Abstract
This paper focuses on the question of, “When is the best time to identify an individual at risk for a treatable genetic condition?” In this review, we describe a framework for considering the optimal timing for pursuing genetic and genomic screening for treatable genetic conditions incorporating a lifespan approach. Utilizing the concept of a carousel that represents the four broad time periods when critical decisions might be made around genetic diagnoses during a person’s lifetime, we describe genetic testing during the prenatal period, the newborn period, childhood, and adulthood. For each of these periods, we describe the objectives of genetic testing, the current status of screening or testing, the near-term vision for the future of genomic testing, the advantages and disadvantages of each approach, and the feasibility and ethical considerations of testing and treating. The notion of a “Genomics Passbook” is one where an early genomic screening evaluation could be performed on each individual through a public health program, with that data ultimately serving as a “living document” that could be queried and/or reanalyzed at prescribed times during the lifetime of that person, or in response to concerns about symptoms of a genetic disorder in that individual.
Keywords: Genetic testing, gene therapy, presymptomatic, screening, newborn, lifespan
1. Introduction
When is the most appropriate time in a lifespan to presymptomatically identify an individual at risk for a treatable genetic condition? Now that gene-targeted therapies (GTT), such as oligonucleotide-based therapeutics, virus-mediated gene replacement strategies, and somatic genome editing, are available, there is the potential to correct up to 90% of known genetic variants associated with human disease (Anzalone et al., 2019). A consideration of the parameters for pondering this question was the focus for a 3-day workshop sponsored by the National Institutes of Health (NIH) held in June 2021 entitled, “Gene-Targeted Therapies: Early Diagnosis and Equitable Delivery” (National Institutes of Health, 2021). On the first day of the workshop, the speakers addressed the “Who, What, and When” of developing a conceptual framework for considering the application of GTT to human disease.
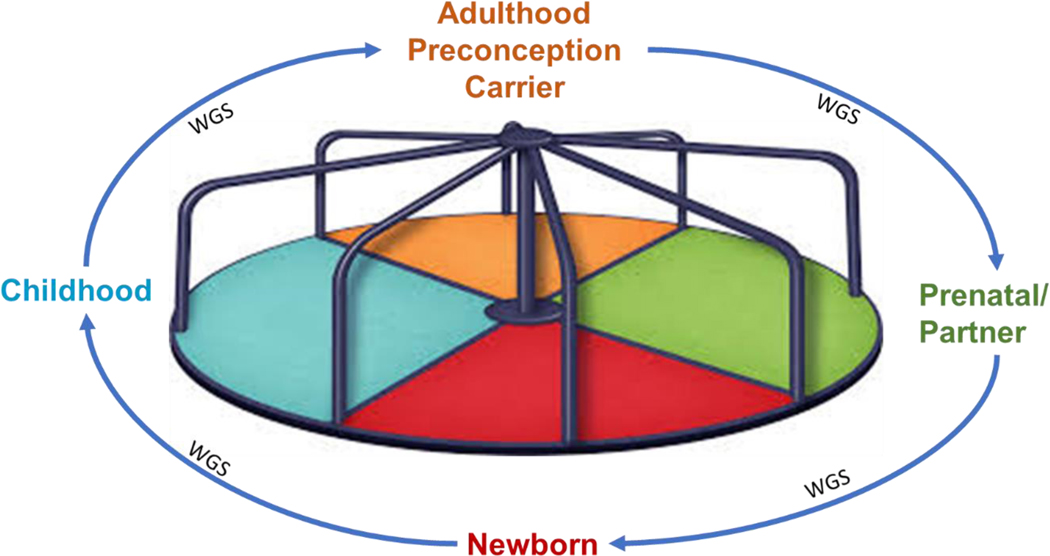
Currently, we find ourselves shifting into a territory where potential GTT treatments for disorders may exist; however, there is no system in place that can effectively and equitably identify individuals that could benefit from such treatments in a timely manner. This article will focus on the issues of “When” to screen for treatable genetic disorders at various stages of human development. The goal of a lifespan approach is to deliver GTT before disease onset or early enough in the disease course to realize a better or optimal outcome. The notion of a “Genomics Passbook” is one where an early whole genome sequencing evaluation could be performed on each individual through a public health program, with that data ultimately serving as a personal “living document,” like a banking account “passbook,” that could be queried and/or reanalyzed at prescribed times during the lifetime of that person, or in response to concerns about symptoms of a genetic disorder in that individual. This approach is similar to the “age-based genetic screening” paradigm proposed by others (Mollison & Berg, 2017), in which discrete excerpts of the genome are targeted and interpreted at appropriate stages of a child’s development for conditions that are relevant to the age of the child, while also involving the child in the assent and consent process, as appropriate. As proposed, however, this approach would most likely involve repeated sampling and periodic reanalysis using targeted screening for conditions with a substantial knowledge base, high actionability, and well-established clinical interventions. In contrast, our approach would be based on an early whole genome sequencing effort with periodic re-analysis and reevaluation at age-appropriate timepoints. A different representation of our approach involves a carousel that is segmented into four different time periods in an individual’s life where screening could be conducted or the results within the “passbook” could be revisited. (Figure 1). The carousel establishes the framework for the discussion that follows.
Figure 1.
The carousel of genetic diagnosis options during critical windows of life development. The carousel represents the four broad categories of time during the lifespan when genetic diagnosis might be valuable in improving health outcomes.
2. Prenatal and preconception
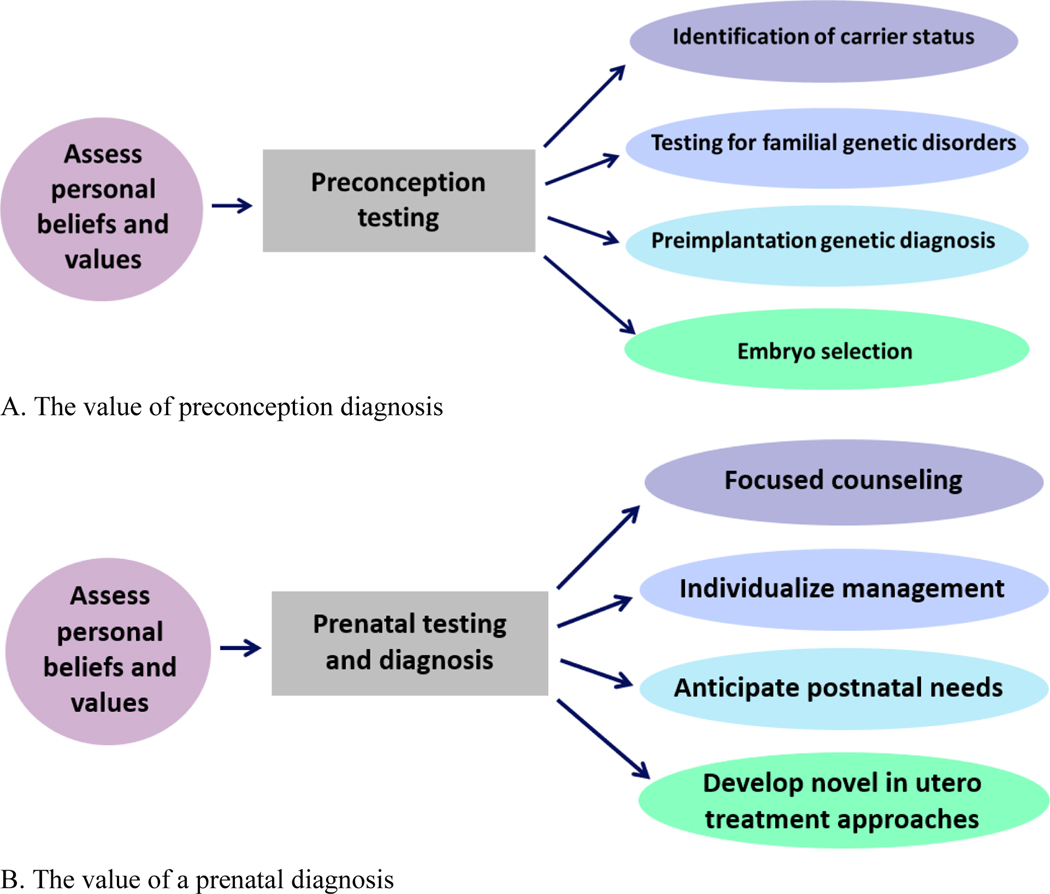
Early-life genomic data can prospectively identify individuals with genetic conditions before the onset of symptoms, with the intention to treat in the prenatal period or prevent in the preconception period. In fact, preconception testing has the potential to identify whether partners are carriers of one of many known genetic disorders that could be passed to their fetus, and subsequent prenatal testing can identify if a fetus carries a genetic diagnosis. In some cases, preimplantation genetic diagnosis and embryo selection can be carried out, typically following genetic counseling, if the couple has resources and a desire to select for implantation of an unaffected embryo (Gregg & Edwards, 2018) (Figure 2A).
Figure 2.
The value of a preconception or prenatal diagnosis. (A) The value of preconception diagnosis; (B) The value of a prenatal diagnosis.
As the cost of next-generation sequencing decreases, more couples may choose to undergo preconception genetic testing for carrier status. The current state of available preconception testing (for adults) includes carrier screening for autosomal recessive and X-linked conditions offered by commercial laboratories. Currently, preconception testing is available to any partners with or without family history of genetic conditions. There has been significant debate about whether there is a role for ethnicity-based carrier screening, focused on conditions known to be more prevalent in a specific population, versus pan-ethnic or even expanded carrier screening, where up to several hundred conditions are screened for simultaneously in a prospective parent. As screening for more conditions has been made available, variants with potential pathogenic significance have been identified even in individuals outside of the presumptive “high-risk” ethnicity groups. In fact, the American College of Obstetricians and Gynecologists has provided guidance on carrier screening and recommends offering expanded carrier screening to all women, regardless of family history or race (“Committee Opinion No. 691: Carrier Screening for Genetic Conditions,” 2017). Some researchers have noted that the best time to offer expanded carrier screening is during preconception or early in the first trimester to increase the likelihood of uptake (Larsen et al., 2019).
In pregnancies where couples chose in vitro fertilization with pre-implantation genetic testing (PGT) of embryos to avoid conceptions with a genetic condition, studies have shown that there is variability in the motivations, decision-making factors, and experiences of the patients who undergo the procedure, and many couples find the process impractical and expensive as well as psychologically demanding (Hughes et al., 2021). Thus, while the options for preconception testing continue to expand, suggesting this might be an ideal time to pursue genetic testing, the implications for a couple at-risk for conceiving an affected fetus create stress and financial burdens for them.
In contrast to pre- or peri-conception genetic testing, prenatal testing and diagnosis can allow for: 1) focused counseling; 2) individualized management; 3) anticipation of postnatal needs; 4) development of novel in utero treatment approaches, particularly for fetuses at known risk for genetic disorders or those with prenatally detected anomalies (Figure 2B). The current state of available prenatal testing of a fetus includes cell-free fetal DNA (cffDNA) evaluation from a maternal blood sample (also known as non-invasive prenatal screening or testing, NIPS or NIPT) to screen, typically for aneuploidies, and chorionic villus sampling (CVS) or amniocentesis for targeted genetic diagnostic testing. Prenatal testing is often reserved for individuals with advanced parental age, a known family history of a genetic condition, a positive screen on cffDNA screening conducted to investigate for aneuploidies or single-gene disorders, or fetal ultrasound abnormalities (Jelin & Vora, 2018; Sabbagh & Van den Veyver, 2020; Shaw et al., 2020; Vermeesch et al., 2016).
Commercial and research laboratories are actively working to determine and validate methods to use cffDNA to screen or even test for conditions beyond the common aneuploidies. While these technologies are currently available, they are not yet broadly implemented. These tests are heavily marketed to pregnant people and healthcare providers, mostly obstetricians, and there has been some controversy about how to accurately interpret positive findings for rare disorders with a low positive predictive value (Kliff & Bhatia, 2022). Since CVS and amniocentesis are still considered invasive testing options, the expansion and implementation of cffDNA technology could increase the number of expectant parents who choose to have genetic screening for autosomal recessive, X-linked, and autosomal dominant conditions, not yet consistently captured by cffDNA screening.
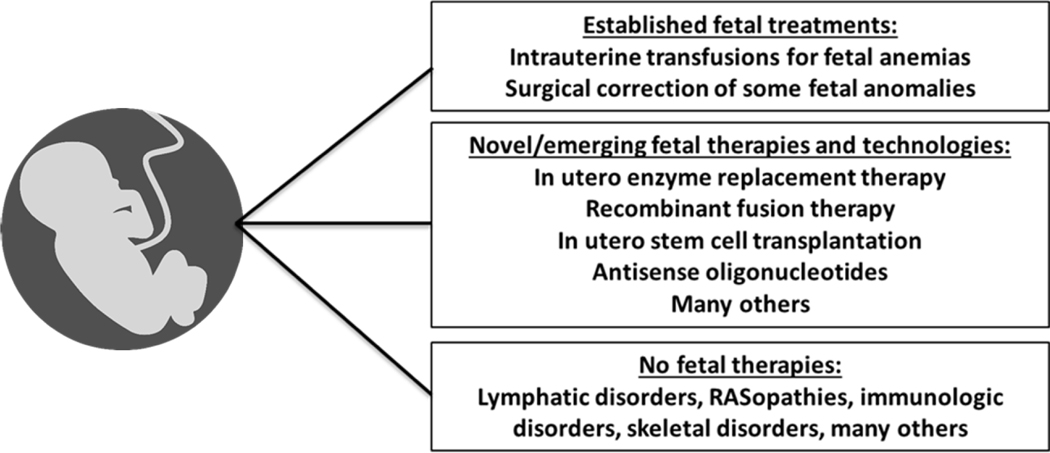
The advantage of identifying a genetic disease affecting a fetus or identifying risk to the expectant couple based on their carrier status is that it may allow for earlier intervention, including in utero treatment options (Figure 3). At present, there are at least three active clinical trials in the United States for treating genetic diseases prenatally: the first is for alpha-thalassemia (NCT02986698) aimed at evaluating the safety of in utero hematopoietic stem cell transplantation in fetuses with alpha-thalassemia major; the second is for eight different early-onset lysosomal storage disorders (LSDs) (NCT04532047) aimed at evaluating the safety of disease-specific in utero enzyme replacement therapy for infantile-onset LSDs (Kreger et al., 2016; Nguyen et al., 2020); and the third is an intra-amniotic administration of a protein to treat X-linked hypohidrotic ectodermal dysplasia (NCT04980638). In the future, these in utero treatment trials may become more widely utilized and may expand to include additional genetic diseases and/or additional treatment modalities, including, but not limited to, a variety of gene therapy approaches, including antisense oligonucleotides (ASO). The disadvantage of uncovering a genetic disease in a fetus or determining an increased risk for genetic disease during procreative testing is that some individuals may not have access to the resources to be tested or treated in order to take advantage of all the medical options currently available, or may find these options overwhelming, anxiety-provoking, or ethically problematic.
Figure 3.
Examples of the evolving field of fetal medicine.
In order to successfully accomplish widespread preconception and prenatal testing, there needs to be extensive education efforts and broader insurance coverage available to more individuals for this service. Expectant parents need to understand the options for preconception testing and the limitations of cffDNA screening that provides information about risks, as opposed to more definitive diagnostic testing (including broader next generation sequencing, microarray, or exome sequencing) that can be accomplished through CVS or amniocentesis. Many insurance companies either do not cover, or only partially cover, the cost of these tests, which can be a barrier for many families. Genetic counselors and physicians need to be accessible and available for pre- and post-testing counseling and management options if a test result is positive.
In the case of any in utero procedure or treatment, there are inherently at least two patients for whom the risk/benefit must be considered, and therefore, ethical questions may arise. The risk to the mother must factor into the calculation when deciding whether to conduct invasive diagnostic genetic testing or to treat with therapies administered through the umbilical cord vein of a fetus. Another ethical issue is uncovering a risk for disease (during preconception counseling) or a diagnostic result (on fetal diagnostic testing) for which there is no clear early treatment or intervention. Lastly, the risk of false positive screening results that are not followed up with appropriate definitive diagnostic testing may result in premature termination of a healthy fetus or other management decisions based on incorrect or unconfirmed prenatal information (Dar et al., 2014), again highlighting the importance of genetics education and understanding about the distinction between screening tests and diagnostic results.
3. Newborn
Newborn screening (NBS) is a universally available public health program run by states to screen and detect newborns with diseases on the “Recommended Uniform Screening Panel” (RUSP). As of 2022, there were 36 core and 26 secondary conditions on the RUSP with the numbers increasing depending on successful nominations (Health Resources & Services Administration, Advisory Committee on Heritable Disorders in Newborns and Children). State program data indicate that > 99% of all newborns are screened for most of the disorders on the RUSP, with some states screening more or less than the RUSP conditions (Urv & Parisi, 2017).
A federal Advisory Committee for Heritable Disorders in Newborns and Children (ACHDNC) was established by the Newborn Screening Saves Lives Act of 2007. The ACHDNC is tasked with making recommendations to the Secretary of Health and Human Services (HHS) for conditions to add to the RUSP, in a process that has been codified and modified slightly over the past decade. For consideration, a condition must be nominated for addition to the RUSP (Figure 4; Health Resources & Services Administration, Condition Nomination and Review). A nomination package can be submitted by anyone, and generally combines input from clinicians, newborn screening staff, researchers, and advocacy group members. Once the nomination is submitted, the package is reviewed for completeness by staff at the Health Resources & Services Administration (HRSA) and referred to a condition review committee. A presentation is made to the ACHDNC and the committee votes on whether or not to advance the condition to full evidence review. The evidence review group (ERG) conducts an extensive assessment of existing data on the natural history of the condition, therapies and clinical outcomes, availability of screening and diagnostic tests, and availability of specialists to treat the condition. The ERG also collects feedback on the public health impact and screening laboratory readiness to implement the test from surveys conducted by the Association of Public Health Laboratories (APHL). The full evidence review is conducted over a 9-month period. After the data are presented to the ACHDNC, a vote is taken to determine whether the committee should recommend that the Secretary of HHS add the condition to the RUSP. The recommendation is then sent to HHS, and the Secretary has 120 days to respond. For conditions added to the RUSP since it was initially established, there is a broad range of time frames that have elapsed from the time a nomination package was submitted to HRSA to when the Secretary of HHS has approved its addition to the RUSP, ranging from 14 months to 4 years; for several conditions, this was only successful on the 2nd or 3rd nomination attempt. Currently, approximately two evidence reviews are conducted each year, a rate which is slow compared to the rate at which therapies are expected to be developed. There is pressure on the newborn screening systems to add disorders at a faster rate (Bailey, 2022; Bailey et al., 2021), but the actual rates of adding new conditions are very dependent on state requirements and resources necessary for implementation of new conditions, which are highly variable. In fact, a visualization of the status of NBS for each condition demonstrates the highly variable rates of uptake by each state within the United States; while some conditions such as spinal muscular atrophy (SMA) have been rapidly adopted, others such as X-linked adrenoleukodystrophy (X-ALD) have languished in adoption rates despite being added to the RUSP earlier (Association of Public Health Laboratories; Figure 5). These delays in adoption often reflect variability among the states with regard to the legal authority to add a condition, the funding required to add new conditions, including ability to hire adequate staff and purchase needed equipment, and the time required to validate a new assay and establish cutoffs for it. The result is inequities in access to testing and diagnosis for these rare but treatable disorders.
Figure 4.
The ACHDNC nomination, review, and decision-making process for adding a new condition to the Recommended Uniform newborn Screening Panel (RUSP). Adapted from source: (Health Resources & Services Administration).
Figure 5.
Variable rates of uptake by state NBS programs for X-linked Adrenoleukodystrophy (X-ALD) and Spinal Muscular Atrophy (SMA). As of August 2022, 23 states do not currently screen for X-ALD despite the condition being added to the RUSP in 2015 (top), whereas only 5 states do not screen for SMA even though it was added to the RUSP in 2018 (bottom). Source: (Association of Public Health Laboratories).
Recent modernizations of NBS have been modest and have led to the development of second-tier testing to increase specificity as well as the use of more molecular-based testing. Conceivably, sequence analysis using high throughput methods could be used to detect numerous monogenic conditions, particularly those without a biochemical marker. A recent publication by experts in NBS evaluated some strategies to truly modernize NBS, but there are no easy answers (Bailey et al., 2021).
Although NBS is the only universally performed screening of almost all individuals, there are some challenges to its utility and expansion. One disadvantage is that NBS results can indicate an increased risk for disease that manifests later in life, beyond the newborn period. In these situations, that baby’s screen could serve as the index case for a family, indicating increased risk for disease in older family members, and requiring cascade testing of these other at-risk family members. There are challenges around decisions regarding informing other family members of this newly identified disease risk. When do you tell? Whom do you tell? How do you ensure disclosure of the information when many family members may be geographically or culturally dispersed, or not in close communication? Additionally, the anticipated potential explosion in available therapies leading to numerous additions to screening panels will significantly impact the genetics provider community. Many areas of the country lack sufficient coverage of medical specialists, dietitians, and genetic counselors to manage current caseloads for screen-positive newborns. Thus, while considering the expansion of NBS conditions, the availability of adequate expertise for management of babies with these new disorders must also be taken into account (Jenkins et al., 2021).
The current approach of adding disorders through the ACHDNC is likely to be slow compared to the rate of available treatments. However, the slowest step in the process can be identification of an appropriate biomarker and development of an assay to identify the NBS condition, traditionally based on a biochemical test. Although high throughput genetic or genomic sequencing is often regarded as a possible solution, the cost, time for testing and variant interpretation, and expertise required are major challenges for implementation of this approach for first-tier NBS screening nationwide, currently. In the future, these barriers may be overcome with automation and regionalization of screening.
A program taking steps to move beyond traditional NBS is the “Early Check” program in North Carolina (Bailey et al., 2019), where voluntary supplemental screening for a select group of rare disorders that are not on the RUSP is made available to parents of infants prior to symptom onset under a partnership with the state NBS program. Initial conditions included in the Early Check program were SMA (prior to its addition to the RUSP), Fragile X syndrome, and Duchenne muscular dystrophy (DMD) (Peay et al., 2022). Such a program could potentially provide an opportunity for gathering evidence to support a RUSP nomination for conditions where the natural history is sparse, the evidence base for the benefit of early screening is limited, or there are no pilot data available.
There are several ethical issues related to NBS and its potential expansion (Goldenberg et al., 2019). One such emerging issue is related to the mandate for NBS and the right of parents to opt-out of screening. All but four states have some mechanism for parents to “opt out” of screening, but there are increasing concerns among some that this testing should be conducted as standard of care, without parental consent. The addition of widescale genomic analyses will likely complicate and magnify these issues, as explored in the Newborn Sequencing in Genomic Medicine and Public Health (NSIGHT) program funded by the National Institutes of Health (Dangouloff et al., 2022; Johnston et al., 2018; National Human Genome Research Institute). Another concern is “ownership” of the DNA, the data, or even the dried blood spot itself. Questions include: How long are these stored? Where and how are they stored? Why are they stored? What are their future uses? What is done with screening data? and Who has access to the data? The implementation of genome-wide analyses in the context of NBS will require a change in NBS practice, and inclusion of an informed consent component is seemingly inevitable. If such practice is moved out of the traditional NBS setting and privatized, i.e., making it available as a fee-for-service, there will not be universal access to this component of screening, and only those with resources will be able to afford it. Furthermore, while NBS is universally conducted, regardless of a family’s ability to pay, the follow-up, diagnostic evaluation, and access to treatments may not be as universal, or if available, perhaps not as timely. In addition, the ability to obtain treatment and manage children requiring complex regimens may be difficult for families with geographic or socioeconomic challenges. NBS prides itself on releasing timely results, but if families are challenged in obtaining treatment for their children, the system fails them. Lastly, while newly available treatments and those under development for rare diseases are highly lauded, the cost to families and the healthcare system may preclude their widespread availability for all who need them. Although the cost of gene-targeted therapies for SMA, for example, is very expensive, thus far, differential access to this lifesaving treatment has not appeared to produce inequities, and cost-effectiveness is relatively high (Shih et al., 2022). Monitoring for such issues is necessary.
4. Childhood
The purpose of screening during childhood (beyond the neonatal period) is to identify children at risk of a genetic disorder prior to the onset of irreversible symptoms. Some of the earliest markers of genetic conditions in childhood are the failure of children to meet growth, developmental, or behavioral milestones. While a newborn has a limited repertoire of developmental tasks, aside from breathing, eating, and exhibiting some primitive reflexes and gross motor skills, childhood affords the opportunity to monitor developmental milestones (gross motor, fine motor, language, social/adaptive) during this dynamic process of child development.
Most of the current childhood screening emphasizes “red flags” or failures to achieve developmental milestones and are typically symptom-based. For example, many pediatricians use the Denver Developmental Screening Test (DDST) or the Ages and Stages Questionnaires (ASQ) from 1 month to up to 6 years to screen for developmental delays, and the Modified Checklist for Autism in Toddlers, Revised (M-CHAT-R) between 16–30 months to screen for symptoms of autism spectrum disorders (ASD). Parent-focused tools include the Centers for Disease Control and Prevention (CDC)’s milestone tracker app with photos and videos of developmental skills and guidance for when parents should discuss their concerns with their child’s medical provider (https://www.cdc.gov/ncbddd/actearly/milestones-app.html). The American Academy of Pediatrics recommends developmental and behavioral screening for all children during well-child visits at 9, 18, and 30 months of age and screening for ASD at 18 and 24 months (https://www.cdc.gov/ncbddd/childdevelopment/screening.html). In particular, between 18 months and 3 years of age, lack of language development becomes a source of potential concern. CDC-developed growth charts and head circumference measurements (Centers for Disease Control and Prevention, Growth Charts) serve as screening tools for failure to thrive, growth delays, or overgrowth, all of which can be associated with genetic disorders. If a concern about growth or development arises in childhood, a referral may be made to a developmental pediatrician, neurologist, or geneticist, a process that can be time-consuming and result in significant delays in diagnosis due to a lack of specialists in many parts of the country and that is impacted by racial and gender disparities due to varying practices among states (Dragojlovic et al., 2020; Kilmer & Boykin, 2022). There is no standardized approach to screening nationwide, and there is a patchwork of specialty providers available to provide timely diagnostic and evaluation services. Due to these constraints, there is often a months-long delay between the initial concerns about developmental delay and eventual genetic diagnosis. This delay may have deleterious consequences on the child if an available treatment is delayed.
If genomic sequencing were instituted at birth or in infancy, every child could potentially have a “Genomics Passbook” or related file as a part of their electronic health record (Figure 6). An advantage of this approach is that the child’s genomic information could be accessed at any time, and could be queried at specified timepoints during development. The information would be owned and controlled by the individual (or their parent/guardian if a child), with opportunities to opt-in or opt-out of future queries, and options for sharing the information with family members, clinicians, and researchers, as requested. Standard ages for re-interpreting the “Genomics Passbook” could be established, at 2 years, 6 years, 10 years, and 14 years, for example, with age-appropriate windows of genes open for evaluation and reporting to healthcare providers and families if pathogenic or likely pathogenic variants were identified, to enable prompt treatment.
Figure 6.
A consolidated “Genomics Passbook” could contain genetic information that follows an individual from prenatal or birth throughout their lifetime.
Another potential use of the “Genomics Passbook” would be to query the data if specific concerns arise. These queries would analyze the existing genomic data for causative variants to explain the child’s condition and might provide diagnostic information very early in disease course, when intervention would be feasible to minimize disease burden. Using this approach in children with developmental or other health concerns might identify disorders that are serious, potentially treatable, and not on current NBS panels. Directly querying existing genomic data would likely minimize the diagnostic delays that are currently all too frequent. For example, a boy with delays in gross motor milestones or who was slow to walk could have a deeper look at his genome for genes associated with muscle disease, such as DMD or a panel of muscular dystrophy-associated genes. Likewise, if a child were to develop epilepsy or hearing loss, the existing genomic data could be evaluated to identify pathogenic variants in genes associated with these conditions. This would enable the sample and/or data that were obtained in the newborn period as a screening tool to also function as a diagnostic tool if the need arose. This approach would likely not only significantly decrease the length of the diagnostic odyssey, but would also yield significant cost savings by obviating the need for additional diagnostic genetic testing.
Advantages of this approach would be the presymptomatic diagnosis and management of many children with potentially treatable (or possibly curable) disorders, resulting in healthcare savings. Disadvantages of this approach include the unintended consequences of identifying potentially untreatable disorders and providing information to families that robs their child of the right to an open future. The identification of medically actionable variants with childhood implications could be both an advantage and a disadvantage. A framework for which genes and variants to report could be the “American College of Medical Genetics and Genomics (ACMG) 73” list of genes for which secondary findings are deemed appropriate for reporting back given their medical actionability (Miller et al., 2022; Miller et al., 2021). In these cases, reporting back pathogenic variants has implications for the child’s future, and can influence positive lifestyle choices in the short-term and long-term, but may also impact the health and welfare of extended family members, particularly for hereditary cancer syndromes. Yet another downside to this approach is the potential to identify a variant with reduced penetrance or a VOUS—variant of uncertain significance--and create stress for families and children who become “patients in waiting” but may never develop manifestations of the condition.
The feasibility of this approach relies on the affordability of genomic sequencing for all children, a willingness to undergo testing, and the personnel and resources to interpret these data in a meaningful way throughout the lifespan. Workforce issues with regard to adequate genetic counselors and geneticists to interpret genomic sequences are significant, as are the reimbursement sources for the costs of periodic reinterpretation throughout the lifespan. In addition, given the rapidity with which sequencing technologies are evolving, yesterday’s whole genome sequence may be deemed far inferior to the sequencing capacity in 5 years’ time, rendering that initial “Genomics Passbook” obsolete and the resources expended to generate it a waste of public dollars. It could also lead to increasing disparities between those with access to these newer technologies and the majority without.
In addition to all of the potential “cons” and the concerns articulated under feasibility and access, there are the added ethical issues of “just because we could do it doesn’t mean we should do it.” Should such a program remain a voluntary one, to avoid stigmatizing children diagnosed presymptomatically with potentially devastating diseases in the future? Concerns about genomic data storage, privacy, and access would also need to be considered before widespread genomic sequencing were adopted in a public health program. And a necessary component would be trust in the healthcare system that operates the program and manages the genomic information to ensure it would not be used for purposes other than improving the health of the individual.
5. Adulthood
Current newborn screening programs have led to improved morbidity and mortality in infants with a subset of rare disorders, but individuals with other treatable genetic disorders may not receive diagnoses until many years after symptoms have developed. As such, many adults may not receive genetic diagnoses or be offered appropriate treatments until their disease has progressed to more advanced stages. Screening adults for treatable genetic disorders, as well as those for which there are expert guidelines for surveillance, has the potential to improve health as well as healthcare spending.
Within unselected populations, undiagnosed genetic disease risk is common. Pathogenic and likely pathogenic variants (P/LP) in the 56–59 (now 73) cancer and cardiac disease genes originally designated by the ACMG as returnable secondary findings have consistently been found in 1–3% of individuals (Amendola et al., 2015; Dorschner et al., 2013; Green et al., 2013; Hoell et al., 2020; Natarajan et al., 2016). Among 21,915 participants in the Electronic Medical Records and Genomics Network Phase III (eMERGE III) network, 3.02% were found to have at least one P/LP in a panel of 78 actionable genes. Retrospective analyses of hospital-based biobanks have revealed individuals with unrecognized monogenic cancer predisposition syndromes, cardiac disorders, and multi-system conditions such as Marfan syndrome and Noonan syndrome (Abul-Husn et al., 2016; Bar-Mashiah et al., 2022; Blout Zawatsky et al., 2021; Buchanan et al., 2018; Damrauer et al., 2019; Haggerty et al., 2019; Manickam et al., 2018; Park et al., 2020; Rosenblum et al., 2020; Wenger et al., 2021). Similar rates of medically actionable findings (2.1–4.1%) were reported in adults who underwent genetic testing for Tier 1 conditions as established by the CDC, which include hereditary breast and ovarian cancer (HBOC), Lynch syndrome, and familial hypercholesterolemia (FH) (Centers for Disease Control and Prevention, Tier 1 Genomics Applications and their Importance to Public Health; Schmidlen et al., 2022). Individuals with other treatable genetic conditions, such as inherited metabolic disorders, may have had false-negative newborn screening, or may have immigrated from countries where NBS is not conducted, suggesting that both classic and subclinical cases of these disorders may also be underdiagnosed (Sirrs et al., 2016; van Wegberg et al., 2021). Taken together, these data suggest that there may be a considerable proportion of adults at risk for genetic disease who might benefit from further evaluation and management.
Delaying evaluation by a medical geneticist or other related specialist may lead to irreversible progression of disease in some adult patients. For example, a patient with Hurler-Scheie syndrome, a lysosomal storage disorder, may experience a range of debilitating symptoms, including hearing loss, joint pain and contractures, or craniofacial changes, before they may be referred to a medical geneticist. Although an approved enzyme replacement therapy is available for this disorder, many of these symptoms will have become irreversible by the time that treatment can be initiated. Proactive screening of individuals at risk for this condition could instead prompt referral to a genetics specialist, leading to appropriate diagnostic biochemical blood laboratory testing, and monitoring for the emergence of related symptoms. When treatment with enzyme replacement therapy is initiated at the first sign of disease, adults with this condition may experience improved quality of life. Furthermore, preempting the lengthy diagnostic odysseys common among rare disease patients, whether children or adults, may eventually diminish healthcare spending by providing a more direct route to appropriate care.
Commercial genetic diagnostic labs are starting to offer consumer-initiated testing, i.e., offering individuals the option to order their own genetic tests (e.g., cancer screening, pharmacogenomics), with results returned directly to them. While these are generally targeted gene panels, their popularity supports the concept that many adults are amenable to the idea of expanded genetic screening as a means to control aspects of their current and future health.
In addition to using existing genomic data in the “Genomics Passbook” as a screening tool, it could also potentially be queried on an as-needed basis for specific concerns. For example, an individual who becomes aware of a personal breast cancer risk due to BRCA1 because of family history could have their data queried for the specific variant. Concerning health symptoms, like the development of kidney disease or early-onset dementia, could similarly result in a data query to identify causative genetic variants. In addition, the data could be accessed for prenatal carrier screening in a prospective parent, or it could even be used as a source for trio testing to help diagnose that person’s child if suspected of having a genetic condition. An additional benefit of screening adults includes the detection of risk alleles that may be actionable such as cancer predisposition and cardiomyopathy variants. Positive results may guide enhanced surveillance to detect these illnesses at stages when treatment is likely to be maximally impactful. Genetic screening for adults, if viewed as a part of routine health maintenance, should be readily incorporated into existing screening guidelines, along with electrocardiogram (EKG), cholesterol levels, and mammography. From a cost-benefit analysis, the cost of genetic testing will likely be lower than that of other currently accepted, and insurance-covered, routine adult screening tests such as mammography. The “Genomics Passbook” for adults would allow autonomy for that person’s ownership of their genomic data but would also serve as a living document that could be shared with family members and healthcare providers with their permission. It could provide varying levels of information depending on the education level of the user, and in order to have maximal benefit to the broader community, the data could be provided in a federated, de-identified manner to rare disease groups and researchers (Figure 6).
While there are obvious benefits to early detection, there are also concerns about screening adults for risk alleles, including the ambiguity about defining a precise risk of disease, which may lead to anxiety. In addition, equitable access to testing and follow-up are necessary components of screening programs. Adult-onset genetic screening is likely to yield information about genetic risk based on specific alleles, which may differ based on genetic ancestry, and which may be less informative in certain populations. A positive result may dictate a need for additional testing, imaging, or other studies that may not be available or accessible to all populations.
6. Discussion
The advent of genetic and genomic sequencing, along with the availability of gene-targeted therapies with high fidelity and potentially broad applicability, has revolutionized the practice of medicine and provided an unprecedented opportunity to intervene for genetic conditions that can be diagnosed prior to the development of irreversible symptoms. But although the costs of genomic sequencing have fallen dramatically, for many individuals, the costs are still prohibitive unless insurance will cover testing for a medical indication. As a consequence, many individuals are not offered routine diagnostic sequencing until it is too late to effectively treat the condition. This paper provides a lifespan perspective to guide decision-making around genomic testing at four critical timepoints, representing a continuity or carousel of genetic diagnostic life stages: prenatal, newborn, childhood, and adulthood. Each timepoint has a set of considerations unique to that period, but there are some shared elements across the lifespan.
While the earliest possible timeframe to provide genetic risk information may be afforded by carrier or preconception screening, that form of testing is typically focused on known conditions with well-defined genetic risks to the offspring, such as Mendelian syndromes or metabolic disorders. Carrier testing in particular is like the “Wild West” due to the ever-expanding number of genes and variants associated with recessive or X-linked disorders that have been added to many panels since more targeted, ancestry-based testing is no longer as reliable for identifying all carriers who may not match the expected race or ethnicity. However, as more diverse cohorts are tested, the possibility of identifying a VOUS increases correspondingly, leading to confusion and anxiety due to unknown risks to the fetus. Nonetheless, preconception testing affords the opportunity to identify carrier status prior to pregnancy and pursue preimplantation genetic diagnosis and embryo selection prior to implantation for those with known risks and the resources to afford such technologies.
Similarly, prenatal screening using cffDNA has exploded in the past decade as screening is being heavily marketed to many pregnant people regardless of age. However, these screening tests may provide a false assurance that a pregnancy is “normal,” when they only screen for a handful or a limited number of conditions; on the other hand, a positive screen must be followed with definitive diagnostic testing, because the majority of assays have relatively low yield and poor positive predictive value, leading to unnecessary worry on the part of prospective parents and/or termination of a healthy pregnancy. For families with known risks, the benefits of focused counseling and pregnancy management can help anticipate the needs of an affected neonate in the perinatal and early prenatal period. Promising fetal therapies are either available or on the horizon, providing the potential to intervene prior to symptom onset for those conditions with very early manifestations.
The newborn period is one of the best-established timeframes for screening for those conditions with neonatal onset for which treatments are currently available. Conventional state NBS programs adhere to a screening paradigm that predominantly uses biochemical or metabolite-based assays, with targeted genetic testing as a part of 2nd tier or confirmatory testing. The process for adding a new condition to the uniform recommended panel of NBS conditions under the auspices of a federal advisory committee is well-established but not necessarily efficient for adding groups of related disorders at one time; moreover, the uptake of new conditions to state screening panels is highly variable, leading to discrepancies in access to diagnosis and treatments. The introduction of genomic sequencing in neonates is one that has been explored through research programs but is not yet readily available for widespread adoption due to practical considerations and ethical issues.
In childhood, screening is based primarily on “red flags” that emerge due to differences in growth and development during critical periods of skills acquisition. With appropriate referrals, genomic testing may be an option that can lead to informative diagnoses, although there is a lag in treatments. A novel concept is that of the “Genomics Passbook” that could follow an individual throughout the lifespan and afford the opportunity to re-query the comprehensive genomic data during critical life periods or when symptoms arise suggestive of a rare or common genetic condition. However, the possibility of secondary findings of unclear significance or identification of variants with incomplete penetrance limit the utility of this approach.
Screening in adulthood also allows for the possibility of genetic diagnosis early during initial manifestations of a disease when treatments might be most effective. In fact, screening for ACMG- or CDC-recommended genes and pathogenic variants known to impact medical care due to high pathogenicity (so-called “secondary findings”) reveals that 2–4% of the adult population carries one of these variants, with implications for extended family members. Again, the concept of the “Genomics Passbook” allows for a living, individualized document that could guide healthcare throughout the person’s lifespan, be queryable at varies life stages, and provide de-identified data to researchers that could increase general knowledge of genetic disease.
Some common threads from all 4 phases of the lifespan approach to genetic testing include the ethical guidance based on the Hippocratic Oath: “First do no harm.” There are risks to identifying low penetrance alleles, VOUS, and incidental or secondary findings that can lead to anxiety for parents and individuals, as well as the “vulnerable child” syndrome where every single symptom is being monitored as a potential manifestation of disease (also known as creating “patients-in-waiting”). In addition, positive findings have implications for other family members and may prompt extensive reflexive testing that can be challenging to coordinate. Ethical issues of diagnosing a condition in the absence of clear-cut treatments that reduce morbidity are also significant, and the costs of pursuing genomic technologies may lead to inequities and issues of accessibility. Finally, workforce issues cannot be underestimated as the limited number of available genetics professionals, including medical and laboratory-based geneticists, genetic counselors, and nurse geneticists, means that many parts of the United States are without access to their expertise, test analytic skills, and counseling.
In spite of these potential perils of genomic screening and diagnosis, the promise of universal genomic screening is tantalizing, and the opportunities for GTT are increasing dramatically. Tackling these identified challenges will facilitate more widespread adoption of genetic testing services at all points during the lifespan. In this way, the answer of “when” to screen for genetic disorders could become “always,” as long as the data are collected in a robust manner, are complete, and can be reevaluated as needed to meet the needs of the individual.
Acknowledgments
We acknowledge Teresa Sparks, Tippi MacKenzie, and Amy Brower for providing the concepts and content for several figures and to Joanne Lumsden for manuscript preparation assistance.
Footnotes
Disclaimer
The content of this publication reflects discussions from a June 2021, 3-day workshop sponsored by the National Institutes of Health (NIH) entitled, “Gene-Targeted Therapies: Early Diagnosis and Equitable Delivery” (National Institutes of Health, 2021). This material should not be interpreted as representing the viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Neurological Disorders and Stroke, or the National Center for Advancing Translational Sciences.
Conflicts of Interest
Nina Gold has been a consultant to Pfizer, Newspring Capital, LLC, and RCG consulting. Melissa Wasserstein has received research funding from Abeona Therapeutics, Alexion Pharmaceuticals, the Ara Parseghian Medical Research Foundation, BioMarin Pharmaceutical, Cure Sanfilippo Foundation, Dana’s Angels Research Trust, Firefly Fund, Noah’s Hope, Orchard Therapeutic, Passage Bio, Sio Gene Therapies, Takeda Pharmaceutical, Travere Therapeutics, and Ultragenyx Pharmaceutical, and consulting fees, speaker fees, research support, and travel reimbursement from Sanofi Genzyme. Melissa Parisi, Michelle Caggana, Jennifer Cohen, Jill Morris, Joseph Orsini, and Tiina Urv have no conflicts to report.
References
- Abul-Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, O’Dushlaine C, Leader JB, Lester Kirchner H, Lindbuchler DM, Barr ML, Giovanni MA, Ritchie MD, Overton JD, Reid JG, Metpally RP, Wardeh AH, Borecki IB, Yancopoulos GD, . . . Murray MF. (2016). Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science, 354(6319). 10.1126/science.aaf7000 [DOI] [PubMed] [Google Scholar]
- Amendola LM, Dorschner MO, Robertson PD, Salama JS, Hart R, Shirts BH, Murray ML, Tokita MJ, Gallego CJ, Kim DS, Bennett JT, Crosslin DR, Ranchalis J, Jones KL, Rosenthal EA, Jarvik ER, Itsara A, Turner EH, Herman DS, . . . Jarvik GP. (2015). Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res, 25(3), 305–315. 10.1101/gr.183483.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, & Liu DR. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature, 576(7785), 149–157. 10.1038/s41586-019-1711-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Public Health Laboratories. Newborn Screening Status for All Disorders. Retrieved August 1, 2022 from https://www.newsteps.org/index.php/resources/data-visualizations/newborn-screening-status-all-disorders [Google Scholar]
- Bailey DB Jr. (2022). A Window of Opportunity for Newborn Screening. Mol Diagn Ther, 26(3), 253–261. 10.1007/s40291-022-00590-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB Jr., Gehtland LM, Lewis MA, Peay H, Raspa M, Shone SM, Taylor JL, Wheeler AC, Cotten M, King NMP, Powell CM, Biesecker B, Bishop CE, Boyea BL, Duparc M, Harper BA, Kemper AR, Lee SN, Moultrie R, . . . Zimmerman SJ. (2019). Early Check: translational science at the intersection of public health and newborn screening. BMC Pediatr, 19(1), 238. 10.1186/s12887-019-1606-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB Jr., Porter KA, Andrews SM, Raspa M, Gwaltney AY, & Peay HL. (2021). Expert Evaluation of Strategies to Modernize Newborn Screening in the United States. JAMA Netw Open, 4(12), e2140998. 10.1001/jamanetworkopen.2021.40998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Mashiah A, Soper ER, Cullina S, Belbin GM, Kenny EE, Lucas AL, & Abul-Husn NS. (2022). CDH1 pathogenic variants and cancer risk in an unselected patient population. Fam Cancer, 21(2), 235–239. 10.1007/s10689-021-00257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blout Zawatsky CL, Shah N, Machini K, Perez E, Christensen KD, Zouk H, Steeves M, Koch C, Uveges M, Shea J, Gold N, Krier J, Boutin N, Mahanta L, Rehm HL, Weiss ST, Karlson EW, Smoller JW, Lebo MS, & Green RC. (2021). Returning actionable genomic results in a research biobank: Analytic validity, clinical implementation, and resource utilization. Am J Hum Genet, 108(12), 2224–2237. 10.1016/j.ajhg.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan AH, Manickam K, Meyer MN, Wagner JK, Hallquist MLG, Williams JL, Rahm AK, Williams MS, Chen ZE, Shah CK, Garg TK, Lazzeri AL, Schwartz MLB, Lindbuchler DM, Fan AL, Leeming R, Servano PO 3rd, Smith AL, Vogel VG, . . . Murray MF. (2018). Early cancer diagnoses through BRCA1/2 screening of unselected adult biobank participants. Genet Med, 20(5), 554–558. 10.1038/gim.2017.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Growth Charts. Retrieved August 1, 2022 from https://www.cdc.gov/growthcharts/index.htm
- Centers for Disease Control and Prevention. Tier 1 Genomics Applications and their Importance to Public Health. Retrieved August 1, 2022 from https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm
- Committee Opinion No. 691: Carrier Screening for Genetic Conditions. (2017). Obstet Gynecol, 129(3), e41–e55. 10.1097/AOG.0000000000001952 [DOI] [PubMed] [Google Scholar]
- Damrauer SM, Chaudhary K, Cho JH, Liang LW, Argulian E, Chan L, Dobbyn A, Guerraty MA, Judy R, Kay J, Kember RL, Levin MG, Saha A, Van Vleck T, Verma SS, Weaver J, Abul-Husn NS, Baras A, Chirinos JA, . . . Do R. (2019). Association of the V122I Hereditary Transthyretin Amyloidosis Genetic Variant With Heart Failure Among Individuals of African or Hispanic/Latino Ancestry. JAMA, 322(22), 2191–2202. 10.1001/jama.2019.17935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangouloff T, Hiligsmann M, Deconinck N, D’Amico A, Seferian AM, Boemer F, & Servais L. (2022). Financial cost and quality of life of patients with spinal muscular atrophy identified by symptoms or newborn screening. Dev Med Child Neurol. 10.1111/dmcn.15286 [DOI] [PubMed] [Google Scholar]
- Dar P, Curnow KJ, Gross SJ, Hall MP, Stosic M, Demko Z, Zimmermann B, Hill M, Sigurjonsson S, Ryan A, Banjevic M, Kolacki PL, Koch SW, Strom CM, Rabinowitz M, & Benn P. (2014). Clinical experience and follow-up with large scale single-nucleotide polymorphism-based noninvasive prenatal aneuploidy testing. Am J Obstet Gynecol, 211(5), 527 e521–527 e517. 10.1016/j.ajog.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Dorschner MO, Amendola LM, Turner EH, Robertson PD, Shirts BH, Gallego CJ, Bennett RL, Jones KL, Tokita MJ, Bennett JT, Kim JH, Rosenthal EA, Kim DS, National Heart L, Blood Institute Grand Opportunity Exome Sequencing, P., Tabor HK, Bamshad MJ, Motulsky AG, Scott CR, . . . Jarvik GP. (2013). Actionable, pathogenic incidental findings in 1,000 participants’ exomes. Am J Hum Genet, 93(4), 631–640. 10.1016/j.ajhg.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragojlovic N, Borle K, Kopac N, Ellis U, Birch P, Adam S, Friedman JM, Nisselle A, Gen CS, Elliott AM, & Lynd LD. (2020). The composition and capacity of the clinical genetics workforce in high-income countries: a scoping review. Genet Med, 22(9), 1437–1449. 10.1038/s41436-020-0825-2 [DOI] [PubMed] [Google Scholar]
- Goldenberg AJ, Lloyd-Puryear M, Brosco JP, Therrell B, Bush L, Berry S, Brower A, Bonhomme N, Bowdish B, Chrysler D, Clarke A, Crawford T, Goldman E, Hiner S, Howell RR, Orren D, Wilfond BS, Watson M, Bioethics, & Legal Workgroup of the Newborn Screening Translational Research, N. (2019). Including ELSI research questions in newborn screening pilot studies. Genet Med, 21(3), 525–533. 10.1038/s41436-018-0101-x [DOI] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, McGuire AL, Nussbaum RL, O’Daniel JM, Ormond KE, Rehm HL, Watson MS, Williams MS, Biesecker LG, American College of Medical, G., & Genomics. (2013). ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med, 15(7), 565–574. 10.1038/gim.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg AR, & Edwards JG. (2018). Prenatal genetic carrier screening in the genomic age. Semin Perinatol, 42(5), 303–306. 10.1053/j.semperi.2018.07.019 [DOI] [PubMed] [Google Scholar]
- Haggerty CM, Damrauer SM, Levin MG, Birtwell D, Carey DJ, Golden AM, Hartzel DN, Hu Y, Judy R, Kelly MA, Kember RL, Lester Kirchner H, Leader JB, Liang L, McDermott-Roe C, Babu A, Morley M, Nealy Z, Person TN, . . . Arany Z. (2019). Genomics-First Evaluation of Heart Disease Associated With Titin-Truncating Variants. Circulation, 140(1), 42–54. 10.1161/CIRCULATIONAHA.119.039573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Resources & Services Administration. Advisory Committee on Heritable Disorders in Newborns and Children. Retrieved August 1, 2022 from https://www.hrsa.gov/advisory-committees/heritable-disorders/index.html
- Health Resources& Services Administration Condition Nomination and Review. Retrieved August 19, 2022 from https://www.hrsa.gov/advisory-committees/heritable-disorders/condition-nomination
- Hoell C, Wynn J, Rasmussen LV, Marsolo K, Aufox SA, Chung WK, Connolly JJ, Freimuth RR, Kochan D, Hakonarson H, Harr M, Holm IA, Kullo IJ, Lammers PE, Leppig KA, Leslie ND, Myers MF, Sharp RR, Smith ME, & Prows CA. (2020). Participant choices for return of genomic results in the eMERGE Network. Genet Med, 22(11), 1821–1829. 10.1038/s41436-020-0905-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Bracewell-Milnes T, Saso S, Jones BP, Almeida PA, Maclaren K, Norman-Taylor J, Johnson M, & Nikolaou D. (2021). A review on the motivations, decision-making factors, attitudes and experiences of couples using pre-implantation genetic testing for inherited conditions. Hum Reprod Update, 27(5), 944–966. 10.1093/humupd/dmab013 [DOI] [PubMed] [Google Scholar]
- Jelin AC, & Vora N. (2018). Whole Exome Sequencing: Applications in Prenatal Genetics. Obstet Gynecol Clin North Am, 45(1), 69–81. 10.1016/j.ogc.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BD, Fischer CG, Polito CA, Maiese DR, Keehn AS, Lyon M, Edick MJ, Taylor MRG, Andersson HC, Bodurtha JN, Blitzer MG, Muenke M, & Watson MS. (2021). The 2019 US medical genetics workforce: a focus on clinical genetics. Genet Med, 23(8), 1458–1464. 10.1038/s41436-021-01162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Lantos JD, Goldenberg A, Chen F, Parens E, Koenig BA, members of the NE, & Policy Advisory B. (2018). Sequencing Newborns: A Call for Nuanced Use of Genomic Technologies. Hastings Cent Rep, 48 Suppl 2, S2–S6. 10.1002/hast.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmer M, & Boykin AA. (2022). Analysis of the 2000 to 2018 autism and developmental disabilities monitoring network surveillance reports: Implications for primary care clinicians. J Pediatr Nurs, 65, 55–68. 10.1016/j.pedn.2022.04.014 [DOI] [PubMed] [Google Scholar]
- Kliff S, & Bhatia A. (2022). When They Warn of Rare Disorders, These Prenatal Tests Are Usually Wrong. The New York Times. https://www.nytimes.com/2022/01/01/upshot/pregnancy-birth-genetic-testing.html [Google Scholar]
- Kreger EM, Singer ST, Witt RG, Sweeters N, Lianoglou B, Lal A, Mackenzie TC, & Vichinsky E. (2016). Favorable outcomes after in utero transfusion in fetuses with alpha thalassemia major: a case series and review of the literature. Prenat Diagn, 36(13), 1242–1249. 10.1002/pd.4966 [DOI] [PubMed] [Google Scholar]
- Larsen D, Ma J, Strassberg M, Ramakrishnan R, & Van den Veyver IB. (2019). The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn, 39(4), 319–323. 10.1002/pd.5434 [DOI] [PubMed] [Google Scholar]
- Manickam K, Buchanan AH, Schwartz MLB, Hallquist MLG, Williams JL, Rahm AK, Rocha H, Savatt JM, Evans AE, Butry LM, Lazzeri AL, Lindbuchler DM, Flansburg CN, Leeming R, Vogel VG, Lebo MS, Mason-Suares HM, Hoskinson DC, Abul-Husn NS, . . . Murray MF. (2018). Exome Sequencing-Based Screening for BRCA1/2 Expected Pathogenic Variants Among Adult Biobank Participants. JAMA Netw Open, 1(5), e182140. 10.1001/jamanetworkopen.2018.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Lee K, Abul-Husn NS, Amendola LM, Brothers K, Chung WK, Gollob MH, Gordon AS, Harrison SM, Hershberger RE, Klein TE, Richards CS, Stewart DR, Martin CL, & documents@acmg.net, A. S. F. W. G. E. a. (2022). ACMG SF v3.1 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med, 24(7), 1407–1414. 10.1016/j.gim.2022.04.006 [DOI] [PubMed] [Google Scholar]
- Miller DT, Lee K, Gordon AS, Amendola LM, Adelman K, Bale SJ, Chung WK, Gollob MH, Harrison SM, Herman GE, Hershberger RE, Klein TE, McKelvey K, Richards CS, Vlangos CN, Stewart DR, Watson MS, Martin CL, & Group ASFW. (2021). Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2021 update: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med, 23(8), 1391–1398. 10.1038/s41436-021-01171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan P, Gold NB, Bick AG, McLaughlin H, Kraft P, Rehm HL, Peloso GM, Wilson JG, Correa A, Seidman JG, Seidman CE, Kathiresan S, & Green RC. (2016). Aggregate penetrance of genomic variants for actionable disorders in European and African Americans. Sci Transl Med, 8(364), 364ra151. 10.1126/scitranslmed.aag2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Human Genome Research Institute. Newborn Sequencing in Genomic Medicine and Public Health (NSIGHT). Retrieved August 1, 2022 from https://www.genome.gov/Funded-Programs-Projects/Newborn-Sequencing-in-Genomic-Medicine-and-Public-Health-NSIGHT [Google Scholar]
- National Institutes of Health. (2021). Gene-Targeted Therapies: Early Diagnosis and Equitable Delivery, online. https://events-support.com/events/Gene-Targeted_Therapies_June_2021 [Google Scholar]
- Nguyen QH, Witt RG, Wang B, Eikani C, Shea J, Smith LK, Boyle G, Cadaoas J, Sper R, MacKenzie JD, Villeda S, & MacKenzie TC. (2020). Tolerance induction and microglial engraftment after fetal therapy without conditioning in mice with Mucopolysaccharidosis type VII. Sci Transl Med, 12(532). 10.1126/scitranslmed.aay8980 [DOI] [PubMed] [Google Scholar]
- Park J, Levin MG, Haggerty CM, Hartzel DN, Judy R, Kember RL, Reza N, Regeneron Genetics C, Ritchie MD, Owens AT, Damrauer SM, & Rader DJ. (2020). A genome-first approach to aggregating rare genetic variants in LMNA for association with electronic health record phenotypes. Genet Med, 22(1), 102–111. 10.1038/s41436-019-0625-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peay HL, Gwaltney AY, Moultrie R, Cope H, Boyea BL, Porter KA, Duparc M, Alexander AA, Biesecker BB, Isiaq A, Check J, Gehtland L, Bailey DB Jr., & King NMP. (2022). Education and Consent for Population-Based DNA Screening: A Mixed-Methods Evaluation of the Early Check Newborn Screening Pilot Study. Front Genet, 13, 891592. 10.3389/fgene.2022.891592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum RE, Ang C, Suckiel SA, Soper ER, Sigireddi MR, Cullina S, Belbin GM, Lucas AL, Kenny EE, & Abul-Husn NS. (2020). Lynch Syndrome-Associated Variants and Cancer Rates in an Ancestrally Diverse Biobank. JCO Precis Oncol, 4. 10.1200/PO.20.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh R, & Van den Veyver IB. (2020). The current and future impact of genome-wide sequencing on fetal precision medicine. Hum Genet, 139(9), 1121–1130. 10.1007/s00439-019-02088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlen TJ, Bristow SL, Hatchell KE, Esplin ED, Nussbaum RL, & Haverfield EV. (2022). The Impact of Proband Indication for Genetic Testing on the Uptake of Cascade Testing Among Relatives. Front Genet, 13, 867226. 10.3389/fgene.2022.867226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J, Scotchman E, Chandler N, & Chitty LS. (2020). PREIMPLANTATION GENETIC TESTING: Non-invasive prenatal testing for aneuploidy, copy-number variants and single-gene disorders. Reproduction, 160(5), A1–A11. 10.1530/REP-19-0591 [DOI] [PubMed] [Google Scholar]
- Shih STF, Keller E, Wiley V, Farrar MA, Wong M, & Chambers GM. (2022). Modelling the Cost-Effectiveness and Budget Impact of a Newborn Screening Program for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. Int J Neonatal Screen, 8(3). 10.3390/ijns8030045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirrs S, Hollak C, Merkel M, Sechi A, Glamuzina E, Janssen MC, Lachmann R, Langendonk J, Scarpelli M, Ben Omran T, Mochel F, Group S-AS, & Tchan MC. (2016). The Frequencies of Different Inborn Errors of Metabolism in Adult Metabolic Centres: Report from the SSIEM Adult Metabolic Physicians Group. JIMD Rep, 27, 85–91. 10.1007/8904_2015_435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urv TK, & Parisi MA. (2017). Newborn Screening: Beyond the Spot. Adv Exp Med Biol, 1031, 323–346. 10.1007/978-3-319-67144-4_19 [DOI] [PubMed] [Google Scholar]
- van Wegberg AMJ, Trefz F, Gizewska M, Ahmed S, Chabraoui L, Zaki MS, Maillot F, van Spronsen FJ, Study Group on Missed, P. K. U., & Missed to, F.-U. (2021). Undiagnosed Phenylketonuria Can Exist Everywhere: Results From an International Survey. J Pediatr, 239, 231–234 e232. 10.1016/j.jpeds.2021.08.070 [DOI] [PubMed] [Google Scholar]
- Vermeesch JR, Voet T, & Devriendt K. (2016). Prenatal and pre-implantation genetic diagnosis. Nat Rev Genet, 17(10), 643–656. 10.1038/nrg.2016.97 [DOI] [PubMed] [Google Scholar]
- Wenger BM, Patel N, Lui M, Moscati A, Do R, Stewart DR, Tartaglia M, Muino-Mosquera L, De Backer J, Kontorovich AR, & Gelb BD. (2021). A genotype-first approach to exploring Mendelian cardiovascular traits with clear external manifestations. Genet Med, 23(1), 94–102. 10.1038/s41436-020-00973-2 [DOI] [PMC free article] [PubMed] [Google Scholar]