Abstract
Background
Hepatitis C remains highly prevalent among people who inject drugs (PWIDs). We propose an integrated approach for screening/diagnostic testing and treatment in 6,665 Viennese PWIDs registered to access opioid agonist therapy (OAT).
Methods
OAT prescriptions were required monthly at one of nine approved authorities, making them ideal platforms for hepatitis C virus (HCV) screening. All PWIDs attending these authorities between January 2019 and March 2020 were offered on-site HCV screening, and consecutive HCV RNA PCR in case of positive HCV serology. In HCV viremic PWIDs, offsite referral to HCV care and treatment according to directly observed therapy (DOT) alongside OAT were performed.
Results
4,327/6,665 (64.9%) individuals were contacted before the COVID-19-related project discontinuation. There were 1,538/4,327 (35.5%) individuals who had participated in the study. HCV serology was available in 1,510/1,538 (98.2%): 795/1,519 (52.6%) had a positive serology, among whom 632 (79.5%) were followed-up with a PCR test. In 8/1,538 (0.5%) additional study participants HCV RNA PCR was assessed without prior serological screening. 239/640 (37.3%) individuals were HCV viremic with 51 (21.3%) having started on direct-acting antivirals (DAAs). 48/51 (94.1%) had completed treatment, among whom 42 (87.5% according to ITT) had achieved sustained virologic response at 12 weeks after completing treatment (SVR12) and 6 (12.5%) had been lost to follow-up after completion of therapy (SVR12 according to mITT: 42/42, 100%). No treatment failures had occurred.
Conclusion
Providing integrated point-of-care HCV screening/diagnostic testing at central OAT approved centers, followed by DOT with DAAs, represents an effective HCV microelimination strategy. While some PWIDs were lost in the cascade to cure and the absolute number of SVR was limited by the COVID-19 pandemic, our approach will allow linkage to care in a large proportion of Viennese PWIDs.
Keywords: HCV, Elimination, Screening, Directly observed therapy, People who inject drugs, PWIDs
Abbreviations
- PWIDs
people who injects drugs
- OAT
opioid agonist therapy
- HCV
hepatitis C virus
- DAA
direct-acting antivirals
- DOT
directly observed therapy
- COVID-19
coronavirus disease
- RNA
ribonucleic acid
- PCR
polymerase chain reaction
- G/P
glecaprevir/pibrentasvir
- SOF/VEL
sofosbuvir/velpatasvir
- SVR
sustained virologic response
- CHC
chronic hepatitis C
- MSM
men-who-have-sex-with-men
- IDU
ongoing injection drug use
- GT
genotype
- TE
transient elastography
- IQR
interquartile range
- CI
confidence interval
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- WHO
World Health Organization
Introduction
Since the introduction of highly effective direct-acting antivirals (DAAs) for hepatitis C (HCV), numerous elimination programs have been initiated worldwide to achieve the WHO public health goal of HCV elimination by 2030.1, 2, 3, 4 While in some high-incidence populations, such as men-who-have-sex-with-men (MSM), the implementation of HCV elimination programs has led to a significant increase in successful HCV cure, comprehensive screening/diagnostic testing and linkage to care represent a major challenge in other difficult-to-reach populations such as people experiencing homelessness, those who are incarcerated and people who inject drugs (PWIDs).5, 6, 7, 8
Therefore, despite international recommendations for routine HCV screening, diagnostic testing, and treatment leading to cure in PWIDs, the prevalence of chronic hepatitis C (CHC) remains high among this population.3,6 HCV screening/diagnostic testing provided at general practitioners and addiction medicine specialists does not produce a sufficient increase in testing among this high-incidence population, as the active advertising of medical services represents a major barrier for most PWIDs.9 Due to the high prevalence of psychiatric co-morbidities and socio-economic challenges such as homelessness, unemployment, and lack of social support, attending appointment-based medical care is a relevant obstacle for PWIDs that also limits treatment once the diagnosis of CHC has been established.1,2 In addition to these individual-level factors, system- and provider-level barriers such as rigid traditional healthcare systems, that are not gentle, accommodating, and understanding of the needs of PWIDs, lack of integrated addiction and HCV care, and physicians lacking adequate training in the care of PWIDs represent even more relevant obstacles in HCV screening/diagnostic testing and treatment among this population.10 Therefore, tailored HCV elimination efforts focusing on these unmet needs of PWIDs are needed to overcome specific barriers in the cascade to HCV cure.5,11,12
In Austria, the prevalence of high-risk, non-recreational opioid use was estimated at 31,000-37,000 (0.3-0.4%) individuals in 2019.13,14 Nationwide, 19,587 individuals were registered to access the Austrian opioid agonist therapy (OAT) program, an initiative to support PWIDs in their attempt to achieve successful abstinence from injection drug use and encourage social reintegration.14 While complete abstinence may not be a possible or a reasonable goal in some PWIDs, the OAT program alongside other harm reduction measures contribute to a reduction in substance-related crime and morbidity and mortality.14, 15, 16 In 2019, 6,665/19,587 (34.0%) of PWIDs registered to use the OAT program were living in Vienna.14,15 Previous microelimination efforts in this cohort had shown that involving low-threshold institutions in the HCV care of PWIDs and using OAT as a vehicle to provide DAA treatment for CHC in the setting of "directly observed therapy" (DOT) had led to excellent adherence and DAA-induced sustained virologic response (SVR) even in PWIDs at high-risk for treatment interruption/discontinuation.1,2,17,18 However, HCV screening/diagnostic testing remains insufficient among PWIDs on OAT in Vienna.3,6,19
This project provides an integrated approach to implement HCV screening/diagnostic testing in PWID routine to achieve comprehensive testing among the Vienna cohort on OAT and thereby to target those individuals who had so far not been reached by other HCV elimination programs. Furthermore, an established approach for DAA treatment among PWIDs on OAT who show a high risk for non-adherence to HCV therapy was used to achieve linkage to care and successful treatment in individuals with CHC.1,2
Methods
Setting
To reduce illegal substance consumption and encourage social reintegration of people with a history of substance use disorder, Austria offers a national substitution program with opioid agonists (Supplement 1, Supplementary Table 1).14,15 PWIDs included in the program are required to pick up their OAT at a defined pharmacy or low-threshold institution on a regular basis, with intervals ranging between once daily and once weekly. The determination of the dispensing intervals as well as the choice of the OAT dispensing institution were made depending on the individual PWID risk of non-adherence to the OAT schedule as assessed by the interdisciplinary team involved in OAT initiation2: PWIDs at highest risk of non-adherence were assigned to a daily schedule while for those expected to be at lower risk of non-adherence, intervals were extended (modified DOT). Individuals in extraordinarily precarious socio-economic situations were assigned to OAT dispensation via the low-threshold facility while routinely OAT was dispensed via a pharmacy. The low-threshold institution involved in this project is a multidisciplinary facility offering integrated medical care, social services, temporary housing, and a syringe-exchange service for PWIDs in Vienna ("Suchthilfe Wien"). OAT must typically be ingested under the supervision of the pharmaceutical or healthcare staff at the pharmacy or at the low-threshold institution, ensuring that OAT is taken by the authorized PWIDs and not used for intravenous injection or sold on the streets (i.e., "directly observed therapy", DOT).
Patient evaluation for and inclusion into the OAT program as well as OAT prescriptions are conducted by addiction medicine specialists. Until the COVID-19 pandemic, PWIDs on OAT who lived in Vienna had to get their long-term OAT prescriptions approved once a month at one of nine local awarding authorities, depending on residence.15 This requirement made awarding authorities an ideal platform for systematic HCV screening among the specific at-risk population of PWIDs on OAT.
Due to their often disadvantageous socio-economic circumstances, including unemployment, homelessness, criminal record, lack of social support and ongoing injection drug use (IDU) or alcoholism, and their high rate of psychiatric co-morbidities, PWIDs are often unable to keep regular appointments or to attend a tertiary care center.1,2 The HCV treatment may only be prescribed by authorized treatment centers in Austria, hence individuals must attend a tertiary care institution to receive antiviral therapy. Currently, the lack of medical system that can provide accessible, flexible, low-threshold care to people struggling with substance use disorders and their myriad of challenges represents a fundamental obstacle to HCV elimination among PWIDs.10 To minimize barriers in PWID linkage to HCV care, an outpatient hepatitis clinic was established at the Ambulatorium Suchthilfe Wien, a low-threshold institution already involved in the dispensation of OAT, to bring easy-access HCV care to PWIDs. The hepatitis clinic is operated by an experienced hepatologist from the Klinik Ottakring, a Viennese tertiary care center authorized for the treatment of HCV and prescription of DAAs. Through this low-barrier hepatitis outpatient clinic, PWIDs are offered liver disease evaluation and hepatitis testing, and in case of HCV viremia, DAA treatment can be prescribed.1,2
When considering the high percentage of PWIDs at high risk for non-adherence to DAA therapy due to socio-economic challenges and psychiatric co-morbidities, OAT has been shown to offer a beneficial anchor for HCV treatment with DAAs. While adherence to schedules and prescriptions may generally be low in PWIDs, adherence to OAT has been shown to be excellent.1,2 In those at high risk of non-adherence to DAA therapy, combining OAT and DAAs to be dispensed and ingested together at the individual patient pharmacy or at the low threshold institution under the supervision of pharmaceutical staff or healthcare workers ("directly observed therapy", DOT) has proved to be an effective measure to increase DAA adherence and achieve sustained virologic response (SVR) rates comparable to HCV infected patients without a history of IDU.1,2
In this study, we present a combined HCV microelimination concept tailored to increase screening and diagnosis rates, linkage to care, and DAA treatment in PWIDs on OAT.
Patients and screening
All PWIDs above 18 years of age who were registered at one of the nine official awarding authorities in Vienna between January 2019 and March 2020 were offered participation in this project.15 There were no relevant exclusion criteria.
A team consisting of a nurse and a social worker was present on-site throughout the opening hours of each awarding authority on an every day basis, for two months per institution. All attending PWIDs were offered counseling on HCV transmission and sterile injection practices, harm-reduction services (e.g. syringe exchange, provision of sterile injection paraphernalia, free-of-charge analysis of substance components, offered by low-threshold facilities like the Suchthilfe Wien and "checkit!" drug checking, respectively), screening/diagnostic testing, and treatment opportunities, and invited to enroll into the study. Furthermore, hepatitits B (HBV) unvaccinated individuals were informed about the recommendation to undergo HBV immunization and received counseling on where this service was provided (e.g. family doctor, outpatient clinics). PWIDs who agreed to participate received a questionnaire to assess previous HCV treatment (yes/no; if yes: timing, institution, substance), previous HCV screening/diagnostic testing (yes/no; if yes: timing, result [unknown HCV antibody/ positive antibody//negative HCV antibody/positive HCV-RNA PCR/negative HCV-RNA PCR]), the individual OAT prescriber as well as the institution distributing OAT to the respective PWIDs. Consecutively, they received HCV antibody screening via a saliva rapid test (OraQuick®).20 In case of a positive HCV antibody result, a diagnostic point-of-care HCV-RNA PCR test (GeneXpert® fingerstick assay) was performed immediately (if the patient was willing to wait until the result of the rapid-saliva screening test was available), or at their next visit the following month.21 Individuals who reported a positive HCV antibody status in the questionnaire had HCV-RNA PCR testing without repeting antibody screening. By using the GeneXpert® fingerstick assay we avoided the need for phlebotomy to acquire blood samples, which increased convenience for PWIDs as well as for the study team as there was no infrastructure/material for venous blood sampling available at the substitution approval centers.21
PWIDs who were HCV viremic and those who reported previously diagnosed and untreated viremic CHC were referred to the hepatitis outpatient clinic of the Ambulatorium Suchthilfe Wien (low-threshold facility) or the Klinik Ottakring (tertiary care center) for pretreatment evaluation. Depending on previous adherence to OAT, expected adherence to DAA, socio-economic status (importantly including availability of stable housing), and health insurance coverage as assessed by the study team on-site, PWIDs with good adherence and those who were in a stable socio-economic situation were preferably assigned to the tertiary care center while those with expected high risk of non-adherence and those who were in a more precarious socio-economic situation were preferably assigned to the low-threshold facility. Of note, all PWIDs were seen by the same experienced hepatologist independent of the institution they were referred to, and pretreatment evaluation did not differ between the tertiary care center and the low-threshold facility. Both institutions are located within the city of Vienna and can be reached easily using the accessible, reliable, and affordable urban public transportation system, hence transportation was not provided during the project. Diagnostic testing, pretreatment workup and DAA treatment were performed according to current guidelines using only licensed pharmacological substances; no financial compensation was provided for PWIDs undergoing HCV testing and/or DAA treatment in this project. However, HCV testing as well as DAA treatment and follow-up visits were provided free-of charge for individuals lacking health insurance coverage at the time of this initiative.
Pretreatment workup
Pretreatment evaluation included medical history (including alcohol consumption habits and ongoing IDU [defined as one or more injections within the three months preceding the evaluation]), socio-economic status (relationship, housing and employment status, criminal record), physical examination, abdominal sonography, standard laboratory testing, serum HCV RNA quantification, HCV genotype (GT), HIV antibody screening, HBV serology (HBsAg, antiHBs, antiHBc), and liver fibrosis stage assessment via transient elastography (TE) fibroscan.1,22, 23, 24 The applied methods were described in detail in a previous publication from our group.1
Antiviral therapy
PWIDs received a fixed-dose combination treatment with either SOF/VEL (400 mg sofosbuvir and 100 mg velpatasvir once daily for 12 weeks) or with G/P (100 mg glecaprevir and 40 mg pibrentasvir, three tablets once daily for 8 weeks, administered with food) according to the drug label. DAA treatment regimen selection was based on PWIDs' individual pretreatment history, co-morbidities, co-medication, and current health insurance reimbursement policies. At the time of the project, costs for DAA treatment were covered by the Austrian health insurance in all HCV viremic patients, independently from the fibrosis stage. In individuals without valid insurance coverage at the time of HCV diagnosis, DAA treatment was kindly provided by pharmaceutical companies throughout the project. Hence, all HCV viremic individuals identified in this project were guaranteed to receive DAA treatment.
Treatment setting
DAA treatment was applied according to the concept of DOT/modified DOT following PWIDs' OAT established schedule of at their individual institution of reference (i.e. pharmacy or low-threshold institution). In case PWIDs did not attend a scheduled OAT appointment and, hence, missed one or more doses of their HCV therapy, DAA treatment was extended for the corresponding number of days to fulfil the required total treatment duration. No home rescue doses of DAA were dispensed aside from the scheduled dispensation according to DOT/modified DOT alongside OAT.
Study endpoints
Primary endpoints of the screening (HCV antibody)/diagnostic testing (HCV RNA) steps included the level of participation among PWIDs in the offered point-of-care HCV testing, and the rate of HCV viremia among the individuals who underwent screening/diagnostic testing.
The primary endpoint of the treatment step was sustained virologic response 12 weeks after DAA treatment completion (SVR12), defined as HCV RNA <15 IU/ml. SVR12 rates were calculated for all the individuals who had received at least one dose of DAA. Separate analyses were performed for all those who had received at least one dose of DAA, excluding those who had not achieved SVR12 due to reasons other than virological failure (i.e. loss to follow-up before SVR12 which could be documented).
Adherence to DAA, frequency of early termination of treatment, and occurrence of serious adverse events were assessed as secondary endpoints.
Post-treatment follow-up
All PWIDs who achieved SVR12 after DAA treatment were invited for HCV RNA PCR follow-up (FU) testing at week 24 and 48 after the end of treatment. Furthermore, all PWIDs contacted through our project were advised to present to the low-threshold institution, Ambulatorium Suchthilfe Wien once a year for an HCV RNA PCR-FU in case of ongoing risk behavior, including injection or nasal drug use, according to current guidelines.25 Additionally, an open-door policy at the low-threshold facility was being upheld to provide HCV RNA PCR-FU on demand for PWIDs, e.g. after potential or known exposure to HCV, independently from recommended routine surveillance intervals.
Statistics
Median/interquartile range (IQR) and absolutes/percentages of a specific characteristic were used to describe continuous variables and categorical variables, respectively. Two-sided 95% confidence intervals (CI) were calculated using the Clopper-Pearson estimation method. Statistical analyses were performed using Microsoft Excel for Mac 2011, Version 16.6.4; IBM SPSS Statistics, Version 27 for Mac; and Prism 9 for macOS, Version 9.3.1 (350), 2021.
Ethical considerations
Screening/diagnostic testing and treatment were conducted according to the currently recommended algorithm of HCV screening, pretreatment evaluation and antiviral therapy in a specific subpopulation of PWIDs participating in the OAT program in Vienna. The protocol was approved by the Institutional Review Board (Ethikkommission der Stadt Wien, EK 16-098-VK) and conducted according to the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. Written informed consent was obtained from all individuals prior to inclusion. While all PWIDs attending the substitution approval centers had access to all steps of the cascade to cure, irrespective of study participation, only those who provided their consent were included in our database and in the consecutive analyses.
Results
During the study period, two of the originally nine awarding authorities in Vienna merged, resulting in a total of eight which could be visited by the study team.15 Between 1st March 2019 and 29th February 2020, six of these eight authorities were investigated according to the study protocol. Due to the outbreak of the COVID-19 pandemic and associated social distancing requirements, the obligation of PWID monthly approval for long-term OAT prescriptions was abandoned and our project had to be stopped prematurely as a monthly in-person visit was no longer required for the approval of new OAT prescriptions.26, 27, 28 The approval process is since beieng carried out via email between the respective OAT prescribing physician and awarding authority, which proved efficient and will therefore be permanently implemented. Importantly, the OAT program was continued throughout the COVID-19 pandemic and beyond, and none of the included PWIDs involuntarily missed their OAT medications.
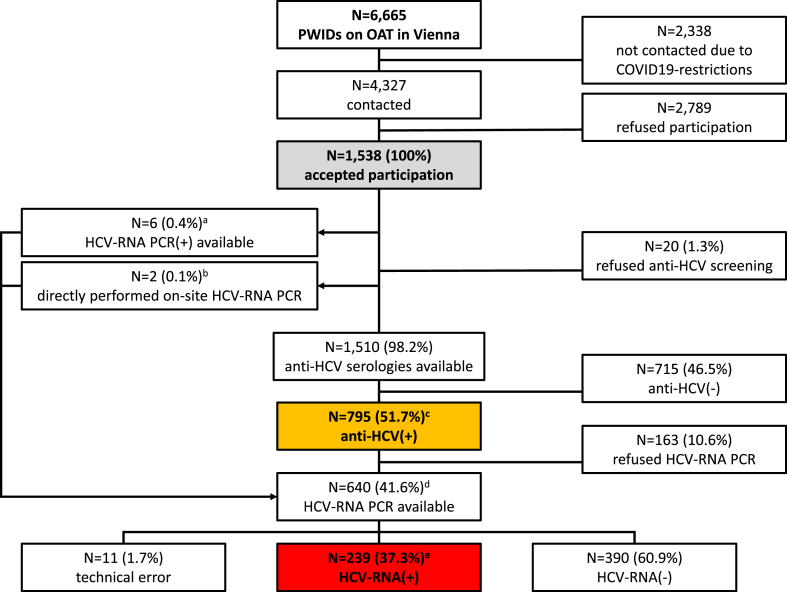
Study population (Fig. 1, Fig. 2, Table 1)
Fig. 1.
Flowchart of the study population.
Abbreviations: PWIDs, people who inject drugs; OAT, opioid agonist therapy; COVID, coronavirus disease; HCV, hepatitis C virus; RNA, ribonucleic acid; PCR, polymerase chain reaction.
a For all 6 individuals a positive HCV RNA PCR result but no HCV serology was available (including n = 1 who declined on-site HCV RNA PCR, n = 1 with positive HCV RNA on-site and n = 4 with positive HCV RNA on-site).
b For 2 individuals neither HCV serology nor HCV RNA PCR were available, but according to the wish of the individuals HCV RNA PCR without prior HCV serology assessment was performed (including n = 1 in whom HCV RNA PCR on-site could not be assessed due to technical issues and n = 1 with positive HCV RNA on-site).
c Including n = 180/795 who underwent HCV serology on-site and n = 615/795 who reported positiveHCV serology from previous testing
d Including n = 632 positive HCV serology and n = 8 without previous HCV serology; n = 595/640 (588 positive HCV serology and 7 without available HCV serology) HCV RNA PCR tests were performed on-site and n = 45/640 (44 positive HCV serology and 1 without available HCV serology) positive HCV RNA were reported from previous testing.
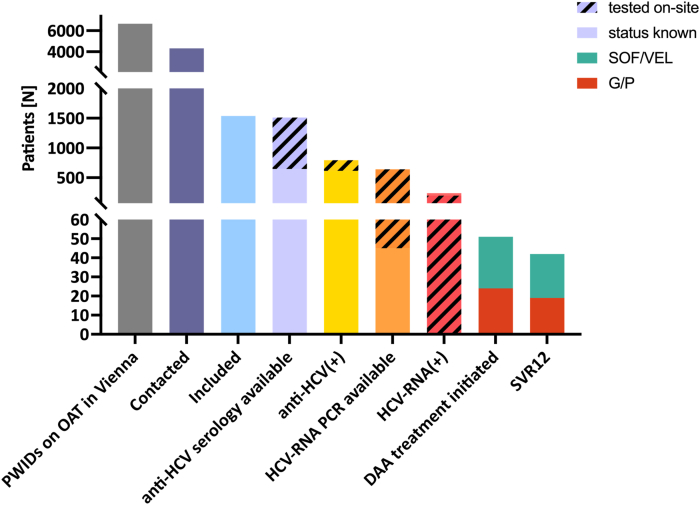
Fig. 2.
Cascade to cure.
Abbreviations: PWIDs, people who inject drugs; OAT, opioid agonist therapy; HCV, hepatitis C virus; RNA, ribonucleic acid; PCR, polymerase chain reaction; DAA, direct-acting antivirals; SVR, sustained virologic response.
Table 1.
Baseline Characteristics of the study population.
| Variable | |
|---|---|
| n (%) | 1,538 (100) |
| sex [n (%)] | |
| male | 1,122 (73.0) |
| female | 416 (27.0) |
| age (years) [median (IQR)] | 40.0 (13.0) |
| reported HCV status [n (%)] | |
| Positive HCV antibody | 620 (40.3)a |
| Positive HCV RNA | 82 (5.3)b |
| HCV treatment-experienced | 314 (20.4)c |
Abbreviations: HCV, hepatitis C virus; RNA, ribonucleic acid.
Including n = 72 with previous HCV-RNA(+).
Including n = 72 with previous anti-HCV(+).
Including n = 93 (29.6%) who had received pegylated interferon and ribavirin, n = 92 (29.3%) who had undergone DAA therapy, and n = 129 (41.1%) in whom the previous treatment regimen could not be determined.
In total, 4,327 (64.9%) of the 6,665 PWIDs who were registered to use the Austrian OAT program in Vienna during the study period were contacted by our team at six of the eight awarding authorities (Fig. 1, Fig. 2). After having received information about HCV screening/diagnostic testing and treatment options as well as the interventions of harm reduction in the case of ongoing IDU, 2,789 (64.5%) study participants chose to participate in the project. Ultimately, 1,538 (35.5%) individuals were registered to take part in the study (Table 1). Among the included individuals, 1,122 (73.0%) were male and the median age was 40.0 (IQR 13.0) years, reflecting the demographic distribution documented in the overall population of PWIDs registered in the Viennese OAT program.15 Previous diagnosis of HCV infection was reported by 630 (41.0%) PWIDs [HCV antibody positive in 620 (40.3%); HCV RNA positive in 82 (5.3%)] and 314 (20.4%) had previously received antiviral treatment for CHC. Among the pretreated population, 93 (29.6%) had received pegylated interferon and ribavirin, 92 (29.3%) DAA therapy, and in 129 (41.1%) the previous treatment regimen could not be determined.
HCV serology screening (Fig. 1, Fig. 2)
Among the 1,538 PWIDs who had consented to participate in the study, 864 (56.2%) received an HCV saliva screening test on-site, of which 180 (20.8%) showed a positive result (Fig. 2).
HCV screening was declined by 646/1,538 (42.0%) individuals because they had previously been tested for HCV (negative HCV serology in n = 31 (4.8%); positive HCV serology in n = 615 (95.2%)). Of the 615 PWIDs who declined HCV antibody screening due to previously diagnosed HCV positivity, 302 (49.1%) had received antiviral treatment. An additional 6/1,538 (0.4%) study participants, all of whom reported to be HCV treatment naïve, declined HCV antibody screening due to previous diagnosis of HCV viremia and directly proceeded to HCV RNA testing for confirmation of chronic infection. Twenty (1.3%) PWIDs who initially consented to taking part in the project eventually declined HCV screening without stating their reasons, while 2 (0.1%) individuals received diagnostic HCV RNA PCR testing without previous HCV serology screening according to their specific request (Fig. 1).
Overall, 1,510/1,538 (98.2%) locally performed and/or individual reported HCV serologies were evaluated during primary screening. Considering the 180 individuals who received a positive HCV serology screening result on-site and the 615 who declined saliva screening due to a previous positive diagnosis of HCV serology, a total of 795/1,510 (52.6%) positive HCV antibody results in PWIDs were recorded during HCV serology screening at the awarding authorities.
HCV RNA PCR follow-up (diagnostic testing) (Fig. 1, Fig. 2)
Among the 795 with positive HCV serology study participants who were identified during the primary screening, -588 (74.0%) received an HCV RNA PCR result (Fig. 2). 389/588 (66.2%) had a negative PCR result, indicating past HCV RNA clearance, and 189 (32.1%) had HCV RNA viremia, while in 10/588 (1.7%) technical problems lead to an inconclusive result (Fig. 1).21 No repeated testing was performed in individuals with inconclusive PCR results. In 8/1,538 (0.5%) PWIDs only HCVRNA PCR without previous antibody screening was assessed in our analysis: 7/8 (87.5%) underwent HCV RNA PCR on-site as 5 of them reported previous diagnosis of HCV viremia and 2 declined HCV antibody screening and specifically requested to directly proceed to HCV RNA PCR diagnostic testing (see "Anti-HCV screening"): one (14.3%) previously HCV viremic PWID had cleared HCV RNA while 5/7 (71.4%) showed a positive PCR on-site, and in 1/7 (14.3%) the PCR was inconclusive due to technical issues.21 No repeated testing was performed in the individual with the inconclusive PCR result. One (12.5%) of the eight individuals declined on-site repetition of HCV RNA PCR diagnostic testing as HCV viremia had been previously diagnosed.
The 44/795 (5.5%) HCV positive antibody PWIDs did not undergo PCR diagnostic testing on-site due to a previous diagnosis of HCV viremia and were directly referred to an HCV treatment center for DAA initiation. One hundre and sixty-three (20.5%) declined HCV RNA PCR testing without stating their reasons.
Overall, a total of 640 PCR tests were assessed through the project, 595 (93.0%) of which were performed on-site at the substitution approval centers. 239/640 (37.3%) of the individuals who were evaluated via HCV RNA PCR showed HCV viremia. One hundred and sixty-three (68.2%) of the identified 239 HCV viremic PWIDs had not undergone HCV RNA PCR evaluation before; 65/163 individuals had never been tested with HCV serology or PCR. Seventy-seven individuals reported having received a positive HCV RNA result in the past (including 33 in whom this positive PCR result was reassessed on-site and 44 who were directly referred to an HCV treatment center for DAA initiation).
Considering the entire cohort of PWIDs evaluated in this project, 239/1,538 (15.5%) HCV viremic individuals were identified.
Linkage to care and characteristics of the treated population (Fig. 2, Table 2)
Table 2.
Characteristics of the DAA-treated population.
| Variable | |
|---|---|
| n (%) | 51 (100) |
| sex [n (%)] | |
| male | 40 (78.4) |
| female | 11 (21.6) |
| age (years) [median (IQR)] | 44.4 (16.8) |
| fibrosis stage [n (%)]a | |
| F0/1 | 18 (35.3) |
| F2 | 10 (19.6) |
| F3 | 9 (17.6) |
| F4 | 14 (27.5) |
| HCV-genotype [n (%)]b | |
| 1 | 21 (45.7) |
| 1a | 17 (33.3) |
| 1b | 4 (7.8) |
| 3 | 23 (50.0) |
| 3a | 12 (23.5) |
| 4 | 2 (4.3) |
| HBV coinfection [n (%)] | 1 (2.0) |
| treatment experienced [n (%)]c | 7 (13.7) |
| OAT medication [n (%)] | |
| morphine | 32 (62.7) |
| morphine hydrochloride | 9 (17.6) |
| methadone | 4 (7.8) |
| levomethadone hydrochloride | 1 (2.0) |
| buprenorphine | 5 (9.8) |
| OAT dispensation | |
| institution [n (%)] | |
| pharmacy | 50 (98.0) |
| low-threshold institution | 1 (2.0) |
| intervals [n (%)] | |
| once weekly | 30 (58.8) |
| twice weekly | 4 (7.8) |
| thrice weekly | 4 (7.8) |
| daily | 13 (25.5) |
| socioeconomic status [n (%)] | |
| own housingd | 34 (82.9) |
| stable relationshipd | 17 (41.5) |
| employmentd | 12 (29.3) |
| hazardous alcohol consumptione | 5 (15.6) |
| ongoing injection drug use | 20 (39.2) |
Abbreviations: DAA, direct-acting antiviral; TE, transient elastography; HCV, hepatitis C virus; HBV, hepatitis B virus; OAT, opioid agonist therapy.
According to transient elastography: F0/F1: TE 0 – 7.1 kPa or APRI ≤0.5; F2: TE 7.2 – 9.4 kPa; F3: TE 9.5 – 12.4 kPa; F4: TE ≥ 12.5.
HCV-genotype not specified in n = 5 (9.8%).
Pretreatment with pegylated interferon and ribavirin in n = 7/7 (100%).
Missing data in n = 10 (19.6%).
Missing data in n = 19 (37.3%).
Among the 239 PWIDs who had HCV viremia with diagnostic PCR testing, 51 (21.3%) could be started on DAA treatment until 19 September 2022: 50 (98.0%) received DOT/modified DOT via a pharmacy and 1 (2.0%) via the low-threshold institution (Fig. 2, Table 2). The one who was treated via the low-threshold institution did not have a valid health insurance coverage at the time of the project, hence OAT was provided by the low-threshold facility and DAA treatment by a pharmaceutical company free of charge. Pretreatment evaluation did not lead to any cases of loss to FU.
Forty (78.4%) of the 51 study participants in whom treatment was initiated were male and the median age was 44.4 (IQR 16.8) years. The most common HCV genotype was GT3 (n = 23, 50.0%), 14 (27.5%) had advanced chronic liver disease according to transient elastography (F4), and 1 (2.0%) showed coinfection with HBV. The PWIDs in whom advanced fibrosis or HBV coinfection were detected received counseling on further treatment and surveillance implications, e.g. the necessity of biennial screening for hepatocellular carcinoma via abdominal sonography in cirrhosis and in HBV coinfection, and the recommendation of HBV treatment evaluation via a tertiary care center on a regular basis in HBV coinfection. All individuals received an index sonography and standard laboratory evaluation during the pretreatment workup at the low-threshold facility, and in cases with evident indications for pharmacotherapy, e.g. diuretic treatment in decompensated cirrhosis, the respective prescriptions were made during the pretreatment evaluation visit at the low-threshold facility. No coinfections with the human immunodeficiency virus were observed among the PWIDs who were started on DAA. Forty-four (86.3%) individuals were treatment-naive while 7 (13.7%) had previously received a combination of pegylated interferon and ribavirin. None of the treated individuals had previously received DAAs.
Among the 41/51 (80.4%) PWIDs for whom socio-economic information was available, 34 (82.9%) reported stable housing and 17 (41.5%) were in a relationship, while 29 (70.7%) were unemployed. 5/32 (15.6%) reported hazardous alcohol consumption and 20/51 (39.2%) stated ongoing IDU.
Treatment outcome and effectiveness of DAA (Fig. 2)
Twenty-four (47.1%) of the 51 PWIDs who were started on DAA were treated with G/P while 27 (52.9%) received SOF/VEL (Fig. 2). In 3 (5.9%) study participants who received SOF/VEL, treatment or post-treatment surveillance is still ongoing. Overall, 42 (87.5%; 95% CI: 74.8-95.3%) of the 48 individuals who finished treatment and post-treatment surveillance achieved SVR12 as of 31 October 2022 according to ITT, including 19 (45.2%) treated with G/P and 23 (54.8%) with SOF/VEL. 6/48 (12.5%) PWIDs were lost to FU after completion of DAA therapy at a pharmacy or a low-threshold facility, 5 (83.3%) of them had received G/P and 1 (16.7%) SOF/VEL.
After exclusion of the individuals who were lost to FU, 42/42 (100%; 95% CI: 91.6-100%) achieved SVR12 according to mITT. Of note, no treatment failures were observed among PWIDs who had completed treatment and post-treatment surveillance, hence the difference in SVR12 according to ITT (19/24 [79.2%] for G/P vs. 23/24 [95.8%] for SOF/VEL) was solely due to a higher number of individuals lost to FU after the end of treatment in the cohort treated with G/P as compared to SOF/VEL, while SVR12 according to mITT was identical at 100% in both groups. During a median FU of 21.5 (IQR 24) weeks, no reinfections were recorded.
Adherence to therapy and early treatment discontinuation
Following the concept of DOT/modified DOT, adherence to DAA therapy was excellent in our study population. Among the 51 PWIDs who started DAA treatment, only 2 (0.2%) of the total 1.268 scheduled drug ingestions were missed and lead to a corresponding prolongation of treatment in the respective individuals. No treatment interruptions due to hospitalization or imprisonment occurred and there were no early treatment discontinuations.
Serious adverse events
No serious adverse events related to DAA therapy were recorded among our study population.
Discussion
The elimination of HCV by 2030 has been declared a global public health goal by the WHO.3 While in 2020 only 11 countries were on track to achieve the set targets, Austria was forecasted to meet the WHO requirements not until 2040 and remained with an estimated HCV prevalence of 0.2% of the population, corresponding to approximately 15,000 individuals with chronic HCV.3,4,19 The prevalence of HCV viremia is especially high in certain populations who are vulnerable to blood-borne virus infections, such as PWIDs.6 However, the early initiation of DAA treatment in PWIDs is key for the prevention of liver disease progression as well as liver-related complications and malignancies, and to avoid onward transmission, especially in PWIDs with ongoing IDU.29 Still, despite the broad availability of DAA in Western Europe and current recommendations for HCV screening among PWIDs, treatment receipt remains insufficient among this population.3,6
Currently, the major remaining barriers in the PWIDs cascade to HCV cure have been shown to be screening, diagnostic testing, two-step diagnostic processes, and linkage to care, often due to the requirement for offsite treatment initiation.5,10 Additionally, reinfection after SVR/spontaneous clearance remains a problem, especially in individuals with ongoing IDU; however, comprehensive HCV treatment among PWIDs will lower the risk of reinfection substantially.9,10 Furthermore, counseling and harm reduction programs including syringe exchange services greatly contribute to maintaining HCV RNA negativity after achieving SVR, even in the setting of ongoing injection substance use.9 Targeting the deficits in the PWID cascade to HCV cure is challenging, yet tailored approaches to treatment have been shown to be effective in this high-incidence community10: As such, adherence to DAA could be significantly improved by the application of integrated treatment approaches such as DOT in various studies.1,2 On the other hand, it must be acknowledged that the assumption of universally low adherence to DAA among PWIDs is often erroneous,30 and that reasonable SVR rates may be achieved even without optimal adherence to therapy.31 However, screening and linkage to care for PWIDs still represent a demanding task to be optimized, which will require a paradigm shift towards the accommodation of PWID needs in medical systems, just like the needs of hearing-impaired individuals are now being accommodated.10 Previous analyses suggest the integration of screening/diagnostic testing and linkage to care in preexisting systems such as primary care, emergency rooms, or harm reduction programs (e.g. syringe exchange services) to increase screening and treatment receipt as well as to comply with economic requirements.32, 33, 34, 35, 36, 37, 38 As demonstrated by the highly successful collaboration in this project, pharmacies represent institutions with great potential to contribute to HCV elimination, not only by supporting treatment adherence but also by expanding the capacity for decentralized screening/diagnostic testing, e.g. by offering point-of-care testing on-site.39 Furthermore, the involvement of PWID peers in HCV screening and treatment can influence the cascade to cure positively and may therefore be a promising tool to be used in future HCV elimination projects.11,40
HCV screening/diagnostic testing at the awarding authorities in Vienna was an effective tool to increase the diagnosis rate in Viennese PWIDs on OAT: 239 of 1,538 study participants included in the research project were identified to be HCV viremic, resulting in a relevant share of 15.5% of PWIDs on OAT in Vienna who remain in need of DAA treatment. However, it must be acknowledged that these calculations likely represent an underestimation of the real HCV prevalence as 163 (10.6%) individuals with positive HCV serology declined confirmatory HCV RNA PCR testing. This drop-off in diagnostic testing owing to our two-step screening approach may be overcome by reflex testing, providing the possibility to perform HCV RNA PCR from the same sample that was used for HCV antibody screening.41 However, single-step reflex testing requires taking a blood sample in every individual, while the easy and convenient approach of saliva-based antibody screening followed by point-of-care confirmatory HCV RNA PCR that was used in our project represents a major strength of this study.42 Among 1,510 PWIDs who underwent HCV serology screening, we found an HCV seropositivity prevalence of 52.6%, which is higher than the positive HCV antibody prevalence of 42.2% discovered through a similar project performed among PWIDs in Southern Italy in 2019.34 Yet of note, the prevalence of HCV viremia was significantly lower among our study population at 37.3% (Fig. 1) as compared to 70.2% among the Italian cohort.34 On the one hand, this finding may be a reflection of the geographical differences in HCV prevalence, which was estimated to be higher in Italy than in Austria with 1% versus 0.2% of the national populations, respectively, in 2020.3 On the other hand, ongoing HCV elimination efforts aimed specifically at PWIDs in Vienna may have led to these substantial differences in HCV viremia prevalence.1,2 Interestingly, a recent study from Australia, who is currently on track to meeting the WHO requirements for HCV elimination by 2030, found HCV viremia rates of 50% among PWIDs with low socio-economic status who were screened for HCV in a point-of-care setting, however, these results may be influenced by a smaller sample size.37 Importantly, 65 (27.2%) PWIDs who were found to be HCV viremic in our study reported having never been tested for HCV before, which is lower than in the Italian cohort where 48.1% were newly diagnosed.34 Overall, 163 (68.2%) of the 239 HCV RNA(+) identified in our study were unaware of their HCV viremia. Given the overall low prevalence of CHC among the general population in Austria, our data supports the current German and European guideline recommendations advising for risk-based HCV screening, e.g. for all PWIDs, that should be repeated and/or changed to HCV RNA PCR testing according to risk behavior such as ongoing IDU and/or HCV antibody positivity due to previous HCV clearance, respectively.25,43,44 The Italian study provided a more comprehensive follow-up of PWIDs who showed a positive HCV serology during the primary screening with 91.2% of the HCV antiody positive individuals receiving HCV RNA PCR diagnostic testing, while only 79.5% of the positve HCV antibody study participants in our project agreed to undergo PCR. However, the study population investigated in our project was much larger than the Italian cohort one with n = 1,510 versus n = 593 individuals respectively.34 While a bias in the data due to the differences in sample size as well as the lower PCR follow-up rate of positive HCV serologies in our cohort may in part cause misleading results, it has to be noted that treatment receipt was excellent in the Italian cohort, while it remained insufficient among our study population so far.34 This clinically relevant drop-off in the cascade to cure may be explained by the offsite referral required to initiate DAA treatment: While HCV screening/diagnostic testing and counseling were performed on-site at the substitution approval centers and PWIDs did not have to go out of their way to receive it, they had to attend a different address, namely Ambulatorium Suchthilfe Wien or Klinik Ottakring, for treatment evaluation. While Ambulatorium Suchthilfe Wien is a low-threshold institution aiming to overcome barriers to treatment for PWIDs and pretreatment evaluation was performed in a single visit there, the geographical change from the substitution approval center to the low-threshold facility alone represents an obstacle in the cascade to HCV cure, which, hence, underlines the need for further decentralization and integration of HCV care in the everyday routine of PWIDs. However, pretreatment evaluation did not lead to any cases of loss to FU in our study. Of note, among the Australian cohort, a similar SVR rate of 21% of HCV viremic individuals was found as compared to our data.37
Unfortunately, the COVID-19 pandemic has greatly interfered with the realization of this project, as only 6 of the total 8 awarding authorities could be investigated. Additionally, social distancing and official governmental restrictions specifically impaired linkage to care in our cohort, which may in part explain the low percentage of treatment initiations among the HCV viremic population. The effects of the COVID-19 pandemic are, however, impacting HCV elimination programs all over the world, and will have to be acknowledged upon the assessment of all global HCV elimination efforts towards the WHO goal.26, 27, 28 Nevertheless, it is to be taken into consideration that the population investigated in this study represents a very difficult-to-reach cohort that has so far escaped all previous HCV elimination initiatives in Vienna.1,2 Therefore, linkage to care can be expected to be specifically challenging and may require repeated recalls and/or the implementation of additional services, like for example a peer-based support program, which was shown to be effective in previous studies.5,11 To close existing gaps - including those provoked by linkage to offsite care - in the HCV elimination cascade for PWIDs, embedding screening and treatment in an established OAT program was shown to be highly effective.45 Furthermore, it must be acknowledged that the Austrian regulation of exclusively tertiary care-mediated DAA prescription represents one of the most relevant obstacles in HCV care for PWIDs. While in this project as well as in previous HCV elimination efforts performed by our group1,2 the regulation was overcome by outsourcing an outpatient hepatitis clinic affiliated with a tertiary care center authorized to prescribe DAA at a low threshold facility, enabling a broader spectrum of healthcare providers. Low threshold facilities to provide DAA treatment prescriptions still remains to be achieved in Austria.
We believe that our study provides a new approach to overcome the barriers to HCV screening/diagnostic testing in Viennese PWIDs. Performing HCV screening/diagnostic testing in a point-of-care setting at the awarding authorities incorporated into the OAT program has facilitated the comprehensive delivery of HCV care to PWIDs who may otherwise never have attended an HCV screening/diagnostic testing institution. Overall, we have engaged a large population at risk of HCV infection in the screening/diagnostic testing process and combined screening/diagnostic testing with the established approach of DOT/modified DOT with DAA to accommodate the needs of PWIDs. Importantly, adherence to DOT/modified DOT was excellent with only 0.2% of the scheduled ingestions being missed by our treated individuals, which led to an aliquot extension of treatment duration in the respective cases.
However, our study also has some limitations. First, only 1,538 (23.1%) of the total 6,665 PWIDs eligible for screening could be included, which was due to the shortened study duration for external reasons, and due to the high rate of PWIDs who declined participation in the project. The initial characterization of the included population is very basic (Table 1), which, however, goes in line with the concept of the project, delivering low-barrier HCV care in a point-of-care setting, and which can be intensified during the pretreatment workup. Second, linkage to care remains low with 21.3% of HCV viremic patients having started DAA therapy. Originally, linkage to care was greatly impaired by COVID-19-associated restrictions but is currently being actively pursued, hence we expect an ongoing increase in treatment initiations. Third, considering that we did not observe any HCV reinfections during FU, it must be pointed out that enforcement of the recommended intervals for HCV RNA PCR-FU was not subject to this project, and as adherence to long-term appointments remains problematic among PWIDs as elaborated in this manuscript, we cannot provide data from standardized assessment points. Therefore, intercurrent HCV reinfection and spontaneous clearance cannot be ruled out. Nevertheless, 31/48 (64.6%) PWIDs who achieved SVR12 had FU HCV RNA PCR tests performed between week 13 and 115 after the end of treatment, which represents a considerable rate of FU testing given the fact that all individuals included in this project were of low socioeconomic status and considered at risk for non-adherence to medical therapy, as represented by the high percentage of daily OAT dispensation among our study population. A fourth limitation of this project is that women represented a rather low percentage (21.6% female vs. 78.4% male DAA recipients) among the treated population. As this distribution mirrors the sex distribution documented among the overall PWID population enrolled in the Viennese OAT program, we do not believe that women suffered from a gender-specific disadvantage in this particular project.15 However, this finding draws attention to the fact that the incidence of HCV among young women marks a change in epidemiology in some parts of the world, and especially women with a history of IDU and/or on OAT may experience risk factors specific to this subpopulation that may have been overlooked so far (e.g. partnership with an older male, being in a relationship with a man who injects drugs, having to exchange sex for drugs to help a male partner obtain drugs, childcare and eldercare responsibilities with lack of childcare at medical appointments/inability to bring children to OAT dispensing visits, etc.).46, 47, 48 Especially for female PWIDs of childbearing age, this deficiency in targeted HCV care implies a potential risk for vertical transmission that may consecutively be overlooked.46 In Austria, vertical infectious disease transmission, namely concerning HIV and HBV, has been effectively targeted through a universal national screening program for pregnant women.49,50 Hence, adding HCV screening to this existing program would be a feasible first step in including this often overlooked but vulnerable population in targeted HCV care.
While there are some promising strategies to increase linkage to care in previously diagnosed HCV viremic PWIDs,5 screening/diagnostic testing in those who declined to take part in low-threshold projects like this will remain a challenge. One option may be the implementation of "opt-out" screening/single-step diagnostic testing scenarios as part of harm reduction services,12 however, a small population of PWIDs - especially those who are not integrated in any systematic programs such as OAT or who do not use services such as syringe exchange - may currently not be able to get treated.
In conclusion, this tailored approach to screening/diagnostic testing and treatment for HCV in Viennese PWIDs was an effective measure to include a population who may otherwise not have been diagnosed. Given the high rate of ongoing IDU among this population, targeted strategies to increase diagnosis rate and treatment receipt are key to avoid further HCV transmission and progressive liver disease. In the absence of an effective HCV vaccine, the combination of OAT, DAA, and harm reduction as applied in this project will be key to facilitate HCV elimination among PWIDs. Our project supports the integration of HCV screening and treatment into preexisting services for PWIDs, which may be transferable to other regions nationally and internationally in the context of HCV elimination.
Financial support
This study was supported by Gilead.
Declaration of competing interest
Schwarz C. (schwarz.caroline1990@gmail.com): received travel support from Gilead, Abbvie, and Gebro; speaking honoraria from Abbvie and Gilead; and served as a consultant for Gilead.
Schubert R. (raphael.schubert@outlook.at): was employed by Suchthilfe Wien gGmbH and received travel support from Gilead.
Schwarz M. (michael.schwarz@meduniwien.ac.at): received speaking honoraria from BMS and travel support from BMS, MSD, Abbvie and Gilead.
Schütz A. (angelika.schuetz@suchthilfe.at): is employed by Suchthilfe Wien gGmbH and has no conflicts of interest.
Jenke A. (jenke.anika@web.de): has no conflicts of interest.
Bauer D. (david.bauer@gesundheitsverbund.at): received travel support from Gilead and Siemens, served as a speaker/advisor for AbbVie and Siemens, and received grant support from Gilead, Philips, and Siemens.
Steinwender B. (benjaminstw@aol.at): was employed by Suchthilfe Wien gGmbH and has no conflicts of interest.
Gutic E. (enisa.gutic@extern.gesundheitsverbund.at): received travel support from Gilead and Abbvie.
Reiberger T. (thomas.reiberger@meduniwien.ac.at): received grant support from Abbvie, Boehringer-Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Boehringer-Ingelheim, Gilead and Roche.
Haltmayer H. (hans.haltmayer@suchthilfe.at): is employed by Suchthilfe Wien gGmbH and has no conflicts of interest.
Gschwantler M. (michael.gschwantler@gesundheitsverbund.at): received grant support from Abbvie, Gilead and MSD; speaking honoraria from Abbvie, Gilead, Intercept, Janssen, BMS, Roche, Norgine, AstraZeneca, Falk, Shionogi, and MSD; consulting/advisory board fees from Abbvie, Gilead, Intercept, Janssen, BMS, Roche, Alnylam, Norgine, AstraZeneca, Falk, Shionogi and MSD; and travel support from Abbvie and Gilead.
Acknowledgements
Nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2023.100338.
Appendix ASupplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Schmidbauer C., Schubert R., Schütz A., et al. Directly observed therapy for HCV with glecaprevir/pibrentasvir alongside opioid substitution in people who inject drugs-First real world data from Austria. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0229239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidbauer C., Schwarz M., Schütz A., et al. Directly observed therapy at opioid substitution facilities using sofosbuvir/velpatasvir results in excellent SVR12 rates in PWIDs at high risk for non-adherence to DAA therapy. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polaris Observatory HCV Collaborators Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022 May;7(5):396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 4.Kåberg M., Weiland O. Hepatitis C elimination - macro-elimination. Liver Int Off J Int Assoc Study Liver. 2020 Feb;40(Suppl 1):61–66. doi: 10.1111/liv.14352. [DOI] [PubMed] [Google Scholar]
- 5.Conway A., Valerio H., Alavi M., et al. A testing campaign intervention consisting of peer-facilitated engagement, point-of-care HCV RNA testing, and linkage to nursing support to enhance hepatitis C treatment uptake among people who inject drugs: the ETHOS engage study. Viruses. 2022 Jul 16;14(7):1555. doi: 10.3390/v14071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidbauer C., Chromy D., Schmidbauer V., et al. Epidemiological trends in HCV transmission and prevalence in the Viennese HIV+ population. Liver Int. 2020;40(4):787–796. doi: 10.1111/liv.14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jachs M., Binter T., Chromy D., et al. Outcomes of an HCV elimination program targeting the Viennese MSM population. Wien Klin Wochenschr. 2021 Jul;133(13–14):635–640. doi: 10.1007/s00508-021-01898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chromy D., Bauer D.J.M., Simbrunner B., et al. The “Viennese epidemic” of acute HCV in the era of direct-acting antivirals. J Viral Hepat. 2022 May;29(5):385–394. doi: 10.1111/jvh.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valerio H., Alavi M., Conway A., et al. Declining prevalence of current HCV infection and increased treatment uptake among people who inject drugs: the ETHOS Engage study. Int J Drug Pol. 2022 Jul;105 doi: 10.1016/j.drugpo.2022.103706. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz T., Horváth I., Fenz L., Schmutterer I., Rosian-Schikuta I., Mårdh O. Interventions to increase linkage to care and adherence to treatment for hepatitis C among people who inject drugs: a systematic review and practical considerations from an expert panel consultation. Int J Drug Pol. 2022 Apr;102 doi: 10.1016/j.drugpo.2022.103588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley D., Murtagh R., Cullen W., et al. Evaluating peer-supported screening as a hepatitis C case-finding model in prisoners. Harm Reduct J. 2019 Jul 5;16(1):42. doi: 10.1186/s12954-019-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartholomew T.S., Tookes H.E., Serota D.P., Behrends C.N., Forrest D.W., Feaster D.J. Impact of routine opt-out HIV/HCV screening on testing uptake at a syringe services program: an interrupted time series analysis. Int J Drug Pol. 2020 Oct;84 doi: 10.1016/j.drugpo.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Statistik Austria. Bevölkerung im Jahresdurchschnitt https://www.statistik.at/statistiken/bevoelkerung-und-soziales/bevoelkerung/bevoelkerungsstand/bevoelkerung-im-jahresdurchschnitt [Internet]. 2019. Available from:
- 14.Horvath Ilonka, Anzenberger Judith, Busch Martin, Gaiswinkler Sylvia, Schmutterer Irene, Schwarz Tanja. Gesundheit Österreich; 2020. Bericht zur Drogensituation 2020. [Google Scholar]
- 15.MA 15 - Fachbereich Aufsicht und Qualitätssicherung. Wiener Substitutionsstatistik. Stadt Wien; 2019. [Google Scholar]
- 16.Gisev N., Bharat C., Larney S., et al. The effect of entry and retention in opioid agonist treatment on contact with the criminal justice system among opioid-dependent people: a retrospective cohort study. Lancet Public Health. 2019 Jul;4(7):e334–e342. doi: 10.1016/S2468-2667(19)30060-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajarizadeh B., Cunningham E.B., Reid H., Law M., Dore G.J., Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(11):754–767. doi: 10.1016/S2468-1253(18)30304-2. [DOI] [PubMed] [Google Scholar]
- 18.Graf C., Mücke M.M., Dultz G., et al. Efficacy of direct-acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 May 23;70(11):2355–2365. doi: 10.1093/cid/ciz696. [DOI] [PubMed] [Google Scholar]
- 19.Polaris Observatory HCV Collaborators . CDA Foundation; 2022. Countries/Territories Achieving Relative or Absolute Impact and Programmatic Targets - HCV.https://cdafound.org/polaris-countries-maps/ [Internet] Available from: [Google Scholar]
- 20.Gao F., Talbot E.A., Loring C.H., et al. Performance of the OraQuick HCV rapid antibody test for screening exposed patients in a hepatitis C outbreak investigation. Tang YW, editor. J Clin Microbiol. 2014 Jul;52(7):2650–2652. doi: 10.1128/JCM.00132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed Z., Mbwambo J., Rwegasha J., et al. In‐field evaluation of Xpert® HCV viral load Fingerstick assay in people who inject drugs in Tanzania. Liver Int. 2020 Mar;40(3):514–521. doi: 10.1111/liv.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwabl P., Bota S., Salzl P., et al. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int Off J Int Assoc Study Liver. 2015 Feb;35(2):381–390. doi: 10.1111/liv.12623. [DOI] [PubMed] [Google Scholar]
- 23.Castéra L., Vergniol J., Foucher J., et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005 Feb;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Chromy D., Schwabl P., Bucsics T., et al. Non-invasive liver fibrosis assessment and HCV treatment initiation within a systematic screening program in HIV/HCV coinfected patients. Wien Klin Wochenschr. 2018 Feb;130(3–4):105–114. doi: 10.1007/s00508-017-1231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL recommendations on treatment of hepatitis C. J Hepatol. 2018;69(2):461–511. doi: 10.1016/j.jhep.2018.03.026. 2018. [DOI] [PubMed] [Google Scholar]
- 26.Blach S., Kondili L.A., Aghemo A., et al. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021 Jan;74(1):31–36. doi: 10.1016/j.jhep.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartl L., Semmler G., Hofer B.S., et al. COVID-19-related downscaling of in-hospital liver care decreased patient satisfaction and increased liver-related mortality. Hepatol Commun. 2021 Oct;5(10):1660–1675. doi: 10.1002/hep4.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pomej K., Scheiner B., Hartl L., et al. COVID-19 pandemic: impact on the management of patients with hepatocellular carcinoma at a tertiary care hospital. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0256544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith D.J., Combellick J., Jordan A.E., Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): a systematic review and meta-analysis. Int J Drug Pol. 2015 Oct;26(10):911–921. doi: 10.1016/j.drugpo.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dore G.J., Altice F., Litwin A.H., et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016 Nov 1;165(9):625–634. doi: 10.7326/M16-0816. [DOI] [PubMed] [Google Scholar]
- 31.Litwin A.H., Lum P.J., Taylor L.E., et al. Patient-centred models of hepatitis C treatment for people who inject drugs: a multicentre, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2022 Dec;7(12):1112–1127. doi: 10.1016/S2468-1253(22)00275-8. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed Z., Scott N., Nayagam S., et al. Cost effectiveness of simplified HCV screening-and-treatment interventions for people who inject drugs in Dar-es-Salaam, Tanzania. Int J Drug Pol. 2022 Jan;99 doi: 10.1016/j.drugpo.2021.103458. [DOI] [PubMed] [Google Scholar]
- 33.Manca F., Robinson E., Dillon J.F., Boyd K.A. Eradicating hepatitis C: are novel screening strategies for people who inject drugs cost-effective? Int J Drug Pol. 2020 Aug;82 doi: 10.1016/j.drugpo.2020.102811. [DOI] [PubMed] [Google Scholar]
- 34.Persico M., Masarone M., Aglitti A., et al. HCV point-of-care screening programme and treatment options for people who use drugs in a metropolitan area of Southern Italy. Liver Int Off J Int Assoc Study Liver. 2019 Oct;39(10):1845–1851. doi: 10.1111/liv.14166. [DOI] [PubMed] [Google Scholar]
- 35.Park J.S., Wong J., Cohen H. Hepatitis C virus screening of high-risk patients in a community hospital emergency department: retrospective review of patient characteristics and future implications. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomon S.S., Quinn T.C., Solomon S., et al. Integrating HCV testing with HIV programs improves hepatitis C outcomes in people who inject drugs: a cluster-randomized trial. J Hepatol. 2020 Jan;72(1):67–74. doi: 10.1016/j.jhep.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howell J., Traeger M.W., Williams B., et al. The impact of point-of-care hepatitis C testing in needle and syringe exchange programs on linkage to care and treatment uptake among people who inject drugs: an Australian pilot study. J Viral Hepat. 2022 May;29(5):375–384. doi: 10.1111/jvh.13664. [DOI] [PubMed] [Google Scholar]
- 38.Saludes V., Antuori A., Folch C., et al. Utility of a one-step screening and diagnosis strategy for viremic HCV infection among people who inject drugs in Catalonia. Int J Drug Pol. 2019 Dec;74:236–245. doi: 10.1016/j.drugpo.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Kherghehpoush S., McKeirnan K.C. The role of community pharmacies in the HIV and HCV care continuum. Explor Res Clin Soc Pharm. 2023 Mar;9 doi: 10.1016/j.rcsop.2022.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falade-Nwulia O., Sacamano P., McCormick S.D., et al. Individual and network factors associated with HCV treatment uptake among people who inject drugs. Int J Drug Pol. 2020 Apr;78 doi: 10.1016/j.drugpo.2020.102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson L.A., Fenton J., Charlton C.L. HCV reflex testing: a single-sample, low-contamination method that improves the diagnostic efficiency of HCV testing among patients in Alberta, Canada. J Assoc Med Microbiol Infect Dis Can J Off Assoc Pour Microbiol Medicale Infect Can. 2022 Jun;7(2):97–107. doi: 10.3138/jammi-2021-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calvaruso V., Bronte F., Ferraro D., et al. Point-of-care HCV RNA testing in the setting of DAA therapy: HCV-FiS (HEpatitis C virus fingerstick study) Liver Int Off J Int Assoc Study Liver. 2019 Dec;39(12):2240–2243. doi: 10.1111/liv.14242. [DOI] [PubMed] [Google Scholar]
- 43.Pawlotsky J.M., Negro F., Aghemo A., et al. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020 Nov;73(5):1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Sarrazin C., Zimmermann T., Berg T., et al. S3-Leitlinie „Prophylaxe, Diagnostik und Therapie der Hepatitis-C-Virus (HCV) -Infektion“: AWMF-Register-Nr.: 021/012. Z Für Gastroenterol. 2018 Jul;56(7):756–838. doi: 10.1055/a-0599-1320. [DOI] [PubMed] [Google Scholar]
- 45.Scherz N., Bruggmann P., Brunner N. Direct-acting antiviral therapy for hepatitis C infection among people receiving opioid agonist treatment or heroin assisted treatment. Int J Drug Pol. 2018 Dec;62:74–77. doi: 10.1016/j.drugpo.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Koneru A., Nelson N., Hariri S., et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission - United States and Kentucky, 2011-2014. MMWR Morb Mortal Wkly Rep. 2016 Jul 22;65(28):705–710. doi: 10.15585/mmwr.mm6528a2. [DOI] [PubMed] [Google Scholar]
- 47.Iversen J., Page K., Madden A., Maher L. HIV, HCV, and health-related harms among women who inject drugs: implications for prevention and treatment. J Acquir Immune Defic Syndr. 2015 Jun 1;69(Suppl 2):S176–S181. doi: 10.1097/QAI.0000000000000659. 1999. 0 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haley D.F., Edmonds A., Ramirez C., et al. Direct-acting antiviral hepatitis C treatment cascade and barriers to treatment initiation among US men and women with and without HIV. J Infect Dis. 2021 Jun 15;223(12):2136–2144. doi: 10.1093/infdis/jiaa686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipp A. Medizinische Universität Graz; 2013. Der österreichische Mutterkindpass als Präventivtool für Kinder. Beteiligungsraten, Stichprobenanalysen und Gedanken zu einer möglichen Kosten-Nutzen-Evaluierung. [Google Scholar]
- 50.Sozialministerium österreich. Mutter-Kind-Pass. https://www.sozialministerium.at/Themen/Gesundheit/Eltern-und-Kind/Mutter-Kind-Pass.html [Internet]. Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.