Abstract
The present study was the first comprehensive investigation of genetic mutation and expression levels of the p53 signaling genes in cutaneous melanoma through various genetic databases providing large datasets. The mutational landscape of p53 and its signaling genes was higher than expected, with TP53 followed by CDKN2A being the most mutated gene in cutaneous melanoma. Furthermore, the expression analysis showed that TP53, MDM2, CDKN2A, and TP53BP1 were overexpressed, while MDM4 and CDKN2B were under-expressed in cutaneous melanoma. Overall, TCGA data revealed that among all the other p53 signaling proteins, CDKN2A was significantly higher in both sun and non-sun-exposed healthy tissues than in melanoma. Likewise, MDM4 and TP53BP1 expressions were markedly greater in non-sun-exposed healthy tissues compared to other groups. However, CDKN2B expression was higher in the sun-exposed healthy tissues than in other tissues. In addition, various genes were expressed significantly differently among males and females. In addition, CDKN2A was highly expressed in the SK-MEL-30 skin cancer cell line, whereas, Immune cell type expression analysis revealed that the MDM4 was highly expressed in naïve B-cells. Furthermore, all six genes were significantly overexpressed in extraordinarily overweight or obese tumor tissues compared to healthy tissues. MDM2 expression and tumor stage were closely related. There were differences in gene expression across patient age groups and positive nodal status. TP53 showed a positive correlation with B cells, MDM2 with CD8+T cells, macrophages and neutrophils, and MDM4 with neutrophils. CDKN2A/B had a non-significant correlation with all six types of immune cells. However, TP53BP1 was positively correlated with all five types of immune cells except B cells. Only TP53, MDM2, and CDKN2A had a role in cutaneous melanoma-specific tumor immunity. All TP53 and its regulating genes may be predictive for prognosis. The results of the present study need to be validated through future screening, in vivo, and in vitro studies.
Keywords: Mutations, expression, p53 signaling genes, cutaneous melanoma
Introduction
Cutaneous melanoma is related to hereditary melanoma and tumor predisposition syndrome and has symptoms including pruritus and exanthema. 1 It is one of the most aggressive and deadly types of skin cancer. Cutaneous melanoma accounts for 3% of all skin cancers but causes 65% of cancer deaths. 2 Cutaneous melanoma incidence has also gradually risen in the last decades, with 96 000 new cases in 2019. Therefore, an understanding of early diagnosis and management of cutaneous melanoma is urgently needed. 3 Tumor protein p53 (TP53) is a well-known tumor gene. The p53 signaling or regulating genes include TP53, Mouse double minute 2 (MDM2), Mouse double minute 4 (MDM4), cyclin-dependent kinase inhibitor 2A (CDKN2A), cyclin-dependent kinase inhibitor 2B (CDKN2B), and TP53-binding protein 1 (TP53BP1). When mutated or differentially expressed, these genes have a role in multiple human cancers.
Earlier data presented a high frequency of mutations in the TP53 for skin cancer.4,5 A single p53 allele mutation has a pro-oncogenic function during skin cancer development through a dominant inhibitory effect on UVB-induced lincRNA-p21 expression and the subsequent evasion of UVB-induced apoptosis. 6 A total of 50% mutations across all skin cancers were reported in TP53. This frequency increased to 90% in xeroderma pigmentosum. A high prevalence of mutant p53 has been reported in metastatic melanoma compared to primary tumors. 7 MDM2 is an E3 ubiquitin ligase, a protein found in the nucleus and, when overexpressed or amplified, may promote tumor development by targeting tumor suppressor proteins (e.g., p53) for proteasomal destruction. MDM2 has a single nucleotide polymorphism 309 associated with multiple kinds of cancers, including non-melanoma skin cancer. 8 Many transcript variants of MDM2, resulting from alternative splicing, are only produced in tumor cells. 9 MDM4 gene also encodes a nuclear protein structurally similar to MDM2 that contains a TP53 binding domain at the N-terminus and a RING finger domain at the C-terminus. 10 These proteins bind, inhibit p53 activity, and are overexpressed in various human cancers.
However, unlike MDM2, which degrades p53, MDM4 protein inhibits p53 by binding its transcriptional activation domain. This protein also interacts with MDM2 protein via the RING finger domain, inhibiting the latter degradation. Therefore, this protein can reverse MDM2-targeted degradation of p53 while maintaining suppression of p53 transactivation and apoptotic functions. 11 Regulation of the protein arginine methyltransferase 5 (PRMT5)-MDM4 axis is critical in responding to CDK4/6 inhibitors in melanoma. 12 CDKN2A loss is a significant event in several cancer types. Still, no targeted therapeutic has been engaged in clinical trials, a few meta-analyses have studied the prognostic impact.13,14 Homozygous deletions inactivate CDKN2A in most cases. Hypermethylation of the CDKN2A promoter region is one of the processes by which the gene can be lost. However, the influence of promoter hypermethylation on prognosis remained a mystery. The CDKN2B gene is located next to the tumor suppressor gene, CDKN2A, and is eliminated in many cancers. This gene codes for a cyclin-dependent kinase inhibitor that binds to CDK4 or CDK6 and prevents CDK kinase activation; as a result, the produced protein acts as a cell growth regulator that limits cell cycle G1 advancement. 15 Transforming growth factor beta (TGFβ) significantly increased the expression of this gene, implying that it plays a role in TGFβ induced growth inhibition. This gene has two alternatively spliced transcript variants, each of which codes for a distinct protein. 16 TP53BP1 promotes checkpoint signaling after DNA damage, acts as a scaffold for the recruitment of DNA damage response proteins to damaged chromatin, and promotes non-homologous end-joining pathways by limiting end resection after a double-strand break.1,17 -20 He et al 21 found that mutations in the TP53BP1 gene are linked to skin cancer.
In summary, mutations and over or/and underexpression of these genes have been related to various cancer types and could also be attributed to cutaneous melanoma. Therefore, the present study investigated the genetic alteration and expression profiles of TP53 signaling genes in cutaneous melanoma through various bioinformatic tools and datasets.
Methods
This work aimed to investigate the mutational and expression landscape of components of the p53 signaling pathway by using modern bioinformatics tools. The current study used the Catalog of Somatic Mutations in Cancer (COSMIC) database, the cBioPortal for Cancer Genomics, the Human Protein Atlas (HPA), the Cancer Genome Atlas (TCGA) data, Gene Expression Profiling Interactive Analysis (GEPIA), and Transcriptional Regulatory Relationships Unveiled by Sentence-based Text mining (TRRUST).
COSMIC
COSMIC (https://cancer.sanger.ac.uk/cosmic) is a publically available database that displays the mutations responsible for various cancer types. 22 Our proposal was solely focused on skin cancer. Further specifications were followed under the database’s requirements.
cBioPortal
cBioPortal (http://cbioportal.org) is an open-access platform for gene mutation analysis, expression pathway analysis, and tissue imaging from patient samples. 23 We chose four different studies and analyzed each report separately. We chose an 826-total sample size from a total of 785 patients.
HPA, TCGA, and GEPIA
The HPA database (http://www.proteinatlas.org) is divided into sections providing details on human protein expression at the cellular, tissue, and organ levels.24-26 We searched TP53 expression in skin cancer cell lines. We retrieved the typical TCGA data on normal tissues that had and had not been exposed to sunlight and information on patient gene expression profiles for clinicopathological and baseline characteristics. The most recent dataset recommended for immune cell type expression was consulted 27
We selected GEPIA for the investigation of TP53 signaling gene expression. It integrates TCGA and GTEx data and uses bioinformatics tools to identify cancer subtypes, driver genes, and other cancer targets and markers. 28 We also evaluated cutaneous melanoma by the platform’s predetermined need. TRRUST (https://www.grnpedia.org/trrust) is a tool for mapping transcriptional regulatory networks and providing details on regulating these connections. 29 The current version, which includes 8444 and 6552 transcription factor target regulatory linkages of 800 human transcriptional factors, was obtained from 11 237 PubMed papers. TRRUST was used to obtain transcriptional regulators for TP53 signaling genes. A thorough search was conducted across various resources, including databases, Pubmed, and other scholarly publishers.
Statistical analysis
The comparative analysis of parameters between the control group and patients was performed using the Student’s t-test and analysis of variance (ANOVA) (GraphPad Prism 6 for Windows, San Diego, California, USA). For all the comparisons, a P ⩽ .05 was considered statistically significant. We selected Log2 (fold-change) under 1.5. Cut-off for TP53 signaling gene expression
Results
Widespread mutations in p53 signaling genes in cutaneous melanoma
The mutational landscape of TP53 and its signaling genes was higher than expected (Figure 1). According to Figure 1A to F, the COSMIC contained 1498 mutations in TP53, ten mutations in CDKN2B, 50 mutations in MDM4, 162 mutations in TP53BP1, and 890 mutations in CDKN2A. The various mutations from cBio cancer genomics are depicted in the G-K of Figure 1. For more data and confirmation, we looked at four separate research studies. The TCGA-Firehose legacy outperformed other studies in these four investigations, registering gene alteration in 52.23% of 448 instances, with profound deletion being the most common type.
Figure 1.
p53 signaling genes mutants identified in skin cancer/cutaneous melanoma. P53 signaling gene mutations in skin cancer patients were generally detected from COSMIC (A-F) and skin cutaneous melanoma from cBio cancer genomics (G-J). (K) Oncoprint for skin cutaneous melanoma derived from cBioPortal.
Expression and p53 signaling genes in cutaneous melanoma
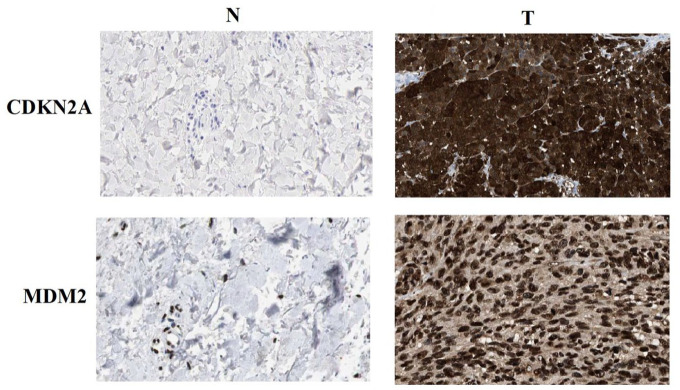
Figure 2 presents the mRNA expression pattern for p53 signaling genes in cutaneous melanoma and healthy tissues (Figure 2A and B). TP53, MDM2, CDKN2A, and TP53BP1 were overexpressed, while MDM4 and CDKN2B were underexpressed in cutaneous melanoma. Compared to normal tissues, both CDKN2A and MDM2 proteins were substantially expressed in melanoma (Figure 3).
Figure 2.
p53 signaling proteins mRNA expression in skin cutaneous melanoma and normal tissues in GEPIA. (A) mRNA expression profile, with red representing tumor tissue and green representing normal tissue. On the box plot, (B) mRNA expression. CDKN2A protein expression in skin cancer and normal tissues. *P = .05 and cutoff = 1.5 for |Log2 (fold-change)|. The level of mRNA expression was shown on a log scale.
Figure 3.
CDKN2A and MDM2 protein expression in melanoma and tumor-adjacent normal tissues. N: tumor-adjacent normal tissues; T: tumor tissues.
Overall, TCGA data revealed that among all the other p53 signaling proteins, CDKN2A was significantly higher in both sun and non-sun-exposed healthy tissues than in the melanoma tissues (Figure 4E). MDM4 and TP53BP1 expressions were significantly (P = .0001) more incredible in non-sun-exposed healthy tissues compared to other groups. However, CDKN2B expression was higher (P = .0001) in the sun-exposed healthy tissues than in other tissues.
Figure 4.
p53 signaling proteins mRNA expression in the sun and non-sun exposed normal tissues in TCGA: (A) mRNA expression in general for both male and female, (B) mRNA expression for TP53 in male versus female, (C) mRNA expression for MDM2 in male versus female, (D) mRNA expression for MDM4 in male versus female, (E) mRNA expression for CDKN2A in male versus female, (F) mRNA expression for CDKN2B in male versus female, and (G) mRNA expression for TP53BP1 in male versus female. ANOVA calculated P values with recommended option Tukey’s multiple comparison test. Error bars indicate S.E.M. *P = .05.
The tissues from sun-exposed and non-sun-exposed males had significantly higher TP53 and CDKN2B expression than tissues from sun-exposed and non-sun-exposed females (P = .0067; 0.0051) (P = .0055; 0.0120). The non-sun-exposed females had significantly higher MDM2 (P = .0042), MDM4 (P = .0217), and TP53BP1 protein expression than non-sun-exposed males (P = .0015). However, CDKN2A (P = .0001) was overexpressed among non-sun-exposed males compared to females (Figure 4B–G). Variations in p53 signaling gene expression were identified across different skin cancer cell lines (Figure 5A). MDM4 was substantially overexpressed in naive B-cells, according to the Monaco dataset (Figure 5B).
Figure 5.
P53 signaling proteins expression in Skin cancer cell lines and immune cell-type expression: (A) expression in skin cancer cell lines (TCGA) and (B) immune cell type expression (Monaco dataset). The Monaco dataset 27 contains data for immune cell types healthy donors’ peripheral blood mononuclear cell (PBMC) fraction using RNA-seq and flow cytometry.
p53 signaling genes expression based on the patient’s baseline and clinicopathological parameters in cutaneous melanoma
Table 1 displays the baseline and clinicopathological parameters of cutaneous melanoma patients. The expression of all 6 p53 signaling genes varied significantly across primary and metastatic tumors. The only gene that was substantially expressed in Asians was TP53. No statistically significant difference was observed among Caucasians, Americans, and Asians (data not shown). All six genes were substantially overexpressed in males as compared to females.
Table 1.
P53 signaling genes expression profile based on patient’s baseline and clinicopathological features.
| Feature | Genes | |||||
|---|---|---|---|---|---|---|
| TP53 | MDM2 | MDM4 | CDKN2A | CDKN2B | TP53BP1 | |
| Sample types | P-value* | P-value* | P-value* | P-value* | P-value* | |
| Primary(n = 104)/metastasis (n = 368) | P > M (1.759970E-01) | P > M (9.452000E-02) | M > P (2.377600E-03) | M > P (1.490210E-02) | P > M (4.840800E-01) | M > P (3.4046999664028E-09) |
| Tumor stage | ||||||
| non. | 0.0132 | non. | non. | non. | non. | |
| Race | ||||||
| Caucasian (n = 438) vs Asian (n = 12) | A > C (8.631700E-02) | C > A (8.403600E-01) | C > A (5.586400E-01) | C > A (1.684430E-01) | C > A (9.841500E-02) | C > A (2.297000E-01) |
| Gender | ||||||
| Male (n = 276) vs female (n = 185) | M > F (5.924200E-02) | M > F (2.875800E-02) | M > F (3.117400E-01) | M > F (8.733600E-01) | M > F (5.525800E-01) | M > F (7.926400E-01) |
| Weight | ||||||
| Normal (n = 77) vs extreme weight (n = 85) | E.W > N (1.776390E-01) | E.W > N (2.173400E-01) | E.W > N (6.752800E-01) | E.W > N (4.682200E-02) | E.W > N (3.416600E-01) | E.W > N (8.456000E-01) |
| Normal vs obese (n = 66) | Ob>N (5.295200E-01) | Ob>N (9.416300E-02) | Ob>N (5.933000E-01) | Ob>N (1.860980E-01) | Ob>N (8.366200E-01) | Ob>N (6.860400E-01) |
| Normal vs extreme obese (n = 10) | E. Ob>N (9.417600E-01) | E. Ob>N (9.987900E-02) | N > E. Ob (9.892200E-01) | E. Ob>N (4.842800E-01) | N > E. Ob (6.566400E-01) | E. Ob>N (5.180800E-01) |
| Extreme weight vs obese | Ob>E.W (4.097800E-02) | E.W>Ob (1.680600E-01) | E.W>Ob (8.552200E-01) | E.W>Ob (9.265600E-01) | E.W>Ob (4.788800E-01) | Ob>E.W (7.937400E-01) |
| Extreme weight vs extreme obese | E. Ob>E.W (3.550800E-01) | E. Ob>E.W (3.829200E-01) | E.W > E. Ob (8.204200E-01) | E. Ob>E.W (7.350600E-01) | E.W > E. Ob 4.125000E-01 | E.W > E. Ob (5.599200E-01) |
| Obese vs extreme obese | E. Ob>Ob (8.049000E-01) | E. Ob>Ob (4.041600E-01) | Ob>E. Ob (7.787600E-01) | E. Ob>Ob (7.290800E-01) | Ob>E. Ob (5.910200E-01) | Ob>E. Ob (7.576200E-01) |
| Age (years) | ||||||
| 21-40 (n = 60) vs 41-60 (n = 185) | 41-60 > 21-40 (2.791000E-01) | 41-60 > 21-40 (1.871400E-01) | 21-40 > 41-60 5.443200E-01 | 41-60 > 21-40 (3.616600E-01) | 41-60 > 21-40 (5.929600E-01) | 21-40 > 41-60 (1.794450E-02) |
| 21-40vs 61-80 (n = 179) | 21-40 > 61-80 (5.490400E-01) | 21-40 > 61-80 (6.974400E-02) | 21-40 > 61-80 (8.036400E-01) | 61-80 > 21-40 (8.347600E-01) | 61-80 > 21-40 (3.837000E-01) | 21-40 > 61-80 (7.215900E-02) |
| 21-40vs 81-100 (n = 31) | 21-40 > 81-100 (1.177190E-01) | 21-40 > 81-100 (4.432800E-01) | 21-40 > 81-100 (7.412000E-01) | 81-100 > 21-40 (3.967600E-01) | 81-100 > 21-40 (8.940400E-01) | 21-40 > 81-100 (4.340000E-01) |
| 41-60vs 61-80 | 41-60 > 61-80 (1.656190E-02) | 41-60 > 61-80 (4.844600E-01) | 41-60 > 61-80 (2.340400E-01) | 61-80 > 41-60 (3.145600E-02) | 41-60 > 61-80 (5.354800E-01) | 41-60 > 61-80 (6.909000E-01) |
| 41-60vs 81-100 | 41-60 > 81-100 (1.318480E-02) | 41-60 > 81-100 (8.335700E-02) | 41-60 > 81-100 (4.427600E-01) | 81-100 > 41-60 (8.903800E-01) | 41-60 > 81-100 (8.033000E-01) | 81-100 > 41-60 (3.983800E-01) |
| 61-80vs 81-100 | 61-80 > 81-100 (1.365790E-01) | 61-80 > 81-100 (3.272400E-02) | 81-100 > 61-80 (8.793600E-01) | 81-100 > 61-80 (9.448000E-02) | 61-80 > 81-100 (6.012400E-01) | 81-100 > 61-80 (5.932200E-01) |
| Nodal status | ||||||
| N0 (n = 235) vs N1 (n = 74) | N0 > N1 (2.536400E-02) | N0 > N1 (9.445800E-01) | N0 > N1 (3.614000E-01) | N1 > N0 (7.666200E-01) | N1 > N0 (4.469800E-01) | N1 > N0 (2.318600E-01) |
| N0 vs N2 (n = 49) | N0 > N2 (7.271800E-02) | N2 > N0 (5.899800E-01) | N0 > N2 (7.931200E-01) | N2 > N0 (9.908800E-01) | N2 > N0 (9.568200E-01) | N2 > N0 (6.343000E-01) |
| N0 vs N3 (n = 56) | N0 > N3 (8.746100E-02) | N3 > N0 (3.730200E-02) | N0 > N3 (1.140770E-01) | N0 > N3 (1.825000E-01) | N0 > N3 (8.549000E-01) | N0 > N3 (1.114120E-02) |
| N1 vs N2 | N1 > N2 (9.014800E-01) | N1 > N2 (5.885000E-01) | N1 > N2 (4.100800E-01) | N2 > N1 (7.699800E-01) | N2 > N1 (5.279600E-01) | N2 > N1 (6.092000E-01) |
| N1 vs N3 | N1 > N3 (7.897400E-01) | N3 > N1 (2.491000E-01) | N1 > N3 (5.374400E-01) | N1 > N3 (1.886150E-01) | N1 > N3 (7.308800E-01) | N1 > N3 (5.256500E-03) |
| N2 vs N3 | N3 > N2 (9.001800E-01) | N2 > N3 (3.065800E-01) | N2 > N3 (2.149600E-01) | N2 > N3 (2.770600E-01) | N2 > N3 (8.390200E-01) | N2 > N3 (4.834400E-02) |
| P53 status | ||||||
| WT (n = 407) vs mutant (n = 66) | WT > mutated (2.50359732945071E-11) | WT > mutated (1.779780E-01) | WT < mutated (8.163000E-01) | WT < mutated (2.11769999999278E-05) | WT < mutated (3.80749999999708E-05) | WT < mutated (5.980800E-03) |
Abbreviations: N0, No regional lymph node metastasis; N1, Metastases in 1 to 3 axillary lymph nodes; N2, Metastases in 4 to 9 axillary lymph nodes; N3, Metastases in 10 or more axillary lymph node.
Normal weight, BMI greater than equal to 18.5 and BMI less than 25; Extreme weight, BMI greater than equal to 25 and BMI less than 30; Obese, BMI greater than equal to 30 and BMI less than 40; Extreme Obese, BMI greater than 40.
Furthermore, all six genes were overexpressed in tumor tissues from extremely overweight or obese compared to healthy tissues. MDM2 expression and tumor stage were closely related. Table 1 shows differences in gene expression across patient age groups and positive nodal status. Patients with high MDM2, CDKN2A, TP53BP1, and low MDM4, CDKN2B, were more likely to have TP53-mutations (Table 1).
Transcription factor targets and correlation of p53 signaling genes with six types of immune cells
Putative transcription factor targets of the p53 signaling genes were investigated using the TRRUST database (Table 2). Forty-two transcription factors, including 16 MDM2, 1 MDM4, 18 CDKN2A, and 11 CDKN2B, were related to the regulation of p53 signaling. A total of 6 types of immune cells concerning p53 signaling genes were investigated using the TIMER tool (Table 3). In cutaneous melanoma, TP53 showed a positive correlation with B cells, MDM2 with CD8+T cells, macrophages and neutrophils, and MDM4 with neutrophils. CDKN2A/B had a non-significant correlation with all six types of immune cells. However, TP53BP1 was positively correlated with all five types of immune cells except B cells. In addition, we reported data on the relationship between p53 signaling pathway genes and anti-tumor immunity in melanoma (Table 4). Only TP53, MDM2, and CDKN2A had a role in cutaneous melanoma-specific tumor immunity.
Table 2.
Key regulated factor of p53 signaling pathway genes in human (TRRUST).
| TF | Description | P-value | Adjusted p-value | Odds ratio | Combined score | Regulated genes |
|---|---|---|---|---|---|---|
| DNMT1 | DNA Methyltransferase 1 | 6.72E-08 | 3.71E-06 | 713.0714 | 11776.65 | CDKN2B;CDKN2A;TP53 |
| ETS2 | ETS Proto-Oncogene 2, Transcription Factor | 7.42E-08 | 3.71E-06 | 688.4483 | 11302.29 | CDKN2A;MDM2;TP53 |
| EZH2 | Enhancer Of Zeste 2 Polycomb Repressive Complex 2 Subunit | 1.48E-07 | 4.92E-06 | 539.3784 | 8483.795 | CDKN2B;CDKN2A;TP53 |
| ETS1 | ETS Proto-Oncogene 1, Transcription Factor | 1.18E-06 | 2.94E-05 | 262.0789 | 3578.235 | CDKN2A;MDM2;TP53 |
| E2F1 | E2F Transcription Factor 1 | 5.80E-06 | 1.16E-04 | 151.626 | 1828.371 | CDKN2A;MDM2;TP53 |
| TP53 | Tumor Protein P53 | 1.06E-05 | 1.64E-04 | 123.1863 | 1410.68 | MDM2;MDM4;TP53 |
| HDAC9 | Histone Deacetylase 9 | 1.15E-05 | 1.64E-04 | 624.3125 | 7103.107 | CDKN2A;TP53 |
| MYCN | MYCN Proto-Oncogene, BHLH Transcription Factor | 7.38E-05 | 8.20E-04 | 231.9884 | 2207.092 | MDM2;TP53 |
| EP300 | E1A Binding Protein P300 | 1.15E-04 | 0.001042 | 184.6296 | 1675.225 | CDKN2B;MDM2 |
| HDAC1 | Histone Deacetylase 1 | 1.85E-04 | 0.001539 | 144.3841 | 1241.262 | CDKN2A;TP53 |
| ESR1 | Estrogen Receptor 1 | 2.12E-04 | 0.001628 | 134.5946 | 1138.746 | MDM2;TP53 |
| SP1 | Sp1 Transcription Factor | 2.48E-04 | 0.001769 | 41.63113 | 345.6725 | CDKN2B;CDKN2A;MDM2 |
| SP3 | Sp3 Transcription Factor | 4.68E-04 | 0.003118 | 89.56306 | 686.7549 | CDKN2B;CDKN2A |
| ZBTB7A | Zinc Finger And BTB Domain Containing 7A | .001799 | 0.00729 | 799.56 | 5053.709 | CDKN2A |
| PAX2 | Paired Box 2 | .001799 | 0.00729 | 799.56 | 5053.709 | TP53 |
| IKBKB | Inhibitor Of Nuclear Factor Kappa B Kinase Subunit Beta | .001799 | 0.00729 | 799.56 | 5053.709 | TP53 |
| SOX6 | SRY-Box Transcription Factor 6 | .001799 | 0.00729 | 799.56 | 5053.709 | TP53 |
| TFAP4 | Transcription Factor AP-4 | .002098 | 0.00729 | 666.2667 | 4108.591 | MDM2 |
| NFATC2 | Nuclear Factor Of Activated T Cells 2 | .002098 | 0.00729 | 666.2667 | 4108.591 | MDM2 |
| ING1 | Inhibitor Of Growth Family Member 1 | .002098 | 0.00729 | 666.2667 | 4108.591 | TP53 |
| ERCC2 | ERCC Excision Repair 2, TFIIH Core Complex Helicase Subunit | .002398 | 0.00729 | 571.0571 | 3445.29 | TP53 |
| PAX8 | Paired Box 8 | .002398 | 0.00729 | 571.0571 | 3445.29 | TP53 |
| ZNF148 | Zinc Finger Protein 148 | .002697 | 0.00729 | 499.65 | 2955.69 | CDKN2A |
| DNMT3A | DNA Methyltransferase 3 Alpha | .002697 | 0.00729 | 499.65 | 2955.69 | CDKN2A |
| HIPK2 | Homeodomain Interacting Protein Kinase 2 | .002697 | 0.00729 | 499.65 | 2955.69 | TP53 |
| SMAD7 | SMAD Family Member 7 | .002697 | 0.00729 | 499.65 | 2955.69 | CDKN2B |
| SMARCA4 | SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily A, Member 4 | .002697 | 0.00729 | 499.65 | 2955.69 | CDKN2A |
| ABL1 | ABL Proto-Oncogene 1, Non-Receptor Tyrosine Kinase | .002997 | 0.007491 | 444.1111 | 2580.412 | TP53 |
| TFDP1 | Transcription Factor Dp-1 | .002997 | 0.007491 | 444.1111 | 2580.412 | TP53 |
| NPM1 | Nucleophosmin 1 | .003296 | 0.007665 | 399.68 | 2284.211 | MDM2 |
| TP73 | Tumor Protein P73 | .003894 | 0.008113 | 333.0333 | 1847.767 | MDM2 |
| KLF5 | Kruppel Like Factor 5 | .003894 | 0.008113 | 333.0333 | 1847.767 | CDKN2B |
| STAT5A | Signal Transducer And Activator Of Transcription 5A | .003894 | 0.008113 | 333.0333 | 1847.767 | CDKN2B |
| NF1 | Neurofibromin 1 | .004193 | 0.008319 | 307.4 | 1682.803 | TP53 |
| NR4A1 | Nuclear Receptor Subfamily 4 Group A Member 1 | .004492 | 0.008319 | 285.4286 | 1542.868 | MDM2 |
| FOSL2 | FOS Like 2, AP-1 Transcription Factor Subunit | .004492 | 0.008319 | 285.4286 | 1542.868 | MDM2 |
| HOXA10 | Homeobox A10 | .004492 | 0.008319 | 285.4286 | 1542.868 | TP53 |
| ING4 | Inhibitor Of Growth Family Member 4 | .004492 | 0.008319 | 285.4286 | 1542.868 | TP53 |
| JUNB | JunB Proto-Oncogene, AP-1 Transcription Factor Subunit | .004492 | 0.008319 | 285.4286 | 1542.868 | CDKN2A |
| ATF3 | Activating Transcription Factor 3 | .004791 | 0.008627 | 266.3867 | 1422.779 | TP53 |
| BCL6 | BCL6 Transcription Repressor | .00509 | 0.008627 | 249.725 | 1318.68 | CDKN2B |
| FOXM1 | Forkhead Box M1 | .00509 | 0.008627 | 249.725 | 1318.68 | CDKN2A |
| HMGA1 | High Mobility Group AT-Hook 1 | .00509 | 0.008627 | 249.725 | 1318.68 | TP53 |
| FOXO3 | Forkhead Box O3 | .005388 | 0.008834 | 235.0235 | 1227.644 | CDKN2B |
| PML | PML Nuclear Body Scaffold | .005687 | 0.008886 | 221.9556 | 1147.411 | TP53 |
| VHL | Von Hippel-Lindau Tumor Suppressor | .005986 | 0.009209 | 210.2632 | 1076.207 | TP53 |
| PAX5 | Paired Box 5 | .006284 | 0.009522 | 199.74 | 1012.625 | TP53 |
| MTA1 | Metastasis Associated 1 | .007179 | 0.010112 | 173.6609 | 857.2875 | CDKN2A |
| HDAC3 | Histone Deacetylase 3 | .007179 | 0.010112 | 173.6609 | 857.2875 | CDKN2A |
| HDAC4 | Histone Deacetylase 4 | .007179 | 0.010112 | 173.6609 | 857.2875 | CDKN2A |
| CREBBP | CREB Binding Protein | .007477 | 0.010243 | 166.4167 | 814.7533 | TP53 |
| SMAD4 | SMAD Family Member 4 | .007776 | 0.010508 | 159.752 | 775.8784 | CDKN2B |
| JUND | JunD Proto-Oncogene, AP-1 Transcription Factor Subunit | .010158 | 0.013366 | 120.9758 | 555.2186 | MDM2 |
| NR3C1 | Nuclear Receptor Subfamily 3 Group C Member 1 | .011347 | 0.014737 | 107.8757 | 483.151 | TP53 |
| KLF4 | Kruppel Like Factor 4 | .011942 | 0.015116 | 102.3333 | 453.1047 | TP53 |
| SIRT1 | Sirtuin 1 | .014316 | 0.017605 | 84.88085 | 360.4389 | TP53 |
| IRF1 | Interferon Regulatory Factor 1 | .015205 | 0.018101 | 79.776 | 333.9551 | TP53 |
| BRCA1 | BRCA1 DNA Repair Associated | .016981 | 0.019518 | 71.20714 | 290.2178 | MDM2 |
| FOS | Fos Proto-Oncogene, AP-1 Transcription Factor Subunit | .016981 | 0.019518 | 71.20714 | 290.2178 | TP53 |
| PPARG | Peroxisome Proliferator Activated Receptor Gamma | .01964 | 0.022067 | 61.32 | 241.0002 | TP53 |
| STAT1 | Signal Transducer And Activator Of Transcription 1 | .02494 | 0.027711 | 47.97831 | 177.1019 | TP53 |
| EGR1 | Early Growth Response 1 | .026114 | 0.028697 | 45.76322 | 166.8193 | TP53 |
| YY1 | YY1 Transcription Factor | .026994 | 0.029342 | 44.23111 | 159.7682 | TP53 |
| MYC | MYC Proto-Oncogene, BHLH Transcription Factor | .029631 | 0.031861 | 40.19192 | 141.4328 | TP53 |
| STAT3 | Signal Transducer And Activator Of Transcription 3 | .041856 | 0.0436 | 28.16028 | 89.3673 | TP53 |
| JUN | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit | .043881 | 0.045238 | 26.81892 | 83.84338 | TP53 |
| RELA | RELA Proto-Oncogene, NF-KB Subunit | .08698 | 0.087536 | 13.12933 | 32.06284 | TP53 |
| NFKB1 | Nuclear Factor Kappa B Subunit 1 | .087536 | 0.087536 | 13.04106 | 31.76415 | TP53 |
Table 3.
Correlation between expressed p53 signaling pathway genes and immune cell infiltration in skin cutaneous melanoma.
| p.cor | P-value | Sig | p.cor | P-value | Sig | ||
|---|---|---|---|---|---|---|---|
| TP53 | MDM2 | ||||||
| Purity | 0.112257815 | .016240811 | ** | Purity | 0.051024468 | .275846546 | n.s. |
| B Cell | 0.014362915 | .761758841 | *** | B Cell | −0.093994959 | .04677365 | * |
| CD8+ T Cell | −0.149811599 | .001665834 | *** | CD8+ T Cell | 0.129100796 | .006820389 | ** |
| CD4+ T Cell | 0.10864395 | .021745863 | ** | CD4+ T Cell | −0.016428867 | .729338059 | n.s. |
| Macrophage | 0.065570972 | .163546292 | n.s. | Macrophage | 0.120285247 | .010396262 | ** |
| Neutrophil | −0.026652297 | .57196018 | n.s. | Neutrophil | 0.215095796 | 3.94E-06 | *** |
| Dendritic Cell | −0.024094405 | .611812095 | n.s. | Dendritic Cell | −0.013807825 | .77120504 | n.s. |
| MDM4 | CDKN2A | ||||||
| Purity | 0.204285521 | 1.05E-05 | *** | Purity | −0.072117954 | .123272657 | n.s. |
| B Cell | 0.06839608 | .148367849 | n.s. | B Cell | 0.08710705 | .065466304 | n.s. |
| CD8+ T Cell | 0.277833693 | 3.32E-09 | *** | CD8+ T Cell | 0.018638128 | .697286324 | n.s. |
| CD4+ T Cell | 0.207039133 | 1.04E-05 | *** | CD4+ T Cell | 0.030854187 | .515739471 | n.s. |
| Macrophage | 0.210331531 | 6.34E-06 | *** | Macrophage | −0.057231383 | .224086919 | n.s. |
| Neutrophil | 0.366586432 | 8.00E-16 | *** | Neutrophil | −0.008441417 | .857958725 | n.s. |
| Dendritic Cell | 0.093403659 | .048684911 | * | Dendritic Cell | 0.08161818 | .085122587 | n.s. |
| CDKN2B | TP53BP1 | ||||||
| Purity | −0.067054428 | .151938207 | n.s. | Purity | 0.094751972 | .042682729 | * |
| B Cell | 0.025157069 | .595369419 | n.s. | B Cell | 0.076126966 | .107586103 | n.s. |
| CD8+ T Cell | 0.059767187 | .211893266 | n.s. | CD8+ T Cell | 0.224972909 | 1.97E-06 | *** |
| CD4+ T Cell | 0.002027688 | .965939064 | n.s. | CD4+ T Cell | 0.193995114 | 3.71E-05 | *** |
| Macrophage | 0.029053666 | .537370587 | n.s. | Macrophage | 0.210537535 | 6.20E-06 | *** |
| Neutrophil | 0.08390466 | .074742463 | n.s. | Neutrophil | 0.378017265 | 8.40E-17 | *** |
| Dendritic Cell | 0.003568796 | .940089717 | n.s. | Dendritic Cell | 0.168697924 | .000345752 | *** |
Table 4.
Studies of the association between p53 signaling pathway genes and anti-tumor immunity in melanoma.
| Gene | Description | Function | Ref. |
|---|---|---|---|
| TP53 | Tumor protein p53 | Promote immunity, Inhibit T cell function essential for immunotherapy | Vilgelm et al 30 ; Guo et al 31 ; Banerjee et al 32 ; Chhabra and Mukherji 33 |
| MDM2 | MDM2 proto-oncogene, E3 ubiquitin protein ligase | Inhibit immunity | Vilgelm et al 30 |
| CDKN2A | cyclin-dependent kinase inhibitor 2A | Deletion of this gene directly correlated with CD8+ T-cell infiltration | Zeng et al 34 ; Horn et al 35 |
p53 signaling genes ontology enrichment
We performed gene ontology analysis of all p53 signaling genes for biological processes, cellular components, and molecular functions (Figure 6). Cyclin-dependent protein serine/threonine kinase inhibitor was the most significantly enriched GO term in molecular function; in the cellular component analysis was the nucleus, and in the biological process analysis was regulation of transcription by RNA polymerase II.
Figure 6.
Top 5 Significant GO enrichment analysis in cellular component terms, biological process terms, and molecular function terms of different expressed p53 signaling genes.
Discussion
Due to its high recurrence and metastatic rates, skin cancer remains a significant concern. 36 The TP53 signaling is implicated in tumor growth, progression, and metastasis. It is the most frequently mutated gene in human cancers. 37 The p53 causes cell cycle arrest, apoptosis, or senescence, which are crucial in tumor suppression. Different in vitro and in vivo models have been designed to study the role of TP53 in skin cancer that revealed contradictory results. 38 The previous individual studies may have less statistical power and clinical implication due to the small sample size. Integrating data from large databases could improve the quality and quantity of the evidence. The present study investigated genetic mutation and expression levels of the 6 TP53 regulating genes to set the direction for future studies and potential therapeutic targets in cutaneous melanoma. According to the current study, the COSMIC and cBioPortal, 37% and 13% of genetic alterations in TP53 were found in skin cancer cases, respectively. TP53 mRNA expression was also higher in cutaneous melanoma than in normal tissues. In mice, TP53 loss rebounds the tumor formation by overexpressing the activated oncogenes. 39 Another report showed that mutations in the TP53 gene were the most common genetic alterations in human malignancies. Further, they concluded that TP53 overexpression was strongly related to all types of skin cancer, including cutaneous melanoma.40,41 The pooled data showed no significant difference in the expression of TP53 among the sun-exposed and non-sun-exposed individuals contradicting individual studies. 42 Patients with high TP53 expression were more likely to have lymph node metastasis in 1 to 3 axillary lymph nodes. According to several studies, TP53 mutations in cutaneous melanoma are linked to increased aggressiveness and a poor prognosis. For instance, TP53 mutations were more prevalent in thick and ulcerated melanomas, which are known to have a worse prognosis than thin and non-ulcerated melanomas, according to a study by Mihic-Probst et al 43 . Similarly, TP53 mutations were linked to decreased survival and a higher risk of metastasis in patients with cutaneous melanoma, according to a study by Jakob et al 44 . However, other studies suggest TP53 mutations may not always be a bad prognostic indicator in cutaneous melanoma. For instance, a survey by Xing et al 45 discovered that while TP53 mutations did not affect survival in patients with advanced melanoma, they were linked to better survival in those with early-stage melanoma. TP53, which has both tumor-suppressive and pro-metastatic properties, plays a complex role in cutaneous melanoma. The impact of TP53 mutations on prognosis and clinical outcomes in melanoma may depend on various factors, including the stage of the disease and other genetic and environmental factors.
MDM2 is a critical negative regulator of the tumor suppressor TP53, playing a unique role in controlling its transcriptional activity, protein stability, and nuclear localization. MDM2 expression is upregulated in multiple cancers, resulting in a loss of TP53-dependent activities, such as apoptosis and cell-cycle arrest. Contrary to many individual studies, present pooled data revealed that the MDM2 gene in cutaneous melanoma represents 3% (17 cases) mutations across 448 subjects, and gene amplification accounted for most changes. In a 2003 study by Alani et al., a patient with multiple primary melanomas had a rare MDM mutation that was thought to be involved in melanoma growth. It has also been noted that MDM2 mutations frequently co-occur with mutations in BRAF and NRAS, two other genes commonly mutated in melanoma. For instance, a study by Hodis et al 46 found that 5.5% of melanomas with NRAS mutations and 2.4% of melanomas with BRAF mutations both had MDM2 mutations. Overall, it needs to be clarified what clinical significance MDM2 mutations in cutaneous melanoma have. In addition, more research is required to understand how they affect the growth and spread of the disease. Patients with high MDM2 were more likely to have lymph node metastasis in 1 to 3 axillary lymph nodes (N1) in 4 to 9 axillary lymph nodes (N2) and ten or more axillary lymph nodes (N3) and was strongly associated with tumor stage. MDM2 serves two functions: an oncogene and a potential target for treatment. On the one hand, increased aggression and a poor prognosis in melanoma have been linked to MDM2 overexpression. For instance, Hussussian et al study from 1994 discovered that MDM2 amplification was more prevalent in advanced melanomas and was linked to a lower survival rate. Similar findings were made by Liu, who found that MDM2 overexpression was connected to melanoma cells’ increased proliferation, invasion, and metastasis. On the other hand, targeting MDM2 has emerged as a promising therapeutic strategy for melanoma. In preclinical melanoma studies, several small-molecule inhibitors of MDM2, including Nutlin-3 and RG7388, have been created and demonstrated to induce p53-dependent apoptosis and tumor suppression. Patients with melanoma are currently participating in clinical trials of MDM2 inhibitors, both as monotherapy and in conjunction with other agents.47,48 In the current study, we found that patients with low MDM2 were likelier to have lymph node metastasis (N1) and TP53 mutations. In conclusion, MDM2 is both an oncogene and a potential therapeutic target in cutaneous melanoma. Targeting MDM2 with small-molecule inhibitors has shown promising preclinical and clinical results, but overexpression of MDM2 is associated with increased aggressiveness and a poor prognosis in melanoma.
MDM4 inhibits TP53′s pro-apoptotic function, allowing human metastatic melanoma to survive. Notably, inhibition of the MDM4-TP53 interaction restored TP53 function in melanoma cells, increasing sensitivity to cytotoxic chemotherapy and inhibitors of the BRAF (V600E) oncogene. 49 Further data from the same research group revealed that MDM4 was overexpressed in ∼65% of human melanomas. There is a controversy between Gembarska et al. and our results as we found the downregulation of MDM4. Therefore, these results need further experimental research to elaborate on the expression events of this gene in cutaneous melanoma. Further, we updated the current record as the data on MDM4 mutations in cutaneous melanoma is rare. Here we present MDM4 bearing 4% mutations in cutaneous melanoma. A study found that specific MDM4 gene polymorphisms were associated with an increased risk of developing cutaneous melanoma. 50 Another study investigated the effects of p53 deletion and MDM4 overexpression on the immortalization and differentiation of human mammary epithelial cells. The authors found that MDM4 overexpression partially rescued the effects of p53 deletion on cell proliferation and differentiation. 51 The role of MDM4 in senescence-associated inflammation in human fibroblasts has been noticed. The authors found that MDM4 was essential for the induction of senescence-associated inflammation and that MDM4 depletion led to reduced inflammation and senescence-associated secretory phenotype (SASP) in these cells. 52 These studies suggest that MDM4 mutations play a role in the development and progression of cutaneous melanoma, potentially through their effects on the p53 pathway and cellular proliferation and differentiation.
CDKN2A is the major high-penetrance susceptibility gene with germline mutations identified in 20%-40% of melanoma families. A positive CDKN2A mutation status has been associated with many affected family members, multiple primary melanomas, and early age at melanoma onset. 53 CDKN2A mutations have been described in families with cutaneous melanoma. 54 Our analysis also revealed that the CDKN2Agene was mutated in 22.44% of patients from COSMIC, and 24% of overall alterations were identified from the cBio cancer platform. In the cBio cancer genomics, the primary alteration type for this gene was deep deletion. Arnoff and El-Deiry 55 revealed that melanoma patients with MDM2 amplification display deep deletions in CDKN2A. Our findings imply that the CDKN2A gene, its other components, and particularly the proteins for which this gene gives processing instructions may play a role in cutaneous melanoma progression. A study published on onco-target demonstrated that the CDKN2A gene is associated with increased susceptibility to developing cutaneous melanoma. 56 Patients with high CDKN2A were likelier to have N2 lymph node metastasis and TP53 mutations. A study looked at the frequency of mutations in CDKN2A and other high-risk melanoma susceptibility genes in patients with various types of melanoma, including cutaneous, uveal, and mucosal melanoma. The authors found that CDKN2A mutations were most common in patients with familial melanoma and multiple primary melanomas. 57 Another study used whole-genome sequencing to identify genetic alterations in various melanoma subtypes, including cutaneous melanoma. The authors found that CDKN2A was one of the most frequently mutated genes in cutaneous melanoma, with mutations occurring in approximately 20% of cases. 58 A review article discusses the role of genetic testing in identifying individuals at high risk for developing melanoma, particularly those with a family history of the disease. The authors note that CDKN2A mutations are essential genetic markers of melanoma risk. They recommend that individuals with a family history of melanoma consider genetic testing to assess their risk. 59 Overall, these studies suggest that CDKN2A mutations are an important genetic risk factor for cutaneous melanoma and may play a role in the development and progression of this disease.
A study published in 2015 showed that CDKN2B loss could contribute to human cancer development. Further observation revealed that it was highly upregulated in benign melanocytic nevi, contributes to maintaining nevus melanocytes in a growth-arrested premalignant state, and is commonly lost in melanoma. 60 We found 1.41% mutations for this gene in COSMIC in the current approach. On the other hand, the genetic alterations score was recorded at 14% from cBioPortal with a significant type of deep deletion. High-resolution genomic profiling was used in a study to locate genetic changes in melanoma tumors, including cutaneous melanoma. With mutations occurring in about 12% of cases, the authors discovered that CDKN2B was one of the most frequently mutated genes in cutaneous melanoma. 61 Recurrent mutations in the GNAQ and GNA11 genes were found by Van Raamsdonk et al 62 in uveal melanoma a small subset of cutaneous melanomas. Additionally, the authors discovered that the loss of CDKN2B expression was connected to these mutations. Following whole-genome sequencing on a group of mucosal melanomas, Furney et al 63 compared the findings to earlier research on cutaneous melanoma. The researchers discovered that cutaneous melanomas were more likely to have CDKN2A mutations, whereas mucosal melanomas were more likely to have CDKN2B mutations. Overall, even though CDKN2B mutations in cutaneous melanoma may not be as frequent as CDKN2A mutations, they are still believed to be involved in the onset and development of this condition. More investigation is required to comprehend the precise mechanisms by which CDKN2B mutations contribute to the pathogenesis of melanoma. Further, we found no significant association between CDKN2B and immune cell infiltration. Patients with low CDKN2B were likelier to have N2 lymph node metastasis and TP53 mutations. These data suggest that it may be involved in melanoma tumorigenesis, although it may not be an effective target.
TP53BP1 was initially identified as a nuclear protein that interacts with the DNA-binding domain of tumor suppressor TP53 and enhances TP53-mediated transcription activation. 64 A direct role of 53BP1 in the cellular response to DNA damage and maintenance of genomic stability has been earlier demonstrated by Stucki 65 . It is a central mediator of DNA damage checkpoint signaling. 66 Huyen 67 concluded that this gene directly participates in repairing DNA double-strand breaks.TP53BP1 has been shown to play an essential role in the etiology of human cancer. 68 Our results revealed 95.97% mutations for this gene in skin cancer from COSMIC alongside 7% significant alterations from cBio cancer genomics. Patients with high TP53BP1 were likelier to have N2 lymph node metastasis and TP53 mutations. This gene may stand out as a critical player in skin cancer. Since this gene is involved in the mediating process of TP53 transcriptional activation, we can say that mutations across this gene may interrupt TP53 login and logout. We further noticed that this gene significantly correlated with the five types of immune cells in our study except the B-cells. Therefore, it is plausible that sequence variation in the regulatory and coding region of the 53BP1 gene might affect its transcription and protein structure and, thus, its biological function in checkpoint signaling and DNA repair, leading to susceptibility to cancers. He et al 21 demonstrated that TP53BP1 gene mutations could prove valuable for skin melanoma research. With mutations occurring in about 10% of cases, Hayward et al 58 discovered that TP53BP1 was one of the most frequently mutated genes in cutaneous melanoma. The function of TP53BP1 in the DNA damage response pathway was examined by Liu et al 69 . The G2/M checkpoint must be activated in response to DNA damage, and the authors discovered that TP53BP1 binds to the protein kinase Chk1. In a study of ovarian carcinomas with BRCA1 mutations, Swisher et al 70 found that tumors with TP53BP1 mutations were more likely to develop resistance to platinum-based chemotherapy. These studies indicate that TP53BP1 mutations may influence cutaneous melanoma development and progression, possibly influencing the DNA damage response pathway. More investigation is required to comprehend the precise mechanisms by which TP53BP1 mutations contribute to the pathogenesis of melanoma. Even though there was much evidence favoring p53 signaling genes in tumor formation and metastasis, genetic mutations in p53 signaling proteins were initially limited to a small number of tumors. Recent deep sequencing investigations have found an unexpectedly high prevalence and frequency of mutations in TP53 and its regulating genes in common human malignancies. Many of these mutations have previously been connected to the development of cancer. Hotspot mutations in TP53 protein genes are among them. Hotspot mutations in TP53 protein genes are among them. In addition, activating mutations in additional p53 signaling genes have been thoroughly characterized with direct cancer implications. Although it is still uncertain if mutations in these genes play a role in cancer start or progression, their somatic mutation rate is much greater than the background mutation rate of the cancer types in which these genetic alterations were discovered. This establishes a compelling argument for the potential significance of these p53 signaling members in cancer and, as a result, lays the groundwork for further research in this intriguing field.
Conclusion
The present analysis indicates that p53 signaling genes play vital roles in cutaneous melanoma. TP53, followed by CDKN2A, were the most frequently mutated genes in the TP53 signaling genes in cutaneous melanoma. Several p53 signaling genes were differentially expressed in cutaneous melanoma compared to healthy, sun-exposed and individuals in a specific gender. The results of the present study need to be further validated in future screening studies, and in vivo or in vitro models could be used for functional analysis.
Footnotes
Author Contributions: Safir Ullah Khan: Conceptualization; Data curation; Formal analysis; Software; Writing – review & editing and wrote the original draft. Zahid Ullah: Conceptualization; Data curation; Methodology. Hadia Shaukat: Methodology; Writing – review & editing. Shiza Unab: Conceptualization; Data curation. Saba Jannat: Writing – review & editing. Waqar Ali: Formal analysis; Data curation. Amir Ali: Validation; Visualization. Muhammad Irfan: Conceptualization; Data curation and Supervision. Muhammad Fiaz Khan: Writing – review & editing and Software; Supervision; Validation. Rodolfo Daniel Cervantes-Villagrana: Supervision; Validation; Visualization.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Liu C, Srihari S, Cao KA, et al. A fine-scale dissection of the DNA double-strand break repair machinery and its implications for breast cancer therapy. Nucleic Acids Res. 2014;42:6106-6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orzan OA, Șandru A, Jecan CR. Controversies in the diagnosis and treatment of early cutaneous melanoma. J Med Life. 2015;8:132-141. [PMC free article] [PubMed] [Google Scholar]
- 3. Naik PP. Cutaneous malignant melanoma: A review of early diagnosis and Management. World J Oncol. 2021;12:7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brash DE, Rudolph JA, Simon JA, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc nut Acud Sci. 1991;88:10124-10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brash DE, Zi~Gliir A, Simon JA, Kunala S. A UV mutation spectrum in the TP53 gene in basal-cell carcinoma of the skin. 83rd annual meeting of the American Association for Cancer Research, San Diego. Proc. Amer. Ass. Cancer Rex. 1992;33. [Google Scholar]
- 6. Hall JR, Messenger ZJ, Tam HW, Phillips SL, Recio L, Smart RC. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015;6:e1700-e1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stretch JR, Gatter KC, Ralfkiaer E, Lane DP, Harris AL. Expression of mutant TP53 in melanoma. Cancer Res. 1991;51:5976-5979. [PubMed] [Google Scholar]
- 8. Yang ZH, Zhou CL, Zhu H, Li JH, He CD. A functional SNP in the MDM2 promoter mediates E2F1 affinity to modulate cyclin D1 expression in tumor cell proliferation. Asian Pac J Cancer Prev. 2014;15:3817-3823. [DOI] [PubMed] [Google Scholar]
- 9. Belluti S, Rigillo G, Imbriano C. Transcription factors in cancer: when alternative splicing determines opposite cell fates. Cells. 2020;9:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang H, Hu L, Qiu W, et al. MDMX exerts its oncogenic activity via suppression of retinoblastoma protein. Oncogene. 2015;34:5560-5569. [DOI] [PubMed] [Google Scholar]
- 11. Bista M, Petrovich M, Fersht AR. MDMX contains an autoinhibitory sequence element. Proc Natl Acad Sci USA. 2013;110:17814-17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. AbuHammad S, Cullinane C, Martin C, et al. Regulation of PRMT5–MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. [published correction appears in Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9644-9645] Proc Natl Acad Sci USA. 2019;116:17990-18000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang B, Li Y, Qi G, et al. Clinicopathological significance of CDKN2A promoter hypermethylation frequency with pancreatic cancer. Sci Rep. 2015;5:13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo JP, Wang J, Huang JH. CDKN2A is a prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma. Biosci Rep. 2021;41:BSR20211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene. 2014;33:1890-1903. [DOI] [PubMed] [Google Scholar]
- 16. Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coffill CR, Lee AP, Siau JW, et al. The TP53-Mdm2 interaction and the E3 ligase activity of Mdm2/Mdm4 are conserved from lampreys to humans. Genes Dev. 2016;30:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacroix M, Riscal R, Arena G, Linares LK, Le Cam L. Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol Metab. 2020;33:2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feroz W, Sheikh AMA. Exploring the multiple roles of guardian of the genome: P53. Egypt J Med Hum Genet. 2020;21:49. [Google Scholar]
- 20. Zhang F, Gong Z. Regulation of DNA double-strand break repair pathway choice: a new focus on 53BP1. J Zhejiang Univ Sci B. 2021;22:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He C, Nan H, Qureshi AA, Han J. Genetic variants in the 53BP1 gene and skin cancer risk. J Investig Dermatol. 2010;130:2850-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805-D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1-pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karlsson M, Zhang C, Méar L, et al. A single–cell type transcriptomics map of human tissues. Sci Adv. 2021;7:eabh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thul PJ, Åkesson L, Wiking M, et al. A subcellular map of the human proteome. Science. 2017;356:eaal3321. [DOI] [PubMed] [Google Scholar]
- 26. Uhlén M, Fagerberg L, Hallström BM, et al.Tissue-based map of the human proteome. Science. 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 27. Monaco G, Lee B, Xu W, et al. RNA-Seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. 2019;26:1627-1640.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98-W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han H, Cho JW, Lee S, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46:D380-D386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vilgelm AE, Pawlikowski JS, Liu Y, et al. Mdm2 and aurora kinase a inhibitors synergize to block melanoma growth by driving apoptosis and immune clearance of tumor cells. Cancer Res. 2015;75:181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo G, Yu M, Xiao W, Celis E, Cui Y. Local activation of p53 in the tumor microenvironment overcomes immune suppression and enhances antitumor immunity. Cancer Res. 2017;77:2292-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Banerjee A, Thyagarajan K, Chatterjee S, et al. Lack of p53 augments anti-tumor functions in cytolytic T cells. Cancer Res. 2016;76:5229-5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chhabra A, Mukherji B. Death receptor-independent activation-induced cell death in human melanoma antigen-specific MHC class I-restricted TCR-engineered CD4 T cells. J Immunol. 2013;191:3471-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng H, Jorapur A, Shain AH, et al. Bi-allelic loss of CDKN2A initiates melanoma invasion via BRN2 activation. Cancer Cell. 2018;34:56-68.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horn S, Leonardelli S, Sucker A, Schadendorf D, Griewank KG, Paschen A. Tumor CDKN2A-Associated JAK2 loss and susceptibility to immunotherapy resistance. J Natl Cancer Inst. 2018;110:677-681. [DOI] [PubMed] [Google Scholar]
- 36. Khan NH, Mir M, Qian L, et al. Skin cancer biology and barriers to treatment: recent applications of polymeric micro/nanostructures. J Adv Res. 2022;36:223-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701-713. [DOI] [PubMed] [Google Scholar]
- 38. Page A, Navarro M, Suarez-Cabrera C, et al. Protective role of p53 in skin cancer: carcinogenesis studies in mice lacking epidermal p53. Oncotarget. 2016;7:20902-20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greenhalgh DA, Wang XJ, Donehower LA, Roop DR. Paradoxical tumor inhibitory effect of p53 loss in transgenic mice expressing epidermal-targeted v-rasHa, v-fos, or human transforming growth factor alpha. Cancer Res. 1996;56:4413-4423. [PubMed] [Google Scholar]
- 40. Weiss J, Heine M, Arden KC, et al. Mutation and expression of TP53 in malignant melanomas. Recent Results Cancer Res. 1995;139:137-154. [DOI] [PubMed] [Google Scholar]
- 41. Benjamin C, Ananthaswamy H. p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol. 2007;224:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Der Pols JC, Xu C, Boyle GM, et al. Expression of p53 tumor suppressor protein in sun-exposed skin and associations with sunscreen use and time spent outdoors: a community-based study. Am J Epidemiol. 2006;163:982-988. [DOI] [PubMed] [Google Scholar]
- 43. Mihic-Probst D, Ikenberg K, Tinguely M, et al. Tumor cell plasticity and angiogenesis in human melanomas. PLoS One. 2014;9:e98624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jakob JA, Bassett RL, Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2014;120:3240-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xing Y, Guo Z, Liu Y, et al. Highly activated p53 contributes to the early stage apoptotic resistance of human melanoma cells under oxidative stress. Oncogene. 2000;19:139-147. [Google Scholar]
- 46. Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ding Q, Zhang Z, Liu JJ, et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013;56:5979-5983. [DOI] [PubMed] [Google Scholar]
- 48. Pappo AS, Patel SP, Kim KB, et al. A phase I study of RG7388, a small-molecule MDM2 antagonist, in patients with advanced malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2017;23:3563-3571. [Google Scholar]
- 49. Gembarska A, Luciani F, Fedele C, et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nat Med. 2012;18:1239-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang L, Li J, Song L. MDM4 gene polymorphism and susceptibility to cutaneous melanoma. Med Oncol. 2012;29:3267-3272. [Google Scholar]
- 51. Weiss MB, Vitolo MI, Mohseni M, Rosenblum J, Landau H, Manfredi JJ. Deletion of p53 in human mammary epithelial cells leads to immortalization and altered differentiation. Stem Cells. 2010;28:409-418. [Google Scholar]
- 52. Zajac M, Moshfegh A, Persson A, et al. MDM4 is an essential factor for senescence-associated inflammation in human fibroblasts. Cell Death Dis. 2019;10:579.31371703 [Google Scholar]
- 53. Rossi M, Pellegrini C, Cardelli L, Ciciarelli V, Di Nardo L, Fargnoli MC. Familial melanoma: Diagnostic and Management Implications. Dermatol Pract Concept. 2019;9:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kannengiesser C, Avril MF, Spatz A, Laud K, Lenoir GM, Bressac-de-Paillerets B. CDKN2A as a uveal and cutaneous melanoma susceptibility gene. Genes Chromosomes Cancer. 2003;38:265-268. [DOI] [PubMed] [Google Scholar]
- 55. Arnoff TE, El-Deiry WS. MDM2/MDM4 amplification and CDKN2A deletion in metastatic melanoma and glioblastoma multiforme may have implications for targeted therapeutics and immunotherapy. Am j cancer res. 2022;12:2102-2117. [PMC free article] [PubMed] [Google Scholar]
- 56. Puig-Butille JA, Escámez MJ, Garcia-Garcia F, et al. Capturing the biological impact of CDKN2A and MC1R genes as an early predisposing event in melanoma and non melanoma skin cancer. Oncotarget. 2014;5:1439-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goldstein AM, Chan M, Harland M, et al. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Res. 2006;66:9818-9828. [DOI] [PubMed] [Google Scholar]
- 58. Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175-180. [DOI] [PubMed] [Google Scholar]
- 59. Kefford R, Bishop JN, Tucker M, et al. Genetic testing for melanoma. Lancet Oncol. 2002;3:653-654. [DOI] [PubMed] [Google Scholar]
- 60. McNeal AS, Liu K, Nakhate V, et al. CDKN2B loss promotes progression from benign melanocytic nevus to melanoma. Cancer Discov. 2015;5:1072-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. New Engl J Med. 2005;353:2135-2147. [DOI] [PubMed] [Google Scholar]
- 62. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Furney SJ, Turajlic S, Stamp G, et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol. 2013;230:261-269. [DOI] [PubMed] [Google Scholar]
- 64. Iwabuchi K, Li B, Massa HF, et al. Stimulation of p53-mediated transcriptional activation by the p53-binding proteins, 53BP1 and 53BP2. J Biol Chem. 1998;273:26061-26068. [DOI] [PubMed] [Google Scholar]
- 65. Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. (2005). MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2015;123:1213-1226. [DOI] [PubMed] [Google Scholar]
- 66. Wang B, Matsuoka S, Carpenter PB, et al. 53BP1, a mediator of the DNA damage checkpoint. Science. 2002;298:1435-1438. [DOI] [PubMed] [Google Scholar]
- 67. Huyen Y, Zgheib O, DiTullio RA, Jr, et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature. 2004;432:406-411. [DOI] [PubMed] [Google Scholar]
- 68. He C, Nan H, Qureshi AA, et al. Genetic variants in the 53BP1 gene and skin cancer risk. J Invest Dermatol 2010;130:2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu Q, Guntuku S, Cui XS, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000;14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 70. Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]