Abstract
Objective:
This review describes the antioxidant activity of flavonoids as a subgroup of polyphenols and a partial or entire substitute for synthetic antioxidants.
Materials and Methods:
All relevant databases were searched using the terms “Phytochemical”, “Polyphenol”, and “Flavonoid”.
Results:
The oxidative reaction caused by free radicals is a reason for food spoilage, which causes unpleasant odor, loss of taste, and damaged tissues. The common antioxidants employed in foods include butylated hydroxyanisole, butylated hydroxytoluene, propyl gallate, and tert-butyl hydroquinone. Despite their high efficiency and potency, synthetic antioxidants have adverse effects on the human body, such as causing mutation and carcinogenicity. A whole and a group of them known as polyphenols possess high antioxidant activity. These compounds are potential antioxidants due to their capabilities such as scavenging free radicals, donating hydrogen atoms, and chelating metal cations. The antioxidant mechanism of action of flavonoids is transferring hydrogen atom to free radicals. Accordingly, the more the flavonoid structure makes the hydrogen transfer faster and easier, the more the flavonoid’s antioxidant power will be. Therefore, the antioxidant activity of the flavonoids with hydroxyl groups in their structure is the highest among different flavonoids.
Conclusion:
In addition to health promotion and some disease prevention effects, various in vitro investigations have indicated that flavonoids possess high antioxidant activity that is comparable with synthetic antioxidants. However, to be commercially available, these compounds should be extracted from a low-price source with a high-performance method.
Key Words: Antioxidant, Flavonoids, Food preservation, Phytochemical, Polyphenol
Introduction
Most food products are made up of various chemical components that are easily oxidized. Lipids (i.e., fats, oils, and waxes) compared to chemical compounds that cause oxidation reactions in most food products, have the most significant potential to lose electrons in general. Lipid auto-oxidation in food products initiated by metal ions exposure, metalloprotein catalysis, light, heat, or ionizing radiation can deteriorate the color, flavor, texture, quality, and safety of food. Food fats are chemically made of triglycerides, and oxidation occurs at the unsaturated sites of the triglycerides, resulting in rancidity (Atta et al., 2017; Latifi et al., 2019). Free radicals are the most frequent oxidants in biological systems. Free radicals are molecules, ions, or atoms, with one or more unpaired electrons in valence shell or outer orbit. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive; as result, it is capable of independent existence. In order to stabilize themselves, unpaired electrons in these free radicals seek and acquire electrons from other substances. When the first assault is paired to the odd electron, another free radical is produced in the process, resulting in a chain reaction. Free radicals cause considerable damage to the macromolecules in body organisms, including deoxyribonucleic acid (DNA), proteins, lipids, and carbohydrates. The damages caused by free radicals are also referred to as oxidative damages (Halliwell, 2012; Hassanpour and Karami, 2021; Nogala-Kalucka et al., 2005; Daneshniya et al., 2020).
Free radicals are frequently obtained from nitrogen, oxygen, and sulfur molecules in biological systems. Such free radicals are the constituents of reactive sulfur species (RSS), reactive nitrogen species (RNS), and reactive oxygen species (ROS) molecular families. Free radicals, such as superoxide anion (O2-•), nitric oxide, hydroxyl radical, per-hydroxyl radical, and other species, such as hydrogen peroxide, singlet oxygen (O2), hypochlorous acid, and peroxynitrite (ONOO-), are forms of ROS. Nitric oxide is converted to RNS by reacting with O2-• to generate ONOO-. By the reaction of thiols with ROS, RSS is quickly produced. The ROS production occurs due to cellular metabolism and functional activities (Atta et al., 2017; Daneshniya, 2020).
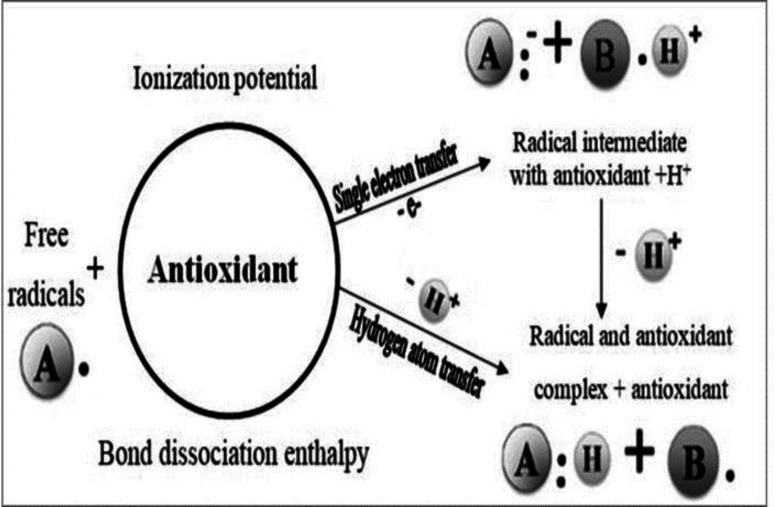
Antioxidants are among the factors that prevent oxidative damage. The structure of antioxidants is such that although they release hydrogen atoms, the reactivity of these atoms is extremely low (Pokorný, 2007). Figure 1 depicts the common schematic mechanism of antioxidants. In general, antioxidants are divided into synthetic and natural groups. Because synthetic antioxidants are cheaper and more accessible and show high efficiency and stability, they were gradually considered alternatives to their natural type after World War II (Halliwell et al., 1995; Afanas' ev et al., 1989). The nonnutritive part of plants (i.e., phytochemicals) has a positive effect on metabolism, cancer, nervous system, wounds, mouth, and teeth. One of the reasons for turning to phytochemicals is their antimicrobial properties; therefore, they are grown in laboratories for easy and extensive access. The adjustment of cultural conditions for propagation shows signs of the future success of this method in order to be used in medicine (Bansal and Priyadarsini, 2021).
Figure 1.
Schematic representation of common antioxidant mechanism
Phytochemicals especially the phenolic compounds are important natural antioxidants and are recognized as excellent antioxidants due to their capacity to scavenge free radicals, donate electrons and hydrogen atoms, and chelate metal cations (Halliwell et al., 1995, Latifi et al., 2021). Currently, synthetic antioxidants are more commonly used in industries, the most common of which are butylated hydroxytoluene (BHT), tert-butyl hydroquinone (TBHQ), butylated hydroxyanisole (BHA), and propyl gallate (P.G.). Several other compounds have been suggested to have antioxidant activity; nevertheless, only a few can be used in foods. Ethoxyquin is a synthetic antioxidant with a nonphenolic structure, the use of which is not permitted for humans, compared to other previously mentioned antioxidants; however, it is used for food preservation in animal feeding (Latifi et al., 2019).
Using antioxidants in foods has been limited by the regulatory laws of one's country or international standards (Dorman et al., 2004). As a result, the consumption of synthetic antioxidants is limited due to their toxic effects and carcinogenicity. Since the carcinogenicity of synthetic antioxidants has been observed, the necessity of using alternatives without adverse effects is becoming increasingly important (Ardabili et al., 2010).
Natural antioxidants have shown strong antimicrobial activity and can be used as a potential alternative to chemical antioxidants. Additionally, many of these compounds are known by the United States Food and Drug Administration (FDA) as safe compounds that are used extensively in food compounds (Prakash B et al., 2020). Therefore, due to increasing consumer concern about synthetic antioxidants due to their toxic effects, the demand for natural antioxidants, especially of plant origin, has increased recently (Formanek et al., Biswas et al., 2004; Jayathilakan et al., 2007). This review study was conducted to investigate the antioxidant activity of phytochemicals, emphasizing on the capacity of flavonoids to be utilized as natural antioxidants in food and examine their capability to substitute for synthetic antioxidants.
Materials and Methods
This review study was conducted to investigate the antioxidant activity of phytochemicals, especially flavonoids, and their potential to entirely or partially substitute for synthetic antioxidants in food products. All relevant databases were searched for the terms “Phytochemical”, “Polyphenol”, “Flavonoid”, “Natural Additive”, and “Antioxidant Activity”.
The data were collected using PubMed, Scopus, Web of Science, Google Scholar, and ScienceDirect databases, related books, and prominent conferences and congresses with various publication dates ranging from 1981 to 2021.
Results
Phytochemicals are bioactive substances that are beneficial for human health. These compounds are categorized by their chemical structures and include polyphenols (i.e., flavonoids and nonflavonoid polyphenols), phytosterols, carotenoids, organosulfur, and betalains. Phytochemicals as natural antioxidants prevent the change in color, taste and odor of food, etc., which is caused by the oxidation process and play an important role in the plant defense system. More than 5000 pigments in plants are considered to be phytochemicals. These compounds can reveal their biological activity through various bioactivities, such as antiviral, antibacterial properties, antioxidant, anti-inflammatory, anticancer, modulation of enzyme activities, stimulation of the immune system, and regulation of cholesterol synthesis, gene expression, and blood pressure.
Phytochemicals can also improve health and prevent diseases (Puri et al., 2012; Yadav and Agarwala, 2011). Due to the presence of these compounds in fruits and vegetable-rich diets, they can delay aging, reduce inflammation and the oxidative stress risk, and decrease the risk of chronic diseases, such as arteriosclerosis, cardiovascular diseases, cancers, cataracts, diabetes, neurological diseases, and cognitive function disorders (Eliassen et al., 2912; Pojer et al., 2013; Tanaka et al., 2012). Vegetables, fruits, legumes, and whole grains are the primary sources of phytochemicals in a diet (Tokusoglu Ö., 2011). There are different methods for extracting phytochemical compounds. These compounds can be extracted from plants through physical and chemical methods, such as ultrasonication, supercritical fluid extraction, solvent extraction, and cold pressing (Puri et al., 2012; Latifi et al., 2019).
It should be pointed out that several phytochemicals are insoluble in aqueous solutions and do not have acceptable oil solubility. These compounds are chemically unstable and decompose promptly after exposure to the external environment due to temperature, oxygen, pH, light, and other reactive substances. Phenolic compounds or polyphenols are important groups of phytochemical compounds in plants (Bravo, 1998; Daneshniya et al., 2021). Phenolic compounds have excellent antioxidant characteristics and are often found in fruits and vegetables (Heim et al., 2002). These compounds include flavonoids, flavanols, anthocyanins, anthraquinones, and their benzoyl and acetylated compounds.
Polyphenols are found in two general classes, namely flavonoids and phenolic acids. Different parts of a plant produce compounds that are mainly phenolic and are known as natural antioxidants. Several compounds, such as flavonoids, coumarins, carotenoids, tocopherols, organic acids, and derivatives of cinnamic acid, are phenolic antioxidants from the plant sources. In this category of compounds, the hydroxyl group is directly bonded to an aromatic hydrocarbon (Amorati and Valgimigli, 2012).
There are at least 8000 structures of phenolic compounds known, the simplest of which is phenol, shown in Figure 2 (Bravo, 1998).
Figure 2.
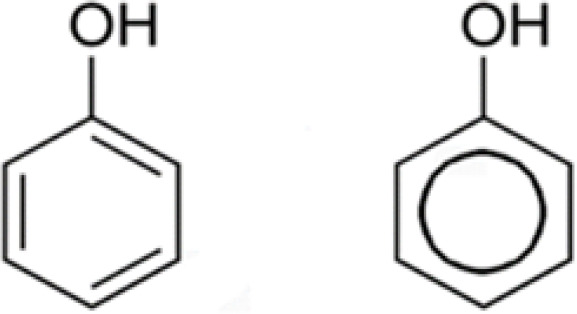
Phenol, the most straightforward component of phenolic compounds
Phenolic compounds are known for their antioxidant activity that depends on the structure, especially the location and number of hydroxyl groups and the nature of substituents on aromatic rings (Balasundram et al., 2006). Phenolic compounds are referred to as health promotion compounds with the capability of preventing chronic heart disease (Harborne and Williams, 2000; Holtekjølen et al., 2008). The antioxidant characteristics of phenolic compounds depend on their capability of donating electrons to trap free radicals by the formation of stable phenoxyl compounds (Lam et al., 2007).
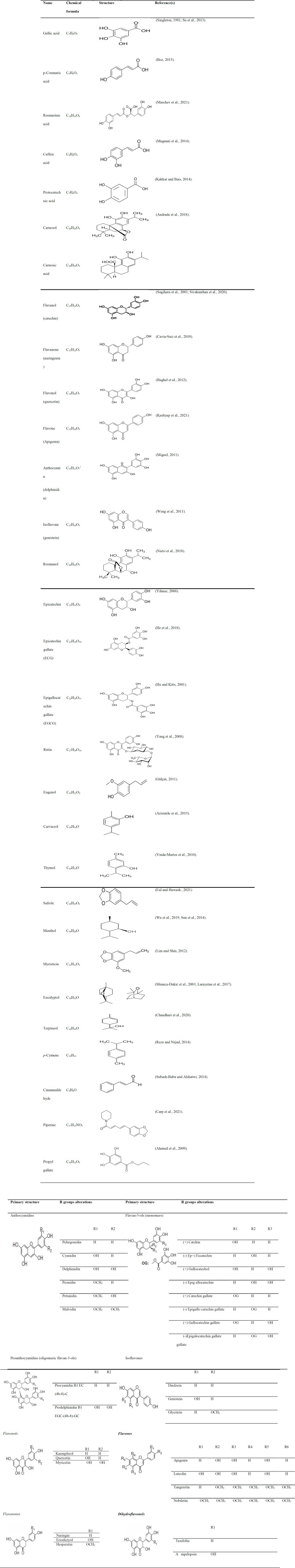
Plants have always been excellent food sources for the consumption of valuable bioactive compounds (Tayel and EL‐TRAS, 2012). Such natural antioxidants are extracted from plants in the form of essential oils and extracts from various sources, such as fruits (e.g. pomegranates, grapes, dates, and kinnow), vegetables (e.g. potatoes, broccoli, drumstick, Indian turmeric, pumpkin, and nettle), and medicinal plants and spices (e.g. rosemary, tea, cinnamon, oregano, common sage, thyme, ginger, peppermint, and clove), and have been assessed for reducing fat oxidation (Devatkal et al., 2009; Huang et al., 2011; Wójciak et al., 2011; Das., 2012; Latifi., 2019; Ebadi et al., 2021). Phenolic compounds are discovered in combination with saccharides (monosaccharides and polysaccharides) bonded to one or more phenolic groups. Although some phenolic compounds are ubiquitous, others are specific to particular plant families and are found in particular plant organs or at specific stages of plant growth (Cheynier, 2012). Table 1 lists important phenolic compounds in plants that have been reported to possess antioxidant activity in in vivo and in vitro studies.
Table 1.
The phenolic compounds with high antioxidant activity
Structures of flavonoids and their antioxidant activity
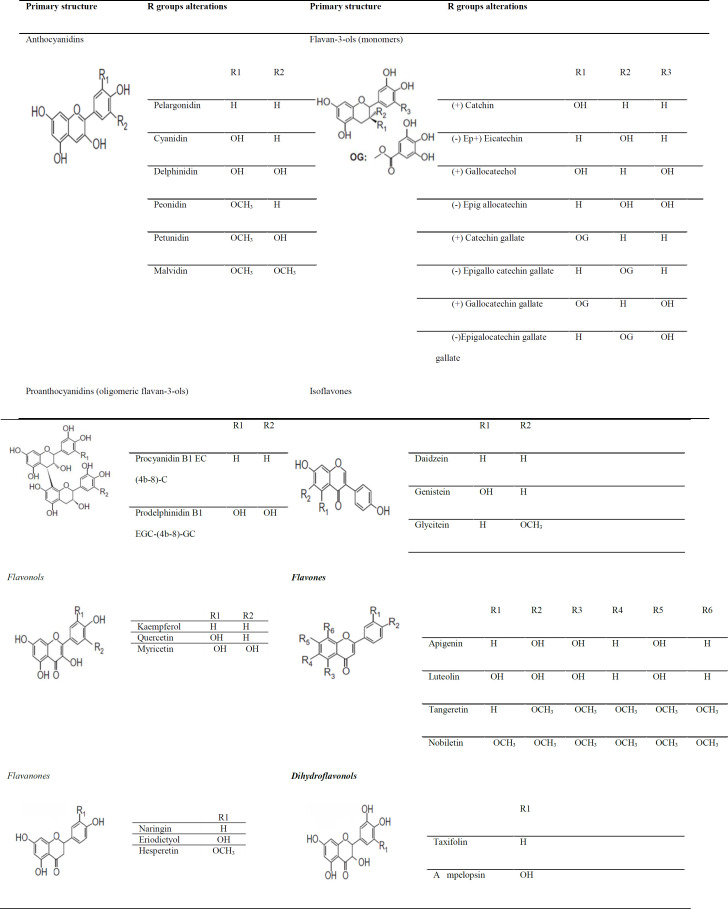
Flavonoids are the largest group of phytochemicals and are considered to be the most widespread group of polyphenols available in vegetables and fruits. Flavonoids have excellent antioxidant properties and can exhibit their antioxidant activity by scavenging free radicals and ROS, chelating metals, and preventing the oxidation of low-density lipoproteins (LDLs) (Heim et al., 2002; Chu et al., 2000). Flavones are the most basic structures of flavonoids. From the structural point of view, all flavonoids possess a C6-C3-C6 carbon skeleton, where the carbon atoms are located in three phenolic A, B, and C rings, and the C ring normally contains oxygen (Pietta, 2000; Ververidiset al., 2007). Figure 3 depicts phenyl-benzopyrone as the basic structure of flavonoids. Flavonoids are found in different families, each of which has different members. The flavonoid families include flavones, isoflavones, flavonols, flavanones, dihydroflavonols, flavan-3-ols (monomers), proanthocyanidins (oligomeric flavan-3-ols), and anthocyanidins. Table 2 shows these families and their members (Laura et al., 2019).
Figure 3.
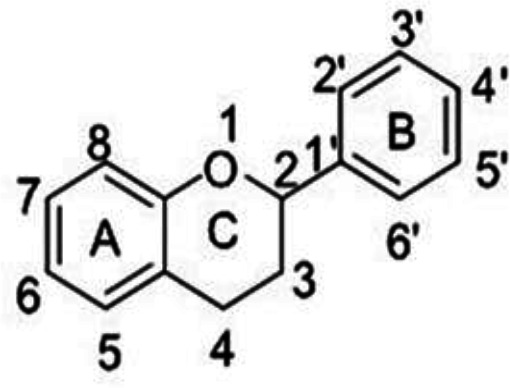
Basic structure of flavonoids (Phenylbenzopyrane)
Table 2.
Flavonoids family
Regulations regarding synthetic and natural antioxidants
There is a need to move away from synthetic antioxidants due to an increasing interest in natural food antioxidants; however, the synthetic or natural origin of the different antioxidants that are applied in the food industry are unknown in the official tables indicating the levels and permissions to use each additive in each food type (Gutiérrez-del-Río et al., 2021). The Codex Alimentarius Commission is the worldwide authority in charge of regulating and establishing the framework of food regulations that guide the use of antioxidants in foods. Since food regulatory systems and legal frameworks regarding using antioxidants as food additives differ by country, these criteria are neither required nor immediately applicable (Manessis et al., 2021).
The European Food Safety Authority of the European Union (E.U.) and the United States FDA are the two primary guiding authorities in charge of regulating the licensing of food additives worldwide (Carocho et al., 2018). Under the E.U., regulation E.C. No 1129/2011 specifies some antioxidants, categorized based on their E-numbers, that are regulated in the “other food additives” section. The natural antioxidants recognized as food additives by the E.U. include rosemary extracts (E392), tocopherols (E306–E309), and ascorbic acid (E300), according to this regulation (European Parliament and Council Commission Regulation, 2011).
Food products and ingredients are monitored by various authorities in the United States, the most relevant of which is the United States FDA. The FDA’s regulations for food items are listed in Title 21 of the Code of Federal Regulations of the United States. There is no distinct category for natural antioxidants in the Code of Federal Regulations, as there is in the European regulations (Oswell et al., 2018; FDA Title 21 Part 172, 2022; FDA Title 21 Part 182, 2021). Although natural antioxidants, such as tocopherols and ascorbic acid, are specifically designated for use in food, the United States regulation is far more comprehensive than the European one and includes additional substances that belong to other categories and still have demonstrated antioxidant activity. Several of the compounds allowed for use as spices, coloring adjuncts, or natural flavorings, such as flavonoids, phloretin glycoside, and carotenoids, such as astaxanthin and carotene, or extracts of sage or rosemary, have been recognized with the antioxidant potential. Astaxanthin, phloretin, and sage extract are not listed as food additives in any category in E.U. regulations, although they are classified for technical applications in United States standards (FDA Title 21 Parts 73, 2018; European Commission Food Additives, 2022). Considering the above-mentioned regulations, it is evident that regardless of the utilization of natural or synthetic antioxidants, the optimal choice of antioxidant for each food matrix should be determined on a case-by-case basis because in vitro antioxidant activities cannot always be generated in the food, and frequent changes occur due to food processing or interactions with other components of food matrix that have prooxidant or antioxidant activities (Barden and Decker, 2016).
Antioxidants in food preservation
Once lipid oxidation in a food matrix is analyzed over time, there is frequently a lag phase, where the deposition of lipid oxidation by-products is moderate. Such lag phase can be associated with the existence of antioxidants in the food matrix, limiting the synthesis of free radicals targeting fatty acids and the less free radical generation that precedes the concentration of ß-scission reactions and hydroperoxides (Gutiérrez-del-Río, 2021). The objective of the food industry is to extend the lag period, where the concentrations of the components that cause rancidity taste are less than human detection limits. When the food matrix lacks natural antioxidants and/or includes significant endogenous prooxidants, the most typical technique used by food manufacturers to limit oxidation is adding antioxidants directly to the food matrix (Gutiérrez-del-Río, 2021).
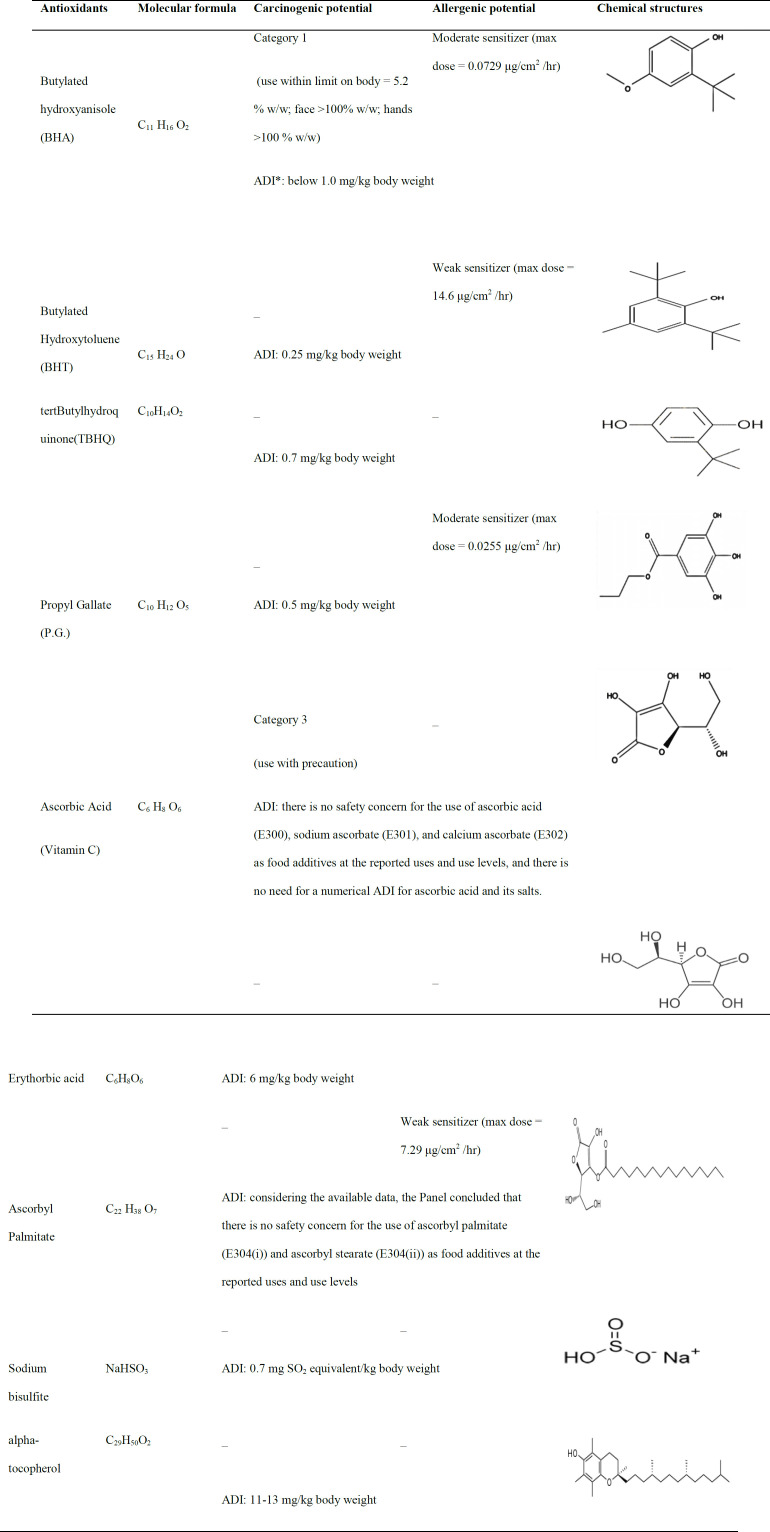
In the context of food science, the phrase “antioxidant” refers to substances that prevent lipid peroxidation (LPO) and other oxidative reactions, thereby preserving the shelf-life extension and freshness of foods. The action method is the same whether the antioxidant is synthetic or natural and comprises O2 quenching, metal chelating, and free radical scavenging (Yang et al., 2018; Lin et al., 2016). Due to their stability, low cost, and wide availability, synthetic antioxidants are frequently employed. Phenolic antioxidants are the most extensively applied synthetic antioxidants in the food industry, with BHT (E321), TBHQ (E319), P.G. (E310), and BHA (E320), as the most common ones. Table 3 shows some critical information regarding the chemical structures and health-related concerns about the consumption of these antioxidants (Kwan et al., 2014; Additives and Nutrient Sources, 2011; Additives and Nutrient Sources, 2012; Additives and Nutrient Sources, 2016; Additives and Nutrient Sources, 2014; Additives and Nutrient Sources, 2015; Additives and Nutrient Sources, 2016; Additives and Nutrient Sources, 2015; Additives and Nutrient Sources, 2016; Nutritional Dietetics Allergy Products, 2015).
Table 3.
Chemical structures of food industry antioxidants
Despite the fact that such synthetic antioxidants are widely used and tightly regulated, their safety should be considered owing to overdose usage and/or misuse; for instance, combining various antioxidants might exacerbate their toxic effects. High concentrations of synthetic chemical antioxidants can induce in vitro toxicity or DNA damage in some tissues (Lourenço et al., 2019; Xu, b2021; Liu and Mabury, 2020; Baran et al., 2021). The food industry is attempting to reduce using synthetic compounds by substituting natural alternatives against consumer concerns that they are being exposed to potentially toxic synthetic chemical compounds through their regular diet. Although plant antioxidants are safe; but for more assurance; toxicity tests should always be performed for all cases (Lourenço et al., 2019).
Structure of flavonoids and their antioxidant properties
Flavonoids are bioactive polyphenol compounds that are found in almost all fruits and vegetables. Aglycones, glycosides, and methylated derivatives are different forms of flavonoids. Aglycones are produced when a hydrogen atom replaces the glycosyl group in a glycoside. Glycosides are composed of two parts, sugars (glycans) and nonsugars (aglycones), and play different roles in plants (Daneshniya et al., 2020). Various species of flavonoids differ in oxidation rate and C-ring substituents; in other words, the existence of one double bond, one carbonyl group, and one hydroxy group in the pyranyl C ring is used to classify them as species and subspecies. The substituents of A and B rings with hydroxy groups are used to identify the available members in a species.
Flavonoids in plants are often derived from glycosylate and play a role in the production of shining blue, reddish-yellow, and reddish-orange shades in leaves, flowers, and fruits. Regardless of the variety of fruits and vegetables, flavonoids are found in seeds, nuts, buds, spices, herbal medications, some drinks, such as wine, especially red and tea wines, and in smaller quantities in beer. The antioxidant properties are among the most important properties of flavonoids. In numerous studies, flavonoid-rich plant extracts have been used to prevent food oxidation (Daneshniya et al., 2020). Despite the many benefits of synthetic antioxidants, compared to those of natural ones, such as their low price, using antioxidants in food has been limited by the regulatory laws of international standards or one country (Dorman et al., 2004). Therefore, the use of synthetic antioxidants is limited due to their toxic effects and carcinogenicity. Synthetic antioxidants have a limit of using 0.02% fat in food (Daneshniya et al., 2020). Since the carcinogenic properties of synthetic antioxidants have been observed, the necessity of the use of alternatives without adverse effects has become increasingly important (Ardabili et al., 2010). Therefore, in recent years, the demand for natural antioxidants, particularly of plant origin, has risen as a consequence of consumers’ concerns regarding such synthetic antioxidants due to their potential toxicological impacts (Jayathilakan et al., 2007).
In general, flavonoids are antioxidants with high efficiency and potency, and as a result, by reducing the oxidation of LDL, they protect the body against cardiovascular diseases. Flavonoids both prevent LPO and act as scavengers of radicals, such as superoxides, lipid peroxides, and hydroxylated compounds, and lead to the inactivation of single oxygen molecules and prevention of the activity of lipoxygenases. The high potency of flavonoids in inhibiting free radicals relates to their ability to transfer a hydrogen atom from a hydroxyl group to the free radical and ultimately stabilize it as follows:
FLOH + R° → FLO° + R.H.
Flavones and catechins are the most powerful flavonoids with antioxidant properties that can protect the body against ROS. The free radical scavenging activity of flavonoids is as follows (Gülçin, 2012):
Myricetin>quercetin>rhamnetin>morin>diosmetin>naringenin>apigenin>catechin>5,7-Dihydroxy-3,3',4'-trimethoxyflavone> robinin > kaempferol > flavone
Different factors affect the antioxidant properties of flavonoids. One of the most important factors is the structure of flavonoids. The presence of hydroxyl and glycosylated groups has a significant effect on the antioxidant activity of flavonoids. The existence of glycosylated groups reduces the antioxidant properties of flavonoids. On the other hand, the presence of hydroxy groups increases the antioxidant properties of flavonoids. For maximum free radical scavenging activity, flavonoids should have a hydroxy group at the 4' and 3' positions of the B ring, a conjugated double bond between carbon 2 and 3, an oxo group at the 4 positions of the C ring, a hydroxy group on carbon 3 in the C ring, and a hydroxy group on carbon 5 in the A ring (Figure 3). The presence of many hydroxy groups, especially in the B ring, leads to an increase in the antioxidant activity of flavonoids. The hydroxyls of the B ring are the first active stations in traversing the oxidation chain. The three structural groups are responsible for determining the scavenging activity of free radicals and antioxidant activity of flavonoids, including a catechol section in the B ring, a conjugated double bond at the positions 2 and 3, an oxo group with the carbonyl group function in the ring C, and the existence of hydroxy groups at the positions 3 and 5 (Gülçin, 2012; Heijnen et al., 2001). Therefore, it is noteworthy that flavonoids can exhibit different antioxidant activities according to their structure.
Flavonoids are classified as nonnutrients in the scientific food realm. They have typically been eliminated from food crops due to their inhibitory effects on digestive enzymes, astringency and bitterness, and poor absorption after intake. However, due to their inclusion in regular meals, several therapeutic benefits, such as antioxidant properties in animal studies, the decrease of cardiovascular disease and high blood pressure, and antiallergic, anti-inflammatory, and anti-diabetic activities, were recognized. As a result, flavonoids are now considered third-order functional components (food factors) that have biological regulatory roles (Terahara, 2015).
Flavonoids are also important in food preservation and industry. The phrase “sustainable intensification” was created by the Royal Society of London, United Kingdom, which denotes current agricultural practices aiming at raising food production while also safeguarding biodiversity and environmental processes. Food waste minimization is one of the most critical elements of “sustainable intensification”. As a result, there is a growing demand for postharvest storage technology and food preservatives research (D'Amelia et al., 2018; Petersen and Snapp, 2015).
Since today’s consumers are concerned about using chemically synthesized preservatives, natural products are prioritized. At the same time, industries are giving a greater focus on using plant-derived antioxidant and antibacterial components to improve shelf life. The antioxidant effect of flavonoids might preserve food stability over time and offer protection against necrotrophic fungi and food-borne diseases. The flavonoids’ antioxidant capacity in food systems is associated with their capacity to prevent lipid autoxidation as a primary cause of food quality degradation and shelf-life reduction (Shahidi and Ambigaipalan, 2015). Flavonoids are capable of donating hydrogen atoms to lipid radicals, resulting in more stable antioxidant radicals that are less susceptible to autoxidation. The antioxidant action mechanisms of flavonoids include direct ROS scavenging, inhibition of ROS generation via the chelation of trace elements (quercetin has iron-stabilizing and iron-chelating effects), or inhibition of free radical-generating enzymes (i.e., microsomal monooxygenase, glutathione S-transferase, nicotinamide adenine dinucleotide phosphate oxidase, and mitochondrial succinoxidase), and activation of antioxidant defenses (i.e., the up-regulation of antioxidant enzymes characterized by radical scavenging capacity). A synergy of some of such mechanisms, such as radical scavenging activity along with some enzymes’ function inhibition, can also occur (Kumar and Pandey, 2013; Agrawal, 2011; Kaleem and Ahmad, 2018).
The capacity of flavonoids to lower the susceptibility of fresh vegetables and fruits to particular postharvest infections might also contribute to their antioxidant effect in extending shelf life (Neha and Pandey-Rai, 2014). Flavonoids, exceptionally high hydroxylated anthocyanins, can prevent the growth of grey mold (produced by Botrytis cinerea), causing the dynamics of the ROS burst to be disrupted during infection. Flavonoids might inactivate and bind proteins and can form complexes that have bacterial cell walls, giving them antibacterial action against numerous pathogens (Zhang et al., 2013; Zhang et al., 2015; Hintz et al., 2015).
Flavonoids have been employed in a variety of dietary applications due to their beneficial characteristics. Flavonoids have been applied as active antioxidant agents in the oxygen-sensitive food packaging industries to extend shelf life and improve the bioactive component levels (López de Dicastillo et al., 2011). Flavonoids have also been applied to minimize lipid oxidation in cooked pork patties and raw mackerel fillets (Viji et al., 2015). Considering all the above-mentioned information regarding the antioxidant potential of flavonoids, it is evident that, in addition to their health-promoting effects, they can be utilized as food additives to extend shelf life.
Discussion
As one of the significant subgroups of polyphenols, flavonoids are crucial secondary chemicals known as phytochemicals generated by plants that are involved in various activities, including growth and development and stress resistance. The high demand and interest in using flavonoids in food processing and their health-promoting effects have resulted in the increased acknowledgment of the positive aspects of flavonoids for human health. It is evident that as an important subset of the phytochemical’s family, flavonoids possess a considerable antioxidant activity that is competitive with synthetic antioxidants. Flavonoids are abundant in vegetables, flowers, and seeds, and approaches have been developed for extracting these compounds from these natural sources for use as food preservatives and additives. However, on a commercial scale, the extraction of flavonoids directly from the aforementioned sources does not seem to be profitable. Therefore, it seems that attempting to find a good source for extraction, such as plant wastes, and designing a high-performance extraction method would be critical steps for these compounds to be applied in food products as preservatives.
Conflicts of interest
The authors have declared that there is no conflict of interest.
Acknowledgment
This study was solely supported by the authors named herein. The authors declare no conflict of interest.
References
- Andrade JM, Faustino C, Garcia C, Ladeiras D, Reis CP, Rijo P. Rosmarinus officinalis L : an update review of its phytochemistry and biological activity. Future Sci OA. 2018;4:FSO283. doi: 10.4155/fsoa-2017-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta EM, Mohamed NH, Silaev AA. Antioxidants: An overview on the natural and synthetic types. Eur Chem Bull. 2017;6:365–375. [Google Scholar]
- Aristatile B, Al‐Numair KS, Al‐Assaf AH, Veeramani C, Pugalendi KV. Protective effect of carvacrol on oxidative stress and cellular DNA damage induced by UVB irradiation in human peripheral lymphocytes. J Biochem Mol Toxicol. 2015;29:497–507. doi: 10.1002/jbt.20355. [DOI] [PubMed] [Google Scholar]
- Amorati R, Valgimigli L. Modulation of the antioxidant activity of phenols by non-covalent interactions. Org Biomol Chem. 2012;10:4147–4158. doi: 10.1039/c2ob25174d. [DOI] [PubMed] [Google Scholar]
- Agrawal AD. Pharmacological activities of flavonoids: A review. Int J Pharm Sci Nanotechnol. 2011;4:1394–1398. [Google Scholar]
- Ardabili AG, Farhoosh R, Khodaparast MH. Frying stability of canola oil in presence of pumpkin seed and olive oils. Eur J Lipid Sci Technol. 2010;112:871–877. [Google Scholar]
- Alamed J, Chaiyasit W, McClements DJ, Decker EA. Relationships between free radical scavenging and antioxidant activity in foods. J Agric Food Chem. 2009;57:2969–2976. doi: 10.1021/jf803436c. [DOI] [PubMed] [Google Scholar]
- Afanas' ev IB, Dcrozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmaco. 1989;38:1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- Bansal A, Priyadarsini C. Medicinal properties of phytochemicals and their production. In: El-Shemy HA, editor. Natural Drugs from Plants . London, IntechOpen: 2021. pp. 401–409. [Google Scholar]
- Baran A, Yildirim S, Ghosigharehaghaji A, Bolat İ, Sulukan E, Ceyhun SB. An approach to evaluating the potential teratogenic and neurotoxic mechanism of BHA based on apoptosis induced by oxidative stress in zebrafish embryo (Danio rerio) Hum Exp Toxicol. 2021;40:425–438. doi: 10.1177/0960327120952140. [DOI] [PubMed] [Google Scholar]
- Barden L, Decker EA. Lipid oxidation in low-moisture food: a review. Crit Rev Food Sci Nutr. 2016;56:2467–2482. doi: 10.1080/10408398.2013.848833. [DOI] [PubMed] [Google Scholar]
- Boz H. p‐Coumaric acid in cereals: presence, antioxidant and antimicrobial effects. Int J Food Sci Technol. 2015;50:2323–2328. [Google Scholar]
- Baghel SS, Shrivastava N, Baghel RS, Agrawal P, Rajput S. A review of quercetin: antioxidant and anticancer properties. World J Pharm Pharm Sci. 2012;1:146–160. [Google Scholar]
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. [Google Scholar]
- Biswas AK, Keshri RC, Bisht GS. Effect of enrobing and antioxidants on quality characteristics of precooked pork patties under chilled and frozen storage conditions. Meat Sci. 2004;66:733–741. doi: 10.1016/j.meatsci.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Carp OE, Moraru A, Pinteala M, Arvinte A. Electrochemical behaviour of piperine Comparison with control antioxidants. Food Chem. 2021;339:128110. doi: 10.1016/j.foodchem.2020.128110. [DOI] [PubMed] [Google Scholar]
- Chaudhari AK, Singh A, Singh VK, Dwivedy AK, Das S, Ramsdam MG, Dkhar MS, Kayang H, Dubey NK. Assessment of chitosan biopolymer encapsulated α-Terpineol against fungal, aflatoxin B1 (AFB1) and free radicals mediated deterioration of stored maize and possible mode of action. Food Chem. 2020;311:126010. doi: 10.1016/j.foodchem.2019.126010. [DOI] [PubMed] [Google Scholar]
- Carocho M, Morales P, Ferreira IC. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci Technol. 2018;71:107–120. [Google Scholar]
- Cheynier V. Phenolic compounds: from plants to foods. Phytochem Rev. 2012;11:153–177. [Google Scholar]
- Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muñiz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. 2010;90:1238–1244. doi: 10.1002/jsfa.3959. [DOI] [PubMed] [Google Scholar]
- Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80:561–566. [Google Scholar]
- Daneshniya M, Maleki MH, Mohammadi MA, Ahangarian K, Kondeskalaei VJ, Alavi H. Antioxidant and antimicrobial activity of ferula species' essential oils and plant extracts and their application as the natural food preservatives. South Asian Res J Nat Prod. 2021;4:1–23. [Google Scholar]
- Daneshniya M. Proceedings of the 2nd International Congress on Science, Engineering &Technology; Maleki MH; Munich, Germany: 2020. [Google Scholar]
- Daneshniya M, Maleki MH. henolic compounds extracted from plants as potential antioxidants,. Proceedings of the 2nd International Congress on Science, Engineering &Technology; 19 October. 2020; Munich, Germany: [Google Scholar]
- D'Amelia V, Aversano R, Chiaiese P, Carputo D. The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem Rev. 2018;17:611–625. [Google Scholar]
- Das AK, Rajkumar V, Verma AK, Swarup D. Moringa oleiferia leaves extract: a natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int J Food Sci Technol. 2012;47:585–591. [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85:155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Dorman HD, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected lamiaceae species grown in turkey. J Agric Food Chem. 2004;52:762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Latifi Z, Daneshniya M. Optimization of the antioxidant effect of ethanolic extract of thistle (Carduus pycnocephalus L) by response surface method and comparison of the antioxidant effect of extract and essential oil on oxidative soybean oil resistance. J Food Sci Technol. 2021;17:151–164. [Google Scholar]
- Eid AM, Hawash M. Biological evaluation of safrole oil and safrole oil nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complement Med Ther. 2021;21:159. doi: 10.1186/s12906-021-03324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA panel on food additives and nutrient sources added to food (ANS. Scientific opinion on the re‐evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives. EFSA J. 2016;14:4438. doi: 10.2903/j.efsa.2022.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA panel on food additives and nutrient sources added to food (ANS. Scientific opinion on the re‐evaluation of erythorbic acid (E 315) and sodium erythorbate (E 316) as food additives. EFSA J. 2016;14:4360. [Google Scholar]
- EFSA panel on food additives and nutrient sources added to food (ANS. Scientific opinion on the re‐evaluation of ascorbyl palmitate (E 304 (i)) and ascorbyl stearate (E 304 (ii)) as food additives. EFSA J. 2015;13:4289. [Google Scholar]
- EFSA panel on dietetic products, nutrition, and allergies (NDA) Scientific opinion on dietary reference values for vitamin E as α‐tocopherol. EFSA J. 2015;13:4149. [Google Scholar]
- EFSA panel on food additives and nutrient sources added to food (ANS) Scientific opinion on the re‐evaluation of propyl gallate (E 310) as a food additive. EFSA J. 2014;12:3642. [Google Scholar]
- Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, Hallmans G. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Nati Cancer Inst. 2013;104:1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA panel on food additives and nutrient sources added to food (ANS) Scientific opinion on the re‐evaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J. 2012;10:2588. [Google Scholar]
- EFSA panel on food additives and nutrient sources added to food (ANS) Scientific opinion on the re‐evaluation of butylated hydroxyanisole–BHA (E 320) as a food additive. EFSA J. 2011;9:2392. [Google Scholar]
- European parliament and council commission regulation (E.U.) No 1129/2011 of 11 November 2011 amending annex II to regulation (E.C.) No 1333/2008 of the european parliament and of the council by establishing a union list of food additives (Text with EEA relevance) [Google Scholar]
- Formanek Z, Kerry JP, Higgins FM, Buckley DJ, Morrissey PA, Farkas J. Addition of synthetic and natural antioxidants to α-tocopheryl acetate supplemented beef patties: effects of antioxidants and packaging on lipid oxidation. Meat Sci. 2001;58:337–341. doi: 10.1016/s0309-1740(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-del-Río I, López-Ibáñez S, Magadán-Corpas P, Fernández-Calleja L, Pérez-Valero Á, Tuñón-Granda M, Miguélez EM, Villar CJ, Lombó F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants (Basel) 2021;10:1264. doi: 10.3390/antiox10081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülçin İ. Antioxidant activity of eugenol: a structure–activity relationship study. J Med Food. 2011;14:975–985. doi: 10.1089/jmf.2010.0197. [DOI] [PubMed] [Google Scholar]
- Hassanpour SH, Karami SZ. Study of the latest discoveries of potential antioxidant compounds to modulate oxidative stress-mediated by free radicals leading to neurotoxicity in PD. Int J Pharmacogn. 2021;8:462–475. [Google Scholar]
- Hassanpour SH, Dehghani MA, Alipour SM, Karami SZ, Dehghani F. Beneficial role of curcumin in diseases treatment. Int J Pharmacogn. 2018;5:327–330. [Google Scholar]
- He J, Xu L, Yang L, Wang X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med Sci Monit. 2018;24:8198–8206. doi: 10.12659/MSM.911175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz T, Matthews KK, Di R. The use of plant antimicrobial compounds for food preservation. Biomed Res Int. 2015;2015:246264. doi: 10.1155/2015/246264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- Gülçin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Huang B, He J, Ban X, Zeng H, Yao X, Wang Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011;87:46–53. doi: 10.1016/j.meatsci.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Hussein MA. A convenient mechanism for the free radical scavenging activity of resveratrol. Int J Phytomed. 2011;3:459–469. [Google Scholar]
- Holtekjølen AK, Bævre AB, Rødbotten M, Berg H, Knutsen SH. Antioxidant properties and sensory profiles of breads containing barley flour. Food Chem. 2008;110:414–421. doi: 10.1016/j.foodchem.2008.02.054. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Heijnen CG, Haenen GR, Van Acker FA, Van der Vijgh WJ, Bast A. Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol In Vitro. 2001;15:3–6. doi: 10.1016/s0887-2333(00)00053-9. [DOI] [PubMed] [Google Scholar]
- Hu C, Kitts DD. Evaluation of antioxidant activity of epigallocatechin gallate in biphasic model systems in vitro. Mol Cell Biochem. 2001;218:147–155. doi: 10.1023/a:1007220928446. [DOI] [PubMed] [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochem. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Murcia MA, Chirico S, Aruoma OI. Free radicals and antioxidants in food and in vivo: what they do and how they work. Crit Rev Food Sci Nutr. 1995;35:7–20. doi: 10.1080/10408399509527682. [DOI] [PubMed] [Google Scholar]
- Jayathilakan K, Sharma GK, Radhakrishna K, Bawa AS. Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chem. 2007;105:908–916. [Google Scholar]
- Kashyap P, Shikha D, Thakur M, Aneja A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: a review. J Food Biochem. 2021;26:e13950. doi: 10.1111/jfbc.13950. [DOI] [PubMed] [Google Scholar]
- Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:952943. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi Y, Chaharlang M, Daneshniya M, Khaki Arani S, Barzanooni M, Boghori P. Antioxidant and antimicrobial properties of methanolic extract of green walnut skin. JFST. 2021;17:127–136. [Google Scholar]
- Lourenço SC, Moldão-Martins M, Alves VD. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. 2019;24:4132. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, Chen H. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21:1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Shin S. Antibacterial and antioxidant activities of the essential oil from the roots of anthriscus sylvestris. Yakhak Hoeji. 2012;56:320–325. [Google Scholar]
- Larayetan RA, Okoh OO, Sadimenko A, Okoh AI. Terpene constituents of the aerial parts, phenolic content, antibacterial potential, free radical scavenging and antioxidant activity of callistemon citrinus (Curtis) skeels (Myrtaceae) from eastern cape province of South Africa. BMC Complement Altern Med. 2017;17:292. doi: 10.1186/s12906-017-1804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Dicastillo C, Nerín C, Alfaro P, Catalá R, Gavara R, Hernández-Muñoz P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J Agric Food Chem. 2011;59:7832–7840. doi: 10.1021/jf201246g. [DOI] [PubMed] [Google Scholar]
- Lam RY, Woo AY, Leung PS, Cheng CH. Antioxidant actions of phenolic compounds found in dietary plants on low-density lipoprotein and erythrocytes in vitro. J Am Coll Nutr. 2007;26:233–342. doi: 10.1080/07315724.2007.10719606. [DOI] [PubMed] [Google Scholar]
- Marchev AS, Vasileva LV, Amirova KM, Savova MS, Koycheva IK, Balcheva-Sivenova ZP, Vasileva SM, Georgiev MI. Rosmarinic acid-from bench to valuable applications in food industry. Trends Food Sci Technol. 2021;117:182–193. [Google Scholar]
- Manessis G, Kalogianni AI, Lazou T, Moschovas M, Bossis I, Gelasakis AI. Plant-derived natural antioxidants in meat and meat products. Antioxidants. 2020;9:1215. doi: 10.3390/antiox9121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani C, Isaac VL, Correa MA, Salgado HR. Caffeic acid: a review of its potential use in medications and cosmetics. Anal Methods. 2014;6:3203–3210. [Google Scholar]
- Miguel MG. Anthocyanins: antioxidant and/or anti-inflammatory activities. J Appl Pharm Sci. 2011;1:7–15. [Google Scholar]
- Mimica-Dukić N, Božin B, Soković M, Mihajlović B, Matavulj M. Antimicrobial and antioxidant activities of three mentha species essential oils. Planta Med. 2003;69:413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- Nieto G, Ros G, Castillo J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis ): a review. Medicines (Basel) 2018;5:98. doi: 10.3390/medicines5030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogala-Kalucka M, Korczak J, Dratwia M, Lampart-Szczapa E, Siger A, Buchowski M. Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycerols during accelerated tests. Food Chem. 2005;93:227–235. [Google Scholar]
- Petersen B, Snapp S. What is sustainable intensification? views from experts. Land Use Policy. 2015;46:1–10. [Google Scholar]
- Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf. 2013;12:483–508. doi: 10.1111/1541-4337.12024. [DOI] [PubMed] [Google Scholar]
- Puri M, Sharma D, Barrow CJ. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012;30:37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Pokorný J. Are natural antioxidants better–and safer–than synthetic antioxidants? Eur J Lipid Sci Technol. 2007;109:629–642. [Google Scholar]
- Queenthy SS, John B. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. Eur J Pharmacol. 2013;718:213–218. doi: 10.1016/j.ejphar.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Oswell NJ, Thippareddi H, Pegg RB. Practical use of natural antioxidants in meat products in the U S a review. Meat Sci. 2018;145:469–479. doi: 10.1016/j.meatsci.2018.07.020. [DOI] [PubMed] [Google Scholar]
- Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Sivakanthan S, Rajendran S, Gamage A, Madhujith T, Mani S. Antioxidant and antimicrobial applications of biopolymers: a review. Food Res Int. 2020;136:109327. doi: 10.1016/j.foodres.2020.109327. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects–a review. J Funct Foods. 2015;18:820–897. [Google Scholar]
- Sun Z, Wang H, Wang J, Zhou L, Yang P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of mentha piperita grown in China. PloS One. 2014;9:e114767. doi: 10.1371/journal.pone.0114767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TR, Lin JJ, Tsai CC, Huang TK, Yang ZY, Wu MO, Zheng YQ, Su CC, Wu YJ. Inhibition of melanogenesis by gallic acid: possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int J Mol Sci. 2013;14:20443–20458. doi: 10.3390/ijms141020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonboli A, Salehi P, Kanani MR, Nejad Ebrahimi S. Antibacterial and antioxidant activity and essential oil composition of grammosciadium scabridum Boiss from Iran. Z Naturforsch C J Biosci. 2005;60:534–535. doi: 10.1515/znc-2005-7-804. [DOI] [PubMed] [Google Scholar]
- Singleton VL. Naturally occurring food toxicants: phenolic substances of plant origin common in foods. Adv Food Res. 1981;27:149–242. doi: 10.1016/s0065-2628(08)60299-2. [DOI] [PubMed] [Google Scholar]
- Terahara N. Flavonoids in foods: a review. Nat Prod Commun. 2015;10:521–528. [PubMed] [Google Scholar]
- Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17:3202–3242. doi: 10.3390/molecules17033202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayel AA, EL‐TRAS WF. Plant extracts as potent biopreservatives for salmonella typhimurium control and quality enhancement in ground beef. J Food Saf. 2012;32:115–121. [Google Scholar]
- Viji P, Binsi PK, Visnuvinayagam S, Bindu J, Ravishankar CN, Srinivasa Gopal TK. Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts as natural preservatives for shelf-life extension of chill stored Indian mackerel. J Food Sci Technol. 2015;52:6278–6289. doi: 10.1007/s13197-015-1788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viuda‐Martos M, Ruiz Navajas Y, Sánchez Zapata E, Fernández‐López J, Pérez‐Álvarez JA. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr J. 2010;25:13–19. [Google Scholar]
- Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N. Biotechnology of flavonoids and other phenylpropanoid‐derived natural products Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol J. 2007;2:1214–1234. doi: 10.1002/biot.200700084. [DOI] [PubMed] [Google Scholar]
- Weng L, Zhang F, Wang R, Ma W, Song Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem Biol Interact. 2019;310:108665. doi: 10.1016/j.cbi.2019.05.031. [DOI] [PubMed] [Google Scholar]
- Wu Z, Tan B, Liu Y, Dunn J, Martorell Guerola P, Tortajada M, Cao Z, Ji P. Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and scotch spearmint. Molecules. 2019;24:2825. doi: 10.3390/molecules24152825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójciak KM, Dolatowski ZJ, Okoń A. The effect of water plant extracts addition on the oxidative stability of meat products. Acta Sci Pol Technol Aliment. 2011;10:175–188. [Google Scholar]
- Xu X, Liu A, Hu S, Ares I, Martínez-Larrañaga MR, Wang X, Martínez M, Anadón A, Martínez MA. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021;353:129488. doi: 10.1016/j.foodchem.2021.129488. [DOI] [PubMed] [Google Scholar]
- Yang X, Sun Z, Wang W, Zhou Q, Shi G, Wei F, Jiang G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci Total Environ. 2018;643:559–568. doi: 10.1016/j.scitotenv.2018.06.213. [DOI] [PubMed] [Google Scholar]
- Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3:10–14. [Google Scholar]
- Yilmaz Y. Novel uses of catechins in foods. Trends Food Sci Technol. 2008;17:64–71. [Google Scholar]
- Zhang Y, De Stefano R, Robine M, Butelli E, Bulling K, Hill L, Rejzek M, Martin C, Schoonbeek HJ. Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant Physiol. 2015;169:1568–1583. doi: 10.1104/pp.15.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelli E, De Stefano R, Schoonbeek HJ, Magusin A, Pagliarani C, Wellner N, Hill L, Orzaez D, Granell A, Jones JDG, Martin C. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr Biol. 2013;23:1094–1100. doi: 10.1016/j.cub.2013.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]