Abstract
Water stress influences plant growth and metabolism. Carnitine, an amino acid involved in lipid metabolism, has been related to responses of plants to abiotic stresses, also modulating their metabolites. Culantro (Eryngium foetidum L.) is a perennial herb, rich in essential oils, native to Latin America, commonly used due to its culinary and medicinal properties. Here, we investigated the effect of exogenous carnitine on morphophysiology and the essential oil profile of culantro plants under water stress. For this, plants were grown under three water conditions: well-watered, drought stress, and re-watered; and sprayed with exogenous carnitine (100 µM) or water (control). Culantro growth was impaired by drought and enhanced by re-watering. Carnitine, in turn, did not reverse drought effects on growth, and impaired the growth of re-watered plants, also improving photosynthetic pigment content. Water conditions and carnitine application changed the essential oil profile of the plants. Drought and re-watering improved the production of eryngial, which was even increased with exogenous carnitine in re-watered plants. In addition, hydroquinone was only produced with the combination of re-watering and carnitine application. The application of exogenous carnitine can be a strategy to induce the production of essential oil compounds with cosmetic and pharmaceutical importance in culantro.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03757-y.
Keywords: Culantro, Drought stress, Medicinal plants, Re-watering, Secondary metabolites profile, Sesquiterpenes
Introduction
Water stress is a main environmental factor affecting the morphophysiology and production of plants (Liang et al. 2019; Soares et al. 2022). Thus, plants have strategies to prevent water loss in order to maintain optimal water balance, such as stomatal closure, osmoregulation, reactive oxygen species (ROS) scavenging, and production of secondary metabolites (Gupta et al. 2020), the latter are organic molecules produced by plants, classified into alkaloids, phenolics, and terpenoids (Takshak and Agrawal 2019). These compounds act as chemical regulators and messengers in plants, playing a fundamental role in fertilization, defense against pathogens and herbivores, and tolerance to abiotic stresses (Böttger et al. 2018). Due to their aromatic and therapeutic properties, they are used for dyes, medicines, artificial flavoring products, nutraceuticals, perfumes, among others (Wink 2015; Kulak et al. 2019).
Culantro (Eryngium foetidum L.) belongs to the Apiaceae family and is a perennial herb native to Central America, with autogamous characteristics and propagation mainly through seeds (Singh et al. 2014; Rodrigues et al. 2022). This species is commonly used in folk medicine to treat burns, earaches, fever, hypertension, constipation, asthma, stomach pain, worms, diarrhea, rheumatism, cramps, and to stimulate appetite (Shavandi et al. 2012; Singh et al. 2013; Rodrigues et al. 2022). Culantro is widely used as a flavoring condiment in foods, having great relevance for the Amazonian food culture (Rodrigues et al. 2022), also having impact on human health, inhibiting the growth of pathogenic bacteria, such as Helicobacter pylori, showing anti-leishmanial activity against Leishmania tarentolae and L. donovani, and being effective against trypanosomes, nematodes, and fungi (Paul et al. 2010; Jaramillo et al. 2011; Rojas-Silva et al. 2014; Mabeku et al 2016). Furthermore, culantro is rich in essential oils stored on its secretory ducts (Bhavana et al. 2013; Rodrigues et al. 2020, 2022), with a characteristic flavor and aroma due an aliphatic aldehyde called eryngial ((E)-2-dodecenal), which is present mostly on the leaves (Quynh and Kubota 2012; Rodrigues et al. 2020).
Mitigation strategies can be used to attenuate water stress in plants (Sai et al. 2016). The application of bioregulators, such as amino acids like proline (Ami et al. 2020; Santos et al 2022a), arginine (Nargesi et al. 2022), and carnitine (Charrier et al. 2012; Turk et al. 2019a, b; Santos et al. 2022b), has been studied. Carnitine is a quaternary ammonium compound involved in metabolic functions like energy metabolism and stress tolerance (Charrier et al. 2012). This amino acid participates in the degradation of triglycerides to fatty acids and their transportation into mitochondria, stimulating respiration, being essential for energy storage, cell structure, and signal transduction (Bourdin et al. 2007; Frank et al. 2015; Oney-Birol 2019; Turk et al. 2019a, b). In addition, exogenous carnitine has been pointed as a bioregulator, modulating plant growth under non-stressful conditions (Charrier et al. 2012; Santos et al. 2022c) and mitigating moderate water stress in plant species, such as arugula (Santos et al. 2022b) and radish (Henschel et al. 2023). This way, the objective of this study was to evaluate the action of carnitine on growth, morphophysiology and the essential oil profile of culantro plants under water stress.
Materials and methods
Experimental location and plant material
The experiment was conducted between January and May 2022, in a greenhouse covered with transparent film, located in the experimental area of the Seedling Production Laboratory of the Center for Human, Social and Agrarian Sciences/Federal University of Paraiba (CCHSA/UFPB), in Bananeiras, Paraiba, Brazil (6° 45ʹ S, 35° 38ʹ W, elevation of 526 m). Culantro seeds (E. foetidum) were, donated by growers from local rural communities in the municipality of Areia, Paraíba, Brazil (6° 57ʹ S, 35° 41ʹ W, elevation of 623 m).
Irrigation and bioregulators treatments
The culantro seeds were sown in trays containing 200 cells. At 35 days after sowing (DAS), when plants presented four fully expanded leaves, they were transplanted to polyethylene bags (22 × 28 cm) containing commercial substrate (Mecplant®, Telêmaco Borba, Brazil). The bags were irrigated until 100% bag capacity (BC) for fifteen days. 50 DAS, the plants were subjected to the treatments: well-watered (80% BC), drought (40% BC), and re-watered (12 days without irrigation with subsequent irrigation with 80% BC, with the application of water (control) or 100 µM carnitine (l-carnitine, Growth Supplements, Tijucas, Brazil) (Santos et al. 2022c), using hand sprayers every 6 days (50, 56, 62, 68, 74, 80, 86, 92, and 98 DAS). The duration of water restriction of 12 days was established based on previous survival tests. Carnitine solution was dissolved in distilled water with addition of the surfactant polysorbate 80 (Tween-80®, 0.03%) (v/v) to increase adhesion to the leaves.
Morphophysiological analysis
At 100 DAS, photosynthetic pigments content, gas exchange parameters, and chlorophyll a fluorescence were measured, and 102 DAS growth parameters were measured. The leaf area, number of leaves, specific leaf area, and root length were determined through image analysis using the software ImageJ (Abramoff et al. 2004). Five plants were collected and separated into shoots and roots using a scalpel and weighed for the determination of the fresh mass. After morphologic measurements, shoots and roots of culantro plants were oven-dried at 65 °C until a constant weight to determine their dry weight. The shoot/root ratio was determined as the dry weight of shoots divided by the dry weight of roots. The total biomass was determined as the sum of dry weight of shoots and roots.
Gas exchange measurements were performed using an open-flow gas exchange system infrared gas analyzer (IRGA, LCpro-SD Portable Photosynthesis System, ADC BioScientific, Hoddesdon, UK). The analyses were made on fully expanded leaves of five plants per treatment between 8 and 11 h a.m. The conditions in the leaf chamber consisted of an ambient temperature and reference CO2 and artificial photosynthetically active radiation of 1000 μmol m−2 s−1 with 10% blue light. The net carbon assimilation rate (A, µmol CO2 m−2 s−1), stomatal conductance (gS, mol H2O m−2 s−1), internal CO2 concentration (Ci, mmol CO2 mol−1 air) and leaf transpiration rate (E, mmol H2O m−2 s−1), and water use efficiency (A/E), carboxylation efficiency (A/Ci) were determined. Light response curves of photosynthesis were studied by varying photosynthetic photon flux density (PPFD) from 0 to 1800 mol m−2 s−1. Light response curve measurements were made on fully expanded leaves of three plants per treatment between 8 and 11 h a.m. From light response curves, were calculated the following parameters: dark respiration (Rdark) (µmol m−2 s−1), apparent quantum yield (mol/mol), light compensation point (LCP) (µmol m−2 s−1), maximum gross assimilation rate (Amax) (µmol m−2 s−1), and light saturation point (LSP) (µmol m−2 s−1).
Levels of photosynthetic pigments were determined according to Santos et al. (2008), with modifications, following the equation of Wellburn (1994). For this, four disks (1 cm2) from fully expanded leaves of four plants per treatment were incubated for 48 h in dark conditions with 7 mL dimethyl sulfoxide (Santos et al. 2008). Then, the extract was read at 480, 649, and 665 nm using a spectrophotometer (GTA-96 UV–Vis, Global Trade Technology, São Paulo, Brazil). The levels of chlorophyll a, chlorophyll b, chlorophyll a/b ratio, total chlorophylls, and total carotenoids were determined.
The relative water content (RWC) was determined according to Barrs and Weatherley (1962), with modifications. Ten leaf disks (1 cm2) were collected from fully expanded leaves of five plants per treatment and immediately weighed (fresh mass, FM). The disks were incubated for 6 h in distilled water, weighed for turgid mass (TM), and oven-dried at 65 °C for 24 h for dry mass (DM) determination. RWC was calculated as [(FM-DM)/(TM-DM)] × 100, and expressed as a percentage (%).
Microextraction of essential oils
Approximately 500 mg of leaves were collected and stored at − 18 °C in test tubes with a screw cap, following Castro et al. (2020), with modifications. After freezing, 1 mL methanol was added to each sample. To accelerate the extraction process, samples were immersed in an ultrasonic bath (Ultra Cleaner 800, UNIQUE) at 40 kHz and room temperature for 10 min. Subsequently, the supernatant was filtered through a sterile cotton wick. Resulting samples of 1 μL clear solution containing the extracted oils were analyzed by gas chromatography.
Qualitative analysis of essential oils
Qualitative analysis of essential oils was carried out on a gas chromatographer coupled to a mass spectrometer (GCMS-QP2010 Plus; Shimadzu, Kyoto, Japan) and an Rtx-5MS® column (Restek, Bellefonte, PA, USA) of 30 m × 0.25 mm, with three technical replicates. The initial oven temperature was 50 °C, where it was maintained for 3 min, followed by an increase of 6 °C min−1 to 240 °C. The injector was operated in split mode (1:10) at 240 °C, and the interface and mass detector were operated at 250 °C.
Helium was used as the carrier gas, with a flow of 1.69 mL min−1. The constituents were identified by comparing the obtained mass spectra with those of the NIST 9.0 database (correlation > 95%) and confirmed by the corresponding retention index (Kováts Index) compared to published data.
Experimental design and statistical analysis
The experiment was in a completely randomized design, in a 2 × 3 factorial scheme (carnitine application × water condition) with two simultaneously repetitions, ten replicates for each combination of water level and carnitine treatment, and the experimental unit composed by one bag with one plant each. For all the analyses, sampling was done through a draw to ensure randomization. The model used for the analysis of variance was: Yijk = μ + Wi + Cj + (WC) ij + Eijk; where: Yijk = are the observations of the dependent variables, μ = the overall mean effect, Wi = effect of the level ith of water factor, Cj = effect of the level ith of carnitine factor, (WC) ij = effect of the interaction ijth water level × carnitine, and Eijk = random error component. The data were tested for normality and homogeneity using the Shapiro–Wilk and Bartlett tests, respectively, subjected to analysis of variance, and the means compared by Tukey’s test (P ≤ 0.05) using the Genes software (Cruz 2016).
Volatiles profile data were submitted to multivariate analysis. The distance between the treatments was determined using canonical discriminant analysis in a three-dimensional scatter plot. The treatments were separated into different groups using the Tocher optimization method and generalized squared interpoint distance of Mahalanobis (D2). The grouping quality was evaluated using the co-optical correlation coefficient (r). The relative contribution of each variable to discriminate treatments was quantified using Singh (1981) criterion.
Results
Drought and exogenous carnitine modulate morphophysiology of culantro plants
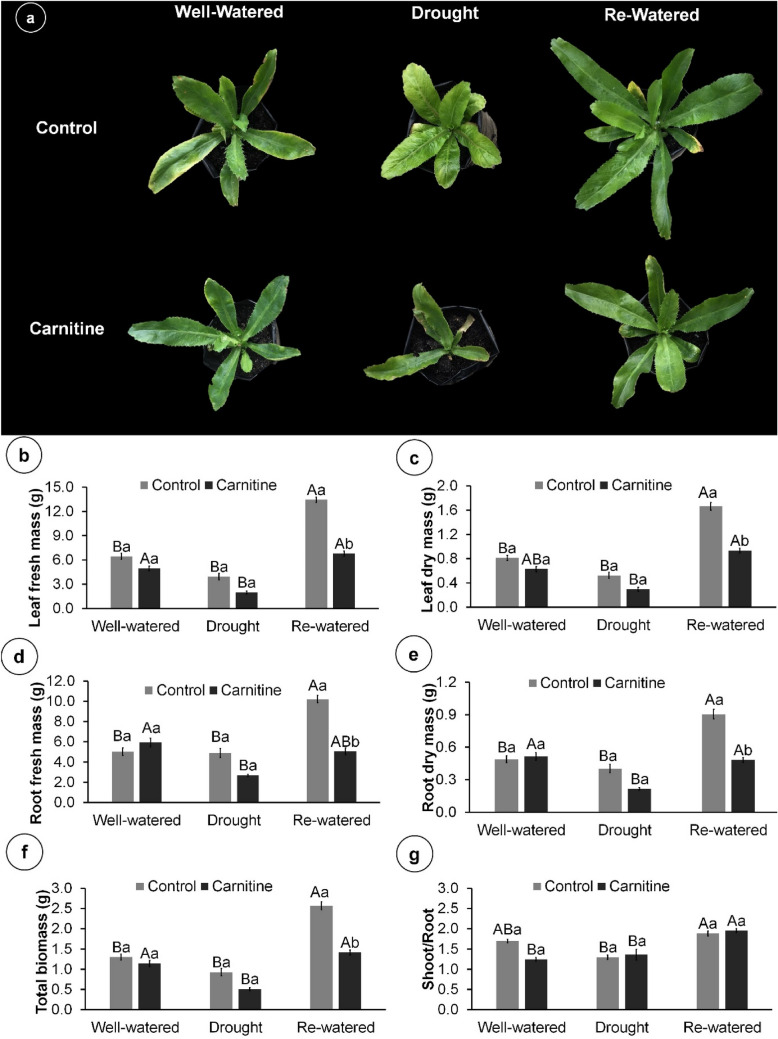
Water levels and carnitine application affected most morphophysiological parameters (Online Resource 1, Supplementary Table 1), with drought stress affecting overall plant growth (Fig. 1a). Re-watered plants doubled leaf dry and fresh mass, root dry and fresh mass, and total biomass compared to well-watered plants (Fig. 1b–f). Carnitine application increased the shoot/root ratio of re-watered compared to well-watered and drought plants. Similarly, in control plants, the highest shoot/root ratio occurred upon re-watering (Fig. 1g).
Fig. 1.
Growth of 102-day-old Eryngium foetidum L. plants sprayed with water or carnitine, and grown under different water conditions. Representative plants of each condition (a); Leaf fresh mass (b); Leaf dry mass (c); Root fresh mass (d); Root dry mass (e); Total biomass (f); and Shoot/root ratio (g). Columns represent the mean of five replicates, and bars represent the standard error. Means followed by the same letter do not differ by Tukey’s test (P ≤ 0.05). Capital letters compare water conditions within carnitine levels, and lowercase letters compare between carnitine and control within each water condition
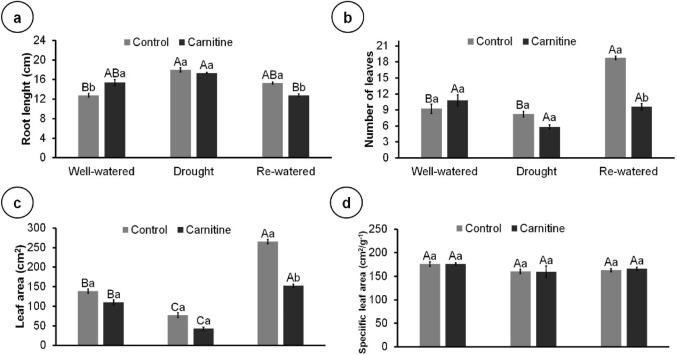
Drought increased root length and decreased the leaf area and number of leaves compared to the well-watered and re-watered plants (Fig. 2a–c). Re-watered treatment doubled the number of leaves and leaf area compared to well-watered treatment; however, these increases were reversed by carnitine application. There was no difference among treatments for specific leaf area (Fig. 2d).
Fig. 2.
Growth parameters of 102-day-old Eryngium foetidum L. plants sprayed with water or carnitine, and grown under different water conditions. Root length (a); number of leaves (b); leaf area (c); and specific leaf area (d). Columns represent the mean of five replicates, and bars represent the standard error. Means followed by the same letter do not differ by Tukey’s test (P ≤ 0.05). Capital letters compare water conditions within carnitine levels, and lowercase letters compare between carnitine and control within each water condition
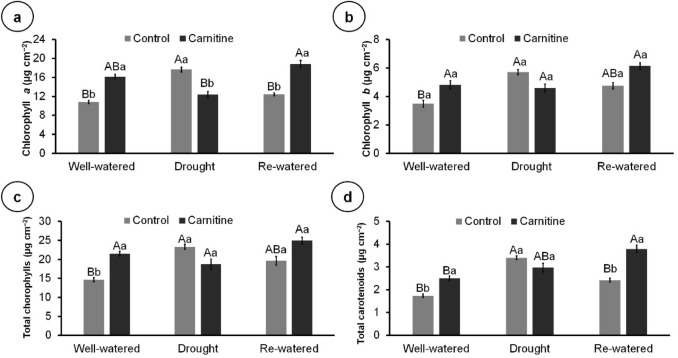
The application of carnitine increased the content of chlorophyll a, total chlorophylls, and total carotenoid in the well-watered plants, and chlorophyll a and total carotenoids in re-watered plants. In contrast, carnitine reduced chlorophyll a in drought stressed plants compared to the control (Fig. 3a–d).
Fig. 3.
Contents of photosynthetic pigments of 100-day-old Eryngium foetidum L. plants sprayed with water or carnitine, and grown under different water conditions. Chlorophyll a (a); Chlorophyll b (b); Total chlorophylls (c); and total carotenoids (d). Columns represent the mean of four replicates, and bars represent the standard error. Means followed by the same letter do not differ by Tukey’s test (P ≤ 0.05). Capital letters compare water conditions within carnitine levels, and lowercase letters compare between carnitine and control within each water condition
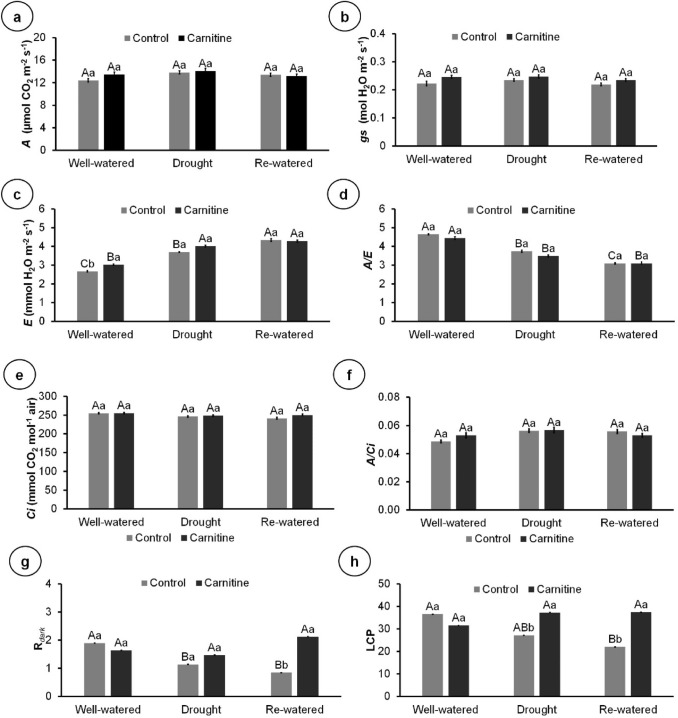
There was no difference among the treatments for carbon assimilation rate, stomatal conductance, internal CO2 concentration, and carboxylation efficiency (Fig. 4a–d). Carnitine application increased respiration in the dark in re-watered treatment, but not in well-watered and drought (Fig. 4e). Similarly, carnitine increased the LCP in re-watered and drought, but not in well-watered plants (Fig. 4f).
Fig. 4.
Gas exchange parameters of 100-day-old Eryngium foetidum L. plants sprayed with water or carnitine, and grown under different water conditions. A, net carbon assimilation rate (a); gs, stomatal conductance (b); E, evapotranspiration rate (c); A/E, water use efficiency (d); Ci, internal CO2 concentration (e); A/Ci, carboxylation efficiency (f); Rdark, dark respiration (g); and LCP, light compensation point (h). Columns represent the mean of four replicates, and bars represent the standard error. Means followed by the same letter do not differ by Tukey’s test (P ≤ 0.05). Capital letters compare water conditions within carnitine levels, and lowercase letters compare between carnitine and control within each water condition
The essential oil profile of culantro is modified by drought and applying carnitine
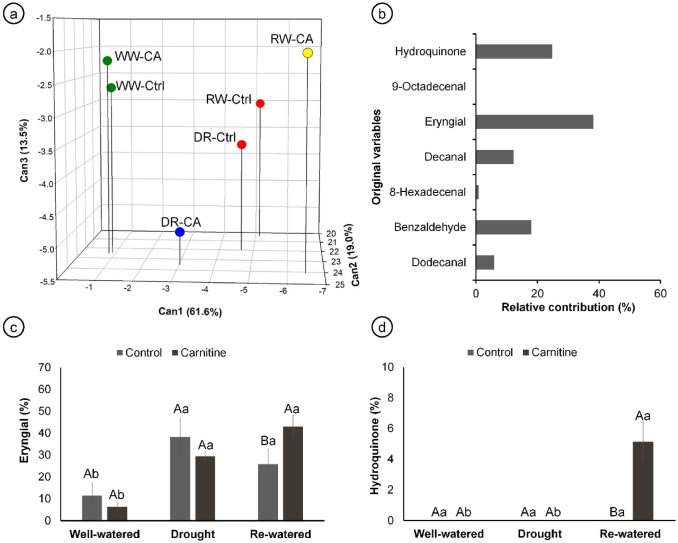
The water levels and the addition of carnitine significantly altered two of the main compounds detected: hydroquinone and eryngial (Online Resource 1, Supplementary Table 1). The first three canonical variables explained 94.1% of the variability among the treatments based on the essential oils profile, allowing for a three-dimensional scatter plot representation (Fig. 5a). The treatments were separated into four groups: group 1 (green circle), well-watered plants; group 2 (red circle), well-watered and drought control plants; group 3 (blue circle), drought plants with carnitine application; and group 4 (yellow circle) re-watered plants with carnitine application.
Fig. 5.
Essential oil profile of 102-day-old Eryngium foetidum L. plants sprayed with water or carnitine, and grown under different water conditions. 3D scatter-plot of the first three canonical variables (% total variance explained by each canonical component is indicated in parentheses; treatments indicated by the same color were assembled into the same group by the Tocher optimization method and the generalized squared interpoint distance of Mahalanobis; WW-Ctrl: well-watered + water application, WW-CA: well-watered + carnitine application, DR-Ctrl: drought + water application, DR-CA: drought + carnitine application, RW-Ctrl: re-watered + water application, RW-CA: re-watered + carnitine application) (a); Relative contributions of the original variables, calculated using the Singh method, to the canonical variables (b); production of eryngial (c); and production of hydroquinone (d). Means followed by the same letter do not differ by Tukey’s test (P ≤ 0.05). Capital letters compare among water conditions, and lowercase letters compare between carnitine and control within each water condition
The relative contributions of the original variables showed that eryngial and hydroquinone were the most prominent compounds, contributing 38.13% and 24.81, respectively, to the total variance (Fig. 5b). Drought and re-watering augmented eryngial content, compared to well-watered plants. In the re-watered, the application of carnitine even increased this compound (Fig. 5c). Hydroquinone, in turn, was only detected in re-watered plants treated with carnitine (Fig. 5d).
Discussion
Water is important for plant growth and food production; thus, it is essential to comprehend plant responses under drought conditions to increase crop production (Henschel et al. 2022). Here, the aerial part growth of culantro plants was impaired by drought and increased by re-watering, while root growth was increased by drought (Fig. 1). Drought-induced reductions in cell division and turgor are responsible for the decrease in leaf area (Tardieu et al. 2014); however, even under water stress, plants can maximize water uptake from the soil by increasing the root growth, which is one of the main survival strategies of plants under drought conditions. During periods of water scarcity, the root system undergoes morphological changes to enhance its ability to absorb water and nutrients, such us deeper roots due increased root growth rate and greater lateral root growth, and these modifications can be attributed to coordinated cell division, elongation, and differentiation events in the root apex (Lynch et al. 2018; Dinneny 2019; Gupta et al. 2020). Here, there probably were changes in cell division and differentiation, as evidenced by the increase in root length.
Re-watering increased the number of leaves and leaf area, which increased the photosynthetic capacity of plants, increasing the fresh and dry mass (Figs. 1, 2, 4). This increase in plant growth after a period of water stress followed by re-watering is an important plant survival strategy, since enhances its adaptation to drought (Sun et al. 2016; Gupta et al. 2020). Here, carnitine not increased root mass (Fig. 1), unlike reported by Lelandais-Brière et al. (2007) and Santos et al. (2022c), in which carnitine application increased root density and growth in Arabidopsis and culantro, respectively. Re-watering altered the shoot/root ratio of the plants compared to drought, leading to more biomass allocated to shoots than roots (Fig. 1). Rapid leaf growth after re-watering is essential to maximize light capture and, consequently, biomass accumulation (Xu and Zhou 2006; Toscano et al. 2014).
The fact that drought reduce leaf growth, resulting in a higher cell density per area (Ren et al. 2019), can explain the increase in pigment levels under this condition. Carnitine application increased the photosynthetic pigments content in well-watered and re-watered (Fig. 3), showing that this compound can act in the signaling of the synthesis pathways of these pigments within the chloroplast. Increased dark respiration in re-watered + carnitine (Fig. 4g) may have occurred due the transport of acyl-CoA to mitochondria during gluconeogenesis, which is mediated by carnitine. This process increases plant respiration, which can contribute to the recovery of growth and regeneration (Steiber et al. 2004). This higher respiration caused by carnitine application also increased LCP, indicating lower light use efficiency (Song et al. 2015).
Secondary metabolites are responsible for plant adaptation and survival, especially under unfavorable conditions (Takshak and Agrawal 2019). Plants modulate their secondary metabolism in response to stress factors, which can improve the quality of medicinal and aromatic plants (Szabó et al. 2017; Costa et al. 2020). Culantro is recognized by its aroma, given by the essential oils (Singh et al. 2014). Water stress induces changes in the profile of essential oils in culantro, and the application of carnitine in these plants under drought induced further qualitative changes in the composition of volatiles (Fig. 5a, b). The compounds that contributed the most to the difference among these profiles were eryngial and hydroquinone (Fig. 5b–d). Amino acids have been reported to alter the profile of secondary metabolites in plants, as demonstrated by Talaat et al. (2014), where the application of tyrosine and phenylalanine resulted in qualitative differences in the essential oils in Ammi visnaga. Amino acids participate in the synthesis of other compounds, such as proteins, vitamins, enzymes, and terpenoids. The canonical discriminant analysis showed that watering treatment and carnitine application interact to determine the essential oil profiles of the culantro plants (Fig. 5a). Martins et al. (2003) and Chandrika et al. (2015) found different profiles of essential oils in culantro depending on the geographic location where it was collected, showing that ambient conditions influence the composition of these compounds. Rodrigues et al. (2020) also reported that mineral fertilizer composition can change the essential oil composition in the leaves and roots of culantro.
Here, drought and re-watering increased the production of eryngial, which is the major component of culantro essential oil (Darriet et al. 2014; Thomas et al. 2017; Rodrigues et al. 2020). Eryngial is an aliphatic and aromatic aldehyde with antibacterial activity and responsible for the flavor and aroma of culantro and other plants (Abiko et al. 2020; Karakaya et al. 2020). In addition, it has anthelmintic, antibacterial, and anti-inflammatory activity, being characterized as a yellowish oil with a pungent odor (Paul et al. 2011; Forbes et al. 2014). Considering that aldehydes, such as eryngial, are derived from α- or β-oxidation of fatty acids (Bridgemohan et al. 2021), and that carnitine is involved in fatty acids catabolism (Bourdin et al. 2007), it may explain the increase in eryngial upon carnitine application found here.
Besides, in re-watered plants, carnitine induced the production of hydroquinone, which is an aromatic phenolic compound derivative from benzene (Cabrera-Alonso et al. 2019; Sun et al. 2021). Hydroquinone is used in the production of antioxidants, agrochemicals, and photographic paper (Jeyanthi et al. 2021). In the cosmetic industry, it is also used as a skin lightener and to treat hyperpigmentation, such as melasma, freckles, senile lentigines, and chloasma (Elferjani et al. 2017). Furthermore, it also shows antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa (Ma et al. 2019; Jeyanthi et al. 2021). Hydroquinone has been found in some plant species, such as Majorana hortensis, Arctostaphylos uva-ursi, Vaccinium vitis idaea, Pyrus communis, and Ecdysanthera rosea (Zhu et al. 2010; Rychlinska and Nowak 2012). However, here we report the presence of hydroquinone in culantro for the first time. It is noteworthy that this compound only appeared with the specific combination of re-watering with carnitine application. The carnitine metabolism in plants and its relation with secondary metabolites are still unclear, but here the application of this amino acid increased the production of eryngial and promoted the production of hydroquinone, indicating that this compound may be related to secondary metabolites pathways.
Conclusion
Culantro plants increase biomass when subjected to a period of drought followed by rehydration. Foliar application of carnitine leads to changes in the synthesis of photosynthetic pigments and essential oil profile, including the induction of hydroquinone production when this application is combined with re-watering. The application of exogenous carnitine can be a strategy to induce the production of essential oil compounds of cosmetic and pharmaceutical importance in culantro. However, more research is needed to understand the action of carnitine in the production of secondary metabolites in plants.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank A. M. Santos and S. M. Santos for kindly donating seeds for the experiments. We also acknowledge the National Council for Scientific and Technological Development (CNPq—Brazil), Research Support Foundation of the State of Paraíba/Federal University of Paraíba (FAPESQ/UFPB), Minas Gerais State Research Foundation (FAPEMIG), and Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarships granted to students.
Author contributions
SKS, JMH, and DSB designed the study; SKS, DSG, AFPO, AMOS, VSM, MHAG, EMM, and RMG performed the experiments and the analyses; SKS, EMM, JMH, and DSB analyzed the data; SKS, EMM, LFV, RMG, JMH, and DSB wrote the article with input from all other authors. All authors read and approved the manuscript.
Funding
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasília, DF, Brazil: Grants no. PQ 304214/2022-1 to DSB and 301858/2023-3 to JMH], Fundação de Apoio à Pesquisa do Estado da Paraíba/Universidade Federal da Paraíba (FAPESQ/UFPB), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), and the Public Call n. 03 Produtividade em Pesquisa PROPESQ/PRPG/UFPB [Grant Proposal code PVO13257-2020 to DSB].
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
References
- Abiko Y, Okada M, Aoki H, Mizokawa M, Kumagai Y. A strategy for repression of arsenic toxicity through nuclear factor E2 related factor 2 activation mediated by the (E)-2-alkenals in Coriandrum sativum L. leaf extract. Food Chem Toxicol. 2020;145:111706. doi: 10.1016/j.fct.2020.111706. [DOI] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Ami K, Planchais S, Cabassa C, et al. Different proline responses of two Algerian durum wheat cultivars to in vitro salt stress. Acta Physiol Plant. 2020;42:21. doi: 10.1007/s11738-019-3004-9. [DOI] [Google Scholar]
- Barr HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust J Biol Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- Bhavana GP, Chandrika R, Thara Saraswathi KJ. Quantitative determination of secondary compounds in populations of Eryngium foetidum L. from India. Int J Curr Sci. 2013;10:1–5. [Google Scholar]
- Böttger A, Vothknecht U, Bolle C, Wolf A. Plant secondary metabolites and their general function in plants. In: Böttger A, Vothknecht U, Bolle C, Wolf A, editors. Lessons on Caffeine, Cannabis & Co. Learning Materials in Biosciences. Cham: Springer; 2018. p. 3:17. [Google Scholar]
- Bourdin B, Adenier H, Perrin Y. Carnitine is associated with fatty acid metabolism in plants. Plant Physiol Biochem. 2007;45:926–931. doi: 10.1016/j.plaphy.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Bridgemohan P, Bridgemohan RS, Mohammed M, Deitch M, Sarran H. Comparative analysis of the odorants of cilantro (Coriandrum sativum L.) and culantro (Eryngium foetidum L.) as aromatic crop mimics of family: Apiaceae. J Hortic Postharvest Res. 2021;4:479–496. doi: 10.22077/JHPR.2021.4260.1203. [DOI] [Google Scholar]
- Cabrera-Alonso R, Guevara E, Ramírez-Elías MG, Moncada B, González FJ. Surface-enhanced Raman scattering of hydroquinone assisted by gold nanorods. J Nanophotonics. 2019;13:036006. doi: 10.1117/1.JNP.13.036006. [DOI] [Google Scholar]
- Castro KM, Batista DS, Silva TD, et al. Water deficit modulates growth, morphology, and the essential oil profile in Lippia alba L. (Verbenaceae) grown in vitro. Plant Cell Tissue Organ Cult. 2020;141:55–65. doi: 10.1007/s11240-020-01766-w. [DOI] [Google Scholar]
- Chandrika R, Saraswathi KJT, Mallavarapu GR. Constituent of the essential oils of the leaf and root of Eryngium foetidum L. from two locations in India. J Essent Oil Bear Plants. 2015;18:349–358. doi: 10.1080/0972060X.2014.960277. [DOI] [Google Scholar]
- Charrier A, Rippa S, Yu A, et al. The effect of carnitine on Arabidopsis development and recovery in salt stress conditions. Planta. 2012;235:123–135. doi: 10.1007/s00425-011-1499-4. [DOI] [PubMed] [Google Scholar]
- Costa ASV, Hott MC, Horn AH. Management of citronella (Cymbopogon winterianus Jowitt ex Bor) for the production of essential oils. SN Applied Sci. 2020;2:1–7. doi: 10.1007/s42452-020-03949-8. [DOI] [Google Scholar]
- Cruz CD. Genes Software-extended and integrated with the R, MATLAB and Selegen. Acta Sci Agron. 2016;38:547–552. doi: 10.4025/actasciagron.v38i3.32629. [DOI] [Google Scholar]
- Darriet F, Andreani S, Cian MC, Costa J, Muselli A. Chemical variability and antioxidant activity of Eryngium maritimum L. essential oils from Corsica and Sardinia Flavour. Fragr J activity of Eryngium maritimum L. essential oils from Corsica and Sardinia. Flavour Fragr J. 2014;29:3–13. doi: 10.1002/ffj.3160. [DOI] [Google Scholar]
- Dinneny JR. Developmental responses to water and salinity in root systems. Annu Rev Cell Dev Biol. 2019;35:239–257. doi: 10.1146/annurev-cellbio-100617-062949. [DOI] [PubMed] [Google Scholar]
- Elferjani HS, Ahmida NH, Ahmida A. Determination of hydroquinone in some pharmaceutical and cosmetic preparations by spectrophotometric method. Int J Sci Res Publ. 2017;6:2219–2224. doi: 10.21275/ART20175678. [DOI] [Google Scholar]
- Forbes WM, Gallimore WA, Mansingh A, Reese PB, Robinson RD. Eryngial (trans-2-dodecenal), a bioactive compound from Eryngium foetidum: its identification, chemical isolation, characterization and comparison with ivermectin in vitro. Parasitology. 2014;141:269–278. doi: 10.1017/s003118201300156x. [DOI] [PubMed] [Google Scholar]
- Frank JA, Moroni M, Moshourab R, et al. Photoswitchable fatty acids enable optical control of TRPV1. Nat Commun. 2015;6:1–11. doi: 10.1038/ncomms8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Rico-Medina A, Caño-Delgado AI. The physiology of plant responses to drought. Science. 2020;368:266–269. doi: 10.1126/science.aaz7614. [DOI] [PubMed] [Google Scholar]
- Henschel JM, Dantas EFO, Soares VA, et al. Salicylic acid mitigates the effects of mild drought stress on radish (Raphanus sativus) growth. Funct Plant Biol. 2022;49:822–831. doi: 10.1071/FP22040. [DOI] [PubMed] [Google Scholar]
- Henschel JM, Dantas EFO, Azevedo-Soares V, et al. Drought stress mitigation by foliar application of L-carnitine and its effect on radish morphophysiology. Physiol Mol Biol Plants. 2023 doi: 10.1007/s12298-023-01308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo BE, Duarte E, Martelo I. Volatile chemical composition of the essential oil from Colombian Eryngium foetidum L. and determination of its antioxidant activity. Rev Cuba De Plantas Medicinales. 2011;16:140–150. [Google Scholar]
- Jeyanthi V, Velusamy P, Kumar GV, Kiruba K. Effect of naturally isolated hydroquinone in disturbing the cell membrane integrity of Pseudomonas aeruginosa MTCC 741 and Staphylococcus aureus MTCC 740. Heliyon. 2021;7:e07021. doi: 10.1016/j.heliyon.2021.e07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaya S, Özdemir Ö, Koca M, et al. Cytotoxic effect and molecular docking studies of essential oils of Cymbocarpum erythraeum (DC.) Boiss. (Apiaceae) as potential inhibitors of cholinesterase. J Essent Oil Res. 2020;32:436–448. doi: 10.1080/10412905.2020.1787884. [DOI] [Google Scholar]
- Kulak M, Ozkan A, Bindak R. A bibliometric analysis of the essential oil-bearing plants exposed to the water stress: how long way we have come and how much further? Sci Hortic. 2019;246:418–436. doi: 10.1016/j.scienta.2018.11.031. [DOI] [Google Scholar]
- Lelandais-Brière C, Jovanovic M, Torres GAM, et al. Disruption of AtOCT1, an organic cation transporter gene, affects root development and carnitine-related responses in Arabidopsis. Plant J. 2007;51:154–164. doi: 10.1111/j.1365-313X.2007.03131.x. [DOI] [PubMed] [Google Scholar]
- Liang G, Bu J, Zhang S, et al. Effects of drought stress on the photosynthetic physiological parameters of Populus× euramericana “Neva”. J for Res. 2019;30:409–416. doi: 10.1007/s11676-018-0667-9. [DOI] [Google Scholar]
- Lynch JP. Rightsizing root phenotypes for drought resistance. J Exp Bot. 2018;69:3279–3792. doi: 10.1093/jxb/ery048. [DOI] [PubMed] [Google Scholar]
- Ma C, He N, Zhao Y, et al. Antimicrobial mechanism of hydroquinone. Appl Biochem Biotechnol. 2019;189:1291–1303. doi: 10.1007/s12010-019-03067-1. [DOI] [PubMed] [Google Scholar]
- Mabeku LBK, Bille BE, Nguepi E. In vitro and in vivo anti-helicobacter activities of Eryngium foetidum (Apiaceae), Bidens pilosa (Asteraceae), and Galinsoga ciliata (Asteraceae) against Helicobacter pylori. BioMed Res Int. 2016 doi: 10.1155/2016/2171032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AP, Salgueiro LR, Cunha AP, et al. Essential oil composition of Eryngium foetidum from S. Tome e Príncipe. J Essent Oil Res. 2003;15:93–95. doi: 10.1080/10412905.2003.9712077. [DOI] [Google Scholar]
- Nargesi M, Sedaghathoor SM, Hashemabadi DS. Effect of arginine, glutamine, humic acid, and fulvic acid spraying on olive cultivars in saline conditions. Plant Physiol Rep. 2022;27:295–307. doi: 10.1007/s40502-022-00661-0. [DOI] [Google Scholar]
- Oney-Birol S. Exogenous L-carnitine promotes plant growth and cell division by mitigating genotoxic damage of salt stress. Sci Rep. 2019;9:17229. doi: 10.1038/s41598-019-53542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul JH, Seaforth CE, Tikasingh T. Eryngium foetidum L., a review. Fitoterapia. 2010;82:302–308. doi: 10.1016/j.fitote.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Quynh CTT, Kubota K. Aroma constituents and enzyme activities of Japanese long coriander leaves (culantro, Eryngium foetidum L.) Food Sci Technol. 2012;18:287–294. doi: 10.3136/fstr.18.287. [DOI] [Google Scholar]
- Ren T, Weraduwage SM, Sharkey TD. Prospects for enhancing leaf photosynthetic capacity by manipulating mesophyll cell morphology. J Exp Bot. 2019;70:1153–1165. doi: 10.1093/jxb/ery448. [DOI] [PubMed] [Google Scholar]
- Rodrigues TLM, Castro GLS, Viana RG, et al. Physiological performance and chemical compositions of the Eryngium foetidum L. (Apiaceae) essential oil cultivated with different fertilizer sources. Nat Prod Res. 2020;35:5544–5548. doi: 10.1080/14786419.2020.1795653. [DOI] [PubMed] [Google Scholar]
- Rodrigues TLM, Silva ME, Gurgel ES, et al. Eryngium Foetidum L. (Apiaceae): a literature review of traditional uses, chemical composition, and pharmacological activities. Evid-Based Complementary Altern Med. 2022 doi: 10.1155/2022/2896895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Silva P, Graziose R, Vesely B, et al. Leishmanicidal activity of a daucane sesquiterpene isolated from Eryngium foetidum. Pharm Biol. 2014;52:398–401. doi: 10.3109/13880209.2013.837077. [DOI] [PubMed] [Google Scholar]
- Rychlinska I, Nowak S. Quantitative determination of arbutin and hydroquinone in different plant materials by HPLC. Not Bot Horti Agrobot. 2012;40:09–113. doi: 10.15835/nbha4027987. [DOI] [Google Scholar]
- Sai SKPV, Sandya V, Manjari S, Ali S. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Santos RP, Cruz ACF, Iarema L, Kuki KN, Otoni WC. Protocolo para extração de pigmentos foliares em porta-enxertos de videira micropropagados. Rev Ceres. 2008;55:356–364. [Google Scholar]
- Santos A, Melo Y, Oliveira L, et al. Exogenous silicon and proline modulate osmoprotection and antioxidant activity in cowpea under drought stress. J Soil Sci Plant Nutr. 2022;22:1692–1699. doi: 10.1007/s42729-022-00764-5. [DOI] [Google Scholar]
- Santos SK, Gomes DS, Santos LWO, et al. Exogenous carnitine mitigates the deleterious effects of mild-water stress on arugula by modulating morphophysiological responses. J Plant Growth Regul. 2022 doi: 10.1007/s00344-022-10868-y. [DOI] [Google Scholar]
- Santos SK, Soares VA, Dantas EFO, et al. Exogenous carnitine application enhances the growth of culantro (Eryngium foetidum) plants. Vegetos. 2022 doi: 10.1007/s42535-022-00438-8. [DOI] [Google Scholar]
- Shavandi MA, Haddadian Z, Ismail MHS. Eryngium foetidum L., Coriandrum sativum and Persicaria ordorata L.: a review. J Asian Sci Res. 2012;2:410–426. [Google Scholar]
- Singh D. The relative importance of characters affecting genetic divergence. Indian J Genet Plant Breed. 1981;41:237–245. [Google Scholar]
- Singh S, Singh DR, Banu S, Salim KM. Determination of bioactives and antioxidant activity in Eryngium foetidum L.: a traditional culinary and medicinal herb. Proc Natl Acad Sci India Sect b Biol Sci. 2013;83:453–460. doi: 10.1007/s40011-012-0141-y. [DOI] [Google Scholar]
- Singh BK, Ramakrishna Y, Ngachan SV. Spiny coriander (Eryngium foetidum L.): a commonly used, neglected spicing-culinary herb of Mizoram, India. Genet Resour Crop Evol. 2014;61:1085–1090. doi: 10.1007/s10722-014-0130-5. [DOI] [Google Scholar]
- Soares VA, Dantas EFO, Santos SK, et al. Effect of salicylic acid on the growth and biomass partitioning in water-stressed radish plants. Vegetos. 2022 doi: 10.1007/s42535-022-00358-7. [DOI] [Google Scholar]
- Song L, Zhang YJ, Chen X, et al. Water relations and gas exchange of fan bryophytes and their adaptations to microhabitats in an Asian subtropical montane cloud forest. J Plant Res. 2015;128:573–584. doi: 10.1007/s10265-015-0721-z. [DOI] [PubMed] [Google Scholar]
- Steiber A, Kerner J, Hoppel CL. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med. 2004;25:455–473. doi: 10.1016/j.mam.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Sun C, Gao X, Chen X, Fu J, Zhang Y. Metabolic and growth responses of maize to successive drought and re-watering cycles. Agric Water Manag. 2016;172:62–73. doi: 10.1016/j.agwat.2016.04.016. [DOI] [Google Scholar]
- Sun X, Kolling DR, Smythers AL, Deal RA. Investigations of the photochemical charge-transfer reduction of uranyl UO22+ (VI) to uranyl UO2+ (V) by benzene-1, 4-diol (1,4–C6H4(OH) 2) and oxalate (C2O42−) by UV–Vis, electron paramagnetic resonance, and luminescence spectroscopies. Inorganica Chim Acta. 2021;525:120451. doi: 10.1016/j.ica.2021.120451. [DOI] [Google Scholar]
- Szabó K, Radácsi P, Rajhárt P, Ladányi M, Németh É. Stress-induced changes of growth, yield and bioactive compounds in lemon balm cultivars. Plant Physiol Biochem. 2017;119:170–177. doi: 10.1016/j.plaphy.2017.07.019. [DOI] [PubMed] [Google Scholar]
- Takshak S, Agrawal SB. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J Photochem Photobiol B. 2019;193:51–88. doi: 10.1016/j.jphotobiol.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Talaat IM, Khattab HI, Ahmed AM. Changes in growth, hormones levels and essential oil content of Ammi visnaga L. plants treated with some bioregulators. Saudi J Biol Sci. 2014;21:355–365. doi: 10.1016/j.sjbs.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F, Parent B, Caldeira CF, Welcker C. Genetic and physiological controls of growth under water deficit. Plant Physiol. 2014;164:1628–2163. doi: 10.1104/pp.113.233353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PS, Essien EE, Ntuk SJ, Choudhary MI. Eryngium foetidum L. essential oils: chemical composition and antioxidant capacity. Medicines. 2017;164:1628–1635. doi: 10.1104/pp.113.233353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano S, Scuderi D, Giuffrida F, Romano D. Responses of Mediterranean ornamental shrubs to drought stress and recovery. Sci Hortic. 2014;178:145–153. doi: 10.1016/j.scienta.2014.08.014. [DOI] [Google Scholar]
- Turk H, Erdal S, Dumlupinar R. Exogenous carnitine application augments transport of fatty acids into mitochondria and stimulates mitochondrial respiration in maize seedlings grown under normal and cold conditions. Cryobiology. 2019;91:97–103. doi: 10.1016/j.cryobiol.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Turk H, Erdal S, Dumlupinar R. Carnitine-induced physio-biochemical and molecular alterations in maize seedlings in response to cold stress. Arch Agron Soil Sci. 2019;66:925–941. doi: 10.1080/03650340.2019.1647336. [DOI] [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. 2015;2:251–286. doi: 10.3390/medicines2030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Zhou GS. Nitrogen metabolism and photosynthesis in Leymus chinensis in response to long-term soil drought. J Plant Growth Regul. 2006;25:252–266. doi: 10.1007/s00344-006-0043-4. [DOI] [Google Scholar]
- Zhu X, Zhang Q, Kong L, Wang F, Luo S. New hydroquinone diglycoside acyl esters and sesquiterpene and apocarotenoid from Ecdysanthera rosea. Fitoterapia. 2010;81:906–909. doi: 10.1016/j.fitote.2010.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.