Abstract
Pterygium is a prevalent ocular disease that can cause discomfort and vision impairment. Early and accurate diagnosis is essential for effective management. Recently, artificial intelligence (AI) has shown promising potential in assisting clinicians with pterygium diagnosis. This paper provides an overview of AI-assisted pterygium diagnosis, including the AI techniques used such as machine learning, deep learning, and computer vision. Furthermore, recent studies that have evaluated the diagnostic performance of AI-based systems for pterygium detection, classification and segmentation were summarized. The advantages and limitations of AI-assisted pterygium diagnosis and discuss potential future developments in this field were also analyzed. The review aims to provide insights into the current state-of-the-art of AI and its potential applications in pterygium diagnosis, which may facilitate the development of more efficient and accurate diagnostic tools for this common ocular disease.
Keywords: pterygium, intelligent diagnosis, artificial intelligence, deep learning, machine learning
INTRODUCTION
The field of medicine is increasingly utilizing artificial intelligence (AI) due to the rapid advancements in computer science and medical technology. AI has already demonstrated exceptional performance in various medical fields, including cancer diagnosis[1], skin diseases[2], cardiology[3], and eye diseases[4]. Pterygium, a common ophthalmic disease caused by conjunctival laxity and cell proliferation, appears triangular, resembling an insect's wings[5]. It can cause visual discomfort, eye fatigue, and dryness[6] and may lead to vision loss if left untreated. Numerous studies have been conducted on the cause of pterygium. However, the cause of pterygium and pathogenesis of pterygium are still unclear[7].
Pterygium surgery has strict indications, and choosing the appropriate time to remove the pterygium can minimize the surgery's damage and the pterygium's recurrence rate[8]. Therefore, early diagnosis and treatment of pterygium are crucial, but the current number of ophthalmologists cannot meet the increasing demand for pterygium diagnosis[9]. The reason for this is that the diagnosis of pterygium relies heavily on the expertise of ophthalmologists in clinical settings. Moreover, training a qualified ophthalmologist is a lengthy and expensive process. Therefore, using AI to assist in primary ophthalmic clinical diagnosis of pterygium has received much attention. By utilizing an AI-based automatic diagnostic model, specialist doctors can concentrate on reviewing and verifying abnormal diagnostic results generated by the AI system, significantly improving diagnostic and therapeutic efficiency[10], and it is expected to become an important future development direction in medical diagnosis[11].
In recent years, there has been an exponential increase in research on using AI to assist in diagnosis and treatment ophthalmic diseases[12]. Several review papers have been published in areas such as diabetic retinopathy (DR) grading[13], macular degeneration[14], and glaucoma[15]. However, there are still few retrospective papers on the use of AI technology in diagnosing pterygium. As far as we are aware, only one paper[16] has investigated and summarized 18 relevant papers. Based on this, this paper collected 33 papers on AI-assisted pterygium diagnosis from 2011 to March 2023, reviewed the current status and development of AI-based pterygium diagnosis technology, and discussed its application prospects and future development trends. The remaining sections of this paper are as follows: first, it introduces the background knowledge of pterygium; then, it presents the history of the clinical diagnosis and treatment of pterygium; next, it elaborates on the development process and research methods of AI-based pterygium diagnosis technology, including image processing, feature extraction, and deep learning models; it then summarizes the application difficulties and challenges of AI-based pterygium diagnosis technology; finally, it provides an outlook on the future research prospects in this field.
CLINICAL BACKGROUND OF PTERYGIUM
Pterygium is a typical eye lesion that mainly occurs at the junction of the nasal conjunctiva and cornea, but can also occur at the junction of the temporal conjunctiva and cornea and may involve the accumulation of sclera tissue[17]. It usually appears as a triangular shape, with a gray-white or light-yellow color. The lesion can spread to the central corneal area, affecting vision, and causing dryness and a foreign body sensation. Because a pterygium is a chronic inflammatory lesion of the eye caused by external stimuli, it is widespread in tropical and subtropical regions[18], with an incidence ranging from 0.074% in Saudi Arabia to 53% in Taiwan, China and a global average incidence of 12%[9]. Although pterygium is not as well-known as “cataracts” or “glaucoma” and does not threaten patient life, it is still a problematic eye disease because it can cause eye irritation, aesthetic defects, and astigmatism as it gradually moves toward the central cornea. Once it extends to the pupil area, it can seriously hinder vision. Large pterygium can even affect eye movement, and invasion of the cornea can cause varying degrees of corneal opacity and other lesions.
Pterygium has been recorded in ancient medical works, including those from ancient Chinese and European medicine. However, it was not until the 20th century that there was a systematic study and analysis of pterygium in the field of ophthalmology. The incidence of pterygium is higher in males and increases with age. In the early stages, pterygium usually has no apparent symptoms. However, as it grows further, patients may experience discomforts such as foreign body sensations, irritation, redness, dryness, inflammation, decreased vision, and astigmatism. Ultraviolet radiation is often considered to be the initiating factor for pterygium. Some studies suggest that the elastic changes in pterygium are similar to the photoaging changes in chronically sun-exposed skin.
Generally, based on different characteristics, it can be divided into true and false pterygium. True pterygium can be further divided into stationary pterygium (which grows up to the edge of the iris, is slightly red, but cannot disappear on its own) and progressive pterygium (which has a prominent head, is highly congested, and has a broad neck and body, grows quickly, and is prone to covering the pupil area in the central cornea). False pterygium is generally caused by local injury and scar formation and does not grow afterward.
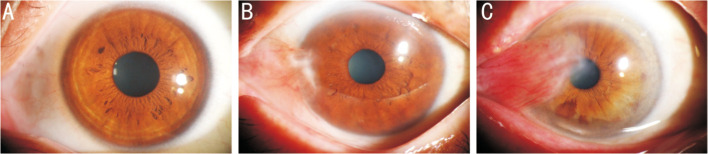
In clinical diagnosis, the most common classification criteria for pterygium are the two categories of pterygium and non-pterygium. It can also be divided into three categories: normal, observation period, and surgical period based on the length or width of the pterygium. Generally, when the length or width is ≥3 mm, it is included in the surgical range[19], as shown in Figure 1. Some clinical experts also classify the severity of pterygium (such as the degree of redness) in a more detailed manner to develop targeted treatment strategies.
Figure 1. Clinical pterygium images.
A: Normal; B: Observation period; C: Surgical period.
Currently, there is still a lack of effective drug therapy for pterygium. As a result, surgical excision remains the most effective method of treatment. However, when the pterygium is larger, there is a high postoperative recurrence rate[20], so it is particularly important to determine the optimal timing for surgery.
The main methods of diagnosing pterygium in clinical practice include eye examination and medical history inquiry. The eye examination is the primary means of diagnosing pterygium. Doctors use equipment such as microscopes and slit lamps to conduct a comprehensive examination of the patient's eyes to determine the size, shape, location, and presence of inflammation of the pterygium. During the examination, doctors also observe the patient's vision, corneal shape, and other conditions to rule out the possibility of other eye diseases. Medical history inquiry is also an essential step in diagnosing pterygium. Doctors will inquire about the patient's medical history, including the time of onset of symptoms, the development process, and whether there have been similar eye diseases in the past, to help determine the etiology and diagnosis of the pterygium.
These diagnostic methods heavily rely on the doctor's personal experience and judgment, which can lead to inaccurate diagnoses and misdiagnoses. With the rapid increase in pterygium patients worldwide, existing ophthalmologists and medical resources cannot meet the increasing demand for diagnosis. Therefore, exploring a more efficient and intelligent diagnostic method is crucial.
TREND OF ARTIFICIAL INTELLIGENCE WITH PTERYGIUM DIAGNOSIS
Due to the challenges and difficulties of diagnosing pterygium solely by physicians, there has been a growing interest in developing an automated diagnostic system based on AI to assist physicians. Currently, AI-based pterygium diagnosis technology is still in its early stages of research, but some promising progress has been made. Figure 2 shows the literature trend on AI-assisted pterygium diagnosis from 2011 to 2022 in Google Scholar. Specifically, we only used “Pterygium” as the keyword to search for literature and conducted manual screening because we found that searching using terms like “pterygium+artificial intelligence” or “pterygium+machine learning” might miss some subtle literature, such as Papa et al[21], Abdani et al[22]. In addition, we included two papers accepted in January 2023 in the count for 2022, as this review paper was written in March 2023. This approach can better reflect the future trend in this field.
Figure 2. Trends of literature related to AI techniques to assist in pterygium diagnosis.
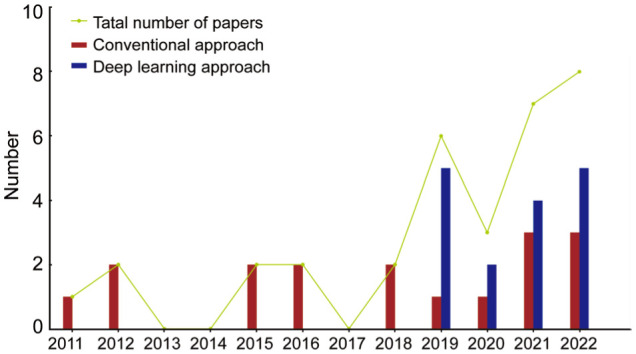
According to Figure 2, intelligent methods were first proposed to diagnose pterygium in 2011, and the literature in this field remained relatively stable in the following period, mainly using traditional machine learning algorithms and computer vision methods. The turning point came in 2016 when Google researchers created an automatic detection model using a deep learning model that could detect DR and macular edema[23]. Subsequently, the idea of using deep learning methods to assist in ophthalmic diagnosis began to spread, such as the deep learning based macular detection algorithm in 2017[24] and the deep learning based glaucoma automatic detection method in 2018[25]. By 2019, this trend had finally spread to pterygium, with a total of 5 papers using deep learning methods to assist in pterygium diagnosis that year, driving the research interest in AI-assisted pterygium diagnosis, which continues to this day. In the following section, this article will provide a detailed introduction to the literature on AI-assisted pterygium diagnosis from two perspectives: traditional intelligent methods and deep learning methods.
SUMMARY OF CONVENTIONAL METHODS APPLIED TO THE INTELLIGENT DIAGNOSIS OF PTERYGIUM
Table 1[21]–[22],[26]–[41] presents 17 papers that use traditional methods to assist in pterygium diagnosis in chronological order, with a summary of each paper. The earliest paper appeared in 2011, in which Papa et al[21] applied their algorithm based on gravitational search and Optimum-Path Forest (OPF) classifier to a binary pterygium dataset, achieving an accuracy of 98.4%. However, unfortunately, the authors did not provide the data source. Gao et al[26] proposed a segmentation method based on color information and visible wavelength images to address the issue of pterygium misdiagnosis as cortical cataract in cataract grading systems. Although both papers did not focus on pterygium as the main task, they indirectly used computer technology to detect pterygium.
Table 1. Summary of research using traditional methods to assist in the intelligent diagnosis of pterygium.
| Year | Task | Sample size | Results | Database | Strength |
| 2011[21] | Classification | 7564 samples with 89 features | Accuracy=98.4% | N/A | The proposed machine learning algorithm was tested on the pterygium dataset. |
| 2012[27] | Segmentation | 58 image datasets of pterygium ASPI | Accuracy=63.4% | Center of Informatics, Federal University of Pernambuco | Propose a digital image processing method for segmenting the iris and pterygium. |
| 2012[26] | Segmentation | 30 images with pterygium | Sensitivity=66.7%; specificity=80.33% | Singapore Malay Eye Study I | Detect pterygium in corneal images to prevent misdiagnosis as cortical cataract. |
| 2015[28] | Segmentation | 58 images of pterygium ASPI | Accuracy=77% | Derived from[26] | Improving iris segmentation accuracy based on normalized HSV. |
| 2015[29] | Segmentation | 58 ASPIs with pterygium | Accuracy=88.18%; sensitivity=70.83%; specificity=93.53% | Derived from[26] | Adaptive nonlinear enhancement method for iris segmentation using sigmoid function. |
| 2016[30] | Severity grading | 68 images with pterygium | ICC=0.898 | N/A | Using online ratings as a benchmark for grading algorithms. |
| 2016[22] | Segmentation | 59 images with pterygium | Accuracy=94%; sensitivity=79.21%; specificity=98.33% | Derived from[26] | Improved segmentation method improves performance. |
| 2018[31] | Classification | 325 images with pterygium | Sensitivity=88.7%; specificity=88.3%; AUC=95.6% | Federal University of Pernambuco | Modify sigmoid function to improve vascular tissue feature extraction. |
| 2018[33] | Classification | 163 images with pterygium | Accuracy=76% | N/A | IntegrateViola Jones method with expert system. |
| 2019[34] | Measure | 120 eyes from 120 primary pterygium patients | ICC=0.995 for intra-observer ICC=0.994 for inter-observer | N/A | Quantification of pterygium area (CPTA) using image analysis software. |
| 2020[35] | Severity grading | 93 cases with primary pterygia | ICC=0.91, 0.922, 0.954 | N/A | Evaluate the reliability of the grading software (PRGS). |
| 2021[32] | Classification | 93 samples of pterygium patients | Accuracy=94.44%; sensitivity=92.14%; specificity=100% | Previous study of Hilmi et al[41] | Using traditional machine learning methods with attribute data to predict pterygium. |
| 2021[36] | Severity grading | 217 slit-lamp images with pterygium | Intra-rater reliability=0.54-0.92 | Singapore National Eye Centre | Grading of pterygium using a combination of conjunctival and corneal parameters. |
| 2021[37] | Classification | 60 images with pterygium | Accuracy=82.5% by Hough transform and 100% by expert system | From the internet and verified by Susi Heryati | Integrating Hough transform and expert system for grading pterygium. |
| 2022[39] | Severity grading | 100 pterygium images with different severity stages | Accuracy=91.4%; specificity=87.7%-92.1%; sensitivity=89.4%-94.5% | Various eye hospitals in Tumakuru | Add DBMF filter in pre-processing section and use fuzzy inference for grading. |
| 2023[38] | Severity grading | 400 histopathological images of pterygium from 40 patients | AUC=0.84-0.95; TPR=81.3%; PPV=82% | Kangwon National University Hospital | Pre-segmentation for regions of interest prior to grading to improve grading accuracy. |
| 2023[40] | Classification | N/A | AUC=0.93 | Gene Expression Omnibus | Prediction based on the correlation between TRP and pterygium development. |
AUC: Area under curve; TPR: True positive rate; PPV: Positive predictive value; ICC: Intraclass correlation coefficient; ASPI: Anterior segment photographed image; N/A: Not mentioned; DBMF: Distance-based mean filter; HSV: Hue saturation value.
In 2012, literature began to emerge that focused on pterygium as the central task. The first study to address the segmentation task was by Mesquita et al[27], who for the first time used digital image processing technology to segment patients' irises for the first time to assist doctors in judging the progress of pterygium disease and determining whether pterygium should be surgically removed. Although the segmentation accuracy could have been higher in this work, the dataset provided by the paper sparked further research: Abdani et al[28] improved the segmentation performance by normalizing the hue saturation value (HSV) color space. In the same year, they proposed an adaptive non-linear enhancement method[29] that uses sigmoid functions to enhance the contrast between different tissues. The accuracy increased from 77% to 88.18%. Later, Abdani et al[22] proposed a segmentation method combining particle swarm optimization with multi-color channels and a three-step thresholding process, achieving a segmentation accuracy of up to 94%.
In addition to segmentation tasks, classification and grading tasks have also received much attention from researchers. We will discuss grading and classification together because grading is a multi-categorization task. Azemin et al[30] first proposed an automatic grading system that divided pterygium into five grades according to Tan et al's[42] grading criteria. Based on obtaining diagnostic scores from different experts through online tables and predicting pterygium images based on external artificial neural networks (ANN), this preliminary attempt yielded promising results intraclass correlation coefficient (ICC)=0.898. Wan Zaki et al[31] evaluated the performance comparison between support vector machines (SVM) and ANN and found that SVM provided better results while improving the blood vessel tissue features algorithm. Jais et al[32] used multiple machine learning models to classify postoperative data of pterygium. Hanifah et al[33] proposed an automatic classification method for pterygium using an expert system and Viola-Jones image processing systems. Radzi et al[34] quantified the area of pterygium using image analysis software to assist clinical doctors in diagnosis. Similarly, Hilmi et al[35] proposed PRGS, a software for grading the degree of redness in pterygium.
In the past three years, more literature has focused on improving traditional methods of diagnosing pterygium. Researchers have tried to integrate segmentation and classification models into automatic systems for better performance. For example, Ting et al[36] evaluated the features of pterygium by relying on external raters and then used machine learning methods to classify and predict the data. Tanazri et al[37] segmented images using the Hough Transform algorithm and classified them based on the severity of symptoms using an expert system. Similarly, Kim et al[38] pre-segmented regions of interest in images to improve the subsequent classification. Apart from the above, Kumar et al[39] graded diagnosis with the help of fuzzy reasoning system. Cai et al[40] provided a new perspective by discovering a connection between transient receptor potential (TRP) and pterygium. They first analyzed microarray data to screen for differentially expressed genes and selected 20 central genes, and then conducted gene correlation analysis to identify five genes related to TRP. Machine learning methods were then used to determine the relationship between these biomarkers and the occurrence of pterygium.
Overall, traditional methods for assisting in diagnosing pterygium have progressed gradually. Initially, image technology was mainly used to segment pterygium lesions for the convenience of clinical doctors' further diagnosis. Subsequently, researchers began to explore directly obtaining prediction results using algorithms, such as using the results of segmentation algorithms as input for expert systems or fuzzy systems and constructing classification software. However, traditional machine learning algorithms often require manual selection and production of features, especially when annotation costs for image segmentation are high. In addition, multi-stage automatic diagnosis systems have high coupling, significantly limiting the generalization and accuracy improvement of the model.
SUMMARY OF DEEP LEARNING METHODS APPLIED TO THE INTELLIGENT DIAGNOSIS OF PTERYGIUM
Table 2[43]–[58] shows a summary of 16 papers that used deep learning methods to assist in diagnosing pterygium in chronological order, along with their summary information. Different from conventional methods, the researchers first tried on the pterygium classification task due to the end-to-end characteristics of deep learning. In their study published in 2019, Zulkifley et al[43] found that automatic detection of pterygium still relied on fundamental machine learning methods at that time and proposed a deep learning model called Pterygium-Net. This network's architecture is similar to VGG-M and uses transfer learning from ImageNet pre-trained models to achieve pterygium classification. They also used a fully convolutional neural network to localize key areas of pterygium. Pterygium-Net was applied to the dataset provided in the paper[31] and performed well in experiments [area under curve (AUC)=97%]. After that, three improved models experimented on the same dataset in three other papers in the same year. These experiments yielded minor improvements in results, including Abdani et al[44], who proposed a compact convolutional neural network achieving an AUC of 98.65%, Lopez and Aguilera[45], who explored the effect of different color channels on classification performance and obtained the best result of AUC=99.4% with RGB format, and Abdani and Zulkifley[46] who further explored DropOut and DropConnect technologies based on the work in[44] and achieved an AUC of 99.5%.
Table 2. Summary of research using deep learning methods to assist in the intelligent diagnosis of pterygium.
| Year | Task | Sample size | Results | Database | Strength |
| 2019[43] | Classification | 60 pterygium images | Sensitivity=95%; specificity=98.3%; AUC=97% | Derived from[32] | Building a multilayer CNN for pterygium detection. |
| 2019[44] | Classification | 60 pterygium images | Accuracy=98.33%; AUC=98.65% | Derived from[32] | Using compact CNN for pterygium classification. |
| 2019[45] | Classification | 325 pterygium images | AUC=99.4% | Derived from[32] | Exploring the impact of different image color formats. |
| 2019[46] | Classification | 60 images of pterygium | AUC=99.5% | Derived from[32] | Add DropOut and DropConnect technologies. |
| 2019[47] | Classification | 844 pterygium and normal anterior segment photographed images | Accuracy=94.09%; F1 (F1 score)=93.93% | Universiti Kebangsaan Malaysia | Explored the best hyperparameters for the DCNN architecture. |
| 2020[48] | Classification | 193 pterygium ASPIs | Accuracy=99.22%; sensitivity=98.45%; specificity=100%; AUC=100% | Padang Terap Community Centre, Kedah | Experimenting with multiple CNN and proposing the VggNet16-wbn model. |
| 2020[56] | Segmentation | 60 pterygium images | Accuracy=92.02%; IoU=0.8381% | Derived from[32] | Adding densely connected layers reduces the zero-gradient decreasing problem. |
| 2021[49] | Classification and segmentation | 482 pterygium images | F1=90.3%; IoU=0.699 | N/A | Development of a new medical image annotation system called EyeHealer. |
| 2021[52] | Classification and severity grading | 171 images with observation group and 110 image with operation group | Accuracy=94.68%; Kappa=85%, F1=99.74%; sensitivity=100%; specificity=99.64% | Affiliated Eye Hospital of Nanjing Medical University. | Building an automatic classification and grading system based on EfficientNet-B6. |
| 2021[54] | Classification | 144 images with observation period (pterygium) and 150 image with surgery period (pterygium) | F1=96.72%, 82.54%, 85.94%; sensitivity=96.72%, 83.87%, 84.62%; specificity=98.43%, 90.48%, 93.5% | Affiliated Eye Hospital of Nanjing Medical University | Propose a lightweight model to assist in the diagnosis of pterygium. |
| 2021[57] | Segmentation | 328 frontal eye images with pterygium | Accuracy=0.9330; IoU=0.8640 | Australian Pterygium Centre | Enhancing segmentation accuracy with spatial pyramid pooling and group convolution modules. |
| 2022[53] | Severity grading | 176 slit-lamp images with pterygium | F1=81.82%–94.34%; sensitivity=80%–89.29%; specificity=91.67%–100%; accuracy=86.67%–90.63%; | Chang Gung Memorial Hospital | Automatic grading of pterygium based on slit-lamp images with deep learning system. |
| 2022[50] | Classification | 1366 pterygium eyes for internal and 5440 study eyes for external | Internal: AUC=99.5% (sensitivity=98.6%; specificity=99.0%). External: AUC=99.1% (sensitivity=95.9%, specificity=98.5%) | Singapore Epidemiology of Eye Diseases | Validate the generalizability of the model using data from different sources. |
| 2022[51] | Classification and segmentation | 172 anterior segment images of pterygium | Accuracy=94.12%; AUC=0.980 | Jiangxi Provincial People's Hospital | Fusing multiple models together to predict and visualize using Grad-CAM. |
| 2022[58] | Segmentation | 367 pterygium anterior segment images | Classification: F1=99%; sensitivity=98.67%; specificity=99.33% Segmentation: MIoU=86.57%; IoU=78.1%; MPA=92.3% | Affiliated Eye Hospital of Nanjing Medical University | Replace the backbone network of PSPNet with ResNet50 while adding feature fusion. |
| 2023[55] | Classification | 8845 slit-lamp images with pterygium, 563 smartphone-based images of pterygium | F1=0.8981; sensitivity=0.8709; specificity=0.9668; AUC=0.9295 | Xiamen Eye Center of Xiamen University | Improved accuracy of pterygium detection and grading via smartphone based on fusion training method. |
AUC: Area under curve; IoU: Intersection over union; MIoU: Mean intersection over union; MPA: Mean pixel accuracy; ASPI: Anterior segment photographed image; CNN: Convolutional neural network; DCNN: Deep convolutional neural network.
It should be noted that although the above experimental results have demonstrated excellent prospects, the pterygium assisted diagnosis based on deep learning is still in its preliminary stage due to the tiny datasets used in these papers. Therefore, many researchers have begun to try on different datasets and improve models based on different characteristics of the datasets. For example, Saad[47] experimented with a deep convolutional neural network on a dataset containing 844 images of pterygium and non-pterygium, while Zamani et al[48] experimented with six pre-trained CNN models on a dataset containing 193 images of pterygium and constructed a deep convolutional neural network named VggNet16-wbn to achieve better performance. Cai et al[49] constructed and publicly released a large ophthalmic image dataset named EyeHealer and conducted preliminary experiments using deep learning models. Fang et al[50] constructed a deep model on a large dataset containing 6806 pterygium images from different sources. They used the Grad-CAM method to visualize the model's focus and provide some interpretability. Similarly, Gan et al[51] used various models to reduce bias and provide better prediction performance than single models and used the Grad-CAM method to interpret which features are responsible for determining outputs.
After making some progress in the classification model of pterygium, researchers have further explored the refinement of classification and portability. Xu et al[52] constructed a three-level classification model for pterygium, including normal, observation, and surgical periods. Hung et al[53] divided pterygium into four severity levels and used a deep model for classification testing. In terms of portability, researchers proposed the idea of using automatic detection systems on mobile phones. Zheng et al[54] first proposed a lightweight three-level deep model for pterygium classification, which laid the foundation for deploying intelligent diagnosis systems on mobile devices. However, they did not consider the differences between the images captured by mobile devices and clinical fundus images. Liu et al[55] proposed a hybrid model that combines slit-lamp image data and mobile phone image data for model training. This further promotes the possibility of landing pterygium intelligent diagnosis systems on mobile devices.
In addition to classification and grading, some scholars use deep learning models to segment pterygium to assist doctors in diagnosis. Abdani et al[56] used the DeepLab model to address the problem of gradient disappearance during training and integrated denser fully connected layers and feedforward layers to construct an automatic segmentation model. They conducted experiments on the dataset provided in reference[31] and achieved good performance. Then, Abdani et al[57] further improved the segmentation model to cater to the characteristics of pterygium based on the previous experiments. They fused spatial pyramid pooling modules and group convolutions to construct a model named Group-PPM-Net, which performed well on a new dataset. Zhu et al[58] replaced the main visual feature extraction network of the PSP-Net and fused pyramid pooling modules to construct an automatic segmentation model for pterygium.
In summary, deep learning methods have made rapid progress in assisting the diagnosis of pterygium in recent years. This is due to the end-to-end training strategy of deep learning that allows models to automatically extract the required features from images, freeing researchers to focus on improving model architecture and experiments. Currently, deep learning based models for pterygium diagnosis tasks can even match the accuracy of ophthalmologists. However, there are still many challenges to implementing a deep learning based diagnostic system, mainly due to a single dataset, oversized models, high computational costs, and the lack of transparency in deep models. Particularly from an ethical perspective, black-box models are unacceptable[59]. Therefore, there is still a long way to go for deep learning based pterygium diagnosis systems.
DIFFICULTIES AND CHALLENGES
The emergence of AI has provided new possibilities to improve the accuracy and efficiency of pterygium diagnosis, and both traditional machine learning and deep learning methods have shown promising performance. However, current literature on AI-assisted pterygium diagnosis still presents some difficulties and challenges that must be addressed. The main challenges in the AI-assisted treatment of pterygium are as follows:
1) Lack of high-quality data: Due to confidentiality agreements, different researchers or institutions can only conduct experiments on their own small datasets. The generalizability of the models to unseen datasets still needs to be determined. 2) Deep learning models often improve model performance by adding more feature fusion and complex networks, which require high computational resources. The strict operating conditions will limit their large-scale promotion in grassroots institutions. 3) The accuracy and controllability of current AI models are inversely proportional, and some AI models (especially deep learning) lack interpretability, which limits their transparency and accountability and raises concerns about their reliability and safety in critical applications such as medical diagnosis and treatment. 4) Lack of standardized grading system: Pterygium has different clinical manifestations, which make it difficult to make accurate comparisons and classifications. For example, the size, thickness, color, and vascular formation of pterygium lesions may vary, making it more difficult to develop uniform grading standards. This heterogeneity poses significant challenges for machine learning algorithms, which rely on large and consistent datasets for training.
In summary, AI has great potential to improve the accuracy and efficiency of pterygium diagnosis and treatment. However, some difficulties and challenges must be addressed to maximize its potential. These include obtaining high-quality data, reducing model computation costs and deployment difficulties, ensuring the transparency and interpretability of AI models, and developing standardized grading systems.
CONCLUSION
Pterygium is a complex eye disease, and timely diagnosis is crucial for its treatment. However, the traditional diagnostic methods used by ophthalmologists are unable to meet the increasing number of patients. With the development of AI technology, using intelligent models to assist in diagnosing pterygium has become a highly researched area in ophthalmology. This paper collects and focuses on 33 pieces of relevant literature on AI-assisted pterygium diagnosis technology, including both traditional machine learning methods and popular deep learning methods. We also summarize the current difficulties and challenges and aim to help more researchers in the pterygium and computer cross-disciplinary field understand this direction's history and progress. Although the current pterygium automatic diagnosis system based on AI is still in the early stages of development, we believe this technology will become one of the important means for diagnosing and treating pterygium in the future. We look forward to more interdisciplinary cooperation to promote this field's rapid development and provide better clinical diagnosis services.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.61906066); Scientific Research Fund of Zhejiang Provincial Education Department (No.Y202250196); Zhejiang Provincial Philosophy and Social Science Planning Project (No.21NDJC021Z); Natural Science Foundation of Ningbo City (No.202003N4072); Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No.SZGSP014); Sanming Project of Medicine in Shenzhen (No.SZSM202011015); Shenzhen Fundamental Research Program (No.JCYJ20220818103207015).
Conflicts of Interest: Chen B, None; Fang XW, None; Wu MN, None; Zhu SJ, None; Zheng B, None; Liu BQ, None; Wu T, None; Hong XQ, None; Wang JT, None; Yang WH, None.
REFERENCES
- 1.Bibault JE, Burgun A, Fournier L, Dekker A, Lambin P. Artificial Intelligence in Medicine. Amsterdam: Elsevier; 2021. Artificial intelligence in oncology; pp. 361–381. [Google Scholar]
- 2.Li ZX, Koban KC, Schenck TL, Giunta RE, Li QF, Sun YB. Artificial intelligence in dermatology image analysis: current developments and future trends. J Clin Med. 2022;11(22):6826. doi: 10.3390/jcm11226826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lareyre F, Behrendt CA, Chaudhuri A, Lee R, Carrier M, Adam C, Lê CD, Raffort J. Applications of artificial intelligence for patients with peripheral artery disease. J Vasc Surg. 2023;77(2):650–658.e1. doi: 10.1016/j.jvs.2022.07.160. [DOI] [PubMed] [Google Scholar]
- 4.Benet D, Pellicer-Valero OJ. Artificial intelligence: the unstoppable revolution in ophthalmology. Surv Ophthalmol. 2022;67(1):252–270. doi: 10.1016/j.survophthal.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghiasian L, Samavat B, Hadi Y, Arbab M, Abolfathzadeh N. Recurrent pterygium: a review. J Curr Ophthalmol. 2022;33(4):367–378. doi: 10.4103/joco.joco_153_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornejo CAM, Levano ERM. Correlation between pterygium and dry eye: diagnostics and risk factors not considered. Arq Bras Oftalmol. 2022;85(6):649–651. doi: 10.5935/0004-2749.2022-0240. [DOI] [PubMed] [Google Scholar]
- 7.Viveiros MMH, Silva MG, da Costa JGM, de Oliveira AG, Rubio C, Padovani CR, Rainho CA, Schellini SA. Anti-inflammatory effects of α-humulene and β-caryophyllene on pterygium fibroblasts. Int J Ophthalmol. 2022;15(12):1903–1907. doi: 10.18240/ijo.2022.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahraki T, Arabi A, Feizi S. Pterygium: an update on pathophysiology, clinical features, and management. Ther Adv Ophthalmol. 2021;13:25158414211020152. doi: 10.1177/25158414211020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezvan F, Khabazkhoob M, Hooshmand E, Yekta A, Saatchi M, Hashemi H. Prevalence and risk factors of pterygium: a systematic review and meta-analysis. Surv Ophthalmol. 2018;63(5):719–735. doi: 10.1016/j.survophthal.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang WH, Shao Y, Xu YW, et al. Guidelines on Clinical Research Evaluation of Artificial Intelligence in Ophthalmology (2023) Guoji Yanke Zazhi (Int Eye Sci) 2023;23(7):1064–1071. doi: 10.18240/ijo.2023.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudry C, Al Hajj H, Arnould L, Mouriaux F. Analysis of international publication trends in artificial intelligence in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2022;260(5):1779–1788. doi: 10.1007/s00417-021-05511-7. [DOI] [PubMed] [Google Scholar]
- 13.Grzybowski A, Brona P, Lim G, Ruamviboonsuk P, Tan GSW, Abramoff M, Ting DSW. Artificial intelligence for diabetic retinopathy screening: a review. Eye (Lond) 2020;34(3):451–460. doi: 10.1038/s41433-019-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong L, Yang Q, Zhang RH, Wei WB. Artificial intelligence for the detection of age-related macular degeneration in color fundus photographs: a systematic review and meta-analysis. EClinicalMedicine. 2021;35:100875. doi: 10.1016/j.eclinm.2021.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murtagh P. Current applications of machine learning in the screening and diagnosis of glaucoma: a systematic review and Meta-analysis. Int J Ophthalmol. 2020;13(1):149–162. doi: 10.18240/ijo.2020.01.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdani SR, Zulkifley MA, Shahrimin MI, Zulkifley NH. Computer-assisted pterygium screening system: a review. Diagnostics. 2022;12(3):639. doi: 10.3390/diagnostics12030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Ge QM, Shu HY, Liao XL, Liang RB, Li QY, Zhang LJ, Gao GP, Shao Y. Decreased retinal microvasculature densities in pterygium. Int J Ophthalmol. 2021;14(12):1858–1867. doi: 10.18240/ijo.2021.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alqahtani J. The prevalence of pterygium in Alkhobar: a hospital-based study. J Fam Community Med. 2013;20(3):159. doi: 10.4103/2230-8229.121980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oner FH, Kaderli B, Durak I, Cingil G. Analysis of the pterygium size inducing marked refractive astigmatism. Eur J Ophthalmol. 2000;10(3):212–214. doi: 10.1177/112067210001000304. [DOI] [PubMed] [Google Scholar]
- 20.Mahar PS, Manzar N. Pterygium recurrence related to its size and corneal involvement. J Coll Physicians Surg Pak. 2013;23(2):120–123. [PubMed] [Google Scholar]
- 21.Papa JP, Pagnin A, Schellini SA, Spadotto A, Guido RC, Ponti M, Chiachia G, Falcao AX. Feature selection through gravitational search algorithm. 2011 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) https://ieeexplore.ieee.org/abstract/document/5946916. Accessd on July 11, 2011. [Google Scholar]
- 22.Abdani SR, Zaki WMDW, Hussain A, et al. Particle swarm optimization-based thresholding for corneal segmentation in pterygium detection. Asian Journal of Information Technology. 2016;15(11):1845–1850. [Google Scholar]
- 23.Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 24.Lee CS, Baughman DM, Lee AY. Deep learning is effective for classifying normal versus age-related macular degeneration OCT images. Ophthalmol Retina. 2017;1(4):322–327. doi: 10.1016/j.oret.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, He Y, Keel S, Meng W, Chang RT, He M. Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology. 2018;125(8):1199–1206. doi: 10.1016/j.ophtha.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Wong DWK, Aryaputera AW, Sun Y, Cheng CY, Cheung C, Wong TY. Automatic pterygium detection on cornea images to enhance computer-aided cortical cataract grading system. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. doi: 10.1109/EMBC.2012.6346950. https://ieeexplore.ieee.org/abstract/document/6346950. Accessed on November 10, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Mesquita RG, Figueiredo EMN. An algorithm for measuring pterygium's progress in already diagnosed eyes. 2012 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) https://ieeexplore.ieee.org/abstract/document/6287988. Accessed on August 30, 2012. [Google Scholar]
- 28.Abdani SR, Zaki WMDW, Mustapha A, Hussain A. Iris segmentation method of pterygium anterior segment photographed image. 2015 IEEE Symposium on Computer Applications & Industrial Electronics (ISCAIE) https://ieeexplore.ieee.org/abstract/document/7298330. Accessed on October 15, 2015. [Google Scholar]
- 29.Abdani SR, Zaki WMDW, Hussain A, Mustapha A. An adaptive nonlinear enhancement method using sigmoid function for iris segmentation in pterygium cases. 2015 International Electronics Symposium (IES) https://ieeexplore.ieee.org/abstract/document/7380813. Accessed on January 14, 2016. [Google Scholar]
- 30.Azemin MZC, Gaffur NA, Hilmi MR, et al. Benchmarked pterygium images for human and machine graders. Journal of Engineering & Applied Sciences. 2016;11:2378–2382. [Google Scholar]
- 31.Wan Zaki WMD, Mat Daud M, Abdani SR, Hussain A, Mutalib HA. Automated pterygium detection method of anterior segment photographed images. Comput Methods Programs Biomed. 2018;154:71–78. doi: 10.1016/j.cmpb.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Jais FN, Azemin MZC, Hilmi MR, Tamrin MIM, Kamal KM. Postsurgery classification of best-corrected visual acuity changes based on pterygium characteristics using the machine learning technique. Sci World J. 2021;2021:6211006. doi: 10.1155/2021/6211006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanifah U, Kusuma PD, Setianingsih C. Detection of pterygium disease using forward chaining and viola jones algorithm. 2018 International Conference on Control, Electronics, Renewable Energy and Communications (ICCEREC) https://ieeexplore.ieee.org/abstract/document/8712102. Accessed on May 13, 2019. [Google Scholar]
- 34.Radzi HM, Khairidzan MK, Zulfaezal CAM, Azrin EA. Corneo-pterygium total area measurements utilising image analysis method. J Optom. 2019;12(4):272–277. doi: 10.1016/j.optom.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilmi MR, Che Azemin MZ, Mohd Kamal K, et al. Reliability of pterygium redness grading software (PRGS) in describing different types of primary pterygia based on appearance. Sains Malays. 2020;49(5):1015–1020. [Google Scholar]
- 36.Ting DSJ, Liu YC, Patil M, Ji AJS, Fang XL, Tham YC, Lee YF, Htoon HM, Mehta JS. Proposal and validation of a new grading system for pterygium (SLIT2) Br J Ophthalmol. 2021;105(7):921–924. doi: 10.1136/bjophthalmol-2020-315831. [DOI] [PubMed] [Google Scholar]
- 37.Tanazri AS, Kusuma PD, Setianingsih C. Detection of pterygium disease using Hough transform and forward chaining. 2021 1st International Conference On Cyber Management And Engineering (CyMaEn) https://ieeexplore.ieee.org/abstract/document/9497292. Accessed on July 30, 2021. [Google Scholar]
- 38.Kim JH, Kim YJ, Lee YJ, Hyon JY, Han SB, Kim KG. Automated histopathological evaluation of pterygium using artificial intelligence. Br J Ophthalmol. 2023;107(5):627–634. doi: 10.1136/bjophthalmol-2021-320141. [DOI] [PubMed] [Google Scholar]
- 39.Kumar HSV, Jayaram MA. Grading Severity of Pterygium using Fuzzy Reasoning. 2022 IEEE International Conference on Data Science and Information System (ICDSIS) https://ieeexplore.ieee.org/abstract/document/9916017. Accessed on October 14, 2022. [Google Scholar]
- 40.Cai YC, Zhou TY, Chen J, Cai XY, Fu Y. Uncovering the role of transient receptor potential channels in pterygium: a machine learning approach. Inflamm Res. 2023;72(3):589–602. doi: 10.1007/s00011-023-01693-4. [DOI] [PubMed] [Google Scholar]
- 41.Hilmi MR, Kamal KM, Ariffin AE, Norazmar NA, Maruziki NN, Musa NH, Mardhiah SN, Azemin MZ, Azami MH, Rahim MASA. Effects of different types of primary pterygium on changes in oculovisual function. Sains Malays. 2020;67:252–270. [Google Scholar]
- 42.Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115(10):1235–1240. doi: 10.1001/archopht.1997.01100160405001. [DOI] [PubMed] [Google Scholar]
- 43.Zulkifley MA, Abdani SR, Zulkifley NH. Pterygium-Net: a deep learning approach to pterygium detection and localization. Multimed Tools Appl. 2019;78(24):34563–34584. [Google Scholar]
- 44.Abdani S R, Zulkifley M A, Hussain A. Compact convolutional neural networks for pterygium classification using transfer learning. 2019 IEEE International Conference on Signal and Image Processing Applications (ICSIPA) https://ieeexplore.ieee.org/abstract/document/8977757. Accessed on February 3, 2020. [Google Scholar]
- 45.Lopez YP, Aguilera LR. Automatic classification of pterygium-non pterygium images using deep learning. Proceedings of the VII ECCOMAS Thematic Conference on Computational Vision and Medical Image Processing. https://linkspringer.53yu.com/chapter/10.1007/978-3-030-32040-9_40. Accessed on September 28, 2019. [Google Scholar]
- 46.Abdani SR, Zulkifley M. Pterygium Screening using Compact Convolutional Neural Networks. Univer Malays. 2019 [Google Scholar]
- 47.Saad AH. Automated pterygium detection in anterior segment photographed images using deep convolutional neural network. Int J Adv Trends Comput Sci Eng. 2019;8(1.6):225–232. [Google Scholar]
- 48.Zamani NSM, Diyana Wan Zaki WM, Huddin AB, Hussain A, Mutalib HA, Ali A. Automated pterygium detection using deep neural network. IEEE Access. 2020;8:191659–191672. [Google Scholar]
- 49.Cai WJ, Xu J, Wang K, et al. EyeHealer: a large-scale anterior eye segment dataset with eye structure and lesion annotations. Prec Clin Med. 2021;4(2):85–92. doi: 10.1093/pcmedi/pbab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang X, Deshmukh M, Chee ML, et al. Deep learning algorithms for automatic detection of pterygium using anterior segment photographs from slit-lamp and hand-held cameras. Br J Ophthalmol. 2022;106(12):1642–1647. doi: 10.1136/bjophthalmol-2021-318866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gan F, Chen WY, Liu H, Zhong YL. Application of artificial intelligence models for detecting the pterygium that requires surgical treatment based on anterior segment images. Front Neurosci. 2022;16:1084118. doi: 10.3389/fnins.2022.1084118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu W, Jin L, Zhu PZ, He K, Yang WH, Wu MN. Implementation and application of an intelligent pterygium diagnosis system based on deep learning. Front Psychol. 2021;12:759229. doi: 10.3389/fpsyg.2021.759229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung KH, Lin C, Roan J, et al. Application of a deep learning system in pterygium grading and further prediction of recurrence with slit lamp photographs. Diagnostics. 2022;12(4):888. doi: 10.3390/diagnostics12040888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng B, Liu YF, He K, et al. Research on an intelligent lightweight-assisted pterygium diagnosis model based on anterior segment images. Dis Markers. 2021;2021:1–8. doi: 10.1155/2021/7651462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu YW, Xu CS, Wang SP, et al. Accurate detection and grading of pterygium through smartphone by a fusion training model. Br J Ophthalmol. 2023:bjo–2022. doi: 10.1136/bjo-2022-322552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdani SR, Zulkifley MA, Moubark AM. Pterygium tissues segmentation using densely connected deeplab. 2020 IEEE 10th symposium on computer applications & industrial electronics (ISCAIE) https://ieeexplore.ieee.org/abstract/document/9108822. Accessed on June 5, 2020. [Google Scholar]
- 57.Abdani SR, Zulkifley MA, Zulkifley NH. Group and shuffle convolutional neural networks with pyramid pooling module for automated pterygium segmentation. Diagnostics. 2021;11(6):1104. doi: 10.3390/diagnostics11061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu SJ, Fang XW, Qian Y, He K, Wu MN, Zheng B, Song JY. Pterygium screening and lesion area segmentation based on deep learning. J Healthc Eng. 2022;2022:1–9. doi: 10.1155/2022/3942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ciravegna G, Barbiero P, Giannini F, Gori M, Liò P, Maggini M, Melacci S. Logic explained networks. Artif Intell. 2023;314:103822. [Google Scholar]