Abstract
The present study was performed to explore the effects of dietary supplementation of hydrolyzed gallotannin (HGT) on intestinal physical barrier, immune function and microbiota structure in yellow-feather broilers. A total of 288 male yellow-feather broilers were randomly allocated to 4 diet treatments: the basal diet (CON) and 3 diets supplemented with 150, 300, and 450 mg/kg HGT for 63 d, respectively, with 6 replicates per treatment and 12 birds per replicate. The findings demonstrated that 300 or 450 mg/kg HGT addition enhanced the expression of duodenal occludin (OCLN) and tight junction protein1 (TJP-1) genes of birds at 21 d of age, and the expression of duodenal and ileal OCLN gene in 63-day-old broilers was upregulated due to 450 mg/kg HGT treatment (P < 0.05). The dietary supplementation of 150 mg/kg HGT strengthened the expression of duodenal IL-6 and IL-4 genes and ileal IL-4 gene of 21-day-old broilers, whereas the expression of jejunal IL1B and IL-6 genes in birds at 63 d of age weakened because of 300 or 450 mg/kg HGT addition (P < 0.05). As for microbial community, the HGT addition altered the cecal microbiota structure of birds at 21 d of age based on analysis of similarities (ANOSIM) test and 450 mg/kg HGT treatment increased the relative abundance of norank Eubacterium coprostanoligenes group at 21 d of age and unclassified Lachnospiraceae at 63 d of age (P < 0.05). In short, diet supplemented with 300 to 450 mg/kg HGT may be the optimal for yellow-feather broilers to enhance intestinal barrier function. Altogether, our study clarified the regulatory role of HGT in broiler intestinal health in earnest, but the underlying mechanism is still unclear. Hence, more research is needed to carry out until the application of HGT as a new functional additive in broiler production.
Key words: tannin, chicken, mechanical barrier, immunity, bacterial community
INTRODUCTION
Novel functional additives are in urgent need to substitute antibiotics, which had been used in husbandry to promote animal intestinal health, improve nutrient absorption and prevent pathogenic bacteria infection before its prohibition of use as a feed additive in European and China (Baumler and Sperandio, 2016). Hydrolyzed gallotannin (HGT), a class of polyphenolic compounds, can bind protein and produce 1 molecule of glucose and 10 molecules of gallic acid when hydrolyzed (Aelenei et al., 2009). Several previous studies indicated that dietary tannins supplementation could exert a positive influence on the health of broilers, especially immune function and antioxidant capacity. A study has shown that there was a significant increase in CD4+CD8+ and γδ+ cell populations in white-feather broilers when supplemented with 500 mg/kg tannins (Ramah et al., 2020). Another recent study reported a causal role of HGT administration in strengthening intestinal barrier of mice suffered from diquat-induced oxidative stress (Wang et al., 2019). In our previous study, we found that dietary supplementation with 150 mg/kg of HGT could improve the growth performance of broilers during the early growth phase (1–21 d of age) and HGT treatment protected against oxidative damage of yellow-feather broilers by increasing the content of liver total antioxidant capacity (T-AOC) and glutathione/glutathione disulfide ratio (GSH/GSSG) (Tong et al., 2022a,b), however, the effect of HGT in intestinal barrier function and microbiota structure was still obscure, and we hypothesized that the sounder intestine might contribute to the growth performance-promoting role of HGT on broilers.
The intestine acts as a defense system to prevent pathogens and toxins from entering the body, and the intestinal barrier system is composed of 4 parts: the physical, chemical, immunological, and microbiological barriers (Anderson et al., 2012). The physical barrier, mainly composed of mucosa epithelial cells and intercellular junctions, is the first line of defense (Odenwald and Turner, 2017). Tight junctions are critical in establishing the interepithelial barrier and maintaining epithelial polarity (Otani and Furuse, 2020). The chemical barrier mainly refers to the mucus layer, which is produced by goblet cells and some epithelial cells, and also serves as the first line of innate defense (Johansson and Hansson, 2016). In addition, digestive enzymes, gastric acid, and bile acids that can inhibit the growth of potential pathogens also play an undoubted role in intestinal chemical barrier (Huang et al., 2021). The immunological barrier consists of intestinal immune tissues (like Peyer's patch), immune cells, and immune factor which maintains immunologic homeostasis by orchestrating immune activation and tolerance (Lecocq et al., 2013). Intestinal microbiota, as the microbiological barrier, is essential in inducing cell renewal, fortifying the mucus layer and stimulating mucosa immunity, thereby affecting the host physiology (Hayes et al., 2018).
Consequently, this study was conducted to investigate the effects of dietary supplementation with HGT on intestinal barrier system of yellow-feathered broilers, in order to promote the use of HGT as a new functional additive to maintain intestinal health of yellow-feather broilers.
MATERIALS AND METHODS
Animals were cared for and handled in accordance with the animal care protocols approved by the Fujian Agriculture and Forestry University Animal Care and Use Ethics Committee (Fuzhou, Fujian, China, approval ID: PZCASFAFU23062).
Experimental Materials
HGT (extracted from gallnut, 50% purity and rice hull as the carrier) was obtained from Fujian Jinhualong Feed Co., Ltd. (Fujian, China). The 1-day-old male broilers were offered by WENS Foodstuff Group Co., Ltd. (Guangdong, China). Feedstuffs for broiler diet were purchased from Fujian Jinhualong Feed Co., Ltd. (Fujian, China).
Experimental Design and Bird Management
A total of 288 one-day-old male broilers (34.10 ± 0.08 g) were randomly assigned to 4 treatments: 1) corn-soybean-based diet (CON), 2) CON diet supplemented with 150 mg/kg HGT, 3) CON diet supplemented with 300 mg/kg HGT, 4) CON diet supplemented with 450 mg/kg HGT, with 6 replicates per group and 12 broilers per replicate. The doses of HGT were selected according to a previous report and the recommended dose of the manufacturer (Tonda et al., 2018). The basal diets formulated according to the feeding standards of Chinese chickens (NY/T33-2004) are showed in Supplementary Table S1. The test period was 63 d. Broilers were reared in cage (1,562.5 cm2/bird) with 23 h of light, ad libitum feeding and water intake. The temperature of the bird room was controlled at 35°C for the first week and then lowered by 2°C to 3°C per wk until 22°C. Additionally, standard immunization procedures were used throughout the experiment.
Sampling Procedure
On d 21 and d 63 of the experiment, 1 broiler from each replicate was randomly chosen to be stunned, exsanguinated, scalded, plucked and sampled. The mucosa of duodenum, jejunum, and ileum was scraped and frozen at −80°C for the detection of digestive enzymes activity and gene expression related to physical and immune barrier function. Cecal digesta were collected and stored at −80°C for 16S rRNA gene sequencing.
Determination of Lipase and α-Amylase Activities
The activities of lipase and α-amylase were determined according to the previous methods (Dahlqvist, 1962; Verduin et al., 1973). The mucosa collected from a middle section of duodenum was fully homogenized with cold PBS (pH = 7.4) with a weight-to-volume ratio of 1:50 and then centrifuged at 1,000 × g for 10 min at 4°C. Total protein content in the intestinal mucosa was evaluated by using a BCA protein quantitative kit purchased from New cell § Molecular Biotech Co. Ltd. (Suzhou, China). The activities of lipase (Catalog number: A054-1-1) and α-amylase (Catalog number: C016-1-1) were determined by using assay kits from Nanjing Jiancheng Institute of Biological Engineering (Najing, China). The absorbance was detected by spectrophotometry (iMark, Bio Rad, Hercules, CA).
Gene Expression Analysis by qPCR
Total RNA was extracted from the duodenal, jejunal, and ileal mucosa by using the Eastep super Total RNA Extraction Kit (Directory number: LS1040) from Shanghai Promega Biological Products Co., Ltd. (Shanghai, China), and a Thermo Scientific NanoDrop 2000 Spectrophotometer (Wilmington) was used to determine the concentration and quality of RNA. Total RNA was reverse-transcribed via using the Eastep RT Master Mix Kit from Shanghai Promega Biological Products Co., Ltd. (Shanghai, China). The cDNA was used for quantitative real-time PCR on an Applied Biosystems. The primer sequences were synthesized by Sango Biotech Co., Ltd. (Shanghai, China) and displayed in Supplementary Table S2. The relative mRNA expression levels of each target gene were analyzed according to the method described by Livak and Schmittgen (2001) and shown as 2−△△CT.
Bacterial DNA Extraction and 16S rRNA Gene Sequencing
The bacterial DNA of digesta was extracted by using Stool DNA Kit (D4015-01, Omega Bio-tek, Norcross, GA). The V3 to V4 regions of the bacterial 16S rRNA genes were amplified with the primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by an ABI GeneAmp 9700 PCR Thermocycler (Waltham, MA). Purified amplicons were pooled in equimolar and paired-end sequenced on an Illumina MiSeq PE300 platform (San Diego, CA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Amplicon sequence variants (ASVs) with 99% similarity cutoff delineated were clustered using divisive Amplicon Denoising Algorithm 2 (DADA2). Rarefaction curves were made using Python 2.7 to assess whether the sequencing volume is sufficient (Gotelli and Colwell, 2001). The unique and common ASVs observed in different groups are showed in Venn diagram. The α-diversity was calculated using accumulated cyclone energy (ACE), Simpson, and Shannon diversity indices by mothur software (Schloss et al., 2009). Principal coordinate analysis (PCoA) based on unweighted UniFrac distance to analysis the similarities between samples and groups, and the intergroup difference analysis was performed by analysis of similarities (ANOSIM) test.
The raw microbial sequencing data have been deposited at the National Center for Biotechnology Information database (NCBI), under accession number PRJNA888548. The data that support the study findings and models are available from the authors upon reasonable request.
Data Analysis and Statistics
Statistical analysis was performed using SPSS 25.0 software (Chicago, IL). The intergroup differences were examined using 1-way ANOVA analysis with Duncan's post hoc test. The linear and quadratic comparisons were applied to determine the dose-effect of HGT on intestinal function of broilers. Specially, statistically significant differences in the relative abundance of the cecal microbiota were determined using Kruskal-Wallis test with Tukey's post hoc test. P value <0.05 was considered statistically significant.
RESULTS
Growth Performance
The effect of HGT supplementation on the growth performance of broilers is presented in Supplementary Table S3 (Tong et al., 2022a,b). Compared with CON group, dietary supplementation with 150 mg/kg HGT significantly decreased the Feed:Gain of broilers at 1 to 21 d of age (P < 0.05), but no obvious effects of HGT treatment on ADFI and ADG were observed. Meanwhile, HGT supplementation had no effects on ADFI, ADG, and Feed:Gain of broilers at 1 to 63 d of age.
Digestive Enzyme Activity
The results of digestive enzyme activity are presented in Table 1. At 21 d of age, the 150 mg/kg HGT supplementation notably increased the lipase activity in duodenum compared with the CON group (P < 0.05). However, α-amylase was not significantly different between groups. At 63 d of age, there were no effects of HGT treatment on α-amylase and lipase activity in duodenum of broilers.
Table 1.
The effect of HGT on digestive enzyme activity in duodenum of broilers.1
| Items | HGT, mg/kg |
SEM |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 150 | 300 | 450 | ANOVA | Linear | Quadratic | ||
| 21 d of age | ||||||||
| α-Amylase, U/g prot | 79.88 | 78.07 | 60.34 | 74.37 | 4.365 | 0.388 | 0.466 | 0.303 |
| Lipase, U/g prot | 0.554b | 1.955a | 0.629b | 0.677b | 0.198 | 0.023 | 0.299 | 0.102 |
| 63 d of age | ||||||||
| α-Amylase, U/g prot | 48.98 | 56.67 | 55.90 | 56.76 | 3.616 | 0.870 | 0.522 | 0.664 |
| Lipase, U/g prot | 1.088 | 1.243 | 1.193 | 1.821 | 0.161 | 0.390 | 0.154 | 0.463 |
Values are means of 6 replicates per treatment with 1 bird each.
Means in the same row without the same superscript differ significantly (P < 0.05).
Gene Expression of Tight Junction Proteins
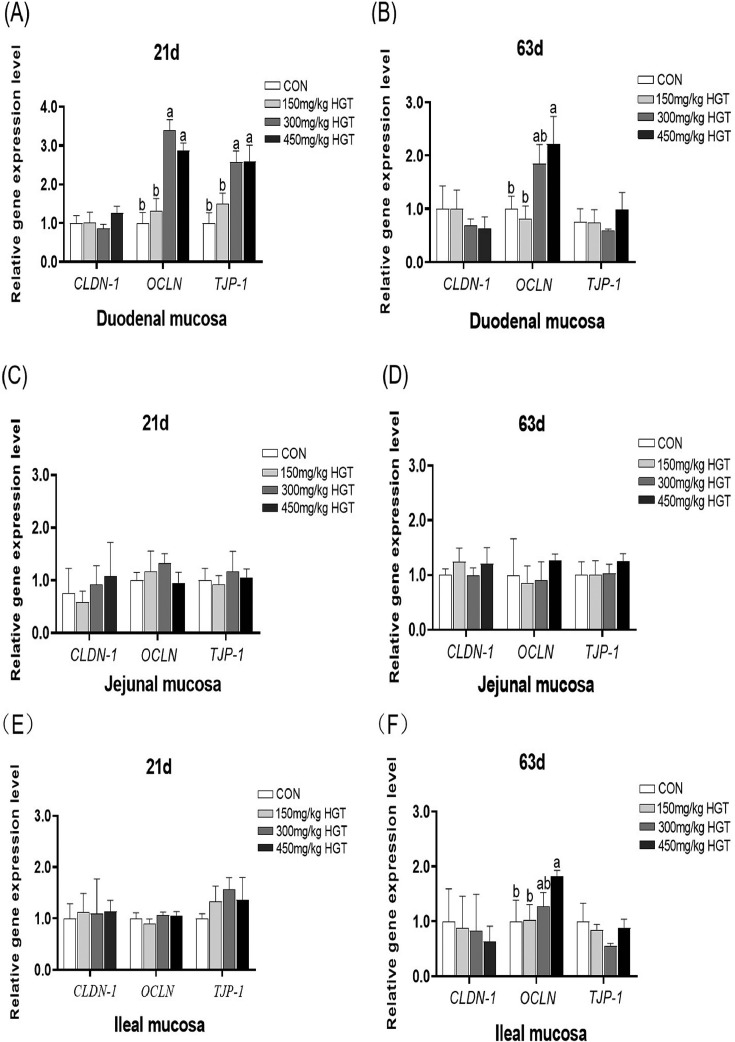
As shown in Figure 1, compared with the CON group, HGT supplementations of 300 or 450 mg/kg could increase the gene expression of occludin (OCLN) and tight junction protein1 (TJP-1) in duodenal mucosa on d 21 (P < 0.05) (Figure 1A). But no significant difference in gene expression of tight junction proteins in jejunal and ileal mucosa of 21-day-old broilers was observed between groups (Figure 1C and E). At 63 d of age, the 450 mg/kg HGT supplementation in the diet of broilers increased the expression of OCLN in duodenal and ileal mucosa compared with the CON group (P < 0.05) (Figure 1B and F). However, there were no special effects of HGT treatment on gene expression of tight junction proteins in jejunal mucosa (Figure 1D).
Figure 1.
The effect of HGT on gene expression of tight junction proteins in intestinal mucosa of broilers. Values are means of 6 replicates per treatment with 1 bird each. CLDN-1, OCLN, and TJP-1 are the genes that encode claudin1, occludin, and tight junction protein1, respectively. CON group: basal diet; 150 mg/kg HGT group: basal diet added with 150 mg/kg HGT; 300 mg/kg HGT group: basal diet added with 300 mg/kg HGT; 450 mg/kg HGT group: basal diet added with 450 mg/kg HGT. a–bMeans in the same row without the same superscript differ significantly (P < 0.05). Error bar stands for SEM.
Gene Expression of Cytokines
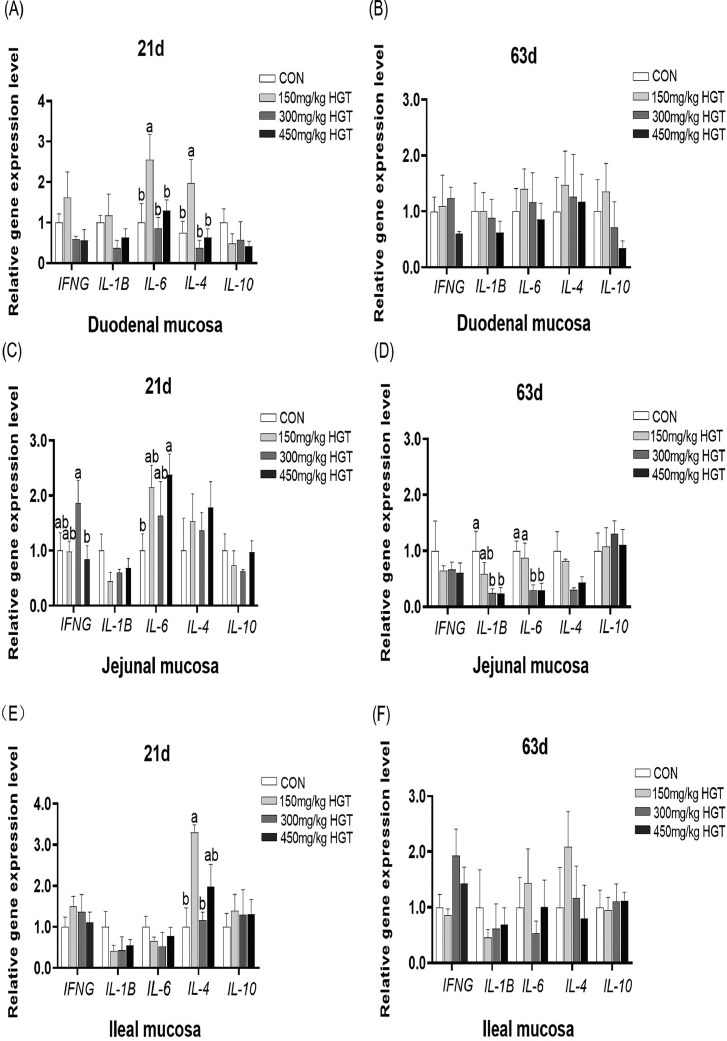
Compared with the CON group, dietary supplementation of 150 mg/kg HGT increased the expression of IL-6 and IL-4 in duodenal mucosa of 21-day-old broilers (P < 0.05) (Figure 2A). Notably, dietary supplementation with 450 mg/kg HGT could obviously increase the expression of IL-6 in jejunal mucosa on d 21 (P < 0.05) (Figure 2C). Interestingly, HGT supplementations of 150 mg/kg could increase the expression of IL-4 in ileal mucosa at 21 d of age (P < 0.05) (Figure 2E).
Figure 2.
The effect of HGT on gene expression of cytokines in intestinal mucosa of broilers. Values are means of 6 replicates per treatment with 1 bird each. Abbreviation: IFNG, interferon gamma. CON group: basal diet; 150 mg/kg HGT group: basal diet added with 150 mg/kg HGT; 300 mg/kg HGT group: basal diet added with 300 mg/kg HGT; 450 mg/kg HGT group: basal diet added with 450 mg/kg HGT. a–bMeans in the same row without the same superscript differ significantly (P < 0.05). Error bar stands for SEM.
The expression of IL1B and IL-6 in jejunal mucosa from 63-day-old broilers fed with 300 and 450 mg/kg HGT was lower than broilers fed with basal diet (P < 0.05) (Figure 2D). However, there were no obvious differences among diets in the HGT of the expression of IFNG, IL1B, IL-6, I-L4, and IL-10 in duodenal and ileal mucosa on d 63 (Figure 2B and F).
Cecal Microbiota
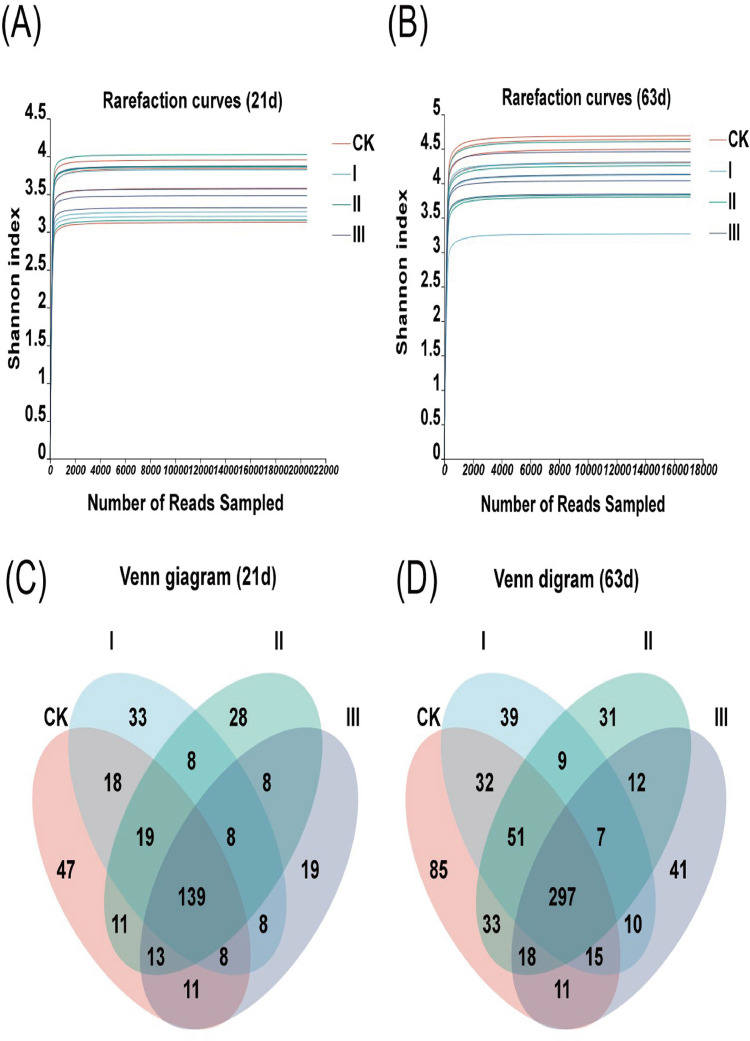
After denoise and quality control, 470,225 and 418,586 16S rRNA gene sequences were obtained from 16 cecal bacterial DNA samples of yellow-feather broilers at 21 d and 63 d of age, respectively. Rarefaction curve analysis showed that the sequencing data were sufficient to reflect the microbial diversity (Figure 3A and B).
Figure 3.
Rarefaction curves of sequencing reads and Venn diagram of ASVs in broilers. Values are means of 4 replicates per treatment with 1 bird each. Group CK: basal diet; Group I: basal diet added with 150 mg/kg HGT; Group Ⅱ: basal diet added with 300 mg/kg HGT; Group Ⅲ: basal diet added with 450 mg/kg HGT.
Besides, 378 and 691 ASVs are obtained based on 99% sequence similarity from all samples on d 21 and d 63, respectively (Figure 3C and D). On d 21, there were 139 shared ASVs and 47, 33, 28, and 19 ASVs unique in the Group CK (0 mg/kg HGT), Group Ⅰ (150 mg/kg HGT), Group Ⅱ (300 mg/kg HGT), and Group Ⅲ (450 mg/kg HGT), respectively (Figure 3C). On d 63, there were 297 ASVs shared among all groups, whereas 85, 39, 31, and 41 ASVs were identified only present in the Group CK, Ⅰ, Ⅱ and Ⅲ, respectively (Figure 3D).
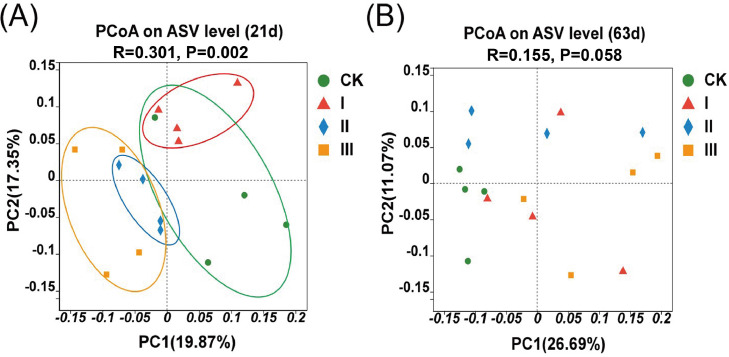
As shown in Table 2, the dietary addition of HGT did not affect the ACE index, Simpson index, and Shannon index of cecum microorganisms in birds at 21 and 63 d of age. As revealed in Figure 4A, PCoA based on unweighted UniFrac distance showed that the distance of samples between groups is far at 21 d of age (P = 0.002), indicating that the microbiota structure between groups were distinguishing. However, the cecal microbiome profiles of 63-day-old broilers were not significantly different between groups (Figure 4B).
Table 2.
Effects of HGT on alpha diversity in cecum microorganism of yellow-feather broilers.1
| Items | HGT, mg/kg |
SEM | P value | |||
|---|---|---|---|---|---|---|
| 0 | 150 | 300 | 450 | |||
| 21 d of age | ||||||
| ACE index | 150.7 | 136.8 | 136.8 | 120.8 | 4.774 | 0.226 |
| Simpson index | 0.065 | 0.062 | 0.053 | 0.054 | 0.006 | 0.571 |
| Shannon index | 3.63 | 3.54 | 3.73 | 3.56 | 0.078 | 0.766 |
| 63 d of age | ||||||
| ACE index | 337.8 | 263.8 | 269.3 | 227.3 | 15.40 | 0.106 |
| Simpson index | 0.026 | 0.056 | 0.057 | 0.045 | 0.007 | 0.178 |
| Shannon index | 4.53 | 4.00 | 4.12 | 4.12 | 0.093 | 0.150 |
Values are means of 4 replicates per treatment with 1 bird each. Abbreviation: ACE, accumulated cyclone energy.
Figure 4.
PCoA analysis based on unweighted UniFrac distance and ANOSIM analysis. Group CK: basal diet; Group I: basal diet added with 150 mg/kg HGT; Group Ⅱ: basal diet added with 300 mg/kg HGT; Group Ⅲ: basal diet added with 450 mg/kg HGT. Abbreviations: ANOSIM, analysis of similarities; ASV, amplicon sequence variants; PCoA, principal coordinates analysis.
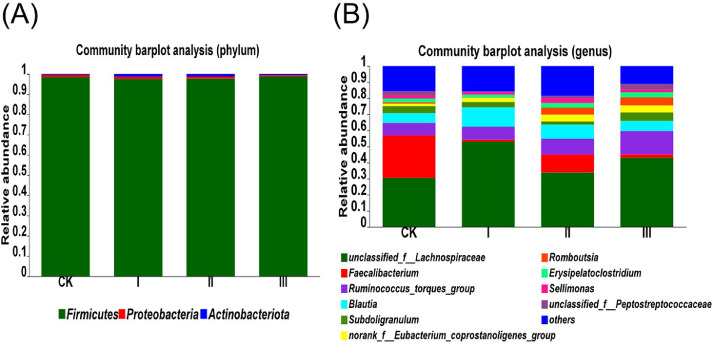
Firmicutes was predominant and Proteobacteria and Actinobacteria were subdominant in cecal microbiota community of 21-day-old broilers. There were no significant differences in the relative abundance of these 3 phyla levels among groups (Table 3 and Figure 5A). At the genus level, the cecal microbiota community were dominated by unclassified Lachnospiraceae, Faecalibacterium, Ruminococcus torques group, Blautia, and Subdoligranulum, but no remarkable intergroup differences were observed. Only the relative abundances of norank Eubacterium coprostanoligenes group in Group Ⅱ and Group Ⅲ were significantly higher than Group CK (P < 0.05) (Table 3 and Figure 5B).
Table 3.
Relative richness of cecal dominant phyla and genera in 21-day-old broilers.1
| Items (%) | HGT, mg/kg |
SEM | P value | |||
|---|---|---|---|---|---|---|
| 0 | 150 | 300 | 450 | |||
| Phylum level | ||||||
| Firmicutes | 98.45 | 97.46 | 97.69 | 98.70 | 0.368 | 0.431 |
| Proteobacteria | 1.13 | 1.13 | 1.08 | 0.66 | 0.260 | 0.830 |
| Actinobacteria | 0.42 | 1.40 | 1.23 | 0.64 | 0.221 | 0.131 |
| Genus level | ||||||
| Unclassified Lachnospiraceae | 30.50 | 53.02 | 33.75 | 43.03 | 3.275 | 0.065 |
| Faecalibacterium | 26.47 | 1.312 | 11.21 | 2.090 | 3.800 | 0.069 |
| Ruminococcus torques group | 7.82 | 7.97 | 9.90 | 14.35 | 1.323 | 0.368 |
| Blautia | 5.99 | 12.03 | 8.66 | 6.34 | 0.925 | 0.057 |
| Subdoligranulum | 4.35 | 3.36 | 2.09 | 5.23 | 1.241 | 0.687 |
| Norank Eubacterium coprostanoligenes group | 1.45b | 2.52ab | 4.22a | 4.42a | 0.410 | 0.010 |
| Romboutsia | 1.00 | 0.27 | 4.34 | 5.08 | 1.015 | 0.056 |
| Erysipelatoclostridium | 2.13 | 1.70 | 2.86 | 3.03 | 0.284 | 0.235 |
| Sellimonas | 2.17 | 1.54 | 3.04 | 1.92 | 0.299 | 0.562 |
| Unclassified Peptostreptococcaceae | 2.42 | 0.42 | 1.28 | 3.26 | 0.643 | 0.524 |
Values are means of 4 replicates per treatment with 1 bird each.
Means in the same row without the same superscript differ significantly (P < 0.05).
Figure 5.
Relative abundance at phylum level (A) and genus level (B) in cecum microbiota of 21-day-old broilers. Values are means of 4 replicates per treatment with 1 bird each. Group CK: basal diet; Group I: basal diet added with 150 mg/kg HGT; Group II: basal diet added with 300 mg/kg HGT; Group Ⅲ: basal diet added with 450 mg/kg HGT.
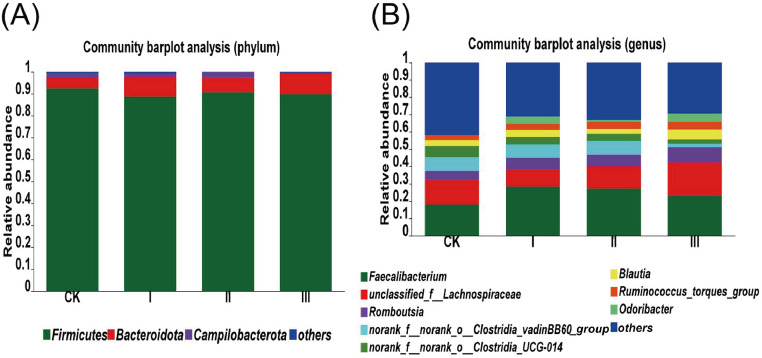
As presented in Table 4 and Figure 6, at the phylum level, the cecal microbiota community in 63-day-old broilers was dominated by Firmicutes, Bacteroidota, and Campylobacterota and their proportions accounted for more than 99%. The relative abundance of Campylobacterota decreased from 1.95 to 0.39% because of 450 mg/kg HGT treatment (P < 0.05). At the genus level, Faecalibacterium, unclassified Lachnospiraceae, Romboutsia, norank Clostridia vadinBB60 group, norank Clostridia UCG-014, and Blautia were dominant genera. The relative abundance of unclassified Lachnospiraceae in Group Ⅲ was significantly higher than Group CK (P < 0.05).
Table 4.
Relative richness of cecal dominant phyla and genera in 63-day-old broilers.1
| Items (%) | HGT, mg/kg |
SEM | P value | |||
|---|---|---|---|---|---|---|
| 0 | 150 | 300 | 450 | |||
| Phylum level | ||||||
| Firmicutes | 92.40 | 88.49 | 90.78 | 90.34 | 0.993 | 0.409 |
| Bacteroidota | 5.02 | 9.43 | 6.57 | 8.91 | 1.029 | 0.309 |
| Campylobacterota | 1.95 | 1.45 | 2.48 | 0.39 | 0.322 | 0.030 |
| Genus level | ||||||
| Faecalibacterium | 17.93 | 28.43 | 28.39 | 22.80 | 2.865 | 0.128 |
| Unclassified Lachnospiraceae | 14.28b | 10.31b | 12.92b | 19.87a | 1.080 | 0.024 |
| Romboutsia | 5.35 | 6.24 | 6.36 | 9.50 | 0.816 | 0.382 |
| Norank Clostridia vadinBB60 group | 7.96 | 6.97 | 7.36 | 1.82 | 1.064 | 0.117 |
| Norank Clostridia UCG-014 | 6.37 | 4.74 | 4.14 | 2.11 | 0.676 | 0.080 |
| Blautia | 3.38 | 4.10 | 2.84 | 5.59 | 0.511 | 0.497 |
| Ruminococcus torques group | 2.94 | 3.78 | 4.14 | 4.58 | 0.306 | 0.343 |
| Parabacteroides | 2.56 | 1.76 | 3.28 | 3.32 | 0.526 | 0.589 |
Values are means of 4 replicates per treatment with 1 bird each.
Means in the same row without the same superscript differ significantly (P < 0.05).
Figure 6.
Relative abundance at phylum level (A) and genus level (B) in cecal microbiota of 63-day-old broilers. Values are means of 4 replicates per treatment with 1 bird each. Group CK: basal diet; Group I: basal diet added with 150 mg/kg HGT; Group Ⅱ: basal diet added with 300 mg/kg HGT; Group Ⅲ: basal diet added with 450 mg/kg HGT.
DISCUSSION
Recently, polyphenol compounds have gained substantial attention because of their antioxidant properties (Surai, 2014). The growth-promoting and health-improving benefits due to phenolic compounds supplementation in broiler diets have been reported in several studies (Starcevic et al., 2015; Mahfuz et al., 2021). Specially, HGT, an endogenous plant phenol, is mainly derived from Chinese gallnuts (Haslam, 1996). Our previous studies indicated that dietary supplementation with 150 mg/kg of HGT could improve the feed conversion ratio (FCR) of broilers during the early growth phase (1–21 d of age), and elevate the content of liver T-AOC and GSH/GSSG (Tong et al., 2022a,b). We speculated that a stronger intestine might facilitate the FCR-promoting role of HGT in broilers, and HGT-generated reinforced antioxidant capacity can also contribute to a healthier intestine. As we expected, in our study, the higher lipase activity in duodenum was observed in the 21-day-old broilers fed with 150 mg/kg HGT. It is widely accepted that the higher digestive enzyme activity indicates the more complete utilization of nutrient (Chen et al., 2022). Similarly, it was found that the tannins at low concentration (200 and 300 mg/d) had a positive effect on the activities of lipase, amylase and trypsin to strengthen nutrient digestion and uptake (Majumdar and Moudgal, 1994). However, Quesada et al. (1995) reported that extracts of pears, lentils and cocoa beans, rich in condensed and hydrolyzed tannins, could inhibit the activity of α-amylase, and the higher the dose, the stronger the inhibition effect. It is common knowledge that the phenolic hydroxyl in tannins acts as the hydrogen donor and the carbonyl peptide in enzyme serves as the hydrogen acceptor. Carbonyl peptide of the enzyme was excessively exposed to the solution of hydrolyzed tannin, resulting in its precipitation and the loss of enzyme activity (Zhu et al., 1997). However, there is a great discrepancy in the affinities between tannins and enzymes. Hence, the reason for the above different results on enzyme activity might be due to the effective content of tannins and the discrepant affinities between tannins and enzymes (Nyamambi et al., 2000).
The interepithelial physical barrier primarily consists of tight junction, adhere junction, and desmosome and is essential to maintain intestinal integrity and sustain the polarity of epithelial cells (Farquhar and Palade, 1963). Herein, tight junction is a multiprotein complex including the transmembrane barrier proteins (claudins, occludin, etc.) and peripheral scaffolding proteins (ZO-1, ZO-2, ZO-3, etc.) (Van Itallie and Anderson, 2014). Claudins were first identified in junctional fraction of chicken liver, and the gene overexpression of Claudins (CLDNs) in fibroblasts was reported sufficient to reconstitute tight junction-like networks of strands (Günzel and Yu, 2013). Additionally, Umeda et al. (2004) observed a marked retardation of tight junction formation when TJP-1 expression of epithelial cell was hampered. Our results showed that dietary supplementation HGT increased the expression of OCLN and TJP-1 in duodenum and ileum mucosa of yellow-feather broilers, especially in broilers from 1 to 21 d of age. Similarly, Liu et al. (2018) found that addition of 2 g/kg HGT in diet strongly increased the expression of TJP-1 in heat-stressed broilers to alleviate the negative effects of heat stress on intestinal function. Previous studies also found that 2.5 and 5 mg/kg pretreated tannins could significantly increase the expression of TJP-1 in diquat-challenged mice, but the 10 mg/kg tannins addition significantly inhibited the expression of CLDN. So the effect of tannins might on intestinal tight junction might partly depends on the physical state of body and the dosage of tannins (Wang et al., 2019). Overall, despite a few inconsistent reports, it is reasonable to speculate that dietary HGT could upregulate the gene expression of tight junction protein, then improving physical barrier function of broilers.
Cytokines play an important role in the modulation of inflammatory response against intestinal mucosal damage (Shen and Turner, 2006). Th2 cytokines such as IL-4 play a crucial role in selective activation of macrophages, whereas IL-6 is a multifunctional cytokine that triggers intracellular signaling pathways to regulate immune response (Rose-John, 2012; Chaudhari et al., 2018). In our study, an increase in the expression of IL-4 and IL-6 on d 21 due to 150 mg/kg HGT supplementation and a decrease in the expression of IL1B and IL-6 on d 63 on account of 300 or 450 mg/kg HGT addition were observed, indicating that HGT had a positive and significant effect on prevention of pathogen invasion during the early growth stage (1–21 d) and an energy-save and production-promotion effect through a reduction of immune response in the late growth stage (22–63 d) of broilers. Consistent with our results, Kawano et al. (2020) discovered that tannins enhanced IL-4 secretion from CD4+ T cells and CD8+ T cells in a dextran sodium salt-induced colitis model of mouse. Additionally, a previous study revealed that 25 mg/kg tannic acid reduced inflammatory response by downregulating the protein expression of toll-like receptor 4 and restraining extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase activation, then inhibiting the expression of Tnfa and Il1b in mice (Sivanantham et al., 2019). Nevertheless, Ramah et al. (2020) found that low-dose (500 mg/kg) tannins resulted in the expansion of CD4+CD8+ and γδ+ cell populations, whereas high-dose (30 g/kg) tannins supplementation generated a smaller T and B cell subsets and inhibited the expression of IL-4 in the spleen. The different results on cytokines expression and secretion might be due to the plant sources of tannins and animal species. Collectively, these observations suggested tannins could be a potential critical immunoregulator of intestinal health in broilers.
A growing body of scientific evidence has demonstrated that polyphenols derived from plants could alter the composition of gut microbial community (Lillehoj et al., 2018). Tannins were reported to induce morphological changes of bacteria in growth phase and to inhibit the microbial proliferation by limiting iron availability to metalloenzymes in microbial cells (Jones et al., 1994). Lee et al. (2010) reported that tannins had antimicrobial activity because there was a lower fecal population of coliform bacteria in weanling pigs fed tannins diets. It was widely accepted that tannins could disrupt membrane function of bacteria by inhibiting the electron transport system and oxidative phosphorylation (Goel et al., 2005). Furthermore, previous studies have described a reduction in genus Bacteroides but certain members of Ruminococcaceae and Lachnospiraceae were increased in broilers fed with tannins (Diaz Carrasco et al., 2018). In our study, dietary HGT supplementation notably affected the composition and structure of cecal microbial community in 21-day-old broilers, and the relative abundance of norank Eubacterium coprostanoligenes group at 21 d of age and unclassified Lachnospiraceae at 63 d of age were raised after 450 mg/kg HGT treatment. Eubacterium coprostanoligenes could decrease serum cholesterol concentration by modulating the conversion of absorbed cholesterol into coprostanol, which plays a key role in cholesterol metabolism homeostasis (Martin et al., 1972; Li et al., 1998; Kriaa et al., 2019). In addition, some studies reported that the Lachnospiraceae family modified the immune statues of their hosts by producing short-chain fatty acids and converting primary bile acids into secondary bile acids (Sorbara et al., 2020), which are considered to have beneficial effects on intestinal health via regulation of gut microbiome community structure (Ridlon et al., 2014). Accordingly, it can be speculated that tannins might alter the relative abundance of beneficial intestinal bacteria but the underlying mechanisms are not to completely understand yet.
CONCLUSIONS
In conclusion, the supplementation of HGT in the diet of broilers could improve intestinal barrier function and regulate the dynamic balance of intestinal flora, thereby promoting the intestinal health of broilers. Overall, dietary supplementation of 300 to 450 mg/kg HGT is recommended for better intestinal function of yellow-feather broilers.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 32202723), Natural Science Foundation of Fujian Province, China (Grant No. 2021J05020), Modern Poultry Industry System of Fujian Province (Grant No. KKE19013A), Science and Technology Innovation Special Fund of Fujian Agriculture and Forestry University (Grant No. CXZX2020054A), Basic Research Project for Public Research Institutes of Fujian Province, China (Grant No. 2023R1024006).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103010.
Appendix. Supplementary materials
REFERENCES
- Aelenei N., Popa M.I., Novac O., Lisa G., Balaita L. Tannic acid incorporation in chitosan-based microparticles and in vitro controlled release. J. Mater. Sci. Mater. Med. 2009;20:1095–1102. doi: 10.1007/s10856-008-3675-z. [DOI] [PubMed] [Google Scholar]
- Anderson R.C., Dalziel J.E., Gopal P.K., Bassett S., Ellis A., Roy N.C. In: Pages 3–30 in Colitis. Masayuki F., editor. IntechOpen; London, UK: 2012. The role of intestinal barrier function in early life in the development of colitis. [Google Scholar]
- Baumler A.J., Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari A.A., Kim W.H., Lillehoj H.S. Interleukin-4 (IL-4) may regulate alternative activation of macrophage-like cells in chickens: a sequential study using novel and specific neutralizing monoclonal antibodies against chicken IL-4. Vet. Immunol. Immunopathol. 2018;205:72–82. doi: 10.1016/j.vetimm.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Chen C.Z., Li P., Wang W.B., Li Z.H. Response of growth performance, serum biochemical parameters, antioxidant capacity, and digestive enzyme activity to different feeding strategies in common carp (Cyprinus carpio) under high-temperature stress. Aquaculture. 2022;548 [Google Scholar]
- Dahlqvist A. A method for the determination of amylase in intestinal content. Scand. J. Clin. Lab. Invest. 1962;14:145–151. doi: 10.3109/00365516209079686. [DOI] [PubMed] [Google Scholar]
- Diaz Carrasco J.M., Redondo E.A., Pin Viso N.D., Redondo L.M., Farber M.D., Fernandez Miyakawa M.E. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed. Res. Int. 2018;2018 doi: 10.1155/2018/1879168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M.G., Palade G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel G., Puniya A.K., Aguilar C.N., Singh K. Interaction of gut microflora with tannins in feeds. Naturwissenschaften. 2005;92:497–503. doi: 10.1007/s00114-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Gotelli N.J., Colwell R.K. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001;4:379–391. [Google Scholar]
- Günzel D., Yu A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J. Nat. Prod. 1996;59:205–215. doi: 10.1021/np960040+. [DOI] [PubMed] [Google Scholar]
- Hayes C.L., Dong J., Galipeau H.J., Jury J., McCarville J., Huang X., Wang X.Y., Naidoo A., Anbazhagan A.N., Libertucci J., Sheridan C., Dudeja P.K., Bowdish D.M.E., Surette M.G., Verdu E.F. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci. Rep. 2018;8:14184. doi: 10.1038/s41598-018-32366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Weng Y., Shen Q., Zhao Y., Jin Y. Microplastic: a potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci. Total Environ. 2021;785 doi: 10.1016/j.scitotenv.2021.147365. [DOI] [PubMed] [Google Scholar]
- Johansson M.E., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.A., McAllister T.A., Muir A.D., Cheng K.J. Effects of sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microb. 1994;60:1374–1378. doi: 10.1128/aem.60.4.1374-1378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Saika K., Takagi R., Matsui M., Matsushita S. Tannic acid acts as an agonist of the dopamine D2L receptor, regulates immune responses, and ameliorates experimentally induced colitis in mice. Brain Behav. Immun. Health. 2020;5 doi: 10.1016/j.bbih.2020.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaa A., Bourgin M., Mkaouar H., Jablaoui A., Akermi N., Soussou S., Maguin E., Rhimi M. Microbial reduction of cholesterol to coprostanol: an old concept and new insights. Catalysts. 2019;9:167. [Google Scholar]
- Lecocq M., Detry B., Guisset A., Pilette C. FcαRI-mediated inhibition of IL-12 production and priming by IFN-gamma of human monocytes and dendritic cells. J. Immunol. 2013;190:2362–2371. doi: 10.4049/jimmunol.1201128. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Shinde P.L., Choi J.Y., Kwon I.K., Lee J.K., Pak S.I., Cho W.T., Chae B.J. Effects of tannic acid supplementation on growth performance, blood hematology, iron status and faecal microflora in weanling pigs. Livest. Sci. 2010;131:281–286. [Google Scholar]
- Li L., Batt S.M., Wannemuehler M., Dispirito A., Beitz D.C. Effect of feeding of a cholesterol-reducing bacterium, Eubacterium coprostanoligenes, to germ-free mice. Lab. Anim. Sci. 1998;48:253–255. [PubMed] [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M.E., Chi F., Cravens R.L., Oh S., Gay C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018;49:76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.W., Li K., Zhao J.S., Deng W. Effects of chestnut tannins on intestinal morphology, barrier function, pro-inflammatory cytokine expression, microflora and antioxidant capacity in heat-stressed broilers. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:717–726. doi: 10.1111/jpn.12839. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahfuz S., Shang Q., Piao X. Phenolic compounds as natural feed additives in poultry and swine diets: a review. J. Anim. Sci. Biotechnol. 2021;12:48. doi: 10.1186/s40104-021-00565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Moudgal R.P. Effect of tannic acid on activities of certain digestive enzymes and alkaline phosphatase in intestine and glucose absorption in adult chickens. J. Appl. Anim. Res. 1994;6:105–112. [Google Scholar]
- Martin W.J., Ravi Subbiah M.T., Kottke B.A., Birk C.C., Naylor M.C. Nature of fecal sterols and intestinal bacterial flora. Lipids. 1972;8:208–215. doi: 10.1007/BF02544637. [DOI] [PubMed] [Google Scholar]
- Nyamambi B., Ndlovu L.R., Read J.S., Reed J.D. The effects of sorghum proanthocyanidins on digestive enzyme activity in vitro and in the digestive tract of chicken. J. Sci. Food Agric. 2000;80:2223–2231. [Google Scholar]
- Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T., Furuse M. Tight junction structure and function revisited. Trends Cell Biol. 2020;30:805–817. doi: 10.1016/j.tcb.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Quesada C., Bartolomé B., Nieto O., Gómez-Cordovés C., Hernandez T., Estrella I. Phenolic inhibitors of α-amylase and trypsin enzymes by extracts from pears, lentils, and cocoa. J. Food Prot. 1995;59:185–192. doi: 10.4315/0362-028X-59.2.185. [DOI] [PubMed] [Google Scholar]
- Ramah A., Yasuda M., Ohashi Y., Urakawa M., Kida T., Yanagita T., Uemura R., Bakry H.H., Abdelaleem N.M., El-Shewy E.A. Different doses of tannin reflect a double-edged impact on broiler chicken immunity. Vet. Immunol. Immunopathol. 2020;220 doi: 10.1016/j.vetimm.2019.109991. [DOI] [PubMed] [Google Scholar]
- Ridlon J.M., Kang D.J., Hylemon P.B., Bajaj J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Turner J.R. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:577–582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- Sivanantham A., Pattarayan D., Bethunaickan R., Kar A., Mahapatra S.K., Thimmulappa R.K., Palanichamy R., Rajasekaran S. Tannic acid protects against experimental acute lung injury through downregulation of TLR4 and MAPK. J. Cell Physiol. 2019;234:6463–6476. doi: 10.1002/jcp.27383. [DOI] [PubMed] [Google Scholar]
- Sorbara M.T., Littmann E.R., Fontana E., Moody T.U., Kohout C.E., Gjonbalaj M., Eaton V., Seok R., Leiner I.M., Pamer E.G. Functional and genomic variation between human-derived isolates of lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe. 2020;28:134–146. doi: 10.1016/j.chom.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic K., Krstulovic L., Brozic D., Mauric M., Stojevic Z., Mikulec Z., Bajic M., Masek T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015;95:1172–1178. doi: 10.1002/jsfa.6805. [DOI] [PubMed] [Google Scholar]
- Surai P.F. Polyphenol compounds in the chicken/animal diet: from the past to the future. J. Anim. Physiol. Anim. Nutr. 2014;98:19–31. doi: 10.1111/jpn.12070. [DOI] [PubMed] [Google Scholar]
- Tonda R.M., Rubach J.K., Lumpkins B.S., Mathis G.F., Poss M.J. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poult. Sci. 2018;97:3031–3042. doi: 10.3382/ps/pey158. [DOI] [PubMed] [Google Scholar]
- Tong Y., Lin Y., Di B., Yang G., He J., Wang C., Guo P. Effect of hydrolyzed gallotannin on growth performance, immune function, and antioxidant capacity of yellow-feather broilers. Animals (Basel) 2022;12:2971. doi: 10.3390/ani12212971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y., Wu S., Li S., Zeng X., Guo S., Guo P., Wang C. Effect of hydrolyzed gallotannin on growth performance, immune and antioxidant capacity of yellow-feather broilers from 1 to 21 d of age. Chin. J. Anim. Sci. 2022;58:251–255. doi: 10.3390/ani12212971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K., Matsui T., Nakayama M., Furuse K., Sasaki H., Furuse M., Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- Van Itallie C.M., Anderson J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014;36:157–165. doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduin P.A., Punt J.M., Kreutzer H.H. Studies on the determination of lipase activity. Clin. Chim. Acta. 1973;46:11–19. doi: 10.1016/0009-8981(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Wang M., Huang H., Liu S., Zhuang Y., Yang H., Li Y., Chen S., Wang L., Yin L., Yao Y., He S. Tannic acid modulates intestinal barrier functions associated with intestinal morphology, antioxidative activity, and intestinal tight junction in a diquat-induced mouse model. RSC Adv. 2019;9:31988–31998. doi: 10.1039/c9ra04943f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Phillipson J.D., Greengrass P.M., Bowery N.E., Cai Y. Plant polyphenols: biologically active compounds or non-selective binders to protein? Phytochemistry. 1997;44:441–447. doi: 10.1016/s0031-9422(96)00598-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.