Abstract
Objectives:
Compared to adult patients undergoing thyroid surgery, pediatric patients have higher rates of hypoparathyroidism often related to parathyroid gland (PG) inadvertent injury or devascularization. Previous studies have shown near-infrared-autofluorescence (NIRAF) can be reliably used intraoperatively for label-free parathyroid identification, but all prior studies have been performed in adult patients. In this study, we assess the utility and accuracy of NIRAF with a fiber-optic probe-based system to identify PGs in pediatric patients undergoing thyroidectomy or parathyroidectomy.
Methods:
All pediatric patients (under 18 years of age) undergoing thyroidectomy or parathyroidectomy were enrolled in this IRB-approved study. The surgeon’s visual assessment of tissues was first noted and the surgeon’s confidence level in the tissue identified was recorded. A fiber-optic probe was then used to illuminate tissues-of-interest with a wavelength of 785 nm and resulting NIRAF intensities from these tissues were measured while surgeon was blinded to results.
Results:
NIRAF intensities were measured intraoperatively in 19 pediatric patients. Normalized NIRAF intensities for PGs (3.63 ± 2.47) were significantly higher than that of thyroid (0.99 ± 0.36, p<0.001) and other surrounding soft tissues (0.86 ± 0.40, p<0.001). Based on the PG identification ratio threshold of 1.2, NIRAF yielded a detection rate of 95.8% (46/48 pediatric PGs)
Conclusion:
Our findings indicate that NIRAF detection can potentially be a valuable and non-invasive technique to identify PGs during neck operations in the pediatric population. To our knowledge, this is the first study in children to assess the accuracy of probe-based NIRAF detection for intraoperative parathyroid identification.
Keywords: parathyroid, autofluorescence, infrared, thyroid, pediatric, adolescent
Lay summary:
Parathyroid glands have been shown to have near infrared auto-fluorescence that is different than surrounding tissues. This study shows the accuracy and efficacy of using near infrared autofluorescence in pediatric patients is similar to the adult population.
Introduction:
Pediatric patients undergoing thyroidectomy are known to have higher rates of endocrine-specific complications compared to adults1. Hypocalcemia is the most common post-operative complication in children undergoing thyroid or parathyroid surgery.2,3 The incidence of transient hypocalcemia (< 6 months) can range between 6 – 47% following pediatric thyroidectomy2,4–10, while permanent hypoparathyroidism can occur in 0.6% – 8% of the cases.11,12 One of the underlying reasons for higher postsurgical hypocalcemia rates in children may be attributed to inadvertent PG injury during the dissection, or unintentional resection, compounded by the difficulty in visualizing diminutive PGs in the neck of children. Moreover the ability to visually identify and confirm PGs in the neck of children is highly subjective and depends on the surgeon’s experience thereby mandating expertise of skilled, high-volume pediatric and head and neck surgeons.13 Given the more daunting challenges in visualizing PGs in children and associated higher rates of postsurgical complications14, there is a need for an objective real-time tool that can guide surgeons for intraoperative PG identification and preservation during pediatric neck operations.
Several light-based (optical) techniques that are noninvasive and provide real-time results have been explored for its feasibility in guiding surgeons identify PGs intra-operatively. However, most of these technologies are technically complex and challenging to translate for routine use in most operating rooms.15,16 These options include optical coherence tomography, dynamic optical contrast imaging, and Raman spectroscopy.15,16 Fluorescence-based techniques on the other hand are simpler and less expensive, and have been shown to have high sensitivity.17–21 Fluorescence occurs in tissues when it has a ‘fluorophore’ that occurs intrinsically (naturally occurring in the target tissue) or is extrinsically introduced (externally synthesized and injected into the patient).16 When a tissue has intrinsic fluorophores, it is deemed ‘autofluorescent’ and detection of this autofluorescence can be performed as a “label-free” technique, eliminating the need to inject contrast agents or targeted-labels into the patient.
In 2011, PGs were demonstrated to exhibit increased near-infrared autofluorescence (NIRAF) compared to other soft tissues in the neck including thyroid, fat, muscle, and trachea.17 Subsequently, several NIRAF detection modalities were developed or investigated for non-invasive label-free PG identification with accuracies as high as 97%.18–21 In 2018, the Food and Drug Administration granted clearance for two NIRAF detection devices – Fluobeam (a handheld camera-based system) and PTeye (a handheld fiber-optic probe-based system) – as adjunct modalities to guide intraoperative PG identification for patients over 18 years of age.16,22,23 In addition, the probe-based modality provides real-time visual and auditory feedback when the fiber-optic probe touches a suspected PG.16,24 Most intraoperative imaging systems require the operating room lights to be off, which can interrupt surgical flow and increase operating room time. In comparison, the probe-based system can measure tissue NIRAF with operating room lights on and provides real-time auditory feedback similar to intraoperative contact-based neuromonitoring devices, which are already being used in many thyroid and parathyroid procedures.
NIRAF detection devices have been studied extensively in adult populations15,16,25,26, but their utility in pediatric populations has not been fully studied. In this study we seek to evaluate the ability of probe-based NIRAF detection to identify PGs in pediatric patients undergoing thyroid or parathyroid operations.
Materials and Methods:
Following approval from Vanderbilt University Medical Center IRB, written informed assent with guardian consent was obtained from all pediatric patients and guardians (<18 years) undergoing thyroid or parathyroid procedures in this study. These procedures included (i) parathyroidectomy and (ii) thyroidectomy including thyroid lobectomy, total thyroidectomy, total thyroidectomy with central neck lymph node dissection, and total thyroidectomy with central and modified lateral neck dissection.
As described earlier, the NIRAF detection device used in this study consists of (i) a display console that encloses a 785 nm laser source with other relevant circuitry, (ii) a research-grade hand-held sterile fiber-optic probe and (iii) a foot-pedal for activating the laser source for tissue measurements (Figure 1A).24 The fiber-optic probe is used to interrogate tissues suspected to be PGs as depicted in Figure 1B. For each patient, a baseline fluorescence measurement was first obtained from 5 different sites of the thyroid gland. Subsequently for each interrogated tissues, ‘detection ratios’ were generated in real-time (Figure 1C) by normalizing the measured NIRAF intensity to the baseline thyroid fluorescence. A detection ratio ≥ 1.2 is classified as PG by the device, which has been based on previous data and investigations.19,24
Figure 1:
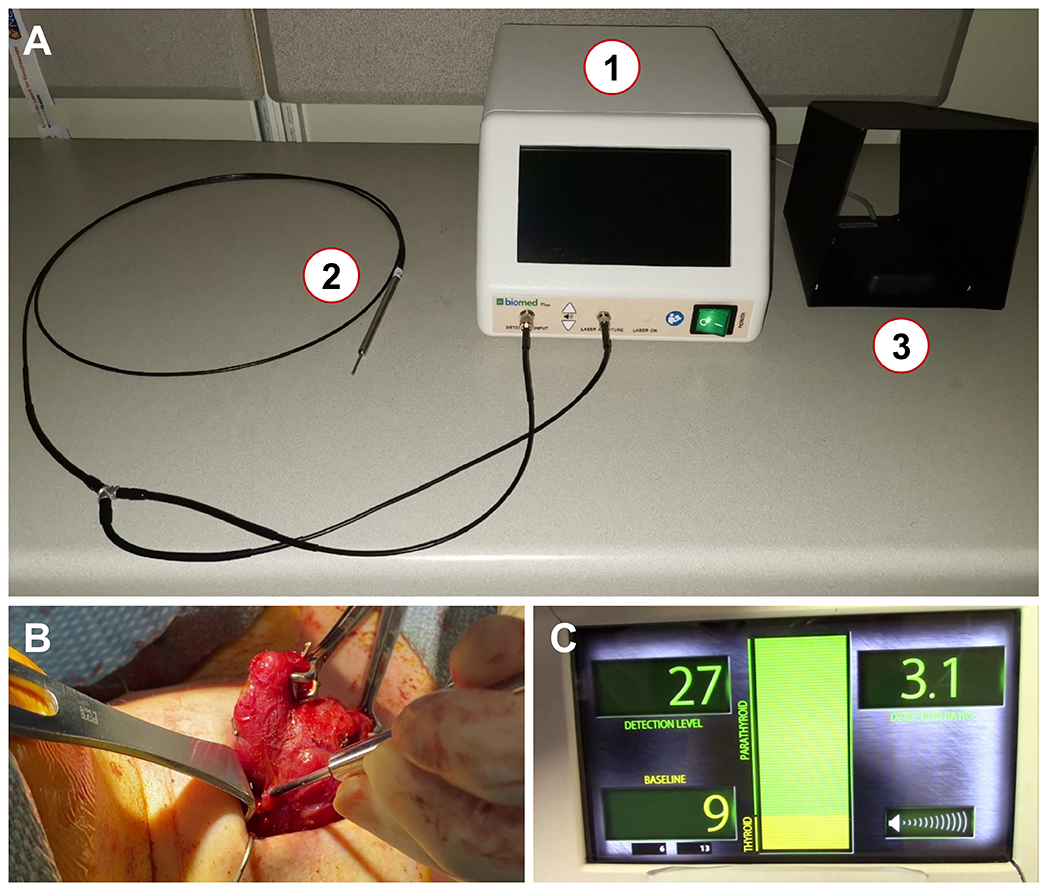
(A) The probe-based near infrared autofluorescence (NIRAF) detection modality (PTeye™) consists of (1) a console that encloses a near infrared (NIR) laser, a photodiode detector and a display that provides information pertaining to tissue NIRAF, (2) a research-grade reusable and sterilizable probe and (3) a foot-pedal to activate the NIR laser for tissue measurements. (B) To measure tissue NIRAF for parathyroid identification, the sterile probe is placed by the surgeon on tissue of interest. (C) The display provides information to the end-user as ‘Detection Level’ – Tissue NIRAF levels, ‘Baseline’ – Baseline NIRAF established by NIRAF measurements obtained on five sites on the thyroid or strap neck muscle (if thyroid is absent), and ‘Detection Ratio’ – Tissue NIRAF normalized to the thyroid ‘Baseline’. A tissue of interest is deemed as ‘parathyroid’ by the device, if the ‘Detection Ratio’ exceeds a value of 1.2, based on a threshold established in the adult population from earlier studies. A Detection Ratio of 3.1 as observed in (C) indicates that the tissue interrogated by the surgeon in (B) has been classified as ‘parathyroid’ by the device.
To quantify performance accuracy of this device, surgeons identified tissues they believed to be PGs along with their level of confidence (recorded as high, medium or low) based on their visual examination. After the surgeon’s confidence level was noted, the device was used to interrogate the tissue and the corresponding detection ratio for the assessed tissue was recorded. The gold standard for tissue validation is histopathology which includes (i) frozen section histology or (ii) permanent histology for excised specimens. If this was not available intraoperatively or post-operatively, the surgeon’s expert opinion was alternatively used for the corroborating identity of the in-situ tissues investigated with the NIRAF modality. While a surgeon’s expert opinion can be subjective and error-prone, it is currently the only option available to verify the identity of in-situ healthy PGs that are typically left intact and are not meant to be biopsied.27 Several earlier studies have already relied on this methodology to validate the performance accuracy of NIRAF detection in adult populations.24,27,28 Therefore for this study, it was not considered a viable proposition to invasively biopsy all healthy PGs for histopathological validation, particularly during pediatric thyroidectomies that carry a higher risk of postsurgical hypocalcemia.1,14 It must further be noted that the surgeon’s expert opinion was considered as gold standard for in-situ tissues only if the surgeon’s confidence was recorded as high (>75% confidence) or medium (50%-75% confidence).27 Tissues identified with ‘low’ confidence (<50% confidence) by the surgeon without corroborating histopathological validation, were excluded from further data analysis.
For this particular study, the surgeon was blinded to the measured NIRAF intensities through the procedure. As the surgeon remained ‘blinded’ to the device output, post hoc interpretation of the device output for each case was categorized as true positive (TP), false positive (FP), true negative (TN), or false negative (FN). Determination of TP, FP, TN, or FN based on the relevant gold standards for in situ or excised tissues in the neck has been described in supplemental Table A. The number of PGs seen by surgeon per patient was expressed as median and corresponding inter-quartile range (IQR). An unpaired two-tailed Student t-test of unequal variance was used to compare NIRAF intensities of various tissues (expressed as mean ± standard deviation), where a p-value < 0.05 was considered significant.
Results:
Nineteen pediatric patients were enrolled in this study with median age of 13 years (Range: 6 – 16 years). Indications for surgery included indeterminate thyroid nodule(s), well-differentiated thyroid cancer, Graves’ disease, hypothyroidism, prophylactic thyroidectomy for MEN2A syndrome and primary hyperparathyroidism. The demographic and clinicopathologic variables for all 19 patients are summarized in Table 1. Depending on the IRB classification of assent in pediatric population based on age, we classified our pediatric population for further analysis into two groups: (i) above 12 years (Age range: 13 – 16 years; n = 11 patients) and (ii) 12 years or below (Age Range: 6 – 12 years; n = 8 patients).
Table 1:
Demographic and clinicopathologic variables of the 19 pediatric patients enrolled in this study (BMI – Body Mass Index, C – Caucasian, AA – African American, MEN2A – Multiple Endocrine Neoplasia 2 syndrome).
| Patient | Gender | Age (years) | Race | BMI (kg/m2) | Disease | Procedure |
|---|---|---|---|---|---|---|
| 1 | Male | 16 | C | 19.9 | Graves’ disease | Total thyroidectomy |
| 2 | Female | 14 | C | 32.6 | Hypothyroidism | Total thyroidectomy |
| 3 | Male | 12 | C | 20.4 | Graves’ disease | Completion thyroidectomy |
| 4 | Female | 12 | C | 17.1 | Papillary thyroid carcinoma with metastasis | Total thyroidectomy with lymph node dissection |
| 5 | Female | 6 | AA | 21.9 | Hyperthyroidism | Total thyroidectomy |
| 6 | Male | 6 | C | 17.7 | MEN2A | Prophylactic total thyroidectomy |
| 7 | Female | 11 | C | 29.7 | Graves’ disease | Total thyroidectomy |
| 8 | Female | 14 | C | 39.1 | Thyroid nodule; Hyperthyroidism | Thyroid lobectomy |
| 9 | Male | 16 | AA | 32.3 | Papillary thyroid carcinoma with metastasis | Total Thyroidectomy with lymph node dissection |
| 10 | Female | 13 | C | 39.1 | Graves’ disease | Total thyroidectomy |
| 11 | Female | 14 | C | 16.4 | Graves’ disease | Total thyroidectomy |
| 12 | Female | 16 | C | 22.9 | Thyroid nodule | Thyroid lobectomy |
| 13 | Male | 13 | AA | 38.2 | Papillary thyroid carcinoma with metastasis | Completion thyroidectomy |
| 14 | Female | 14 | C | 22.2 | Hyperthyroidism | Total thyroidectomy |
| 15 | Male | 10 | C | 18.2 | Thyroid nodule | Thyroid lobectomy |
| 16 | Female | 16 | C | 22 | Multinodular Goiter | Total thyroidectomy |
| 17 | Female | 12 | C | 19.9 | Hyperthyroidism | Total thyroidectomy |
| 18 | Male | 14 | C | 41.5 | Primary Hyperparathyroidism | Parathyroidectomy |
| 19 | Female | 8 | C | 17.5 | Thyroid nodule | Thyroid lobectomy |
Forty-eight tissues believed to be PGs were interrogated using the device. A median of 3 PGs were visualized per patient for interrogation by the surgeon (IQR: 2 – 3 PGs per patient). The number of PGs visualized by surgeons in children over 12 years of age were 29 PGs (Median: 3 PGs per patient, IQR: 2 – 3.5 PGs per patient). For children 12 years of age or under, surgeons were able to visualize 19 PGs (Median: 2 PGs per patient, IQR: 2 – 3 PGs per patient). As illustrated in Figure 2, mean normalized NIRAF intensities for PGs (3.63 ± 2.47) in the overall dataset were significantly higher than the thyroid (0.99 ± 0.36, p<0.001) and other surrounding tissues-fat, muscle, lymph node, thymus (0.86 ± 0.40, p<0.001). Normalized NIRAF intensities for PGs were consistently higher than non-parathyroid tissues across all patients (Figure 3), except in Patient #12, where no PGs were identified. Patient #18 with primary hyperparathyroidism exhibited high NIRAF only in the healthy ‘cap’ of the diseased PG, with negligible NIRAF in the adenomatous areas (Figure 4A, 4C). We also observed that normalized NIRAF for PGs for children below 12 years were significantly lower at 2.78 ± 1.20, compared to PGs for children above 12 years which was 4.03 ± 2.80 (p=0.006).
Figure 2:
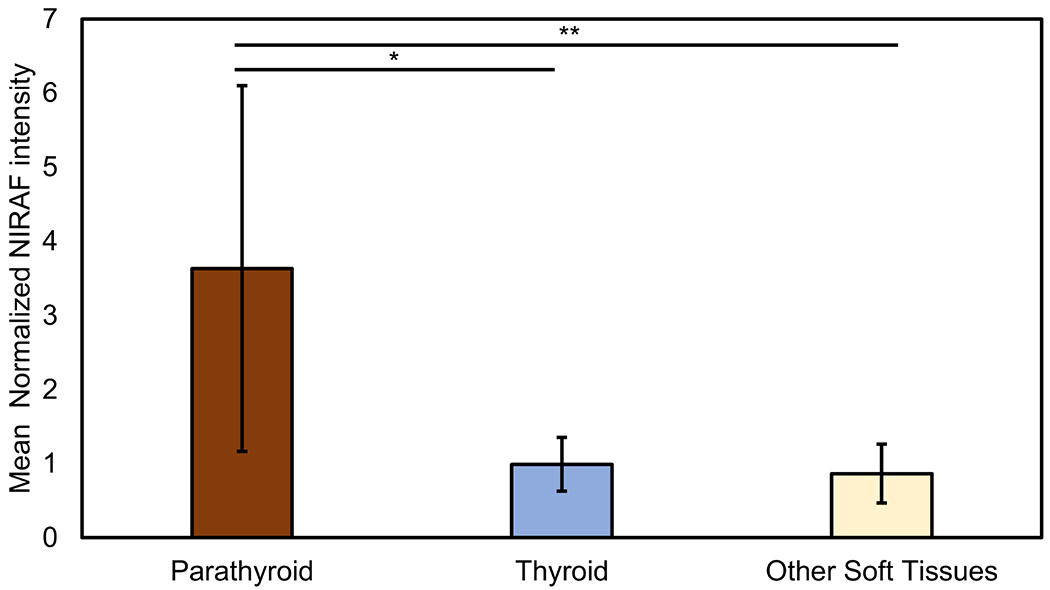
Comparison of near infrared autofluorescence (NIRAF) intensities (normalized to thyroid NIRAF) among various tissues in the neck – parathyroid, thyroid and other soft tissues – for the enrolled pediatric cohort. (Other soft tissues – fat, muscle, trachea, thymus, lymph nodes). For each category, the bar plots and corresponding error bars represent the mean and standard deviation of the calculated values. *p-value < 0.05, parathyroid versus thyroid NIRAF, **p-value < 0.05, parathyroid versus other soft tissue NIRAF.
Figure 3:
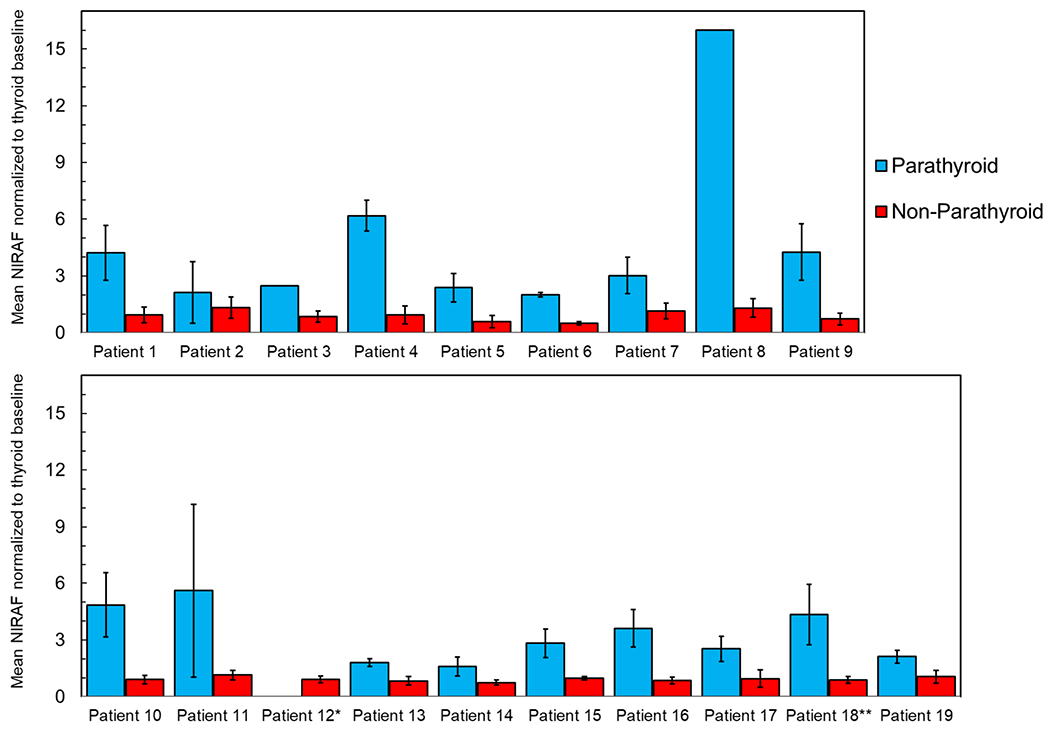
Inter-patient and intra-patient variability of tissue near infrared autofluorescence (NIRAF) measured in-situ from parathyroid and non-parathyroid tissues in a pilot cohort of 19 pediatric patients. For each patient, the bar plots and corresponding error bars represent the mean and standard deviation of ‘tissue NIRAF normalized to the thyroid baseline’ in that patient (same as the Detection Ratio described in Figure 1). Parathyroid tissues exhibited consistently higher NIRAF levels than non-parathyroid tissues, except in Patient 12. *No PGs was seen by surgeon during the procedure in Patient 12. **Only NIRAF from the healthy PGs in Patient 18 was included in the data analysis, while NIRAF from the diseased parathyroid adenoma in the same patient was excluded.
Figure 4:
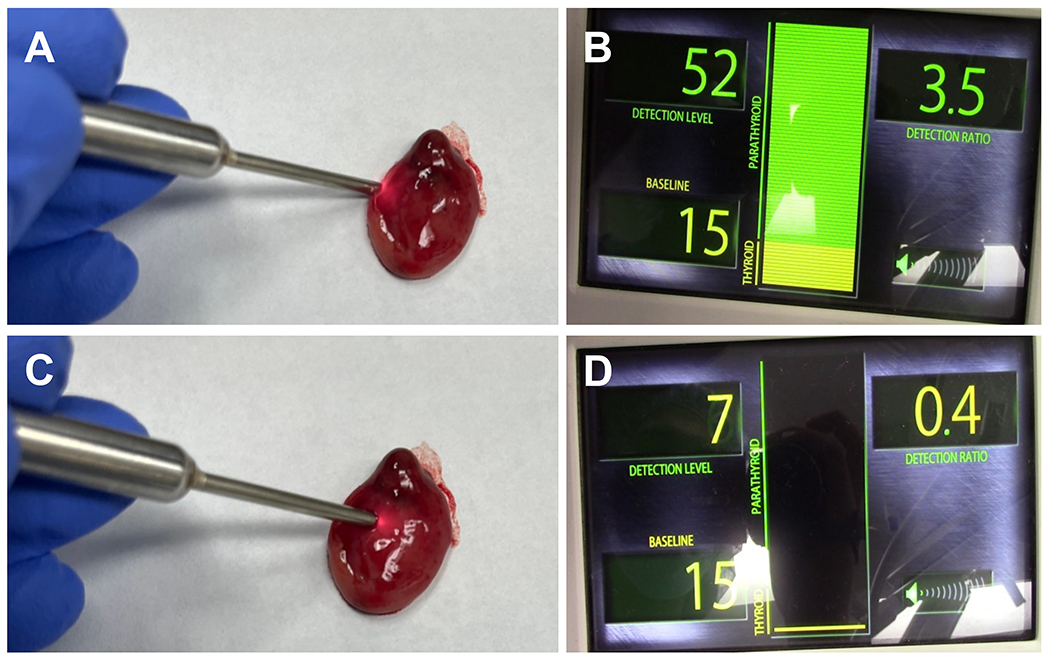
Parathyroid glands (PGs) in Patient 18 (age = 14 years) with primary hyperparathyroidism was evaluated by PTeye™ (described in Figure 1) (A, B) NIRAF of a diseased left superior PG ex vivo which demonstrates high NIRAF in the ‘healthy cap’ of the diseased PG and (C, D) very low NIRAF in the adenomatous area of the diseased PG
With a NIRAF detection ratio threshold of 1.219,24, 95.8% sensitivity (46/48 PGs) and 78.4% specificity were achieved for PG identification. The positive predictive value (PPV) was 60.5% and negative predictive value (NPV) was 98.2%, with an overall performance accuracy of 82.9%. For each patient we show the surgeon’s confidence level reported when identifying the parathyroid gland candidates in Table 2. The “low confidence” identifications were excluded from the analysis unless frozen section analysis or histology were performed on the specimen. As seen in Table 3, increasing the detection ratio threshold to 1.5 and 1.8 markedly reduced the false positive rate and improved the positive predictive value, enhancing performance accuracies to 91.4% and 96.3% respectively. However, the sensitivity in PG detection also dropped to 93.8% (45/48 PGs) and 89.6% (43/48 PGs) as the detection threshold was raised to 1.5 and 1.8 respectively.
Table 2:
Distribution of parathyroid candidates identified with high, medium and low confidence by participant surgeons. (LND – lymph node dissection, FSA – frozen section analysis)
|
Patient No. |
Procedure | No. of parathyroid candidates identified with HIGH confidence by surgeon | No. of parathyroid candidates identified with MEDIUM confidence by surgeon | No. of parathyroid candidates identified with LOW confidence by surgeon* |
|---|---|---|---|---|
| Patient 1 | Total thyroidectomy | 2 | 1 | 1 |
| Patient 2 | Total thyroidectomy | 1 | 2 | 1 |
| Patient 3 | Completion hemi-thyroidectomy | 1 | 0 | 0 |
| Patient 4 | Total thyroidectomy + LND | 1 | 1 | 0 |
| Patient 5 | Total thyroidectomy | 1 | 0 | 1 |
| Patient 6 | Prophylactic total thyroidectomy | 3 | 0 | 2 |
| Patient 7 | Total thyroidectomy | 2 | 2 | 0 |
| Patient 8 | Thyroid lobectomy | 1 | 0 | 0 |
| Patient 9 | Total thyroidectomy + LND | 2 | 1 | 1 |
| Patient 10 | Total thyroidectomy | 2 | 2 | 0 |
| Patient 11 | Total thyroidectomy | 1 | 3 | 0 |
| Patient 12 | Thyroid lobectomy | 0 | 0 | 0 |
| Patient 13 | Completion thyroidectomy | 0 | 1 | 0 |
| Patient 14 | Total thyroidectomy | 0 | 3 | 1 |
| Patient 15 | Thyroid lobectomy | 2 | 0 | 1 |
| Patient 16 | Total thyroidectomy | 1 | 3 | 0 |
| Patient 17 | Total thyroidectomy | 0 | 3 | 0 |
| Patient 18 | Parathyroidectomy | 2 | 1 | 0 |
| Patient 19 | Thyroid lobectomy | 0 | 2 | 0 |
| Total | 22 | 25 | 8 | |
| (%) | 40% (22/55) | 45.5% (25/55) | 14.5% (8/55) | |
(excluded from analysis with NIRAF modality, unless FSA/histology was performed)
Table 3:
Performance accuracy metric for the near infrared autofluorescence (NIRAF) detection device for parathyroid identification in the enrolled pediatric cohort. Performance accuracies were assessed at various thresholds (1.2 – 1.8). True positives, true negatives, false positives and false negatives were determined based on the criteria described in Supplemental Table A.
| Number of pediatric patients enrolled for study | 19 patients | ||
| Number of parathyroid glands (PGs) tested with device | 48 PGs | ||
| Number of non-parathyroid tissue sites tested with device – (thyroid, fat, muscle, thymus, trachea and lymph nodes) | 139 non-parathyroid sites | ||
| Device threshold for positive PG identification | ≥ 1.2 * | ≥ 1.5 | ≥ 1.8 |
| Number of true positives | 46 | 45 | 43 |
| Number of false positives | 30 | 13 | 4 |
| Device threshold for negative PG identification | < 1.2 * | < 1.5 | < 1.8 |
| Number of true negatives | 109 | 126 | 135 |
| Number of false negatives | 2 | 3 | 5 |
| Device performance metrics at assigned device threshold | 1.2 * | 1.5 | 1.8 |
| Sensitivity (%) or PG detection rate | 95.8 | 93.8 | 89.6 |
| Specificity (%) | 78.4 | 90.6 | 98.6 |
| Positive predictive value (%) | 60.5 | 77.6 | 95.6 |
| Negative predictive value (%) | 98.2 | 97.7 | 96.5 |
| False positive rate (%) | 21.6 | 9.4 | 1.4 |
| False negative rate (%) | 4.2 | 6.2 | 10.4 |
| Accuracy | 82.9 | 91.4 | 96.3 |
| Kappa | 0.62 | 0.79 | 0.9 |
Post-operative outcomes were documented for each patient in Table 4, particularly the immediate post-operative, post-operative day 1, and serum calcium and parathyroid hormone (PTH) levels at clinic follow-up. Out of 14 patients that had a total thyroidectomy or completion thyroidectomy, 7/14 (50%) had an immediate post-operative (drawn over 20 minutes after thyroid gland removed) low PTH. Six of the 7 recovered to a normal PTH level with patient 16 not following up at our institution for their PTH levels, though they had correction of their calcium level. Patient 5 had return of PTH in the normal range, though had hypocalcemia at follow up while still taking calcium supplementation. All but one patient (13/14) returned to normal calcium levels at the time of post-operative follow up and were no longer taking calcium supplementation. At our institution, our post-operative protocol will only initiate post-operative calcium supplementation if patient has a low PTH or calcium level below 8.5.
Table 4:
Correlative chart depicting number of parathyroid glands (PGs) identified, number of inadvertently excised PGs and post-operative outcomes of hypoparathyroidism or hypocalcemia in the 14 patients that underwent thyroidectomies. TT – Total Thyroidectomy, LND – Lymph node Dissection, pTT – prophylactic total thyroidectomy, CT – completion thyroidectomy, PTH – parathyroid hormone, Ca – calcium, POD1 – postoperative Day 1)
| PTH levels (22 - 88 pg/ml) | Ca levels (8.4 - 10.5 mg/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Procedure | No. of PGs identified by surgeon with medium/high confidence | No. of inadvertently excised PGs | Immediate PTH | POD1 PTH | Latest visit PTH | Immediate Ca | POD1 Ca | Latest visit Ca | Outcome for hypoparathyroidism or hypocalcemia, if present | |
| Patient 1 | TT | 3 | 0 | 45 | 36 | - | 8.5 | 8.8 | - | Uneventful |
| Patient 2 | TT | 3 | 1 | 6 | - | 23 | 9.4 | 8.5 | 8.9 | Resolved |
| Patient 3 | CT | 1 | 0 | 41 | 34 | 43 | 7.7 | 10 | 9.9 | Resolved |
| Patient 4 | TT + LND | 2 | 0 | 6 | 5 | 32 | 8 | 5.9 | 9.3 | Resolved |
| Patient 5 | TT | 2 | 1 | 6 | 4 | 38 | 9.3 | 7.2 | 7.8 | Unresolved* |
| Patient 6 | pTT | 3 | 0 | 28 | 32 | 48 | 9.6 | 9 | 10 | Uneventful |
| Patient 7 | TT | 4 | 0 | 45 | - | - | 9.4 | 7.9 | 9.7 | Resolved |
| Patient 9 | TT | 3 | 0 | 15 | 17 | 65 | 8.6 | 7.7 | 9.4 | Resolved |
| Patient 10 | TT | 4 | 0 | 28 | 13 | 60 | 9.7 | 9.5 | 10.4 | Resolved |
| Patient 11 | TT | 4 | 0 | 5 | 20 | 9.4 | 8.2 | 9.7 | Resolved | |
| Patient 13 | CT | 1 | 0 | 107 | 35 | 74 | 8.7 | 8.7 | 9.7 | Uneventful |
| Patient 14 | TT | 3 | 0 | 10 | 12 | 48 | 10 | 7.8 | 9.4 | Resolved |
| Patient 16 | TT | 4 | 0 | 17 | - | - | 8.6 | 8.7 | 10 | Resolved** |
| Patient 17 | TT | 3 | 0 | 48 | 29 | - | 8.6 | 8.2 | 9.5 | Uneventful |
Patient 5 – Despite their follow-up PTH returning to normal levels, their calcium level has remained low while on calcium supplementation.;
Patient 16 – Family offered to follow up with pediatric endocrinology at our institution and chose not to, so no follow up appointment PTH values available
Due to the limitations in biopsies during pediatric neck operations, it must however be noted that only 5 specimens interrogated by the device were histologically validated within the dataset. Upon assessing the performance accuracy purely based on histological validation, the device demonstrated 100% accuracy where it successfully discriminated all 5 specimens, which were histologically validated to be 3 parathyroids, 1 thymic tissue and 1 lymph node.
Discussion:
Given the rates of incidental parathyroidectomy and hypocalcemia following pediatric thyroidectomy 11,29, ancillary methods are needed for real-time intraoperative PG identification and preservation. To our knowledge, this is the first study to successfully demonstrate that probe-based NIRAF detection can be used for intraoperative PG identification in pediatric patients. Probe-based NIRAF yielded 95.8% sensitivity in this cohort of pediatric patients, similar to results reported in adults.24,27,28 Normalized NIRAF intensities for PGs were consistently and significantly higher than the thyroid and surrounding tissues (p<0.001). These normalized NIRAF intensity values (3.63, ± 2.47) of the PGs in our pediatric patients appear to be congruent with the mean or median normalized NIRAF intensity of adult population PGs (3.55 ± 0.27 to 4.89 ± 3.06) reported in prior studies.24,28,30 Similarly, our inter-patient and intra-patient variability of NIRAF shown in Figure 3, with PG NIRAF being constantly elevated over non-parathyroid NIRAF, is consistent with the inter-patient and intra-patient NIRAF variability reported with PTeye in a cohort of 35 adult paitents.24 While there were no patients under 6 years of age in this cohort, NIRAF intensities were consistently elevated in PGs compared to non-parathyroid tissues across the age groups enrolled in this study i.e., 6 – 16 years (Figure 3).
Although the ratio threshold of 1.2 for PG identification (established in adults)19,24 yielded a favorable performance accuracy of 82.9%, higher ratio thresholds of 1.8 further improved the device accuracy to 96.3%. Therefore, the NIRAF detection ratio threshold for PG identification in children may be higher than in adults, but to determine this, a much larger subset of pediatric patients will be needed. While NIRAF of PGs were reliably higher than other soft tissues in the neck, another notable finding was that PGs in younger children under 12 years yielded lower NIRAF detection ratios (2.78 ± 1.20) than PGs in children older than 12 years of age (4.03 ± 2.80). With the variability of NIRAF intensity among adults and different pediatric age groups, it is possible that the intensity of the fluorophore responsible for NIRAF in PGs may be linked to growth and development, while potentially correlating with age of the patient. So far, the fluorophore that is responsible for NIRAF in PGs has not been elucidated. A potential candidate could be lipofuscin, which are brownish-yellow pigmented granules that collect in tissues with age or ‘wear-and-tear’. Lipofuscin has been reported to have a broad fluorescence range that extends into the near infrared region beyond 800 nm31 and has also been reported to be present in the cytoplasm of active chief cells in PGs.32 Future investigations for identifying the moiety responsible for NIRAF may not only inform clinical decision-making but could also shed insight into PG physiology.
Out of the 19 patients in our cohort, 14 of them had a completion thyroidectomy or total thyroidectomy where PTH and calcium levels were monitored post-operatively. Seven out of these 14 had a low PTH level immediately after surgery and required oral calcium supplementation with calcium carbonate and +/−calcitriol depending on our institution protocol. Only patient 4 required one dose of post-operative IV calcium due to precipitous drop in their POD1 Ca level, as they also had a concurrent central and lateral neck dissection. All but one patient is off of their calcium supplementation at the time of clinic follow up. Patient 5 is still taking calcium carbonate and calcitriol with a persistently low calcium level despite daily adherence. Their PTH has returned to “normal” levels but functionally the patient has hypoparathyroidism since the level of PTH cannot support a normal serum calcium without supplementation.
A key aspect to be borne in mind while relying on NIRAF detection modalities is that NIR wavelengths can only penetrate tissues down to a depth of few millimeters.24 As a result, the technique will not be able to identify a ‘missing’ PG that may be deeply located under fat or intra-thyroidal. The utility of the described probe-based approach would rely on a surgeon’s anatomical knowledge and experience to first locate a prospective ‘PG’ tissue. After locating the candidate tissue, the surgeon may need to carefully expose the potential PG for interrogation with the device, for successful parathyroid identification without compromising PG perfusion and viability.16 While NIRAF-based approaches are valuable for identifying PGs, the technique itself will not be able to determine if the visualized PG is vascularized or not. In such circumstances, surgeons may rely on visual assessment and accrued experience to assess PG perfusion/viability.24 Nonetheless, NIRAF detection can still be very useful in neck operations for identifying inadvertently excised PG(s) on the resected thyroid specimen, following which the parathyroid tissue can be autotransplanted.27 An example of this would be patient 5 in our cohort where one of the PGs was identified with NIRAF ex-vivo.
Since probe-based NIRAF detection was being interrogated for the first time in the pediatric population, the participant surgeons were ‘blinded’ from the device output, so as to not influence surgical outcome of the procedure. As the ‘blinded’ nature of this study prevented intraoperative feedback to the surgeon, further investigations are required to determine the true benefits of this technology for pediatric thyroid and parathyroid surgeries. When used in a non-blinded manner, as indicated for the adult population by the FDA, this system can provide real-time feedback as an intraoperative adjunct to a surgeon’s own assessment. Consequently, the technology could potentially reduce use of tissue aspirate for PTH level analysis, and may reduce the use of frozen section analyses. An additional key advantage is that these modalities could improve a surgeon confidence in identifying PGs.27 Kiernan et al. found that using NIRAF detection improved surgeon confidence in identifying PGs regardless of their level of experience, which included a surgeon with 18 years of endocrine surgery experience as well as a surgeon with 2 years of independent practice. This study also found the modality was useful as a teaching tool for their surgical trainees, aided by the real-time feedback on the trainee’s interpretation of the anatomy.27 This resulted in the trainees reporting high confidence in identifying PGs when using NIRAF detection devices, such as PTeye27. However, as with any intraoperative tool used for diagnostics or surgical guidance, appropriate risk mitigation strategies must be followed to counter false positives or false negatives that could arise with NIRAF detection modalities used for PG identification.16,24,27 One possible pitfall seen in the adult population has been the use of NIRAF in adenomatous/diseased PGs.16 In PG adenomas the healthy ‘cap’ of the diseased PG can exhibit high NIRAF with sometimes negligible NIRAF in the adenomatous areas, which was also the case in our patient #18 (14 years old male).
This study unveils the potential of utilizing NIRAF to accurately identify PGs with the aim to reduce post-operative complications associated with inadvertent injury to healthy PGs during pediatric thyroid and parathyroid surgery. Larger prospective studies at multiple high-volume centers and across all ages are necessary to truly assess the merit of NIRAF-based technologies in pediatric neck operations.
Conclusion:
Pediatric parathyroid tissues demonstrate similar NIRAF properties as adults. Our findings demonstrate the scope of NIRAF detection or non-invasive intraoperative PG identification during pediatric neck operations. Further determination is required to identify an appropriate detection threshold for PG identification, particularly in the pediatric population. Future studies are mandatory to investigate whether this technique can prospectively minimize the risk of post-operative transient or permanent hypoparathyroidism in children undergoing neck operations.
Supplemental Table A: Explanation of true positive, false positive, true negative, and false negative as described in this study, which was utilized for determining device accuracy.
Supplementary Material
Acknowledgements:
Dr. G. Thomas, Mr. P. A. Willmon, Dr. C. Solόrzano and Dr. A. Mahadevan-Jansen were supported by the National Institute of Health under Grant No. R01CA212147.
Footnotes
Conflicts of interest: Prof. Anita Mahadevan-Jansen and Vanderbilt University have a licensing agreement with AiBiomed (Santa Barbara, CA) for the described technology. A. Mahadevan-Jansen was previously an equity holder at AiBiomed. The described technology has been acquired by Medtronic (Dublin, Ireland). Other authors have no conflicts of interest to declare
Meeting Presentation: This manuscript was previously presented at the 79th Annual American Society of Pediatric Otolaryngology meeting in Dallas, TX on May 1st, 2022
Level of Evidence: Level 4
References:
- 1.Sosa JA, Tuggle CT, Wang TS et al. Clinical and Economic Outcomes of Thyroid and Parathyroid Surgery in Children. The Journal of Clinical Endocrinology & Metabolism 2008; 93:3058–3065. [DOI] [PubMed] [Google Scholar]
- 2.Baumgarten HD, Bauer AJ, Isaza A, Mostoufi-Moab S, Kazahaya K, Adzick NS. Surgical management of pediatric thyroid disease: Complication rates after thyroidectomy at the Children’s Hospital of Philadelphia high-volume Pediatric Thyroid Center. Journal of Pediatric Surgery 2019; 54:1969–1975. [DOI] [PubMed] [Google Scholar]
- 3.Kao K-T, Ferguson EC, Blair G, Chadha NK, Chanoine J-P. Risk factors for the development of hypocalcemia in pediatric patients after total thyroidectomy – A systematic review. International Journal of Pediatric Otorhinolaryngology 2021; 143:110666. [DOI] [PubMed] [Google Scholar]
- 4.Scholz S, Smith JR, Chaignaud B, Shamberger RC, Huang SA. Thyroid surgery at Children’s Hospital Boston: a 35-year single-institution experience. Journal of Pediatric Surgery 2011; 46:437–442. [DOI] [PubMed] [Google Scholar]
- 5.Freire AV, Ropelato MG, Ballerini MG et al. Predicting hypocalcemia after thyroidectomy in children. Surgery 2014; 156:130–136. [DOI] [PubMed] [Google Scholar]
- 6.Kundel A, Thompson GB, Richards ML et al. Pediatric Endocrine Surgery: A 20-Year Experience at the Mayo Clinic. The Journal of Clinical Endocrinology & Metabolism 2014; 99:399–406. [DOI] [PubMed] [Google Scholar]
- 7.Francis GL, Waguespack SG, Bauer AJ et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015; 25:716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Masiakos PT, Gaz RD et al. Pediatric thyroidectomy in a high volume thyroid surgery center: Risk factors for postoperative hypocalcemia. Journal of Pediatric Surgery 2015; 50:1316–1319. [DOI] [PubMed] [Google Scholar]
- 9.Hanba C, Svider PF, Siegel B et al. Pediatric Thyroidectomy: Hospital Course and Perioperative Complications. Otolaryngology–Head and Neck Surgery 2017; 156:360–367. [DOI] [PubMed] [Google Scholar]
- 10.Yu YR, Fallon SC, Carpenter JL et al. Perioperative determinants of transient hypocalcemia after pediatric total thyroidectomy. Journal of Pediatric Surgery 2017; 52:684–688. [DOI] [PubMed] [Google Scholar]
- 11.Nordenström E, Bergenfelz A, Almquist M. Permanent Hypoparathyroidism After Total Thyroidectomy in Children: Results from a National Registry. World Journal of Surgery 2018; 42:2858–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesson DE, Johnson BL, Barclay C et al. Thyroid surgery outcomes at a children’s hospital: The value of a multidisciplinary team approach. Journal of Pediatric Surgery 2022; 57:622–629. [DOI] [PubMed] [Google Scholar]
- 13.Stack BC, Twining C, Rastatter J et al. Consensus Statement by the American Association of Clinical Endocrinology (AACE) and the American Head and Neck Society Endocrine Surgery Section (AHNS) on Pediatric Benign and Malignant Thyroid Surgery. Endocrine Practice 2021; 27:174–184. [DOI] [PubMed] [Google Scholar]
- 14.Wang TS, Roman SA, Sosa JA. Predictors of outcomes following pediatric thyroid and parathyroid surgery. Current Opinion in Oncology 2009; 21. [DOI] [PubMed] [Google Scholar]
- 15.Hartl D, Obongo R, Guerlain J, Breuskin I, Abbaci M, Laplace-Builhé C. Intraoperative parathyroid gland identification using autofluorescence: pearls and pitfalls. World J Surg Surgical Res 2019; 2:1166. [Google Scholar]
- 16.Solórzano CC, Thomas G, Berber E et al. Current state of intraoperative use of near infrared fluorescence for parathyroid identification and preservation. Surgery 2021; 169:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paras C, Keller M, Mahadevan-Jansen A, White L, Phay J. Near-infrared autofluorescence for the detection of parathyroid glands. Journal of Biomedical Optics 2011; 16:067012. [DOI] [PubMed] [Google Scholar]
- 18.Falco J, Dip F, Quadri P, de la Fuente M, Rosenthal R. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. Journal of the American College of Surgeons 2016; 223:374–380. [DOI] [PubMed] [Google Scholar]
- 19.McWade MA, Sanders ME, Broome JT, Solórzano CC, Mahadevan-Jansen A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016; 159:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SW, Song SH, Lee HS et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands With Autofluorescence Imaging. The Journal of Clinical Endocrinology & Metabolism 2016; 101:4646–4652. [DOI] [PubMed] [Google Scholar]
- 21.Kahramangil B, Dip F, Benmiloud F et al. Detection of Parathyroid Autofluorescence Using Near-Infrared Imaging: A Multicenter Analysis of Concordance Between Different Surgeons. Annals of Surgical Oncology 2018; 25:957–962. [DOI] [PubMed] [Google Scholar]
- 22.The United States Food and Drug Administration. FDA permits marketing of two devices that detect parathyroid tissue in real-time during surgery. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624982.htm. Accessed November 3, 2018.
- 23.Voelker R Devices Help Surgeons See Parathyroid Tissue. JAMA 2018; 320:2193. [DOI] [PubMed] [Google Scholar]
- 24.Thomas G, McWade MA, Paras C et al. Developing a Clinical Prototype to Guide Surgeons for Intraoperative Label-Free Identification of Parathyroid Glands in Real Time. Thyroid 2018; 28:1517–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbaci M, De Leeuw F, Breuskin I et al. Parathyroid gland management using optical technologies during thyroidectomy or parathyroidectomy: A systematic review. Oral Oncology 2018; 87:186–196. [DOI] [PubMed] [Google Scholar]
- 26.Solórzano CC, Thomas G, Baregamian N, Mahadevan-Jansen A. Detecting the Near Infrared Autofluorescence of the Human Parathyroid: Hype or Opportunity? Annals of Surgery 2020; 272:973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiernan CM, Thomas G, Baregamian N, Solόrzano CC. Initial clinical experiences using the intraoperative probe-based parathyroid autofluorescence identification system—PTeye™ during thyroid and parathyroid procedures. Journal of Surgical Oncology 2021; 124:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas G, Squires MH, Metcalf T, Mahadevan-Jansen A, Phay JE. Imaging or Fiber Probe-Based Approach? Assessing Different Methods to Detect Near Infrared Autofluorescence for Intraoperative Parathyroid Identification. Journal of the American College of Surgeons 2019; 229:596–608.e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziai H, Dixon P, Berman G, Campisi P, Wasserman JD. Incidental Parathyroidectomy Among Pediatric Patients Undergoing Thyroid Surgery. The Laryngoscope 2022; (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 30.Thomas G, Solorzano CC, Baregamian N, et al. Comparing intraoperative parathyroid identification based on surgeon experience versus near infrared autofluorescence detection-A surgeon-blinded multi-centric study. American Journal of Surgery 2021; 222(5):944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taubitz T, Fang Y, Biesemeier A, Julien-Schraermeyer S, Schraermeyer U. Age, lipofuscin and melanin oxidation affect fundus near-infrared autofluorescence. EBioMedicine 2019; 48:592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munger BL, Roth SI. The cytology of the normal parathyroid glands of man and Virginia deer: a light and electron microscopic study with morphologic evidence of secretory activity. The Journal of Cell Biology 1963; 16:379–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


