Abstract
The objective of the current study was to ascertain the effect of using ginger powder or frankincense oil at different levels on the production rate, biochemical properties of blood, and immune response of laying Japanese quail housed from 12 to 21 wk of age. Three hundred sexually mature quail (200 females and 100 males) were distributed among 5 groups at 12 wk of age in a completely randomized design. Group 1: received basal diet not including additives and acted as a control group. Groups 2 and 3: received a basal diet with 250 and 500 mg ginger/kg feed, respectively. Groups 4 and 5: received a basal diet with 200 and 400 mg frankincense oil/kg feed, respectively. Results revealed that egg production parameters of laying Japanese quail were not influenced by ginger or frankincense oil added in all groups tested during experimental periods except at the time (15–18 wks.). Moreover, egg weight was significantly increased only during the period (15–18 wks.) and total period (12–21 wk of age), and group 5 recorded the highest weight during the 2 periods studied. Feed additives did not impact feed consumption or feed conversion ratio (FCR). Except for WBCs, Hb, and monocytes, treatments impacted blood hematological parameters. Also, blood serum parameters were influenced by feed additives, except total protein, albumin, globulin, and creatinine were not influenced. Moreover, histological examination of the spleen was influenced by feed additives. It is concluded that utilizing frankincense oil or ginger powder in diets of laying Japanese quails enhanced blood serum properties and improved reproductive and productive performance.
Key words: ginger powder, frankincense oil, Japanese quail, production performance, antioxidant activity
INTRODUCTION
Livestock production aims to produce a high-quality product at a low price. In addition to the increasing demand for domestic animal meat, continuous, active, and targeted health care is required to prevent the development of diseases. The poultry industry is under increasing pressure to produce high-quality products and high consumer volumes. Antibiotics have been utilized worldwide as antibacterial, growth-promoting feed additives to control and prevent disease-causing bacteria in the intestinal mucosa to drive meat and egg production (Abdelnour et al., 2019, 2020; Abd El-Hack et al., 2020, 2022; Attia et al., 2020; Swelum et al., 2021). However, leftovers from the overuse of antibiotics in chicken production have become unfavorable (Chandrakar et al., 2023) and the spread of antibiotic-resistant microorganisms in humans (Shahin et al., 2002). In January 2006, the European Union prohibited using antibiotics as growth enhancers (Eckert et al., 2010). Grassy excerpts are herbal-obtained materials blended with animal feed to enhance growing production and output quality (Emwas et al., 2019, 2020, 2021, 2023; Chandra et al., 2021a,b). They have been categorized with a remark about their origins and vital basics: extracts, seasoning, main oils (lipophilic compounds obtained from the vapor distillation of herbs), and olive (complexes gotten from nonhydrolyzed solvents) (Windisch et al., 2007).
Ginger roots (Zingiber officinale) have been utilized for flavoring or flavor-enhancing treatments. Increased poultry production, enhanced mash flavor and palatability, increased nutrient intake, and advanced stomach enzyme outflow are all benefits of using ginger instead of an antibiotic growth-promoting agent (Kothari et al., 2019). Every antibiotic or fully artificial material applied like a feed additive in the domestic animal ration is under debate for its possible toxic influence. In addition, there is a forward way for products of natural origin to decrease the utilization of synthetic materials (Zhang et al., 2009). As a result, medicinal grass or its derivatives are considered safe and offer a workable option for addressing client wants and managing marker competitions (Ali et al., 2008; Onu, 2010).
Shirin and Jamuna (2010) reported that ginger is remarkable as it is wealthy in nutrients like amino acids, minerals, fatty acids and vitamins. Also, ginger includes a lot of bioactive composites, such as terpenes and phenolic materials. Most phenolic chemicals are gingerol, gingerdione, gingerdiol, shogaol, and paradol (Zhao et al., 2011; Stoner 2013, Liu et al., 2019), which are strong intestinal mucous membrane animators (Dieumou et al., 2009). Also, ginger has shown several bioactive activities, such as antioxidants (Nile and Park, 2015), and helpful impacts on health by reducing free radical harm and rising cardiovascular cases (Verma et al., 2004; El-Kashif, 2022).
This study aimed to estimate the effects of natural feed additives (ginger or frankincense oil) as a phytogenic additive and naturalistic antioxidant on productive performance, serum metabolites and antioxidant activity of Japanese quail.
MATERIALS AND METHODS
Experimental Design and Diets
This experiment was conducted at the Poultry Research Laboratory, part of the Faculty of Agriculture (Saba Basha) at Alexandria University. After 2 wk of observation, 300 Japanese quail birds (200 females and 100 males) were chosen and constructed on an egg production average of greater than 70%. They were then randomly assigned to 5 experimental groups, each containing 60 birds (40 hens and 20 males), and each was divided into 5 replicates with 12 birds (8 hens and 4 males). Quails were housed in wire-laying pens (2 hens and 1 male) from the open house system for the duration of the experiment, which begging at 12 wk of age and finished at 21 wk of age.
Isocaloric and isonitrogenous rations were formulated in Table 1. Renewed feed was only kept for a week at a time and was blended every week. For the 9-wk experiment, both feed and water were ad libitum available. Throughout the laying term, all laying hens were exposed to 16 h of constant light every day. All experimental birds were raised in the same environmental, sanitary, and administrative environments.
Table 1.
structure and analysis of the main experimental ration.
| Ingredients | % |
|---|---|
| Yellow corn | 60.50 |
| Soybean meal (44%) | 22.60 |
| Concentrate (50%) 1 | 10.00 |
| Di-calcium phosphate | 0.40 |
| Limestone | 5.50 |
| Sunflower oil | 1.00 |
| Total | 100 |
| Calculated analyses2: | |
| Crude protein, % | 20.08 |
| ME (Kcal/ Kg diet) | 2,939.89 |
| Ether extract, % | 2.60 |
| Crude fiber, % | 3.04 |
| Methionine, % | 0.71 |
| Methionine + cystine, % | 0.90 |
| Lysine, % | 1.15 |
| Calcium, % | 2.58 |
| Av. phosphorus | 0.40 |
Concentrate: ME (K cal/kg) 2,870, crude protein 50%, crude fiber 1.51%, crude fat 1.54%, calcium 4.29%, phosphorus 2.39%, NaCl 0.8%, methionine 4.6%, methionine and cystine 5.38%, lysine 3.90%.
According to NRC (1994).
The experimental groups were: Group1) basal diet with no supplement and acted as a control group, Group 2) basal diet with 250 mg ginger/Kg of diet, Group 3) basal diet with 500 mg ginger/Kg diet, Group 4) basal diet with 200 mg frankincense oil/Kg of diet and group 5) basal diet with 400 mg frankincense oil/Kg of ration.
Egg Production Traits
The egg number was recorded daily and the laying rate was calculated for each pen within each experimental group during the experimental period. Also, eggs were individually weighed to the nearest gram during the experimental period. The egg mass of each experimental group was calculated throughout the experiment. Every pen's Feed intake and conversion ratio (FCR) were recorded weekly during the experimental time.
Blood Sampling
Six chickens fasting from every treatment were randomly chosen and slaughtered after the trial. Before being slaughtered, a flank vein sample containing about 3 mL of blood was taken. Two equal portions of collected blood samples were split. To define the hematological parameter, the first fraction was gathered using heparin as an anticoagulant (0.1 mL of heparin to 1 mL of blood). The serum was separated from the second fraction by centrifuging for 15 min at 4,000 rpm while coagulating. The serum was recovered and then frozen at -18°C for examination.
All biochemical tests (including those for total protein, alkaline phosphatase, albumin, aspartate aminotransferase, and alanine aminotransferase, total lipids, total cholesterol, low-density lipoproteins [LDL], high-density lipoproteins [HDL], triglycerides, creatinine, and uric acid) were performed. Malondialdehyde (MDA) and total antioxidant capacity peroxidase measurements were made utilizing industrial kits from Bio-Diagnostics, Egypt (www.bio-diagnostic.com); serum globulin levels were calculated by deducting albumin values from total protein values; and phagocytic activity (PA) was assessed following Kawahara et al. (1991). One milliliter of cetranic blood taken from treated and control quails were mixed in a water bath heated to between 23 and 25 degrees Celsius with 50 micrograms of Candida albicans (formerly modified to 1 g of Candida albicans per 100 mL of saline solution). After 3 to 5 h, blood smears were stained with Giemsa solution, and the percentage of macrophages that contained intracellular macrophages was used to determine the amount of phagocytosis as a proportion of PA, per (PA). Using the phagocytosis index (PI), the numeral of phagocytic organisms in phagocytic cells was calculated and categorized (PI) (Nurhayati et al., 2022). Results were given as means ± Se. Differences were evaluated using the Student t test (Mishra et al., 2019).
Phagocytic activity (PA) = Percentage of phagocytic cells containing yeast cells.
As previously illustrated, the lymphocytic transformation test (LTT) was carried out with a few minor modifications (Bosisio, 1992). In brief, as previously mentioned, 6 hens from each group were divided into 2 groups at 21 wk. The PBMCs were then gathered in RPMI-1640 growth media (not including phenol red, with NaHCO3, Sigma, Cairo, Egypt) to which 0.25 g/100 mL HEPES, 100 IU/mL of benzyl penicillin, and 100 g/mL of streptomycin sulphate were inserted. Trypan blue dye exclusion was utilized to ascertain the viability of the cells. The cells were then modified to have 5 × 106 viable cells per milliliter and plated in 96-well flat-bottomed tissue culture plates (Nunclon, Denmark). In duplicate wells, along with unstimulated control wells, cells were encouraged (10 g/mL) with either Con-A (Sigma-Aldrich, St. Louis, MO) or CIAV antigen (whole pure CIAV-A inactivated virus) dissolved in RPMI. At 5% CO2 tension, the plates were incubated at 37°C in a humid environment. All of the wells were filled with 20 l (5 mg/mL) of MTT (3-[4, 5-Dimethyl thiazolyl]-2, 5-diphenyl tetrazolium bromide) solution after 72 h, which was added and incubated for another 4 h. The plate was then centrifuged at 1,200 rpm for 15 min. All of the wells' supernatant was delicately removed. DMSO (150 l/well) removed the MTT formazan from the cells. At 510 nm for the test and 650 nm for the reference, the wells' optical densities (OD) were measured. Divide the OD value of stimulated by the OD of un-stimulated to get the stimulation index (SI).
Histological Examination
Each bird's abdominal cavity was opened after it was slaughtered, and its livers and spleens were each given a macroscopic and histological examination. The histology slide was prepared using the method listed below. Chicken tissue was taken and set in 10% formalin for a full day. Next, it was successfully dehydrated in rising ethanol concentrations, cleaned in xylene, and finally saturated in paraffin (Khenenou et al., 2017). The blocks were sliced with a microtome to a thickness of 2 μm. Sections were put into a floating bath that was 37 degrees Celsius. After that, they were adhered to the slides and heated to dry. The sections were stained using the hematoxylin and eosin procedure (Khenenou et al., 2013). Under a microscope, the microscopic investigation was clarified and examined.
Statistical Analyses
The gathered data were statistically estimated with Duncan's Multiple Range Test (Duncan, 1955) and the 1-way ANOVA procedure of SPSS (Windows Version of SPSS, release 16) to determine the significant changes among the means.
The model used was:
Where: Yij = An observation treatment.
μ = Overall mean.
Ti = the i-treatment effect (I =1—5).
e ij = The random error.
RESULTS
Production Performance
In all groups over the investigated experimental periods, except the period (15–18 wk), the addition of ginger or frankincense oil had no impact on the egg-laying rate (%) of laying quail hens (Table 2). The number of eggs was only significantly (P < 0.05) impacted during that time (15–18 wk). When the hens were 15 to 18 wk old, it was apparent that group 4 had a better EN (P < 0.05) (0.8 chickens/d) than the other groups (Table 2). Also, the period (15–18 wk) and the entire time were the only ones where the weight of the egg was significantly impacted (P < 0.05). Comparing the group 5 to the other groups, it was found that they had the largest (P < 0.05) egg weights (15.23 and 14.71 g, respectively) during 2 time periods (15–18 and 12–21 wk of age) (Table 2). Only during the interval was egg mass considerably (P < 0.05) influenced (15–18 wk). According to the study, group 4 had a higher EM (11.47 g/hen/d) at 15 to 18 wk of age than other groups (P < 0.05). Table 2 presented that the addition of ginger or frankincense oil did not affect feed intake or FCR in all treatments during all studies of the experimental periods.
Table 2.
Effect of ginger and frankincense oil on production performance of Japanese quail laying hens from 12 to 21 wk of age.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Ginger powder |
Frankincense oil |
|||||
| Items | Control | 0.250 mg | 0.500 mg | 0.200 mg | 0.400 mg | P-value |
| Egg laying rate (%) | ||||||
| W12–W15 | 79.89 ± 0.70 | 78.57 ± 2.55 | 76.46 ± 3.70 | 79.89 ± 2.69 | 76.46 ± 2.61 | 0.784 |
| W15–W18 | 76.99 ± 1.65ab | 74.34 ± 1.91b | 76.98 ± 0.46ab | 79.89 ± 0.70a | 73.81 ± 2.29b | 0.017 |
| W18–W21 | 74.07 ± 5.54 | 75.13 ± 2.07 | 77.25 ± 0.95 | 75.13 ± 1.73 | 71.16 ± 0.70 | 0.656 |
| W12–W21 | 76.98 ± 1.98 | 76.01 ± 0.71 | 76.90 ± 1.02 | 78.31 ± 1.15 | 73.81 ± 1.80 | 0.309 |
| Egg number (hen/ d) | ||||||
| W12–W15 | 0.80 ± 0.01 | 0.79 ± 0.02 | 0.76 ± 0.04 | 0.80 ± 0.03 | 0.76 ± 0.03 | 0.726 |
| W15–W18 | 0.77 ± 0.02ab | 0.74 ± 0.02b | 0.77 ± 0.01ab | 0.80 ± 0.01a | 0.74 ± 0.02b | 0.012 |
| W18–W21 | 0.74 ± 0.05 | 0.75 ± 0.02 | 0.77 ± 0.01 | 0.75 ± 0.02 | 0.71 ± 0.01 | 0.635 |
| W12–W21 | 0.77 ± 0.02 | 0.76 ± 0.01 | 0.77 ± 0.01 | 0.78 ± 0.01 | 0.74 ± 0.02 | 0.291 |
| Average egg weight (g) | ||||||
| W12–W15 | 13.44 ± 0.37 | 13.53 ± 0.50 | 13.16 ± 0.34 | 13.44 ± 0.25 | 13.93 ± 0.21 | 0.660 |
| W15–W18 | 13.71 ± 0.38b | 13.95 ± 0.12b | 14.27 ± 0.52ab | 14.37 ± 0.37ab | 15.23 ± 0.33a | 0.019 |
| W18–W21 | 13.72 ± 0.33 | 13.79 ± 0.09 | 13.99 ± 0.48 | 14.59 ± 0.55 | 15.05 ± 0.86 | 0.375 |
| W12–W21 | 13.62 ± 0.35b | 13.76 ± 0.23b | 13.81 ± 0.23b | 14.11 ± 0.24ab | 14.71 ± 0.29a | 0.014 |
| Egg mass (g/hen/d) | ||||||
| W12–W15 | 10.75 ± 0.38 | 10.64 ± 0.69 | 10.05 ± 0.45 | 10.72 ± 0.17 | 10.63 ± 0.20 | 0.763 |
| W15–W18 | 10.55 ± 0.20ab | 10.37 ± 0.24b | 10.98 ± 0.34ab | 11.47 ± 0.20a | 11.24 ± 0.41ab | 0.018 |
| W18–W21 | 10.13 ± 0.51 | 10.37 ± 0.31 | 10.82 ± 0.48 | 10.96 ± 0.37 | 10.72 ± 0.71 | 0.755 |
| W12–W21 | 10.47 ± 0.02 | 10.46 ± 0.20 | 10.62 ± 0.21 | 11.05 ± 0.22 | 10.87 ± 0.44 | 0.439 |
| Feed consumption (g/hen/d) | ||||||
| W12–W15 | 31.95 ± 1.18 | 31.88 ± 0.77 | 31.05 ± 0.37 | 32.84 ± 0.49 | 31.27 ± 0.56 | 0.495 |
| W15–W18 | 32.22 ± 0.09 | 32.52 ± 0.39 | 32.35 ± 0.67 | 32.29 ± 0.28 | 32.44 ± 0.62 | 0.991 |
| W18–W21 | 32.08 ± 0.80 | 32.21 ± 0.40 | 31.70 ± 0.30 | 32.63 ± 0.27 | 31.86 ± 0.86 | 0.820 |
| W12–W21 | 32.09 ± 0.30 | 32.20 ± 0.51 | 31.70 ± 0.22 | 32.58 ± 0.32 | 31.86 ± 0.63 | 0.643 |
| Feed conversion ratio (g feed/g egg) | ||||||
| W12–W15 | 2.98 ± 0.09 | 3.03 ± 0.24 | 3.10 ± 0.11 | 3.06 ± 0.08 | 2.94 ± 0.11 | 0.932 |
| W15–W18 | 3.06 ± 0.06 | 3.14 ± 0.08 | 2.96 ± 0.14 | 2.82 ± 0.07 | 2.89 ± 0.15 | 0.294 |
| W18–W21 | 3.17 ± 0.08 | 3.11 ± 0.08 | 2.94 ± 0.13 | 2.99 ± 0.11 | 3.01 ± 0.29 | 0.839 |
| W12–W21 | 3.07 ± 0.03 | 3.08 ± 0.10 | 2.99 ± 0.08 | 2.95 ± 0.09 | 2.95 ± 0.18 | 0.841 |
Different superscripts within a row indicate significant differences (P < 0.05).
Blood Indices
Blood Hematological Parameters
The data in Table 3 showed that red blood cell and packed cell volume levels were significantly lower in the control diet than in the remaining groups. Group 4 had the highest levels of red blood cells and packed cell volume (Table 3). On the other hand, white blood cell and hemoglobin levels were not significantly impacted (Table 3). Moreover, the data in Table 3 reported that all differential leukocytes of layer Japanese quail chickens studied were affected using different levels of ginger or frankincense oil except for monocyte cells. Groups 3 and 4 had the highest heterophil levels (P = 0.001) (Table 3). The results in Table 4 showed that the blood parameters studied (PA, PI%, and LTT) were influenced by the addition of various levels of ginger or frankincense oil. The pest values of hematological blood parameters tested were recorded in groups 4, 2, and 5 for PA, PI%, and LTT, respectively.
Table 3.
Effect of ginger and frankincense oil on hematological parameters of Japanese quail laying hens at 21 wk of age.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Ginger powder |
Frankincense oil |
|||||
| Items | Control | 0.250 mg | 0.500 mg | 0.200 mg | 0.400 mg | P-value |
| WBCs (103/mm3) | 19.90 ± 0.79 | 23.72 ± 2.00 | 24.60 ± 2.06 | 24.14 ± 2.18 | 24.28 ± 2.17 | 0.417 |
| RBCs (millions/mm3) | 1.52 ± 0.19b | 2.34 ± 0.18a | 2.40 ± 0.13a | 2.55 ± 0.22a | 2.37 ± 0.04a | 0.002 |
| Hb (g/dL) | 8.44 ± 0.66 | 10.08 ± 0.73 | 10.38 ± 0.68 | 10.68 ± 1.22 | 10.12 ± 0.12 | 0.305 |
| PVC (%) | 24.24 ± 1.19c | 33.58 ± 1.15ab | 32.46 ± 3.13b | 33.22 ± 2.12ab | 38.64 ± 0.23a | 0.001 |
| Lymphocytes | 44.60 ± 1.29a | 37.20 ± 1.62b | 35.40 ± 1.29b | 36.80 ± 1.11b | 37.00 ± 0.45b | 0.001 |
| Heterophils | 49.60 ± 1.29b | 56.60 ± 1.57a | 58.00 ± 1.14a | 57.00 ± 1.30a | 57.00 ± 0.45a | 0.001 |
| H/L ratio | 1.12 ± 0.06b | 1.54 ± 0.11a | 1.65 ± 0.09a | 1.56 ± 0.08a | 1.54 ± 0.03a | 0.001 |
| Monocytes | 5.20 ± 0.20 | 6.00 ± 0.32 | 5.80 ± 0.20 | 5.80 ± 0.20 | 5.60 ± 0.24 | 0.203 |
Different superscripts within a row indicate significant differences (P < 0.05).
Table 4.
Effect of ginger and frankincense oil on phagocytic activity (PA) phagocytic index (PI %) and lymphocytes transformation test (LTT) of Japanese quail lying hens at 21 wk of age.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Ginger powder |
Frankincense oil |
|||||
| Items | Control | 0.250 mg | 0.500 mg | 0.200 mg | 0.400 mg | P-value |
| PA | 16.40 ± 0.81b | 17.40 ± 0.40b | 21.00 ± 0.71a | 21.40 ± 0.40a | 20.80 ± 0.58a | 0.001 |
| PI (%) | 1.26 ± 0.08c | 1.74 ± 0.16a | 1.74 ± 0.09a | 1.68 ± 0.06ab | 1.42 ± 0.07bc | 0.006 |
| LTT | 19.80 ± 0.80b | 21.60 ± 0.75ab | 21.20 ± 0.58ab | 21.00 ± 0.55ab | 22.80 ± 0.58a | 0.051 |
Different superscripts within a row indicate significant differences (P < 0.05).
Blood Serum Properties
The studied serum parameters total protein, albumin, globulin, alkaline phosphates, total lipids, cholesterol, LDL, HDL, triglycerides, creatinine, uric acid, GOT and GPT were not impacted using different levels of ginger or frankincense oil except for alkaline phosphate (Table 5). Compared to other treatments, a lower alkaline phosphate level was observed in group 5 (frankincense oil 0.40 mg/kg diet).
Table 5.
Effect of ginger and frankincense oil on blood biochemical parameters of Japanese quail laying hens at 21 wk of age.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Ginger powder |
Frankincense oil |
|||||
| Items | Control | 0.250 mg | 0.500 mg | 0.200 mg | 0.400 mg | P-value |
| Total protein (g/dL) | 3.36 ± 0.11 | 3.54 ± 0.26 | 3.93 ± 0.03 | 3.68 ± 0.27 | 3.61 ± 0.29 | 0.490 |
| Albumin (g/dL) | 2.10 ± 0.12 | 2.05 ± 0.10 | 2.37 ± 0.07 | 2.10 ± 0.11 | 2.02 ± 0.08 | 0.167 |
| Globulin (g/dL) | 1.25 ± 0.09 | 1.49 ± 0.17 | 1.56 ± 0.05 | 1.58 ± 0.17 | 1.59 ± 0.23 | 0.543 |
| Alkaline phosphates (IU/L) | 32.67 ± 1.45a | 21.33 ± 0.33b | 21.33 ± 0.88b | 21.67 ± 0.33b | 20.67 ± 0.33b | 0.001 |
| Total lipids (mg/dL) | 286.33 ± 5.93a | 283.00 ± 1.14ab | 281.00 ± 4.51ab | 264.33 ± 2.67bc | 256.33 ± 0.33c | 0.023 |
| Cholesterol (mg/dL) | 115.00 ± 0.52a | 108.67 ± 1.33abc | 110.33 ± 0.33ab | 101.00 ± 0.58bc | 99.40 ± 0.83c | 0.024 |
| LDL (mg/dL) | 78.67 ± 1.93ab | 75.67 ± 1.45ab | 83.67 ± 0.33a | 70.33 ± 1.76b | 69.67 ± 0.88b | 0.031 |
| HDL (mg/dL) | 17.00 ± 0.58b | 24.67 ± 0.88a | 22.00 ± 1.53a | 23.00 ± 1.53a | 23.00 ± 1.53a | 0.015 |
| Triglycerides (mg/dL) | 50.00 ± 1.15a | 40.67 ± 1.76b | 32.00 ± 1.53d | 37.33 ± 1.76bc | 32.93 ± 1.44cd | 0.001 |
| Creatinine (mg/dL) | 0.37 ± 0.03 | 0.40 ± 0.12 | 0.50 ± 0.06 | 0.37 ± 0.03 | 0.43 ± 0.12 | 0.759 |
| Uric acid (mg/dL) | 4.67 ± 0.09a | 4.07 ± 0.03b | 4.10 ± 0.06b | 4.07 ± 0.03b | 4.10 ± 0.06b | 0.001 |
| GOT (U/L) | 18.33 ± 1.20b | 23.00 ± 0.58ab | 21.00 ± 1.53b | 28.67 ± 1.67a | 24.00 ± 1.53ab | 0.042 |
| GPT (U/L) | 12.83 ± 0.84b | 16.10 ± 0.40ab | 14.70 ± 1.07b | 20.07 ± 2.57a | 16.80 ± 1.07ab | 0.042 |
Different superscripts within a row indicate significant differences (P < 0.05).
The results presented in Table 5 presented that the tested serum lipids were substantially affected by using different diets, including the tested feed additives. The values of all studied serum lipid traits were improved using the feed additives during the experiment as the serum lipid values except for high-density lipoprotein (group 2, Ginger 0.25 mg/kg) were reduced using the additives in the present trait compared to the different groups.
Table 5 displays the impact of ginger and frankincense oil on the levels of creatinine (mg/dL), uric acid (mg/d), glutamic-oxaloacetic transaminase (GOT, U/L), and Glutamic-pyruvic transaminase (GPT, U/L) in the serum of Japanese laying hens at age 21 wk. The treated birds had lower uric acid levels. The uric acid levels in group 2 and group 4 were the lowest (P = 0.001). Moreover, the therapy group experienced changes in sGPT and sGOT. Group 4 was when serum GPT and GOT were found to be at their maximum levels (28.67 and 20.07, respectively). However, dietary feed additive supplements did not impact creatinine levels.
The results presented in Table 6 show all antioxidant properties. The highest values for total antioxidant capacity (mmol/L) and serum glutathione peroxidase (mmU/mL) were recorded in group 5 (frankincense oil 0.40/kg feed). On the other hand, blood MDA values were significantly reduced by using the tested feed additives. A lower value was observed in group 4 (frankincense oil 0.20 mg/kg feed) compared to the other treatments.
Table 6.
Effect of ginger and frankincense oil on serum total antioxidant capacity, serum glutathione peroxidase and serum malondialdehyde of Japanese quail lying hens at 21 wk of age.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Ginger powder |
Frankincense oil |
|||||
| Items | Control | 0.250 mg | 0.500 mg | 0.200 mg | 0.400 mg | P-value |
| Total antioxidant capacity (mM/L) | 0.66 ± 0.01b | 0.86 ± 0.07a | 0.91 ± 0.08a | 0.91 ± 0.02a | 0.92 ± 0.04a | 0.017 |
| Glutathione peroxidase (mU/mL) | 33.69 ± 0.98d | 39.00 ± 0.30c | 41.71 ± 0.79b | 45.07 ± 1.19a | 47.38 ± 0.52a | 0.001 |
| Malondialdehyde (nmol/mL) | 8.62 ± 0.96a | 8.05 ± 0.35a | 5.63 ± 0.58b | 5.13 ± 0.36b | 5.21 ± 0.35b | 0.003 |
Different superscripts within a row indicate significant differences (P < 0.05).
Histological Examination
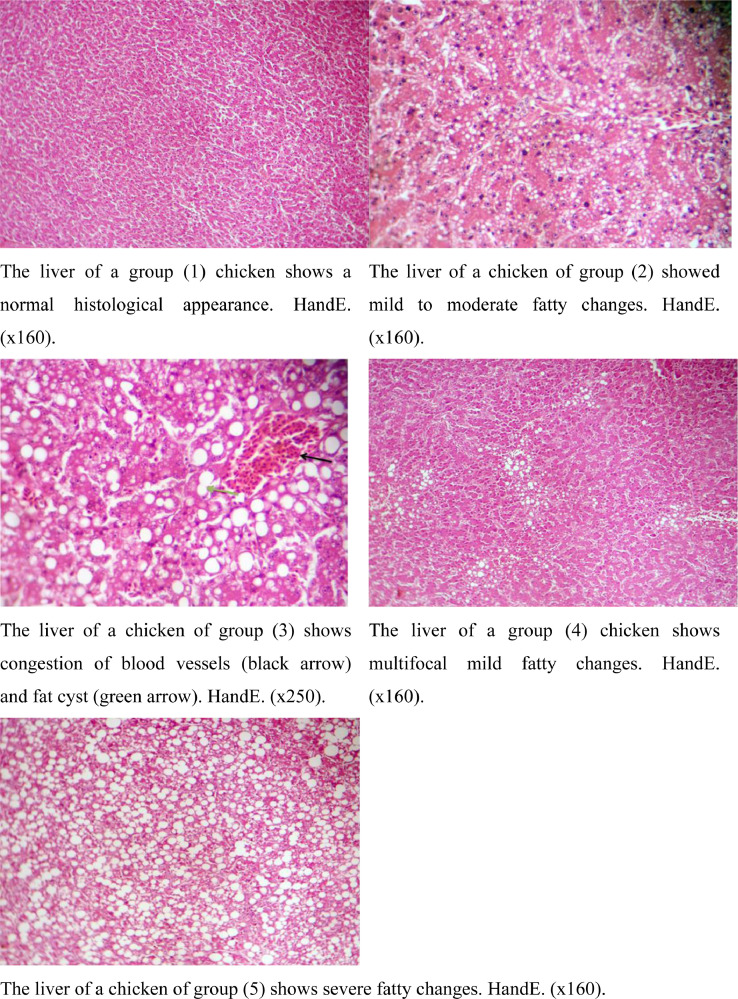
The histological evolution of the liver and spleen after 9 wk of treatments is illustrated in Figures 1 and 2. Birds fed ginger powder and frankincense oil at all levels indicates that the histopathology examination of the spleen is normally compared to the control. In contrast, histopathology examination of the liver of birds fed ginger powder and frankincense oil at all levels indicates that there was congestion of blood vessels, moderate to severe fatty changes, multifocal mild fatty changes and fat cysts compared to the control diets.
Figure 1.
Histological examination of the spleen after 9 wk from treatments (at 21 wk of age).
Figure 2.
Histological examination of the liver after 9 wk from treatments (at 21 wk of age).
DISCUSSION
Production Performance
The results presented in Table 2 showed the impacts of using ginger roots and different grades of frankincense oil on the number, weight, and mass of eggs laid by laying quail birds. The results showed that adding ginger and frankincense oil to the diets of laying quail birds improved various periods of trial egg production characteristics. Our results are partly in line with those of El-Kashif and Roshdy (2022), who found that egg production and number were considerably (P ≤ 0.05) greater in treatments receiving feed having various grades of ginger root than in the control group. Along the same lines, egg weight and mass were considerably higher in the same treatments over 3 age phases and the entire reproductive period (P ≤ 0.05).
These findings concur with those of Abdel-Galil and Mahmoud (2015), who detected a significant increase in the egg weight, mass and number after ginger was added to quail feed at rates of 0.25, 0.5, and 0.75%. Also, Kumar et al. (2019) also established the same findings when they examined the impact of ginger root powder supplements on leghorn coats at 0.5 and 1%. Moreover, Zomrawi et al. (2014) and Malekizadeh et al. (2012) revealed a reduction in egg weight due to giving ginger root powder at various grades. Herve et al. (2019) discovered that main ginger oil enhanced quail egg weight, like Núñez-Torres et al. (2021), who found an enhancement in egg weight after utilizing 0.2, 0.4, and 0.6% ginger flour on laying quails. Wen et al. (2019) discovered that birds administered ginger extract significantly increased egg mass and weight. According to Akbarian et al. (2014), a quails-fed diet with 0.25, 0.5, and 0.75% ginger produced eggs of a higher quality.
Sittiya et al. (2017) indicated a nonsignificant reduction in egg production because of supplementation with dry ginger fermented at 500 ppm. The cause for this advance is that ginger contains major nutrients similar to fatty acids, vitamins, amino acids and minerals, for instance, selenium, zinc, iron, magnesium, calcium, and vitamins C and E (Shirin and Jamuna, 2010). In addition, ginger too includes a lot of bioactive materials such as phenols and terpenes. Phenolic composites have a variety of physiological effects, including antioxidant (Nile and Park, 2015), antianxiety (Vishwakarma et al., 2002), anti-inflammatory (Zhang et al., 2016), antidiabetic (El-Amin et al., 2006), and beneficial impacts for health by decreasing free radical destruction and developing cardiovascular case (Verma et al., 2004), anticancer (Citronberg et al., 2013). Wang and Wang (2005) reported ginger also treats upper respiratory infections. It also significantly improves fertility (Grzanna et al., 2005; Manallah, 2012).
Blood Parameters
Results in Table 5 revealed that numerous blood characteristics improved in the diets containing various amounts of ginger and frankincense oil compared to the control group. According to the current study's findings, the total protein, albumin, and globulin levels were insignificant by the diets evaluated. When ginger essential oil was used in laying Japanese quail at rates of 50, 100, and 150 mL/kg, Herve et al. (2019) noticed a significant rise in total protein, albumin, and globulin. El-Kashef (2022), who documented ginger usage in male quails' diets by 0.25, 0.50, and 0.75%, observed the same trend.
Also, using ginger reduced serum LDL and total cholesterol levels while raising HDL levels across the board compared to the control group. Our findings concur with Salmanzadeh (2015), Zewail et al. (2016), Swain et al. (2017), Herve et al. (2018), Herve et al. (2019), Asghar et al. (2021), El-Kashif and Roshdy (2022) all described that ginger significantly reduced total cholesterol, HDL, and LDL. Due to ginger's high amount of unsaturated fatty acids, which might increase intestinal cholesterol release, plasma cholesterol levels may have decreased. Moreover, triglyceride and cholesterol levels responded well to ginger's powerful antilipidemia effects (Jang et al., 2007). Consequently, it may prevent cholesterol synthesis using substances like hydroxymethylglutaryl coenzyme A as a mechanism of action (Saeid et al., 2010), to prevent the creation of cholesterol specifically in the liver (Manju et al., 2006).
Moreover, Herve et al. (2018) verified that treated quail had lower MDA serum levels, transaminases, total cholesterol and triglycerides. In exposed birds used as controls, serum levels of total protein, globulin, and antioxidant enzymes were higher. Additionally, Herve et al. (2019) obtained that oral administration of 100 or 150 mL/kg body weight of ginger root extracted essential oil for laying Japanese quail resulted in significantly lower cholesterol levels (serum or egg) without any negative consequences. They also observed that the characteristics of serum total cholesterol and LDL were significantly reduced through 100 or 150 mL/kg body weight of ginger root extract essential oil compared to the control group. According to An et al. (2019), only the adjusted plasma SOD merit, which lowers the MDA rate of birds, was affected by ginger extract, which did not affect the activity of GSH-PX and TAOC. Moreover, ginger extract only favorably increased the lysozyme (LZM) act and did not change total protein levels, albumin, or serum globulin. Moreover, Ghasemi and Taherpour (2015) noted that mannan-oligosaccharide added to diet and ginger main oil (100 mg/kg) reduced cholesterol readings.
Histological Examination
Results showed that when compared to the control group, the birds administered ginger powder and frankincense oil at all levels exhibited improved spleen histology. These results are consistent with those made by Daudu and Mohammed (2020), who found that adding ginger by-product meal that had been treated with an exogenous enzyme to broiler feed, enhanced the histology of the chickens' spleens. Moreover, Amer et al. (2023) noted that feeding frankincense oil to broiler chicks significantly increased the immunoexpression of the CD3 and CD20 genes in the spleen. By activating B- and T-lymphocytes and producing IgG and IgM antibodies, frankincense oil supplements help the body fight off bacterial and viral infections (Al-Yasiry and Kiczorowska, 2016). Without a doubt, the bioactive component profile of frankincense oil contributes to its immunomodulatory effects. Frankincense oil contains a bioactive substance called farnesol that possesses antibiofilm, fungicidal, antitumor, and anticancer effects (Wang et al., 2020). According to Sachivkina et al. (2022), farnesol boosted resistance to yeast-like fungi, indicating its antibacterial properties (Zawrotniak et al., 2019). Moreover, the manufacturing of prostaglandin, lipoxygenase, and leukotriene is inhibited by sesquiterpene components such as α-farnesene, which modulates the anti-inflammatory response (Miguel, 2010).
Ginger is an anti-inflammatory that boosts the spleen's and immune systems' function. Ginger can be stated that antioxidants like ginger inhibit the creation of free radicals because of the oxidation of fatty acids, causation a final enhancement in the purpose of the humoral system. This is because ginger comprises antioxidants, which are thought to protect the fatty acids of the humoral immune system (Taghdisi and Hejazi, 2019).
Contrarily, compared to the control diets, a closer look at the histology of the liver of the ginger powder and frankincense oil supplementation shows that there was blood vessel congestion, moderate to severe fatty changes, multifocal mild fatty changes, and fat cysts. From these outcomes, it might be concluded that adding ginger powder and frankincense oil to quail diets may harm the liver and should be used cautiously. These results imply that excess consumption of ginger may be toxic to the liver. These outcomes are harmonious with those made by (Nwaopara et al., 2007; Udo-Affah et al., 2014), who found that consuming too much ginger could lead to hepatic necrosis.
CONCLUSIONS
This study demonstrated that feeding ginger powder at level 500 mg/kg diet or frankincense oil at level 200 mg/ diet as natural feed additives to Japanese quail laying hens improved their productive and reproductive performance and progressed most studied blood serum properties compared to control birds.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large group Research Project under grant number RGP2/144/44.
Author contributions: All authors contributed equally to this work. All authors read and approved the final version of this manuscript.
DISCLOSURES
All authors have no conflicts of interest to disclose.
REFERENCES
- Abd El-Galil K., Mahmoud H.A. Effect of ginger roots meal as feed additives in laying Japanese quail diets. J. Am. Sci. 2015;11:154–173. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Shaheen H., Samak D., Othman S.I., Allam A.A., Sitohy M. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals. 2020;10:452. doi: 10.3390/ani10030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour S.A., Abd El-Hack M.E., Alagawany M., Farag M.R., Elnesr S.S. Beneficial impacts of bee pollen in animal production, reproduction and health. J. Anim. Physiol. Anim. Nutr. 2019;103:477–484. doi: 10.1111/jpn.13049. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., Abd El-Hack M.E., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Italian J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Akbarian A., Golian A., Ahmadi A.S., Moravej H. Effects of ginger root (Zingiber officinale) on egg yolk cholesterol, antioxidant status and performance of laying hens. J. Appl. Ani. Res. 2014;39:19–21. [Google Scholar]
- Al-Amin Z.M., Thomson M., Al-Qattan K.K., Peltonen-Shalaby R., Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006;96:660–666. doi: 10.1079/bjn20061849. [DOI] [PubMed] [Google Scholar]
- Al-Yasiry A.R.M., Kiczorowska B. Frankincense–therapeutic properties. Adv. Hygiene Expert. Med. 2016;70:380–391. doi: 10.5604/17322693.1200553. [DOI] [PubMed] [Google Scholar]
- Ali B.H., Blunden G., Tanira M.O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem. Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Amer S.A., Gouda A., Saleh G.K., Nassar A.H., Abdel-Warith A.W.A., Younis E.M., Altohamy D.E., Kilany M.S., Davies S.J., Omar A.E. Dietary frankincense (Boswellia serrata) oil modulates the growth, intestinal morphology, the fatty acid composition of breast muscle, immune status, and immunoexpression of CD3 and CD20 in broiler chickens. Animals. 2023;13:971. doi: 10.3390/ani13060971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S., Liu G., Guo X., An Y., Wang R. Ginger extract enhances antioxidant ability and immunity of layers. Anim. Nutr. 2019;5:407–409. doi: 10.1016/j.aninu.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M.U., Rahmana A., Hayata Z., Rafique M.K., Badard I.H., Yara M.K., Ijaza M. Exploration of Zingiber officinale effects on growth performance, immunity and gut morphology in broilers. Brazilian J. Biol. 2021;83 doi: 10.1590/1519-6984.250296. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Alagawany M.M., Farag M.R., Alkhatib F.M., Khafaga A.F., Abdel-Moneim A.M.E., Abd El-Hack M.E. Phytogenic products and phytochemicals as a candidate strategy to improve tolerance to coronavirus. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.573159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosisio E. Effect of the flavanolignans of Silybum marianum L. On lipid peroxidation in rat liver microsomes and freshly isolated hepatocytes. Pharmacol. Res. 1992;25:147–165. doi: 10.1016/1043-6618(92)91383-r. [DOI] [PubMed] [Google Scholar]
- Chandra K., Al-Harthi S., Sukumaran S., Almulhim F., Emwas A.-H., Atreya H.S., Jaremko Ł., Jaremko M. NMR-based metabolomics with enhanced sensitivity. RSC Adv. 2021;11:8694–8700. doi: 10.1039/d1ra01103k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra K., Harthi S., Almulhim F., Emwas A.-H., Jaremko L., Jaremko M. The robust NMR toolbox for metabolomics. Mol. Omics. 2021;1:1–9. doi: 10.1039/d1mo00118c. [DOI] [PubMed] [Google Scholar]
- Chandrakar C., Shakya S., Patyal A., Bhonsle D., Pandey A.K. Detection of antibiotic residues in chicken meat from different agro-climatic zones of Chhattisgarh, India by HPLC-PDA and human exposure assessment and risk characterization. Food Control. 2023;148 [Google Scholar]
- Citronberg J., Bostick R., Ahearn T., Turgeon D.K., Rufn M.T., Djuric Z., Sen A., Brenner D.E., Zick S.M. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res. 2013;6:271–281. doi: 10.1158/1940-6207.CAPR-12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudu O.M., Mohammed B. Morphometry and histopathology of broiler chickens fed ginger by-product meal treated with exogenous enzyme. Trop. Subtrop. 2020;23:1–10. [Google Scholar]
- Dieumou F.E., Teguia A., Kuiate J.R., Tamokou J.D., Fonge N.B., Dongmo M.C. Effects of ginger (Zingiber officinale) and garlic (Allium sativum) essential oils on growth performance and gut microbial population of broiler chickens. Livest. Res. Rural Dev. 2009:21. [Google Scholar]
- Duncan D.B. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- Eckert N., Lee J., Hyatt D., Stevens S., Anderson S., Anderson P., Beltran R., Schatzmayr G., Mohnl M., Caldwell D. Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets. J. Appl. Poult. Res. 2010;19:59–67. [Google Scholar]
- El-kashef M.M.A. Evaluation of using Ginger (Zingiber Officinale) on growth performance, carcass characteristics, blood biochemistry and immune responses of quail birds. Egypt. Poult. Sci. 2022;42:199–212. [Google Scholar]
- El-kashef M.M.A., Roshdy A.R. Impact of using ginger (Zingiber Officinale) in laying quail diets on egg production, egg quality and blood parameters. Egypt. Poult. Sci. 2022;42:419–435. [Google Scholar]
- Emwas A-HM., Al-Rifai N., Szczepski K., Alsuhaymi S., Rayyan S., Almahasheer H., Jaremko M., Brennan L., Lachowicz JI. You are what you eat: application of metabolomics approaches to advance nutrition research. Foods. 2021;10:1249. doi: 10.3390/foods10061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emwas A.-H., Alghrably M., Al-Harthi S., Poulson B.G., Szczepski K., Chandra K., Jaremko M. Pages 83–106 in Nuclear Magnetic Resonance. IntechOpen; London, UK: 2019. New advances in fast methods of 2d nmr experiments. [Google Scholar]
- Emwas A.H.M., Alsuhaymi S., Al-Nemi R., Jaremko M. Compound-specific 1D 1H NMR pulse sequence selection for metabolomics analyses. ACS Omega. 2023;8:23651–23663. doi: 10.1021/acsomega.3c01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emwas A.H., Szczepski K., Poulson B.G., Chandra K., McKay R.T., Dhahri M., Jaremko M. NMR as a “gold standard” method in drug design and discovery. Molecules. 2020;25:4597. doi: 10.3390/molecules25204597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi H.A., Taherpour K. Comparison of broiler performance, blood biochemistry, hematology and immune response when feed diets were supplemented with ginger essential oils or mannan-oligosaccharide. Iran. J. Vet. Med. 2015;9:195–205. [Google Scholar]
- Grzanna R., Lindmark L., Frondoza C.G. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food. 2005;8:125–132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- Herve T., Raphaël K.J., Ferdinand N., Herman N.V., Marvel N.M.W., D’Alex T.C., Vitrice F.T.L. Effects of ginger (Zingiber officinale, Roscoe) Essential oil on growth and laying performances, serum metabolites, and egg yolk antioxidant and cholesterol status in laying Japanese quail. J. Vet. Med. 2019;2019 doi: 10.1155/2019/7857504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve T., Raphaël K.J., Ferdinand N., Vitrice L., Tiwa F., Gaye A., Moyo N. Growth performance, serum biochemical profile, oxidative status, and fertility traits in male Japanese quail fed on ginger (Zingiber officinale, roscoe) essential oil. Vet. Med. Int. 2018;2018 doi: 10.1155/2018/7682060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I., Ko Y., Kang S., Lee C. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007;134:304–315. [Google Scholar]
- Kawahara E., Ueda T., Nomura S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. 1991;26:213–214. [Google Scholar]
- Khenenou T., Boughrara M., Melizi M., Lamraoui R. Histomorphological study of the bursae of fabricius of broiler chickens during gumboro disease in Algeria area. Global Vet. 2017;18:132–136. [Google Scholar]
- Khenenou T., Melizi M., Benzaoui H., Bennoune O., Ibrir M. Histological changes in liver and pectoral muscles of broiler chickens slaughtered with and without naming of Allah. Int. J. Poult. Sci. 2013;12:550–552. [Google Scholar]
- Kothari D., Lee W.D., Niu K.M., Kim S.K. The genus Allium as poultry feed additive: a review. Animals. 2019;9:1032. doi: 10.3390/ani9121032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Arya R.S., Dhuria R.K., Nehra R., Jain D. Effect of supplementation of ginger root powder and black cumin seed powder on performance of white leghorn layers. J. Anim. Res. 2019;9:683–688. [Google Scholar]
- Liu Y., Liu J., Zhang Y. Research progress on chemical constituents of zingiber officinale roscoe. Bio. Res. Int. 2019;9:5370823. doi: 10.1155/2019/5370823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekizadeh M., Moeini M.M., Ghazi S. The effects of different levels of ginger (Zingiber officinale Rosc) and turmeric (Curcuma longa Linn) rhizomes powder on some blood metabolites and production performance characteristics of laying hens. J. Agr. Sci. Tech. 2012;14:127–134. [Google Scholar]
- Manallah A. Universit´e Ferhat Abbas de S´etif; Algeria: 2012. Antioxidant and Anticoagulant Activities of Olea-Europaea L Olive Pulp Polyphenols, [M´Emoire Pour Obtenir Le Diplˆome de Magister] [Google Scholar]
- Manju V., Viswanathan P., Nalini N. Hypolipidemic effect of ginger in 1,2-dimethyl hydrazine-Induced experimental colon carcinogenesis. Toxicol. Mech. Methods. 2006;16:461–472. doi: 10.1080/15376520600728811. [DOI] [PubMed] [Google Scholar]
- Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P., Singh U., Pandey C.M., Mishra P., Pandey G. Application of student's t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019;22:407–411. doi: 10.4103/aca.ACA_94_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile S.H., Park S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crop. Prod. 2015;70:238–244. [Google Scholar]
- NRC . National Research Council, National Academy Press; Washington, DC: 1994. Pages 33–34 in Nutrition Requirements of Poultry. [Google Scholar]
- Núñez-Torres O.P., Elizabeth D.A.V., Ismael A.S.R., Margarita C.Q.S. Ginger supplementation in quail as a nutritional alternative in egg production and quality. J. Selva. Andina. Anim. Sci. 2021;8:90–101. [Google Scholar]
- Nurhayati A.P.D., Rihandoko A., Fadlan A., Ghaissani S.S., Jadid N., Setiawan E. Anti-cancer potency by induced apoptosis by molecular docking P53, caspase, cyclin D1, cytotoxicity analysis and phagocytosis activity of trisindoline 1, 3 and 4. Saudi Pharm. J. 2022;30:1345–1359. doi: 10.1016/j.jsps.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaopara A.O., Odike M.A.C., Inegbenebor U., Nwaopara S.O., Ewere G.I. A Comparative study on the effects of excessive consumption of ginger, clove, red pepper and black pepper on the histology of the liver. Pak. J. Nutr. 2007;6:524–527. [Google Scholar]
- Onu P. Evaluation of two herbal spices as feed additives for finisher broilers. Biotechnol. Anim. Husb. 2010;26:383–392. [Google Scholar]
- Sachivkina N., Vasilieva E., Lenchenko E., Kuznetsova O., Karamyan A., Ibragimova A., Zhabo N., Molchanova M. Reduction in pathogenicity in yeast-like fungi by farnesol in quail model. Animals. 2022;12:489. doi: 10.3390/ani12040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeid J.M., Mohamed A.B., Al-Baddy M.A. Effect of aqueous extract of ginger (Zingiber officinale) on blood biochemistry parameters of broiler. Int. J. Poult. Sci. 2010;9:944–947. [Google Scholar]
- Sahin O., Morishita T.Y., Zhang Q. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 2002;3:95–105. doi: 10.1079/ahrr200244. [DOI] [PubMed] [Google Scholar]
- Salmanzadeh M. Does dietary ginger rhizome (Zingiber officinale) supplementation improve the performance, intestinal morphology and microflora population, carcass traits and serum metabolites in Japanese quail? Eur. Poult. Sci. 2015;79:1–10. [Google Scholar]
- Shirin A.P.R., Jamuna P. Chemical composition and antioxidant properties of ginger root (Zingiber officinale) J. Med. Plant. Res. 2010;4:2674–2679. [Google Scholar]
- Sittiya J., Khonyoung D., Yamauchi K., Yamauchi K. Preliminary study: egg production performance, egg quality and blood plasma cholesterol concentration in laying hens fed dietary dried fermented ginger and/or fermented corncob powder. Int. J. Food Sci. Nutr. 2017;3:1–5. [Google Scholar]
- Stoner G.D. Ginger: is it ready for prime time? Cancer Prev. Res. 2013;6:257–262. doi: 10.1158/1940-6207.CAPR-13-0055. [DOI] [PubMed] [Google Scholar]
- Swain P., Mohapatra L.M., Sethy K., Sahoo P.R., Nayak S.M., Patro P., Behera K., Pradhan C.R. Effect of ginger and garlic supplements on growth and haemato-biochemical profile of Japanese quail (Coturnix Coturnix Japonica) Explor. Anim. Med. Res. 2017;7:77–83. [Google Scholar]
- Swelum A.A., El-Saadony M.T., Abdo M., Ombarak R.A., Hussein E.O., Suliman G., Abd El-Hack M.E. Nutritional, antimicrobial and medicinal properties of Camel's milk: a review. Saudi J. Biol. Sci. 2021;28:3126–3136. doi: 10.1016/j.sjbs.2021.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghdisi A., Hejazi S. The effect of Zingiber officinale on the spleen tissue and antibody titer of broiler chickens. J. Morphol. Sci. 2019;36:046–050. [Google Scholar]
- Udo-Affah G., Kebe E.O., Obasee P.P., Isaac V.N. Extract of ginger (Zingiber officinale) on the histology of the spleen using adult male rats. J. Biol. Agric. Healthcare. 2014;4:259–267. [Google Scholar]
- Verma S.K., Singh M., Jain P., Bordia A. Protective effect of ginger, Zingiber officinale Rosc on experimental atherosclerosis in rabbits. Indian J. Exp. Biol. 2004;42:736–738. [PubMed] [Google Scholar]
- Vishwakarma S.L., Pal S.C., Kasture V.S., Kasture S.B. Anxiolytic and antiemetic activity of Zingiber officinale. Phytother. Res. 2002;16:621–626. doi: 10.1002/ptr.948. [DOI] [PubMed] [Google Scholar]
- Wang X., He H., Liu J., Xie S., Han J. Inhibiting roles of farnesol and HOG in morphological switching of Candida albicans. Am. J. Transl. Res. 2020;12:6988. [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang Z. Studies of commonly used traditional medicine-ginger. Zhongguo Zhong Yao Za Zhi China. 2005;30:1569–1573. [PubMed] [Google Scholar]
- Wen C., Gu Y., Tao Z., Cheng Z., Wang T., Zhou Y. Effects of ginger extract on laying performance, egg quality, and antioxidant status of laying hens. Animals. 2019;9:857. doi: 10.3390/ani9110857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of herbal extracts as feed additives for swine and poultry. J. Anim. Sci. 2007;86:140–148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Zawrotniak M., Wojtalik K., Rapala-Kozik M. Farnesol, a quorum-sensing molecule of Candida albicans triggers the release of neutrophil extracellular traps. Cells. 2019;8:1611. doi: 10.3390/cells8121611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeweil H.S., Abd El-Rahman M.H.A., Dosoky W.M., Abu Hafsa S.H., Abdulhamid A.B.A. Effects of ginger and bee propolis on the performance, carcass characteristics and blood constituents of growing Japanese quail. Egypt. Poult. Sci. 2016;36:143–159. [Google Scholar]
- Zhang M., Viennois E., Prasad M., Zhang Y., Wang L., Zhang Z., Han M.K., Xiao B., Xu C., Srinivasan S. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.F., Yang Z.B., Wang Y., Yang W.R., Jiang S.Z., Gai G.S. Effect of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. J. Poult. Sci. 2009;88:2159–2166. doi: 10.3382/ps.2009-00165. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang Z.B., Yang W.R., Wang Y., Jiang S.Z., Zhang G.G. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poult. Sci. 2011;90:1720–1727. doi: 10.3382/ps.2010-01280. [DOI] [PubMed] [Google Scholar]
- Zomrawi W.B., Abdel-Atti K.A., Dousa B.M., Mohammed K.E., G A. Cholesterol and egg characteristic. Int. J. Livest. Res. 2014;4:42–47. [Google Scholar]