Author's summary
While coronary physiology and plaque vulnerability have been considered distinct attributes of coronary artery disease, evaluated from different diagnostic modalities, clinical data and underlying pathophysiology have demonstrated that there is a strong connection between hemodynamic properties and vulnerable plaque. This link suggests that using invasive physiological indexes to identify functional significance may also be somewhat useful in detecting plaque vulnerability. Furthermore, emerging data have suggested the independent and synergistic prognostic value between a physiology-based approach and a plaque-based approach, and understanding the prognostic relevance between the two can optimize risk prediction and treatment decision-making.
Keywords: Coronary artery disease, Atherosclerosis
Abstract
In the catheterization laboratory, the measurement of physiological indexes can help identify functionally significant lesions and has become one of the standard methods to guide treatment decision-making. Plaque vulnerability refers to a coronary plaque susceptible to rupture, enabling risk prediction before coronary events, and it can be detected by defining a certain type of plaque morphology on coronary imaging modalities. Although coronary physiology and plaque vulnerability have been considered different attributes of coronary artery disease, the underlying pathophysiological basis and clinical data indicate a strong correlation between coronary hemodynamic properties and vulnerable plaque. In prediction of coronary events, emerging data have suggested independent and additional implications of a physiology-based approach to a plaque-based approach. This review covers the fundamental interplay between coronary physiology and plaque morphology during disease progression with clinical data supporting this relationship and examines the clinical relevance of physiological indexes in prediction of clinical outcomes and therapeutic decision-making along with plaque vulnerability.
INTRODUCTION
The physiological assessment of coronary artery disease (CAD) in the catheterization laboratory, including fractional flow reserve (FFR) or non-hyperemic pressure ratio (NHPR) measurement, can identify functionally significant lesions that cannot be reliably detected by invasive coronary angiography alone.1),2),3) Currently, a physiology-based approach has become one of the standard methods for revascularization decision-making.4) In the meantime, imaging modalities have been used to identify vulnerable plaque susceptible to rupture, thus enabling risk prediction before coronary events.5),6) Although functional significance and morphological plaque characteristics have traditionally been regarded as distinct characteristics of CAD, the pathophysiological basis and emerging clinical data have shown a close association between coronary hemodynamics and plaque vulnerability.7) Therefore, it is essential to understand the clinical value and pathophysiological basis of coronary physiology in relation to plaque vulnerability and the functional significance of coronary lesions. In this review, we aimed to explore the fundamental interplay between coronary hemodynamics and plaque morphology during disease progression, provide supporting clinical data for their relationship, and examine the independent and additive prognostic implications of a physiology-based approach along with plaque vulnerability.
PATHOPHYSIOLOGICAL BASIS FOR CORONARY HEMODYNAMIC PROPERTIES AND PLAQUE VULNERABILITY INTERACTION
Defining plaque vulnerability
Acute coronary syndrome is a leading cause of death worldwide,8) with over two-thirds of cases attributable to plaque rupture, in which a plaque ruptures suddenly and leads to the formation of a thrombus and myocardial infarction.9),10) Plaque vulnerability typically refers to a specific type of plaque associated with a high risk of acute coronary events. Thin cap fibroatheroma (TCFA), defined as a thin fibrous cap (<65 μm) with a large lipid pool or necrotic core, has been identified as a vulnerable plaque based on postmortem histopathological studies.11) Microcalcifications, inflammatory infiltrates, such as activated macrophages, cholesterol crystals, and intraplaque hemorrhage, are additional precursors of ruptured plaque.12) Various invasive and non-invasive coronary imaging modalities, including optical coherence tomography (OCT), intravascular ultrasound (IVUS), coronary computed tomography (CT) angiography (CCTA), or near-infrared spectroscopy (NIRS), can identify high-risk plaque features as surrogate markers for vulnerable plaque and clinical outcomes.12),13),14),15),16) Additionally, plaque quantity has been proposed as a determinant of the prevalence and prognostic significance of vulnerable plaque. The prevalence of OCT-derived TCFA correlates with anatomical severity in three-vessel OCT studies.17),18) The relationship between high-risk plaque characteristics and adverse cardiovascular events depended on the presence of a large amount of plaque in several studies,14),19),20) implying that plaque quality and quantity should be considered in defining vulnerable plaque. Moreover, plaque morphology observed at a single time point cannot fully determine the fate of plaque.21) The progression from atherosclerosis to vulnerable plaque and subsequent coronary events is a dynamic process involving continuous interaction of a plaque with the surrounding coronary anatomy and environment, particularly with hemodynamic properties.7)
Role of hemodynamic properties in vulnerable plaque development
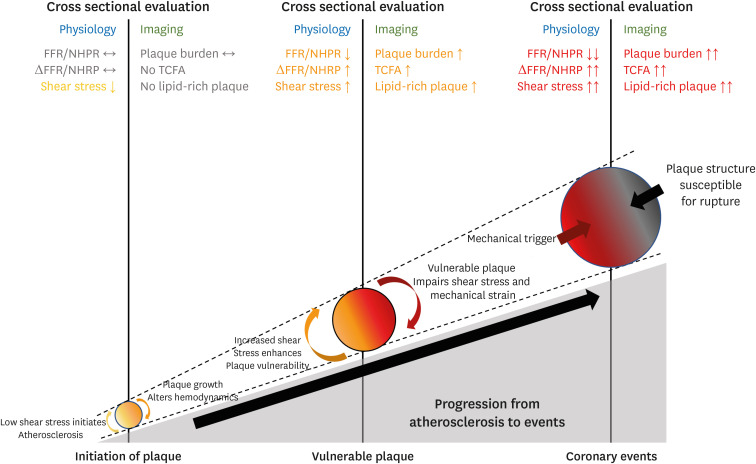
Wall shear stress, the normalized tangential force exerted on the vessel wall, regulates the pro-inflammatory pathway perceived by endothelial cells.22) At specific sites within the arterial system exposed to low wall shear stress, atherosclerosis can be initiated by increased inflammatory activity involving the interaction of circulating inflammatory cells and adhesion molecules expressed on the activated endothelial cells.23) This results in the transdifferentiation of monocytes into foams cells and the formation of the fatty streak, which initiates atherosclerotic plaque formation.24) As the plaque grows and luminal narrowing progresses, shear stress increases in the upstream segment of a lesion, and high wall shear stress can promote plaque vulnerability.25),26),27) High wall shear stress can inhibit smooth muscle cell proliferation and induce apoptosis, resulting in the thinning of the fibrous cap and a reduction in total fibrous tissue.28),29) Furthermore, high wall shear stress is associated with vascular inflammation, larger necrotic core, and platelet activation, adhesion, and aggregation, which can increase the risk of rupture.30),31) Ultimately, at the stage of coronary events, excessive mechanical strain can weaken the fibrous cap and cause plaque rupture,26),32),33) thereby increasing the probability of fatal coronary events associated with vulnerable plaque.7) Therefore, hemodynamic properties should be regarded as an essential component of coronary events and a surrogate marker for plaque vulnerability throughout the natural progression of atherogenesis to clinical events (Figure 1).
Figure 1. Coronary events developed by the pathophysiological interplay between coronary hemodynamic properties and plaque morphology.
Throughout the atherosclerosis progress, coronary hemodynamic properties and plaque morphology continually interact with each other at every step, ultimately leading to subsequent coronary events. At each step, hemodynamic and plaque aspects of coronary lesions can be correlated, which explains the association between physiological indexes and plaque vulnerability on cross-sectional evaluation.
FFR = fractional flow reserve; NHPR = non-hyperemic pressure ratio; TCFA = thin cap fibroatheroma.
CLINICAL EVIDENCE FOR ASSOCIATION BETWEEN PHYSIOLOGICAL CHARACTERISTICS AND PLAQUE VULNERABILITY
In vivo association between coronary hemodynamics and vulnerable plaque
In vivo studies have demonstrated the relationship between coronary hemodynamics and plaque vulnerability. An initial prospective study using a combination of IVUS and coronary angiography in human coronary arteries reported that non-obstructive lesions with low wall shear stress were associated with increased plaque thickness and outward remodeling.34) This finding was confirmed by the Prediction of Progression of Coronary Artery Disease and Clinical Outcome Using Vascular Profiling of Shear Stress and Wall Morphology (PREDICTION) study, which reported that larger plaque burden and low wall shear stress were independent predictors of plaque progression and luminal narrowing requiring coronary revascularization.35) Another prospective IVUS study further validated the association between low wall shear stress and plaque progression and constrictive remodeling. However, high wall shear stress was related to greater necrotic core progression and expansive remodeling, resulting in a more vulnerable plaque phenotype.27) Furthermore, low and high wall shear stress had a greater predictive value than plaque burden for plaque progression or plaque phenotype,36),37) demonstrating the significant effect of hemodynamics on plaque formation and destabilization.38) Wall shear stress was consistently associated with high-risk plaque features on other imaging modalities. Lipid-rich plaque identified by NIRS and OCT, which are exposed to low wall shear stress, were associated with accelerated plaque growth.39),40) Low shear stress can predict active microcalcifications detected by 18F-NaF positron emission tomography.41) In obstructive lesions, high wall shear stress in the proximal segments was an independent predictor of the presence of OCT-derived TCFA.42) This relationship of low and high wall shear stress with adverse plaque was similarly demonstrated using CCTA-derived adverse plaque characteristics, including low-attenuation plaque positive remodeling, spotty calcification, or napkin-ring sign.43),44),45),46)
Complex association between physiological indexes and plaque quality and quantity
In addition to substantial clinical evidence for the link between hemodynamic properties surrounding a coronary lesion and its vulnerability, several studies investigated the association of physiological indexes that can be measured in the catheterization laboratory with plaque vulnerability (Table 1),47),48),49) demonstrating the prevalence of OCT-derived TCFA was higher in lesions with low coronary pressure or flow indexes. A recent study expanded the association between various physiological indexes and the CCTA-derived plaque quantity and quality.50) High disease burden, characterized by a large plaque burden and small minimum lumen area (MLA), was predicted by resting and hyperemic pressure, and coronary flow reserve. However, resting and hyperemic pressure and microvascular resistance were associated with adverse plaque, defined by low attenuation plaque, and positive remodeling.50) When the association of plaque characteristics with physiological indexes was investigated, impaired myocardial blood flow on [15O]H2O positron emission tomography and FFR were predicted by partially calcified plaques, low attenuation plaque, and positive remodeling, with a value that was incremental over stenosis severity.51) Moreover, in the prediction of FFR ≤0.80, the best plaque metrics interrogated by the machine learning algorithm were MLA, percent atheroma volume, fibrofatty and necrotic core volume, plaque volume, proximal left anterior descending coronary artery lesion, and remodeling index, indicating a complex interaction between luminal stenosis, plaque quantity, and quality, and functional significance.52) How physiological indexes representing a functional status are associated with plaque vulnerability can be appreciated by the correlation of translesional pressure drop with local hemodynamic parameters32),53) and the independent relationship between per-vessel coronary pressure and flow with local shear stress.54),55) Therefore, the use of invasive physiological indexes to define functional significance can also be associated to the identification of plaque vulnerability to some extent.56)
Table 1. Association of physiological indexes with plaque vulnerability.
| Study | Publication year | Number of lesions (patients) | Prevalence of OCT-derived TCFAs, n (%) | p value | |
|---|---|---|---|---|---|
| Usui et al.47) | 2018 | 382 (340) | FFR >0.79 | 15 (11.7) | 0.02 |
| FFR 0.72–0.79 | 14 (10.8) | ||||
| FFR <0.72 | 27 (21.8) | ||||
| Hoshino et al.48) | 2020 | 473 (419) | FFR >0.80 & CFR >2.0 | 8 (7.8) | <0.01 |
| FFR >0.80 & CFR ≤2.0 | 6 (23.1) | ||||
| FFR ≤0.80 & CFR >2.0 | 14 (8.2) | ||||
| FFR ≤0.80 & CFR ≤2.0 | 38 (22.0) | ||||
| Zuo et al.49) | 2021 | 132 (126) | QFR >0.93 | 5 (19.2) | <0.01 |
| QFR 0.85–0.93 | 4 (13.8) | ||||
| QFR ≤0.85 | 14 (50.0) | ||||
CFR = coronary flow reserve; FFR = fractional flow reserve; OCT = optical coherence tomography; QFR = quantitative flow ratio; TCFA = thin cap fibroatheroma.
INDEPENDENT PROGNOSTIC IMPLICATIONS OF CORONARY HEMODYNAMICS ON CORONARY EVENTS
Independent role of coronary hemodynamics on coronary events
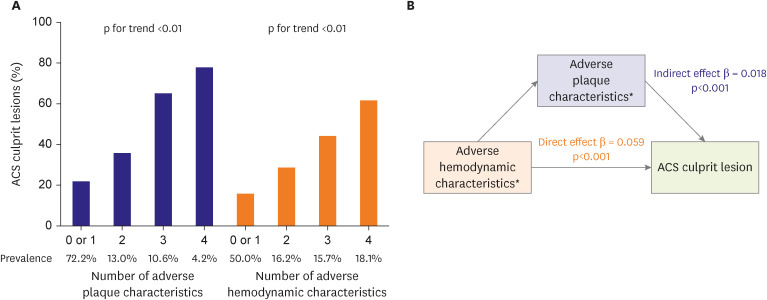
Given the pathophysiological basis and clinical evidence between coronary physiology and plaque vulnerability, the next question would be whether the hemodynamic assessment is a surrogate marker for plaque vulnerability or an independent predictor for future coronary events. In the Exploring the MEchanism of Plaque Rupture in Acute Coronary Syndrome Using Coronary CT Angiography and computationaL Fluid Dynamic (EMERALD) study that compared CCTA-derived plaque and hemodynamic characteristics between non-culprit and culprit lesions in patients with acute coronary syndrome (ACS),57) the likelihood of culprit lesions significantly increased with the increasing number of adverse plaque characteristics and adverse hemodynamic characteristics (i.e., FFRCT ≤0.80, ΔFFRCT ≥0.06, wall shear stress ≥154.7 dyn/cm2, and axial plaque stress ≥1,606.6 dyn/cm2) (Figure 2). Using the number of adverse plaque characteristics as a mediator in a mediation analysis revealed that adverse hemodynamic characteristics had direct and indirect effects on ACS culprit lesions (Figure 2). These findings indicate that hemodynamic properties may have an independent prognostic impact and a mediating impact through plaque vulnerability on plaque rupture events. Moreover, this notion is supported by previous studies demonstrating a correlation between the shear stress concentration and the plaque rupture site.25),58) High wall shear stress is independently associated with plaque rupture and erosion in patients with ACS.59) Increased plaque structural stress is also associated with a higher risk of ruptured plaque,60) and high wall shear stress derived from three-dimensional quantitative coronary angiography predicted plaque progression61) and impending myocardial infarction (MI).62),63) Therefore, the current evidence suggests that hemodynamic properties can serve as independent prognostic factors for coronary events as well as indicators for vulnerable plaque.
Figure 2. Prognostic value of coronary hemodynamic properties in relation to plaque characteristics.
In the EMERALD study that included 216 lesions from 72 patients with ACS, lesion characteristics were compared between 66 culprit and 150 non-culprit lesions on CCTA taken before ACS events. (A) The correlation of the number of adverse plaque characteristics (i.e., low-attenuation plaque, positive remodeling, spotty calcification, and napkin-ring sign) and adverse hemodynamic characteristics (i.e., FFRCT ≤0.80, ΔFFRCT ≥0.06, WSS ≥154.7 dyn/cm2, and axial plaque stress ≥1,606.6 dyn/cm2) with the proportion of culprit lesions is shown. (B) The mediation analysis investigated the direct and indirect impact of adverse hemodynamic characteristics on ACS culprit lesions mediated by adverse plaque characteristics.
ACS = acute coronary syndrome; CCTA = coronary computed tomography angiography; CT = computed tomography; EMERALD = Exploring the MEchanism of Plaque Rupture in Acute Coronary Syndrome Using Coronary CT Angiography and computationaL Fluid Dynamic; FFRCT = CCTA-derived fractional flow reserve; WSS = wall shear stress.
*The number of adverse plaque or hemodynamic characteristics is shown.
Outcome comparison between physiology- and plaque-based approach
The relationship between coronary hemodynamics with coronary events was similarly supported by the clinical data evaluating the relationship between physiological indexes and outcomes. In the international pooled registry with patients who underwent invasive flow and pressure measurement, the 5-year incidence of cardiac death or target vessel MI was higher in patients with low coronary flow reserve or low FFR.64) When treatment decision-making was guided by physiological indexes, revascularized low FFR lesions showed a lower risk of cardiac death or MI than medically treated low FFR lesions in an individual patient-level meta-analysis,65) indicating the efficacy of physiological indexes-based revascularization in treating patient’s vulnerability. In this context that both physiology and plaque assessment may be used to identify and treat the patient’s vulnerability, a recent randomized controlled study tested the non-inferiority of FFR-guided treatment strategy (revascularization if FFR ≤0.80) to the IVUS-guided treatment strategy (revascularization if MLA <3 mm2 or 3 mm2< MLA ≤4 mm2 with plaque burden ≥70%) in patients with intermediate stenosis and demonstrated comparable 2-year outcomes of all-cause death, MI, and revascularization.66) A previous network meta-analysis evaluated the similarity in outcomes following physiology- and imaging-guided treatment, which strongly supports the independent prognostic impact of each modality on clinical outcomes.67) Ongoing studies will shed more light on the direct comparability of physiology-guided and plaque vulnerability-guided treatment.
INTEGRATIVE PHYSIOLOGY AND PLAQUE ASSESSMENT AND FUTURE DIRECTIONS
Additive prognostic value of combined physiological and plaque assessment
Since physiological assessment offers prognostic implications independent of plaque vulnerability, obtaining hemodynamic properties and plaque morphology may provide the most comprehensive prognostic data. Several studies have shown the efficacy of integrative physiology and plaque assessment in predicting clinical outcomes. In lesions with FFR >0.80, which can be safely deferred from revascularization according to the current guideline,4) OCT-derived TCFA was present in approximately 25% of lesions and was associated with an increased risk of a composite of cardiac death, target vessel MI, clinically driven target lesion revascularization or hospitalization due to unstable angina until 5-years among patients with diabetes.68),69) This finding was consistent in that IVUS, or CCTA-based adverse plaque features were also independent prognostic indicators in deferred lesions with FFR >0.80.52),70),71) Similarly, in lesions without high-risk plaque, adverse hemodynamic characteristics can distinguish culprit lesions from non-culprit lesions of ACS events,53) indicating that physiological indexes and plaque features can complement each other to define high-risk lesions that a single modality cannot capture. Furthermore, several studies reported that abnormal physiological indexes and adverse plaque features could predict future clinical events synergistically. The likelihood of ACS culprit lesions was higher in lesions with both adverse plaque and hemodynamic characteristics than in those with none.57),72) The risk of vessel-specific composite outcomes was increased proportionally with FFR ≤0.80, high local and global burden, and adverse plaque.73) In a recent report from the first-in-human study of CCTA-derived FFR, the predictability for per-lesion and vessel 10-year outcomes was highest when plaque and hemodynamic predictors were added to baseline clinical and lesion characteristics, suggesting that physiological and plaque components may contribute synergistically even very long-term coronary events.74)
Future perspectives of hemodynamic approach for plaque vulnerability and clinical outcomes
Since standard physiological indexes, including FFR or NHPR, are per-vessel indicators of functional significance, they are limited for assessing the lesion-specific risk for future coronary events.7),21) Recently, novel hemodynamic indexes that can be used in the catheterization laboratory for better lesion-level evaluation, including plaque vulnerability, increased predictability for outcomes, and optimized guiding for appropriate treatment decision-making, have been introduced (Table 2). ΔFFR is a simplified index defined as the difference between the proximal and distal FFR. Several studies have demonstrated that ΔFFR may have additive predictive values for plaque vulnerability and clinical outcomes to FFR.53),57),74) The strength of ΔFFR is its simplicity in that ΔFFR can be obtained using well-defined FFR pullback estimation without additional analysis tools. The prognostic value of ΔFFR or ΔNHPR will be demonstrated further in ongoing studies, such as the Prognostic Impact of Lesion-specific Hemodynamic Index in Patients With Coronary Artery Disease (PRIME-FFR) study (NCT05250557) and the Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect: Guided Physiologic Stenting (DEFINE GPS) study (NCT04451044). Pressure pullback gradient (PPG), calculated by the combined maximal pressure gradient and the functional lesion length, can characterize a physiological pattern of a vessel as physiological focal or diffuse CAD.75) Given that percutaneous coronary intervention (PCI) is a local treatment that relieves the pressure gradient of a lesion, the derivation of PPG, in addition to FFR, is expected to define further suitable PCI candidates that benefit from revascularization.76) Radial wall strain is a new index representing lesion-specific biomechanical properties and vessel wall distortion during the heartbeat cycle. Recent studies have shown that high maximal radial wall strain along the lesion segment was associated with a higher prevalence of vulnerable plaque and an increased risk of lesion progression and clinical events in non-ischemic lesions.77),78),79) With these evolving physiological approaches for plaque vulnerability and clinical outcomes, physicians will be in a better position to make decisions and administer treatment to patients with CAD.
Table 2. New physiological indexes representing plaque vulnerability and lesion-specific hemodynamics properties.
| Hemodynamic metrics | Derivation | Physiological implications | Clinical evidence |
|---|---|---|---|
| ΔFFR or ΔNHPR | Proximal FFR or NHPR − Distal FFR or NHPR | • A lesion-specific pressure drop | • ΔFFR was correlated with lesion-specific hemodynamic properties surrounding a plaque, such as pressure gradient, wall shear stress, or axial plaque stress.53) |
| • High ΔFFR (≥0.06) was associated with a higher risk of ACS and long-term clinical events.57),74) | |||
| PPG |
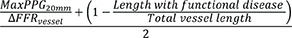
|
• A physiological disease pattern (focal vs. diffuse) of a vessel | • Revascularization for focal physiological disease, defined by high PPG, was related to better improvement in post-PCI FFR and larger minimal stent area.76) |
| RWSmax | Maximal RWS  along the lesion segment along the lesion segment |
• A quantified coronary strain of a lesion | • RWSmax was correlated with OCT-derived lipid-rich plaque and a prevalence of TCFA.77) |
| • Among intermediate coronary lesions, high RWSmax predicted lesion progression.78) | |||
| • In deferred lesions with QFR >0.80 in the FAVOR III China study, high RWSmax was predictive of 1-year clinical events.79) |
ACS = acute coronary syndrome; FFR = fractional flow reserve; NHPR = non-hyperemic pressure ratio; OCT = optical coherence tomography; PCI = percutaneous coronary intervention; PPG = pullback pressure gradient; QFR = quantitative flow ratio; RWSmax = maximal radial wall strain; TCFA = thin cap fibroatheroma.
CONCLUSION
Plaque vulnerability and subsequent acute coronary events are determined by the continuous interaction between plaque morphology and the hemodynamic properties of the surrounding plaque, which is the basis for the correlation of abnormal physiological indexes with the presence of vulnerable plaque. A coronary physiology-based approach provides an independent and additional prognostic value to a plaque-based approach. Using integrative physiology and plaque assessment, along with emerging physiological indexes, will allow for improved risk assessment and treatment decision-making in the management of CAD.
Footnotes
Funding: Dr. Bon-Kwon Koo received an Institutional Research Grant from Abbott Vascular, Philips Volcano, Boston Scientific, and HeartFlow.
Conflict of Interest: Seokhun Yang has no financial conflicts of interest.
Data Sharing Statement: The data generated in this study is available from the corresponding author(s) upon reasonable request.
- Conceptualization: Yang S, Koo BK.
- Data curation: Yang S, Koo BK.
- Formal analysis: Yang S, Koo BK.
- Funding acquisition: Koo BK.
- Investigation: Yang S, Koo BK.
- Methodology: Yang S, Koo BK.
- Project administration: Koo BK.
- Supervision: Koo BK.
- Validation: Yang S, Koo BK.
- Visualization: Koo BK.
- Writing - original draft: Yang S, Koo BK.
- Writing - review & editing: Yang S, Koo BK.
References
- 1.Kogame N, Ono M, Kawashima H, et al. The impact of coronary physiology on contemporary clinical decision making. JACC Cardiovasc Interv. 2020;13:1617–1638. doi: 10.1016/j.jcin.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Hwang D, Yang S, Zhang J, Koo BK. Physiologic assessment after coronary stent implantation. Korean Circ J. 2021;51:189–201. doi: 10.4070/kcj.2020.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JM, Doh JH, Nam CW, Shin ES, Koo BK. Functional approach for coronary artery disease: filling the gap between evidence and practice. Korean Circ J. 2018;48:179–190. doi: 10.4070/kcj.2017.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 5.Tomaniak M, Katagiri Y, Modolo R, et al. Vulnerable plaques and patients: state-of-the-art. Eur Heart J. 2020;41:2997–3004. doi: 10.1093/eurheartj/ehaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KY, Chang K. Understanding vulnerable plaques: current status and future directions. Korean Circ J. 2019;49:1115–1122. doi: 10.4070/kcj.2019.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Koo BK, Narula J. Interactions between morphological plaque characteristics and coronary physiology: from pathophysiological basis to clinical implications. JACC Cardiovasc Imaging. 2022;15:1139–1151. doi: 10.1016/j.jcmg.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 9.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014;11:379–389. doi: 10.1038/nrcardio.2014.62. [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Montarello NJ, Hoogendoorn A, et al. Multimodality intravascular imaging of high-risk coronary plaque. JACC Cardiovasc Imaging. 2022;15:145–159. doi: 10.1016/j.jcmg.2021.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 14.Erlinge D, Maehara A, Ben-Yehuda O, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet. 2021;397:985–995. doi: 10.1016/S0140-6736(21)00249-X. [DOI] [PubMed] [Google Scholar]
- 15.Prati F, Romagnoli E, Gatto L, et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur Heart J. 2020;41:383–391. doi: 10.1093/eurheartj/ehz520. [DOI] [PubMed] [Google Scholar]
- 16.Chang HJ, Lin FY, Lee SE, et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J Am Coll Cardiol. 2018;71:2511–2522. doi: 10.1016/j.jacc.2018.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian J, Dauerman H, Toma C, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol. 2014;64:672–680. doi: 10.1016/j.jacc.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 18.Araki M, Soeda T, Kim HO, et al. Spatial distribution of vulnerable plaques: comprehensive in vivo coronary plaque mapping. JACC Cardiovasc Imaging. 2020;13:1989–1999. doi: 10.1016/j.jcmg.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Cheng JM, Garcia-Garcia HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J. 2014;35:639–647. doi: 10.1093/eurheartj/eht484. [DOI] [PubMed] [Google Scholar]
- 20.Schuurman AS, Vroegindewey MM, Kardys I, et al. Prognostic value of intravascular ultrasound in patients with coronary artery disease. J Am Coll Cardiol. 2018;72:2003–2011. doi: 10.1016/j.jacc.2018.08.2140. [DOI] [PubMed] [Google Scholar]
- 21.Kaul S, Narula J. In search of the vulnerable plaque: is there any light at the end of the catheter? J Am Coll Cardiol. 2014;64:2519–2524. doi: 10.1016/j.jacc.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Gijsen F, Katagiri Y, Barlis P, et al. Expert recommendations on the assessment of wall shear stress in human coronary arteries: existing methodologies, technical considerations, and clinical applications. Eur Heart J. 2019;40:3421–3433. doi: 10.1093/eurheartj/ehz551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 24.Yahagi K, Kolodgie FD, Otsuka F, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98. doi: 10.1038/nrcardio.2015.164. [DOI] [PubMed] [Google Scholar]
- 25.Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol. 2008;51:645–650. doi: 10.1016/j.jacc.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Gijsen FJ, Wentzel JJ, Thury A, et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am J Physiol Heart Circ Physiol. 2008;295:H1608–H1614. doi: 10.1152/ajpheart.01081.2007. [DOI] [PubMed] [Google Scholar]
- 27.Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 28.Kolpakov V, Gordon D, Kulik TJ. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995;76:305–309. doi: 10.1161/01.res.76.2.305. [DOI] [PubMed] [Google Scholar]
- 29.Casey PJ, Dattilo JB, Dai G, et al. The effect of combined arterial hemodynamics on saphenous venous endothelial nitric oxide production. J Vasc Surg. 2001;33:1199–1205. doi: 10.1067/mva.2001.115571. [DOI] [PubMed] [Google Scholar]
- 30.Kenagy RD, Fischer JW, Davies MG, et al. Increased plasmin and serine proteinase activity during flow-induced intimal atrophy in baboon PTFE grafts. Arterioscler Thromb Vasc Biol. 2002;22:400–404. doi: 10.1161/hq0302.105376. [DOI] [PubMed] [Google Scholar]
- 31.Bark DL, Jr, Ku DN. Wall shear over high degree stenoses pertinent to atherothrombosis. J Biomech. 2010;43:2970–2977. doi: 10.1016/j.jbiomech.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Choi G, Lee JM, Kim HJ, et al. Coronary artery axial plaque stress and its relationship with lesion geometry: application of computational fluid dynamics to coronary CT angiography. JACC Cardiovasc Imaging. 2015;8:1156–1166. doi: 10.1016/j.jcmg.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka A, Imanishi T, Kitabata H, et al. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation. 2008;118:2368–2373. doi: 10.1161/CIRCULATIONAHA.108.782540. [DOI] [PubMed] [Google Scholar]
- 34.Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108:438–444. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 35.Stone PH, Saito S, Takahashi S, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126:172–181. doi: 10.1161/CIRCULATIONAHA.112.096438. [DOI] [PubMed] [Google Scholar]
- 36.Corban MT, Eshtehardi P, Suo J, et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis. 2014;232:271–276. doi: 10.1016/j.atherosclerosis.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 37.Stone PH, Maehara A, Coskun AU, et al. Role of low endothelial shear stress and plaque characteristics in the prediction of nonculprit major adverse cardiac events: the PROSPECT study. JACC Cardiovasc Imaging. 2018;11:462–471. doi: 10.1016/j.jcmg.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 38.Bajraktari A, Bytyçi I, Henein MY. High coronary wall shear stress worsens plaque vulnerability: a systematic review and meta-analysis. Angiology. 2021;72:706–714. doi: 10.1177/0003319721991722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman EMJ, De Nisco G, Kok AM, et al. Wall shear stress-related plaque growth of lipid-rich plaques in human coronary arteries: an near-infrared spectroscopy and optical coherence tomography study. Cardiovasc Res. 2023;119:1021–1029. doi: 10.1093/cvr/cvac178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varshney AS, Coskun AU, Siasos G, et al. Spatial relationships among hemodynamic, anatomic, and biochemical plaque characteristics in patients with coronary artery disease. Atherosclerosis. 2021;320:98–104. doi: 10.1016/j.atherosclerosis.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelsey LJ, Bellinge JW, Majeed K, et al. Low endothelial shear stress is associated with high-risk coronary plaque features and microcalcification activity. JACC Cardiovasc Imaging. 2021;14:2262–2264. doi: 10.1016/j.jcmg.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto N, Vengrenyuk Y, Fuster V, et al. Relationship between high shear stress and OCT-verified thin-cap fibroatheroma in patients with coronary artery disease. PLoS One. 2020;15:e0244015. doi: 10.1371/journal.pone.0244015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hetterich H, Jaber A, Gehring M, et al. Coronary computed tomography angiography based assessment of endothelial shear stress and its association with atherosclerotic plaque distribution in-vivo. PLoS One. 2015;10:e0115408. doi: 10.1371/journal.pone.0115408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JB, Choi G, Chun EJ, et al. Computational fluid dynamic measures of wall shear stress are related to coronary lesion characteristics. Heart. 2016;102:1655–1661. doi: 10.1136/heartjnl-2016-309299. [DOI] [PubMed] [Google Scholar]
- 45.Kay FU, Canan A, Abbara S. Future directions in coronary CT angiography: CT-fractional flow reserve, plaque vulnerability, and quantitative plaque assessment. Korean Circ J. 2020;50:185–202. doi: 10.4070/kcj.2019.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dwivedi A, Al’Aref SJ, Lin FY, Min JK. Evaluation of atherosclerotic plaque in non-invasive coronary imaging. Korean Circ J. 2018;48:124–133. doi: 10.4070/kcj.2017.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Usui E, Yonetsu T, Kanaji Y, et al. Optical coherence tomography-defined plaque vulnerability in relation to functional stenosis severity and microvascular dysfunction. JACC Cardiovasc Interv. 2018;11:2058–2068. doi: 10.1016/j.jcin.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Hoshino M, Usui E, Sugiyama T, Kanaji Y, Yonetsu T, Kakuta T. Prevalence of OCT-defined high-risk plaque in relation to physiological characteristics by fractional flow reserve and coronary flow reserve. Rev Esp Cardiol (Engl Ed) 2020;73:331–332. doi: 10.1016/j.rec.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Zuo W, Sun R, Zhang X, et al. The association between quantitative flow ratio and intravascular imaging-defined vulnerable plaque characteristics in patients with stable angina and non-ST-segment elevation acute coronary syndrome. Front Cardiovasc Med. 2021;8:690262. doi: 10.3389/fcvm.2021.690262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S, Hoshino M, Koo BK, et al. Relationship of plaque features at coronary CT to coronary hemodynamics and cardiovascular events. Radiology. 2022;305:578–587. doi: 10.1148/radiol.213271. [DOI] [PubMed] [Google Scholar]
- 51.Driessen RS, Stuijfzand WJ, Raijmakers PG, et al. Effect of plaque burden and morphology on myocardial blood flow and fractional flow reserve. J Am Coll Cardiol. 2018;71:499–509. doi: 10.1016/j.jacc.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 52.Yang S, Koo BK, Hoshino M, et al. CT angiographic and plaque predictors of functionally significant coronary disease and outcome using machine learning. JACC Cardiovasc Imaging. 2021;14:629–641. doi: 10.1016/j.jcmg.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Yang S, Choi G, Zhang J, et al. Association among local hemodynamic parameters derived from CT angiography and their comparable implications in development of acute coronary syndrome. Front Cardiovasc Med. 2021;8:713835. doi: 10.3389/fcvm.2021.713835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalykakis GE, Antonopoulos AS, Pitsargiotis T, et al. Relationship of endothelial shear stress with plaque features with coronary CT angiography and vasodilating capability with PET. Radiology. 2021;300:549–556. doi: 10.1148/radiol.2021204381. [DOI] [PubMed] [Google Scholar]
- 55.Wong CC, Javadzadegan A, Ada C, et al. Fractional flow reserve and instantaneous wave-free ratio predict pathological wall shear stress in coronary arteries: implications for understanding the pathophysiological impact of functionally significant coronary stenoses. J Am Heart Assoc. 2022;11:e023502. doi: 10.1161/JAHA.121.023502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang S, Koo BK. Physiology versus imaging-guided revascularization: where are we in 2023? JACC Asia. 2023 doi: 10.1016/j.jacasi.2023.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JM, Choi G, Koo BK, et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC Cardiovasc Imaging. 2019;12:1032–1043. doi: 10.1016/j.jcmg.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 58.Fukuyama Y, Otake H, Seike F, et al. Potential relationship between high wall shear stress and plaque rupture causing acute coronary syndrome. Heart Vessels. 2023;38:634–644. doi: 10.1007/s00380-022-02224-7. [DOI] [PubMed] [Google Scholar]
- 59.Thondapu V, Mamon C, Poon EK, et al. High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc Res. 2021;117:1974–1985. doi: 10.1093/cvr/cvaa251. [DOI] [PubMed] [Google Scholar]
- 60.Costopoulos C, Huang Y, Brown AJ, et al. Plaque rupture in coronary atherosclerosis is associated with increased plaque structural stress. JACC Cardiovasc Imaging. 2017;10:1472–1483. doi: 10.1016/j.jcmg.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bourantas CV, Zanchin T, Torii R, et al. Shear stress estimated by quantitative coronary angiography predicts plaques prone to progress and cause events. JACC Cardiovasc Imaging. 2020;13:2206–2219. doi: 10.1016/j.jcmg.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 62.Candreva A, Pagnoni M, Rizzini ML, et al. Risk of myocardial infarction based on endothelial shear stress analysis using coronary angiography. Atherosclerosis. 2022;342:28–35. doi: 10.1016/j.atherosclerosis.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Kumar A, Thompson EW, Lefieux A, et al. High coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J Am Coll Cardiol. 2018;72:1926–1935. doi: 10.1016/j.jacc.2018.07.075. [DOI] [PubMed] [Google Scholar]
- 64.van de Hoef TP, Lee JM, Boerhout CK, et al. Combined assessment of FFR and CFR for decision making in coronary revascularization: from the multicenter international ILIAS registry. JACC Cardiovasc Interv. 2022;15:1047–1056. doi: 10.1016/j.jcin.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann FM, Omerovic E, Fournier S, et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: meta-analysis of individual patient data. Eur Heart J. 2019;40:180–186. doi: 10.1093/eurheartj/ehy812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koo BK, Hu X, Kang J, et al. Fractional flow reserve or intravascular ultrasonography to guide PCI. N Engl J Med. 2022;387:779–789. doi: 10.1056/NEJMoa2201546. [DOI] [PubMed] [Google Scholar]
- 67.Iannaccone M, Abdirashid M, Annone U, et al. Comparison between functional and intravascular imaging approaches guiding percutaneous coronary intervention: a network meta-analysis of randomized and propensity matching studies. Catheter Cardiovasc Interv. 2020;95:1259–1266. doi: 10.1002/ccd.28410. [DOI] [PubMed] [Google Scholar]
- 68.Kedhi E, Berta B, Roleder T, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. 2021;42:4671–4679. doi: 10.1093/eurheartj/ehab433. [DOI] [PubMed] [Google Scholar]
- 69.Fabris E, Berta B, Hommels T, et al. Long-term outcomes of patients with normal fractional flow reserve and thin-cap fibroatheroma. EuroIntervention. 2023;18:e1099–e1107. doi: 10.4244/EIJ-D-22-00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JM, Choi KH, Koo BK, et al. Prognostic implications of plaque characteristics and stenosis severity in patients with coronary artery disease. J Am Coll Cardiol. 2019;73:2413–2424. doi: 10.1016/j.jacc.2019.02.060. [DOI] [PubMed] [Google Scholar]
- 71.Cho YK, Hwang J, Lee CH, et al. Influence of anatomical and clinical characteristics on long-term prognosis of FFR-guided deferred coronary lesions. JACC Cardiovasc Interv. 2020;13:1907–1916. doi: 10.1016/j.jcin.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 72.Park J, Lee JM, Koo BK, et al. Relevance of anatomical, plaque, and hemodynamic characteristics of non-obstructive coronary lesions in the prediction of risk for acute coronary syndrome. Eur Radiol. 2019;29:6119–6128. doi: 10.1007/s00330-019-06221-9. [DOI] [PubMed] [Google Scholar]
- 73.Yang S, Koo BK, Hwang D, et al. High-risk morphological and physiological coronary disease attributes as outcome markers after medical treatment and revascularization. JACC Cardiovasc Imaging. 2021;14:1977–1989. doi: 10.1016/j.jcmg.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Yang S, Lesina K, Doh JH, et al. Long-term prognostic implications of hemodynamic and plaque assessment using coronary CT angiography. Atherosclerosis. 2023;373:58–65. doi: 10.1016/j.atherosclerosis.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Collet C, Sonck J, Vandeloo B, et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol. 2019;74:1772–1784. doi: 10.1016/j.jacc.2019.07.072. [DOI] [PubMed] [Google Scholar]
- 76.Mizukami T, Sonck J, Sakai K, et al. Procedural outcomes after percutaneous coronary interventions in focal and diffuse coronary artery disease. J Am Heart Assoc. 2022;11:e026960. doi: 10.1161/JAHA.122.026960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong H, Li C, Gutiérrez-Chico JL, et al. Radial wall strain: a novel angiographic measure of plaque composition and vulnerability. EuroIntervention. 2022;18:1001–1010. doi: 10.4244/EIJ-D-22-00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang ZQ, Xu B, Li CM, et al. Angiography-derived radial wall strain predicts coronary lesion progression in non-culprit intermediate stenosis. J Geriatr Cardiol. 2022;19:937–948. doi: 10.11909/j.issn.1671-5411.2022.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tu S, Xu B, Chen L, et al. Short-term risk stratification of non-flow-limiting coronary stenosis by angiographically derived radial wall strain. J Am Coll Cardiol. 2023;81:756–767. doi: 10.1016/j.jacc.2022.11.056. [DOI] [PubMed] [Google Scholar]