Abstract
Childhood maltreatment is a leading risk factor for psychopathology, though it is unclear why some develop risk averse disorders, such as anxiety and depression, and others risk-taking disorders including substance abuse. A critical question is whether the consequences of maltreatment depend on the number of different types of maltreatment experienced at any time during childhood or whether there are sensitive periods when exposure to particular types of maltreatment at specific ages exert maximal effects. Retrospective information on severity of exposure to ten types of maltreatment during each year of childhood was collected using the Maltreatment and Abuse Chronology of Exposure scale. Artificial Intelligence predictive analytics were used to delineate the most important type/time risk factors. BOLD activation fMRI response to threatening versus neutral facial images was assessed in key components of the threat detection system (i.e., amygdala, hippocampus, anterior cingulate, inferior frontal gyrus and ventromedial and dorsomedial prefrontal cortices) in 202 healthy, unmedicated, participants (84M/118F, 23.2 ± 1.7 years old). Emotional maltreatment during teenage years was associated with hyperactive response to threat whereas early childhood exposure, primarily to witnessing violence and peer physical bullying, was associated with an opposite pattern of greater activation to neutral than fearful faces in all regions. These findings strongly suggest that corticolimbic regions have two different sensitive period windows of enhanced plasticity when maltreatment can exert opposite effects on function. Maltreatment needs to be viewed from a developmental perspective in order to fully comprehend its enduring neurobiological and clinical consequences.
INTRODUCTION
According to the Adverse Childhood Experiences (ACEs) and similar studies, childhood maltreatment and household dysfunction account for 30%, 45%, 54%, and 64% of the population attributable risk for anxiety disorders (1), childhood onset psychiatric disorders (1), depression (2) and drug addiction (3), respectively. Understanding how maltreatment becomes biologically embedded to enhance risk is a pressing concern and several studies have reported replicable alterations in an array of brain regions in maltreated individuals including: the hippocampus, amygdala, prefrontal and sensory cortices (See (4, 5) for review). Not surprisingly, most of the regions identified are involved in threat response (4, 5).
Epidemiological studies indicated that risk for various psychiatric and medical disorders increases monotonically in relation to the number of ACEs and this has been interpreted as a consequence of cumulative burden (1–3). A compelling alternative is that the most important determinant is exposure to specific types of adversity during developmental sensitive periods (6). In this scenario a monotonic relationship emerges because exposure to more types of maltreatment increases risk of experiencing a critical type of maltreatment at a critical age (7). The difference between these perspectives is important as the cumulative burden hypothesis suggests that the key concern is allostatic load (8) while a sensitive period hypothesis is consistent with maltreatment targeting specific neurodevelopmental processes such as synaptic overproduction or pruning. From the cumulative burden perspective reducing exposure to any type of adversity would diminish risk to some degree while a sensitive period perspective has the potential to identify particular types of adversity at specific ages which if prevented might markedly reduce risk.
Some evidence has emerged in support of the sensitive period hypothesis by showing both prospectively and retrospectively that maltreatment during one developmental stages may be more consequential than during another stage on risk for psychopathology (7, 9), cortisol regulation (10, 11) and brain volume (12–15). We also recently reported that retrospectively assessed prepubertal versus post-pubertal maltreatment appeared to be associated with opposite effects on adult amygdala response to threatening faces versus neutral geometric shapes (16). This finding is consistent with the observations in some studies of larger (12, 17–19) and in other studies of smaller amygdala volumes (20–23) in maltreated participants, which may vary based on age at the time of scanning (15). Of critical concern is whether this age-dependent association between adversity and functional consequence is specific to the amygdala or if it is a general pattern that applies to other stress-susceptible brain regions which are more consistently associated with reduced volumes in maltreated individuals (4, 5).
Hence, the aim of this study was to identify in this sample whether there were distinct windows of vulnerability when exposure to childhood maltreatment was associated with either a blunted or augmented fMRI blood oxygenation level-dependent (BOLD) response to angry/fearful faces versus neutral faces in corticolimbic regions of interest (ROIs) involved in threat detection.
This information is particularly important as a blunted threat response has been associated with impaired ability to detect threat and has been observed in individuals with substance use (24) and conduct disorders (25). In contrast, an exaggerated response has been reported in individuals with anxiety disorders (26), autistic spectrum disorder (27) and post-traumatic stress (28, 29).
METHODS AND MATERIALS
Participants
This study was approved by Partners Healthcare Institutional Review Board and recruitment methods and amygdala imaging results from this sample were recently reported (30). Briefly, medically healthy, right-handed, unmedicated participants between 20–25 years of age were selected based on maltreatment history. Exclusion criteria included neurologic diseases, head trauma with more than 5 minutes loss of consciousness or exposure to 3 or more unrelated forms of adversity. We included an approximately equal number of participants exposed to 0, 1, 2, and 3 or more types of maltreatment. Informed written consent was obtained from all participants.
Assessment of Maltreatment and Psychopathology
The Maltreatment and Abuse Chronology of Exposure (MACE) scale was used to retrospectively evaluate severity of exposure to childhood sexual abuse, parental verbal abuse, parental non-verbal emotional abuse, parental physical abuse, witnessing interparental violence, witnessing violence towards siblings, peer emotional bullying, peer physical bullying, emotional neglect and physical neglect across 18 years of childhood. The MACE consists of 52 items selected using Item Response Theory and each type of maltreatment has a severity score that ranges from 0 to 10. Subjects indicated whether they experienced a given event and checked off each year of occurrence. MACE provides good to excellent reliability at each age and each type of maltreatment as well as excellent overall reliability (r = .91) (31). Additional information on reliability is provided in the Supplement. Data on family income, parental education, and perceived financial sufficiency during childhood (1= much less than enough money to meet our needs; 5 = much more than enough money to meet our needs) were also collected. Structured clinical interviews for DSM-IV Axis I and II psychiatric disorders were used for diagnoses. The assessment interviews were conducted by mental health professionals (psychiatrists, Ph.D. psychologists, clinical nurse specialists).
fMRI Task
Participants performed a block-designed implicit Emotional Face Processing Task (32) with three blocks of negative (i.e., angry or fearful) faces and three blocks of neutral faces interleaved with seven blocks of geometric shapes. Each block lasted 36s, containing six face or shape trios presented for 4s with a 2s interstimulus interval. Subjects were instructed to match the target image at the top of the screen with one of two simultaneously presented images appearing at the bottom of the screen. All simultaneously presented faces displayed the same expression.
Image Acquisition and Data Processing
Neuroimaging was performed on a TIM Trio Scanner (3T; Siemens AG, Siemens Medical Solutions) using a 32-element phased-array radiofrequency reception coil. A high-resolution 3D T1-weighted sequence was conducted for anatomical registration (TR time = 2,100 ms, TE = 2.25 ms, flip angle = 12°, 1.0×1.0×1.3 mm voxels, 3D matrix 256×256×170 mm field of view, 128 repetitions). Functional data were acquired using a T2-weighted echo planar imaging sequence (TR = 3,000 ms, TE = 30 ms, flip angle = 76.1°, 42 slices, 3.5×3.5×3.5 mm voxel size, 3D matrix 64×64×141.24 field of view, 208 repetitions).
fMRI data were preprocessed using FMRI Expert Analysis Tool (FEAT version 4.1.6, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Preprocessing steps included non-brain removal, slice-timing correction, image realignment, coregistration with T1 images, transformation to Montreal Neurological Institute (MNI) space, spatial smoothing using a 5 mm FWHM Gaussian kernel and intensity normalization. Head movement greater than 3 mm led to exclusion of the data from further analysis. The mean BOLD signal time-series as well as time course data were extracted using the FSL fslmeants command. Degree of neural activation was computed using general linear models convolved with a canonic hemodynamic response function to extract BOLD estimates. Individuals processing the fMRI data were blind to the clinical and exposure history of the participants.
Region-of-Interest Analyses of fMRI activation
ROIs selected for analysis were key components of a threat detection and response circuit based on work by LeDoux (33–35), Mujica-Parodi and colleagues (36), Herringa et al (37, 38) and our earlier models (4, 39). In addition to the amygdala, ROIs were: (i) the fusiform gyrus which is a visual association area that plays and important role in facial recognition; (ii) the inferior frontal gyrus pars triangularis (IFGPT) that has been proposed to set a threshold as to whether or not an image is threatening (36); (iii) the ventromedial prefrontal cortex (vmPFC) that receives information from the IFGPT and inhibits amygdala response (36, 40); (iv) the dorsal anterior cingulate (dACC) that can enhance amygdala response to threat (41); (v) the dorsal medial prefrontal cortex (dmPFC) that appears to regulated vmPFC versus dACC influences on the amygdala (41); and (vi) the hippocampus that receives input from the fusiform gyrus and IFGPT and helps bring contextual information to the circuit (42). The locations of these bilateral ROIs are delineated in Figure 1 along with a circuit diagram which illustrates their key interconnections.
Figure 1. The seven anatomically predefined regions of interest (ROIs) and their interconnections.
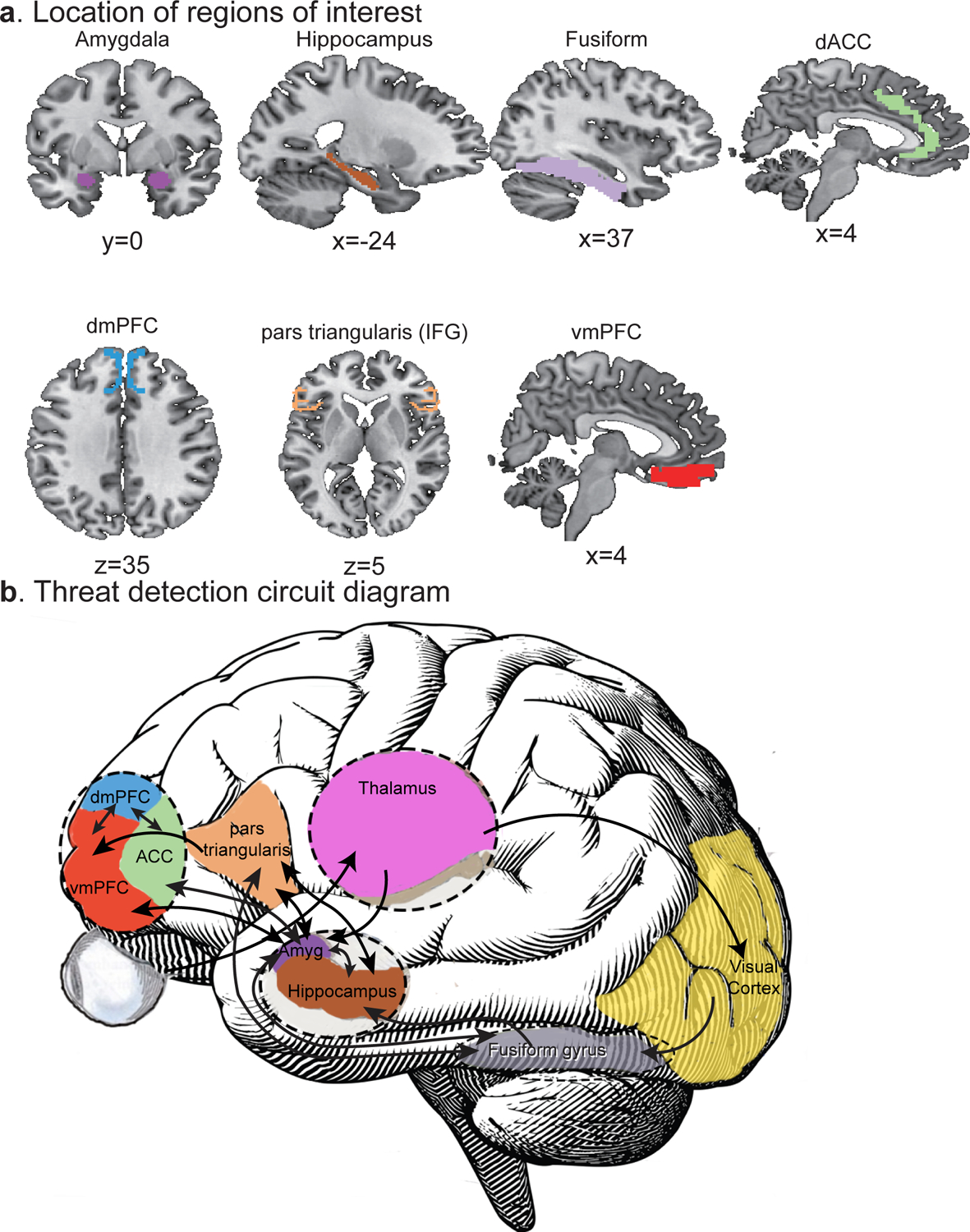
a. The bilateral amygdala and hippocampus ROIs were created with the anatomical 60% FSL Harvard-Oxford maximum likelihood subcortical atlases (64). The bilateral fusiform ROIs were taken from the Automated Anatomical Labeling (AAL) atlas (65). Predefined Brodmann’s area templates provided ROIs for dACC (BA 32), dmPFC (BA 8 and 9), IFGPT (BA 45) and vmPFC (BA 11, 12, 24, 25 and 32) but we excluded the BA32 dACC component from the vmPFC. b. Circuit diagram indicating the major interconnections between these regions in detection of visual threats down to the level of the amygdala. Abbreviations: dACC – dorsal anterior cingulate cortex, dmPFC – dorsomedial prefrontal cortex, IFGPT – inferior frontal gyrus, pars triangularis, vmPFC – ventromedial prefrontal cortex.
Random Forest Regression with Conditional Inference Trees
The aim of this analysis was to identify the most important risk factors for an enhanced or blunted BOLD response to threat. Conventional analytic methods are inappropriate given the substantial collinearity between exposure levels at adjacent ages. Instead, we used random forest regression with conditional inference trees (RFR-CIT, cforest in R package party), which is an AI strategy that is highly resistant to collinearity (43). We found this algorithm to be superior to other algorithms in identifying underlying predictor variables in MACE data using Monte-Carlo simulations and we, and others, have used it in previous sensitive period studies (7, 12–14, 16, 44, 45). The dataset was randomly split into a training set (63.3% of the participants) and withheld test set (36.7%) to test the models’ predictive accuracy. The variable importance (VI) of each risk factor was assessed by sequentially permuting each risk factor and refitting the model to assess the degree to which the loss of information due to permutation worsened fit. VI for each risk factor was defined as the increase in mean square error following permutation and averaged over 50 random splits of the data. To determine the significance of these VI measures, the overall process was repeated 1000 times using reshuffled outcome values to derive the random chance importance values for each variable. The significance for each regressor was assessed non-parametrically as the probability of obtaining a value equal to or greater than the actual VI measure by random chance in the reshuffled data, which provides a more conservative measure that the z test difference between chance and observed VI measures with Bonferroni correction.
We are unaware of any published power analyses for RFR-CIT for sample size determination for identify underlying predictor variables. Hence, we ran a series of Monte-Carlo simulations using actual exposure data and simulated outcomes to determine power to correctly identify multiple early and late childhood predictor variables drawing N=202 participants at random from a population of N=2092. Predictor variables that accounted for 10–11% of the variance in the total population were correctly identified in the smaller samples with power of 0.79±0.11 (mean±SD, range 0.6 – 0.85).
One disadvantages of RFR-CIT is that it does not provide an indication of the nature of the relationship between the predictor and outcome. We used the saved random forest to delineate this relationship, as we have done in prior studies (12, 13, 16), by varying degree of exposure to the significant risk factors while holding all other variables constant at their modal value to produce a predicted dose-dependent outcome. Measures of severity, multiplicity and duration of maltreatment across childhood, as well as gender, age, parental education and perceived financial sufficiency were included as additional risk factors.
Code availability.
The R code for determining significant type/time risk factors using random forest regression with conditional inference trees has been deposited on Zenodo (doi: 10.5281/zenodo.5273055) and made open source as has the R code used to generate the supplementary tables (doi: 10.5281/zenodo.7340449). The remaining code is included in the supplement as R Markdown files.
RESULTS
Demographics
The sample consisted of 202 participants (84M/118F, 23.2 ± 1.7 years old) recruited from urban and suburban Boston areas. Altogether, 69% described themselves as White, 16% as Asian, 9% as Black and 6% as mixed or other races and 13% indicated that they were of Hispanic ethnicity. Twenty-six percent reported no exposure to maltreatment whereas 23%, 19% and 32% reported exposure to 1, 2 or 3 or more types of maltreatment, respectively.
There were enough participants with reported exposure to analyze effects of physical neglect, emotional neglect and parental non-verbal emotional abuse across all ages, parental physical abuse from age 2 on, parental verbal abuse from age 4 on, peer emotional bullying from age 5 on, and peer physical bullying from age 7 on. Assessment of witnessing interparental violence was limited to ages 6–15 and witnessing violence to sibling to ages 5–13. The major limitation is that effects of sexual abuse could only be gauged from age 12 on.
Altogether 29.4%, 26.9%, 17.9% of the participants had lifetime histories of major depression, one or more anxiety disorders, and one or more personality disorders, respectively. Adjusted odds of meeting lifetime criteria for these disorders were 4.81-fold (95% CI 2.28–10.12), 2.36-fold (95% CI 1.17–4.77) and 2.39-fold (95% CI 1.05–5.46) higher in participants reporting exposure to 2 or more types of maltreatment versus 0 or 1 types.
Identification of Underlying Risk Factors
As seen in Figure 2, RFR-CIT identified a modest array of recollected events with relatively high VI values. Those meeting criteria for significance are listed in Table 1. The number of significant risk factors varied from 6 for the fusiform gyrus to 11 for the dACC and vmPFC. In none of these ROIs did the number of types, overall severity or duration of maltreatment emerge as significant risk factors, nor did parental education or perceived financial sufficiency.
Figure 2. Sensitive period analyses.

Random forest regression with conditional trees indicating the importance of ten types of childhood maltreatment across ages on fMRI BOLD response in key cortical and subcortical regions involved in treat detection and response. Mean importance is defined as the increase in mean square error of the fit following permutation of each variable. Abbreviations EN - emotional neglect; NVEA - nonverbal emotional abuse; Peer_E - peer emotional bullying; Peer_P - peer physical bullying; Phys - parental physical abuse; PN - physical neglect; PVA - parental verbal abuse; SexA - sexual abuse; WIPV - witnessing interparental violence; and Wsib - witnessing violence toward siblings.
Table 1.
Important predictors of functional activation to emotional faces > neutral faces within regions of interest.
| Predictors | Age | Random Forest Regression | Dose-response Relationship | ||
|---|---|---|---|---|---|
| Importance | p Value | Direction | |||
| Amygdala | Peer Physical Bullying | 6 | 0.79 | 0.019 | ⬇ |
| Witnessing IPV | 7 | 0.49 | 0.029 | ⬇ | |
| Witnessing IPV | 9 | 0.92 | 0.008 | ⬇ | |
| Non-verbal Emotional Abuse | 9 | 1.44 | 0.005 | ⬇ | |
| Physical Neglect | 9 | 0.49 | 0.04 | ⬆ | |
| Peer Physical Bullying | 11 | 1.07 | 0.026 | ⬇ | |
| Emotional Neglect | 14 | 0.71 | 0.025 | ⬆ | |
| Peer Emotional Abuse | 17 | 0.75 | 0.039 | ⬆ | |
| Parental Verbal Abuse | 17 | 0.77 | 0.031 | ⬆ | |
| Parental Verbal Abuse | 18 | 0.76 | 0.04 | ⬆ | |
| Hippocampus | Witnessing IPV | 9 | 0.30 | 0.039 | ⬇ |
| Peer Emotional Abuse | 9 | 1.02 | 0.031 | ⬇ | |
| Peer Physical Bullying | 10 | 0.69 | 0.034 | ⬇ | |
| Emotional Neglect | 14 | 0.47 | 0.039 | ⬆ | |
| Parental Verbal Abuse | 15 | 1.15 | 0.016 | ⬆ | |
| Parental Verbal Abuse | 16 | 0.75 | 0.031 | ⬆ | |
| Parental Verbal Abuse | 17 | 0.82 | 0.038 | ⬆ | |
| Dorsal anterior cingulate cortex | Physical Neglect | 2 | 0.97 | 0.015 | ⬇ |
| Peer Physical Bullying | 6 | 0.50 | 0.021 | ⬇ | |
| Witnessing IPV | 9 | 0.78 | 0.011 | ⬇ | |
| Witnessing Viol. Sibling | 12 | 0.27 | 0.034 | ⬇ | |
| Emotional Neglect | 13 | 1.95 | 0.004 | ⬆ | |
| Emotional Neglect | 14 | 2.39 | 0.000 | ⬆ | |
| Parental Verbal Abuse | 15 | 1.41 | 0.008 | ⬆ | |
| Parental Verbal Abuse | 16 | 1.57 | 0.005 | ⬆ | |
| Parental Verbal Abuse | 17 | 2.03 | 0.005 | ⬆ | |
| Emotional Neglect | 17 | 0.77 | 0.015 | ⬆ | |
| Parental Verbal Abuse | 18 | 2.67 | 0.005 | ⬆ | |
| Ventromedial prefrontal cortex | Physical Neglect | 2 | 0.69 | 0.032 | ⬇ |
| Emotional Neglect | 6 | 0.54 | 0.033 | ⬇ | |
| Peer Physical Abuse | 6 | 0.51 | 0.027 | ⬇ | |
| Witnessing IPV | 9 | 0.28 | 0.033 | ⬇ | |
| Peer Physical Abuse | 11 | 1.62 | 0.008 | ⬇ | |
| Emotional Neglect | 14 | 0.89 | 0.012 | ⬆ | |
| Parental Verbal Abuse | 15 | 0.76 | 0.035 | ⬆ | |
| Parental Verbal Abuse | 16 | 1.06 | 0.023 | ⬆ | |
| Parental Verbal Abuse | 17 | 1.17 | 0.018 | ⬆ | |
| Peer Emotional Abuse | 17 | 0.77 | 0.042 | ⬆ | |
| Parental Verbal Abuse | 18 | 1.64 | 0.014 | ⬆ | |
| Dorsomedial prefrontal cortex | Witnessing IPV | 7 | 0.63 | 0.017 | ⬇ |
| Peer Physical Bullying | 11 | 1.01 | 0.013 | ⬇ | |
| Emotional Neglect | 13 | 0.70 | 0.027 | ⬆ | |
| Emotional Neglect | 14 | 0.73 | 0.018 | ⬆ | |
| Emotional Neglect | 17 | 0.58 | 0.036 | ⬆ | |
| Peer Emotional Abuse | 17 | 1.27 | 0.014 | ⬆ | |
| Parental Verbal Abuse | 18 | 1.42 | 0.016 | ⬆ | |
| Inferior frontal gyrus pars triangularis | Peer Physical Bullying | 6 | 1.48 | 0.003 | ⬇ |
| Peer Physical Bullying | 10 | 0.77 | 0.02 | ⬇ | |
| Peer Physical Bullying | 11 | 0.56 | 0.033 | ⬇ | |
| Parental Verbal Abuse | 12 | 0.77 | 0.03 | ⬆ | |
| Parental Verbal Abuse | 13 | 0.79 | 0.028 | ⬆ | |
| Peer Physical Bullying | 13 | 0.67 | 0.038 | ⬇ | |
| Parental Verbal Abuse | 15 | 1.84 | 0.002 | ⬆ | |
| Parental Verbal Abuse | 17 | 0.53 | 0.045 | ⬆ | |
| Parental Verbal Abuse | 18 | 0.76 | 0.03 | ⬆ | |
| Fusiform gyrus | Witnessing Viol. Sibling | 6 | 0.27 | 0.047 | ⬇ |
| Parental Verbal Abuse | 7 | 0.69 | 0.028 | ⬇ | |
| Peer Physical Bullying | 10 | 0.57 | 0.040 | ⬇ | |
| Parental Verbal Abuse | 14 | 1.78 | 0.004 | ⬆ | |
| Parental Verbal Abuse | 15 | 0.81 | 0.025 | ⬆ | |
| Parental Verbal Abuse | 17 | 0.65 | 0.036 | ⬆ | |
Abbreviations: IPV - Interparental Violence; Viol. – Violence
The dose-response relationship between severity of exposure to significant risk factor and degree of change in BOLD response to threatening versus neutral faces is illustrated in Figure 3. Briefly, 10 significant risk factors were identified for the amygdala, five of which were associated with a blunted response and involved exposures between ages 6 – 11 years. Four of the 5 risk factors associated with an enhanced response were reported to occur between ages 14–18. Types of maltreatment associated with a blunted response included peer physical bullying, witnessing interparental violence and non-verbal emotional abuse. Types of maltreatment associated with an enhanced response were primarily emotional neglect, parental verbal abuse and peer emotional bullying.
Figure 3. Dose-response relation between statistically significant risk factors and scaled fMRI BOLD response.

The stored random forest models were used to estimate the direction of change in functional activation to negative minus neutral faces resulting from varying the degree of exposure to the significant risk factors while holding all other risk factors constant. The horizontal axis indicates the risk factor level for each risk factor (range 0 – 10). The first column presents dose-response curves for significant risk factors occurring at ages 1–6 years, the second for ages 7–10, third for ages 11–14 and fourth for ages 15–18 years. See Fig 2 for abbreviations. The identity of each predictor consists of the abbreviated maltreatment type and age of occurrence.
For the hippocampus there were three risk factors for blunted response which involved witnessing interparental violence or peer physical or emotional bullying at 9–10 years and four risk factors for enhanced response that included emotional neglect at age 14 and parental verbal abuse at ages 15–17 years. For the dACC there were 4 risk factors associated with a blunted response that occurred between ages 2–12 and involved physical neglect, peer physical bullying, witnessing interparental violence and witnessing violence to siblings. Exposures associated with an enhanced response occurred between ages 13–18 and involved emotional neglect or parental verbal abuse.
This basic pattern was true for the other ROIs. Enhanced response for the dmPFC, vmPFC, IFGPT and fusiform gyrus involved exposures to emotional neglect, parental verbal abuse or peer emotional bullying within the 12–18-year age range. Blunted response was primarily associated in the dmPFC, IFGPT and fusiform gyrus with exposures between 6 – 11 years of age and involved witnessing interparental violence, witnessing violence to siblings, and peer physical bullying. Blunted response in the vmPFC was associated with physical neglect, emotional neglect, witnessing interparental violence and peer physical bullying between ages 2 – 11 years. Overall, 59 out of 61 identified ‘predictor’ variables followed a pattern with risk factors for blunted response occurring at earlier ages than risk factors for enhanced response.
Mean motion was 0.05 ± 0.03 cm. There were no significant associations between degree of movement and regional BOLD response to negative versus neutral faces (ACC r = −0.003, p = .96; amygdala r = .038, p = .59; dmPFC r = −.027, p = .71; fusiform r = −.03, p = .66; hippocampus r = .0015, p = .98; IFG par triangularis r = .09, p = .19; vmPFC r = −0.005, p = .94). Including motion as a predictor variable in the random forest regression had no meaningful effect on the type / time risk factors identified (Supplement Table S1).
Group Differences in Bold Time Course
To visualize differences in response based on type and timing of maltreatment we created three groups for each ROI (Fig. 4). One group included participants who reported exposures to any of the early type and time risk factors associated with a blunted response but not to any of the later risk factors. A second group consisted of participants who reported exposure to any of the later risk factors associated with an enhanced response but not the earlier risk factors, and the third group consisted of participants with no exposure to either set of risk factors. Group differences were assessed using linear mixed models with B-splines and a compound symmetry correlation matrix. (See Supplement R Markdown 2).
Figure 4. Differential time courses of fMRI-BOLD activation to negative faces minus neutral faces.

The percent MR signal change (mean ± sem) from baseline to negative minus neutral faces within regions of interest. Participants were divided into an earlier exposure group who reported exposure to any of the significant early regionally-specific risk factors associated with an attenuated response (but not any of the significant later exposure risk factors), a later exposure group who reported exposure to any of the significant later regionally-specific risk factors associated with an enhanced response (but not any of the significant early exposure risk factors), and unexposed group who reported no exposure to any of the significant early or later type/time risk factors. The graph extends for the duration of each fMRI trial block and presents the mean of the differences in degree of BOLD activation to threatening versus neutral faces at each MRI pulse sequence repetition time.
Overall, there were significant group × time interaction effects in each region for the three-group comparisons, as well as for each of the possible two-group comparisons.
The most striking difference can be seen in the early exposure groups which had a rapid drop in relative signal strength which was apparent within 3–12 seconds, and persisted for 15 – 24 seconds, in most regions. In contrast, the late exposure groups had a progressive increase in their differential response which reached its maximum level at 12 sec in the hippocampus and fusiform gyrus, at 18 sec in the amygdala, dACC, vmPFC and IFGPT and at 21 sec in the dmPFC.
The unexposed group had a significantly smaller increase in signal strength change than the late only group, and there was an initial drop in signal strength in the IFGPT and hippocampus. Peak increases in signal strength were observed at 18 seconds in the fusiform gyrus and at 27 seconds in the amygdala, dACC, dmPFC, vmPFC and hippocampus.
Sample sizes for the three groups varied by region. On average there were N = 61 (range 53–77) participants in the unexposed groups, N = 16 (range 10–27) in the early only exposure groups and N = 73 (range 53–97) in the late only exposure groups. Demographic, behavioral, and clinical differences between the three regionally-specific exposure groups are provided in the Supplement (Tables S2 – S8).
Differential Response to Negative Versus Neutral Faces
Time course data were analyzed using mixed effects models with B-splines and a first order autocorrelation structure (See Supplement R Markdown 3 for statistical comparisons). Three patterns were clear in the amygdala, dACC, vmPFC, and dmPFC (Fig. 5). First, a significant differential response to negative versus neutral faces emerged more rapidly in the maltreatment groups than the unexposed group. Second, there was a greater relative increase in signal strength to negative versus neutral faces in the late exposure versus unexposed group. Third, the early only group had a reversed response pattern showing a greater signal strength increase to neutral versus negative faces.
Figure 5. Time courses of fMRI-BOLD activation to negative faces and neutral faces.

The percent MR signal change (mean ± sem) to both negative and neutral faces within regions of interest. Participants were divided into an earlier exposure group who reported exposure to any of the significant early regionally-specific risk factors associated with an attenuated response (but not any of the significant later exposure risk factors), a later exposure group who reported exposure to any of the significant later regionally-specific risk factors associated with an enhanced response (but not any of the significant early exposure risk factors), and an unexposed group who reported no exposure to any of the significant early or later type/time risk factors.
In the fusiform gyrus the first two patterns were observed, but in the early only group there was no evidence of a significant differential response to negative versus neutral faces. Patterns one and two were also seen in the hippocampus. In the early exposure only group there was a trend for an initial neutral > negative response followed by a brief but significant negative > neutral response at 18 seconds.
In the IFGPT the early and late exposure groups had the expected opposite response patterns. However, the unexposed group was similar to the early only group in having a significantly greater increase in signal strength to the neutral versus negative faces during the first 15 seconds.
Association With Psychopathology
McCrory and colleagues (46) proposed that functional abnormalities in threat processing would act as a latent vulnerability, increasing risk for developing psychiatric disorders rather than directly producing symptoms. We used logistic regression modeling to assess whether differences in regional BOLD response to negative versus neutral faces was associated with lifetime history of anxiety disorders. The most parsimonious model was established by eliminating non-significant predictor variables whose removal did not lead to a significant worsening of fit. Predictors in the most parsimonious model were age, vmPFC (z = 8.61, p = .0034) and IFGPT (z = 14.37, p = .00015) BOLD response. A 1 SD increase in negative vs neutral BOLD response in the vmPFC was associated with a marked reduction in risk for an anxiety disorder diagnosis (adjusted odds ratio = 0.15, 95% CI 0.04 – 0.56) whereas a 1 SD increase in negative vs neutral BOLD response in the IFGPT was associated with a marked increase in risk (adjusted odds ratio = 5.58, 95% CI 2.17 – 14.35).
In general, participants in the regionally specific late only groups had an increased risk for: MDD (all regions); one or more personality disorders (all regions); one or more anxiety disorders (amygdala, hippocampus, vmPFC, fusiform); an eating disorder (hippocampus, dACC, vmPFC, fusiform); panic disorder (dACC, IFGPT, fusiform); ADHD (hippocampus) and a substance use disorder (fusiform). There were too few participants in the early only exposure groups to draw firm conclusions, but this experience was associated with increased risk for obsessive compulsive disorder (amygdala, dmPFC) relative to unexposed participants. Within most ROIs the early only group had reduced risk for MDD relative to the late only group (Tables S2–S8).
DISCUSSION
A number of important observations emerged in this study. First, these finding support a sensitive period rather than allostatic load model as exposure to particular types of maltreatment at specific ages were the significant risk factors for altered fMRI response rather than multiplicity or overall severity.
Second, exposure during early versus later periods of childhood were associated with opposite alterations in BOLD response to negative versus neutral faces. The finding that measures of cumulative burden or socioeconomic factors were not significant risk factors makes sense given that adversity could be associated with either an enhanced or reversed response pattern depending on timing. Hence, overall measures of maltreatment, such as an ACE score, which do not provide information on timing, may be inadequate to fully understand the potential neurobiological consequences of childhood maltreatment.
Similarly, McLaughlin and Sheridan (47, 48) proposed that the consequences of maltreatment can be largely understood by dichotomizing experience into either threat (presence of experiences that represent a threat to one’s physical integrity) or deprivation (absence of expected social and cognitive experiences). However, the types of maltreatment that emerged as the most important risk factors cut across these dimensions and the proposed threat / deprivation dichotomy provided no insight into the nature of experiences associated with heightened or blunted responses; underscoring the critical importance of developmental timing. In addition, timing of exposure may be an important determinant of whether adversity leads to accelerated (49) or delayed maturation (50) of brain regions involved in emotional regulation.
We have proposed that maltreatment produces phenotypic adaptations that facilitate survival and reproductive success but may be maladaptive in other contexts (5, 16). From this perspective it makes sense that exposure during adolescence would enhance BOLD response to threat as older children can use this information to mount an effective fight or flight response. It also makes adaptive sense for this response to be blunted or reversed by exposure during early stages of development as infants, toddlers and elementary school students are not equipped to mount an effective flight or fight response and a blunted or reversed response may help to preserve the attachment bond to episodically abusive parents. The central nucleus of the amygdala interconects with the paraventricular nucleus of the hypothalamus, which plays a critical role in the regulation of autonomic activity and studies have shown that amygdala activation is associated with increased sympathetic nervous system response (51, 52). Our hypothesis is that a reversal in BOLD response in participants with early only exposure may be associated with a reduction in sympathetic and an increase in parasympathetic tone to threat, which has been observed as a stress response in some maltreated individuals (53, 54). An enhanced parasympathetic response could be potentially adaptive in signaling submission and in facilitating crying and self-soothing (55).
Third, there were marked group differences in regional time course of response. An engineering control model by Mujica-Parodi et al (36) proposed that the IFGPT acts as an informational set point, gating signals between the vmPFC – amygdala negative feedback loop and a visual cortical information-gathering loop. However, group-specific temporal response patterns suggest that information may be processed quite differently in the three groups.
An important observation was that these ROIs appeared to have not one but two sensitive periods. Briefly, sensitive periods denote a time frame of enhanced plasticity when a brain region may be maximally shaped by environment or experiential factors (56). Much of what we know about the influence of early experience emerged through research on unimodal sensory regions which have a single extended critical period (57, 58). The present finding suggests that corticolimbic regions involved in affective processing may have two separate windows, likely occurring during prepubertal periods of synaptic overproduction and again during post-pubertal periods of pruning, and that exposure to adversity during these sensitive periods may result in opposite effects on brain function. The opening and closing of sensitive periods appears to be related to developmental changes in GABAeregic innervation and to emergence of ‘molecular brakes’ and perineuronal nets that attenuate plasticity (59). The possible role of gonadal and adrenal hormones is unclear.
The key limitation of this study was that maltreatment was assessed through retrospective self-report, which may lack accuracy. There is relatively little overlap between individuals identified as maltreated by Child Protective Services (CPS) and adults retrospectively reporting maltreatment (60), which may occur in part because most cases are never reported to CPS, the tendency of CPS to focus on early childhood and racial and social class biases in reporting to CPS (61) plus the potential hesitancy of adults to reveal exposure or their lack of ability to recall certain distressing events (60). Nevertheless, both prospective and retrospective studies identify similar clinical outcomes and alterations in biomarkers (60), though, retrospectively recalled maltreatment is typically a more powerful predictor of psychopathology than prospectively assessed exposure (60). We are not aware of any prospective studies that have collected as detailed information on type and timing of major forms of abuse and neglect as we have garnered retrospectively and doing so may not be ethical without intervening in certain cases; producing a situation in which prospective assessments may alter outcomes. Hence, this limitation may be difficult to surmount and highlights an advantage of retrospective assessments, particularly those taking place in emerging adults when participants are close enough to childhood to have clear memories but are no longer subject to mandated reporting requirements. Prospective studies however are necessary to establish cause and effect relationships and to identify underlying mechanisms. Another important limitation is that the impact of some types of maltreatment, particularly sexual abuse before age 12, could not be assessed in this sample.
Clinically, these findings may help us to understand why some maltreated individuals develop risk-averse disorders associated with enhanced amygdala response to threat, whereas others develop risk-taking disorders associated with a blunted threat response. Our sensitive period findings may be useful in the design of neurodevelopmental optimization programs that deliver timed interventions to mitigate risk for psychopathology in at risk youths (62). They strongly underscore the need to study maltreatment from a developmental perspective in order to fully understand its clinical and neurobiological consequences and highlight the need to take maltreatment into account when researching the relationship between psychopathology and brain function (63).
Supplementary Material
Acknowledgments:
This work was supported by the National Institutes of Health grants (Grant No. MH-091391, MH-113077, DA-017846, HD-0794841 [to MHT]), ANS Research Foundation ([to MHT]), National Natural Science Foundation of China (Grant No. 32000755 [to JZ]) and Natural Science Foundation of Zhejiang Province (Grant No. LQ21C090008 [to JZ]).
Cynthia E. McGreenery and Elizabeth Bolger, MA, Developmental Biopsychiatry Research Program, McLean Hospital, Belmont, Massachusetts, assisted with recruitment and assessment of study participants and Michael Rohan, PhD, and Gordana Vitaliano, MD, PhD, Brain Imaging Center, McLean Hospital, Belmont, Massachusetts, provided technical and clinical support. All of these individuals received compensation for their help.
Footnotes
Disclosures
MHT created the MACE scale used to collect data on type and timing of exposure to maltreatment used in this study. However, there is no financial conflict as this scale was placed into the public domain and it is fully available and free to use. The other authors declare that they have no competing interests.
Data and materials availability
All anonymized data used in these analyses have been deposited on Zenodo (doi: 10.5281/zenodo.5271714 and zenodo.7340449) and are open source.
References
- 1.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37(3):268–77. [DOI] [PubMed] [Google Scholar]
- 3.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111(3):564–72. [DOI] [PubMed] [Google Scholar]
- 4.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17(10):652–66. [DOI] [PubMed] [Google Scholar]
- 5.Teicher MH. Scars that won’t heal: the neurobiology of child abuse. Sci Am. 2002;286(3):68–75. [DOI] [PubMed] [Google Scholar]
- 6.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–91. [DOI] [PubMed] [Google Scholar]
- 7.Khan A, McCormack HC, Bolger EA, McGreenery CE, Vitaliano G, Polcari A, et al. Childhood Maltreatment, Depression, and Suicidal Ideation: Critical Importance of Parental and Peer Emotional Abuse during Developmental Sensitive Periods in Males and Females. Front Psychiatry. 2015;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–24. [DOI] [PubMed] [Google Scholar]
- 9.Kaplow JB, Widom CS. Age of onset of child maltreatment predicts long-term mental health outcomes. J Abnorm Psychol. 2007;116(1):176–87. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA 3rd. Causal effects of the early caregiving environment on development of stress response systems in children. Proc Natl Acad Sci U S A. 2015;112(18):5637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schalinski I, Teicher MH, Rockstroh B. Early neglect is a key determinant of adult hair cortisol concentration and is associated with increased vulnerability to trauma in a transdiagnostic sample. Psychoneuroendocrinology. 2019;108:35–42. [DOI] [PubMed] [Google Scholar]
- 12.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teicher MH, Anderson CM, Ohashi K, Khan A, McGreenery CE, Bolger EA, et al. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage. 2018;169:443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog JI, Thome J, Demirakca T, Koppe G, Ende G, Lis S, et al. Influence of Severity of Type and Timing of Retrospectively Reported Childhood Maltreatment on Female Amygdala and Hippocampal Volume. Sci Rep. 2020;10(1):1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luby JL, Tillman R, Barch DM. Association of Timing of Adverse Childhood Experiences and Caregiver Support With Regionally Specific Brain Development in Adolescents. JAMA Netw Open. 2019;2(9):e1911426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH. Association of Prepubertal and Postpubertal Exposure to Childhood Maltreatment With Adult Amygdala Function. JAMA Psychiatry. 2019;76(8):843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons-Ruth K, Pechtel P, Yoon SA, Anderson CM, Teicher MH. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav Brain Res. 2016;308:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 2011;108(34):14324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med. 2011;165(12):1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korgaonkar MS, Antees C, Williams LM, Gatt JM, Bryant RA, Cohen R, et al. Early exposure to traumatic stressors impairs emotional brain circuitry. PLoS One. 2013;8(9):e75524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Dam NT, Rando K, Potenza MN, Tuit K, Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry. 2014;71(8):917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61(11):1306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71(6):627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–34. [DOI] [PubMed] [Google Scholar]
- 27.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2014;9(1):106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma. Biol Psychiatry. 2017;81(12):1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety. 2012;29(5):449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH. Association of Prepubertal and Postpubertal Exposure to Childhood Maltreatment With Adult Amygdala Function. JAMA Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teicher MH, Parigger A. The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One. 2015;10(2):e0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–23. [DOI] [PubMed] [Google Scholar]
- 33.LeDoux JE, Brown R. A higher-order theory of emotional consciousness. Proc Natl Acad Sci U S A. 2017;114(10):E2016–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeDoux JE, Pine DS. Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. Am J Psychiatry. 2016;173(11):1083–93. [DOI] [PubMed] [Google Scholar]
- 35.LeDoux JE. Synaptic Self: how our brains become who we are. Harmondsworth, England: Viking Penguin; 2002. [Google Scholar]
- 36.Mujica-Parodi LR, Cha J, Gao J. From Anxious to Reckless: A Control Systems Approach Unifies Prefrontal-Limbic Regulation Across the Spectrum of Threat Detection. Front Syst Neurosci. 2017;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110(47):19119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(4):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170(10):1114–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur J Neurosci. 2006;24(10):2917–22. [DOI] [PubMed] [Google Scholar]
- 41.Wolf RC, Herringa RJ. Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41(3):822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14(5):1070–9. [DOI] [PubMed] [Google Scholar]
- 43.Strobl C, Boulesteix A-L, Zeileis A, Hothorn T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC bioinformatics. 2007;8(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomoda A, Polcari A, Anderson CM, Teicher MH. Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS One. 2012;7(12):e52528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schalinski I, Teicher MH, Nischk D, Hinderer E, Muller O, Rockstroh B. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCrory EJ, Gerin MI, Viding E. Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry - the contribution of functional brain imaging. J Child Psychol Psychiatry. 2017;58(4):338–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci. 2014;18(11):580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110(39):15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keding TJ, Heyn SA, Russell JD, Zhu X, Cisler J, McLaughlin KA, et al. Differential Patterns of Delayed Emotion Circuit Maturation in Abused Girls With and Without Internalizing Psychopathology. Am J Psychiatry. 2021;178(11):1026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang TT, Simmons AN, Matthews SC, Tapert SF, Bischoff-Grethe A, Frank GK, et al. Increased amygdala activation is related to heart rate during emotion processing in adolescent subjects. Neurosci Lett. 2007;428(2–3):109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage. 2005;24(3):751–62. [DOI] [PubMed] [Google Scholar]
- 53.Iffland B, Klein F, Rosner R, Renneberg B, Steil R, Neuner F. Cardiac reactions to emotional words in adolescents and young adults with PTSD after child abuse. Psychophysiology. 2020;57(1):e13470. [DOI] [PubMed] [Google Scholar]
- 54.Portnoy J, Cui N, Raine A, Frazier A, Rudo-Hutt AS, Liu J. Autonomic nervous system activity and callous-unemotional traits in physically maltreated youth. Child Abuse Negl. 2020;101:104308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharman LS, Dingle GA, Vingerhoets A, Vanman EJ. Using crying to cope: Physiological responses to stress following tears of sadness. Emotion. 2020;20(7):1279–91. [DOI] [PubMed] [Google Scholar]
- 56.Hubel DH, Wiesel TN. Early exploration of the visual cortex. Neuron. 1998;20(3):401–12. [DOI] [PubMed] [Google Scholar]
- 57.Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10(1):138–45. [DOI] [PubMed] [Google Scholar]
- 58.Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35(10):1540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laham BJ, Gould E. How Stress Influences the Dynamic Plasticity of the Brain’s Extracellular Matrix. Front Cell Neurosci. 2021;15:814287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baldwin JR, Reuben A, Newbury JB, Danese A. Agreement Between Prospective and Retrospective Measures of Childhood Maltreatment: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2019;76(6):584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards F, Wakefield S, Healy K, Wildeman C. Contact with Child Protective Services is pervasive but unequally distributed by race and ethnicity in large US counties. Proc Natl Acad Sci U S A. 2021;118(30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luby JL, Baram TZ, Rogers CE, Barch DM. Neurodevelopmental Optimization after Early-Life Adversity: Cross-Species Studies to Elucidate Sensitive Periods and Brain Mechanisms to Inform Early Intervention. Trends Neurosci. 2020;43(10):744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teicher MH, Gordon JB, Nemeroff CB. Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol Psychiatry. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. [DOI] [PubMed] [Google Scholar]
- 65.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All anonymized data used in these analyses have been deposited on Zenodo (doi: 10.5281/zenodo.5271714 and zenodo.7340449) and are open source.


