Abstract
Grape juice is a widely consumed fruit due to its bioactive compounds, minerals, and aroma components. Our objective was to investigate ultrasound treatment of black grape juice affects its bioactive components due to using response surface methodology (RSM) and artificial neural network (ANN) optimization. At the same time, mineral components, sugar components, organic acids, and volatile aroma profiles were compared in black grape juice treated with thermal and ultrasound pasteurization. ANN showed superior predictive values (>99%) to RSM. Optimal combinations were obtained at 40 °C, 12 min, and 65% amplitude for thermosonication. Under these conditions, phenolic, flavonoid, antioxidant activity, and anthocyanin values were 822.80 mg GAE/L, 97.50 mg CE/L, 24.51 mmol Trolox/L, and 368, 81 mg of mv-3-glu/L, respectively. Thermosonicated grape juice (TT-BGJ) was tested against black grape juice (P-BGJ) produced with conventional thermal methods. This study investigated the effects of thermal pasteurization and thermosonication on black grape juice bioactive compounds and minerals, aroma profile, and sensory evaluation. Thermosonication affected the aroma profile less, 329.98 μg/kg (P-BGJ) and 495.31 μg/kg (TT-BGJ). TT-BGJ was detected to contain seven different mineral elements (Mn, K, Fe, Mg, Cu, Zn, and Na). Thermosonication caused an increase in Fe, Zn, Mn, and K minerals. Panelists generally liked the TT-BGJ sample. These results suggest that the thermosonication process may potentially replace the traditional black grape juice processing thermal process.
Keywords: Black grape juice, Thermosonication, Artificial neural network, Mineral, Aroma profile
1. Introduction
Currently, due the increasing health awareness of consumers, there are great developments in the production of functional and natural foods for the food market [1]. Grapes are one of the largest fruit crops harvested worldwide [2]. Grapes have high sugar levels, high levels of acids, pectin, various aromatic compounds, antioxidants, edible fibers, vitamins, and minerals. Grape juice is one of the many end product varieties of grapes [3]. The grape juice is widely produced in China, France, United America, South Africa, Italy, Chile, Iran, Turkey, Spain, and Argentina [4].
The literature mentions that black grapes (Vitis vinifera L.) are rich in flavonoids such as anthocyanins, which are natural antioxidants that combat oxidative stress, and prevent the occurrence of cardiovascular disorders and neurodegenerative diseases [5]. Thus, black grape juice is becoming more attractive to consumers of these health properties [6]. In response to human wants, researchers are now investigating new technology that is less aggressive to the nutritional and freshness value of food [7]. Thermal processing technology is the oldest and most commonly used process to inhibit pathogens and extend juice's shelf life, but it also damages the beneficial nutrients [8,9]. Ultrasound (frequency of 20 kHz or more) is a non-thermal physical technique with high environmental protection, low cost, high efficiency, and low energy consumption widely used for sterilization, detection, extraction, and other processes [10]. Furthermore, there is various research in the literature showing that ultrasound technology increased the quality of juice, with examples including ougan [11], tangerine [12], cape gooseberry [13], mulberry [14], Acai [15], watermelon [16,17], prebiotic orange [18], strawberry [19], and grapefruit juices [7].
Response surface methodology (RSM) is used to indicate the significance level of each factor through statistical analysis and to analyze interactions between factors that influence experimental response values [20]. Artificial neural network (ANN) is designed as a prediction tool that has higher efficiency, can simulate various systems, and provides flexibility and accuracy in experimental data fitting and nonlinear correlation estimation compared to RSM [21]. RSM and ANN have gained popularity in many studies, such as apple juice [22], cashew apple juice [23], Chironji (Buchanania lanzan) fruit juice [24], Bael Fruit Juice [25], orange juice [26] garlic [27].
To our knowledge, there is no study in the literature about how ultrasound treatment of black grape juice affects its bioactive compounds using RSM and ANN optimization. Therefore, the research aimed to investigate ultrasound treatment of black grape juice involves its bioactive components (antioxidant activity, total flavonoid content, total phenolic content, and total anthocyanin content) due to adaptive RSM and ANN optimization. In addition, in this study, phenolic compounds, organic acids, sugar components, volatile aroma profile, and mineral components were compared in black grape juice treated with ultrasound and thermal pasteurization.
2. Material
Black grapes (Vitis vinifera L.) were collected from vineyard areas in the Sarkoy district of Tekirdag province in 2021–2022 under producer conditions. The grapes were first cleaned. Before juice extraction, grapes were washed in cold tap water and drained. Damaged grapes were discarded. Then, to minimize the microorganism loads in grape skin, washing was performed with apple cider vinegar. The cleaned grapes were crushed with a commercial blender (Waring Blender, USA). Black grape juice was purified of grape seeds and coarse particles using a single layer of sterilized muslin. The glass bottles where the grape juices will be stored were sterilized by autoclaving. Sterile bottles (100 mL) were used to transferred freshly-squeezed black grape juice. The black grape juice was stored at −18 ± 1 °C for later use. Analyzes were carried out by dissolving in a water bath at room temperature. The study coded black grape juice without any treatment as a control (untreated black grape juice, BGJ).
2.1. Thermal pasteurization and thermosonication treatments
Black grape juice sample was pasteurized for 2 min after the internal temperature reached 85 °C with a water bath (Wisd-Model WUC-D06H, Daihan, Wonju, Korea). This temperature and time interval were determined based on previously reported results [28]. The thermal pasteurized sample was coded as black grape juice (P-BGJ). Ultrasonic processor (Hielscher Ultrasonics Model UP200St, Berlin, Germany) at a frequency of 26 kHz and 200 W was used to obtain thermosonication treated (TS) juice sample. Ultrasound parameters studied included amplitudes (60%, 70%, 80%, 90% and 100%), processing duration (8, 9, 10, 11 and 12 min), and temperature (40, 45, 50, 55 and 60 °C) in constant mode. An ice-water bath was used to prevent overheating during ultrasound processing. After TS, the black grape juice samples were cooled and were kept at −18 ± 1 °C until analysis (TT-BGJ).
2.2. Modelling procedure for response
The effect of thermosonication technology on the TPC, TFC, TAC, and TEAC in black grape juice is investigated with RSM and ANN. While amplitude (X3, 60–100%), temperature (X1, 40–60 °C), and time (X2, 8–12 min) were independent factors, antioxidant activity, total flavonoid content (TFC; mg CE/L), total phenolic content (TPC; mg GAE/L), and total anthocyanin content (TAC; mg mv-3-glu/L) were response variables. For RSM, Minitab software (19th version, State College, PA, USA) was used implenting central composite design (CCD) to optimize thermosonication processing of black grape juice. After CCD design, the number of experiments were found 20, performed in triplicate, and the results are given in Table 1. The following quadratic-polynomial formula was used to create the equation models (Equation (1):
| (1) |
Table 1.
Experimental and predicted responses of RSM and ANN and results of BGJ and P-BGJ.
| Run no | Independent variables |
Dependent variables |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temputure (X1) | Time (X2) | Amplitude (X3) | TPC (mg GAE/L) |
TFC (mg CE/L) |
Antioxidant TEAC (mmol Trolox/L) |
TAC (mg of mv-3-glu/L) |
|||||||||
| Experimental data | RSM predicted | ANN predicted | Experimental data | RSM predicted | ANN predicted | Experimental data | RSM predicted | ANN predicted | Experimental data | RSM predicted | ANN predicted | ||||
| 1 | 60 | 8 | 80 | 804.28 ± 5.26 | 804.16 | 804.26 | 94.62 ± 1.24 | 94.54 | 94.45 | 25.12 ± 0.15 | 25.04 | 25.12 | 364.52 ± 1.22 | 364.16 | 364.50 |
| 2 | 55 | 9 | 90 | 808.71 ± 2.66 | 807.09 | 808.70 | 94.62 ± 0.45 | 94.81 | 94.62 | 24.38 ± 0.68 | 24.50 | 24.38 | 363.52 ± 1.78 | 363.83 | 363.52 |
| 3 | 45 | 11 | 90 | 804.55 ± 1.48 | 804.57 | 804.56 | 94.45 ± 1.24 | 94.58 | 94.45 | 24.84 ± 0.02 | 24.82 | 24.84 | 367.26 ± 0.56 | 367.53 | 367.26 |
| 4 | 45 | 9 | 70 | 824.20 ± 5.54 | 823.34 | 824.18 | 96.92 ± 0.78 | 97.05 | 96.92 | 23.38 ± 0.35 | 23.29 | 23.38 | 363.14 ± 1.44 | 363.18 | 363.20 |
| 5 | 55 | 11 | 70 | 802.65 ± 6.22 | 802.29 | 802.67 | 94.32 ± 0.34 | 94.32 | 94.32 | 23.95 ± 0.28 | 23.89 | 23.80 | 364.51 ± 1.45 | 364.74 | 364.51 |
| 6 | 55 | 11 | 90 | 807.88 ± 2.78 | 809.83 | 808.23 | 95.65 ± 1.28 | 95.51 | 95.65 | 24.80 ± 0.06 | 24.78 | 24.80 | 364.55 ± 2.78 | 364.59 | 364.60 |
| 7 | 55 | 9 | 70 | 805.79 ± 1.22 | 807.00 | 805.78 | 94.80 ± 2.1 | 94.72 | 94.80 | 24.05 ± 0.54 | 24.12 | 24.05 | 364.29 ± 1.16 | 364.78 | 364.22 |
| 8 | 50 | 8 | 80 | 811.85 ± 4.24 | 812.16 | 812.85 | 95.50 ± 1.55 | 95.40 | 95.52 | 24.15 ± 0.01 | 24.17 | 24.15 | 364.40 ± 0.55 | 364.71 | 364.62 |
| 9 | 45 | 11 | 70 | 809.60 ± 6.76 | 812.45 | 809.58 | 95.88 ± 0.66 | 95.74 | 95.88 | 24.43 ± 0.06 | 24.35 | 24.43 | 366.18 ± 1.49 | 366.63 | 366.17 |
| 10 | 45 | 9 | 90 | 806.55 ± 5.67 | 808.01 | 806.48 | 94.82 ± 1.77 | 94.81 | 94.82 | 23.29 ± 0.24 | 23.24 | 23.29 | 363.42 ± 2.16 | 363.28 | 363.60 |
| 11 | 50 | 10 | 80 | 804.65 ± 2.87 | 804.55 | 804.53 | 94.35 ± 0.55 | 94.52 | 94.56 | 24.86 ± 0.75 | 24.56 | 24.55 | 364.85 ± 3.84 | 365.01 | 365.22 |
| 12 | 50 | 10 | 80 | 804.15 ± 3.74 | 804.55 | 804.53 | 94.65 ± 1.22 | 94.52 | 94.56 | 24.55 ± 0.07 | 24.56 | 24.55 | 364.85 ± 1.24 | 365.01 | 365.22 |
| 13 | 50 | 10 | 80 | 804.65 ± 2.63 | 804.55 | 804.53 | 94.65 ± 0.76 | 94.52 | 94.56 | 24.55 ± 0.05 | 24.56 | 24.55 | 365.40 ± 1.65 | 365.01 | 365.22 |
| 14 | 50 | 10 | 60 | 821.10 ± 1.87 | 820.29 | 821.10 | 96.47 ± 0.45 | 96.43 | 96.47 | 22.85 ± 0.23 | 22.91 | 22.85 | 366.24 ± 0.42 | 365.83 | 366.24 |
| 15 | 50 | 8 | 80 | 811.85 ± 2.66 | 812.16 | 811.85 | 95.52 ± 0.22 | 95.40 | 95.52 | 24.15 ± 0.46 | 24.17 | 24.15 | 364.85 ± 2.18 | 364.71 | 364.63 |
| 16 | 50 | 10 | 100 | 812.81 ± 4.75 | 812.50 | 810.51 | 95.53 ± 1.54 | 95.37 | 95.40 | 23.80 ± 0.07 | 23.77 | 23.80 | 365.85 ± 4.23 | 365.78 | 365.85 |
| 17 | 40 | 10 | 80 | 814.80 ± 3.58 | 813.81 | 814.83 | 96.10 ± 2.18 | 95.98 | 96.10 | 23.25 ± 0.04 | 23.36 | 23.25 | 362.44 ± 1.55 | 362.32 | 362.44 |
| 18 | 50 | 10 | 80 | 804.80 ± 5.43 | 804.55 | 804.53 | 94.68 ± 1.24 | 94.52 | 94.56 | 24.45 ± 0.45 | 24.56 | 24.55 | 365.40 ± 1.22 | 365.01 | 365.22 |
| 19 | 50 | 10 | 80 | 805.65 ± 3.65 | 804.55 | 804.53 | 94.62 ± 1.67 | 94.52 | 94.56 | 24.45 ± 0.45 | 24.56 | 24.55 | 365.40 ± 1.25 | 365.01 | 365.22 |
| 20 | 50 | 12 | 80 | 805.65 ± 3.40 | 804.00 | 805.66 | 94.78 ± 1.35 | 94.78 | 94.78 | 25.44 ± 0.05 | 25.51 | 25.44 | 369.24 ± 2.65 | 368.92 | 369.23 |
| Predictive capacity comparison of RSM and ANN models for five response variables | R2 | 0.96 | 0.99 | 0.97 | 0.99 | 0.98 | 0.98 | 0.96 | 0.99 | ||||||
| RMSE | 1.12 | 0.63 | 0.12 | 0.08 | 0.10 | 0.08 | 0.29 | 0.14 | |||||||
| ADD (%) | 0.1 | 0.04 | 0.11 | 0,05 | 0,3 | 0,13 | 0,07 | 0,02 | |||||||
| TT-BGJ | 40 | 12 | 65 | 822.80 | 97.50 | 24,51 | 368,81 | ||||||||
| BGJ | 792.53 | 92.41 | 22.77 | 357.44 | |||||||||||
| P-BGJ | 775.28 | 87.14 | 20.15 | 344.86 | |||||||||||
TPC: Total phenolic content; TFC: Total flavonoid content; TAC:Total anthocyanin content; GAE: Gallic acid equivalent; CE: Catechin equivalent; mv-3-glu: malvidin 3-monoglucoside; BGJ (untreated black grape juice); P-BGJ (thermal pasteurized black grape juice); TT-BGJ (Thermosonication-treated black grape juice). R2: Coefficient of determination; RMSE: Root mean square error; AAD: Absolute average deviation; RSM: Response surface methodology; ANN: Artificial neural network. Results are presented mean ± standard deviation (n = 3).
The definition of this formula is as follows: the dependent variable (y); the intercept term (βo); the first order (linear) equation coefficient (βi); the quadratic equation coefficient (βii); the two-factor cross-interaction coefficient (βij); and independent variables Xi and Xj.
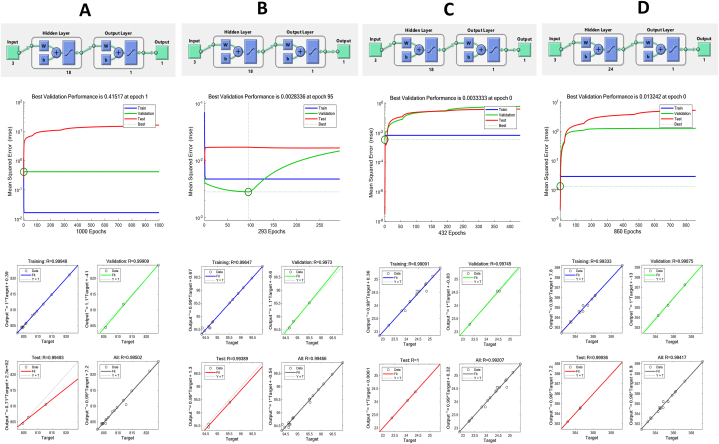
ANN was implemented using the MATLAB Neural Network Toolbox (MATLAB Version R2020b-Mathworks Inc., Natick, MA, USA) to optimize thermosonication processing of black grape juice. The model parameters from our previous study were used [21]. The ANN learning rates applied to bioactive components (antioxidant activity, TFC, TPC, and total anthocyanin content) were set as 0.415, 0.003, 0.003 and 0.013, respectively. ANNs were trained using 1000 iterations. 1000, 293, 432, and 860 were the best performing epochs of the responses. The plot regression function was used measured the performance of the system. The generated neuaral network input, hidden layers and output layers were different for each bioactive component response (Fig. 1A–D).
Fig. 1.
A) TPC, B) TFC, C) Antioxidant, and D) TAC, optimal architecture of developed ANN model, performance plot for the ANN model, the actual values versus the predicted values using the ANN.
To clarify the performance of the models, determination coefficient (R2), root mean square error (RMSE) and absolute average deviation (AAD) were compared between RSM and ANN models. The formulas are written as follows (Equations (2), (3), (4):
| (2) |
| (3) |
| (4) |
where n, YAverage, YPredicted, and YExperimental, are number of data points, the average of data, the predicted value, and the experimental value, respectively. The validity and accuracy of the model were measured based on R2, AAD, and RMSE.
2.3. Determination of bioactive compounds
Methods of bioactive components were used for the optimization of the process conditions. After the optimization, measurements were made again using the optimization conditions in the best parameters. TPC determination was based on the Folin-Ciocalteu method [29]. The calorimetric method determined total flavonoid content (TFC) [30]. Total monomeric anthocyanin content (TAC) was investigated using the pH differential differences method [31]. Absorbances were measured at 528 nm (λvis-max). Trolox equivalent antioxidant activity capacity (TEAC) assay was determined using the method described by Re et al. (1999) using a spectrophotometer (Spectrum Instrument, SP-UV/VIS–300SRB, Australia) [32]. TPC, TFC, TAC, and TEAC results are expressed as mg gallic acid equivalents per L, mg catechin equivalents per L, mg of malvidine-3-O-glucoside (mv-3-glu) equivalents per L, mmol of Trolox equivalent per L, respectively.
2.4. Analysis of volatile compounds
Black grape juice samples were analyzed for volatile components, as described before by Yikmis et al. (2021) (2). The volatile contents of black grape juice samples were identified by calculating the retention index (RI) for each compound using an n-alkane series (C10–C26) under the same conditions. The peak identifications were based on a comparison of the mass spectra of unknown compounds with those in Wiley 8 and NIST 05 mass spectral laboratory. Quantification of volatile compounds was performed from relative abundances as follows (Equation (5):
| (5) |
here, C: Mean relative abundance; Cis: is the concentration of internal standard; Ais: is the peak area of internal standard; and Ac: is the peak area of the compound.
2.5. Determination of organic acid and sugar composition
Organic acid and sugar composition were analyzed by high-performance liquid chromatography (HPLC, model 1260 Infinity LC, Agilent Technologies, Santa Clara, CA, USA) according to the method described by Coelho et al. with some modifications [33]. The black grape juice sample (500 μL) was filtered through a 0.45 μm disc syringe filter, and a 20 μL volume was injected. The ion exchange column was an Agilent Hi-Plex H (300 × 7.7 mm). The temperature of the column was 65 °C while the RID flow cell was maintained at 35 °C. The flow rate applied was 0.6 mL min−1 with a run time of 20 min. The phase was 10.0 mML−1 H2SO4 in ultrapure water. Standard solutions were injected to obtain the retention time for each compound. For the sugars sucrose, fructose, and glucose, RID carried out detection. Detection was conducted in the DAD at 210 nm to determine tartaric, citric, and malic acids. Results are given as g/L.
2.6. Sensory evaluation
The method of sensory evaluation described by of Yikmis (2019) with slight some modifications were used. Trained panelists (32) from Tekirdag Namik Kemal University, participated in the sensory assessment of overall acceptability, mouthfeel, appearance, color, aroma, smell, aroma, color, odor intensity, flavor, and taste [34]. The attributes were scored from 1 (dislike extremely) to 9 (like extremely). In order to explain the differences between the samples, the panelists were asked to evaluate with a 9-point hedonic scale using different codes. They were informed about the study aims and provided written consent to perform the sensory analysis, according to the ethical guidelines of the laboratory of nutrition and dietetics.
2.7. ICP-MS-based mineral compound analysis
The mineral analysis (Fe, Zn, Cu, Mg, K, Na, and Mn) of the black grape juice samples was completed with inductively-coupled plasma optical emission spectroscopy (ICP-OES) (Thermo Scientific iCap 6000 Dual view, Thermo Scientific, Cambridge, England). The sample was dissolved using a microwave digestion system (Berghof Instruments, Speedwave, Germany).
The 3-stage microwave digestion method recommended by the manufacturer was followed for elemental analysis. For the microwave digestion system, hydrogen peroxide (H2O2) solution (Merck, Darmstadt, Germany) and nitric acid (Sigma-Aldrich Corp, St Louis, USA) were used. The dissolution was performed using the method of Sezer et al. with slight modifications (Sezer et al., 2019). Each sample (3 mL) was accurately transferred into Teflon digestion vessels, and 4 mL of 65% (m/v) HNO3 solution and 1 mL of 35% (m/v) H2O2solution were added.
2.8. Statistical analysis
The experimental results are expressed as mean ± standard deviation (SD) of three replicates for each treatment. Data analysis was performed using SigmaPlot 12.0 Software (Systat Software, Inc., USA), and SPSS 22.0 software (SPSS Inc., Chicago, USA). Analysis of variance (one-way ANOVA) followed by Tukey's multiple comparison post hoc test was performed to compare samples. Principal component analysis (PCA) of volatile compounds data was performed using JMP (12.2.0 SAS Institute, Inc., Cary, NC, USA). Values of p < 0.05 were considered significant.
3. Results and discussion
3.1. Optimization of TPC, TFC, TEAC, and TAC
The experimental and predicted results for thermosonication treatments of black grape juice samples with different amplitude levels, temperatures, and times on TPC, TFC, antioxidant activity, and TAC values are showed in Table 1. After thermosonication, the results were subjected to a second-order polynomial regression model. Equations for TPC, TFC, TAC, and antioxidant activity results are given below (Equations (6), (7), (8), (9)).
| (6) |
| (7) |
| (8) |
| (9) |
The analysis of variance for TFC, TPC, antioxidant activity, and TAC as a result of RSM was given Table 2. The F values of the models were determined as 27.36, 45.58, 50.62 and 27.02, respectively, and this shows that the developed polynomial model is significant. The coefficient of determination, adjusted R2 and R2 values were over 96%, respectively, which showed that the model was suitable for the experimental results (Table 2). The effect of three independent variables (amplitude, time, and temperature) on black grape juice bioactive attributes was explained by the significance coefficient (p < 0.05) of the quadratic polynomial model.
Table 2.
Corresponding p-values of linear, interaction and quadratic terms of regression coefficients obtained by RSM of responses for TPC and DPPH experiments.
| Source | DF | TPC (mg GAE/L) |
DPPH (mg TEAC/mL) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Adj SS | Adj MS | F-Value | p-Value | Adj SS | Adj MS | F-Value | p-Value | ||
| Model | 9 | 1374.39 | 152.71 | 56.66 | 0.000 | 0.031597 | 0.003511 | 76.12 | 0.000 |
| Linear | 3 | 326.22 | 108.742 | 40.34 | 0.001 | 0.006922 | 0.002307 | 50.03 | 0.000 |
| X1 | 1 | 108.34 | 108.339 | 40.2 | 0.001 | 0.000476 | 0.000476 | 10.31 | 0.024 |
| X2 | 1 | 120.53 | 120.528 | 44.72 | 0.001 | 0.001762 | 0.001762 | 38.21 | 0.002 |
| X3 | 1 | 97.36 | 97.357 | 36.12 | 0.002 | 0.004684 | 0.004684 | 101.57 | 0.000 |
| Square | 3 | 906.64 | 302.215 | 112.13 | 0.000 | 0.01805 | 0.006017 | 130.46 | 0.000 |
| X12 | 1 | 37.77 | 37.774 | 14.01 | 0.013 | 0.001385 | 0.001385 | 30.03 | 0.003 |
| X22 | 1 | 653.48 | 653.476 | 242.45 | 0.000 | 0.008202 | 0.008202 | 177.84 | 0.000 |
| X32 | 1 | 224.55 | 224.554 | 83.31 | 0.000 | 0.010628 | 0.010628 | 230.45 | 0.000 |
| 2-Way Interaction | 3 | 141.52 | 47.173 | 17.5 | 0.004 | 0.006624 | 0.002208 | 47.88 | 0.000 |
| X1*X2 | 1 | 35.88 | 35.88 | 13.31 | 0.015 | 0.000289 | 0.000289 | 6.27 | 0.054 |
| X1*X3 | 1 | 104.04 | 104.04 | 38.6 | 0.002 | 0.004619 | 0.004619 | 100.14 | 0.000 |
| X2*X3 | 1 | 1.6 | 1.598 | 0.59 | 0.476 | 0.001717 | 0.001717 | 37.22 | 0.002 |
| Error | 5 | 13.48 | 2.695 | 0.000231 | 0.000046 | ||||
| Lack-of-Fit | 3 | 13.41 | 4.47 | 136.71 | 0.007 | 0.000169 | 0.000056 | 1.81 | 0.375 |
| Pure Error | 2 | 0.07 | 0.033 | 0.000062 | 0.000031 | ||||
| Total | 14 | 1387.86 | 0.031828 | ||||||
| R2 | 99.03% | 99.28% | |||||||
| Adj R2 | 97.28% | 97.97% | |||||||
| Pred. R2 | 84.53% | 91.09% | |||||||
X1: Tempature; X2: Time; X3: Amplitude; Adj SS: sums of squares; Adj MS: Adjusted mean squares; DF: Degree of freedom; TPC: Total phenolic content; DPPH: 1,1-Diphenyl-2-picrylhydrazyl radical; GAE: Gallic acid equivalent; TEAC: Trolox equivalent antioxidant capacity; p-values less than 0.05 indicate model terms are significant.
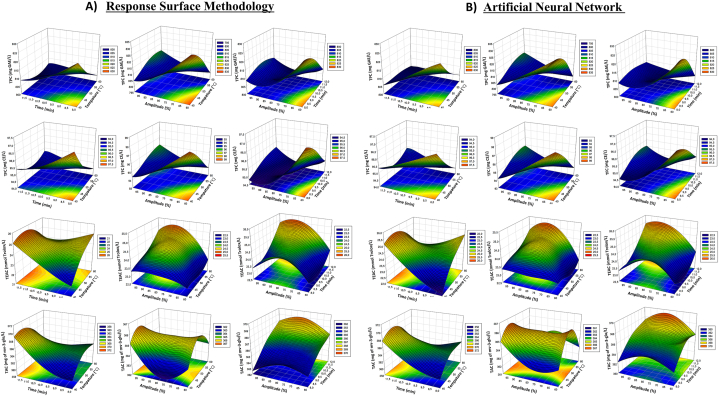
To determine the effects of independent variables on bioactive attributes (TPC, TFC, antioxidant activities and TAC), response surface graphs (3-dimensional) were created to determine the optimum values for the bioactive components. The effect of thermosonication parameters on TPC is illustrated in the response surface plots shown in Fig. 2A–B. In particular, the increase in duration and amplitude positively affected TPC, consistent with previous studies about the increased phenolic compounds [35,36]. A 3.8% increase after thermosonication treatment compared to the BGJ sample (Table 1). In the P-BGJ sample, a decrease of 2.2% was observed compared to the BGJ sample. It was determined that thermosonication treatment is superior to thermal pasteurization, as in the studies applied to samples of fresh pomegranate juice [37], black carrot juice [38] and beetroot (Beta vulgaris L.) juice [39]. The influence of thermosonication parameters on TFC is illustrated in the response surface plots shown in Fig. 2A–B. Thermal pasteurization further destroyed the total flavonoid content of black grape juice (6% reduction). With thermosonication treatment, the amount of TFC increased by 5.5%. Similar increases were observed in orange juice whey drinks [40] and black, white, and red currant juices [41].
Fig. 2.
Response surface plots (3D) for total phenoic content, total flavonoid content, total anthocyanin content, and TEAC analysis as a function of significant interaction factors for A) RSM and B) ANN.
The influence of thermosonication parameters on total antioxidants is illustrated in the response surface plots shown in Fig. 2A–B. Thermosonication caused an increase (7.6% increase) in the antioxidant activity content of black grape juice, while thermal pasteurization caused a decrease (13% decrease). Increasing the ultrasound application parameters was reported to increase the antioxidant activity in black carrot juice, as in our study [38]. A similar result was observed in other studies where thermosonication of purple cactus pear juice and amora (Spondius pinnata) juice caused an increase in antioxidant activity values compared to heat treatment. The influence of thermosonication parameters on TAC is illustrated in the response surface plots shown in Fig. 2A–B. Thermosonication parameters did not cause the degradation of anthocyanins. Thermal pasteurization resulted in a reduction of 12.58 mg of mv-3-glu/L in the BGJ sample. Li et al. found that red radish had increased antioxidant levels in their study of different thermosonication treatments, but it caused some deterioration in anthocyanins [42]. Tiwari et al. reported that ultrasound applied to grape juice could be chosen to preserve anthocyanin retention [43]. In the juices obtained from blue honeysuckle (Lonicera caerulea L.), anthocyanin levels were increased with ultrasound treatment [44]. The increase of bioactive compounds with thermosonication is due to the cavitation phenomenon. At the same time, the increase in TFC and TPC associated with the production of OH radicals during ultrasound may cause hydroxylation of phenolic compounds in aromatic rings at different positions, including para-, meta- and ortho positions, or the transfer of bound configuration of phenolic compounds as a result of cell wall breakage [45,46].
The 3D response surfaces show that the interaction of independent parameters affects the bioactive components in black grape juice. While keeping the third independent parameter at the center point, Fig. 2A–B indicates the effect of two independent parameters simultaneously. It was observed that thermosonication temperature did not significantly affect the increased bioactive components in black grape juice. Numerical optimization was performed to obtain the optimum combination of temperature, time and amplitude values to achieve maximum bioactive yield from black grapes. All three independent variables were kept within the required ranges, and the black grape juice bioactive increase was maximized. Optimal combinations were obtained at 40 °C, 12 min and 65% amplitude. Under these conditions, TPC, TFC, antioxidant, and TAC values were 822.80 mg GAE/L, 97.50 mg CE/L, 24.51 mmol Trolox/L, and 368, 81 mg of mv-3-glu/L, respectively (Table 1).
The validation values revealed that the slight variation or % difference between the predicted values and the trial response data from the optimum conditions confirmed the validity of the proposed optimum thermosonication conditions. So, the predicted model is applicable to optimize thermosonication conditions for bioactive components (TPC, TFC, antioxidant, and TAC).
3.2. RSM and ANN model comparison
AAD, MSE, and R2 were determined to compare the ANN and RSM models (Table 3). The higher R2 value for the ANN model compared to the RSM model, valid for all bioactive components, indicates better prediction ability and accuracy for the ANN model. When the RSME values for the ANN model are examined, TFC, TPC, TAC, and antioxidant values were measured as 0.08, 0.63, 0.14, and 0.08, respectively. The RSME values for the ANN model were lower than for RSM. When the ADD values for the ANN model are examined, TPC, TFC, antioxidant and TAC values were measured as 0.05, 0.05, 0.13 and 0.02, respectively. Compared to the RSM model, the ANN model had better ADD results. The results for all modeling showed that RSM and ANN models had good accuracy and predictive ability; but it was determined that the ANN model is superior to the RSM model. In the optimization of processes, as in our study, ANN showed superiority over RSM in general [47,48].
Table 3.
Experimental values (data) of confirmation of predicted values.
| Parameters | Experimental values | Predicted values | % Difference |
|---|---|---|---|
| TPC (mg GAE/L) | 838.24 | 822.80 ± 5.18 | 1.88 |
| TFC (mg CE/L) | 96.37 | 97.50 ± 1.44 | 1.16 |
| Antioxidant TEAC (mmol Trolox/L) | 24.28 | 24.51 ± 0.67 | 0.94 |
| TAC (mg of mv-3-glu/L) | 359.76 | 368.81 ± 4.55 | 2.45 |
TPC: Total phenolic content; TFC: Total flavonoid content; TAC:Total anthocyanin content; GAE: Gallic acid equivalent; CE: Catechin equivalent; TEAC: Trolox equivalent antioxidant capacity; mv-3-glu: malvidin-3-glucoside. Results are presented mean ± standard deviation (n = 3).
3.3. Aroma profiles
A total of 31, 26, and 29 volatile compounds were detected in the untreated black grape juice (BGJ), thermal pasteurized black grape juice (P-BGJ), and thermosonication-treated black grape juice (TT-BGJ), respectively (Table 4). While the highest aroma compound amount was 735.87 μg/kg in the BGJ sample, 329.98 μg/kg, and 495.31 μg/kg in P-BGJ and TT-BGJ samples, respectively. In total, 8 aldehyde compounds were identified in the samples, with 2-hexenal, hexenal, and acetaldehyde being dominant. Among the aldehydes, 2-hexenal was the most abundant in all samples. The highest 2-hexenal concentration was found in the BGJ sample with 182.22 μg/kg, followed by TT-BGJ with 122.74 μg/kg and P-BGJ with 80.88 μg/kg. The only ketone compound detected in black grape juice samples was 6-methyl-5-hepten-2-one. The highest amount of 6-methyl-5-hepten-2-one was 9.29 μg/kg detected in the BGJ sample, followed by 6.58 μg/kg and 4.51 μg/kg in the TT-BGJ and P-BGJ samples, respectively. 1-Hexanol, ethanol, and 3-methyl-1-butanol were the highest alcohol compounds detected in all samples. The concentrations of these predominant alcohol compounds decreased with pasteurization and thermosonication treatments. In total, four esters were found in black grape juice samples. Ethyl hexanoate was the most abundant ester in BGJ and TT-BGJ samples, while ethyl acetate was the most abundant ester in P-BGJ samples. In the acids group, six acids were identified in black grape juice samples with total concentrations of 172.89 μg/kg (BGJ), 75.48 μg/kg (P-BGJ), and 115.33 μg/kg (TT-BGJ). Limonene and ρ-cymene were recovered from black grape juice samples. Limonene was the most abundant terpene in all samples. The totals for acid, aldehyde, terpene, ketone, ester, and alcohol aroma compounds after thermal pasteurization of black grape juice were lower than after thermosonication treatment. Cheng et al. (2020) reported the same results for mandarin (Citrus unshiu) juice, while Yikmis et al. (2022) found similar results for pomegranate juice [37,49]. The good effects on aroma components can be related to possible synergistic impacts of cavitation and temperature during thermosonication treatment [50]. Some volatile compounds disappeared after pasteurization and thermosonication treatment. Previous works reported similar results [21,37,51].
Table 4.
Determination of volatile profiles of BGJ, P- BGJ, and TT- BGJ.
| Organic volatile compounds (RI) | Samples |
||
|---|---|---|---|
| BGJ (μg/kg) | P-BGJ (μg/kg) | TT-BGJ (μg/kg) | |
| Acetaldehyde (820) | 11.24 ± 0.53a | 5.68 ± 0.62b | 7.91 ± 0.30c |
| 2-methyl butanal (904) | 3.49 ± 0.28a | 1.76 ± 0.13b | 2.12 ± 0.23b |
| 3-methyl butanal (920) | 3.40 ± 0.16a | n.d. | 1.51 ± 0.18b |
| Hexanal (1081) | 34.08 ± 0.80a | 13.24 ± 1.10b | 21.89 ± 1.47c |
| 2-Hexenal (1224) | 182.22 ± 9.09a | 80.88 ± 3.34b | 122.74 ± 5.10c |
| Nonanal (1396) | 5.24 ± 0.10a | n.d. | 5.30 ± 0.23a |
| Decanal (1502) | 6.69 ± 0.47a | 2.43 ± 0.35b | 4.11 ± 0.36c |
| Benzaldehyde (1542) | 4.79 ± 0.18a | 1.88 ± 0.19b | 1.67 ± 0.11b |
| Total aldehids | 251.12 ± 8.39a | 105.85 ± 3.53b | 167.22 ± 7.17c |
| Ethanol (929) | 74.36 ± 4.07a | 29.96 ± 1.12b | 60.71 ± 2.23c |
| 2-methyl-1-propanol (1091) | 1.31 ± 0.16a | n.d. | n.d. |
| 2-methyl-1-butanol (1198) | 8.39 ± 0.33a | 5.12 ± 0.34b | 4.69 ± 0.46b |
| 3-methyl-1-butanol (1204) | 48.99 ± 2.47a | 19.44 ± 0.95b | 30.82 ± 1.92c |
| 1-Hexanol (1356) | 96.72 ± 5.01a | 56.68 ± 2.17b | 65.18 ± 2.74b |
| 1-Heptanol (1460) | 1.81 ± 0.08a | n.d. | 1.21 ± 0.10b |
| 2-Ethyl-1-hexanol (1496) | 4.71 ± 0.18a | 1.27 ± 0.18b | 2.52 ± 0.33c |
| Linalool (1548) | 1.92 ± 0.22a | n.d. | 1.48 ± 0.16a |
| 1-Octanol (1562) | 3.08 ± 0.19a | 1.86 ± 0.16b | n.d. |
| 1-Nonanol (1662) | 2.69 ± 0.08a | 0.89 ± 0.06b | 3.03 ± 0.30b |
| Total alcohols | 243.96 ± 10.9a | 115.2 ± 1.27b | 169.62 ± 1.09c |
| Acetic acid (1458) | 5.11 ± 0.23a | 2.49 ± 0.18b | 2.89 ± 0.35b |
| 2-Methyl propanoic acid (1568) | 19.48 ± 1.61a | 10.19 ± 0.42b | 13.57 ± 0.86b |
| Hexanoic acid (1851) | 51.78 ± 2.77a | 20.1 ± 1.22b | 38.78 ± 2.02c |
| Octanoic acid (2064) | 35.46 ± 3.08a | 15.26 ± 1.24b | 22.76 ± 2.29b |
| Nonanoic acid (2162) | 18.64 ± 0.97a | 8.78 ± 0.52b | 11.61 ± 1.23b |
| Decanoic acid (2248) | 42.43 ± 2.54a | 18.68 ± 1.17b | 25.72 ± 3.41b |
| Total acids | 172.89 ± 3.26a | 75.48 ± 1.11b | 115.33 ± 5.59c |
| Ethyl acetate (886) | 13.42 ± 1.28a | 7.75 ± 0.51b | 7.32 ± 0.34b |
| Butyl acetate (1070) | 5.89 ± 0.31a | 3.22 ± 0.35b | 4.96 ± 0.31a |
| Ethyl hexanoate (1236) | 18.49 ± 1.35a | 7.63 ± 0.55b | 12.4 ± 0.87c |
| Ethyl octanoate (1442) | 11.69 ± 0.38a | 7.01 ± 0.74b | 6.89 ± 0.19b |
| Total esters | 49.48 ± 3.32a | 25.6 ± 0.66b | 31.56 ± 0.65c |
| Limonene (1196) | 5.34 ± 0.25a | 1.98 ± 0.21b | 3.06 ± 0.30c |
| ρ-cymene (1274) | 3.79 ± 0.11a | 1.36 ± 0.24b | 1.94 ± 0.12b |
| Total terpenes | 9.13 ± 0.36a | 3.34 ± 0.45b | 5.00 ± 0.18c |
| 6-Methyl-5-hepten-2-one (1342) | 9.29 ± 0.20a | 4.51 ± 0.30b | 6.58 ± 0.47c |
| Total ketones (mg/kg) | 9.29 ± 0.20a | 4.51 ± 0.30b | 6.58 ± 0.47c |
RI: Retention Index; n.d: not determined; BGJ (untreated black grape juice); P-BGJ (thermal pasteurized black grape juice); TT-BGJ (Thermosonication-treated black grape juice). Results are presented mean ± standard deviation (n = 3). Values with the different letters within line are significantly different (p < 0.05).
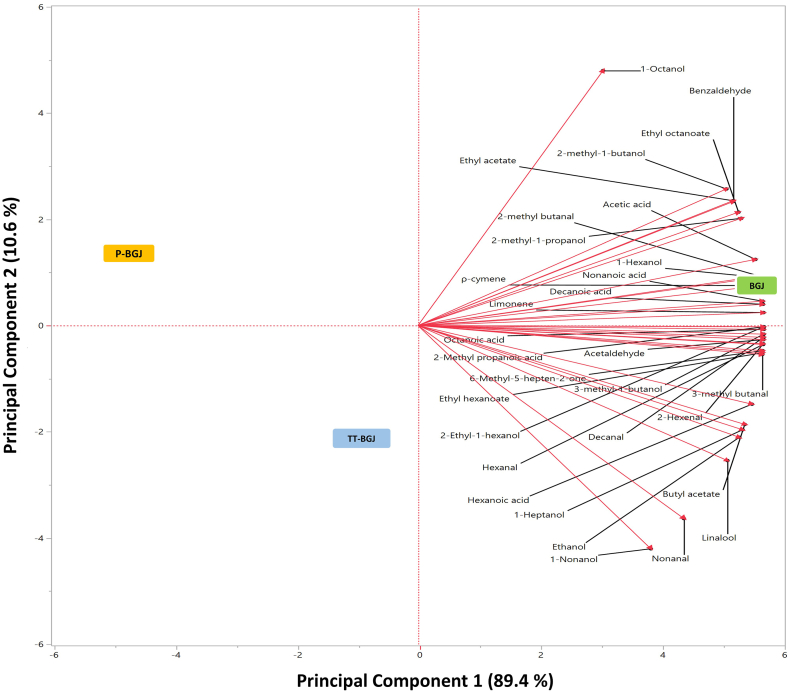
Principal component analysis (PCA) evaluated the differences between BGJ, P-BGJ, and TT-BGJ black grape juice samples regarding volatile compounds. PCA is suitable for grouping volatile compounds according to their special location and distinguishing black grape juice samples. The PCA plot in Fig. 3 shows the distribution of samples for compounds. Eigenvector values in the score graph evaluating all black grape juice samples were obtained as 100% (PC1 = 89.4% and PC2 = 10.6%). TT-BGJ was negatively loaded on PC1 and PC2. When BGJ was positively loaded on PC1 and PC2, P-BGJ was negatively loaded on PC1 and positively loaded on PC2.
Fig. 3.
PCA bi-plot of volatile compounds in black grape juice samples. BGJ: untreated black grape juice; P-BGJ: thermal pasteurized black grape juice; TT-BGJ: thermosonication-treated black grape juice.
3.4. Organic acid and sugar content
The results for organic acid contents analyses of BGJ, P-BGJ, and TT-BGJ samples are shown in Table 5. When it comes to grape juices, following sugars, organic acids are the main solids and are influenced by many factors such as climatic region, sunlight exposure, grape variety, cultural practices, maturity index, and soil chemistry [52]. The main acids present in BGJ, P-BGJ, and TT-BGJ were tartaric acid, with values of 5.02 ± 0.18, 4.28 ± 0.13, and 4.65 ± 0.10 g L−1, respectively. Similarly to this result, Coelho et al. reported that the main acid present in grape juice was tartaric acid, with concentrations ranging from 4.02 ± 0.01 to 5.38 ± 0.01 [33]. There was no significant decrease in malic acid, tartaric acid, and citric acid content in TT-BGJ. Tokatli Demirok (2022), found similar effects for organic acid, and statistically significant differences were not detected (p > 0.05) in control and ultrasound-treated mallow vinegar [53]. There were no significant differences in the concentrations of organic acid in sonication-treated prebiotic cranberry juice [54], similar to our study. There was a significant (p < 0.05) decrease in tartaric acid content in thermal pasteurized black grape juice. Contrary to our results, Margean et al. (2020) found significantly higher contents (p < 0.05) of tartaric acid in the pasteurized and high-power ultrasound (20 kHz, 70% amplitude, 10 min) treated red grape juice [2].
Table 5.
Organic acid and sugar contents results of samples BGJ, P-BGJ and TT-PGJ.
| Sample | Sugar Composition |
Organic Acid Composition |
||||
|---|---|---|---|---|---|---|
| Sucrose (g/L) | Fructose (g/L) | Glucose (g/L) | Citric acid (g/L) | Malic acid (g/L) | Tartaric acid (g/L) | |
| BGJ | 0.22 ± 0.04a | 97.79 ± 2.64a | 90.88 ± 1.07a | 0.14 ± 0.01a | 2.19 ± 0.21a | 5.02 ± 0.18a |
| P-BGJ | 0.18 ± 0.02a | 86.54 ± 1.58b | 83.98 ± 1.64b | 0.09 ± 0.02a | 1.86 ± 0.06a | 4.28 ± 0.13b |
| TT-BGJ | 0.14 ± 0.01a | 91.34 ± 2.59ab | 87.01 ± 1.73ab | 0.11 ± 0.01a | 1.85 ± 0.08a | 4.65 ± 0.10ab |
P BGJ (untreated black grape juice); P-BGJ (thermal pasteurized black grape juice); TT-BGJ (Thermosonication-treated black grape juice). Results are presented mean ± standard deviation (n = 3). Values with the different letters within the column are significantly different (p < 0.05).
Results regarding the effect of sonication and thermal pasteurization treatments on fructose, glucose, and sucrose are shown in Table 5. Fructose was the main sugar in black grape juice samples, followed by glucose and sucrose. Dutra et al. (2021) reported glucose was the main sugar in whole grape juice, followed by fructose and maltose [55]. After ultrasound treatment, an insignificant decrease in fructose, sucrose, and glucose was detected compared to the control black grape juice sample. Contrary to our results, Erdal et al. (2022) reported that sonication processing caused a significant decrease in the concentration of sucrose and glucose in gilaburu vinegar [56]. In the report by Abid et al. (2014), the opposite effects were seen; they found significant increases in glucose, sucrose, and fructose amounts in all sonicated apple juice samples compared to controls [8]. Silva et al. (2020) found increasing sucrose content with 300, 600, and 1200 W high-intensity ultrasound treatment of prebiotic orange juice [18]. Another study reported that increases in glucose and sucrose were detected after ultrasound treatment applied to grapefruit juice [7]. There was a significant (p < 0.05) decrease in fructose and glucose content in thermal pasteurized black grape juice compared to control black grape juice. Thermal processing of fruit juices leads to sugar and vitamin loss, browning reactions, and causes loss of the quality of fruit juices [57].
3.5. Sensory analyses
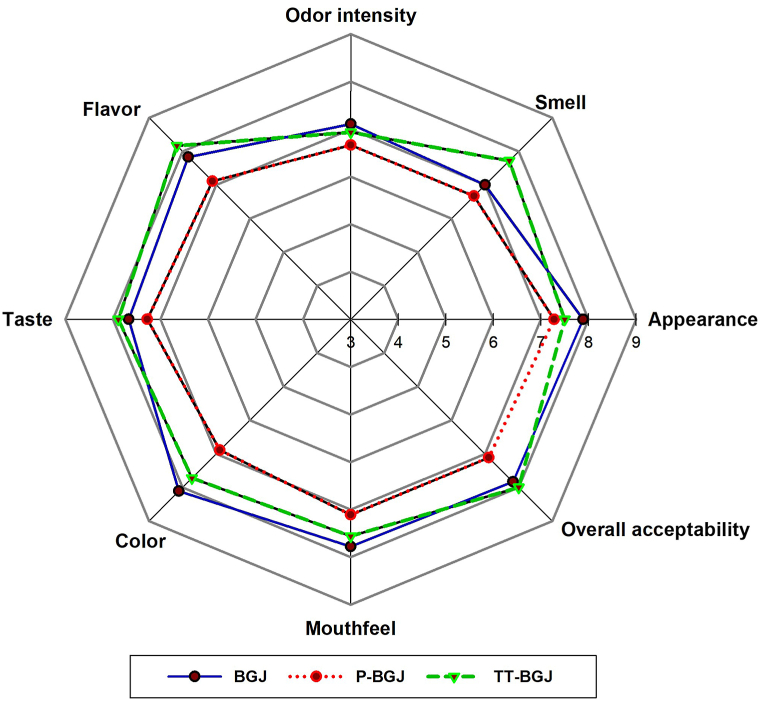
The sensory evaluation test evaluated appearance, smell, odor, flavor, taste, color, mouthfeel, and overall acceptability features. Fig. 4 reports the results of sensory analysis values for black grape juices subjected to different processes. As shown in Fig. 4, odor was not affected by processes applied to the black grape juices. On the other hand, appearance, flavor, taste, color, mouthfeel and overall acceptability were significantly affected by the thermal pasteurization process. Moreover, thermosonication treatment affected the appearance, smell, flavor, taste, mouthfeel features significantly (p < 0.05) (Fig. 4). There were significant increases for panelist approval and points in terms of smell, mouthfeel and flavor properties with the thermosonication process. According to the hedonic scale, there were significant decreases in the points for color, appearance, and overall acceptability with the thermal pasteurization process, while no change in these properties was detected with the thermosonication process. These results were on par with insignificant differences for the color scores of ultrasound-treated blueberry–grape–pineapple–cantaloupe juice and soursop juice [58,59]. Also, Yildiz and Aadil (2022) stated that high-intensity ultrasound showed good performance for the prevention of color degradation in mango fruit pieces [60]. Munir et al. (2022) reported that maximum points for overall acceptability were determined for the treatment using 21 J/g ultrasound processing technique compared to microwave and high-pressure processing in cheddar, similar to this study. They reported that the 41 J/g ultrasound-treated sample received lower score than the 21 J/g ultrasound-treated sample for sensory properties [61]. Erkaya et al. (2015), found the sensory properties of the ultrasound-treated fermented milk drink were better than thermally-treated samples [62]. Likewise, the panelists acceptance of ultrasound and UV combinations was higher than for thermally-pasteurized pineapple juice in terms of taste, overall acceptance, and flavor [63]. Contrary to this study, the appearance of ultrasound-treated sohiong (Prunus nepalensis L.) juice samples was reported to be significantly lower (p ≤ 0.05) than native samples [64]. Sensory properties are among the most useful and significant parameters to determine the quality of end food products [61]. Ultrasound treatment can change the juice structure with health effects [65].
Fig. 4.
Sensory analysis values chart for black grape juices. BGJ: untreated black grape juice; P-BGJ: thermal pasteurized black grape juice; TT-BGJ: thermosonication-treated black grape juice.
3.6. ICP-MS mineral compound analysis results
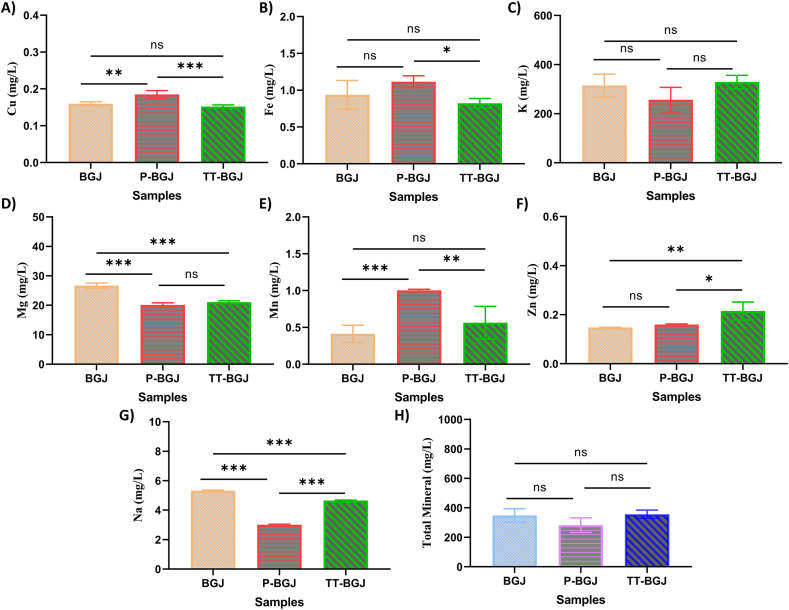
The elemental composition of the black grape juice samples obtained via ICP-OES is shown in Fig. 5A–H. Mn, K, Fe, Cu, Mg, Zn and Na mineral elements were detected in the black grape juice samples. The K, Mg, Na, Fe, Mn, Cu, and Zn levels in the untreated black grape juice samples were 314.81, 26.70, 5.32, 0.82, 0.41, 0.16, and 0.15 mg/L, respectively. Total mineral content was determined as 348.49 mg/L. Although grape variety has a significant influence on the mineral concentration, the concentration of K was the highest among the seven elements, which is consistent with previous studies [66,67].
Fig. 5.
Cu (A), Fe (B), K (C), Mg (D), Mn (E), Zn (F), Na (G), and total mineral (H) of untreated black grape juice (BGJ), thermal pasteurized black grape juice (P-BGJ), thermosonication-treated black grape juice (TT-BGJ). Letters atop bars indicate statistically significant differences (ns: no significant; *p < 0.05; **p < 0.01; ***p < 0.001), (n = 3 ± SD).
Mineral content is an important quality criterion affecting many properties of fruits, like pH, acidity, Brix, and sensory properties, and it can be affected by the processes applied to fruit juice [68]. As shown in Fig. 5A–G, although there were no significant changes in K content and total mineral content, significant differences were detected in the Ca, Mg, Mn, Zn, and Na contents. After thermal pasteurization (P), while Cu and Mn contents increased, Mg and Na contents decreased significantly (p < 0.001). As the thermosonication process was applied, a statistically significant decrease in Mg and Na contents was noted (p < 0.001). There were no significant changes in Ca, Fe, Zn, and total mineral contents due to thermal pasteurization. Cu, Fe, K, and Mn and total mineral contents were not significantly altered when the black grape juice samples were exposed to the thermosonication process. As a result, Cu, Fe, and Mn contents were increased in the thermally-pasteurized black grape juice sample (P-BGJ). Our study is in good agreement with the study which found that ultrasound and pasteurization treatments affected the mineral content of fruit juice [69]. Yikmis et al. (2022) stated that there were no statistically significant changes in levels of Fe in vinegar samples after ultrasound and pasteurization treatments (p > 0.05). Manzoor et al. (2020) reported that there was a significant increase in K content and significant decreases in Fe, Ca and Zn in the spinach; but, the opposite results were found for these elements in present research [70]. In fact, the effect of cavitation, which is an outcome of ultrasound treatment, on minerals is not fully understood; however, the migration of some minerals from the cells into the solution and the increase in the activity of substances in the cells may lead to an increase in mineral content. It is recommended that further studies be performed to better understand mineral matter variations and the sonochemical process.
4. Conclusion
Black grape juice is an important fruit juice due to its bioactive components, nutritional content, and aroma profile. Bioactive components of black grape juice were increased by thermosonication processing. The RSM and ANN estimates showed high similarity with the experimental results. RSM and ANN models were used for increased and had superior features, while ANN was more predictive. It was determined that thermosonication preserves the aroma and mineral content better than thermal pasteurization. Black grape juice processed by thermosonication had superior taste. The current study implied that thermosonication as a good alternative to thermal pasteurization could be used to enhance bioactive compenents of black grape juice. In future studies, evaluating the amino acid content, phenolic compounds, and health effects such as anticancer and antimicrobial properties is recommended.
Ethics statement
The approval statement through an Ethical Committee is not applicable in this case because in our country sensory tests doesn't require ethical approval. Sensory experiments were performed according to established ethical guidelines and informed consent was obtained from the participants.
Author contribution statement
Seydi Yıkmıs: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Nazan Tokatlı Demirok: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Okan Levent: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Demet Apaydin: Performed the experiments; Wrote the paper.
Data availability statement
The authors do not have permission to share data.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Bendaali Y., Vaquero C., González C., Morata A. Elaboration of an organic beverage based on grape juice with positive nutritional properties. Food Sci. Nutr. 2022;10:1768–1779. doi: 10.1002/FSN3.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margean A., Lupu M.I., Alexa E., Padureanu V., Canja C.M., Cocan I., Negrea M., Calefariu G., Poiana M.A. An overview of effects induced by pasteurization and high-power ultrasound treatment on the quality of red grape juice. 2020. Mol. 2020, Vol. 25, Page 1669. 25. [DOI] [PMC free article] [PubMed]
- 3.El Kersh D.M., Hammad G., Donia M.S., Farag M.A. A comprehensive review on grape juice beverage in context to its processing and composition with future perspectives to maximize its value. Food Bioprocess Technol. 2022 doi: 10.1007/s11947-022-02858-5. [DOI] [Google Scholar]
- 4.FAO, Food and Agriculture Organization 2011 Production... - Google Akademik. 2011 [Google Scholar]
- 5.Paun N., Botoran O.R., Niculescu V.C. Total phenolic, anthocyanins HPLC‐DAD‐MS determination and antioxidant capacity in black grape skins and blackberries: a comparative study. Appl. Sci. 2022;12 doi: 10.3390/APP12020936. [DOI] [Google Scholar]
- 6.Salah Eddine N., Tlais S., Alkhatib A., Hamdan R. 2020. Effect of Four Grape Varieties on the Physicochemical and Sensory Properties of Unripe Grape Verjuice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aadil R.M., Zeng X.A., Wang M.S., Liu Z.W., Han Z., Zhang Z.H., Hong J., Jabbar S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food Sci. Technol. 2015;50:1144–1150. doi: 10.1111/IJFS.12767. [DOI] [Google Scholar]
- 8.Abid M., Jabbar S., Wu T., Hashim M.M., Hu B., Lei S., Zeng X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014;21:93–97. doi: 10.1016/J.ULTSONCH.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Danışman G., Arslan E., Kırca Toklucu A. Kinetic analysis of anthocyanin degradation and polymeric colour formation. Czech J. Food Sci. 2015:103–108. [Google Scholar]
- 10.Erihemu M., Wang F., Zhang D., Wang M., Zhao N., Cui G., Gao J., Guo Q., Zhang Optimization of the process parameters of ultrasound on inhibition of polyphenol oxidase activity in whole potato tuber by response surface methodology. Lebensm. Wiss. Technol. 2021;144 doi: 10.1016/J.LWT.2021.111232. [DOI] [Google Scholar]
- 11.Gao X., Feng T., Liu E., Shan P., Zhang Z., Liao L., Ma H. Ougan juice debittering using ultrasound-aided enzymatic hydrolysis: impacts on aroma and taste. Food Chem. 2021;345 doi: 10.1016/J.FOODCHEM.2020.128767. [DOI] [PubMed] [Google Scholar]
- 12.Tokatlı Demirok N., Yıkmış S. Combined effect of ultrasound and microwave power in tangerine juice processing: bioactive compounds, amino acids, minerals, and pathogens. Processes. 2022;10:2100. doi: 10.3390/PR10102100. 10 (2022) 2100. [DOI] [Google Scholar]
- 13.Ordóñez-Santos L.E., Martínez-Girón J., Arias-Jaramillo M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017;233:96–100. doi: 10.1016/J.FOODCHEM.2017.04.114. [DOI] [PubMed] [Google Scholar]
- 14.Kwaw E., Ma Y., Tchabo W., Sackey A.S., Apaliya M.T., Xiao L., Wu M., Sarpong F. Ultrasonication effects on the phytochemical, volatile and sensorial characteristics of lactic acid fermented mulberry juice. Food Biosci. 2018;24:17–25. doi: 10.1016/j.fbio.2018.05.004. [DOI] [Google Scholar]
- 15.de Souza Carvalho L.M., Lemos M.C.M., Sanches E.A., da Silva L.S., de Araújo Bezerra J., Aguiar J.P.L., das Chagas do Amaral Souza F., Alves Filho E.G., Campelo P.H. Improvement of the bioaccessibility of bioactive compounds from Amazon fruits treated using high energy ultrasound. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105148. [DOI] [PubMed] [Google Scholar]
- 16.Yıkmış S. Sensory, physicochemical, microbiological and bioactive properties of red watermelon juice and yellow watermelon juice after ultrasound treatment. J. Food Meas. Char. 2020;14:1417–1426. doi: 10.1007/s11694-020-00391-7. [DOI] [Google Scholar]
- 17.Levent O., Yikmiş S., Tokatlı Demirok N. Optimization of bioactive components in fresh red watermelon juice of ultrasound assisted extraction conditions with response surface methodology. J. Agric. Fac. Gaziosmanpasa Univ. 2022;39:113–119. doi: 10.55507/gopzfd.1138189. [DOI] [Google Scholar]
- 18.Silva E.K., Arruda H.S., Pastore G.M., Meireles M.A.A., Saldaña M.D.A. Xylooligosaccharides chemical stability after high-intensity ultrasound processing of prebiotic orange juice. Ultrason. Sonochem. 2020;63 doi: 10.1016/J.ULTSONCH.2019.104942. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Wang J., Ye J., Vanga S.K., Raghavan V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control. 2019;96:128–136. doi: 10.1016/J.FOODCONT.2018.09.007. [DOI] [Google Scholar]
- 20.Zhang H., Li H., Zhang Z., Hou T. 2021. Optimization of Ultrasound-Assisted Extraction of Polysaccharides from Perilla Seed Meal by Response Surface Methodology: Characterization and in Vitro Antioxidant Activities. [DOI] [PubMed] [Google Scholar]
- 21.Yıkmış S., Bozgeyik E., Levent O., Aksu H. Organic cherry laurel (Prunus laurocerasus) vinegar enriched with bioactive compounds with ultrasound technology using artificial neural network (ANN) and response surface methodology (RSM): antidiabetic, antihypertensive, cytotoxic activities, volatile profile and optical microstructure. J. Food Process. Preserv. 2021;45 doi: 10.1111/JFPP.15883. [DOI] [Google Scholar]
- 22.Abdullah S., Pradhan R.C., Aflah M., Mishra S. Efficiency of tannase enzyme for degradation of tannin from cashew apple juice: modeling and optimization of process using artificial neural network and response surface methodology. J. Food Process. Eng. 2020;43:1–10. doi: 10.1111/jfpe.13499. [DOI] [Google Scholar]
- 23.Emeko H.A., Olugbogi A.O., Betiku E. Appraisal of artificial neural network and response surface methodology in modeling and process variable optimization of oxalic acid production from cashew apple juice: a case of surface fermentation. Bioresources. 2015;10:2067–2082. doi: 10.15376/biores.10.2.2067-2082. [DOI] [Google Scholar]
- 24.Pradhan D., Abdullah S., Pradhan R.C. Chironji (Buchanania lanzan) fruit juice extraction using cellulase enzyme: modelling and optimization of process by artificial neural network and response surface methodology. J. Food Sci. Technol. 2021;58:1051–1060. doi: 10.1007/s13197-020-04619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonawane A., Pathak S.S., Pradhan R.C. Optimization of a process for the enzymatic extraction of nutrient enriched Bael fruit juice using artificial neural network (ANN) and response surface methodology (RSM) Int. J. Fruit Sci. 2020;20:S1845–S1861. doi: 10.1080/15538362.2020.1834898. [DOI] [Google Scholar]
- 26.Vahedi Torshizi M., Azadbakht M., Kashaninejad M. A study on the energy and exergy of Ohmic heating (OH) process of sour orange juice using an artificial neural network (ANN) and response surface methodology (RSM) Food Sci. Nutr. 2020;8:4432–4445. doi: 10.1002/fsn3.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciric A., Krajnc B., Heath D., Ogrinc N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020;135 doi: 10.1016/j.fct.2019.110976. [DOI] [PubMed] [Google Scholar]
- 28.Yıkmış S., Aksu H. Effects of natamycin and ultrasound treatments on red grape juice. Eff. Natamycin Ultrasound Treat. Red Grape Juice. 2020;29:1012–1024. [Google Scholar]
- 29.Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 30.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 31.Giusti M., Wrolstad R. Curr. Protoc. Food Anal. Chem. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2001. Characterization and measurement of anthocyanins by UV-visible spectroscopy. [DOI] [Google Scholar]
- 32.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 33.Coelho E.M., da Silva Padilha C.V., Miskinis G.A., de Sá A.G.B., Pereira G.E., de Azevêdo L.C., dos Santos Lima M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018;66:160–167. doi: 10.1016/J.JFCA.2017.12.017. [DOI] [Google Scholar]
- 34.Yıkmış S. Investigation of the effects of non-thermal, combined and thermal treatments on the physicochemical parameters of pomegranate (punica granatum L.) juice. Food Sci. Technol. Res. 2019;25:341–350. doi: 10.3136/FSTR.25.341. [DOI] [Google Scholar]
- 35.Kaur B., Panesar P.S., Anal A.K. Standardization of ultrasound assisted extraction for the recovery of phenolic compounds from mango peels. J. Food Sci. Technol. 2022;59:2813–2820. doi: 10.1007/S13197-021-05304-0/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadioli Tarone A., Keven Silva E., Dias de Freitas Queiroz Barros H., Baú Betim Cazarin C., Roberto Marostica Junior M. High-intensity ultrasound-assisted recovery of anthocyanins from jabuticaba by-products using green solvents: effects of ultrasound intensity and solvent composition on the extraction of phenolic compounds. Food Res. Int. 2021;140 doi: 10.1016/J.FOODRES.2020.110048. [DOI] [PubMed] [Google Scholar]
- 37.Yıkmış S., Ozer H., Levent Okan, Başak ·, Çöl G., Erdal B., Yıkmış S. Effect of thermosonication and thermal treatments on antidiabetic, antihypertensive, mineral elements and in vitro bioaccessibility of bioactive compounds in freshly squeezed pomegranate juice. J. Food Meas. Char. 2022;2022:1–19. doi: 10.1007/S11694-022-01402-5. [DOI] [Google Scholar]
- 38.Hasheminya S.M., Dehghannya J. Non-thermal processing of black carrot juice using ultrasound: intensification of bioactive compounds and microbiological quality. Int. J. Food Sci. Technol. 2022;57:5848–5858. doi: 10.1111/IJFS.15901. [DOI] [Google Scholar]
- 39.Ramírez-Melo L.M., Cruz-Cansino N. del S., Delgado-Olivares L., Ramírez-Moreno E., Zafra-Rojas Q.Y., Hernández-Traspeña J.L., Suárez-Jacobo Á. Optimization of antioxidant activity properties of a thermosonicated beetroot (Beta vulgaris L.) juice and further in vitro bioaccessibility comparison with thermal treatments. Lebensm. Wiss. Technol. 2022;154 doi: 10.1016/J.LWT.2021.112780. [DOI] [Google Scholar]
- 40.Oliveira G.A.R., Guimarães J.T., Ramos G.L.P.A., Esmerino E.A., Pimentel T.C., Neto R.P.C., Tavares M.I.B., Sobral L.A., Souto F., Freitas M.Q., Costa L.E.O., Cruz A.G. Benefits of thermosonication in orange juice whey drink processing. Innovat. Food Sci. Emerg. Technol. 2022;75 doi: 10.1016/J.IFSET.2021.102876. [DOI] [Google Scholar]
- 41.Kidoń M., Narasimhan G. Effect of ultrasound and enzymatic mash treatment on bioactive compounds and antioxidant capacity of black, red and white currant juices. Molecules. 2022;27:318. doi: 10.3390/MOLECULES27010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W., Gong P., Ma H., Xie R., Wei J., Xu M. Ultrasound treatment degrades, changes the color, and improves the antioxidant activity of the anthocyanins in red radish. Lebensm. Wiss. Technol. 2022;165 doi: 10.1016/J.LWT.2022.113761. [DOI] [Google Scholar]
- 43.Tiwari B.K., Patras A., Brunton N., Cullen P.J., O'Donnell C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010;17:598–604. doi: 10.1016/J.ULTSONCH.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Dadan M., Grobelna A., Kalisz S., Witrowa-Rajchert D. The impact of ultrasound-assisted thawing on the bioactive components in juices obtained from blue honeysuckle (Lonicera caerulea L. Ultrason. Sonochem. 2022;89 doi: 10.1016/J.ULTSONCH.2022.106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dias D. da R.C., Barros Z.M.P., de Carvalho C.B.O., Honorato F.A., Guerra N.B., Azoubel P.M. Effect of sonication on soursop juice quality. LWT--Food Sci. Technol. 2015;62:883–889. doi: 10.1016/j.lwt.2014.09.043. [DOI] [Google Scholar]
- 46.Abid M., Jabbar S., Wu T., Hashim M.M., Hu B., Lei S., Zhang X., Zeng X. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013;20:1182–1187. doi: 10.1016/J.ULTSONCH.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Yu H.C., Huang S.M., Lin W.M., Kuo C.H., Shieh C.J. Comparison of artificial neural networks and response surface methodology towards an efficient ultrasound-assisted extraction of chlorogenic acid from Lonicera japonica. Molecules. 2019;24:2304. doi: 10.3390/MOLECULES24122304. 2019, Vol. 24, Page 2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashyap P., Riar C.S., Jindal N. Optimization of ultrasound assisted extraction of polyphenols from Meghalayan cherry fruit (Prunus nepalensis) using response surface methodology (RSM) and artificial neural network (ANN) approach. J. Food Meas. Char. 2021;15:119–133. doi: 10.1007/S11694-020-00611-0/TABLES/5. [DOI] [Google Scholar]
- 49.Cheng C.X., Jia M., Gui Y., Ma Y. Comparison of the effects of novel processing technologies and conventional thermal pasteurisation on the nutritional quality and aroma of Mandarin (Citrus unshiu) juice. Innovat. Food Sci. Emerg. Technol. 2020;64 doi: 10.1016/J.IFSET.2020.102425. [DOI] [Google Scholar]
- 50.Lino D.L., Guimarães J.T., Ramos G.L.P.A., Sobral L.A., Souto F., Neto R.P.C., Tavares M.I.B., Sant'Anna Celso, Esmerino E.A., Mársico E.T., Freitas M.Q., Flores E.M.M., Raices R.S.L., Campelo P.H., Pimentel T.C., Cristina Silva M., Cruz A.G. Positive effects of thermosonication in Jamun fruit dairy dessert processing. Ultrason. Sonochem. 2022;86 doi: 10.1016/J.ULTSONCH.2022.106040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doguer C., Yıkmış S., Levent O., Turkol M. Anticancer effects of enrichment in the bioactive components of the functional beverage of Turkish gastronomy by supplementation with purple basil (Ocimum basilicum L.) and the ultrasound treatment. J. Food Process. Preserv. 2021;45 doi: 10.1111/JFPP.15436. [DOI] [Google Scholar]
- 52.Granato D., de Magalhães Carrapeiro M., Fogliano V., van Ruth S.M. Effects of geographical origin, varietal and farming system on the chemical composition and functional properties of purple grape juices: a review. Trends Food Sci. Technol. 2016;52:31–48. doi: 10.1016/J.TIFS.2016.03.013. [DOI] [Google Scholar]
- 53.Tokatli Demirok N. Sonication processing of mallow vinegar: effects on the bioactive compounds, amino acids, organic acid, sugar, mineral and microstructure. Food Sci. Technol. 2022;42 doi: 10.1590/FST.67122. [DOI] [Google Scholar]
- 54.Gomes W.F., Tiwari B.K., Rodriguez Ó., de Brito E.S., Fernandes F.A.N., Rodrigues S. Effect of ultrasound followed by high pressure processing on prebiotic cranberry juice. Food Chem. 2017;218:261–268. doi: 10.1016/j.foodchem.2016.08.132. [DOI] [PubMed] [Google Scholar]
- 55.M. da C.P. Dutra. Viana A.C., Pereira G.E., R. de C.M.R. Nassur. M. dos S. Lima Whole, concentrated and reconstituted grape juice: impact of processes on phenolic composition, “foxy” aromas, organic acids, sugars and antioxidant capacity. Food Chem. 2021;343 doi: 10.1016/J.FOODCHEM.2020.128399. [DOI] [PubMed] [Google Scholar]
- 56.Erdal B., Yıkmış S., Demirok N.T., Bozgeyik E., Levent O. Effects of non-thermal treatment on gilaburu vinegar (viburnum opulus L.): polyphenols, amino acid, antimicrobial, and anticancer properties. Biology. 2022;11:926. doi: 10.3390/BIOLOGY11060926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damasceno L.F., Fernandes F.A.N., Magalhães M.M.A., Brito E.S. Non-enzymatic browning in clarified cashew apple juice during thermal treatment: kinetics and process control. Food Chem. 2008;106:172–179. doi: 10.1016/J.FOODCHEM.2007.05.063. [DOI] [Google Scholar]
- 58.Kalsi B.S., Singh S., Alam M.S. Influence of ultrasound processing on the quality of guava juice. J. Food Process. Eng. 2022 doi: 10.1111/JFPE.14163. [DOI] [Google Scholar]
- 59.Xie X., Wang X., Bi X., Ning N., Li M., Xing Y., Che Z. Effects of ultrafiltration combined with high-pressure processing, ultrasound and heat treatments on the quality of a blueberry–grape–pineapple–cantaloupe juice blend. Int. J. Food Sci. Technol. 2022;57:4368–4379. doi: 10.1111/IJFS.15763. [DOI] [Google Scholar]
- 60.Yildiz G., Aadil R.M. Comparative analysis of antibrowning agents, hot water and high-intensity ultrasound treatments to maintain the quality of fresh-cut mangoes. J. Food Sci. Technol. 2022;59:202–211. doi: 10.1007/S13197-021-05001-Y/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munir M., Nadeem M., Ali B., Sultan M., Kanwal R., Al‐jumayi H.A., Algarni E.H.A., Alnofeai M.B., Mahmoud S.F. Investigating the impact of ultrasound, microwave, and high-pressure processing of milk on the volatile compounds and sensory properties of cheddar cheese. Agric. For. 2022;12:577. doi: 10.3390/AGRICULTURE12050577. 12 (2022) 577. [DOI] [Google Scholar]
- 62.Erkaya T., Başlar M., Şengül M., Ertugay M.F. Effect of thermosonication on physicochemical, microbiological and sensorial characteristics of ayran during storage. Ultrason. Sonochem. 2015;23:406–412. doi: 10.1016/j.ultsonch.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Anjaly M.G., Prince M.V., Warrier A.S., Lal A.M.N., Mahanti N.K., Pandiselvam R., Thirumdas R., Sreeja R., Rusu A.V., Trif M., Kothakota A. Design consideration and modelling studies of ultrasound and ultraviolet combined approach for shelf-life enhancement of pine apple juice. Ultrason. Sonochem. 2022;90 doi: 10.1016/J.ULTSONCH.2022.106166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pradhan D., Himakshi Baishya, Amit, Das B. Effect of ultrasound, centrifugation, and enzymatic pretreatments on phytochemical properties and membrane fouling during microfiltration of Sohiong (Prunus nepalensis L.) juice. Food Sci. Biotechnol. 2022;31:1443–1450. doi: 10.1007/s10068-022-01129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rojas M.L., Kubo M.T., Miano A.C., Augusto P.E. Ultrasound processing to enhance the functionality of plant-based beverages and proteins. Curr. Opin. Food Sci. 2022;48 doi: 10.1016/J.COFS.2022.100939. [DOI] [Google Scholar]
- 66.Pepi S., Coletta A., Crupi P., Leis M., Russo S., Sansone L., Tassinari R., Chicca M., Vaccaro C. Geochemical characterization of elements in Vitis vinifera cv. Negroamaro grape berries grown under different soil managements. Environ. Monit. Assess. 2016;188:1–15. doi: 10.1007/S10661-016-5203-9/FIGURES/7. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu H., Akamatsu F., Kamada A., Koyama K., Iwashita K., Goto-Yamamoto N. Effects of variety and vintage on the minerals of grape juice from a single vineyard. J. Food Compos. Anal. 2022;107 doi: 10.1016/J.JFCA.2021.104377. [DOI] [Google Scholar]
- 68.Huang X., Wang H., Luo W., Xue S., Hayat F., Gao Z. Prediction of loquat soluble solids and titratable acid content using fruit mineral elements by artificial neural network and multiple linear regression. Sci. Hortic. 2021:278. doi: 10.1016/J.SCIENTA.2020.109873. [DOI] [Google Scholar]
- 69.Yıkmış S., Erdal B., Bozgeyik E., Levent O., Yinanç A. Evaluation of purple onion waste from the perspective of sustainability in gastronomy: ultrasound-treated vinegar. Int. J. Gastron. Food Sci. 2022;29 doi: 10.1016/J.IJGFS.2022.100574. [DOI] [Google Scholar]
- 70.Manzoor M.F., Ahmed Z., Ahmad N., Aadil R.M., Rahaman A., Roobab U., Rehman A., Siddique R., Zeng X.A., Siddeeg A. Novel processing techniques and spinach juice: quality and safety improvements. J. Food Sci. 2020;85:1018–1026. doi: 10.1111/1750-3841.15107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.