Summary
Plastic pollution and climate change are two major environmental focuses. Having the forming potential due to ambient plastic pollution, the environmental fate of microplastics shall be inevitably impacted by global warming. This manuscript discusses the destiny of environmental microplastics and characterizes their fate considering the framework of the planetary boundary. The major routes for microplastic discharge include the release of microplastic stored in the ice into the sea when the ice melts as a result of global temperature increase, flushing of the plastic/microplastic debris from the shorelines into the adjacent water bodies as a result of increased rainfall, redistribution of the microplastics away from the source of plastic debris as a result of increased wind, and accumulation of microplastics in the soil as a result of drought. A perspective on the impact of climate change and microplastic pollution on aquatic and soil organisms was discussed as well.
Subject areas: Environmental science, Global change, Environmental monitoring
Graphical abstract
Environmental science; Global change; Environmental monitoring
Introduction
Plastic pollution and climate change are two major global threats that impact the pedosphere, hydrosphere, and atmosphere of the environment. With the influence of climate change, the elevated temperature leads to extreme weather events such as glacier thawing and increased ocean temperature, causing sea-level rise and ocean acidification.1 Even worse, the atmospheric temperature increase in some regions exceeded 1.5°C, only half a degree away from the 2°C temperature rise threshold set by the 26th UN Conference of the Parties.2 On the other hand, global production of plastics increased from 1.3 million tons in 1950 to 359 million tons in 2018.3,4 Total plastic waste can reach 634 million tons by 2025.5 Global plastic production is on the rise and improper waste management leads to the ubiquity of plastic litter in the environment. Once exposed to the environment, plastic waste can be sideswiped into microplastics via photodegradation, mechanical impact, ambient weathering, or microbial degradation.6,7 Therefore, it is important to understand the environmental fate and consequential impact of microplastics on the organisms since their wide distribution and persistence in the environment is a critical issue of concern.
Environmental plastic could be classified as macroplastic (size >5 mm) or microplastic (size <5 mm). Microplastics may be further categorized into primary microplastic (originally manufactured to a size <5 mm) or secondary microplastic (degraded macroplastics exposed to various environmental conditions).8,9 Depending on their types, shapes, sizes, and composition, microplastics impose different toxic levels on the environment and ecology. Microplastics with smaller sizes are more toxic in general as they are more susceptible to uptake by aquatic organisms as well as plants.10,11 While several review papers assess the transport of microplastics into coastal shorelines and water bodies (e.g., Browne,12 Luo et al; ,13 Zhou et al; 14), the influences of climate change on the microplastic formation and/or distribution are not discussed in detail. Therefore, this manuscript throws light on how climate change impacts microplastics’ fate and environmental consequences.
Climate change intensifies the impact of microplastics on the environment by influencing their degradation, distribution, and interaction with ecosystems. Understanding these impacts is crucial for developing effective strategies to address the microplastic problem in the face of climate change. Climate change has various effects on the fate of microplastics in the environment. Increasing temperatures can expedite the degradation of plastic, resulting in enhanced fragmentation and the release of smaller microplastic particles.9 Additionally, elevated temperatures facilitate the physical breakdown of plastics through thermal degradation, rendering them more susceptible to other degradation processes.15
Climate change can also lead to more frequent and intense extreme weather events like storms, hurricanes, and floods, which can transport and disperse microplastics across larger areas, thereby increasing their distribution in the environment.16 Moreover, climate change contributes to the rise of sea levels and ocean acidification.17 This combination raises the vulnerability of coastal areas to plastic pollution, while also influencing the breakdown and degradation mechanisms of plastics in marine environments. Changes in ocean currents and circulation patterns, driven by climate change, can further impact the transport and dispersion of microplastics. Altered currents have the potential to carry microplastics over extensive distances, thus increasing the likelihood of contamination in previously unaffected regions.18 Additionally, climate change disrupts marine ecosystems, affecting the behavior, reproduction, and feeding patterns of organisms that interact with microplastics. The rising temperatures and alterations in ocean chemistry can disrupt the exposure of marine species to microplastics, leading to the accumulation of microplastics within the food chain.19,20,21
Overall, comprehending these effects is crucial for developing effective strategies to address and mitigate the microplastic issue in the context of climate change. There are only a limited number of available literature that discuss the link between climate change and plastic/microplastic pollution. For example, Welden et al.22 and Ford et al.23 examined the impact of climate change on marine plastic pollution and how plastic pollution and climate change are fundamentally linked, i.e., how plastic generation aggravates greenhouse gas emission and climate change. Li et al.24 reviewed the effect of environmental and anthropogenic factors on the distribution of microplastics in freshwater systems. Zhang et al.25 studied the impact of climate change and eutrophication on microplastic distribution in shallow lakes. Similarly, Huang et al.26 explored microplastic pollution in the aquatic environment and its impact on human health. However, most of these review studies focused on the fate of microplastic in the aquatic environment. In contrast, this manuscript aims to discuss the fate of microplastics in the total environment including the pedosphere, hydrosphere, and atmosphere under the influence of climate change.
Given the previous context, the main objectives of this study are to 1) delineate the destiny of the environmental microplastics, we need to understand what the major sinks for microplastics are to assess the fate of microplastic in the environment; 2) illustrate the environmental impacts of climate change outcomes, namely temperature, rainfall, drought, and wind on the fate of microplastics. This section discusses how various climatic factors impact the microplastic sinks as well as the (re)distribution of the microplastic in the environment; 3) provide a perspective on the effect of climate change parameters and microplastic accumulation on aquatic and soil organisms. The research gaps are identified, and future research directions are proposed. The impact of climate change on microplastic was considered less when experimental works were designed or field monitoring studies were involved. Microplastic transport across the terrestrial environment, aquatic environment, and the atmosphere follows a transport route that is similar to any given biogeochemical cycle,27,28 but a deeper exploration of the impact of climate change on microplastic is missing.
Our previous work, Haque and Fan,29 covers the fate and transport of microplastics in the environment as a whole, specifically addressing the hydrosphere, pedosphere, and atmosphere. Its aim focused on providing a comprehensive understanding of the fate and transport of microplastics in these three zones, along with their ecotoxicological impact on aquatic and soil communities. It also deliberates future research directions and plastic waste management strategies to control microplastic pollution. In contrast, this study primarily explores the impact of climate change parameters (temperature, rainfall, drought, and wind) on the fate of microplastics in the environment. The main objectives involve defining microplastic destiny, examining the influence of climate change outcomes on microplastic fate and redistribution, and providing a perspective on the effects of climate change parameters and microplastic accumulation on aquatic and soil organisms. While both works address the fate and transport of microplastics, the work by Haque and Fan29 provided a broader overview of the environmental fate in the hydrosphere, pedosphere, and atmosphere, while this current manuscript focuses more specifically on the influence of climate change parameters and their effects on microplastic fate and distribution. Additionally, this manuscript highlights the gaps in current research regarding the impact of climate change on microplastics, aiming to deepen the understanding of these interactions and mechanisms.
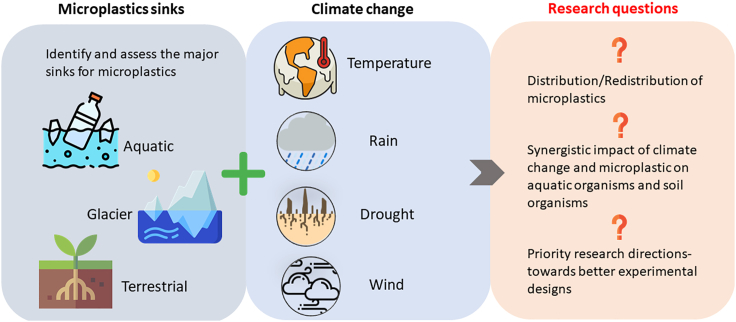
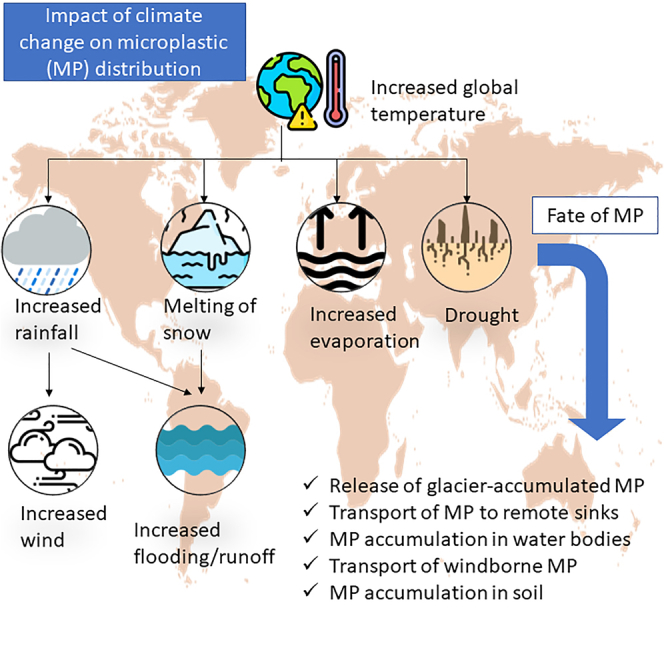
This study proposes a perspective on the effect of climate change parameters (i.e., temperature, rainfall, drought, and wind) on microplastic fate (dispersion/distribution/redistribution). Each climatic factor was examined and discussed to provide a deeper insight into possible interactions and mechanisms between microplastic and various environmental systems. This review also explores the knowledge and scientific gaps in the current undergoing of experimental designs, which hardly considered the climate change impact to assess the threats of microplastics to the environment. Figure 1 illustrates the objective of this study: the effect of climate change parameters (temperature, rainfall, drought, and wind) on microplastic fate in the environment.
Figure 1.
Schematic representation of the goal and objective of this study: the effect of climate change parameters (temperature, rainfall, drought, and wind) on microplastic fate in the environment
Destiny of environmental microplastics
The generation of plastic waste is inevitable as proof of human survival and modernization. Once released into the ambient environment, plastics may be regarded as mismanaged plastic waste, which becomes a potential source for microplastic development. Environmental weathering is the major mechanism responsible for microplastic formation. Generally, natural plastic degradation is a slow process (i.e., thousands of years), which explains why there are limited studies to evaluate the degradation of plastic and the formation of microplastic under environmental conditions.30 Environmental weathering results in plastic degradation, breaking long-chain polymers into smaller ones. Weathering mechanisms including abiotic degradation such as UV radiation, heat, and chemical reactions lead to plastic breakdown or fragmentation via mechanical stress or chemical oxidation.6,7
Another notable degradation mechanism is the biotic degradation caused by enzymatic processes as well as biodisintegration in which plastics are fragmented into small pieces, for example, the composting processes.31 Detailed reviews of the degradation pathways of different types of plastic were given by Shah et al.,32 Singh and Sharma,33 and Zhang et al.9 The microplastic crisis would be worsened as a result of global warming and climate change. The interplay between these two pressing issues creates an unhealthy cycle that intensifies the impact on ecosystems and environmental health. Table 1 summarizes the different plastic degradation pathways, along with how climate change can aggravate the microplastic crisis. One of the primary mechanisms by which global warming exacerbates the microplastic crisis is through the acceleration of abiotic degradation processes. As temperatures rise, plastic materials break down at an increased rate, leading to the release of even smaller microplastic particles into the environment.34 This not only amplifies the contamination levels but also makes it more challenging to mitigate their effects.
Table 1.
Plastic degradation pathways and their connections with climate change factors
| Degradation pathway | Climate change factor | Degradation mechanism | Connection with climate change factor | Reference |
|---|---|---|---|---|
| Mechanical degradation | Wind and ocean current, thawing of snow. | External forces result in the mechanical breakdown of plastics. | Plastics on coastal shores collide with rocks and sand due to wind and ocean movement. In colder areas, freezing and thawing of ice degrade the accumulated plastics. | Zhang et al.,9 Cooper and Corcoran16 |
| Thermal degradation | Temperature | When plastics absorb heat, their polymeric chains break down, releasing radicals. These radicals react with oxygen, forming hydroperoxide, which further breaks down into hydroxyl and alkoxy radicals. These radicals contribute to the formation of aldehydes, ketones, esters, or alcohols, leading to plastic degradation. | Plastic debris on coastal shores undergoes thermo-oxidative degradation due to increased temperatures. They undergo gradual thermal degradation along with enhanced photodegradation due to prolonged exposure to sunlight. | Torikai et al.37 Kamweru et al.15 |
| Chemical degradation | Acid rain (sulfur dioxide, nitrogen dioxide, ozone, and volatile organic compounds present in the atmosphere). | These pollutants can degrade plastics directly or facilitate radical formation through photochemical reactions. Sulfur dioxide and nitrogen dioxide, through UV excitation and reaction with oxygen, can promote ozone formation, which can break the carbon double bonds in plastic polymers through a chain scission mechanism. | Acid rain can result in enhanced plastic degradation of the plastic debris present on terrestrial soils. It can also alter the pH of the water environment and result in plastic degradation. | Lee and Li,17 Hocker et al.38 Krupa and Manning39 |
| Photodegradation | Increased UV radiations. | Plastic photodegradation occurs when exposed to both high-energy UVB (290–315 nm) and medium-energy UVA (315–400 nm) sunlight. This process involves the formation of free radicals and oxidation of plastic polymers, leading to the creation of peroxides that break down into alkoxy and hydroxyl radicals. | Prolonged exposure of plastic litter on terrestrial soil or coastal shorelines to increased UV radiations will enhance plastic degradation. | Zhang et al.9 Gewert et al.40 |
Climate change also contributes to the problem of microplastic pollution through its influence on ocean currents and weather patterns. Rising sea levels and altered ocean currents can transport microplastics over greater distances, spreading contamination to previously unaffected regions. Additionally, extreme weather events such as hurricanes and storms can cause plastic debris to be distributed over vast areas, further increasing the dispersion of microplastics.35 Moreover, global warming impacts the ecological balance of marine ecosystems, affecting the organisms that interact with microplastics. Rising temperatures and ocean acidification disrupt the reproductive cycles and behaviors of marine species, potentially altering their exposure to microplastics.36 This disruption can lead to the accumulation of microplastics within the food chain, posing threats to both aquatic organisms and humans who rely on marine resources. To address the worsening microplastic crisis, concerted efforts are required to mitigate the impacts of global warming and plastic pollution.
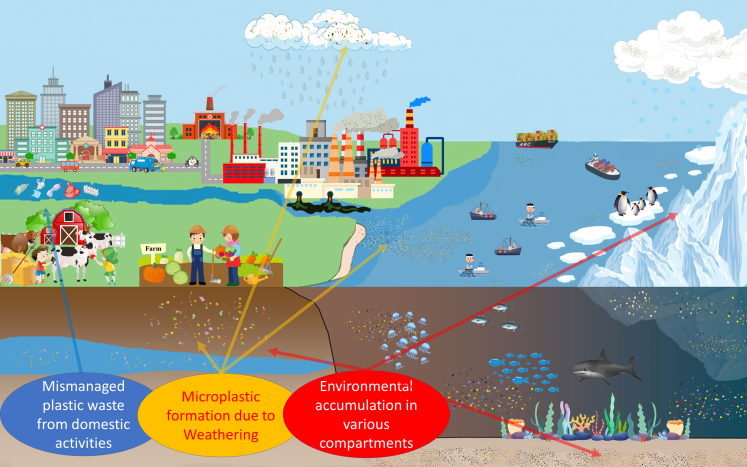
In Figure 2, a graphical illustration of the destiny of environmental microplastics is presented. Originating from mismanaged plastic waste, a substantial portion of environmental microplastics forms after experiencing weathering process, followed by redistribution through atmospheric and oceanic currents. Subsequently, atmospheric deposition and gravitation process results in microplastic accumulation in various environmental compartments. A more detailed description of the microplastic accumulation in aquatic habitats (including sediments), glacier habitats (including ice caps in remote zones), and terrestrial soils can be referred to in the supplementary materials.
Figure 2.
Origins, distribution, and accumulation of environmental microplastics
As stated previously, the atmospheric current or oceanic circulation is the major driving force that displaces microplastics to distant areas before their accumulation in various environmental compartments. Therefore, the fate of environmental microplastics can be classified into two categories: the one that is suspended in the circulated currents and the other that settles due to depository or gravitation processes. The criteria that control the microplastics in suspension or deposition may be diverse and complicated. Theoretically, the specific weight, shape, and surrounding environment are the major factors that determine the possibility of sedimentation of suspended microplastics. The environmental conditions such as flow speed of the climatic currents, buoyancy, and viscosity of the carrying media are also required to consider for assessing the fate of environmental microplastics.
By inspecting the available studies in the literature, most of the research articles were conducted based on their pre-defined research domains, i.e., local studies, regional investigations, or global evaluations. To be more specific, most of the studies consist of experimental works focusing on pre-determined purposes such as environmental monitoring, mechanisms of microplastic degradation, transport, toxicological implications of microplastics, etc. A compilation of these local studies was applied to deducing the investigation concerning a large-scale issue, such as regional or planetary explorations depending on the number and geographical distribution of the individual study cases.
Microplastic pollution has been an issue of concern since decades ago, and its impact on the environment has been repetitively emphasized considering the global distribution and persistence of microplastics. In the literature, many review studies (please refer to the supplementary material for these studies) keep on underlining the long-term implications of microplastics on ecological integrity and environmental quality from a global perspective, showing a future trend of exploring microplastic issues using the framework of planetary boundaries.
The concept of planetary boundary framework was first suggested by Rockström et al.,41 who proposed a framework to address limits to the impacts of human activities on the Earth system, which may not be able to function sustainably beyond these limits. To apply the planetary boundary approach successfully, three key factors need to be considered: (1) the scale of human action about the sustaining capacity of the Earth, (2) the understanding of the essential terrestrial processes including human actions, and (3) the resilience and its links to complex dynamics and self-regulation of living systems. This research approach became well recognized and its application can be found in various databases when addressing global concerning issues, including sustainability, nutrient cycle, water use, and so many others. Also, Steffen et al.42 indicated the importance of cross-scale interactions and the regional-level heterogeneity of the environmental processes when addressing global issues using the framework of planetary boundaries. In their study, two core planetary boundaries (nine boundaries were proposed in their original study)—climate change and biosphere integrity—were identified to have the potential to drive the Earth system into a new state.
However, very few studies (the study by Arp et al.43 is one of the limited examples) have been found for microplastic study from a global perspective. In line with the previous discussion, the framework of planetary boundaries could be an excellent tool and forward-thinking approach for microplastic research. The planetary boundaries framework identifies nine critical Earth system processes that, if crossed, could lead to irreversible and detrimental environmental changes. In short, the framework of planetary boundaries advocates the philosophy of limits-to-grow, limiting the environmental stresses to support the system growth of the Earth. As we can observe nowadays, many global issues of the environment prevail (e.g., climate change, nutrient cycles, water supply, the impact of atmospheric aerosol, etc.), not being confined within one identified planetary boundary. Therefore, cross-discipline thinking coupled with a planetary boundary framework could be attractive when tackling the global environmental issue of microplastics.
Additionally, solving microplastic issues requires diverse expertise. With the implementation of the planetary boundary approach, collaboration among experts from different fields, such as ecology, chemistry, geology, and social sciences could be fostered. While the use of the planetary boundary approach holds much promise, challenges exist in implementing it effectively. Obtaining precise data on microplastics’ distribution, behavior, and long-term impacts is demanding due to their ubiquity and minute size.
Impact of climate change on microplastic’s fate
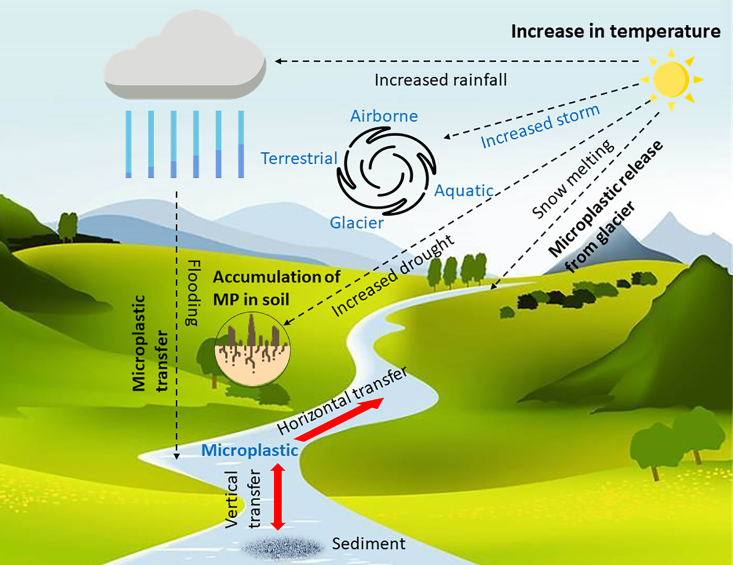
The rise in atmospheric temperature leads to extreme weather events such as glacier thawing, gusty wind, augmented thunderstorms, severe drought, and increased rainfall.1 In the case of glacier thawing, the microplastic stored in the ice would be released into the sea,18 implying that the microplastics with a density lower than that of the surrounding water tend to be carried away by ocean currents and redistributed over long distances, thus carrying the microplastics released from the melted snow into the aquatic habitat. In addition, once the microplastic reaches the freshwater zones, it will have a higher probability to sink to the bottom because of the smaller density of fresh water compared to saline waters. In the case of increased rainfall, coastal flooding would occur in addition to the sea-level rise as a result of glacier thawing,1 which implies that the plastic debris/microplastics will be prone to be flushed from the shorelines into the water bodies. As a consequence, the plastic debris would be further subjected to mechanical weathering by ocean currents, resulting in the formation of microplastics.6 The microplastics may undergo further degradation or storage in the aquatic habitat. Therefore, climate change shall not only impact the fate of microplastic in the environment but also facilitate the degradation of environmental microplastics. Most of the literature studies on microplastics discuss the effect of microplastics on aquatic or soil biota; however, this manuscript takes a step further to discuss the ecotoxicological impact of microplastics on the environment under the influence of climate change.
As a result of ice melting that increases the velocity of oceanic currents, the gusty wind and augmented thunderstorms under the influence of temperature rise will impact the fate of microplastic as well. The changes in the wind pattern may alter the surface water movement, thus redistributing the microplastics away from the source of plastic debris. This phenomenon makes it challenging to understand the ultimate distribution of microplastic in the environmental matrix. In addition to the horizontal surface water movement, strong wind events also cause vertical mixing of the water,44,45 resuspending the settled microplastics in the seabed to the water surface. Floods, storms, and extreme weather events can lead to the spreading of microplastics to areas without plastic pollution, exposing pollution-free zones to microplastics. Microplastics are not only translocated from rivers to oceans46 but also from aquatic regions to terrestrial soils.47 Increased global temperature results in droughts that may entrap microplastics in soil.48 Therefore, assessing the risk posed by microplastics necessitates understanding the impact of each of these climate change parameters on the fate of microplastics, as discussed in the following sections. Table 2 summarizes the important works on climate change-related microplastic studies, and Figure 3 represents a schematic of the fate of microplastics under the influence of climate change parameters.
Table 2.
Summarized results of microplastic-climate change studies
| Location | Sample collection | Climatic factor | Analysis | Result summary | Reference |
|---|---|---|---|---|---|
| Sanggou Bay (China) | Seawater, sediments, and cultured oysters (Crassostrea gigas). | Typhoon | The seawater was treated with sodium hydroxide to remove the organic matter, followed by sieving and drying the collected MP at 60°C. The sediments were dried at 60°C, followed by treatment with hydrogen peroxide to remove the organic matter, and suspended in saturated sodium chloride to separate the MP by density difference method and dried on a Petri dish at 60°C (two-step extraction method). The soft tissues of the oysters, including the digestive tract were removed and MP was extracted using the two-step extraction method. MP was characterized using a microscope, FTIR, and SEM. | MP concentration in the seawater and sediments increased by 40% after the occurrence of typhoons. This implies the impact of climatic factors on the fate of MP. MP of size 0.05–0.1 mm was found in the tissues extracted from oysters, which is smaller than the size prior to the typhoon (0.1–0.5 mm). MPs were characterized as PP, PS, and PET. SEM scan shows the presence of heavy metals in the oysters’ tissues, suggesting the possibility of MP acting as carriers of heavy metals and enhancing their assimilation by marine organisms. | Wang et al.73 |
| Northern Gulf of Mexico | Sandy sediments were collected from coastal nesting sites for turtles (n = 10). | Temperature | MP was recovered from the sand samples using the density separation method using a saturated salt solution. MP was characterized based on shape, size, and color. | The presence of MP and high temperature have a negative effect on the incubating nests and hatching process for turtles. | Beckwith et al.36 |
| Mallnow, Lebus (Germany) | Sandy loam soil was used for the experimental design using polyacrylic fibers. | Temperature | Water stable aggregate (%) of the soil was determined. | This laboratory study confirmed that the presence of MP fibers and increased temperature (28°C compared to the control of 25°C) decreased the percentage of water-stable aggregates, resulting in poor soil quality. | Liang et al.79 |
| Laboratory experiment (Bangladesh) | Nile tilapia fish were exposed to MP polyamide and different temperatures of 30°C, 33°C, and 36°C. | Temperature | The fish samples underwent analysis to assess their hemato-biochemical parameters, identify erythrocytic abnormalities, and examine the histopathology of their gills and intestines. MP was extracted using hydrogen peroxide followed by filtration. MP was characterized under a microscope. | At temperatures greater than 36°C, fish ingested the highest amount of polyamide, and their hemoglobin and RBC number decreased as compared to fish samples not exposed to MP. | Hasan et al.80 |
| Mississippi Sound (Gulf of Mexico) | Water samples (n = 10) | Flood | MPs were extracted using sieving techniques and characterized using fluorescence microscopy and laser direct infrared. | As a result of flooding and freshwater inflow, MP concentration decreased, indicating the horizontal distribution of the MP entrained in the surface water. Oysters, found in the sampling site, were exposed to polyester, acrylates/polyurethanes, polyamide, polypropylene, polyethylene, and polyacetal. | Scircle et al.81 |
| Ho Chi Minh City (Vietnam) | Dry and wet atmospheric 3fallout samples (n = 3) | Wind and Rain | MP was retrieved using the density separation method and characterized using a stereomicroscope and FTIR. | Atmospheric fallouts confirmed the presence of MP (70–900 pieces/m2), indicating the role of wind and rain in the fate of MP. MP was predominated by fibers and sizes ranging from 300 to 5000 μm. | Truong et al.78 |
| Trent University Environmental Wind Tunnel (TEWT), Ontario, (Canada) | Freestream wind speed was sampled. | Wind | An experiment was set up using quartz (mimicking sand found near coastal shores) and soil as the substrate bed, and MP (PE and PS) of different shapes, sizes, and densities were added. The wind velocity was controlled to study the velocity profile and fate of MP. | MP fibers were found to be more prone to transport by wind compared to microbeads. | Bullard et al.82 |
| Shiraz, southwest Iran. | 6 h after the dust storm, dust samples were collected from windscreens or backscreens of 5–10 cars from 22 locations. | Dust storm | The dust samples were sieves, and MPs were extracted using the density separation method with zinc chloride followed by vacuum filtration. The MP was characterized using a microscope, Raman spectrometer, and SEM-EDX. | The majority of particles were fibrous, with an average diameter of approximately 20 μm, and more than 60% of them were shorter than 100 μm in length. HYSPLIT modeling, satellite imagery, and geochemical signatures of regional dust particles indicate that the deserts of Saudi Arabia are the primary distant and transboundary source of microplastics (MPs). This implies that dust storms play a crucial role in the transportation and redistribution of MPs in arid and semi-arid environments. | Abbassi et al.83 |
| Laboratory study (Italy) | The growth of Lepidium sativum (garden cress) was investigated. | Acid rain | Lepidium sativum was exposed to artificial acid rain (pH 4.5 made of sulfuric acid and nitric acid) and PET. The effect of PET and acid rain on the plant was determined in terms of pigment change, antioxidant concentration, and aminolevulinic acid and proline determination. | PET and acid rain negatively affected L. sativum resulting in lower pigment, aminolevulinic acid, and proline production. | Pignattelli et al.84 |
| Beijing, China | Rainfall runoffs and urban pipeline rainwater runoffs were collected from main roads, office complexes, residential areas, and agricultural experiment stations. | Rain | MP was retrieved using digestion with hydrogen peroxide followed by filtration. They were characterized using a stereomicroscope and μ- FTIR. | MP levels in pipeline rainwater runoff varied from 1.6 to 29.6 MP per liter, with 0.7–6.0 MP per liter originating from rainfall, accounting for 24%–77.4% of the total. The majority of MPs were smaller than 1 mm, comprising 38% in rainfall and 44.5% in rainwater runoff. It was concluded that urban pipeline rainwater runoff is a major route for land-based MP transport to surface waters. | Zhang et al.85 |
| Tai Po, Lam Tsuen River, Hong Kong (China) | Water samples. | Rain | MP was retrieved using digestion with hydrogen peroxide followed by sieving. They were characterized using FTIR. | MP concentration in the river surface water was twice the amount found in the coastal sea surface water after a three-day rainfall period, and the microplastic amount decreased to one-tenth after 2 h suggesting the washing off of the microplastic from the river to the coastal sea. | Cheung et al.56 |
| Nam Co Basin (Tibetan Plateau) | Rain fallout, lake water, glacial runoff, and non-glacial runoff. | Wind and Temperature | MP was retrieved using digestion followed by sieving. They were characterized using micro-FTIR. | The water sample contained fragments and fibers of MP. PP was found in the glacial runoff. Air mass trajectory analysis showed that MP can be transported over a distance of 800 km, and glacial and non-glacial runoff could contribute to ∼560 kg of MP. | Dong et al.50 |
| Greenhouse experiment (Germany) | Plant, soil, and fungal samples were collected. | Drought | Onions were grown in sandy loam soil, PS fibers, and arbuscular mycorrhizal fungi. Water treatment was controlled to mimic the drought conditions. Root traits, plant biomass, fungal colonization, and soil aggregation were measured. | The presence of microfibers increased the plant biomass of onion shoots and arbuscular mycorrhizal fungi colonization in soil, favoring root symbionts’ growth. | Lehmann et al.64 |
Figure 3.
Fate of microplastic under the impact of different climate change parameters
Temperature
As stated previously, the parent and degraded plastic waste would be transported by surface runoffs to freshwaters, and thereafter to the oceans.49 An increase in atmospheric temperature would increase plastic degradation in their transport processes. The impact of global warming on microplastic movement was investigated by characterizing microplastics in the rainfall, melting glaciers, as well as non-glacial runoff. One trillion pieces of microplastics at most were estimated to be released from sea ice into the Arctic Ocean in a decade due to ice melting.18 Microplastic pollution in a remote lake basin on the Tibetan plateau resulted from glacial melting and the released microplastics were usually light weighted and small sized.50 Another noted example is the microplastic pollution in the lakes of Svalbard due to the melting of arctic ice.51 Meanwhile, global warming increases the soil temperature of the permafrost as well,52 leading to the release of microplastics into the soil and water bodies due to melting processes.53 The annual freeze-thaw cycle numbers would cause further degradation of the microplastics into nanoplastics due to light irradiation or physical erosion.54 The nanoplastics have smaller particle sizes and are readily absorbed by soil organisms. They would easily percolate into the deep soil layers and the groundwater to affect the soil biogeochemical cycle.53 Also, the rivers originating from the glaciers or snow caps can transfer the microplastics into downstream water bodies. Yang et al.55 reported that an alpine river originating from the Himalayas between China and Nepal contained microplastic in the flow (102–302 microplastics per cubic meter) as well as in its sediment (30–85 microplastic per cubic meter).
Rain
Increased rainfall results in enhanced surface water runoffs that translocate the microplastics from terrestrial soils to the aquatic environment.46 In a climatic event of three-day rainfall, the microplastic concentration in the river surface water was twice the amount found in the coastal seawater in Hong Kong, and the number of microplastics decreased to one-tenth after 2 h, suggesting the microplastics being washed off from the river to the coastal sea.56 Xia et al.57 indicated that microplastic concentration increased from 7.4 microplastic per liter to 30 microplastic per liter (∼4 times higher) in the surface water after heavy rainfall. Rainfall can move atmospheric microplastics into the aquatic environment by trapping them in the raindrops.58 Also, rainfall may affect the hydrodynamics of water flow and resuspend the microplastics in the sediments,59 showing an inverse relationship between sediment and suspended microplastics. Several noted examples of microplastic transport due to rainfall events are summarized in Table 2. As mentioned in a previous section, coral reefs can outgrow microplastics and serve as a sink to store microplastics. Acid rain can lead to coral reef bleaching and increase the release of the stored microplastics, rendering the microplastic redistribution.60
Drought
A laboratory study using a sandy wet-dry cycle in a soil column to mimic rainy-dry seasons confirms the vertical movement of the microplastic, showing a linear relationship between the soil depth and the number of wet-dry cycles.61 However, a field-scale study is required to confirm the laboratory observations. Despite the negative effects on aquatic organisms and soil organisms,20,21,62,63 microplastics have been shown to possess positive effects on plant communities in a study when the soil system suffers from drought.64 Due to limited enzymatic activities in soil, drought can reduce nutrient cycling and microplastics can diminish the nutrient loss by less leaching (∼70%).48 Soil water content is another factor that influences the fate of microplastics. Microplastics can move deep down into the groundwater or be washed off to the surface water bodies in case of heavy rainfall. In the case of drought, microplastics would stay in the soil pores and aggregate.48,65 Such an aggregation phenomenon improves not only the soil water-holding capacity during drought but also the nutrient retention in the soil, increasing their availability for plant uptake. For example, the presence of microfibers increased the plant biomass of onion shoots and arbuscular mycorrhizal fungi colonization in soil, favoring root symbionts’ growth.64 The positive effect of the microplastic in drought might occur on plants like onions, but a negative effect was observed on wheat or grass.65,66 More research is needed to fill this knowledge gap on how certain plants react in the presence of microplastics during drought.
Wind
The atmosphere offers a pathway to move the microplastic over tens of kilometers to distant areas and wind speed facilitates the transport of microplastics from the terrestrial zones to their ultimate sinks,67,68,69 such as the Arctic,70 the Tibetan Plateau,71 French Pyrenees,67 as well as to the urban areas in the western United States69 and European shorelines.72 For example, typhoons in China increased the concentration of microplastics in its neighboring aquatic environment.73 The wind translocated microplastics to the Antarctic ice sink, and the microplastic was considered an airborne pollutant that migrated to distant areas up to the Antarctic snow cap.74 Airborne microplastics are known to have direct radiative effects75 and are a contributing factor to local atmospheric heating or cooling. Their light-absorbing properties enable a further increase in the local atmospheric temperature.76 In the polar regions, airborne microplastics would result in cryosphere melting which released the entrapped microplastics from ice. Dong et al.50 estimated that airborne transport could be the traveling mode of microplastics to the remote lake basin in the Tibetan Plateau (alpine lake environment). There is a positive correlation among wind speed, microplastic transport, and microplastic accumulation in surface water.77 Once reaching remote locations, atmospheric microplastics would travel along with rainfall into ambient waters and snow beds, or reach different cities.78
In the environmental matrix, the aquatic habitat may serve as the primary sink, where microplastics can suspend in the water column, sink to the bedrock, and accumulate in sediments, depending on the properties of the microplastic and environmental conditions. The increased rainfall and wind due to global warming would enhance the water circulation to facilitate microplastic transport to distant aquatic zones such as the Arctic sea. Increased wind velocity leads to the atmospheric transport of microplastics to glacier habitats. Elevated temperature propels the loss of accumulated microplastic from the melted ice caps. Flooding and drought determine the fate of terrestrial soil microplastics which either move between the marine habitat and terrestrial soils (due to flooding) or remain entrapped in the soil (due to drought).
Impact of climate change and microplastic pollution on aquatic and soil organisms
While the impact of climate change on the fate of microplastic in the environment is evident, microplastic pollution and climate change together can have an ecotoxicological effect on aquatic and soil organisms. The impact of microplastics on aquatic and soil organism has been well reported. In the open literature, the investigations by Egbeocha et al.,19 Wright et al.,20 Guzzetti et al.,21 Rillig and Bonkowski,62 and Guo et al.63 are a few of the available studies that reviewed the impact of microplastics on soil and aquatic organisms. Meanwhile, Prata et al.86 explored the impact of airborne microplastics on humans. However, most of these studies did not consider the combined impact of climate change and microplastics on ecotoxicity and the ecosystem.
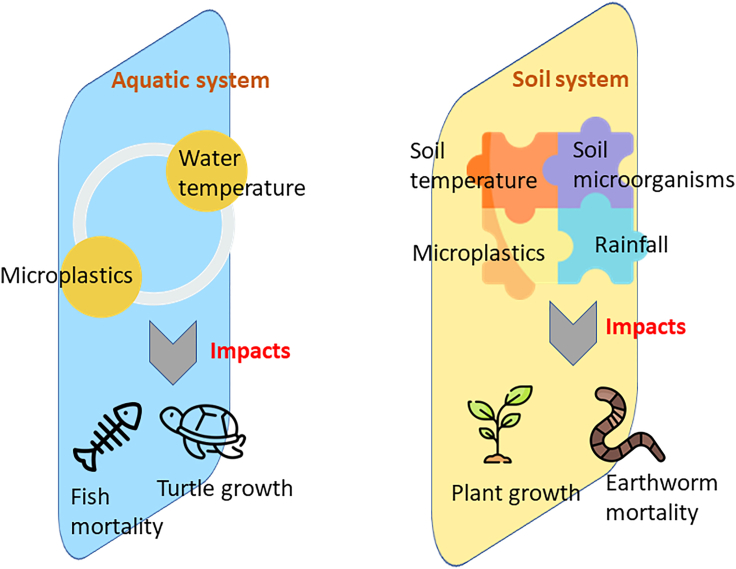
In this section, we presented the perspective regarding the impact of climate change and microplastic on organisms using relevant evidence from the literature, as illustrated in Figure 4. Microplastics reach the Arctic seas, either by airborne transmission or via glacier melting (i.e., various microplastic transportation pathways), exposing the arctic seabirds, fish, and benthic organisms to microplastics.87,88,89 In another scenario, at the temperature of 20°C, the presence of microplastic does not significantly affect the fish (Pomatoschistus microps, a common species found in the estuaries and coastal waters of Northern Europe and the Mediterranean Sea) mortality (8%, not a significant difference). However, as the water temperature rises to 25°C as a result of global warming, 33% of fish mortality occurred due to temperature increase and microplastic presence.90 Seemingly, climate change and microplastic generation may impact the survival of aquatic organisms, which affects the population of aquatic species, either altering their number or pushing them to the edge of extinction. Other noted examples also indicated that the global temperature increase has a cascading effect on marine turtles’ numbers; microplastic increases the dune’s temperature and consequently inhibits turtle growth.36 Microplastics have various mechanisms through which they can raise the temperature of dunes. Firstly, their dark color, typically black or dark brown, enables them to absorb and retain more solar radiation than natural sand particles. The heightened absorption of sunlight results in elevated surface temperatures in areas where microplastics accumulate.91 Secondly, microplastics can modify the physical characteristics of dunes. When mixed with sand, microplastics can alter the surface texture, diminishing the dune’s reflectivity, also known as albedo. A lower albedo means that more solar energy is absorbed rather than reflected into the atmosphere, which further elevates the temperature.92 Additionally, microplastics can influence the thermal conductivity of dune sediments. Compared to natural sand, microplastics possess lower thermal conductivity, which means they are less effective at transferring heat. Consequently, microplastics may act as insulators, trapping heat and causing temperatures to rise within the dune environment.93 The combined effects of enhanced solar absorption, reduced albedo, and modified thermal conductivity resulting from the presence of microplastics collectively contribute to increased temperatures in dunes where these particles are present. Loss of coral reefs due to coral bleaching is also concerning,60 which not only releases the stored microplastic back into the sea but also results in coral extinction.
Figure 4.
Impact of climate change factors and microplastic pollution on aquatic and soil organisms
Biofilm formation on the surface of microplastics is an often-observed phenomenon, where various photosynthetically active bacteria (for example, Rhodobacteraceae spp.) or cyanobacteria grow on the microplastic surface.94 The formation of biofilms depends on environmental parameters such as temperature, light, nutrient availability, water circulation, as well as hydrodynamic conditions.95 In the extreme events of climate change, such as higher temperature, intense light irradiation, and enhanced ocean circulation, the biofilm formation on the microplastic varies, and the carbon-storing capacity of the oceans would be impaired by altering the circulation of organic matter and nutrients.96 Additionally, sediment resuspension due to enhanced ocean recirculation would enhance lake eutrophication by resuspension of the nutrients.25
The effect of global warming and microplastic accumulation in the soil impacts the soil’s biophysical properties such as bulk density and water-holding capacity, which further affects the microbial activities as well as stability to form aggregates.97 Cracking in the soil as the plastic filming increases the rate of evaporation and leads to soil moisture loss,98 affecting the plant’s growth (de Souza Machado et al., 2019).99 Knowing that the effect of microplastic on the soil ecosystem has not been investigated as widely as the effect on the aquatic ecosystem, we discuss the impact of microplastics on the soil environment under the influence of climate change. High microplastic concentration in soil (e.g., greater than 1000 mg per 100 g soil) is likely to inhibit the growth of earthworms.100 When polyethylene terephthalate (PET), a major constituent in plastic bottles and plastic bags, enters the soil system, it can impose chronic toxicity on plants. For example, garden cress (grass) growth was inhibited, along with reduced biomass and leaf number, and unbalanced total chlorophyll content affecting the photosynthesis performing potential of the plant when exposed to PET and acid rain.84
At a higher temperature, the existence of microplastics in the soil can increase aggregate formation in the presence of soil fungi (i.e., Mucor fragilis) as the growth rate of fungi is enhanced, resulting in the release of the binding agents that hold the soil particles together.79 The soil aggregate formation was affected by the fungi in the soil. For example, Chaetomium angustispirale is known to degrade the cellulose in the soil at a higher temperature, reducing aggregation in soil.101 Although the reason why the microplastics do not negatively impact the soil’s aggregate-forming capability of the fungi is not well explored, one probable reason might be that the microplastic, especially the microfiber, can entangle the soil particles together and increase aggregation. The aggregation might serve as a radiative surface to increase the soil temperature and improve fungi growth. However, there is a threshold temperature up to which fungi can produce the optimum amount of binding agents; thereafter, a further increase in soil temperature above the threshold would decrease fungal activity. O’Brien et al.102 employed duckweed as the sample plant to explore the interaction between the plant-microbe-climate change microplastic, using a mixture of microbes from its microbiome, temperature, and carbon dioxide as the climate change parameters, and tire wears leachates as the microplastic. In the absence of microplastics at a higher temperature and carbon dioxide concentration, a positive mutual symbiotic relationship between the microbes and duckweed was observed, and this relationship shifted to negativity in the presence of microplastic. Anyhow, knowledge of the microplastic presence in soil is still needed to draw deeper insights into the interaction among the microplastic, soil, and fungi.102
In summary, the presence of microplastics in the aquatic or terrestrial soil habitat poses a threat to aquatic and soil organisms, and its ecotoxicological effect is enhanced due to changing climate change parameters. The existence of climate change and microplastic pollution together impacts the organisms inherent to the glacier habitat since the microplastics will no longer be entrapped in the ice. The presence of microplastics and climate change impact seems to have a negative ecotoxicological effect on aquatic organisms. However, it may also have a positive effect in some soil habitats, depending on the soil environmental conditions and the presence of certain soil microbes. Therefore, understanding the combined effect of climate change and microplastic pollution on the environment and its biota is of prime importance.
Based on the review presented in this study, major inferences are discussed herein. The aquatic habitat is threatened as a sink for microplastics in the current scenario of climate change due to several factors. Firstly, rising temperatures and increased UV radiation can accelerate the degradation of plastics, leading to the release of more microplastic particles into the aquatic environment. This increases the overall abundance and concentration of microplastics in water bodies. Secondly, climate change can alter ocean currents and circulation patterns, affecting the transport and distribution of microplastics. Changes in currents can result in the accumulation of microplastics in specific areas, such as coastal regions or gyres, posing a higher risk to aquatic organisms in those habitats.
Additionally, extreme weather events like storms and floods, which are becoming more frequent and intense due to climate change, can cause the redistribution of microplastics. These events can transport microplastics over long distances and introduce them to new aquatic ecosystems, increasing the potential for contamination. Furthermore, climate change-induced sea-level rise can exacerbate the problem by bringing more plastic waste from coastal areas into the aquatic environment. Coastal regions are often hotspots for plastic pollution, and as sea levels rise, more plastic debris can enter rivers and estuaries, ultimately reaching the oceans. The combined effects of climate change and microplastic pollution can disrupt the ecological balance of aquatic habitats. Microplastics can be ingested by marine organisms, leading to negative physiological effects, reduced feeding efficiency, and altered reproductive processes. These phenomena can have cascading impacts on the entire aquatic food chain, from primary producers to higher level predators, ultimately threatening the health and biodiversity of the ecosystem. In conclusion, climate change intensifies the threats posed by microplastics to aquatic habitats by accelerating plastic degradation, altering ocean currents, increasing the frequency of extreme weather events, and promoting the influx of plastic waste from coastal areas. Understanding and addressing these challenges are crucial to safeguarding the integrity and resilience of aquatic ecosystems in the face of climate change and microplastic pollution.
Rising temperatures associated with climate change lead to the accelerated melting of glaciers. As glaciers melt, a large quantity of water, along with the microplastics trapped within them, is released into downstream environments, resulting in the redistribution of microplastics from glacier habitats to other ecosystems. Glacier habitats have traditionally acted as sinks for microplastics, trapping them over long periods. However, as glaciers recede due to climate change, the stored microplastics are released, contributing to the contamination of downstream environments such as rivers, lakes, and oceans. The thawing of glaciers also exposes previously buried microplastics, which can further contribute to their release into the environment. These microplastics can be transported by meltwater and carried over long distances, potentially reaching ecologically sensitive areas. The implications of microplastic release from melting glaciers are concerning for both the environment and organisms that inhabit these ecosystems. Microplastics can negatively impact aquatic organisms, including fish, invertebrates, and other wildlife, through ingestion, disrupting their feeding habits, reproductive systems, and overall health. Therefore, the accelerated melting of glaciers driven by climate change not only threatens the integrity of these habitats but also exacerbates the distribution of microplastics, amplifying the environmental challenges posed by plastic pollution.
Based on the review presented in this study, it was inferred that the terrestrial habitat is impacted as a sink for microplastics in several ways. Firstly, the increased frequency and intensity of extreme weather events, such as heavy rainfall and flooding, can result in the transport of microplastics from land to water bodies. The microplastics that were originally present on land can be carried by runoff and enter rivers, lakes, and oceans. Secondly, rising temperatures can accelerate the degradation of plastic in terrestrial environments. As temperatures increase, the physical breakdown of plastics through thermal degradation can occur more rapidly. This can lead to the fragmentation of larger plastic items into smaller microplastic particles, which can then be carried by wind or water currents and transported to different locations.
Furthermore, changes in precipitation patterns and drought conditions associated with climate change can affect the distribution and transport of microplastics in terrestrial habitats. During dry periods, microplastics may accumulate in soil and sediment, making them more susceptible to wind erosion and subsequent transport to other areas. Conversely, heavy rainfall events can cause soil erosion and the release of microplastics into water bodies. Additionally, climate change can alter the behavior and distribution of organisms in terrestrial ecosystems, which indirectly affect the fate of microplastics by influencing the interactions between microplastics and biota. Overall, the combination of increased transport through runoff, accelerated degradation, changes in precipitation patterns, and shifts in ecosystem dynamics poses a significant threat to the terrestrial habitat as a sink for microplastics in the current scenario of climate change.
Outlook on priority research directions
From the discussion previously, it is evident that microplastic pollution and climate change impacts are inherently linked. Therefore, future research direction should focus on their synergistic impact on the environment. Microplastics are ubiquitous, finding their way from the terrestrial systems to aquatic and marine systems, and eventually, reaching the Arctic seas and remote snow caps via oceanic and atmospheric circulations. The fate and distribution of microplastic seem to be governed by climatic factors (e.g., temperature, rainfall, drought, and wind) and microplastic composition (i.e., type, size, density, and chemical composition). A comprehensive understanding of these determinants across the environmental matrices as well as their magnitude of effect is important when assessing the fate of microplastic under ever-changing environmental scenarios.
Here are the prioritized research directions concluded from the review presented in this study.
-
•
With the increasing impact of climate change on the environment, monitoring the fate of microplastics in various habitats becomes critical. Since aquatic habitat is the primary sink for microplastics, understanding the mechanism governing the distribution of microplastics in the aquatic ecosystem should be one of the top research priorities. Laboratory-scale studies can be conducted to verify the horizontal and vertical distribution of microplastics within the aquatic habitat.
-
•
As global warming continues to be a significant threat to the earth, many actions have been taken to curb the influence of climate change, such as adopting renewable sources of energy or sustainable use of land.103 However, glacier melting and the release of microplastics are still inevitable due to the increase in global temperatures, since glacier snow has been identified as another ultimate sink for microplastics. Therefore, monitoring microplastic redistribution from melting ice caps should be conducted as well. Similarly, future research on terrestrial soil habitat should also include monitoring microplastics in the pedosphere. Understanding the fate of microplastic in the soil as well as its impact on soil biota, especially plants, is important to prevent microplastic from reaching humans via the food chain.
-
•
Though we have discussed the possible routes of microplastic transport in this manuscript, there are limited experimental studies to confirm the transport pathway through water, soil, or air.104 Experimental studies on the fate of microplastics should be encouraged. Laboratory-scale experimental designs can include water column or soil media studies to understand the fate of microplastic in the habitats. Also, a closed air-box system can be designed to understand the impact of wind speed and flow direction on microplastics. These experiments can further be assembled into a complex system to understand the interactions between the three environmental media (i.e., water, soil, and air) on microplastic fate. Parameters such as precipitation rate, wind velocity, soil types, and types of microplastics can be manipulated to examine the interactions between these factors. The integrated experimental system would provide knowledge on the widespread dispersal of microplastic not only to the remote glacier regions but also to distinct areas which are not even susceptible to plastic pollution. Laboratory-scale experiments can verify concepts, but their validation needs to be confirmed at the field scale. Therefore, field environmental monitoring should be conducted as well.
-
•
Another prioritized research direction should encourage studies on the biological and chemical interactions of the microplastic with their environmental matrix (aquatic and terrestrial habitats). The aquatic and soil biota, primarily microorganisms, can attach to the microplastics and excrete extracellular polysaccharides that lead to microplastic aggregation, resulting in microplastic sinking to the seabed or getting retained in the soil. Experimental studies should be conducted to explore the mechanisms for the above-mentioned phenomena. Additionally, the parameters for risk assessment of microplastic exposure to aquatic and soil organisms need to be explored under the influence of climate change.
-
•
Integrating planetary boundaries into microplastic research is crucial for a comprehensive understanding of the environmental implications and effective management of microplastics.43 Planetary boundaries define the limits within which human activities should operate to maintain a stable and resilient Earth system. By incorporating these boundaries into microplastic research, we can ensure that our efforts align with broader sustainability goals and minimize the negative impacts on the environment. These boundaries encompass various dimensions such as climate change, biodiversity loss, freshwater use, land system change, and chemical pollution. Therefore, future research on microplastics should acknowledge the interconnections between different planetary boundaries and consider the potential trade-offs and synergies addressing microplastic pollution and other environmental challenges. Taking a holistic approach, researchers should incorporate the concept of planetary boundaries into the design of their experiments and investigations. This involves recognizing the specific environmental dimensions and impacts associated with microplastics and assessing how they align with the planetary boundaries framework. For example, researchers should evaluate the potential chemical pollution caused by microplastics and examine its implications in relation to the boundary related to chemical pollution. Furthermore, it is crucial to develop methodologies that allow for the quantification of the impacts of microplastics within the identified planetary boundaries. This could include assessing the concentration and distribution of microplastics in different environmental compartments, evaluating their effects on biodiversity and ecosystem functioning, and considering their contribution to chemical pollution.
In summary, the following research directions could be pursued.
-
•
Investigate the long-term trends and patterns of microplastic pollution in different environmental matrices (e.g., water, soil, air) under various climate change scenarios to understand the magnitude and trajectory of microplastic contamination.
-
•
Explore the role of microplastics as the carriers and vectors of other pollutants in the environment, and assess how climate change influences the bioavailability and transfer of these pollutants through interactions with microplastics.
-
•
Examine the potential effects of microplastics on ecosystem functioning and services under different climate change scenarios, including impacts on nutrient cycling, carbon sequestration, and overall ecosystem resilience.
-
•
Develop predictive modeling frameworks to assess the combined impacts of microplastic pollution and climate change on designated ecosystems and vulnerable species, considering both direct and indirect effects.
-
•
Investigate innovative technologies for the detection, monitoring, and removal of microplastics in various environmental compartments, considering the influence of climate change on the effectiveness of these methods.
-
•
Assess the economic and societal implications of microplastic pollution and climate change interactions, including the costs of mitigation, remediation, and potential damages to ecosystem services, human health, and industry’s reliance on natural resources.
-
•
Explore the social and cultural dimensions of microplastic pollution and climate change, including public awareness, perception, and behavior toward plastic consumption, waste management, and climate change adaptation.
-
•
Examine the effectiveness of policy interventions and regulations in addressing microplastic pollution and mitigating climate change impacts, and identify opportunities for improved governance and international cooperation.
-
•
Foster interdisciplinary research collaborations to integrate ecological, environmental, and social sciences, as well as engineering and technology, to develop holistic solutions for addressing the intertwined challenges of microplastic pollution and climate change.
These research directions can move the efforts toward a comprehensive understanding of the complex interactions between microplastic pollution and climate change, ultimately informing strategies and actions to mitigate their combined impacts on the environment. These recommended prioritized research directions should be able to provide deeper insights into the impact of climate change on the fate of microplastic among its various habitats, as well as its synergistic ecotoxicological effects.
To study the effects of climate change on plastics, particularly in extreme conditions, researchers can employ accelerated weathering experiments using model microplastics. In these experiments, the researchers simulate the environmental conditions associated with climate change to understand how plastics behave and interact with the surrounding environment.
-
•
Choose plastic materials commonly found in the environment, such as polyethylene or polypropylene, as they exist in a significant portion of environmental plastic pollution. The size, shape, and composition of the plastic samples should be considered to ensure they resemble real-world scenarios.
-
•
Create controlled environments that simulate extreme conditions associated with climate change. Factors to consider include temperature, UV radiation levels, humidity, and exposure to air or water. These conditions can be achieved using environmental chambers, solar simulators, temperature-controlled incubators, or outdoor exposure setups.
-
•
Decide on the duration of exposure to extreme conditions, considering the accelerated nature of the research. This involves the selection of an appropriate time frame that reflects the anticipated effects of long-term climate change within a feasible experimental period.
-
•
Assess the physical and chemical changes occurring in the plastic samples under extreme conditions. This may include measuring degradation rates, changes in mechanical properties, fragmentation, surface morphology alterations, and chemical breakdown of the plastic materials. Techniques such as spectroscopy, microscopy, thermal analysis, and mechanical testing can be used for characterization.
-
•
Investigate the environmental impacts of plastic under extreme conditions. This includes studying the leaching of additives, the release of microplastics, and their interactions with organisms and ecosystems. The investigation may include conducting toxicity assessments, evaluating biological effects, and observing ecological changes caused by plastic exposure in simulated extreme conditions.
-
•
Compare the results obtained from the simulated extreme conditions with observations from real-world environments experiencing climate change. This helps validate the relevance and accuracy of the research findings and provides insights into the potential effects of climate change on plastics in different contexts.
By following these steps, researchers can effectively mimic the effects of climate change on plastics and gain valuable insights into the behavior, degradation, and environmental consequences of plastics under extreme conditions. This knowledge can inform strategies for mitigating plastic pollution and guide policy-making efforts aimed at addressing the challenges posed by climate change and plastic waste.
Acknowledgments
This work is supported by the Ministry of Science and Technology, Taiwan under the grant numbers MOST 108-2313-B-002-026 and 109-2313-B-002 -049 -MY2.
Author contributions
F.H.: Formal analysis, Investigation, Writing-Original Draft, Writing-Review & Editing, Visualization. C.F.: Conceptualization, Methodology, Project administration, Writing-Original Draft, Writing-Review & Editing.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107649.
Supplemental information
References
- 1.IPCC . In: IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Pörtner H.-O., Roberts D.C., Masson-Delmotte V., Zhai P., Tignor M., Poloczanska E., Mintenbeck K., Alegría A., Nicolai M., Okem A., et al., editors. 2019. In press. [Google Scholar]
- 2.NOAA National Centers for Environmental Information State of the Climate: Global Climate Report for January 2022. 2022. https://www.ncdc.noaa.gov/sotc/global/202201 retrieved on April 1, 2022 from.
- 3.Plastics Europe E.P. Plastics—The Facts 2019. An Analysis of European Plastics Production, Demand and Waste Data. Plast Eur. 2016 [Google Scholar]
- 4.Shanmugam V., Das O., Neisiany R.E., Babu K., Singh S., Hedenqvist M.S., Berto F., Ramakrishna S. Polymer recycling in additive manufacturing: an opportunity for the circular economy. Mater. Circ. Econ. 2020;2:11. [Google Scholar]
- 5.Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 6.Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parashar N., Hait S. Plastics in the time of COVID-19 pandemic: protector or polluter? Sci. Total Environ. 2021;759 doi: 10.1016/j.scitotenv.2020.144274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrady A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017;119:12–22. doi: 10.1016/j.marpolbul.2017.01.082. [DOI] [PubMed] [Google Scholar]
- 9.Zhang K., Hamidian A.H., Tubić A., Zhang Y., Fang J.K., Wu C., Lam P.K. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021;274 doi: 10.1016/j.envpol.2021.116554. [DOI] [PubMed] [Google Scholar]
- 10.Huang D., Tao J., Cheng M., Deng R., Chen S., Yin L., Li R. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard Mater. 2021;407 doi: 10.1016/j.jhazmat.2020.124399. [DOI] [PubMed] [Google Scholar]
- 11.Mateos-Cárdenas A., van Pelt F.N.A.M., O'Halloran J., Jansen M.A.K. Adsorption, uptake and toxicity of micro- and nanoplastics: Effects on terrestrial plants and aquatic macrophytes. Environ. Pollut. 2021;284 doi: 10.1016/j.envpol.2021.117183. [DOI] [PubMed] [Google Scholar]
- 12.Browne M.A. Sources and pathways of microplastics to habitats. Marine anthropogenic litter. 2015:229–244. [Google Scholar]
- 13.Luo W., Su L., Craig N.J., Du F., Wu C., Shi H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ. Pollut. 2019;246:174–182. doi: 10.1016/j.envpol.2018.11.081. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q., Bauden M., Andersson R., Hu D., Marko-Varga G., Xu J., Sasor A., Dai H., Pawłowski K., Said Hilmersson K., et al. The distribution and characteristics of microplastics in coastal beaches and mangrove wetlands. J. Transl. Med. 2020;18:77–92. doi: 10.1186/s12967-020-02254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamweru P.K., Ndiritu F.G., Kinyanjui T.K., Muthui Z.W., Ngumbu R.G., Odhiambo P.M. Study of temperature and UV wavelength range effects on degradation of photo-irradiated polyethylene films using DMA. J. Macromol. Sci., Part B. 2011;50(7):1338–1349. [Google Scholar]
- 16.Cooper D.A., Corcoran P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai. Mar. Pollut. Bull. 2010;60:650–654. doi: 10.1016/j.marpolbul.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Lee Q.Y., Li H. Photocatalytic degradation of plastic waste: a mini review. Micromachines. 2021;12:907. doi: 10.3390/mi12080907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obbard R.W., Sadri S., Wong Y.Q., Khitun A.A., Baker I., Thompson R.C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth's Future. 2014;2:315–320. [Google Scholar]
- 19.Egbeocha C.O., Malek S., Emenike C.U., Milow P. Feasting on microplastics: ingestion by and effects on marine organisms. Aquat. Biol. 2018;27:93–106. [Google Scholar]
- 20.Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Guzzetti E., Sureda A., Tejada S., Faggio C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018;64:164–171. doi: 10.1016/j.etap.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Welden N.A., Lusher A.L. Impacts of changing ocean circulation on the distribution of marine microplastic litter. Integrated Environ. Assess. Manag. 2017;13:483–487. doi: 10.1002/ieam.1911. [DOI] [PubMed] [Google Scholar]
- 23.Ford H.V., Jones N.H., Davies A.J., Godley B.J., Jambeck J.R., Napper I.E., Suckling C.C., Williams G.J., Woodall L.C., Koldewey H.J. The fundamental links between climate change and marine plastic pollution. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150392. [DOI] [PubMed] [Google Scholar]
- 24.Li W., Li X., Tong J., Xiong W., Zhu Z., Gao X., Li S., Jia M., Yang Z., Liang J. Effects of environmental and anthropogenic factors on the distribution and abundance of microplastics in freshwater ecosystems. Sci. Total Environ. 2023;856 doi: 10.1016/j.scitotenv.2022.159030. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Liang J., Zeng G., Tang W., Lu Y., Luo Y., Xing W., Tang N., Ye S., Li X., Huang W. How climate change and eutrophication interact with microplastic pollution and sediment resuspension in shallow lakes: A review. Sci. Total Environ. 2020;705:135979. doi: 10.1016/j.scitotenv.2019.135979. [DOI] [PubMed] [Google Scholar]
- 26.Huang W., Song B., Liang J., Niu Q., Zeng G., Shen M., Deng J., Luo Y., Wen X., Zhang Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124187. [DOI] [PubMed] [Google Scholar]
- 27.Horton A.A., Dixon S.J. Microplastics: An introduction to environmental transport processes. WIREs Water. 2018;5:e1268. [Google Scholar]
- 28.Brahney J., Mahowald N., Prank M., Cornwell G., Klimont Z., Matsui H., Prather K.A. Constraining the atmospheric limb of the plastic cycle. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2020719118. e2020719118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haque F., Fan C. Fate and Impacts of Microplastics in the Environment: Hydrosphere, Pedosphere, and Atmosphere. Environments. 2023;10:70. [Google Scholar]
- 30.Lin Z., Jin T., Zou T., Xu L., Xi B., Xu D., He J., Xiong L., Tang C., Peng J., et al. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022;304 doi: 10.1016/j.envpol.2022.119159. [DOI] [PubMed] [Google Scholar]
- 31.Kawai F. In: Encyclopedia of Polymeric Nanomaterials. Kobayashi S., Müllen K., editors. Springer; 2015. Biodegradation of Polymers (Bioassimilation, Biomineralization, Biodisintegration, Compost), Overview. [Google Scholar]
- 32.Shah A.A., Hasan F., Hameed A., Ahmed S. Biological degradation of plastics: a comprehensive review. Biotechnol. Adv. 2008;26:246–265. doi: 10.1016/j.biotechadv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Singh B., Sharma N. Mechanistic implications of plastic degradation. Polym. Degrad. Stabil. 2008;93:561–584. [Google Scholar]
- 34.Shen M., Huang W., Chen M., Song B., Zeng G., Zhang Y. (Micro) plastic crisis: un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020;254 [Google Scholar]
- 35.Horton A.A., Barnes D.K. Microplastic pollution in a rapidly changing world: Implications for remote and vulnerable marine ecosystems. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.140349. [DOI] [PubMed] [Google Scholar]
- 36.Beckwith V.K., Fuentes M.M.P.B. Microplastic at nesting grounds used by the northern Gulf of Mexico loggerhead recovery unit. Mar. Pollut. Bull. 2018;131:32–37. doi: 10.1016/j.marpolbul.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Torikai A., Takeuchi A., Nagaya S., Fueki K. Photodegradation of polyethylene: Effect of crosslinking on the oxygenated products and mechanical properties. Polym. Photochem. 1986;7:199–211. [Google Scholar]
- 38.Hocker S., Rhudy A.K., Ginsburg G., Kranbuehl D.E. Polyamide hydrolysis accelerated by small weak organic acids. Polymer. 2014;55:5057–5064. [Google Scholar]
- 39.Krupa S.V., Manning W.J. Atmospheric ozone: formation and effects on vegetation. Environ. Pollut. 1988;50:101–137. doi: 10.1016/0269-7491(88)90187-x. [DOI] [PubMed] [Google Scholar]
- 40.Gewert B., Plassmann M.M., MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts. 2015;17:1513–1521. doi: 10.1039/c5em00207a. [DOI] [PubMed] [Google Scholar]
- 41.Rockström J., Steffen W., Noone K., Persson A., Chapin F.S., III, Lambin E., Lenton T.M., Scheffer M.F.C., Schellnhuber H.J., et al. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecol. Soc. 2009;12:32. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 42.Steffen W., Richardson K., Rockström J., Cornell S.E., Fetzer I., Bennett E.M., Biggs R., Carpenter S.R., de Vries W., de Wit C.A., et al. Planetary boundaries: Guiding human development on a changing planet. Science. 2015;347 doi: 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- 43.Arp H.P.H., Kühnel D., Rummel C., MacLeod M., Potthoff A., Reichelt S., Rojo-Nieto E., Schmitt-Jansen M., Sonnenberg J., Toorman E., Jahnke A. Weathering plastics as a planetary boundary threat: exposure, fate, and hazards. Environ. Sci. Technol. 2021;55:7246–7255. doi: 10.1021/acs.est.1c01512. [DOI] [PubMed] [Google Scholar]
- 44.Kukulka T., Proskurowski G., Morét-Ferguson S., Meyer D.W., Law K.L. The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 2012;39 [Google Scholar]
- 45.Reisser J., Slat B., Noble K., Du Plessis K., Epp M., Proietti M., de Sonneville J., Becker T., Pattiaratchi C. The vertical distribution of buoyant plastics at sea. Biogeosci. Discuss. 2014;11 [Google Scholar]
- 46.Napper I.E., Baroth A., Barrett A.C., Bhola S., Chowdhury G.W., Davies B.F.R., Duncan E.M., Kumar S., Nelms S.E., Hasan Niloy M.N., et al. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021;274 doi: 10.1016/j.envpol.2020.116348. [DOI] [PubMed] [Google Scholar]
- 47.Allen S., Allen D., Moss K., Le Roux G., Phoenix V.R., Sonke J.E. Examination of the ocean as a source for atmospheric microplastics. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lozano Y.M., Aguilar-Trigueros C.A., Onandia G., Maaß S., Zhao T., Rillig M.C. Effects of microplastics and drought on soil ecosystem functions and multifunctionality. J. Appl. Ecol. 2021;58:988–996. [Google Scholar]
- 49.Gündoğdu S., Çevik C., Ayat B., Aydoğan B., Karaca S. How microplastics quantities increase with flood events? An example from Mersin Bay NE Levantine coast of Turkey. Environ. Pollut. 2018;239:342–350. doi: 10.1016/j.envpol.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 50.Dong H., Wang L., Wang X., Xu L., Chen M., Gong P., Wang C. Microplastics in a Remote Lake Basin of the Tibetan Plateau: Impacts of Atmospheric Transport and Glacial Melting. Environ. Sci. Technol. 2021;55:12951–12960. doi: 10.1021/acs.est.1c03227. [DOI] [PubMed] [Google Scholar]
- 51.Luoto T.P., Rantala M.V., Kivilä E.H., Nevalainen L., Ojala A.E. Biogeochemical cycling and ecological thresholds in a High Arctic lake (Svalbard) Aquat. Sci. 2019;81:1–6. [Google Scholar]
- 52.Teufel B., Sushama L. Abrupt changes across the Arctic permafrost region endanger northern development. Nat. Clim. Change. 2019;9:858–862. [Google Scholar]
- 53.Chen X., Huang G., Gao S., Wu Y. Effects of permafrost degradation on global microplastic cycling under climate change. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- 54.Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- 55.Yang L., Luo W., Zhao P., Zhang Y., Kang S., Giesy J.P., Zhang F. Microplastics in the Koshi River, a remote alpine river crossing the Himalayas from China to Nepal. Environ. Pollut. 2021;290 doi: 10.1016/j.envpol.2021.118121. [DOI] [PubMed] [Google Scholar]
- 56.Cheung P.K., Hung P.L., Fok L. River microplastic contamination and dynamics upon a rainfall event in Hong Kong, China. Environ. Process. 2019;6:253–264. [Google Scholar]
- 57.Xia W., Rao Q., Deng X., Chen J., Xie P. Rainfall is a significant environmental factor of microplastic pollution in inland waters. Sci. Total Environ. 2020;732:139065. doi: 10.1016/j.scitotenv.2020.139065. [DOI] [PubMed] [Google Scholar]
- 58.Abbasi S. Microplastics washout from the atmosphere during a monsoon rain event. Journal of Hazardous Materials Advances. 2021;4 [Google Scholar]
- 59.Baldwin A.K., Corsi S.R., Mason S.A. Plastic debris in 29 Great Lakes tributaries: relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016;50:10377–10385. doi: 10.1021/acs.est.6b02917. [DOI] [PubMed] [Google Scholar]
- 60.Haapkylä J., Unsworth R.K.F., Flavell M., Bourne D.G., Schaffelke B., Willis B.L. Seasonal rainfall and runoff promote coral disease on an inshore reef. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Connor D., Pan S., Shen Z., Song Y., Jin Y., Wu W.M., Hou D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019;249:527–534. doi: 10.1016/j.envpol.2019.03.092. [DOI] [PubMed] [Google Scholar]
- 62.Rillig M.C., Bonkowski M. Microplastic and soil protists: a call for research. Environ. Pollut. 2018;241:1128–1131. doi: 10.1016/j.envpol.2018.04.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo J.J., Huang X.P., Xiang L., Wang Y.Z., Li Y.W., Li H., Cai Q.Y., Mo C.H., Wong M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020;137 doi: 10.1016/j.envint.2019.105263. [DOI] [PubMed] [Google Scholar]
- 64.Lehmann A., Leifheit E.F., Feng L., Bergmann J., Wulf A., Rillig M.C. Microplastic fiber and drought effects on plants and soil are only slightly modified by arbuscular mycorrhizal fungi. Soil Ecol. Lett. 2020;4:32–44. [Google Scholar]
- 65.Lozano Y.M., Rillig M.C. Effects of microplastic fibers and drought on plant communities. Environ. Sci. Technol. 2020;54:6166–6173. doi: 10.1021/acs.est.0c01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boots B., Russell C.W., Green D.S. Effects of microplastics in soil ecosystems: above and below ground. Environ. Sci. Technol. 2019;53:11496–11506. doi: 10.1021/acs.est.9b03304. [DOI] [PubMed] [Google Scholar]
- 67.Allen S., Allen D., Phoenix V.R., Le Roux G., Durántez Jiménez P., Simonneau A., Binet S., Galop D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019;12:339–344. [Google Scholar]
- 68.Zhang Y., Gao T., Kang S., Sillanpää M. Importance of atmospheric transport for microplastics deposited in remote areas. Environ. Pollut. 2019;254 doi: 10.1016/j.envpol.2019.07.121. [DOI] [PubMed] [Google Scholar]
- 69.Brahney J. United States. 2020 June. 2017-2019. Wet and dry plastic deposition data for western US National Atmospheric Deposition Program sites. [DOI] [Google Scholar]
- 70.Bergmann M., Mützel S., Primpke S., Tekman M.B., Trachsel J., Gerdts G. Mützel Sophia, Primpke Sebastian, Mine B. Tekman, Jürg Trachsel, Gunnar Gerdts, White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019;5:eaax1157. doi: 10.1126/sciadv.aax1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y., Gao T., Kang S., Allen S., Luo X., Allen D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143634. [DOI] [PubMed] [Google Scholar]
- 72.Browne M.A., Galloway T.S., Thompson R.C. Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010;44:3404–3409. doi: 10.1021/es903784e. [DOI] [PubMed] [Google Scholar]
- 73.Wang J., Lu L., Wang M., Jiang T., Liu X., Ru S. Typhoons increase the abundance of microplastics in the marine environment and cultured organisms: a case study in Sanggou Bay, China. Sci. Total Environ. 2019;667:1–8. doi: 10.1016/j.scitotenv.2019.02.367. [DOI] [PubMed] [Google Scholar]
- 74.Aves A.R., Revell L.E., Gaw S., Ruffell H., Schuddeboom A., Wotherspoon N.E., LaRue M., McDonald A.J. First evidence of microplastics in Antarctic snow. Cryosphere. 2022;16:2127–2145. [Google Scholar]
- 75.Revell L.E., Kuma P., Le Ru E.C., Somerville W.R.C., Gaw S. Direct radiative effects of airborne microplastics. Nature. 2021;598:462–467. doi: 10.1038/s41586-021-03864-x. [DOI] [PubMed] [Google Scholar]
- 76.Evangeliou N., Grythe H., Klimont Z., Heyes C., Eckhardt S., Lopez-Aparicio S., Stohl A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020;11:3381. doi: 10.1038/s41467-020-17201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jong M.C., Tong X., Li J., Xu Z., Chng S.H.Q., He Y., Gin K.Y.H. Microplastics in equatorial coasts: Pollution hotspots and spatiotemporal variations associated with tropical monsoons. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127626. [DOI] [PubMed] [Google Scholar]
- 78.Truong T.N.S., Strady E., Kieu-Le T.C., Tran Q.V., Le T.M.T., Thuong Q.T. Microplastic in atmospheric fallouts of a developing Southeast Asian megacity under tropical climate. Chemosphere. 2021;272:129874. doi: 10.1016/j.chemosphere.2021.129874. [DOI] [PubMed] [Google Scholar]
- 79.Liang Y., Lehmann A., Ballhausen M.B., Muller L., Rillig M.C. Increasing temperature and microplastic fibers jointly influence soil aggregation by saprobic fungi. Front. Microbiol. 2019;10:2018. doi: 10.3389/fmicb.2019.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hasan J., Siddik M.A., Ghosh A.K., Mesbah S.B., Sadat M.A., Shahjahan M. Increase in temperature increases ingestion and toxicity of polyamide microplastics in Nile tilapia. Chemosphere. 2023;327 doi: 10.1016/j.chemosphere.2023.138502. [DOI] [PubMed] [Google Scholar]
- 81.Scircle A., Cizdziel J.V., Tisinger L., Anumol T., Robey D. Occurrence of microplastic pollution at oyster reefs and other coastal sites in the Mississippi Sound, USA: impacts of freshwater inflows from flooding. Toxics. 2020;8:35. doi: 10.3390/toxics8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bullard J.E., Ockelford A., O'Brien P., McKenna Neuman C. Preferential transport of microplastics by wind. Atmos. Environ. 2021;245 [Google Scholar]
- 83.Abbasi S., Rezaei M., Ahmadi F., Turner A. Atmospheric transport of microplastics during a dust storm. Chemosphere. 2022;292 doi: 10.1016/j.chemosphere.2021.133456. [DOI] [PubMed] [Google Scholar]
- 84.Pignattelli S., Broccoli A., Piccardo M., Terlizzi A., Renzi M. Effects of polyethylene terephthalate (PET) microplastics and acid rain on physiology and growth of Lepidium sativum. Environ. Pollut. 2021;282:116997. doi: 10.1016/j.envpol.2021.116997. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J., Ding W., Zou G., Wang X., Zhao M., Guo S., Chen Y. Urban pipeline rainwater runoff is an important pathway for land-based microplastics transport to inland surface water: A case study in Beijing. Sci. Total Environ. 2023;861:160619. doi: 10.1016/j.scitotenv.2022.160619. [DOI] [PubMed] [Google Scholar]
- 86.Prata J.C., da Costa J.P., Lopes I., Duarte A.C., Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020;702 doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- 87.Fang C., Zheng R., Zhang Y., Hong F., Mu J., Chen M., Song P., Lin L., Lin H., Le F., Bo J. Microplastic contamination in benthic organisms from the Arctic and sub-Arctic regions. Chemosphere. 2018;209:298–306. doi: 10.1016/j.chemosphere.2018.06.101. [DOI] [PubMed] [Google Scholar]
- 88.Morgana S., Ghigliotti L., Estévez-Calvar N., Stifanese R., Wieckzorek A., Doyle T., Christiansen J.S., Faimali M., Garaventa F. Microplastics in the Arctic: a case study with sub-surface water and fish samples off Northeast Greenland. Environ. Pollut. 2018;242:1078–1086. doi: 10.1016/j.envpol.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Hamilton B.M., Bourdages M.P.T., Geoffroy C., Vermaire J.C., Mallory M.L., Rochman C.M., Provencher J.F. Microplastics around an Arctic seabird colony: particle community composition varies across environmental matrices. Sci. Total Environ. 2021;773 doi: 10.1016/j.scitotenv.2021.145536. [DOI] [PubMed] [Google Scholar]
- 90.Fonte E., Ferreira P., Guilhermino L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016;180:173–185. doi: 10.1016/j.aquatox.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Kang S., Cong Z., Schmale J., Sprenger M., Li C., Yang W., Gao T., Sillanpää M., Li X., et al. Light-absorbing impurities enhance glacier albedo reduction in the southeastern Tibetan Plateau. J. Geophys. Res. Atmos. 2017;122:6915–6933. [Google Scholar]
- 92.Zhang X., Jiao Z., Zhao C., Qu Y., Liu Q., Zhang H., Tong Y., Wang C., Li S., Guo J., et al. Review of Land Surface Albedo: Variance Characteristics, Climate Effect and Management Strategy. Rem. Sens. 2022;14:1382. [Google Scholar]
- 93.Fuentes M.M.P.B., Beckwidth V., Ware M. The effects of microplastic on the thermal profile of sand: implications for marine turtle nesting grounds. Front. Mar. Sci. 2023;10 [Google Scholar]
- 94.Oberbeckmann S., Löder M.G.J., Labrenz M. Marine microplastic-associated biofilms–a review. Environ. Chem. 2015;12:551–562. [Google Scholar]
- 95.Chen X., Xiong X., Jiang X., Shi H., Wu C. Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere. 2019;222:856–864. doi: 10.1016/j.chemosphere.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 96.Shen M., Ye S., Zeng G., Zhang Y., Xing L., Tang W., Wen X., Liu S. Can microplastics pose a threat to ocean carbon sequestration? Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110712. [DOI] [PubMed] [Google Scholar]
- 97.de Souza Machado A.A., Lau C.W., Till J., Kloas W., Lehmann A., Becker R., Rillig M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018;52:9656–9665. doi: 10.1021/acs.est.8b02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wan Y., Wu C., Xue Q., Hui X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019;654:576–582. doi: 10.1016/j.scitotenv.2018.11.123. [DOI] [PubMed] [Google Scholar]
- 99.de Souza Machado A.A., Lau C.W., Kloas W., Bergmann J., Bachelier J.B., Faltin E., Becker R., Görlich A.S., Rillig M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019;53:6044–6052. doi: 10.1021/acs.est.9b01339. [DOI] [PubMed] [Google Scholar]
- 100.Cao D., Wang X., Luo X., Liu G., Zheng H. Vol. 61. IOP Publishing; 2017. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. (InIOP conference series: earth and environmental science). No. 1. [Google Scholar]
- 101.Yadav L.S., Bagool R.G. Isolation and screening of cellulolytic Chaetomium sp. from deteriorated paper samples. International Journal of Current Microbiology and Applied Science. 2015;4:629–635. [Google Scholar]
- 102.O'Brien A.M., Lins T.F., Yang Y., Frederickson M.E., Sinton D., Rochman C.M. Microplastics shift impacts of climate change on a plant-microbe mutualism: Temperature, CO2, and tire wear particles. Environ. Res. 2022;203 doi: 10.1016/j.envres.2021.111727. [DOI] [PubMed] [Google Scholar]
- 103.Fawzy S., Osman A.I., Doran J., Rooney D.W. Strategies for mitigation of climate change: a review. Environ. Chem. Lett. 2020;18:2069–2094. [Google Scholar]
- 104.Qi R., Jones D.L., Li Z., Liu Q., Yan C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.