Abstract
The coordination of tissue-level polarity with organism-level polarity is crucial in development, disease, and regeneration. Here, we characterize a new example of large-scale control of dynamic remodeling of body polarity. Exploiting the flexibility of the body plan in regenerating planarians, we used mirror duplication of the primary axis to show how established tissue-level polarity adapts to new organism-level polarity. Characterization of epithelial planar cell polarity revealed a remarkable reorientation of tissue polarity in double-headed planarians. This reorientation of cilia occurs even following irradiation-induced loss of all stem cells, suggesting independence of the polarity change from the formation of new cells. The presence of the two heads plays an important role in regulating the rate of change in overall polarity. We further present data that suggest that the nervous system itself adapts its polarity to match the new organismal anatomy as revealed by changes in nerve transport driving distinct regenerative outcomes. Thus, in planaria tissue-level polarity can dynamically reorient to match the organism-level anatomical configuration.
Keywords: Polarity, Neural polarity, Cilia, PCP, Planaria
1. Introduction
A fundamental question in biology is how spatial patterns are coordinated across scales in an organism during development. Adapting polarity is a particular challenge as it requires interactions across scales, from an individual cell’s cytoskeletal structure to the body-wide axial orientation. As such few adult systems have the ability to change polarity after it has already been established. Regenerative systems are one of the naturally occurring cases where established parts of an organism are placed into novel spatial and signaling contexts and have to adapt to regrow lost body parts. In particular, individual cells have to be placed and oriented with respect to global tissue patterns. Such regenerative systems therefore allow us to understand how established polarity can be altered in response to changes in the large-scale pattern of the organism. We sought to address the question of how cellular polarity adapts on the whole-organism scale during the regeneration of new morphologies in the highly regenerative planarian flatworms. This question is of fundamental biological interest and is also relevant to bioengineering and regenerative medicine, which seek to apply cell-level signals in order to achieve large-scale anatomical outcomes.
Regeneration is a biological process that enables organ remodeling and repair in a number of organisms. A prominent model of regeneration is the planarian flatworm, which has the ubiquitous ability to regenerate all tissues in its body. Inducing the formation of double-headed animals with mirror-image duplication of the anterior-posterior axis from fully formed single-headed animals provides an opportunity to study how existing tissues adapt to new polarity cues. This allows interrogation of a new aspect of polarity setting compared to the standard developmental systems often used to study tissue polarity, such as Drosophila wings and Xenopus embryos (Devenport, 2014; Werner and Mitchell, 2012b). While the regenerative process in planarians is well described (Reddien, 2018), we address here two key knowledge gaps. First, it is not known how directional and positional informational cues interact during regeneration – specifically, how tissue-level polarity becomes coordinated with changes in organism-level polarity. Second, long-term repatterning in response to damage has received comparatively little attention; here, we characterize such changes and explore the mechanisms that drive them. Finally, while the presence of neural tissues is known to be permissive for regeneration across phyla, the patterning roles of head-derived signals are not known.
In addition to forming new structures, planarian regeneration requires that the remaining tissues adjust to their new position in the organism in a process termed repatterning or morphallaxis (Adell et al., 2010; Morgan, 1901; Reddien and Sanchez Alvarado, 2004). In normal planarian regeneration, this process involves the appropriate scaling of pre-existing organs to the animal’s new size, reestablishment of the correct length/width ratio of the animal, and adaptation of the expression patterns of positional control genes (PCGs) as tissues adapt to their new position within the organism (Witchley et al., 2013). This repatterning process happens in parallel to the regrowth of missing tissues via blastema formation and cell differentiation but often takes longer than the simple replacement of lost cells. Repatterning is most apparent in animals induced to regenerate with atypical morphologies, such as the axial duplication in double-headed planarians (Durant et al., 2017; Oviedo et al., 2010) where large sections of tissue in the original posterior of the animal undergo anteriorization.
While there are a number of mechanisms for setting polarity, planar cell polarity (PCP) signaling is the most common. PCP is a highly conserved pathway which consists of a core group of proteins, including the transmembrane receptors Frizzled, Flamingo, and Vang, as well as the intracellular effectors Dishevelled, Diego and Prickle (Devenport, 2014). The factors are distributed in a polarized manner on the opposing membranes of the cells, either anterior/posterior or proximal/distal depending on the system, and the contact with the neighboring cells through these transmembrane proteins allows for the establishment of large-scale polarity. PCP signaling plays a key role in establishing and maintaining polarity in many tissues, for example in cancer suppression (Lee and Vasioukhin, 2008) and the setting of polarity in multiciliated epithelia (Brooks and Wallingford, 2014; Meunier and Azimzadeh, 2016; Spassky and Meunier, 2017).
Cilia are microtubule-based structures that grow out of basal bodies attached to the cell membrane and which, through a dynein motor driven process, perform a beating motion. The establishment of ciliary polarity, via the orientation of the basal body attachment, relies on both molecular signals from PCP transmitted via the cytoskeleton (Kunimoto et al., 2012; Wallingford, 2010; Werner et al., 2011; Werner and Mitchell, 2012a), as well as hydrodynamic cues (Chien et al., 2018; Mitchell et al., 2007). Ciliary beat orientation is thus a sensitive and convenient readout of the underlying epithelial planar organization. While cilia in single-celled organisms can reverse their beat directions (Iwadate and Suzaki, 2004; Noguchi et al., 2005), there have not been any reports of cilia in multiciliated epithelial cells changing their beat direction after it has been established during development.
Multiciliated epithelia fulfill many physiological functions (Spassky and Meunier, 2017), including the clearing of mucus in the airway (Konishi et al., 2016), the movement of cerebrospinal fluid in the brain ventricles in mammals (Ohata and Alvarez-Buylla, 2016), and motility in the larval forms of many marine animals (Veraszto et al., 2017). In planarians, the multiciliated epithelium on the ventral surface is responsible for the main mode of movement of the worm, allowing smooth gliding (Azimzadeh and Basquin, 2016; Azimzadeh et al., 2012; Rompolas et al., 2013; Rompolas et al., 2010; Rustia, 1925). Classical observational data (Pearl, 1903; Rustia, 1925), showed that in planarians with abnormal morphologies, such as in double-headed or double-tailed animals, cilia polarity is aligned with the polarity of the new body plan. Recent work using both modern molecular tools and modelling confirmed this alignment of the cilia rootlet orientation with the body axis on a subcellular level and showed that PCP and Ft/Ds signaling sets up the polarity fields that give rise to the global alignment of the cilia both in wildtype planaria and in ones with abnormal morphologies (Basquin et al., 2019; Vu et al., 2019). However, the dynamics of the underlying polarity rearrangements and the mechanism by which new morphologies are translated into effects on polarity during the regeneration of abnormal morphologies had not previously been explored. The well-described link between PCP signaling and neural development (Goodrich, 2008; Tissir and Goffinet, 2010), and our recent work revealing a dependence of the formation of new body plans in planarians on nerve transport (Pietak et al., 2019), led us to also investigate how the polarity of the nervous system is affected by atypical body morphologies and how signals from the nervous system impact cilia orientation.
Here, we uncover and characterize the remarkable remodeling of the ventral multiciliated epithelium in double-headed planarians, during which cilia beat orientation across large tissue sections is progressively reoriented independent of cell turnover. Alongside this reorientation of the multiciliated epithelium, we observed that the overall nervous system polarity is reoriented progressively towards the midpoint of the animal. These results reveal how nervous system structure and cilia-driven flow adapt so that pre-existing tissues can accommodate a drastically different body architecture.
2. Material and methods
2.1. Planaria colony care
Planaria of the species Dugesia japonica were maintained in a colony at 13 °C in Poland Spring water, fed once a week with calf liver paste and cleaned twice a week, as described in (Oviedo et al., 2008). Animals were maintained at 13 °C for the time course of the entire experiment to prevent fissioning and were therefore taken from the cold-adapted colony at 13 °C, which is continuously kept at this temperature. Animals were starved for 1 week before use in experiments and for the course of the entire experiment to reduce variability due to metabolic state.
2.2. Double-head induction
Double-headed planaria were generated by excision of pharynx and pre-tail fragments from planaria. These fragments were placed in 127 μM Octanol for 3 days before the solution was replaced with Poland Spring water and allowed to regenerate at 20 °C (Durant et al., 2017). Double-head regenerative outcomes were scored 7 days after cutting by the presence of a head, marked by at least one eye spot, on either end of the fragment. Double-head animal age was calculated based on the original cut, i.e. freshly regenerated DHs when they are selected, are here termed Day 7 DHs.
2.3. Flow assay
Flow assays were performed based on original experiments in (Rustia, 1925). To detect cilia-driven flow, planaria were placed in a small dish with water, ventral-side up, so that they attached to the water surface. Carmine powder (Sigma-Aldrich, Darmstadt, Germany) was sprinkled on top of the worms. Powder and slime build-up was repeatedly removed using a paint brush. Movement of the powder particles was recorded on a Nikon AZ100M Multizoom Macroscope with a 0.5× objective and side-illumination from a Volpi IntraLED lightsource, using an Andor DL-604M camera. Movement was recorded at a frame rate of 100 ms. Animals were observed until powder accumulation reflecting cilia-driven flow had happened at least twice. Animals were washed 3 times in Poland Spring water to remove remaining carmine powder. Animals were maintained at 13 °C for duration of the assessment and were not fed.
Position of the collision zone was measured in ImageJ, where worm length and length from second head to midpoint of the powder collection point was measured. Both values were measured in worms which had even extension across the entire length of their body. Particle image velocimetry (PIV) was performed using Matlab (Mathworks, Natick, MA) with the PIVlab program (Thielicke and Stamhuis, 2014, 2019). For the analysis, 2 s segments were selected in which the worm exhibited little muscle movement and PIV was calculated in an interrogation window of 64 × 48 × 32 pixels for subsequent passes of the program, run on all frames, smoothed and all frames in the 2 s segment were averaged to give flow pattern.
2.4. Animal manipulations
Animals were irradiated using Nordion Gamma Cell 1000 Irradiator with a Cesium-137 radiation source with a dose of 200 Gy achieved via 30 min exposure at Day 7, unless otherwise specified. Primary and secondary head identity was determined in animals at Day 7 based on development of head structures (eye size, pigmentation and auricle development) as well as overall movement pattern of the animal.
Decapitations were performed at the base of the head, directly underneath the auricles, at Day 7. Lateral cutting was performed at the respective positions between the two heads in animals placed ventral side up, so that ventral nerve cords were visible and cut as far across the body as necessary to sever both nerve cords, or to the midpoint for single VNC cutting. Nerve cord deviation was performed by cutting perpendicular to the head-head axis at the given point up to the midline of the worm and then continued along the midline, as described in (Pietak et al., 2019). In both cases cuts were reinforced every day for 7 days and worms were maintained at 13 °C during cutting and regeneration. Regenerative outcomes were scored at Day 14.
Half-head removal, both lateral and perpendicular, was performed in DHs at Day 7 following irradiation. The secondary head of the animals was cut in all these cases. For lateral head removal a cut was placed downward between the eyes and then perpendicular at the base of the head to remove half of the head. For the perpendicular half-head removal, the cutting plane was perpendicular to the body axis and set at the plane of the eyes. Cuts were confirmed using synapsin staining. Cuts bisecting the brain were performed in DHs at Day 7 following irradiation, targeting the secondary head. The cut was placed downwards between the eyes to the base of the head. The cut was re-enforced for 3 days following the initial cut.
Internal tissue removal was performed using square glass capillaries of a 1 mm2 inner diameter and 0.1 mm wall thickness (VitroCom, Mountain Lakes, NJ), using a fine paint brush to remove the cut tissue from the middle of the animal. Double-headed animals at Day 7 and Day 49 were cut perpendicular to the head-head axis either at the midpoint between the two heads, halfway between the midpoint and the head, i.e. at 25% body length, and directly at the base of either the primary or secondary head. Fragments regenerated at 20 °C for 14 days before scoring regenerative outcomes by counting number of single-headed and double-headed regenerates. Brightfield pictures of animals were taken on a Nikon SMZ1500.
2.5. Drug treatments
200 proof Ethanol (VWR, Radnor, PA) was used at 3% in Poland Spring water to briefly disrupt cilia (Stevenson and Beane, 2010) by incubating for 1 h before washing out.
2% Carboxy-methylcellulose (Sigma-Aldrich) solution was prepared in Poland Spring water and stirred at room temperature until fully dissolved. Worms were suspended in the Carboxy-methylcellulose solution and only removed before flow analysis. Worms were washed 3 times for 5 min in Poland Spring water after removal from Carboxy-methylcellulose to remove remaining solution.
2.6. Fixation and immunohistochemistry
Animals were fixed at the described time point using an 2% HCl treatment for 2 min followed by incubation in Carnoy’s fixative (60% Ethanol, 30% Chloroform, 10% Acetic Acid) on ice for 2 h, before washing with Methanol at −20 °C and overnight bleaching with 10% H2O2 in Methanol. Fixed samples were stained using the VSI InSitu Pro robot (Intavis, Cologne, Germany), specifically rehydrated in PBSTx (1x PBS with 0.3% Triton X-100), blocked in 10% Goat serum in PBSTx + B (1x PBS with 0.3% Triton X-100 and 10% BSA) for 6 h, stained in primary and secondary antibodies (10 h at 4 °C for both) and washed with PBSTx (2 × 20 min and 1 × 1 h). The primary antibody used were - for synapsin, mouse-anti-synapsin (SYNORF1) antibody at 1:50 (Developmental Studies Hybridoma Bank (DSHB), University of Iowa), for dividing cells, rabbit-anti-phospho-Histone H3 (Ser 10) clone MC463 (Millipore Sigma) at 1:250, and for cilia, mouse-anti-acetylated tubulin (Sigma) at 1:1000. Secondary antibody used was goat-anti-mouse IgG (H + L) Cross-Adsorbed-Alexa 555 (ThermoFisher) at 1:400 and goat-anti-rabbit-HRT 1:100, with TSA-Alexa 488 amplification for the phosphor-Histone H3 antibody. All antibodies were diluted in PBSTx + B. Anti-SYNORF1 was deposited to the DSHB by E. Buchner (DSHB Hybridoma Product 3C11 (anti-SYNORF1)) (Klagges et al., 1996). Samples were mounted in Vectashield® hard set mounting medium (Vector Laboratories, Burlingame, CA) and imaged on a Leica SP8 confocal (Leica, Mannheim, Germany) with HyD detector, 488 nm and 552 nm diode light source, and 10x NA = 0.4 (Leica HC PL APO CS2) or 25x water-immersion NA = 0.95 (Leica HC Fluotar L) objectives.
2.7. Image analysis, data analysis and statistical information
Sample sizes were chosen in accordance with standards of the field. Repeat numbers and sample sizes are reported in the respective figure legends. Animals taken from the same colony unit and treated at the same time and investigated in parallel are considered technical replicates, while animals from a different colony unit investigated at a different time represent biological replicates. Animals were randomly selected out of large colony units and randomly assigned into treatment groups. Data analysis was performed blinded. All image analysis, measurements and image post-processing were performed in FIJI ImageJ (Rueden et al., 2017; Schindelin et al., 2012). Data analysis and plotting was done in GraphPad Prism V8.0 (GraphPad, San Diego, CA). All datapoints are plotted where reasonable, otherwise center and dispersion measures are defined in the respective figure legends. All data was included in all analyses. All plotted data can be found in Supplementary Dataset 1.
Unless otherwise specified, multiple t-tests with false discovery rate (FDR) approach using the two-stage step-up method of Benjamini, Krieger and Yekutieli with an FDR = 1%, not assuming equal SD, were performed to analyze datasets. All other datasets were analyzed using a Welsh’s two-tailed t-test. Significance threshold was set to p < 0.01. Statistical test data are given in respective Supplementary Tables.
3. Results
3.1. Ciliary beat orientation reveals progressive adaptation to new organismal polarity
Double-headed (DH) planarians with mirrored axial polarity can be created via a variety of methods, including manipulation of bioelectric signaling (Durant et al., 2017; Oviedo et al., 2010) or interference with the Wnt signaling pathway (Petersen and Reddien, 2008). Newly regenerated DHs (7 days after transection of the single-headed animal) of the species Dugesia japonica show developmental differences between their two heads: the head regenerated from the anterior blastema is more fully regenerated than the head positioned on the original tail end of the animal, based on criteria such as eye development, head shape, and blastema coloration (Fig. 1A i–iii). We refer to the anterior blastema-derived head as the primary head, while the posterior blastema-derived head is termed the secondary head. The primary head orientation dominates the body movement (Video 1). The morphological differences between the two heads reduce over two to three weeks, eventually leading to both sides being equal in appearance and having equal control over the body movement after about one month (Fig. 1Aiv–vi, Video 2). The growth of a secondary head represents an enormous deviation in organismal polarity, and the length of time required to anteriorize the formerly posterior tissue adjacent to the secondary head provides a unique opportunity to assess the long-term repatterning processes.
Fig. 1. Flow driven by the multiciliated epithelium can be tracked and epithelial polarity dynamically remodels in DH animals.
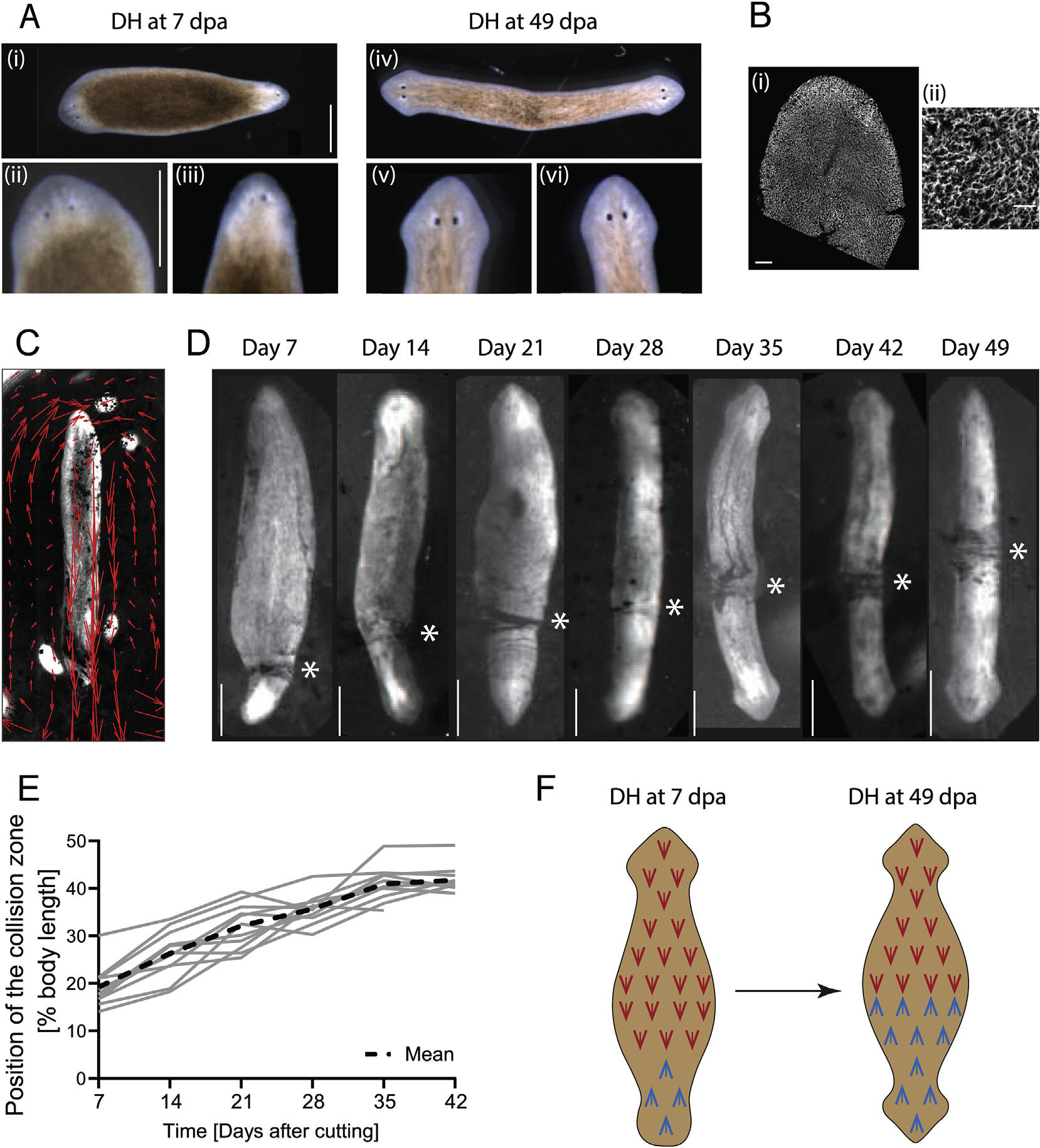
A) (i) Morphological differences in head development in DHs can be observed at Day 7 (days after amputation – dpa), revealing that the primary head (ii) is more developed than the secondary head (iii). (iv) In DHs at Day 49 both heads (v, vi) are equally developed. Scale bars 1 mm. B) Cilia of the multiciliated ventral surface epithelium stained with antibody against acetylated-tubulin at (i) low and (ii) high magnification. Scale bar 100 μm in (i) and 25 μm in (ii). C) Flow driven by cilia beat visualized with carmine powder and tracked with particle image velocimetry (PIV) in a single-headed animal (red arrows). D) Opposing flow directions in DH animals lead to the accumulation of particles at the point where the opposing flow fields meet. This “collision zone” (asterisk) repositions towards the middle of the animal from Day 7 until Day 49. E) Plot of position of the collision zone as percentage of total body length for 12 animals individually tracked over time, measured every 7th day, and mean curve (dashed line). N = 12, with 5 repeats showing similar pattern. F) Schematic representing the change in cilia orientation in the posterior half of the DH as the cilia orientation changes over time.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
The difference in movement patterns between DHs at Day 7 and Day 49 led us to investigate the underlying changes in the multiciliated ventral epithelium, the tissue that drives movement in planarians (Fig. 1B) (Azimzadeh et al., 2012; King and Patel-King, 2016; Rompolas et al., 2013). Since cilia beat orientation in multiciliated epithelia serves as a convenient and reliable read-out of underlying tissue polarity (Wallingford, 2010), we adapted an assay to visualize cilia beat orientation in living planarians (Pietak et al., 2019; Rustia, 1925). In this assay, worms were placed ventral side up, attached to the underside of the water surface through surface tension, carmine powder was sprinkled on top of the water, and movement of the powder driven by the cilia was observed (Fig. 1C, Video 3). Known disruptions of cilia beat function, such as reduction of temperature or 3% ethanol treatment (Stevenson and Beane, 2010), showed immediate cessation or significant reduction of flow, validating our assay (Fig. S1). In normal single-headed worms, we observed a complete concordance between the polarity of the epithelium as detected by tracked powder flow and the antero-posterior axial patterning (Fig. 1C).
We then tracked the cilia-driven flow in DH animals, which exhibited a clear mirroring of the ciliary polarity, as previously described (Rustia, 1925; Vu et al., 2019). Due to the opposing beat direction in DH animals, the powder used to visualize the flow accumulated at a point along the animal’s body (Fig. 1D). We termed this point the “collision zone”, which marks the symmetry point of the ciliated epithelium in the animal. Interestingly we observed that in DHs at 7 days after cutting, the collision zone is located at the base of the secondary head (Fig. 1D, Video 4). However, over time, the collision zone progressively shifts along the length of the animal until it reaches the midpoint around 42 days after cutting (Fig. 1D, Video 4). The timing of this process is highly conserved across all DHs (Fig. 1E). The cilia beat direction reorientation progresses at a relatively consistent speed from Day 7 to Day 35, before slowing down between Day 35 and Day 42 and stalling following Day 42 (Fig. S2A). The animals were not fed for the duration of the entire experiment, but their size remained relatively unchanged until at least Day 42, with some shrinkage observed after Day 42 (Fig. S2B). This constant size until Day 42, at which time point the reorientation is essentially complete, indicates that the observed reorientation of the flow pattern does not results from unequal growth on one side of the animal but rather from changes to the existing tissues. The cilia reorientation occurs at the same relative speed in animals of different sizes (Fig. S2C), suggesting that the reorientation rate is scaling with the size of the animal. Thus, we discovered that the posterior half of DH worms dynamically adapts its planar polarity over multiple weeks to match the new axial body plan (Fig. 1F).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
3.2. The signal driving cilia reorientation propagates slowly across the body
The strikingly long timeframe during which cilia change their beat orientation in newly-established DHs poses the question of when the signal to reorient is sent. It could be that the signal driving this reorientation is slowly distributed across the animal, but the reorientation is rapid once the signal is received. It is also possible that the cells of the epidermis require time to adapt to a signal that is present throughout the body from the time of DH induction. To address this, we cut out tissue from the middle of the DH animal at Day 7 post-amputation (Fig. 2A and B) to force the formation of new ciliated epidermis in the middle of the body while the original reorientation is still in progress. If the information is already present throughout the animal, the newly regenerated cells could be expected to regenerate with the correct orientation in place, leading to a faster reorientation or irregular flow pattern in DHs with internal tissue removal. This was not the case. We observed no difference between control animals and DHs with internal tissue removal in both cilia reorientation speed (Fig. 2B, Supplementary Table 1) and collision zone/flow pattern (Video 5). This suggests that the signal responsible for the correct pattern is transmitted slowly across the worm rather than epithelial cells slowly adapting to already present information.
Fig. 2. Reorientation of cilia does not rely on cell turnover and is unaffected by blockage of cilia beating.
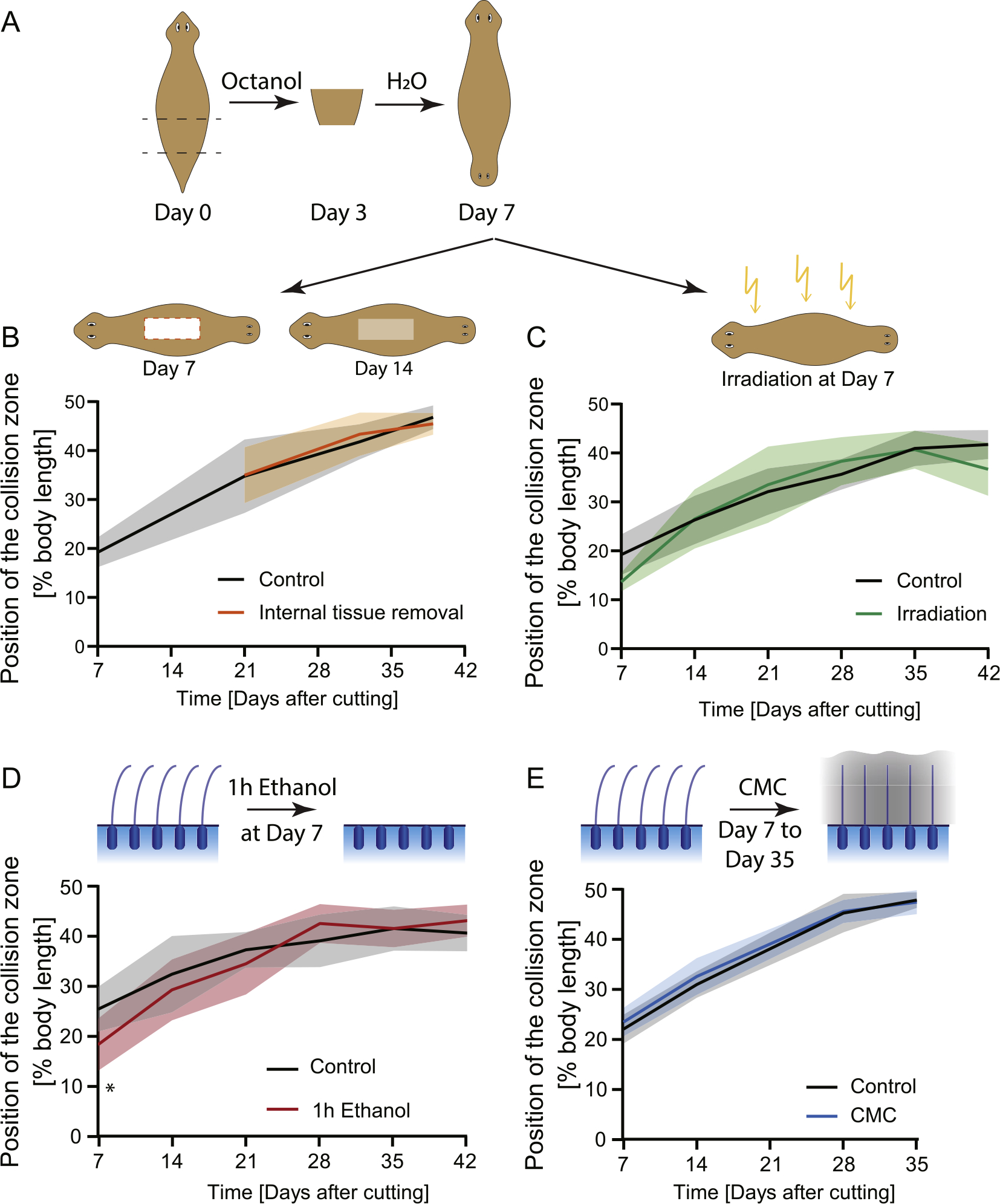
A) Experimental scheme showing the induction of DH animals and subsequent treatments. B) Position of the collision zone over time in animals that had a large portion of tissue removed from their interior (orange line) at Day 7, compared to controls (black); there is no difference in reorientation speed, n = 12. C) Cilia reorientation in animals treated with high levels of radiation to prevent new cell formation (green), compared to controls (black); there is no significant difference in cilia reorientation speed, n = 12, with 3 repeats showing same pattern. D) Position of the collision zone in DHs which were treated with 3% ethanol for 1 h at Day 7 to remove external cilia (red); there is no difference in cilia reorientation compared to control (black), except at Day 7 immediately following treatment. n = 12. E) Position of the collision zone in DHs which were incubated in 2% Carboxymethylcellulose (CMC) solution from Day 7 to Day 35 (blue), showing no difference compared to controls (black). n = 12 with 2 repeats showing the same pattern. The mean of all samples is plotted. Shaded area represents standard deviation. All experimental worms were compared to age-matched controls.
However, this process of slowly transmitted reorientation information across the newly formed double-headed animal may be dependent on tissue type. De novo DH pharynx formation (two pharynges with opposing orientations) can be observed in DH animals between Day 7 and Day 10 (Fig. S3). The positioning of the newly forming pharynges in single-headed planarians relies on the localized expression of position control genes (PCG) (Adler et al., 2014). The pharynges in the DH animals form in the correct position in relation to the DH body proportion and in the appropriate relative orientation. They do not shift their position significantly over time after Day 10 (Fig. S3). This suggests that the signals setting the directionality of planar tissue polarity in the multiciliated epithelium to be in concordance with the new axial morphology may not propagate at the same rate as the resetting of the PCG expression domains in DHs, which determine pharynx positioning. Whether these two signaling systems are independent or PCG signaling precedes and lies upstream of PCP signaling remains to be explored.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
3.3. Cilia reorientation in the ventral epithelium is not dependent on cell turnover
To investigate whether the observed change in cilia beat direction during DH formation requires the replacement of wrongly oriented cells with new, correctly oriented cells, we treated animals 7 days after DH induction (as soon as both heads were discernable and regeneration was mostly completed) with high doses of radiation (~200 Gy), which is known to kill all stem cells (neoblasts) and thereby prevent all subsequent formation of new cells in planaria (Wagner et al., 2011). We confirmed the success of the irradiation in each experimental round by cutting some irradiated animals and checking for a lack of regenerative response. Further we confirmed irradiation success by death of the DH experimental animals around 45 days after irradiation. The 45 days period between irradiation and death of the animals is slightly longer than that previously reported for other planarian species (Wagner et al., 2011) and this difference is most likely due to species differences and our animals being maintained at 13 °C.
Complete removal of neoblasts following irradiation was further confirmed by absence of staining for actively dividing cells (Fig. S4), which shows that no new cells are forming in irradiated animals after Day 7. It is possible that a few progenitors that fate committed just prior to irradiation might still give rise to some new epithelial cells, but the differentiation of progenitor cells in planaria is a fairly rapid process (Abnave et al., 2017). Therefore, even if the early reorientation response is in part driven by new epithelial cell formation after irradiation, this would not last for the entirety of the reorientation process and at later timepoints irradiated animals should not be able to generate new epithelial tissue. However, we observed no difference in the cilia reorientation speed of these irradiated animals compared to age-matched controls over the entire experimental period (Fig. 2C, Supplementary Table 2); moreover, irradiated animals had collision zones positioned almost at the midpoint before they died. The full reorientation of the flow pattern observed in irradiated animals over the long time frame strongly suggests that cilia reorientation in developing DHs happens via intracellular reorientation of the pre-existing cilia rather than via replacement of misoriented cells as it is not dependent on neoblast activity. While our observations and measurements were done at the tissue level and do not reveal details of the process of cilia orientation in individual cells, the fact that flow patterns change strongly in irradiated animals, which cannot form new cells, suggests that cilia reorientation is taking place in existing cells.
3.4. Cilia reorientation progresses independent of cilia beating
To investigate how the reorientation of cilia occurs in existing cells, we first removed the protrusive components of the cilia using a low dose ethanol treatment (Stevenson and Beane, 2010). DHs were treated at Day 7 for 1 h with 3% ethanol and did not show different cilia reorientation speeds compared to untreated controls except at Day 7, which was measured only 4 h after ethanol treatment. The difference at Day 7 is likely due to incomplete regeneration of cilia in this time frame (Fig. 2D, Supplementary Table 3). This suggests that cilia reorientation cannot be induced by removal and rebuilding of the external cilia structures as the reformation of the cilia is a fast process, taking only a number of hours, while the reorientation takes weeks. At the same time, these data show that a brief intervention that removes the external cilia portion does not trigger the reorientation and does not lead to changes in reorientation speed in the long term.
Work in developmental systems has shown that hydrodynamic forces play a critical role in aligning cilia alongside molecular signaling factors (Guirao et al., 2010). These observations prompted us to investigate whether blocking the ability of cilia to beat through incubation of animals in a highly viscous medium would impact the timeline of the cilia reorientation in developing DHs. We therefore maintained DH animals continuously in a 2% solution of Carboxy-methylcellulose (CMC), which is highly viscous and did not allow for cilia-driven flow (Supplemental Video 6), from Day 7 to Day 35, and only removed them briefly once a week for flow measurements.
This placement in a highly viscous solution did not impact the rate at which the cilia beat direction changes in developing DHs compared to control animals (Fig. 2E, Supplementary Table 4). This indicates that hydrodynamic factors are unlikely to play a strong role in the cilia reorientation in DH planarians and it appears to be a molecularly driven process.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
3.5. Cilia reorientation is dependent on the presence of the head
Given the recent characterization of the importance of brain-derived signals in morphogenesis in development (Herrera-Rincon et al., 2017), and the observation that cilia reorientation begins at the new head and extends from there towards the middle of the body (Fig. 1D), we hypothesized that one or both brains in a DH animal may be driving cilia reorientation.
To test the influence of the primary and secondary head on cilia reorientation, DHs at Day 7 were irradiated and then decapitated (Fig. 3A). Irradiation was performed a few hours prior to amputation to prevent regrowth of the removed tissue. As previously shown (Fig. 2C), irradiation does not, by itself, impact the progression of cilia reorientation. However, removal of the primary head following irradiation led to a significant increase in reorientation speed, with the collision zone being positioned significantly further towards the animal’s midsection in decapitated animals compared to controls at all timepoints from Day 14 onward (Fig. 3B and D, Video 7, Supplementary Table 5). The removal of the secondary head lead to a reduction of the cilia reorientation rate, with the collision zone advancing significantly less at all timepoints after Day 14 in treated animals compared to controls (Fig. 3C and D, Video 7, Supplementary Table 6). This indicates that the two heads control cilia reorientation, with the secondary head driving reorientation and the primary head opposing it.
Fig. 3. The presence of the heads controls cilia reorientation.
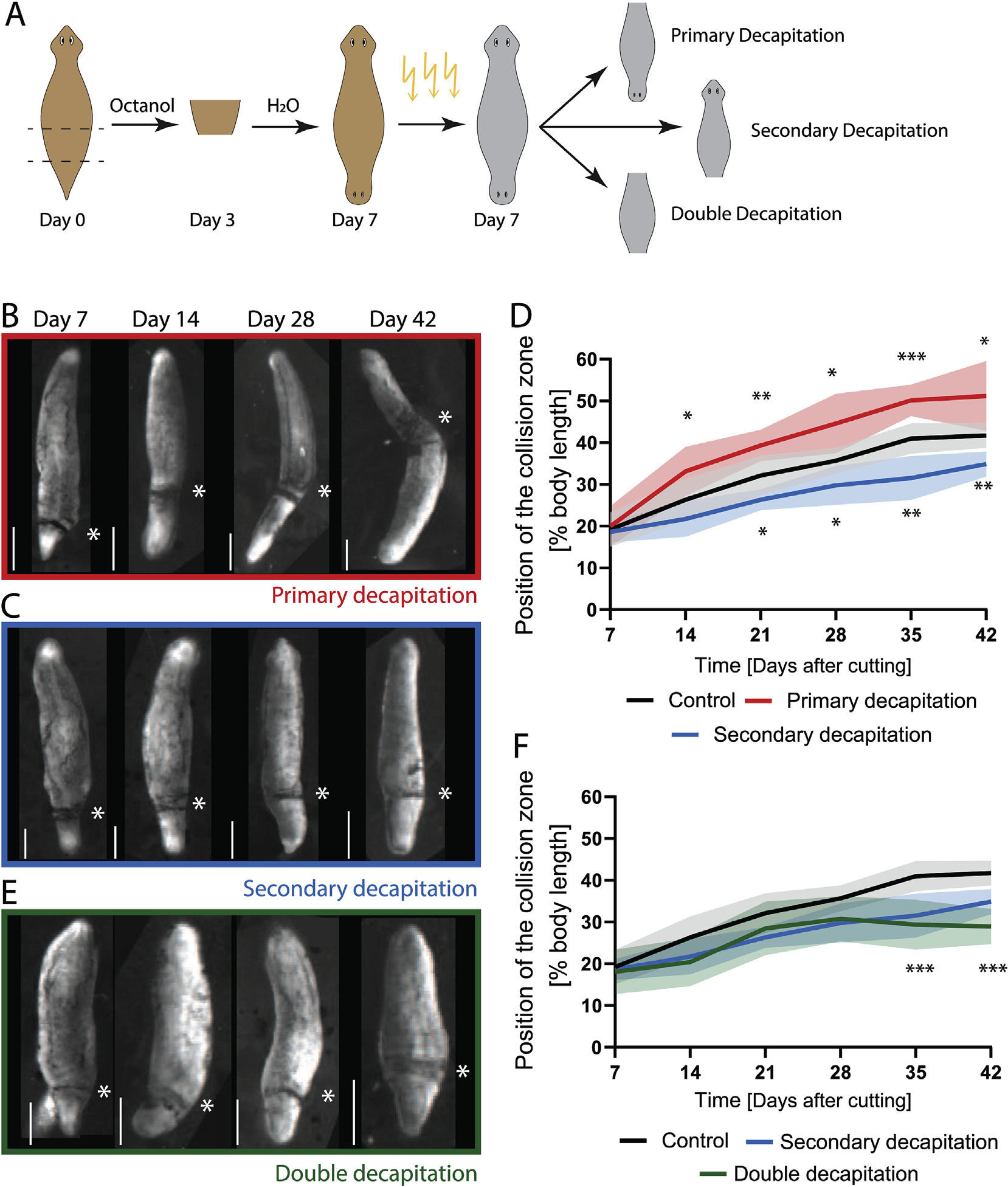
A) Experimental scheme showing induction of DHs by Octanol treatment, followed by irradiation at Day 7 and subsequent removal of either the primary, secondary or both heads. B) Position of the collision zone (asterisk) in DH animals, which were irradiated and decapitated at the primary head at Day 7, at Day 7, 14, 28 and 42 showing the advance of the collision zone across the midpoint at Day 42. C) Position of the collision zone (asterisk) in DH animals, which were irradiated and decapitated at the secondary head at Day 7, at same time points as above. D) Quantification of the position of the collision zone in control animals (black curve) and in animals with either the primary head (red) or the secondary head (blue) removed at Day 7 following irradiation. Removal of the primary head significantly speeds up cilia reorientation, while removal of the secondary head reduces the speed. E) Position of the collision zone (asterisk) in DH animals, which were irradiated and double-decapitated at Day 7, at same timepoints as above. F) Quantification of the position of collision zone in animal with both heads removed (green), compared to control (black) and secondary decapitation (blue). *p < 0.01, **p < 0.001, ***p < 0.0001. N = 12, with 2 repeats showing same pattern. The mean of all samples is plotted. Shaded area represents standard deviation. All experiments are paired with their respective controls. Scale bar 1 mm.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
Considering our finding that both primary and secondary head contribute to remodeling, we hypothesized that the relative smaller size of the secondary brain might be affecting its ability to drive cilia reorientation. We measured primary and secondary brain size using immunostaining for synapsin to visualize the brains at different timepoints and quantified the length of the brain (Fig. 4A and B). We observed that early during DH development, between Day 7 and Day 21, there is great variability in the relative brain lengths between the two heads of the same animal. This indicates that some animals have a much more developed primary brain compared to their secondary brain, while other DH animals have evenly sized brains already at Day 7. The variability reduced progressively from Day 7 to Day 21, when it reached stable values that did not change over the next 22 days until Day 43. We did not observe any correlation between large differences in relative brain size and either positional origin of the fragments cut to induce DHs within the original single-headed (SH) animal (Fig. S5A) or overall DH size (Fig. S5B). Given the highly consistent rate at which cilia reorientation processes across all DHs and the simultaneous diversity of brain sizes, it appears unlikely that the secondary brain size would affect ciliary reorientation. In order to magnify the differences in relative brain size over development, we investigated the brain size of DHs at Day 28 that had been irradiated at Day 7. Irradiation stopped the brain development and maintained the diversity of brain sizes observed at Day 7 until Day 28, making them significantly different from non-irradiated animals at Day 21 and Day 30 (Fig. 4B, Supplementary Table 7). Since irradiated animals had their cilia reorienting at the same rate as controls (Fig. 2C), the smaller brain size of the secondary brain is not responsible for slower reorientation rates.
Fig. 4. Brain size in DHs changes over time.
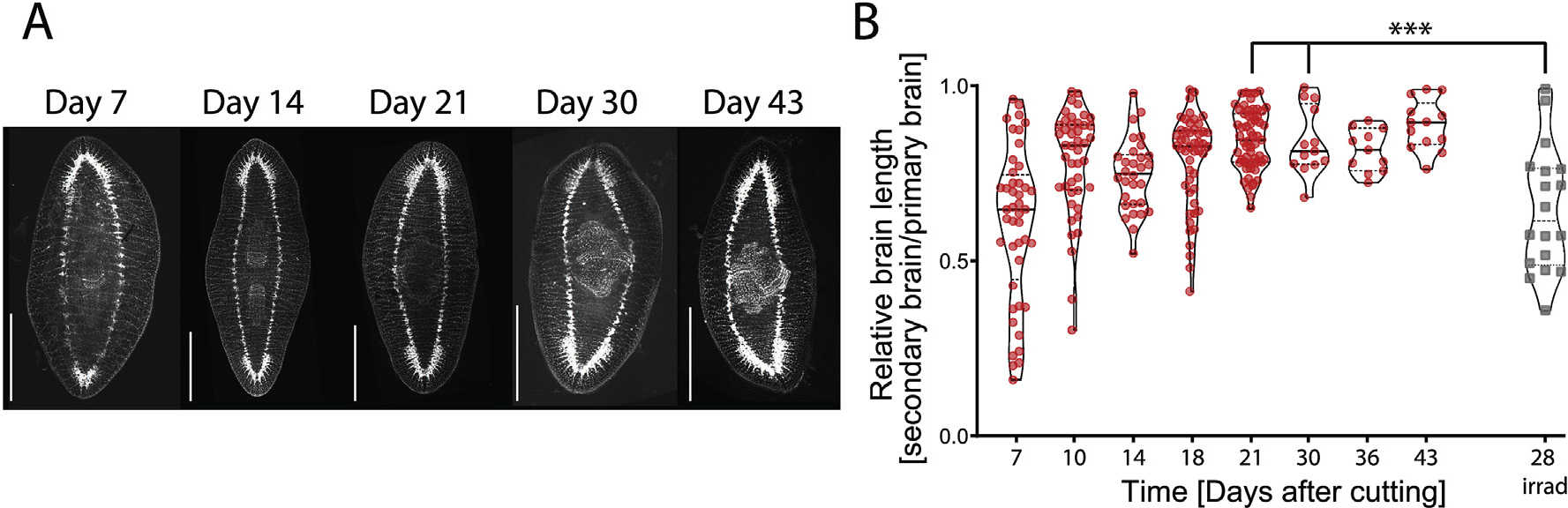
A) Staining of the nervous system of DH planarians from Day 7 to Day 43 using synapsin antibody staining shows the different sizes of the brain in the primary and secondary head at the early time points. Animals are oriented with the primary head towards the top. B) Quantification of relative brain length in secondary and primary brain over time in untreated double-headed animals (red), and animals irradiated at Day 7 and stained at Day 28 (grey), which shows a reduction in the variability of brain size over time, except for irradiated samples. Brain length was measured in synapsin stains from base of brain where ventral nerve cords (VNCs) widen, to the tip of the brain, where the two halves meet. Scale bar 1 mm ***p < 0.0001.
To establish whether the reorientation process can take place when both heads are absent, we removed both heads of the DH at Day 7 following irradiation to prevent head regrowth. Removal of both heads resulted in cilia reorientation progressing significantly slower than in controls, to a similar extent as observed for the removal of only the secondary head (Fig. 3E and F, Supplementary Table 8). The fact that removal of both heads and removal of only the secondary head give such similar results indicates that the secondary head serves as the main driving force in reorienting the cilia beat pattern – removal of the inhibitory force of the primary brain is not sufficient for the reorientation to occur in the absence of the driving force of the secondary brain. At the same time, the collision zone in these double-decapitated animals moves from an average position of 18% body length to 29% body length between Day 7 and Day 42. This may indicate that some head-independent process is able to induce some level of cilia reorientation or reflect an effect induced before the secondary head was amputated.
For a limited number of DH animals in which the primary head was removed, we observed that the secondary head dominated reorientation to the point where the collision zone crossed the midpoint of the animal. Irradiated and decapitated animals, however, died before a sufficient number showed the collision zone crossing the midpoint, preventing a firm conclusion as to whether removal of the primary head could allow secondary-head-mediated cilia reorientation to progress beyond the midpoint far into the primary half of the animal. We therefore took non-irradiated animals and amputated the primary head at Day 7 and then every other day to maintain a worm with no primary head without relying on irradiation. Care was taken to only remove blastema tissue in subsequent amputations. These DHs, which were continuously decapitated on the primary head side, showed reorientation of the cilia leading to the collision zone crossing the midpoint and reaching a collision zone at 86% body length in the most advanced example, while the collision zone in controls did not cross the midpoint (Fig. S6, Video 8, Supplementary Table 9). Reversal of cilia beat orientation across the whole animal, i.e. collision zone reaching 100%, was not observed, because the animals were not of a sufficient size to allow the assay to be continued after Day 63. The steady slope of the collision zone position curve in the continuously decapitated animals however suggests that full reversal of cilia polarity could be achieved. This indicated that there is no set midpoint marked independent of head presence.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
The importance of the head in controlling cilia reorientation led us to investigate whether removal of the anterior pole of the animal, known to express the gene notum and to be crucial for tissue organization in planarians (Petersen and Reddien, 2011), would prevent cilia reorientation. We found that removal of just the tip of the secondary head in an animal irradiated before amputation to prevent regrowth of the tissue did not impact cilia reorientation (Fig. S7, Supplementary Table 10). This data suggests that notum signaling is not required for the changes in beat direction in the ciliated epithelium. This observation also reveals that changes to cilia reorientation do not occur in response to any kind of injury.
It is clear that the presence of the secondary head is crucial in driving the cilia reorientation in the developing DH. To determine whether this is only true during the initial establishment of the new cilia orientation or whether the presence of the head is required for maintenance of the midpoint, we performed decapitations following irradiation in DHs at Day 49. In these animals the collision zone had reached the midpoint before they were decapitated. In the 21 days following decapitation at Day 49, we did not observe any change in the position of the collision zone (Fig. S8, Video 9, Supplementary Table 11). Similarly, decapitating irradiated SH animals did not lead to a change in flow pattern (Video 10). These data indicate that once the position of the collision zone is established, the presence of the head is no longer required for maintaining its position or organizing cilia beat pattern. Overall, it appears that each head provides an influence during early establishment of cilia orientation, but once patterning is complete it is not required to maintain the cilia orientation.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
3.6. An intact brain is required for cilia reorientation
We then investigated whether removing part of the head would affect the progression of cilia reorientation (Fig. 5A). We first removed half of the secondary head laterally (Fig. 5A and B) following irradiation at Day 7 to prevent regrowth of amputated tissue. In DHs with only half the secondary head removed cilia reorientation progressed at the same speed as in animals that had the complete secondary head removed (Fig. 5B, Supplementary Table 12). Notably, we never observed an angled collision zone, which would have indicated that the two lateral halves of the worm are regulated independently from each other by the brain hemispheres (Fig. S9). We also removed half of the head perpendicular to the head-head axis (cut at the eye plane, Fig. 5C), which lead to an equivalent reduction in cilia reorientation speed as the lateral half-head removal (Fig. 5C compared to 5B, Supplementary Table 13). These data indicate that presence of half the head is not sufficient to complete the function necessary to drive cilia reorientation, independent of which half of the head is removed.
Fig. 5. An intact brain is critical for driving cilia reorientation.
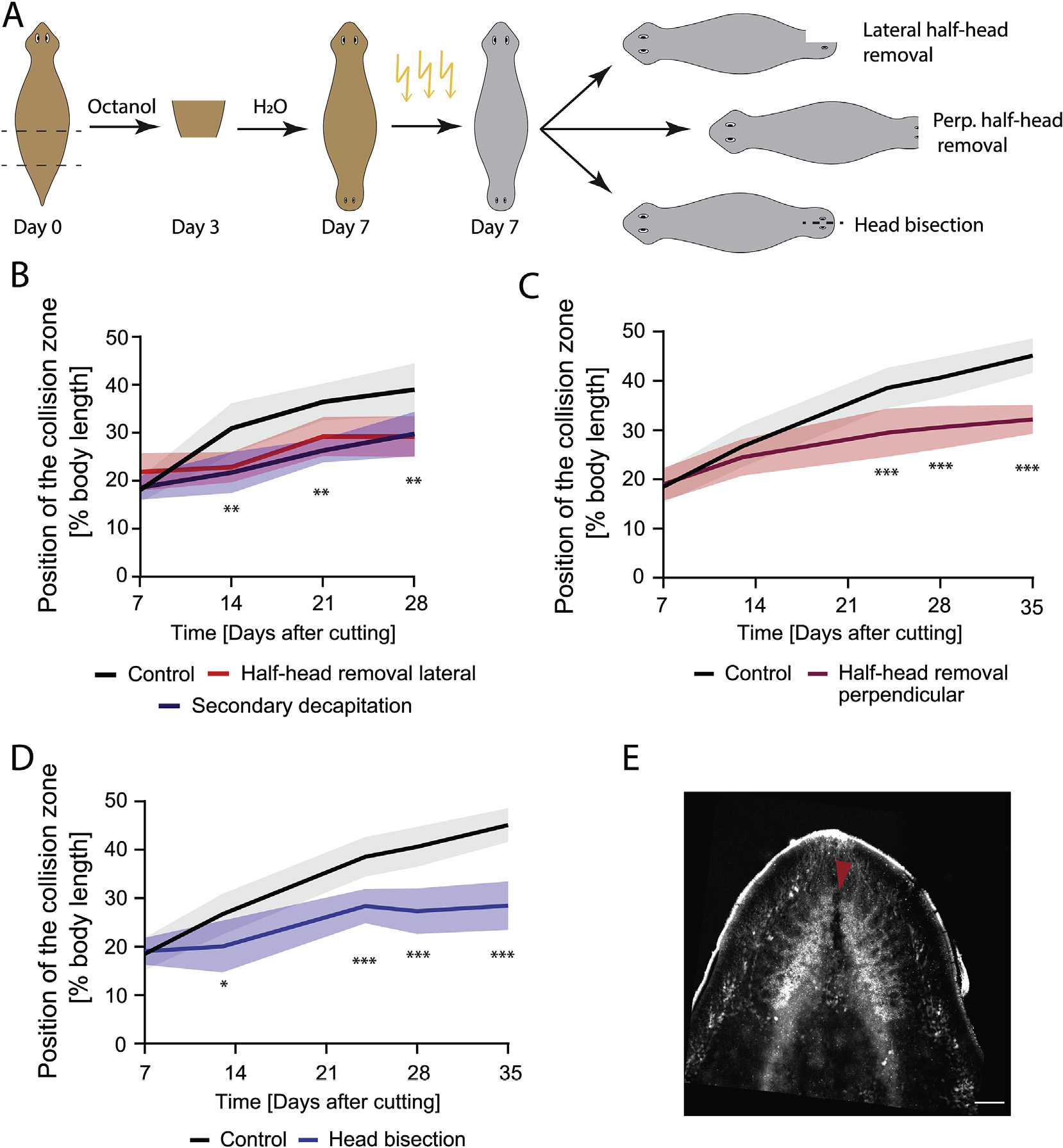
A) Experimental scheme showing induction of the DHs via Octanol treatment, followed by irradiation to remove neoblasts at Day 7. Cuts to remove parts of the brain or bisect the head were performed several hours after irradiation. B) DHs with the lateral half of the secondary head removed following irradiation at Day 7 (red) showed significantly reduced cilia reorientation speed compared to control (black) to the same extent as animals with the entire secondary head removed (blue). C) DHs with the frontal half of the secondary head removed following irradiation at Day 7 (red) showed significantly reduced cilia reorientation speed compared to control (black). D) DHs with secondary head bisected laterally between the eyes without tissue removal following irradiation at Day 7 (blue) reoriented their cilia significantly slower compared to controls (black). E) Synapsin stain of brain following head bisection without tissue removal. Red arrow highlighting disruption of the brain due to the head bisecting cut. *p < 0.01, **p < 0.001, ***p < 0.0001. N = 12. Plotted is mean of all samples, with standard deviation as shaded area for all experiments with their age-matched controls. Scale bar 100 μm.
Given that small intact brains are capable of driving cilia reorientation, while removal of half the head significantly slowed reorientation, we asked whether any injury to the brain was sufficient to reduce cilia reorientation by bisecting the two halves of the brain in the secondary head via a cut made from the head tip to between the eyes at Day 7 (Fig. 5D and E). This was done in animals that had been irradiated hours before the cutting was performed to prevent the regrowth of any lost tissue. The cut persisted due to the inability to replace cells following irradiation and was sufficient to significantly reduce cilia reorientation to a rate similar to that observed with removal of half or the entire head (Fig. 5D compared to Fig. 5B, Supplementary Table 14). Since we were unable to disrupt brain function by another mechanism, it remains possible that the disruption of another anterior structure is responsible for controlling flow patterns. The sum of our different disruptions to the head region however suggests that it is likely the intact state of the brain, rather than its overall size, that is responsible for controlling cilia reorientation.
3.7. The signals that determine cilia reorientation are transmitted along axial structures
Given the important instructive role of the intact brain in cilia reorientation in developing DHs, we sought to understand by which pathway the signal driving cilia reorientation is sent from the brain to the rest of the body. To test whether the signal diffused through the body or was transmitted along axial structures, we irradiated DHs at Day 7 and then cut them below either the primary or the secondary head (Fig. 6 Ai, Bi and Ci). The wounds healed, reforming the majority of the physical connection between head and body (Fig. 6 Aii, Bii, Cii) and allowing for diffusion to take place normally. Using synapsin staining, we showed that following these cuts the ventral nerve cords (VNCs) were severed and the connections did not reform in any of the tested animals due to irradiation-driven lack of new cell formation (Fig. 6Aiv, v compared to Biv, v and Civ, v and Fig. S10). External wound healing suggests that epidermal connections are reformed, but we did not explore whether muscle connections are reestablished. We found that this axial cut at either the primary or secondary head phenocopied the effect of entire head removal (Fig. 6D, Video 11). Compared to controls (Fig. 6A), animals with a lateral cut behind the primary head (Fig. 6B) reoriented their cilia significantly faster at all timepoints following Day 14 compared to controls (Fig. 6D, Supplementary Table 15). At the same time, DHs in which the body was partially cut behind the secondary head (Fig. 6C) reoriented their cilia significantly slower, with the collision zone significantly less advanced at all timepoints following Day 14 (Fig. 6D, Supplementary Table 16). These data support the hypothesis that the factors driving the cilia reorientation are not diffusing through the mesenchyme but are transported along axial structures disrupted by the cut, such as the VNCs or the musculature.
Fig. 6. Remodeling-inducing signals from the brain are transmitted across the body.
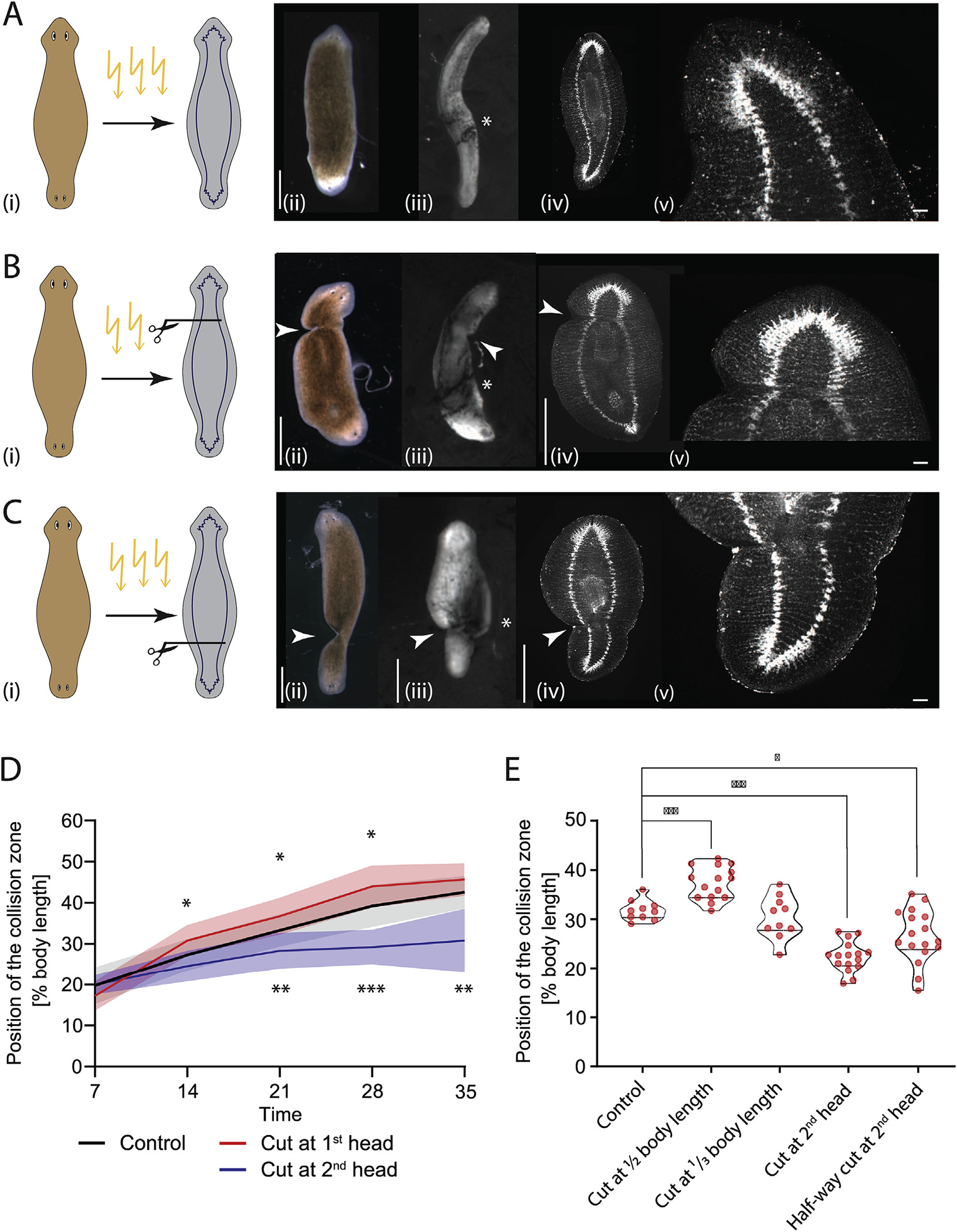
A) Uncut control animals: sketch of DHs showing irradiation of DHs at Day 7 with CNS scheme (i), in brightfield at Day 7 (ii), flow assay result of the same animal at Day 28 (iii), synapsin stain in the same animal at Day 28 (iv), and higher magnification of intact VNCs (v). B) Irradiated DH with cut at primary head: sketch showing cut position with CNS (i), in brightfield at Day 7 (ii), flow assay result of the same animal at Day 28 (iii), synapsin stain in the same animal at Day 28 (iv), and higher magnification of cut VNCs confirming severance (v). C) Irradiated DH with cut at secondary head: sketch of cut position with CNS (i), in brightfield at Day 7 (ii), flow assay result of the same animal at Day 28 (iii), matching synapsin stain at Day 28 (iv), and higher magnification of cut VNCs confirming severance (v). D) Quantification of position of the collision zone in animals with VNCs cut at either the primary head (red) or the secondary head (blue) at Day 7 following irradiation, showing significant difference compared to control (black). N = 12. Plotted is mean of all samples, with standard deviation as shaded area, all experiments with their age-matched controls. E) Position of collision zone at Day 21 in animals treated at Day 7 compared to controls, with animals with cut at midpoint, cut at 1/4th body length, cut at base of 2nd head, as well as animals with cut to midline at base of 2nd head – all in the secondary half. *p < 0.01, **p < 0.001, ***p < 0.0001. Arrowheads mark the cut sites. Scale bar 1 mm, in insets 100 μm.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ydbio.2020.08.009
To further confirm that the factors driving cilia reorientation travel the length of the body in long axial structures and to ascertain the spatial dynamics of this influence, we performed lateral cuts at different positions along the head-head axis following irradiation at Day 7 and observed the position of the collision zone at Day 21 (Fig. 6E, Supplementary Table 17). When the DHs were cut in the middle of the body, the collision zone was positioned significantly further towards the midpoint compared to controls, mirroring cuts at the base of the primary head, consistent with the signal from the primary head having to be transmitted into the secondary half of the animal to block the cilia reorientation in that part of the body. Cuts at 1/3rd body length did not significantly differ from controls (Fig. 6E, Supplementary Table 17), likely because the cut was placed close to the position of the collision zone at the timepoint of cutting, while animals with cuts between the secondary head and the collision zone (cut at the base of the secondary head) had a significantly lower position of the collision zone (Fig. 6E, Supplementary Table 17).
We also performed lateral cuts which only went to the midline, disrupting only one of the VNCs at the base of the secondary head in irradiated worms. In these DHs we found that the depth of the cut did not impact its effect, with a cut severing most of the body resulting in the same reorientation delay as a cut only to the midline (Fig. 6E). This result implicates the VNCs as the axial structures required for transmission of the molecules leading to cilia reorientation, as disruption of one VNC is likely to disrupt nerve signaling, consistent with our observations following half-brain removal (Fig. 5). It remains possible, however, that the effect of the cuts on other structures, such as disruption of the musculature or induction of epithelial defects, play a crucial role in the observed changes in flow pattern.
3.8. Nervous system in double-headed worms adapts to new morphology over time
Given that our above-presented data indicated that the nervous system may play an important role in the adaptation of the tissue polarity of the ventral epithelium, we next explored whether the polarity of the nervous system itself is affected in these repatterning DHs. Our previous work showed that morphogen transport in the nervous system plays an important role in determining regenerative outcomes and that mature DHs have a morphogen transport field that is mirror-symmetrical along the midline (Pietak et al., 2019). In the previous work, we showed that in mature DHs different cutting planes led to distinct regenerative outcomes based on whether the fragment included the midpoint of the animal, where we suggest the two halves of the nervous system meet. Fragments containing the midpoints always regenerate as DHs, while any non-midpoint fragments regenerated as single-headed animals (SHs) (Pietak et al., 2019). Given that the symmetry point of the multiciliated epithelium shifts between immature and mature DHs, we asked whether the symmetry point of the nervous system is similarly affected by assaying its ability to instruct polarity during regeneration.
Our nerve-transport data suggest that if changes to the polarity of the nervous system take place, the regenerative outcomes of identical amputations performed in 7-day old DHs and 49-day old DHs will be different as the underlying transport field is distinct (Fig. 7A). We performed amputations at three different planes: a narrow decapitation directly at the base of one of the heads (around 1/6th of the body length), a cut positioned 1/4th of the way along the head-head axis, and a cut bisecting the DH animals in half. While the decapitations lead to the same regenerative outcomes in both mature and immature DHs, cuts positioned at 1/4th of the body length and at the middle of the body lead to distinct regenerative outcomes for immature and mature DHs.
Fig. 7. The CNS’ influence over polarity during regeneration changes spatially over time.
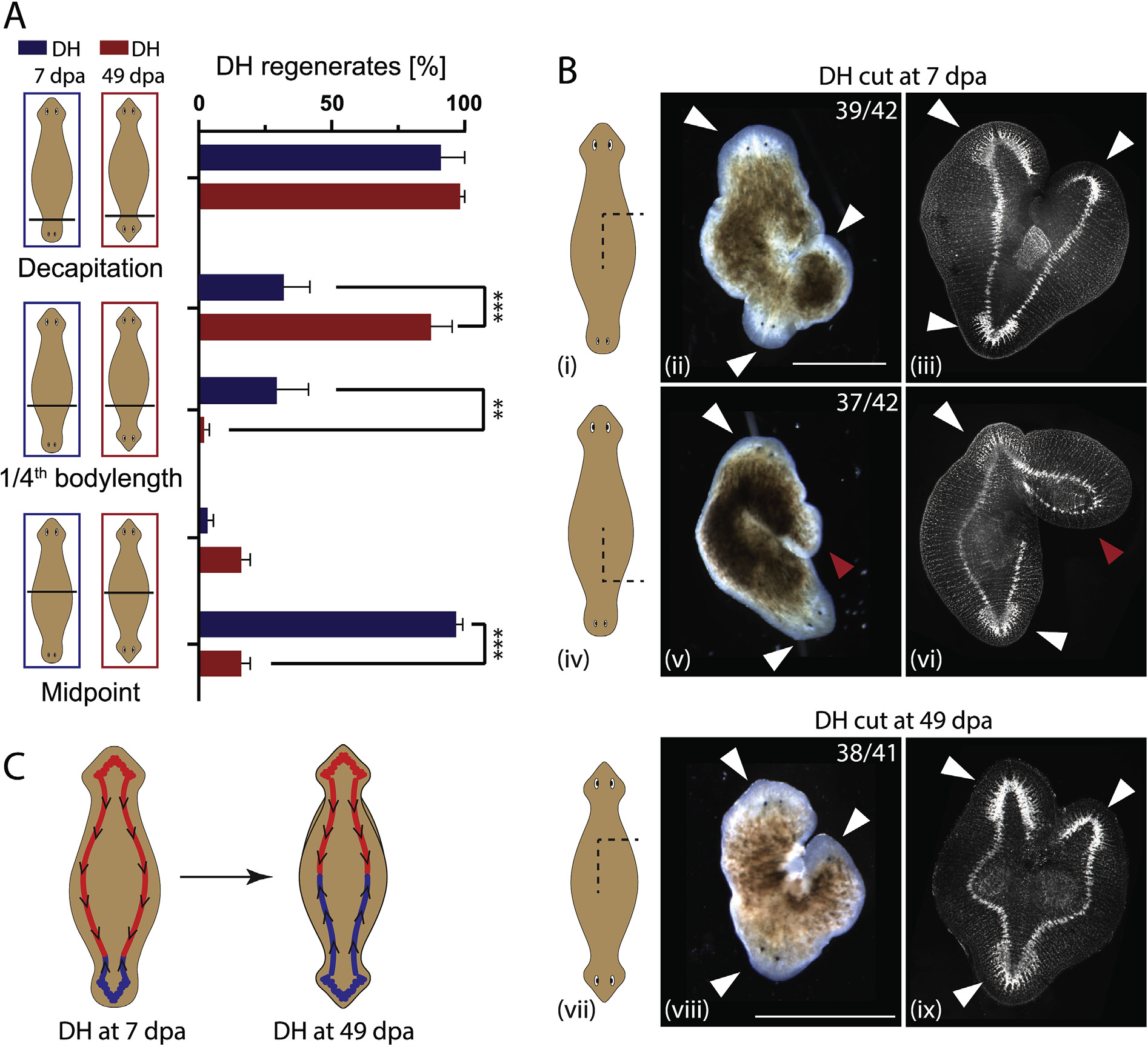
A) Percentage of DH regenerates from DHs cut at various locations along the body axis. Cuts were performed either in animals at Day 7 (blue) or Day 49 (red) (days after amputation – dpa). Decapitation resulted in 96% and 98% DH for Day 7 and Day 49 animals, respectively. Fragment consisting of 3/4th of the body regenerated at 28% vs. 87% DHs, while the 1/4th fragments regenerated only 29% and 2% DHs for Day 7 and Day 49, respectively. Midway cuts gave 16% DH regenerates for both halves of DHs at Day 49, while DHs at Day 7 had 3% of the primary half regenerating as DHs, while 97% of the secondary halves regenerates as DHs. N = 4, with 15 worms per replicate. B) Regenerative outcomes of induced side-outgrowths facing either (i-iii) towards the primary head or (iv-vi) towards the secondary head in DHs at Day 7 and (vii-ix) in DHs at Day 49, shown as sketch (left column), in brightfield (middle column) and in synapsin antibody stain, visualizing the nervous system (right column). Frequency of the observed outcomes is given in each panel. White arrow – head regeneration, red arrow – tail regeneration. C) Model of the nervous system polarity in double-headed animals at Day 7 and Day 49, representing the change in orientation in the secondary half of the animal as it adapts to the new morphology. **p < 0.01, ***p < 0.0001, plotted are mean values with error bars representing standard deviation. Scale bars 1 mm.
For the cuts positioned at 1/4th of the body length the larger fragment (containing the midpoint) regenerated significantly more often as a DH in mature DHs compared to immature DHs (87% vs. 31%, p < 0.0001, Supplementary Table 18). In immature DHs, both the 1/4th and the 3/4th fragments regenerated as DHs at similar percentages (29% vs. 31%). This suggests that when a cut is placed at around 1/4th of the way from the secondary to the primary head, in approximately one third of the cases the symmetry point of the nervous system is destroyed via cutting, leading to just SH regeneration, while in the other two-thirds of cases the symmetry point is located in either one of the two fragments leading to a regenerative outcome of one DH and one SH, suggesting that the location of the symmetry point of the nervous system at Day 7 is located around 1/4th of the body length away from the secondary head. When mature DHs were cut in half, both halves regenerated mostly as SHs (84% SHs), while for immature DHs the two halves behaved very differently. The half containing the primary head almost always regenerated as SH (96%), while the half containing the secondary head almost always regenerated as a DH (97%) (Fig. 7A). These data reveal that the symmetry point of the DH nervous system is positioned very off-center in an immature DH, somewhere around the 1/4th body length point, while it is positioned in the middle in mature DHs. This supports the hypothesis that the nervous system polarity adapts in parallel with the polarity of the ciliated epithelium. The position of the symmetry point of the nervous system in DHs at Day 7 suggested by these experiments at around 1/4th of the body length is consistent with the cilia-flow collision zone located at 27% of body length on average at Day 7 (Fig. 1).
To further explore the hypothesis that the underlying orientation of the morphogen transport fields in the VNCs in immature and mature DHs is different, we generated side-wounds innervated with a single VNC in both mature and immature DHs (Fig. 7B). In the immature DHs, where a distinction between the primary and secondary head could be identified visually, the wounds were induced either facing towards the primary or the secondary head, at about 1/3rd of the body length away from the head they were facing towards. The induced outgrowths were scored for either head or tail identity using both appearance and synapsin staining to visualize the nervous system structures. In immature DHs, side outgrowths facing towards the primary head regenerated with a head 93% of the time (39/42, Fig. 7 Bi–iii), while the outgrowths facing towards the secondary head regenerated as tails 88% of the time (37/42, Fig. 7 Biv–vi). In mature DHs, no distinction between primary and secondary head remains apparent to guide the positioning of cuts, therefore the comparative outcome to the immature DH result would be 50% heads and 50% tail regenerates in the side outgrowths, as samples are an equal mix of cuts facing the primary and the secondary head. However, we observed 93% head outgrowths, consistent with a repatterning of the nervous system occurring as DHs mature to make the two halves of the animal equivalent (Fig. 7B vii–ix). Taken together, these data reveal that the instructive influence of the CNS over the type of anatomical structures that will be formed after amputation changes over a much longer timescale than regeneration itself: in a mature double-headed animal, the CNS and tissue polarity remodel progressively (Fig. 7C). Thus, the progressive remodeling of tissue polarity affects not only the epidermis but also the instructive aspects of the CNS.
4. Discussion
Regeneration is a remarkable process because it orchestrates a multitude of activities of many individual cells (migration, differentiation, proliferation, and shape change) toward a specific large-scale anatomical outcome. Order is simultaneously regulated at multiple scales and levels of organization, including cells, tissues, and whole-body axes. Planarian regeneration illustrates the ability of some systems to (re) establish organs and physiological signaling systems in precise spatial coordination. This requires information about direction (alignment of planar polarity) as well as position (which organ to make at what location), but it is still very poorly understood how tissues adjust to changes in this information during regeneration. This is important not only for cell and developmental biology, but also for biomedicine. For example, planar polarity disruption is a known factor in carcinogenesis (Lee and Vasioukhin, 2008). Likewise, in the case of embryonic left-right patterning, significant birth defects occur when the cell-level alignment in organs such as the heart does not match the mirror-image inversion of body asymmetry (Delhaas et al., 2004). Here, we exploited the planarian model system to study the plasticity of tissue polarity and its relation to whole organism polarity in the developing double-headed planaria, and the ways in which existing tissues are remodeled in place to achieve the concordance between tissue polarity and organismal polarity.
We present evidence that planarians, which implement atypical body morphologies following interventions in normal regenerative signaling pathways, adjust the polarity of their existing tissues to align with these new morphologies on long timescales. Double-headed planarians possess, in addition to their normal head positioned on the original anterior end, a secondary head positioned on the original posterior, and internally exhibit a complete mirroring of their axis along the midpoint. This system gives us the opportunity to explore how cilia orientation changes in response to changes of overall organismal polarity. We show that cilia beat direction reverses in the portion of the double-headed animals that switches from a posterior to an anterior identity over a period of around 40 days. This long timeframe places this process outside of the normal regenerative timeframe commonly explored in studies with planarians, highlighting the importance of monitoring remodeling events past the time when the damaged or missing tissues have been repaired or replaced.
In recent elegant work, methods to quantify the orientation of cilia in planaria were developed using antibody labelling and large-scale automated microscopy (Vu et al., 2019). Using these tools opened the door for an exploration of the molecular factors regulating the polarity and it was shown that core PCP and Ft/Ds pathways form a polarity field that regulates cilia orientation both along the head-tail axis and towards the margins respectively (Vu et al., 2019). Further work showed how the cytoskeleton controls the orientation of epidermal cilia on the molecular level at the cilia rootlet (Basquin et al., 2019). These very interesting papers focused on a detailed molecular mechanistic explanation of the factors regulating cilia polarity, but due to the limitations of the tools available in the planaria field, no observations of the dynamics of pattern changes could be made using the tools established in these papers, as they require fixation of the animals to visualize the cilia. We were interested in exploring the dynamics of the reorientation of the cilia-driven flow, and therefore chose the more coarse-grained tissue--level approach of assessing flow patterns.
We are particularly interested in the dynamics of flow changes, as the orientation of cilia is usually thought to be set during development or regeneration and is assumed not to change once established. Here we have identified a system in which the dynamic change of cilia-driven flow can be explored thanks to the flexibility of the planaria body plan. By preventing formation of new cells via irradiation, we can separate new tissue growth from the plasticity of intact planarian tissues. We found that inhibition of new cell formation did not impact the reorientation of cilia, indicating that the reorientation of the cilia most likely takes place in pre-existing multiciliated cells. We hypothesize that the reorientation of tissue is furthermore linked to the organism-scale polarity via signals from the brain that may be transmitted along the ventral nerve cords (VNCs), given that removal of the heads and lateral incisions that bisect the VNCs affect the speed and extent of the cilia reorientation similarly. We cannot rule out that other structures transmit these signaling factors, such as gap-junction-linked epidermal cells or the musculature that spans the length of the body. The requirement for signals from the brain makes a transport of signaling factor along the VNCs a likely solution, as no transfer of the signal between different cell types would be necessary.
The formation of an additional brain in the double-headed animal may provide an intrinsic signaling source to drive the required cilia reorientation through the nervous system spanning the body. Our finding that bisection of the brain, without removal of tissue, results in the same magnitude of inhibition of the cilia reorientation as removal of the entire head, suggests that the production of the reorientation signal relies on the intactness of the brain. Consistent with this idea, the overall size of the brain does not appear to influence in the rate of cilia reorientation as long as it is intact, as indicated by irradiated animals with normal cilia reorientation even though they have very differently sized brains.
While signals from the brain are the major drivers of cilia reorientation, a small amount of reorientation continues to happen after removal of both heads, which can be interpreted either as signal sent before decapitation continuing to be put into effect, or as a secondary system capable of driving limited cilia reorientation via a different mechanism. This mechanism can be speculated to consist of local PCP signaling, which is transmitted from cell to cell, leading to slower cilia reorientation than that mediated by a neural path. We have hypothesized elsewhere that neural connections may have evolved in part to provide an optimized version of pre-neural signaling events operating in pattern regulation (Fields et al., 2019), so perhaps the cilia reorientation that occurs in the absence of both head is an example of less efficient, ancestral non-neural signaling.
We find that during DH maturation the collision zone can be shifted across the midline through continuous removal of the primary head, suggesting that there is no fixed midpoint. While we were unable to keep the worms alive long enough to test whether full reversal of cilia beat direction across the entire animal is possible, our data suggest that it is. Our data illustrate that there is no secondary underlying signal setting the midpoint of the epithelium but that this midpoint solely arises in normal DHs from the balance of signaling forces from the two heads. At the same time, there appears to be a window of time after which the orientation of the cilia is set and can no longer be affected by removal of the animal’s head, as demonstrated by the lack of change in the position of the collision zone in mature DHs that had one head removed. This is a surprising observation as such windows of opportunity are commonly only seen in development and do not translate easily to highly regenerative systems such as planaria. We hypothesize that each regeneration-inducing event triggers a whole cascade of regenerative processes, each with their own timeline and windows of plasticity during which they can occur. Once these windows close, the organism returns to a stable state until the next triggering event, at which point the windows can be reopened. Similarly, signals from the brain do not appear to be important for maintaining cilia orientation in single-headed animals. This suggests that there is a window of plasticity during which signals from the brain can fully determine the polarity of the epithelium, but that once this window closes, cilia orientation is set, likely until changes induced by the regeneration of damaged or lost tissue occurs. This observation is reminiscent of data in developmental systems, where cilia orientation and tissue polarity are set in certain developmental timeframes (Boisvieux--Ulrich et al., 1991; Tung and Yeh-Tung, 1940; Twitty, 1928). What signals determine this plasticity in planarians remain to be addressed in future work.
Importantly, the link between global organ distribution and underlying tissue polarity exists in the nervous system too, not only in the epidermis. Our previous work showed how the global transport direction of the nervous system determines regenerative outcomes (Pietak et al., 2019). Here we observed that in maturing DHs, the symmetry point of the transport field of the nervous system shifts progressively to the middle of the animal, as revealed by the distinct regenerative outcomes of the same cuts along the body axis in immature and mature DHs. This suggests that the nervous system changes its overall polarity to adapt to the new, artificially induced morphologies. This is an extreme example of neuronal plasticity, opening new questions about what signals drive the changes in neural polarity and how correct and functional connections can re-form without interfering with CNS functions necessary for the animal to survive and continue its normal activity.
This work uses the dynamic body plan of the planarians as a way to explore multi-scale polarity. While organ structures are formed by the activity of cells, this bottom-up, self-assembly mechanism is complemented by top-down controls in which axial polarity drives changes of tissue-level directionality. The dynamic regeneration and repatterning of adult tissues in planarians offer an exciting addition to investigations of polarity in developmental systems. The mechanism of reorientation of the ciliated epithelium under the control of signals from the brain further highlights the importance of the nervous system as an overarching organizing system to coordinate and set polarity in regeneration and repatterning. At the same time the polarity control by the nervous system may be a bi-directional process, in which the nervous system acts both as a mediator of remodeling signals to the epithelium and at the same time is the target of remodeling itself. Beyond planarians, these findings suggest approaches that exploit brain-like signaling to understand and manipulate multi-scale order in bioengineering and developmental contexts.
Supplementary Material
Acknowledgements
The authors would like to thank Anna Kane, Joshua Finkelstein, Alexis Pietak, and all members of the Levin lab for thoughtful discussions on this project. We thank Nicolas Spitzer, Mansi Shrivastava, and James Monaghan for critical reading and comments on the manuscript. We thank Junji Morokuma and Hans Gonzembach for planaria colony maintenance. This research was supported by the Allen Discovery Center program through The Paul G. Allen Frontiers Group (12171), and by the National Institutes of Health Research Infrastructure grant NIH S10 OD021624.
Footnotes
Declaration of competing interest
The authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ydbio.2020.08.009.
References
- Abnave P, Aboukhatwa E, Kosaka N, Thompson J, Hill MA, Aboobaker AA, 2017. Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development 144, 3440–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell T, Cebria F, Salo E, 2010. Gradients in planarian regeneration and homeostasis. Cold Spring Harb. Perspect. Biol. 2, a000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler CE, Seidel CW, McKinney SA, Sanchez Alvarado A, 2014. Selective amputation of the pharynx identifies a FoxA-dependent regeneration program in planaria. eLife 3, e02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J, Basquin C, 2016. Basal bodies across eukaryotes series: basal bodies in the freshwater planarian Schmidtea mediterranea. Cilia 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J, Wong ML, Downhour DM, Sanchez Alvarado A, Marshall WF, 2012. Centrosome loss in the evolution of planarians. Science 335, 461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basquin C, Ershov D, Gaudin N, Vu HT, Louis B, Papon JF, Orfila AM, Mansour S, Rink JC, Azimzadeh J, 2019. Emergence of a bilaterally symmetric pattern from chiral components in the planarian epidermis. Dev. Cell 51, 516–525 e515. [DOI] [PubMed] [Google Scholar]
- Boisvieux-Ulrich E, Sandoz D, Allart J-P, 1991. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol. Cell. 72, 3–14. [DOI] [PubMed] [Google Scholar]
- Brooks ER, Wallingford JB, 2014. Multiciliated cells. Curr. Biol. 24, R973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YH, Srinivasan S, Keller R, Kintner C, 2018. Mechanical strain determines cilia length, motility, and planar position in the left-right organizer. Dev. Cell 45, 316–330 e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaas T, Decaluwe W, Rubbens M, Kerckhoffs R, Arts T, 2004. Cardiac fiber orientation and the left-right asymmetry determining mechanism. Ann NY Acad Sci 1015, 190–201. [DOI] [PubMed] [Google Scholar]
- Devenport D, 2014. The cell biology of planar cell polarity. J. Cell Biol. 207, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant F, Morokuma J, Fields C, Williams K, Adams DS, Levin M, 2017. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys. J. 112, 2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields C, Bischof J, Levin M, 2019. Morphological coordination: a common ancestral function unifying neural and non-neural signaling. Physiology in Print. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, 2008. The plane facts of PCP in the CNS. Neuron 60, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, Spassky N, 2010. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341–350. [DOI] [PubMed] [Google Scholar]
- Herrera-Rincon C, Pai VP, Moran KM, Lemire JM, Levin M, 2017. The brain is required for normal muscle and nerve patterning during early Xenopus development. Nat. Commun. 8, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwadate Y, Suzaki T, 2004. Ciliary reorientation is evoked by a rise in calcium level over the entire cilium. Cell Motil. Cytoskelet. 57, 197–206. [DOI] [PubMed] [Google Scholar]
- King SM, Patel-King RS, 2016. Planaria as a model system for the analysis of ciliary assembly and motility. Methods Mol. Biol 1454, 245–254. [DOI] [PubMed] [Google Scholar]
- Klagges BRE, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E, 1996. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J. Neurosci. 16, 3154–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Gotoh S, Tateishi K, Yamamoto Y, Korogi Y, Nagasaki T, Matsumoto H, Muro S, Hirai T, Ito I, Tsukita S, Mishima M, 2016. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Reports 6, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto K, Yamazaki Y, Nishida T, Shinohara K, Ishikawa H, Hasegawa T, Okanoue T, Hamada H, Noda T, Tamura A, Tsukita S, Tsukita S, 2012. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell 148, 189–200. [DOI] [PubMed] [Google Scholar]
- Lee M, Vasioukhin V, 2008. Cell polarity and cancer–cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 121, 1141–1150. [DOI] [PubMed] [Google Scholar]
- Meunier A, Azimzadeh J, 2016. Multiciliated cells in animals. Cold Spring Harb. Perspect. in Biol. 8, a028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, Kintner C, 2007. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447, 97–101. [DOI] [PubMed] [Google Scholar]
- Morgan TH, 1901. Regeneration. Macmillan, New York. [Google Scholar]
- Noguchi M, Kitani T, Ogawa T, Inoue H, Kamachi H, 2005. Augmented ciliary reorientation response and cAMP-dependent protein phosphorylation induced by glycerol in triton-extracted Paramecium. Zool. Sci. 22, 41–48. [DOI] [PubMed] [Google Scholar]
- Ohata S, Alvarez-Buylla A, 2016. Planar organization of multiciliated ependymal (E1) cells in the brain ventricular epithelium. Trends Neurosci. 39, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, Levin M, 2010. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 339, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Nicolas CL, Adams DS, Levin M, 2008. Establishing and maintaining a colony of planarians. CSH Protoc. 2008, pdb prot5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl R, 1903. The movements and reactions of fresh-water planarians: a study in animal behaviour. J. Cell Sci. 46. [Google Scholar]
- Petersen CP, Reddien PW, 2008. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319, 327–330. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW, 2011. Polarized activation of notum at wounds inhibits Wnt function to promote planarian head regeneration. Science 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietak A, Bischof J, LaPalme J, Morokuma J, Levin M, 2019. Neural control of body-plan axis in regenerating planaria. PLoS Comput. Biol. 15, e1006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, 2018. The cellular and molecular basis for planarian regeneration. Cell 175, 327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Sanchez Alvarado A, 2004. Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20, 725–757. [DOI] [PubMed] [Google Scholar]
- Rompolas P, Azimzadeh J, Marshall WF, King SM, 2013. Analysis of ciliary assembly and function in planaria. Methods Enzymol. 525, 245–264. [DOI] [PubMed] [Google Scholar]
- Rompolas P, Patel-King RS, King SM, 2010. An outer arm Dynein conformational switch is required for metachronal synchrony of motile cilia in planaria. Mol. Biol. Cell 21, 3669–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW, 2017. ImageJ 2: ImageJ for the next generation of scientific image data. BMC Bioinf. 18, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustia CP, 1925. The control of biaxial development in the reconstitution of pieces of Planaria. J. Exp. Zool. 42, 111–142. [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Meunier A, 2017. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423. [DOI] [PubMed] [Google Scholar]
- Stevenson CG, Beane WS, 2010. A low percent ethanol method for immobilizing planarians. PloS One 5, e15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielicke W, Stamhuis EJ, 2014. PIVlab – towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. J. Open Res. Software 2. [Google Scholar]
- Thielicke W, Stamhuis EJ, 2019. PIVlab - Time-Resolved Digital Particle Image Velocimetry Tool for MATLAB, 12 ed. figshare. [Google Scholar]
- Tissir F, Goffinet AM, 2010. Planar cell polarity signaling in neural development. Curr. Opin. Neurobiol. 20, 572–577. [DOI] [PubMed] [Google Scholar]
- Tung T-C, Yeh-Tung Y, 1940. Experimental studies on the determination of polarity of ciliary action of anuran embryos. H. Vaillant-Carmanne. [Google Scholar]
- Twitty, 1928. Experimental studies on the ciliary action of amphibian embryos. J. Exp. Zool. 50, 319–344. [Google Scholar]
- Veraszto C, Ueda N, Bezares-Calderon LA, Panzera A, Williams EA, Shahidi R, Jekely G, 2017. Ciliomotor circuitry underlying whole-body coordination of ciliary activity in the Platynereis larva. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu HT, Mansour S, Kucken M, Blasse C, Basquin C, Azimzadeh J, Myers EW, Brusch L, Rink JC, 2019. Dynamic polarization of the multiciliated planarian epidermis between body plan landmarks. Dev. Cell 51, 526–542 e526. [DOI] [PubMed] [Google Scholar]
- Wagner DE, Wang IE, Reddien PW, 2011. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, 2010. Planar cell polarity signaling, cilia and polarized ciliary beating. Curr. Opin. Cell Biol. 22, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, Mitchell BJ, 2011. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 195, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Mitchell BJ, 2012a. Planar cell polarity: microtubules make the connection with cilia. Curr. Biol. 22, R1001–1004. [DOI] [PubMed] [Google Scholar]
- Werner ME, Mitchell BJ, 2012b. Understanding ciliated epithelia: the power of Xenopus. Genesis 50, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchley JN, Mayer M, Wagner DE, Owen JH, Reddien PW, 2013. Muscle cells provide instructions for planarian regeneration. Cell Rep. 4, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


