Abstract
Humans’ everyday experience of the world is influenced by our mood. Moods are consciously accessible affective states that extend over time that are characterized by their valence and arousal. They also likely have a long evolutionary heritage and serve as an important adaptive affective mechanism. When they become maladaptive or overly biased, pathological affective states such as depression can emerge. Despite the importance of moods for human experience, little is known about their causal neurobiological mechanisms. In humans, methodological and interpretational limitations prevent causal investigations into the origins of mood, highlighting the importance of animal models. Nonhuman primates that share key neuroanatomical, affective, and social features with humans will be essential to uncovering their foundation. Identifying and validating mood-like states in animals is, however, challenging not least because mood is a human construct requiring verbal communication. Here we outline a theoretical framework for animal models of human mood, drawing upon established psychological literature where it exists before reviewing the extant studies of non-human primate models of mood-like states.
Keywords: Neurobiology, Animal models, Mood, Affect
Human mood provides color to our daily experiences. An engaging conversation with a good friend over a morning coffee can leave a lasting air of pleasantness as we go about our day; a board meeting in which budgets are cut and hard-working colleagues are fired is likely to sour our mood considerably. The presence of things and people whom we enjoy is typically experienced as positive and the presence of things and people whom we loathe is typically experienced as negative. Momentary affect – encoding stimuli or other people as positive or negative, as calming or arousing – can extend over prolonged durations, creating moods.
At their best, moods provide important adaptive information about our relationship with the world in terms of what is good for us and bad for us. Not all moods, however, are adaptive. At their worst, moods can be detrimental to our wellbeing. Indeed, mood disorders, most notably depression, are a one of the leading causes of disability worldwide (Depression and Other Common Mental Disorders Global Health Estimates, 2017). This problem is exacerbated by the chronic nature of mood disorders, which often last many years and for which there is no single cure. Despite decades of study, new effective treatments and interventions for mood disorders remain elusive. In part, this stems from the fact that the biological mechanisms that generate healthy moods are largely unknown for a number of reasons. First, apart from a few isolated exceptions (reviewed below), assessments in humans are limited to correlations between people’s mood experiences and neuroimaging measures that are unable to reveal causal mechanisms. Second, determining the causal mechanisms of mood requires manipulating neural circuits, which requires experiments in nonhuman animals (herein, animals). Animal studies have primarily focused on two types of affect-related experiments – those that present stimuli very quickly for which the affective response time window evaluated is very brief (e.g., delivery of a few drops of juice) and those that generate major manipulations to the individual that cause life-long changes to affective neural systems (e.g. early life stress inductions), neither of which are homologous with human mood states. Additionally, a significant portion of research into affect-related processes in animals utilize rodents, which may or may not be representative of all facets of human mood systems due to their different social structures, ecological niches, evolutionary histories, and neurobiology.
Our goal here is to begin to address what is and what is not known about how mood is controlled at a neurobiological level, highlighting assumptions that have been made in the animal literature relative to ideas about the basis of mood and affect in the human literature. To do that, we begin by operationalizing definitions of the phenomenon of mood and making clear what can and cannot be studied in animal models – an important first step in translational neuroscience. We draw on work in animal models in which brief momentary affect and temporarily extended mood-like states can be manipulated experimentally to understand the neural mechanisms. We focus specifically on nonhuman primates as a model, owing to their complex social structures, highly differentiated brains, as well as their shared ecological niche and evolutionary heritage with humans. We finish by highlighting major outstanding questions and chart a path towards determining how moods are generated and regulated.
Mood in Humans, Temporarily Extended Affective States in Other Animals
One of the major challenges for translational affective neuroscience is defining or operationalizing phenomena of interest. This is important as it means that the same phenomena can be studied across species and makes clear when homologs do not exist. Defining phenomena for the study of mood and mood disorders is particularly challenging because in humans, the word mood is used to refer to many different sensations, and mood is typically assumed to be available to conscious awareness (for a discussion see Russell, 2005). The extent to which nonhuman animals are able to consciously access and assess their internal states is unclear. Even in cases where evidence exists that nonhuman animals can use and act on internal state information (for example, the contents of their memories; Kornell et al., 2007; Middlebrooks and Sommer, 2012; Rosati and Santos, 2016; Templer and Hampton, 2012), it remains unclear whether they have secondary reflexive awareness of those states, a capacity that healthy humans certainly do have (see Lambie and Marcel, 2002, for a discussion of different ‘levels’ of consciousness in affective and emotional experience).
Nevertheless, the capacity to experience states that are characterized by valence (hedonics) and arousal (activation), like moods, are likely evolutionarily old and conserved across some species (Bliss-Moreau, 2017). For that reason, we distinguish between moods and what we call “temporally extended affective states”. Moods are consciously experienced and thus an individual can be reflexively aware of them (e.g., “I feel depressed”); as a result, by definition, they only occur in animals with the capacity for consciousness.1 Temporally extended affective states (TEAS) are moods without conscious experience and therefore can occur in animals without the capacity for consciousness.2 Thus, TEAS form the basis of mood and are our operational definition of these affective states.
Affect as the foundation of mood, TEAS, and emotions.
We define TEAS as affective states that extend over some period of time in order to differentiate them from affective states that are fleeting (see below for further discussion of the temporal aspect) (Figure 1). As discussed, affect is an omnipresent psychological state that is characterized by valence (hedonics) and arousal (arousal) (Barrett and Bliss-Moreau, 2009; Barrett and Russell, 1999; Russell, 2005, 2003 for a discussions of affect). Affect is thought to be homologous in mammals (Barrett, 2017; Barrett and Bliss-Moreau, 2009; Bliss-Moreau, 2017) and likely other animals, opening the possibility of studying affect in species other than humans.3 Affect comes to be psychologically realized via the integration of sensory information about the individual’s external environment (sensory information; exteroceptive information) with information about the individual’s internal environment (physiological information; interoceptive information). Together this results in an integrated representation that provides information to guide action in terms of what is good or bad for the individual. This integration of exteroceptive and interoceptive information allows for allostasis – the predictive regulation of physiology. In this way, affect serves as a sort of barometer representing an individual’s place in the world, providing information about what that individual should do next to optimize physiological and psychological functioning. Affect is the foundation of both mood and TEAS, as well as discrete emotions like happiness, sadness, and fear4.
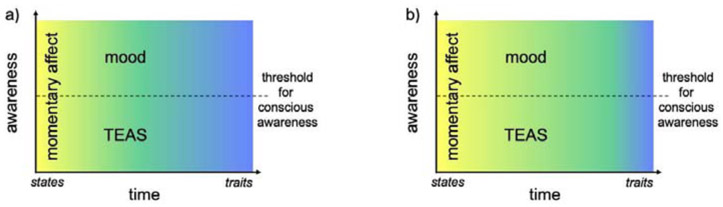
Figure 1: The relationship between affective phenomena and time scale.
Momentary affect is related to temporally quick or fleeting experience that can either be conscious or unconscious. At longer timescales, temporally extended affective states (TEAS) are differentiated from moods on the basis of conscious awareness. This temporal distinction can be mapped to the state (yellow) to trait (blue) continuum, where states are temporally bound and traits are general characteristics of individuals that are not bounded in time. We note that the temporal boundary between momentary affective states and TEAS/mood, represented here as the gradient transition between yellow and green, as well as the temporal boundary between state and trait, represented here as the gradient transition between yellow and blue, is not clear based on the literature and could be differentially positioned, as depicted in panels a and b.
Affect reflects the momentary status of an individual's place and fit in the environment. Consequently, affect is constantly changing as shifts occur both in the environment and, or, in an individual’s physiology. Typically, momentary updates to affect give the individual new information about how to act or what to do in a given situation. Affective states can be fleeting or can extend over prolonged periods; when human affective states extend over time and awareness to be felt consciously, we refer to them as moods (Gross, 1998; Larsen, 2000; Parkinson et al., 1996; Russell, 2005). Their extension over time is thought to reflect ongoing allostatic (physiological) processes that reflect the individual’s attempts to adapt or adaptation to the environment, changing and, or shifting intensity as the environment changes (for a similar argument, see Larsen 2001). In that way, these temporarily extended states provide information about our ability to face threats and challenges in the environment (Morris, 1992). Importantly, while most discussions of the psychology of affect and mood stipulate that they reflect internal physiological information and, or, environmental demands in the form of exteroceptive information, neither affect nor mood need have an object or be ‘about something’ (Gross, 1998; Larsen, 2000; Russell, 2005). Instead, both phenomena can be free floating, seemingly lacking a cause or object because interoceptive processes that are critical for the generation of affect are generated on a slower timescale than exteroceptive processes that provide information about the sensory world. This feature is rarely recognized in neuroscientific studies, which present discrete stimuli to induce changes in affect/mood and thus inherently link those states to the presented sensory stimuli (e.g., in humans, studies that present affect-related stimuli like emotion faces or words to induce mood, see Quigley et al., 2014 for a review on mood induction techniques).
Using temporal features to distinguish momentary affect from TEAS becomes particularly important when evaluating nonhuman animals, and in particular, the nonhuman primate (e.g., calling into question where the temporal boundary is between an affect state and a TEAS). There is a substantial debate regarding what constitutes a moment in affective experience – that is how an affective experience is temporally bound. Debate in particular surrounds the time course via which affective and emotional experiences unfold and people have the psychological experience of oscillating between experiences or perceive experiences to co-occur (Hoemann et al., 2017; Norris et al., 2010; Russell, 2017; Russell and Carroll, 1999). Bounding affective experiences in time to distinguish what is a momentary affect state from a mood state (in humans) may be an important philosophical undertaking but one that is well beyond the scope of this paper. For the purposes of discussion here, and in order to establish a model that can be applied to animals but has translational relevance to humans, we distinguish momentary affect from TEAS based primarily on the methodologies used to evaluate both in animals. Studies of momentary affect typically deliver an affect-related stimulus and evaluate behavior and biology concurrent with its presentation; often the presentation of the stimulus is very brief (e.g., a 100 ms shock or delivery of 10 uL of juice). In contrast, studies of TEAS deliver an affect-related stimulus and evaluate its impact on behavior and physiology both during and following its presentation to evaluate how TEAS unfold over time. We operationalize studies of affect versus TEAS in animals as related to the period of time in which the response is evaluated. On this view, briefly presented affective stimuli (e.g., shocks) may produce longer lasting TEAS (e.g., because they perturb physiology across longer durations or because after their initial presentation the animal anticipates the next shock). The TEAS may occur when the object of the initial affective response is no longer present and thus TEAS may be “objectless” (Russell, 2005).
The relationship between TEAS and traits
The importance of, and lack of clarity around the use of, timescales for differentiating affect-related phenomena is also germane to the distinction between states and traits made in the human mood literature. This distinction (e.g., state versus trait anxiety; (Spielberger et al., 1999)) is relevant for translational studies but made less often in the animal literature. States are momentary or temporary behaviors, actions, or experiences that may have some temporal duration, but from which an individual will return to some sort of baseline (noting that ‘baselines’ are not static but may change over time). Traits reflect general patterns of an individual’s behavior, action, and experience that represent the starting place (or ‘baseline’) for subsequent momentary experiences (Figure 1). The human literature widely recognizes that states and traits are related to each other – to wit, a person who is consistently in a particular state may be said to have a particular trait. For example, the human trait extraversion is characterized by high levels of positive affect and sociability (although the relationship between the two is complex, see Lucas et al., 2008; Lucas and Diener, 2001). Repeated states of positive affect therefore contribute to the trait extraversion. Further, a person’s ‘baseline’ may change over time. For example, an increased prevalence of negative mood states may lead to depression which itself becomes the ‘baseline’ from which subsequent affective states arise. Where the temporal boundary between state and trait, or even healthy mood and disorder, is drawn may ultimately be somewhat arbitrary. In the clinical literature the Diagnostic and Statistical Manual 5 assigns temporal features to mood disorders, but they are only one piece of a multi-faceted criteria set used for diagnosis (e.g., major depressive disorder requires symptomology including depressed mood or loss of interest/ pleasure for at least 2 weeks; persistent depressive disorder requires depressed mood for most of the day over most days during a 2 year period; APA, 2013).
For the purposes of discussion of the neurobiology of TEAS, the focus of this paper, we operationalize traits as long lasting behavioral and experiential patterns from which momentary behaviors and experiences arise. States are therefore momentary behaviors and experiences that may be temporally extended, rising to the level of TEAS but from which individuals return to their baseline (trait) functioning eventually. The sequelae of states may be such that a new momentary state arises before an individual returns to baseline. This is consistent with the idea that mood states drive the perception of and response to mood-relevant information creating a sort of ‘momentum’ between states (Eldar et al., 2016).
A Snapshot of the Neurobiology of Mood in Humans
Studies of the neurobiology of human mood, by and large, have been carried out in either 1) individuals who are experiencing disordered mood or 2) in healthy people under different experimental conditions using short term assessments (for literature and meta-analytic reviews: Drevets, 1998; Drysdale et al., 2017; Kaiser et al., 2015; Lindquist et al., 2016, 2012; Price and Drevets, 2009). The latter of these may be more consistent with affect or emotion than mood (given the short time scales; e.g., following mood inductions with music or autobiographical recall). Of the available methods, these have typically used electroencephalogram (EEG), positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) to glean insights into the brain regions involved in and brain activity dynamics of mood states. Collectively this body of work has demonstrated that there are brain regions that function as key hubs in larger networks that contribute to mood states (including, subcallosal anterior cingulate cortex (ACC), hippocampus, insula cortex, and amygdala). These large scale networks – often called functional or intrinsic networks – are linked to affect and mood and can be elucidated via the analysis of functional MRI data (Barrett and Satpute, 2013; Drysdale et al., 2017; Kaiser et al., 2015; Kleckner et al., 2017; Lindquist et al., 2016; Seeley et al., 2007; Touroutoglou et al., 2015, 2012). Thus, this work has been essential for constraining the areas involved in regulating mood to parts of prefrontal cortex and limbic system and also to demonstrating large scale patterns of activity in neural networks.
Despite the many insights garnered from neuroimaging studies in humans, neuroimaging approaches are de facto limited with regard to the temporal resolution of their phenomena of study (Sejnowski et al., 2014). Functional neuroimaging via MRI has a time scale of seconds and may provide important information about the dynamics of large-scale brain networks during mood states. But what functional MRI is actually measuring – that is, how activity of single neurons or multi-neuron ensembles scale to MRI signals – is still debated (Logothetis, 2008). Scalp EEG and magnetoencephalography (MEG) provide higher fidelity temporal signals than MRI, but themselves have some limitations. Scalp EEG is limited to recording from neuroanatomical regions on the surface of the brain, constraining it to cortex, although MEG has the capacity to record neuronal activity across the brain. Neither method is able to provide information about dynamics at the level of the activity of individual neurons or groups of neurons that happen on the order of milliseconds or oscillatory neural activity between areas that happens at frequencies of less than a second. Insights into the specific patterns of sub-second changes in neural activity that are associated with moods are largely lacking. For example, nowhere is this need for sub-second resolution more apparent than in the refinement of deep brain stimulation (DBS) approaches for the treatment mood disorders (Mayberg et al., 2005). High frequency DBS that is routinely used in the treatment of depression and Parkinson’s disease alters neural activity of interconnected circuits by functionally lesioning an area (Benabid, 2003). If we knew the specific pathways affected and aberrant neural dynamics that are engaged in depression, it might be possible to correct their failures with more targeted stimulation to reduce unwanted side effects (Rudebeck et al., 2019).
In contrast with the methodologies discussed above, invasive human electrophysiological studies do have the capacity to reveal information about the firing of single neurons, groups of neurons, and the oscillatory activity between brain areas with high temporal fidelity. However, these studies are only carried out in humans in very limited circumstances: typically, intracranial electrodes are placed in people with extremely debilitating cases of epilepsy in order to map seizure foci. In the period before a seizure occurs, these electrodes can also be used to understand neural dynamics related to cognitive and affective processes. A meta-analysis demonstrated that positive and negative affective states were supported by broadly distributed networks with hubs in neocortex, limbic and paralimbic regions, and subcortical nuclei (Guillory and Bujarski, 2014). Two recent studies of intracranial activity in humans build upon the findings of that meta-analysis (and the papers that it reviews) and now provide initial insights into the patterns of neural activity that support mood.
Kirkby and colleagues (Kirkby et al., 2018) identified a network centering on the amygdala and hippocampus which was related to the valence of patients’ moods while they were undergoing intracranial monitoring for epilepsy. In 13 out of the 21 patients in the study, increased variability of coordinated activity (β-frequency coherence at 13-30 Hz) between the amygdala and hippocampus was correlated with decreases in positive mood. No other areas or frequency bands exhibited similar relationship to mood. In an effort to develop a predictive neural model of mood, Sani and colleagues (Sani et al., 2018) recorded from multiple neural regions thought to be involved in mood (orbitofrontal cortex, anterior cingulate cortex, insula cortex, amygdala, and hippocampus, among other cortical regions) while tracking patients mood via self-report. Using individual level data, they were able to build a model for each patient that predicted mood across time (hours and days). Importantly, regional activity that predicted mood varied across patients – with some patients’ moods predicted by activity in the orbitofrontal cortex alone (N=3 of 7), one patient’s mood was predicted by activity in the amygdala alone, and another patient’s mood was predicted by activity in the hippocampus alone. The two remaining patients’ models included multiple areas – orbitofrontal cortex, dorsal anterior cingulate cortex and ventral anterior cingulate cortex for the first; and dorsal anterior cingulate cortex, hippocampus, and superior and middle frontal gyrus for the second.5 While the algorithms relied on different activity features across the patients, the best decoding of mood was made from lower frequency neural oscillations.
While providing foundational insight into the neural basis of moods, it is probably wise to treat findings from human intracranial recordings with caution. Patients with epilepsy have comorbidities and the areas likely involved in regulating moods are also those directly involved in the pathology of epilepsy, namely limbic structures (for a review, Onat et al., 2013). Finally, the small number of participants in these studies were physically restricted to a hospital bed, while receiving significant medication to manage pain produced by brain surgery.
Animal models and their inherent assumptions
Issues associated with studying the neural basis of mood in humans highlight the need for complementary approaches that allow the ability to probe, quantify, and causally manipulate biological systems in minute detail from the level of genes and epigenetic changes through to systems-level neurobiology and behavior. Animal models afford the ability to carry out such experiments, although they come with their own challenges, often in the form of assumptions that are made about how the model maps to human phenomena. The efficacy of the animal model becomes questionable in cases where the assumptions are false or where there is a paucity of research to support the assumptions.
Assumption 1: Overt animal behaviors map to discrete affective states in a one-to-one manner.
The first set of assumptions that animal model studies of human affect-related phenomena often make is about the mapping of overt behaviors to affect, moods, and discrete emotions. Often this is done without proper consideration of the face and construct validity of those measures. It is often assumed that if a behavior in animals looks like X (e.g., an emotion, a mental state, etc.) and X=Y in humans, then the behavior in question equals Y in the animal being studied as well. For example, many primates (including humans) generate a facial behavior in which the corners of the mouth are pulled back to expose the teeth (see Bliss-Moreau and Moadab, 2017 for a review). This facial behavior in monkeys has historically been referred to as the ‘fear grimace’ and evaluated (often, simply counted) as an index of fear. The behavior, however, occurs in many different contexts, including those that have nothing to do with submission or fear (e.g., during mating; (Allen and Lemmon, 1981) and accumulating evidence demonstrates that its meaning is context dependent (Beisner and McCowan, 2014). Thus, they are best interpreted as a complex social signal rather than as a vertical signal of an internal state (Bliss-Moreau and Moadab, 2017). Further, behaviors generated by animals are often confused for the constructs being studied, or the constructs that scientists are attempting to study (e.g., measuring a startle response and calling it ‘fear’) (for a similar argument, LeDoux, 2013). Part of this challenge is that behavioral outputs in both animals and humans, even very ‘simple’ behaviors (for a review, Maren et al., 2013), are variable and context dependent (A = B in Context 1, but A = C in Context 2)). Another part of this challenge arises because unlike humans, animals do not have the ability to verbally report on their internal states.
This challenge can be addressed via the use of what we have called translational tools (Bliss-Moreau et al., 2017; Bliss-Moreau, 2017) – tools that that can be deployed in humans and animals in the exact same form and without modification or anthropomorphic assumptions and that relate to affect in a specific way. These tools include measures of co-recorded sympathetic and parasympathetic autonomic nervous system activity that appear to track with affect in nonhuman animals (Bliss-Moreau et al., 2013)6, behavioral reactivity indices that quantify the intensity or magnitude of the affective response (generally, rather than recording specific behaviors thought to be indicative of affect or emotions like ‘freezing’) (Bliss-Moreau et al., 2011; Bliss-Moreau and Baxter, 2018; Bliss-Moreau and Moadab, 2016), and specific reflex-like behaviors, such as startle (Lang and Davis, 2006). In all of these methodological cases, there are direct homologs in human physiology and behavior that do not require anthropomorphism to animal affective states. Take for example, the case of autonomic nervous system physiology. In 2013, we documented that when we induced affective states in rhesus monkeys by having them watch videos of other monkeys that varied in valance, both parasympathetic and sympathetic activity of the heart tracked with the valence of the stimuli in patterns that are consistent with the human literature. Specifically, as stimuli ranged from negative to positive, parasympathetic activity (indexed by respiratory sinus arrythmia) increased and sympathetic activity (indexed by pre-ejection period) decreased (Bliss-Moreau et al., 2013). Similar patterns are observed in humans (for a meta-analysis Cacioppo et al., 2000). For example, respiratory sinus arrythmia is positively correlated with expressivity of positive emotions (Wang et al., 2013) and people with greater levels of trait positive affective experiences have higher respiratory sinus arrythmia than those with lower levels of trait positive affect (Oveis et al., 2009; Wang et al., 2013; although see for a discussion of heterogeneity in these effects see Overbeek et al., 2012).
In cases where specific reflex-like behaviors are recorded, we interpret them as evidence of an affective response, which may be characterized as having a magnitude/intensity or duration, but not a specific affect (e.g., negative affect) or specific emotion (e.g., ‘fear’). For example, imagine that the dependent variable for an experiment is whole-body startle in response to electric shocks. If an animal generates a whole-body startle response of 1V in response to shock A and 2V to shock B we would say that shock B generates an affective response that has two times the magnitude of shock B. In situations like this, shocks are often assumed to generate a negative affective response and often discussed as producing ‘fear’ (LeDoux, 2013). This inferential jump – between evidence of the affective response and it being negative affect or fear – represents a significant and important assumption, especially given heterogeneity in the human population with response to stimuli that are presumed to be negative (including having positive or pleasant experiences while watching others suffer, Cikara et al., 2011; or from physical injury, Nock, 2010) and shared neurobiology (Leknes and Tracey, 2008).
Assumption 2: The neurobiology of affect and affect-related phenomena is evolutionarily old and conserved across species.
A second set of assumptions often present in translational affective neuroscience is the assumption that neural structures that are responsible for affect-related phenomena (e.g., structures in the limbic system) are evolutionarily old and thus have been largely conserved; MacLean, 1990), at least across mammals. Perhaps the persistence assumption is part of the reason the vast majority of neurobiology studies of affect-related phenomena in animal models have used rodents. Decades of work in rodent models have elucidated circuitry associated with momentary affect responses both to negative and positive stimuli (for reviews: Kringelbach and Berridge, 2009; LeDoux, 2000). Trait-like changes to TEAS can be induced in rodents through immunological challenge (as has been done in humans) or by subjecting animals to stressful situations (for reviews Hodes et al., 2015; Krishnan and Nestler, 2008; Nestler and Hyman, 2010; Takahashi et al., 2018). In one of the most widely used paradigms, social defeat stress, mice are repeatedly paired with older mice who are liable to attack and aggressively interact with them, either because of age or sex differences (for a review Nestler and Hyman, 2010). Following these experiences, a proportion of the attacked mice exhibit a prolonged pattern of behavior indicative of a TEAS that may be akin to depression (these are often referred to as susceptible mice): they are uninterested in social interaction and other previously rewarding stimuli or situations. The induction of TEAS in this paradigm results in a host of biological changes in at nearly all levels of analysis from genetics to behavior. At the level of single neuron activity, mice exhibiting depression-like TEAS have altered activity patterns across a broad network of areas (Hultman et al., 2018), including a specific pattern of dopaminergic cell activity. Notably, optogenetic insertion of the pattern of dopaminergic firing observed in susceptible mice induces a depression-like TEAS in mice unaffected by social defeat stress (Chaudhury et al., 2012). In summary, social defeat stress and similar rodent models have enabled fundamental insight into the neurobiology of mood-like states in mice at nearly every level of study from genetics, epigenetics, single neuron physiology, circuit physiology, and behavior (Russo and Nestler, 2013).
Translating findings from rodent studies of affect or TEAS directly to humans is, however, not straightforward. Even extrapolating between rodent and non-human primates in the study of neurobiology of affect is not straightforward. It has been known for some time that there are differences in cortical neuroanatomy between rodents and non-human primates (Preuss, 1995; Wise, 2008), but more recently the variation between rodent and nonhuman primate subcortical structures implicated in mood has begun to be appreciated. For example, the relative size and composition of rat and monkey amygdala nuclei differ (Chareyron et al., 2011). Some monkey amygdala nuclei are not consistently larger than rat amygdala nuclei, such that the central and medial nuclei are only slightly larger (8 and 4 times larger, respectively), while the lateral, basal, and accessory basal nuclei are substantially larger (~37 times larger on average). Notably, neuron and glia density are also lower in the monkey than the rodent (Chareyron et al., 2011). Despite these differences, the temporal trajectories for postnatal amygdala nucleus development is similar in the rat and monkey (Chareyron et al., 2012a, 2012b). The functional significance of these differences and similarities is largely unknown.
A new study in marmoset monkeys (Wallis et al., 2017) also illustrates functional dissociation in anatomical regions in rodents and non-human primates. In rodents, prelimbic cortex, a division of the rodent medial frontal cortex, is required for the generating defensive behaviors related to threats, whereas another part of medial frontal cortex, infralimbic cortex, is required for storing memories about which stimuli lead to threats and thus generate defensive responses (for review and discussion: Baldi and Bucherelli, 2015; Quirk and Beer, 2006; Sierra-Mercado et al., 2011). In marmosets, by contrast, the opposite pattern is true. Furthermore, pharmacologically increasing activity of the area in marmosets thought to be homologous to rodent infralimbic cortex leads to reduced motivation for rewards, a pattern not observed in rodents (Wallis et al., 2017). These divergent findings reflect the marked differences in anatomical connections of the frontal cortex and subcortical structures between the two species, as well as enlargement and differentiation of the prefrontal cortex in primates. Prefrontal cortex may be particularly important for TEAS because evidence suggests medial prefrontal cortex maintains a representation of potential affective stimuli even when they are no longer physically present (Alexander et al., 2019; Powell and Ginsberg, 2005; Rudebeck et al., 2014). While variation in prefrontal cortex anatomy is likely critical for differences across rodents and primates, cortex expansion across the whole brain including areas of parietal and temporal cortex involved in social perception may also result in important differences (Hill et al., 2010; Sherwood et al., 2012). Thus, parts of the cortex in primates have taken on new functions given the differences in ecological niche, social structures, and millions of years of divergent evolution. If we are to make progress in understanding the neural basis of human moods, nonhuman primates are likely the next best thing to humans.
The Neurobiology of Temporally Extended Affective States in Nonhuman Primates
By comparison to the work in rodents, much less is known about the neurobiology of TEAS in non-human primates. The vast majority of neurobiological studies on nonhuman primates have focused on momentary affect (discussed in brief above), with little focus on how such states extend in time, highlighting a critical need for the study of temporal dynamics in the primate brain. Nevertheless, these studies have revealed important information about the neural hubs and temporal dynamics of momentary affective states that set the stage for greater consideration of temporal dynamics in TEAS. For example, brief aversive stimuli such as airpuffs or appetitive stimuli such as sweet liquids are signaled by punctate changes in the firing rates of neurons in amygdala, ventral striatum, orbitofrontal, and anterior cingulate cortex of monkeys (Costa et al., in press; Gothard et al., 2007; Matsumoto et al., 2007; Morrison et al., 2011; Paton et al., 2006; Rudebeck et al., 2013). These neural signals are dynamically modulated as animals learn which specific environmental stimuli predict the delivery of aversive or appetitive stimuli, mirroring learning mechanisms described by behaviorists. Neural activity in these areas is also influenced by the costs that might have to be incurred before pleasant stimuli are delivered, such as a delay or an effortful action meaning that they integrate both costs and benefits of different courses of action (Kennerley et al., 2009) and may be modulated by social context (Chang et al., 2012; Rakoczy et al., 2014; Watson and Platt, 2012). While these studies have been related to momentary affect, their main impact has been to inform understanding of neural basis of foraging behaviors in animals and provide a basis for economic behaviors in humans (for a review, Rushworth et al., 2011).
In relation to the neurobiology of TEAS, a small number of studies in nonhuman primates have looked at how factors like satiety or fatigue that accumulate during ongoing behavior influence neural activity (Bouret and Richmond, 2010; Critchley and Rolls, 1996). Amongst a number of areas, the ventromedial prefrontal cortex appears to be especially important for tracking these states. The activity of neurons in this area appear to track an animals’ progress through a session and their willingness to work (San-Galli et al., 2016). The issue with these studies is that it is challenging to disentangle the influence of reward satiety or fatigue from the core concepts that make up TEAS, namely valence and arousal.
In part to get around these issues, another set of studies in nonhuman primates have delivered specific patterns of electrical micro-stimulation to either dorsal ACC or striatum while nonhuman primates make approach-avoidance decisions (Amemori et al., 2018; Amemori and Graybiel, 2012). Because stimulation is counterbalanced across time (i.e. either early or late in a session) it is possible to dissociate changes in TEAS from reward satiety and task-related fatigue. Using this approach, the researchers could also look for temporally extended changes in decision-making that extend after micro-stimulation has ended. Any such long-lasting change would be indicative of a TEAS. Micro-stimulation of either dorsal ACC or striatum predominantly, although not always induced a negative bias in decision-making away from seeking rewards associated with aversive air puffs. Importantly, the effects were cumulative such that increasing lengths of stimulation led to longer periods of negative bias that persisted after stimulation ended. In addition, micro-stimulation of striatum that led to a negative bias during decision-making that was associated with a specific pattern of LFP activity, increased beta-oscillations. This finding potentially provides a specific neural mechanism associated with negative bias in decision-making. What neural mechanisms are associated with positive TEAS and whether micro-stimulation could be used to control the balance between positive and negative states will be a key avenue for future research.
Despite the paucity of a literature on the neurobiology of TEAS in healthy animals, a number of promising animal models of human mood-related psychopathology exist and some have begun to elucidate important information about neural mechanisms that may be relevant to TEAS. Such models typically rely on identifying behavioral patterns that are consistent with behaviors generated by humans with anxiety or depression, conferring face validity, and then interrogate the biological mechanisms that support the generation of such behavior. These models are often discussed as representing “anxious temperament” (Kalin, 2017; Kalin and Shelton, 2003), or “depression in monkeys” and while accumulating evidence demonstrates their validity relative to the human conditions, it is impossible to determine whether the phenomenological aspects across species are comparable. For that reason, we conceptualize these models as being models of trait TEAS rather than anxiety or depression per se. Trait TEAS studies either capitalize on naturally occurring variation in TEAS (e.g., animals high and low in anxiety) or studies that create long term changes to TEAS via experimental manipulation (e.g., via early life stress).
Macaques vary in their spontaneous generation of behaviors associated with high arousal negative TEAS and this variation is considered a phenotypic model for human anxiety and, or, behavioral inhibition. One particularly fruitful avenue of research has been to characterize macaques relative to the behaviors that they generate whichh are thought to be indicative of anxiety in humans. Such data can be used as evidence of trait TEAS. The advantage here is that variation in behavior and biology in animals that generate many of those behaviors as compared to those who do not can then be interrogated (for a review of nonhuman primate models, see Capitanio, 2018).
Characterizing animals in terms of trait TEAS varies by laboratory. Capitanio and colleagues (for a review Capitanio, 2018), evaluate 3-4 month old rhesus monkey infants via their BioBehavioral Assessment program which includes a standardized battery of affect-related and attention tasks, behavioral observations, and biological assays (e.g., cortisol) carried out over a 25 hour period. Behavioral responses are scaled (z-scored) within each annual birth cohort but can also be compared to historical data dating back many years. Infants who generate few affective responses (including vocalizations, facial behaviors, etc.) during the first behavioral observation (immediately after being relocated to the test room on Day 1) and who have a high frequency of producing vigilance behaviors during the 25-hour test period are said to have a “behaviorally-inhibited” phenotype. Behaviorally inhibited infants differ from non-behaviorally inhibited infants on a number of TEAS-relevant behavioral and physiological indices when tested both as infants and as juveniles. As juveniles, behaviorally inhibited animals, compared to non-behaviorally inhibited animals, generated more anxiety related behaviors when relocated to a new space (a test of anxiety), spent less time with their mothers and other adult females engaged in behaviors indicative of close social relationships (e.g., grooming) (Chun and Capitanio, 2016) and greater social stress induced depression-related behavior (Hennessy et al., 2014). They were also more likely to have hyper-responsive airways Capitanio et al., 2011; Chun et al., 2013). A combination of the components of behavioral inhibition from the BioBehavioral Assessment evaluations and cortisol responses to stress correctly classified 95% of the sample based on their airway response (Capitanio et al, 2011; Capitanio, 2018).
A similar approach has been taken to identify individual differences in defense-related behaviors, namely ‘freezing’, in response to anxiogenic stimuli, such as unknown humans or snakes (for a review Kalin, 2017; Kalin and Shelton, 2003). Animals with high numbers of these behaviors are said to have “anxious temperaments” which has been linked to alterations in brain metabolism in prefrontal cortex, the amygdala and anterior hippocampus (for a review Kalin, 2017). Animals with this phenotype have higher levels of activity in the amygdala and hippocampus as indexed via PET (Shackman et al., 2017, 2013). For some animals the phenotype is consistent over time (Shackman et al., 2017) and the phenotypic relationship to variation in function of the amygdala appears to be heritable (Fox et al., 2018, 2015). In humans, identification of behavioral inhibition or anxious temperament in childhood is associated with increased risk of going on to develop anxiety disorders or depression (for a review: Fox et al., 2005).
Macaques also develop a trait TEAS phenotype that is thought to be a model for human depression. While such a phenotype can occur spontaneously (without experimental manipulation; see Willard and Shively, 2012 for a discussion), models of persistent low arousal negative TEAS in nonhuman primates typically rely on experimental manipulations that are thought to be stressful, including manipulations of the early social environment (e.g., rearing in contexts with limited social interactions), manipulations of the adult social environment (e.g., single housing for brief durations of time), or manipulations of access to food (e.g., ‘variable foraging’ paradigms) (see Willard and Shively, 2012; Worlein, 2014 for reviews; but also Hennessy et al., 2017; Hennessy et al., 2014). Animals who generate behaviors consistent with this phenotype exhibit persistent low arousal negative trait TEAS evidenced by disengagement from the environment (reduced ingestion, movement around enclosure), hunched posture, increased risk of coronary artery atherosclerosis, alterations in metabolism and inflammation, lower weight/body mass, and higher heart rates (Hennessy et al., 2017; Hennessy et al., 2014; Shively et al., 2009, 2002; Xu et al., 2015; for a review Shively, 2017; Willard and Shively, 2012). Further, increased behavioral reactivity at 3-4 months of age (discussed above) predicted the magnitude of the behavioral phenotype for depression (namely hunched posture) later in life when relocated from a large social group to individual housing. The number of seconds that animals spent in the depressed-like posture (Hennessy et al., 2014) was similarly correlated with infant affective reactivity. This constellation of behavioral traits suggests that the anxiety-related phenotype early in life might predispose animals for depression-related experiences later in life.
There have only been a small number of neurobiological studies of animals with the low arousal negative trait TEAS phenotype, and none, to our knowledge, have included direct recordings from brain areas to elucidate neural dynamics in TEAS (reviewed in Shively, 2017). They do, however, point to the same set of neural hubs thought to be involved in momentary affect, human mood, and studies of monkey models of anxiety (discussed above). For example, compared to controls animals with the depression-like phenotype exhibit reduced serotonin receptor density in the raphe nucleus, amygdala, and anterior cingulate cortex (Shively et al., 2006) as well as reduced hippocampus volume (Willard et al., 2009). Clearly, further neurobiological study of macaques exhibiting state and trait TEAS is essential to uncovering the neural basis of this phenomena.
Conclusion and Future Directions
Our review highlights that the opportunities and challenges for studying state and trait TEAS in animals, focusing on nonhuman primates. As we note, we did not selectively review the nonhuman primate TEAS literature, rather it is the case that very little is known about how affect unfolds over time in the primate brain. Part of the challenge of this work is that it has relied on assumptions about homologies in neurobiology and behavior, and inferences about the mapping of behaviors to affective states, that may be unfounded or at the very least require additional empirical investigation. Here we set out the theoretical basis by which mood-like states in animals can be studied, highlighting key differences between TEAS, momentary affective responses (states), and TEAS-related traits (Figure 1). Further we emphasize that by using translational tools (e.g., cardiac physiology) to index TEAS during neural recording or ongoing behavior, and considering time as a major factor contributing to affect (that is, the temporal component of TEAS), it may be possible to bridge the nonhuman primate and human literatures and uncover the neural basis of mood-like states and speed forward the science of the neurobiology of mood. Connecting nonhuman primate and human work in order to fill the gaps in the literature on affective states will ultimately advance understanding of momentary human moods and persistent human mood-related pathology. Doing so has the potential to further the development of effective treatments and interventions to promote human health.
Highlights.
In humans, moods serve an important adaptive function, but their neural basis is unknown.
Identification of mood-like states in animals requires both behavioral and physiological markers.
Determining the neural basis of moods requires both correlative and causal studies in primates.
Acknowledgments
Preparation of this manuscript was supported by R21MH112539 to both EBM and PHR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Typically, mood is differentiated from other affect-related phenomena like emotions in terms of features like time course and duration, function, and objectlessness (or not being ‘about’ something). That said, it is often the case that the term mood is used without clearly defining it and, or, without indicating how it is the same or different from other affect-related phenomena (for a recent example, see Eldar et al., 2016).
For the purposes of this paper, we are agnostic about the process by which TEAS becomes mood. One possibility is that having the capacity for conscious awareness of TEAS generates mood, in an additive or constructed way. Another possibility is the mood is emergent from TEAS and consciousness.
Even single cell organisms generate behaviors that may demonstrate rudimentary affective processing. For example, bacteria and viruses engage in chemotaxis, moving towards stimuli that have the potential to benefit them (e.g., food sources) and away from stimuli that have the potential to harm them (e.g., concentrated acids) (Bliss-Moreau et al., 2018; Webre et al., 2003; Wolanin and Stock, 2004)(Webre et al., 2003; Wolanin and Stock, 2004; for more on this argument see Bliss-Moreau et al., 2018).
A detailed discussion of emotions and how affect forms the basis of emotions is beyond the scope of this paper. Briefly, psychological models posit that when conceptual knowledge about emotions is bound to affect via attentional processes and language, an affective state can materialize into an emotion (Barrett, 2014; Lindquist, 2013). For example, a person might experience a negative, high arousal affective state while watching two people fight. That affective state could become anger if the experiencer were to identify that one of the people had been racially profiled or fear if the provocateur were to turn his attention to the experiencer (for experimental evidence that illustrates this point see Lindquist and Barrett, 2008). The affective state in both of these situations would be the same although the emotion would be different.
It is important to note that the specific area in which the electrodes were placed varied, so while the results speak to individual differences, some variance may be accounted for by methodological variation.
Heart rate is the most widely used metric in nonhuman primate studies of affect (Bliss-Moreau et al., under review), but is influenced by activity in both the sympathetic and parasympathetic nervous systems (Brownley et al., 2000) and thus provides only an indication that there has been a change to autonomic nervous system activity but not an indication of the specific pattern or system controlling that change.
Contributor Information
Eliza Bliss-Moreau, University of California, Davis.
Peter H. Rudebeck, Icahn School of Medicine at Mount Sinai
References
- Alexander L, Gaskin PLR, Sawiak SJ, Cockcroft GJ, Clarke HF, Roberts AC, Fryer TD, Hong YT, 2019. Fractionating Blunted Reward Processing Characteristic of Anhedonia by Over-Activating Primate Subgenual Anterior Cingulate Cortex Article Fractionating Blunted Reward Processing Characteristic of Anhedonia by Over-Activating Primate Subgenual Anterior Cingulate Cortex. Neuron 101, 307–320.e6. 10.1016/j.neuron.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen ML, Lemmon WB, 1981. Orgasm in female primates. Am. J. Primatol 1, 15–34. 10.1002/ajp.1350010104 [DOI] [PubMed] [Google Scholar]
- Amemori K. ichi, Amemori S, Gibson DJ, Graybiel AM, 2018. Striatal Microstimulation Induces Persistent and Repetitive Negative Decision-Making Predicted by Striatal Beta-Band Oscillation. Neuron 99, 829–841.e6. 10.1016/j.neuron.2018.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemori KI, Graybiel AM, 2012. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat. Neurosci 15, 776–785. 10.1038/nn.3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA, 2013. DSM-5 Desk Reference, Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Publishing, Inc. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Baldi E, Bucherelli C, 2015. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci. Biobehav. Rev 53, 160–190. 10.1016/J.NEUBIOREV.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Barrett LF, 2017. How Emotions are Made: The Secret Life of the Brain. Houghton Mifflin Harcourt, New York. [Google Scholar]
- Barrett LF, 2014. The Conceptual Act Theory: A Précis. Emot. Rev 6, 292–297. 10.1177/1754073914534479 [DOI] [Google Scholar]
- Barrett LF, Bliss-Moreau E, 2009. Affect as a psychological primitive. Adv. Exp. Soc. Psychol 41, 167–218. 10.1016/S0065-2601(08)00404-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Russell JA, 1999. The Structure of Current Affect. Curr. Dir. Psychol. Sci 8, 10–14. 10.1111/1467-8721.00003 [DOI] [Google Scholar]
- Barrett LF, Satpute AB, 2013. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol 23, 361–372. 10.1016/J.CONB.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, McCowan B, 2014. Signaling context modulates social function of silent bared-teeth displays in rhesus macaques (Macaca mulatta). Am J Primatol 76, 111–121. 10.1002/ajp.22214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, 2003. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol 13, 696–706. 10.1016/J.CONB.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau Eliza, 2017. Constructing nonhuman animal emotion. Curr. Opin. Psychol 17, 184–188. 10.1016/j.copysyc.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG, 2011. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci 125, 848–858. 10.1037/a0025757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Baxter MG, 2018. Estradiol treatment in a nonhuman primate model of menopause preserves affective reactivity. Behav. Neurosci 132, 224–229. 10.1037/bne0000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Machado CJ, Amaral DG, 2013. Macaque Cardiac Physiology Is Sensitive to the Valence of Passively Viewed Sensory Stimuli. PLoS One 8. 10.1371/journal.pone.0071170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, 2017. The Faces Monkeys Make, in: Fernandes-Dols J-M, Russell JA (Eds.), The Science of Facial Expression. Oxford University press, New York, NY, pp. 153–171. [Google Scholar]
- Bliss-Moreau E, Moadab G, 2016. Variation in behavioral reactivity is associated with cooperative restraint training efficiency. J Am Assoc Lab Anim Sci 55, 41–49. [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Capitanio JP, 2017. Maternal rearing environment impacts autonomic nervous system activity. Dev Psychobiol 59, 551–556. 10.1002/dev.21513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Williams LA, Karaskiewicz CL, 2018. The Evolution of Emotion in Social Context, in: Shackelford TK, Weekes-Shackelford VA (Eds.), The Encyclopedia of Evolutionary Psychological Science. Springer, New York, New York. 10.1007/978-3-319-16999-6_2459-1 [DOI] [Google Scholar]
- Bouret S, Richmond BJ, 2010. Ventromedial and orbital prefrontal neurons differentially encode internally and externally driven motivational values in monkeys. J. Neurosci 30, 8591–8601. 10.1523/JNEUROSCI.0049-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownley KA, Hurwitz BE, Schneiderman N, 2000. Cardiovascular psychophysiology, in: Cacioppo JT, Tassinary LG, Berntson GG (Eds.), Handbook of Psychophysiology. Cambridge University Press, New York, NY, pp. 224–264. [Google Scholar]
- Cacioppo JT, Bernston GG, Larsen JT, Poehlmann KM, Ito TA, 2000. The psychophysiology of emotion, in: Lewis M, Haviland-Jones JM (Eds.), Handbook of Emotions. Guilford Press, New York, pp. 173–191. [Google Scholar]
- Capitanio JP, 2018. Behavioral Inhibition in Nonhuman Primates: The Elephant in the Room, in: Behavioral Inhibition. Springer International Publishing, Cham, pp. 17–33. 10.1007/978-3-319-98077-5_2 [DOI] [Google Scholar]
- Capitanio JP, Miller LA, Schelegle ES, Mendoza SP, Mason WA, Hyde DM, 2011. Behavioral Inhibition Is Associated With Airway Hyperresponsiveness but not Atopy in a Monkey Model of Asthma. Psychosom. Med 73, 288–294. 10.1097/PSY.0b013e3182155c83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Gariépy J-F, Platt ML, 2012. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci 16, 243–250. 10.1038/nn.3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Banta Lavenex P, Amaral DG, Lavenex P, 2011. Stereological analysis of the rat and monkey amygdala. J Comp Neurol 519, 3218–3239. 10.1002/cne.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Amaral DG, Lavenex P, 2012a. Postnatal development of the amygdala: A stereological study in macaque monkeys. J Comp Neurol 520, 1965–1984. 10.1002/cne.23023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Lavenex P, 2012b. Postnatal development of the amygdala: a stereological study in rats. J Comp Neurol 520, 3745–3763. 10.1002/cne.23132 [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai H-C, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han M-H, 2012. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536. 10.1038/nature11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun K, Capitanio JP, 2016. Developmental consequences of behavioral inhibition: a model in rhesus monkeys (Macaca mulatta ). Dev. Sci 19, 1035–1048. 10.1111/desc.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun K, Miller LA, Schelegle ES, Hyde DM, Capitanio JP, 2013. Behavioral Inhibition in Rhesus Monkeys (Macaca mulatta) Is Related to the Airways Response, but Not Immune Measures, Commonly Associated with Asthma. PLoS One 8, e71575. 10.1371/journal.pone.0071575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M, Bruneau EG, Saxe RR, 2011. Us and Them: Intergroup Failures of Empathy. Curr. Dir. Psychol. Sci 20, 149–153. 10.1177/0963721411408713 [DOI] [Google Scholar]
- Critchley HD, Rolls ET, 1996. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J. Neurophysiol 75, 1673–1686. 10.1152/jn.1996.75.4.1673 [DOI] [PubMed] [Google Scholar]
- Depression and Other Common Mental Disorders Global Health Estimates, 2017. Geneva. [Google Scholar]
- Drevets WC, MD, 1998. Functional Neuroimaging Studies of Depression: The Anatomy of Melancholia. Annu. Rev. Med 49, 341–361. 10.1146/annurev.med.49.1.341 [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey B, Dubin MJ, Liston C, 2017. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med 23, 28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Rutledge RB, Dolan RJ, Niv Y, 2016. Mood as Representation of Momentum. Trends Cogn. Sci 10.1016/j.tics.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Birn RM, Shackman AJ, Alexander AL, Kalin NH, 2018. Functional Connectivity within the Primate Extended Amygdala Is Heritable and Associated with Early-Life Anxious Temperament. J. Neurosci 38, 7611–7621. 10.1523/JNEUROSCI.0102-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, Converse AK, Alexander A, Davidson RJ, Blangero J, Rogers J, Kalin NH, 2015. Intergenerational neural mediators of early-life anxious temperament. Proc. Natl. Acad. Sci 112, 9118–9122. 10.1073/PNAS.1508593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM, 2005. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annu. Rev. Psychol 56, 235–262. 10.1146/annurev.psych.55.090902.141532 [DOI] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG, 2007. Neural Responses to Facial Expression and Face Identity in the Monkey Amygdala. J. Neurophysiol 97, 1671–1683. 10.1152/jn.00714.2006 [DOI] [PubMed] [Google Scholar]
- Gross JJ, 1998. The Emerging Field of Emotion Regulation: An Integrative Review. Rev. Gen. Psychol 2, 271–299. 10.1037/1089-2680.2.3.271 [DOI] [Google Scholar]
- Guillory SA, Bujarski KA, 2014. Exploring emotions using invasive methods: Review of 60 years of human intracranial electrophysiology. Soc. Cogn. Affect. Neurosci 9, 1880–1889. 10.1093/scan/nsu002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Chun K, Capitanio JP, 2017. Depressive-like behavior, its sensitization, social buffering, and altered cytokine responses in rhesus macaques moved from outdoor social groups to indoor housing. Soc. Neurosci 12, 65–75. 10.1080/17470919.2016.1145595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy Michael B., McCowan B, Jiang J, Capitanio JP, 2014. Depressive-like behavioral response of adult male rhesus monkeys during routine animal husbandry procedure. Front. Behav. Neurosci 8, 309. 10.3389/fnbeh.2014.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D, 2010. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. U. S. A 107, 13135–40. 10.1073/pnas.1001229107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M, Russo SJ, 2015. Neuroimmune mechanisms of depression. Nat. Neurosci 18, 1386–1393. 10.1038/nn.4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoemann K, Gendron M, Feldman Barrett L, Feldman L, 2017. Mixed emotions in the predictive brain. Curr. Opin. Behav. Sci 15, 51–57. 10.1016/j.cobeha.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman R, Ulrich K, Sachs BD, Blount C, Carlson DE, Ndubuizu N, Bagot RC, Parise EM, Vu MAT, Gallagher NM, Wang J, Silva AJ, Deisseroth K, Mague SD, Caron MG, Nestler EJ, Carin L, Dzirasa K, 2018. Brain-wide Electrical Spatiotemporal Dynamics Encode Depression Vulnerability. Cell 173, 166–180.e14. 10.1016/j.cell.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, 2017. Mechanisms underlying the early risk to develop anxiety and depression: A translational approach. Eur. Neuropsychopharmacol 27, 543–553. 10.1016/J.EURONEURO.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, 2003. Nonhuman Primate Models to Study Anxiety, Emotion Regulation, and Psychopathology. Ann. N. Y. Acad. Sci 1008, 189–200. 10.1196/annals.1301.021 [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD, 2009. Neurons in the Frontal Lobe Encode the Value of Multiple Decision Variables. J. Cogn. Neurosci 21, 1162–1178. 10.1162/jocn.2009.21100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby LA, Luongo FJ, Lee MB, Nahum M, Van Vleet TM, Rao VR, Dawes HE, Chang EF, Sohal VS, 2018. An Amygdala-Hippocampus Subnetwork that Encodes Variation in Human Mood. Cell 175, 1688–1700.e14. 10.1016/J.CELL.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Feldman Barrett L, 2017. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav 1, 0069. 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornell N, Son LK, Terrace HS, 2007. Transfer of Metacognitive Skills and Hint Seeking in Monkeys. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC, 2009. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn. Sci. 13, 479–487. 10.1016/j.tics.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2008. The molecular neurobiology of depression. Nature 455, 894–902. 10.1038/nature07455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie JA, Marcel AJ, 2002. Consciousness and the varieties of emotion experience: A theoretical framework. Psychol. Rev 109, 219–259. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, 2006. Emotion, motivation, and the brain: Reflex foundations in animal and human research. Prog. Brain Res 156, 3–29. 10.1016/S0079-6123(06)56001-7 [DOI] [PubMed] [Google Scholar]
- Larsen RJ, 2000. Toward a Science of Mood Regulation. Psychol. Inq 11, 129–141. 10.1207/S15327965PLI1103_01 [DOI] [Google Scholar]
- LeDoux JE, 2013. The slippery slope of fear. Trends Cogn. Sci 17, 155–156. 10.1016/J.TICS.2013.02.004 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 2000. Emotion circuits in the brain. Annu Rev Neurosci 23, 155–184. 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I, 2008. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci 9, 314–320. 10.1038/nrn2333 [DOI] [PubMed] [Google Scholar]
- Lindquist KA, 2013. Emotions emerge from more basic psychological ingredients: A modern psychological constructionist model. Emot. Rev 5, 356–368. 10.1177/1754073913489750 [DOI] [Google Scholar]
- Lindquist KA, Barrett LF, 2008. Constructing emotion: The experience of fear as a conceptual act. Psychol. Sci 19, 898–903. 10.1111/j.1467-9280.2008.02174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF, 2016. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cereb Cortex 26, 1910–1922. 10.1093/cercor/bhv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF, 2012. The brain basis of emotion: A meta-analytic review. Behav. Brain Sci 35, 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, 2008. What we can do and what we cannot do with fMRI. Nature 453, 869–878. 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Lucas RE, Diener E, 2001. Understanding extraverts’ enjoyment of social situations: The importance of pleasantness. J. Pers. Soc. Psychol 81, 343–356. 10.1037/0022-3514.81.2.343 [DOI] [PubMed] [Google Scholar]
- Lucas RE, Le K, Dyrenforth PS, 2008. Explaining the extraversion/positive affect relation: Sociability cannot account for extraverts’ greater happiness. J. Pers 76, 385–414. 10.1111/j.1467-6494.2008.00490.x [DOI] [PubMed] [Google Scholar]
- MacLean PD, 1990. The triune brain in evolution : role in paleocerebral functions. Plenum Press. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I, 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14, 417–428. 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Abe H, Tanaka K, 2007. Medial prefrontal cell activity signaling prediction errors of action values. Nat. Neurosci 10, 647–656. 10.1038/nn1890 [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH, 2005. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron 45, 651–660. 10.1016/J.NEURON.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Middlebrooks PG, Sommer MA, 2012. Neuronal Correlates of Metacognition in Primate Frontal Cortex. Neuron 75, 517–530. 10.1016/j.neuron.2012.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris WN, 1992. A functional analysis of the role of mood in affective systems. - PsycNET, in: Clark MS (Ed.), Review of Personality and Social Psychology, No. 13. Sage Publications, Inc., Thousand Oaks, CA, US, pp. 256–293. [Google Scholar]
- Morrison SE, Saez A, Lau B, Salzman CD, 2011. Different Time Courses for Learning-Related Changes in Amygdala and Orbitofrontal Cortex. Neuron 71, 1127–1140. 10.1016/J.NEURON.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, 2010. Animal models of neuropsychiatric disorders. Nat. Neurosci 13, 1161–1169. 10.1038/nn.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, 2010. Self-Injury, Annu. Rev. Clin. Psychol [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, Cacioppo JT, 2010. The current status of research on the structure of evaluative space. Biol. Psychol 84, 422–436. 10.1016/J.BIOPSYCHO.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat FY, van Luijtelaar G, Nehlig A, Snead OC, 2013. The involvement of limbic structures in typical and atypical absence epilepsy. Epilepsy Res. 103, 111–123. 10.1016/J.EPLEPSYRES.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D, 2009. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion 9, 265–270. 10.1037/a0015383 [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, van Boxtel A, Westerink JHDM, 2012. Respiratory sinus arrhythmia responses to induced emotional states: Effects of RSA indices, emotion induction method, age, and sex. Biol. Psychol 91, 128–141. 10.1016/j.biopsycho.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Parkinson B, Totterdell P, BRiner RB, Reynolds S, 1996. Changing moods: The psychology of moods and mood regulation. Addison-Wesley, Essex, England. [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD, 2006. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, 865–870. 10.1038/nature04490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Ginsberg JP, 2005. Single unit activity in the medial prefrontal cortex during pavlovian heart rate conditioning: Effects of peripheral autonomic blockade. Neurobiol. Learn. Mem 84, 200–213. 10.1016/J.NLM.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Preuss TM, 1995. Do Rats Have Prefrontal Cortex? The Rose-Woolsey-Akert Program Reconsidered. J. Cogn. Neurosci 7, 1–24. 10.1162/jocn.1995.7.1.1 [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC, 2009. Neurocircuitry of Mood Disorders, Neuropsychopharmacology. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley KS, Lindquist KA, Barrett LF, 2014. Inducing and measuring emotion and affect: Tips, tricks, and secrets, in: Reis HT, Judd C (Eds.), Handbook of Research Methods in Personality and Social Psychology. Oxford University Press, New York. [Google Scholar]
- Quirk GJ, Beer JS, 2006. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol 16, 723–727. 10.1016/J.CONB.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Rakoczy H, Clüver A, Saucke L, Stoffregen N, Gräbener A, Migura J, Call J, 2014. Apes are intuitive statisticians. Cognition 131, 60–68. 10.1016/j.cognition.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Rosati AG, Santos LR, 2016. Spontaneous Metacognition in Rhesus Monkeys. Psychol. Sci 27, 1181–1191. 10.1177/0956797616653737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Mitz AR, Chacko RV, Murray EA, 2013. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80, 1519–1531. 10.1016/J.NEURON.2013.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SEV, Murray EA, Rudekbeck PH, Putn, Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SEV, Murray EA, 2014. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc. Natl. Acad. Sci 111, 5391–5396. 10.1073/pnas.1317695111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Rich EL, Mayberg HS, 2019. From bed to bench side: Reverse translation to optimize neuromodulation for mood disorders. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1902287116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE, 2011. Frontal Cortex and Reward-Guided Learning and Decision-Making. Neuron 70, 1054–1069. 10.1016/J.NEURON.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Russell JA, 2017. Mixed Emotions Viewed from the Psychological Constructionist Perspective. Emot. Rev 9, 111–117. 10.1177/1754073916639658 [DOI] [Google Scholar]
- Russell JA, 2005. Emotion in Human Consciousness Is Built on Core Affect, Journal of Consciousness Studies. [Google Scholar]
- Russell JA, 2003. Core affect and the psychological construction of emotion. Psychol. Rev 110, 145–172. 10.1037/0033-295X.110.1.145 [DOI] [PubMed] [Google Scholar]
- Russell JA, Carroll JM, 1999. On the bipolarity of positive and negative affect. Psychol. Bull 125, 3–30. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ, 2013. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci 14, 609–625. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Galli A, Varazzani C, Abitbol R, Pessiglione M, Bouret S, 2016. Primate Ventromedial Prefrontal Cortex Neurons Continuously Encode the Willingness to Engage in Reward-Directed Behavior. Cereb. Cortex 28, 73–89. 10.1093/cercor/bhw351 [DOI] [PubMed] [Google Scholar]
- Sani OG, Yang Y, Lee MB, Dawes HE, Chang EF, Shanechi MM, 2018. Mood variations decoded from multi-site intracranial human brain activity. Nat. Biotechnol 36, 954. 10.1038/nbt.4200 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control., Journal of Neuroscience. Society for Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski TJ, Churchland PS, Movshon JA, 2014. Putting big data to good use in neuroscience. Nat. Neurosci. 10.1038/nn.3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH, 2013. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc. Natl. Acad. Sci 110, 6145–6150. 10.1073/pnas.1214364110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Oakes TR, Davidson RJ, Kalin NH, 2017. Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Mol. Psychiatry 22, 724–732. 10.1038/mp.2016.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, 2017. Depression in captive nonhuman primates: Theoretical underpinnings, methods, and application to behavioral management, in: Schapiro SJ (Ed.), Handbook of Primate Behavioral Management. CRC Press, Boca Raton, FL, pp. 115–125. 10.1201/9781315120652-8 [DOI] [Google Scholar]
- Shively CA, Friedman DP, Gage H, et al. , 2006. Behavioral depression and positron emission tomography–determined serotonin 1a receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry 63, 396–403. 10.1001/archpsyc.63.4.396 [DOI] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL, 2009. Stress, depression, and coronary artery disease: Modeling comorbidity in female primates. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Williams JK, Laber-Laird K, Anton RF, 2002. Depression and coronary artery atherosclerosis and reactivity in female Cynomolgus monkeys. Psychosom. Med 64, 699–706. 10.1097/01.PSY.0000021951.59258.C7 [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ, 2011. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538. 10.1038/npp.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, Marsh BJ, 1999. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI)., in: The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd Ed. Lawrence Erlbaum Associates Publishers, Mahwah, NJ, US, pp. 993–1021. [Google Scholar]
- Takahashi A, Flanigan ME, McEwen BS, Russo SJ, 2018. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front. Behav. Neurosci 12, 56. 10.3389/fnbeh.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer VL, Hampton RR, 2012. Rhesus monkeys (Macaca mulatta) show robust evidence for memory awareness across multiple generalization tests. Anim. Cogn 15, 409–419. 10.1007/s10071-011-0468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF, 2012. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage 60, 1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Lindquist KA, Dickerson BC, Barrett LF, 2015. Intrinsic connectivity in the human brain does not reveal networks for ‘basic’ emotions. Soc. Cogn. Affect. Neurosci 10, 1257–1265. 10.1093/scan/nsv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis CU, Cardinal RN, Alexander L, Roberts AC, Clarke HF, 2017. Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proc. Natl. Acad. Sci 114, E4075–E4084. 10.1073/pnas.1620115114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lu W, Qin R, 2013. Respiratory sinus arrhythmia is associated with trait positive affect and positive emotional expressivity. Biol. Psychol 93, 190–196. 10.1016/j.biopsycho.2012.12.006 [DOI] [PubMed] [Google Scholar]
- Watson KK, Platt ML, 2012. Social signals in primate orbitofrontal cortex. Curr. Biol 22, 2268–2273. 10.1016/J.CUB.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webre DJ, Wolanin PM, Stock JB, 2003. Bacterial chemotaxis. Curr. Biol 13, R47–R49. 10.1016/S0960-9822(02)01424-0 [DOI] [PubMed] [Google Scholar]
- Willard SL, Friedman DP, Henkel CK, Shively CA, 2009. Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology 34, 1469–1475. 10.1016/J.PSYNEUEN.2009.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Shively CA, 2012. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis). Am. J. Primatol 74, 528–542. 10.1002/ajp.21013 [DOI] [PubMed] [Google Scholar]
- Wise SP, 2008. Forward frontal fields: Phylogeny and fundamental function. Trends Neurosci. 31, 599–608. 10.1016/j.tins.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolanin PM, Stock JB, 2004. Bacterial chemosensing: Cooperative molecular logic. Curr. Biol 14, 486–487. 10.1016/j.cub.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Worlein JM, 2014. Nonhuman primate models of depression: Effects of early experience and stress. ILAR J. 55, 259–273. 10.1093/ilar/ilu030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Wu Q, Xie L, Gong W, Zhang J, Zheng P, Zhou Q, Ji Y, Wang T, Li X, Fang L, Li Q, Yang D, Li J, Melgiri ND, Shively C, Xie P, 2015. Macaques exhibit a naturally-occurring depression similar to humans. Sci. Rep 5, 9220. 10.1038/srep09220 [DOI] [PMC free article] [PubMed] [Google Scholar]