Abstract
Objective
Synthetic expanded polytetrafluoroethylene (ePTFE) grafts are known to be susceptible to bacterial infection. Results from preclinical and clinical studies of bioengineered human acellular vessels (HAVs) have shown relatively low rates of infection. This study evaluates the interactions of human neutrophils and bacteria with ePTFE and HAV vascular conduits to determine whether there is a correlation between neutrophil-conduit interactions and observed differences of their infectivity in vivo.
Methods
A phase III comparative clinical study between investigational HAVs (n = 177) and commercial ePTFE grafts (n = 178) used for hemodialysis access (ClinicalTrials.gov Identifier: NCT02644941) was evaluated for conduit infection rates followed by histological analyses of HAV and ePTFE tissue explants. The clinical histopathology of HAV and ePTFE conduits reported to be infected was compared with immunohistochemistry of explanted materials from a preclinical model of bacterial contamination. Mechanistic in vitro studies were then conducted using isolated human neutrophils seeded directly onto HAV and ePTFE materials to analyze neutrophil viability, morphology, and function.
Results
Clinical trial results showed that the HAV had a significantly lower (0.93%; P = .0413) infection rate than that of ePTFE (4.54%). Histological analysis of sections from infected grafts explanted approximately 1 year after implantation revealed gram-positive bacteria near cannulation sites. Immunohistochemistry of HAV and ePTFE implanted in a well-controlled rodent infection model suggested that the ePTFE matrix permitted bacterial infiltration and colonization but may be inaccessible to neutrophils. In the same model, the HAV showed host recellularization and lacked detectable bacteria at the 2-week explant. In vitro results demonstrated that the viability of human neutrophils decreased significantly upon exposure to ePTFE, which was associated with neutrophil elastase release in the absence of bacteria. In contrast, neutrophils exposed to the HAV material retained high viability and native morphology. Cocultures of neutrophils and Staphylococcus aureus on the conduit materials demonstrated that neutrophils were more effective at ensnaring and degrading bacteria on the HAV than on ePTFE.
Conclusions
The HAV material seems to demonstrate a resistance to bacterial infection. This infection resistance is likely due to the HAV's native-like material composition, which may be more biocompatible with host neutrophils than synthetic vascular graft material.
Keywords: Tissue-engineered blood vessels, Synthetic vascular grafts, Expanded polytetrafluoroethylene (ePTFE), Bacterial infection, Neutrophils
Clinical Relevance
Clinical trial results to date have shown that human acellular vessels may be a promising alternative to synthetic conduits in certain indications based on mechanical function, host remodeling, and low infection rates. Resistance to infection may be inherent to the human acellular vessel based on its biological composition, which supports the migration and normal function of a patient's own cells after implantation.
Article Highlights.
-
•
Type of Research: In vitro studies combined with rat and human explant histopathology
-
•
Key Findings: A phase III comparative clinical trial results from 355 patients showed that human acellular vessels (HAV) had a significantly lower (0.93%; P = .0413) infection rate than that of expanded polytetrafluoroethylene (ePTFE) grafts (4.54%). In vitro studies of neutrophil viability combined with evaluation of preclinical and clinical tissue explants show that the biocompatibility of the HAV with host immune cells supports infection resistance and may explain clinically observed low rates of HAV infection. The ePTFE graft matrix induces cell death in neutrophils in vitro and appears to have limited host immune cell infiltration despite bacterial colonization in vivo.
-
•
Take Home Message: Unlike synthetic ePTFE, the native-like composition of HAV supports neutrophil viability and function, which may explain its superior resistance to bacterial infection.
Vascular graft infection represents a major clinical and economic burden in the United States, with tremendous impact on patient quality of life and an estimated cost of $640 million per year.1 Complications of vascular graft infection include graft removal or revision, anastomotic disruption, bacteremia spread, amputation, sepsis, and death.2, 3, 4 Specifically, vascular graft infection is associated with a 24% risk of amputation and a 6% risk of mortality.5, 6, 7 Synthetic vascular grafts, typically derived from expanded polytetrafluoroethylene (ePTFE) or polyethylene terephthalate, in particular, suffer from infection rates that can be as high as 28% when used for dialysis access8 and between 20% and 35% when used to repair vascular trauma.9,10 Relatedly, bacterial infection is the second leading cause of death in patients on hemodialysis,3,11 and synthetic grafts are not recommended for implantation into potentially contaminated sites,2,12 such as those resulting from penetrating vascular trauma13,14 or some intra-abdominal repairs.15
Bioengineered blood vessels may offer a promising off-the-shelf alternative to synthetic grafts in certain indications.16 The human acellular vessel (HAV) is an investigational tissue-engineered blood vessel that is generated using human vascular cells seeded onto a biodegradable scaffold and cultured in a sterile bioreactor with biochemical and biomechanical stimuli.17 After a final decellularization process removes living cells and antigens, the remaining 40-cm-long, 6-mm diameter acellular cylindrical conduit is primarily composed of human collagens and capable of being stored, readily available, for ≤18 months with refrigeration. Clinical use of the HAV for hemodialysis access,18 bypass in peripheral artery disease,19 and arterial repair in vascular trauma20,21 have shown that the HAV is a nonimmunogenic and mechanically robust vascular conduit with long-term (>5 year) durability,22,23 evolves to resemble a native blood vessel through host recellularization and remodeling,24 and has relatively low infection rates.18,19,21 Specifically, when used for dialysis access in 60 patients in 2 phase II trials (ClinicalTrials.gov Identifier: NCT01744418 and NCT01840956), only 1 HAV infection was observed, with an overall infection rate of 1.3% per patient-year of exposure.18 This infection rate is well below that reported for historical ePTFE performance of 9%.25 To date, no infections of the HAV have been reported in clinical trials (ClinicalTrials.gov Identifier: NCT02887859 and NCT01872208) for peripheral arterial disease.22 Finally, implantation of the HAV in likely contaminated sites has occurred in expanded access cases, including replacement of infected ePTFE21 and polyethylene terephthalate synthetic grafts,26 with no subsequent reported incidence of infection.
In addition to clinical evidence of low infection rates, the HAV was shown to be significantly more resistant than ePTFE to both gram-positive and gram-negative bacterial infection using an established in vivo rodent infection model.27 Collectively, these findings suggest that an inherent difference in these materials contributes to their susceptibility of infection. Contaminated synthetic ePTFE grafts often develop bacterial biofilms, such as those secreted by Staphylococcus or Pseudomonas strains, which enable persistent and intractable infection despite prolonged antibiotic therapy.28,29 These biofilms help to shield bacteria from host cellular defense mechanisms.30 At the onset of infection, the innate immune response is activated and leukocytes such as neutrophils and macrophages are mobilized to help contain and eliminate invading pathogens. Neutrophils migrate toward and destroy bacteria using a sophisticated process that includes the expulsion of a sticky web-like complex called neutrophil extracellular traps (NETs), which contain nuclear material and antimicrobial enzymes, including neutrophil elastase, to ensnare and degrade pathogens.31
Previous studies from other investigators have demonstrated that the synthetic composition of ePTFE interferes with neutrophil chemosensory migration32 and viability,33 which may impact neutrophil clearance of bacteria and contribute to the observed higher infection rates for implanted ePTFE. Because it is composed of a native-like human extracellular matrix and has shown capacity for host recellularization and remodeling in patients,24 we hypothesized that the HAV may provide a more favorable environment for neutrophil viability and function. In this study, histological analyses of explanted HAV and ePTFE samples from prior preclinical and clinical studies were performed to characterize the localization of neutrophils and bacteria. Controlled in vitro studies were then conducted to directly compare the survival, function, and phenotype of human neutrophils on HAV and ePTFE to evaluate potential mechanisms for the observed differences in resistance to bacterial infection.
Methods
Evaluation of clinical HAV and ePTFE explants
A phase III, prospective, multicenter, and randomized clinical trial (ClinicalTrials.gov Identifier: NCT02644941) was conducted to compare the use of the HAV to that of an ePTFE graft as an arteriovenous conduit for hemodialysis access in patients with end-stage renal failure. This study was conducted in full conformity with the Declaration of Helsinki and the International Council for Harmonization requirements for Good Clinical Practice. The independent ethics committee of each participating clinical center approved the protocol and each patient provided written informed consent before enrollment. A total of 177 patients received a 6-mm diameter HAV, and 178 patients received a 6-mm diameter ePTFE graft (Gore PROPATEN Vascular Graft, W. L. Gore & Associates, Inc., Phoenix, AZ; or Bard Impra Vascular Graft, Bard Peripheral Vascular, Inc., Tempe, AZ) based on random allocation. Patients were followed for 24 months after implantation at routine study visits regardless of patency status. Determination of implant infection was adjudicated by an independent Clinical Events Committee based on clinical, laboratory, and pathological findings. Calculated infection rates from patent conduits being cannulated for hemodialysis during the study were compared using a Poisson regression model with the treatment group and the randomization stratification variable as factors. This analysis was conducted using a two-sided test at a significance level of alpha = 0.05.
Sections of HAV or ePTFE and adjacent tissue were occasionally resected during surgical interventions to typically treat stenosis or infection. Explant samples were fixed in 10% formalin and sent to Inotiv (Morrisville, NC) for histopathological examination after processing and staining (hematoxylin and eosin and Gram stains) using standard protocols. Immunohistochemical staining24 was performed on tissue cross-sections after 20 minutes of antigen retrieval (sodium citrate buffer at 75°C). Samples were stained with anti-Staphylococcus aureus (S.aureus) (NOVUS NB100-64,499, diluted 1:100) and anti-neutrophil elastase (R&D Systems MAB91671-100, diluted 1:50) overnight at 4°C. Fluorescent-conjugated secondary antibodies (Invitrogen A11001 and A11012, diluted 1:200) and 4′,6-diamidino-2-phenylindole (Thermo Fisher Scientific 62247, diluted 1:1000) were applied for 1 hour at room temperature. Brightfield or immunofluorescence imaging was performed using an Olympus BX41 microscope and DP74 camera with cellSens software (Olympus, Tokyo, Japan) or a Nikon TE2000U microscope and Photometrics IRIS 9 camera with ImageJ software (National Institutes of Health, Bethesda, MD), respectively.
Histology of tissue explants from preclinical infection model
As previously reported,27 a rodent infection model was used to compare the susceptibility of the HAV and ePTFE to bacterial infection and evaluate host cellular response. Briefly, 21 adult male Sprague Dawley rats were anesthetized and 1 cm2 samples of either ePTFE (Advanta VXT ePTFE Vascular Graft, Atrium Medical Corporation, Hudson, NH) or HAV were inserted within dorsal subcutaneous pockets and then directly inoculated with a solution of 107 colony-forming units of gram-positive S.aureus (ATCC #25923) bacteria. Two weeks later, the sections of HAV or ePTFE were explanted, fixed in 10% formalin, paraffin embedded, and sectioned for Gram staining and immunohistochemistry similar to that of clinical explants.
Human neutrophil isolation
Peripheral venous whole blood (approximately 30 mL) was collected into tubes containing acid citrate dextrose anticoagulant from consenting and healthy donors (n = 6 individuals). Neutrophils were isolated using immunomagnetic negative selection with the EasySep Direct Human Neutrophil Isolation Kit (STEMCELL Technologies, Vancouver, Canada). Neutrophils were then pelleted via centrifugation (235 ×g for 10 minutes) and resuspended in Hank's Balanced Salt Solution (HBSS) supplemented with Ca2+ and Mg2+ (Thermo Fisher Scientific). Quantification and viability of the purified human neutrophils was assessed using a LUNA-FL cell counter (Logos Biosystems, Gyeonggi-do, South Korea) and then neutrophils were diluted to a concentration of 1 million cells/mL. Microscopic examination of nuclear morphology by Hoechst staining after each isolation confirmed high purity of polymorphonuclear cells.
Seeding human neutrophils onto HAV, ePTFE, and control substrates
A 10-mm biopsy punch was used to cut out circular discs of ePTFE, HAV, and a cell adhesive thermoplastic (ACLAR, Electron Microscopy Services, Hatfield, PA)34 as a control material. Materials were affixed to the bottoms of culture plate wells using biologically inert silicone high vacuum grease (Dow Corning, Midland, MI). Based on prior assay optimization, approximately 750,000 freshly isolated neutrophils were seeded onto each sample material in 750 μL of HBSS with Ca2+ and Mg2+ and placed into a 37°C incubator similar to previous studies.33,35 To induce neutrophil activation and cell death, phorbol-12-myristate-13-acetate (PMA, EMD Millipore, Burlington, MA) was added in some control wells at a final concentration of 100 ng/mL.31,36
Evaluation of neutrophil viability
Neutrophils were prepared for live/dead cell imaging by the application of NucBlue Live and NucGreen Dead staining reagents (Invitrogen) directly into wells 30 minutes after seeding onto substrates (HAV, ePTFE, or control with and without PMA addition). At 1, 3, and 5 hours after seeding, samples were imaged using an upright fluorescence microscope (Nikon 80i) equipped with a Photometrics IRIS 9 camera. The neutrophil death rate was quantified using the dead cell number (green nuclei) divided by the total cell number (blue nuclei) via ImageJ software.
Quantification of neutrophil viability by lactate dehydrogenase release
At 1, 3, and 5 hours after seeding neutrophils on the HAV, ePTFE, or control substrates, with and without the addition of PMA, 200 μL of supernatant was extracted from each sample well for quantification of lactate dehydrogenase (LDH) cellular release using the CyQUANT LDH Cytotoxicity Kit (Invitrogen) and an Infinite 200 Pro plate reader (Tecan, Männedorf, Switzerland) for absorbance measurements at 490 nm and 680 nm. Data were collected using neutrophils isolated from three different volunteers across four separate experiments.
Real-time monitoring of neutrophil elastase release
Neutrophil elastase release from neutrophils on HAV, ePTFE, or control substrates, with and without PMA addition, was monitored every 20 minutes for 5 hours by changes in the fluorescence of MeOSuc-AAPV-AMC (ENZO, BML-P224-0005, 100 μM final concentration added to each well)37 using an Infinite 200 Pro plate reader at 37°C (excitation 380 nm and emission 460 nm). To enhance the detectability of neutrophil elastase release, deoxyribonuclease 1 (DNase 1, EN0521; Thermo Fisher Scientific) was added into the cell culture solutions at 20 μg/mL to facilitate disintegration of NETs.37 Substrate samples with seeded neutrophils but no addition of MeOSuc-AAPV-AMC and DNase 1 were monitored for removal of background fluorescence. Data was collected using neutrophils isolated from six different volunteers in six separate experiments with three replicates per condition in each experiment.
Immunostaining for neutrophil elastase and apoptosis markers
After 1, 3, and 5 hours of culture, neutrophils seeded onto the HAV, ePTFE, or control substrates were fixed with 4% paraformaldehyde (Thermo Fisher Scientific) for 15 minutes and then rinsed with phosphate-buffered saline (Thermo Fisher Scientific) in preparation for immunofluorescence staining. Following standard protocols,24 samples were stained for neutrophil elastase (R&D Systems, Cat. No. MAB91671-100, diluted 1:50) and cleaved caspase-3 (Cell Signaling Technology, Danvers, MA; Cat. No. 9661, diluted 1:200) followed by fluorescent conjugated secondary antibodies (Invitrogen, Cat. No. A11001 and A11012, each diluted 1:200) and 4′,6-diamidino-2-phenylindole (Thermo Fisher Scientific 62247, diluted 1:1000) before imaging.
Electron microscopy of neutrophil and bacterial coculture on materials
Freshly isolated human neutrophils and bacteria (S.aureus AH2547) were cocultured according to Lu et al.38 A solution of 5 × 105 colony-forming units of bacteria in 10% serum were seeded into each well (96-well plate) containing samples of either HAV or ePTFE affixed to the bottom. Thirty minutes later, 5 × 105 neutrophils resuspended in HBSS buffer with Ca2+ and Mg2+ were added to one-half of the sample wells followed by incubation at 37°C for 5 hours. Samples were then gently washed, fixed, and processed using standard techniques24 prior to imaging by Field Emission Scanning Electron Microscopy (FE-SEM, Zeiss Supra 25) at the Microscopy Services Laboratory (University of North Carolina at Chapel Hill, Chapel Hill, NC).
Results
Clinical infection rate and histopathology of HAV and ePTFE explants
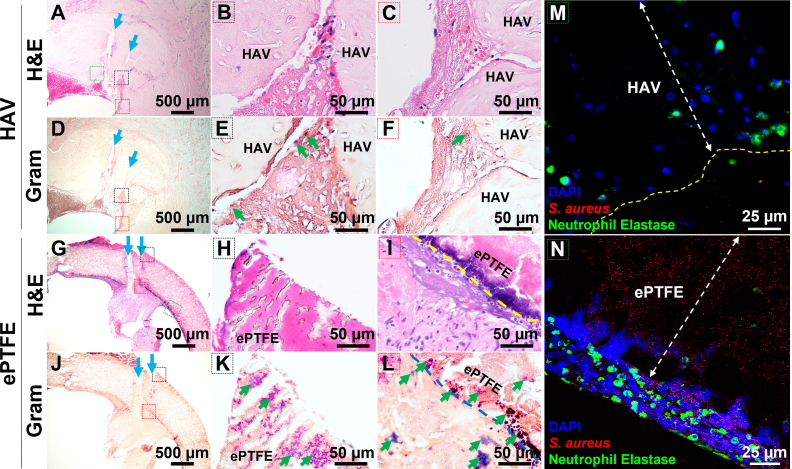
In a phase III study that directly compared the performance of the HAV vs ePTFE as arteriovenous conduits for dialysis, 2 conduit infection events occurred in the HAV cohort; whereas, 10 conduit infection events were observed in the ePTFE cohort as adjudicated by an independent clinical events committee. These results translated to a statistically lower (P = .0413) infection rate of 0.93% per 100 patient-years for HAV recipients vs 4.54% per 100 patient-years for ePTFE recipients. Fig 1 shows representative images of tissue sections taken from an infected HAV and ePTFE graft approximately 1 year after implantation (HAV, 54 weeks; ePTFE, 56 weeks). Hematoxylin and eosin images show multiple cannulation tracts from dialysis access through the wall of both the HAV and ePTFE graft (Fig 1, A and G, blue arrows). Near these sites, Gram staining revealed the presence of gram-positive bacteria that likely were introduced during the cannulation events. Specifically, bacteria was found inside the wall (Fig 1, K) and within luminal pannus tissue (Fig 1, L) of the ePTFE graft, which also did not have much host cell infiltration (Fig 1, H). No bacteria were identified inside the wall of the HAV explant, but small clusters of bacteria were found within the thrombus and fibrous tissue that developed in the cannulation tracts (Fig 1, E and F). Localization of neutrophils and S.aureus by immunostaining showed that neutrophils were found inside the wall of the HAV explant (Fig 1, M, green) which, similar to Gram staining, also had little to no observable positive staining of S. aureus bacteria within the HAV. A dense population of neutrophils (Fig 1, N, green) near extracellular nuclear material (Fig 1, N, blue), suggesting a potential loss of cell viability, was observed adjacent to but not within the wall of the ePTFE graft, which contained S.aureus bacteria (Fig 1, N, red).
Fig 1.
Representative hematoxylin and eosin (H&E), Gram stain, and immunofluorescence images of explanted human acellular vessels (HAV) and expanded polytetrafluoroethylene (ePTFE) dialysis access conduits, determined to be infected approximately 1 year after implantation in a phase III clinical trial. H&E images show multiple cannulation tracts (blue arrows) from dialysis access through the wall of both the HAV (A-C) and ePTFE graft (G-I). A few clusters of gram-positive bacteria (dark blue) were identified in the thrombus and fibrous tissue but not seen within the wall of the HAV explant (D-F, green arrows). Numerous clusters of bacteria were found inside the wall (K) and within the luminal pannus tissue (L) of the ePTFE graft. The black and red dashed line boxes on the low-magnification images (A, D, G J) correspond with regions shown in color labeled high-magnification H&E and gram stain images. Immunostaining for neutrophils (green) and Staphylococcus aureus bacteria (red) in the HAV (M) and ePTFE (N) explants are shown at high magnification near regions identified with green dashed-line boxes in low-magnification images (A, G). Neutrophils were typically found both outside and inside of the HAV (M), but only along the exterior and not within the contaminated ePTFE graft (N). 4′,6-Diamidino-2-phenylindole (DAPI) stained cell nuclei (blue) as well as diffuse extracellular nuclear material into edge of ePTFE (N) which suggested a loss of neutrophil viability or NETosis at this interface.
Bacterial contamination and neutrophil responses to ePTFE and HAV implants in rodent infection models
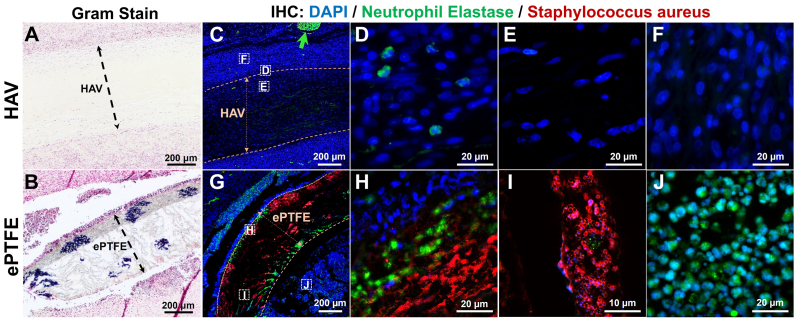
Sections of ePTFE and/or HAV were implanted bilaterally in the dorsum of rats and inoculated with a controlled dose of S.aureus for evaluation of abscess formation and microbial recovery at 2 weeks, as previously described.27 Representative staining of an ePTFE and HAV sample explanted from the same animal showed that no bacteria could be identified by Gram staining (Fig 2, A) or S.aureus immunostaining (Fig 2, C) in the HAV sample. Conversely, Gram staining (Fig 2, B, dark blue) and S.aureus immunostaining (Fig 2, G, red) of the explanted ePTFE material revealed numerous areas of gram-positive S.aureus infiltration into the interstices of the ePTFE matrix.
Fig 2.
Representative Gram stain images and immunohistochemical (IHC) localization of neutrophils and Staphylococcus aureus (S. aureus) from a human acellular vessel (HAV) and expanded polytetrafluoroethylene (ePTFE) explant 2 weeks after implantation in a rodent infection model. Gram-positive S.aureus (dark blue clusters) were identified within interstices of ePTFE (B) but not seen within or around HAV explant (A). Immunostaining for neutrophil elastase (green) and S.aureus (red) is shown in (C-F) and (G-J). The HAV explant had only a few intact neutrophils on the tissue and graft interface (D), but host cells (blue nuclei) infiltrated into the entire HAV wall (C and E), unlike that of the ePTFE wall (G). Numerous clusters of S.aureus were found within the ePTFE wall (G and I), but no bacteria were detected in the HAV (C). Some positive neutrophil elastase staining was observed just at the edge of the ePTFE wall, but relatively few intact nuclei were found to be associated with this staining (H). The tissue surrounding the actively contaminated ePTFE explant (J) had a high density of neutrophil elastase-positive neutrophils compared with that of the HAV implant (F). The green arrow in (C) shows autofluorescent red blood cells.
Neutrophil elastase and S.aureus costaining (Fig 2, G-J) showed that, in general, neutrophils were localized around the exterior and not within the wall of the ePTFE explants (Fig 2, J, green), similar to that seen in clinical explants. There was no presence of S.aureus-positive staining on the outside of the ePTFE, perhaps indicating that neutrophils were able to migrate to and eliminate bacteria outside the graft material but were unable to infiltrate and eliminate bacterial contamination within the ePTFE wall (Fig 2, G-I, red circles). Within the outer edge of the ePTFE grafts, we observed positive neutrophil elastase staining but, interestingly, it was not usually associated with intact nuclei (Fig 2, H, green) suggestive of neutrophil death upon migration into ePTFE.
In contrast, at 2 weeks after implantation there were essentially no identifiable bacteria in the HAV explants and correspondingly very few neutrophils were seen within or surrounding the HAV (Fig 2, C-F, green). These observations were similar to those made in other explanted HAV examined microscopically despite some bacteria enumerated through microbial recovery methods.27 Numerous non-neutrophil host cells recellularized the HAV wall (Fig 2, C and E, blue nuclei), a finding consistent with previous nonclinical and clinical studies of in vivo HAV remodeling.17,24 Explanted ePTFE graft material had only a thin layer of cell infiltration into the edges of the synthetic material (Fig 2, H, blue nuclei). Whether host cells were inhibited from infiltrating into the narrow interstices of ePTFE or lost viability upon contact with the synthetic ePTFE in these samples is unknown but was explored in our subsequent in vitro studies. Overall, these histological findings suggest that the synthetic matrix in the wall of the ePTFE graft permits bacterial infiltration and colonization but may be inaccessible and uninhabitable for invasion of neutrophils.
Neutrophil viability and morphology after contact with HAV and ePTFE
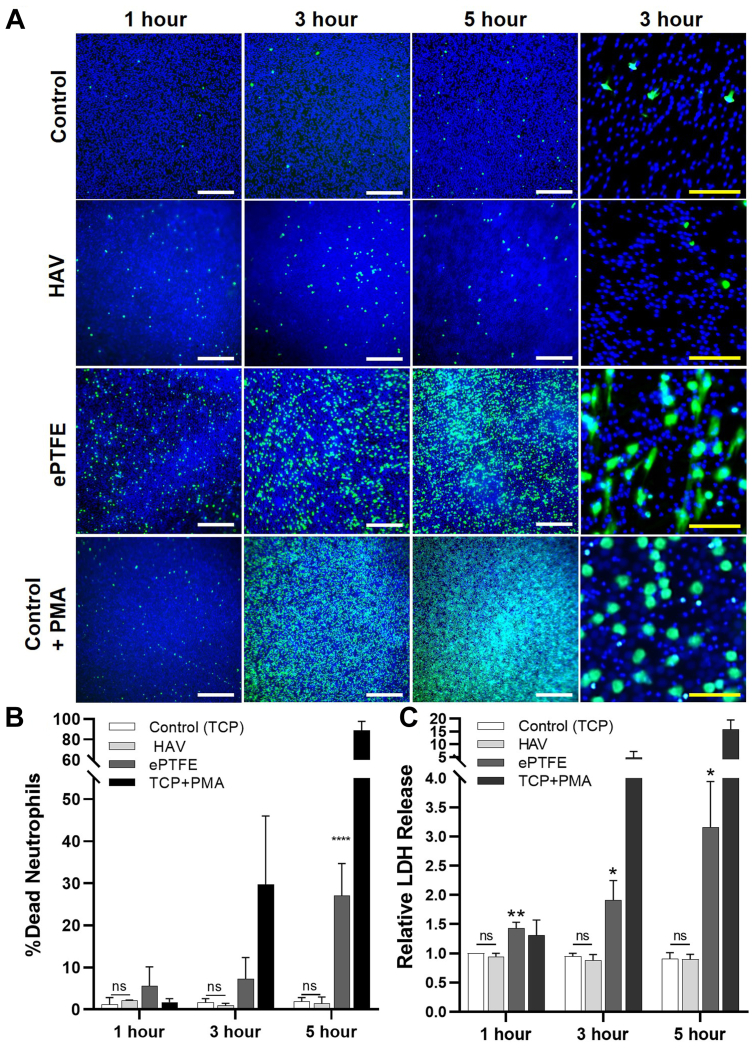
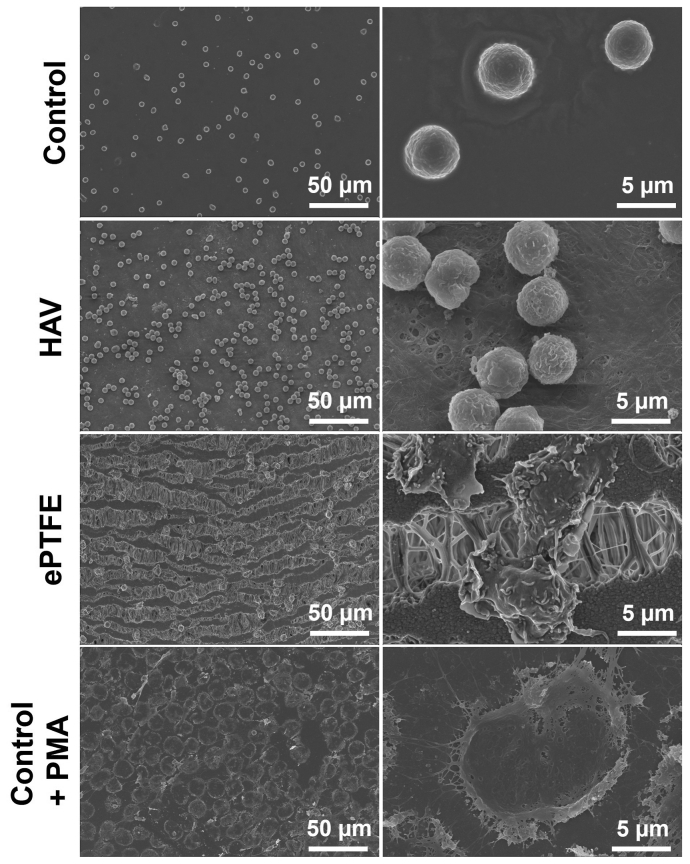
To further evaluate the interaction of the HAV or ePTFE with neutrophils in a controlled setting, in vitro studies were performed using freshly isolated human neutrophils seeded onto HAV, ePTFE, and control substrates. To visualize neutrophil viability on each material at 1, 3, and 5 hours after seeding, a live/dead cell staining assay was performed and analyzed via fluorescence microscopy (Fig 3, A). Overall, there was no observable difference in the number of dead neutrophils (a few green nuclei) seeded onto control and HAV samples across all time points, and these neutrophils retained their round shape (Fig 4). In contrast, neutrophils seeded onto ePTFE underwent a progressive increase in cell death (ie, a higher proportion of green to blue nuclei) over time. These dying cells frequently had an irregular shape with pseudopodia (Fig 3, A, 3-hour timepoint and Fig 4, SEM images). The addition of PMA caused a dramatic increase in neutrophil death, as expected, starting at 3 and 5 hours after seeding. These dying neutrophils swelled as shown by the larger sized green nuclei and blue nuclei (Figs 3, A, and 4). Nuclear changes such as swelling and fusion of nuclear lobes, followed by an increase in membrane permeability, were also observed within minutes to hours of PMA treatment in previous studies.39,40
Fig 3.
Neutrophil viability after seeding onto human acellular vessel (HAV), expanded polytetrafluoroethylene (ePTFE), and control substrates. Evaluation of neutrophil viability by (A) imaging and (B) quantification of live (blue nuclei)/dead (green nuclei) cell staining, as well as LDH release (C) at 1, 3, and 5 hours after seeding onto HAV, ePTFE, and control coverslip substrates, with and without phorbol-12-myristate-13-acetate (PMA). White scale bars are 500 μm; yellow scale bars are 50 μm. Data shown as mean ± standard deviation from at least five studies. Significance displayed between ePTFE and HAV (∗∗∗∗P < .001) and analyzed using a two-way analysis of variance followed by Tukey's multiple comparisons test. There was no statistical difference (ns) between the HAV and control at all time points evaluated.
Fig 4.
Neutrophil morphology after contact with human acellular vessel (HAV) and expanded polytetrafluoroethylene (ePTFE). Representative scanning electron microscopy (SEM) images at 3 hours after seeding onto control, HAV, ePTFE, and control with phorbol-12-myristate-13-acetate (PMA) (100 ng/mL).
To statistically analyze neutrophil viability on each material, neutrophil death rates were calculated from the live/dead staining images (Fig 3, B). Neutrophils on the control and HAV samples exhibited a <2% death rate; whereas, death rate on the ePTFE samples reached 27.2 ± 7.6% at 5 hours, which was significantly higher than with the HAV (P < .001). Neutrophil cytotoxicity on the materials was further analyzed using an LDH assay (Fig 3, C). Results from multiple LDH release experiments showed that neutrophils had the same viability when seeded onto the HAV as the neutrophils seeded onto the control coverslip material (P > .05) at all time points analyzed (1, 3, and 5 hours). Supportive of the live/dead staining results, LDH release was significantly higher even at 1 hour after seeding and increased with time when neutrophils were in contact with ePTFE. As expected, LDH release from neutrophils increased dramatically in control substrate samples after PMA treatment.
Neutrophil elastase release from neutrophils after contact with HAV and ePTFE
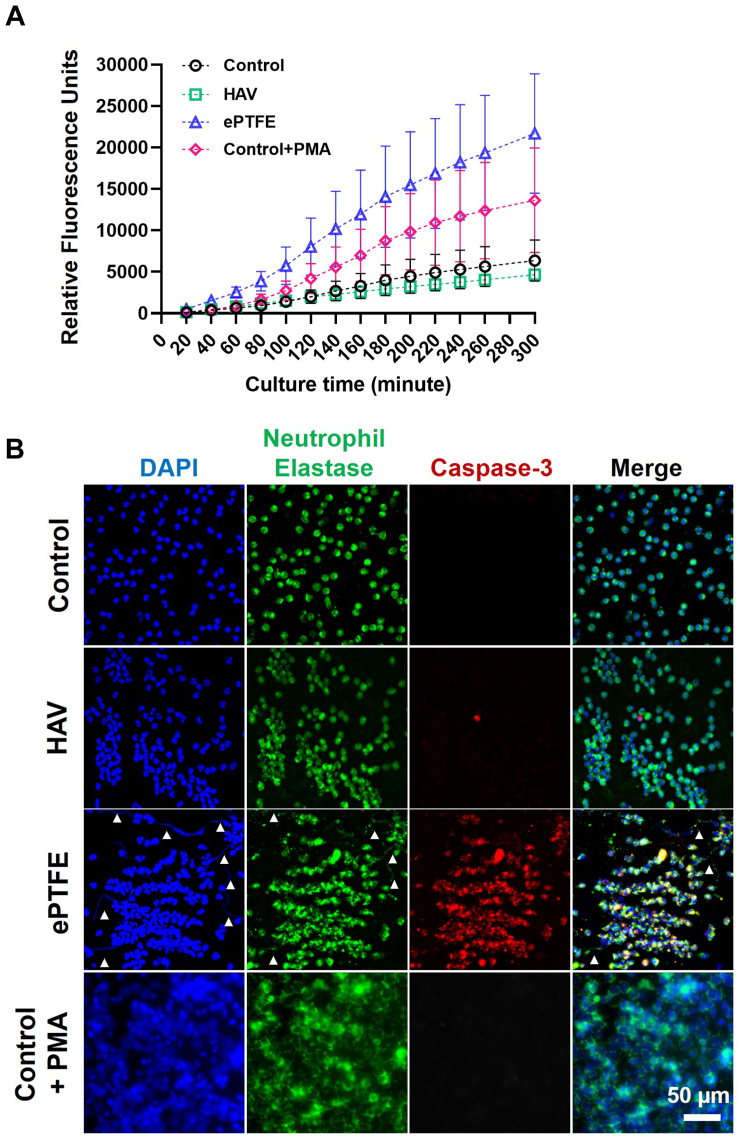
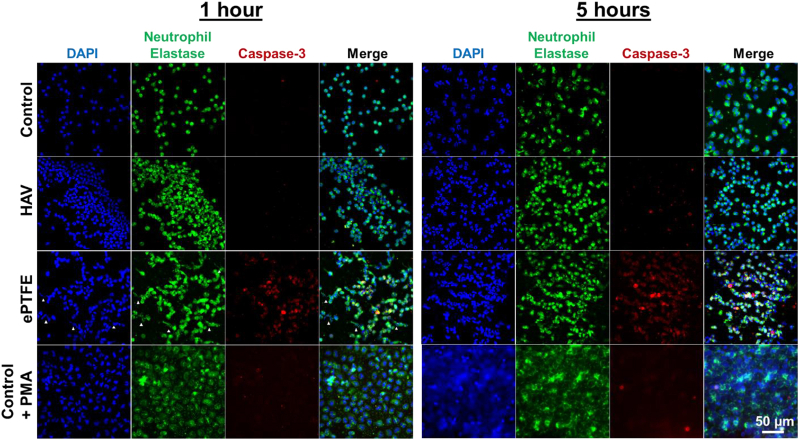
Neutrophil elastase release, as detected by MeOSuc-AAPV-AMC fluorescence, was low for neutrophils seeded onto HAV during the 5-hour culture period (Fig 5, A). In these samples, neutrophil elastase remained within the cytoplasm and nuclei retained a typical multilobular structure (Fig 5, B, and Supplementary Fig). In contrast, neutrophils on ePTFE released elastase, reaching levels that were approximately five times higher than those observed on the HAV at 5 hours (Fig 5, A). NET formation in the absence of pathogenic stimuli was also observed on ePTFE as shown by extracellular release of nuclear material and neutrophil elastase (Fig 5, B, and Supplementary Fig, white arrow heads). This observation is consistent with the detection of higher levels of extracellular neutrophil elastase measured by fluorescence in the MeOSuc-AAPV-AMC assay and may be indicative of an ePTFE-triggered NETosis or apoptosis cell death pathway. Neutrophils seeded onto ePTFE also expressed the apoptosis marker cleaved caspase-3, which increased with time in culture (Fig 5, B, and Supplementary Fig, red). There were little to no cleaved caspase-3-positive neutrophils on the HAV at 1 and 3 hours after seeding and only a few caspase-3-positive cells at 5 hours.
Fig 5.
Neutrophil elastase release after contact with human acellular vessel (HAV) and expanded polytetrafluoroethylene (ePTFE). (A) Real-time monitoring of neutrophil elastase release by MeOSuc-AAPV-AMC fluorescence in the presence of DNAse I after seeding neutrophils onto HAV, ePTFE, and control substrates, with and without phorbol-12-myristate-13-acetate (PMA) stimulation. Data are mean ± standard error from six experiments with three replicates for each condition. (B) Representative images of neutrophil elastase (green) and caspase-3 (red) immunostaining at 3 hours after seeding onto control, HAV, ePTFE, and control with PMA (100 ng/mL). Nuclei (blue) counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The white arrowheads in ePTFE samples show extracellular DNA or neutrophil elastase within neutrophil extracellular traps (NETs).
Supplementary Fig.
Representative images of neutrophil elastase (green) and caspase-3 (red) immunostaining at 1 and 5 hours after seeding onto control, human acellular vessel (HAV), expanded polytetrafluorethylene (ePTFE), and control with phorbol-12-myristate-13-acetate (PMA) (100 ng/mL). Nuclei (blue) counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 50 μm. The white arrowheads in ePTFE samples show the leaking DNA or neutrophil elastase.
In the PMA controls, a large proportion of neutrophil elastase seemed to be retained within the swollen neutrophil membrane containing enlarged spherical nuclei (Figs 4 and 5, B). This observation may explain why a lower level of extracellular neutrophil elastase was detected in the PMA controls compared with that from the ePTFE samples in the MeOSuc-AAPV-AMC studies. Interestingly, neutrophils seeded onto control coverslips and treated with PMA did not seem to express cleaved caspase-3, suggesting that cell death observed by live/dead imaging and LDH release occurred through a nonapoptotic pathway. Overall, these results indicated that ePTFE but not HAV can induce NET formation, elastase release, and subsequent cell death in neutrophils upon contact.
Neutrophils and bacteria coculture on HAV and ePTFE
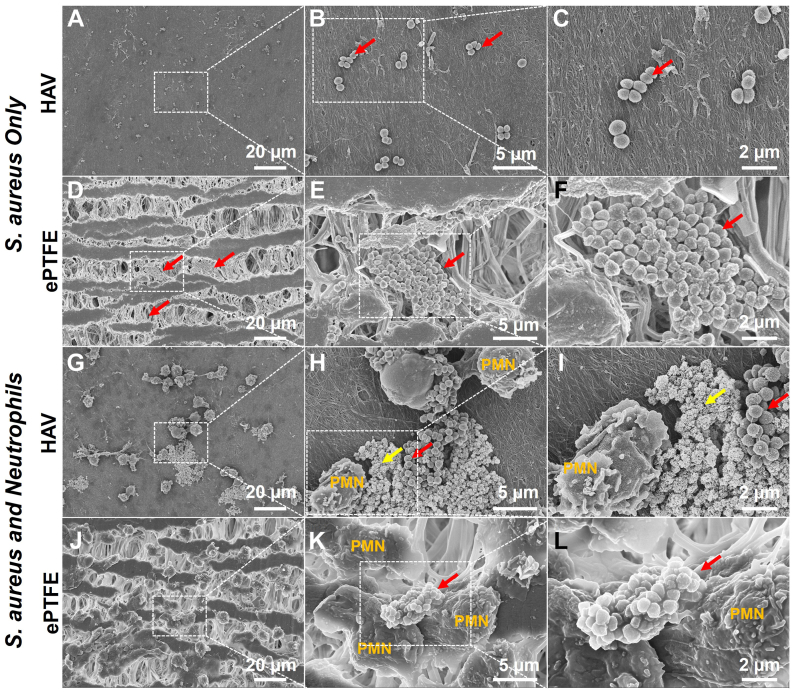
To test the ability of human neutrophils to phagocytose bacteria on HAV and ePTFE, S.aureus was seeded onto the material surface for 30 minutes, then neutrophils were added directly for 5 hours of coculture. For the control samples, S.aureus was seeded onto the materials without neutrophils. As shown in Fig 6, the S.aureus bacteria was found to be denser and within the grooves and interstices of ePTFE as compared with the more isolated bacteria on the HAV. Neutrophils on the HAV were also observed forming NETs31,41 that ensnared bacteria which showed signs of bacterial cell lysis, membrane damage, and cytoplasmic leakage (Fig 6, H and I, yellow arrow). In contrast, although some neutrophils seeded onto the ePTFE showed signs of NET formation based on morphology, they seemed to be less directly adjacent to and engaged with bacteria. Many of the bacteria aggregated near neutrophils appeared to have retained their membrane integrity (Fig 6, K and L) as opposed to those near neutrophils on HAV samples. These findings may indicate that, after 5 hours of coculture, neutrophils seeded onto the HAV were more functional in migrating toward and eliminating bacteria than those seeded onto ePTFE.
Fig 6.
Neutrophil and bacteria coculture on human acellular vessel (HAV) and expanded polytetrafluoroethylene (ePTFE). Representative scanning electron microscopy (SEM) images of S.aureus with and without freshly isolated neutrophils after 5 hours of seeding onto HAV and ePTFE. Red arrows indicate S.aureus bacteria. Clusters of bacteria scattered on surface of HAV (A-C), but accumulated within the interstitial nodes of the ePTFE matrix (D-F). Polymorphonuclear neutrophils (PMN) on HAV formed NETs with ensnared bacteria (G). Bacteria with cytoplasmic leakage and membrane integrity loss are seen near PMNs on the HAV (H and I, yellow arrows), indicating bacterial cell death. Bacteria near PMNs on ePTFE often had normal morphology (L) and did not seem to be within NETs (J and K), suggesting that, by 5 hours, PMNs on ePTFE were less effective than those on HAV at eliminating bacteria. The magnified images on the right are of the area depicted by dashed boxes in images on the left.
Discussion
Here we report that, in a clinical comparison of HAV and ePTFE used as arteriovenous conduits for hemodialysis access, a setting with a high risk for infection,8 the HAV had a significantly lower rate of infection than that of ePTFE, despite comparable patency rates. Not surprisingly, bacteria found within explanted sections of an infected HAV and ePTFE graft were associated with sites of cannulation, defects that are permanent in ePTFE but are actively repaired in HAV conduits.24 Bacteria were observed at greater amounts both within the wall and in tissue surrounding ePTFE. Unlike the HAV, the ePTFE explant had minimal host cell infiltration, even after 1 year of implantation, despite dense populations of cells, including neutrophils, surrounding the infected ePTFE graft. Interestingly, these findings were supported by immunostaining of ePTFE and HAV samples from the rodent infection model. We found dense populations of neutrophils surrounded ePTFE grafts that were contaminated with S.aureus, but very few neutrophils with intact nuclei were found within the ePTFE matrix. The presence of neutrophil elastase without nuclei within a narrow band along the outer edge of the ePTFE suggested that neutrophils migrated to the contamination but were unable to survive when attempting to invade the ePTFE. In contrast, no bacteria but numerous host cells, including some neutrophils, were found both in the surrounding tissue as well as within the HAV consistent with prior clinical histopathology.24
In this study, controlled in vitro studies were implemented to define the impact of the HAV and ePTFE material on human neutrophil survival and function to evaluate potential mechanisms for the observed differences in resistance to bacterial infection. Neutrophils play a vital role in combatting infection, especially at infection onset, by migrating toward and degrading bacteria through the release of NETs containing nuclear material, reactive oxygen species, and proteolytic enzymes, including neutrophil elastase.31,36 Here we observed that the viability of human neutrophils decreased significantly upon exposure to ePTFE, a process that also stimulated neutrophil elastase release in the absence of bacteria (Fig 3, Fig 4, Fig 5, Fig 6). These results are supported by other studies that demonstrated contact with ePTFE inhibits neutrophil function32,35 and induces neutrophil death.33 Interestingly, neutrophil activation in whole blood exposed to ePTFE has been shown to induce NET formation that directly increased thrombogenesis.42 Thus, the stimulation of NETosis may be linked to thrombosis and inflammation in ePTFE vascular grafts, even without concomitant infection. Uncontrolled and amplified NET release is believed to promote autoimmune disorders, cancer, atherosclerosis, and vasculitis.43, 44, 45 Recent evidence has also linked increased thrombotic events and inflammation in patients with coronavirus disease 2019 with dysregulation of NETosis.46,47
Examination of neutrophil and S.aureus cocultures demonstrated that bacteria were preferentially clustered within the interstices of ePTFE, as compared with the more isolated bacteria found on the HAV (Fig 6). Moreover, when associated with neutrophils on HAV, these bacteria seemed to be ensnared in NETs40 and showed signs of bacterial cell lysis, membrane damage, and cytoplasmic leakage (Fig 6, H and I, yellow arrows). As observed in the absence of bacteria (Fig 5, B), NET formation occurred in some neutrophils cocultured with bacteria on ePTFE, but these NETs were often not directly adjacent to or did not contain bacteria. In general, bacteria on ePTFE, even when in direct contact with neutrophils (Fig 6, L), seemed to be relatively normal and healthy. These findings, in addition to our results from LDH release assays, live/dead cell staining, and cleaved capase-3 immunostaining, support the theory that contact with ePTFE dramatically decreases neutrophil viability and, consequently, bactericidal function. Neutrophils exposed to the HAV material, in contrast, retained high viability, native morphology, and function.
The limitations of this study include not evaluating the response of other immune cells known to be involved in pathogen clearance, such as macrophages, to HAV and ePTFE in both in vitro and in vivo experiments. Within the rodent infection model, the materials were implanted subcutaneously rather than directly into arterial circulation as would be possible in larger animal models. Thus, these results may not be fully representative of the vascular environment and clinical applications. An additional limitation of these experiments was the singular end point at 2 weeks. Earlier explants may have allowed for the ability to capture more evidence of neutrophil-mediated bacterial clearance within the HAV. The focus of this study was a direct comparison of HAV with synthetic ePTFE, but additional studies using native vein, cryopreserved allografts, and xenografts would be beneficial to evaluate neutrophil response and infection resistance of these biological materials compared with that of HAV. Although allografts and xenografts are reported to have better infection resistance than that of synthetic grafts, their risk for calcification or degradation are concerning for mechanical durability.12 Based on our results and >5 years of use in clinical trial patients,22,23 the HAV may offer both infection resistance and long-term durability.
Conclusions
Our clinical, preclinical, and in vitro studies suggest that the infection resistance of HAV may be attributed to the biocompatibility of its native-like composition of human extracellular matrix proteins to host cells, including neutrophils. In contrast, the synthetic ePTFE matrix seems to permit bacterial infiltration and colonization, but is inaccessible and inhospitable to neutrophils, which may explain higher rates of infection in ePTFE grafts compared to HAVs. Thus, in addition to its off-the-shelf availability, mechanical integrity, and capacity for remodeling by host cells after implantation, the low susceptibility to bacterial infection that we have observed in the HAV warrant further study of its potential as a preferred option in surgical locations or indications that require high resistance to bacterial infection, such as hemodialysis access and vascular trauma.
Author Contributions
Conception and design: JW, SB, HP, LN, RK
Analysis and interpretation: JW, SB, HP, LN, RK
Data collection: JW, SB, GL, RK
Writing the article: JW, SB, GL, RK
Critical revision of the article: JW, SB, GL, HP, LN, RK
Final approval of the article: JW, SB, GL, HP, LN, RK
Statistical analysis: JW, RK
Obtained funding: LN
Overall responsibility: RK
Acknowledgments
The authors thank Kelly Kirker and Garth James (Montana State University) for support with neutrophil and bacteria co-culture, Kristen White (UNC-Chapel Hill) for SEM assistance, Jason Keller (Humacyte, Inc.) for histological sectioning, and Gina (Jiahui) Chen (NC State University) for assisting with neutrophil studies.
Footnotes
Humacyte, Inc. supported this study.
Author conflict of interest: J.W. reports ownership of stock or stock options and employment. S.K.F.B. reports ownership of stock or stock options and employment. G.S.L. reports ownership of stock or stock options and employment. H.L.P. reports ownership of stock or stock options and employment. L.E.N. reports ownership of stock or stock options, employment, and board membership. R.D.K. reports ownership of stock or stock options and employment.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix
References
- 1.Kilic A., Arnaoutakis D.J., Reifsnyder T., et al. Management of infected vascular grafts. Vasc Med. 2016;21:53–60. doi: 10.1177/1358863X15612574. [DOI] [PubMed] [Google Scholar]
- 2.Wilson W.R., Bower T.C., Creager M.A., et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134:412–460. doi: 10.1161/CIR.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 3.Benrashid E., Youngwirth L.M., Mureebe L., Lawson J.H. Operative and perioperative management of infected arteriovenous grafts. J Vasc Access. 2017;18:13–21. doi: 10.5301/jva.5000613. [DOI] [PubMed] [Google Scholar]
- 4.Schutte W.P., Helmer S.D., Salazar L., Smith J.L. Surgical treatment of infected prosthetic dialysis arteriovenous grafts: total versus partial graft excision. Am J Surg. 2007;193:385–388. doi: 10.1016/j.amjsurg.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Piano G. Infections in lower extremity vascular grafts. Surg Clin North Am. 1995;75:799–809. doi: 10.1016/s0039-6109(16)46700-9. [DOI] [PubMed] [Google Scholar]
- 6.Antonios V.S., Noel A.A., Steckelberg J.M., et al. Prosthetic vascular graft infection: a risk factor analysis using a case-control study. J Infect. 2006;53:49–55. doi: 10.1016/j.jinf.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Zamani N., Sharath S.E., Barshes N.R., Braun J.D., Kougias P. Long-term outcomes of lower extremity graft preservation using antibiotic beads in patients with early deep wound infections after major arterial reconstructions. J Vasc Surg. 2020;71:1315–1321. doi: 10.1016/j.jvs.2019.06.192. [DOI] [PubMed] [Google Scholar]
- 8.Bachleda P., Kalinova L., Utikal P., Kolar M., Hricova K., Stosova T. Infected prosthetic dialysis arteriovenous grafts: a single dialysis center study. Surg Infect. 2012;13:366–370. doi: 10.1089/sur.2011.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson J.D., Houston Rt, Morrison J.J., Gifford S.M., Rasmussen T.E. A retrospective cohort comparison of expanded polytetrafluorethylene to autologous vein for vascular reconstruction in modern combat casualty care. Ann Vasc Surg. 2015;29:822–829. doi: 10.1016/j.avsg.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Guerrero A., Gibson K., Kralovich K.A., et al. Limb loss following lower extremity arterial trauma: what can be done proactively? Injury. 2002;33:765–769. doi: 10.1016/s0020-1383(01)00175-9. [DOI] [PubMed] [Google Scholar]
- 11.Tatterton M.R., Homer-Vanniasinkam S. Infections in vascular surgery. Injury. 2011;42(Suppl 5):S35–S41. doi: 10.1016/S0020-1383(11)70131-0. [DOI] [PubMed] [Google Scholar]
- 12.Chakfé N., Diener H., Lejay A., et al. Editor's choice–European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg. 2020;59:339–384. doi: 10.1016/j.ejvs.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Fox C.J., Gillespie D.L., O'Donnell S.D., et al. Contemporary management of wartime vascular trauma. J Vasc Surg. 2005;41:638–644. doi: 10.1016/j.jvs.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Martin L.C., McKenney M.G., Sosa J.L., et al. Management of lower extremity arterial trauma. J Trauma. 1994;37:591–598. doi: 10.1097/00005373-199410000-00012. discussion: 8-9. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor S., Andrew P., Batt M., Becquemin J.P. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg. 2006;44:38–45. doi: 10.1016/j.jvs.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 16.Niklason L.E., Lawson J.H. Bioengineered human blood vessels. Science. 2020;370 doi: 10.1126/science.aaw8682. [DOI] [PubMed] [Google Scholar]
- 17.Dahl S.L., Kypson A.P., Lawson J.H., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 18.Lawson J.H., Glickman M.H., Ilzecki M., et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016;387:2026–2034. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutowski P., Gage S.M., Guziewicz M., et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020;72:1247–1258. doi: 10.1016/j.jvs.2019.11.056. [DOI] [PubMed] [Google Scholar]
- 20.Morrison J.J., McMahon J., DuBose J.J., Scalea T.M., Lawson J.H., Rasmussen T.E. Clinical implementation of the humacyte human acellular vessel: implications for military and civilian trauma care. J Trauma Acute Care Surg. 2019;87(Suppl 1):S44–S47. doi: 10.1097/TA.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 21.Lauria A.L., Kersey A.J., Propper B.W., et al. Preliminary experience with the human acellular vessel: a descriptive case series detailing early use of a bioengineered blood vessel for arterial repair. Ann Vasc Surg. 2022;87:100–112. doi: 10.1016/j.avsg.2022.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Gutowski P., Guziewicz M., Ilzecki M., et al. 6-Year outcomes of a phase 2 study of human-tissue engineered blood vessels for peripheral arterial bypass. JVS Vasc Sci. 2022;4 doi: 10.1016/j.jvssci.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakimowicz T., Przywara S., Turek J., et al. Five year outcomes in patients with end stage renal disease who received a bioengineered human acellular vessel for dialysis access. EJVES Vasc Forum. 2022;54:58–63. doi: 10.1016/j.ejvsvf.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkton R.D., Santiago-Maysonet M., Lawson J.H., et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halbert R.J., Nicholson G., Nordyke R.J., Pilgrim A., Niklason L. Patency of ePTFE arteriovenous graft Placements in hemodialysis patients: systematic literature review and meta-analysis. Kidney360. 2020;1:1437–1446. doi: 10.34067/KID.0003502020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guth C., Naslund T. Surgical management of an infected external iliac artery interposition graft with a bioengineered human acellular vessel. J Vasc Surg Cases Innov Tech. 2022;8:111–114. doi: 10.1016/j.jvscit.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkton R.D., Prichard H.L., Santiago-Maysonet M., Niklason L.E., Lawson J.H., Dahl S.L.M. Susceptibility of ePTFE vascular grafts and bioengineered human acellular vessels to infection. J Surg Res. 2018;221:143–151. doi: 10.1016/j.jss.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Archer N.K., Mazaitis M.J., Costerton J.W., Leid J.G., Powers M.E., Shirtliff M.E. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence. 2011;2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van de Vyver H., Bovenkamp P.R., Hoerr V., et al. A novel mouse model of staphylococcus aureus vascular graft infection: noninvasive imaging of biofilm development in vivo. Am J Pathol. 2017;187:268–279. doi: 10.1016/j.ajpath.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Papayannopoulos V. Neutrophils facing biofilms: the battle of the barriers. Cell Host Microbe. 2019;25:477–479. doi: 10.1016/j.chom.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Brinkmann V., Reichard U., Goosmann C., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 32.Chang C.C., Lieberman S.M., Moghe P.V. Quantitative analysis of the regulation of leukocyte chemosensory migration by a vascular prosthetic biomaterial. J Mater Sci Mater Med. 2000;11:337–344. doi: 10.1023/a:1008925722623. [DOI] [PubMed] [Google Scholar]
- 33.Nadzam G.S., De La Cruz C., Greco R.S., Haimovich B. Neutrophil adhesion to vascular prosthetic surfaces triggers nonapoptotic cell death. Ann Surg. 2000;231:587–599. doi: 10.1097/00000658-200004000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingsley R.E., Cole N.L. Preparation of cultured mammalian cells for transmission and scanning electron microscopy using Aclar film. J Electron Microsc Tech. 1988;10:77–85. doi: 10.1002/jemt.1060100110. [DOI] [PubMed] [Google Scholar]
- 35.Chang S., Popowich Y., Greco R.S., Haimovich B. Neutrophil survival on biomaterials is determined by surface topography. J Vasc Surg. 2003;37:1082–1090. doi: 10.1067/mva.2003.160. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs T.A., Abed U., Goosmann C., et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podolska M.J., Mahajan A., Hahn J., et al. Treatment with DNases rescues hidden neutrophil elastase from aggregated NETs. J Leukoc Biol. 2019;106:1359–1366. doi: 10.1002/JLB.3AB0918-370R. [DOI] [PubMed] [Google Scholar]
- 38.Lu T., Porter A.R., Kennedy A.D., Kobayashi S.D., DeLeo F.R. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J Innate Immun. 2014;6:639–649. doi: 10.1159/000360478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takei H., Araki A., Watanabe H., Ichinose A., Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59:229–240. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- 40.Fadeel B., Åhlin A., Henter J.-I., Orrenius S., Hampton M.B. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood. 1998;92:4808–4818. [PubMed] [Google Scholar]
- 41.Li J., Wang G., Zhu H., et al. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci Rep. 2014;4:4359. doi: 10.1038/srep04359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling C., Fischer M., Maitz M.F., Werner C. Neutrophil extracellular trap formation upon exposure of hydrophobic materials to human whole blood causes thrombogenic reactions. Biomater Sci. 2017;5:1998–2008. doi: 10.1039/c7bm00458c. [DOI] [PubMed] [Google Scholar]
- 43.Fuchs T.A., Brill A., Duerschmied D., et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masucci M.T., Minopoli M., Del Vecchio S., Carriero M.V. The Emerging role of neutrophil extracellular traps (NETs) in Tumor progression and Metastasis. Front Immunol. 2020;11:1749. doi: 10.3389/fimmu.2020.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong S.L., Demers M., Martinod K., et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillot C., Favresse J., Mullier F., Lecompte T., Dogne J.M., Douxfils J. NETosis and the immune System in COVID-19: mechanisms and potential treatments. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.708302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y., Chen X., Liu X. NETosis and neutrophil extracellular traps in COVID-19: Immunothrombosis and beyond. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.838011. [DOI] [PMC free article] [PubMed] [Google Scholar]