Summary
GFR reaches a new baseline, primarily correlating with nephron-mass preservation, 1–12 months after partial nephrectomy (PN). However, does the ipsilateral GFR experience subsequent decline, and does acute ischemic injury has long-term effect on the operated kidney? 319 patients with two kidneys and unilateral clamped PN were analyzed. All had preoperative, new-baseline, and latest follow-up imaging/serum creatinine levels. Annual ipsilateral GFR decline rate (AIGDR) was defined as new-baseline GFR minus latest follow-up GFR normalized by new-baseline GFR, per year. Spectrum score was used to reflect the degree of acute ischemic injury in the operated kidney. 100 subjects searching for health screening served as controls. Predictive factors for AIGDR were assessed. The median AIGDR was 2.25%, significantly higher than controls (0.88%, p = 0.036). With some contralateral hypertrophy, the global annual GFR decline was similar to that of controls (0.81% vs. 0.88%, p = 0.7). Spectrum score correlated significantly with AIGDR (p = 0.037). These results support that acute ischemic injury has long-term effect on the operated kidney.
Subject areas: Endocrinology, Public health, Pathophysiology, Medical endocrinology
Graphical abstract
Highlights
-
•
Our study mainly focuses on the long-term change of the operated kidney after clamped PN
-
•
We found a subsequent ipsilateral GFR decline greater than natural process
-
•
Ischemia may have long-term impact on the operated kidney
Endocrinology; Public health; Pathophysiology; Medical endocrinology
Introduction
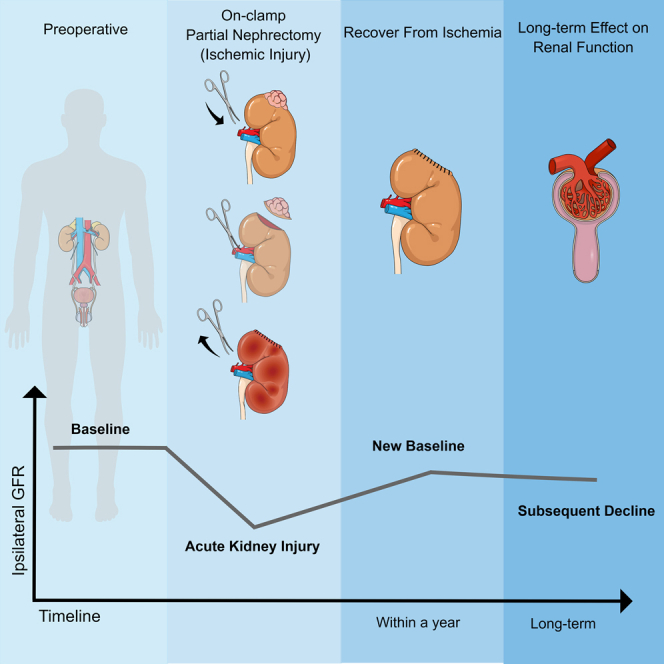
Partial nephrectomy (PN) is the reference standard for the management of small renal masses, and exploration of conducting PN for more complex renal mass is ongoing, mainly because it optimizes functional outcomes.1,2,3 Off-clamp or minimally ischemic PN techniques have been reported in selective patients with potentially better renal function outcome.4,5 Preoperative superselective transarterial tumor embolization was reported as a safe and reliable procedure before performing PN to achieve zero ischemia with good functional results and comparable oncological outcomes.6,7,8 However, the expense of this additional invasive procedure and the lack of interventional radiology expertise in many centers restricted the generalizability of this technique.9 Overall off-clamp and minimally ischemic techniques are technically demanding, with potential for increased blood loss, and require considerable experience with PN surgery.5 Therefore, in most cases, the surgeons clamp the renal artery for clear visualization, which leads to acute ischemic insult in the operated kidney. Acute ischemic injury in the operated kidney after PN is often masked by a functional contralateral kidney, and there was no practical method to assess the degree of this until 2016. In 2016, our team proposed a novel scoring system, entitled Spectrum score, to reflect the degree of acute ischemic injury in the operated kidney for patients with two kidneys and unilateral clamped PN.10 This score ideally ranges from 0 to 1, with a score of 1 representing temporary complete shutdown of the ipsilateral kidney with serum creatinine rising to the level of function only provided by the contralateral kidney and a score of 0 representing only minimal rise of the serum creatinine reflecting only minimal loss of parenchymal volume in the ipsilateral kidney. Although we found that higher Spectrum score was associated with worse short-term function recovery, the long-term effect of acute ischemic injury on the kidney that underwent clamped PN was not explored in that study.
GFR of the operated kidney reaches a new baseline (entitled NBGFR), primarily correlating with nephron-mass preservation, 1–12 months after PN. Autorino and colleagues reported a multicenter PN cohort with mean percentage eGFR loss of 10.31% at 1 year postoperatively, which could be predicted by preoperative eGFR, sex, ischemia technique, and percentage eGFR loss at discharge.11 However, the fate of operated kidney beyond new baseline, as well as the subsequent change of contralateral and global GFR is not clear. Most studies in this domain have focused on the global GFR and report marginal recovery in the first year after PN and relatively stable GFR thereafter.12,13,14 Only a few studies have used serial nuclear renal scans (NRSs) to estimate the split renal function (SRF) and provide focused analysis of each kidney.15 However, previous results have been inconsistent, probably due to restricted numbers of patients and limited follow-up.
Our previous study using paired normal parenchyma revealed histological deterioration and increased collagen deposition of the preserved renal parenchyma several years after PN, which correlated with presence of comorbidities but not ischemia duration, type, and acute kidney injury (AKI).16 However, the number of patients under analysis was necessarily limited, and further research regarding the long-term functional outcomes of the operated kidney as well as better assessment of AKI using Spectrum score is greatly needed, ideally with comparison to normal controls, which was not possible in our prior study.
In the current study, our objective was to analyze a substantial cohort of patients with two functional kidneys managed with clamped PN, with special focus on the kidney exposed to ischemia, and specifically its fate over the next few years after reaching the NBGFR. For this study we used parenchymal volume analysis (PVA) to define SRF, which appears to be more accurate than NRS,17 and we used Spectrum score to reflect the degree of acute ischemic injury in the operated kidney. We have also incorporated a control group to assess the significance of the observed functional changes.
Results
Patient and tumor characteristics
Table 1 summarizes patient/tumor characteristics. Median tumor diameter was 3.5 cm, and median R.E.N.A.L was 8; 85 (26%) tumors had low complexity, 165 (52%) intermediate complexity, and 69 (22%) high complexity. The median ischemia duration was 26 min (interquartile range [IQR]:19–32). Warm ischemia was used in 243 patients (median duration 26 min), and hypothermia in 76 patients (median duration 27 min). Median preoperative serum creatinine was 0.8 mg/dL, and global eGFR was 126.3 mL/min/1.73m2. Two hundred and eighty-six patients (90%) had preoperative GFR≥90 mL/min/1.73m2. Median body mass index (BMI) was 23.6 kg/m2, and 88 patients (28%) had hypertension (HTN), diabetes (DM), and/or preexisting chronic kidney disease (CKD). Median Spectrum score was 0.22 (IQR: 0.06–0.39). Our study cohort (n = 319) had comparable baseline characteristics as the overall PN cohort (n = 2725) at our center during this time frame except for more robotic surgery, slightly higher R.E.N.A.L score (8 vs. 7), and marginally increased ischemia time (26 vs. 25 min) (See Table S1).
Table 1.
Baseline characteristics of patients
| Variable | Clinical features |
|---|---|
| No. patients | 319 |
| Age (year) (median, IQR) | 50 (39–59) |
| Male (n, %) | 206 (65) |
| BMI (kg/m2) (median, IQR) | 23.6 (21.4–25.7) |
| Comorbidities (HTN/DM/CKD) (n, %) | 88 (28) |
| Pre-PN global eGFR (ml/min/1.73m2) (median, IQR) | 126.3 (108.4–146.4) |
| Surgical approach | |
| No. open (%) | 82 (26) |
| No. laparoscopic (%) | 84 (26) |
| No. robotic (%) | 153 (48) |
| Tumor size (cm) (median, IQR) | 3.5 (2.5–5.0) |
| R.E.N.A.L score (median, IQR) | 8 (6–9) |
| 4–6 (n, %) | 85 (26) |
| 7–9 (n, %) | 165 (52) |
| 10–12 (n, %) | 69 (22) |
| Ischemic type | |
| No. warm (%) | 243 (76) |
| No. cold (%) | 76 (24) |
| Ischemic time (min) (median, IQR) | 26 (19–32) |
| Interval between new baseline and latest follow-up (year) (median, IQR) | 1.7 (1.1–3.0) |
| Spectrum score (median, IQR) | 0.22 (0.06–0.39) |
BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range; PN, partial nephrectomy; R.E.N.A.L, (R)adius (tumor size as maximal diameter), (E)xophytic/endophytic properties of tumor, (N)earness of tumor deepest portion to collecting system or sinus, (A)nterior (a)/posterior (p)descriptor, and (L)ocation relative to polar line.
Subsequent GFR decline after new baseline
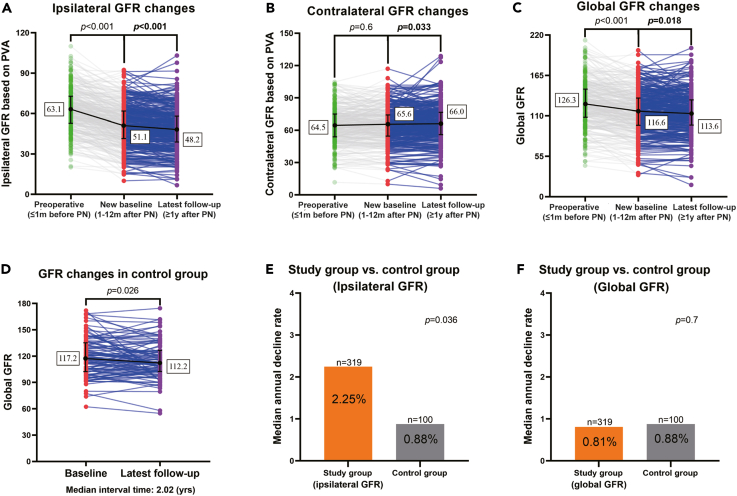
Detailed functional data globally and for each kidney are shown in Table 2 with comparison to controls. Our main focus was on subsequent changes in function after NBGFR was reached. With a median interval time (new baseline to latest follow-up) of 1.7 years (IQR = 1.1–3.0), the ipsilateral GFR dropped from 51.1 mL/min/1.73m2 to 48.2 mL/min/1.73m2, with an annual decline rate of 2.25%. Simultaneously, the contralateral GFR increased slightly (from 65.6 to 66.0 mL/min/1.73m2) and the global GFR decreased from 116.6 mL/min/1.73m2 to 113.6 mL/min/1.73m2 (Figure 1; Table 2). Sixty-eight percent of patients (216/319) experienced GFR decline of the ipsilateral kidney after reaching NBGFR.
Table 2.
GFR at various time points (baseline, new baseline, and latest follow-up) in patients and controls
| Variable | Preoperative | NEW BASELINE | LATEST FOLLOW-UPa | p valueb |
|---|---|---|---|---|
| GFR (ml/min/1.73m2) (median, IQR) | ||||
| Total | 126.3 (108.4–146.4) | 116.6 (97.6–134.3) | 113.6 (97.9–131.8) | 0.018 |
| Ipsilateral | 63.1 (52.9–72.9) | 51.1 (41.5–61.6) | 48.2 (39.0–58.0) | <0.001 |
| Contralateral | 64.5 (54.0–74.8) | 65.6 (54.6–73.9) | 66.0 (56.2–76.4) | 0.033 |
| Control group (n=100) | Baseline | Latest follow-up | p value |
|---|---|---|---|
| GFR (ml/min/1.73m2) (median, IQR) | 117.2 (103.0–133.8) | 112.2 (102.8–126.3) | 0.026 |
IQR, interquartile range.
Median follow-up was 1.7 years from new baseline GFR to latest GFR for each patient.
p value was calculated based on the comparison between New Baseline and Latest Follow-up.
Figure 1.
The change of GFR (ml/min/1.73m2) in study group (preoperative, new baseline and latest follow-up) and control group
(A) Ipsilateral GFR dropped sharply in the short-term after PN and then showed mild decline beyond new baseline GFR. Data are presented as the individuals and the median with interquartile range.
(B) Contralateral GFR showed compensation beyond new baseline. Data are presented as the individuals and the median with interquartile range.
(C) Global GFR decreased both at short-term and beyond new baseline. Data are presented as the individuals and the median with interquartile range.
(D) Global GFR decline in control group over time (n = 100). Data are presented as the individuals and the median with interquartile range.
(E) Annual GFR decline rate beyond new baseline GFR was significantly higher in the operated kidney than control group.
(F) Annual decline rate of global GFR beyond new baseline was similar between study group and control group.
For the control group, the median age was 49.8 years old and 31% had HTN, DM, and/or preexisting CKD, comparable to the study group (all p > 0.05). With an interval of 2 years (IQR = 1.9–2.2, p = 0.8, compared with the study group) for the control group, the global GFR declined from 117.2 to 112.2 mL/min1.73m2, representing a median annual decline of 0.88%, significantly lower than that of the ipsilateral kidney in the study cohort (2.25%, p = 0.036). However, the annual decline rate of global GFR was analogous between study and control groups (0.81% vs. 0.88%, p = 0.7).
Factors associated with AIGDR
We evaluated factors correlating with AIGDR. As shown in Figure 2, age, ischemia time/type, and comorbidity all failed to correlate with AIGDR, while Spectrum score which was used to reflect the degree of acute ischemic injury correlated significantly (R2 = 0.0127, p = 0.045). Patients with higher Spectrum score had increased AIGDR, even after adjusted for age and comorbidities (coefficient = 3.358, p = 0.037) (Table 3).
Figure 2.
Factors correlated with annual subsequent ipsilateral GFR decline rate
(A–E) Correlation between annual GFR decline rate beyond new baseline GFR and (A) age, (B) ischemic time, (C) Spectrum score, (D) ischemic type, and (E) comorbidities of HTN, DM, and/or preexisting CKD. Spectrum score: this score ideally ranges from 0 to 1, with a score of 1 representing temporary complete shutdown of the ipsilateral kidney with serum creatinine rising to the level of function only provided by the contralateral kidney and a score of 0 representing only minimal rise of the serum creatinine reflecting only minimal loss of parenchymal volume in the ipsilateral kidney.
Table 3.
Multivariable analysis of predictors of annual percent ipsilateral GFR decline
| Variables | Coefficient | 95% CI | p value |
|---|---|---|---|
| Age | −0.060 | (-0.149, 0.030) | 0.19 |
| HTN/DM/CKD (Y; N) | 0.553 | (-1.915, 3.022) | 0.7 |
| Spectrum score | 3.358 | (0.208, 6.507) | 0.037 |
CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension.
Discussion
Short-term functional recovery of the operated kidney after clamped PN to its NBGFR has been studied extensively in recent years, with most studies showing that the degree of parenchymal volume preservation is the key determinant, while type and duration of ischemia play secondary roles.18,19,20 However, the subsequent fate of the kidney exposed to ischemia has not been well studied, with some speculating that it may be frailer and more vulnerable to functional decline in the subsequent years. Furthermore, any potential impact of this on the contralateral kidney and the global GFR also remains unclear. In this study we used semi-automated software to estimate SRF and provide a focused analysis of the functional status of each kidney at the time that NBGFR was established, and more importantly, our main focus and analysis were on changes subsequent to that. Our objectives were to evaluate the subsequent degree of functional change in each kidney and globally to determine whether exposure to ischemia impacted functional evolution of the ipsilateral kidney.
Our main findings are that the ipsilateral kidney experienced a median annual GFR decline of 2.25%, significantly higher than the 0.88% annual functional decline observed in controls due to the natural aging process. In contrast, the global GFR declined at a rate similar to controls, in part due to some degree of contralateral hypertrophy. The main predictor of GFR decline in the kidney exposed to ischemia was Spectrum score which was used to reflect the degree of acute ischemic injury, while ischemia type/duration and other comorbidities failed to associate. Our study thus provides a focused look at the long-term evolution of functional outcomes in both kidneys in patients undergoing clamped PN and will stimulate further research into this domain.
A previous study from our group evaluated histologic and morphologic changes in the ipsilateral renal parenchyma in the years after clamped PN with exposure to ischemia.16 However, that study has limitations including lacking of computed tomography (CT) imaging and SRF to determine Spectrum score, necessarily limited patient number, and lacking of normal control. The current study is an extension of our previous one, which included a substantial cohort, provided a control group, and evaluated acute ischemic injury using Spectrum score. Our results suggested that acute ischemic injury does have long-term effect on the operated kidney. This is supported by higher annual GFR decline rate of the operated kidney than control (2.25% vs. 0.88%) and the association between Spectrum score and annual ipsilateral GFR decline rate. Ischemia duration failed to associate with annual ipsilateral GFR decline rate, which implies that longer ischemia time does not equate to severer acute ischemic injury as the tolerance of human kidney to ischemic insult varies among individuals. Our main conclusion that ischemia insult does have long-term effect on the operated kidney is consistent with consensus in nephrology that AKI can predispose to CKD and also accorded with the finding in animal model that kidney ischemic injury lead to continuous GFR decline.21,22 However, the degree of damage that ischemia brings to the operated kidney is somewhat moderate, and with the compensation from the contralateral kidney, the global GFR declines in a similar rate as natural-aging progress, whereas a recent prospective study using serial renal scintigraphy to evaluate SRF failed to detect a statistically significant subsequent decline in the ipsilateral GFR.15 However, the number of subjects in this previous study was limited (n = 54). Further studies will be required to address this discordance.
In solitary kidney that underwent PN, degree of AKI seems to be not associated with long-term GFR decline. Zabell and colleague reported a group of solitary kidneys that underwent PN with long-term follow-up. Their results showed that the median change ratio (long-term GFR normalized by NBGFR) in this setting was 1.0 in patients with no AKI, 1.0 in patients with grade 1 and 2 AKI, and 1.1 in patients with grade 3 AKI.23 This study also indicated that renal function of solitary kidney exposed to moderate ischemia beyond new baseline is quite stable, which implied that solitary kidney (with lower global GFR) may be more tolerant to ischemic insult, probably due to lack of compensation from the contralateral kidney. It has been reported that lower preoperative global GFR “encouraged” compensation of renal function after acute function loss. In 2015, Zabor and colleagues found that radical nephrectomy (RN) patients with preoperative GFR less than 60 mL/min/1.73m2 were more likely to have postoperative eGFR recovered to their preoperative level by 2 years following surgery.24 In 2017, a multicenter cooperative study of 1,928 patients further confirmed that patients with a lower preoperative GFR had an increased chance of function recovery.25 However, this hypothesis maybe appropriate only for moderate kidney injury (i.e., median ischemia time of 26 min in our study) and needs to be verified in further studies. For renal cancer in solitary kidneys or completely endophytic renal masses, percutaneous ablation which was reported to be associated with reduced complication rate may be considered.26
In our previous study, we found that comorbidities, such as HTN/DM/preexisting CKD, were the main predictors of histological deterioration of the preserved renal parenchyma after clamped PN.16 This study included 38 Chinese and 27 American patients, and the incidence of such comorbidities was much higher (55%). In the current study, which only included Chinese patients with median age of only 50 years, the comorbidity rate was substantially lower (28%) and this may have contributed to our finding that comorbidities failed to associate with long-term ipsilateral GFR decline. The current study population also had much higher preoperative global GFR (126.3 mL/min/1.73m2) than what was observed in the American population (76 mL/min/1.73m2).17
Limitations of the study
Our study has limitations including retrospective design with concern about selection bias. During the study period, a total of 2,725 PNs were performed at our center. Only 319 patients (12%) were included in this analysis due to inclusion criteria that required imaging and serum creatinine levels at 3 distinct time points. However, the study patients had similar baseline characteristics as the overall population, suggesting that it was representative. Proper follow-up interval to reflect the trend of postoperative GFR is necessary, which was designated as 3 distinct time points in the current study due to the retrospective data. Nevertheless, our results can still reflect the changes of GFR to some extent based on the assumption that functional recovery occurred within a year after PN and remained debated thereafter. A prospective setting regarding regular evaluation of GFR would be more definitive in the future research. Our study is single center and evaluated a relatively healthy and young Chinese population with low incidence of comorbidity, which might affect its generalizability. The interval time from new baseline to latest follow-up was somewhat limited at about 2 years. As such our analysis is best considered hypothesis generating, and a multicenter study focusing on the subsequent ipsilateral GFR decline after PN with longer follow-up would be more definitive.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| MIMICS 17.0 | Materialise | https://www.materialise.com/en/healthcare/mimics-innovation-suite/mimics |
| SPSS 25.0 | IBM | https://www.ibm.com/analytics/spss-statistics-software |
| GraphPad-Prism 8.3.0 | GraphPad-Software | https://www.graphpad.com/ |
Resource availability
Lead contact
Further information and requests for resources and data should be directed to and will be fulfilled by the lead contact, Zhiling Zhang, MD, PhD (zhangzhl@sysucc.org.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
This retrospective study was approved by the Institutional Review Board and Ethical Committee of Sun Yat-Sen University Cancer Center (B2022-619-01). Informed consent was waived by our Institutional Review Board because of the retrospective nature of our study. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All participants are Asian, other demographic information including age and gender are provided in Table 1.
Method details
Patient population
With institutional-review-board approval, retrospective review of 2725 patients who had two functional kidneys managed with unilateral clamped-PN was performed. 319 patients qualified for analysis, which required availability of CT or MR imaging and serum creatinine levels at all 3 time-points: ≤1 month before PN (preoperative), 1-12 months after PN (new-baseline), and ≥1 year after PN (latest follow-up). The baseline-characteristics of the study cohort (n=319) were compared with the entire PN cohort (n=2725) to assess generalizability (See Table S1). A group of 100 subjects searching for health screening was used as control to assess the natural decline of global GFR.
PN procedures
Decisions about surgical approach and warm/cold ischemia were made by the primary surgeon based on individual patient/tumor characteristics. PN was conducted as summarized as follow: Gerota’s fascia is opened in an area far from the tumor to find the capsule, and dissection is performed along the renal capsule until the mass is exposed. The renal artery was always occluded; the vein was clamped selectively. The tumor resected along the margin using cold scissors. Renorrhaphy is performed in two layers. After the completion of renorraphy, the hilum is unclamped.27,28
R.E.N.A.L. score
Demographic/clinicopathologic/perioperative parameters and relevant comorbidities were obtained by retrospective review, and R.E.N.A.L. complexity was defined as follow: R.E.N.A.L. score consists of (R)adius (tumor size as maximal diameter), (E)xophytic/endophytic properties of tumor, (N)earness of tumor deepest portion to collecting system or sinus, (A)nterior (a)/posterior (p)descriptor, and (L)ocation relative to polar line. Of the 5 components 4 are scored on a 1, 2 or 3-point scale with the 5th indicating the anterior or posterior location of the mass relative to the coronal plane of the kidney.29
Spectrum score
Spectrum score was used to evaluate the degree of acute ischemic injury in the operated kidney. Estimations were determined based on split function and percent parenchymal mass preserved in the ipsilateral kidney: serum creatinine (SCr)ideal-peak: expected peak SCr presuming no ischemic injury; and SCrworstcase-peak: expected peak SCr presuming temporary complete nonfunction of the ipsilateral kidney. The acute ipsilateral renal dysfunction spectrum score was defined: (observed peak SCr - SCrideal-peak)/(SCrworstcase-peak- SCrideal-peak).10
Ipsilateral SRF based on parenchymal volume analysis (PVA)
Preoperative/postoperative ipsilateral/contralateral parenchymal volumes were evaluated at 3-mm intervals from the venous phase. Standard renal mass protocol or abdominal CT protocols were used for preoperative and postoperative imaging, respectively. Semi-automated software (MIMICS 17.0, Materialise, Leuven-Belgium) was used for image evaluation. Freehand scripting was used to define the area of interest at each 3–5 level, which was then fill to a contiguous 3D object automatically and summed to yield: (1) the total volume of functioning renal parenchyma and tumor, (2) the tumor alone, and (3) the volume of the functioning renal parenchyma post-operatively.18 The collecting system/vessels/cysts/sinus fat and tumour were excluded from all parenchymal volume measurements. Ipsilateral SRF was based on PVA and defined as ipsilateral parenchymal volume normalized by total parenchymal volume of both kidneys.17 To verify that PVA has analogous utility for estimation of SRF in patients with renal masses compared with NRS, we compared ipsilateral GFR based on PVA and NRS in 87 patients preoperatively and 64 at new baseline who had both PVA and NRS (See Figure S1).
Estimation of ipsilateral GFR and definition of subsequent GFR decline
Global eGFR was estimated using the Modification of Diet in Renal Disease study (MDRD) equation.30 Preoperative/postoperative ipsilateral eGFR values were obtained by multiplying the global eGFR by the SRF derived from PVA. Ipsilateral GFRs obtained ≤1 month before PN, 1-12 months after PN, and ≥1 year after PN were defined as preoperative, new-baseline and latest follow-up GFR of the operated kidney, respectively. Annual ipsilateral GFR decline rate (AIGDR) was defined as (NBGFR minus latest follow-up GFR) divided by NBGFR normalized by interval time from new baseline to latest follow-up.
Quantification and statistical analysis
Continuous variables were expressed as median (IQR) and categorical variables as number (percentage). The paired t-test or Wilcoxon matched pairs signed rank test were used for testing paired data while unpaired data were compared using unpaired t-test or Mann-Whitney. Categorical variables were compared using chi-square. Factors associated with annual subsequent GFR decline rate were evaluated by univariable and multivariable linear regression analysis, using an Enter method. All p-values were two-tail and p<0.05 considered significant. Data were analyzed using SPSS-version-25.0 (SPSS Inc., Chicago, IL, USA) and GraphPad-Prism version-8.3.0 (GraphPad-Software, LLC, San Diego, CA, USA).
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 82203288, 82203320, 81972382, 82273031, 82273311, and 81872091), Medical Scientific Research Foundation of Guangdong Province (A2022492), China National Postdoctoral Program for Innovative Talents (NO. BX20220363), and the Natural Science Foundation for Distinguished Young Scholars of Guangdong Province (No. 2021B1515020077).
Author contributions
Zhiling Zhang, Chunping Yu, Zhaohui Zhou, and Longbin Xiong: Conceptualization, Methodology, Project administration. Zhaohui Zhou, Kang Ning, and Zhiyong Li: Data curation, Writing- Original draft preparation, Software, Formal analysis. Longbin Xiong, Huiming Liu, Yixin Huang, and Xin Luo: Visualization, Investigation. Zhiyong Li, Huiming Liu, Yulu Peng, Chunping Yu, Hui Han, Shengjie Guo, and Pei Dong: Acquisition of data, Supervision, Administrative or technical support. Kang Ning, Lijie Chen, Binglei Ma, Xiangpeng Zou, Wensu Wei, and Cheng Luo: Software, Validation, Acquisition of data. Fangjian Zhou and Zhiling Zhang: Writing- Reviewing and Editing. Zhiling Zhang, Fangjian Zhou and Chunping Yu: Funding acquisition.
Declaration of interests
The authors declare no competing interests.
Published: August 12, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107610.
Contributor Information
Chunping Yu, Email: ychp_83@163.com.
Zhiling Zhang, Email: zhangzhl@sysucc.org.cn.
Supplemental information
Data and code availability
-
•
The individual patient data reported in this study cannot be deposited in a public repository because these data are confidential medical records. To request access, contact Zhiling Zhang, MD, PhD (zhangzhl@sysucc.org.cn) for de-identified summary data.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Ljungberg B., Bensalah K., Canfield S., Dabestani S., Hofmann F., Hora M., Kuczyk M.A., Lam T., Marconi L., Merseburger A.S., et al. Eau guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Campbell S., Uzzo R.G., Allaf M.E., Bass E.B., Cadeddu J.A., Chang A., Clark P.E., Davis B.J., Derweesh I.H., Giambarresi L., et al. Renal mass and localized renal cancer: aua guideline. J. Urol. 2017;198:520–529. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 3.Cerrato C., Patel D., Autorino R., Simone G., Yang B., Uzzo R., Porpiglia F., Capitanio U., Porter J., Beksac A.T., et al. Partial or radical nephrectomy for complex renal mass: a comparative analysis of oncological outcomes and complications from the rosula (robotic surgery for large renal mass) collaborative group. World J. Urol. 2023;41:747–755. doi: 10.1007/s00345-023-04279-1. [DOI] [PubMed] [Google Scholar]
- 4.Simone G., Ferriero M., Papalia R., Costantini M., Guaglianone S., Gallucci M. Zero-ischemia minimally invasive partial nephrectomy. Curr. Urol. Rep. 2013;14:465–470. doi: 10.1007/s11934-013-0359-0. [DOI] [PubMed] [Google Scholar]
- 5.Simone G., Gill I.S., Mottrie A., Kutikov A., Patard J.J., Alcaraz A., Rogers C.G. Indications, techniques, outcomes, and limitations for minimally ischemic and off-clamp partial nephrectomy: a systematic review of the literature. Eur. Urol. 2015;68:632–640. doi: 10.1016/j.eururo.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Gallucci M., Guaglianone S., Carpanese L., Papalia R., Simone G., Forestiere E., Leonardo C. Superselective embolization as first step of laparoscopic partial nephrectomy. Urology. 2007;69:642–645. doi: 10.1016/j.urology.2006.10.048. discussion 645-646. [DOI] [PubMed] [Google Scholar]
- 7.Simone G., Papalia R., Guaglianone S., Forestiere E., Gallucci M. Preoperative superselective transarterial embolization in laparoscopic partial nephrectomy: technique, oncologic, and functional outcomes. J. Endourol. 2009;23:1473–1478. doi: 10.1089/end.2009.0334. [DOI] [PubMed] [Google Scholar]
- 8.Simone G., Papalia R., Guaglianone S., Carpanese L., Gallucci M. Zero ischemia laparoscopic partial nephrectomy after superselective transarterial tumor embolization for tumors with moderate nephrometry score: long-term results of a single-center experience. J. Endourol. 2011;25:1443–1446. doi: 10.1089/end.2010.0684. [DOI] [PubMed] [Google Scholar]
- 9.Cadeddu J.A. Re: zero ischemia laparoscopic partial nephrectomy after superselective transarterial tumor embolization for tumors with moderate nephrometry score: long-term results of a single-center experience. J. Urol. 2012;187:1226. doi: 10.1016/j.juro.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., Zhao J., Dong W., Aguilar Palacios D., Remer E.M., Li J., Demirjian S., Zabell J., Campbell S.C. Acute ipsilateral renal dysfunction after partial nephrectomy in patients with a contralateral kidney: spectrum score to unmask ischemic injury. Eur. Urol. 2016;70:692–698. doi: 10.1016/j.eururo.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Crocerossa F., Fiori C., Capitanio U., Minervini A., Carbonara U., Pandolfo S.D., Loizzo D., Eun D.D., Larcher A., Mari A., et al. Estimated glomerular filtration rate decline at 1 year after minimally invasive partial nephrectomy: a multimodel comparison of predictors. Eur. Urol. Open Sci. 2022;38:52–59. doi: 10.1016/j.euros.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.H., Park Y.H., Kim Y.J., Kang S.H., Byun S.S., Kwak C., Hong S.H. Perioperative and long-term renal functional outcomes of robotic versus laparoscopic partial nephrectomy: a multicenter matched-pair comparison. World J. Urol. 2015;33:1579–1584. doi: 10.1007/s00345-015-1488-5. [DOI] [PubMed] [Google Scholar]
- 13.Dawidek M.T., Chan E., Boyle S.L., Sener A., Luke P.P. Assessing time of full renal recovery following minimally invasive partial nephrectomy. Urology. 2018;112:98–102. doi: 10.1016/j.urology.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim D.K., Jang Y., Lee J., Hong H., Kim K.H., Shin T.Y., Jung D.C., Choi Y.D., Rha K.H. Two-year analysis for predicting renal function and contralateral hypertrophy after robot-assisted partial nephrectomy: a three-dimensional segmentation technology study. Int. J. Urol. 2015;22:1105–1111. doi: 10.1111/iju.12913. [DOI] [PubMed] [Google Scholar]
- 15.Porpiglia F., Fiori C., Bertolo R., Morra I., Russo R., Piccoli G., Angusti T., Podio V. Long-term functional evaluation of the treated kidney in a prospective series of patients who underwent laparoscopic partial nephrectomy for small renal tumors. Eur. Urol. 2012;62:130–135. doi: 10.1016/j.eururo.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Xiong L., Nguyen J.K., Peng Y., Zhou Z., Ning K., Jia N., Nie J., Wen D., Wu Z., Roversi G., et al. What happens to the preserved renal parenchyma after clamped partial nephrectomy? Eur. Urol. 2022;81:492–500. doi: 10.1016/j.eururo.2021.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Ye Y., Tanaka H., Wang Y., Campbell R.A., Aguilar Palacios D., Dewitt-Foy M.E., Mahmood F.A., Eltemamy M., Remer E.M., Li J., Campbell S.C. Split renal function in patients with renal masses: utility of parenchymal volume analysis vs nuclear renal scans. BJU Int. 2020;125:686–694. doi: 10.1111/bju.14997. [DOI] [PubMed] [Google Scholar]
- 18.Mir M.C., Campbell R.A., Sharma N., Remer E.M., Simmons M.N., Li J., Demirjian S., Kaouk J., Campbell S.C. Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology. 2013;82:263–268. doi: 10.1016/j.urology.2013.03.068. [DOI] [PubMed] [Google Scholar]
- 19.Simmons M.N., Hillyer S.P., Lee B.H., Fergany A.F., Kaouk J., Campbell S.C. Functional recovery after partial nephrectomy: effects of volume loss and ischemic injury. J. Urol. 2012;187:1667–1673. doi: 10.1016/j.juro.2011.12.068. [DOI] [PubMed] [Google Scholar]
- 20.Lane B.R., Russo P., Uzzo R.G., Hernandez A.V., Boorjian S.A., Thompson R.H., Fergany A.F., Love T.E., Campbell S.C. Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J. Urol. 2011;185:421–427. doi: 10.1016/j.juro.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 21.Chawla L.S., Eggers P.W., Star R.A., Kimmel P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Kumar S., Dolzhenko E., Alvarado G.F., Guo J., Lu C., Chen Y., Li M., Dessing M.C., Parvez R.K., et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zabell J., Isharwal S., Dong W., Abraham J., Wu J., Suk-Ouichai C., Palacios D.A., Remer E., Li J., Campbell S.C. Acute kidney injury after partial nephrectomy of solitary kidneys: impact on long-term stability of renal function. J. Urol. 2018;200:1295–1301. doi: 10.1016/j.juro.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Zabor E.C., Furberg H., Mashni J., Lee B., Jaimes E.A., Russo P. Factors associated with recovery of renal function following radical nephrectomy for kidney neoplasms. Clin. J. Am. Soc. Nephrol. 2016;11:101–107. doi: 10.2215/CJN.04070415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zabor E.C., Furberg H., Lee B., Campbell S., Lane B.R., Thompson R.H., Antonio E.C., Noyes S.L., Zaid H., Jaimes E.A., Russo P. Long-term renal function recovery following radical nephrectomy for kidney cancer: results from a multicenter confirmatory study. J. Urol. 2018;199:921–926. doi: 10.1016/j.juro.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandolfo S.D., Beksac A.T., Derweesh I., Celia A., Schiavina R., Bianchi L., Costa G., Carbonara U., Loizzo D., Lucarelli G., et al. Percutaneous ablation vs robot-assisted partial nephrectomy for completely endophytic renal masses: a multicenter trifecta analysis with a minimum 3-year follow-up. J. Endourol. 2023;37:279–285. doi: 10.1089/end.2022.0478. [DOI] [PubMed] [Google Scholar]
- 27.Kaouk J.H., Khalifeh A., Hillyer S., Haber G.P., Stein R.J., Autorino R. Robot-assisted laparoscopic partial nephrectomy: step-by-step contemporary technique and surgical outcomes at a single high-volume institution. Eur. Urol. 2012;62:553–561. doi: 10.1016/j.eururo.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Uzzo R.G., Novick A.C. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J. Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 29.Kutikov A., Uzzo R.G. The r.e.n.a.l. Nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y.C., Zuo L., Chen J.H., Luo Q., Yu X.Q., Li Y., Xu J.S., Huang S.M., Wang L.N., Huang W., et al. Modified glomerular filtration rate estimating equation for chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The individual patient data reported in this study cannot be deposited in a public repository because these data are confidential medical records. To request access, contact Zhiling Zhang, MD, PhD (zhangzhl@sysucc.org.cn) for de-identified summary data.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.