Abstract
BACKGROUND
Few studies have assessed U.S. cardiometabolic health trends—optimal levels of multiple risk factors and absence of clinical cardiovascular disease (CVD)—or its impact on health disparities.
OBJECTIVES
The purpose of this study was to investigate U.S. trends in optimal cardiometabolic health from 1999 to 2018.
METHODS
We assessed proportions of adults with optimal cardiometabolic health, based on adiposity, blood glucose, blood lipids, blood pressure, and clinical CVD; and optimal, intermediate, and poor levels of each component among 55,081 U.S. adults in the National Health and Nutrition Examination Survey.
RESULTS
In 2017–2018, only 6.8% (95% CI: 5.4%−8.1%) of U.S. adults had optimal cardiometabolic health, declining from 1999–2000 (P trend = 0.02). Among components of cardiometabolic health, the largest declines were for adiposity (optimal levels: from 33.8% to 24.0%; poor levels: 47.7% to 61.9%) and glucose (optimal levels: 59.4% to 36.9%; poor levels: 8.6% to 13.7%) (P trend <0.001 for each). Optimal levels of blood lipids increased from 29.9% to 37.0%, whereas poor decreased from 28.3% to 14.7% (P trend <0.001). Trends over time for blood pressure and CVD were smaller. Disparities by age, sex, education, and race/ethnicity were evident in all years, and generally worsened over time. By 2017–2018, prevalence of optimal cardiometabolic health was lower among Americans with lower (5.0% [95% CI: 2.8%−7.2%]) vs higher education (10.3% [95% CI: 7.6%−13.0%]); and among Mexican American (3.2% [95% CI: 1.4%−4.9%]) vs non-Hispanic White (8.4% [95% CI: 6.3%−10.4%]) adults.
CONCLUSIONS
Between 1999 and 2000 and 2017 and 2018, U.S. cardiometabolic health has been poor and worsening, with only 6.8% of adults having optimal cardiometabolic health, and disparities by age, sex, education, and race/ethnicity. These novel findings inform the need for nationwide clinical and public health interventions to improve cardiometabolic health and health equity.
Keywords: cardiometabolic health, diabetes, health disparities, metabolic syndrome, obesity
The concept of cardiometabolic health, rather than disease, represents an important advance for promoting health and health equity.1,2 Rather than focusing on abnormal levels of isolated risk factors and disease conditions, cardiometabolic health is characterized by optimal levels of multiple risk factors jointly. The concept of cardiometabolic health can also increase assessment and understanding of health inequities, by age, sex, race/ethnicity, and socioeconomic status.3–5 These issues have been further highlighted by the striking relationships between poor cardiometabolic health and more severe COVID-19 outcomes, especially in disadvantaged populations.6
However, national trends and disparities in cardiometabolic health are not well established. Prior studies have generally focused on abnormal levels of isolated risk factors,4,5,7–9 rather than on optimal levels that jointly define health. Declines in cardiovascular mortality have been slowing, and even reversing in some age groups, over the past 10 years,10,11 suggesting the potential for worsening trends in risk factors including underlying cardiometabolic health. In addition, prior studies have often not assessed population inequities in these conditions in detail, essential to quantify and address health disparities.
We investigated trends in optimal, intermediate, and poor cardiometabolic health among U.S. adults, overall and in key population subgroups, based on nationally representative data from 1999 to 2018. The findings aim to inform priorities for improving cardiometabolic health and health equity in the United States.
METHODS
STUDY DESIGN AND PARTICIPANTS.
We assessed 55,081 U.S. adults age 20+ years from the 10 most recent cycles of the National Health and Nutrition Examination Survey (NHANES, 1999–2000 to 2017–2018), accounting for the complex survey design and sampling weights to be representative of the noninstitutionalized U.S. population. NHANES collects demographic, socioeconomic, dietary, and health-related data and standardized physical examination and laboratory measures. NHANES was approved by the Institutional Review Board at the National Center for Health Statistics, and all participants provided informed written consent.
CARDIOMETABOLIC HEALTH OUTCOMES.
Optimal cardiometabolic health was defined by optimal levels of adiposity, blood glucose, blood lipids, blood pressure (BP), and absence of prior clinical CVD events, adapted from the American Heart Association (AHA) definition of ideal cardiovascular health with extension to include metabolic health (Table 1).12 We also assessed the optimal, intermediate, and poor levels of each component separately. So that changes over time in the clinical definitions of intermediate BP (or lipids) and corresponding treatment targets would not influence the results, we used the most recent clinical practice definitions consistently throughout the analysis, including in both earlier and later periods. In other words, our analysis used one consistent definition for each risk factor across all years, to avoid the problem of evolving national guidelines over time affecting misclassification of individuals.
TABLE 1.
Definitions of Metrics for Each Cardiometabolic Component, Categorized by Optimal, Intermediate, and Poor Levelsa
| Cardiometabolic Component | Optimal | Intermediate | Poor |
|---|---|---|---|
| Adiposityb | BMI <25 kg/m2 AND WC ≤88 cm (women)/WC ≤102 cm (men) | BMI 25–30 kg/m2 AND WC ≤88 cm (women)/WC ≤102 cm (men) | BMI >30 kg/m2 OR WC >88 cm (women)/WC>102 cm (men) |
| Blood glucosec,d | FPG <100 mg/dL AND HbA1c <5.7% AND NOT taking diabetes medication | FPG 100–125 mg/dL OR HbA1c 5.7%−6.4% OR FPG <100 mg/dL AND HbA1c <5.7% AND taking diabetes medication | FPG ≥126 mg/dL OR HbA1c ≥6.5% |
| Blood lipidse,f | TC:HDL <3.5:1 AND NOT taking lipid-lowering medication | TC:HDL 3.5–5:1 OR TC:HDL <3.5:1 AND taking lipid-lowering medication | TC:HDL >5:1 |
| Blood pressureg | SBP <120 AND DBP <80 AND NOT taking BP-lowering medications | (SBP 120–139 OR DBP 80–89) OR (SBP <120 AND DBP <80 AND taking BP-lowering medications) | SBP ≥140 OR DBP ≥90 |
| History of CVDh | None of the listed conditions | Angina only | One or more of CHD, myocardial infarction, heart failure, stroke |
We separately evaluated metabolic syndrome as defined by the ATPIII/National Heart, Lung, and Blood Institute guidelines as presence of 3 or more of the following: HDL-C <40 mg/dL (men)/<50 mg/dL (women) or on drug treatment for reduced HDL-C; triglycerides ≥150 mg/dL or on drug treatment for elevated triglycerides; blood pressure ≥130 SBP or ≥85 DBP or on antihypertensive drug treatment with a history of hypertension; WC ≥102 cm (men)/ ≥88 cm (women); and fasting plasma glucose ≥100 mg/dL or on drug treatment for elevated glucose.14
In sensitivity analyses, BMI thresholds for Asian American individuals were as follows: optimal: BMI <23 kg/m2, intermediate: 23–27.5 kg/m2, and poor: BMI >27.5 kg/m2, based on guidelines from National Institute for Health and Care Excellence, United Kingdom, World Health Organization, and American Diabetes Association.13
FPG and HbA1c levels from the American Diabetes Association37 FPG values before 2015–2016 NHANES survey cycles were corrected to account for differences in measurement instruments used. See Supplemental Text 1 for further details.
Self-reported history of diabetes was not separately included in the definitions.
TC:HDL levels from Calling et al.38
In sensitivity analyses, we evaluated LDL-cholesterol and triglyceride levels instead of the TC:HDL ratio. Optimal: LDL<100 mg/dL, TG <150 mg/dL, and not taking lipid-lowering medication; intermediate: LDL 100–159 mg/dL or TG 150–174 mg/dL, regardless of lipid-lowering medication usage or LDL <100 mg/dL and TG <150 mg/dL and taking lipid-lowering medication; and poor: LDL ≥160 mg/dL or TG ≥175 mg/dL or taking lipid-lowering medication.39
Blood pressure levels from Unger et al.40
Prior CHD, myocardial infarction, heart failure, or stroke based on self-reported history. Presence of angina based on having both a positive Rose Questionnaire or use of anti-anginal medication.
BMI = body mass index; CHD = coronary heart disease; CVD = cardiovascular disease; DBP = diastolic blood pressure; FPG = Fasting Plasma Glucose; HbA1c = Hemoglobin A1c; LDL = low-density lipoprotein; SBP = systolic blood pressure; TC:HDL = total cholesterol to high-density lipoprotein ratio; TG = triglycerides; WC = waist circumference.
In sensitivity analyses, body mass index (BMI) thresholds for Asian American individuals were defined using alternative guidelines from the National Institute for Health and Care Excellence, United Kingdom, World Health Organization, and American Diabetes Association.13 We also assessed low-density lipoprotein cholesterol (LDL-C) and triglycerides as an alternative metric to total cholesterol:high-density lipoprotein cholesterol ratio (TC:HDL) ratio for blood lipids in sensitivity analyses, as triglycerides are measured only on a fasting subsample of the NHANES cohort, and LDL-C is a calculated value and inaccurate in the presence of high serum triglycerides. As a secondary endpoint, we also evaluated metabolic syndrome (MetS) as defined by the AHA/National Heart, Lung, and Blood Institute.14
POPULATION SUBGROUPS.
We investigated findings in subgroups stratified by age (20–34, 35–49, 50–64, ≥65 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic, Asian/other), education (<high school graduate, high school graduate, some college or Associate’s degree, college graduate or above), and income (income-to-poverty ratio <1.3, 1.3–2.99, and ≥3, representing the ratio of family income, adjusted for family size, to the federal poverty level). Race/ethnicity results were combined for Asian and other race/ethnicity categories due to small samples and lack of separation of these categories in earlier survey cycles. Race/ethnicity was self-identified by each participant based on standardized response categories. All subgroup analyses (except for by age) were age-standardized to the age proportions in the 2017–2018 U.S. adult population.
STATISTICAL ANALYSES.
The percentage of adults with optimal cardiometabolic health; optimal, intermediate, or poor levels of each the 5 components; and MetS were estimated using survey-weighted proportions. The mean counts of optimal, intermediate, or poor levels of the 5 components were estimated using survey-weighted mean counts. Significance of overall time trends was assessed by adding the survey cycle as an ordinal variable in survey-weighted models; and between population subgroups, by a survey-weighted Wald F test to evaluate the set of multiplicative interaction terms between survey cycle (ordinal) and each sociodemographic factor (indicator categories). Significance of absolute differences between any 2 cycles or between subgroups was determined by assessing whether the central value for one estimate fell within the 95% CIs for the other estimate, approximating the Wald test. To address missingness in cardiometabolic health components, imputation incorporated all demographics, risk factors, and conditions in the dataset. Sensitivity analyses used a more liberal approach in which missing dichotomous self-reported variables (ie, medication usage, history of CVD) were assumed to be normal (optimal), and participants with missing biometric outcomes (eg, BP) were excluded. Analyses were performed with RStudio version 4.0.0 and Stata/SE (15.1), with 2-sided alpha-level = 0.05.
RESULTS
PARTICIPANT CHARACTERISTICS.
From 1999 to 2000 to 2017 to 2018, the proportion of U.S. adults age 65þ years increased from 15.8% to 20.4%, whereas those aged 20–34 years declined from 31.8% to 27.6% (Table 2). The proportion identifying as non-Hispanic White adults decreased from 70.3% to 62.2%, and those identifying as Other races (including Asian and multiracial) increased from 4.4% to 10.5%. Educational attainment grew, whereas family income distributions were relatively stable.
TABLE 2.
Characteristics of U.S. Adultsa by NHANES Survey, 1999 to 2018
| No. of Participants (Weighted %)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999–2000 (n = 4,880) | 2001–2002 (n = 5,411) | 2003–2004 (n = 5,041) | 2005–2006 (n = 4,979) | 2007–2008 (n = 5,935) | 2009–2010 (n = 6,218) | 2011–2012 (n = 5,560) | 2013–2014 (n = 5,769) | 2015–2016 (n = 5,719) | 2017–2018 (n = 5,569) | |
| Age group, y | ||||||||||
| 20–34 | 1,290 (31.8) | 1,476 (30.1) | 1,365 (29.2) | 1,513 (28.1) | 1,382 (27.5) | 1,551 (27.7) | 1,490 (27.6) | 1,482 (27.7) | 1,476 (27.7) | 1,270 (27.6) |
| 35–49 | 1,170 (32.3) | 1,372 (33.4) | 1,166 (31.3) | 1,252 (31.2) | 1,479 (30.6) | 1,638 (29.2) | 1,366 (27.5) | 1,507 (27.1) | 1,418 (25.9) | 1,230 (24.7) |
| 50–64 | 1,028 (20.1) | 1,100 (21.4) | 1,016 (22.3) | 1,025 (23.5) | 1,518 (25.2) | 1,506 (25.4) | 1,454 (27.3) | 1,474 (26.5) | 1,447 (25.9) | 1,569 (27.3) |
| ≥65 | 1,392 (15.8) | 1,463 (15.2) | 1,494 (17.1) | 1,189 (17.2) | 1,556 (16.8) | 1,523 (17.7) | 1,250 (17.7) | 1,306 (18.7) | 1,378 (20.4) | 1,500 (20.4) |
| Sex | ||||||||||
| Male | 2,269 (47.7) | 2,536 (47.8) | 2,418 (47.9) | 2,387 (48.1) | 2,910 (48.2) | 3,006 (48.2) | 2,740 (48.0) | 2,758 (48.1) | 2,747 (48.1) | 2,702 (48.1) |
| Female | 2,611 (52.3) | 2,875 (52.2) | 2,623 (52.1) | 2,592 (51.9) | 3,025 (51.8) | 3,212 (51.8) | 2,820 (52.0) | 3,011 (51.9) | 2,972 (51.9) | 2,867 (51.9) |
| Race/Ethnicity | ||||||||||
| Mexican American | 1,282 (6.4) | 1,113 (7.2) | 985 (7.8) | 1,003 (8.0) | 1,033 (8.4) | 1,140 (8.6) | 540 (7.7) | 767 (9.2) | 995 (8.9) | 735 (8.8) |
| Other Hispanic | 310 (8.1) | 237 (6.0) | 152 (3.6) | 154 (3.4) | 666 (4.9) | 632 (5.0) | 578 (6.6) | 508 (5.6) | 768 (6.4) | 517 (7.0) |
| Non-Hispanic White | 2,214 (70.3) | 2,858 (71.4) | 2,689 (72.1) | 2,495 (71.8) | 2,761 (69.4) | 2,976 (67.9) | 2,041 (66.4) | 2,472 (65.8) | 1,863 (63.8) | 1,935 (62.2) |
| Non-Hispanic Black | 910 (10.8) | 1,012 (10.9) | 994 (11.2) | 1,123 (11.5) | 1,227 (11.3) | 1,122 (11.4) | 1,455 (11.5) | 1,177 (11.4) | 1,198 (11.4) | 1,298 (11.4) |
| Otherc | 164 (4.4) | 191 (4.5) | 221 (5.4) | 204 (5.3) | 248 (6.1) | 348 (7.1) | 946 (7.8) | 845 (8.0) | 895 (9.5) | 1,084 (10.5) |
| Education level | ||||||||||
| Less than HS graduate | 1,909 (24.1) | 1,677 (19.6) | 1,494 (18.5) | 1,398 (17.8) | 1,863 (20.5) | 1,781 (19.1) | 1,335 (16.8) | 1,249 (15.3) | 1,365 (14.6) | 1,121 (11.3) |
| HS graduate | 1,102 (26.0) | 1,270 (25.2) | 1,272 (27.0) | 1,182 (25.0) | 1,464 (25.5) | 1,430 (22.9) | 1,169 (20.2) | 1,305 (21.8) | 1,239 (20.7) | 1,330 (27.2) |
| Some college or AA degree | 1,073 (27.4) | 1,384 (29.5) | 1,357 (31.3) | 1,419 (31.3) | 1,506 (28.8) | 1,746 (30.4) | 1,658 (32.1) | 1,771 (32.7) | 1,693 (32.5) | 1,780 (30.6) |
| College graduate or above | 796 (22.5) | 1,080 (25.7) | 918 (23.2) | 980 (25.9) | 1,102 (25.2) | 1,261 (27.7) | 1,398 (30.9) | 1,444 (30.2) | 1,422 (32.3) | 1,338 (30.8) |
| Ratio of family income to povertyd | ||||||||||
| <1.30 | 1,540 (23.9) | 1,483 (21.9) | 1,453 (20.9) | 1,324 (17.7) | 1,867 (21.7) | 2,158 (22.8) | 2,029 (25.7) | 2,014 (25.5) | 1,898 (21.9) | 1,633 (21.1) |
| 1.30–2.99 | 1,571 (29.6) | 1,699 (27.7) | 1,694 (30.4) | 1,613 (29.7) | 2,026 (30.2) | 1,919 (28.7) | 1,605 (27.9) | 1,602 (27.1) | 1,935 (30.7) | 1,945 (28.6) |
| ≥3.00 | 1,769 (46.4) | 2,229 (50.4) | 1,894 (48.7) | 2,042 (52.6) | 2,042 (48.1) | 2,141 (48.5) | 1,926 (46.4) | 2,153 (47.4) | 1,886 (47.4) | 1,991 (50.2) |
| Metabolic health characteristics, mean ± SD | ||||||||||
| Waist circumference, cm | 95.5 ± 15.8 | 95.9 ± 15.8 | 97.5 ± 15.6 | 97.7 ± 16.3 | 98 ± 16.2 | 98.3 ± 16.3 | 98.8 ± 16.1 | 99.7 ± 16.8 | 100.4 ± 16.8 | 101.0 ± 17.5 |
| Body mass index, kg/m2 | 28 ± 6.3 | 28 ± 6.3 | 28.2 ± 6.3 | 28.6 ± 6.8 | 28.5 ± 6.6 | 28.8 ± 6.7 | 28.7 ± 6.7 | 29.1 ± 7.1 | 29.4 ± 7.0 | 29.8 ± 7.3 |
| HbA1c, % | 5.4 ± 1.0 | 5.5 ± 0.9 | 5.5 ± 0.8 | 5.5 ± 0.9 | 5.6 ± 0.9 | 5.6 ± 0.9 | 5.6 ± 0.9 | 5.6 ± 1.0 | 5.7 ± 1.0 | 5.7 ± 0.9 |
| Fasting plasma glucose, mg/dL | 103.2 ± 33.1 | 103.7 ± 31.2 | 103.5 ± 29.8 | 103.0 ± 27.8 | 105.9 ± 32.2 | 105.9 ± 30.3 | 106.7 ± 30.9 | 107 ± 33.4 | 107.9 ± 32.7 | 108.8 ± 32.0 |
| Total cholesterol, mg/dL | 202.8 ± 40.3 | 201.8 ± 44.1 | 201.4 ± 42.9 | 198.5 ± 41.8 | 196.7 ± 40.9 | 195.6 ± 41.4 | 195 ± 41.0 | 189.5 ± 41.9 | 192.1 ± 42.4 | 188.7 ± 40.5 |
| HDL-cholesterol, mg/dL | 50.4 ± 15.4 | 52.0 ± 15.6 | 54.1 ± 15.9 | 54.5 ± 16.2 | 52.0 ± 15.9 | 53.1 ± 16.7 | 53.0 ± 15.2 | 53.2 ± 16.3 | 55.5 ± 18.7 | 53.6 ± 15.4 |
| LDL-cholesterol, mg/dL | 127.9 ± 42.6 | 123.5 ± 43.4 | 121.8 ± 44.4 | 118.4 ± 43.2 | 119.9 ± 42.6 | 118.4 ± 42.8 | 117.1 ± 41.8 | 112.5 ± 41.7 | 113.2 ± 43.3 | 112.3 ± 42.0 |
| Triglycerides, mg/dL | 121.7 ± 109.0 | 132.3 ± 156.3 | 126.8 ± 117.3 | 128.1 ± 111.7 | 123.4 ± 108.3 | 120.7 ± 105.1 | 124.7 ± 102.5 | 119.4 ± 120.0 | 118.4 ± 102.4 | 114.8 ± 96.9 |
| Total cholesterol: HDL ratio | 4.4 ± 1.6 | 4.2 ± 1.5 | 4.0 ± 1.5 | 3.9 ± 1.3 | 4.1 ± 1.6 | 4.0 ± 1.5 | 3.9 ± 1.3 | 3.9 ± 1.4 | 3.8 ± 1.5 | 3.8 ± 1.4 |
| Systolic blood pressure, mm Hg | 123.2 ± 19.2 | 122.9 ± 19.0 | 123.4 ± 18.9 | 122.8 ± 18.4 | 121.9 ± 17.2 | 120.8 ± 17.3 | 121.9 ± 17.1 | 121.9 ± 17.1 | 123.5 ± 17.2 | 123.8 ± 18.1 |
| Diastolic blood pressure, mm Hg | 72.2 ± 12.9 | 71.8 ± 13.1 | 70.8 ± 13.0 | 70.1 ± 13.1 | 70.6 ± 12.3 | 69.4 ± 12.5 | 71.2 ± 12.0 | 69.7 ± 12.2 | 70.1 ± 11.8 | 72.4 ± 12.3 |
| History of cardiovascular diseasee | ||||||||||
| Heart failure | 174 (2.1) | 192 (2.2) | 198 (2.6) | 183 (2.6) | 222 (2.4) | 176 (2.0) | 189 (2.8) | 184 (2.6) | 218 (2.5) | 206 (2.4) |
| CHD | 211 (3.3) | 248 (3.3) | 270 (4.0) | 209 (3.5) | 262 (3.3) | 259 (3.2) | 202 (3.2) | 238 (3.7) | 245 (3.5) | 271 (4.2) |
| Myocardial infarction | 227 (3.5) | 262 (3.0) | 277 (4.1) | 217 (3.6) | 284 (3.3) | 263 (3.2) | 203 (3.2) | 230 (3.3) | 251 (3.4) | 271 (3.6) |
| Stroke | 191 (2.3) | 205 (2.3) | 215 (2.8) | 197 (3.0) | 258 (3.2) | 228 (2.6) | 229 (2.9) | 202 (2.9) | 209 (2.7) | 275 (3.3) |
| Angina | 330 (6.4) | 361 (5.8) | 329 (5.8) | 313 (5.4) | 337 (4.7) | 321 (4.3) | 310 (4.6) | 247 (4.1) | 326 (5.0) | 352 (5.2) |
| Cardiometabolic medications | ||||||||||
| Diabetes medication, yes | 397 (4.9) | 421 (5.4) | 458 (6.5) | 428 (6.5) | 686 (7.8) | 666 (7.9) | 652 (8.5) | 677 (9.7) | 769 (10.3) | 833 (11.0) |
| Lipid-lowering medication, yes | 423 (7.8) | 605 (9.7) | 733 (12.6) | 768 (14.3) | 1,194 (16.6) | 1,227 (17.3) | 1,101 (18.6) | 1,227 (20.7) | 1,167 (18.6) | 1,328 (19.8) |
| Blood pressure medication, yes | 1,217 (18.7) | 1,422 (19.8) | 1,527 (24.2) | 1,379 (24.3) | 1,967 (26.6) | 1,964 (26.9) | 1,778 (27.6) | 1,849 (29.2) | 1,909 (29.3) | 2,007 (29.5) |
Values are n (%), unless specified as mean ± SD.
Missing values for all demographic, risk factor, and conditions criteria were imputed, incorporating all available demographics, risk factors, and conditions data in the dataset.
Percentages were adjusted for NHANES survey weights to represent the national U.S. population of noninstitutionalized adults.
Other includes race/ethnicity other that non-Hispanic White, non-Hispanic Black, and Hispanic, including multiracial.
Represents the ratio of family income to the federal poverty threshold, adjusting for household size. A higher ratio indicates a higher level of income.
Prior heart failure, CHD, myocardial infarction, or stroke based on self-reported history of these conditions. Presence of angina based on the presence of both a positive Rose Questionnaire or use of anti-anginal medication.
AA = Associate of Arts degree; CHD = coronary heart disease; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; HS = high school; LDL = low-density lipoprotein; NHANES = National Health and Nutrition Examination Survey.
TRENDS IN OPTIMAL CARDIOMETABOLIC HEALTH.
In 2017–2018, only 6.8% (95% CI: 5.4%−8.1%) of U.S. adults had optimal cardiometabolic health, as defined by optimal levels of all 5 cardiometabolic components (Central Illustration, Supplemental Table 1). From 1999–2000 to 2017–2018, the mean number of optimal levels of the 5 factors among U.S. adults decreased from 2.5 (95% CI: 2.4–2.6) to 2.2 (95% CI: 2.1–2.3) (Supplemental Table 2). At the same time, the mean number of intermediate levels increased from 1.4 (95% CI: 1.3–1.4) to 1.6 (95% CI: 1.5–1.6), whereas the mean number of poor levels remained stable (1.1 [95% CI: 1.0–1.2] and 1.2 [95% CI: 1.1–1.3], respectively) (Supplemental Tables 3 and 4).
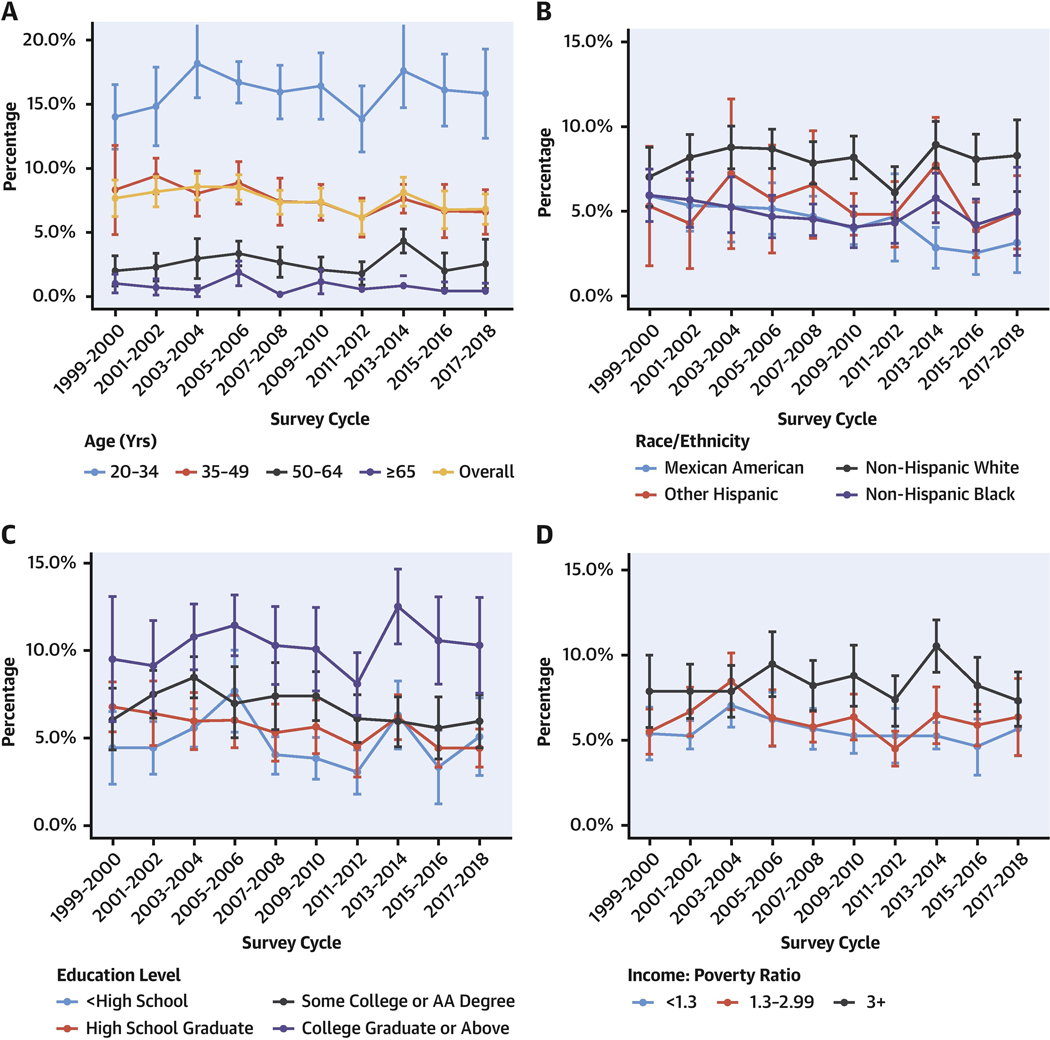
CENTRAL ILLUSTRATION. Prevalence of Optimal Cardiometabolic Health Among U.S. Adults, 1999 to 2018.
Survey-weighted national proportion (line) and 95% CIs (error bars) are shown (A) overall and by age, (B) race/ethnicity, (C) education level, and (D) income level. Optimal cardiometabolic health was defined as optimal levels for adiposity, blood glucose, blood lipids, blood pressure, and prior CVD (Table 1). Prevalence estimates were adjusted for NHANES survey weights and age-standardized to the 2017–2018 survey cycle age proportions for subgroup analyses. For race/ethnicity, adults identifying as Asian/other were removed from the figure because of large uncertainty in estimates. The findings show declining cardiometabolic health among U.S. adults between 1999 and 2018, with optimal cardiometabolic health generally less common at older vs younger ages, in lower vs higher educated adults, in lower vs higher income adults, and in Mexican American and non-Hispanic Black adults vs adults of other races. AA = Associate of Arts; CVD = cardiovascular disease; NHANES = National Health and Nutrition Examination Survey.
Within population subgroups in 2017–2018, optimal cardiometabolic health was less likely among adults age 65+ years (0.4% [95% CI: 0.0%−1.0%]) vs 20–34 years (15.3% [95% CI: 11.6%−19.1%]) and among men (3.1% [95% CI: 1.9%−4.4%]) vs women (10.4% [95% CI: 8.2%−12.6%]) (Central Illustration, Supplemental Table 1). Over time, prevalence of optimal cardiometabolic health modestly increased among non-Hispanic White adults (from 7.0% [95% CI: 5.2%−8.8%] in 1999–2000 to 8.4% [95% CI: 6.3%−10.4%] in 2017–2018), but modestly decreased for Mexican American, other Hispanic, non-Hispanic Black, and other race adults. Although prevalence of optimal cardiometabolic health varied little by income, adults with lower education were only one-half as likely to have optimal cardiometabolic health (5.0% [95% CI: 2.8%−7.2%]) compared with adults with higher education (10.3% [95% CI: 7.6%−13.0%]) in 2017–2018. Declining national trends in cardiometabolic health were generally similar over time by age, sex, education, and income, but with worsening disparities over time by race/ethnicity (P trend for interaction = 0.01).
Similar findings were seen for counts of optimal, intermediate, and poor cardiometabolic components. Older adults, men, and adults with lower educational attainment were each more likely to have lower counts of optimal levels and higher counts of poor levels (Figures 1 and 2, Supplemental Figure 1, Supplemental Tables 2 to 4). Disparities by education level declined over time, with modest improvements in mean counts of poor components in less educated adults, but modest worsening in more educated adults (P interaction for trend = 0.01). Mexican American adults had lower counts of optimal cardiometabolic components in 2017–2018 (2.0 [95% CI: 1.9–2.1]), compared with non-Hispanic White (2.3 [95% CI: 2.2–2.4]) and other race/ethnicities (2.2 [95% CI: 2.1–2.3]) (Supplemental Table 2), whereas Mexican American and non-Hispanic Black adults had higher counts of poor components (1.3 [95% CI: 1.2–1.5] and 1.3 [95% CI: 1.2–1.4], respectively) than non-Hispanic White (1.2 [95% CI: 1.1–1.3]), other Hispanic (1.2 [95% CI: 1.1–1.3]) and other race/ethnicities adults (1.1 [95% CI: 1.0–1.2]) (Supplemental Table 4). Differences in counts of optimal, intermediate, and poor cardiometabolic components were more similar by income (Supplemental Tables 2 to 4).
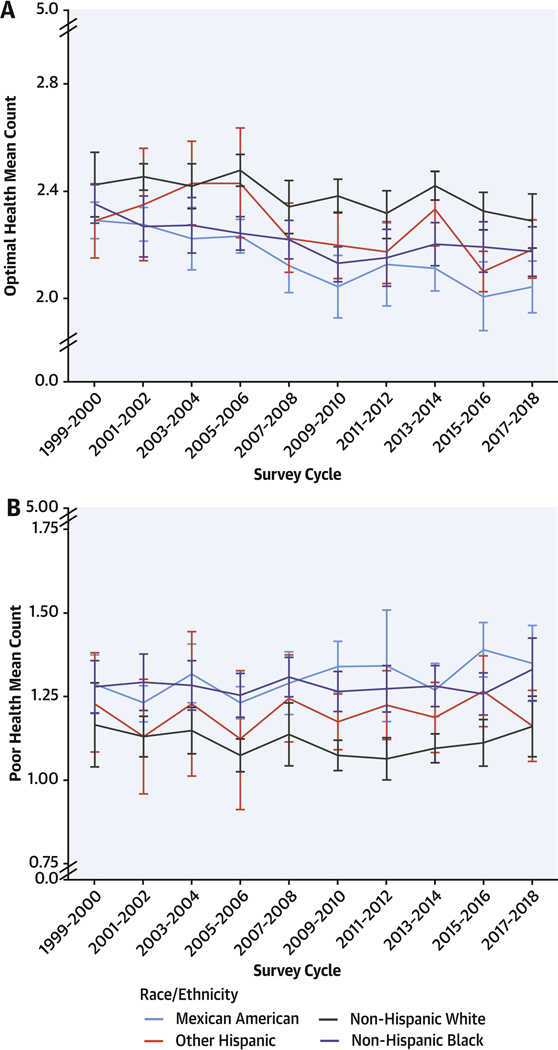
FIGURE 1. Mean Counts of Cardiometabolic Components by Race/Ethnicity, 1999 to 2018.
Survey-weighted national means (line) and 95% CIs (error bars) for U.S. adults are shown for counts of (A) optimal and (B) poor levels for 5 cardiometabolic components: adiposity, blood glucose, blood lipids, blood pressure, and prior CVD (see Table 1 for definitions). Mean counts were adjusted for NHANES survey weights and age-standardized to the 2017–2018 survey cycle age proportions. U.S. adults identifying as Asian or other (including multiracial) were removed from the figure because of large uncertainty in estimates (see Supplemental Tables 2 to 4 for optimal, intermediate, and poor counts by all race/ethnicity categories). The findings show differences in counts of optimal, intermediate, and poor cardiometabolic components by race/ethnicity. Mexican American adults had lower counts of optimal components compared with non-Hispanic White and other race/ethnicity adults, whereas Mexican American and non-Hispanic Black adults had higher counts of poor components than non-Hispanic White, other Hispanic, and other race/ethnicity adults in 2017–2018. CVD = cardiovascular disease; NHANES = National Health and Nutrition Examination Survey.
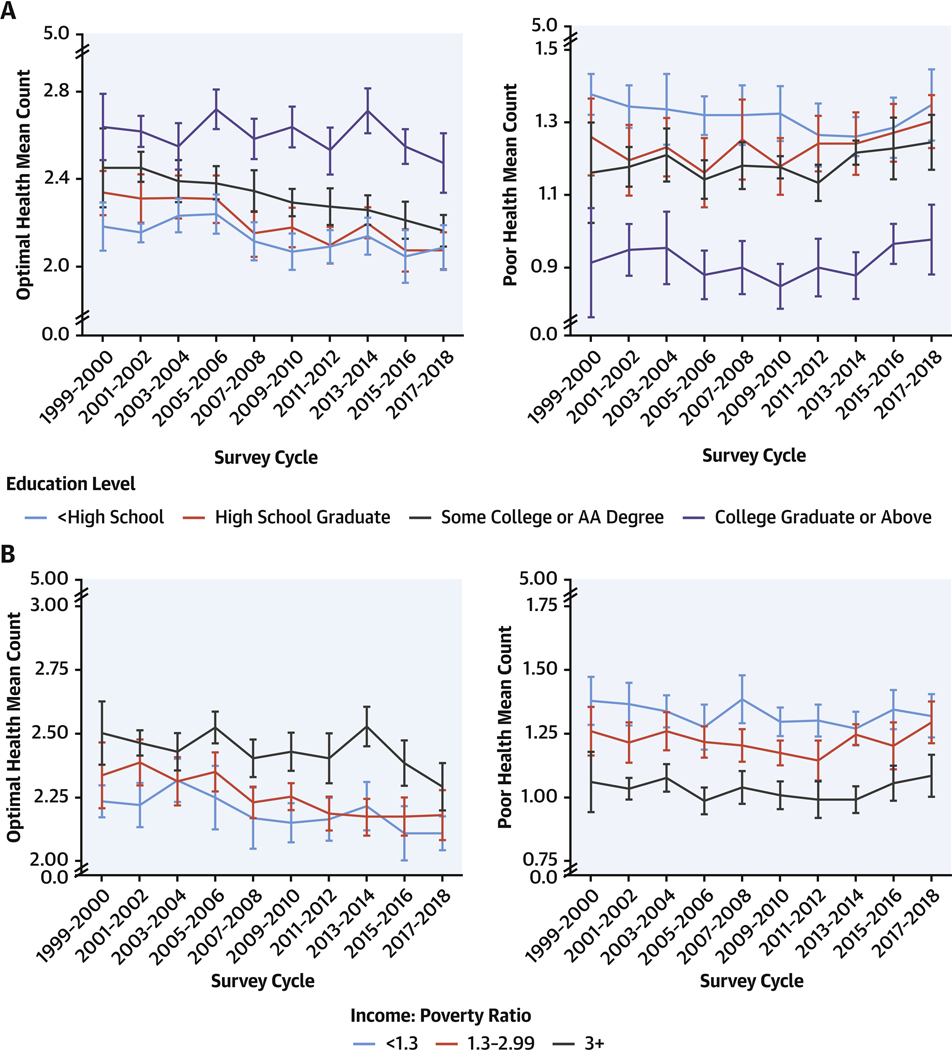
FIGURE 2. Mean Counts of Cardiometabolic Components by Education and Income, 1999 to 2018.
Survey-weighted national mean (line) and 95% CIs (error bars) for U.S. adults are shown for counts of optimal and poor levels for 5 cardiometabolic components: adiposity, blood glucose, blood lipids, blood pressure, and prior CVD (see Table 1 for definitions) by (A) education level and (B) income: poverty ratio. Mean counts were adjusted for NHANES survey weights and age-standardized to the 2017–2018 survey cycle age proportions. The findings show U.S. adults with lower educational attainment were more likely to have lower counts of optimal levels and higher counts of poor levels, with declining disparities by education level between 1999 and 2018. Abbreviations as in Figure 1.
TRENDS IN INDIVIDUAL CARDIOMETABOLIC COMPONENTS.
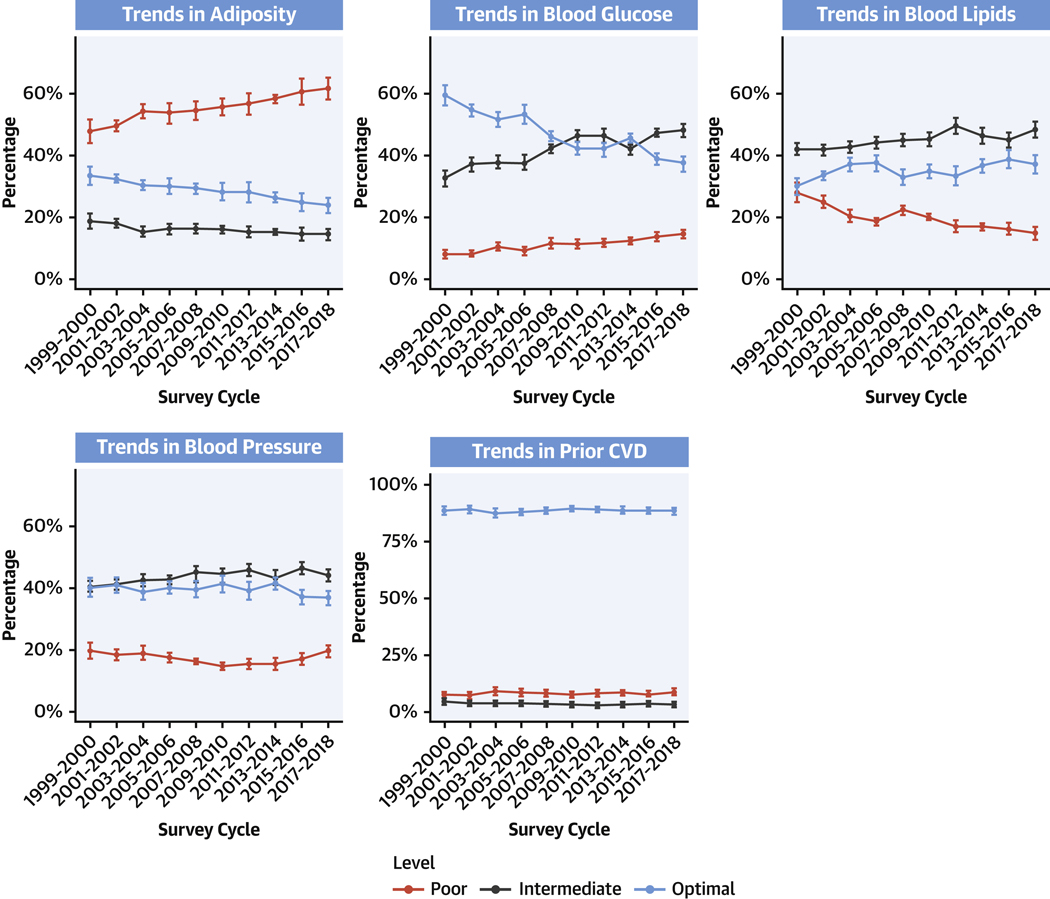
Evaluating the 5 cardiometabolic components, levels of adiposity and blood glucose demonstrated the largest declines from optimal over time. Between 1999–2000 and 2017–2018, prevalence of optimal levels of adiposity decreased from 33.8% (95% CI: 30.9%−36.6%) to 24.0% (95% CI: 21.5%−26.4%), whereas prevalence of optimal glucose levels decreased from 59.4% (95% CI: 56.0%−62.7%) to 36.9% (95% CI: 34.5%−39.2%) (P trend <0.001 each). In parallel, poor levels of adiposity increased from 47.7% (95% CI: 43.8%−51.5%) to 61.9% (95% CI: 58.6%−65.2%); and glucose, from 8.6% (95% CI: 7.2%−10.0%) to 13.7% (95% CI: 12.4%−14.9% (P trend <0.001 each) (Figure 3, Supplemental Table 5).
FIGURE 3. Trends in 5 Major Cardiometabolic Components Among U.S. Adults, 1999 to 2018.
Survey-weighted national proportions (lines) and 95% CIs (error bars) are shown for optimal, intermediate, and poor levels for each cardiometabolic component: adiposity, blood glucose, blood lipids, blood pressure, and prior CVD (see Table 1 for definitions). Prevalence estimates were adjusted for NHANES survey weights to represent the national U.S. population of noninstitutionalized adults. The findings show worsening levels (eg, higher prevalence of poor levels along with lower prevalence of optimal levels) of adiposity and glucose, and to a lesser extent blood pressure, among U.S. adults from 1999 to 2018. Prevalence of CVD remained fairly stable, whereas optimal and intermediate levels of blood lipids improved. Abbreviations as in Figure 1.
Optimal BP levels decreased more modestly, from 40.3% (95% CI: 37.2%−43.5%) to 36.5% (95% CI: 34.2%−38.7%) (P trend = 0.03), whereas poor levels remained stable, from 19.2% (95% CI: 16.7%−21.7%) to 19.5% (95% CI: 17.6%−21.4%) (P trend = 0.11). In contrast, blood lipids showed increases in prevalence of optimal levels, from 29.9% (95% CI: 26.8%−33.0%) to 37.0% (95% CI: 34.0%−39.9%), and declines in poor levels from 28.3% (95% CI: 25.2%−31.4%) to 14.7% (95% CI: 12.6%−16.8%) (P trend <0.001 each). This was largely due to steady declines in TC and LDL-C concentrations during this period. HDL-C concentrations were generally stable between 2003 and 2018 (values from 1999–2002 were slightly lower, but not directly comparable because of differing methods for their measurement in NHANES).15 Among individuals defined as having intermediate BP based on medication use, approximately 40% had on-treatment levels that would otherwise have been considered optimal, with little change in this proportion between 2000–2001 and 2017–2018 (Supplemental Table 6). Among individuals defined as having intermediate lipids based on medication use, the proportion with on-treatment levels that would otherwise have been considered optimal increased over the period between 1999–2000 and 2017–2018, to 63.3% in the most recent cycle using TC:HDL (or 53.0% using LDL-C and triglycerides). Prevalence of prior clinical CVD (myocardial infarction, CHD, heart failure, or stroke), angina, and absence of any CVD remained relatively stable.
TRENDS IN MetS.
From 1999–2000 to 2017–2018, prevalence of MetS increased from 36.2% (95% CI: 33.2%−39.1%) to 47.3% (95% CI: 45.3%−49.3%) (P trend <0.001) (Supplemental Figure 2, Supplemental Table 7). In 2017–2018, prevalence of MetS was higher among older (aged 65þ years: 78.1% [95% CI: 75.9%80.4%]) vs younger adults (ages 20–34 years: 23.1% [95% CI: 19.5–26.6%]) (Supplemental Figure 3, Supplemental Table7); among those with lower (49.1% [95% CI: 44.7%−53.6%]) vs higher (39.3% [95% CI: 34.9%−43.6%]) education (Supplemental Figure 4, Supplemental Table 7); and among those having lower (50.6% [95% CI: 47.0%−54.2%]) vs higher (44.2% [95% CI: 40.9%−47.6%]) income (Supplemental Figure 5, Supplemental Table 7). Differences by sex were small (Supplemental Figure 6). By race/ethnicity, Mexican American adults generally had the highest rates of MetS (52.2% [95% CI: 48.1%−56.2%] in 2017–2018), whereas other Hispanic and non-Hispanic White adults had the lowest rates (45.9% [95% CI: 41.9%50.0%] and 46.6% (95% CI: 42.9%−50.2%], respectively) (Supplemental Figure 7, Supplemental Table 7). Increasing trends over time in MetS were generally similar in each population subgroup (P trend-interaction ≥0.05 for each).
SENSITIVITY ANALYSES.
Findings were generally similar in sensitivity analyses, including when excluding those with missing biometric values, assuming normal values for those with missing dichotomous endpoints (Supplemental Tables 8 and 9), using differing BMI cutpoints for Asian adults, and evaluating LDL-C and triglycerides in place of TC:HDL (Supplemental Figures 8 and 9, Supplemental Text 2).
DISCUSSION
Based on nationally representative U.S. data, in 2017–2018, fewer than 1 in 14 adults (6.8%) had optimal cardiometabolic health, characterized by healthy levels of weight, BP, glucose, lipids, and clinical CVD. Over a 20-year period, cardiometabolic health among U.S. adults significantly worsened, with more people having intermediate and/or poor levels of cardiometabolic components, and fewer having optimal levels. These declines were primarily related to worsening levels of adiposity and glucose, and to a lesser extent, BP. Prevalence of prior clinical CVD remained fairly stable, whereas optimal and intermediate levels of blood lipids improved. Throughout this period, optimal cardiometabolic health was generally less common, and poor levels more common, at older vs younger ages, in men vs women, in lower vs higher educated adults, in lower vs higher income adults, and in Mexican American and non-Hispanic Black adults vs adults of other races. Over time, prevalence of optimal cardiometabolic health also declined more among Mexican American adults vs adults of other race/ethnicities (with modest increases in non-Hispanic White adults), and otherwise disparities present in 1999–2000 remained generally persistent throughout this period.
For adiposity and blood glucose, our demonstration of declining optimal levels coupled with increasing poor levels indicates a “double hit” for national cardiometabolic health. Among all cardiometabolic components in 2017–2018, optimal levels were lowest, and poor levels highest, for adiposity. These results demonstrate a dire situation for the health of the U.S. population, and an urgent need for clinical and public health strategies that prioritize obesity treatment as well as prevention, especially given its foundational role in aggravating each of the other cardiometabolic components.
The identified substantial rises in poor and intermediate levels of blood glucose, and declines in optimal levels, are likely explained by observed trends in adiposity.16,17 In contrast, we found that BP levels worsened much more modestly, whereas optimal and intermediate levels of blood lipids actually increased. BP medication usage nearly doubled over the study period (18.7% in 1999–2000 vs 29.5% in 2017–2018), likely at least partly contributing to modest trends in BP levels. The increase in intermediate levels of blood lipids is similarly partly driven by increased use of lipid-lowering medications (7.8% in 1999–2000 vs 19.8% in 2017–2018). In addition, observed declines in TC and LDL-C concentrations may partly be artifactual, as obesity and diabetes lead to atherogenic dyslipidemia with lower LDL particle size (and therefore measured LDL-C concentrations) without declines in numbers of atherogenic ApoB particles.18
Our findings also highlight and quantify disparities in optimal cardiometabolic health, including by age, sex, education, income, and race/ethnicity. In particular, our findings provide new insights into differences in cardiometabolic health by income and education level in the United States. For example, although trends and prevalence of optimal cardiometabolic health varied little by income, significant disparities were observed in optimal cardiometabolic health by education level. Of note, we also identified declining disparities by education level in counts of poor risk factors over time, suggesting the importance of other public health efforts outside of the education system in driving improvements in these health outcomes over time. Overall, our findings indicate that educational level is a stronger differentiator of cardiometabolic health status than income level, supporting public health efforts that aim to increase educational attainment for all.
Observed disparities in cardiometabolic health by race/ethnicity are consistent with prior reports of disparities in prevalence and trends over time of MetS and diabetes by race/ethnicity.8,19 A growing body of research indicates that social determinants of health are closely linked to and may drive these health disparities, including differences in jobs and wages, education access and quality, food access and quality, transportation, economic stability, social support networks, community crime and violence, neighborhood built environment, and health care access and quality.20–23 Our findings add to the evidence base on the continuing need to assess and address the interconnectedness of race/ethnicity, social determinants of health, and disparities in cardiometabolic health in the United States.
At the same time, we found that cardiometabolic health is worsening in every population subgroup evaluated. Our findings of persistent disparities over this 20-year period demonstrate the failure of current clinical, public health, and community approaches to effectively address health inequities.
The scope of problems identified in our analysis supports a need for new multisectoral approaches to address clinical care of cardiometabolic components, the food and built environment, and supportive policy and business innovations at the population level.24–30 In addition, the identified health disparities support prioritization of population-level interventions toward traditionally marginalized groups. Major new national investments in foundational and translational research, public and private health care, leveraging of federal nutrition assistance programs, use of regulatory powers, and increased cross-government coordination can each help improve cardiometabolic health and associated health disparities.17,24,26,28,31–34
Compared with the AHA’s definition of ideal cardiovascular health,12 the present investigation of optimal, intermediate, and poor cardiometabolic health includes more detailed assessment of adiposity, blood glucose, blood lipids, BP, and clinical CVD, and does not include primary lifestyle risk factors like current smoking status, physical activity, and diet. We included several updates to the health factors in the AHA Cardiovascular Health score, such as including waist circumference to define adiposity, given its utility especially among individuals with intermediate BMI levels; incorporating alternative BMI cutpoints for Asian American individuals; using the TC:HDL ratio rather than TC alone given growing prevalence and importance of atherogenic dyslipidemia; and using new, updated systolic and diastolic BP cutpoints for poor, intermediate, and optimal levels, aligned with the latest evidence of clinical risk.
Prior U.S. studies of multiple cardiometabolic components typically focused on continuous values (eg, mean BP levels) or prevalence of poor levels (eg, hypertension, MetS).5,7–9,19,35 For example, a recent study assessed U.S. trends in cardiovascular risk factors by sociodemographic factors, assessing population mean levels of cardiometabolic biomarkers and risk for atherosclerotic CVD.7 Such analyses miss changes in levels of optimal and also intermediate components, which are important and informative. For example, we found that, between 1999 and 2018, poor levels of blood glucose increased by an absolute difference of 5.1 percentage points, but optimal levels decreased by 22.5 percentage points (due to an increase in intermediate levels). Similarly, poor levels of BP remained relatively stable during this period, but optimal levels decreased by 3.8 percentage points. Thus, assessment of cardiometabolic health, including optimal, intermediate, and poor levels, provides more insight into the full spectrum of health and disease and their changes over time. Another prior study aggregated data from 2009 to 2016 and found that only 12.2% of U.S. adults had optimal levels of waist circumference, blood glucose, BP, triglycerides, and HDL-C; however, this study did not assess trends over time.36 Our investigation builds on and extends these prior findings by evaluating optimal, intermediate, and poor levels of 5 major cardiometabolic components jointly over time, using the most up-to-date national data, and evaluating comparative trends and disparities in key population subgroups.
STUDY STRENGTHS.
We leveraged standardized laboratory measures, physical examination, and interview data from NHANES, facilitating evaluation of national trends in cardiometabolic health over 20 years. We assessed optimal, intermediate, and poor levels of 5 major cardiometabolic components together and separately, providing a comprehensive and granular picture of cardiometabolic health. Because the clinical definitions for treating BP and lipid levels have changed over time, we used the most recent clinical practice definitions consistently throughout the analysis, to avoid the problem of evolving national guidelines causing misclassification of individuals. Exploratory analyses suggested that the proportion of adults with “on-treatment” optimal BP levels was relatively stable over this period, whereas the proportion with “on-treatment” optimal lipid levels increased over time. The latter finding could be explained by: 1) greater use of higher intensity lipid-lowering medications developed over this time, bringing more individuals under control; or 2) an increase in lipid-lowering medication use over time among people with lower lipid levels, for example, due to either greater physician and patient awareness and practice, or changing guidelines.
We investigated trends and differences across key sociodemographic subgroups, providing new data on national health disparities. Sensitivity analyses assessed alternative methods for handling missing values, or specific blood lipid and adiposity definitions, as well as whether findings were driven by demographic shifts.
STUDY LIMITATIONS.
The NHANES cross-sectional sampling provides data on the population, not on any 1 person over time. Fasting metrics like plasma glucose and triglycerides had higher levels of missing values, as these data were collected on subsamples of respondents. Medication usage may be partly misclassified. However, we used multiple criteria to classify poor, intermediate, and optimal levels of each component, reducing the impact of any 1 measure alone and the potential for misclassification, and performed sensitivity analyses testing alternative approaches to handling missing values.
CONCLUSIONS
Cardiometabolic health among U.S. adults significantly declined between 1999–2000 and 2017–2018, with only 6.8% having optimal cardiometabolic health by 2017–2018, and with significant differences by age, sex, race/ethnicity, and education. These findings renew the call for clinical, public health, and policy interventions to improve cardiometabolic health and health equity in the United States.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN SYSTEMS-BASED PRACTICE:
Among adults in the United States, optimal cardiometabolic health as defined by adiposity, blood glucose, blood lipids, BP, and clinical CVD decreased from 7.7% in 1999–2000 to 6.8% in 2017–2018, with disparities related to age, sex, race/ethnicity, and level of education.
TRANSLATIONAL OUTLOOK:
These findings emphasize the need for targeted strategies to improve the cardiometabolic health of the U.S. population.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This research was supported by the National Institutes of Health and the National Heart, Lung, and Blood Institute, Bethesda, Maryland (grant 2R01HL115189–06A1 to Dr Mozaffarian). The funding agency did not contribute to the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. All authors have received research funding from the National Institutes of Health. Dr Mozaffarian has received additional research funding from the Gates Foundation, the Rockefeller Foundation, and Vail Institute for Global Research; is on the scientific advisory board for Beren Therapeutics, Calibrate, DayTwo (ended 6/20), Elysium Health, Filtricine, Foodome, HumanCo, January Inc., Perfect Day, Season, and Tiny Organics; all outside the submitted work. Dr Wong has membership in the U.S. Preventive Services Task Force, outside the submitted work. Ms Lauren has received personal fees from Abt Associates and the Centers for Disease Control and Prevention, both outside the submitted work.
ABBREVIATIONS AND ACRONYMS
- AHA
American Heart Association
- BMI
body mass index
- BP
blood pressure
- CVD
cardiovascular disease
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- MetS
metabolic syndrome
- NHANES
National Health and Nutrition Examination Survey
- TC
total cholesterol
- TC:HDL
total cholesterol: high-density lipoprotein cholesterol ratio
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Listen to this manuscript’s audio summary by Editor-in-Chief Dr Valentin Fuster on www.jacc.org/journal/jacc.
REFERENCES
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. 10.1161/circulationaha.109.192703 [DOI] [PubMed] [Google Scholar]
- 2.Labarthe DR, Kubzansky LD, Boehm JK, Lloyd-Jones DM, Berry JD, Seligman ME. Positive cardiovascular health: a timely convergence. J Am Coll Cardiol. 2016;68(8):860–867. 10.1016/j.jacc.2016.03.608 [DOI] [PubMed] [Google Scholar]
- 3.Pool LR, Ning H, Lloyd-Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J Am Heart Assoc. 2017;6(9):e006027. 10.1161/JAHA.117.006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: an analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab. 2018;20(3): 667–671. 10.1111/dom.13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. 10.5888/pcd14.160287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021;10(5):e019259. 10.1161/jaha.120.019259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in cardiovascular risk factors in US adults by race and ethnicity and socioeconomic status, 1999–2018. JAMA. 2021;326(13):1286–1298. 10.1001/jama.2021.15187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 9.Palmer MK, Toth PP. Trends in lipids, obesity, metabolic syndrome, and diabetes mellitus in the United States: an NHANES analysis (2003–2004 to 2013–2014). Obesity. 2019;27(2):309–314. 10.1002/oby.22370 [DOI] [PubMed] [Google Scholar]
- 10.Sidney S, Quesenberry CP Jr, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1(5):594–599. 10.1001/jamacardio.2016.1326 [DOI] [PubMed] [Google Scholar]
- 11.Shah NS, Lloyd-Jones DM, O’Flaherty M, et al. Trends in cardiometabolic mortality in the United States, 1999–2017. JAMA. 2019;322(8):780–782. 10.1001/jama.2019.9161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121(4):586–613. 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 13.Araneta MRG, Kanaya AM, Hsu WC, et al. Optimum BMI cut points to screen Asian Americans for type 2 diabetes. Diabetes Care. 2015;38(5): 814–820. 10.2337/dc14-2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112(17):2735–2752. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention: National Center for Health Statistics. 2010. National Health and Nutrition Examination Survey: 2001–2002 data documentation, codebook, and frequencies. Choesterol - total, HDL, LDL triglycerides, second exam. Accessed June 27, 2021. https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/L13_2_B.htm
- 16.Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS ONE. 2013;8(7):e65174. 10.1371/journal.pone.0065174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2): 187–225. 10.1161/circulationaha.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(suppl 1):s68–s71. 10.2337/diacare.27.2007.S68 [DOI] [PubMed] [Google Scholar]
- 19.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526–2528. 10.1001/jama.2020.4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuru-Jeter AM, Michaels EK, Thomas MD, Reeves AN, Thorpe RJ Jr, LaVeist TA. Relative roles of race versus socioeconomic position in studies of health inequalities: a matter of interpretation. Annu Rev Public Health. 2018;39:169–188. 10.1146/annurev-publhealth-040617-014230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker RJ, Strom Williams J, Egede LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci. 2016;351(4):366–373. 10.1016/j.amjms.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuevas AG, Chen R, Slopen N, et al. Assessing the role of health behaviors, socioeconomic status, and cumulative stress for racial/ethnic disparities in obesity. Obesity (Silver Spring). 2020;28(1):161–170. 10.1002/oby.22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. Washington, DC: The National Academies Press; 2019. https://nap.nationalacademies.org/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving [PubMed] [Google Scholar]
- 24.Mozaffarian D, Angell SY, Lang T, Rivera JA. Role of government policy in nutrition-barriers to and opportunities for healthier eating. BMJ. 2018;361:k2426. 10.1136/bmj.k2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruss SM, Nhim K, Gregg E, Bell M, Luman E, Albright A. Public health approaches to type 2 diabetes prevention: the US National Diabetes Prevention Program and beyond. Curr Diabetes Rep. 2019;19(9):78. 10.1007/s11892-019-1200-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downer S, Berkowitz SA, Harlan TS, Olstad DL, Mozaffarian D. Food is medicine: actions to integrate food and nutrition into healthcare. BMJ. 2020;369: m2482. 10.1136/bmj.m2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Division for Heart Disease and Stroke Prevention. (2020). National Hypertension Control Roundtable. Accessed June 27, 2021. https://www.cdc.gov/dhdsp/programs/hypertension-roundtable.htm
- 28.Lee Y, Mozaffarian D, Sy S, et al. Health impact and cost-effectiveness of volume, tiered, and absolute sugar content sugar-sweetened beverage tax policies in the United States: a microsimulation study. Circulation. 2020;142(6):523–534. 10.1161/circulationaha.119.042956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenney EL, Mozaffarian RS, Long MW, et al. Limiting television to reduce childhood obesity: cost-effectiveness of five population strategies. Child Obes. 2021;17(7):442–448. 10.1089/chi.2021.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon BN, Ugwoaba UA, Brockmann AN, Ross KM. Associations between the built environment and dietary intake, physical activity, and obesity: a scoping review of reviews. Obes Rev. 2021;22(4):e13171. 10.1111/obr.13171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mande J, Willett W, Auerbach J, et al. 2020. Report of the 50th Anniversary of the White House Conference on Food, Nutrition, and Health: honoring the past, taking actions for our future. Boston, MA. Accessed June 26, 2021. https://sites.tufts.edu/foodnutritionandhealth2019/ [Google Scholar]
- 32.Fleischhacker SE, Woteki CE, Coates PM, et al. Strengthening national nutrition research:rationale and options for a new coordinated federal research effort and authority. Am J Clin Nutr. 2020;112(3):721–769. 10.1093/ajcn/nqaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson-Stuttard J, Kypridemos C, Collins B, et al. Estimating the health and economic effects of the proposed US Food and Drug Administration voluntary sodium reformulation: microsimulation cost-effectiveness analysis. PLoS Med. 2018;15(4): e1002551. 10.1371/journal.pmed.1002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Government Accountability Office. 2021. Chronic health conditions: federal strategy needed to coordinate diet-related efforts. Accessed June 26, 2021. https://www.gao.gov/assets/gao-21-593.pdf
- 35.Shin D, Kongpakpaisarn K, Bohra C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int J Cardiol. 2018;259:216–219. 10.1016/j.ijcard.2018.01.139 [DOI] [PubMed] [Google Scholar]
- 36.Araujo J, Cai J, Stevens J. Prevalence of optimal metabolic health in American adults: National Health and Nutrition Examination Survey 2009–2016. Metab Syndr Relat Disord. 2019;17(1): 46–52. 10.1089/met.2018.0105 [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. 2021. Understanding A1c: diagnosis. Accessed June 16, 2021. https://www.diabetes.org/a1c/diagnosis
- 38.Calling S, Johansson S-E, Wolff M, Sundquist J, Sundquist K. The ratio of total cholesterol to high density lipoprotein cholesterol and myocardial infarction in women’s health in the Lund area (WHILA): a 17-year follow-up cohort study. BMC Cardiovasc Disord. 2019;19(1):239. 10.1186/s12872-019-1228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 40.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357. 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.