Abstract
Introduction
CDI is a recurrent disease that is treated with antibiotics, but patients commonly experience repeat infections with significant impacts on hospital budgets and patient health quality. Standard of care management includes the antibiotics, vancomycin and fidaxomicin, which frequently provide clinical response, but do not avoid recurrence of Clostridioides difficile infection (rCDI). These recurrent infections occur due to dysbiosis of the colonic microbiota. One adjunctive therapeutic approach is to restore the deficient gastrointestinal flora using fecal microbiota transplantation (FMT) or live biotherapeutic products (LBP) when given after standard of care antimicrobials, which have been successful in reducing repeat infections with success rates up to 88%. FMT or LBP can be given by various routes.
Methods
Two groups of subjects aged ≥18 years with at least one previous CDI episode within the previous 36 months completed self-administered online surveys to assess the acceptability of an LBP administered rectally. Group 1 consisted of LBP-recipients who had received RBL (REBYOTA) rectally as part of the Phase III PUNCH CD3 clinical trial. Group 2 consisted of LBP-naïve subjects who volunteered to participate and had experienced CDI within the prior 36 months but had no history of receiving FMT or LBP therapy.
Results
LBP-recipients considered rectal administration easy (96%) and quick (94%), while 98% of respondents considered the lack of need for bowel preparation appealing. Most LBP-recipients (96%) wished they had earlier access to RBL. Most LBP-naïve subjects (87%) were likely or somewhat likely to consider a rectally administered treatment and 80% preferred a treatment option that does not require bowel preparation. Many of these subjects (76%) expressed interest in finding out about new treatment options for rCDI.
Discussion
LBP-recipients and LBP-naïve subjects alike felt that rectal delivery of microbiome therapy is not only acceptable but highly interesting as a treatment avenue.
Keywords: Clostridioides difficile infection, patient preference, quality of life, fecal microbiota transplant, live biotherapeutic product
Plain Language Summary
Fecal microbiota transplantation (FMT) and live biotherapeutic products (LBP) are emerging as treatment options for recurrent infections. These products can be administered rectally. While LBP are screened and standardized products, FMT products are not. Patients with recurrent Clostridioides difficile infection considered the rectal route of delivery to be quick, convenient, acceptable, and lacking issues such as need for bowel preparation.
Introduction
Clostridioides difficile infection (CDI) affects almost 500,000 US patients annually.1 The current guideline-recommended antibiotic therapies, such as vancomycin or fidaxomicin, are inadequate to avoid recurrent CDI (rCDI).2 Dysbiosis, or disruption of the gastrointestinal microbiome, is often caused by misuse of antibiotics; this creates an environment which enables C. difficile spores to germinate causing diarrheal disease and other potential complications including sepsis and toxic megacolon. Restoration of the healthy microbiome is essential for reduction of rCDI, which carries a significant clinical burden given that 25% of initial CDI cases result in a first recurrence and, of these, up to 60% experience further recurrences.3
The use of live biotherapeutic products (LBP) to restore the disturbed microbiota is gaining acceptance as a useful adjunctive therapy following standard of care antimicrobials.4,5 LBP involves the transfer of screened standardized healthy donor fecal material into a patient’s gastrointestinal tract to restore microbial diversity and an array of benefits such as colonization resistance against various pathogens, immune modulation, gut barrier integrity, and metabolic processes.
FMT can be administered via different routes including intranasal,6 oral,7 upper gastrointestinal tract,8 colonoscopy,9 and rectal enema.10 The aim of this study was to ascertain how two different groups of CDI patients felt regarding the rectal route of delivery of a single-dose microbiota restoration product.
Materials and Methods
All subjects were aged ≥18 years and had at least one previous CDI episode within the previous 36 months. LBP-recipients consisted of patients without sepsis or prior surgery who had received RBL (REBYOTA) rectally as part of the Phase III PUNCH CD3 clinical trial and were questioned following its administration. LBP-naïve subjects consisted of subjects who volunteered to participate and had experienced CDI within the prior 36 months but had no history of receiving FMT or LBP therapy. These subjects provided demographic data including age, gender, location, type of health insurance, number of CDI episodes in the prior 36 months, and relevant medical history such as previous intestinal surgery or sepsis.
Using quantitative methods, each subject completed one of two self-administered online surveys designed to assess feelings about rectal administration of an LBP, which inherently does not carry any of the potential limitations associated with administration via colonoscopy, which requires anesthesia, use of an endoscopy suite, and risks associated with colonoscopy such as bleeding, infection transfer, and perforation. LBP-recipients who had received RBL, a rectally administered product, answered four questions with five possible responses, while the LBP-naïve subjects answered five questions with 4–5 possible responses for each question. Additional questions were asked of the LBP-naïve subjects about concerns of returning infection and the disruption of CDI on their lives. Responses were collected using an anonymized database via a dedicated link. Data were collected between June 2022 and January 2023. LBP-experienced subjects provided informed consent as per the PUNCH CD3 trial protocol, while the LBP-naïve subjects were compensated for their time but blinded as to the sponsor.
The study received an exemption waiver from WCG IRB, Puyallup, WA.
Results
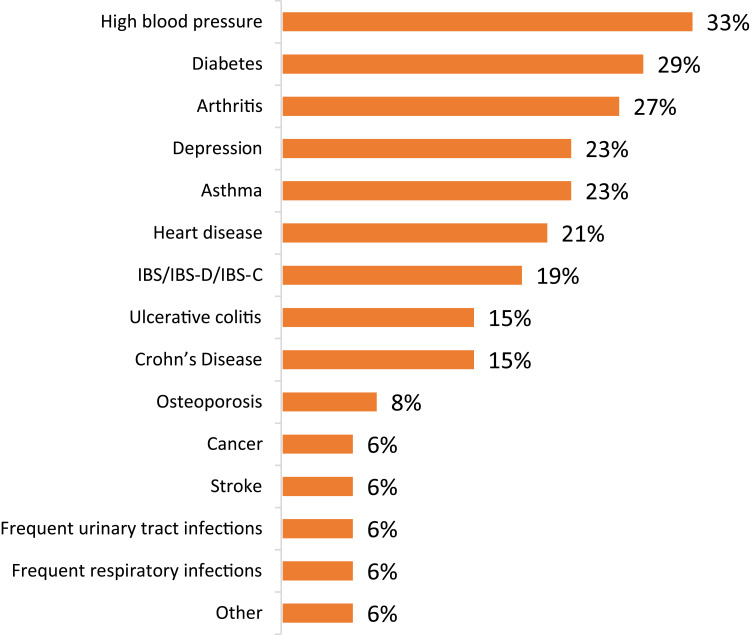
Forty-six PUNCH CD3 LBP-recipients and 100 LBP-naïve subjects responded to the questionnaire. LBP-recipients consisted of an older population than LBP-naïve subjects with a higher percentage of female respondents (Table 1). Within the previous 3–12 months, 45% of LBP-naïve subjects had experienced CDI, and 66% had CDI in the prior 12 months. Additionally, 24% of the LBP-naïve subjects had undergone gastrointestinal surgery, and 25% had been diagnosed with sepsis as a result of CDI; of those who had been diagnosed with sepsis, 40% have had gastrointestinal surgery. Finally, 52% of the LBP-naïve subjects reported that they had chronic disease, with high blood pressure and diabetes being the most common (Figure 1).
Table 1.
Participant Demographics and Baseline Characteristics
| LBP-Recipients (n=46)* | LBP-Naïve Patients (n=100) | |
|---|---|---|
| Age (years) | ||
| Mean | 62 | 46 |
| Gender, n (%) | ||
| Male | 14 (30) | 42 (42) |
| Female | 32 (70) | 58 (58) |
| Complications, n (%) | ||
| Sepsis | 0 (0) | 25 (25) |
| Bowel surgery | 0 (0) | 24 (24) |
Note: *On-going clinical trial demographic data not reported yet.
Figure 1.
Comorbidities among the LBP-naïve subjects (% subjects).
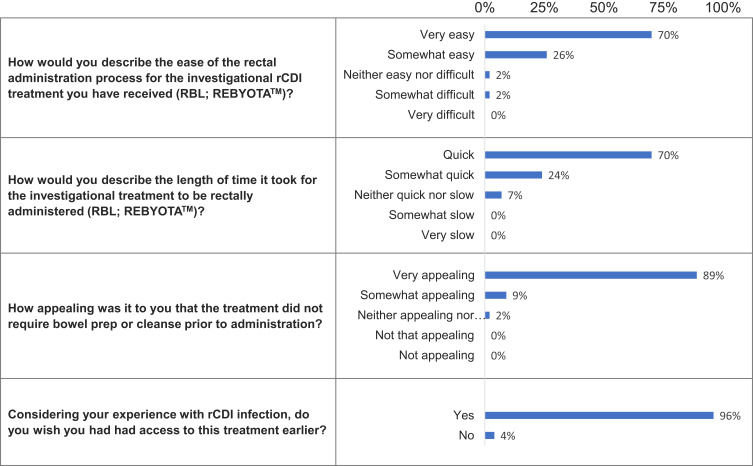
Questions and responses for the LBP-recipients are illustrated in Figure 2. Almost all patients described the process of RBL rectal administration as easy (96%) and quick (94%). The lack of need for bowel preparation was considered appealing by 98% of respondents. Most patients (96%) wished they had earlier access to RBL and the following reasons were noted: 35% reported that prior therapies were less or ineffective; 13% believed the disease would have improved sooner with earlier treatment; and 9% stated the treatment had improved their quality of life. Furthermore, CDI had negatively impacted quality of life in 52% of patients with a range of negative physical symptoms including extreme pain, dehydration, and loss of muscle tone, and there were also reports of emotional and psychological impacts.
Figure 2.
LBP-recipient questions and responses.
Abbreviation: rCDI, recurrent Clostridioides difficile infection.
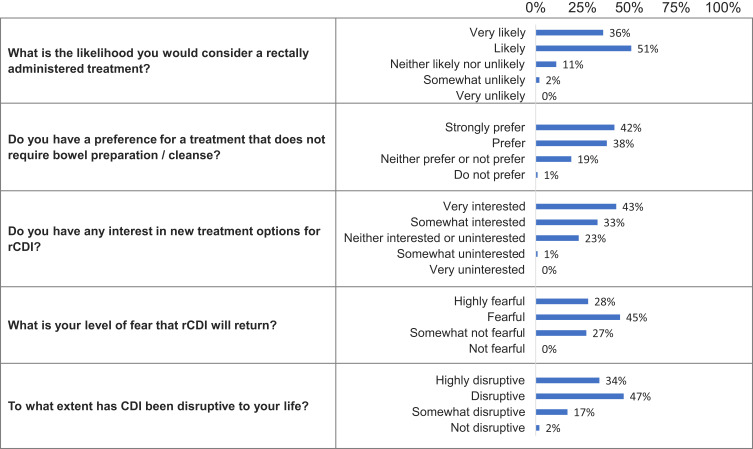
The LBP-naïve subjects’ questions and responses are shown in Figure 3. Most LBP-naïve subjects (87%) were likely or somewhat likely to consider a rectally administered treatment, with only 2% somewhat unlikely to consider it, and none very unlikely to consider it. Those who were likely to consider a rectally administered treatment reported fear of recurrence with 54% stating they were “very worried” and 32% stating they were “worried” about CDI recurrence. Disruption of life was also a major concern with those very likely to consider rectal administration.
Figure 3.
LBP-naïve subject questions and responses.
Abbreviations: CDI, Clostridioides difficile infection; rCDI, recurrent Clostridioides difficile infection.
Among the LBP-naïve subjects, most (80%) prefer a treatment option that does not require bowel preparation. It was noted that those who strongly preferred not to undergo bowel preparation were more likely to have had sepsis. Many of these subjects (76%) expressed interest in finding out about new treatment options for rCDI. Those who were very interested were older, had other chronic medical diseases, regarded CDI as being highly disruptive to their lives, and had a history of sepsis (43%).
Finally, most LBP-naïve subjects were fearful of a CDI recurrence (73%) and considered CDI to be disruptive to their lives (81%).
Discussion
Current treatment recommendations for CDI include vancomycin and fidaxomicin;2 however, these antimicrobials can contribute to gastrointestinal flora disruption, leading to the elimination of critical bacteria such as Bacteroidetes and Firmicutes and driving the proliferation of organisms like Proteobacteria (Escherichia coli, Klebsiella species), which have detrimental impacts to human health. These antibiotics are unable to prevent spore germination, which unfortunately contributes to a predisposition for recurrence.
Recurrence of CDI has not only been shown to impact patients clinically with the symptomatology of infection, but it is also a predictor for other poor health outcomes including increased risk of sepsis and surgery within one year of infection.11 Moreover, rCDI has a profound effect on psychological and social parameters,12 as well as increased hospital readmissions, healthcare utilization, and associated economic consequences.13 Avoidance of recurrences is essential to limit these negative issues.
Restoration of the eubiotic or healthy state can be achieved through live FMT, which has become one approach to microbiome restoration. The rudimentary methodologies of FMT lack standardization and some techniques have been associated with significant safety alerts,14 including risks associated with administering living microbes into patients with impaired immunity and dysregulated intestinal epithelial barrier function, as well as the possible transmission of multidrug-resistant or highly pathogenic organisms.15 Therefore, LBPs with well-overseen clinical trials assessing efficacy and safety represent an important evolution in this therapeutic technique that will hopefully make this intervention more widely available to patients.
Important factors to consider when selecting an LBP include the potential for interactions with other medications, the number of capsules required on a daily or repeat basis, the ease of administration, patient comfort, pre-treatment requirements (eg, bowel preparation, anesthesia, endoscopy suite requirement, possible hospitalization), and the need for subsequent microbiome therapy.
Recently, the FDA approved REBYOTA (fecal microbiota, live-jslm RBL) (Ferring, NJ), a rectally instilled suspension of screened biological material that includes a broad array of microorganisms. As an LBP, REBYOTA is manufactured according to good manufacturing procedures, stored in a strictly controlled environment, and is thoroughly screened as per FDA requirements.
Another microbiota restoration product was recently approved by the FDA, VOWST (SER 109, SERES Therapeutics), while VE303 (Vedanta Biosciences) is in development. These products are delivered orally and have different administration considerations such as the number of capsules four day for 3 days, need for bowel preparation, taken on empty stomach and frequency of delivery. Accounting for these various factors, we interviewed patients with a history of rCDI for their opinions on the acceptability of a rectally administered biological product such as REBYOTA. Overall, the vast majority of patients felt that the administration of REBYOTA was quick, easy, and appealing. Most of these patients wished they had access to the product earlier as it would have avoided many of the issues associated with rCDI such as anxiety, depression, social limitations, and lost productivity.
Our questionnaire revealed that LBP-naïve CDI patients reported having significant disruption to their lives, and virtually all respondents feared recurrence. Consequently, most of these subjects were open to using a rectally administered therapy as they were interested in a new treatment option that does not require bowel preparation.
A limitation of this study was the size of the two cohorts; however, the consistency of responses suggests that this was not a major problem. Other limitations include self-reporting bias, use of an unvalidated survey, and possibly the selection of study participants that may include some bias towards those who were interested in novel therapies.
Conclusion
In summary, LBP-recipients and LBP-naïve subjects alike felt that delivery of microbiome therapy directly to the site of the infection by a route that is convenient as a single dose, straightforward, and not complicated by the need for bowel preparation, anesthesia, or an endoscopy suite, is not only acceptable but highly interesting as a treatment avenue. Increased patient access to a rectally administered LBP, such as REBYOTA, could therefore have significant impacts on quality of life, healthcare utilization, medical costs, and more.
Acknowledgments
The authors thank Angela Donald ND MSc for editorial assistance. The abstract of this paper was presented as Poster #590R at the 2023 MAD-ID Annual Antimicrobial Stewardship Meeting in Orlando, Florida, from May 8–11.
Funding Statement
No funding was received for the writing of this manuscript. The study was supported by Ferring Pharmaceuticals.
Disclosure
Paul Feuerstadt declares the following interests: Ferring Pharmaceuticals: Consultant, Speaker’s bureau; SERES Pharmaceuticals: Consultant, Speaker’s bureau; Takeda Pharmaceuticals: Consultant, Speaker’s bureau; Merck and Co.: Consultant and Regeneron Pharmaceuticals: Consultant.
Caterina Oneto declares the following interests: research collaborations with Rebiotix, Seres Therapeutics, AbbVie, Salix, Ferring, Intercept, Exact Sciences, Janssen, and Vedanta and serving on speaker bureaus for AbbVie, Salix, Bristol Myers Squibb, and Pfizer.
Glenn Tillotson declares the following interests: employee of GST Micro, consultant to Spero Therapeutics, Dynavax Pharmaceuticals and Ferring Pharmaceuticals, and personal fees from AbbVie, BMS and Ferring Pharmaceuticals.
Nicholas W. Van Hise declares the following interests: Scientific Advisory Board participation for Basilea, Shionogi, AbbVie and grants from Ferring Pharma, grants from Rebiotix, grants from Finch, outside the submitted work. NVH was the lead provider on the Ferring Phase III clinical trial.
References
- 1.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029–e1044. doi: 10.1093/cid/ciab549 [DOI] [PubMed] [Google Scholar]
- 3.Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(2):S154–61. doi: 10.1093/cid/cis462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection. Ann Intern Med. 2016;165(9):609–616. doi: 10.7326/M16-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowle C. Faecal microbiota transplantation: a review of FMT as an alternative treatment for clostridium difficile infection. Biosci Horiz. 2016;9:1–14. [Google Scholar]
- 6.Aas J, Gessert CE, Bakken JS. Recurrent clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585. doi: 10.1086/367657 [DOI] [PubMed] [Google Scholar]
- 7.Allegretti J, Fischer M, Papa E, et al. Su1738 fecal microbiota transplantation delivered via oral capsules achieves microbial engraftment similar to traditional delivery modalities: safety, efficacy and engraftment results from a multi-center cluster randomized dose-finding study. Gastroenterology. 2016;150:S540. [Google Scholar]
- 8.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 9.Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012;40(6):643–648. doi: 10.1007/s15010-012-0307-9 [DOI] [PubMed] [Google Scholar]
- 10.Schwan A, Sjolin S, Trottestam U, Aronsson B. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2(8354):845. doi: 10.1016/S0140-6736(83)90753-5 [DOI] [PubMed] [Google Scholar]
- 11.Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med. 2021;9:2050312120986733. doi: 10.1177/2050312120986733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lurienne L, Bandinelli P-A, Galvain T, et al. Perception of quality of life in people experiencing or having experienced a Clostridioides difficile infection: a US population survey. J Patient Rep Outcomes. 2020;4(1):14. doi: 10.1186/s41687-020-0179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feuerstadt P, Stong L, Dahdal DN, et al. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ. 2020;23(6):603–609. doi: 10.1080/13696998.2020.1724117 [DOI] [PubMed] [Google Scholar]
- 14.FDA. Information pertaining to additional safety protections regarding use of fecal microbiota for transplantation – screening and testing of stool donors for multi-drug resistant organisms; 2019. Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/information-pertaining-additional-safety-protections-regarding-use-fecal-microbiota-transplantation. Accessed February 27, 2023.
- 15.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437 [DOI] [PubMed] [Google Scholar]