Abstract
The surface of the small bowel mucosa is covered more than any other section of the digestive canal; however, the overall prevalence of small bowel tumors of the whole gastrointestinal tract is evidently low. Owing to the improvement in endoscopic techniques, the prevalence of small bowel tumors has increased across multiple countries, which is mainly due to an increase in duodenal tumors. Superficial non-ampullary duodenal epithelial tumors (SNADETs) are defined as tumors originating from the non-ampullary region in the duodenum that share similarities and discrepancies with their gastric and colorectal counterparts in the pathogenesis and clinicopathologic characteristics. To date, white light endoscopy (WLE) remains the cornerstone of endoscopic diagnosis for SNADETs. Besides, narrow-band imaging (NBI) techniques and magnifying endoscopy (ME) have been widely used in the clinic and endorsed by multiple guidelines and consensuses for SNADETs’ evaluation. Confocal laser endomicroscopy (CLE), endocytoscopy (ECS), and artificial intelligence (AI) are also up-and-coming methods, showing an exceptional value in the diagnosis of SNADETs. Similar to the endoscopic treatment for colorectal polyps, the choices for SNADETs mainly include cold snare polypectomy (CSP), endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and laparoscopic endoscopic cooperative surgery (LECS). However, owing to the narrow lumen, rich vascularity, weak muscle layer, abundant Brunner’s gland, and the hardship of endoscope control, the duodenum ranks as one of the most dangerous operating areas in the digestive tract. Therefore, endoscopists must anticipate the difficulties in endoscopic maneuverability, remain aware of the increased risk of complications, and then select the appropriate treatment according to the advantages and disadvantages of each method.
Key words: superficial non-ampullary duodenal epithelial tumors, endoscopic diagnosis, endoscopic treatment
Introduction
In general, superficial non-ampullary duodenal epithelial tumors (SNADETs) are rarer than other gastrointestinal (GI) tumors; however, studies performed in recent years have reported an increase in the incidence of these lesions.[1] Notably, endoscopy has adopted an irreplaceable role in the stepwise understanding of SNADETs. With the improved availability of endoscopic screening and advances in endoscopic techniques,[2] a broader investigation of the diagnosis and treatment of SNADETs has emerged.[3,4] Recently, the European Society of Gastrointestinal Endoscopy (ESGE) issued the very first clinical practice guideline on SNADETs.[5] Nevertheless, current diagnostic and therapeutic strategies still have room for improvement. This review seeks to shed light on the current status of endoscopic modalities for the diagnosis and treatment of SNADETs and discuss the remaining challenges.
Clinical features of SNADETs
SNADETs are defined as tumors originating from the non-ampullary region in the duodenum, consisting of dysplastic glandular epithelium, which may show intestinal-type or pyloric gland differentiation (also called gastric-type).[6] In epidemiology, the estimated prevalence of non-ampullary duodenal adenoma is 0.03%–0.40%,[7, 8, 9] and the incidence of non-ampullary duodenal cancer is 23.7 per 1,000,000 person-years in the up-to-date Japanese survey study.[10] Most of the lesions are detected incidentally by endoscopic examinations as they are usually small and asymptomatic. However, large lesions can cause obstructive symptoms or complaints related to tumor progression and metastasis.[11] Only very few cases have been reported from the onset of metastatic symptoms.[12] SNADETs are less often sporadic than their ampullary counterparts, as approximately 60% of SNADETs occur in patients with familial adenomatous polyposis (FAP).[13] Besides, other predisposing genetic syndromes, including MUTYH-associated polyposis (MAP), Lynch syndrome (LS), and Peutz–Jeghers syndrome (PJS), are associated with an increased risk of SNADETs.[14, 15, 16] Therefore, when SNADET is detected in a patient, an extra colonoscopy is strongly recommended.[17] Further, specific management strategies for these polyposis syndromes are discussed in another guideline from ESGE.[18]
The revised Vienna classification (VCL) (Table 1) is now a well-established clinicopathologic parameter for evaluating the malignant levels of SNADETs. This parameter generally divides the lesions into mucosal low-grade adenoma (VCL 3) and mucosal high-grade adenoma/carcinoma (VCL 4/5).[19] Most of the lesions are found to be VCL 3 at the initial diagnosis, and some could progress to VCL 4/5.[20] As mentioned above, SNADETs can be further subdivided into intestinal-type and gastric-type based on immunohistochemical (IHC) staining: CDX2 and MUC2 for intestinal-type and MUC5AC and MUC6 for gastric-type.[21,22] Accordingly, the pathogenesis from adenoma to adenocarcinoma differs between intestinal-type and gastric-type.[23] Similar to colorectal cancer (CRC), the adenoma– carcinoma sequence model involves tumor progression in SNADETs of intestinal-type. In contrast, the gastric-type seems to progress de novo, which is associated with the Wnt/β-catenin pathway. Many studies have confirmed the differences in the clinicopathologic characteristics between the two types of SNADETs,[24, 25, 26] as shown in Figure 1.
Table 1.
The revised Vienna classification of gastrointestinal epithelial neoplasia
| Category | Diagnosis |
|---|---|
| 1 | Negative for neoplasia |
| 2 | Indefinite for neoplasia |
| 3 | Mucosal low-grade neoplasia |
| Low-grade adenoma | |
| Low-grade dysplasia | |
| 4 | Mucosal high-grade neoplasia |
| 4.1 | High-grade adenoma/dysplasia |
| 4.2 | Noninvasive carcinoma (carcinoma in situ) |
| 4.3 | Suspicious for invasive carcinoma |
| 4.4 | Intramucosal carcinoma |
| 5 | Submucosal invasion by carcinoma |
Figure 1.
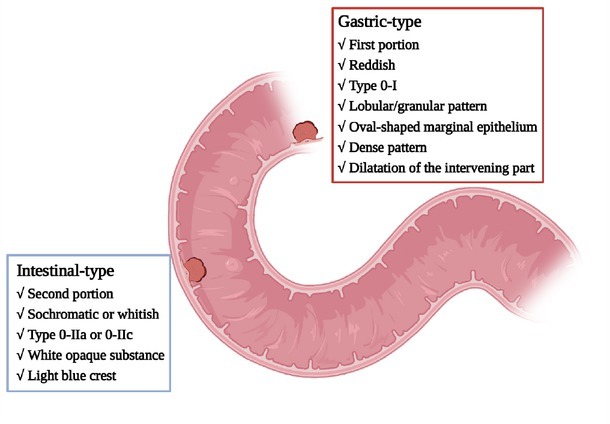
Summary of the clinicopathologic characteristics of gastric-type and intestinal-type SNADETs. SNADETs: superficial non-ampullary duodenal epithelial tumors.
Endoscopic diagnosis for SNADETs
White light endoscopy and biopsy
White light endoscopy (WLE) and biopsy are the most fundamental and essential means of diagnosing SNADETs.[27] In WLE, the Paris endoscopic classification is widely used to describe the macroscopic types of SNADETs, namely, pedunculated (0-Ip), sessile (0-Is), slightly elevated (0-IIa), flat (0-IIb), slightly depressed (0-IIc), and excavated (0-III).[28] According to several studies, size, color, macroscopic type, and biopsy results are important assessment indicators for endoscopic diagnosis. For example, a surveillance study (46 lesions followed up for ≥ 6 months) reported that a lesion with a diameter ≥ 20 mm and its diagnosis as high-grade dysplasia (HGD) at the first biopsy are two key indicators of a higher risk of progression to adenocarcinoma.[20] In another multicenter case series involving 396 SNADETs, HGD and superficial adenocarcinoma (SAC; including carcinoma in situ and invasive carcinomas) were recognized to more likely present with a dimeter > 5 mm and red color.[29] Furthermore, Kakushima et al.[30] proposed a simple endoscopic scoring system to differentiate between VCL 3 and VCL 4 or higher SNADETs (Table 2), where a score ≥ 3 points indicated the histology of VCL 4 or higher, with sensitivity and specificity of 88% and 86%, respectively. In the follow-up study, the researchers mentioned extra endoscopic features of submucosal invasion under WLE, including submucosal tumor-like appearance (defined as a smooth or gentle elevation at the margin of a lesion) and location at the oral side of the ampulla, even if the lesion is ≤ 10 mm.[31] Overall, WLE remains the cornerstone of endoscopic diagnosis for SNADETs, as it is still a fundamental skill for every endoscopist. However, biopsy seems to be less recommended as it can affect the visual observation and the sequential treatment procedure.[32] Besides, Kinoshita et al.[33] revealed that the accuracy of SNADETs biopsy was unsatisfactory, with a sensitivity of 37.5% and specificity of 83.1%. Tsuji et al.[34] also showed that the sensitivity and specificity were 89% and 14%, respectively, and that optical findings would be obstructed by the preceding biopsy injuries. Additionally, both studies emphasized that the unexpected fibrosis after biopsy might increase the difficulty in treatment, with predetermined endoscopic mucosal resection (EMR) being converted to endoscopic submucosal dissection (ESD). Collectively, WLE and biopsy play significant roles in the endoscopic diagnosis of SNADETs owing to their convenience and feasibility during endoscopic screen. Nonetheless, both WLE and biopsy require plenty of experience to avoid misdiagnosis, especially the biopsy procedure. Endoscopists must have considerable expertise to handle an exact specimen with minimal interference in the subsequent endoscopic treatment. Given the inadequacy, more novel tools have been introduced to complement the existing methods, and these techniques will be discussed in the next part.
Table 2.
A simple endoscopic scoring system for SNADETs[30]
| Endoscopic finding | Score |
||
|---|---|---|---|
| 0 | 1 | 2 | |
| Lesion diameter | < 10 mm | ≥ 10 mm | |
| Color | White | Isochromatic | Red |
| Macroscopic type | Is, Ip, IIa without depression | Any type with depression or mixed type | |
| Nodularity | Uniform | Heterogeneous or none |
SNADETs: superficial non-ampullary duodenal epithelial tumors.
Narrow band imaging and magnifying endoscopy
Narrow-band imaging (NBI) techniques and magnifying endoscopy (ME) have been widely used in the clinic, as endorsed by multiple guidelines and consensuses for diagnosing GI tumors.[35,36] For SNADETs, NBI and ME have shown unique value in differentiating neoplastic lesions from non-neoplastic lesions, acting as an important complementary tool to WLE.[37,38] Many researchers have proposed their original diagnostic algorithms for SNADETs using ME-NBI, which achieved excellent performances for distinguishing VCL 4/5 lesions from VCL 3 lesions.[39, 40, 41] In particular, the absence of white opaque substance (WOS) and lack of milk-white mucosa (MWM) findings were significantly associated with VCL 4/5 lesions.[42,43] Based on the WLE scoring system, Ishii et al.[44] developed another scoring system using ME- NBI. Besides the diameter and color of the lesion, they included the surface pattern and vessel pattern (observed by ME-NBI) into the scoring system, as shown in Table 3, where a score ≥ 3 served as the cut-off for VCL 4/5 lesions, with a sensitivity and specificity of 95% and 93%, respectively. The application of ME-NBI opens a chapter in the endoscopic diagnosis for SNADETs. Additionally, chromoendoscopy was introduced as an adjuvant tool to provide more details of the lesion with “a colorful perspective,” which ultimately has become a valuable aid to WLE and ME-NBI.[45] Crystal violet (CV) staining, accompanied with ME-NBI, has been confirmed to be the most suitable choice for SNADETs.[46] For instance, Toya et al.[47] designed a diagnostic algorithm of ME-NBI with CV staining to distinguish VCL 4/5 from VCL 3. Briefly, they subdivided the surface patterns into four categories (a convoluted pattern, a leaf-like pattern, a reticular/sulciolar pattern, a pinecone pattern) and organized two structure patterns (regular pattern and irregular pattern). In their theory, VCL 4/5 lesions presented with multiple surface patterns or irregular structure patterns, while VCL 3 lesions were more likely to manifest single surface patterns and regular structure patterns. Regretfully, due to the lack of larger-scale analysis, more investigations are required to demonstrate the diagnostic value of this algorithm.
Table 3.
Endoscopic findings included in the scoring system[44]
| Variables | 0 | 1 | 2 |
|---|---|---|---|
| Diameter | < 10 mm | 10–<20 mm | ≥ 20 mm |
| Color | White/isochromatic | Red | |
| Surface pattern | Regular | Irregular | |
| Vessel pattern | Regular | Irregular |
Confocal laser endomicroscopy, endocytoscopy, and artificial intelligence
Confocal laser endomicroscopy (CLE) and endocytoscopy (ECS) are novel endoscopic techniques that provide an unprecedented high-resolution assessment of GI mucosal histology at the cellular and sub-cellular level, offering high hopes of achieving “optical biopsies” of nearly any accessible endoluminal surface.[48,49] To date, two types of CLE have been introduced, endoscopic-based CLE (eCLE) and probe-based CLE (pCLE), with pCLE appearing more practical and helpful in the duodenum.[50] Shahid et al.[51] elucidated the high diagnostic efficacy of pCLE in both ex vivo pathology and in vivo duodenal polyps. Likewise, Tahara et al.[52] indicated that the dark epithelium and distorted crypt structure are characteristic pCLE features of neoplasia and cancer in SNADETs, with a sensitivity and specificity of nearly 100%. In terms of ECS, Hirose et al.[53] illustrated that ECS diagnosis with methylene blue staining could achieve a high accuracy to predict the histology of SNADETs. Moreover, Muramoto et al.[54] created an original ECS classification for the diagnosis of SNADETs based on cell nuclear morphology, which showed significantly superior diagnostic effects compared to preoperative biopsy. Nevertheless, with the requirements of expensive equipment and extra proficiency, gaining popularity at all levels of the health sector is not an easy task, thereby immensely constraining the scale-up. Beyond the exciting updates in facilities, artificial intelligence (AI) has gradually become a rising star in the field of endoscopic diagnosis.[55] Inoue et al.[56] made the first attempt to apply convolutional neural networks to the detection of SNADETs in endoscopic images, which required 12 s to identify the lesions in 399 photos, with an accuracy of 94.7%. Besides various electronic and information technologies, the biological molecule is becoming an integral part of research on many other GI diseases.[57, 58, 59] Accordingly, we believe that it will also optimize the diagnosis of SNADETs. With the rapid advances in both “hardware” and “software,” the technological revolution will contribute to the increasing awareness and recognition of SNADETs.
Endoscopic treatment for SNADETs
Similar to the endoscopic treatment for colorectal polyps, the choices for SNADETs mainly include cold snare polypectomy (CSP), EMR, underwater EMR (UEMR), ESD, and laparoscopic endoscopic cooperative surgery (LECS).[60, 61, 62] However, characterized by a narrow lumen, rich vascularity, weak muscle layer, abundant Brunner’s gland, and the hardship of endoscope control, the duodenum ranks as one of the most dangerous operating areas in the digestive tract.[63] Furthermore, unlike the intensive experience in colonoscopy,[64] the experience of endoscopic treatment in the duodenum remains limited. Therefore, endoscopists must anticipate the difficulties in endoscopic maneuverability, be aware of the increased risk of complications, and select the appropriate treatment according to the advantages and disadvantages of each method (Table 4).
Table 4.
Characteristics and indications of endoscopic treatments
| Treatment methods | Advantages | Disadvantages | Recommended indications |
|---|---|---|---|
| CSP | Simple, safe, low incidence of complications | Incomplete resection and local recurrence | Lesion size < 6 mm, especially suitable for FAP |
| EMR (UEMR) | Convenient, relatively low incidence of complications | Relatively low en bloc resection rate | Lesion size ≤ 20 mm |
| ESD | High en bloc resection rate irrespective of lesion size | High incidence of complications and proficiency requirement | Lesion size > 20 mm |
| LECS | Less invasive compared to pancreaticoduodenectomy and secure defect closure | Postoperative obstruction and not suitable for lesions adjacent to ampulla | Lesion size > 20 mm and endoscopically unresectable |
CSP: cold snare polypectomy; EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; FAP: familial adenomatous polyposis; LECS: laparoscopic endoscopic cooperative surgery; UEMR: underwater EMR.
EMR and UEMR
EMR is a well-established method for removing GI tumors. According to the ESGE guidelines, EMR is the first choice of endoscopic resection for SNADETs.[5] Generally, the procedure refers to the isolation of the lesion via a submucosal fluid injection and snare excision of the isolated dysplastic lesion. Basically, lesions ≤20 mm can be removed en bloc by EMR, and most endoscopists can perform this procedure.[65] The high rates of complete resection (range 90%–100 %) were confirmed by multiple studies.[66, 67, 68, 69, 70] Despite simple manipulation, the complications cannot be completely avoided. Given the difficulties of endoscopic therapy in the duodenum, several additional studies revealed higher complications rates than lesions elsewhere in the digestive tract, as the morbidities of bleeding and perforation ranged 0–22.2% and 0–4.8%, respectively.[71, 72, 73, 74] Moreover, a recent study showed that en bloc resection was almost impossible for lesions ≥30 mm.[75] Dealing with these giant lesions, the operators may perform piecemeal resection in EMR procedures. With the requirement for piecemeal resection, larger lesions often result in a higher risk of local recurrence (range 6%–23%). In fact, the highest incidence of recurrence was 37% in a retrospective study.[70,76-78] Nonetheless, EMR retains an important role in endoscopic resection, regardless of its shortcomings. Moreover, endoscopists have identified various measures to improve this technique. UEMR counts as a reliable advanced technique that originated from conventional EMR. UEMR was first introduced by Binmoeller et al.[79] for managing SNADETs. In conventional EMR, the lumen is permanently dilated by air, causing the duodenum wall to be thinner and particularly vulnerable to collision damage. In UEMR, filling with water can retain the thickness and configuration of the duodenum, which helps to reduce the thermal injury. Of note, the water pressure also helps terminate bleeding. Besides, water can eliminate the need for submucosal injection, with a “floating” effect on the mucosa and submucosa.[80, 81, 82] For instance, Kiguchi et al.[83] compared the clinical outcomes between UEMR and conventional EMR and found that UEMR could notably assist in the avoidance of ESD conversion. Notably, no significant differences were observed between the complication rates of the two groups. Nevertheless, UEMR exhibited a relatively low R0 resection rate and en bloc resection rate than EMR. Altogether, the outcome of conventional EMR might be overestimated as the difficult cases were excluded because of the conversion to ESD in the study. Besides, the researchers suggested that devices dedicated to UEMR procedures should be developed. In a follow-up study, the researchers demonstrated the feasibility of partial submucosal injection technique combining UEMR (PI-UEMR) for SNADETs.[84] To remove the lesion with non-lifting sign during UEMR, they administered a submucosal injection on the difficult side (mostly anal side) of the lesion and then resected the lesion by recognizing a sufficient margin. In 30 patients who underwent PI-UEMR, only one case of immediate bleeding occurred without any other delayed complications. In addition, UEMR was shown to be appropriate for treating residual lesions after previous endoscopic resection.[85,86] Altogether, EMR and the derived techniques (like UEMR and PI-UEMR) are top selections, while ESD is more suitable for large and complex lesions, as discussed in the next section.
Endoscopic submucosal dissection
ESD for SNADETs is more complex than for lesions from other locations along the GI tract. In fact, the ESGE guidelines suggest ESD should be considered for exclusive indications only in the hands of an expert.[5] Usually, ESD is the first strategy of choice for lesions > 20 mm.[60] Besides, lesions presenting non-lifting conditions are always eventually removed by ESD, despite the initial procedure.[87] However, ESD is regarded as a “double-edged sword” due to its reliable removal efficacy and high incidence of complications, even in experienced endoscopy units.[88,89] In terms of the advantages, duodenal ESD was proved to achieve an en bloc resection rate greater than 90 %, even for lesions larger than 20 mm.[90,91] Moreover, multiple comparative studies showed that duodenal ESD could reach higher R0 resection rates for giant lesions, with no significant differences found in long-term outcomes between ESD and EMR.[91, 92, 93] Nevertheless, the higher risk of procedure-related complications is the limiting factor. As observed in multiple studies, the bleeding rate increases up to 46% and the perforation rate ranges from 13 % to 50 %. Even more, 17% of the cases accepted additional surgery in a study.[88,90,94–96] The exact reasons can be summarized as follows: (1) the narrow lumen of the duodenum restricts the reverse method of manipulation; (2) deep intubation into the duodenum shortens the operable parts for endoscopists, causing great difficulty in operational stability; (3) the C-loop structure of the duodenum makes it easy to slip off the target; (4) abundant Brunner’s gland impairs the effectiveness of submucosal injection; (5) rich blood supply and active intestinal peristalsis obstruct the dissection; (6) poor expandability of the duodenal mucosa makes it difficult to close the defect; (7) the thin muscularis propria and muscularis mucosa are easily damaged and induce perforation; and (8) the transfer to surgery faces extra challenges. Therefore, the discovery of effective endoscopic methods is markedly desired, and many researchers have made meaningful attempts to attenuate these complications. For example, covering the wound with polyglycolic acid (PGA) sheets helped to prevent delayed perforation after duodenal ESD.[97,98] Some endoscopists also suggest indwelling endoscopic nasobiliary and pancreatic duct drainage (ENBPD) tubes for incomplete closed lesions;[99] this is because ENBPD is thought to be a helpful prophylaxis for protecting the duodenal mucosa from the erosion of bile and pancreatic juice.[100] Bleeding is another main complication of duodenal ESD, which appears even more commonly than perforation. Overall, guaranteeing closure of the mucosal defects is the essential solution.[101] Typically, the application of endoscopic clipping is the most convenient and primary method of preventing bleeding after duodenal ESD.[102] Recently, an over-the-scope clip (OTSC) system was reported to help close the defect and reduce delayed bleeding after duodenal ESD.[103,104] Subtly, the coordination of endoscopic clipping and suturing has become a highlight, which fully unfolded the craft and creativity of endoscopists.[105, 106, 107] Remarkable efforts have also been made to improve endoscopic instruments for cutting, with an aim to decrease electrical injury in ESD procedures. Hook knife and scissors-type knife (clutch cutter) have been acknowledged by many endoscopists as practicable and safe equipment in duodenal ESD.[108, 109, 110, 111] Thoughtfully, ensuring maneuverability in the confined room has been an issue for endoscopists. The double-balloon endoscope has been described as a helpful tool to stabilize the operation of the endoscope tip, which can be especially suitable for duodenal ESD.[112] Many researchers have also emphasized the value of various types of traction techniques in duodenal ESD. Goda et al.[113] revealed the efficacy of ring-shaped thread counter traction, which could provide sufficient operation view in duodenal ESD. Tashima et al.[114] performed traction-assisted ESD with dental floss and a clip for a lesion with severe fibrosis and used multiple clip-and-thread traction to remove a large lesion located in the duodenal bulb.[115] With a better operation view, the rate of bleeding and perforation can be effectively reduced. In summary, the better safety and more effortless procedure of EMR lead to a higher priority for removing SNADETs. Furthermore, ESD remains of vital indispensability for large and complex lesions; however, endoscopists must be aware of procedure-related complications.
CSP and LECS
Compared to EMR and ESD, CSP and LECS are less popular; however, both can be exploited in some special situations to gain desirable effects. CSP is a physical method that uses a snare without an electrical current, ultimately reducing the potential injury caused by electrically induced heat. Relatively, the solo physical force of the snare limits the removal efficacy. Thus, the ESGE guidelines suggest that CSP is only suitable for small and nonmalignant SNADETs (<6 mm in size).[5] In particular, CSP is appropriate for FAP patients with numerous and small duodenal polyps, owing to its simplicity and safety.[116, 117, 118] Nevertheless, the relatively high incomplete resection rate is an obvious shortcoming of CSP, leading to its limited application in SNADETs.[119,120] LECS is a creative approach first introduced by Hiki et al.[121] for GI stromal tumor dissection. Technically, the procedure involves laparoscopic resection with endoscopic guidance and endoscopic resection with surgical repair.[122,123] In theory, LECS should fulfill both fewer complications related to endoscopic resection and slighter damage than traditional surgery.[124,125] LECS requires high-level cooperation between endoscopists and surgeons to achieve safe management of SNADETs, which virtually raises the threshold for medical facilities. Although LECS has been reported to exhibit an exemplary safety and efficacy for reducing adverse events and recurrence,[123,126] more extensive prospective studies are still needed to prove these conclusions.
Conclusions and outlook
Currently, endoscopic management of GI tumors is advancing with remarkable momentum. Due to the rarity, SNADETs, especially sporadic cases, still lack standardized diagnostic criteria and treatment approaches. To improve diagnostic levels, internationally recognized criteria should be established according to the advances in optical techniques. As mentioned above, multiple choices are available for treating SNADETs; yet the final decision is frequently based on the endoscopist’s personal experience. Further, the complications related to the endoscopic procedure remain as challenges. Therefore, more consensus guidelines are urgently needed to normalize the workflow of the endoscopic treatment for SNADETs. As it stands, EMR can be first considered for the endoscopic resection of SNADETs. For larger lesions, especially those with the possibility of deep invasion, ESD and LECS are more suitable after detailed and rigorous preoperative assessment of efficacy and safety. As presented in this review, many leading-edge methods have been applied practically to prevent complications. Ultimately, the endoscopists must carefully manage SNADETs and conduct more extensive investigations to determine whether the technique will help improve outcomes.
Footnotes
Author Contributions
Zheng Zhao, Yue Jiao, and Shuilong Guo conceived and designed this study. Zheng Zhao and Shuyue Yang searched for and extracted data from the included articles and drafted the manuscript. Anni Zhou and Guiping Zhao critically revised the manuscript. Peng Li and Shutian Zhang supervised the project.All authors have read and agreed to the published version of the manuscript.
Source of Funding
This work was supported by the National Natural Science Foundation of China (82070575) and Beijing Municipal Natural Science Foundation (J180010).
Conflict of Interest
None declared.
Contributor Information
Prof. Peng Li, Email: lipeng@ccmu.edu.cn.
Prof. Shutian Zhang, Email: zhangshutian@ccmu.edu.cn.
References
- 1.Fang Y, Ding X. Current status of endoscopic diagnosis and treatment for superficial non-ampullary duodenal epithelial tumors. Scand J Gastroenterol. 2021;56:604. doi: 10.1080/00365521.2021.1900384. - [DOI] [PubMed] [Google Scholar]
- 2.Sato S, Fushimi S, Tahata Y, Mizutamari H, Mimori N, Kato Y. Feasibility of Endoscopic Screening for Upper Gastrointestinal Malignancy in a Comprehensive Health Checkup. Intern Med. 2021;60:1493. doi: 10.2169/internalmedicine.6020-20. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim CH, Cho YS. Nonampullary duodenal adenoma:Current understanding of its diagnosis, pathogenesis, and clinical management. World J Gastroenterol. 2016;22:853. doi: 10.3748/wjg.v22.i2.853. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlovic-Markovic A, Dragasevic S, Krstic M, Stojkovic Lalosevic M, Milosavljevic T. Assessment of Duodenal Adenomas and Strategies for Curative Therapy. Dig Dis. 2019;37:374. doi: 10.1159/000496697. - [DOI] [PubMed] [Google Scholar]
- 5.Vanbiervliet G, Moss A, Arvanitakis M, Arnelo U, Beyna T, Busch O. Endoscopic management of superficial nonampullary duodenal tumors:European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:522. doi: 10.1055/a-1442-2395. et al. - [DOI] [PubMed] [Google Scholar]
- 6.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182. doi: 10.1111/his.13975. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483. doi: 10.3109/00365529409092458. et al. - [DOI] [PubMed] [Google Scholar]
- 8.Jung SH, Chung WC, Kim EJ, Kim SH, Paik CN, Lee BI. Evaluation of non-ampullary duodenal polyps:Comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2010;16:5474. doi: 10.3748/wjg.v16.i43.5474. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhatib AA. Sporadic nonampullary tubular adenoma of the duodenum:Prevalence and patients’ characteristics. Turk J Gastroenterol. 2019;30:112. doi: 10.5152/tjg.2018.17823. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida M, Yabuuchi Y, Kakushima N, Kato M, Iguchi M, Yamamoto Y. The incidence of non-ampullary duodenal cancer in Japan:The first analysis of a national cancer registry. J Gastroenterol Hepatol. 2021;36:1216. doi: 10.1111/jgh.15285. et al. - [DOI] [PubMed] [Google Scholar]
- 11.Kakushima N, Ono H, Yoshida M, Takizawa K, Tanaka M, Kawata N. Characteristics and risk factors for sporadic non-ampullary duodenal adenocarcinoma. Scand J Gastroenterol. 2017;52:1253. doi: 10.1080/00365521.2017.1369563. et al. - [DOI] [PubMed] [Google Scholar]
- 12.Tulsi R, Ul Haque MM, Hanif FM, Devi A, Mubarak M, Hassan Luck N. Metastasis of Duodenal Adenocarcinoma to the Urinary Bladder Presenting as Hematuria. J Transl Int Med. 2021;9:143. doi: 10.2478/jtim-2021-0010. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas:sporadic versus familial disease. J Gastrointest Surg. 2010;14:229. doi: 10.1007/s11605-009-1091-4. - [DOI] [PubMed] [Google Scholar]
- 14.Hurley JJ, Thomas LE, Walton SJ, Thomas-Gibson S, Haycock A, Suzuki N. The impact of chromoendoscopy for surveillance of the duodenum in patients with MUTYH-associated polyposis and familial adenomatous polyposis. Gastrointest Endosc. 2018;88:665. doi: 10.1016/j.gie.2018.04.2347. et al. - [DOI] [PubMed] [Google Scholar]
- 15.Hammoudi N, Dhooge M, Coriat R, Leblanc S, Barret M, Bordacahar B. Duodenal tumor risk in Lynch syndrome. Dig Liver Dis. 2019;51:299303. doi: 10.1016/j.dld.2018.10.005. et al. [DOI] [PubMed] [Google Scholar]
- 16.Carbone R, Rovedatti L, Lenti MV, Furlan D, Errichiello E, Gana S. Histologic heterogeneity and syndromic associations of non-ampullary duodenal polyps and superficial mucosal lesions. Dig Liver Dis. 2021;53:1647. doi: 10.1016/j.dld.2021.03.011. et al. - [DOI] [PubMed] [Google Scholar]
- 17.Maruoka D, Arai M, Ishigami H, Okimoto K, Saito K, Minemura S. Sporadic nonampullary duodenal adenoma/carcinoma is associated with not only colon adenoma/carcinoma but also gastric cancer:association of location of duodenal lesions with comorbid diseases. Scand J Gastroenterol. 2015;50:333. doi: 10.3109/00365521.2014.1003399. et al. - [DOI] [PubMed] [Google Scholar]
- 18.van Leerdam ME, Roos VH, van Hooft JE, Dekker E, Jover R, Kaminski MF. Endoscopic management of polyposis syndromes:European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019;51:877. doi: 10.1055/a-0965-0605. et al. - [DOI] [PubMed] [Google Scholar]
- 19.Dixon MF. Gastrointestinal epithelial neoplasia:Vienna revisited. Gut. 2002;51:130. doi: 10.1136/gut.51.1.130. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada K, Fujisaki J, Kasuga A, Omae M, Kubota M, Hirasawa T. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer:A study of follow-up surveillance. Am J Gastroenterol. 2011;106:357. doi: 10.1038/ajg.2010.422. et al. - [DOI] [PubMed] [Google Scholar]
- 21.Wagner PL, Chen YT, Yantiss RK. Immunohistochemical and molecular features of sporadic and FAP-associated duodenal adenomas of the ampullary and nonampullary mucosa. Am J Surg Pathol. 2008;32:1388. doi: 10.1097/PAS.0b013e3181723679. - [DOI] [PubMed] [Google Scholar]
- 22.Chen ZM, Scudiere JR, Abraham SC, Montgomery E. Pyloric gland adenoma:An entity distinct from gastric foveolar type adenoma. Am J Surg Pathol. 2009;33:186. doi: 10.1097/PAS.0b013e31817d7ff4. - [DOI] [PubMed] [Google Scholar]
- 23.Niwa A, Kuwano S, Tomita H, Kimura K, Orihara Y, Kanayama T. The different pathogeneses of sporadic adenoma and adenocarcinoma in non-ampullary lesions of the proximal and distal duodenum. Oncotarget. 2017;8:41078. doi: 10.18632/oncotarget.17051. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hijikata K, Nemoto T, Igarashi Y, Shibuya K. Extra-ampullary duodenal adenoma:A clinicopathological study. Histopathology. 2017;71:200. doi: 10.1111/his.13192. - [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Shimoda T, Abe M, Kakushima N, Kawata N, Takizawa K. Clinicopathological characteristics of non-ampullary duodenal tumors and their phenotypic classification. Pathol Int. 2019;69:398. doi: 10.1111/pin.12829. et al. - [DOI] [PubMed] [Google Scholar]
- 26.Akazawa Y, Ueyama H, Tsuyama S, Ikeda A, Yatagai N, Komori H. Endoscopic and Clinicopathological Features of Superficial Non-Ampullary Duodenal Tumor Based on the Mucin Phenotypes. Digestion. 2021;102:663. doi: 10.1159/000508040. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner A, Zandanell S, Kiesslich T, Neureiter D, Klieser E, Holzinger J. Systematic Review on Optical Diagnosis of Early Gastrointestinal Neoplasia. J Clin Med. 2021;10:2794. doi: 10.3390/jcm10132794. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Workshop PI. The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, Stomach, and Colon. Gastrointestinal Endoscopy. 2003;58:S3. doi: 10.1016/s0016-5107(03)02159-x. - [DOI] [PubMed] [Google Scholar]
- 29.Goda K, Kikuchi D, Yamamoto Y, Takimoto K, Kakushima N, Morita Y. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan:Multicenter case series. Dig Endosc. 2014;2:23. doi: 10.1111/den.12277. et al. 26 Suppl. - [DOI] [PubMed] [Google Scholar]
- 30.Kakushima N, Yoshida M, Iwai T, Kawata N, Tanaka M, Takizawa K. A simple endoscopic scoring system to differentiate between duodenal adenoma and carcinoma. Endosc Int Open. 2017;5:E763. doi: 10.1055/s-0043-113567. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takinami M, Kakushima N, Yoshida M, Sasaki K, Takizawa K, Yabuuchi Y. Endoscopic features of submucosal invasive non-ampullary duodenal carcinomas. J Gastroenterol Hepatol. 2020;35:821. doi: 10.1111/jgh.14870. et al. - [DOI] [PubMed] [Google Scholar]
- 32.Tsuji S, Doyama H, Tsuji K, Tsuyama S, Tominaga K, Yoshida N. Preoperative endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors, including magnifying endoscopy. World J Gastroenterol. 2015;21:11832. doi: 10.3748/wjg.v21.i41.11832. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinoshita S, Nishizawa T, Ochiai Y, Uraoka T, Akimoto T, Fujimoto A. Accuracy of biopsy for the preoperative diagnosis of superficial nonampullary duodenal adenocarcinoma. Gastrointest Endosc. 2017;86:329. doi: 10.1016/j.gie.2016.12.007. et al. - [DOI] [PubMed] [Google Scholar]
- 34.Tsuji S, Doyama H, Tsuyama S, Dejima A, Nakashima T, Wakita S. Does previous biopsy lead to cancer overdiagnosis of superficial non-ampullary duodenal epithelial tumors? Endosc Int Open. 2021;9:E58. doi: 10.1055/a-1293-7487. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526. doi: 10.1111/den.12644. et al. - [DOI] [PubMed] [Google Scholar]
- 36.Committee AT, Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502. doi: 10.1016/j.gie.2014.12.022. et al. e1-e16. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura N, Goda K, Tajiri H, Ikegami M, Nakayoshi T, Kaise M. Endoscopic features of nonampullary duodenal tumors with narrowband imaging. Hepatogastroenterology. 2010;57:462. - [PubMed] [Google Scholar]
- 38.Mizumoto T, Sanomura Y, Tanaka S, Kuroki K, Kurihara M, Yoshifuku Y. Clinical usefulness of magnifying endoscopy for non-ampullary duodenal tumors. Endosc Int Open. 2017;5:E297. doi: 10.1055/s-0043-103681. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikuchi D, Hoteya S, Iizuka T, Kimura R, Kaise M. Diagnostic algorithm of magnifying endoscopy with narrow band imaging for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;2:16. doi: 10.1111/den.12282. 26 Suppl. - [DOI] [PubMed] [Google Scholar]
- 40.Kakushima N, Yoshida M, Yamaguchi Y, Takizawa K, Kawata N, Tanaka M. Magnified endoscopy with narrow-band imaging for the differential diagnosis of superficial non-ampullary duodenal epithelial tumors. Scand J Gastroenterol. 2019;54:128. doi: 10.1080/00365521.2018.1557740. et al. - [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki Y, Takeuchi Y, Kanesaka T, Kanzaki H, Kato M, Ohmori M. Differentiation between duodenal neoplasms and non-neoplasms using magnifying narrow-band imaging - Do we still need biopsies for duodenal lesions? Dig Endosc. 2020;32:84. doi: 10.1111/den.13485. et al. - [DOI] [PubMed] [Google Scholar]
- 42.Nakayama A, Kato M, Takatori Y, Shimoda M, Mizutani M, Tsutsumi K. How I do it:Endoscopic diagnosis for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2020;32:417. doi: 10.1111/den.13538. et al. - [DOI] [PubMed] [Google Scholar]
- 43.Hara Y, Goda K, Hirooka S, Mitsuishi T, Ikegami M, Sumiyama K. Association between Endoscopic Milk-White Mucosa, Epithelial Intracellular Lipid Droplets, and Histological Grade of Superficial Non-Ampullary Duodenal Epithelial Tumors. Diagnostics (Basel) 2021;11:769. doi: 10.3390/diagnostics11050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishii R, Ohata K, Sakai E, Takita M, Minato Y, Muramoto T. Simple scoring system for the diagnosis of superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2021;33:399. doi: 10.1111/den.13762. et al. - [DOI] [PubMed] [Google Scholar]
- 45.Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS, Matsuda T. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2015;64:121. doi: 10.1136/gutjnl-2013-306503. et al. - [DOI] [PubMed] [Google Scholar]
- 46.Toya Y, Endo M, Akasaka R, Urushikubo J, Gonai T, Asakura K. Clinicopathological Features and Magnifying Chromoendoscopic Findings of Non-Ampullary Duodenal Epithelial Tumors. Digestion. 2018;97:219. doi: 10.1159/000485505. et al. - [DOI] [PubMed] [Google Scholar]
- 47.Toya Y, Endo M, Oizumi T, Akasaka R, Yanai S, Kawasaki K. Diagnostic algorithm of magnifying endoscopy with crystal violet staining for non-ampullary duodenal epithelial tumors. Dig Endosc. 2020;32:1066. doi: 10.1111/den.13640. et al. - [DOI] [PubMed] [Google Scholar]
- 48.Yun SH, Kwok SJJ. Light in diagnosis, therapy and surgery. Nat Biomed Eng. 2017;1:1. doi: 10.1038/s41551-016-0008. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy:A Prospective Study. Ann Intern Med. 2018;169:357. doi: 10.7326/M18-0249. et al. - [DOI] [PubMed] [Google Scholar]
- 50.Pittayanon R, Imraporn B, Rerknimitr R, Kullavanijaya P. Advances in diagnostic endoscopy for duodenal, including ampullary, adenoma. Dig Endosc. 2014;2:10. doi: 10.1111/den.12244. 26 Suppl. - [DOI] [PubMed] [Google Scholar]
- 51.Shahid MW, Buchner A, Gomez V, Krishna M, Woodward TA, Raimondo M. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging in detection of dysplasia in duodenal polyps. J Clin Gastroenterol. 2012;46:382. doi: 10.1097/MCG.0b013e318247f375. et al. - [DOI] [PubMed] [Google Scholar]
- 52.Tahara T, Horiguchi N, Terada T, Yamada H, Yoshida D, Okubo M. Diagnostic utility of probe-based confocal laser endomicroscopy in superficial non-ampullary duodenal epithelial tumors. Endosc Int Open. 2019;7:E1515. doi: 10.1055/a-0999-5282. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirose T, Kakushima N, Furukawa K, Furune S, Ishikawa E, Sawada T. Endocytoscopy Is Useful for the Diagnosis of Superficial Nonampullary Duodenal Epithelial Tumors. Digestion. 2021;102:895. doi: 10.1159/000516512. et al. - [DOI] [PubMed] [Google Scholar]
- 54.Muramoto T, Ohata K, Sakai E, Inamoto R, Kurebayashi M, Takayanagi S. A new classification for the diagnosis of superficial non-ampullary duodenal epithelial tumors using endocytoscopy:A prospective study. J Gastroenterol Hepatol. 2021;36:3170. doi: 10.1111/jgh.15585. et al. - [DOI] [PubMed] [Google Scholar]
- 55.Wu L, Shang R, Sharma P, Zhou W, Liu J, Yao L. Effect of a deep learning-based system on the miss rate of gastric neoplasms during upper gastrointestinal endoscopy:a single-centre, tandem, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:700. doi: 10.1016/S2468-1253(21)00216-8. et al. - [DOI] [PubMed] [Google Scholar]
- 56.Inoue S, Shichijo S, Aoyama K, Kono M, Fukuda H, Shimamoto Y. Application of Convolutional Neural Networks for Detection of Superficial Nonampullary Duodenal Epithelial Tumors in Esophagogastroduodenoscopic Images. Clin Transl Gastroenterol. 2020;11:e00154. doi: 10.14309/ctg.0000000000000154. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S, Zhang X, Walline JH, Yu X, Zhu H. Comparing the Performance of the ABC, AIMS65, GBS, and pRS Scores in Predicting 90-day Mortality Or Rebleeding Among Emergency Department Patients with Acute Upper Gastrointestinal Bleeding: A Prospective Multicenter Study. J Transl Int Med. 2021;9:114. doi: 10.2478/jtim-2021-0026. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu Z, Shen HQ, Fu PH, Chen M. Screening of long non-coding RNAs markers in plasma of children with chronic gastritis. Chronic Dis Transl Med. 2020;6:62. doi: 10.1016/j.cdtm.2020.01.001. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weissman S, Belyayeva A, Sharma S, Aziz M, Elias S, Tabibian JH. SARS-CoV-2 and Acute Diverticulitis: the Expanding Gastrointestinal Manifestations of COVID-19 Infection. J Transl Int Med. 2021;9:59. doi: 10.2478/jtim-2021-0019. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ochiai Y, Kato M, Kiguchi Y, Akimoto T, Nakayama A, Sasaki M. Current Status and Challenges of Endoscopic Treatments for Duodenal Tumors. Digestion. 2019;99:21. doi: 10.1159/000494408. et al. - [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Zhao Y, Li P, Zhang S. Advances in The Application of Regenerative Medicine in Prevention of Post-endoscopic Submucosal Dissection for Esophageal Stenosis. J Transl Int Med. 2022;10:28. doi: 10.2478/jtim-2022-0011. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang K, Zhao X, Chen X, Gao Y, Yu J, Wu L. Analysis of Digestive Endoscopic Results During COVID-19. J Transl Int Med. 2021;9:38. doi: 10.2478/jtim-2021-0006. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501. doi: 10.3748/wjg.v20.i35.12501. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin SY, Yaow CYL, Ng CH, Wong NW, Tham HY, Chong CS. Different position from traditional left lateral for colonoscopy? A meta-analysis and systematic review of randomized control trials. Chronic Dis Transl Med. 2021;7:27. doi: 10.1016/j.cdtm.2020.09.002. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gotoda T. Endoscopic resection of early gastric cancer:the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561. doi: 10.1097/01.mog.0000239873.06243.00. - [DOI] [PubMed] [Google Scholar]
- 66.Kuroki K, Sanomura Y, Oka S, Yorita N, Kurihara M, Mizumoto T. Clinical outcomes of endoscopic resection for superficial non-ampullary duodenal tumors. Endosc Int Open. 2020;8:E354. doi: 10.1055/a-0998-3708. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein A, Nayyar D, Bahin FF, Qi Z, Lee E, Williams SJ. Endoscopic mucosal resection of large and giant lateral spreading lesions of the duodenum:success, adverse events, and long-term outcomes. Gastrointest Endosc. 2016;84:688. doi: 10.1016/j.gie.2016.02.049. et al. - [DOI] [PubMed] [Google Scholar]
- 68.Jamil LH, Kashani A, Peter N, Lo SK. Safety and efficacy of cap-assisted EMR for sporadic nonampullary duodenal adenomas. Gastrointest Endosc. 2017;86:666. doi: 10.1016/j.gie.2017.02.023. - [DOI] [PubMed] [Google Scholar]
- 69.Valerii G, Tringali A, Landi R, Boskoski I, Familiari P, Bizzotto A. Endoscopic mucosal resection of non-ampullary sporadic duodenal adenomas:A retrospective analysis with long-term follow-up. Scand J Gastroenterol. 2018;53:490. doi: 10.1080/00365521.2018.1438508. et al. - [DOI] [PubMed] [Google Scholar]
- 70.Tomizawa Y, Ginsberg GG. Clinical outcome of EMR of sporadic, nonampullary, duodenal adenomas:A 10-year retrospective. Gastrointest Endosc. 2018;87:1270. doi: 10.1016/j.gie.2017.12.026. - [DOI] [PubMed] [Google Scholar]
- 71.Zou J, Chai N, Linghu E, Zhai Y, Li Z, Du C. Clinical outcomes of endoscopic resection for non-ampullary duodenal laterally spreading tumors. Surg Endosc. 2019;33:4048. doi: 10.1007/s00464-019-06698-x. et al. - [DOI] [PubMed] [Google Scholar]
- 72.Hara Y, Goda K, Dobashi A, Ohya TR, Kato M, Sumiyama K. Short- and long-term outcomes of endoscopically treated superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2019;25:707. doi: 10.3748/wjg.v25.i6.707. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh A, Siddiqui UD, Konda VJ, Whitcomb E, Hart J, Xiao SY. Safety and efficacy of EMR for sporadic, nonampullary duodenal adenomas:a single U.S. center experience (with video) Gastrointest Endosc. 2016;84:700. doi: 10.1016/j.gie.2016.03.1467. et al. - [DOI] [PubMed] [Google Scholar]
- 74.Navaneethan U, Lourdusamy D, Mehta D, Lourdusamy V, Venkatesh PG, Sanaka MR. Endoscopic resection of large sporadic non-ampullary duodenal polyps:Efficacy and long-term recurrence. Surg Endosc. 2014;28:2616. doi: 10.1007/s00464-014-3512-z. - [DOI] [PubMed] [Google Scholar]
- 75.Probst A, Freund S, Neuhaus L, Ebigbo A, Braun G, Goelder S. Complication risk despite preventive endoscopic measures in patients undergoing endoscopic mucosal resection of large duodenal adenomas. Endoscopy. 2020;52:847. doi: 10.1055/a-1144-2767. et al. - [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;2:50. doi: 10.1111/den.12273. 26 Suppl. - [DOI] [PubMed] [Google Scholar]
- 77.Maruoka D, Arai M, Kishimoto T, Matsumura T, Inoue M, Nakagawa T. Clinical outcomes of endoscopic resection for nonampullary duodenal high-grade dysplasia and intramucosal carcinoma. Endoscopy. 2013;45:138. doi: 10.1055/s-0032-1325799. et al. - [DOI] [PubMed] [Google Scholar]
- 78.Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps:Characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754. doi: 10.1016/j.gie.2009.11.043. - [DOI] [PubMed] [Google Scholar]
- 79.Binmoeller KF, Shah JN, Bhat YM, Kane SD.. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video) Gastrointest Endosc. 2013;78:496. doi: 10.1016/j.gie.2013.03.1330. - [DOI] [PubMed] [Google Scholar]
- 80.Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S.. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video) Gastrointest Endosc. 2012;75:1086. doi: 10.1016/j.gie.2011.12.022. - [DOI] [PubMed] [Google Scholar]
- 81.Curcio G, Granata A, Ligresti D, Tarantino I, Barresi L, Liotta R. Underwater colorectal EMR:Remodeling endoscopic mucosal resection. Gastrointest Endosc. 2015;81:1238. doi: 10.1016/j.gie.2014.12.055. et al. - [DOI] [PubMed] [Google Scholar]
- 82.Uedo N, Nemeth A, Johansson GW, Toth E, Thorlacius H. Underwater endoscopic mucosal resection of large colorectal lesions. Endoscopy. 2015;47:172. doi: 10.1055/s-0034-1390749. - [DOI] [PubMed] [Google Scholar]
- 83.Kiguchi Y, Kato M, Nakayama A, Sasaki M, Mizutani M, Tsutsumi K. Feasibility study comparing underwater endoscopic mucosal resection and conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor < 20 mm. Dig Endosc. 2020;32:75360. doi: 10.1111/den.13524. et al. [DOI] [PubMed] [Google Scholar]
- 84.Takatori Y, Kato M, Masunaga T, Kubosawa Y, Mizutani M, Kiguchi Y. Feasibility Study of Partial Submucosal Injection Technique Combining Underwater EMR for Superficial Duodenal Epithelial Tumors. Dig Dis Sci. 2022;67:971. doi: 10.1007/s10620-021-06925-3. et al. - [DOI] [PubMed] [Google Scholar]
- 85.Shichijo S, Uedo N, Takeuchi Y, Iwagami H, Ohmori M, Inoue S. Underwater endoscopic mucosal resection of residual duodenal tumor. Endoscopy. 2019;51:E329. doi: 10.1055/a-0919-4357. et al. - [DOI] [PubMed] [Google Scholar]
- 86.Iwagami H, Takeuchi Y, Yamasaki Y, Nakagawa K, Ohmori M, Matsuno K. Feasibility of underwater endoscopic mucosal resection and management of residues for superficial non-ampullary duodenal epithelial neoplasms. Dig Endosc. 2020;32:565. doi: 10.1111/den.13541. et al. - [DOI] [PubMed] [Google Scholar]
- 87.Kakushima N, Yoshida M, Yabuuchi Y, Kawata N, Takizawa K, Kishida Y. Present Status of Endoscopic Submucosal Dissection for Non-Ampullary Duodenal Epithelial Tumors. Clin Endosc. 2020;53:652. doi: 10.5946/ce.2019.184. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shibagaki K, Ishimura N, Kinoshita Y. Endoscopic submucosal dissection for duodenal tumors. Ann Transl Med. 2017;5:188. doi: 10.21037/atm.2017.03.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Committee AT, Maple JT, Abu Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:1311. doi: 10.1016/j.gie.2014.12.010. et al. - [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto S, Yoshida Y. Selection of appropriate endoscopic therapies for duodenal tumors:An open-label study, single-center experience. World J Gastroenterol. 2014;20:8624. doi: 10.3748/wjg.v20.i26.8624. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yahagi N, Kato M, Ochiai Y, Maehata T, Sasaki M, Kiguchi Y. Outcomes of endoscopic resection for superficial duodenal epithelial neoplasia. Gastrointest Endosc. 2018;88:676. doi: 10.1016/j.gie.2018.05.002. et al. - [DOI] [PubMed] [Google Scholar]
- 92.Santos-Antunes J, Baldaque-Silva F, Marques M, Lopes J, Carneiro F, Macedo G. Real-life evaluation of the safety, efficacy and therapeutic outcomes of endoscopic submucosal dissection in a Western tertiary centre. United European Gastroenterol J. 2018;6:702. doi: 10.1177/2050640618755237. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez-Cuadrado-Robles E, Queneherve L, Margos W, Shaza L, Ivekovic H, Moreels TG. Comparative analysis of ESD versus EMR in a large European series of non-ampullary superficial duodenal tumors. Endosc Int Open. 2018;6:E1008. doi: 10.1055/a-0577-7546. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoteya S, Yahagi N, Iizuka T, Kikuchi D, Mitani T, Matsui A. Endoscopic submucosal dissection for nonampullary large superficial adenocarcinoma/adenoma of the duodenum:Feasibility and long-term outcomes. Endosc Int Open. 2013;1:2. doi: 10.1055/s-0033-1359232. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270. doi: 10.1111/j.1443-1661.2009.00908.x. et al. - [DOI] [PubMed] [Google Scholar]
- 96.Hoteya S, Furuhata T, Takahito T, Fukuma Y, Suzuki Y, Kikuchi D. Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Non-Ampullary Superficial Duodenal Tumor. Digestion. 2017;95:36. doi: 10.1159/000452363. et al. - [DOI] [PubMed] [Google Scholar]
- 97.Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;2:46. doi: 10.1111/den.12280. 26 Suppl. - [DOI] [PubMed] [Google Scholar]
- 98.Doyama H, Tominaga K, Yoshida N, Takemura K, Yamada S. Endoscopic tissue shielding with polyglycolic acid sheets, fibrin glue and clips to prevent delayed perforation after duodenal endoscopic resection. Dig Endosc. 2014;2:41. doi: 10.1111/den.12253. 26 Suppl. - [DOI] [PubMed] [Google Scholar]
- 99.Fukuhara S, Kato M, Iwasaki E, Sasaki M, Tsutsumi K, Kiguchi Y. Management of perforation related to endoscopic submucosal dissection for superficial duodenal epithelial tumors. Gastrointest Endosc. 2020;91:1129. doi: 10.1016/j.gie.2019.09.024. et al. - [DOI] [PubMed] [Google Scholar]
- 100.Fukuhara S, Kato M, Iwasaki E, Machida Y, Tamagawa H, Kawasaki S. External drainage of bile and pancreatic juice after endoscopic submucosal dissection for duodenal neoplasm:Feasibility study (with video) Dig Endosc. 2021;33:977. doi: 10.1111/den.13907. et al. - [DOI] [PubMed] [Google Scholar]
- 101.Kato M, Ochiai Y, Fukuhara S, Maehata T, Sasaki M, Kiguchi Y. Clinical impact of closure of the mucosal defect after duodenal endoscopic submucosal dissection. Gastrointest Endosc. 2019;89:87. doi: 10.1016/j.gie.2018.07.026. et al. - [DOI] [PubMed] [Google Scholar]
- 102.An JY, Kim BW, Kim JS, Park JM, Kim TH, Lee J. The Use of Endoscopic Clipping in Preventing Delayed Complications after Endoscopic Resection for Superficial Non-Ampullary Duodenal Tumors. Clin Endosc. 2021;54:563. doi: 10.5946/ce.2020.109. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tashima T, Ohata K, Sakai E, Misumi Y, Takita M, Minato Y. Efficacy of an over-the-scope clip for preventing adverse events after duodenal endoscopic submucosal dissection:A prospective interventional study. Endoscopy. 2018;50:487. doi: 10.1055/s-0044-102255. et al. - [DOI] [PubMed] [Google Scholar]
- 104.Ohata K, Sakai E, Suzuki Y, Takayanagi S, Kurebayashi M, Kimoto Y. Risk factors of delayed bleeding after endoscopic resection of superficial non-ampullary duodenal epithelial tumors and prevention by over-the-scope and conventional clipping. Dig Endosc. 2021;33:390. doi: 10.1111/den.13729. et al. - [DOI] [PubMed] [Google Scholar]
- 105.Nomura T, Sugimoto S, Tsuda N, Matsushima R, Oyamada J, Kamei A. Mucosal defect closure after duodenal endoscopic submucosal dissection using the reopenable-clip over the line method. JGH Open. 2021;5:831. doi: 10.1002/jgh3.12577. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nishizawa T, Akimoto T, Uraoka T, Mitsunaga Y, Maehata T, Ochiai Y. Endoscopic string clip suturing method:A prospective pilot study (with video) Gastrointest Endosc. 2018;87:1074. doi: 10.1016/j.gie.2017.11.007. et al. - [DOI] [PubMed] [Google Scholar]
- 107.Nishizawa T, Ochiai Y, Uraoka T, Akimoto T, Mitsunaga Y, Goto O. Endoscopic slip-knot clip suturing method:Prospective pilot study (with video) Gastrointest Endosc. 2017;85:433. doi: 10.1016/j.gie.2016.07.047. et al. - [DOI] [PubMed] [Google Scholar]
- 108.Ishii N, Akiyama H, Suzuki K, Fujita Y. Safety and Efficacy of Endoscopic Submucosal Dissection for Non-Ampullary Duodenal Neoplasms: A Case Series. ACG Case Rep J. 2015;2:146. doi: 10.14309/crj.2015.36. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Minoda Y, Akahoshi K, Otsuka Y, Kubokawa M, Motomura Y, Oya M. Endoscopic submucosal dissection of early duodenal tumor using the Clutch Cutter:A preliminary clinical study. Endoscopy. 2015;47 doi: 10.1055/s-0034-1392209. et al. Suppl 1 UCTN:E267-8. [DOI] [PubMed] [Google Scholar]
- 110.Dohi O, Yoshida N, Naito Y, Yoshida T, Ishida T, Azuma Y. Efficacy and safety of endoscopic submucosal dissection using a scissors-type knife with prophylactic over-the-scope clip closure for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2020;32:904. doi: 10.1111/den.13618. et al. - [DOI] [PubMed] [Google Scholar]
- 111.Nishimura T, Kuwai T, Yamaguchi T, Kohno H, Ishaq S. Usefulness and safety of a scissors-type knife in endoscopic submucosal dissection for nonampullary duodenal epithelial tumors. VideoGIE. 2017;2:287. doi: 10.1016/j.vgie.2017.06.012. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamamoto H, Miura Y. Duodenal ESD:conquering difficulties. Gastrointest Endosc Clin N Am. 2014;24:235. doi: 10.1016/j.giec.2013.11.007. - [DOI] [PubMed] [Google Scholar]
- 113.Goda Y, Mori H, Kobara H, Masaki T. Efficacy of sufficient operation view by ring-shaped thread counter traction for safer duodenal ESD. Minim Invasive Ther Allied Technol. 2018;27:327. doi: 10.1080/13645706.2018.1455706. - [DOI] [PubMed] [Google Scholar]
- 114.Tashima T, Nonaka K, Kurumi H, Fujii Y, Tanisaka Y, Ryozawa S. Successful traction-assisted endoscopic submucosal dissection using dental floss and a clip for a huge superficial nonampullary duodenal epithelial tumor with severe fibrosis (with video) JGH Open. 2019;3:179. doi: 10.1002/jgh3.12118. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tashima T, Miyaguchi K, Tanisaka Y, Fujita A, Ryozawa S. Successful duodenal endoscopic submucosal dissection using multiple clip-and-thread traction for a large tumor located in the duodenal bulb. VideoGIE. 2021;6:178. doi: 10.1016/j.vgie.2020.12.005. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamada K, Takeuchi Y, Ishikawa H, Tonai Y, Matsuura N, Ezoe Y. Feasibility of Cold Snare Polypectomy for Multiple Duodenal Adenomas in Patients with Familial Adenomatous Polyposis:A Pilot Study. Dig Dis Sci. 2016;61:2755. doi: 10.1007/s10620-016-4165-7. et al. - [DOI] [PubMed] [Google Scholar]
- 117.Patel NJ, Ponugoti PL, Rex DK. Cold snare polypectomy effectively reduces polyp burden in familial adenomatous polyposis. Endosc Int Open. 2016;4:E472. doi: 10.1055/s-0042-104114. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hamada K, Takeuchi Y, Ishikawa H, Ezoe Y, Arao M, Suzuki S. Safety of cold snare polypectomy for duodenal adenomas in familial adenomatous polyposis:A prospective exploratory study. Endoscopy. 2018;50:511. doi: 10.1055/s-0043-124765. et al. - [DOI] [PubMed] [Google Scholar]
- 119.Matsuura N, Takeuchi Y, Yamashina T, Ito T, Aoi K, Nagai K. Incomplete resection rate of cold snare polypectomy:A prospective single-arm observational study. Endoscopy. 2017;49:251. doi: 10.1055/s-0043-100215. et al. - [DOI] [PubMed] [Google Scholar]
- 120.Kawamura T, Takeuchi Y, Asai S, Yokota I, Akamine E, Kato M. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps:A multicentre randomised controlled trial (CRESCENT study) Gut. 2018;67:1950. doi: 10.1136/gutjnl-2017-314215. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729. doi: 10.1007/s00464-007-9696-8. et al. - [DOI] [PubMed] [Google Scholar]
- 122.Ohata K, Murakami M, Yamazaki K, Nonaka K, Misumi N, Tashima T. Feasibility of endoscopy-assisted laparoscopic full-thickness resection for superficial duodenal neoplasms. ScientificWorldJournal. 2014;2014:239627. doi: 10.1155/2014/239627. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ichikawa D, Komatsu S, Dohi O, Naito Y, Kosuga T, Kamada K. Laparoscopic and endoscopic co-operative surgery for non-ampullary duodenal tumors. World J Gastroenterol. 2016;22:10424. doi: 10.3748/wjg.v22.i47.10424. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yanagimoto Y, Omori T, Jeong-Ho M, Shinno N, Yamamoto K, Takeuchi Y. Feasibility and Safety of a Novel Laparoscopic and Endoscopic Cooperative Surgery Technique for Superficial Duodenal Tumor Resection:How I Do It. J Gastrointest Surg. 2019;23:2068. doi: 10.1007/s11605-019-04176-2. et al. - [DOI] [PubMed] [Google Scholar]
- 125.Nunobe S, Ri M, Yamazaki K, Uraoka M, Ohata K, Kitazono I. Safety and feasibility of laparoscopic and endoscopic cooperative surgery for duodenal neoplasm:A retrospective multicenter study. Endoscopy. 2021;53:1065. doi: 10.1055/a-1327-5939. et al. - [DOI] [PubMed] [Google Scholar]
- 126.Ojima T, Nakamori M, Nakamura M, Hayata K, Katsuda M, Takifuji K. Laparoscopic and Endoscopic Cooperative Surgery Versus Endoscopic Submucosal Dissection for the Treatment of Low-Risk Tumors of the Duodenum. J Gastrointest Surg. 2018;22:935. doi: 10.1007/s11605-018-3680-6. et al. - [DOI] [PubMed] [Google Scholar]


