that involves planting two or more species (or cultivars) simultaneously in the same field in a variable order (row or rowless)” [37]. In this context, a system where farmers grew a single maize (Zea mays L.) crop the fields under the influence of the crop intensification program (CIP) was hypothetically considered as a monocropping system. Then, a system where farmers grew maize (Zea mays L.) crop in a mixture with at least one additional crop (e.g., beans, soja, Irish potatoes, etc) with or without agroforestry species in the single field without caring about any field patterns was considered as a mixed cropping or indigenous intercropping system. Thus, the soil and yield data were collected in these fields where the two systems were lazed respectively.
2.3. Data type, sources, and collection methods
Data type, sources, collection and analysis methods were solely planned based on the particularity of each activity. At first, it was for determining soil properties and SOC Stocks in monocropping and mixed cropping systems. Secondarily, it was the simulation of maize biomass and grain yields in monocropping compared to mixed cropping systems in the past 20 years (2001–2021). As integrated approaches employ both primary and secondary data, the two types of data were collected. Primary data originated from soil laboratory and soil profile study. Secondary data on climate trends for the 20 years (2001–2021) were collected from Rwanda Meteorology Agency (RMA); and maize yield data were from the Rwanda Agriculture and Animal Resources Board (RAB), National Institute of Statistics of Rwanda (NISR) and Sector Agronomists respectively. Herewith, the measured maize crop yield data of the past 20 years (2001–2021) were collected from RAB‒station in Kayonza District while observed maize yield data were from the NISR and sector agronomists.
2.4. Research design, sampling techniques, and data analysis
2.4.1. Determination of selected soil physico-chemical properties and SOC stocks in MnC and MxC systems
Existing LULC categories were identified using a topographic map (1:1,000,000) obtained from Rwanda Land Management and Use Authority (RLMUA) and Google Earth online imagery and Digital Elevation Model (DEM: 30 m × 30 m resolution). Following this, physical observations were made to confirm the basic information about the major cropping system types at 1000 m between two sampling points. A stratified simple random sampling technique was exercised to collect soil samples at the field via 2 cropping systems (i.e., mono-cropped and mixed-cropped maize fields) [38]. Herewith, transects were established along the slope at 1000 m-interval crossing two cropping systems. Besides, random sample points were established in areas that are not covered by the transect, where homogeneity was addressed. A sample plot of 2 m × 2 m was used in all cropping systems (Ullah and Al-Amin, 2012). Soil samples were taken from the four corners and center of the 2 m × 2 m plot by using Auger. The samples were taken from 2 soil depths, 0–30 cm and 30–60 cm [39,40], of soil matrix from two cropping systems, 4 transects per cropping system, 3 plots per each transect within 2 sectors of the study area. The sampled soil had been put into a bucket, and thoroughly mixed. The mixture was split into four sub-samples, and then one sub-sample was a representative of the entire 2 m × 2 m-plot (named a composite sample). Forty-eight (48) disturbed composite soil samples were collected. Composite soil samples were made, put in plastic bags, and transported to the laboratory for organic C (%) analysis and other parameters. On the other hand, 1 undisturbed soil sample was taken from the center of each plot using a core sampler for bulk density estimation [38]. Respectively, 48 undisturbed soil samples were collected and well-treated, and analyzed in the soil laboratory. The soil sample was air-dried and passed through a 2 mm sieve to obtain the fine fraction for chemical analysis except organic carbon and total nitrogen in which case the samples were crashed further to pass through a 0.5 mm mesh sieve. Soil texture was determined by the Bouyoucos hydrometer method as described in Ref. [41]. The dry bulk density of the soils was determined using the core method as described in Ref. [[40], [42]] in which case the core samples were dried in an oven set at a temperature of 105 °C to a constant weight. The bulk density was obtained by dividing the oven-dry mass by the volume of the respective cores, following Equation 1
| (1) |
where MoDs is the mass of the oven-dry soil (g); Vt is the total volume of the soil core (cm3), calculated from . Where r is the internal radius of the cores measured using a caliber (cm), and h is the height of the cores measured using a hand tape; is a constant that is equal to 22/7.
Soil pH in water was measured in soil–water (1:2.5) suspension using a pH meter [43]. The SOC content was determined following the Walkley-Black oxidation method [38]. Total nitrogen was determined by the micro-Kjeldahl digestion, distillation, and titration method [44].
The Soil Organic Carbon Stock (SOCS) was calculated based on the soil organic carbon contents obtained from laboratory analysis. The SOCS for each layer was calculated as described by Ref. [45] in the 2 layers (0–30 and 30–60 cm) following Equation (2),
| (2) |
where: SOCS: SOC stock is the soil organic carbon per unit area (t C ha−1); SOC concentration, is the carbon concentration in the soil layer (%), which means 1gC/100 g soil. To convert SOC (%) into (t C. t−1soil), by using international measurement units (1 ton = 1,000 kg = 1m3; and 1 ha = 10,000m2). BD is bulk density (t soil m−3); D is the depth of the sample layer (m); i … n represent the number of layers [i.e., 0–0.3 m and 0.3–0.6 m]; CFM is percent mass course fragmented matter >2 mm sieve and the multiplying factor 104 is required to express the result in a correct unit. Coarse fractions were determined during the preparation and handling of samples after the repeated crushing of clods by hand, mechanical grinding, drying, and sieving until the samples were sieved and passed through a 2 mm sieve. Thus, the coarse fractions were weighed and the proportions were determined using Equation (3).
| (3) |
where: WCF is the percentage weight of coarse fragments [46]; WTS is the weight of the total soil sample. The SOC stock density in mineral soil was calculated based on the fixed depth method using carbon concentration. The depth of 60 cm for each cropping system was considered a fixed depth.
Excel spreadsheets were used to cater, organize, validate, and store data from the soil laboratory according to the cropping systems and soil depths. The obtained data were tested for normality. The data for individual depths were subjected to ANOVA (analysis of variance). The least significant difference (LSD) was used to separate significantly different means from each cropping system at P < 0.05. Besides, statistical differences were tested using two-way ANOVA to identify whether differences in soil attributes between the two cropping systems and sampling depths are significant or not following the generalized linear model (GLM) procedure of SPSS version 20.0 for Windows.
2.4.2. Simulation of maize biomass and grain yields in MnC compared to MxC systems in the past 20 Years (2001–2021)
Throughout different studies, simulation of maize biomass and grain yields was performed using different models such as AquaCrop, CROPWAT, EPIC phase, APEX-CUTE, CERES-Maize, among others. Many of these models like CERES-Maize do not simulate biomass and grain yield under the impacts of water stress to which AquaCrop model do [47]. AquaCrop is a crop simulation model which explains the relationship between the soil, plant, and atmosphere. This starts from the root zone, the plant extracts water and nutrients, which play a big role in the variability of crop biomass and yield [48,48,49]. AquaCrop model predicts crop productivity, water requirement, and use efficiency and identifies the conditions where water is a major limiting factor for crop production [50,51]. This model simulates the yield response to water, via the entailed relationship between crop yield and crop water use, resulting from water supplied either from rainfall or irrigation during the entire growing period [52]. The boundaries of this model start from Aquifers, soil, plant, and the upper part of the atmosphere which provide ETo and supply CO2 and energy for crop growth [49]. AquaCrop model differs from other crop simulation models by the fact of using canopy cover instead of leaf area index [48]. It has been parameterized and tested on different types of crops [[53], [54], [55]]. To efficiently simulate biomass and yield, the AquaCrop model needs to be calibrated and validated based on field data to assess its robustness in different climatic conditions [48]. Different studies on agricultural research in different countries using calibrated and validated AquaCrop models have been done, but they are still limited in Rwanda.
2.4.2.1. Biomass simulation and carbon sequestration potential
This study used two types of climate data for biomass simulation and C sequestration potential. The first one is observed in long-term daily climate data of Kayonza district, from 2001 to 2021, which were obtained from Rwanda Meteorology Agency (RMA). The climate data were filled for gaps based on standard procedures. This daily observed baseline climate data was used as an input for the AquaCrop model to simulate the biomass development of maize (Zea mays L.) varieties grown in the Kayonza District under past and current climate conditions. The past data ranged from 2001 to 2020. The observed and current climate data were collected at Kawangire meteorological station representing Rukara and Gahini sectors in Kayonza District. The 20-years (2001–2021) measured yield data were obtained from RAB-Kayonza Station chaired in Kayonza district. The soil characteristics, such as soil rooting depth, field capacity, the soil moisture content in the root zone, permanent wilting point, water retention in the fine soil fraction at saturation, and the hydraulic conductivity of the soil at saturation were obtained also from RAB-Kayonza Station. These soil characteristics were verified in the field by myself where I conducted a soil profile study at each 2 m × 2 m sampled plots spaced at 1 km apart. Crop (maize) specific parameters were considered in water stress conditions [56]. Indeed, the 20 years-observed maize grain yield data were obtained both from the National Institute of Statistics of Rwanda (NISR) and the sector agronomists, for calibration and validation of the AquaCrop model (version 6.1) [57]. Nevertheless, I missed the observed biomass yield data in this institution and elsewhere in Rwanda. In addition, the model requires agronomic and land management input data (including land management features like mulching practices, irrigation, and water harvesting systems, among others), which were additionally recorded at the field for simulating maize biomass and grain yields, and C sequestration potential. The more a cropping system (either MnC or MxC) fixes atmospheric CO2, the more it substantially store much C, with high biomass and grain yields [58].
2.4.2.2. Agronomic and management input data for AquaCrop model
The AaquaCrop model requires plant, soil, and crop management-related parameters for running the simulation [59]. Those plant-related parameters fall under two groups. The first group pertains to those parameters affected by crop management, while the second group relates to cultivar-specific parameters. Maximum effective rooting depth is the parameter related to soil-crop characteristics. In this study, it included soil physical and chemical characteristics, such as rooting depth, the soil water content in the root zone, bulk density, soil porosity, gravel, soil texture, soil organic carbon, carbon in gravel (>2 mm size), total nitrogen, pH water, and soil horizon width [60]. These were obtained by profile study, where all soil parameters to run the model were generated. Land management characteristics, including crop management (i.e., soil fertility, fertilization, mulches, tillage practices, conservation measures, and rain-water harvesting) and irrigation types (whether farrow, sprinkle, or drip) [60]. Irrigation and rain-water harvesting systems were not considered (they were free from the landscape).
2.4.2.3. Model calibration and validation
The model was parameterized and tested for many crops [[61], [62], [63]], with various studies showing that the model is capable of simulating canopy cover (CC), biomass development and grain yield of different crops, and their respective cultivars, grown under varying conditions [64]. This leads to the establishment of crop-specific conservative parameters [56]. Thus, in this study, while retaining the crop-specific conservative parameters, the AquaCrop model was calibrated for the maize varieties grown in Kayonza District. Model performance was assessed by using the following statistical parameters: Nash-Sutcliffe efficiency index (NSE) (Equation (4)), mean absolute error (MAE) (Equation (5)), root mean square error normalized (RMSEN) (Equation (6)), and Willmott's index (d) (Equation (7)). Willmott's index (d) provides a single index of model performance that encompasses bias and variability. The closer the index value tends to the unity, the better the agreement between the two variables that are being compared and vice versa [65] cited by Ref. [62], while RMSEN and MAE were used to evaluate the model prediction errors. The RMSEN, MAE, d, and E were computed according to Refs. [56,66]:
| (4) |
| (5) |
| (6) |
The d-index was calculated as [65]:
| (7) |
where Oi is the observed yield value and O is the mean observed value of Oi, Si is the simulated yield value, while Mi is the measured value, M is the mean measured value of Mi and n represents the number of observations (n = 20). When NSE and d get closer to unity, and RMSEN and MAE approach zero, they represent positive indicators of model performance. The simulation is considered excellent if RMSEN is less than 10%; it is good if it comes between 10 and 20%; reasonable when it comes between 20 and 30%; and poor when it is greater than 30% [67,68]. The coefficient of efficiency (NSE) shows how much the overall deviation between observed and simulated values depart from the overall deviation between observed values (Oi) and their mean observed value (O). The value of NSE can range from negative infinity (–∞) to +1, and the model estimation efficiency increases as NSE gets closer to +1.
2.4.2.4. Determination of biomass and grain yield methods
According to Ref. [68], the relationship between biomass and grain yields is clarified in the AquaCrop model by using the original equation from the FAO I and D Paper No. 33 approach of Doorenbos and Kassam (1979) cited in Ref. [68] for calculating the crop biomass based on the amount of water transpired, and the crop yield as the proportion of biomass in harvested yield. Under the continuum between soil water, climate, and crop phenology through photosynthesis by intercepting solar radiation, AquaCrop estimates the relationship between biomass production and water consumed through transpiration. Reduced transpiration and water stress proportionally reduce yields [69]. AquaCrop model uses the equation here below to estimate crop biomass production directly from actual crop transpiration through a water productivity parameter [69], following Equation (8),
| (8) |
where B is the biomass produced cumulatively (kg. m−2), Tri is the crop transpiration (either mm or m3 per unit surface), with the summation (∑) locations (i …. n) in which the biomass is produced, and WP is the water productivity normalized at ETo and CO2 (either kg of biomass per m2 and mm, or kg of biomass per m3 of water transpired). For many crops, this biomass (B) is used to calculate yield (Y; kg. ha−1) thereby multiplying with harvest index (HI) using Equation (9),
| (9) |
3. Results and discussion
3.1. Determination of selected soil properties and SOC stocks in MnC and MxC systems
3.1.1. Effects of MnC and MxC systems on selected physical properties of soils
As depicted in Table 2 the bulk density showed no significant (p ≥ 0.05) differences by cropping systems and soil depths. Likewise, the interaction effect of the cropping system and soil depth on bulk density was not significant (Table 2). Across the two soil depths, however, the bulk density was increased from topsoil to subsoil: high values were recorded in the topsoil of mixed-cropped fields compared to that of mono-cropped fields (Table 1). The constancy of soil bulk density following the cropping system in the Rukara and Gahini sectors is due to minimum long-term disturbances by farmers, who were historically absent from the area [it was a buffer zone of the Akagera National Park encroached by farmers]. The fact of the bulk density increased from topsoil to subsoil is inherently harnessed by the long-term alluvial deposition from the mountainous parts of the country following the Nile River. These have been confirmed by Refs. [[70], [71], [72]] also reported no significant soil bulk density under different cropping systems that varied through depths. The results of a 2-year experiment conducted by Ref. [73], showed that the organic materials applied into the soil decreased bulk density and increased SOC. Besides, a significant (p < 0.05) higher clay content was recorded at the 30–60 cm soil depth of the two cropping systems, very high for monocropping, with r equals to 0.211 compared to mixed cropping systems, that it increased from topsoil to subsoil. In contrast, a higher sand content was measured in soils of the mixed cropping systems (45.5%) with no significant difference (p ≥ 0.05) across the two soil depths. However, the highest values were observed in the top layer of each of the two cropping systems. This increase in clay and silt contents in subsoil originated from the translocation process [70]. Accordingly, silt content was not significantly (p ≥ 0.05) higher in mixed cropping systems than in monocropping systems, and remained almost constant across the two depths. The volumetric water content (VWC) was not significantly (p ≥ 0.05) affected by both cropping systems and depths. However, it was relatively higher in MxC fields (42%) than that in monocropping (38%) and increased through depths with r = 0.072.
Table 2.
Two-way ANOVA for bulk density (g/cm³), clay (%) sand (%), silt (%), VWC (%) under different cropping systems.
| Source of Variation |
d.f |
BD |
Clay |
Sand |
Silt |
VWC |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | Sig | MS | Sig | MS | Sig | MS | Sig | MS | Sig | ||
| Cropping system | 1 | 0.00 | 0.66 | 160.42 | 0.04* | 109.40 | 0.06 | 4.87 | 0.59 | 227.05 | 0.49 |
| Depth Land use × Depth |
1 | 0.02 | 0.20 | 7.59 | 0.65 | 13.50 | 0.51 | 0.84 | 0.82 | 1162.04 | 0.12 |
| 1 | 0.00 | 0.97 | 0.00 | 1.00 | 0.04 | 0.97 | 0.03 | 0.96 | 16.69 | 0.85 |
MS = Mean square; Sig = sign or p-value; VWC = volumetric water content; * the mean difference is significant at the 0.05 level.
Table 1.
Soil physical properties by cropping systems and depths.
|
BD (%) |
Clay (%) |
Sand (%) |
Silt (%) |
VWC (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Depth (cm) |
Depth (cm) |
Depth (cm) |
Depth (cm) |
Depth (cm) |
|||||||||||
| CS | 0–30 | 30–60 | Overall | 0–30 | 30–60 | Overall | 0–30 | 30–60 | Overall | 0–30 | 30–60 | Overall | 0–30 | 30–60 | Overall |
| MxC | 1.13 | 1.16 | 1.14 | 27.8 | 28.4 | 28.1 | 45.9 | 45.1 | 45.5 | 26.3 | 26.5 | 26.4 | 38.5 | 45.9 | 42.2 |
| MnC | 1.12 | 1.14 | 1.13 | 31.1 | 31.7 | 31.4 | 43.1 | 42.4 | 42.8 | 25.8 | 25.9 | 25.8 | 35.7 | 40.9 | 38.3 |
CS = cropping system; MxC = mixed cropping; MnC = monocropping; BD = Bulk density; VWC = Volumetric water content.
3.1.2. Effects of MnC and MxC systems on selected chemical properties of soils
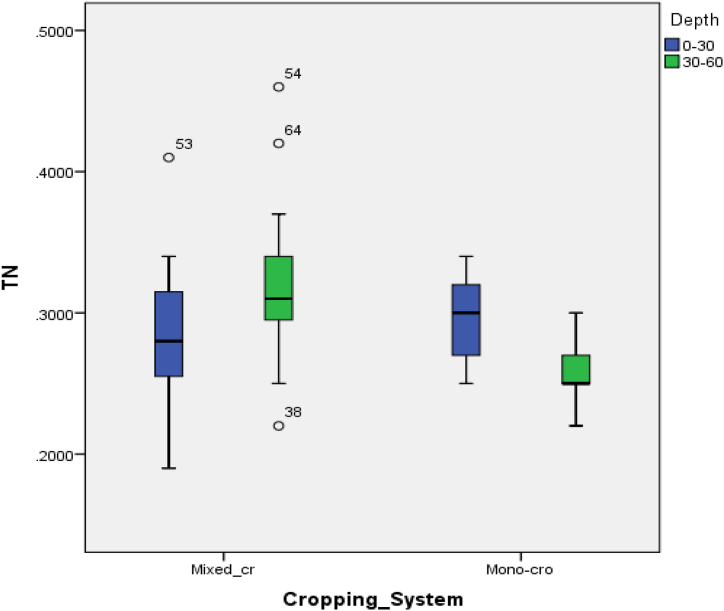
Table 3 shows that the highest soil pH values were observed in mixed-cropped fields compared to mono-cropped fields. By depths, a low pH value was found in top-soils (pHw = 5.61) of mono-cropped fields and pHw = 5.99 of mixed-cropped fields; and a low value of pHw = 5.25 was in sub-soils of mono-cropped fields compared to mixed cropped fields (pHw = 6.1). As indicated in Table 4, the soil pH was highly significantly (p < 0.001) influenced by the cropping system; but not by soil depths and their interaction (p ≥ 0.05). The highest soil pH values observed in mixed cropping (pH = 6.1) compared to that of mono-cropping systems (pH = 5.25) were also reported by Ref. [74]. The scholars intended to evaluate the effect of eight different cropping systems on selected soil chemical properties in lowland areas of Southeastern Nigeria, they found that mixed cropping systems significantly enhanced the soil pH higher than the sole/monocropping systems. This relative increase in pH could be related to the incorporated crop straw decayed products and organic manure [74], the availability of basic cations [71], and the release of OH− ions [73]. The organic substances not only revamp soil pH but also enhance its buffering capacity, hence improving soil acidification resistance and cation exchange capacity [75]. The low soil pH found in mono-cropped fields may be the intense weathering of parental materials, and organic and chemical fertilizers applied by farmers [72]. The lower soil pH could originate from applied mineral N (e.g., Urea, Di-ammonium phosphate (DAP), NPK17,17,17, etc) and mineral S fertilizers, which decomposed and release H+, NH4+, Al3++H+ (total acidity), Al3+ and Fe3+ contents into the soil solution, and hence the soil become acidic [[76], [77], [78]]. According to Ref. [79], the higher soil pH found in mixed-cropped fields was rated good while moderate (5.0–6.0) for mono-cropped fields. Moreover, decreasing pH values at pH < 5.5 [e.g., the pH = 5.25 of 30–60 cm depth of mono-cropping in this study] resulted in the increase in Mn and Zn in the soil [72]. Consequently, soil acidification could significantly be increased harnessing the intent of the Government of Rwanda of increasing the amount of applied chemical fertilizers from 32 kg ha−1yr−1 to 75 kg ha−1yr−1 by 2024 dominated in maize monocropping systems [80]. The SOC showed highly significant (p < 0.001) differences in means following cropping systems and depths; and significant (p < 0.05) differences in the interaction between cropping system and depths. The highest SOC values (SOC = 2.25%) were found in mixed cropped fields while the lowest SOC = 1.74% was in mono-cropped fields. Following soil depths, the topsoils generally pooled more carbon in both cropping systems, wherein mixed cropping outpaced monocropping by the rate of about 20% of the overall SOC; while mixed cropping accounted for over 82% of overall SOC on monocropping in sub-soils (Table 3) [74,81]. reported that mixed cropping systems of maize significantly revamped SOC and SOCS. The availability of SOC in the subsoil layers shows how these soil layers are substantial pools for the long-term preservation of SOC [26,71]. A 7-year experiment conducted by Ref. [82] reported an increment of 4% ± 1% in SOC in mixed crops over sole crops in the top 20 cm equal to sequestered C of 184 ± 86 kg C ha−1 yr−1. Mixed cropping systems have been included in strategies for revamping C pools and crop productivity previously suggested by Ref. [83]. The scholar stated that an increase of 1 ton of soil carbon pool of degraded cropland soils may increase crop yield by 10–20 kg. ha−1 for maize [83]. Moreover, mixed-cropped soils had shown to have higher organic C and other nutrients, microbial biomass, and enzyme activities than the mono-cropped soils which were associated with a decline in disease severity [84]. However, the ecological effects and biological functions of the crops in mixed cropping systems are still critical, since mixed cropping systems with higher yields do not inevitably reproduce better soil health [84]. For instance, mixed cropping systems reported a significant increase in total shoot biomass relative to sole cropping, using Lucerne (Medicago sativa) and cocksfoot (Dactylis glomerata), but the N2O production rate also increased, critically showing doubts on the nature of these intercropping designs for soil and environmental health maintenance [85]. The total Nitrogen (TN) showed a significant (p < 0.05) difference in means by cropping systems and depths; and a highly significant difference (p < 0.001) following the interaction (Table 4). A significant overall TN amount (0.3% N) was obtained in mixed cropped fields compared to 0.28% N in monocropped fields. By depths, a high value (0.3% N) was obtained in the topsoils of monocropping compared to mixed cropping systems (0.29% N); while 0.26%N was found in sub-soils of mono-cropped fields compared to the 0.32%N in the same layer of mixed cropped fields (Table 3). A substantially higher amount of TN was found in mixed-cropped fields and its top layer could be brought by organic matter originating from decayed products of intercrops [75]. reported about a 25% increment of TN from decayed straw products and manure. The strong positive correlation of the TN on the SOC (r = 0.72) and SOCS in the topsoils could be staked on the presence of organic matter and root biomass containing much N-fixing species mixed cropping with maize such as beans. As reported by Ref. [82], the increase of 11% ± 1% TN was greater in mixed cropping than in monocropping showing a difference in N sequestration of 45 ± 10 kg N ha−1 yr−1; and the 23% increment of the total root biomass in mixed crops over sole crops. However, the negative correlation of the TN with the BD (r = −0.04) and sand particles (r = −0.09) of topsoils may be due to denitrification followed by leaching of nitrogen to subsoils during rainy periods occurred after the mineralization process in drought conditions. This was confirmed by Radersma and Smit in their experimental research on fodder radish residues with or without paper pulp, their results 65% TN was lost due to denitrification and 70% losses due to N leaching [86]. Accordingly, [88] reported the effectiveness of mixed cropping systems on N leaching rather than monoculture in fields with N fertilizer over-dose. [89] pointed out that cover crops are prominent to reduce N leaching. Likely to clay which has r = −0.07, the poor correlation of TN on SOCS in 30–60 depth (r = −0.08) could be resulted in the complexation and adsorption of nitrogen on clay's reactive surfaces that inhibit it to avail in soil solution; and/or ingestion of organic C by soil fauna and flora for maintaining body mass and seeking energy and the certain amount lost in respiration as carbon dioxide (CO2). As was testified by Allred and his/her fellows' experimental results showed the rationale of clay's (kaolinite) anion adsorption for affecting NO3− mobility in low pH soils with limited amounts of organic matter [89]. In contrast, the overall C: N ratio showed highly significant (p < 0.001) values which were higher (7.5%) in mixed-cropped soils than in mono-cropped soils (6.1%). The depths elucidated highly significant (p < 0.01) C: N ratios (8.95%) in the top layer of mixed-cropped fields compared to 7.8% in the same layer of monocropped fields; whereas the subsoil layer accounted for highly significant (p < 0.01) C: N ratios (6.16%) in mixed-cropped soils and 4.4% in mono-cropped soils. These support C: N ratio having r = −0.2 in subsoils which decreased from the r = 0.0 of the top-soils in mixed cropping systems, whilst a similar positive decline in correlation was observed from r = 0.47 (0–30 cm) to r = 0.02 (30–60 cm) in monocropping systems. However, the C: N ratios were not significantly shown differences in means following the interaction between cropping systems and depths. These show that N leached amount was much lower in mixed cropped fields than in mono-cropped fields. This was confirmed by [90] and [91] that the C: N ratio reduced while feeding soil microorganisms through the immobilization process (loss of N in the form of a gas), in contrast, by the mineralization process (decomposition of SOCS for searching C for building a body and energy when the C: N is lesser than the natural carrying capacity of 24:1).
Table 3.
Selected soil chemical properties based on cropping systems and depths.
| pHw | SOC (%) | TN (%) | C: N ratio (%) | ||||||||
| Depth (cm) | Depth (cm) | Depth (cm) | Depth (cm) | ||||||||
| CS | 0–30 | 30–60 | 0–30 | 30–60 | Overall | 0–30 | 30–60 | Overall | 0–30 | 30–60 | Overall |
| MnC | 5.61 | 5.25 | 2.35 | 1.12 | 1.74 | 0.30 | 0.26 | 0.28 | 7.84 | 4.39 | 6.11 |
| MxC | 5.99 | 6.10 | 2.56 | 1.94 | 2.25 | 0.29 | 0.32 | 0.30 | 8.95 | 6.16 | 7.55 |
CS = cropping system, MxC = mixed cropping, MnC = monocropping; TN = total nitrogen; pHw = pH in water.
Table 4.
Two-way ANOVA for selected soil chemical properties and SOCS (tC. ha−1) under two cropping systems.
| Source of |
d.f |
pH |
SOC |
TN |
C: N |
SOCS |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variation | MS | Sig | MS | Sig | MS | Sig | MS | Sig | MS | Sig | |
| Cropping system | 1 | 5.58 | 0.00*** | 3.81 | 0.00*** | 0.0090 | 0.02* | 30.35 | 0.00*** | 3477.3 | 0.00*** |
| Depth | 1 | 0.01 | 0.89 | 12.83 | 0.00*** | 0.0075 | 0.03* | 203.12 | 0.00** | 9163.1 | 0.00*** |
| Land use × Depth | 1 | 0.78 | 0.18 | 1.39 | 0.02* | 0.0210 | 0.00*** | 1.59 | 0.39 | 1532.3 | 0.015* |
*The mean difference is at 0.05, ** at the 0.01, and *** at the 0.001 significance level, respectively.
Table 5 includes the adoption frequency indicates that the availability of monocropping and mixed cropping systems was differently, inherently, and unevenly distributed in farmlands of farmers due to drought conditions that frequently occurred in agricultural season B. The results indicate that mixed cropping systems were dominantly practiced at 81% whilst monocropping systems were adopted at 19% of total maize field accounts. These show the preference of the majority of farmers in the area.
Table 5.
Frequency, and some selected soil physico-chemical properties about different cropping systems through soil depths (mean ± SE).
| Parameter | Land Use | Frequency (Frequency, %) | Sample size (N) | Mean |
Std Deviation |
|---|---|---|---|---|---|
| BD (g/cm3) | MxC | 78 (81) | 96 (100) | 1.14 (±0.01) | 0.101 |
| MnC | 18 (19) | 96 (100) | 1.13 (±0.02) | 0.076 | |
| Clay (%) | MxC | 78 (81) | 96 (100) | 28.08 (±0.73)c | 6.442 |
| MnC | 18 (19) | 96 (100) | 31.39 (±0.89)c | 3.775 | |
| Sand (%) | MxC | 78 (81) | 96 (100) | 45.51 (±0.66) | 5.846 |
| MnC | 18 (19) | 96 (100) | 42.78 (±0.85) | 3.606 | |
| Silt (%) | MxC | 78 (81) | 96 (100) | 26.41 (±0.47) | 4.157 |
| MnC | 18 (19) | 96 (100) | 25.83 (±0.78) | 3.294 | |
| pH (H2O) | MxC | 78 (81) | 96 (100) | 6.05 (±0.07)a | 0.652 |
| MnC | 18 (19) | 96 (100) | 5.43 (±0.15)a | 0.621 | |
| SOC (%) | MxC | 78 (81) | 96 (100) | 2.25 (±0.07)b | 0.582 |
| MnC | 18 (19) | 96 (100) | 1.74 (±0.18)b | 0.779 | |
| TN (%) | MxC | 78 (81) | 96 (100) | 0.31 (±0.005)c | 0.045 |
| MnC | 18 (19) | 96 (100) | 0.28 (±0.008)c | 0.036 | |
| C: N | MxC | 78 (81) | 96 (100) | 7.55 (±0.23)d | 2.018 |
| MnC | 18 (19) | 96 (100) | 6.11 (±0.53)d | 2.247 | |
| VWC (%) | MxC | 78 (81) | 96 (100) | 42.22 (±2.48) | 21.881 |
| MnC | 18 (19) | 96 (100) | 38.28 (±4.89) | 20.742 |
BD = bulk density; TN = total nitrogen; SOC = soil organic carbon; C: N = carbon-nitrogen ratio; SOCS = Soil organic carbon stock; VWC = volumetric water content; MxC = mixed cropping; MnC = mono-cropping. For each parameter, different letters indicate significant differences (p < 0.05) for cropping systems at each depth, respectively.
3.2. Determination of soil organic carbon stocks and density under MnC and MxC systems
Table 5 indicated that the SOC Stocks were significantly (p < 0.001) high in mixed cropped fields (SOCS = 74.8 tC ha−1), and tend to closely and consistently increase at SD = 19.6 tC ha−1 compared to mono-cropped fields. As was shown also in Table 6, this highly significantly different (p < 0.001) higher SOC stock amount was accounted for in the MxC fields than in mono-cropped fields. By depths, 0–30 cm depth showed higher SOC stocks than it was at the 30–60 cm depth in all cropping systems (Table 6). Shifting from mixed cropping to monocropping has led to 41.2% of the mean SOC stock losses. The mean SOC stock loss between two cropping systems (20.7 tC. ha−1) resulted from the division of the deviation sum of changes in SOC stock of monocropping systems (14.8 tC. ha−1) with mixed cropping systems (35.5 tC. ha-1) stated in Table 6. This loss may be associated with seasonal tillage practices in monocropping without fallowing; which are different from mixed cropping where intercrops act as fallow and re-fund C that might be lost through the sequestration process. Throughout the soil profile (0–60 cm depth), the soil organic carbon stock density (SOCSD) was higher in mixed-cropped soils than in mono-cropped soils with a difference of 30.9 tC. ha−1 pedogenic C stock (Table 6).
Table 6.
SOCS and SOCSD (Mean ± S.E) concerning cropping systems and soil depths.
| Frequency (Frequency in%) |
Depth |
SOCS |
SOCSD at 60 cm-depth |
F |
P-value |
|
|---|---|---|---|---|---|---|
| Cropping system | (Composite samples = 96) | (cm) | (tC. ha−1 | (tC. ha−1) | ||
| MxC system | 9 (9.4) | 0–30 | 74.8 (±2.5) | – | – | – |
| 9 (9.4) | 30–60 | 60.0 (±2.5) | 134.9 (±4.6) | – | – | |
| MnC system | 39 (40.6) | 0–30 | 69.6 (±5.3) | – | – | – |
| 39 (40.6) | 30–60 | 34.4 (±5.3) | 104.0 (±9.7) | – | – | |
| SOCD (tC. ha−1) * CS | ANOVA between MnC and MxC combined | 8.456 | 0.006** | |||
Source: Own computations from primary data collected in 2021; CS: cropping system; S.E: standard error
3.3. Variance and correlation analyses for selected soil properties and SOC stocks
The SOCS showed large variations in mono-cropped fields (CV = 45.8%) with little (CV = 25.8%) variations in mixed-cropped fields. The soil organic stock was negatively skewed (rsk = −0.55) and positively enriched (rkt = 0.002) in mixed cropped fields whilst it was positively skewed at rsk = 0.26, and left-tailed and concentrated (rkt = −1.4) in mono-cropped fields (Table 7). As elucidated in Table 8 and Table 9 of the two-way ANOVA, the mean difference of soil pHw, SOC, SOCS, and C: N ratio was highly significantly (p < 0.01) influenced by CS systems at r = 0.353, r = 0.309 and r = 0.383, respectively; whilst the TN was significant at p < 0.05, with r = 0.218 (Appendix 7). The TN was positively correlated with silt (r = 0.24), C: N ratio (r = 0.0), pHw (r = 0.02), SOC (r = 0.72), and SOCS (r = 0.19) in top soils, and with BD (r = 0.07), silt (r = 0.32), and SOC (r = 0.26) in subsoils in MxC systems (Table 8). Meanwhile, the TN was positively linked to C: N ratio (r = 0.47), pHw (r = 0.54), silt (r = 0.29), SOC (r = 0.86), and SOCS (r = 0.46) in top soils, and with C: N ratio (r = 0.02), clay (r = 0.33), pHw (r = 0.23), SOC (r = 0.2), and SOCS (r = 0.18) in sub-soils of monocropping systems (Table 9). This soil property showed a positive influence on the SOCS in the top soils, and with a weak positive correlation (r = 0.18) in sub-soil under mono-cropped fields, which is lower than that was found in mixed cropped fields (r = 0.3) (Table 6). The TN was negatively interlinked with top soils' BD (r = −0.04), clay (r = −0.07), and sand particles (r = −0.09), and the C: N ratio (r = −0.2) of subsoils, clay (r = −0.18), pHw (r = −0.03), and sand particles (r = −0.04). It generally showed a strong positive correlation with topsoil SOC and silt, and tends to decrease SOCS towards the subsoil layer in two cropping systems; while showing a strong negative correlation with C: N ratio (r = −0.2) and clay (r = −0.18) in mixed cropping systems (Table 8). In these cropping systems, the TN content significantly increases with the increase in depths unlikely to mono-cropped fields where it decreases with the decline in depths (Appendix 2). The C: N ratio was highly significantly influenced by cropping systems, and significantly (p < 0.05) soil depths. The interaction between cropping systems and depths significantly (p < 0.01) enhances the C pool into soils, except for the C: N ratio (p ≥ 0.05) (Table 4). A higher significant C stock loss was found in monocropped fields compared to mixed cropped fields (Appendix 1). Through these two depths, C: N ratio showed almost a slight decline trend downward under both cropping systems (Appendix 2). This ratio showed a highly significant negative correlation (r = −0.34**) with the subsoil's BD and no significant negative correlation (r = −0.32) to the topsoil's BD in mixed cropped fields (Table 8) as did to monocropped fields (Table 8). The C: N ratio presented a highly significant correlation (r = 0.68**) with the topsoil's SOC, and a positive one with the subsoil's SOC and the SOCS in the top soils (r = 0.5) and subsoils (r = 0.4). The SOCS was highly significantly (p < 0.001) influenced by cropping systems and soil depths as well as the interaction (p < 0.01) (Table 4). The property showed a significant deviation from mixed-cropped to mono-cropped soils (mean diff. = 15.4***) (Appendix 7). The subsoil C: N ratio showed a highly significant positive correlation with the SOC stocks in the two cropping systems [with r = 0.83** in mixed-cropped fields, and r = 0.91** in mono-cropped fields]. The SOC showed a strong positive correlation with SOCS. This correlation increases through depths in contrast to that found in mixed-cropped fields where it decreases with depths (i.e., the topsoil layer's correlation is higher than that found in the sub-soil layer) (Table 8). In the top layer of mixed-cropped fields, the SOCS were highly positively interlinked to the C: N ratio (r = 0.41), clay (r = 0.23), silt (r = 0.39*), SOC (r = 0.18), and SOC (r = 0.42**) whilst the BD, with r = −0.48** in top-soils and r = −0.34* in sub-soils, and the sand contents (r = −0.33*) deposited in sub-soils strongly showed a significant negative influence with the SOC stocks (Table 8). In addition, the SOC and C: N showed a higher positive correlation with SOC stocks in mixed cropped fields. The presence of clay contents positively significantly (r = 0.38*) influenced SOCS in sub-soils of mixed cropping systems (Table 8). This may have resulted in the cheluviation of organic C held on the clay reactive surfaces to form a clay-organic C complex (e.g., halloysite chelates organic compounds into the soil). This has been supported by [92] who showed that clay minerals like kaolinite and halloysite intercalate with polar organic compounds to form an interlayer 1:1 layer silicate complex. The sand contents showed a highly significant negative correlation (−0.04**) to volumetric water content in the top soils of mixed cropping systems. This may be due to very low holding capacity and lack of adhesive forces to hold water molecules in their inherent chemical structures. Indeed, sand contents were positively significantly linked to SOCS in the top soils and negative ones in the sub-soils of mixed cropping (MxC) systems, respectively (Table 7).
Table 7.
Statistics of the SOC stocks for MnC versus MxC systems.
| Soil organic carbon stocks (tC. ha−1) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cropping System | Min | Max | Mean | Median | Range | rsk | rkt | SD | CV (%) |
| Mixed cropping | 23.2 | 103.9 | 67.4 | 71.1 | 80.7 | −0.5 | 0.0 | 17.4 | 25.8 |
| Mono-cropping | 20.8 | 91.8 | 52.0 | 49.4 | 71.0 | −0.3 | −1.4 | 23.8 | 45.8 |
SD = standard deviation; rsk. = Coefficient of Skewness, rkt = Coefficient of Kurtosis; CV = Coefficient of variation.
Table 8.
Pearson correlation analysis results of soil physico-chemical properties for the MxC system.
| Depth (cm) | BD | C: N | Clay | pHw | Sand | Silt | SOC | SOCS | TN | VWC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | 0–30 | 1 | |||||||||
| 30–60 | 1 | ||||||||||
| C: N | 0–30 | −0.32 | 1 | ||||||||
| 30–60 | −0.34** | 1 | |||||||||
| Clay | 0–30 | −0.10 | −0.1 | 1 | |||||||
| 30–60 | −0.11 | 0.3 | 1 | ||||||||
| pHw | 0–30 | 0.08 | 0.2 | 0.07 | 1 | ||||||
| 30–60 | 0.14 | 0.0 | 0.06 | 1 | |||||||
| Sand | 0–30 | 0.17 | −0.2 | −0.79** | −0.13 | 1 | |||||
| 30–60 | 0.20 | −0.2 | −0.76** | −0.06 | 1 | ||||||
| Silt | 0–30 | −0.08 | 0.44** | −0.49** | 0.07 | −0.16 | 1 | ||||
| 30–60 | −0.12 | −0.1 | −0.45** | 0.00 | −0.25 | 1 | |||||
| SOC | 0–30 | −0.20 | 0.68** | −0.16 | 0.15 | −0.17 | 0.49 | 1** | |||
| 30–60 | −0.30 | 0.9 | 0.19 | −0.02 | −0.24 | 0.06 | 1 | ||||
| SOCS | 0–30 | −0.48** | 0.41* | 0.23 | 0.01 | 0.39* | 0.18 | 0.42** | 1 | ||
| 30–60 | −0.34* | 0.83** | 0.38* | 0.1 | −0.33* | −0.12 | 0.81** | 1 | |||
| TN | 0–30 | −0.04 | 0.0 | −0.07 | 0.02 | −0.09 | 0.24 | 0.72 | 0.19 | 1 | |
| 30–60 | 0.07 | −0.2 | −0.18 | −0.03 | −0.04 | 0.32 | 0.26 | −0.08 | 1 | ||
| VWC | 0–30 | 0.09 | −0.1 | 0.07 | −0.37 | −0.04** | −0.06 | 0.05 | 0.00 | 0.14 | 1** |
| 30–60 | −0.04 | −0.1 | 0.12 | −0.34 | −0.04 | −0.12 | 0.06 | 0.07 | 0.22 | 1 |
*Correlation is significant at the 0.05 level (2-tailed); **. Correlation is significant at the 0.01 level (2-tailed).
Table 9.
Pearson correlation analysis results of soil physico-chemical properties for MnC system.
| Depth (cm) | BD | C: N | Clay | pHw | Sand | Silt | SOC | SOCS | TN | VWC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | 0–30 | 1 | |||||||||
| 30–60 | 1 | ||||||||||
| C: N | 0–30 | −0.73* | 1 | ||||||||
| 30–60 | −0.53 | 1 | |||||||||
| Clay | 0–30 | 0.11 | 0.06 | 1 | |||||||
| 30–60 | 0.16 | −0.04 | 1 | ||||||||
| pHw | 0–30 | −0.38 | 0.39 | −0.58 | 1 | ||||||
| 30–60 | −0.19 | 0.18 | −0.60 | 1 | |||||||
| Sand | 0–30 | −0.41 | 0.32 | −0.65 | 0.23 | 1 | |||||
| 30–60 | −0.54 | 0.04 | −0.55 | 0.03 | 1 | ||||||
| Silt | 0–30 | 0.35 | −0.46 | −0.45 | 0.44 | −0.38 | 1 | ||||
| 30–60 | 0.38 | 0.01 | −0.52 | 0.62 | −0.43 | 1 | |||||
| SOC | 0–30 | −0.7* | 0.85** | −0.10 | 0.54 | 0.17 | −0.08 | 1* | |||
| 30–60 | −0.51 | 0.98** | 0.03 | 0.19 | −0.03 | 0.00 | 1 | ||||
| SOCS | 0–30 | −0.3 | 0.62 | −0.01 | −0.04 | 0.44 | −0.5 | 0.64 | 1* | ||
| 30–60 | −0.38 | 0.91** | 0.1 | −0.00 | 0.11 | −0.22 | 0.93 | 1** | |||
| TN | 0–30 | −0.45 | 0.47 | −0.17 | 0.54 | −0.07 | 0.29 | 0.86 | 0.46 | 1 | |
| 30–60 | −0.02 | 0.02 | 0.33 | 0.23 | −0.33 | −0.02 | 0.20 | 0.18 | 1 | ||
| VWC | 0–30 | 0.12 | 0.30 | −0.35 | 0.26 | 0.51 | −0.17 | 0.06 | 0.3 | −0.20 | 1 |
| 30–60 | 0.04 | −0.27 | −0.16 | 0.11 | 0.39 | −0.22 | −0.31 | −0.19 | −0.14 | 1 |
*. Correlation is significant at the 0.05 level (2-tailed); **. Correlation is significant at the 0.01 level (2-tailed).
3.4. Simulation of maize biomass and grain yields in MnC compared to MxC systems in the past 20 years (2001─2021) in Kayonza district
3.4.1. Perspectives on a changing climate in the Eastern Province of Rwanda
Fig. 2 shows the variations of precipitations and temperatures in Eastern Province [93]. The province receives a mean annual precipitation of between 700 mm and 1100 mm, herewith a mean annual temperature ranging between 20 °C and 22 °C [93]. From 1961 to 2016, the temperature increase has been shown to rise between 1.4 °C and 2.6 °C in the province. Besides, Rwanda experienced a significant decline in rainfall due to the impacts of the El Niño Southern Oscillation; hence, the eastern regions were particularly stymied by dry spells [93]. The Ministry of Foreign Affairs of the Netherlands (MinFAN) and Rwanda Meteorology Agency (RMA) reported the eastern regions of Rwanda will experience frequent rainfall deficits in the coming years harnessing the results of average annual rainfall prediction models from 2000 to 2050 [23].
Fig. 2.
Climate change observed in the Eastern Province from 1901 to 2021.
3.4.2. AquaCrop model performance indicators
These model performance indicators were solely employed on maize grain yields [due to a lack of observed biomass yields in Rwanda which required me to conduct a study for the entire 20 years period from the year 2021]. However, the performance of the model on grain yields shaped my insights on biomass yields. As indicated in Table 10, the statistical indicators for the performance of the model, such as MAE, RMSE, RMSEN, NSE, and d were assessed. The MAE was 29%, and RMSE was 28% in all cropping systems; RMSEN was 8.9% in MnC systems and 7.7% in mixed cropping systems. Accordingly, the NSE was −0.88 in MnC while −0.14 in mixed cropping systems. The Willmott's index (d) was respectively 71.5% in monocropping systems whilst 74% in mixed cropping systems. This testifies to the excellent performance of the AquaCrop model (version 6.1) used in this study. As this index shows the model performance that involves bias and variability, it is consistent and tends to unity. Thus, there is a better agreement between observed and simulated yields.
Table 10.
Statistical indicators for AquaCrop model performance.
| Index | MnC | MxC |
| MAE | 0.290 | 0.290 |
| RMSE | 0.284 | 0.284 |
| RMSEN | 0.089 | 0.077 |
| NSE | −0.880 | −0.138 |
| d | 0.715 | 0.743 |
3.4.3. Simulated biomass and grain yield for 20 years (2001–2021) in the study area
Fig. 3 shows that the biomass and grain yield from mixed cropping systems were highly significantly (p < 0.001) higher than the yields from monocropping systems in the entire 20-year tenure (Appendix 8). The mean biomass of the mixed cropping systems averagely reached 6.492 t ha-1 loaded at (rkt = 2.9; rsk = −1.5) in; whereas that of monocropping systems yielded 5.565 t ha-1 with a varying trend at (rkt = 0.9; rsk = −0.6). The mean grain yield was 3.227 t ha-1 that accrued at rkt = 3.7 and rsk = −1.8 in mixed cropping systems, whilst that of monocropping systems declined to 2.756 t ha-1 (rkt = 2.1; rsk = −1.2) respectively at 95% confidence interval. The results of this study showed large variation in biomass and grain yields of monocropping systems; whilst the stable biomass and grain yields were found in mixed cropping systems.
Fig. 3.
Changes in simulated biomass and grain yields in MnC and MxC systems (2001–21).
The assessment of long-term experiments of pure mixed cropping systems in comparison with monocropping systems on biomass and grain yield trend, stability, and sustainability is unclear. As an intercropping sub-set, harnessing to its definition [36], the results of this study seem to be that of [94] who found that maize-mucuna and maize-cowpea intercropping significantly increased forage and grain yields compared to that of sole maize cropping system in the 3-years-experimental study in Murehwa, Zimbabwe. These results also were supported by the results of [95] who found that soja-bean-mixed cropping increased maize grain yield by 22% and stable year-to-year greater than in maize monocultures, in the study conducted in 4 long-term (10−16 years) experiments. And the results of [96] pointed out the larger maize cobs and increased yields though grains had a small size in simultaneously mixed cropping of two bean lines compared to the monocropping system. However, this result differs from that of [97] showed the highest grain yield in monocropping compared to that of the maize-beans-mixture. The overall yield of the potato-maize mixture was greater than that of monocropping in Tigray, however, the sole maize cropping grain yield was higher than the yield in mixed cropping [98]. As indicated in the research of [99] the drought declined the sole maize grain yield under fertilized conditions, but the yield had not reduced in the pigeon-pea-maize mixture. The latter outperformed maize-monocropping in terms of protein content. Hence, the increase in biomass and grain yields in mixed cropping systems suggests that this cropping system sequesters many C and N contents and stores them into maize straws and grains as well as into other mixed residues compared to the monocropping system. However, there is limited literature on N content dynamics and the C: N ratio in maize grain yield of monocropping compared to mixed cropping systems under drought conditions. This has substantial impacts on food nutritious contents that need to be addressed under a changing climate.
3.4.4. Simple linear regression of rainfall with simulated biomass and grain yields in MnC and MxC systems for the past 20 years (2001–2021)
Fig. 4, Fig. 5 show the simple linear regression of rainfall with simulated maize biomass and grain yields in monocropping against mixed cropping systems for the past 20 years (2001–2021). A regressed rainfall showed a higher positive slope of simulated maize biomass yields in mixed cropping than the positive slope of simulated maize biomass yields in monocropping systems, respectively (Fig. 4); and a higher positive slope of simulated maize grain yield in mixed cropping systems than the positive one of simulated maize grain yield in monocropping systems (Fig. 5).
Fig. 4.
Regression of rainfall with maize biomass yield in MnC and MxC systems for the past 20 years (2001–2021).
Fig. 5.
Regression of Rainfall with maize grain yield of MnC and MxC system for the past 20 years (2001–2021).
On the other hand, the regressed annual mean temperature with maize biomass yields showed a negative slope in mixed cropping systems; and a negative slope with the maize grain yields in monocropping and mixed cropping systems, respectively.
4. Conclusion
Climate change has been clarified as a major global environmental threat that may jeopardize food and nutrition security, socio-economic human activities and other civilization sectors, biodiversity, and different ecosystems. In Rwanda, the monocropping system was criticized to do not incorporate environmental sustainability settings into its core and fails to address climate change impacts and food insecurity. As an indigenous option, the mixed cropping system was also criticized to produce poor sole crop yield, but many studies confirmed that it sustainably increases diverse yields and income. As it is shown by the results of this study, the selected soil chemical properties showed that the mixed cropping systems highly significantly increased soil pH, SOC, TN, C: N ratio, and SOC stocks compared to the monocropping systems. Mixed cropping had more C sequestration and CO2-mitigation potentials than monocropping as it stored much C amount in the soil. This resulted in improving soil health and quality under the mixed cropping systems. Again, mixed cropping systems showed a highly significant increase in maize biomass and grain yields than monocropping systems. It is; therefore, mixed cropping systems have proven to be more sustainable, stable, and beneficial to smallholder farmers than monocropping systems in the face of drought hazards in the Kayonza district. Yet, there are limited studies on N-biochemistry dynamics and concentrations in soil pools, maize biomass, and grain yields in mixed cropping versus monocropping systems. Long-term experimental studies on the comparative analysis of mixed cropping systems (or indigenous intercropping) and modern farming technologies under a changing climate are still scant. Therefore, long-term experimental studies on the C: N ratio should also be conducted to analyze and compare mixed cropping systems with modern farming technologies on C and N pools in soils, biomass and grain yields, to solve the problem of food nutritional imbalance caused by drought hazards and rising temperature. Environmentally sound, as C pools were monitored and analyzed in the two cropping systems in this study, N-biochemistry dynamics should also be conducted thereby comparing its environmental pools and impacts to both below-and above-ground ecotones.
Author contributions
Hashakimana Léonidas conceived and designed the experiments, performed the experiments, soil surveying, availed reagents, materials, performed laboratory analysis and avail the data; analyzed and interpreted the data, and wrote the paper. Tessema Toru conceived and designed the experiments; supported during data analysis and interpretation; and contributed to the write-up of the paper. Niyitanga Fidel contributed to the field data collection and the write-up of the paper. Cyamweshi Rusanganwa Athanase contributed to the data analysis and interpretation. Mukuralinda Athanase provided soil sampling tools and reagents for laboratory analysis.
Funding
We highly recognize the funding provided by the Africa Center of Excellence for Climate Smart Agriculture and Biodiversity Conservation (Funding number: P151847) at Haramaya University, Ethiopia from the World Bank, United States (Funding Number: IDA 57940). We also immensely acknowledge the financial support provided by PHARMAPHILOS Ltd, Rwanda.
Declaration of competing interest
There are no potential competing interests with co-authors, the funder, and somebody else on this paper.
Acknowledgments
We greatly acknowledge the technical support from the Rwanda Agriculture and Animal Resources Development Board (RAB) and the CIFOR-ICRAF Rwanda Country Directorate. We highly recognize the service provided by the officials of Kayonza District and its lower local government authorities to us for data collection during the semi-lockdown of the COVID-19 pandemic.
APPENDICES
Appendix 1. Changes of SOCS in cropping systems and depths
Appendix 2. Changes of SOCS in MnC and MxC systems concerning depths
Appendix 3. Changes of TN in cropping systems throughout depths
Appendix 4. Descriptive statistics of SOCS (tC/ha) under the effects of MnC and MxC systems
| Cropping_system | Depth | Mean | Std. Deviation |
|---|---|---|---|
| Mixed_cropping | 0–30 | 74.83 | 13.66 |
| 30–60 | 60.03 | 17.67 | |
| Total | 67.43 | 17.37 | |
| Mono-cropping | 0–30 | 69.64 | 16.313 |
| 30–60 | 34.38 | 15.57 | |
| Total | 52.01 | 23.84 | |
| Total | 0–30 | 73.86 | 14.15 |
| 30–60 | 55.22 | 19.91 | |
| Total | 64.54 | 19.57 |
Appendix 5. Two-way ANOVA of SOCS (tC/ha) under the presence of MnC and MxC systems
| Source of variation | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 13342.466a | 3 | 4447.489 | 17.768 | .000 |
| Intercept | 208645.556 | 1 | 208645.556 | 833.548 | .000 |
| Cropping_system | 3477.326 | 1 | 3477.326 | 13.892 | .000 |
| Depth | 9163.139 | 1 | 9163.139 | 36.607 | .000 |
| Cropping_system * Depth | 1532.314 | 1 | 1532.314 | 6.122 | .015 |
| Error | 23028.543 | 92 | 250.310 | ||
| Total | 436245.360 | 96 | |||
| Corrected Total | 36371.010 | 95 |
a. R Squared = 0.367 (Adjusted R Squared = 0.346) at 0.05; Sig = p-value; df = Degrees of freedom; F F-value.
Appendix 6. Estimated means and pairwise comparisons of cropping systems
|
Estimated marginal means |
Pairwise comparisons |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CS | Mean( ± SE) | L.B | U. B | Diff. in CS | Mean Diff. | Sig. | L.B | U. B | |
| BD | MxC | 1.14 (±0.01) | 1.12 | 1.17 | MxC-MnC | 0.01 | 0.66 | −0.04 | 0.06 |
| MnC | 1.13 (±0.02) | 1.09 | 1.18 | MnC-MxC | −0.01 | 0.66 | −0.06 | 0.04 | |
| C: N | MxC | 7.55 (±0.165) | 7.23 | 7.88 | MxC-MnC | 1.44* | 0.00 | 0.69 | 2.20 |
| MnC | 6.11 (±0.343) | 5.43 | 6.79 | MnC-MxC | −1.44* | 0.00 | −2.20 | −0.69 | |
| Clay | MxC | 28.08 (±0.69) | 26.70 | 29.45 | MxC-MnC | 3.31* | 0.04 | 0.14 | 6.48 |
| MnC | 31.389 (±1.44) | 28.53 | 34.25 | MnC-MxC | −3.31* | 0.04 | −6.48 | −0.14 | |
| pHw | MxC | 6.05 (±0.07) | 5.90 | 6.19 | MnC-MxC | −0.62* | 0.00 | −0.95 | −0.28 |
| MnC | 5.43 (±0.15) | 5.13 | 5.73 | MxC-MnC | 0.62* | 0.00 | 0.28 | 0.95 | |
| Sand | MxC | 45.51 (±0.63) | 44.26 | 46.76 | MnC-MxC | −2.74 | 0.06 | −5.62 | 0.15 |
| MnC | 42.78 (±1.31) | 40.18 | 45.38 | MxC-MnC | 2.74 | 0.06 | −0.15 | 5.62 | |
| Silt | MxC | 26.41 (±0.46) | 25.50 | 27.32 | MnC-MxC | −0.58 | 0.59 | −2.68 | 1.53 |
| MnC | 25.83 (±0.96) | 23.93 | 27.73 | MxC-MnC | 0.58 | 0.59 | −1.53 | 2.68 | |
| SOC | MxC | 2.25 (±0.06) | 2.14 | 2.36 | MnC-MxC | −0.51* | 0.00 | −0.77 | −0.26 |
| MnC | 1.74 (±0.12) | 1.51 | 1.97 | MxC-MnC | 0.51* | 0.00 | 0.26 | 0.77 | |
| SOCS | MxC | 67.43 (±1.9) | 63.52 | 71.35 | MnC-MxC | −15.42 | 0.00 | −23.75 | −7.09 |
| MnC | 52.01 (±5.6) | 40.15 | 63.87 | MxC-MnC | 15.42 | 0.00 | 7.09 | 23.75 | |
| TN | MxC | 0.30 (±0.005) | 0.29 | 0.31 | MnC-MxC | −0.03* | 0.02 | −0.05 | 0.00 |
| MnC | 0.28 (±0.009) | 0.26 | 0.30 | MxC-MnC | 0.03* | 0.02 | 0.00 | 0.05 | |
| VWC | MxC | 42.22 (±2.45) | 37.36 | 47.08 | MnC-MxC | −3.94 | 0.49 | −15.17 | 7.29 |
| MnC | 38.28 (±5.1) | 28.16 | 48.40 | MxC-MnC | 3.94 | 0.49 | −7.29 | 15.17 | |
CS= Cropping system, MnC = mono-cropping system, MxC = mixed cropping system, SE = standard error of means, L.B = lower bound, UB = upper bound.
Based on estimated marginal means: *. The mean difference is significant at the 0.05 level.
b. Adjustment for multiple comparisons: Bonferroni.
Appendix 7. Tests of normality of unseparated means in two cropping systems
| CS | Kolmogorov-Smirnova |
Shapiro-Wilk |
|||||
|---|---|---|---|---|---|---|---|
| Statistic | d.f | Sig. | Statistic | d.f | Sig. | ||
| BD | MxC | 0.157 | 78 | 0.000 | 0.918 | 78 | 0.000 |
| MnC | 0.136 | 18 | 0.200* | 0.952 | 18 | 0.459 | |
| C: N | MxC | 0.053 | 78 | 0.200* | 0.992 | 78 | 0.891 |
| MnC | 0.163 | 18 | 0.200* | 0.890 | 18 | 0.039 | |
| Clay | MxC | 0.083 | 78 | 0.200* | 0.965 | 78 | 0.029 |
| MnC | 0.200 | 18 | 0.056 | 0.895 | 18 | 0.046 | |
| pHw | MxC | 0.075 | 78 | 0.200* | 0.975 | 78 | 0.122 |
| MnC | 0.149 | 18 | 0.200* | 0.926 | 18 | 0.166 | |
| Sand | MxC | 0.095 | 78 | 0.079 | 0.974 | 78 | 0.110 |
| MnC | 0.304 | 18 | 0.000 | 0.696 | 18 | 0.000 | |
| Silt | MxC | 0.123 | 78 | 0.005 | 0.970 | 78 | 0.066 |
| MnC | 0.156 | 18 | 0.200* | 0.920 | 18 | 0.132 | |
| SOC | MxC | 0.060 | 78 | 0.200* | 0.988 | 78 | 0.676 |
| MnC | 0.194 | 18 | 0.073 | 0.910 | 18 | 0.086 | |
| SOCS | MxC | 0.093 | 78 | 0.094 | 0.967 | 78 | 0.042 |
| MnC | 0.166 | 18 | 0.200* | 0.918 | 18 | 0.119 | |
| TN | MxC | 0.082 | 78 | 0.200* | 0.966 | 78 | 0.035 |
| MnC | 0.191 | 18 | 0.080 | 0.936 | 18 | 0.249 | |
| VWC | MxC | 0.173 | 78 | 0.000 | 0.885 | 78 | 0.000 |
| MnC | 0.181 | 18 | 0.124 | 0.903 | 18 | 0.065 | |
*. This is a lower bound of the true significance.
a. Lilliefors significance correction.
CSs = cropping systems, MnC = mono-cropping system, MxC = mixed cropping system.
| Contrast-test |
F-test |
Measures of correlation |
Levene's Test of Equality of error variances |
Model fit |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | F | Sig. | R | R2 | F | df1 | df2 | Sig. | R2 | Adj.R2 | |
| BD | 0.995 | 0.321 | 0.201 | 0.655 | 0.046 | 0.002 | 0.876 | 3 | 92 | 0.456 | 0.02 | −0.012 |
| C: N | 67.047 | 0.000 | 14.338 | 0.000 | 0.266 | 0.071 | 1.047 | 3 | 92 | 0.376 | 0.547 | 0.532 |
| Clay | 0.123 | 0.727 | 4.303 | 0.041 | 0.211 | 0.045 | 1.047 | 3 | 92 | 0.376 | 0.047 | 0.016 |
| pH(H2O) | 0.553 | 0.459 | 13.349 | 0.000 | 0.353 | 0.124 | 1.028 | 3 | 92 | 0.384 | 0.142 | 0.114 |
| Sand | 0.244 | 0.622 | 0.244 | 0.622 | 0.192 | 0.037 | 2.418 | 3 | 92 | 0.071 | 0.042 | 0.01 |
| Silt | 0.296 | 0.588 | 0.296 | 0.588 | 0.057 | 0.003 | 0.47 | 3 | 92 | 0.704 | 0.004 | −0.029 |
| SOC | 51.716 | 0.000 | 15.805 | 0.000 | 0.308 | 0.095 | 0.082 | 3 | 92 | 0.97 | 0.448 | 0.430 |
| SOCS | 13.892 | 0.000 | 9.937 | 0.002 | 0.309 | 0.096 | 1.293 | 3 | 92 | 0.281 | 0.367 | 0.346 |
| TN | 0.302 | 0.584 | 5.477 | 0.021 | 0.218 | 0.048 | 0.900 | 3 | 92 | 0.445 | 0.200 | 0.173 |
| VWC | 1.238 | 0.269 | 0.486 | 0.488 | 0.072 | 0.005 | 0.424 | 3 | 92 | 0.737 | 0.032 | 0.000 |
Appendix 8. Maize biomass and grain yields (t.ha−1) in MnC and MxC systems in 20 years (2001–2021)
| Simulated Biomass |
Measured Biomass |
Simulated Yield |
Measured Yield |
Observed Yields |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (t/ha) |
(t/ha) |
(t/ha) |
(t/ha) |
(t/ha) |
||||||
| Year | MnC | MxC | MnC | MxC | MnC | MxC | MnC | MxC | MnC | MxC |
| 2001 | 5.467 | 6.413 | 5.505 | 6.417 | 2.733 | 3.206 | 2.752 | 3.208 | 3.010 | 3.483 |
| 2002 | 5.495 | 6.45 | 5.391 | 6.430 | 2.747 | 3.225 | 2.695 | 3.215 | 3.015 | 3.493 |
| 2003 | 5.523 | 6.484 | 5.651 | 6.436 | 2.761 | 3.242 | 2.825 | 3.218 | 3.030 | 3.511 |
| 2004 | 5.376 | 6.429 | 5.325 | 6.411 | 2.66 | 3.206 | 2.635 | 3.197 | 2.945 | 3.491 |
| 2005 | 5.436 | 6.471 | 5.152 | 6.469 | 2.696 | 3.232 | 2.555 | 3.231 | 2.970 | 3.506 |
| 2006 | 5.627 | 6.573 | 5.571 | 6.577 | 2.813 | 3.287 | 2.785 | 3.289 | 3.075 | 3.549 |
| 2007 | 5.616 | 6.412 | 5.570 | 6.434 | 2.808 | 3.206 | 2.785 | 3.217 | 3.070 | 3.47 |
| 2008 | 5.57 | 6.539 | 5.277 | 6.577 | 2.771 | 3.269 | 2.625 | 3.288 | 3.047 | 3.545 |
| 2009 | 5.034 | 5.517 | 5.044 | 4.677 | 2.429 | 2.668 | 2.434 | 2.262 | 2.760 | 2.999 |
| 2010 | 5.499 | 6.296 | 5.463 | 6.459 | 2.714 | 3.126 | 2.696 | 3.207 | 2.985 | 3.397 |
| 2011 | 5.682 | 6.663 | 5.644 | 6.657 | 2.841 | 3.330 | 2.822 | 3.327 | 3.099 | 3.588 |
| 2012 | 5.662 | 6.484 | 5.602 | 6.510 | 2.831 | 3.242 | 2.801 | 3.255 | 3.090 | 3.501 |
| 2013 | 5.490 | 6.575 | 5.705 | 6.605 | 2.706 | 3.274 | 2.812 | 3.289 | 2.990 | 3.560 |
| 2014 | 5.385 | 6.466 | 5.514 | 6.492 | 2.624 | 3.184 | 2.687 | 3.197 | 2.925 | 3.475 |
| 2015 | 5.581 | 6.511 | 5.711 | 6.545 | 2.751 | 3.220 | 2.815 | 3.237 | 3.025 | 3.494 |
| 2016 | 4.937 | 5.59 | 4.886 | 5.628 | 2.305 | 2.633 | 2.281 | 2.651 | 2.655 | 2.983 |
| 2017 | 5.892 | 6.869 | 5.786 | 6.845 | 2.941 | 3.440 | 2.888 | 3.428 | 3.200 | 3.699 |
| 2018 | 5.745 | 6.79 | 5.880 | 6.760 | 2.847 | 3.389 | 2.914 | 3.374 | 3.115 | 3.657 |
| 2019 | 5.972 | 6.871 | 5.750 | 6.889 | 2.986 | 3.436 | 2.875 | 3.445 | 3.245 | 3.695 |
| 2020 | 5.950 | 6.971 | 5.972 | 6.843 | 2.967 | 3.485 | 2.978 | 3.421 | 3.225 | 3.743 |
| 2021 | 5.915 | 6.955 | 5.758 | 6.909 | 2.943 | 3.478 | 2.865 | 3.455 | 3.205 | 3.740 |
Appendix 9. Paired Samples Test of biomass and grain yields in MnC versus MxC systems
| Paired Statistics | Mean | SD | SE Mean | ||
|---|---|---|---|---|---|
| B_MnC (t/ha) | 5.564 | 0.264 | 0.058 | ||
| B_MxC (t/ha) | 6.492 | 0.366 | 0.080 | ||
| Y_MnC (t/ha) | 2.756 | 0.164 | 0.036 | ||
| Y_MxC (t/ha) | 3.228 | 0.217 | 0.047 | ||
| Paired Samples Correlations | Correlations | P-value | |||
| B_MnC (t/ha) and B_MxC (t/ha) | 0.941 | 0.00 | |||
| Y_MnC (t/ha) and Y_MxC (t/ha) | 0.95 | 0.00 | |||
| Paired Samples Test | d.f | Mean | SD | SE Mean | P-value |
| B_MnC (t/ha) - B_MxC (t/ha) | 20 | −0.927 | 0.148 | 0.032 | 0.000 |
| Y_MnC (t/ha) - Y_MxC (t/ha) | 20 | −0.472 | 0.080 | 0.017 | 0.000 |
B: Biomass yield; Y: Grain Yield.
References
- 1.MANAGE . Telangana State; India: 2018. Climate Smart Agriculture and Advisory Services: Approaches and Implications for Future.http://www.manage.gov.in/publications/discussion [Online]. Available: papers/MANAGE-Discussion Paper-1.pdf. [Accessed Jan. 10, 2022. [Google Scholar]
- 2.FAO Climate-Smart agriculture sourcebook. Food and Agriculture Organization of the United Nations (FAO), Rome, Italy. 2013;3(2) doi: 10.3224/eris.v3i2.14. [DOI] [Google Scholar]
- 3.FAO . Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2017. Soil Organic Carbon: the Hidden Potential. [Google Scholar]
- 4.FAO . second ed. Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2017. Agriculture Sourcebook Summary Climate-Smart Agriculture.www.fao.org/climate-smart-agriculture-sourcebook [Online]. Available: Sept. 10, 2021. [Google Scholar]
- 5.Williams P.A., Crespo O., Abu M. Adapting to changing climate through improving adaptive capacity at the local level – the case of smallholder horticultural producers in Ghana. Clim. Risk Manag. 2019;23(September 2018):124–135. doi: 10.1016/j.crm.2018.12.004. [DOI] [Google Scholar]
- 6.WBG Shock waves: managing the impacts of climate change on poverty. Washington, DC , USA: International Bank for Reconstruction and Development / The World Bank 1818 H Street NW, Washington, DC. 2016;59(1) doi: 10.1596/978-1-4648-0673-5. [DOI] [Google Scholar]
- 7.Abegunde V.O., Sibanda M., Obi A. The dynamics of climate change adaptation in sub-Saharan Africa: a review of climate-smart agriculture among small-scale farmers. Climate. 2019;7:11. doi: 10.3390/cli7110132. [DOI] [Google Scholar]
- 8.Byishimo P. Assessment of climate change impacts on crop yields and farmers' adaptation measures: a case of Rwanda. J. Chem. Inf. Model. 2018;53(9):1689–1699. [Google Scholar]
- 9.FAO The climate is changing. Food and agriculture must too. Appropr. Technol. 2016;43(4):15–16. [Online]. Available: [Google Scholar]
- 10.OECD/FAO . In: Makurira E., editor. vol. 181. Rome; 2016. Agriculture in sub-saharan Africa: prospects and challenges for the next decade, chapter 2, Part I; pp. 35–48. (Water Productivity in Rainfed Agriculture). [DOI] [Google Scholar]
- 11.Kedir U., Ahemad A. The effect of climate change on yield and quality of wheat in Ethiopia : a review. J. Environ. Earth Sci. 2017;7(12):46–52. ISSN 2224–3216/ISSN 2225-0948. [Google Scholar]
- 12.Warner K.E. The remarkable decrease in cigarette smoking by American youth: further evidence. Prev. Med. Reports. 2015;2:259–261. doi: 10.1016/j.pmedr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karamage F., Zhang C., Ndayisaba F., Shao H., Kayiranga A., Fang X., Nahayo L., Nyesheja E.K., Tian G. Extent of cropland and related soil erosion risk in Rwanda. Sustain. Times. 2016;8(7):1–19. doi: 10.3390/su8070609. [DOI] [Google Scholar]
- 14.Rizinjirabake F., Tenenbaum D.E., Pilesjö P. Sources of soil dissolved organic carbon in a mixed agricultural and forested watershed in Rwanda. Catena. 2019;181(May) doi: 10.1016/j.catena.2019.104085. [DOI] [Google Scholar]
- 15.REMA . Kigali; Rwanda: 2013. The Assessment of Economic Impacts of the 2012 Wet Season Flooding in Rwanda. [Google Scholar]
- 16.Wfp Rwanda. 2018. Comprehensive Food Security and Vulnerability Analysis,” Wold Food Programme (WFP), Rome, Italy, 2018.http://www.wfp.org/food-security [Online]. Available: [Google Scholar]
- 17.World Bank Rwanda systematic country diagnostic. Rwanda Syst. Ctry. Diagnostic. 2019 doi: 10.1596/32113. [DOI] [Google Scholar]
- 18.FAO . Italy; Rome: 2021. “Rwanda at a Glance,” Food and Agriculture Organization of the United States (FAO)http://www.fao.org/rwanda/our-office-in-rwanda/rwanda-at-a-glance/en/ [Google Scholar]
- 19.World Bank, CIAT . “Climate-Smart Agriculture in Rwanda.” CSA Country Profile for Africa, Asia, and Latin America and Caribbean Series. The World Bank; Washington D. C: 2015. pp. 1–16. [Online]. Available: //efaidnbmnnnibpcajpcglclefindmkaj/https://climateknowledgeportal.worldbank.org/sites/default/files/2019-06/CSA%20RWANDA%20NOV%2018%202015.pdf. (Accessed 19 August 2023) [Google Scholar]
- 20.IFAD . Kigali, Rwanda; 2019. Rwanda Kayonza Irrigation and Integrated Watershed Management Project (KIIWP1) Project Design Report (PDR) [Google Scholar]
- 21.MINAGRI Final-year Report 2016-2017,” Kigali, Rwanda. 2017 www.minagri.gov.rw [Online]. Available: [Google Scholar]
- 22.FAO . Rome; Italy: 2020. What Is Soil Carbon Sequestration,” Food and Agriculture Organization of the United States (FAO)http://www.fao.org/soils-portal/soil-management/soil-carbon-sequestration/en/ [Google Scholar]
- 23.MinFAN . Kigali; Rwanda: 2018. Climate Change Profile Rwanda,” Ministry of Foreign Affairs of the Netherlands (MinFAN) [Google Scholar]
- 24.IFAD . Rome, Italy; 2019. Republic of Rwanda-Country Strategic Opportunities Programme 2019-2024: for Review. [Online]. Available: EB 2019/126/R.13/Rev.1. [Google Scholar]
- 25.Habumugisha V., Mourad K.A., Hashakimana L. The effects of trees on soil chemistry. Curr. Environ. Eng. Dec. 2018;6(1):35–44. doi: 10.2174/2212717806666181218141807. [DOI] [Google Scholar]
- 26.Lal R., Smith P., Jungkunst H.F., Mitsch W.J., Lehmann J., Nair R.P.K., McBratney A.B., De Moraes Sá J.C., Schneider J., Zinn Y.L., Skorupa A.L.A., Zhang H.L., Minasny B., Srinivasrao C., Ravindranath N.H. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018;73(6):145A–152A. doi: 10.2489/jswc.73.6.145A. [DOI] [Google Scholar]
- 27.FAO . Food and Agriculture Organization of the United Nations (FAO) vol. 52. Springer; Rome, Italy: 2018. Climate smart agriculture: building resilience to climate change. [DOI] [Google Scholar]
- 28.Gaba S., Lescourret F., Boudsocq S., Enjalbert J., Hinsinger P., Journet E.P., Navas M.L., Wery J., Louarn G., Malézieux E., Pelzer E., Prudent M., Ozier-Lafontaine H. Multiple cropping systems as drivers for providing multiple ecosystem services: from concepts to design. Agron. Sustain. Dev. 2015;35(2):607–623. doi: 10.1007/s13593-014-0272-z. [DOI] [Google Scholar]
- 29.Lizarazo C.I., Tuulos A., Jokela V., Mäkelä P.S.A. vol. 4. July; 2020. pp. 1–15. (Sustainable Mixed Cropping Systems for the Boreal-Nemoral Region). [DOI] [Google Scholar]
- 30.FAO . Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2018. Cropping System Diversification in Eastern and Southern Africa. 978-92-5-130981-0; CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 31.Waha K., Müller C., Bondeau A., Dietrich J.P., Kurukulasuriya P., Heinke J., Lotze-Campen H. Adaptation to climate change through the choice of cropping system and sowing date in sub-Saharan Africa. Global Environ. Change. 2013;23(1):130–143. doi: 10.1016/j.gloenvcha.2012.11.001. [DOI] [Google Scholar]
- 32.WFP, “Rwanda IR-EMOP . Kigali, Rwanda; 2016. Food Assistance to Drought Affected Population in Eastern Rwanda-Project Document Part 1 : Information Note,” Wold Food Programme (WFP) [Google Scholar]
- 33.Winowiecki L.A., Bargués-Tobella A., Mukuralinda A., Mujawamariya P., Ntawuhiganayo E.B., Mugayi A.B., Chomba S., Vågen T.-G. Assessing soil and land health across two landscapes in eastern Rwanda to inform restoration activities. SOIL. 2021;7(11):767–783. doi: 10.5194/soil-7-767-2021. [DOI] [Google Scholar]
- 34.USAID . Kigali; Rwanda: 2018. Soil Pre-test Report: Rwanda Rural Sanitation - Isuku Iwacu Activity. [Google Scholar]
- 35.NISR-SAS . Kigali, Rwanda; 2019. Seasonal Agricultural Survey - SAS 2019 Final Report. [Google Scholar]
- 36.Paustian K., Collier S., Baldock J., Burgess R., Creque J., DeLonge M., Dungait J., Ellert B., Frank S., Goddard T., Govaerts B., Grundy M., Henning M., Izaurralde R.C., Madaras M., McConkey B., Porzig E., Rice C., Searle R., Seavy N., Skalsky R., Mulhern W., Jahn M. Quantifying carbon for agricultural soil management: from the current status toward a global soil information system. Carbon Manag. 2019;10(6):567–587. doi: 10.1080/17583004.2019.1633231. Nov. 02. Taylor and Francis Ltd. [DOI] [Google Scholar]
- 37.Ullah M.R., Al-Amin M. Above- and below-ground carbon stock estimation in a natural forest of Bangladesh. J. For. Sci., vol. 2012. 2012;58(8):372–379. https://www.agriculturejournals.cz/web/jfs.htm?type=article&id=103_2011-JFS [Online]. Available: [Google Scholar]
- 38.Bispo A., Andersen L., Angers D.A., Bernoux M., Brossard M., Cécillon L., Comans R.N.J., Harmsen J., Jonassen K., Lamé F., Lhuillery C., Maly S., Martin E., Mcelnea A.E., Sakai H., Watabe Y., Eglin T.K. Accounting for carbon stocks in soils and measuring GHGs emission fluxes from soils: do we have the necessary standards? Front. Environ. Sci. 2017;5(JUL):1–12. doi: 10.3389/fenvs.2017.00041. [DOI] [Google Scholar]
- 39.Van Reeuwijk L. vol. 119. International Soil Renference and Information Centre (ISRIC); Wageningen, Netherlands: 2002. Procedures for Soil Anlysis. [Google Scholar]
- 40.Blake G.R., Hartge K.H. Bulk density. Encycl. Earth Sci. Ser. 1986;9(11718):74–75. doi: 10.1017/cbo9780511545993.016. [DOI] [Google Scholar]
- 41.McLean E.O. ASA-SSSA, 677 S. Segoe Rd., Madison, WI 53711; Columbus, Ohio, USA: 1982. Soil pH and Lime Requirement Determination, 2nd Ed., Vol. 9, No. 9. [DOI] [Google Scholar]
- 42.Bremner J., Mulvaney C. Nitrogen—total. Methods of soil analysis. Part 2. Chemical and Microbiological Properties-Agronomy Monograph no. 9. 1982;9(9):595–624. https://acsess.onlinelibrary.wiley.com/doi/pdf/10.2134/agronmonogr9.2.2ed.c31 Iowa State University, Ames, Iowa, USA. [Online]. Available. [Google Scholar]
- 43.Page-Dumroese D., Harvey A., Jurgensen M. A guide to soil sampling and analysis on the national forests of the inland northwest United States. Gen. Tech. Rep. - US Dep. Agric. For. Serv., no. INT-GTR. 1995;326 doi: 10.5962/bhl.title.100094. [DOI] [Google Scholar]
- 44.Hsiao T.C., Heng L., Steduto P., Rojas-Lara B., Raes D., Fereres E. Aquacrop-The FAO crop model to simulate yield response to water: III. Parameterization and testing for maize. Agron. J. 2009;101(3):448–459. doi: 10.2134/agronj2008.0218s. [DOI] [Google Scholar]
- 45.FAO . Rome, Italy; 2018. Measuring and Modelling Soil Carbon Stocks and Stock Changes in Livestock Production Systems:– Guidelines for Assessment (Draft for Public Review),” Food and Agriculture Oragnization of the United Nations (FAO)www.fao.org/publications [Online]. Available: [Google Scholar]
- 46.Raes D., Steduto P., Hsiao T.C., Fereres E. Reference Manual: AquaCrop, Version 6.0. No. May. Food and Agriculture Oragnization of the United Nations (FAO) Rome; Italy: 2018. Chapter 2: users guide AquaCrop reference manual.www.fao.org/publications [Online]. Available. [Google Scholar]
- 47.Feng Q., Xu J., Zhang Y., Li X., Xu J., Han H., Ning T., Lal R., Li Z. CO2 fixation in above-ground biomass of summer maize under different tillage and straw management treatments. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-17247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alishiri M., Shojaei A., Abdekhodaie M.J., Yeganeh H. Synthesis and characterization of biodegradable acrylated polyurethane based on poly(ε-caprolactone) and 1,6-hexamethylene diisocyanate. Mater. Sci. Eng. C. 2014;42:763–773. doi: 10.1016/j.msec.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 49.Msongaleli B., Rwehumbiza F., Tumbo S.D., Kihupi N. Sorghum yield response to changing climatic conditions in semi-arid Central Tanzania: evaluating crop simulation model applicability. Agric. Sci. 2014;5(10):822–833. doi: 10.4236/as.2014.510087. [DOI] [Google Scholar]
- 50.Xu J., Bai W., Li Y., Wang H., Yang S., Wei Z. Water Manag.; 2019. Modeling Rice Development and Field Water Balance Using AquaCrop Model under Drying-Wetting Cycle Condition in Eastern China,” Agric. [DOI] [Google Scholar]
- 51.Van Gaelen H., Tsegay A., Delbecque N., Shrestha N., Garcia M., Fajardo H., Miranda R., Vanuytrecht E., Abrha B., Diels J., Raes D. A semi-quantitative approach for modelling crop response to soil fertility: evaluation of the AquaCrop procedure. J. Agric. Sci. 2015;153(7):1218–1233. doi: 10.1017/S0021859614000872. [DOI] [Google Scholar]
- 52.Willmott C.J., Ackleson S.G., Davis R.E., Feddema J.J., Klink K.M., R Legates D., O'Donnel J., Rowe C.M. Statistics for the evaluation and comparison of models. J. Geophys. Res. 1985;90(9):8995–9005. doi: 10.1016/0198-0254(86)91285-9. [DOI] [Google Scholar]
- 53.Aziz M., Rizvi S.A., Sultan M., Bazmi M.S.A., Shamshiri R.R., Ibrahim S.M., Imran M.A. Simulating cotton growth and productivity using AquaCrop model under deficit irrigation in a semi-arid climate. Agric. For. 2022;12(2):1–18. doi: 10.3390/agriculture12020242. [DOI] [Google Scholar]
- 54.Silva V.P.R., Silva R.A., Maciel G.F., Braga C.C., Silva Junior J.L.C., Souza E.P., Almeida R.S.R., Silva M.T., Holanda R.M. Calibration and validation of the AquaCrop model for the soybean crop grown under different levels of irrigation in the Motopiba region, Brazil. Ciência Rural. 2018;48(1):1–8. doi: 10.1590/0103-8478cr20161118. [DOI] [Google Scholar]
- 55.FAO . Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2012. Crop Yield Response to Water. [Google Scholar]
- 56.Steduto P., Raes D., Hsiao T.C., Fereres E. Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 1979. 3.1 AquaCrop: Concepts, Rationale and Operation. [Google Scholar]
- 57.Gikonyo N.F., Dong X., Mosongo P.S., Guo K., Liu X. Long-term impacts of different cropping patterns on soil physico-chemical properties and enzyme activities in the low land plain of North China. Agronomy. 2022;12(2):1–18. doi: 10.3390/agronomy12020471. [DOI] [Google Scholar]
- 58.Tessema T., Kibebew K. Carbon stock under major land use/land cover types of Hades sub-watershed, eastern Ethiopia. Carbon Balance Manag. 2019;14(1):1–14. doi: 10.1186/s13021-019-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bizuhoraho T., Kayiranga A., Manirakiza N., Mourad K.A. The effect of land use systems on soil properties; A case study from Rwanda. Sustain. Agric. Res. 2018;7(2):30. doi: 10.5539/sar.v7n2p30. [DOI] [Google Scholar]
- 60.Abbasi M.K., Tahir M.M. Economizing nitrogen fertilizer in wheat through combinations with organic manures in Kashmir, Pakistan. Agron. J. 2012;104(1):169–177. doi: 10.2134/agronj2011.0264. [DOI] [Google Scholar]
- 61.Nwite J.C., Nebo A.I., Mailili T.Y., V Ilorah C. Middle East journal of agriculture research. Middle East J. Agric. Res. 2022;11(1):197–205. doi: 10.36632/mejar/2022.11.1.16. [DOI] [Google Scholar]
- 62.Pan X.Y., Shi R.Y., Hong Z.N., Jiang J., He X., Xu R.K., Qian W. Characteristics of crop straw-decayed products and their ameliorating effects on an acidic Ultisol. Arch. Agron Soil Sci. 2021;67(12):1708–1721. doi: 10.1080/03650340.2020.1805104. [DOI] [Google Scholar]
- 63.Brady N.C., Weil R.R. fifteenth ed. Pearson Education, Inc.; 2016. The Nature and Properties of Soils. [Google Scholar]
- 64.Bucagu C., Vanlauwe B., Giller K.E. Managing Tephrosia mulch and fertilizer to enhance coffee productivity on smallholder farms in the Eastern African Highlands. Eur. J. Agron. 2013;48:19–29. doi: 10.1016/j.eja.2013.02.005. [DOI] [Google Scholar]
- 65.Mbonigaba J.J.M., Culot M. Reclamation of an acidic soil of Rwanda's central upland by composts based on natural vegetation biomass. Rwanda J. 2009;17(1):64–81. [Google Scholar]
- 66.Msanya B.M., Kimaro D.N., Kimbi G.G., Kileo E.P., Mbogoni J.J. Land resources inventory and suitability assessment for the major land use types in Morogoro urban district , Tanzania. Soils L. Resour. Morogoro Rural Rural Urban Dist. 2001;4(6):73. [Google Scholar]
- 67.Primature-NST1 . Government of Rwanda (GoR); Kigali, Rwanda: 2017. 7 Years Government Programme: National Strategy for Transformation (NST1) (2017–2024). Prime Minister's Office (Primature) [Google Scholar]
- 68.Ferreira R.V., Tavares R.L.M., Furquim-de-Medeiros S., Guerra da-Silva A., Fernandes-da-Silva-Júnior J. Carbon stock and organic fractions in soil under monoculture and sorghum bicolor–urochloa ruziziensis intercropping systems. Bragantia. 2020;79(3):425–433. doi: 10.1590/1678-4499.20200042. [DOI] [Google Scholar]
- 69.Cong W., Hoffland E., Li L., Six J., Sun J.-H. Intercropping enhances soil carbon and nitrogen. Glob. Chang. Biol. 2014:1. doi: 10.1111/gcb.12738. –12. [DOI] [PubMed] [Google Scholar]
- 70.Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004;304(5677):1623–1627. doi: 10.1126/science.1097396. [DOI] [PubMed] [Google Scholar]
- 71.Yang T., Siddique K.H.M., Liu K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020;23(5):12–13. doi: 10.1016/j.gecco.2020.e01118. [DOI] [Google Scholar]
- 72.Graf D.R.H., Saghaï A., Zhao M., Carlsson G., Jones C.M., Hallin S. Lucerne (Medicago sativa) alters N 2 O-reducing communities associated with cocksfoot (Dactylis glomerata) roots and promotes N 2 O production in intercropping in a greenhouse experiment. Soil Biol. Biochem. 2019;137 doi: 10.1016/j.soilbio.2019.107547. June. [DOI] [Google Scholar]
- 73.Radersma S., Smit A.L. Assessing denitrification and N leaching in a field with organic amendments. NJAS - Wageningen J. Life Sci. 2011;58(1–2):21–29. doi: 10.1016/j.njas.2010.06.001. [DOI] [Google Scholar]
- 74.Nie S.W., Eneji A.E., Chen Y.Q., Sui P., Huang J.X., Huang S.M. Nitrate leaching from maize intercropping systems with N fertilizer over-dose. J. Integr. Agric. 2012;11(9):1. doi: 10.1016/S2095-3119(12)60156-7. [DOI] [Google Scholar]
- 75.Nouri A., Lukas S., Singh S., Singh S., Machado S. When do cover crops reduce nitrate leaching? A global meta-analysis. Glob. Chang. Biol., no. December 2021. 2022:4736–4749. doi: 10.1111/gcb.16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.USDA Carbon to nitrogen ratios in cropping systems. 2011 http://www.nrcs.usda.gov [Online]. Available: [Google Scholar]
- 77.Swangjang K. Soil carbon and nitrogen ratio in different land use. 2015 International Conference on Advances in Environment Research. 2013;87(9):36. doi: 10.7763/IPCBEE. [DOI] [Google Scholar]
- 78.Chesworth W., Arbestain M.C., Macías F., Spaargaren O., Mualem O., Morel‐Seytoux Y.H.J., Horwath W.R., Almendros G., Grossl P.R., Sparks D.L., Fairbridge R.W., Singer A., Weaver C.E.B., Theng K.G. Encyclopedia of Soil Science. 2016. Clay‐organic interactions; pp. 144–150. [Google Scholar]
- 79.Souza J.L.M., Oliveira C.T., Rosa S.L.K., Tsukahara R.Y. Calibration and validation of the aquacrop model to estimate maize production in campos gerais, paraná state, Brazil. Rev. Bras. Meteorol. 2020;35(2):243–253. doi: 10.1590/0102-7786352001. [DOI] [Google Scholar]
- 80.B. R. Waghmode, “Lecture No. 05 & 06; Chapter No 9. Cropping Systems-Meaning, Types/classification of Cropping Systems, Advantages and Disadvantages of Each Copping Systems,”UAS, Dharwad..
- 81.Mutsamba E.F., Nyagumbo I., Mupangwa W. Forage and maize yields in mixed crop-livestock farming systems: enhancing forage and maize yields in mixed crop-livestock systems under conservation agriculture in sub-humid Zimbabwe. NJAS - Wageningen J. Life Sci. 2020;92(11):1–9. doi: 10.1016/j.njas.2019.100317. [DOI] [Google Scholar]
- 82.Li X.-F., Wang Z.-G., Bao X.-G., Sun J.-H., Yang S.-C., Wang P., Wang C.-B., Wu J.-P., Liu X.-R., Tian X.-L., Wang Y., Li J.-P., Xia H.-Y., Mei P.-P., Wang X.-F., Zhao J.-H., Yu R.-P., Zhang W.-P., Che Z.-X., Gui L.-G., Callaway R.M., Tilman D., Li L. Long-term increased grain yield and soil fertility from intercropping. Nat. Sustain. 2021;4(10):1–8. doi: 10.1038/s41893-021-00767-7. [DOI] [Google Scholar]
- 83.Suárez J.C., Anzola J.A., Contreras A.T., Salas D.L., Vanegas J.I., Urban M.O., Beebe S.E., Rao I.M. Influence of simultaneous intercropping of maize-bean with input of inorganic or organic fertilizer on growth, development, and dry matter partitioning to yield components of two lines of common bean. Agronomy. 2022;12(5):6–20. doi: 10.3390/agronomy12051216. [DOI] [Google Scholar]
- 84.Kasenge V., Kyamanywa S., Taylor D.B., Bigirwa G., Erbaugh J.M. Farm-level evaluation of monocropping and intercropping impacts and maize yields and returns in iganga district-Uganda. East. Afr. J. Rural Dev. 2004;17(1):1–10. [Google Scholar]
- 85.Kidane B.Z., Hailu M.H., Haile H.T. Maize and potato intercropping: a technology to increase productivity and profitability in tigray. Open Agric. 2017;2(1):411–416. doi: 10.1515/opag-2017-0044. [DOI] [Google Scholar]
- 86.Renwick L.L.R., Kimaro A.A., Hafner J.M., Rosenstock T.S., Gaudin A.C.M. Maize-pigeonpea intercropping outperforms monocultures under drought. Front. Sustain. Food Syst. 2020;4(12):6–7. doi: 10.3389/fsufs.2020.562663. [DOI] [Google Scholar]