Abstract
Objectives:
Giant cell arteritis (GCA) afflicts older adults who may have age- and comorbidity-related risks for infection and is treated with immunosuppressants that increase risk of infection. We examined GCA treatment patterns and rates of serious infections in two real-world cohorts in the U.S.
Methods:
We identified two GCA cohorts using two U.S. health insurance databases, Medicare (public, 2007–2017) and MarketScan (commercial, 2015–2019), by applying a validated claims-based algorithm with positive predictive value 79.0% for GCA. We required age ≥50 years and assessed baseline comorbidities, dispensing of immunosuppressants and prophylactic antibiotics, and vaccine administration. We calculated incidence rates (IR) of serious infections, defined as bacterial or viral infections requiring hospitalization based on primary inpatient diagnosis code. Multivariable Cox proportional hazards models estimated hazard ratios for risk of serious infection for prespecified covariates.
Results:
The Medicare cohort included 734 patients, 28% male, mean age 77.1; the MarketScan cohort included 1022 patients, 30% male, mean age 68.4. More than 85% used prednisone ≥60mg daily at index date and <10% used tocilizumab. Serious infections developed in 27.9% of Medicare and 7.2% of MarketScan patients: IR per 100 person-years = 10.7 (95% CI 9.3, 12.2) in Medicare and 6.3 (95% CI 5.0, 7.9) in MarketScan. Older age and higher frailty score were significantly associated with increased risk for serious infection.
Conclusion:
In these two U.S. GCA cohorts, high-dose glucocorticoids were the most common initial treatment, and over 25% of Medicare and 7% of MarketScan patients developed serious infection during follow-up. Older age and higher frailty score were associated with higher risk of serious infections, though maximum daily prednisone dose was not. Pneumocystic jiroveci pneumonia was rare in two GCA cohorts despite infrequent use of prophylactic antibiotics.
Keywords: GCA, giant cell arteritis, serious infection, glucocorticoids, treatment
INTRODUCTION
Serious infections are a major cause of morbidity and mortality among older adults [1]. Giant cell arteritis (GCA) afflicts adults aged 50 years or older and requires treatment with glucocorticoids and/or other immunosuppressants, which raises questions about possible increases in infectious risk [2, 3]. Older adults are susceptible to infection for numerous reasons including decreased immune function (immunosenescence), nutritional deficits, and anatomical and physiologic changes of ageing that facilitate infection [4]. Common comorbidities in older adults, including hypertension, diabetes, and cancer, also predispose to infection [5, 6].
Glucocorticoids, the mainstay of GCA treatment for decades, increase the risk of infection in other autoimmune diseases [7, 8]. GCA has historically been treated with high-dose prednisone (e.g. 40–60mg daily) to induce remission, followed by a gradual taper over at least 12 months to maintain remission. In 2016, a small randomized, double-blind, placebo-controlled trial reported the efficacy of intravenous tocilizumab, an interleukin-6 alpha receptor antagonist, in the induction and maintenance of remission in patients with giant cell arteritis [9]. In 2017, subcutaneous tocilizumab demonstrated efficacy for treating GCA in combination with a 26-week glucocorticoid taper in a large randomized, placebo-controlled Phase 3 trial (GiACTA) [10], providing the basis for the subsequent world-wide marketing authorization of subcutaneous tocilizumab for the treatment of GCA. GiACTA demonstrated that tocilizumab treatment in GCA allowed more rapid tapering of glucocorticoids compared to conventional glucocorticoid monotherapy, without an increase in adverse events [10]. Treatment patterns of tocilizumab use for GCA since its approval in the U.S. have not been evaluated.
Potential infectious risks of immunosuppressive treatment for GCA may be mitigated by preventive measures including vaccination and use of prophylactic anti-infective medications. The US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices and the European Alliance of Associations for Rheumatology (EULAR) recommend pneumococcal vaccination and annual influenza vaccination for GCA patients; live-attenuated virus vaccination against herpes zoster is recommended for those using prednisone 20mg/day or less [11, 12]. Prophylaxis against Pneumocystis jiroveci pneumonia is commonly used in the care of patients with systemic rheumatic disease, with a number needed to treat of 19 when the risk of Pneumocystis jiroveci pneumonia is 6.2% or greater; however, data on the risk of Pneumocystis jiroveci pneumonia in GCA are lacking [13, 14].
The primary objectives of this study were to examine GCA treatment patterns and to determine rates of serious infections and risk factors for serious infections in two real-world cohorts in the U.S.
METHODS
Study design and data source
Using claims data from two U.S. health insurance databases -- Medicare (public, 2007–2017) linked with electronic health records (EHR), and MarketScan (commercial, 2015–2019) -- we selected two cohorts of GCA patients identified via a validated claims-based algorithm requiring ≥2 diagnosis codes for GCA (ICD-9 446.5 or ICD-10 M31.5, M31.6) plus dispensing of prednisone ≥40mg/day for ≥14 days within 14 days of the second diagnosis code (positive predictive value 79.0%) [15]. We required age ≥50 years and selected patients with incident GCA by excluding those with GCA diagnosis code in in the [−365, −31] days prior to index date (Figure 1). The Medicare GCA cohort included Medicare enrollees who were treated at Partners HealthCare, a medical network in Massachusetts that includes Brigham and Women’s Hospital, Massachusetts General Hospital, and affiliated community hospitals and primary care centers. The MarketScan GCA cohort was identified from a nationwide sample of patients aged ≥50 years with commercial health insurance [16]. The MarketScan database contains longitudinal health care information representing individuals enrolled in various employer-sponsored commercial health plans across the United States. Both Medicare-EHR linked and MarketScan databases contain longitudinal health care information including hospital admissions, emergency department visits, outpatient visits, as well as pharmacy dispensing.
Figure 1.
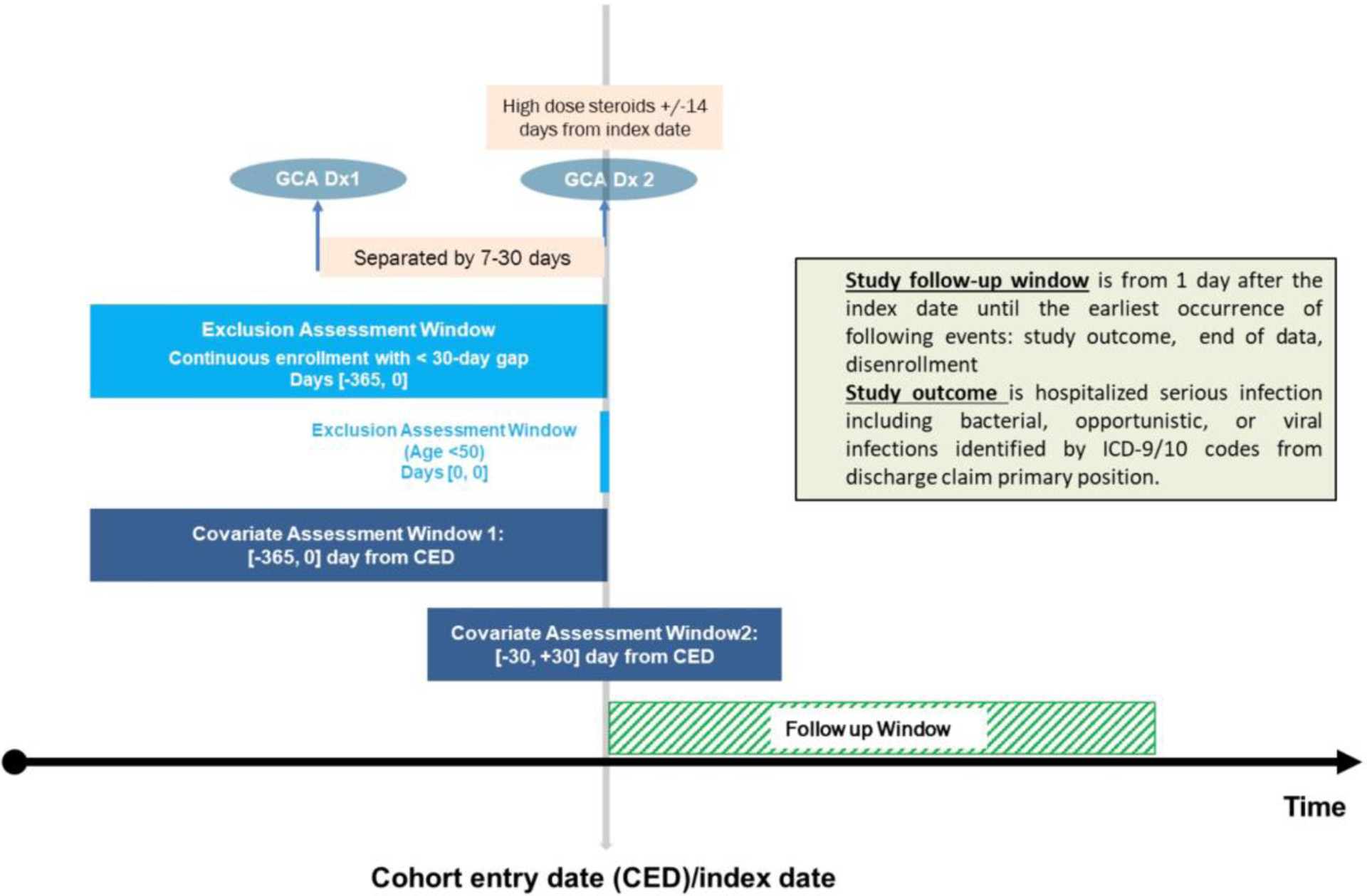
Study design diagram
Follow-up time started on the day after the second GCA diagnosis code (i.e., index date) and ended at the earliest of the following: end of cohort follow-up (12/31/2017 in Medicare; 9/7/2019 in MarketScan), insurance disenrollment, or death. We required at least 365 days continuous enrollment in the respective cohorts for covariate assessment.
Outcomes
The primary outcome was a composite endpoint of serious infection, defined as bacterial or viral infection requiring hospitalization identified through primary ICD-9/10 codes from hospital encounters. Secondary outcomes included categories of serious infection that were not mutually exclusive: bacterial, pulmonary (including Pneumocystis jiroveci pneumonia), urinary tract, and skin/soft tissue. Herpes zoster infection identified through ambulatory and inpatient diagnosis codes was also considered a secondary outcome regardless of whether hospitalization was required, as in prior work [17]. We also separately evaluated incidence rates of Pneumocystis jiroveci pneumonia.
Covariates
Covariates were assessed during the 365-day baseline period prior to and including index date. We required one or more diagnosis codes during the baseline period to define each comorbidity. A claims-based frailty index (CFI) was used to estimate the risk for adverse health outcomes and higher health care utilization among older patients. The CFI can range from 0 to 1, with higher values indicating greater frailty. We categorized the CFI into robust (<0.15), prefrail (0.15–0.24), mildly frail (0.25–0.34), and moderately to severely frail (>0.34) [16, 18]. A combined comorbidity score was a single numerical score that combines 20 elements from the Charlson index and the Elixhauser system to summarize patients’ co-morbid conditions [19, 20]. Healthcare utilization was represented by the total number of ambulatory visits and number of hospitalizations. Temporal artery biopsy procedure codes were recorded from ambulatory and hospitalized encounters in the 30 days around index date. We extracted dates of influenza, pneumococcal, and herpes zoster vaccine administration in the year prior to index date and during the year after index date. Prophylaxis against Pneumocystis jiroveci pneumonia (trimethoprim-sulfamethoxazole, dapsone, or atovaquone) was assessed in the 30 days after index date. Filled prescriptions for glucocorticoids, intravenous or subcutaneous tocilizumab, and methotrexate were assessed in 30-day blocks starting from 14 days before index date through end of follow-up. The first block was +/− 14 days from the index date (29 days), and subsequent blocks were measured in 30 days. We calculated the mean daily glucocorticoid dose in prednisone equivalents (mg/day) and use of tocilizumab (yes/no) in each 30-day block.
Statistical analysis
Baseline characteristics were summarized using descriptive statistics. We estimated incidence rates (IR) and 95% confidence intervals (CI) for the primary and secondary outcomes in each cohort. We also estimated IR for the primary outcome in the first year after index date. We determined baseline covariates of interest a priori as risk factors for serious infection, including age, sex, calendar year of index date, combined comorbidity score, frailty score, cancer, hospitalization, number of ambulatory visits, Pneumocystis jiroveci prophylaxis, vaccinations, and maximum daily glucocorticoid dose. A multivariable Cox proportional hazards model estimated hazard ratios (HR) and 95% CI for these prespecified baseline risk factors for serious infection among GCA patients in each cohort. Because pneumococcal and herpes zoster vaccinations are not administered annually, a sensitivity analysis excluded these two vaccinations during baseline. The Partners HealthCare Institutional Review Board approved all aspects of this study and granted a waiver of patient consent (protocol #2019P001602).
RESULTS
We identified 734 GCA patients forming the Medicare cohort (mean age 77.1 (SD 7.4) years, 27.5% male, mean follow-up 31 (SD 30) months) and 1022 GCA patients forming the MarketScan cohort (mean age 68.4 (SD 10.9) years, 29.8% male, mean follow-up 14 (SD 11) months). Hypertension was the most frequent comorbidity (80.1% Medicare, 72.2% MarketScan) followed by hyperlipidemia, cancer, coronary artery disease and diabetes (Table 1). Ambulatory visits with a rheumatologist were more common in the Medicare cohort than in the MarketScan cohort. Temporal artery biopsy was performed in more than 80% of the Medicare cohort; ESR and CRP were also tested in more than 80%. Advanced imaging modalities to evaluate for large vessel vasculitis were infrequently ordered within 30 days of GCA index date in either cohort.
Table 1.
Characteristics of GCA cohorts
| Medicare cohort (n=734) | MarketScan cohort (n=1022) | |
|---|---|---|
| Baseline demographics and comorbidities | ||
| Age | 77.1 (7.4) | 68.4 (10.9) |
| Male | 27.5 | 29.8 |
| Geographic region | ||
| Northeast | 88.2 | 16.4 |
| Midwest | <2.0+ | 18.5 |
| South | 9.7 | 25.6 |
| West | 1.5 | 11.4 |
| Baseline comorbidities | ||
| Hypertension | 80.1 | 72.2 |
| Hyperlipidemia | 70.7 | 61.4 |
| Cancer | 38.3 | 24.3 |
| Coronary artery disease | 33.9 | 25.4 |
| Diabetes | 34.6 | 31.0 |
| Stroke or TIA | 22.3 | 24.3 |
| Chronic kidney disease | 19.2 | 13.0 |
| Diverticulitis | 15.0 | 12.9 |
| Rheumatoid arthritis | 13.4 | 11.3 |
| Chronic liver disease | 7.8 | 6.8 |
| Herpes zoster | 2.7 | 3.5 |
| Claims-based frailty index category | ||
| Robust | 24.0 | 34.0 |
| Prefrail | 48.9 | 52.6 |
| Mildly frail | 21.7 | 11.8 |
| Moderately/severely frail | 5.4 | 1.6 |
| Combined comorbidity score | 2.9 (3.2) | 2.1 (2.6) |
| Number of hospitalizations | 0.7 (1.1) | 0.5 (0.8) |
| Number of rheumatology visits | 1.4 (2.2) | 0.8 (1.7) |
| GCA diagnostics within 30 days of index date | ||
| Temporal artery biopsy performed | 83.7 | 79.7 |
| ESR test ordered | 92.0 | 62.3 |
| CRP test ordered | 81.7 | 54.6 |
| CT angiogram ordered | 4.4 | 5.5 |
| MR angiogram ordered | <2.0+ | 1.0 |
| PET-CT ordered | 1.5 | 0.7 |
| GCA-related treatments within 30 days of index date | ||
| Prednisone maximum dose ≥60mg daily* | 87.1 | 90.5 |
| Tocilizumab (intravenous or subcutaneous) | <2.0+ | 4.5 |
| Methotrexate | 2.7 | 4.4 |
| Trimethoprim-sulfamethoxazole, atovaquone, or dapsone | 13.2 | 10.0 |
| Vaccines in the year before index date | ||
| Influenza vaccine | 64.5 | 27.7 |
| Pneumococcal vaccine** | 28.3 | 33.2 |
| Herpes zoster vaccine** | <2.0+ | 1.7 |
| Vaccines in the year after index date | ||
| Influenza vaccine | 56.0 | 22.1 |
| Pneumococcal vaccine** | 32.8 | 27.0 |
| Herpes zoster vaccine** | <2.0+ | 0.6 |
Presented as % or mean (standard deviation)
All patients were prescribed prednisone ≥40mg daily
Not an annually-administered vaccine
The Centers for Medicare and Medicaid prohibits reporting of data with cell size <11
All subjects received glucocorticoids with a prednisone equivalent dose ≥40mg per day, according to the algorithm for classifying GCA subjects. The initial dose of prednisone was ≥60mg daily in 87.1% of Medicare and 90.5% of MarketScan subjects. Figure 2 illustrates the mean daily prednisone equivalent dose during each month of follow-up. In Medicare, the mean daily prednisone dose was <20mg daily within 3 months after GCA index date; 209 of 499 patients remaining in the dataset were taking no prednisone at 12 months (41.9%). At 24 months after index date, the mean prednisone was <5mg daily among those still in the dataset. Among 62 subjects with at least 7 years of follow-up, the pattern of glucocorticoid use was initial high-dose treatment with a taper in the first 1–2 years, followed by a period of no or minimal glucocorticoid use, followed by resuming low-dose prednisone around year 6.5. In MarketScan, the mean daily prednisone dose was <20mg daily within 10 months after GCA index date; 294 of 526 patients remaining in the dataset were taking no prednisone at 12 months (55.9%). Over half of GCA patients tapered completely off prednisone by 15 months in Medicare and 10 months in MarketScan cohorts.
Figure 2.
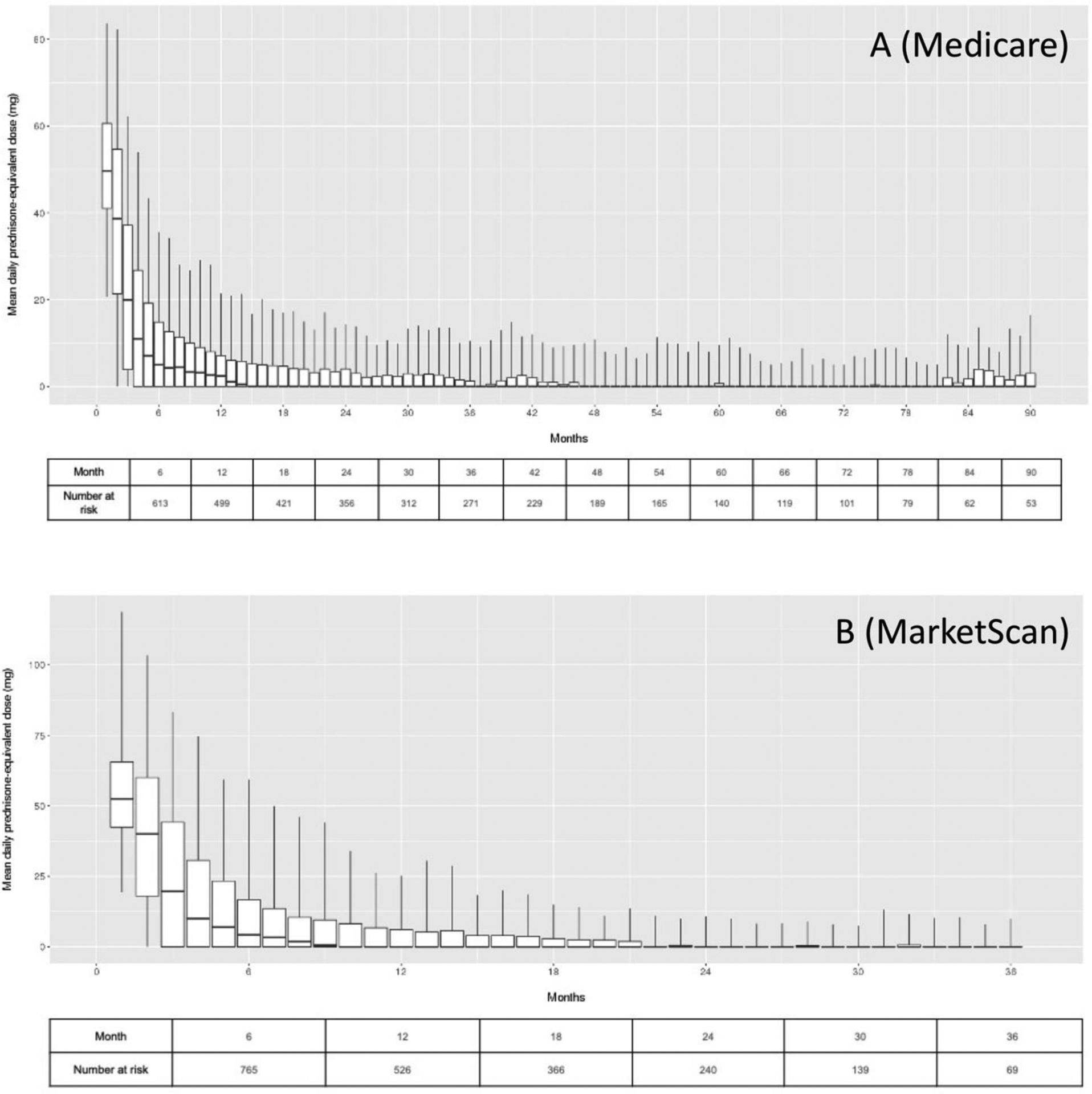
Patterns of glucocorticoid use in (A) Medicare and (B) MarketScan GCA cohorts.
Tocilizumab use was infrequent in both cohorts from index date throughout study follow-up. GCA index date occurred during or after 2017 – the year of US FDA approval for tocilizumab for GCA treatment – for 16.5% of the Medicare cohort and 65.9% of the MarketScan cohort. Among subjects with index date during or after 2017, <4% of Medicare and 6.7% of MarketScan subjects used tocilizumab within 30 days of index date; the frequency of tocilizumab use slightly increased during the first three months after index date but remained <10%.
Use of influenza, pneumococcal, and herpes zoster vaccination differed between cohorts. Influenza vaccination in the year prior to GCA index date was more common in the Medicare (64.5%) than MarketScan (27.7%) cohort. In the year prior to index date, approximately 30% of patients in each cohort received a pneumococcal vaccine and <2% received a herpes zoster vaccine. Prescriptions filled for trimethoprim-sulfamethoxazole, atovaquone, or dapsone within 30 days of index date were uncommon in both cohorts (<15%).
Table 2 presents crude incidence rates for serious infections and by category of serious infection. Serious infections occurred in 205 Medicare subjects during 1919 person-years (incidence rate per 100 person-years = 10.7, 95% CI 9.3, 12.2), and in 74 MarketScan subjects during 1173 person-years (incidence rate per 100 person-years = 6.3, 95% CI 5.0, 7.9). Bacterial infections were the most common type of serious infection (Medicare incidence rate per 100 person-years = 6.6, 95% CI 5.6, 7.7; MarketScan incidence rate per 100 person-years = 4.8, 95% CI 3.7, 6.1). When evaluating serious infections only during the first year after index date, the incidence rate was 18.9 (95% CI 15.7, 22.7) per 100 person-years in Medicare and 8.2 (95% 6.3, 10.5) per 100 person-years in MarketScan. Figure 3 presents cumulative incidence curves for serious infections and categories of infection. The incidence rate for Pneumocystis jiroveci pneumonia was 0.08 (95% CI 0.02, 0.27) per 100 person-years in Medicare and 0.08 (95% CI 0.01, 0.38) per 100 person-years in MarketScan.
Table 2.
Crude serious infection rates in GCA cohorts
| Medicare cohort (n=734) | MarketScan cohort (n=1022) | |||
|---|---|---|---|---|
| events/p-y | IR (95% CI) per 100 p-y | events/p-y | IR (95% CI) per 100 p-y | |
| Serious infection | 205/1919 | 10.7 (9.3, 12.2) | 74/1173 | 6.3 (5.0, 7.9) |
| Category of serious infection (not mutually exclusive) | ||||
| Bacterial | 141/2143 | 6.6 (5.6, 7.7) | 57/1193 | 4.8 (3.7, 6.1) |
| Pulmonary | 81/2220 | 3.6 (2.9, 4.5) | 20/1225 | 1.6 (1.0, 2.5) |
| Herpes zoster | 66/2242 | 2.9 (2.3, 3.7) | 37/1210 | 3.1 (2.2, 4.2) |
| Urinary tract | 43/2311 | 1.9 (1.4, 2.5) | 10/1237 | 0.8 (0.4, 1.4) |
| Skin and soft tissue | 28/2315 | 1.2 (0.8, 1.7) | 6/1239 | 0.5 (0.2, 1.0) |
IR: incidence rate p-y: person-years
Figure 3.
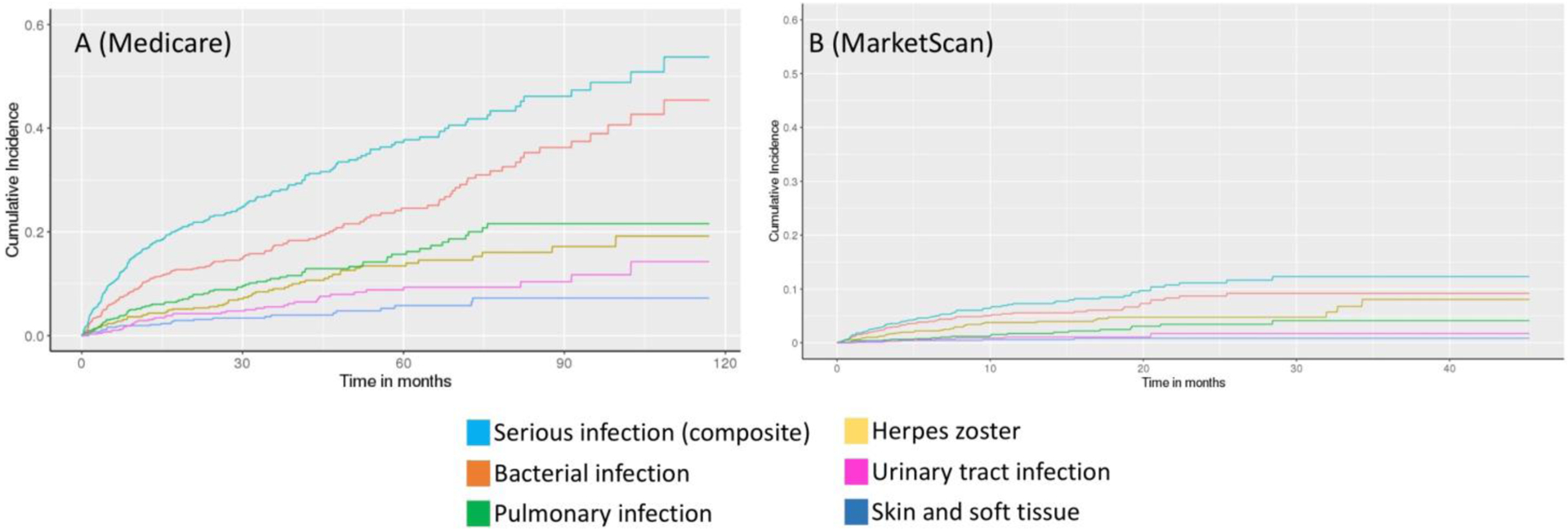
Cumulative incidence curves for serious infection and categories of serious infection in (A) Medicare and (B) MarketScan GCA cohorts. Categories of serious infection are not mutually exclusive.
Multivariable Cox proportional hazards models identified several factors associated with increased risk for serious infection in GCA subjects. Older age was associated with greater risk for serious infection in Medicare (HR 1.03, 95% CI 1.01, 1.05) and MarketScan (HR 1.04, 95% CI 1.02, 1.06) in an adjusted model (see Table 3). Moderate/severe frailty score was also an independent risk factor in adjusted models, with two- to four-fold increased risk for infection. Results were similar in a sensitivity analysis excluding herpes zoster vaccination and pneumococcal vaccination in the year before index date.
Table 3.
Multivariable-adjusted hazard ratios and 95% confidence intervals for risk of serious infection in GCA cohorts
| Medicare cohort | MarketScan cohort | |
|---|---|---|
| Age, years | 1.03 (1.01, 1.05) | 1.04 (1.02, 1.06) |
| Male sex | 1.20 (0.87, 1.66) | 1.53 (0.93, 2.51) |
| Combined comorbidity score | 1.04 (0.98, 1.10) | 1.03 (0.92, 1.14) |
| Claims-based frailty index category | ||
| Robust | 0.47 (0.30, 0.75) | 0.52 (0.27, 1.03) |
| Prefrail | 1.00 (ref) | 1.00 (ref) |
| Mildly frail | 1.37 (0.95, 1.97) | 1.06 (0.51, 2.17) |
| Moderately/severely frail | 2.51 (1.38, 4.57) | 4.54 (1.44, 14.29) |
| Cancer | 1.25 (0.93, 1.69) | 1.02 (0.59, 1.77) |
| Hospitalization (yes/no) | 1.13 (0.82, 1.57) | 0.99 (0.58, 1.70) |
| Number of office visits | 1.00 (0.99, 1.01) | 1.01 (0.98, 1.03) |
| Influenza vaccination | 1.08 (0.80, 1.46) | 1.04 (0.51, 2.10) |
| Pneumococcal vaccination | 0.95 (0.62, 1.45) | 0.91 (0.46, 1.76) |
| Herpes zoster vaccination | 2.09 (0.76, 5.72) | n/a |
| Pneumocystis prophylaxis | 1.13 (0.74, 1.72) | 1.28 (0.60, 2.72) |
| Maximum daily prednisone equivalent, mg* | 1.35 (0.93, 1.95) | 1.00 (0.77, 1.30) |
Model adjusted for all covariates in the table and calendar year of index date
log-transformed maximum daily prednisone equivalent
DISCUSSION
Glucocorticoids were the most common treatment among >1700 patients with incident GCA enrolled in public or commercial health insurance plans in the U.S. Glucocorticoid dosing was characterized by initially high doses (prednisone ≥40mg/day) followed by tapering doses, with at least half of GCA patients tapered completely off prednisone by 15 months in Medicare and 10 months in MarketScan. Some patients resumed low-dose glucocorticoids following a period of no glucocorticoid use. Recurrent use of high-dose glucocorticoids was infrequent after index date. Tocilizumab was initiated by <10% of patients with a new diagnosis of GCA occurring between calendar years 2017 and 2019.
Serious infections represent a substantial problem in these GCA cohorts. Rates of serious infections (10.7 per 100 person-years in Medicare; 6.3 per 100 person-years in MarketScan) were similar to the rate of serious infections in a clinic-based cohort of 486 GCA patients (11.1 per 100 patient-years) and a cohort of 325 patients with ANCA-associated vasculitis (9.1 per 100-person years), a systemic small-vessel vasculitis that is associated with high morbidity and mortality [21, 22]. Serious infection rates were highest in the first year after index date in GCA (18.9 per 100 person-years in Medicare), which was also comparable to the first year after ANCA-associated vasculitis diagnosis in a previous report (22.1 per 100 person-years) [21]. Previous studies of serious infection in GCA have also reported that risks are greatest in the first year after diagnosis, and have identified higher mean daily glucocorticoid dose – especially in the first six months – as a risk factor for serious infection [22–27].
In the present study, older age was an independent risk factor for serious infection in GCA patients in adjusted Cox models, with a 3–4% increased risk for each additional year of age at GCA diagnosis. Maximum daily prednisone dose was not an independent risk factor, which is likely due to the small variability in maximum glucocorticoid dose; all patients were initially treated with high-dose glucocorticoids per the study definition of GCA. Prior studies that used MarketScan administrative claims data employed a different definition of GCA than the present study – for example, they did not require high-dose glucocorticoid prescription around index date – and adjusted for time-varying glucocorticoid dose after GCA index date; these studies identified cumulative glucocorticoid dose as a risk factor for serious infection [24, 28]. We did not adjust for glucocorticoid dose after index date, as this is likely a mediator of the relationship between GCA and serious infection and therefore such adjustment is expected to attenuate the effect estimate. Future studies to evaluate the risk of infections related to the pace of glucocorticoid tapering, and comparing infectious risks in GCA patients using glucocorticoid monotherapy versus glucocorticoids plus tocilizumab will clarify treatment-related risk of infection.
A number of differences between the two cohorts in the present study may explain the observed higher crude rate of serious infection in Medicare than MarketScan. The GCA patients in MarketScan were substantially younger (mean age 68.4) than those in the Medicare cohort (mean age 77.1). This is likely because Medicare enrollees are typically >65 years old, while MarketScan generally includes adults of all ages enrolled in commercial health plans though in the present study we required age ≥50. Correspondingly, moderate/severe frailty was more common in the Medicare cohort (5.4%) than the MarketScan cohort (1.6%). Other metrics of worse health status, such as the combined comorbidity score and number of hospitalizations during baseline, were also more common in the Medicare cohort. While patients in the Medicare cohort more frequently tapered to prednisone <20mg within the first 3 months than patients in the MarketScan cohort – which might be expected to associate with lower infectious risk in the Medicare cohort -- the MarketScan cohort tended to taper completely off prednisone (month 10) sooner than the Medicare cohort (month 15). We hypothesize that the above-mentioned factors contributed to the higher crude observed rate of serious infection in the Medicare cohort compared to the MarketScan cohort. The multivariable HRs demonstrate that most of the covariates that were imbalanced between the two cohorts produced similar effect estimates. Moderate/severe frailty stands out as one factor that was potentially more influential in the MarketScan cohort, though the 95% confidence intervals overlap.
Use of prophylaxis against Pneumocystis jiroveci pneumonia was uncommon in both cohorts (<15%). Notably, the incidence rate for Pneumocystis jiroveci pneumonia was low in both cohorts (approximately 0.08 per 100 person-years). Several case series of Pneumocystis jiroveci pneumonia in GCA patients similarly suggested that this opportunistic infection is rare in GCA, and may be more common when GCA is treated with methotrexate in combination with glucocorticoids [22, 29, 30]. The low incidence rate of Pneumocystis jiroveci pneumonia is notable seeing as prophylaxis with trimethoprim-sulfamethoxazole can cause adverse drug events including rash, renal dysfunction, and cytopenias [13]. The number needed to treat to prevent Pneumocystis jiroveci pneumonia among GCA patients is likely be higher than 19 and warrants future study.
Annual influenza vaccination rates were suboptimal in both cohorts: 64.5% in Medicare and 27.7% in MarketScan in the year before index date, though information on vaccination may have been missing if patients received immunizations without billing through insurance. Influenza vaccination rates were slightly lower in the year after GCA index date in each cohort, which is concerning given that patients were being treated with immunosuppressive medication for GCA. Pneumococcal vaccination rates were similar before and after GCA index date, and these are not administered on an annual schedule which makes comparison difficult. Use of herpes zoster vaccination was very infrequent in both cohorts both before and after GCA index date.
The limitations of this study include lack of access to laboratory, pathology, and imaging results. While we assessed the use and dose of glucocorticoids by filled pharmacy claims, we do not have data on actual intake of these medication. We were able to identify serious infections using inpatient and outpatient claims data. Although we could not verify the diagnosis of GCA using medical record review or temporal artery biopsy data, we applied a validated claims-based algorithm with a high PPV of 79.0% for GCA [15].
CONCLUSION
This study identifies increased risk of serious infection in GCA patients of similar magnitude to the risk in ANCA-associated vasculitis. Glucocorticoid monotherapy remained the most common treatment for GCA from 2017–2019 in these two large U.S. cohorts. Glucocorticoids were tapered off within 10–15 months in the majority of patients. While recurrent use of low-dose glucocorticoids was common several years after the initial GCA diagnosis, recurrent use of high-dose glucocorticoids was rare. Older age and higher frailty score were associated with higher risk of serious infections, though maximum daily prednisone dose was not. Pneumocystic jiroveci pneumonia was rare in two GCA cohorts despite infrequent use of prophylactic antibiotics.
Key messages.
Older age and frailty significantly increased risk for serious infection in GCA patients.
Serious infection rates in GCA were similar to rates in ANCA-associated vasculitis.
Pneumocystic jiroveci pneumonia was rare in two GCA cohorts despite infrequent use of prophylactic antibiotics.
Funding:
This study was supported by Roche Protocol #SG41180. Dr. Tedeschi receives support from the National Institutes of Health (K23 AR075070).
Conflicts of interest:
Tedeschi, Jin, Vine, Lee: No conflict of interest.
Pethoe-Schramm is employed by F. Hoffmann-La Roche.
Yau is employed by F. Hoffmann-La Roche/Genentech.
Kim received research grants from Pfizer, F. Hoffmann-La Roche, AbbVie, and Bristol-Myers Squibb to Brigham and Women’s Hospital.
REFERENCES
- [1].Wiese AD, Griffin MR, Stein CM, Schaffner W, Greevy RA, Mitchel EF Jr., et al. Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ Open 2018;8(6):e020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: A systematic review. JAMA 2016;315(22):2442–58. [DOI] [PubMed] [Google Scholar]
- [3].Buttgereit F, Matteson EL, Dejaco C. Polymyalgia rheumatica and giant cell arteritis. JAMA 2020;324(10):993–4. [DOI] [PubMed] [Google Scholar]
- [4].Gavazzi G, Krause K-H. Ageing and infection. The Lancet Infectious Diseases 2002;2(11):659–66. [DOI] [PubMed] [Google Scholar]
- [5].Donnelly JP, Nair S, Griffin R, Baddley JW, Safford MM, Wang HE, et al. Association of diabetes and insulin therapy with risk of hospitalization for infection and 28-day mortality risk. Clin Infect Dis 2017;64(4):435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qian J, Heywood AE, Karki S, Banks E, Macartney K, Chantrill L, et al. Risk of herpes zoster prior to and following cancer diagnosis and treatment: A population-based prospective cohort study. J Infect Dis 2019;220(1):3–11. [DOI] [PubMed] [Google Scholar]
- [7].Pappas DA, Hooper MM, Kremer JM, Reed G, Shan Y, Wenkert D, et al. Herpes zoster reactivation in patients with rheumatoid arthritis: Analysis of disease characteristics and disease-modifying antirheumatic drugs. Arthritis Care Res (Hoboken) 2015;67(12):1671–8. [DOI] [PubMed] [Google Scholar]
- [8].Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: Associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 2006;54(2):628–34. [DOI] [PubMed] [Google Scholar]
- [9].Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. The Lancet 2016;387(10031):1921–27. [DOI] [PubMed] [Google Scholar]
- [10].Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377(4):317–28. [DOI] [PubMed] [Google Scholar]
- [11].Furer V, Rondaan C, Heijstek M, van Assen S, Bijl M, Agmon-Levin N, et al. Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (aiird): A systemic literature review informing the 2019 update of the eular recommendations for vaccination in adult patients with aiird. RMD Open 2019;5(2):e001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Centers for Disease C. General best practice guidelines for immunization: Best practices guidance of the advisory committe on immunization practices. March 8, 2021. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html.
- [13].Schmajuk G, Jafri K, Evans M, Shiboski S, Gianfrancesco M, Izadi Z, et al. Pneumocystis jirovecii pneumonia (pjp) prophylaxis patterns among patients with rheumatic diseases receiving high-risk immunosuppressant drugs. Semin Arthritis Rheum 2019;48(6):1087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for pneumocystis pneumonia (pcp) in non-hiv immunocompromised patients. Cochrane Database Syst Rev 2014(10):CD005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee H, Tedeschi SK, Chen SK, Monach PA, Kim E, Liu J, et al. Identification of acute giant cell arteritis in real-world data using administrative claims-based algorithms. ACR Open Rheumatol 2021;3(2):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin KJ, Glynn RJ, Singer DE, Murphy SN, Lii J, Schneeweiss S. Out-of-system care and recording of patient characteristics critical for comparative effectiveness research. Epidemiology 2018;29(3):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kang EH, Jin Y, Tong AY, Desai RJ, Kim SC. Risk of serious infection among initiators of tumor necrosis factor inhibitors plus methotrexate versus triple therapy for rheumatoid arthritis: A cohort study. Arthritis Care Res (Hoboken) 2020;72(10):1383–91. [DOI] [PubMed] [Google Scholar]
- [18].Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci 2019;74(8):1271–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64(7):749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun JW, Rogers JR, Her Q, Welch EC, Panozzo CA, Toh S, et al. Adaptation and validation of the combined comorbidity score for icd-10-cm. Med Care 2017;55(12):1046–51. [DOI] [PubMed] [Google Scholar]
- [21].Rathmann J, Jayne D, Segelmark M, Jonsson G, Mohammad AJ. Incidence and predictors of severe infections in anca-associated vasculitis: A population-based cohort study. Rheumatology (Oxford) 2020. [DOI] [PubMed] [Google Scholar]
- [22].Schmidt J, Smail A, Roche B, Gay P, Salle V, Pellet H, et al. Incidence of severe infections and infection-related mortality during the course of giant cell arteritis: A multicenter, prospective, double-cohort study. Arthritis Rheumatol 2016;68(6):1477–82. [DOI] [PubMed] [Google Scholar]
- [23].Best JH, Kong AM, Unizony S, Tran O, Michalska M. Risk of potential glucocorticoid-related adverse events in patients with giant cell arteritis: Results from a USA-based electronic health records database. Rheumatol Ther 2019;6(4):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gale S, Wilson JC, Chia J, Trinh H, Tuckwell K, Collinson N, et al. Risk associated with cumulative oral glucocorticoid use in patients with giant cell arteritis in real-world databases from the USA and uk. Rheumatol Ther 2018;5(2):327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Durand M, Thomas SL. Incidence of infections in patients with giant cell arteritis: A cohort study. Arthritis Care Res (Hoboken) 2012;64(4):581–8. [DOI] [PubMed] [Google Scholar]
- [26].Udayakumar PD, Chandran AK, Crowson CS, Warrington KJ, Matteson EL. Hospitalized infections in giant cell arteritis: A population-based retrospective cohort study. J Rheumatol 2014;41(12):2447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wilson JC, Sarsour K, Collinson N, Tuckwell K, Musselman D, Klearman M, et al. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (gca): A nested case-control analysis. Semin Arthritis Rheum 2017;46(6):819–27. [DOI] [PubMed] [Google Scholar]
- [28].Broder MS, Sarsour K, Chang E, Collinson N, Tuckwell K, Napalkov P, et al. Corticosteroid-related adverse events in patients with giant cell arteritis: A claims-based analysis. Semin Arthritis Rheum 2016;46(2):246–52. [DOI] [PubMed] [Google Scholar]
- [29].Kermani TA, Ytterberg SR, Warrington KJ. Pneumocystis jiroveci pneumonia in giant cell arteritis: A case series. Arthritis Care Res (Hoboken) 2011;63(5):761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Berger CT, Greiff V, John S, Koenig KF, Bigler MB, Recher M, et al. Risk factors for pneumocystis pneumonia in giant cell arteritis: A single-centre cohort study. Clin Exp Rheumatol 2015;33(2 Suppl 89):S-122–5. [PubMed] [Google Scholar]


