Highlights
-
•
Photobacterium damselae ssp. piscicida (Phdp) growth is inhibited by probiotics.
-
•
Phdp is virulent to Artemia inducing oxidative stress and ultrastructural damage.
-
•
Probiotics administration prior to infection ameliorates oxidative stress response.
-
•
Probiotics improve intestinal ultrastructure and survival of Artemia post infection.
Keywords: Free vibration, Spatially closed-coupled plates with curved edges, Arbitrary connection and boundary conditions, Virtual spring stiffness functions, Jacobian differential quadrature method, Hammer modal test
Abstract
The effects of Photobacterium damselae ssp. piscicida (Phdp) on immune responses and intestinal ultrastructure of Artemia franciscana following infection and their amelioration by the probiotic bacteria Bacillus subtilis, Lactobacillus plantarum and Lactococcus lactis were evaluated. Pathogen growth inhibition in coculture with each probiotic and its virulence against Artemia were confirmed with an LC50 of 105 CFU mL−1. Phdp administration to Artemia at sublethal levels resulted in depletion of superoxide dismutase, glutathione reductase, glutathione transferase and phenoloxidase activities, extensive lipid peroxidation and reduced survival. Following a combined administration of each probiotic and the pathogen, enzyme activities and survival were significantly higher, while lipid peroxidation was reduced, compared to the infected group with no probiotic treatment (P < 0.05). The transmission electron microscopy study revealed that pathogen infection resulted in disarranged and fragmented microvilli, formation of empty or pathogen containing cytoplasmic vacuoles and damaged mitochondria. In the probiotic-treated and Phdp-infected series, intestinal cells showed normal appearance, except for the presence of pathogen-containing vacuoles and highly ordered but laterally stacked microvilli. The results of the present study indicate that Phdp induces cell death through an oxidative stress response and probiotics enhance Artemia immune responses to protect it against the Phdp induced damage.
1. Introduction
Aquaculture, a prominent sector in the supply of food, faces the adverse effects of mass mortality due to bacterial diseases of cultured organisms, increasing the disease burden and thus affecting wildlife welfare [1]. Pathogens originate from the introduced fish stocks and their propagation is augmented by stress and high stocking density [2]. Vibriosis and pseudotuberculosis pose as the most prevalent bacterial diseases in marine aquaculture [3]. The causative agent of pseudotuberculosis, which is often referred to as photobacteriosis or pasteurellosis, is Photobacterium damselae ssp. piscicida (Phdp). The disease, named after the characteristic white granulomatous lesions [4] that appear on the surface of kidney and spleen, leads to high mortality due to vertical transmission from brood stock to larvae [5]. Following infection, necrotic changes appear in the spleen, liver, intestine, and gills of fish [6]. The fact that photobacteriosis is related to an increase in water temperature led to the suggestion of low temperature maintenance as a strategy to control disease [7]. Treatment with antimicrobials is often employed [4], implicating the possible development of pathogens resistance [8]. To this end, preventive strategies efficient against fish bacterial diseases, such as vaccines and the use of probiotics, are recommended. The efficacy of vaccines against Phdp, although controversial [9], is enhanced in the presence of immunostimulants [10]. In the case of small fish, vaccination is performed by immersion, which is considered inferior to vaccination by injection [11].
An effective and environmentally friendly approach to prevent fish diseases is the administration of probiotic bacteria with beneficial effects on the growth and pathogen resistance of the host organism as well as positive effects in water quality [12], [13], [14]. Probiotics are defined as “microbial supplements that affect beneficially the host animal by improving its health when consumed in adequate amounts” [15,16]. In aquaculture the employed probiotics include lactic acid bacteria (LAB) and strains of the genus Bacillus [17], Vibrio and Pseudomonas [12,[18], [19], [20]]. Efficient delivery of probiotics to the target organism is performed using Artemia, the live fish and shrimp larvae food, as their vehicle [21,22]. However, the protective effects of probiotics are mainly focused on their action against vibrioses caused by various strains of Vibrio in fish [23], shrimp [24] and Artemia [21,22,[25], [26], [27]]. Even though Phdp has been characterized as a fish pathogen [28], infections have been confirmed in invertebrates, as well. However, studies are limited to the black tiger shrimp, Penaeus monodon and P. indicus with LD50 values of 2 × 102 to 5 × 105 CFU [29], Octopus vulgaris which was resistant to experimental infection by injection [30] and the lined seahorse Hippocampus erectus with a cumulative mortality of 25% on the 7th day after the disease was first observed in a farm and to 90% following experimental infection by injection with 2.6 × 105 CFU/mL [31].
Many aquaculture species, mainly fish and shrimp species rely on zooplankton especially during their early life stages, since it is palatable and easily digested. Artemia is the zooplankton of choice as a live starter feed due to its high nutritional value and its ability to be used as a carrier of nutrients, therapeutics or probiotics using the technique of bioencapsulation [32]. The beneficial effects of probiotics on cultured organisms have been documented rendering probiotics a beneficial mediator in aquaculture [33]. The delivery of probiotics by their bioencapsulation in Artemia nauplii has been reported to benefit various species of cultured fish [13,34] and shrimp [35]. Moreover, our previous data confirmed the beneficial effects of probiotics on Dicentrarchus labrax larvae following their administration through Artemia and infection with V. anguillarum [21,22]. Despite the importance of Artemia nauplii and metanauplii as a live feed in aquaculture and the fact that the microbial load of the feed may be transferred to the prey [36], data on the infection of Artemia by Phdp and its protection are scarce. In fact, they are limited to the in vitro effect of microalgae against Vibrio anguillarum and Phdp [37] or Vibrio alginolyticus [38] and the reduction of the microbial load of Artemia following microalgae administration to nauplii cultured under non-axenic conditions. Hence, the effects of a Phdp infection on Artemia welfare and its protection by probiotics have not been studied. The immune enhancing action of the probiotics B. subtilis, L. plantarum and L. lactis on Artemia and the offered protection against a V. anguillarum challenge have been previously documented [26]. These three probiotics are all known producers of bacteriocins, which are antibacterial peptides that inhibit the growth of similar bacterial strains and present immunomodulatory and anticarcinogenic activity [39], [40], [41]. Moreover, the production of lysozymes by B. subtilis [42,43] as well as the ability of the bacterium to accumulate selenium which promotes immunomodulation [44], have also been reported. On the other hand, L. plantarum, presents tolerance to acidic pH and adhesion capacity to intestinal mucosa, excellent antioxidant, and antimicrobial properties, which enable this probiotic to exert beneficial effects on the host health [45]. The production of a variety of important products such as acids and bacteriocins including nisin, which are active against a wide variety of Gram-positive bacteria as well as cancer cells, have nominated L. lactis a microbial cell factory, able to boost host immune system in humans [46,47]. Ιnvertebrates lack adaptive immunity and rely on their innate immune response to confront pathogen infection [48,49]. The innate immune response of invertebrates includes phagocytosis and coagulation of hemocytes, production of antimicrobial peptides (AMPs), reactive nitrogen species (RNS), reactive oxygen species (ROS) and the melanin formation pathway [49,50]. ROS production, the most widely used mechanism in both vertebrates and invertebrates is balanced by antioxidant enzymes [49]. The role of oxidative stress related enzymes and the melanin formation pathway as well as of intestinal ultrastructure have been studied in Artemia nauplii, against pathogens of the genus Vibrio, including the protective effect of probiotics [21,22,[25], [26], [27]]. Since a route of Phdp infection is the intestinal tract [51], the pathogen can be transmitted through feed, and due to its great binding capacity, it results in necrotic changes in fish intestines [6]. The morphology of Artemia gut was studied in detail in the developmental stage of nauplii under non-axenic conditions [52], following exposure to the marine fungus Haliphthoros milfordensis [53], following administration of the probiotic Aeromonas hydrophila [54] and in a gnotobiotic system [55]. The only report on ultrastructural observations under pathological conditions is following an infection with Vibrio campbellii [56]. However, the infection of Artemia by Phdp as well as the possible ameliorative effect of probiotics have not been studied. The aim of this study was: (1) to determine the effect of an experimental infection with Phdp on the survival, antioxidant enzyme activity, lipid peroxidation and intestinal ultrastructure of Artemia nauplii and (2) to evaluate the effect of the probiotics B. subtilis, L. plantarum and L. lactis, either alone or in combination with a Phdp infection, on these parameters.
2. Materials and methods
2.1. Bacterial strains and culture conditions
The bacterial strains that were used as probiotics, B. subtilis, L. plantarum and L. lactis were a gift from the Department of Microbiology (Aristotle University of Thessaloniki, Greece) and were previously identified by 16S rRNA sequencing as B. subtilis NCIB 3610 (DCM10), L. plantarum strain 2035 (SM-I) and L. lactis RKG 1–319 respectively. Cultures of L. plantarum and L. lactis were grown in brain heart infusion (BHI) broth, of B. subtilis in Luria Bertani (LB) broth, and media were purchased from AppliChem (Ottoweg 4, and d-64,291 Darmstadt, Germany). All microorganisms were cultivated at 29±1 °C in a rotary shaker with mild agitation for 24 h prior to use. Glycerol stocks of all microorganisms were prepared in the appropriate broth medium including glycerol 25% (v/v) and they were kept at − 80 °C. The Greek strain of the pathogen Photobacterium damselae ssp. piscicida (Phdp) used for challenge experiments was a gift from Dr G. Savvidis (Greek Agricultural Organization Demeter) previously isolated from infected seabass and characterized as G9 [57]. Phdp was grown in BHI broth supplemented with 1.5% NaCl, at 25 °C, for 48 h [58]. The optical density of bacterial cultures was monitored at 630 nm and stock cultures in 20% glycerol were maintained at −80 °C for long term storage.
2.2. Animals
Experiments were performed on A. franciscana nauplii originating from cysts (E. G. grade, Great Salt Lake strain, batch 11.119.03, INVE HELLAS S.A., Athens, Greece). Following hydration and decapsulation with hypochlorite solution, the cysts were washed, suspended in autoclaved artificial seawater (35 gL−1, pH 8.7) and allowed to hatch for 24 h under continuous aeration and illumination at 28 ± 1 °C [26]. Instar I nauplii collected at 24 h, were rinsed and transferred to fresh autoclaved seawater (15 nauplii mL−1). From 48 h onwards the nauplii were fed daily on yeast processed inactivated and kindly provided by Prof. Sorgeloos (Ghent University), the culture medium was renewed daily, and samples were collected at 144 h for biochemical evaluation.
2.3. Pathogen inhibition assays
The probiotic strains were tested for antagonistic effects against Phdp in broth co-culture assays as previously described [21]. For each probiotic, 104 CFU were added to 1 ml of BHI broth containing 2% NaCl and 104 CFU of the pathogen Phdp. The cultures were monitored for 72 h, namely from the onset to the end of the stationary phase. Monocultures of each probiotic and of the pathogen were included, an average CFU mL−1 was calculated by plating on BHI agar containing 2% NaCl, followed by morphological identification of each bacterium and counting. All cultures were performed in triplicate and comparisons were performed among probiotic-probiotic + Phdp series and Phdp- probiotic + Phdp series.
2.4. Administration of probiotics to Artemia nauplii
Each probiotic was administered separately to Artemia nauplii in three experimental series, one for each probiotic, as described previously [26]. Briefly, each probiotic was cultured for 24 h, centrifuged; bacteria were harvested, resuspended, and titrated to determine the contained CFU per mL of suspension. Then the appropriate bacterial suspension volume was added to the Artemia culture (48 h nauplii) to provide 2 × 105 CFU of probiotic per mL of Artemia culture medium, and this comprised one dose of probiotic. Two doses of each probiotic, were administered daily for two consecutive days, corresponding to the administration of 4 × 105 CFU per mL of Artemia culture medium of each probiotic daily and of 8 × 105 CFU per mL of Artemia culture medium in total. These administration scheme previously provided best results in terms of Artemia growth, survival, and biochemical parameters [26]. Survival was recorded throughout the experiment (48–96 h). Artemia metanauplii were collected at 144 h to be used for biochemical evaluations or fixation and nauplii that received no probiotic treatment provided control samples. The presence of each probiotic in Artemia was confirmed by plating of a metanauplii homogenate in BHI agar in the L. plantarum and L. lactis series and LB agar in the B. subtilis series, incubation at 37 °C for 24–72 h and characterization by colony morphology observation and staining (Gram and catalase tests).
2.5. Determination of LC50 following a challenge of Artemia with Phdp
The pathogenicity of Phdp was evaluated by the method of Verschuere et al. [59] with certain modifications. Briefly, triplicates of four different Artemia cultures were employed, and were challenged with 2 × 106, 2 × 105, 2 × 104, 2 × 103 or 2 × 102 CFU mL−1 of the pathogen at 96 h, to allow administration of probiotics in further experiments, while a fifth culture not challenged with the pathogen served as control. Survival was recorded daily under a stereoscope and LC50 was calculated at 24 and 48 h of exposure.
2.6. Experimental infection of Artemiawith Phdp
The experimental infection of Artemia was performed at 96 h of culture with one dose of Phdp corresponding to 1 × 104 CFU mL−1 of Artemia culture medium [60]. The pathogen was cultured in BHI broth supplemented with 1.5% NaCl for 48 h, bacteria were harvested by centrifugation and 200 μL of bacterial pellet were suspended in 2 mL culture medium. The culture titre was determined in 100 μL of the bacterial suspension by preparing serial dilutions in tubes containing 900 μL of culture medium, transferring 100 μL and plating. An OD630 nm∼0.9 of the initial culture corresponded to approximately 109 bacteria cells/mL [61]. Artemia metanauplii that received no pathogen served as control and survival was monitored throughout the experiment (24–144 h). At the end of the experiment metanauplii were collected to be used directly for biochemical evaluation or to be submitted to fixation for microscopy observations.
The infection of Artemia treated with probiotics with Phdp was performed at 96 h metanauplii in three separate experimental series, one for each probiotic. To this end, metanauplii were collected following the administration of each probiotic for two consecutive days (48h–96 h) as described above, washed, and transferred to clean sterile seawater. Then one dose of Phdp corresponding to 1 × 104 CFU mL−1 of Artemia culture medium was administered. As control served individuals that did not receive any probiotic treatment or challenge (control series), while as infection control served metanauplii that were not fed with probiotics but were challenged with the pathogen (Phdp series). All experiments were performed in triplicate and Artemia survival was monitored throughout the experiment (24−144 h). Artemia metanauplii were collected at the end of the experiment (144 h) and used for further evaluation. The presence of the pathogen in the nauplii was confirmed at the end of each experiment by plating a homogenate of the nauplii in BHI broth containing 2% NaCl on the plates prepared using the corresponding medium.
2.7. Biochemical evaluation of Artemia metanauplii
In all experiments fresh homogenates of animals collected at the end of the experiment (144 h) were prepared, either in ice-cold phosphate buffers 50 mM, pH= 7.0 with 0.1% TRITON X-100 phosphate buffer were used for the determination of superoxide dismutase (SOD), glutathione reductase (GR) and glutathione transferases (GST) [62] or in a mixture 1:1 (v/v) of A: 0.85% (w/v) NaCl-2.5 mM EDTA and B: Triton –X 1% (v/v) −10 mM CaCl2 for the determination of phenoloxidase (PO) [63]. Following centrifuging (13,000 rpm, 10 min, 4 °C) the determination of enzymatic activities was performed spectrophotometrically. The activity of SOD was performed according to Beyer and Fridovich [64] using a standard curve constructed with 0 to 200 U of commercial SOD (S9697, Sigma-Aldrich Co. LLC.). The amount of enzyme that results in a 50% inhibition of NBT reduction corresponds to one unit of SOD. The method of Carlberg and Mannervik [65] was used for the determination of GR activity monitoring the oxidation of NADPH at 340 nm. GST activity was determined using the method of Habig et al. [66] by monitoring the formation of a CDNB-glutathione conjugate at 340 nm. Phenoloxidase activity was measured by the method of Fan et al. [67] in dark vials using 100 mM sodium acetate- citric acid buffer, pH 7.1, following the addition of chymotrypsin (5 mg/mL, 10 min, room temperature) for the conversion of pro-phenoloxidase to phenoloxidase. Then 200 μL of l-Dopa (4 mg mL−1) were added, the samples were incubated at room temperature in a shaker and the formation of dopachrome was monitored at 490 nm at 0 and at 40 min. One unit of PO activity corresponded to an increase of absorbance by 0.001 per min over the 40 min period. The protein concentration of all extracts was determined by the Bradford method employing fresh BSA solutions (0–500 μg) and plotting a standard curve of protein ( μg) vs OD 595 nm, constructed for each Coomassie Brilliant Blue G-250 reagent [68]. The activity of all enzymes tested is expressed in Units / mg of protein.
The levels of lipid peroxidation were measured by the malondialdehyde (MDA) method [69] following the formation of an MDA adduct with 2-thiobarbituric acid (TBA) at 535 nm. Lipid peroxidation was estimated using a standard curve prepared for each TCA-TBA-HCl reagent and expressed as μmoles MDA per g of tissue. The number of alive nauplii was monitored throughout the experiment and survival (%) was calculated.
2.8. Transmission electron microscopy (TEM) of Artemia
Τhe study of the intestinal morphology using TEM was performed according to the method of Gunasekara et al. [55] with certain modifications regrading shorter sample treatment with osmium tetroxide, repeated washes, post staining of samples instead of sections, an additional dehydration step in 100% ethanol and gradual embedding for a total of about 52 h. In detail, Artemia metanauplii collected at 144 h were transferred to glass vials filled with Karnovsky's fixative in 50 mM sodium cacodylate buffer (pH 7.4) and were allowed to fix overnight at 4 °C. After two washing steps in the same buffer, 15 min each, post fixation was performed in 1% osmium tetroxide for 1 h at 4 °C. Following washes in the same buffer, the samples were stained with 0.5% uranyl acetate 0.5% for 16–18 h at 4 °C. After removal of the staining solution two wash steps with sodium cacodylate buffer (pH 7.4) were performed. After dehydration by immersion in 50%, 75% and 95% ethanol for 15 min and twice in 100% for 10 min in room temperature, embedding in SPURR's resin commenced by immersion in ethanol: resin 3:1 (v/v) for 24 h at 4 °C, then in ethanol: resin 2:1 (v/v) for 24 h at 4 °C, at ethanol: resin 1:1 (v/v) for 24 h at room temperature under stirring. Then the specimens were transferred to fresh ethanol: resin 1:1 (v/v) and incubated for 24 h at 4 °C followed by another immersion in fresh ethanol: resin 1:1 (v/v) for 24 h at room temperature under stirring. Afterwards, the specimens were transferred in Beem capsules containing 100% resin and stirred for 3–4 h at room temperature. Finally, the samples were incubated in an oven (70 °C) for at least 8 h. Thin sections were obtained using a Reichert-Jung Ultracut E ultramicrotome (Reichert, Inc. Depew, NY, USA) and they were examined under optical microscope following staining with toluidine blue 0.5% to determine the fixation quality and locate the area of interest. Ultrathin sections (70–90 nm) were placed on copper grids coated with formvar and the specimens were examined with a JEOL JEM 1011 (JEOL Ltd., Tokyo, Japan) at 80 kV. Micrographs were obtained using a digital camera Gatan ES500W (Erlangshen ES500W; Gatan Inc.).
2.9. Statistical analysis
All experiments and analyzes were performed in three replicate experiments and two separate determinations were performed in two samples from each replicate, resulting in six repeats. Results in figures represent the mean ± standard deviation (SD). Statistical analysis of the data was performed using one-way analysis of variance (ANOVA) followed by Tuckey post-hoc test. Statistical significance was set at a level of 0.05.
3. Results
3.1. Pathogen inhibition assays
The probiotic strains were tested for antagonistic effects on Phdp growth in broth co-culture assays with the pathogen for 72 h. The significant inhibition of pathogen growth that was observed, amounted at 48 h, which appears to be the end of the stationary phase for the pathogen, to 47%, 46%, and 49% in the B. subtilis (Fig. 1A), L. plantarum (Fig. 1B) and L. lactis (Fig. 1C) series respectively compared to control growth (P < 0.001). On the other hand, Phdp also inhibited the growth of probiotics with B. subtilis (P = 0.057) and L. plantarum (P = 0.056), which was not significantly different compared to control, and the growth reduction at the end of the experiment (72 h) amounted to 5% and 11% correspondingly. However, L. lactis growth was significantly lower than the corresponding control at 72 h (P = 0.0036) and inhibition of growth amounted to 21%. Although in all cases the pathogen growth was reduced from 0 to 72 h of co-culture compared to control growth, elimination of the pathogen was not observed.
Fig. 1.
Growth inhibition in coculture assays of Phdp with (A) B. subtilis, (B) L. plantarum, (C) L. lactis. Growth of Phdp alone is indicated by black columns and solid black trendline, growth of Phdp in the presence of the probiotic with patterned columns and dashed black trendline, growth of probiotic alone with white columns and gray trendline, growth of the probiotic in the presence of Phdp with gray columns and dashed gray trendline. Each value is mean ± SD, of four separate samples originating from two replicate experiments. Asterisks indicate significant difference (P < 0.05) among relevant groups.
3.2. Determination of Phdp LC50
In a preliminary set of experiments a wide range of both lethal and sublethal concentrations of bacteria from 102 to 109 CFU mL−1 were employed. At 106 CFU mL−1 mortality was 56% at 24 h and 79% at 48 h, while at higher concentrations mortality was over 80% at 24 h and 100% at 48 h. Hence the concentration range of 102 to 106 was examined and the results of this experiment were used to conduct further analysis. The graph of the pathogen concentration logarithm to the base 10 versus mortality (%) provided a trendline equation of y = 15.712x - 41.213 (R2 = 0.946) when the 24 h mortality was considered and of y = 18.18x - 40.434 (R2 = 0.990) for the 48 h mortality series. The 24h-LC50 amounted to 105.9, while the 48h-LC50 values provided the value of 105 (Fig. 2A). Calculations by logit and probit analysis for the 48 h-LC50 provided values of 105.12, and 105.19 respectively (Fig. 2B, C). Since the best goodness of fit was observed in the 48 h-LC50, 48 h observations and the sublethal concentration of 104 CFU mL−1 were used in further experiments.
Fig. 2.
LC50 calculations following a 48 h exposure to pathogen, using (A) trendline equation (R2 = 0.990, df 4, p = 0.0004), (B) logit (R2 = 0.945, df 3, p = 0.027) and (C) probit analysis (R2 = 0.945, df 3, p = 0.027). The values provided in parentheses were calculated by regression analysis in excel. 48h-LC50 values were estimated to be 105, 105.12 and 105.19 respectively. For each concentration a total of six replicates (2 replicates per container X 3 independent experiments) were used (mean ± SD) for survival determinations and LC50 calculations.
3.3. Biochemical and immunological evaluation of Artemia
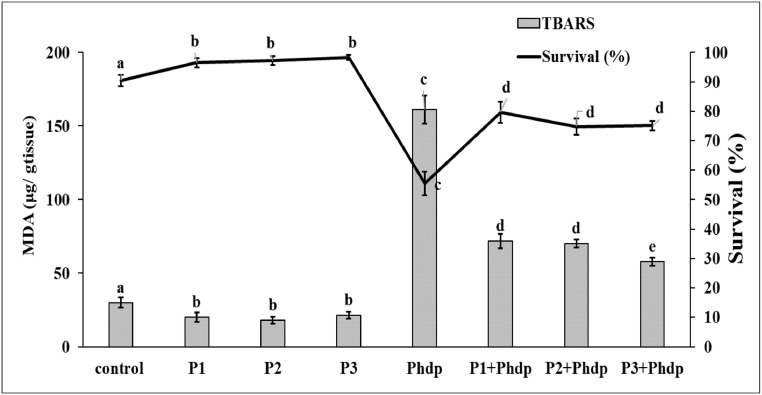
The activity of all enzymes tested at the end of the experiment (144 h) was significantly higher than control values following the administration of probiotics (Fig. 3, series P1, P2 and P3 corresponding to B. subtilis, L. plantarum, and L. lactis respectively). The only exceptions were the activity of PO in the activity of SOD in the B. subtilis series (P1, Fig. 3C) and in the L. plantarum series (P2, Fig. 3D), which although they appeared higher than control values, the difference was not significant (P = 0.317, P = 0.166 respectively). On the other hand, lipid peroxidation appeared significantly reduced (P = 0.038, P = 0.008, P = 0.001 in the L. lactis, B. subtilis and L. plantarum series respectively) and survival significantly increased in all probiotic series (P = 0.003, P = 0.042, P = 0.017 in the L. lactis, L. plantarum and B. subtilis series respectively) (P1, P2, P3, Fig. 4). Experimental infection with the pathogen (Phdp series, Fig. 3) resulted in a significant decrease in the activity of all enzymes tested compared to the corresponding control values (P < 0.001). The largest decrease in activity was observed for SOD (Phdp series, Fig. 3C) amounting to 96.5% of the control value, and PO presented the lowest activity decrease amounting to 85.6% (Phdp series, Fig. 3D). Pathogen administration resulted in a marked increase in lipid peroxidation compared to control and amounting to 161.1 ± 9.6 μg MDA/ g of tissue, that corresponds to levels five-fold higher than the control value. Moreover, the experimental infection with Phdp resulted in decreased Artemia survival amounting to 55.5 ± 7.02% (Phdp series, Fig. 4).
Fig. 3.
Biochemical evaluation of pathogen, probiotic, or combined treatments on enzymatic activities. (A) GR (black bars), (B) GST (gray bars), (C) SOD (white bars), (D) PO (patterned bars) P1: B. subtilis, P2: L. plantarum, P3: L. lactis, Phdp: pathogen, P1 + Phdp, P2 + Phdp, P3 + Phdp: combined treatments with B. subtilis, L. plantarum, or L. lactis respectively, followed by administration of Phdp. Each point is the mean ± SD of six separate samples originating from three replicate experiments. Different letters denote significant difference (P < 0.05) compared to control.
Fig. 4.
Lipid peroxidation and Artemia survival. The effect of pathogen, probiotic, or combined treatments on lipid peroxidation (bars) expressed as MDA ( μg/ g tissue) and on Artemia survival (line) is shown. P1: B. subtilis, P2: L. plantarum, P3: L. lactis, Phdp: pathogen, P1 + Phdp, P2 + Phdp, P3 + Phdp: combined treatments with B. subtilis, L. plantarum, or L. lactis respectively, followed by administration of Phdp. Each point is the mean ± SD of six separate samples originating from three replicate experiments. Different letters denote significant difference (P < 0.05) compared to control.
In the groups that were treated with a probiotic and then subjected to experimental infection with Phdp, all the enzymes’ activities were significantly higher compared to the Phdp series (P < 0.001). However, compared to control values the enzyme activities were significantly lower in most cases (P < 0.001). The exceptions were the activity of GR in the B. subtilis series (P = 0.997, Fig. 3A: P1 + Phdp), GST activity in L lactis series (P = 0.104, Fig. 3B:P3 + Phdp), and SOD activity in B. subtilis (P = 0.547, Fig. 3C: P1 + Phdp) and L. plantarum series (P = 0.245, Fig. 3C: P2 + Phdp), which were not significantly different from control values. A reduction in GST activity in the probiotic treated and infected Artemia individuals compared to control, was observed only in B. subtilis (Fig. 3B, P1 + Phdp) and L. plantarum series (Fig. 3B, P2 + Phdp) amounting to 26.1% and 27.8% of control value respectively, while in the L. lactis series GST activity was higher than control by 8.1% (Fig. 3B, P3 + Phdp). In the case of PO best protection was offered by treatment with B. subtilis with the reduction amounting to 27.2% of control, followed by L. lactis (39.8% reduction) and L. plantarum (55.9% reduction). In the case of GR B. subtilis offered best protection with the reduction in GR activity amounting to only 3.6% of control, followed and L. plantarum and L. lactis series in which the reduction in GR activity compared to control was 18% and 20% respectively. Regarding lipid peroxidation, decreased values were determined in the three probiotic series following experimental infection compared to the corresponding value in infection series (Phdp). The reduction in lipid peroxidation amounted to 64%, 56% and 55% of the infection series (Fig. 4: Phdp) in the L. lactis, L. plantarum and B. subtilis series respectively (Fig. 4: P + Phdp). However, the peroxidation of lipids in the probiotic treated and infected series was significantly higher than control values (P < 0.001).
Although Artemia survival appeared greatly improved in the experimental infection followed probiotic administration series (Fig. 4, P1 + Phdp, P2 + Phdp, P3 + Phdp series) in comparison to the series receiving no probiotic treatment (P < 0.001, Fig. 4, Phdp series), it was significantly lower than control (P < 0.001). However, the reduction in survival, which amounted to 39% of control survival in Phdp group, was only 15% in the B. subtilis series and 20% of in the L. plantarum and L. lactis series.
3.4. Artemia intestinal ultrastructure
Observations of sections at the midgut to hindgut level revealed typical columnar cells, each encompassing a central nucleus and abundant mitochondria (Fig. 5A). Cell junctions resembling septate desmosomes were present towards the apical surface (Fig. 5B), circular muscles were attached to the basal membrane (Fig. 5A) and normal microvilli extended from the apical surface (Fig. 5A, C).
Fig. 5.
Transmission electron micrographs of gnotobiotic Artemia metanauplii gut. (A) Midgut epithelial cells with N: Nucleus, MV: microvilli, M: mitochondria, C: circular muscle and J: cell junction possibly septate desmosome; (B) junction; (C) microvilli. Scale Bars: A (5 μm), B (200 nm) and C (0.5 μm).
Experimental infection with Phdp resulted in shorter cells, rather cuboid at side view (Fig. 6A). Prominent vacuoles were observed in the cytoplasm, some of which contained electron dense material, probably representing pathogen cells, while mitochondria appeared damaged with electron dense deposits (Fig. 6B). Cell junctions were intact but shorter than in uninfected cells (Fig. 6C; cf. Fig. 5B) and structures resembling actin pedestals were formed (Fig. 6C). In addition, microvilli appeared to be sparse, disarranged, with electron dense tips (Fig. 6D) and intense fragmentation (Fig. 6E).
Fig. 6.
Ultrastructural changes of gnotobiotic Artemia metanauplii gut following infection with Phdp. (A) Midgut epithelial cells with N: Nucleus, MV: microvilli, C: circular muscle and J: cell junction, V: vacuole, Vp: vacuole with electron dense inclusion, possibly pathogen (B) arrows indicate damaged mitochondria with electron dense deposits, (C) intact cell junction, AP: actin pedestal, (D) fragmented microvilli with electron dense tips, (E) microvilli fragments. Scale bars: A (2 μm), B (0.5 μm), C and D (0.2 μm), E (0.5 μm).
The administration of probiotics had no adverse effect on the ultrastructure of columnar epithelial cells, which were connected with intact long junctions and bore a dense array of long microvilli on their apical surface (Fig. 7A, B, C). The nuclei, appearing enlarged in some cells, were centrally located, exhibiting even chromatin distribution (Fig. 7A), while the cytoplasm contained numerous intact mitochondria (Fig. 7C). A circular muscle attached to the basal membrane and in some cases a longitudinal striated muscle located at the periphery of the basement membrane of intestinal cells were observed (Fig. 7A).
Fig. 7.
Transmission electron micrographs of the midgut to hindgut section of gnotobiotic Artemia metanauplii gut following administration of probiotic (left) and following administration of probiotic and Phdp infection (right). A, D: L. plantarum, B, E: B. subtilis, C, F: L. lactis. 1, 4 Epithelial cells with N: Nucleus, C: circular muscle, L: Longitudinal muscle, J: cell junction, M: mitochondria, V: vacuole, MV: microvilli, 2: asterisk denotes probiotic adjacent to the microvilli, 5: Pathogen adjacent to the microvilli, 3: intact mitochondria, 6: Vp: vacuoles with electron dense inclusions possibly pathogen, intact cell junction (J), clustered microvilli (MV). Microvilli appear clustered only in the series employing probiotic treatments followed by infection. Scale bars: A (5 μm), B, C and E (0.5 μm), D and F (2 μm).
The intestinal ultrastructure in the probiotic treated and challenged with Phdp series was normal, with columnar cells connected with intact junctions and bearing a centrally located nucleus with even chromatin distribution (Fig. 7D). The cytoplasm contained abundant intact mitochondria (Fig. 7E), as well as vacuoles, most of them containing electron dense material, possibly corresponding to pathogen remnants (Fig. 7F). Ordered dense arrays of microvilli were observed, exhibiting a particular stacking-like clustering (Fig. 7D, E, F).
4. Discussion
The present study provides evidence that the immune defenses and the intestinal ultrastructure of Artemia franciscana are adversely affected by Phdp and that probiotic bacteria ameliorate these effects. Infections caused by Phdp constitute one of the most challenging issues for marine aquaculture contributing to several mortality cases. Τhe Phdp strains exhibit high pathogenicity due to the abundance of virulence genes and the production of the siderophore piscibactin [70], and present some level of antibiotic resistance [71]. Hence, the use of alternative methods, such as probiotics, to control or inhibit infection becomes a priority.
4.1. Inhibition of Phdp growth by probiotics
Data on the efficacy of probiotics against Phdp refer to the effect of autochthonous probiotics and their protective action in fish [12,[72], [73], [74]]. The non-autochthonous probiotics employed in the present study inhibited in vitro the pathogen growth in co-culture, while retaining their own growth, hence posing as suitable candidates to be used against Phdp. The only exception was L. lactis, the growth of which appeared reduced compared to control (P < 0.05). Growth inhibition of this probiotic might be attributed to the salt concentration in the media. The halophilic Gram-negative bacterium Phdp requires salt for its growth [58] while L. lactis is sensitive to the presence of salt [75]. The effective inhibition of the pathogen by the three probiotics employed in the present study allowed further experiments.
4.2. Virulence of Phdp against Artemia
Since Phdp is considered a fish pathogen, another significant factor to be determined was the virulence of Phdp against Artemia. The virulence of Phdp against the live fish feed Artemia has been confirmed in the present study and 48 h-LC50 amounted to 105 CFU mL−1. This is the first report on Phdp induced mortality on Artemia, including the determination of its LC50. While Phdp was not pathogenic when tested in mice (LD50 > 108 CFU), toxicity was evident for gilthead seabream, rainbow trout and turbot following intraperitoneal injection, with the LD50 values ranging between 103 and 106 live CFU depending on the strain and the host [76]. The pathogenesis of pasteurellosis has been attributed to Phdp extracellular products, since they were highly toxic to fish, with the LD50 ranging from 1 to 4.6 pg protein per g fish) [77]. In sea bass experimentally infected with Phdp, all the virulent strains resulted in 100% mortality following intraperitoneal injection of 107 CFU [78]. On the other hand, an LD50 of 9.75 × 105CFU per g of body weight has been reported for P. vannamei following infection with Phdp by reverse gavage [79]. Although intraperitoneal or intramuscular injection of the pathogen is the preferred method to determine fish response to infection, the route of infection can influence the response of the host since it does not consider pathogen barriers such as the mucosa and the skin. However, following infection of the Senegalese sole (Solea senegalensis) with Phdp by intraperitoneal administration (104 CFU per fish) or by immersion (107 CFU mL−1), mortality was observed only in the case of IP inoculation [80]. Since intraperitoneal or intramuscular administration is not possible in Artemia, immersion was the route of infection in the present study. Our 48 h-LC50 values of 105 CFU mL−1 are comparable with the values reported following intraperitoneal administration to fish. Similar values of 104 CFU mL−1 of Photobacterium phosphoreum have been reported to be pathogenic against Artemia nauplii when administered at 103 CFU mL−1, causing mortalities with values of about 38% [60]. Based on the determined 48 h-LC50 value, infection in further experiments was performed using one dose of 1 × 104 CFU mL−1 of Artemia culture medium, that is a sublethal concentration of the pathogen slightly higher than 48 h-LC25 amounting to 1 × 103.6.
4.3. Probiotics, Phdp and their combination affect Artemia biochemical parameters
The biochemical evaluations performed in the present study indicated a stimulatory action of probiotics, since treatment with probiotics without a following infection resulted in an increase in the activity of the oxidative enzymes tested. The fact that only the activities of GR in the B. subtilis series, GST activity in L. lactis series and SOD activity in B. subtilis and L. plantarum series were not significantly different from control values indicates a different action of each probiotic and a different response of the host organism 48 h following probiotic administration. The increased enzyme activities at the end of the experiment (144 h) compared to control values is in accordance with our previous results on the increased enzyme activity at 96 h, a time point that corresponds to the end of probiotic administration [26]. Similar results on the effect of probiotics on enzymatic activity have been reported for the iridescent shark [81], the rainbow trout [82], tilapia [83], the white shrimp [84], the mud crab Scylla paramamosian [85,86] and the red swamp crayfish [87]. The decreased lipid peroxidation which appeared in the probiotic series, has been also observed previously for the sole Solea senegalensis following treatment with autochthonous bacteria as probiotics [73]. Our results indicate that probiotic action is retained 48 h after end of probiotic administration (96 h). The host organism, namely Artemia, responds in a different manner to each probiotic, with the increased antioxidant enzyme activity suggesting an immunostimulatory effect of probiotics. The production of different active substances by each probiotic, namely lysozyme [41,42], bacteriocins [39] and selenium nanoparticles [44,88,89] by B. subtilis, organic acids and bacteriocins including nisin by L. lactis [41] and organic acids, plantaricin, hydrogen peroxide, phenolic compounds, and surfactants by L. plantarum [90] might contribute to this variable effect among the probiotics employed. In fact, the addition of lysozyme significantly decreased the MDA content, and increased (SOD), activity, improving gur health in piglets [91], plantaricin presented a synergistic action with lactic acid against A. hydrophila [92] and nisin was reported to boost growth performance and increases infection clearance rate in humans by upregulating innate and acquired immune responses [93].
The diminished activity of antioxidant enzymes in combination with the reduced survival in the infected group are indicative of the potency of the pathogen against Artemia. Following infection with a pathogen, reactive oxygen species are produced to confront it [94], but their overproduction may result in severe cell damage or even death [95]. Marine invertebrates depend on the oxidative stress response to confront environmental stressors such as pollutants, increased stocking densities and infections from pathogens [96]. The main defense mechanisms in Artemia against infection, as in other invertebrates, are the innate immune responses due to the lack of adaptive immunity mediated by immunoglobulins, T- and B-cell receptors in vertebrates [97]. The innate immune responses include the generation of reactive oxygen species (ROS) and the activation of the prophenoloxidase system [48]. Since excessive ROS production may result in oxidative damage of many cell constituents and even cell death, the imperative maintenance of redox homeostasis is performed by the antioxidant system, in collaboration with the glutathione system to offer cells protection from oxidative stress induced damage [98,99]. The diminished activity of the antioxidant enzymes observed in the present study, might have resulted in their inability to restrict the damage induced by the ROS. A similar enzyme activity depletion has been reported following infection of meager (Argyrosomus regius) and the sole with Phdp [100], as well as following Artemia infection with V. alginolyticus [101] or V. anguillarum [26] with enzyme depletion being accompanied by a marked lipid peroxidation and decreased survival of Artemia. Accumulation of products of lipid peroxides has also been reported for gilthead seabream infected with Phdp [102]. In fact, lipid peroxide products such as lipid hydroperoxide, hydroxy lipids, 4‑hydroxy-2-hexenal, and propanal have been suggested as indices of fish health [103]. The oxidation products of lipids are considered disease mediators [104] since excessive oxidation of lipids adversely affects cellular membranes as well as proteins and nucleic acids. Although enzymes with glutathione as the co-substrate normally eliminate toxic lipid peroxides, their inactivation results in lipid peroxides accumulation which in turn may lead to cell death.
Regarding infection following probiotic administration, the fact that all enzyme activities were significantly higher compared to the Phdp series and certain activities were not significantly different from the non-infected control indicates the protective action of probiotics. This is further supported by the decreased lipid peroxidation in the three probiotic series following experimental infection, compared to the infection series. This protective action of probiotics resulted in a greatly improved Artemia survival. Similar protective probiotic action has been reported for the Phdp infected meager Argyrosomus regius following treatment with algae [100] and of Litopenaeus stylirostris following treatment with the probiotic Pediococcus acidilactici [105]. The protective effects varied among the probiotic strains employed, possibly due to the production of different substances by each strain [41,42,90]. These compounds might be responsible for the effects of probiotics on Artemia intestinal cells morphology.
4.4. Probiotics effects on Artemia intestinal ultrastructure in health and in disease
The ultrastructural observations of Artemia metanauplii midgut, characterized by the presence of the circular muscle, epithelial columnar cells equipped with a microvilli-rich brush border, connected via junctions, and containing a central nucleus and numerous mitochondria, are in accordance with previous images on the alimentary tract of gnotobiotic Artemia nauplii [54,55]. The administration of probiotics resulted in normal intestinal ultrastructure with a dense array of long microvilli on the columnar epithelial cells that were connected with intact and elongated junctions. Our results are in accordance with observations on the action of probiotics on the intestinal barrier, which include closer cell connection of epithelial cells by increasing production of proteins involved in tight junction signaling, through increased expression of their genes [106,107]. Enlargement of nucleus, besides being key change in malignant cells, is also a characteristic change of benign cells reacting to inflammatory stimuli [108]. The immunostimulatory effect of probiotics indicated by our biochemical results favor the hypothesis of probiotics acting as stimulants of innate immunity [106,107]. However, the numerous intact mitochondria and the reduced lipid peroxidation support the absence of extensive and uncontrolled ROS production.
The Phdp induced effects, including the shortening and disarrangement of microvilli with possible fragmentation and the change in cell shape to a rounder one, are indicative of an apoptotic cell death [108,109]. A similar shortening or even absence of microvilli was observed following a Vibrio proteolyticus infection of gnotobiotic Artemia [58]. The electron dense tips of the microvilli observed in the present study, following Phdp infection, are possibly related to the reported presence of certain receptors within microvilli, rendering them able to serve as signaling centers [110]. The formation of vacuoles, some of them containing a pathogen like structure, has also been observed following invasion of fish epithelial cells by Phdp [111]. The pathogen has been reported to be viable in such vacuoles for 48 h post cell invasion, and the internalization of the pathogen was considered to depend on F-actin. Such mechanisms of internalization are required for certain Gram-negative pathogens, including Escherichia coli, which manipulates the host cell actin cytoskeleton leading to the formation of actin pedestals [112]. The formation of a structure resembling an actin pedestal observed in the present study supports this hypothesis. The observed damaged mitochondria corroborate our biochemical results indicating oxidative stress. Moreover, they resemble the damaged mitochondria previously observed in MDR1 deficient mice and the authors suggested that the resulting intestinal inflammation was due to reduced SOD activity [113].
The normal appearance of the intestinal ultrastructure in the probiotic-treated and challenged with Phdp series, with cells connected with intact junctions containing abundant intact mitochondria as well as vacuoles, most of which contained pathogen remnants, supports the protective action of probiotics. Although the microvilli appeared dense and orderly arrayed, the most striking finding was their local stacking. A normal microvilli morphology but with altered orientation was also observed following probiotic administration to malnourished mice [114]. The brush border, apart from serving as a processing and transport machinery, plays a significant part in host defense and clustering of microvilli occurs by means of their connection through thread-like links [115]. Pathogen adhesion is achieved following disruption of the microvilli and hence the electrostatic protective barrier of host cells [116]. It appears that the enhancement of the mucosal barrier, induced by probiotics, contributes to a normal cell morphology and function.
5. Conclusion
The present study indicates that the administration of B. subtilis, L. plantarum or L. lactis protect Artemia against a following Phdp infection by enhancing its innate immune responses and phenoloxidase activity as well as by the preservation of the intestinal mucosal barrier. Although pathogen elimination mechanisms by the probiotics require further investigation, the beneficial effects of the probiotics employed have been demonstrated and certain mechanisms implicating ROS enzymes and phenoloxidase have been elucidated. The modulation of Artemia innate immune mechanisms by probiotics contributes to reduced oxidative damage, normal intestinal ultrastructure, and increased survival.
5.1. Future prospective
The promising effects of probiotics on resistance of Artemia metanauplii against the detrimental effects of a Phdp infection, encourage further research on other aquatic invertebrates and fish. Due to the sensitivity of shrimp and other invertebrate cultured organisms to diseases in combination with the primary nature of their immune system, the development of a means to control disease is of utmost importance. Consequently, probiotics may serve not only as an environmentally friendly dietary supplement promoting growth of the host but also act as an alternative for antibiotics, especially in marine aquaculture. For the application of probiotics in aquaculture the prerequisite of the production, preservation, and quality validation of large quantities of bacteria, in accordance with relevant guidelines, is still an issue to be confronted. The effects of probiotics greatly depend on the species or even strain employed. Therefore, the optimum probiotic strain, or the combination of different strains in mixed-culture systems and the administration dose to cultured organisms should be defined. The use of probiotics against aquaculture diseases might also include studies on the control of parasites and viruses. Finally, the clarification of the molecular mechanisms underlying the interactions of probiotics with the host as well as the ecological importance remains to be elucidated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors wish to extent their gratitude to INVE HELLAS S.A., who provided the Artemia cysts for the experiments.
Data availability
Data will be made available on request.
References
- 1.Lafferty K.D., Harvell C.D., Conrad J.M., Friedman C.S., Kent M.L., Kuris A.M., Powell E.N., Rondeau D., Saksidan S.M. Infectious diseases affect marine fisheries and aquaculture economics. Ann. Rev. Mar. Sci. 2015;7:471–496. doi: 10.1146/annurev-marine-010814-015646. [DOI] [PubMed] [Google Scholar]
- 2.Bouwmeester M.M., Goedknegt M.A., Poulin R., Thieltges D.W. Collateral diseases: aquaculture impacts on wildlife infections. J. Appl. Ecol. 2021;58(3):453–464. doi: 10.1111/1365-2664.13775. [DOI] [Google Scholar]
- 3.Toranzo A.E., Magariños B., Romalde J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. 2004;246:37–61. doi: 10.1016/j.aquaculture.2005.01.002. [DOI] [Google Scholar]
- 4.Magariños B., Romalde J.L., Bandín I., Fouz B., Toranzo A.E. Phenotypic, antigenic, and molecular characterization of Pasteurella piscicida strains isolated from fish. Appl. Environ. Microbiol. 1992;58(10):3316–3322. doi: 10.1128/aem.58.10.3316-3322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cascarano M.C., Stavrakidis-Zachou O., Mladineo I., Thompson K.D., Papandroulakis N., Katharios P. Mediterranean aquaculture in a changing climate: temperature effects on pathogens and diseases of three farmed fish species. Pathogens, 2021;10(9):1205. doi: 10.3390/pathogens10091205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulos C., Bakopoulos V., Zolota V., Dimitriadis G.J. Histpathological findings after sea bass exposure to extracellular products of Photobacterium damsella subsp.piscicida in vivo. Aquac. Res. 2004;35(10):931–936. doi: 10.1111/j.1365-2109.2004.01086.x. [DOI] [Google Scholar]
- 7.Magariños B., Couso N., Noya M., Merino P., Toranzo A.E., Lamas J. Effect of temperature on the development of pasteurellosis in carrier gilthead seabream (Sparus aurata) Aquaculture. 2001;195:17–21. doi: 10.1016/S0044-8486(00)00547-0. [DOI] [Google Scholar]
- 8.Schar D., Zhao C., Wang Y., Larsson D.G.J., Gilbert M., Van Boeckel T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021;12:5384. doi: 10.1038/s41467-021-25655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreoni F., Magnani M. Photobacteriosis: prevention and diagnosis. J. Immunol. Res. 2014 doi: 10.1155/2014/793817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spinos E., Kokkoris G.D., Bakopoulos V. Prevention of sea bass (Dicentrarchus labrax, L. 1758) photobacteriosis and vibriosis. Long term efficacy study of intraperitoneally administered bivalent commercial vaccines. Aquaculture. 2017;471:172–184. doi: 10.1016/j.aquaculture.2017.01.017. [DOI] [Google Scholar]
- 11.Bøgwald J., Dalmo R.A. Vol. 7. MDPI AG; 2019. Review on immersion vaccines for fish: an update; p. 627. (Microorganisms). Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez Falcón A., Padilla D., Real F., Ramos Sosa M.J., Acosta-Hernández B., Sánchez Henao A., García-Álvarez N., Rosario Medina I., Silva Sergent F., Déniz S., Martín-Barrasa J.L. Screening of new potential probiotics strains against Photobacterium damselae subsp. piscicida for marine aquaculture. Anim. MDPI. 2021;11:2029. doi: 10.3390/ani11072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoseinifar S.H., Sun Y.Z., Wang A., Zhou Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018;9:2429. doi: 10.3389/fmicb.2018.02429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauhan A., Singh R. Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis. 2019;77:99–113. doi: 10.1007/s13199-018-0580-1. [DOI] [Google Scholar]
- 15.Guarner F., Schaafsma G.J. Probiotics. Int. J. Food Microbiol. 1998;39:237–238. doi: 10.1016/S0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 16.FAO/WHO Probiotics in food: health and nutritional properties and guidelines for evaluation . World Health Organization: Food and Agriculture Organization of the United Nations; Rome: 2006. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1–4 October 2001. [Google Scholar]
- 17.Ringø E., Van Doan H., Lee S.H., Soltani M., Hoseinifar S.H., Harikrishnan R., Song S.K. Probiotics, lactic acid bacteria and bacilli: interesting supplementation for aquaculture. J. Appl. Microbiol. 2020;129:116–136. doi: 10.1111/jam.14628. [DOI] [PubMed] [Google Scholar]
- 18.Verschuere L., Rombaut G., Sorgeloos P., Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000;64(4):655–671. doi: 10.1128/MMBR.64.4.655-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balcázar J.L., de Blas I., Ruiz-Zarzuela I., Cunningham D., Vendrell D., Múzquiz J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006;114(3–4):173–186. doi: 10.1016/j.vetmic.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Balcázar J.L., Vendrell D., De Blas I., Ruiz-Zarzuela I., Gironés O., Múzquiz J.L. Ιn vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet. Microbiol. 2007;122:373–380. doi: 10.1016/j.vetmic.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Touraki M., Karamanlidou G., Karavida P., Chrysi K. Evaluation of the probiotics Bacillus subtilis and Lactobacillus plantarum bioencapsulated in Artemia nauplii against vibriosis in European sea bass larvae (Dicentrarchus labrax, L.) World J. Microbiol. Biotechnol. 2012;28(6):2425–2433. doi: 10.1007/s11274-012-1052-z. [DOI] [PubMed] [Google Scholar]
- 22.Touraki M., Karamanlidou G., Koziotis M., Christidis I. Antibacterial effect of Lactococcus lactis subsp. lactis on Artemia franciscana nauplii and Dicentrarchus labrax larvae against the fish pathogen Vibrio anguillarum. Aquacult. Int. 2013;21:481–495. doi: 10.1007/s10499-012-9579-4. [DOI] [Google Scholar]
- 23.Yilmaz S., Yilmaz E., Dawood M.A.O., Ringø E., Ahmadifar E., Abdel-Latif H.M.R. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: a review. Aquaculture. 2022;547 doi: 10.1016/j.aquaculture.2021.737514. [DOI] [Google Scholar]
- 24.Abdel-Latif H.M.R., Yilmaz E., Dawood M.A.O., Ringø E., Ahmadifar E., Yilmaz S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: a review. Aquaculture. 2022;551 doi: 10.1016/j.aquaculture.2022.737951. [DOI] [Google Scholar]
- 25.Villamil L., Figueras A., Planas M., Novoa B. Control of Vibrio alginolyticus in Artemia culture by treatment with bacterial probiotics. Aquaculture. 2003;219:43–56. doi: 10.1016/S0044-8486(02)00515-X. [DOI] [Google Scholar]
- 26.Giarma E., Amanetidou E., Toufexi A., Touraki M. Defense systems in developing Artemia franciscana nauplii and their modulation by probiotic bacteria offer protection against a Vibrio anguillarum challenge. Fish Shellfish Immunol. 2017;66:163–172. doi: 10.1016/j.fsi.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Lamari F., Sadok K., Bakhrouf A., Gatesoupe F.J. Selection of lactic acid bacteria as candidate probiotics and in vivo test on Artemia nauplii. Aquacult. Int. 2014;22:699–709. doi: 10.1007/s10499-013-9699-5. [DOI] [Google Scholar]
- 28.Romalde J.L. Photobacterium damselae subsp. piscicida: an integrated view of a bacterial fish pathogen. Int. Microbiol. 2002;5:3–9. doi: 10.1007/s10123-002-0051-6. [DOI] [PubMed] [Google Scholar]
- 29.Vaseeharan B., Sundararaj S., Murugan T., Chen J.C. Photobacterium damselae ssp. damselae associated with diseased black tiger shrimp Penaeus monodon Fabricius in India. Lett. Appl. Microbiol. 2007;45:82–86. doi: 10.1111/j.1472-765X.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- 30.White D.M., Valsamidis M.A., Bakopoulos V. In vitro hemocyte phagocytosis activation after experimental infection of common octopus, Octopus vulgaris (Cuvier, 1797) with Photobacterium damselae subsp. piscicida or Vibrio alginolyticus at different temperatures and infection routes. J. Invertebr. Pathol. 2022;191 doi: 10.1016/j.jip.2022.107754. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S., Zhang S., Bai X., Zhang L., Liu J., Ke K. Pathogenic Photobacterium Sp. induce mortality in the lined seahorse (Hippocampus erectus): first case report from China. Isr. J. Aquac. - Bamidgeh. 2022;74:1–10. doi: 10.46989/001c.33603. [DOI] [Google Scholar]
- 32.Kandathil Radhakrishnan D., AkbarAli I., Schmidt B.V., John E.M., Sivanpillai S., Thazhakot Vasunambesan S. Improvement of nutritional quality of live feed for aquaculture: an overview. Aquac. Res. 2020;51:1–17. doi: 10.1111/are.14357. [DOI] [Google Scholar]
- 33.Hasan K.N., Banerjee G. Recent studies on probiotics as beneficial mediator in aquaculture: a review. JoBAZ. 2020;81:53–69. doi: 10.1186/s41936-020-00190-y. [DOI] [Google Scholar]
- 34.Wuertz S., A Schroeder, Wanka K.M. Probiotics in fish nutrition—long-standing household remedy or native nutraceuticals? Water (Basel) 2021;13(10):1348. doi: 10.3390/w13101348. [DOI] [Google Scholar]
- 35.Butt U.D., Lin N., Akhter N., Siddiqui T., Li S., Wu B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021;114:263–281. doi: 10.1016/j.fsi.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Vadstein O., Attramadal K.J.K., Bakke I., et al. Managing the microbial community of marine fish larvae: a holistic perspective for larviculture. Front. Microbiol. 2018;9:1820. doi: 10.3389/fmicb.2018.01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makridis P., Costa R., Dinis M.T. Microbial conditions and antimicrobial activity in cultures of two microalgae species, Tetraselmis chuii and Chlorella minutissima, and effect on bacterial load of enriched Artemia metanauplii. Aquaculture. 2006;255:76–81. doi: 10.1016/j.aquaculture.2005.12.010. [DOI] [Google Scholar]
- 38.Olsen A.I., Olsen Y., Attramadal Y., Christie K., Birkbeck T.H., Skjermo J., Vadstein O. Effects of short-term feeding of microalgae on the bacterial flora associated with juvenile Artemia franciscana. Aquaculture. 2000;190:11–25. doi: 10.1016/S0044-8486(00)00396-3. [DOI] [Google Scholar]
- 39.Karagiota A., Tsitsopoulou H., Tasakis R.N., Zoumpourtikoudi V., Touraki M. Characterization and quantitative determination of a diverse group of Bacillus subtilis subsp. Subtilis NCIB 3610 antibacterial peptides. Probiotics Antimicrob. Proteins. 2021;13(2):555–570. doi: 10.1007/s12602-020-09706-y. [DOI] [PubMed] [Google Scholar]
- 40.da Silva Sabo S., Vitolo M., González J.M.D., Oliveira R.P.S. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res. Int. 2014;64:527–536. doi: 10.1016/j.foodres.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 41.Tasakis R.N., Touraki M. Identification of bacteriocins secreted by the probiotic Lactococcus lactis following microwave-assisted acid hydrolysis (MAAH), amino acid content analysis, and bioinformatics. Anal. Bioanal. Chem. 2018;410(4):1299–1310. doi: 10.1007/s00216-017-0770-3. [DOI] [PubMed] [Google Scholar]
- 42.Naveed M., Tianying H., Wang F., Yin X., Chan M.W.H., Ullah A., Xu B., Aslam S., Ali N., Abbas Q., et al. Isolation of lysozyme producing Bacillus subtilis strains, identification of the new strain Bacillus subtilis BSN314 with the highest enzyme production capacity and optimization of culture conditions for maximum lysozyme production. Curr. Res. Biotechnol. 2022;4:290–301. doi: 10.1016/j.crbiot.2022.06.002. [DOI] [Google Scholar]
- 43.Naveed M., Wang Y., Yin X., Chan M.W.H., Aslam S., Wang F., Xu B., Ullah A. Purification, characterization and bactericidal action of lysozyme, isolated from Bacillus subtillis BSN314: a disintegrating effect of lysozyme on gram-positive and gram-negative bacteria. Molecules. 2023;28:1058. doi: 10.3390/molecules28031058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ullah A., Mu J., Wang F., Chan M.W.H., Yin X., Liao Y., Mirani Z.A., Sebt-e-Hassan S., Aslam S., Naveed M., et al. Biogenic selenium nanoparticles and their anticancer effects pertaining to probiotic bacteria—a review. Antioxidants. 2022;11:1916. doi: 10.3390/antiox11101916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Gonzalez N., Battista N., Prete R., Corsetti A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms. 2021;9(2):349. doi: 10.3390/microorganisms9020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaur S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015;6:272. doi: 10.3389/fphar.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song A.A.L., In L.L.A., Lim S.H.E., et al. A review on Lactococcus lactis: from food to factory. Microb. Cell Fact. 2017;16:55. doi: 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyholm S.V., Graf J. Knowing your friends: invertebrate innate immunity fosters beneficial bacterial symbioses. Nat. Rev. Microbiol. 2012;10:815–827. doi: 10.1038/nrmicro2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Söderhäll K., Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 50.Yu B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 51.Bogwald J., Dalmo R.A. In: Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics. Merrifield D., Ringø E., editors. John Wiley & Sons, Ltd; 2014. Gastrointestinal pathogenesis in aquatic animals; pp. 53–74. [DOI] [Google Scholar]
- 52.Hootman S.R., Conte F.P. Fine structure and function of the alimentary epithelium in Artemia salina nauplii. Cell Tissue Res. 1974;155:423–436. doi: 10.1007/BF00227006. [DOI] [PubMed] [Google Scholar]
- 53.Overton S.V., Bland C.E. Infection of Artemia Salina by Haliphthoros Milfordensis: a scanning and transmission electron microscope study. J. Invertebr. Pathol. 1981;37:249–257. doi: 10.1016/0022-2011(81)90083-5. [DOI] [Google Scholar]
- 54.Asanka Gunasekara R.A.Y.S., Rekecki A., Baruah K., Bossier P., Van den Broeck W. Evaluation of probiotic effect of Aeromonas hydrophila on the development of the digestive tract of germ-free Artemia franciscana nauplii. J. Exp. Mar. Biol. Ecol. 2010;393:78–82. doi: 10.1016/j.jembe.2010.07.006. [DOI] [Google Scholar]
- 55.Asanka Gunasekara R.A.Y.S., Rekecki A., Cornillie P., Cornelissen M., Sorgeloos P., Simoens P., Bossier P., Van den Broeck W. Morphological characteristics of the digestive tract of gnotobiotic Artemia franciscana nauplii. Aquaculture. 2011;321:1–7. doi: 10.1016/j.aquaculture.2011.07.037. [DOI] [Google Scholar]
- 56.Asanka Gunasekara R.A.Y.S., A R., Defoirdt T., Rekecki A., Decostere A., Cornelissen M., Sorgeloos P., Bossier P., Van den Broeck W. Light and transmission electron microscopy of Vibrio campbellii infection in gnotobiotic Artemia franciscana and protection offered by a yeast mutant with elevated cell wall glucan. Vet. Microbiol. 2012;158:337–343. doi: 10.1016/j.vetmic.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 57.Kvitt H., Ucko M., Colorni A., Batargias C., Zlotkin A., Knibb W. Photobacterium damselae ssp. piscicida: detection by direct amplification of 16S rRNA gene sequences and genotypic variation as determined by amplified fragment length polymorphism (AFLP) Dis. Aquat. Org. 2002;48:187–195. doi: 10.3354/dao048187. [DOI] [PubMed] [Google Scholar]
- 58.Nitzan S., Shwartsburd B., Heller E.D. The effect of growth medium salinity of Photobacterium damselae subsp. piscicida on the immune response of hybrid bass (Morone saxatilis x M. chrysops) Fish Shellfish Immunol. 2004;16:107–116. doi: 10.1016/S1050-4648(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 59.Verschuere L., Heang H., Criel G., Sorgeloos P., Verstraete W. Selected bacterial strains protect Artemia spp. from the pathogenic effects of Vibrio proteolyticus CW8T2. Appl Environ Microbiol. 2000;66(3):1139–1146. doi: 10.1128/AEM.66.3.1139-1146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prayitno S.B., Latchford J.W. Experimental infections of crustaceans with luminous bacteria related to Photobacterium and Vibrio. Effect of salinity and pH on infectiosity. Aquaculture. 1995;132:105–112. doi: 10.1016/0044-8486(94)00374-W. [DOI] [Google Scholar]
- 61.Nagano I., Inoue S., Kawai K., Oshima S. Repeatable immersion infection with Photobacterium damselae subsp. piscicida reproducing clinical signs and moderate mortality. Fish Sci. 2009;75:707–714. doi: 10.1007/s12562-009-0099-8. [DOI] [Google Scholar]
- 62.Nunes B., Carvalho F., Guilhermino L. Effects of widely used pharmaceuticals and a detergent on oxidative stress biomarkers of the crustacean Artemia parthenogenetica. Chemosphere. 2006;62:581–594. doi: 10.1016/j.chemosphere.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Rojas-García C.R., Hasanuzzaman A.F.M., Sorgeloos P., Bossier P. Cell wall deficient Saccharomyces cerevisiae strains as microbial diet for Artemia larvae: protective effects against vibriosis and participation of phenoloxidase. J. Exp. Mar. Biol. Ecol. 2008;360:1–8. doi: 10.1016/j.jembe.2008.01.008. [DOI] [Google Scholar]
- 64.Beyer W.F., Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 65.Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 66.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- 67.Fan T., Jing Z., Fan X., Yu M., Jiang G. Purification and characterization of phenoloxidase from brine shrimp Artemia sinica. Acta Biochim. Biophys. Sin. (Shanghai). 2011;43:722–728. doi: 10.1093/abbs/gmr061. [DOI] [PubMed] [Google Scholar]
- 68.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 69.Buege J.A., Aust S.D. Microsomal lipid peroxidation Methods. Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 70.de la Fuente M.C., Segade Y., Valderrama K., Rodríguez J., Jiménez C. Convergent total synthesis of the siderophore piscibactin as its Ga3+ complex. Orga. lett., 2021;23:340–345. doi: 10.1021/acs.orglett.0c03850. [DOI] [PubMed] [Google Scholar]
- 71.Lattos A., Giantsis I.A., Tsavea E., Kolygas M., Athanassopoulou F., Bitchava K. Virulence genes and in vitro antibiotic profile of Photobacterium damselae strains, isolated from fish reared in Greek aquaculture facilities. Animals. 2022;12:3133. doi: 10.3390/ani12223133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Díaz-Rosales P., Arijo S., Chabrillón M., Alarcón F.J., Tapia-Paniagua S.T., Martínez-Manzanares E., Balebona M.C., Moriñigo M.A. Effects of two closely related probiotics on respiratory burst activity of Senegalese sole (Solea senegalensis, Kaup) phagocytes, and protection against Photobacterium damselae subsp. piscicida. Aquaculture. 2009;293:16–21. doi: 10.1016/j.aquaculture.2009.03.050. [DOI] [Google Scholar]
- 73.Peixoto M.J., Domingues A., Batista S., Gonçalves J.F.M., Gomes A.M., Cunha S., Valente L.M.P., Costas B., Ozório R.O.A. Physiopathological responses of sole (Solea senegalensis) subjected to bacterial infection and handling stress after probiotic treatment with autochthonous bacteria. Fish Shellfish Immunol. 2018;83:348–358. doi: 10.1016/j.fsi.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 74.Xing C.F., Hu H.H., Huang J.B., Fang H.C., Kai Y.H., Wu Y.C., Chi S.C. Diet supplementation of Pediococcus pentosaceus in cobia (Rachycentron canadum) enhances growth rate, respiratory burst and resistance against photobacteriosis. Fish Shellfish Immunol. 2013;35:1122–1128. doi: 10.1016/j.fsi.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 75.Smith W.M., Pham T.H., Lei L., Dou J., Soomro A.H., Beatson S.A., Dykes G.A., Turner M.S. Heat resistance and salt hypersensitivity in Lactococcus lactis due to spontaneous mutation of llmg_1816 (gdpP) induced by high-temperature growth. Appl. Environ. Microbiol. 2012;78:7753–7759. doi: 10.1128/AEM.02316-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Magariños B., Santos Y., Romalde J.L., Rivas C., Barja J.L., Toranzo A.E. Pathogenic activities of live cells and extracellular products of the fish pathogen Pasteurella piscicida. J. Gen. Microbiol. 1992;138:2491–2498. doi: 10.1099/00221287-138-12-2491. [DOI] [PubMed] [Google Scholar]
- 77.Paolini A., Ridolfi V., Zezza D., Cocchietto M., Musa M., Pavone A., Conte A., Giorgetti G. Vaccination trials of sea bass (Dicentrarchus labrax) against pasteurellosis using oral, intraperitoneal and immersion methods. Vet. Ital. 2005;41:137–144. PMID: 20437375. [PubMed] [Google Scholar]
- 78.do Vale A., Marques F., Silva M.T. Apoptosis of sea bass (Dicentrarchus labrax L.) neutrophils and macrophages induced by experimental infection with Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 2003;15:129–144. doi: 10.1016/s1050-4648(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z., Shi C., Wang H., Wan X., Zhang Q., Song X., Li G., Gong M., Ye S., Xie G., Huang J. A novel research on isolation and characterization of Photobacterium damselae subsp. damselae from Pacific white shrimp, Penaeus vannamei, displaying black gill disease cultured in China. J. Fish Dis. 2020;43(5):551–559. doi: 10.1111/jfd.13153. [DOI] [PubMed] [Google Scholar]
- 80.Núñez-Díaz J.A., Fumanal M., Mancera J.M., Moriñigo M.A., Balebona M.C. Two routes of infection with Photobacterium damselae subsp. piscicida are effective in the modulation of the transcription of immune related genes in Solea senegalensis. Vet. Immunol. Immunopathol. 2016;179:8–17. doi: 10.1016/j.vetimm.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Gobi N., Malaikozhundan B., Sekar V., Shanthi S., Vaseeharan B., Jayakumar R., Khudus Nazar A. GFP tagged Vibrio parahaemolyticus Dahv2 infection and the protective effects of the probiotic Bacillus licheniformis Dahb1 on the growth, immune and antioxidant responses in Pangasius hypophthalmus. Fish Shellfish Immunol. 2016;52:230–238. doi: 10.1016/j.fsi.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 82.Giannenas I., Karamaligas M., Margaroni I., Pappas E., Mayer P., Encarnação E. Karagouni effect of dietary incorporation of a multi-strain probiotic on growth performance and health status in rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 2015;41:119–128. doi: 10.1007/s10695-014-0010-0. [DOI] [PubMed] [Google Scholar]
- 83.Welker T.L., Lim C. Use of probiotics in diets of Tilapia. J. Aquac. Res. Dev. 2011;S1:014. doi: 10.4172/2155-9546.S1-014. [DOI] [Google Scholar]
- 84.Wang Y., Gu Q. Effect of probiotics on white shrimp (Penaeus vannamei) growth performance and immune response. Mar. Biol. Res. 2010;6:327–332. doi: 10.1080/17451000903300893. [DOI] [Google Scholar]
- 85.Wu H.J., Sun L.B., Li C.B., Li Z.Z., Zhang Z., Wen X.B., Hu Z., Zhang Y.L., Li S.K. Enhancement of the immune response and protection against Vibrio parahaemolyticus by indigenous probiotic Bacillus strains in mud crab (Scylla paramamosain) Fish Shellfish Immunol. 2014;41:156–162. doi: 10.1016/j.fsi.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 86.Yang Q., Lü Y., Zhang M., Gong Y., Li Z., Tran N.T., He Y., Zhu C., Lu Y., Zhang Y. Lactic acid bacteria, Enterococcus faecalis Y17 and Pediococcus pentosaceus G11, improved growth performance, and immunity of mud crab (Scylla paramamosain) Fish Shellfish Immunol. 2019;93:135–143. doi: 10.1016/j.fsi.2019.07.050. [DOI] [PubMed] [Google Scholar]
- 87.Zhu L., Kong Y., Chang X., Feng J., Wang X., Hou L., Zhao X., Pei C., Kong X. Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture. 2023;562 doi: 10.1016/j.aquaculture.2022.738765. [DOI] [Google Scholar]
- 88.Ullah A., Yin X., Wang F., Xu B., Mirani Z.A., Xu B., Chan M.W.H., Ali A., Usman M., Ali N., Naveed M. Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities. Molecules. 2021;26(18):5559. doi: 10.3390/molecules26185559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ullah A., Mirani Z.A., Binbin S., Wang F., Chan M.W.H., Aslam S., Yonghong L., et al. An elucidative study of the anti-biofilm effect of selenium nanoparticles (SeNPs) on selected biofilm producing pathogenic bacteria: a disintegrating effect of SeNPs on bacteria. Process Biochem. 2022;126:98–107. doi: 10.1016/j.procbio.2022.12.031. [DOI] [Google Scholar]
- 90.Rocchetti M.T., Russo P., Capozzi V., Drider D., Spano G., Fiocco D. Bioprospecting antimicrobials from Lactiplantibacillus plantarum: key factors underlying its probiotic action. Int. J. Mol. Sci. 2021;22(21):2076. doi: 10.3390/ijms222112076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu X., Huang P., Cui X., Li X., Sun J., Ji Q., Wei Q., Huang Y., Li Z., Bao G., et al. Effects of dietary coated lysozyme on the growth performance, antioxidant activity, immunity and gut health of weaned piglets. Antibiotics. 2022;11(11) doi: 10.3390/antibiotics11111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y., Wei Y., Shang N., Li P. Synergistic inhibition of plantaricin E/F and lactic acid against aeromonas hydrophila LPL-1 reveals the novel potential of class IIb bacteriocin. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.774184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saleena L.A.K., Teo M.Y.M., How Y.H., In L.L.A., Pui L.P. Immunomodulatory action of Lactococcus lactis. J. Biosci. Bioeng. 2023;135(1):1–9. doi: 10.1016/j.jbiosc.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 94.Li H., Zhou X., Huang Y., Liao B., Cheng L., Ren B. Reactive oxygen species in pathogen clearance: the killing mechanisms, the adaption response, and the side effects [published correction appears in front. Microbiol. 2021;11 doi: 10.3389/fmicb.2020.622534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchi S., Giorgi C., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Missiroli S., Patergnani S., Poletti F., Rimessi A., Duszynski J., Wieckowski M.R., Pinton P. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012 doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chaitanya R.K., Shashank K., Sridevi P. Oxidative stress in invertebrate systems. Free Rad. Dis. 2016 doi: 10.5772/64573. [DOI] [Google Scholar]
- 97.Wang J.X., Vasta G.R. Introduction to special issue: pattern recognition receptors and their roles in immunity in invertebrates. Dev. Comp. Immunol. 2020;109 doi: 10.1016/j.dci.2020.103712. [DOI] [PubMed] [Google Scholar]
- 98.Trestrail C., Nugegoda D., Shimeta J. Invertebrate responses to microplastic ingestion: reviewing the role of the antioxidant system. Sci. Total Environ. 2020;734 doi: 10.1016/j.scitotenv.2020.138559. [DOI] [PubMed] [Google Scholar]
- 99.Matés J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 100.Peixoto M.J., Salas-Leitón E., Brito F., Pereira L.F., Svendsen J.C., Baptista T., Pereira R., Abreu H., Reis P.A., Gonçalves J.F.M., de R.O., Ozório A. Growth performance, metabolic rates and health in meagre (Argyrosomus regius) subjected to pathogen infection. J. Appl. Phycol. 2017;29:433–447. doi: 10.1007/s10811-016-0917-1. [DOI] [Google Scholar]
- 101.Rojas-García C.R., Sorgeloos P., Bossier P. Phenoloxidase and trypsin in germ-free larvae of Artemia fed with cooked unicellular diets: examining the alimentary and protective effects of putative beneficial bacterium, yeast and microalgae against vibriosis. J. Exp. Mar. Biol. Ecol. 2009;381:90–97. doi: 10.1016/j.jembe.2009.08.001. [DOI] [Google Scholar]
- 102.Santos P., Peixoto D., Ferreira I., Passos R., Pires P., Simões M., Pousão-Ferreira P., Baptista T., Costas B. Short-term immune responses of gilthead seabream (Sparus aurata) juveniles against Photobacterium damselae subsp. piscicida. Int. J. Mol. Sci. 2022;23:1561. doi: 10.3390/ijms23031561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tanaka R., Shigeta K., Y.Sugiura H.Hatate, Matsushita T. Accumulation of hydroxyl lipids and 4-Hydroxy-2-hexenal in live fish infected with fish diseases. Lipids. 2014;49:385–396. doi: 10.1007/s11745-013-3875-2. [DOI] [PubMed] [Google Scholar]
- 104.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017;482:419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castex M., Lemaire P., Wabete N., Chim L. Effect of probiotic Pediococcus acidilactici on antioxidant defenses and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol. 2010;28:622–631. doi: 10.1016/j.fsi.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 106.Gou H.Z., Zhang Y.L., Ren L.F., Li Z.J., Zhang L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.929346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pagnini C., Saeed R., Bamias G., Arseneau K.O., Pizarro T.T., Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. PNAS. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oberhammer F.A., Hochegger K., Fröschl G., Tiefenbacher R., Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J. Cell Biol. 1994;126:827–837. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kroemer G., Galluzzi L., Vandenabeele P., et al. Classification of cell death: recommendations of the nomenclature committee on cell death. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orbach R., Su X. Surfing on membrane waves: microvilli, curved membranes, and immune signaling. Front. Immunol. 2020;11:2187. doi: 10.3389/fimmu.2020.02187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.López-Dóriga M.V., Barnes A.C., N.M.S Dos Santos, Ellis A.E. Invasion of fish epithelial cells by Photobacterium damselae subsp. piscicida: evidence for receptor specificity, and effect of capsule and serum. Microbiology. 2000;146:21–30. doi: 10.1099/00221287-146-1-21. [DOI] [PubMed] [Google Scholar]
- 112.Brown M.D., Bry L., Li Z., Sacks D.B. Actin pedestal formation by enteropathogenic Escherichia coli is regulated by IQGAP1, calcium, and calmodulin. J. Biol. Chem. 2008;283:35212–35222. doi: 10.1074/jbc.M803477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ho G.T., Aird R.E., Liu B., Boyapati R.K., Kennedy N.A., Dorward D.A., Noble C.L., Shimizu T., Carter R.N., Chew E.T.S., Morton N.M., Rossi A.G., Sartor R.B., Iredale J.P., Satsangi J. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 2018;11:120–130. doi: 10.1038/mi.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shukla G., Singh S., Verma A. Oral administration of the probiotic Lactobacillus casei ameliorates gut morphology and physiology in malnourished-giardia intestinalis-infected BALB/c mice. ISRN Parasitol. 2013 doi: 10.5402/2013/762638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crawley S.W., Shifrin D.A., Jr NE.Grega-Larson, McConnell R.E., Benesh A.E., Mao S., Zheng Y., Zheng Q.Y., Nam K.T., Millis B.A., Kachar B., Tyska M.J. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell. 2014;157:433–446. doi: 10.1016/j.cell.2014.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bennett K.M., Walker S.L., Lo D.D. Epithelial microvilli establish an electrostatic barrier to microbial adhesion. Infect. Immun. 2014;82:2860–2871. doi: 10.1128/IAI.01681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.