Abstract
Background and Objectives
Triptolide (TP) is known to impair testicular development and spermatogenesis in mammals, but the mechanism of the side effects still needs to be investigated. The aim of the research is to confirm whether TP can cause autophagy in TM3 Leydig cells and the potential molecular pathway in vitro.
Methods
TM3 Leydig cells are used to investigate the molecular pathway through Western blot, detection of apoptosis, transmission electron microscopy for autophagosomes and so on.
Results
The data show that TP treatment resulted in the decreasing of the viability of TM3 cells due to the increased apoptosis. Treated with TP, the formation of autophagosomes, the decrease in P62, and the increase in the conversion of LC3-I to LC3-II suggested the induction of autophagy. The induction of autophagy has accompanied the activation of the mTOR/P70S6K signal pathway. The viability of the TM3 cells was further inhibited when they were co-treated with autophagy inhibitor, chloroquine (CQ).
Conclusion
All these data suggest that autophagy plays a very important role in antagonizing TM3 cell apoptosis during the TP exposure.
Key words: autophagy, triptolide, TM3
Introduction
Infertility is a growing problem and about 8%–12% of reproductive-aged couples suffer worldwide,[1] with approximately 25%–30% having male-factor infertility.[2] In a long-term clinical observation, it has been found that reproductive issues associated with herbal therapies have increased recently, especially in patients with autoimmune disorders and cancer.[3]
Triptolide (TP) is a major active ingredient of Tripterygium wilfordii Hook F,[4] which exhibits various biological functions, including immunosuppression,[5] antitumor activity,[6] antiinflammatory,[7] neurotrophic, and neuroprotective effects.[8] However, TP has many side effects that can cause damage to multiple organs.[9] Furthermore, its reproductive toxicity has been proven serious, which limits its clinical application.
It has been demonstrated that TP can reduce estradiol and progesterone levels and increase follicle-stimulating hormone (FSH) and luteinising hormone (LH) levels,[10,11] even reduce the relative weights of the ovary and uterus, and increase follicular atresia.[12] In male rats, TP is capable of reducing the weight of the testis and epididymis, the motility and viability of sperm,[13] and increases instances of sperm deformity.[14] TP has not been shown to cause pathological changes in Leydig cells or the epididymal epithelium.[15] However, another study showed that TP exposure suppressed the marker enzymes of spermatogenesis and testosterone.[14,16] In brief, TP not only affects female reproduction, but also causes spermatogenesis dysfunction.
Testosterone is a major androgen that plays an important role in maintaining normal sexual function, spermatogenesis, and fertility in adult males. Leydig cells, one of the somatic cells in the testis, are located in the seminiferous tubules. Ninety-five percent of androgens are secreted by Leydig cells in normal adult male individuals. The steroid-synthesizing acute regulatory (StAR) protein, located on the mitochondrial membrane of cells, plays an important role in regulating steroid hormone synthesis.[17] The synthesis of steroid hormones requires precursor cholesterol to be transported from the mitochondrial outer membrane to the mitochondrial inner membrane. This is the limiting step in the synthesis of steroid hormones. After entering the mitochondria, cholesterol is cleaved by cytochrome P450 cholesterol side chain lyase and then hydroxylated to pregnenolone to synthesize testosterone.[18] It has been reported that testosterone levels affect spermatogenesis.[19] Abnormal spermatogenesis, a common pathophysiological process, is associated with male infertility. TP causes Leydig cell apoptosis,[20] but whether it can cause testicular steroidogenic toxicity and further affect spermatogenesis requires further investigation.
Autophagy is a metabolic process that maintains cell homeostasis and prevents the accumulation of harmful mutations. It is accompanied by other forms of cell death, such as apoptosis.[21] A previous review showed that TP can inhibit the proliferation of spermatogenic cells and induce cell apoptosis through oxidative stress, but whether TP acts on Leydig cells and its related mechanisms was not investigated. Based on these findings, we attempted to confirm whether TP can cause autophagy in TM3 Leydig cells and investigate the potential molecular pathway to explain how TP influences male infertility in vitro.
Materials and methods
Reagents
Cell culture medium, Dulbecco’s Modified Eagle Medium: F12 (DMEM/F12), and phosphate buffer solution (PBS) were purchased from Hyclone (USA). Fetal bovine serum was obtained from BI (Biological Industries, Israel), and TP was purchased from Beijing ChengZhiKewWei Biological Engineering (Beijing, China). Chloroquine (CQ) (cas: 5063-05), dimethyl sulfoxide (DMSO) (cas: 67-68-5), and monodansyl-cadaverine (MDC) were purchased from Sigma-Aldrich (USA). β-Actin (TA-09) and β-tubulin (TA-10) antibodies were purchased from Zhongshanjinqiao; anti-LC3B monoclonal antibody (#2775), anti-P62 monoclonal antibody (#8025), anti-mammalian rapamycin target protein (mTOR) monoclonal antibody (#2983), anti-p-mTOR monoclonal antibody (#5536) , anti-StAR monoclonal antibody (#8449), anti-p70s6k monoclonal antibody(#2708), anti-p-p70s6k monoclonal antibody (#9234) , anti-4E-BP1 monoclonal antibody (#9644) , and anti-p-4E-BP1 antibody (#2855) were purchased from Cell Signaling Technology (USA). The cell-counting kit (CCK)-8 and Annexin V-FITC/propidium iodide (PI) apoptosis assay kits were obtained from Kaiji Biological Technology (Nanjing, China).
Cell culture
TM3 cells were obtained from Professor Haoshu Luo of China Agricultural University. DMEM/F12 enriched with 10% fetal bovine serum was used for the cell culture. All cells were maintained at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Cell viability assay
TP was dissolved in DMSO at a concentration of 200 mmol/L as a stock solution and stored at –20°C until use. To evaluate the toxic effect of TP on cell growth, TM3 cells were plated at a density of 4 × 103 cells/well in 96-well culture plates. After the cell attachment density was approximately 50–60%, the medium was discarded and replaced with 100 μL of the fresh serum-free medium containing various concentrations (25, 50, 100, 150, and 200 nmol/L) of TP and five replicate wells were set in each group and cultured for 12, 24, 36, and 48 h. After the treatment, the cell viability was determined the CCK-8 assay. Briefly, the culture medium was removed and 100 μL of fresh serum-free medium containing 10% CCK-8 was added to each well and incubated for 1 h at 37°C in the dark. Absorbance was then measured at 450 nm.
Detection of apoptosis
Apoptosis was quantified using an Annexin V-FITC/PI apoptosis kit. TM3 cells were seeded at a density of approximately 20%–30% on 6-cm plates. When the attachment density was approximately 60%–70%, the cells were treated with varying concentrations of TP (50, 100, and 200 nmol/L) or a control (0.1% DMSO). Twenty-four hours later, TM3 cells were rinsed once with PBS, digested with trypsin lacking EDTA, centrifuged at 800 rpm/min for 5 min, washed twice with cold PBS, and stained with 5 μL PI and 5 μL Annexin V-FITC in 500 μL of binding buffer in the dark at room temperature for 15 min. Cell apoptosis was determined using a FACScan flow cytometer (Becton-Dickinson, USA).
Monodansyl cadaverine (MDC) staining
MDC can be used to specifically detect the formation of autophagic vacuoles in macrophages and acidic organelles. Chemical staining with MDC was used to detect the acidic vesicular organelles. TM3 cells were seeded at a density of 20%–25% in a 35-mm confocal glass bottom dish. After attachment density was approximately 60%–70%, the cells were treated with varying concentrations of TP (50, 100, and 200 nmol/L) or a control (0.1% DMSO) for 24 h. The medium was discarded and 2 mL complete medium containing 50 μmol/L monodansyl cadaverine was added and incubated for 30 min at 37°C in the dark, washed three times with PBS, and observed under a laser confocal microscope.
Transmission electron microscopy (TEM) for autophagosomes as an evidence of autophagy
TEM is a common methods for monitoring autophagy. TM3 cells were plated at a density of approximately 20%–30% on 10-cm plates. After attachment density was approximately 60%–70%, the cells were treated with various concentrations of TP (50, 100, 200 nmol/L) or a control (0.1% DMSO) for 24 h. The cells were harvested, washed, and fixed with 1% glutaraldehyde at 4°C. Samples were further postfixed in 1% osmium tetroxide, dehydrated in serial acetone, and embedded in Epon 812 resin; the ultrathin sections were stained with uranyl acetate and lead citrate, and then observed and photographed under a transmission electron microscope (JEM-1230, JEOL, Tokyo, Japan) at 80 kV.
Western blot analysis
TM3 cells were plated at a density of approximately 20%–30% on 6-cm plates. After attachment density was approximately 60%–70%, the cells were treated with various concentrations of TP (50, 100, and 200 nmol/L) of a control (0.1% DMSO) for 24 h. Cells were washed with precooled PBS, RIPA lysate was added, centrifuged at 12,000 rpm for 10 min at 4°C, and the supernatant was collected. The protein concentration was determined using the BCA method (Beyotime Biotechnology, China). The protein was adjusted to an equal concentration with bromophenol blue and a lysate, and denatured at a high temperature. Equal amounts of protein (20–40 μg/lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, electrophoresis was performed at 80 V for 30 min and 120 V for 90 min, and then the gel was transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, USA). The membranes were blocked with 5% BSA or skim milk solution for 1 h at room temperature. The membranes were incubated overnight with monoclonal primary antibodies in Tris-buffered saline with 0.1% Tween20 detergent (TBST) solution. The following primary antibodies were used: β-actin, β-tubulin, LC3B, P62, p-mTOR, mTOR, p-p70s6k, p70s6k, p-4EBP1, 4EBP1 (1:1000 in TBST), and StAR (1:500 in TBST). After being washed three times with TBST, the membranes were incubated with secondary HRP-conjugated anti-rabbit/mouse IgG antibody (1:5000 in TBST) for 1 h at room temperature. Finally, the protein bands were visualized using an enhanced chemiluminescence detection system. The data were normalized to the corresponding internal reference β-actin or β-tubulin. Immunoblotting densitometry analysis was performed using ImageJ software version 1.4 . All samples were analyzed in triplicate.
Autophagy inhibitor treatment
Chloroquine, a specific autophagy inhibitor, was prepared as a 50-mmol/L stock solution in DMSO. For analysis of autophagy inhibitors, cultured cells were cotreated with the inhibitor at a final concentration of 50 mmol/L with 0.1% DMSO for 24 h. Control cells were treated with 0.1% DMSO.
Statistical analysis
Statistical analysis was performed using the statistical software SPSS version 24.0 (Chicago, IL, USA). All values are presented as the mean ± standard deviation. The data were analyzed using one-way ANOVA. Statistical significance was set at P < 0.05. All data were obtained from at least three independent experiments.
Results
TP inhibits activity of TM3 cells in vitro
To determine the cytotoxicity of TP in vitro, TM3 cells were treated with TP at various concentrations for 12, 24, 36, and 48 h. Cell viability was measured using CCK-8 cell proliferation assay. As shown in Figure 1, TP inhibited TM3 cell growth in a dose- and time-dependent manner. When cells were exposed to 100 nmol/L TP for 24 h, the cell viability was 58%, resulting in a significant decrease compared to the control group. At the maximum concentration tested (200 nmol/L), the cell viability decreased to 74%, 35%, 19%, and 10% at 12, 24, 36, and 48 h, respectively, indicating that the cytotoxicity of TP is time-dependent. Results represent the average of three independent experiments.
Figure 1.
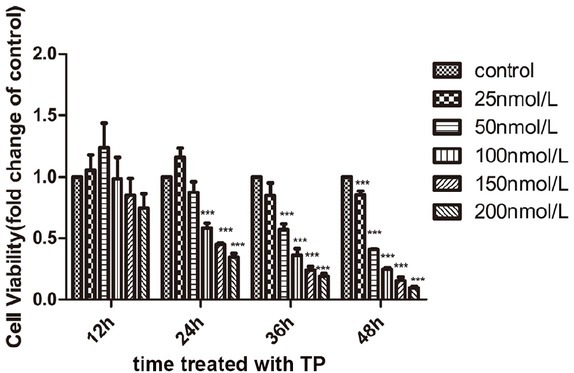
TP inhibits cell activity of TM3 cell. TM3 cells were treated with various concentrations of TP for 12, 24, 36, and 48 h or a control (0.1% DMSO) and then measured using CCK-8 proliferation assay. The results are expressed as mean ± SD (***P < 0.01 vs. control, n = 3). TP: triptolide; DMSO: dimethyl sulfoxide.
TP induces apoptosis of TM3 cells in vitro
To elucidate the mechanism by which TP causes cell death in TM3 cells, we monitored one of the apoptosis markers, Annexin, using V-FITC/PI staining. Cells were treated with various concentrations of TP for 24 h and then harvested for the apoptosis assay. The cytotoxic effects of TP were confirmed using flow cytometry. The cells in early apoptosis (Annexin V+/PI-) fell into the lower right quadrant, and the late apoptosis cells (Annexin V-/PI+) fell into the upper right quadrant. As shown in Figure 2A–2D, the apoptosis rate increased after treatment with TP (50, 100, and 200 nmol/L) for 24 h in a concentration-dependent manner. Our results also indicated that the apoptosis rate was significantly increased in response to 200 nmol/L TP compared to the control group (Figure 2E).
Figure 2.
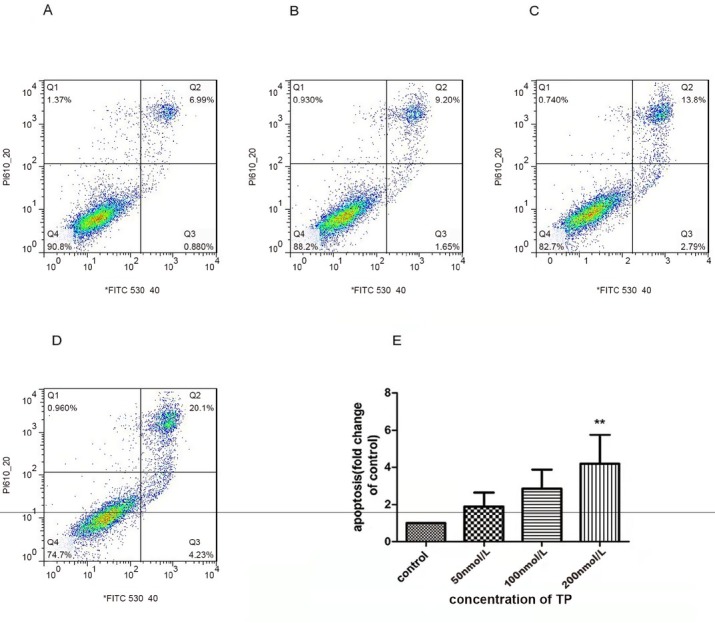
Effects of TP on cell apoptosis in the TM3 cells. A–D. Apoptosis in TM3 cells was detected after 24 h of treatment with TP at various concentrations (B: 50 nmol/L; C:100 nmol/L; D: 200 nmol/L) or a control (A : 0.1% DMSO) using Annexin V-FITC/PI binding and measured using flow cytometry analysis. E: The chart illustrates apoptosis proportion from three independent experiments (**P < 0.05 vs. control group, n = 3). TP: triptolide; DMSO: dimethyl sulfoxide.
Effect of TP on StAR expression of TM3 cell
TP inhibited StAR expression in TM3 cells. As shown in Figure 3, StAR expression in TM3 cells exposed to 100 or 200 nmol/L TP for 24 h significantly reduced compared to the control.
Figure 3.
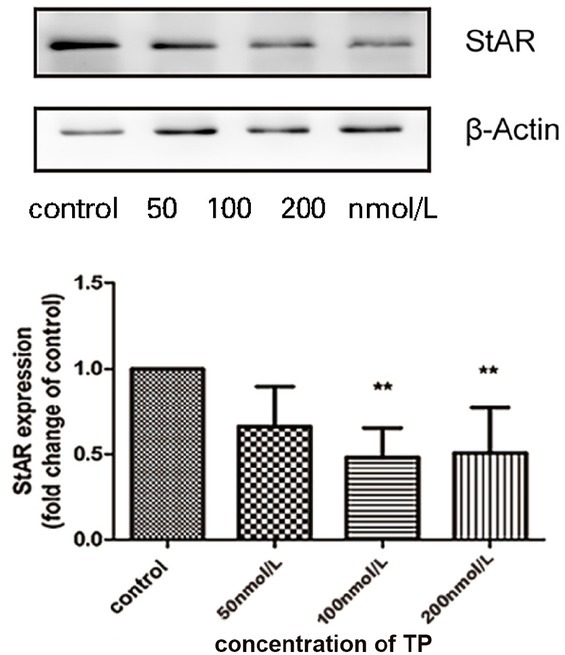
Effect of TP on StAR expression of TM3 cell. TM3 cells were treated with TP (50, 100, and 200 nmol/L) or a control (0.1% DMSO) for 24 h. Bar graphs are representative of three independent experiments (**P< 0.05 vs. control group, n = 3). TP: triptolide; StAR: steroid-synthesizing acute regulatory; DMSO: dimethyl sulfoxide.
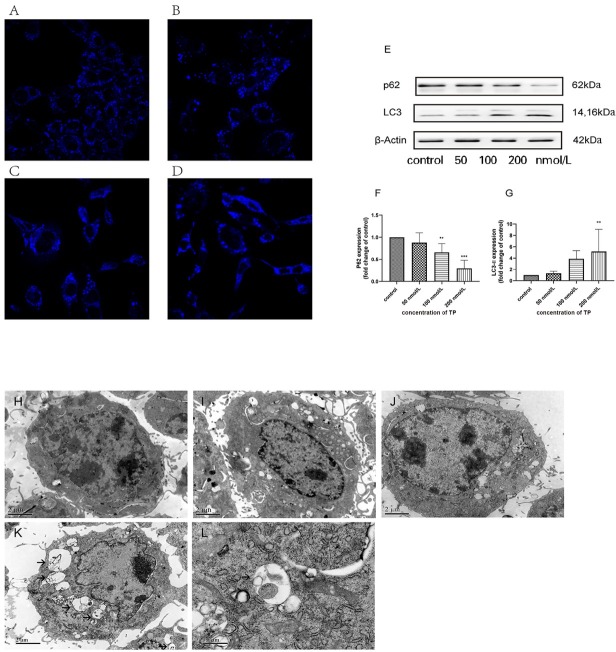
TP increases autophagy of TM3 cell in vitro
The formation of autophagosomes during autophagy can be detected by classical methods, including MDC staining, western blotting, and electron microscopy.
MDC is a fluorescent dye that can be absorbed by cells and is displayed on autophagy vesicles. MDC-labeled bubble vesicles can be used to evaluate the level of autophagy in TM3 cells. MDC staining demonstrated that the treated groups exhibited higher fluorescence intensity and more MDC-labeled puncta compared to the control group. As shown in Figure 4A–4D, these results indicate that TP treatment increased autophagy in TM3 to some extent.
Figure 4.
High concentration TP triggers autophagy in TM3 cells. A–D. To characterize autophagy, we used MDC staining to observe the acidic compartment in TP-treated TM3 cell. Cells were treated with various concentration for 24 h, (A) control, (B) 50 nmol/L, (C) 100 nmol/L, (D) 200 nmol/L. E. Expression of autophagy-related protein following treatment with TP (50, 100, and 200 nmol/L) for 24 h. F–G: Bar graphs are representative of three independent experiments. (**P < 0.05,***P < 0.01 vs. control group, n = 3). H–L. Ultrastructural evidence of TP-induced autophagy of TM3 cells. TM3 cells were treated with control (H), 50 nmol/L TP (I), 100 nmol/L TP (J), 200 nmol/L TP (K) for 24 h. Autophagic vacuoles were visualized using TEM (12,000× magnification). L. The classic structure (15,000× magnification) treated with 200 nmol/L TP. The autophagic vacuoles are indicated by arrows. Scale bar: H-K=2 μm, L=0.5 μm. TP: triptolide; TEM: Transmission electron microscopy.
Furthermore, we evaluated the LC3-II and p62 protein levels by western blotting. The data (Figure 4E–4G)) showed that compared with the control group, the protein p62 decreased and the ratio of LC3-II/LC3-I increased with TP treatment (50, 100, and 200 nmol/L) for 24 h. These western blots were consistent with the fluorescence results, indicating that TP induced autophagy in TM3 cells.
Autophagy was morphologically defined by TEM in TM3 cells to confirm that it was induced by TP treatment. As depicted in Figure 4H–4L, TM3 cells treated with TP (100 and 200 nmol/L) exhibited several autophagosomes that were absent in the control cells. These results indicated that TP induced autophagy in TM3 cells.
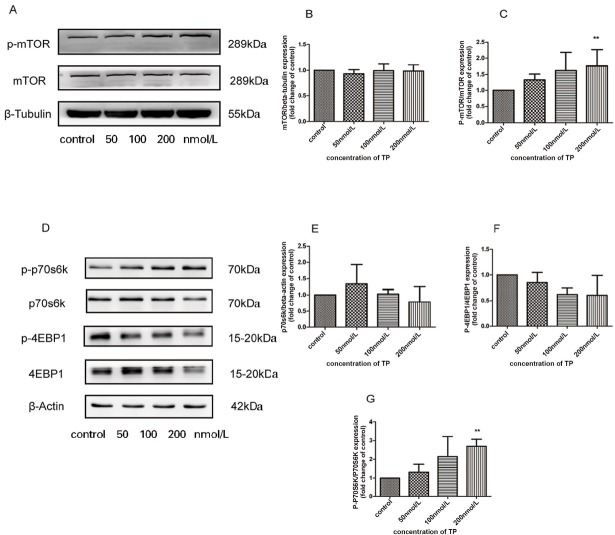
Effect of TP on the activation of mTOR signaling in cultured TM3 cells
To assess the involvement of the mTOR signaling pathway in TP-induced apoptosis and autophagy of TM3, the expression levels of both total and phosphorylated proteins were evaluated by western blot analysis. TM3 cells were treated with various concentrations TP (50, 100, and 200 nmol/L) or a control (0.1% DMSO) for 24 h. As shown in Figure 5, we observed the activation of p-mTOR and p-p70s6k in a dose-dependent manner, and a significant difference was observed in 200 nmol/L group. However, we observed no change in the total mTOR and p70s6k concentrations after TP treatment. Suppression of p-4EBP1 was observed in TM3 cells, but there was no significant difference after treatment for 24 h.
Figure 5.
TP activates mTOR signaling pathway in cultured TM3 cells. Western blot analysis was used to detect the proteins expression.A: Expression of p-mTOR, mTOR protein following treatment with TP (50, 100, 200 nmol/L for 24 h.B-C: Bar graphs were representative of three independent expriments.D: Exrpession of p-p70s6k, p70s6k, p-4EBP1,and 4EBP1 protein following treatment with TP (50, 100, 200 nmol/L for 24 h.E-G: Bar graphs were representative of three independent expriments. Data were shown as mean±SD. (**P ﹤ 0.05 vs. control group, n = 3). TP: triptolide.
Autophagy inhibitor treatment
After exposure to the autophagy inhibitor CQ, we detected P62 protein by western blotting. As shown in Figure 6, no differences in p62 protein were apparent between the CQ and control groups. No significant difference was noted between the TP treatment and TP-CQ cotreatment groups. In addition, flow cytometry was used to detect apoptosis. No significant difference was observed in the CQ group compared to the control group. Although apoptosis increased in the TP-CQ cotreatment group compared to the TP treatment group, the difference was not statistically significant. CCK-8 was used to measure the cell viability, and no significant difference was observed between the CQ group and the control group. Compared with the TP group, cell viability was significantly reduced in the TP-CQ cotreatment group.
Figure 6.
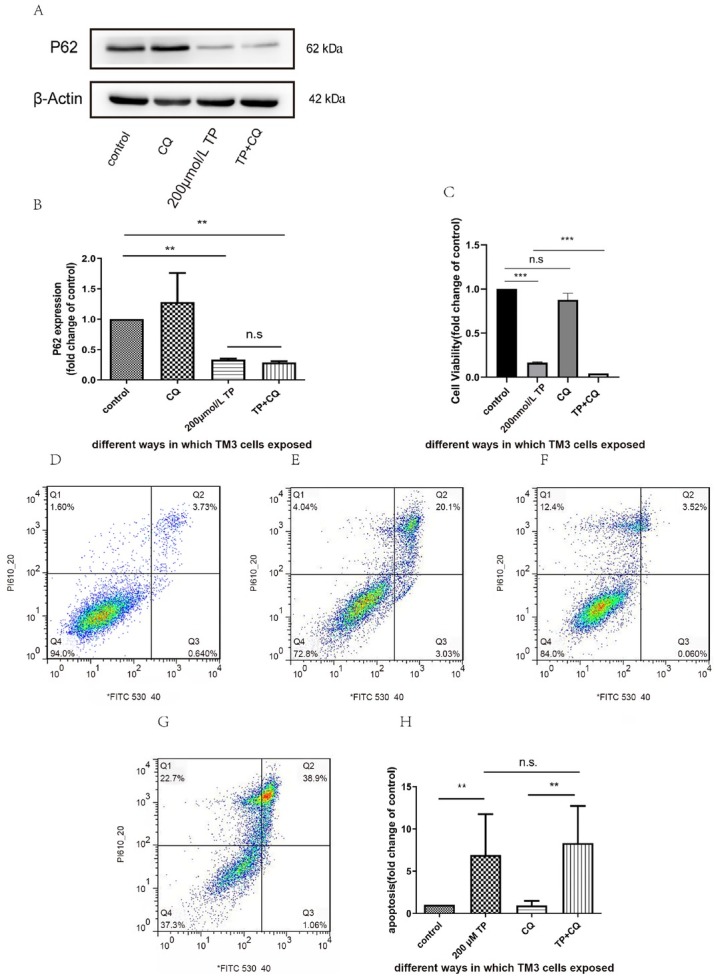
Effects of CQ on TP-induced apoptosis. A–B: TM3 cells were pretreated with 50 nmol/L of CQ for 1 h, followed by 200 nmol/L TP treatment for 24 h. Western blot analysis and relative densitometry of P62 protein. Values are expressed as fold mean ± SD. C: Cell viability was measured using the CCK-8 assay. The results are presented as mean ± SD. D-H: TM3 cell were stained with Annexin V and propidium iodide, and the apoptotic was determined using flow cytometry (D: control, E: 200 μmol/L TP, F: CQ, G: TP+CQ,H:Bar graphs were representative of three independent expriments). The results were presented as mean±SD. **P ﹤ 0.05, ***P ﹤ 0.01 vs. control group, n = 3. CQ: chloroquine; TP: triptolide. n.s: no significant.
Discussion
Autophagy is highly conserved during evolution and is a metabolic process in which eukaryotic cells degrade intracellular components through lysosomes, maintaining cell homeostasis and cell survival.[22] Studies have confirmed that autophagy plays an important role not only in cancer, [23] neurodegeneration,[24] and cardiovascular disease,[25] but also in male reproductive dysfunction,[26] especially in the formation of abnormal sperm.[27] Spermatogenesis from the proliferation and differentiation of undifferentiated spermatogonial stem cells is based on the complete function of the tissue structure.[28, 29, 30] In addition to supporting cells, Leydig cells play an important role in maintaining spermatogenesis[31, 32, 33] and are affected by various chemicals and drug metabolism. [34, 35, 36, 37]
TP is a widely used immunosuppressive agent that has adverse effects on the male reproductive system, as well as hepatotoxicity and nephrotoxicity.[38] It has been reported that TP can reduce the cAMP content in HCG-induced cells and then disrupt the cAMP/protein kinase A (PKA)-mediated expression of estrogen synthase enzymes, leading to reduced estradiol synthesis.[39] TP can bind to the active site of human estrogenic 17-β-hydroxysteroid dehydrogenase and human estrogen receptor.[40] TP is observed on the inhibition of the mRNA expression of most genes related to steroigenesis.[41,42] All that provide new views into the impact of steroidogenesis on the severe reproductive toxicity of TP. The steroidal toxicity of TP might be mainly due to disruption of direct cytotoxicity, which would lead to reduced sex steroid synthesis and reproductive dysfunction.[42] However, there have been no reports of autophagy in the testes after TP exposure. We used TM3 cells as a model to explore the effects and molecular mechanisms of TP on male reproduction in vitro. In this study, we first investigated whether TP can induce autophagy activation in TM3 cells (Figure 4), which is accompanied by activation of the mTOR signaling pathway (Figure 5), and we found that inhibition of autophagy by CQ further reduced cell viability.
We found that TP not only inhibited the viability of TM3 cells, but also decreased the expression of StAR (Figure 3). These results are consistent with those of the previous studies reporting that inhibition of TM3 cell viability may affect testosterone expression.[20] In addition, studies have shown that TP significantly inhibits cell viability at 50 nmol/L and significant apoptosis (Figure 2) at higher concentrations (200 nmol/L). Thus, there may be other mechanisms involved in regulating cell viability, and autophagy may be one of them. Studies have also shown that Leydig cell autophagy is involved in the synthesis and regulation of testosterone. [43]
According to previous studies, microtubule-associated protein light chain 3 (LC3) is an important biomarker of autophagy and is present in two forms: the cytosolic, LC3-I, and the membrane-bound form, LC3-II.[44] The cytosolic LC3-I is converted into LC3-II when the autophagy process is activated, so the level of autophagy can be measured by the amount of LC3-II. In this study, we found that TP could significantly increase the accumulation of LC3-II and accelerate the decomposition of P62, meanwhile the number of acidic vesicles increased. TEM was used as another method to identify autophagy (Figure 4), as this is ‘gold standard’ of autophagy identification. [44] Based on these results, we concluded that TP induced autophagy in TM3 Leydig cells.
To further explore the connections between cytotoxicity, autophagy, and apoptosis, we used CQ as an autophagy inhibitor which blocked the last step of the autophagy pathway.[45] CQ, a lysosomal lumen alkalizer, blocks the autophagic process by impairing lysosomes.[46] CQ has been reported to be the only clinically relevant autophagy inhibitor. It is widely used as an antimalarial and antirheumatic agent.[47] CQ disrupts the pH value of acidic vesicles, prevents the fusion of autophagosomes with lysosomes, and subsequently impairs autophagic vesicle clearance. [47] TP-induced cell death was accelerated by CQ through inhibition of autophagy (Figure 6C), but apoptosis of TM3 Leydig cell toxicity could not be mitigated by autophagy (Figure 6D-H). This indicated that autophagy could protect cells, which is in agreement with previous research.[48]
Autophagy stabilizes the intracellular environment and maintains cell survival by synthesizing balanced anabolism and catabolism.[49] The mTOR signaling pathway is also a major signal transduction cascade involved in cell metabolism, proliferation, and survival,[50] and plays an important role in autophagy.[51] Our results showed that the mTOR/P70S6K signaling pathway was activated (Figure 5). A previous study reported that the activation of mTOR phosphorylates the p70 ribosomal subunit protein S6 kinase p70S6K, which could promote protein synthesis.[52] Studies have shown that activation of this pathway could have an antiapoptotic function through a series of phosphorylations.[53] At the same time, some studies have confirmed that this pathway contributes to spermatogenesis[55] and participates in the self-renewal of spermatogonial stem cells. [55] Therefore, we considered that activation of the mTOR signaling pathway is associated with abnormal cell survival conditions. In contrast, our results showed that activated autophagy had a protective effect on TP-induced cytotoxicity (Figure 6). To our knowledge, mTOR is an important negative regulator of autophagy, [56] and our results conflicted with this view. The signaling pathway is complex in regulating the development of autophagy. Perhaps TP-induced autophagy does not work through mTOR, but this requires further research.
Conclusion
Our study demonstrates that TP inhibits cell viability and expression of StAR protein. It is the first time that TP has been reported to induce autophagy activation in TM3 cells and the autophagy plays a very important role in antagonizing TM3 cell apoptosis during TP exposure. This provides a novel molecular mechanism responsible for the regulation of TM3 cells following TP exposure.
Footnotes
Source of Funding
This work was funded by a grant from the Natural Science Foundation of Beijing Municipality (Grant Serial No:7142158).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Nowak I, Wilczynska K, Radwan P, Wisniewski A, Krasinski R, Radwan M. Association of Soluble HLA-G Plasma Level and HLA-G Genetic Polymorphism With Pregnancy Outcome of Patients Undergoing in vitro Fertilization Embryo Transfer. Front Immunol. 2019;10:2982. doi: 10.3389/fimmu.2019.02982. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz DJ, Teloken P, Shoshany O. Male infertility-The other side of the equation. Aust Fam Physician. 2017;46:641–6. [PubMed] [Google Scholar]
- 3.Li S, Li C, Cheng X, Liu X, Han M. Research Progress of Male Reproductive Toxicity of Chinese Materia Medicas. Evid Based Complement Alternat Med. 2019;2019:7249679. doi: 10.1155/2019/7249679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs in R&D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Qi Q, Li Q, Zhu H, Lu H, Yang X, Wu Y. Triptolide analog LLDT-8 ameliorates psoriasis-like dermatitis in BALB/c mice via suppressing the IL-36α signaling pathway. Pharmacol Res. 2021;169:105678. doi: 10.1016/j.phrs.2021.105678. et al. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Ma L, Zhou GB. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules. 2011;16:5283–97. doi: 10.3390/molecules16065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyle GW, Hoyle CI, Chen J, Chang W, Williams RW, Rando RJ. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;298:L830–6. doi: 10.1152/ajplung.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Zhang WJ, Wang XM. Triptolide with potential medicinal value for diseases of the central nervous system. CNS Neurosci Ther. 2013;19:76–82. doi: 10.1111/cns.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xi C, Peng S, Wu Z, Zhou Q, Zhou J. Toxicity of triptolide and the molecular mechanisms involved. Biomed Pharmacother. 2017;90:531–41. doi: 10.1016/j.biopha.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Dhar P, Singla N. Effect of triptolide on reproduction of female lesser bandicoot rat, Bandicota bengalensis. Drug Chem Toxicol. 2014;37:44858. doi: 10.3109/01480545.2014.884111. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Jiang Z, Liu L, Zhang Y, Zhang S, Xiao J. Triptolide induces adverse effect on reproductive parameters of female Sprague-Dawley rats. Drug Chem Toxicol. 2011;34:1–7. doi: 10.3109/01480541003774358. et al. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Y, Sun H, Li Y, Shao M, Han P, Yu X. Exposure to triptolide affects follicle development in NIH mice: Role of endoplasmic reticulum stress in granulosa cell apoptosis. Hum Exp Toxicol. 2017;36:82–92. doi: 10.1177/0960327116638725. et al. [DOI] [PubMed] [Google Scholar]
- 13.Hikim AP, Lue YH, Wang C, Reutrakul V, Sangsuwan R, Swerdloff RS. Posttesticular antifertility action of triptolide in the male rat: evidence for severe impairment of cauda epididymal sperm ultrastructure. J Androl. 2000;21:431–7. [PubMed] [Google Scholar]
- 14.Huang ZJ, Que HQ, Peng HY, Lin S, Guo SM, Qian LP. [Reproductive toxicity of triptolide and its mechanism in male rats] Zhongguo Zhong Yao Za Zhi. 2015;40:4655–9. [PubMed] [Google Scholar]
- 15.Ni B, Jiang Z, Huang X, Xu F, Zhang R, Zhang Z. Male reproductive toxicity and toxicokinetics of triptolide in rats. Arzneimittel-Forschung. 2008;58:673–80. doi: 10.1055/s-0031-1296570. et al. [DOI] [PubMed] [Google Scholar]
- 16.Ma B, Qi H, Li J, Xu H, Chi B, Zhu J. Triptolide disrupts fatty acids and peroxisome proliferator-activated receptor (PPAR) levels in male mice testes followed by testicular injury: A GC-MS based metabolomics study. Toxicology. 2015;336:84–95. doi: 10.1016/j.tox.2015.07.008. et al. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zou Z, Yang Z, Jiang S, Lu Y, Wang D. HIF 1 inhibits StAR transcription and testosterone synthesis in murine Leydig cells. J Mol Endocrinol. 2018;62:1–13. doi: 10.1530/JME-18-0148. et al. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Q, Yi H, Huang L, An Q, Wang H. Reduced testosterone and Ddx3y expression caused by long-term exposure to arsenic and its effect on spermatogenesis in mice. Environ Toxicol Pharmacol. 2018;63:84–91. doi: 10.1016/j.etap.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Di Guardo F, Vloeberghs V, Bardhi E, Blockeel C, Verheyen G, Tournaye H. Low Testosterone and Semen Parameters in Male Partners of Infertile Couples Undergoing IVF with a Total Sperm Count Greater than 5 Million. J Clin Med. 2020;9:3824. doi: 10.3390/jcm9123824. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Yu Q, Zhao F, Ji J, Jiang Z, Chen X. Protection of Quercetin against Triptolide-induced apoptosis by suppressing oxidative stress in rat Leydig cells. Chem Biol Interact. 2015;240:38–46. doi: 10.1016/j.cbi.2015.08.004. et al. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–75. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher LE, Williamson LE, Chan EY. Advances in Autophagy Regulatory Mechanisms. Cells. 2016;5:24. doi: 10.3390/cells5020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron. 2017;93:1015–34. doi: 10.1016/j.neuron.2017.01.022. et al. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, He Q, Gao Z, Redington AN. Atg5 knockdown induces age-dependent cardiomyopathy which can be rescued by repeated remote ischemic conditioning. Basic Res Cardiol. 2021;116:47. doi: 10.1007/s00395-021-00888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Xu Y, Zhang Y, Chen Y, Chang G, An G. Deciphering the autophagy regulatory network via single-cell transcriptome analysis reveals a requirement for autophagy homeostasis in spermatogenesis. Theranostics. 2021;11:5010–27. doi: 10.7150/thno.55645. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin J, Ni B, Tian ZQ, Yang F, Liao WG, Gao YQ. Regulatory effects of autophagy on spermatogenesis. Biol Reprod. 2017;96:525–30. doi: 10.1095/biolreprod.116.144063. [DOI] [PubMed] [Google Scholar]
- 28.Jiang XH, Bukhari I, Zheng W, Yin S, Wang Z, Cooke HJ. Blood-testis barrier and spermatogenesis: lessons from genetically-modified mice. Asian J Androl. 2014;16:572–80. doi: 10.4103/1008-682X.125401. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moraveji SF, Esfandiari F, Taleahmad S, Nikeghbalian S, Sayahpour FA, Masoudi NS. Suppression of transforming growth factor-beta signaling enhances spermatogonial proliferation and spermatogenesis recovery following chemotherapy. Hum Reprod. 2019;34:2430–42. doi: 10.1093/humrep/dez196. et al. [DOI] [PubMed] [Google Scholar]
- 30.Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol. 2016;59:10–26. doi: 10.1016/j.semcdb.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Tian L, Li X, Wang Y, Chen Q, Li X, Ge RS. Oncostatin M stimulates immature Leydig cell proliferation but inhibits its maturation and function in rats through JAK1/STAT3 signaling and induction of oxidative stress in vitro. Andrology. 2022;10:354–66. doi: 10.1111/andr.13109. et al. [DOI] [PubMed] [Google Scholar]
- 32.Rebourcet D, Darbey A, Monteiro A, Soffientini U, Tsai YT, Handel I. Sertoli Cell Number Defines and Predicts Germ and Leydig Cell Population Sizes in the Adult Mouse Testis. Endocrinology. 2017;158:2955–69. doi: 10.1210/en.2017-00196. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Oliveira SA, Cerri PS, Sasso-Cerri E. Impaired macrophages and failure of steroidogenesis and spermatogenesis in rat testes with cytokines deficiency induced by diacerein. Histochem Cell Biol. 2021:1–21. doi: 10.1007/s00418-021-02023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang FC, Wang SC, Chang MM, Pan BS, Wong KL, Cheng KS. Midazolam activates caspase, MAPKs and endoplasmic reticulum stress pathways, and inhibits cell cycle and Akt pathway, to induce apoptosis in TM3 mouse Leydig progenitor cells. Onco Targets Ther. 2018;11:1475–90. doi: 10.2147/OTT.S154442. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Xu W, Wu J, Zhang D, Abou-Shakra A, Di L. Nicotine induced autophagy of Leydig cells rather than apoptosis is the major reason of the decrease of serum testosterone. Int J Biochem Cell Biol. 2018;100:30–41. doi: 10.1016/j.biocel.2018.05.001. et al. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Wang J, Zhao T, Wei Y, Han L, Shen L. LncRNAs activate longevity regulation pathway due to aging of Leydig cells caused by DEHP exposure: A transcriptome-based study. Ecotoxicol Environ Saf. 2021;209:111798. doi: 10.1016/j.ecoenv.2020.111798. et al. [DOI] [PubMed] [Google Scholar]
- 37.Mohan UP, P BT, Iqbal STA, Arunachalam S. Mechanisms of doxorubicin-mediated reproductive toxicity - A review. Reprod Toxicol. 2021;102:80–9. doi: 10.1016/j.reprotox.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Li XX, Du FY, Liu HX, Ji JB, Xing J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. J Ethnopharmacol. 2015;162:238–43. doi: 10.1016/j.jep.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Liu L, Mu X, Jiang Z, Zhang L. Effect of triptolide on estradiol release from cultured rat granulosa cells. Endocr J. 2012;59:473–81. doi: 10.1507/endocrj.ej11-0407. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Z, Huang X, Huang S, Guo H, Wang L, Li X. Sex-Related Differences of Lipid Metabolism Induced by Triptolide: The Possible Role of the LXRα/SREBP-1 Signaling Pathway. Front Pharmacol. 2016;7:87. doi: 10.3389/fphar.2016.00087. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu LY, Wu W, Cheng R, Sun LX, Jiang ZZ, Zhang LY. Toxic effects of triptolide on adrenal steroidogenesis in H295R cells and female rats. J Biochem Mol Toxicol. 2019;33:e22394. doi: 10.1002/jbt.22394. et al. [DOI] [PubMed] [Google Scholar]
- 42.Zheng N, Wei A, Wu T, Long L, Yang H, Li H. Triptolide and atorvastatin synergistically promote hepatotoxicity in cultured hepatocytes and female Sprague-Dawley rats by inhibiting pregnane X receptor-mediated transcriptional activation of CYP3A4. Toxicol Lett. 2021;342:85–94. doi: 10.1016/j.toxlet.2021.02.008. et al. [DOI] [PubMed] [Google Scholar]
- 43.Zhao T, Wang J, Wu Y, Han L, Chen J, Wei Y. Increased m6A modification of RNA methylation related to the inhibition of demethylase FTO contributes to MEHP-induced Leydig cell injury. Environ Pollut. 2021;268:115627. doi: 10.1016/j.envpol.2020.115627. et al. [DOI] [PubMed] [Google Scholar]
- 44.Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Fedele AO, Proud CG. Chloroquine and bafilomycin A mimic lysosomal storage disorders and impair mTORC1 signalling. Biosci Rep. 2020;40 doi: 10.1042/BSR20200905. BSR20200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Zheng W, Bian X, Yuan Y, Gu J, Liu X. Zearalenone induces apoptosis and cytoprotective autophagy in primary Leydig cells. Toxicol Lett. 2014;226:182–91. doi: 10.1016/j.toxlet.2014.02.003. et al. [DOI] [PubMed] [Google Scholar]
- 47.Goel P, Gerriets V. Chloroquine.StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021 StatPearls Publishing LLC. 2021 [Google Scholar]
- 48.Zhou J, Xi C, Wang W, Yang Y, Qiu Y, Huang Z. Autophagy plays an important role in triptolide-induced apoptosis in cardiomyocytes. Toxicol Lett. 2015;236:168–83. doi: 10.1016/j.toxlet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361–5. doi: 10.1038/nature14587. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Bari MAA, Xu P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann N Y Acad Sci. 2020;1467:3–20. doi: 10.1111/nyas.14305. [DOI] [PubMed] [Google Scholar]
- 52.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng RH, Zhang WW, Ji YN, Bai XJ, Yan CP, Wang J. Exogenous supplement of glucagon like peptide-1 protects the heart against aortic banding induced myocardial fibrosis and dysfunction through inhibiting mTOR/p70S6K signaling and promoting autophagy. Eur J Pharmacol. 2020;883:173318. doi: 10.1016/j.ejphar.2020.173318. et al. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Huang L, Geng Y, He J, Chen X, Xu H. Rapamycin inhibits spermatogenesis by changing the autophagy status through suppressing mechanistic target of rapamycin-p70S6 kinase in male rats. Mol Med Rep. 2017;16:4029–37. doi: 10.3892/mmr.2017.7120. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma F, Zhou Z, Li N, Zheng L, Wu C, Niu B. Lin28a promotes self-renewal and proliferation of dairy goat spermatogonial stem cells (SSCs) through regulation of mTOR and PI3K/AKT. Sci Rep. 2016;6:38805. doi: 10.1038/srep38805. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]