Abstract
Objectives
The appropriate strategy for enteral feeding in critically ill patients still remains controversial. Therefore, we conducted this meta-analysis to compare the effect of intermittent versus continuous enteral feeding method for critically ill patients.
Methods
Electronic databases including PubMed, Embase, Scopus, and Cochrane Library were searched up to April 10th, 2023 for randomized controlled trials evaluating the effect of intermittent versus continuous enteral feeding for critically ill patients. The primary outcomes were feeding intolerances, including diarrhea, vomiting, distension, constipation, gastric retention, and aspiration pneumonia. The secondary outcomes were mortality in intensive care unit (ICU), length of stay in ICU, and achievement of nutritional goal.
Results
Thirteen studies with a total of 884 patients were analyzed in this meta-analysis. Overall, the use of intermittent enteral feeding was associated with higher incidence of diarrhea (OR 1.66, 95%CI 1.13 to 2.43, I2 = 16%) and distension (OR 2.29, 95%CI 1.16 to 4.51, I2 = 0%), lower incidence of constipation (OR 0.58, 95%CI 0.37 to 0.90, I2 = 0%), and longer length of ICU stay (MD 1.09, 95%CI 0.53 to 1.64, I2 = 0%). Moreover, no significant difference was identified for other outcome measures.
Conclusion
In critically ill patients, the implementation of intermittent enteral feeding was associated with higher incidence of diarrhea and distension, longer length of ICU stay, but lower occurrence of constipation. Nevertheless, the absence of sufficient high-quality randomized controlled clinical trials precludes any definitive conclusions regarding the optimal approach to enteral feeding in this population. There is an imperative need for more studies to further assess the efficacy of the two enteral feeding strategies.
Keywords: intermittent feeding strategy, continuous feeding strategy, enteral nutrition, critically ill, meta - analysis
Introduction
Nutritional support is important for critically ill patients in intensive care unit (ICU), adequate nutritional therapy can improve clinical outcomes associated with malnutrition (1, 2). The methods of nutritional support have been widely described, including enteral nutrition (EN) and parenteral nutrition (PN) (3). EN is safer and more cost-effective than PN, and EN has the potential advantage of maintaining gastrointestinal tract integrity to prevent bacterial translocation (4, 5). Thus, current clinical guidelines promote the early implementation of EN for critically ill patients (2). However, critically ill patients are commonly affected by gastrointestinal dysfunction due to various factors such as post-operative ileus, gastric stasis, gut hypoperfusion, and administration of certain antibiotics and sedatives (6). These factors lead to an increased occurrence of feeding intolerance, which in turn hinders the successful implementation of enteral nutrition. Thus, there is an urgent need for further research to establish the optimal method of delivering EN and to address the discrepancies between guidelines and clinical practices (7).
There are two major methods for EN administration: continuous and intermittent enteral feeding. Continuous feeding uses a constant rate hourly by an electric feeding pump for 24 h per day (8). The infusion speed is relatively low and is therefore theorized as a safer enteral nutrition delivery strategy particularly for patients with an intolerance of enteral feeding (9). However, continuous enteral feeding was associated with a reduction in patient mobility and alterations in gastrointestinal hormone secretion, which may further lead to long-term metabolic complications such as hyperglycemia and insulin resistance (10, 11). Conversely, intermittent feeding delivers nutrition multiple times per day (generally four to six times a day) by a feeding pump, syringe, or gravity pump (8). It has been considered as a more physiological approach compared to continuous strategy, as it provides patients with greater mobility, increases protein synthesis, helps maintain regular secretion and digestion of gastrointestinal hormones (11, 12). Nevertheless, current clinical guideline and studies indicated that intermittent feeding increased the risk of feeding intolerance for critically ill patients (2, 6, 13, 14). A recent randomized trial indicated that compared with intermittent feeding, continuous feeding significantly improved the achievement of target nutrition requirements (15). The effectiveness and safety of the two enteral feeding methods for in critically ill patients still remain controversial, it is imperative to determine the optimal enteral nutrition strategy. Therefore, we aimed to perform a meta-analysis of randomized controlled trials (RCTs) to compare the effect of intermittent versus continuous enteral feeding in critically ill patients.
Methods
Study selection
This meta-analysis was performed according to the updated PRISRMA statement (16), the PRISRMA checklist is shown in Supplementary material 1. We preregistered our study protocol in Open Science Framework.1 Two authors (JQ, XX) searched the PubMed, Embase, Scopus, and Cochrane Library for relevant studies in English up to April 10th, 2023. The search algorithms included “intermittent,” “continuous,” “critically ill,” and “randomized.” The details of the search strategies are presented in Supplementary material 2.
Inclusion criteria
Studies fulfilled the inclusion criteria were included:
Type of study: randomized trials;
Population: critically ill patients required enteral nutritional support;
Intervention: intermittent enteral feeding;
Comparison: continuous enteral feeding;
Outcomes: trial must have reported at least one outcome of interest as detailed below.
Outcomes
The primary outcomes were feeding intolerances, including diarrhea, vomiting, distension, constipation, gastric retention, and aspiration pneumonia. The secondary outcomes were mortality in ICU, length of stay in ICU, and achievement of nutritional goal. The definitions of outcomes were the same as in the included trials.
Data extraction and quality assessment
Two authors (JQ, LH) separately screened all retrieved studies, then extracted the relevant information (first author or study name, publication years, study design, population, intervention and control methods). Each clinical outcome of this meta-analysis was also extracted from each included study.
Two authors (JQ, LH) adopted the Cochrane risk of bias tool (17) to assess the methodological quality of including studies. Any disagreement between the two authors was resolved by a consensus after discussing with a third adjudicator (XX).
Statistical synthesis and analysis
The computation of pooled odds ratios (OR) with corresponding 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) with 95% CI for continuous outcomes. The heterogeneity was assessed by the Higgins inconsistency (I2) statistics (18). Substantial heterogeneity was identified when I2 value>30% and a random-effects model was employed to perform the analysis, otherwise a fixed-effects model would be used. In addition, publication bias was evaluated through the use of the funnel plot and Egger’s regression test (19). Furthermore, a sensitivity analysis was performed through the consecutive exclusion of each study to investigate the effect of individual studies. All statistical analyses and assessments of bias risk were conducted by Review Manager Version 5.3, “meta” package in R software (version 4.3.1).
Results
Study characteristics
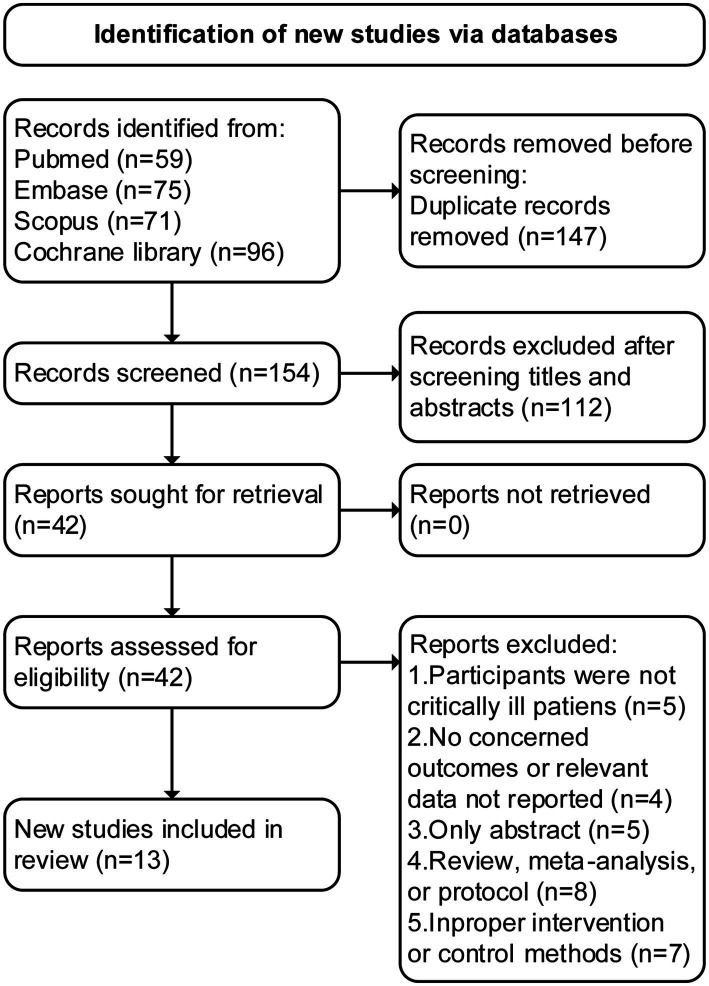
An initial search of the literature resulted in the identification of 301 articles, of which 147 were deemed as duplicates and excluded. Through the screening of abstracts, an additional 112 studies were excluded. Following a thorough evaluation of the full text, 29 additional studies were excluded for various reasons. Finally, 13 RCTs (13, 15, 20–30) met the inclusion criteria and were included in this study (see Figure 1 for detailed follow chart).
Figure 1.
PRISMA 2020 flow diagram for the meta-analysis.
The characteristics of the included studies are presented in Table 1. A total of 884 patients were analyzed, with 444 patients receiving intermittent enteral feeding and 440 patients receiving continuous enteral feeding during the respective study periods. The study periods ranged from 1 to 7 days. The number of patients in each study ranged from a minimum of 18 up to 160, and sample size of all included studies were relatively small (<100 patients per arm) (31). Patients were typically receiving enteral nutrition support in a mixed ICU. Seven studies reported the illness severity scores, with the average APACHE II score ranging from 12 to 28. The study durations ranged from 1 to 14 days, most of included studies compared the two feeding regimens in a 7-day study period.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study and year | Design | Number of patients (Intermittent/Continuous) | Population | Characteristics (Intermittent/Continuous) | Study arm | Study period | Clinical outcomes | |
|---|---|---|---|---|---|---|---|---|
| Intermittent | Continuous | |||||||
| Lee et al. (15) | Unblinded, single-center | 49/50 | Patients ≥20 years of age, required mechanical ventilation | Age: 66.2/67.5; Male (%): 67.3/66.0; BMI: 22.0/23.3; APACHE II: 27.7/28.6 | 1 h for 4 times/day, initial rate was 150 ml/h | 24 h/day, initial rate was 25 ml/h | 7 days | Feeding intolerances, achievement of nutritional goal, ICU mortality, length of ICU stay |
| Ren et al. (27) | Single-blinded, single-center | 32/30 | Patients admitted to ICU and fed through gastric tubes | Age: 66/55; Male (%): 53/63; BMI: 24/23; APACHE II: 19/16 | 2 h for 3 times/day | 24 h/day | 7 days | Feeding intolerances, ICU mortality, length of ICU stay |
| Zhu et al. (13) | Single-blinded, single-center | 40/38 | Patients ≥18 years of age, diagnosed with hemorrhagic stroke, GCS ≤12 | Age: 59.9/59.6; Male: 55.3/47.5; BMI: 24.6/24.1 | 0.5–1 h for 4 times/day | 24 h/day, maximum rate was 100 ml/h | 7 days | Feeding intolerances |
| McNelly et al. (25) | Single-blinded, multicenter | 62/59 | Patients ≥18 years of age, required mechanical ventilation for ≥48 h | Age: 55.2/60.3; Male: 66.1/67.8; APACHE II: 23.1/20.2 | Six bolus feeds, one bolus every 4 h | Total volume of feed administered over 24 h | 10 days | Feeding intolerances, achievement of nutritional goal requirement, ICU mortality |
| Nasiri et al. (26) | Triple-blinded, single-center | 20/20 | Patients aged between 18 to 65 years, admitted to the ICUs with the diagnosis of sepsis | Age: 54.6/53.0; Male: 38.2/35.3 | 18 h/day, with night rest for 6 h | 24 h/day | 3 days | Feeding intolerances |
| Kadamani et al. (22) | Unblinded, single-center | 15/15 | Patients aged between 20 and 80 years, received mechanical ventilation and EN for ≥72 h | Age: 61.6/64.7; Male: 66.7/60.0; BMI: 23.3/23.1; APACHE II: 16.0/20.3 | 10–15 min every 4–6 h | 24 h/day | 3 days | Feeding intolerances |
| Tavares de Araujo et al. (30) | Unblinded, single-center | 18/23 | Patients over 18 years of age, under clinical treatment in an intensive care unit | Age: 68.9/61.3; Male: 55.6/60.9; BMI: 24.6/22.3; APACHE II: 20.7/22.4 | 18 h/day, with night rest for 6 h | 24 h/day | 5 days | Feeding intolerances, achievement of nutritional goal, ICU mortality |
| Maurya et al. (24) | Unblinded, single-center | 20/20 | Adult male patients age of 20–60 years with history of head injury requiring mechanical ventilatory support | Age: 40.2/40.7; Male: 100/100; BMI: 22.0/20.6 | 3 times/day | 24 h/day | 1 day | Feeding intolerances |
| MacLeod et al. (23) | Unblinded, single-center | 79/81 | Patients over 18 years of age, admitted to the trauma intensive care unit, required mechanical ventilation ≥48 h | Age: 44.6/48.4; Male: 67.1/74.1; APACHE II: 12/14 | 100 ml of formula within 30–60 min every 4 h | 24 h/day, initial rate was 20 ml/h | 7 days | Feeding intolerances, achievement of nutritional goal, ICU mortality, length of ICU stay |
| Chen et al. (21) | Unblinded, single-center | 56/51 | Patients over 20 years of age, admitted to the trauma intensive care unit, required mechanical ventilation | Male: 76.8/76.5 | 125 ml of formula every 4 h | 24 h/day, initial rate was 25 ml/h | 7 days | Feeding intolerances |
| Serpa et al. (28) | Unblinded, single-center | 14/14 | Critically ill patients admitted to ICU because of clinical or surgical emergencies | Age: 64.9/69.6; Male: 50.0/64.3 | 1 h for 6 times/day | 24 h/day | 3 days | Feeding intolerances, ICU mortality, length of ICU stay |
| Steevens et al. (29) | Unblinded, single-center | 9/9 | Multiple trauma patients age of 18–70 years admitted to ICU, expected to need enteral nutrition ≥5 days | Age: 35.9/37.3; Male: 55.6/77.8; BMI: 27.5/25.4 | 125 ml within 15 min every 4 h | 24 h/day, initial rate was 25 ml/h | 7 days | Feeding intolerances, achievement of nutritional goal |
| Bonten et al. (20) | Unblinded, single-center | 30/30 | All mechanically ventilated patients over 15 years of age, admitted to ICU | Age: 68/65; Male: 53.3/63.3; APACHE II: 17/19 | 18 h/day, with night rest for 6 h | 24 h/day | 14 days | Feeding intolerances, ICU mortality |
BMI, body mass index; APACHE II, acute physiology and chronic health evaluation II; ICU, intensive care unit; GCS, Glasgow coma scale; EN, enteral nutrition.
Quality assessment
The results of quality assessment (Figure 2) showed that most included trials were rated as high risk of bias, largely due to a lack of blinding and allocation concealment. Furthermore, 12 trials did not report the blinding method for outcome assessment, which may result in an underestimation or overestimation of the true effect.
Figure 2.
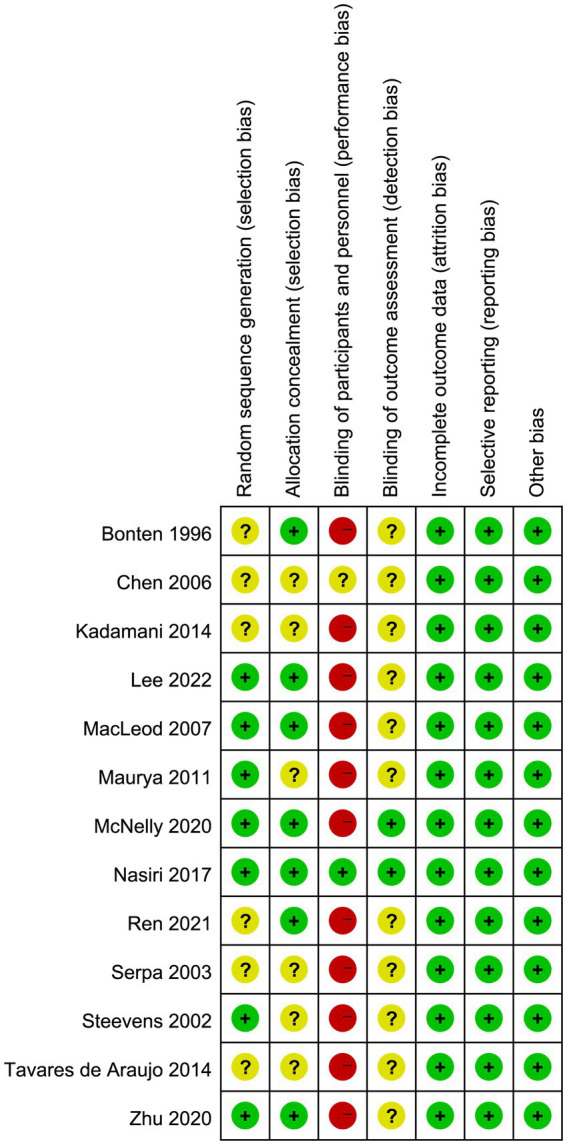
Assessment of quality by the Cochrane risk of bias tool.
In addition, we conducted an assessment of publication bias utilizing the Egger’s test and funnel plot, and the results did not indicate a significant risk of publication bias (Egger’s test, p > 0.05; Supplementary material 3).
Primary outcomes
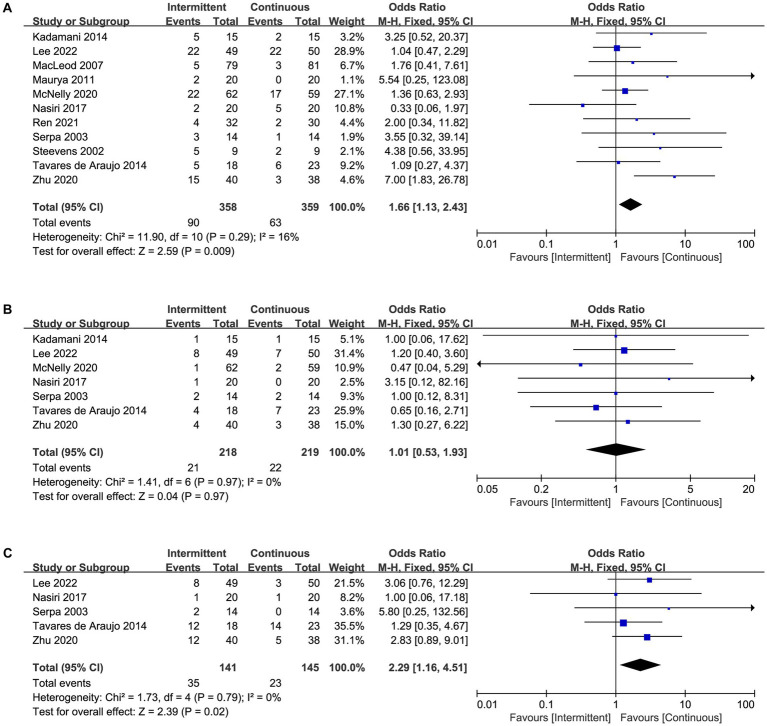
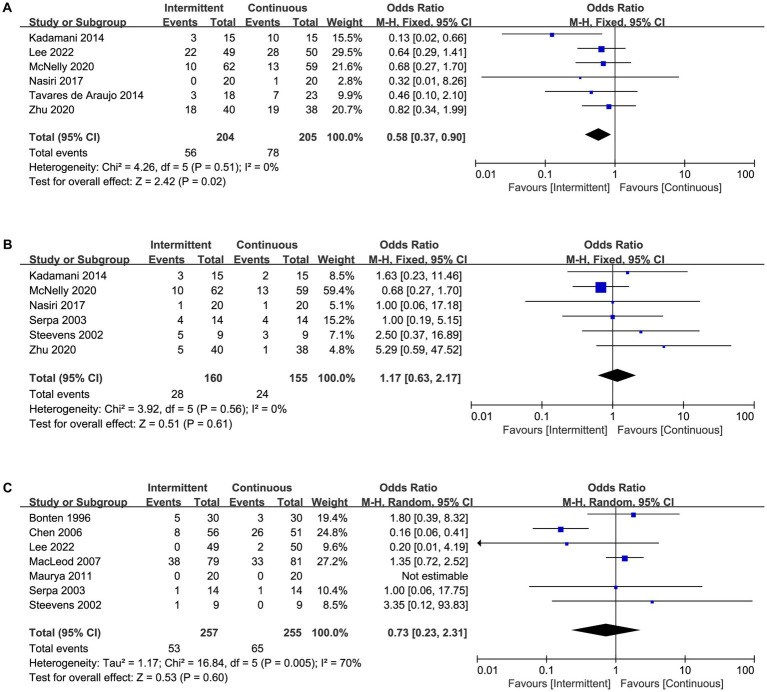
A total of 12 trials reported the incidence of feeding intolerances (Supplementary material 4), including 11 trials reported diarrhea (Figure 3A), seven trials reported vomiting (Figure 3B), five trials reported distension (Figure 3C), six trials reported constipation (Figure 4A), six trials reported gastric retention (Figure 4B), and seven trials reported aspiration pneumonia (Figure 4C). Overall, the pooled data showed that the use of intermittent enteral feeding was associated with higher incidence of diarrhea (OR 1.66, 95%CI 1.13 to 2.43, I2 = 16%, Figure 3A) and distension (OR 2.29, 95%CI 1.16 to 4.51, I2 = 0%, Figure 3C), lower incidence of constipation (OR 0.58, 95%CI 0.37 to 0.90, I2 = 0%, Figure 4A). No statistically significant difference was identified between intermittent and continuous enteral feeding for vomiting (OR 1.01, 95%CI 0.53 to 1.93, I2 = 0%, Figure 3B), gastric retention (OR 1.17, 95%CI 0.63 to 2.17, I2 = 0%, Figure 4B), and aspiration pneumonia (OR 0.73, 95%CI 0.23 to 2.31, I2 = 70%, Figure 4C).
Figure 3.
Forest plot showing the difference between intermittent versus continuous enteral feeding for (A) diarrhea, (B) vomiting, and (C) distension.
Figure 4.
Forest plot showing the difference between intermittent versus continuous enteral feeding for (A) constipation, (B) gastric retention, and (C) aspiration pneumonia.
However, when we assessed the effect of every single trial on the pooled result by leave-one-out sensitivity analysis, we found that exclusion of several studies might change the results. The incidence of diarrhea became no statistical significance when we excluded the trial by Zhu et al. (13) (Supplementary material 3). Similarly, excluding the trial by Lee et al. (15), Zhu et al. (13), or Kadamani et al. (20) changed the results of distension and constipation (Supplementary material 3). The sensitivity analysis showed that the results were not robust enough. Furthermore, no trial was found to have an undue influence on other outcomes as the effect size remained statistically significant on the exclusion of any trial (Table 2).
Table 2.
Main findings of our meta-analysis.
| Outcome | N | Result |
|---|---|---|
| Primary outcomes | ||
| Diarrhea | 11 | OR 1.66, 95%CI 1.13 to 2.43, I2 = 16% |
| Vomiting | 7 | OR 1.01, 95%CI 0.53 to 1.93, I2 = 0% |
| Distension | 5 | OR 2.29, 95%CI 1.16 to 4.51, I2 = 0% |
| Constipation | 6 | OR 0.58, 95%CI 0.37 to 0.90, I2 = 0% |
| Gastric retention Low | 6 | OR 1.17, 95%CI 0.63 to 2.17, I2 = 0% |
| Aspiration pneumonia | 7 | OR 0.73, 95%CI 0.23 to 2.31, I2 = 70% |
| Secondary outcomes | ||
| ICU mortality | 7 | OR 1.49, 95%CI 0.97 to 2.27, I2 = 0% |
| Length of ICU stay | 4 | MD 1.09, 95%CI 0.53 to 1.64, I2 = 0% |
| Achievement of nutritional goal | 5 | OR 1.99, 95%CI 0.67 to 1.45, I2 = 22% |
OR, odds ratio; MD, mean difference; CI, confidence interval; ICU, intensive care unit.
Secondary outcomes
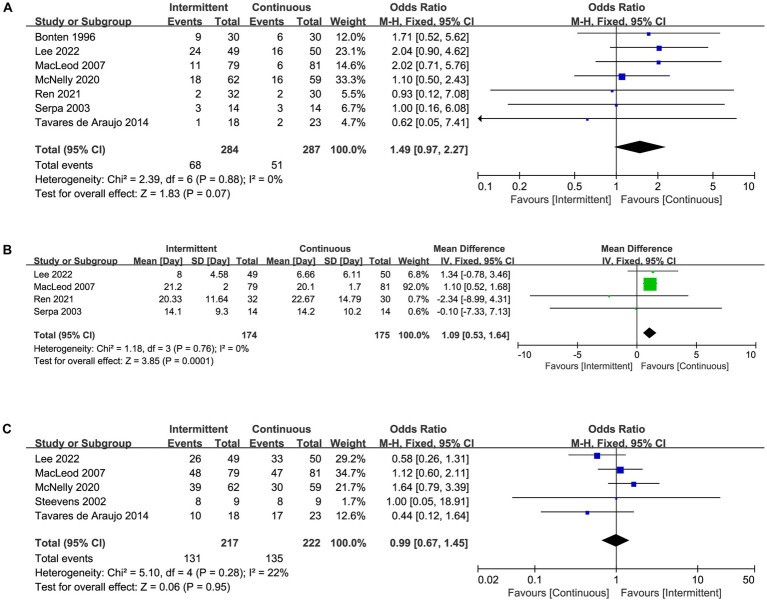
Mortality in ICU was reported in seven trials (Figure 5A), length of ICU stay was reported in four trials (Figure 5B), and achievement of nutritional goal was assessed in five trials (Figure 5C). There was a statistically significant difference between intermittent and continuous enteral feeding, with a prolonged length of ICU stay in patients receiving intermittent enteral feeding (MD 1.09, 95%CI 0.53 to 1.64, I2 = 0%, Figure 5B). No statistically significant difference was observed for mortality in ICU (OR 1.49, 95%CI 0.97 to 2.27, I2 = 0%, Figure 5A) or achievement of nutritional goal (OR 1.99, 95%CI 0.67 to 1.45, I2 = 22%, Figure 5C). Nevertheless, the difference in length of ICU became no statistically significant if we excluded the trial by MacLeod et al. (23), indicated the result was not robust enough.
Figure 5.
Forest plot showing the difference between intermittent versus continuous enteral feeding for (A) mortality in ICU, (B) length of ICU stay, (C) achievement of nutritional goal.
Discussion
Nutritional support is one of the important parts of treatments for critically ill patients (32). Despite its importance, there is limited evidence comparing the intermittent and continuous enteral feeding methods in clinical practice. In this study, we conducted a comprehensive review of existing literature that compares the effect of intermittent and continuous enteral feeding on feeding intolerance and clinical outcomes in critically ill patients. The findings of our meta-analysis indicated that intermittent enteral feeding was associated with increased risk of diarrhea and distension, as well as a longer ICU stay. Furthermore, the ICU mortality was higher in patients receiving intermittent feeding than others receiving continuous feeding, but the difference did not reach statistical significance. Conversely, a lower incidence of constipation was observed in the intermittent feeding group. However, there were no other differences in the incidence of vomiting, gastric retention, and aspiration pneumonia. Critically ill patients have high risk of developing feeding intolerance. Studies have shown that among critically ill patients, the rate of feeding intolerance can exceed 30 percent (33). In current clinical practice, both intermittent and continuous enteral feeding are commonly utilized and widely accepted as effective methods for delivering prescribed diets (9). However, current evidence indicates that each administration method may present its own pros and cons (11). Since the current evidence regarding the effects of the two administration methods was limited, clinicians may tend to select the method with a lower risk of adverse events. Therefore, in this comprehensive and up to date meta-analysis, we revealed that there was no statistically significant difference in the incidence of other feeding intolerance symptoms, such as vomiting, gastric retention, and aspiration pneumonia. Furthermore, despite intermittent feeding allowing for more time for patients to engage in rehabilitation training, which can be beneficial for recovery, the ICU mortality rate was higher in the intermittent feeding group. Even if the difference did not reach statistical significance, the result is of notable interest. A probable reason is the higher incidence of diarrhea and distension in patients receiving intermittent feeding may result in more severe complications. This result suggested that continuous feeding might be a preferable enteral nutrition administration strategy for critically ill adults to reduce feeding intolerance incidence, which is consistent with the recommendation of ASPEN guidelines (34). However, since both feeding methods may give rise to some form of feeding intolerances and gastroenterological complications, current practice should balance these potential adverse events for individualized patient care to mitigate potential adverse events (35). The latest consensus (36, 37) advocated shifting from the ‘one size fits all’ feeding approach to one where individualization may be the key factor in driving positive outcomes for critically ill patients. When determining individualized nutrition interventions for critically ill patients, the dietitian and clinicians should consider the patient population, the individual patient’s nutritional status and the expected outcome and formulate an appropriate feeding regimen based on this.
Some of the findings of our meta-analysis are in contrast to previous studies. A recent meta-analysis (35) showing no difference in mortality, diarrhea, increased gastric residuals, pneumonia, and bacterial colonization between intermittent and continuous enteral feeding methods. Ma et al. (14) reported a meta-analysis of 14 RCTs that found intermittent feeding provided significantly more calories, increased the risk of high gastric residual volume and aspiration, reduced the incidence of constipation as compared to continuous feeding. Nevertheless, the majority of studies included by Ma et al. (14) were predominantly local data where practices may differ from worldwide feeding practices, the results may not be fully appropriate and executable internationally. Compared with previous meta-analyses, our study performed a comprehensive and up to date search of the worldwide literature, included more recent RCTs (15, 27) that met the inclusion criteria. The results of our research are approximately consistent with the previous studies, but we found that the intermittent enteral feeding was associated with higher incidence of diarrhea and distension. The outcome measure for nutritional intake in our meta-analysis was the proportion of achievement the nutritional goal, which is a more meaningful measure of nutritional adequacy, as opposed to total calories.
Notably, the current study found a higher incidence of constipation in the continuous feeding group compared to the intermittent feeding group, which was consistent with the conclusion of previous studies (14, 35). This may be attributed to colonic sensorimotor disturbance, which is a well-established cause of constipation (38). Gut motility is partly dependent on stimulation from intraluminal contents, which is more effectively achieved through intermittent feeding that delivers a larger amount of intraluminal contents within a certain period, and can thereby stimulate intestinal smooth muscle contractions (39). Conversely, continuous feeding is delivered at a slower rate and with smaller volumes, which may not effectively stimulate postprandial patterns of gastrointestinal motility and lead to reduced antral hypomotility (8). Furthermore, prolonged bed rest is a proved risk factor for constipation (40), and continuous feeding patients are often restricted to bed due to the non-interruptible nature of enteral nutrition, which may also contribute to the higher incidence of constipation in this group (41).
In practice, various factors may influence the options of feeding modalities. Previous meta-analysis by Ma et al. (14) indicated that intermittent administration has been linked to a potentially higher daily caloric intake (MD 184.81, 95% CI 56.61 to 313.01), although our own study did not detect difference in the achievement of nutritional goal between intermittent and continuous feeding groups. Interruptions in enteral nutrition delivery due to patient care or diagnostic testing may contribute to this difference. Furthermore, intermittent administration may allow for faster achievement of nutritional goals, particularly in cases where continuous administration is gradually initiated. The impact of these factors on patient outcomes remains uncertain. Moreover, intermittent administration may have the added benefit of minimizing patient immobility associated with additional tubing, particularly in a hospital setting.
Limitations
However, our meta-analysis has several limitations. First of all, due to the apparent distinction between the two feeding strategies, blinding of participants and investigators was difficult to implement. Our meta-analysis included RCTs with moderate to high levels of bias and small sample sizes (number of participants <100 per arm), thereby limiting the scope of definitive conclusions that can be drawn (31). Hence, caution must be exercised while interpreting the findings of such studies.
Second, included studies varied in the definitions for assessing the outcomes of interest (e.g., distension, gastric retention, and aspiration pneumonia), which could cause indirectness of evidence. Such heterogeneity in outcome definitions warrants careful consideration while interpreting and synthesizing the results from these studies.
Moreover, several studies did not provide sufficient details regarding the determination of the nutritional requirements of the patients, the formulas employed for the calculation of these requirements, and other factors that may influence these calculations, including the potential inclusion of propofol. The absence of such crucial information may undermine the ability to fully appraise the study findings.
Conclusion
In this updated meta-analysis of 13 RCTs, we found that intermittent enteral feeding in critically ill patients was associated with high occurrence of feeding intolerance, including diarrhea and distension. However, there is a higher risk of constipation associated with continuous enteral feeding. Based on available evidence, continuous enteral feeding may be more appropriate for patients at higher risk of feeding intolerance. Moreover, we advocate for additional high-quality RCTs with longer durations and more diverse clinical outcomes to more effectively validate the effects of both continuous and intermittent feeding methods on enteral nutrition.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JQ and LH conceived the idea, performed the analysis, and drafted the initial draft writing of this paper. XX, CX, and XD contributed to the collection and interpretation of data. KZ helped to frame the idea of the study and provided technical support. LH contributed to the revision of this paper, and the final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1214774/full#supplementary-material
References
- 1.de Vries MC, Koekkoek WK, Opdam MH, van Blokland D, van Zanten AR. Nutritional assessment of critically ill patients: validation of the modified NUTRIC score. Eur J Clin Nutr. (2018) 72:428–35. doi: 10.1038/s41430-017-0008-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38:48–79. doi: 10.1016/j.clnu.2018.08.037, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Hill A, Elke G, Weimann A. Nutrition in the intensive care unit-a narrative review. Nutrients. (2021) 13:2851. doi: 10.3390/nu13082851, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, Zhang K, Cui W, Hong Y, Zhang Z. The effect of enteral versus parenteral nutrition for critically ill patients: a systematic review and meta-analysis. J Clin Anesth. (2018) 51:62–92. doi: 10.1016/j.jclinane.2018.08.008, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Phillips MS, Ponsky JL. Overview of enteral and parenteral feeding access techniques: principles and practice. Surg Clin North Am. (2011) 91:897–911, ix. doi: 10.1016/j.suc.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 6.Tatsumi H. Enteral tolerance in critically ill patients. J Intensive Care. (2019) 7:30. doi: 10.1186/s40560-019-0378-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wischmeyer PE. Overcoming challenges to enteral nutrition delivery in critical care. Curr Opin Crit Care. (2021) 27:169–76. doi: 10.1097/MCC.0000000000000801, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel JJ, Rosenthal MD, Heyland DK. Intermittent versus continuous feeding in critically ill adults. Curr Opin Clin Nutr Metab Care. (2018) 21:116–20. doi: 10.1097/MCO.0000000000000447, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Ichimaru S. Methods of enteral nutrition Administration in Critically ill Patients: continuous, cyclic, intermittent, and bolus feeding. Nutr Clin Pract. (2018) 33:790–5. doi: 10.1002/ncp.10105, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Marik PE. Feeding critically ill patients the right 'whey': thinking outside of the box. A personal view. Ann Intensive Care. (2015) 5:51. doi: 10.1186/s13613-015-0051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bear DE, Hart N, Puthucheary Z. Continuous or intermittent feeding: pros and cons. Curr Opin Crit Care. (2018) 24:256–61. doi: 10.1097/MCC.0000000000000513 [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury AH, Murray K, Hoad CL, Costigan C, Marciani L, Macdonald IA, et al. Effects of bolus and continuous nasogastric feeding on gastric emptying, small bowel water content, superior mesenteric artery blood flow, and plasma hormone concentrations in healthy adults: a randomized crossover study. Ann Surg. (2016) 263:450–7. doi: 10.1097/SLA.0000000000001110, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu W, Jiang Y, Li J. Intermittent versus continuous tube feeding in patients with hemorrhagic stroke: a randomized controlled clinical trial. Eur J Clin Nutr. (2020) 74:1420–7. doi: 10.1038/s41430-020-0579-6, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Cheng J, Liu L, Chen K, Fang Y, Wang G, et al. Intermittent versus continuous enteral nutrition on feeding intolerance in critically ill adults: a meta-analysis of randomized controlled trials. Int J Nurs Stud. (2021) 113:103783. doi: 10.1016/j.ijnurstu.2020.103783, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Lee HY, Lee JK, Kim HJ, Ju DL, Lee SM, Lee J. Continuous versus intermittent enteral tube feeding for critically ill patients: a prospective, randomized controlled trial. Nutrients. (2022) 14:664. doi: 10.3390/nu14030664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonten M, Gaillard CA, Hulst R, Leeuw P, Geest S, Stobberingh EE, et al. Intermittent enteral feeding: the influence on respiratory and digestive tract colonization in mechanically ventilated intensive-care-unit patients. Am J Respir Crit Care Med. (1996) 154:394–9. doi: 10.1164/ajrccm.154.2.8756812, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Chen YC, Chou SS, Lin LH, Wu LF. The effect of intermittent nasogastric feeding on preventing aspiration pneumonia in ventilated critically ill patients. J Nurs Res. (2006) 14:167–80. doi: 10.1097/01.JNR.0000387575.66598.2a, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Kadamani I, Itani M, Zahran E, Taha N. Incidence of aspiration and gastrointestinal complications in critically ill patients using continuous versus bolus infusion of enteral nutrition: a pseudo-randomised controlled trial. Aust Crit Care. (2014) 27:188–93. doi: 10.1016/j.aucc.2013.12.001, PMID: [DOI] [PubMed] [Google Scholar]
- 23.MacLeod JB, Lefton J, Houghton D, Roland C, Doherty J, Cohn SM, et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. (2007) 63:57–61. doi: 10.1097/01.ta.0000249294.58703.11, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Maurya I, Pawar M, Garg R, Kaur M, Sood R. Comparison of respiratory quotient and resting energy expenditure in two regimens of enteral feeding - continuous vs. intermittent in head-injured critically ill patients. Saudi J Anaesth. (2011) 5:195–201. doi: 10.4103/1658-354X.82800, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. (2020) 158:183–94. doi: 10.1016/j.chest.2020.03.045 [DOI] [PubMed] [Google Scholar]
- 26.Nasiri M, Farsi Z, Ahangari M, Dadgari F. Comparison of intermittent and bolus enteral feeding methods on enteral feeding intolerance of patients with Sepsis: a triple-blind controlled trial in intensive care units. Middle East J Dig Dis. (2017) 9:218–27. doi: 10.15171/mejdd.2017.77, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren CJ, Yao B, Tuo M, Lin H, Wan XY, Pang XF. Comparison of sequential feeding and continuous feeding on the blood glucose of critically ill patients: a non-inferiority randomized controlled trial. Chin Med J. (2021) 134:1695–700. doi: 10.1097/CM9.0000000000001684, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serpa LF, Kimura M, Faintuch J, Ceconello I. Effects of continuous versus bolus infusion of enteral nutrition in critical patients. Rev Hosp Clin Fac Med Sao Paulo. (2003) 58:9–14. doi: 10.1590/S0041-87812003000100003 [DOI] [PubMed] [Google Scholar]
- 29.Steevens EC, Lipscomb AF, Poole GV, Sacks GS. Comparison of continuous vs intermittent nasogastric enteral feeding in trauma patients: perceptions and practice. Nutr Clin Pract. (2002) 17:118–22. doi: 10.1177/0115426502017002118, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Tavares de Araujo VM, Gomes PC. Caporossi C: enteral nutrition in critical patients; should the administration be continuous or intermittent? Nutr Hosp. (2014) 29:563–7. doi: 10.3305/nh.2014.29.3.7169, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. (2013) 17:R2. doi: 10.1186/cc11919, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue X, Li M, Wang Y, Zhang J, Wang X, Kan L, et al. Nutritional support and clinical outcome of severe and critical patients with COVID-19 pneumonia. Front Nutr. (2020) 7:581679. doi: 10.3389/fnut.2020.581679, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gungabissoon U, Hacquoil K, Bains C, Irizarry M, Dukes G, Williamson R, et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr. (2015) 39:441–8. doi: 10.1177/0148607114526450, PMID: [DOI] [PubMed] [Google Scholar]
- 34.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2016) 40:159–211. doi: 10.1177/0148607115621863, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Heffernan AJ, Talekar C, Henain M, Purcell L, Palmer M, White H. Comparison of continuous versus intermittent enteral feeding in critically ill patients: a systematic review and meta-analysis. Crit Care. (2022) 26:325. doi: 10.1186/s13054-022-04140-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reintam Blaser A, Rooyackers O, Bear DE. How to avoid harm with feeding critically ill patients: a synthesis of viewpoints of a basic scientist, dietitian and intensivist. Crit Care. (2023) 27:258. doi: 10.1186/s13054-023-04543-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wischmeyer PE, Bear DE, Berger MM, De Waele E, Gunst J, McClave SA, et al. Personalized nutrition therapy in critical care: 10 expert recommendations. Crit Care. (2023) 27:261. doi: 10.1186/s13054-023-04539-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharucha AE, Lacy BE. Mechanisms, evaluation, and Management of Chronic Constipation. Gastroenterology. (2020) 158:1232–1249.e3. doi: 10.1053/j.gastro.2019.12.034, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato M, Shibata C, Kikuchi D, Ikezawa F, Imoto H, Kakyo M, et al. Effect of viscosity of enteral nutrient on gut motility and hormone secretion in dogs. Hepato-Gastroenterology. (2011) 58:36–41. PMID: [PubMed] [Google Scholar]
- 40.Iovino P, Chiarioni G, Bilancio G, Cirillo M, Mekjavic IB, Pisot R, et al. New onset of constipation during long-term physical inactivity: a proof-of-concept study on the immobility-induced bowel changes. PLoS One. (2013) 8:e72608. doi: 10.1371/journal.pone.0072608, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erichsén E, Milberg A, Jaarsma T, Friedrichsen M. Constipation in specialized palliative care: factors related to constipation when applying different definitions. Support Care Cancer. (2016) 24:691–8. doi: 10.1007/s00520-015-2831-5, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.