Abstract
Purpose
Colorectal cancer is one of the most common causes of cancer-related death, and its main site of metastasis is the liver. The surgical method used for metastases of colorectal cancer in the liver varies according to the lobe affected, as does the prognosis. However, there is a lack of relevant basic research. Therefore, a good animal model is needed for basic studies of metastases from colorectal cancer to the different lobes of the liver.
Methods
A CT26 colon cancer cell line transfected with a virus expressing green fluorescent protein was inoculated into BALB/C mice via the spleen. Tumor formation in the liver lobes was observed under a fluorescence microscope according to which portal vein branch was ligated and according to clamping time. The differential formation of metastatic lesions in the different lobes was then compared with physical anatomy. Serum samples were used to detect the changes in liver function postoperatively.
Results
Ligation and resection of the spleen 1 min after injection of the CT26 cells and release of the vessel clamp 1 min after splenectomy created an ideal tumor-bearing mouse model with little effect on liver function. Selective clamping of each portal vein branch and splenic injection of a CT26 cell line successfully established a selective liver lobe tumor-bearing model of colorectal cancer with distinct characteristics.
Conclusion
This model provides an opportunity for investigation of the mechanisms of metastasis of colorectal cancer to different lobes of the liver and may provide a basis for clinical treatment.
Keywords: colorectal cancer, liver metastases, selective tumor-bearing model, mouse
Introduction
Colorectal cancer (CRC) is one of the most common causes of cancer-related death worldwide, especially when metastatic disease is included. The most common site of metastasis from CRC is the liver. 1 More than 25% of patients with CRC have liver metastases at the time of diagnosis or develop them shortly after diagnosis.2,3 At present, hepatectomy is the most effective treatment for localized colorectal metastases. In recent years, the indications for treatment of CRC metastases to the liver have expanded. Unfortunately, despite advances in surgery, tumors can be surgically removed in only about 25% of patients. 4 Indications for resection of liver metastases are based primarily on tumor-related factors, such as tumor number, size, and distribution in the liver. 5 However, there has been little research discussion on whether the location of hepatic metastatic lesions affects prognosis, and the relevant basic research is limited. Kuo et al report that in colorectal cancer, centrally located liver metastasis represents a poor prognostic factor after hepatectomy, and is associated with early recurrence. 6 Therefore, the exact mechanism by which the location of hepatic metastatic lesions affects prognosis needs to be explored in animal models. However, a suitable selective liver lobe tumor-bearing animal model of CRC has not been established or validated.
There are already some animal models of liver cancer. First, direct injection of cancer cells via the portal vein is an ideal method for establishing metastases of CRC in the liver. However, because of the thin portal vein in mice, surgery and hemostasis are difficult and the postoperative survival rate is low. 7 Another commonly used method for forming hepatic metastases is to implant tumor tissue directly into the liver. 8 However, this method destroys the original morphological structure of the liver and makes hemostasis difficult, which limits the application of this animal model. Injection of tumor cells into the spleen is considered to be the best choice because it not only ensures the integrity of the liver tissue but also avoids the risk of bleeding. The present study aimed to provide an effective animal model for researchers to conduct basic research on the different liver lobe metastases of colorectal cancer.
Materials and Methods
Mice
BALB/C male mice purchased from Paisiao (Hangzhou, China) were used in this study. All mice were maintained under specific pathogen-free conditions in our laboratory (23 °C, 12 h light/dark cycle, 50% humidity, and libitum access to food and water). The mice underwent surgery when they weighed 20–25 g and were 6–8 weeks of age.
ARRIVE Guidelines Statement
The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE 2.0 guidelines. 9
Ethical Approval
The study protocol was approved by the ethics committee of Shaoxing People's Hospital (approval no. 2022Z006) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals. 10
Cell Culture
A CT26 colon cancer cell line was cultured in RPMI (Roswell Park Memorial Institute) 1640 dual antibody medium (Haoxin Biotechnology, China) supplemented with 10% fetal bovine serum (Haoxin Biotechnology) in an incubator maintained at 37 °C in an atmosphere containing 5% carbon dioxide with a relative humidity of 95%.
Virus Transfection
The virus expressing green fluorescent protein was purchased from Genechem (Shanghai, China). Virus transfection was performed as follows. CT26 cells were cultured in 6-well plates at a concentration of 2 × 105 per plate and prepared for transfection. The CT26 cells had a multiplicity of infection of 20. The virus was then transfected into the CT26 cells using HitransG P transfection reagent (25×; Genechem) to improve transfection efficiency. After incubation for 16 h, the RPMI 1640 complete medium was refreshed. After 48–72 h of incubation, the cells were observed by fluorescence microscopy, and the transfection efficiency was evaluated.
Anesthetic and Analgesic Regimens
The mice were placed in an anesthesia box and induced anesthesia was induced by inhaling isoflurane at a concentration of 3 Vol%, and then maintained by inhaling isoflurane at a concentration of 1 Vol%. 11 Confirm the level of anesthesia by pinching the foot reflex. Provide analgesia using subcutaneously applied buprenorphine (0.01 mg/kg) during the surgical intervention.
Determination of Optimal Time for Clamping of Each Portal Vein Branch
The mice were randomly divided into six groups (five mice per group). The right hepatic portal vein branch was clamped using a microvascular clamp, and 200 µL of a suspension containing CT26 cells transfected with the virus were injected into the spleen using a 1-mL syringe at a cell concentration of 4 × 106/mL. Splenectomy was performed at 1 min and at 3 min after injection of the CT26 cells in the different groups. The clamp was released at 0.5 min, 1 min, or 3 min after splenectomy. In each mouse, the abdominal wall was sutured layer by layer. The postoperative survival time was recorded in each group. Two necessary conditions were set: (1) tumor cell growth detected in the pathological sections of all liver lobes except for the right lobe and (2) a postoperative survival time of longer than 2 weeks. When both conditions were met at the same time, the shortest portal vein branch clamping time was determined to be the best (Figure 1a).
Figure 1.
Establishment of a selective liver lobe tumor-bearing mouse model of colorectal cancer. (a) Determination of the optimal portal vein branch clamping time. (b) Clipping of the portal vein branches in the different hepatic lobes.
Selective Liver Lobe Tumor-Bearing Mouse Model of CRC
Mice were randomly divided into four groups (20 mice per group). The right hepatic portal vein branch was clamped in the first group, the left and middle hepatic portal vein branches were clamped in the second group, no portal vein branch was clamped in the third group, normal saline was injected through the spleen in the fourth group (control group). The best portal vein branch clamping time was used (Figure 1b). Next, 200 µL of a suspension containing CT26 cells at a concentration of 4 × 106/mL was injected via the spleen, causing the mice to develop tumors in specific lobes of the liver.
Liver Function and Liver Lobe Weight
On postoperative days 1, 3, 7, and 14, five mice in each group were anesthetized for collection of blood through the orbital venous plexus. Serum samples were used to measure changes in aspartate transaminase and alanine transaminase levels after surgery.
The mice were anesthetized with isoflurane at an inhalation concentration of 3 Vol%, and the cervical vertebrae were dislocated after anesthesia. The liver tissue was surgically removed from each mouse after it had been euthanized. Non-tumor-bearing and tumor-bearing liver lobes were weighed and analyzed.
Histopathology Study
Fresh liver tissue was taken for frozen sections. The slides were then stained with hematoxylin and eosin and examined under a light microscope (TS100F, Nikon, Tokyo, Japan) with NIS-Elements image analysis software (Nikon).
Statistical Analysis
All statistical analyses were performed using GraphPad version 8.00 (GraphPad Software Inc., San Diego, CA). A p-value <0.05 was considered statistically significant.
Results
Establishment of a Colon Cancer Cell Line Stably Expressing Green Fluorescent Protein
After transfection with a virus expressing green fluorescent protein, the CT26 cells were observed by fluorescence microscopy (Figure 2). The results showed that the CT26 cells were successfully transfected with a transfection efficiency of more than 80%.
Figure 2.
The CT26 cell line under blank and fluorescent vision (×100).
Best Portal Vein Branch Clamping Time
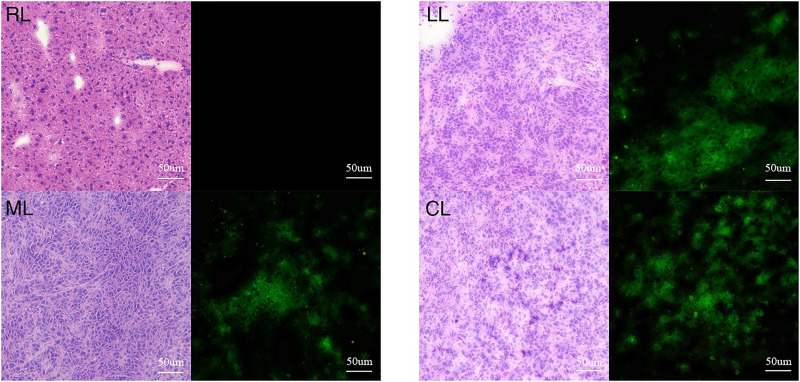
Observation of the mouse liver tissue sections under the fluorescence microscope showed that ligating and resecting the spleen 1 min after injection of the cells and releasing the vessel clamp 1 min after splenectomy resulted in an ideal tumor-bearing mouse model (Figure 3). All mice survived for more than 2 weeks.
Figure 3.
Mouse liver tissue sections under blank and fluorescent vision (×100). The liver tissue on the right contains normal hepatocytes with no bioluminescence in the fluorescence field. The other liver tissues (LL, ML, CL) show low tissue differentiation with bioluminescence in the fluorescence field. RL: right lobe; LL: left lobe; ML: middle lobe; CL: caudate lobe.
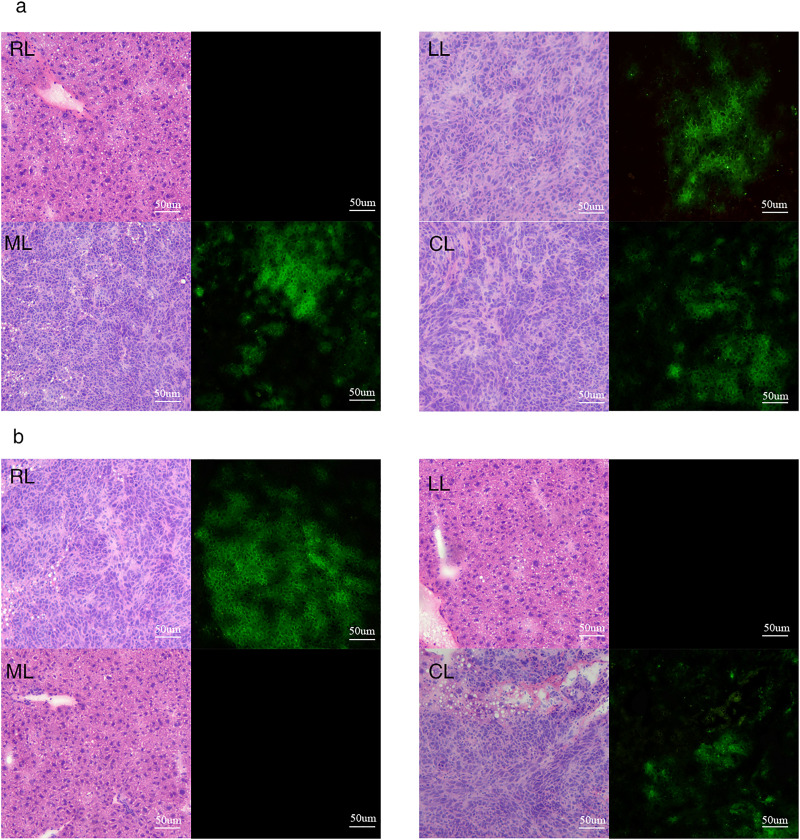
Successful Creation of a Selective Liver Lobe Tumor-Bearing Mouse Model of CRC
The mouse livers were dissected on days 1, 3, 7, or 14 after surgery, and significant changes in liver tissue were found (Figure 4). Fluorescence detection of mouse liver tissue sections from the groups in which the right hepatic portal vein branch was clamped and the left and middle hepatic portal vein branches were clamped revealed bioluminescence only in specific liver lobes (Figure 5). This finding confirmed successful establishment of a selective liver lobe tumor-bearing mouse model of CRC.
Figure 4.
Physical anatomy after surgery. (a) Changes in the liver on postoperative days 1, 3, 7, and 14 in the group in which the right hepatic portal vein branch was clamped. (b) Changes in the liver on postoperative days 1, 3, 7, and 14 in the group in which the left and middle hepatic portal vein branches were clamped.
Figure 5.
Mouse liver tissue sections under blank and fluorescent vision (×100). (a) Fourteen days after clamping the right hepatic portal vein branch. The right liver lobe was not tumor-bearing. The rest of the liver lobe contained tumors. (b) Fourteen days after clamping the left and middle hepatic portal vein branches. The left and middle liver lobes were not tumor-bearing. The rest of the liver lobe contained tumors. RL: right lobe; LL: left lobe; ML: middle lobe; CL: caudate lobe.
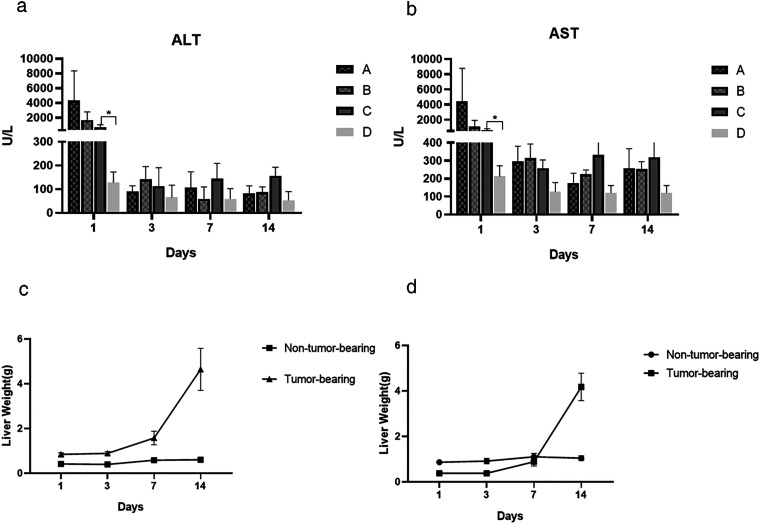
Changes in Indices of Liver Function
One day after injection of the CT26 cells, the indices of liver function increased significantly compared with injection of normal saline; however, there was no statistical significance in liver function among injection of the CT26 cells groups (Figure 6a, 6b). It indicated that the portal vein branch clamping time selected had little effect on liver function in mice, and the injection of CT26 cells has a great effect on liver function.
Figure 6.
Changes in liver function and weight of the different liver lobes after surgery (n = 5 mice per group). (a, b) Evaluation of aspartate transaminase and alanine transaminase in the different groups of mice (A, the right hepatic portal vein branch was clamped; B, the left and middle hepatic portal vein branches were clamped; C, the portal vein was not clamped; D, normal saline was injected through the spleen) of mice on different days after the operation. *P < 0.05. (c) Trend of different hepatic lobe weight in mice on different days after right hepatic portal vein clamping. (d) Trend of different hepatic lobe weight in mice on different days after left and middle hepatic portal vein clamping. ALT: Alanine aminotransferase; AST: Aspartate transaminase.
Changes in Liver Tissue Weight
In the groups that underwent selective portal vein branch clamping, the weight of the tumor-bearing liver lobes increased significantly after 3 days, with a peak in the rate of increase after 7 days; however, the weight of the non-tumor-bearing liver lobes increased slightly after 3 days but showed no significant change after 7 days (Figure 6c, 6d).
Discussion
CRC accounts for approximately 10% of all cancer diagnoses and cancer-related deaths worldwide each year. 12 The high rate of CRC metastasis to the liver and the prognosis of metastatic liver cancer have been a main focus of research. But few studies have investigated why there are differences in the progression and prognosis of metastasis of CRC to the different liver lobes. This has prompted researchers to develop suitable animal models in which they can study the disease process.
In our experiments, we used the CT26 cell line, which shares molecular characteristics with aggressive, undifferentiated, refractory human CRC cells. 13 By transfecting with a virus expressing green fluorescent protein into CT26 cells, we were able to observe tumor growth in each liver lobe more easily under a fluorescence microscope.
CT26 cells are not only easy to injection into the spleen but also have strong tumor-forming potential. Considering that the spleen is fragile and prone to bleeding and formation of splenic tumors after injection of tumor cell lines, we finally opted for ligation and resection of the spleen. Besides, the resection of the spleen also avoids flow of the tumor cells remaining in the spleen into the normal liver lobe after the vascular clamp was loosened.
Given that this experiment was the first attempt to develop this model, there was no appropriate portal vein branch clamping time that could be used as a reference. We first explored the time between tumor cell injection and splenectomy, allowing us to determine the shortest time required for cells to enter the liver and form a tumor. At the same time, we explored the interval between splenectomy and release of the vascular clamp that was needed to ensure that all injected cells flowed into the targeted liver lobe, so as to avoid formation of tumors in the designated non-tumor-bearing liver lobe. We found that resection of the spleen 1 min after injection of the CT26 cells was sufficient for tumor formation in the liver. Releasing the clamp 30 s after splenectomy could lead to tumor formation in the non-tumor-bearing lobe; however, releasing the clamp after 1 min achieved what we expected. Therefore, the spleen was ligated and excised 1 min after injection of the tumor cells, and the vascular clamp was released 1 min after splenectomy, which we considered to be the optimal clamping time for the portal vein branches. This procedure can minimize the damage to the mouse liver caused by clamping a portal vein branch while ensuring the success of the experiment.
Finally, we selectively clamped the right hepatic portal vein branch and the left and middle hepatic portal vein branches separately to allow formation of CT26 tumor cell metastasis in the different hepatic lobes, thus providing a reliable animal model for the study of metastasis of CRC in the liver.
The issues when using mice as model animals are their anatomy of the liver. Fundamentally, mice have heavily lobulated livers consisting of four major lobe groups called the right lateral, median, left lateral, and caudate. Each lobe has its own portal venous and arterial supply and a separate bile duct and all four lobes are connected to the inferior caval vein with individual hepatic veins. This is different from human liver anatomy.
Conclusion
In conclusion, the selective liver lobe CT26 tumor-bearing mouse model established in this study has similarities with the metastases of CRC that occur in the different liver lobes in patients. The CT26 cell line has great potential for metastasis in the liver. Our model of CRC liver metastasis in the various hepatic lobes can be constructed successfully by clamping the different portal vein branches and injecting tumor cells via the spleen. Overall, we believe that this animal model will provide a useful tool for basic studies of metastases of CRC to the liver.
Abbreviations:
- CRC
Colorectal cancer
- RL
Right lobe
- LL
Left lobe
- ML
Middle lobe
- CL
Caudate lobe
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
Footnotes
ARRIVE Guidelines Statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Authors’ Note: The senior authors, Zheqi Han and Biying Qiu, contributed equally to this work.
Data Availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Basic Public Welfare Research Project of Zhejiang Province, (grant number LGF22H160035).
Institutional Animal Care and Use Committee Statement: All experiments were conducted with approval of the Shaoxing People's Hospital Institutional Animal Care and Use Committee (licence No.2022Z006).
ORCID iD: Zhiyang Zhu https://orcid.org/0000-0001-9076-7288
References
- 1.Bhullar DS, Barriuso J, Mullamitha S, Saunders MP, O'Dwyer ST, Aziz O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine. Feb 2019;40:363‐374. doi: 10.1016/j.ebiom.2019.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. Aug 2006;244(2):254‐259. doi: 10.1097/01.sla.0000217629.94941.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. Jan 15 2018;18(1):78. doi: 10.1186/s12885-017-3925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. Nov 4 2014;14:810. doi: 10.1186/1471-2407-14-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akgul O, Cetinkaya E, Ersoz S, Tez M. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. May 28 2014;20(20):6113‐6122. doi: 10.3748/wjg.v20.i20.6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo IM, Huang SF, Chiang JM, et al. Clinical features and prognosis in hepatectomy for colorectal cancer with centrally located liver metastasis. World J Surg Oncol. Mar 4 2015;13:92. doi: 10.1186/s12957-015-0497-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares KC, Foley K, Olino K, et al. A preclinical murine model of hepatic metastases. J Vis Exp. 2014;91:51677. doi: 10.3791/51677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budai A, Fulop A, Hahn O, et al. Animal models for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): Achievements and future perspectives. Eur Surg Res. 2017;58(3-4):140‐157. doi: 10.1159/000453108 [DOI] [PubMed] [Google Scholar]
- 9.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol. Aug 2020;177(16):3617‐3624. doi: 10.1111/bph.15193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guide for the Care and Use of Laboratory Animals. 8th ed. 2011. The National Academies Collection: Reports funded by National Institutes of Health.
- 11.Herrmann K, Flecknell P. Retrospective review of anesthetic and analgesic regimens used in animal research proposals. ALTEX. 2019;36(1):65‐80. doi: 10.14573/altex.1804011 [DOI] [PubMed] [Google Scholar]
- 12.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. Nov 2018;68(6):394‐424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 13.Castle JC, Loewer M, Boegel S, et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics. Mar 13 2014;15(1):190. doi: 10.1186/1471-2164-15-190 [DOI] [PMC free article] [PubMed] [Google Scholar]