Abstract
Chronic wound management is extremely challenging because of the persistence of biofilm-forming pathogens, such as Pseudomonas aeruginosa and Staphylococcus aureus, which are the prevailing bacterial species that co-infect chronic wounds. Phage therapy has gained an increased interest to treat biofilm-associated infections, namely when combined with antibiotics. Here, we tested the effect of gentamicin as a co-adjuvant of phages in a dual species-biofilm wound model formed on artificial dermis. The biofilm-killing capacity of the tested treatments was significantly increased when phages were combined with gentamicin and applied multiple times as multiple dose (three doses, every 8 h). Our results suggest that gentamycin is an effective adjuvant of phage therapy particularly when applied simultaneously with phages and in three consecutive doses. The multiple and simultaneous dose treatment seems to be essential to avoid bacterial resistance development to each of the antimicrobial agents.
Keywords: Pseudomonas aeruginosa, Staphylococcus aureus, Phage-antibiotic combination, Gentamicin, Phage-antibiotic synergy (PAS), Sequential treatment, Dual-species biofilm, Artificial wound model, Wound infection
1. Introduction
Biofilm formation in wounds is considered a major barrier to successful treatments and contributes to the high global cost of chronic wound management [1]. It leads to impaired epithelialization, and microorganisms embedded in these biofilms show reduced susceptibility to antimicrobial agents [2], delaying the healing process [3]. Pseudomonas aeruginosa and Staphylococcus aureus are the most common species in chronic wounds [4,5]. These pathogens coexist in multi-species biofilms, and their association can result in higher virulence and increased tolerance to antimicrobial agents [6,7]. Phage therapy is a promising approach to tackle infectious diseases [8]. However, several studies have raised concerns about phage therapy directed against biofilm-related infections [9], particularly due to the fast emergence of phage resistance [10]. Therefore, there has been an increased interest in using antibiotics as adjuvants of phage-therapy [11]. Gentamicin (GEN) is an aminoglycoside antibiotic that can be used for topical application to treat chronic wounds [12]. Recent clinical studies reveal that topical GEN application reduces the duration of wound healing [13], however, treatments should be limited in duration due to concerns about antibiotic resistance [14].
We have previously shown that the sequential combination of a Pseudomonas-specific phage EPA1 and GEN resulted in P. aeruginosa eradication in biofilms formed in standard laboratory conditions [15]. However, it is generally recognized that standard laboratory conditions do not always accurately reflect the infectious microenvironment, and the use of model systems that more closely resemble the in vivo situation is recommended [16].
In the present study, we designed new combined phage-antibiotic therapy protocols and application strategies, using phages targeting both P. aeruginosa and S. aureus with the combination of GEN as an adjuvant of phage therapy, in an in vitro artificial wound model.
2. Results
2.1. Isolation and characterization of a new S. aureus infecting phage SAFA
A new S. aureus infecting virus, designated phage SAFA, was isolated from a sewage plant in Braga, Portugal. This phage has an icosahedral head that is 95 nm in diameter, and a contractile tail of approximately 232 × 23 nm in diameter, resembling the morphology of a myovirus (Fig. S1). Phage SAFA could propagate on 13 out of 20 S. aureus strains investigated (65%) with moderate to high Efficiency of Plating (EOP) (Table S1). This phage has a latent period of 25 min, and an average burst size of 64 progeny phages per infected cell (Fig. S2).
Phage SAFA has a linear double-stranded DNA genome of 148,740 bp in size, and comparative genomics show that SAFA is very similar to many other staphylococcal phages of the Kayvirus genus. SAFA is presumably virulent and does not encode any genes associated with lysogeny or virulence. This suggests that SAFA is potentially safe for therapeutic purposes.
2.2. Establishing dual-species biofilm on the artificial dermis
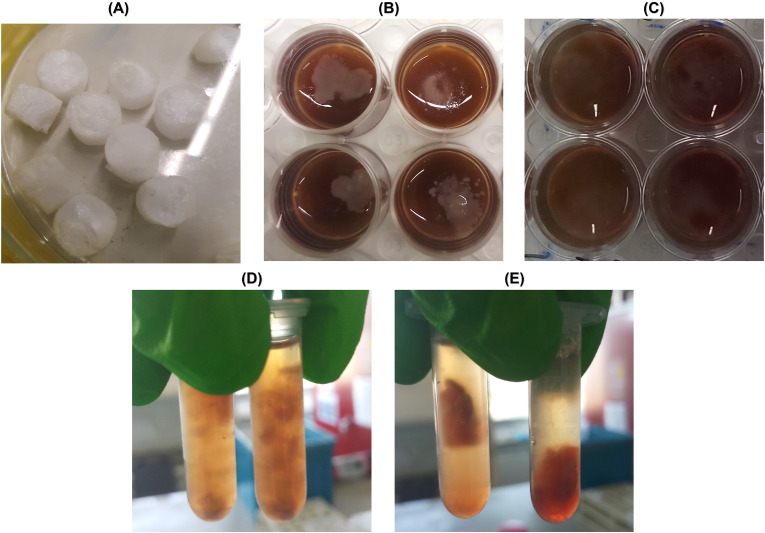
To assess the anti-biofilm activity of the antimicrobials (phages and GEN), dual-species biofilms of P. aeruginosa and S. aureus were formed in an in vitro wound model containing an artificial dermis (AD) (Fig. 1A). After 24 h, biofilm populations consisted of 1.13 × 109 CFU/mL of P. aeruginosa and 2.43 × 108 CFU/mL of S. aureus (Fig. 2) [[17], [18], [19]]. Images of the colonized wound model show visible bacterial colonization on the upper part of the dermis with a darkened colour change of growth medium after 24 h of biofilm formation (Fig. 1B). When the incubation time was extended to 48 h, an additional colour change in the medium and an increase in surface colonization were observed (Fig. 1C), concurrently, dermal fragmentation was evident (Fig. 1D); however, this phenomenon was not present in simultaneous treatments (SIM) of AD samples (Fig. 1E).
Fig. 1.
Macroscopic images of wound biofilm model used. (A) AD (B) P. aeruginosa and S. aureus infected AD after 24 h of biofilm formation. (C) P. aeruginosa and S. aureus infected AD (non-treated control) after 48 h of biofilm formation (D) Untreated control (48 h) dermis after being transferred to 2 mL microcentrifuge tubes containing saline solution (E) Treated AD (48 h, the treatment details are in section 0) after being transferred to 2 mL microcentrifuge tubes containing saline solution.
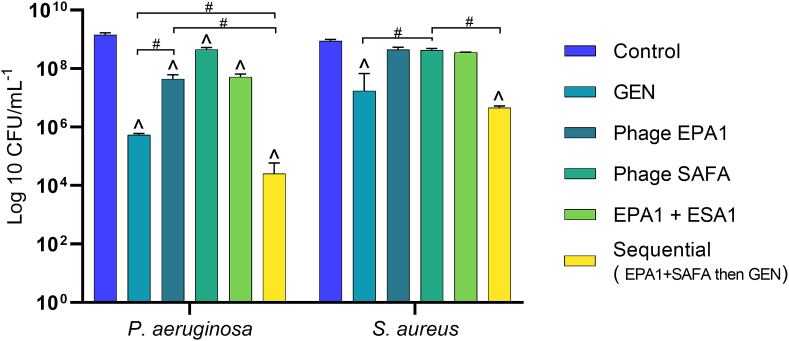
Fig. 2.
The number of P. aeruginosa and S. aureus CFU recovered after single-dose treatment of 24 h old dual-species biofilms. EPA1+SAFA: phage EPA1 and SAFA were applied simultaneously at MOI of 1. Sequential means that phage EPA1 and SAFA were applied simultaneously at MOI of 1; subsequently GEN was applied (4 μg/mL, i.e. the MIC for P. aeruginosa) with a 6 h delay. (^) Statistical differences between the control and treated biofilms. (#) Statistical differences between the compared treatment groups. Statistical differences were determined by two-way repeated-measures analysis of variance (ANOVA) with Tukey’s multiple comparison tests (p < 0.001). Values are the average of three technical repeats in duplicate, error bars indicate standard deviation.
2.3. Single-dose administration of sequential phages-antibiotic combination showed bacterial killing in dual-species biofilm
The activity of phage EPA1, phage SAFA, and GEN alone or in combinations was tested in the dual-species biofilms. The 6-h treatments resulted in a modest reduction of the biofilm populations (Fig. S3). Phage EPA1 treatment reduced the P. aeruginosa population by 1.5 log reduction, while phage SAFA did not produce a significant reduction in the S. aureus population when compared to the control. The anti-biofilm activity was not altered when phages EPA1 and SAFA were applied simultaneously. Treatment with GEN alone led to a modest reduction of the numbers of P. aeruginosa (1.0 log reduction) and S. aureus (0.9 log reduction) (Fig. S3).
In dual-species biofilms, after 24 h of treatments, phage EPA1 alone reduced the P. aeruginosa population by 1.5 log reduction, however, phage SAFA did not significantly reduce the S. aureus population. The killing activity of the simultaneous application of the two phages (EPA1+SAFA) was similar to their single treatments (Fig. 2). The effect of treatment with GEN alone was more pronounced after 24 h compared to 6 h treatment and resulted in a population reduction of 3.4 and 1.7 logof P. aeruginosa and S. aureus, respectively (Fig. 2). When EPA1+SAFA and GEN were applied sequentially (first EPA1+SAFA, followed by GEN 6 h later), biofilm reductions of 4.8 and 2.3 log reduction were observed for P. aeruginosa and S. aureus, respectively.
2.4. Administration of multiple doses of phage(s) or/and antibiotic significantly reduced both P. aeruginosa and S. aureus populations in dual-species biofilms
To develop more efficient treatment strategies, both phages (EPA1+SAFA) and the antibiotic (GEN) were administered in three doses (in different combinations and sequences) every 8 h for a total of 24 h (Table S2). To explore the most efficient combinations, a total of 27 antimicrobial treatment regimens were designed and tested on dual-species biofilms formed in 24-well plates. The most promising combinations (12 out of 27 treatments) were selected to test in the in vitro wound model (Fig. 3, Table S2).
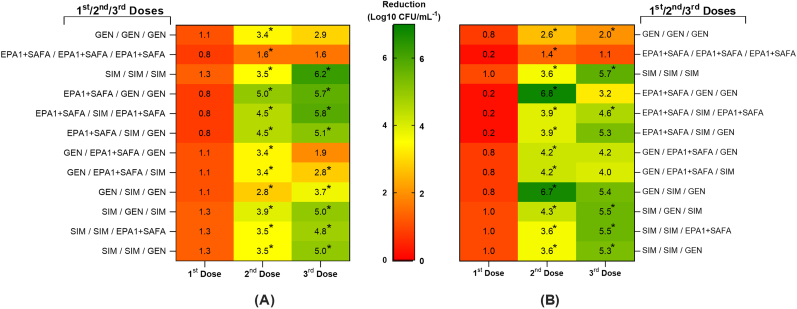
Fig. 3.
Heat map representing the log reduction of (A) P. aeruginosa and (B) S. aureus in dual-species biofilm after multiple treatments. The middle legend bar indicates the colour change according to log reduction reductions, with log reduction reductions increasing from red to green. first dose, second dose, and third dose indicate the order of treatment. The 24 h old dual-species biofilms were treated for 24 h in total (3 treatments of 8 h). The prefix “SIM” indicates the simultaneous application of phage EPA1, SAFA (at MOI of 1) and GEN (4 μg/mL, i.e. the MIC for P. aeruginosa) treatments. (^) Statistical differences between the control and treated biofilms. (*) Statistical differences between the current and previous dose-treated biofilms. Statistical differences were determined by two-way repeated-measures analysis of variance (ANOVA) with Tukey’s multiple comparison tests (p < 0.001). Values are the average of three technical repeats in duplicate. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The pre-formed dual-species biofilms were initially exposed to either of three treatments for 8 h, i.e. EPA1+SAFA, GEN, and the combination of EPA1+SAFA and GEN. After this first treatment, P. aeruginosa populations were reduced by 0.8, 1.1, and 1.3 log reduction, while S. aureus populations were reduced by 0.2, 0.8, and 1.0 log reduction, respectively (Fig. 3). The second dose resulted in additional biofilm reduction for P. aeruginosa and S. aureus, the total biofilm reductions at this stage ranged from 1.1 to 5.0 log reduction for P. aeruginosa and from 1.6 to 6.8 log reduction for S. aureus (Fig. 3). The highest reduction for both species was observed when treatment with EPA1+SAFA was followed by GEN treatment, while the lowest reduction was observed when treatment with EPA1+SAFA was followed by another EPA1+SAFA treatment (Fig. 3). The most effective treatment regimen was obtained following multiple doses of EPA1+SAFA + GEN (SIM), with a 6.2 log reduction for P. aeruginosa and 5.7 log reduction for S. aureus (Fig. 3). The combinations EPA1+SAFA/SIM/GEN, SIM/GEN/SIM, and SIM/SIM/GEN also led to more than 5 log reduction for both bacterial species (Fig. 3). Some treatment regimens resulted in biofilm regrowth, most probably as a result development of resistance. This is particularly relevant in the case of multiple dose administration of the antibiotic and the phages alone.
3. Discussion
Increasing evidence suggests that phages are useful in the treatment of wound-associated infections, and phage therapy can be highly effective when administered appropriately, as demonstrated in standard laboratory conditions, as well as in vivo animal models and even in human patients (reviewed in Refs. [20,21]). Although treatments with single phages or phage cocktails have shown promising results [[22], [23], [24], [25]], recent studies have suggested that the use of antibiotics as phage adjuvants are more effective against biofilm-related infections [[26], [27], [28], [29]].
In the present study, we tested the anti-biofilm activity of two phages targeting P. aeruginosa and S. aureus alone and combined with gentamicin in different treatment regimens in an in vitro dual-species biofilm model of chronic wound infection [30,31] and found that the sequential treatment with phages (EPA1+SAFA) and antibiotic (GEN) led to significantly higher biofilm reductions than those obtained with single treatments.
The antimicrobial agents were also applied in multiple dose regimens with different combination strategies. The obtained reductions ranged from 1.9 to 5.2 log, suggesting that the order and frequency of application influence the treatment outcome.
The application of GEN as the first dose treatment, followed by phages usually led to low reductions. Phages rely on host mechanisms to facilitate their replication and antibiotics may adversely impact these essential mechanisms. For example, antibiotics that target the protein synthesis can alter the outcome of bacteria–phage interactions by interfering with the production of phage-encoded counter-defense proteins [32]. GEN targets protein synthesis and inhibits phage replication [15], therefore phage efficacy is compromised when it is added first. However, when GEN is applied simultaneously with phages, the rapid killing activity of phages can probably overcome the antagonistic effect of GEN on the activity of the phage against the biofilm, at least in the initial stages after application. Furthermore, the application of both antimicrobials in multiple doses can lead to a complementary effect in which phages target preferentially antibiotic resistant bacteria, and antibiotics kill phage resistant cells.
The use of single antimicrobial agents in consecutive doses, be it phages or the antibiotic, was very ineffective. In fact, when GEN was used in three consecutive treatments, a regrowth in the biofilm population was observed (Fig. 3). The same was observed for consecutive applications of phages (Fig. 3). If phages do not manage to kill a sufficient number of bacteria quickly, this may result in the proliferation of bacteriophage-insensitive mutants (BIMs) [33,34]. Bacteria possess or can quickly develop different mechanisms to escape viral infections, such as alteration or loss of receptors [10], secretion of substances that prevent phage adhesion to the bacterial pathogen like outer membrane vesicles [35], blocking phage DNA injection, and inhibition of phage replication and release [36]. Nonetheless, phages and antibiotics use different mechanisms of action [37]. This feature can make their combination very effective against biofilms. When phages and antibiotics are used simultaneously or sequentially, bacteria have a low chance of evolving resistance against both at the same time [38].
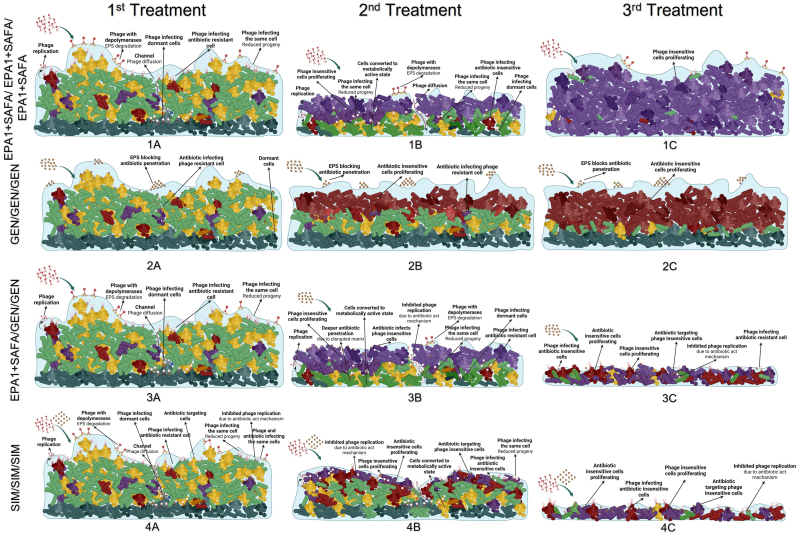
The possible mechanisms involved in the biofilm treatment with multiple doses of antibiotics or phages alone and in combination are summarized in Fig. 4. Here we hypothesise that in a multi-dose treatment with simultaneous application of phages and antibiotics, the bacterial population is exposed to multiple stresses at the same time and is unlikely to be able to recover or evolve resistance.
Fig. 4.
Schematic presentation of antimicrobial treatments. The row A represents first dose treatment; row B represents second dose treatment; row C represents third dose treatment. EPA1+SAFA/EPA1+SAFA/EPA1+SAFA (1A, 1B, 1C) represent multiple dose treatment regimens of phages at a MOI of 1. In the first dose treatment, phages disrupt and penetrate the biofilm matrix and infect the bacteria cells, helping the penetration of larger molecules such as nutrients. The additional second and third doses of phage treatment continue to target phage-sensitive cells. However, BIM cells proliferate and dominate the biofilm population. GEN/GEN/GEN (2A, 2B, 2C) represent 3 multiple dose treatment regimens of GEN at MIC for P. aeruginosa, 4ug/mL. In the first dose treatment, GEN infects sensitive cells in the upper layer of biofilm. However, single GEN treatment results in GEN-insensitive cell proliferation. The evolved bacteria can proliferate and dominate the biofilm population, rendering the second and third antibiotic treatments ineffective. EPA1+SAFA/GEN/GEN (3A, 3B, 3C) represent multiple dose treatment regimens of antimicrobials: EPA1+SAFA, GEN, and GEN, respectively. In the first dose of treatment, phages disrupt and penetrate the biofilm matrix and infect the bacteria cells. It helps the penetration of larger molecules such as nutrients and antibiotics. However, initial phage treatment induces BIM cell proliferation. The following GEN treatments targets proliferating BIMs and GEN-sensitive cells. Nonetheless, GEN treatments can inhibit phage replication and result in reduced phage efficiency. SIM/SIM/SIM (4A, 4B, 4C) represent multiple dose treatment regimens of the simultaneous combination of EPA1+SAFA and GEN at MOI of 1 and MIC value (4ug/mL, i.e. the MIC for P. aeruginosa). Phages disrupt and penetrate the biofilm matrix and infect the bacteria cells. It helps the penetration of larger molecules such as nutrients and antibiotics. Phages and antibiotics use different mechanisms of action. Following the first dose of treatment, the proliferating phage- or GEN-insensitive cells are targeted by another antimicrobial agent, which is supplied to the environment by the second and third doses of treatment.
Our work shows that, the in vitro wound model can be used to test the efficacy of phages against chronic wounds and that results obtained in this in vivo-like model may differ from those obtained in other in vitro models. This observation reiterates the importance of using relevant models that capture important aspects of host physiology and the infectious microenvironment when evaluating innovative anti-biofilm strategies [16,39]. Our data indicate that gentamicin is an effective adjuvant of phage therapy, particularly when applied simultaneously with phages in a multiple-dose treatment, to minimise the effect of resistance mechanisms. Moreover, our results suggest that antibiotics can be effective adjuvants for phage therapy against chronic wound infections. However, the order and frequency of the applied antimicrobials (phages or antibiotics) is important for an optimal treatment outcome.
4. MATERIAL and METHODS
4.1. Bacterial strains and culture conditions
The bacterial strains P. aeruginosa PAO1 (DSM22644) and S. aureus ATCC 25923 are reference strains obtained from the German Collection of Microorganisms and Cell Cultures and American Type Culture Collection, respectively. Seventeen additional clinical S. aureus isolates, and two culture collection strains were kindly provided by the LPhage Laboratory in CEB (University of Minho, Braga, Portugal, Table S1) and were also used in this study. All strains were grown in Tryptic Soy Broth (TSB, VWR Chemicals), Tryptic Soy Agar (TSA; VWR Chemicals), or in TSA soft overlays (TSB with 0.6% agar) at 37 °C. Pseudomonas isolation agar (PSA; Becton, Dickinson) was used to enumerate P. aeruginosa cells, and mannitol salt agar (MSA; Neogene) was used to enumerate S. aureus cells in dual-species biofilms.
4.2. Phage isolation and production
Phage SAFA was isolated from effluent samples of raw sewage obtained in a waste-water treatment plant in Braga, Portugal, using the enrichment protocol described before [40]. Briefly, 100 mL of the effluent was mixed with 100 mL of double-strength TSB and with 10 μL of each of the exponentially grown S. aureus strains (Table S1) and incubated at 37 °C, at 120 rpm (BIOSAN ES-20/60, Riga, Latvia) overnight. Suspensions were further centrifuged (15 min, 9000×g, 4 °C), and the supernatants were filtered through a 0.22 μm polyethersulfone (PES) membrane (ThermoFisher Scientific, Massachusetts, USA). The presence of phages was confirmed by performing spot assays on bacterial lawns. The prepared plates were further incubated overnight at 37 °C, and the presence of inhibition halos was observed. When phage plaques appeared, successive rounds of single plaque purification were carried out until purified plaques were observed, reflected by a single plaque morphology.
The purified phage was produced by using the double agar layer method, as described before [33]. Briefly, 100 μL of a phage suspension at 108 PFU/mL were spread on P. aeruginosa PAO1 or S. aureus ATCC 25923 lawns for overnight incubation at 37 °C. If full lysis was observed, plates were further incubated at 4 °C for 6 h at 120 rpm (BIOSAN PSU-10i), with 2 mL of SM Buffer (100 mM NaCl, 8 mM MgSO4, 50 mM Tris/HCl, pH 7.5) to resuspend the phage particles. The liquid phase was collected and centrifuged (15 min, 9000×g, 4 °C), and the supernatants were filtered through a 0.22 μm PES membrane. Purified phages were stored at 4 °C for further use.
4.3. Electron microscopy
Phage suspension was sedimented by centrifugation (25,000×g, 60 min, 4 °C) using a ScanSpeed 1730R centrifuge (Labogene, Lillerød, Denmark). The pellet was further washed in tap water by repeating the centrifugation step. Subsequently, phage suspension was deposited on copper grids with a carbon-coated Formvar carbon film on a 200 square mesh nickel grid, stained with 2% uranyl acetate (pH 4.0) and examined using a Jeol JEM 1400 transmission electron microscope (TEM) (Tokyo, Japan) [15].
4.4. Phage host range and efficiency of plating determination phage
The host range of SAFA was determined with the spot test method [15] using the strains listed in Table S1. Briefly, 100 μL of each overnight bacterial culture was added to 5 mL of TSB-soft agar and poured onto TSB agar plates. 10 μL of serial 10-fold dilutions of the phage suspension was spotted on the bacterial lawns and plates were incubated at 37 °C overnight. The efficiency of plating (EOP) was calculated by dividing the titer of the phage (PFU/mL) obtained for each isolate by the titer determined in the propagating bacteria. EOP was recorded as high (>10%), moderate (0.01–9%) or low (<0.01%) [15].
4.5. Genome sequencing and in silico analysis
The DNA of the Staphylococcus phage SAFA was extracted according to the standard phenol-chloroform-isoamyl alcohol methods, as described elsewhere [41]. The DNA sample was used for library construction using the Illumina Nextera XT library preparation kit. The generated DNA libraries were sequenced in the lllumina MiSeq platform, using 250bp paired-end sequencing reads. Next, reads were assembled de novo with Geneious R9, and manually inspected. SAFA genome was annotated using RAST [42]. The function of proteins was manually inspected using BLASTP. tRNAscan-SE was used to predict tRNAs [43]. For comparative studies, pairwise alignments were made using BLASTN or BLASTP.
4.6. Biofilm formation in microtiter plates
For the in vitro assessment of antimicrobial efficacy, 48 h old dual-species biofilm were formed in 24-polystyrene well plates (Orange Scientific, Braine-l’Alleud, Belgium) as previously described [15]. Briefly, to initiate biofilm formation, one bacterial colony (P. aeruginosa or S. aureus) was incubated in TSB overnight in an orbital shaker (120 rpm, BIOSAN ES-20/60) at 37 °C. For establishing mono-species biofilms, 10 μL of the starter culture was transferred into 24-well plates containing 990 μL of fresh TSB media. The plates were incubated for 24 h in an orbital shaker incubator (120 rpm, BIOSAN ES-20/60) at 37 °C. After 24 h, half of the growth medium (500 μL TSB, 1:1, v:v) was replaced with fresh TSB and plates were incubated for an additional 24 h. For dual-species biofilms, S. aureus cells were inoculated prior to P. aeruginosa addition. Thus, biofilms were initiated with 10 μL of the overnight culture of S. aureus (∼108 CFU/mL) in 990 μL TSB and incubated for 24 h in an orbital shaker (120 rpm) at 37 °C. After that, half of the growth medium (500 μL TSB, 1:1, v:v) was replaced with TSB including 10 μL of the starter culture of P. aeruginosa (∼108 CFU/mL, 1:49, v/v) and incubated for additional 24 h. In mono and dual-species biofilms, the supernatant was aspirated, and the wells were washed twice with saline solution (0.9% NaCl (w/v)) to remove planktonic bacteria. Biofilms were scraped of the plate in saline solution (1 mL) using a micropipette tip, and the number of culturable cells was determined using plate counts [43].
4.7. Biofilm formation in the in vitro wound model
For the wound model, we used the previously prepared two-layer (upper and lower) AD substrate as described elsewhere [33]. Dual-species biofilms were grown on an AD with minor modifications to the previously described chronic wound biofilm model [31]. Briefly, ADs were placed in the 24-well microtiter plate, and 500 μL of Bolton Broth with 50% plasma (Sigma–Aldrich) and 5% freeze-thaw laked horse blood was added to the ADs. Then, the same amount of growth medium was added into the wells. Next, 10 μL of the overnight culture of P. aeruginosa and S. aureus (∼108 CFU/mL) were spotted simultaneously on the upper part of each AD and incubated at 37 °C overnight.
4.8. Biofilm challenge
Dual-species biofilms formed on AD were treated with the antimicrobials; alone, in simultaneous (EPA1+SAFA + GEN) or sequential combinations (first EPA1+SAFA and then GEN with 6 h delay) for 24 h. Briefly, 10 μL of antimicrobials were added to the AD at final concentration of 4 μg/mL (MIC of GEN for P. aeruginosa PAO1) and at MOI of 1 for phages. Plates were incubated at 37 °C for 24 h. Then, treated and untreated (control) ADs were transferred into tubes containing 10 mL saline solution, the sessile cells were removed from the AD by three cycles of vortexing (30 s) and sonication (30 s; Branson 3510; Branson Ultrasonics Corp, Danbury, CT) and the number of CFU/biofilm was determined by plate counting.
To develop more efficient treatment strategies, 27 different treatment variables were initially tested on dual-species biofilms formed on 24-well polystyrene plates (Table S2). Briefly, biofilms were washed twice with the saline solution and GEN (at 1x MIC for P. aeruginosa, 4 μg/mL) and EPA1+SAFA (at MOI 1) were applied in TSB according to the order as described in Table S2. Following the CFU counting, the most promising variables were selected and tested on dual-species biofilm formed on ADs. The same protocol was applied to treat and enumerate the cells as described above in AD treatment. However, instead of the single-dose treatment, the multiple dose treatments were applied every 8 h for a total of 24 h, and the number of viable cells was enumerated by plate counting.
4.9. Statistical analysis
In all the assays, averages and standard deviations were determined based on 3 independent experiments (n = 3) performed in duplicate. The results of the assays were compared using two-way analysis of variance (ANOVA) by applying the Tukey’s multiple comparisons tests using Prism 9.0.0 for Windows. Plots were obtained using Prism 9 (GraphPad, La Jolla, CA, USA). Means and standard deviations (SD) were calculated with the software. Differences among conditions were considered statistically significant when p < 0.001.
4.10. Accession number
SAFA genome was deposited in GenBank database under the accession number OP651044.
Funding
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit, and Project PTDC/BIA-MIC/2312/2020. Ergun Akturk is recipient of a FCT PhD grant with the reference PD/BD/135254/2017. Luís D. R. Melo acknowledges funding from the FCT through the Scientific Employment Stimulus Program (2021.00221. CEECIND).
Ethical approval
Not required.
CRediT authorship contribution statement
Ergun Akturk: Conceptualization, Investigation, Methodology, Formal analysis, Visualization, Resources, Writing – original draft, Writing – review & editing. Luís D.R. Melo: Conceptualization, Methodology, Validation, Supervision, Funding acquisition, Writing – review & editing. Hugo Oliveira: Software, Formal analysis, Writing – original draft. Aurélie Crabbé: Conceptualization, Supervision. Tom Coenye: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision. Joana Azeredo: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge Professor Hermínia de Lencastre and Professor Oto Melter for gently providing some of the strains used in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioflm.2023.100147.
Contributor Information
Tom Coenye, Email: tom.coenye@ugent.be.
Joana Azeredo, Email: jazeredo@deb.uminho.pt.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Han G., Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James G.A., Swogger E., Wolcott R., Pulcini E.D., Secor P., Sestrich J., et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 3.Maslova E., Eisaiankhongi L., Sjöberg F., McCarthy R.R. Burns and biofilms: priority pathogens and in vivo models. NPJ Biofilms Microbiomes. 2021;7:1–9. doi: 10.1038/s41522-021-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirketerp-Møller K., Jensen P., Fazli M., Madsen K.G., Pedersen J., Moser C., et al. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol. 2008;46:2717–2722. doi: 10.1128/JCM.00501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazli M., Bjarnsholt T., Kirketerp-Møller K., Jørgensen B., Andersen A.S., Krogfelt K.A., et al. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol. 2009;47:4084–4089. doi: 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLeon S., Clinton A., Fowler H., Everett J., Horswill A.R., Rumbaugh K.P. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect Immun. 2014;82:4718–4728. doi: 10.1128/IAI.02198-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briaud P., Camus L., Bastien S., Doléans-Jordheim A., Vandenesch F., Moreau K. Coexistence with Pseudomonas aeruginosa alters Staphylococcus aureus transcriptome, antibiotic resistance and internalization into epithelial cells. Sci Rep. 2019;9:1–14. doi: 10.1038/s41598-019-52975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatfull G.F., Dedrick R.M., Schooley R.T. Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med. 2022;73:197–211. doi: 10.1146/annurev-med-080219-122208. [DOI] [PubMed] [Google Scholar]
- 9.Jurado A., Fernández L., Rodríguez A., García P. Understanding the mechanisms that drive phage resistance in staphylococci to prevent phage therapy failure. Viruses. 2022;14 doi: 10.3390/v14051061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pires D.P., Dötsch A., Anderson E.M., Hao Y., Khursigara C.M., Lam J.S., et al. A genotypic analysis of five P. aeruginosa strains after biofilm infection by phages targeting different cell surface receptors. Front Microbiol. 2017;8:1229. doi: 10.3389/fmicb.2017.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Nieuwenhuyse B., Van der Linden D., Chatzis O., Lood C., Wagemans J., Lavigne R., et al. Bacteriophage-antibiotic combination therapy against extensively drug-resistant Pseudomonas aeruginosa infection to allow liver transplantation in a toddler. Nat Commun. 2022;13:5725. doi: 10.1038/s41467-022-33294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooley J., Obaidi N., Diaz V., Anselmo K., Eriksson E., Carlsson A.H., et al. Delivery of topical gentamicin cream via platform wound device to reduce wound infection—a prospective, controlled, randomised, clinical study. Int Wound J. 2023;20:1426–1435. doi: 10.1111/iwj.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P., Long Z., Yu Z., Liu P., Wei D., Fang Q., et al. The efficacy of topical gentamycin application on prophylaxis and treatment of wound infection: a systematic review and meta‐analysis. Int J Clin Pract. 2019;73 doi: 10.1111/ijcp.13334. [DOI] [PubMed] [Google Scholar]
- 14.Heuer H., Krögerrecklenfort E., Wellington E.M.H., Egan S., Elsas J.D., Overbeek L., et al. Gentamicin resistance genes in environmental bacteria: prevalence and transfer. FEMS Microbiol Ecol. 2002;42:289–302. doi: 10.1111/j.1574-6941.2002.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 15.Akturk E., Oliveira H., Santos S.B., Costa S., Kuyumcu S., Melo L.D.R., et al. Synergistic action of phage and antibiotics: parameters to enhance the killing efficacy against mono and dual-species biofilms. Antibiotics. 2019;8 doi: 10.3390/antibiotics8030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjarnsholt T., Whiteley M., Rumbaugh K.P., Stewart P.S., Jensen P., Frimodt-Møller N. The importance of understanding the infectious microenvironment. Lancet Infect Dis. 2022;22:e88–e92. doi: 10.1016/S1473-3099(21)00122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman L.R., Déziel E., D’Argenio D.A., Lépine F., Emerson J., McNamara S., et al. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2006;103 doi: 10.1073/pnas.0606756104. 19890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer K.L., Aye L.M., Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin Z., Yang L., Qu D., Molin S., Tolker-Nielsen T. Pseudomonas aeruginosa extracellular products inhibit staphylococcal growth, and disrut established biofilms produced by staphylococcus epidermidis. Microbiology (N Y) 2009;155:2148–2156. doi: 10.1099/mic.0.028001-0. [DOI] [PubMed] [Google Scholar]
- 20.Steele A., Stacey H.J., de Soir S., Jones J.D. The safety and efficacy of phage therapy for superficial bacterial infections: a systematic review. Antibiotics. 2020;9:1–14. doi: 10.3390/antibiotics9110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto A.M., Cerqueira M.A., Bañobre-Lópes M., Pastrana L.M., Sillankorva S. Bacteriophages for chronic wound treatment: from traditional to novel delivery systems. Viruses. 2020;12 doi: 10.3390/v12020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pires D.P., Vilas Boas D., Sillankorva S., Azeredo J. Phage therapy: a step forward in the treatment of Pseudomonas aeruginosa infections. J Virol. 2015;89:7449–7456. doi: 10.1128/jvi.00385-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rostkowska O.M., Międzybrodzki R., Miszewska-Szyszkowska D., Górski A., Durlik M. Treatment of recurrent urinary tract infections in a 60-year-old kidney transplant recipient. The use of phage therapy. Transpl Infect Dis. 2021;23 doi: 10.1111/tid.13391. [DOI] [PubMed] [Google Scholar]
- 24.Schmerer M., Molineux I.J., Bull J.J. Synergy as a rationale for phage therapy using phage cocktails. PeerJ. 2014;2014 doi: 10.7717/peerj.590. e590–e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaVergne S., Hamilton T., Biswas B., Kumaraswamy M., Schooley R.T., Wooten D. Phage therapy for a multidrug-resistant acinetobacter baumannii craniectomy site infection. Open Forum Infect Dis. 2018;5 doi: 10.1093/ofid/ofy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghaee B.L., Mirzaei M.K., Alikhani M.Y., Mojtahedi A., Maurice C.F. Improving the inhibitory effect of phages against pseudomonas aeruginosa isolated from a burn patient using a combination of phages and antibiotics. Viruses. 2021;13 doi: 10.3390/v13020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engeman E., Freyberger H.R., Corey B.W., Ward A.M., He Y., Nikolich M.P., et al. Synergistic killing and re-sensitization of pseudomonas aeruginosa to antibiotics by phage-antibiotic combination treatment. Pharmaceuticals. 2021;14:1–17. doi: 10.3390/ph14030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comeau A.M., Tétart F., Trojet S.N., Prère M.F., Krisch H.M. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordillo Altamirano F.L., Kostoulias X., Subedi D., Korneev D., Peleg A.Y., Barr J.J. Phage-antibiotic combination is a superior treatment against Acinetobacter baumannii in a preclinical study. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brackman G., Coenye T. In vitro and in vivo biofilm wound models and their application. Adv Exp Med Biol. 2016;897:15–32. doi: 10.1007/5584_2015_5002. [DOI] [PubMed] [Google Scholar]
- 31.Brackman G., Garcia-Fernandez M.J., Lenoir J., de Meyer L., Remon J.P., de Beer T., et al. Dressings loaded with cyclodextrin–hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol Biosci. 2016;16:859–869. doi: 10.1002/mabi.201500437. [DOI] [PubMed] [Google Scholar]
- 32.Pons B.J., Dimitriu T., Westra E.R., van Houte S. Antibiotics that affect translation can antagonize phage infectivity by interfering with the deployment of counter-defenses. Proc Natl Acad Sci USA. 2023;120 doi: 10.1073/pnas.2216084120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons E.L., Bond M.C., Koskella B., Drescher K., Bucci V., Nadell C.D. Biofilm structure promotes coexistence of phage-resistant and phage-susceptible bacteria. mSystems. 2020;5 doi: 10.1128/msystems.00877-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto G., Minnich S.A., Hovde C.J., Oliveira H., Smidt H., Almeida C., et al. The interactions of bacteriophage Ace and Shiga toxin-producing Escherichia coli during biocontrol. FEMS Microbiol Ecol. 2021;97 doi: 10.1093/femsec/fiab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augustyniak D., Olszak T., Drulis-Kawa Z. Outer membrane vesicles (OMVs) of Pseudomonas aeruginosa provide passive resistance but not sensitization to LPS-specific phages. Viruses. 2022;14 doi: 10.3390/v14010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seed K.D. Battling phages: how bacteria defend against viral attack. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres-Barceló C., Hochberg M.E. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol. 2016;24:249–256. doi: 10.1016/j.tim.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhry W.N., Concepcion-Acevedo J., Park T., Andleeb S., Bull J.J., Levin B.R. Synergy and order effects of antibiotics and phages in killing pseudomonas aeruginosa biofilms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vyas H.K.N., Xia B., Mai-Prochnow A. Clinically relevant in vitro biofilm models: a need to mimic and recapitulate the host environment. Biofilm. 2022;4 doi: 10.1016/j.bioflm.2022.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melo L.D.R., Brandão A., Akturk E., Santos S.B., Azeredo J. Characterization of a new Staphylococcus aureus Kayvirus harboring a lysin active against biofilms. Viruses. 2018;10:1–16. doi: 10.3390/v10040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J., Russel W.D. vol. 3. Cold Spring Harboc Laboratory Press; 2000. p. 999. (Molecular cloning, 3-volume set : a laboratory manual). [Google Scholar]
- 42.Overbeek R., Olson R., Pusch G.D., Olsen G.J., Davis J.J., Disz T., et al. The SEED and the rapid annotation of microbial genomes using subsystems Technology (RAST) Nucleic Acids Res. 2014;42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan P.P., Lin B.Y., Mak A.J., Lowe T.M. TRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021;49:9077–9096. doi: 10.1093/nar/gkab688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.