Abstract
Pathogenic parasites of the Trichomonas genus are causative agents of sexually transmitted diseases affecting millions of individuals worldwide and whose outcome may include stillbirths and enhanced cancer risks and susceptibility to HIV infection. Trichomonas vaginalis relies on imported purine and pyrimidine nucleosides and nucleobases for survival, since it lacks the enzymatic activities necessary for de novo biosynthesis. Here we show that T. vaginalis additionally lacks homologues of the bacterial or mammalian enzymes required for the synthesis of the nicotinamide ring, a crucial component in the redox cofactors NAD+ and NADP. Moreover, we show that a yet fully uncharacterized T. vaginalis protein homologous to bacterial and protozoan nucleoside hydrolases is active as a pyrimidine nucleosidase but shows the highest specificity toward the NAD+ metabolite nicotinamide riboside. Crystal structures of the trichomonal riboside hydrolase in different states reveals novel intermediates along the nucleoside hydrolase–catalyzed hydrolytic reaction, including an unexpected asymmetry in the homotetrameric assembly. The active site structure explains the broad specificity toward different ribosides and offers precise insights for the engineering of specific inhibitors that may simultaneously target different essential pathways in the parasite.
Keywords: Trichomonas vaginalis, NAD, pyrimidine, enzyme structure, X-ray crystallography, drug design
Trichomonas vaginalis is a flagellate, extracellular protozoan parasite that is responsible for one of the most common (>170 million cases reported/year worldwide) sexually transmitted infections, trichomoniasis (1). T. vaginalis colonizes the urogenital tract of both women and men, and although most cases are asymptomatic, the infection often correlates with concomitant pathogens, such as Chlamydia or Neisseria gonorrheae. Unresolved trichomoniasis enhances the susceptibility to several conditions including pelvic inflammatory disease, papillomavirus infection, the risk of cervical and prostate cancer, and the transmission of HIV. Trichomoniasis-associated premature birth due to membrane rupture and other adverse outcomes in pregnant women are also of major concern. T. vaginalis interferes via various mechanisms with the neutrophile-mediated immune response of the host that seldom completely resolves the infection (2), and more than 70% of the trichomoniasis cases persist over time if left untreated. The recommended antiparasitic therapy, and the only one approved for use in the United States, employs nitroimidazole prodrugs such as metronidazole that require reduction to reactive nitrogen species. Several reports hinted to the ability of T. vaginalis to develop resistance to this drug, with around 5% of the cases in the United States but with peaks of 17% of isolates in countries such as Papua New Guinea being refractory to treatment (3). Although the level of nitroimidazole resistance is limited, the availability of a single approved drug for the treatment of this parasitic disease represents a potential source of danger, and thus the characterization of alternative targets to be exploited for the design of specific drugs toward T. vaginalis infections is a cogent requirement.
The known auxotrophy of the parasite for both purine and pyrimidine bases (4, 5) makes the nucleoside uptake and salvage pathway an attractive target for drug design. Nucleosides are a privileged form for the entry of nitrogenous bases in cells that can take advantage of equilibrative nucleoside transporters to cross cell membranes (6). The T. vaginalis purine and pyrimidine nucleoside transporters have been recently characterized, and their expression is similar in both metronidazole-resistant and sensitive strains (7). The intracellular salvage of purine ribonucleosides in the parasite involves direct formation of the mononucleotide through the action of purine nucleoside kinases (4, 8). No purine phosphoribosyltransferase (PRTase) activity has been reported in T. vaginalis, indicating that, unlike in most other organisms, the Trichomonas nucleoside phosphorylases (NPs) act in the reverse direction catalyzing in vivo the condensation of ribose 1-phosphate and the free nitrogenous base to the nucleoside (4, 9). Pyrimidine metabolism in the Trichomonas genus has been investigated both in the human parasite (5) and the cattle pathogen Tritrichomonas foetus (10). Uracil PRTase activity is very high in cellular extracts of the latter, and the similar kinetics of uracil or uridine incorporation in mononucleotides indicates that exogenously acquired uridine is converted to uracil before transformation to the corresponding mononucleotide (5). Instead, in T. vaginalis the conversion of uracil to the mononucleotide proceeds via the NP-catalyzed condensation with ribose 1-phosphate followed by phosphorylation through the action uridine kinase (11). Thus, pyrimidine metabolism in the Trichomonas genus has a distinct structure compared with most organisms and most notably with the mammalian hosts.
Hydrolytic or phosphorolytic N-glycosidic bond scission has been proposed as a likely route for the conversion of uridine to uracil in T. vaginalis. The parasite’s genome contains four genes encoding for proteins bearing the amino terminal aspartate-rich fingerprint sequence (DXDXXXDD) that is characteristic of enzymes with nucleoside hydrolase (NH) activity (12, 13). Two purine-preferring NH enzymes have been characterized (14, 15, 16), and they likely represent a central pathway for purine bases salvage in the Trichomonas metabolic network. A third enzyme involved in uridine hydrolysis to uracil has been identified, and lead compounds with inhibitory activity have been discovered (17). Interestingly, H+/K+ ATPase inhibitors such as omeprazole and rabeprazole that are effective in vitro against T. vaginalis (18), albeit with discordant reported potencies, displayed Ki values as low as 0.3 μM for this enzyme (19). Taken together, these findings raise the intriguing possibility that interfering with the parasite’s NH-mediated uracil uptake metabolic network may be an effective pharmaceutical strategy.
Here, we report the full enzymatic characterization of the T. vaginalis pyrimidine-preferring NH, to further define the structure of the parasite nucleoside salvage pathways and guide the improvement of inhibitors with potential antitrichomonal activity. Although both uridine and cytidine are substrates for the enzyme, the highest catalytic efficiency is observed with NAD+ precursor nicotinamide riboside (NR), likely indicating its concomitant involvement in the redox cofactor components salvage. This is also corroborated by metagenomics analyses that highlight the lack of homologues to the bacterial and eukaryotic enzymes involved in de novo pyridine ring synthesis, thus suggesting an NAD+ auxotrophy of the parasite. The X-ray structural analysis of the enzyme, here renamed TvRH (Riboside Hydrolase) to acknowledge its broader activity on diverse ribosides, in the unliganded state and bound to active site ligands reveals new intermediate steps in the catalytic cycle of group I NHs and offers high-resolution templates for the design of inhibitors with antiparasitic potential.
Results
T. vaginalis lacks crucial enzyme for pyrimidine and pyridine nucleotide biosynthesis
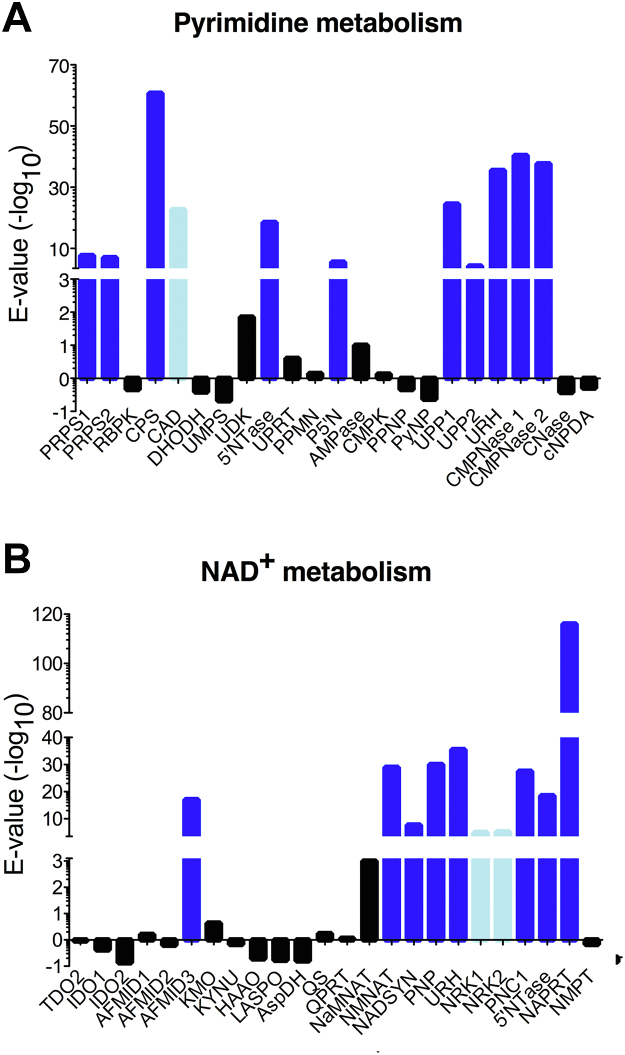
We performed homology searches to identify differences existing between the N-ribosides salvage pathways of T. vaginalis and mammalian hosts. Previous label incorporation and enzymatic studies on Trichomonas cell extracts showed the inability of the parasite to synthesize purine and pyrimidine bases. We performed a genome-wide search for homologues of eukaryotic and bacterial enzymes involved in pyrimidine ring synthesis and salvage. Although a homologue of the aspartate carbamoyltransferase domain of the trifunctional carbamoyl-phosphate synthetase 2-aspartate transcarbamylase-dihydroorotase protein was identified, currently annotated as ornithine carbamyltransferase, T. vaginalis is clearly unable to synthesize the orotate base (Fig. 1A and Table S1), confirming also at the genetic level the defective pyrimidine anabolism in the parasite (Fig. S1A). Thus, the pyrimidine uptake and salvage are attractive pathways for the design of drugs against this parasite. Next, we performed the same analysis for the enzymes involved in NAD+ synthesis and salvage. No homologues of the enzymes that catalyze the rate-limiting steps in the anabolic pathway from L-aspartate (bacteria) or L-tryptophan (eukaryotes) to nicotinate were identified (Fig. 1B and Table S1). Based on the current knowledge on the NAD+ biosynthetic pathways, we conclude that T. vaginalis likely requires exogenous pyridine-containing compounds, such as nicotinamide or its riboside, to synthesize the cofactors NAD+ and NADP via the salvage pathway (Fig. S1B). This finding is corroborated by previous reports that parasites of the Trichomonas genus require exogenously provided nicotinamide to sustain growth (20). The presence of three N-ribohydrolase-encoding genes in T. vaginalis confirms the necessity for highly diversified enzymes devoted to nitrogenous base recycling to promote cell survival.
Figure 1.
Trichomonas vaginalis lacks homologues of enzymes necessary for nucleobase and pyridine synthesis. The indicated enzymes involved in pyrimidine and pyridine metabolism were individually aligned against the T. vaginalis genome using tBLASTn and the returned −log(E-values) are plotted, shown as blue bars when >3. The Expect value E is a measure of the significance of the match. A, identification of pyrimidine-metabolizing enzymes. The human multifunctional CAD protein sequence identified significant homology with a Trichomonas gene in the aspartate carbamoyltransferase domain only, and the corresponding bar is colored light blue. B, identification of NAD+-metabolizing enzymes. The Trichomonas homologue of the human NRK1 and NRK2 kinases, also shown in light blue, is a AAA domain protein with several insertions compared with the human homologue and divergent at the characteristic β-hairpin lining the catalytic site. 5'NTase, 5′-nucleotidase; AFMID, kynurenine formamidase; AMPase, AMP phosphorylase; AspDH, aspartate dehydrogenase; CAD, Gln-dependent carbamoyl-phosphate synthase/aspartate carbamoyltransferase/dihydroorotase; CMPK, cytidine monophosphate kinase; CMPNase, cytidine deaminase 1,2; CNase, cytosine/isoguanine deaminase; cNPDA, 2′,3′-cyclic-nucleotide 2′-phosphodiesterase; CPS, carbamoyl-phosphate synthase-1; DHODH, dihydroorotate dehydrogenase; HAAO, 3-hydroxyanthranilate 3,4-dioxygenase; IDO1/2, indoleamine 2,3-dioxygenase 1,2; KMO, kynurenine 3-monooxygenase; KYNU, kynureninase; LASPO, L-aspartate oxidase; NADSYN, ammonia-dependent NAD+ synthetase; NaMNAT, nicotinate mononucleotide adenylyltransferase; NAPRT, nicotinate phosphoribosyltransferase; NMNAT, nicotinamide/nicotinic acid mononucleotide adenylyltransferase; NMPT, nicotinamide phosphoribosyltransferase; NRK 1,2, nicotinamide riboside kinase 1,2; P5′N, pyrimidine 5′-nucleotidase; PNC1, nicotinamidase 1; PNP, purine nucleoside phosphorylase; PPMN, pyrimidine/purine nucleotide 5′-monophosphate nucleosidase; PPNP, purine/pyrimidine nucleoside phosphorylase; PRPS, ribose-phosphate pyrophosphokinase 1,2; PyNP, pyrimidine nucleoside phosphorylase; QPRT, nicotinate-nucleotide pyrophosphorylase; QS, quinolinate synthase; RBPK, ribose 1,5-bisphosphate phosphokinase; TDO2, tryptophan 2,3-dioxygenase 2; UDK, uridine-cytidine kinase; UMPS, uridine 5′-monophosphate synthase; UPP, uridine phosphorylase 1,2; UPRT, uracil phosphoribosyltransferase; URH, pyrimidine-specific ribonucleoside hydrolase. The gene and corresponding proteins used in the homology search as well as the matches with highest homology in the T. vaginalis genome are listed in Table S1.
The third NH-like protein from T. vaginalis
The T. vaginalis TVAG_092730 gene encodes for a protein (UniProt A2FTT0) of 347 amino acids that contains the “NH fingerprint” aspartate-rich N-terminal sequence (8DCDPGHDD16). The protein also contains two histidine residues (His83 and His262) that are known to participate in substrate binding and catalysis in members of the NH structural homology group I that includes homotetrameric nonspecific and pyrimidine-preferring enzymes. Only one of the two tyrosine residues required for purine nucleoside hydrolysis is present, thus suggesting that pyrimidine nucleosides may be the preferred substrates (21). Compared with the group I NHs so far characterized, the Trichomonas protein here considered is larger in size than its bacterial and protozoan homologues (347 versus ∼315 amino acids) (Fig. S2). We cloned the open reading frame in the pET21a vector, overexpressed the protein in Escherichia coli cells, and purified the recombinant protein to homogeneity using metal affinity and size exclusion chromatography (SEC). The engineered N-terminal His6 peptide was removed by partial proteolysis using thrombin, followed by a second SEC step to remove the protease and cleaved peptide. The elution volume of TvRH from SEC is consistent with a homotetrameric quaternary structure (Fig. S3), strengthening its assignment to the group I NH.
Substrate preference of TvRH confirms involvement in pyrimidine salvage
The steady-state kinetic analysis of TvRH carried out at 30 °C showed a clear preference for the uridine substrate, which is hydrolyzed with a turnover number 40 times greater than for cytidine (Table 1). Purine nucleosides are poor substrates for the TvRH enzyme, with Km values exceeding 1 mM and turnover numbers lower than 4·10−2 s−1. Substituents at position 5 of the uracil ring have a minor effect on the Michaelis’ constant but modulate the rate of substrate hydrolysis. Indeed, the electron-withdrawing fluorine increases the turnover number, while the other halogens display an opposite and comparable effect. The 5-methyluridine (5mUR) nucleoside, a breakdown product from tRNA molecules, is hydrolyzed slowly. The exquisite specificity for the ribose moiety of substrates is shown by the 40-fold slower hydrolysis of thymidine, a 2′-deoxynucleoside, coupled to a 37-fold increase in Km compared with 5mUR. Taken together, the steady-state kinetic analysis of TvRH shows that the enzyme retains the ribosyl-discriminating properties of previously characterized NHs, requiring all hydroxyls to be present for maximal activity. The synthetic substrate para-nitrophenyl riboside (pNPR; (2R, 3R, 4S, 5R)-2-(4-nitrophenoxy)-5-hydroxymethyl-tetrahydrofurane-3,4-diol (para-nitrophenyl β-D-ribofuranoside)) is rapidly hydrolyzed by TvRH, underscoring how the catalytic power of the enzyme is achieved through distortion of the ribose moiety to an oxonium ion geometry, similarly to what was determined for the inosine-uridine-preferring nucleoside hydrolase (IUNH) from Crithidia fasciculata. The negative charge that develops in the nitrogenous base leaving group in the NH-catalyzed reaction is stabilized through interactions with two active site histidine residues (22, 23). The effect on the turnover number of the different substituents at the position 5 of the uracil ring correlates with the inductive effect, indicating that a partial negative charge also likely develops at the pyrimidine ring in the transition state of the TvRH-catalyzed hydrolytic reaction. The enzyme has the highest turnover number for the synthetic O-riboside pNPR that does not require leaving group protonation (24) and is thus able to efficiently distort the ribosyl moiety conformation to the transition state geometry (25). Indeed, comparing the rates of the uncatalyzed and catalyzed reaction for pNPR, the ΔΔG of the transition state due to ribose distortion at 278 K, amounts to −13.8 kcal/mol, a value similar to that of the Crithidia IUNH enzyme (22). The products of the hydrolytic reaction ribose and uracil are poor inhibitors of the TvRH, with Ki values 5.2 and 65.2 mM, respectively (Fig. S4A). However, while ribose is a competitive inhibitor, the pyrimidine base acts with a noncompetitive mechanism, as judged from double reciprocal plots (Fig. S4B). These values are consistent with the enzyme catalyzing in vivo the hydrolytic reaction and limited product inhibition under normal growth conditions.
Table 1.
Steady-state kinetic parameters of TvRH with different substrates
| Substrate or inhibitor | kcat (s−1) | Km (μM) | kcat/Km (s−1 M−1) | Kis or Ki(app) (mM) |
|---|---|---|---|---|
| Uridine | 9.3 ± 0.27 | 39 ± 3 | 2.4·105 | |
| Cytidine | 0.19 ± 0.003 | 33 ± 1 | 5.7·103 | |
| Guanosine | <0.04 | 1200 ± 110 | 23.3 | |
| Inosine | <0.04 | 1980 ± 126 | 14.1 | |
| Adenosine | <0.04 | 4200 ± 264 | 6.7 | |
| p-Nitrophenylriboside | 391 ± 2.0 | 13 ± 1 | 3.0·107 | |
| 5-Fluorouridine | 12.6 ± 0.6 | 17 ± 2 | 7.7·105 | |
| 5-Chlorouridine | 2.3 ± 0.1 | 11 ± 2 | 2.1·105 | |
| 5-Bromouridine | 2.1 ± 0.1 | 20 ± 1 | 1.0·105 | |
| 5-Methyluridine | 7.5·10−2 ± 3·10−3 | 65 ± 7 | 1.1·103 | |
| Thymidine | 1.9·10−3 ± 3·10−4 | 2400 ± 216 | 0.78 | |
| Ribose | 5.2 ± 0.47 | |||
| Uracil | 65.2 ± 2.1 |
Errors reported are standard errors from the fit. The inhibition constant Kis was obtained from experiments using at least five substrate concentrations at each of three inhibitor concentrations. The inhibition constant was obtained by fitting the data to the equation for either competitive inhibition or noncompetitive inhibition. Data were fitted using the program GraFit (Erithacus).
TvRH is a highly efficient nicotinamide riboside hydrolase
With the aim of assessing the catalytic efficiency of TvRH at the temperature of the host, we repeated the kinetic analysis at 37 °C in phosphate buffer at pH 6.5, using uridine and the pyridine-containing NR as substrates. Under the assay conditions, NR is the best substrate for TvRH, with a 3-fold greater catalytic efficiency (kcat/Km) compared with uridine as a substrate (Table 2 and Fig. S4C). The TvRH-catalyzed NR hydrolysis is characterized by a greater turnover number (10.7 compared with 2.0 s−1), with a higher Km value (99 versus 58.6 μM) when compared with uridine. Thus, TvRH is active with comparable efficiencies on the pyrimidine nucleoside uridine and the pyridine-containing NAD+ metabolite NR. This finding suggests that TvRH may have a dual role in both the recycling of pyrimidine nucleobases as well as in providing an essential component for redox cofactor biosynthesis in the parasite. This additional involvement of TvRH in recycling NAD+ components, which based on our metagenomics analysis is an essential pathway for Trichomonas cells, strengthens the view of the enzyme as an attractive target for the design of antitrichomonal agents.
Table 2.
N-ribosidase activity of TvRH at 37 °C and pH 6.5
| Substrate | kcat (s−1) | Km (μM) | kcat/Km (s−1 M−1) |
|---|---|---|---|
| Nicotinamide riboside | 10.9 ± 0.24 | 99.1 ± 8.2 | 1.1·105 |
| Uridine | 2.0 ± 0.08 | 58.6 ± 7.1 | 0.34·105 |
The steady-state kinetic parameters were obtained from the direct fit of initial velocities to the Michaelis–Menten equation using GraphPad Prism. Errors reported are standard errors from the fit.
Crystallographic analysis of TvRH
To gain detailed insights on the active site characteristics of TvRH for inhibitor design we determined five X-ray structures of the enzyme crystallized under different conditions (Table S2). The TvRH enzyme was crystallized both with and without the hexahistidine tag, and the complexes with glycerol and the slowly hydrolyzed 5-methyluridine substrate were obtained by diffusion. The His-tagged TvRH is bound to one bicine molecule from the crystallization buffer at each active site. Moreover, a crystal of the complex between TvRH and the reaction product D-ribose was obtained by cocrystallization, incubating the enzyme with a high concentration of the aldopentose to overcome the low affinity (Kd = 5.2 mM). The latter cocrystals are affected by a strong pseudocentering of the crystal lattice, due to a molecular 2-fold axis parallel to the crystallographic symmetry element, which affects the reflection intensity distribution and the final refinement statistics. Nevertheless, the electron density maps clearly show the presence of ribose in all four independent polypeptide chains as well as a defined conformation for the regions that are flexible in other TvRH structures (see below). The various crystal forms obtained in this study differ in the number of TvRH monomers in the asymmetric unit, ranging from two to eight, but in all cases a conserved homotetrameric arrangement of the protomers was observed.
TvRH is a member of the NH structural homology group I
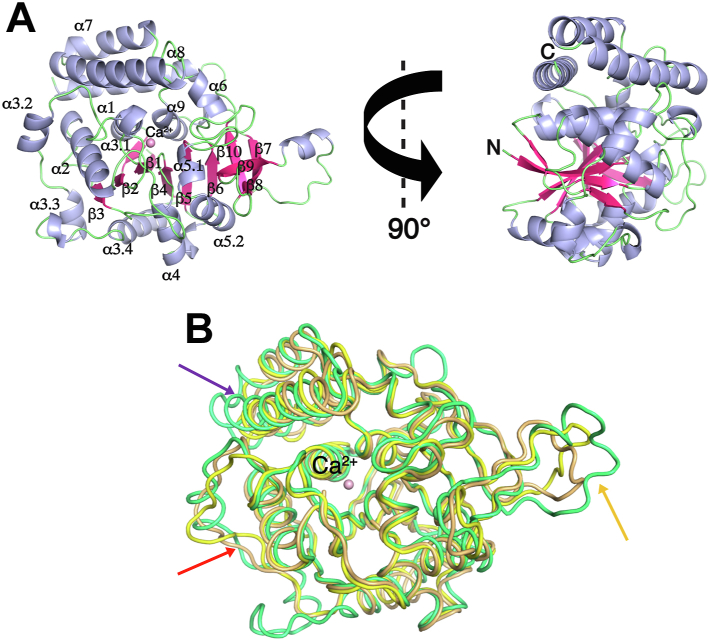
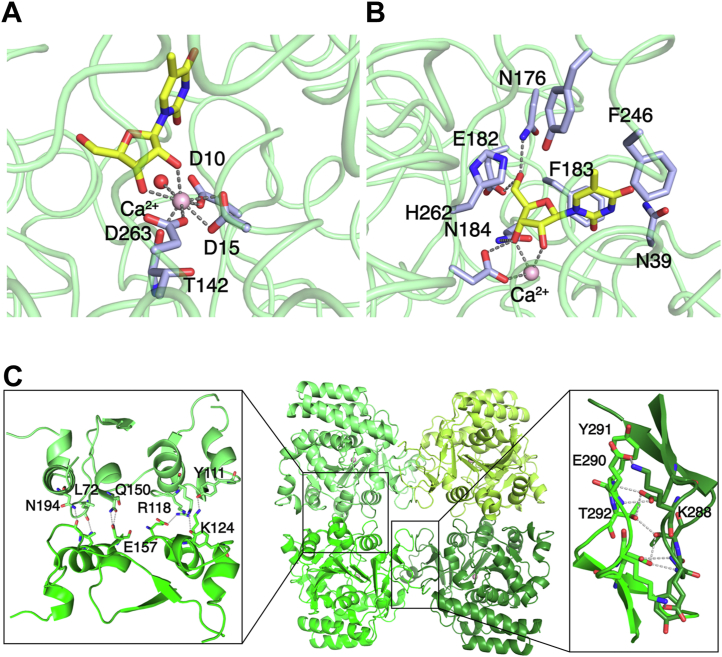
The TvRH enzyme adopts the group I NH fold (Fig. 2A), with an open (α, β) structure composed of a central mixed eight-stranded β-sheet surrounded by α-helices, a four-helix domain that completes the active site, and two antiparallel β strands that mediate monomer–monomer interactions. The crossover loop connecting strand β3 to helix α3 and the α8 helix is flexible and could be fully traced in electron density maps only in selected subunits either when stabilized by crystal contacts or through interactions with active site ligands. The main structural differences compared with other group I NHs are limited to the conformation of the crossover region linking strands β3 and β4, which in TvRH includes two additional short helical segments (Fig. 2B). The cavity located at the topological switch point between strands β1 and β4 is lined by three aspartate residues (Asp10, Asp15, and Asp263) that together with the main chain carbonyl of Thr142 and three water molecules participate in the octacoordination of the Ca2+ ion that is characteristic of all NHs (Fig. 3A). One of the water molecules may be replaced by the hydroxyl of serendipitous active site ligands, such as bicine or glycerol. Two water molecules are displaced by the O2′ and O3′ of ribosyl-containing ligands. The outer portion of the active site of TvRH that interacts with the leaving group is amphiphilic, structured with both hydrophobic (Cys79, Ile82, Ile177, and Phe183) and hydrophilic (Asn176, Tyr250, and His262) side chains (Fig. 3B).
Figure 2.
Crystal structure of TvRH.A, two orthogonal views showing the overall structure of the TvRH monomer with α-helices shown as light blue ribbons and β-strands as hotpink arrows. Secondary structure elements are labeled following the scheme proposed for NHs (13). Loop regions are shown as light green tubes, and the active site calcium ion as a pink sphere. B, the structures of the individual monomers of TvRH bound to ribose (light green), the C. fasciculata IUNH bound to a transition state–like inhibitor (2MAS, yellow), and the E. coli RihB in complex with the substrate inosine (3B9X, orange) are superimposed. The arrows indicate the region where TvRH is dissimilar, namely, the β3–α3 crossover loop (red), the α7–α8 helices (purple), and the β9–β10 loop (orange).
Figure 3.
Structural difference between TvRH and NH proteins.A, octacoordination of the active site Ca2+ ion in TvRH in complex with 5mUR. The coordination geometry is conserved in all structures determined. B, direct interactions between the ribosyl moiety of 5mUR substrate and the TvRH active site. Residues within 5 Å of the substrate are shown as sticks with carbon atoms colored light blue. C, the TvRH homotetrameric structure, with the molecular 2-fold axes aligned horizontally, vertically, and perpendicular to the plane. Details of the interactions at the minor (left) and major (right) interfaces are shown, with interacting residues depicted as sticks and potential hydrogen bonding interactions as dashed lines.
TvRH displays a homotetrameric quaternary structure with D2 (222) symmetry characteristic of group I NHs, with two distinct intermolecular interaction surfaces (Fig. 3C). The major surface, involving strands β8, β9 and the following loop (amino acids 281–309), the loop between strand β5 and helix α5.1 (167–177), and the C terminus of helix α8 (252–254), spans 1080 ± 90 Å2 and includes 11 hydrogen bonds and 2 salt bridges between the subunits. The minor surface buries a total of 880 ± 20 Å2, composed by residues from the β3–α3 loop (65–72 and 115–124), two kinked α-helices following strands β4 (143–154) and β5 (187–194), and leads to the formation of four intersubunit hydrogen bonds (Fig. 3C). A detailed analysis of the conformations of the tetramer subunits reveals an asymmetry of the two monomers interacting via the major surface, and thus the quaternary structure of TvRH is best described as a dimer of dimers. This quaternary structure of TvRH was observed in all crystal forms obtained, and in the case of the tetragonal crystals a molecular symmetry axis is coincident with the crystallographic 2-fold axis; thus, a dimer is found in the asymmetric unit.
Structures of catalytic intermediates bound to TvRH
Since 5mUR is a substrate of TvRH characterized by a low turnover number (7.5·10−2 s−1), we performed cryotrapping experiments (21) by soaking the compound into the tetragonal TvRH crystals, followed by rapid cooling to 100 K in liquid N2. The electron density maps showed full occupancy of both the ribose and base moieties of the nucleoside in the two independent polypeptides in the asymmetric unit of the crystal (Fig. 4A). The two TvRH chains in the asymmetric unit of the complex superimpose with little differences, except for the β3–α3 loop that could not be traced into electron density maps in one subunit, and maintain the same open conformation as in the ligand-free enzyme in the second subunit (Fig. 4B). The partially ordered loop is involved in intermolecular contacts with a symmetry-related molecule also mediated by a Ni2+ ion from the crystallization buffer, and thus its movement is likely hindered by the proximal polypeptide. Hence, the intermolecular contacts in the tetragonal TvRH crystals that involve the β3–α3 loop likely prevent the attainment of the catalytically competent closed enzyme structure and the observed conformation of the substrate as well as enzyme–substrate contacts are representative of an intermediate pre-Michaelis complex along the reaction coordinate. Indeed, the 5mUR molecule is bound to the TvRH active site with the ribosyl moiety in a C1′-exo conformation, with the nitrogenous base oriented equatorially. This conformation is unlike the ones observed for the iminoribitol-based inhibitors bound to the C. fasciculata IUNH and the E. coli RihA and RihB (26, 27, 28), where the ribose is in the C4′-endo conformation and the aglycone is oriented axially (Fig. 4C). Binding of the ribosyl moiety of 5mUR is achieved through coordination of the Ca2+ ion by the O2′ and O3′ hydroxyls and direct hydrogen bonds between O2′ and Thr142, Asn184, and Asp263, and O5′ with Asn176 and Glu182 (Fig. 4B). Although these residues are strictly conserved in the bacterial RihB where they interact with the corresponding atoms of inosine substrate, the positioning of the ribosyl moiety differs (Fig. 4D) and results in a distinct nucleoside conformation. The nitrogenous base is involved only in one polar interaction between the N3 atom and the side chain of residue Asn39 of TvRH. Proximal residues Phe183 and Phe246 provide a hydrophobic environment to accommodate the aromatic pyrimidine base.
Figure 4.
Conformation of TvRH-bound substrates and ligands.A, omit electron density (mFo–DFc, ɸc) contoured at 3.0 σ showing 5mUR bound at the TvRH active site. Polar contacts are shown as black dashed lines. Residues involved in Ca2+ ion (depicted as a light pink sphere in all panels) coordination are detailed in Figure 3B. B, contacts between the 5mUR substrate and TvRH. The β3–α3 is in an open conformation. In (A and B), the amino acid residues that are within 5 Å of the ligand are shown as light blue sticks. C, the structures of the complexes of TvRH with 5mUR (carbon atoms colored green), Crithidia IUNH with pAPIR (PDB code 2MAS, yellow), and E. coli RihB with inosine (PDB code 3B9X, magenta) were superimposed showing the aglycones in both the pAPIR inhibitor and inosine molecules are axially oriented and the β3–α3 loop is closed while the thymine base is oriented axially when bound to the TvRH active site with an open β3–α3 loop. D, the positioning of the ribosyl moiety of 5mUR at the active site differs compared with other homologous ligands bound to the parasitic and enterobacterial NHs, as does the five-membered ring conformation.
In the product-bound structure, the ribose ring assumes a C4′-exo puckering in all four subunits (Figs. 5A and S5A), different from what was seen for the substrate 5mUR and similar to what was previously observed both in the E. coli RihA–ribose complex (29) and in the C. fasciculata IUNH bound to a transition state–like inhibitor (26). Unexpectedly, in one monomer of each product-bound TvRH dimer the β3–α3 loop is found in the closed conformation (Fig. 5B), with the imidazole ring of residue His83 within hydrogen bonding distance to the O1′ atom of ribose, while in the opposing subunit it is flexible as previously seen in the E. coli RihA–ribose complex (29). When this loop is in the closed conformation, residue Ile82 is nested in a pocket lined by the side chains of the α8 helix residues Phe246, Tyr250, Val253, and Phe254, stabilizing perhaps surprisingly a tight structure of the active site (Fig. 5C).
Figure 5.
Structural rearrangements along the TvRH reaction coordinate.A, contacts between the reaction product ribose and TvRH, underscoring the closed conformation of the β3–α3 loop and the movement of residue Asn39. The amino acid residues that are within 5 Å of the ligand are shown as light blue sticks. B, superposition of TvRH monomers from the 5mUR-bound and ribose-bound crystal structures. The backbone of both monomers is colored green. The regions differing in conformation are colored orange (5mUR complex) and blue (ribose). Arrows indicate the β3–α3 loop (red), α8 helix (purple), and β9–β10 loop (orange), respectively. C, hydrophobic interactions between Ile82 from the β3–α3 loop and a hydrophobic patch on helix α8 stabilize the closed conformation. The ribose molecule and Ca2+ ion are shown for reference. D, binding of glycerol at the TvRH active site mimics part of the ribosyl–enzyme interactions.
A third TvRH structure with one molecule of the serendipitous ligand glycerol bound to each of the four subunits was also determined. Binding of glycerol to the TvRH enzyme active site apparently stabilizes the closed conformation, since the α8 helix or β3–α3 loop could be traced into the electron density maps. In TvRH, glycerol mimics the position of the O5′, C5′, C4′, C3′, and O3′ atoms of ribose with its hydroxyls within hydrogen bonding distance from the side chains of residues Asp13, Asn179, Glu185, Asn187, and Asp266. The positioning of the glycerol molecule parallels that seen in the enterobacterial NH RihB (30) (Figs. 5D and S5B). No specific contacts are established between glycerol and either the α8 helix or β3–α3 loop, and it is thus unclear what factors induce the ordered conformation of these two protein segments.
Structural asymmetry in TvRH dimers
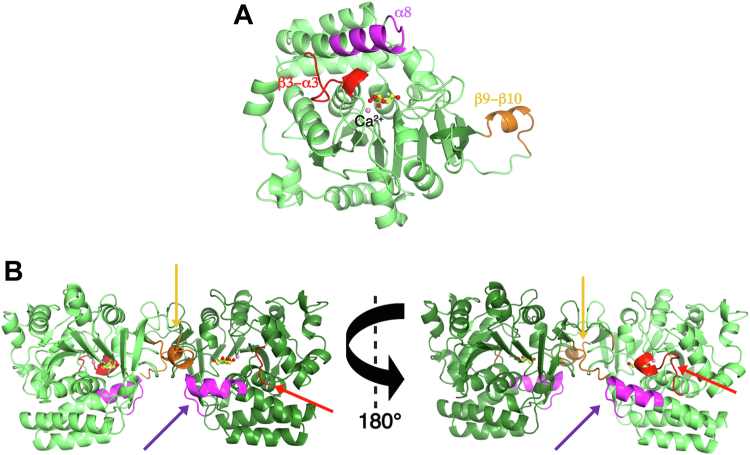
The α8 helix in group I NHs borders the active site and contributes residues that are crucial for substrate specificity and catalysis (21, 26, 27). This helix undergoes a swiveling movement and ordering of its C-terminal portion upon substrate binding and establishes hydrogen bonding and hydrophobic contacts that are essential for transition state stabilization. In TvRH, the α8 helix shows similar flexibility, albeit its N-terminal part is better defined and does not undergo significant conformational changes in the different crystal forms. In the ribose-bound and monoclinic glycerol-bound structures, the C-terminal end of the helix (residue 245–257) differs in the two monomers forming dimers via the major interaction surface. Indeed, within such dimers the terminal portion of the helix is ordered in one subunit, extending toward the active site, and not traceable into electron density maps, and thus highly flexible, in the other. When in the closed conformation, the α8 helix interacts with amino acids from the β9–β10 region (amino acids from 298 to 313) of the neighboring dimer subunit (Fig. 6, A and B and Movie S1). In TvRH this segment contains an insertion compared with the group I NHs so far characterized and folds as a short helix when in the ordered conformation (Fig. 6A). Based on the structures here determined, we conclude that TvRH has a distinct structural heterogeneity with respect to the β3–α3 loop and α8 helix, and the binding of a molecule to the active site selects one specific conformation that optimally complements the chemical characteristics of the ligand, inducing short-range conformational rearrangements in the neighboring subunit.
Figure 6.
Asymmetry in the TvRH dimers.A, the structure of the TvRH monomer shown as a cartoon representation highlighting the β3–α3 loop (red), α8 helix (purple), and β9–β10 loop (orange) that differ in conformation within a TvRH dimer. B, two views of a TvRH dimer obtained through the major interaction surface. When the β3–α3 loop is flexible (left view), the β9–β10 loop is ordered in a helical conformation and the α8 helix is bent away from the active site. When the β3–α3 loop is in a fixed, closed conformation (right) the α8 helix leans toward the active site and the β9–β10 loop is flexible.
It is worth noting that the pH of the crystallization solutions apparently correlates with the possibility to observe a closed β3–α3 loop, since the glycerol-bound and ribose-bound TvRH crystals were obtained at a lower pH (4.85) compared with the other structures (7.5–8.0) where the loop is either flexible or blocked in a specific conformation by crystal contacts (Fig. S6). However, the asymmetry of the TvRH dimers, displaying alternatively this loop as either flexible or closed, indicates that the protonation of His83 is not the sole factor that stabilizes the tight conformation.
The closed TvRH active site structure complements the natural substrates
The high turnover number for the NR does not allow cryotrapping of this substrate in TvRH crystals, as it is rapidly hydrolyzed to products. Thus, we resorted to molecular modeling of the enzyme–uridine and enzyme–NR complexes using the structure of the C. fasciculata IUNH bound to the transition state–like inhibitor pAPIR ((2R, 3R, 4S, 5R)-2-(4-aminophenyl)-5-hydroxymethyl-pyrrole-3,4-diol (para-aminophenyl iminoribitol)) as a template. First, we superimposed the Crithidia enzyme structure (26) onto the closed form of glycerol-bound TvRH observed in the monoclinic crystals. Then, we modeled a molecule of NR with a C4′-endo ribosyl conformation and axial nitrogenous base matching that of pAPIR, thus distinct from that observed for 5mUR bound to the enzyme and matching the puckering of the enzyme-bound pentose molecule, with the rationale that this ribose conformation recapitulates the structural features of the transition state of the hydrolytic reaction. The active site of TvRH has a dual character, with one half lined by hydrophobic residues Cys79, Ile82, Ile177, and Phe183 and the other by the polar side chain groups of Asn176, Tyr250, and His262 (Fig. 7A). The 3-carboxamide group in NR can be likely positioned in this region of the binding cavity, taking advantage of hydrogen bonding interactions with residues Asn176 and Tyr250. Instead, when uridine is positioned at the TvRH active site, the shape of the cavity clearly indicates that the O2 carbonyl of the nitrogenous base can be positioned between His83 and His262 (Fig. 7B), highly conserved amino acid residues that have been implicated in catalysis in the Crithidia and E. coli isozymes. Notably, the observed closed conformation of TvRH is sterically compatible with the modeled substrate and likely represents the correct enzyme conformation to promote riboside hydrolysis.
Figure 7.
Models of Michaelis complexes of TvRH.A, section of the molecular surface of TvRH in the closed conformation from the glycerol-bound structure with NR modeled at the active site based on the C. fasciculata IUNH–inhibitor complex. In the model, residue Asn176 is positioned close to the carboxyamide group of the nicotinamide ring, which is also approached by the side chain of Tyr250. B, same as (A), with a molecule of the substrate UR (right) modeled in the active site. The side chains of His83 and Tyr250 are within hydrogen bonding distance of the O2 and O4 carbonyl oxygens of the uracil base, respectively. In both panels, residues within 5 Å of the modeled substrate molecule are shown as light blue sticks. Parts of the structure have been omitted for clarity. The distances reported in the panels are measured in the model obtained as described and without energetic optimization and are thus not to be interpreted as experimentally determined values.
Discussion
Enzymes that catalyze the N-glycosidic bond hydrolysis of N-ribosides have long been known and primarily characterized for their activity on classic RNA components. Yet it is becoming increasingly clear that NH activity is broader and includes also other ribosides that have been characterized as metabolic intermediates in other pathways. One of the most striking examples is the yeast NH URH1p, originally described as a uridine hydrolase, which is also involved in the hydrolysis of exogenously derived NR to promote NAD+ synthesis and sirtuin activation (31, 32). Our findings also show that the T. vaginalis enzyme, previously described as a uridine-preferring NH, is not only able to hydrolyze the N-glycosidic bond in pyrimidine nucleosides but also shows the highest specificity toward NR. Hence, the TvRH can participate in both pyrimidine nucleoside and NAD+ salvage.
Since T. vaginalis lacks genes encoding the crucial enzymatic activities required for purine and pyrimidine nucleotide biosynthesis, the inhibition of nucleoside salvage enzymes has been proposed as a strategy for the development of new therapeutic agents. Indeed, our analysis of the Trichomonas genome confirms that orthologues of the central enzymes in pyrimidine and pyridine ring biosynthesis are absent. The presence of UPRTase activity in T. foetus suggests that pyrimidine nucleosides are converted to uracil and subsequently to UMP and the activity of TvRH on uridine may be central in providing the free base for this reaction. However, in T. vaginalis UPRTase enzymatic activity was absent, and indeed we could not identify uprt-like gene products, and thus conversion of uracil to UMP requires a reverse-NP reaction and uridine kinase activities. Hence, the definition of the precise role and importance of NH activity in trichomonatids’ pyrimidine metabolism requires further study.
The correct NAD+ supplies in living cells are typically maintained through a balanced action between de novo synthesis from amino acid precursors, aspartate or tryptophane, and salvage from degraded or uptaken pyridine-containing compounds such as nicotinamide, nicotinic acid, as well as their corresponding ribosides. Our study uncovered that in Trichomonas genomes no homologues of the bacterial and eukaryotic enzymes involved in the initial, crucial steps of the de novo synthesis of the pyridine base were present. This finding suggests that members of this parasite’s genus may be auxotrophic for the crucial redox metabolite NAD+. Indeed, earlier work showed that growth of the trichomonatid Trichomonas gallinae was reduced by 86% when nicotinamide was omitted from the growth culture (20), suggesting its inability to synthesize pyridines de novo. The here demonstrated activity of TvRH on the NR substrate supports its additional involvement in the metabolism of exogenously derived pyridine ring in the form of ribosides such as NR for NAD+ biosynthesis, as seen, for instance, in Saccharomyces cerevisiae. This cofactor is central in numerous cellular pathways, either directly or as a precursor of compounds such as cyclic ADP-ribose. In yeast, exogenous NR is primarily metabolized through hydrolysis over phosphorolysis (31, 32). NR released from cells during the inflammatory response against Trichomonas infection may thus be imported into the parasites’ cells through equilibrative nucleoside transporters and efficiently cleaved into ribose and nicotinamide by TvRH. This reaction would provide the substrate for phosphoribosyl transfer toward the synthesis of nicotinamide mononucleotide, whose condensation with ATP via an adenyltransferase-catalyzed reaction yields the final salvage product, NAD+ (Fig. S7). Since no genes encoding for proteins with riboside hydrolase characteristics have been identified in mammals (30), the here presented structural and enzymatic characterization clearly corroborates the definition of TvRH as an attractive target for the development of antitrichomonal agents, interfering simultaneously with the pyrimidine and nicotinamide recycling. It remains to be experimentally determined whether nicotinic acid, nicotinamide, or their ribosides are the most relevant pyridine-containing species that are available to Trichomonas cells during infection. It is also tempting to speculate that the metabolic pathways described here could also be exploited against other parasites, such as Giardia intestinalis, whose genomes include NH-like genes.
While the overall structure of the Trichomonas riboside hydrolase displays high similarity to known group I NHs, an unprecedented asymmetry in the TvRH dimer of dimers in the glycerol- and ribose-bound crystal structures is apparent. Indeed, within each dimer the α8 helix and β3–α3 loop are alternatively in an open/flexible or closed conformation. The significance and the structural basis for such asymmetry are both unclear. Indeed, no long-range cross talk between the monomers is apparent from the analysis of the conformation of the amino acid residues at the protein–protein interfaces. One possibility is that the enzyme displays half-of-the-sites reactivity, as demonstrated for the group II purine-specific inosine-adenosine-guanosine-preferring nucleoside hydrolase (IAGNH) from Trypanosoma vivax (33, 34). Kinetic analysis of site-specific mutants targeting the amino acid residues involved in the intermolecular contacts will provide further insights into the mechanistic relevance of the observed structural asymmetry.
The conformational flexibility of group I NHs is likely advantageous for the interaction with ribosides via the active site Ca2+ ion at the bottom of the catalytic cavity, providing facilitated access. Subsequently, the interactions between the substrate’s aglycone and the protein lead to a tightening of these regions, providing a mechanism for substrate selection and shielding of the reaction center. Substrate distortion is a crucial process for the IUNH from C. fasciculata, and this is accomplished through enzyme–substrate contacts that induce a ribosyl conformation similar to the oxonium ion at the transition state (25, 26). This distortion imposes an axial orientation of the nitrogenous base with respect to the pentose ring, largely induced by the contacts with His83 (His82 in the Crithidia enzyme) and the preceding alkylic amino acid side chain (26). In the complex between TvRH and 5mUR the β3–α3 loop is in an open conformation, and these contacts could not be established, allowing an axial orientation of the thymine base in the nucleoside that is closer to the low-energy conformation attainable in solution. Thus, the TvRH–5mUR complex here determined likely represents the structure of an initial enzyme–substrate complex along the coordinate of the NH-catalyzed reaction that requires the conformational “tightening” of the β3–α3 loop to induce substrate distortion to the transition state geometry. An alternative possibility is a conformational selection of the substrate from the conformers in solution followed by loop closure. These findings are in line with the proposed role of this loop movement in determining the turnover number of the E. coli RihB NH enzyme (28).
Only a limited number of group I NH-specific inhibitors have been reported (26, 35, 36), largely due to a stronger initial interest in IAGNHs for the development of antitrypanosomal drugs. Indeed, C-glycosides such as the immucillins (37) or N-aryl iminoribitols (38) display high selectivity toward IAGNHs and are effective in killing trypanosomes both in vitro and in vivo (39, 40). Owing to the differences in active site dimensions and composition between group I and II NHs, immucillins are generally lower-potency inhibitors of pyrimidine-preferring NHs, with Ki values in the mid-micromolar range (21). Moreover, immucillins are potent inhibitors of human purine nucleoside phosphorylases and it is thus highly desirable to avoid such off-target effects in the perspective of treatment of persons with trichomoniasis. The now available structures of TvRH may guide the synthesis of riboside analogues targeting the different enzyme conformation to achieve optimal selectivity. The TvRH active site is largely hydrophobic; thus, aromatic moieties can adequately complement the cavity and provide significant entropic and enthalpic contribution to the binding energy. We suggest that appropriate functional groups positioned to establish interactions with active site residues Asn39, His83, Tyr250, and His262 will likely yield highly specific inhibitors of the Trichomonas TvRH enzyme. At present, rabeprazole and analogous molecules have shown an inhibitory effect of TvRH activity (19). It is, however, unclear, based on the here presented experimental structures, how such molecules could fit the NH active site. Further structural analysis of TvRH bound to these compounds is currently ongoing, along with several classes of fragment inhibitors identified in fragment screens, and will provide further information on the amino acid residues that can be engaged to develop high-affinity inhibitors.
Experimental procedures
Meta-analysis of the nucleoside and NAD+ salvage pathways in T. vaginalis
Key enzymes for “pyrimidine metabolism” and “nicotinate and nicotinamide metabolism” were selected from the KEGG pathways (https://www.genome.jp/kegg/pathway.html). T. vaginalis-restricted tBLASTn searches were performed using the corresponding protein sequences from reference organisms (Homo sapiens, S. cerevisiae, Arabidopsis thaliana, and E. coli; the search also included homologues from Pseudonocardia eucalypti, Methanococcus jannaschii, and Thermococcus siculi). The BLAST expected-value (E-value) threshold was set to 10 to allow for the retrieval of low-homology alignments (i.e., E-value ≥ 0.001). The E-value of the highest scoring pairwise alignment for each probed enzyme homologue is reported in Figure 1, A and B. Queries using amino acid sequences of the available proteins from Toxoplasma gondii or T. foetus did not yield better scores. NH-like proteins were inferred from homology searches using the amino acid sequences of the enterobacterial RihA/YbeK, RihB/YeiK, and RihC/YaaF proteins.
Cloning and expression of the TVAG_092730 gene
Synthetic DNA encoding the T. vaginalis RH enzyme (TVAG_092730) and an N-terminal hexahistidine tag followed by a thrombin cleavage site (GenScript) was cloned into a pET-21a vector (Novagen) using the NdeI and BamHI restriction enzymes. The plasmid was transformed into BL21(DE3) E. coli competent cells (Novagen) and plated on Lysogeny Broth (LB) and grown overnight in the presence of 50 μg/ml ampicillin. A single colony was used to inoculate 10 ml of LB medium containing ampicillin, and the culture was grown with shaking overnight at 37 °C. A 1:30 dilution was then performed into fresh LB medium and grown to an A600 of 0.8, and then 45 ml was added to 1.45 l of fresh LB medium and grown at 37 °C with shaking to an A600 of 0.9. TvRH expression was induced by addition of 0.1 mM isopropyl β-D-thiogalactopyranoside (IPTG) for 18 h at 22 °C in a shaking incubator. Cells were harvested by centrifugation at 4000 rpm and washed once with buffer comprising 20 mM potassium phosphate and 0.3 M KCl at pH 6.5. A total of 18.5 g of cell paste was stored at −80 °C until purification.
Protein purification
Cells were thawed and suspended in 20 ml of buffer comprising 50 mM potassium phosphate, 0.3 M KCl, 0.2 mM DTT, 2 mg/ml lysozyme, and four cOmplete mini protease inhibitor cocktail tablets (Roche) at pH 6.5. After 30 min at 37 °C, 2 mM MgCl2 and 0.01 mg/ml DNase (Sigma) were added. After 15 min at 37 °C and 15 min on ice, cells were lysed via sonication using a Microson XL200 sonicator. The cell lysate was then centrifuged for 30 min at 20,000g followed by ultracentrifugation for 2 h at 50,000 rpm at 4 °C. The protein supernatant was applied to a HiTrap Chelating HP 5-ml column (Cytiva) connected to a Biologic Dual-flow FPLC system. The column was washed with 25 mM phosphate, 0.3 M KCl, and 0.05 M imidazole at pH 7.2 before eluting protein using the same buffer with a linear gradient of 0.05 to 0.7 M imidazole. Fractions with TvRH activity were pooled, concentrated to 1.0 ml using a 10 kDa Vivaspin 15R concentrator (Sartorius) and applied to a Superdex G200 size-exclusion chromatography column (Cytiva) equilibrated with buffer comprising 25 mM phosphate and 0.3 M KCl at pH 7.2. Fractions with TvRH activity were pooled and concentrated to 12.0 mg/ml, and aliquots were flash frozen and stored at −80 °C. The final yield of protein was 39.6 mg. The affinity tag removal was performed by digesting TvRH with thrombin (Merck) at a ratio of 10 U per mg of recombinant protein for 18 h at 4 °C, followed by SEC as above. Protein purity was determined by SDS-PAGE, and concentration was measured using UV-visible spectrophotometry using a computed value of ε280 = 41,370 M−1·cm−1 based on the amino acid sequence.
Steady-state enzymatic characterization
Colorimetric reducing sugar assays (41) and continuous spectrophotometric assays (42) were performed at 30 °C in 50 mM Mes, 0.3 M KCl at pH 6.2 using a Shimadzu UV-2401PC UV-vis spectrophotometer with a six-cell position Peltier temperature controller, controlled by UV-Probe software as described previously. Data were analyzed using GraFit or GraphPad Prism data analysis software. The reducing sugar assay measures the amount of ribose released in the reaction (41). The initial rates of product formation in the continuous assays were determined by taking advantage of the variation in absorbance (Δε) due to the difference in molar absorption between each nucleoside and its corresponding free nucleobase at a given wavelength (42). The Δε values (mM−1 cm−1) and wavelengths used were cytidine, −2.18 at 270 nm; 5-bromouridine, −1.94 at 278 nm; 5-chlorouridine, 8.77 at 277 nm; 5-fluorouridine, −1.86 at 269 nm; 5-methyluridine, −2.0 at 267 nm; p-nitrophenyl riboside, 2.0 at 402 nm; thymidine, −1.85 at 267 nm; uridine, −2.18 at 262 nm; nicotinamide riboside, −2.9 at 262 nm.
Crystallization
Before crystallization, the TvRH buffer was exchanged to 20 mM Tris pH 7.2, 50 mM KCl by SEC. Single crystals of TvRH were obtained using the hanging drop (unliganded NH) or sitting drop (complex with D-ribose) vapor diffusion method at 18 °C using the Oryx8 crystallization robot (Douglas Instruments). The His6-tagged TvRH was crystallized mixing equal volumes of protein (4 mg/ml) and a precipitant solution composed of 0.1 M bicine pH 8.5, 20% (w/v) PEG 5000 monomethyl ether, and 3% sucrose. The untagged TvRH at 5 mg/ml was mixed with equal volumes of a solution containing 90 mM piperazine-N-N′-bis-(2-ethanesulfonic acid) (Pipes) pH 7.0, 90 mM MgCl2, 45 mM KCl, 1 mM NiSO4, and 14% (w/v) PEG 5000 monomethyl ether. These crystals were also diffused with the slowly hydrolyzed substrate 5-methyluridine (5mUR) added in the solid state to the stabilizing solution containing a single crystal of TvRH in a 10-μl drop for 10 to 60 min at 18 °C. A third crystal form of unliganded TvRH was obtained by mixing at 1:1 ratio the TvRH protein (10 mg/ml) to a solution with 200 mM ammonium citrate dibasic, 15% w/v PEG 5000 monomethyl ether, 17.5% (v/v) glycerol at pH 4.85. The complex between TvRH and ribose was prepared by cocrystallizing the untagged enzyme (10 mg/ml) with a 2000-fold molar excess of ribose dissolved in water with a precipitant solution containing 200 mM ammonium citrate dibasic pH 4.85, 15% PEG 3300.
X-ray structural analysis
Crystals were transferred to an appropriate stabilizing solution for cryoprotection consisting of the appropriate precipitant solution brought to either 40% (w/v) sucrose (for His6-TvRH, the P4322 TvRH crystal form and the 5mUR–TvRH complex) or 30% (v/v) glycerol (the P21 TvURH crystal form and the TvRH–ribose complex) and rapidly dipped in liquid N2 where they were maintained until data collection. Diffraction data were measured at beamlines ID23-1, ID23-2, and ID30B at the European Synchrotron Radiation Facility (Grenoble, F) and I04 at the Diamond Light Source using the oscillation method. Data were indexed, integrated, scaled, and merged using XDS (43) and AIMLESS (44) as implemented in the autoProc (45) package. Phases for the dataset obtained from His6-tagged TvRH crystals were calculated using the molecular replacement technique as implemented in Phaser (46) with the E. coli YeiK/RihB (Protein Data Bank [PDB] 1Q8F, 30.2% amino acid sequence identity) monomer as a search model after removal of water molecules, ions, and ligands. The structure was automatically rebuilt and the amino acid sequence assigned with AutoBuild, part of the Phenix (47) package, followed by manual adjustment of the model into σA-weighted (2mFo-DFc, ɸc) and (mFo-DFc, ɸc) maps using the program Coot (48). After rebuilding of one monomer, the molecular replacement and automated rebuilding procedure were repeated yielding a substantially complete TvRH structure with two tetramers in the crystal asymmetric unit. The model was improved in rounds of restrained refinement, using local noncrystallographic symmetry restraints and refining individual isotropic B-factors and TLS groups as implemented in REFMAC5 (49) and Phenix, followed by manual rebuilding into electron density maps in Coot. The stereochemical quality of the structure was continuously monitored using Molprobity (50). The structures of the untagged TvRH crystals and the ligand complexes were determined using molecular replacement and the previously obtained TvRH monomer structure as search model. Manual rebuilding and refinement were carried out as described above. The parallel decrease of Rcrys and Rfree was closely monitored to minimize overrefinement for all structures. The inclusion of the maximum resolution of the measured diffraction intensities that yielded an improvement in the model was established with the paired refinement technique as implemented in PDB_REDO (51). Visual analysis and figure preparation were carried out with PyMol (http://www.pymol.prg). The analyses of surfaces and intermolecular contacts were performed using QtPISA (52). Data collection and refinement statistics are presented in Table S1.
Molecular modeling
The molecular structures of NR and UR were modeled based on the pAPIR (2-(4-aminophenyl)-5-hydroxymethyl-pyrrole-3,4-diol) conformation bound to the Crithidia IUNH (PDB 2MAS). The molecular structure of the inhibitor was modified to include all the atoms of the ribosides substrates. Then, each structure was minimized in vacuo using the UFF force field with the steepest descent algorithm, constraining the ribose and N-glycosidic bond orientation as implemented in Avogadro (53). The substrates were positioned in the TvRH active site matching the position of the pAPIR molecule after superposition of the Crithidia IUNH enzyme in Coot, and the torsion angle of the N-glycosidic bond was adjusted manually to minimize steric clashes.
Data availability
The refined models and the structure factors have been deposited with the Protein Data Bank with the following accession codes: native TvRH, 8OI7; TvRH bound to 5-methyluridine, 8OI9; TvRH bound to ribose, 8OIA; TvRH bound to glycerol, 8OIB; His-tagged TvRH bound to bicine, 8OIC. The available DOIs associated with the data collections are https://data.esrf.fr/doi/10.15151/ESRF-DC-1110614788 (TvRH-ribose complex) and https://data.esrf.fr/doi/10.15151/ESRF-DC-1110614797 (TvRH-glycerol).
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors wish to thank the beamline scientists at ESRF (Grenoble, F) and DLS (Didcot, UK) for their highly skilled support; Dr Alan Schoenfeld for assistance with gene cloning and transformation; Dr Flavio di Pisa for assistance with the soaking experiments, data collection, and initial data processing for the tetragonal crystal form; Paola Tornaghi for technical support; and lab members for helpful discussions.
Author contributions
M. P. and D. W. P. methodology; D. W. P. validation; M. P., G. S. G., F. K., M. M. N., D. W. P., and B. J. S. formal analysis; M. P., G. S. G., F. K., M. M. N., D. W. P., B. J. S., and M. D. investigation; D. W. P., B. J. S., and M. D. resources; M. D. writing–original draft; M. P. and B. J. S. writing–review & editing; M. P. and M. D. visualization; D. W. P., B. J. S., and M. D. supervision; B. J. S. and M. D. funding acquisition.
Funding and additional information
Research reported in this publication was supported by the NIAID, National Institutes of Health under Award Number R15AI128585 to B. J. S., and in part by AIRC grant IG 25764 to M. D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Joseph Jez
Contributor Information
Brian J. Stockman, Email: bstockman@adelphi.edu.
Massimo Degano, Email: degano.massimo@hsr.it.
Supporting information
References
- 1.Rowley J., Vander Hoorn S., Korenromp E., Low N., Unemo M., Abu-Raddad L.J., et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019;97:548–562P. doi: 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan C.M., de Miguel N., Johnson P.J. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem. 2011;51:161–175. doi: 10.1042/bse0510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunne R.L., Dunn L.A., Upcroft P., O'Donoghue P.J., Upcroft J.A. Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis. Cell Res. 2003;13:239–249. doi: 10.1038/sj.cr.7290169. [DOI] [PubMed] [Google Scholar]
- 4.Heyworth P.G., Gutteridge W.E., Ginger C.D. Purine metabolism in Trichomonas vaginalis. FEBS Lett. 1982;141:106–110. doi: 10.1016/0014-5793(82)80026-4. [DOI] [PubMed] [Google Scholar]
- 5.Heyworth P.G., Gutteridge W.E., Ginger C.D. Pyrimidine metabolism in Trichomonas vaginalis. FEBS Lett. 1984;176:55–60. doi: 10.1016/0014-5793(84)80910-2. [DOI] [PubMed] [Google Scholar]
- 6.Boswell-Casteel R.C., Hays F.A. Equilibrative nucleoside transporters-a review. Nucleosides Nucleotides Nucleic Acids. 2017;36:7–30. doi: 10.1080/15257770.2016.1210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natto M.J., Miyamoto Y., Munday J.C., AlSiari T.A., Al-Salabi M.I., Quashie N.B., et al. Comprehensive characterization of purine and pyrimidine transport activities in Trichomonas vaginalis and functional cloning of a trichomonad nucleoside transporter. Mol. Microbiol. 2021;116:1489–1511. doi: 10.1111/mmi.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munagala N.R., Wang C.C. Adenosine is the primary precursor of all purine nucleotides in Trichomonas vaginalis. Mol. Biochem. Parasitol. 2003;127:143–149. doi: 10.1016/s0166-6851(02)00330-4. [DOI] [PubMed] [Google Scholar]
- 9.Munagala N., Wang C.C. The purine nucleoside phosphorylase from Trichomonas vaginalis is a homologue of the bacterial enzyme. Biochemistry. 2002;41:10382–10389. doi: 10.1021/bi026025n. [DOI] [PubMed] [Google Scholar]
- 10.Wang C.C., Verham R., Tzeng S.F., Aldritt S., Cheng H.W. Pyrimidine metabolism in Tritrichomonas foetus. Proc. Natl. Acad. Sci. U. S. A. 1983;80:2564–2568. doi: 10.1073/pnas.80.9.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C.C., Cheng H.W. Salvage of pyrimidine nucleosides by Trichomonas vaginalis. Mol. Biochem. Parasitol. 1984;10:171–184. doi: 10.1016/0166-6851(84)90005-7. [DOI] [PubMed] [Google Scholar]
- 12.Degano M., Gopaul D.N., Scapin G., Schramm V.L., Sacchettini J.C. Three-dimensional structure of the inosine-uridine nucleoside N-ribohydrolase from Crithidia fasciculata. Biochemistry. 1996;35:5971–5981. doi: 10.1021/bi952999m. [DOI] [PubMed] [Google Scholar]
- 13.Degano M. Structure, oligomerization and activity modulation in N-ribohydrolases. Int. J. Mol. Sci. 2022;23:2576. doi: 10.3390/ijms23052576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam R., Barbarovich A.T., Caravan W., Ismail M., Barskaya A., Parkin D.W., et al. Druggability of the guanosine/adenosine/cytidine nucleoside hydrolase from Trichomonas vaginalis. Chem. Biol. Drug Des. 2018;92:1736–1742. doi: 10.1111/cbdd.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck S., Muellers S.N., Benzie A.L., Parkin D.W., Stockman B.J. Adenosine/guanosine preferring nucleoside ribohydrolase is a distinct, druggable antitrichomonal target. Bioorg. Med. Chem. Lett. 2015;25:5036–5039. doi: 10.1016/j.bmcl.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Muellers S.N., Nyitray M.M., Reynarowych N., Saljanin E., Benzie A.L., Schoenfeld A.R., et al. Structure-guided insight into the specificity and mechanism of a parasitic nucleoside hydrolase. Biochemistry. 2022;61:1853–1861. doi: 10.1021/acs.biochem.2c00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auletta S., Caravan W., Persaud J.K., Thuilot S.F., Brown D.G., Parkin D.W., et al. Discovery of ligand-efficient scaffolds for the design of novel Trichomonas vaginalis uridine nucleoside ribohydrolase inhibitors using fragment screening. ACS Omega. 2019;4:16226–16232. doi: 10.1021/acsomega.9b02472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Villanueva J., Romo-Mancillas A., Hernández-Campos A., Yépez-Mulia L., Hernández-Luis F., Castillo R. Antiprotozoal activity of proton-pump inhibitors. Bioorg. Med. Chem. Lett. 2011;21:7351–7354. doi: 10.1016/j.bmcl.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Shea T.A., Burburan P.J., Matubia V.N., Ramcharan S.S., Rosario I., Parkin D.W., et al. Identification of proton-pump inhibitor drugs that inhibit Trichomonas vaginalis uridine nucleoside ribohydrolase. Bioorg. Med. Chem. Lett. 2014;24:1080–1084. doi: 10.1016/j.bmcl.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Jones I., Smith B.F. Certain B complex vitamins as growth-promoting factors for Trichomonas gallinae. Exp. Parasitol. 1959;8:509–514. doi: 10.1016/0014-4894(59)90039-6. [DOI] [PubMed] [Google Scholar]
- 21.Iovane E., Giabbai B., Muzzolini L., Matafora V., Fornili A., Minici C., et al. Structural basis for substrate specificity in group I nucleoside hydrolases. Biochemistry. 2008;47:4418–4426. doi: 10.1021/bi702448s. [DOI] [PubMed] [Google Scholar]
- 22.Gopaul D.N., Meyer S.L., Degano M., Sacchettini J.C., Schramm V.L. Inosine-uridine nucleoside hydrolase from Crithidia fasciculata. Genetic characterization, crystallization, and identification of histidine 241 as a catalytic site residue. Biochemistry. 1996;35:5963–5970. doi: 10.1021/bi952998u. [DOI] [PubMed] [Google Scholar]
- 23.Minici C., Cacciapuoti G., De Leo E., Porcelli M., Degano M. New determinants in the catalytic mechanism of nucleoside hydrolases from the structures of two isozymes from Sulfolobus solfataricus. Biochemistry. 2012;51:4590–4599. doi: 10.1021/bi300209g. [DOI] [PubMed] [Google Scholar]
- 24.Mazzella L.J., Parkin D.W., Tyler P.C., Furneaux R.H., Schramm V.L. Mechanistic diagnoses of N-ribohydrolases and purine nucleoside phosphorylase. J. Am. Chem. Soc. 1996;118:2111–2112. [Google Scholar]
- 25.Horenstein B.A., Parkin D.W., Estupiñán B., Schramm V.L. Transition-state analysis of nucleoside hydrolase from Crithidia fasciculata. Biochemistry. 1991;30:10788–10795. doi: 10.1021/bi00108a026. [DOI] [PubMed] [Google Scholar]
- 26.Degano M., Almo S.C., Sacchettini J.C., Schramm V.L. Trypanosomal nucleoside hydrolase. A novel mechanism from the structure with a transition-state inhibitor. Biochemistry. 1998;37:6277–6285. doi: 10.1021/bi973012e. [DOI] [PubMed] [Google Scholar]
- 27.Garau G., Muzzolini L., Tornaghi P., Degano M. Active site plasticity revealed from the structure of the enterobacterial N-ribohydrolase RihA bound to a competitive inhibitor. BMC Struct. Biol. 2010;10:14. doi: 10.1186/1472-6807-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornili A., Giabbai B., Garau G., Degano M. Energy landscapes associated with macromolecular conformational changes from endpoint structures. J. Am. Chem. Soc. 2010;132:17570–17577. doi: 10.1021/ja107640u. [DOI] [PubMed] [Google Scholar]
- 29.Muzzolini L., Versées W., Tornaghi P., Van Holsbeke E., Steyaert J., Degano M. New insights into the mechanism of nucleoside hydrolases from the crystal structure of the Escherichia coli YbeK protein bound to the reaction product. Biochemistry. 2006;45:773–782. doi: 10.1021/bi0511991. [DOI] [PubMed] [Google Scholar]
- 30.Giabbai B., Degano M. Crystal structure to 1.7 a of the Escherichia coli pyrimidine nucleoside hydrolase YeiK, a novel candidate for cancer gene therapy. Structure. 2004;12:739–749. doi: 10.1016/j.str.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Belenky P., Racette F.G., Bogan K.L., McClure J.M., Smith J.S., Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Belenky P., Christensen K.C., Gazzaniga F., Pletnev A.A., Brenner C. Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. J. Biol. Chem. 2009;284:158–164. doi: 10.1074/jbc.M807976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Versées W., Decanniere K., Pellé R., Depoorter J., Brosens E., Parkin D.W., et al. Structure and function of a novel purine specific nucleoside hydrolase from Trypanosoma vivax. J. Mol. Biol. 2001;307:1363–1379. doi: 10.1006/jmbi.2001.4548. [DOI] [PubMed] [Google Scholar]
- 34.Versées W., Goeminne A., Berg M., Vandemeulebroucke A., Haemers A., Augustyns K., et al. Crystal structures of T. vivax nucleoside hydrolase in complex with new potent and specific inhibitors. Biochim. Biophys. Acta. 2009;1794:953–960. doi: 10.1016/j.bbapap.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Boutellier M., Horenstein B.A., Semenyaka A., Schramm V.L., Ganem B. Amidrazone analogues of D-ribofuranose as transition-state inhibitors of nucleoside hydrolase. Biochemistry. 1994;33:3994–4000. doi: 10.1021/bi00179a028. [DOI] [PubMed] [Google Scholar]
- 36.Parkin D.W., Limberg G., Tyler P.C., Furneaux R.H., Chen X.Y., Schramm V.L. Isozyme-specific transition state inhibitors for the trypanosomal nucleoside hydrolases. Biochemistry. 1997;36:3528–3534. doi: 10.1021/bi962319v. [DOI] [PubMed] [Google Scholar]
- 37.Evans G.B., Schramm V.L., Tyler P.C. The immucillins: design, synthesis and application of transition- state analogues. Curr. Med. Chem. 2015;22:3897–3909. doi: 10.2174/0929867322666150821100851. [DOI] [PubMed] [Google Scholar]
- 38.Goeminne A., Berg M., McNaughton M., Bal G., Surpateanu G., Van der Veken P., et al. N-Arylmethyl substituted iminoribitol derivatives as inhibitors of a purine specific nucleoside hydrolase. Bioorg. Med. Chem. 2008;16:6752–6763. doi: 10.1016/j.bmc.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 39.Giannese F., Berg M., Van der Veken P., Castagna V., Tornaghi P., Augustyns K., et al. Structures of purine nucleosidase from Trypanosoma brucei bound to isozyme-specific trypanocidals and a novel metalorganic inhibitor. Acta Crystallogr. D Biol. Crystallogr. 2013;69:1553–1566. doi: 10.1107/S0907444913010792. [DOI] [PubMed] [Google Scholar]
- 40.Berg M., Kohl L., Van der Veken P., Joossens J., Al-Salabi M.I., Castagna V., et al. Evaluation of nucleoside hydrolase inhibitors for treatment of African trypanosomiasis. Antimicrob. Agents Chemother. 2010;54:1900–1908. doi: 10.1128/AAC.01787-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkin D.W., Horenstein B.A., Abdulah D.R., Estupiñán B., Schramm V.L. Nucleoside hydrolase from Crithidia fasciculata. Metabolic role, purification, specificity, and kinetic mechanism. J. Biol. Chem. 1991;266:20658–20665. [PubMed] [Google Scholar]
- 42.Parkin D.W. Purine-specific nucleoside N-ribohydrolase from Trypanosoma brucei brucei. Purification, specificity, and kinetic mechanism. J. Biol. Chem. 1996;271:21713–21719. [PubMed] [Google Scholar]
- 43.Kabsch W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans P.R., Murshudov G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013;69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vonrhein C., Flensburg C., Keller P., Sharff A., Smart O., Paciorek W., et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 2011;67:293–302. doi: 10.1107/S0907444911007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Echols N., Headd J.J., et al. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106. doi: 10.1016/j.ymeth.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murshudov G.N., Skubák P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen V.B., Arendall W.B., Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joosten R.P., Long F., Murshudov G.N., Perrakis A. The PDB_REDO server for macromolecular structure model optimization. IUCrJ. 2014;1:213–220. doi: 10.1107/S2052252514009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krissinel E. Stock-based detection of protein oligomeric states in jsPISA. Nucleic Acids Res. 2015;43:W314–W319. doi: 10.1093/nar/gkv314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanwell M.D., Curtis D.E., Lonie D.C., Vandermeersch T., Zurek E., Hutchison G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The refined models and the structure factors have been deposited with the Protein Data Bank with the following accession codes: native TvRH, 8OI7; TvRH bound to 5-methyluridine, 8OI9; TvRH bound to ribose, 8OIA; TvRH bound to glycerol, 8OIB; His-tagged TvRH bound to bicine, 8OIC. The available DOIs associated with the data collections are https://data.esrf.fr/doi/10.15151/ESRF-DC-1110614788 (TvRH-ribose complex) and https://data.esrf.fr/doi/10.15151/ESRF-DC-1110614797 (TvRH-glycerol).