Summary
Background
Cytokines and chemokines play a critical role in the response to infection and vaccination. We aimed to assess the longitudinal association of COVID-19 vaccination with cytokine and chemokine concentrations and trajectories among people with SARS-CoV-2 infection.
Methods
In this longitudinal, prospective cohort study, blood samples were used from participants enrolled in a multi-centre randomised trial assessing the efficacy of convalescent plasma therapy for ambulatory COVID-19. The trial was conducted in 23 outpatient sites in the USA. In this study, participants (aged ≥18 years) were restricted to those with COVID-19 before vaccination or with breakthrough infections who had blood samples and symptom data collected at screening (pre-transfusion), day 14, and day 90 visits. Associations between COVID-19 vaccination status and concentrations of 21 cytokines and chemokines (measured using multiplexed sandwich immunoassays) were examined using multivariate linear mixed-effects regression models, adjusted for age, sex, BMI, hypertension, diabetes, trial group, and COVID-19 waves (pre-alpha or alpha and delta).
Findings
Between June 29, 2020, and Sept 30, 2021, 882 participants recently infected with SARS-CoV-2 were enrolled, of whom 506 (57%) were female and 376 (43%) were male. 688 (78%) of 882 participants were unvaccinated, 55 (6%) were partly vaccinated, and 139 (16%) were fully vaccinated at baseline. After adjusting for confounders, geometric mean concentrations of interleukin (IL)-2RA, IL-7, IL-8, IL-15, IL-29 (interferon-λ), inducible protein-10, monocyte chemoattractant protein-1, and tumour necrosis factor-α were significantly lower among the fully vaccinated group than in the unvaccinated group at screening. On day 90, fully vaccinated participants had approximately 20% lower geometric mean concentrations of IL-7, IL-8, and vascular endothelial growth factor-A than unvaccinated participants. Cytokine and chemokine concentrations decreased over time in the fully and partly vaccinated groups and unvaccinated group. Log10 cytokine and chemokine concentrations decreased faster among participants in the unvaccinated group than in other groups, but their geometric mean concentrations were generally higher than fully vaccinated participants at 90 days. Days since full vaccination and type of vaccine received were not correlated with cytokine and chemokine concentrations.
Interpretation
Initially and during recovery from symptomatic COVID-19, fully vaccinated participants had lower concentrations of inflammatory markers than unvaccinated participants suggesting vaccination is associated with short-term and long-term reduction in inflammation, which could in part explain the reduced disease severity and mortality in vaccinated individuals.
Funding
US Department of Defense, National Institutes of Health, Bloomberg Philanthropies, State of Maryland, Mental Wellness Foundation, Moriah Fund, Octapharma, HealthNetwork Foundation, and the Shear Family Foundation.
Introduction
Inflammation plays an important role in the pathogenesis of COVID-19.1 The phenomenon of a cytokine storm during the acute phase of SARS-CoV-2 infection might be life-threatening when the damage caused by the immune response to infection outweighs the immediate anti-viral benefit.2 The elevation of cytokine and chemokine concentrations during the acute phase has been found to be associated with increased risk of disease severity and mortality.3 Patients with COVID-19 have significantly higher concentrations of inflammatory mediators than healthy controls.4 Donors of convalescent plasma who have recently recovered from COVID-19 have also been shown to have elevated concentrations of cytokines and chemokines compared with pre-pandemic controls.5 Elevated concentrations of IL-6 and persistent inflammation can also last for months and might be related to the development of post-COVID-19 conditions.6,7
Vaccination is the most promising method to combat the COVID-19 pandemic. mRNA and adenovirus vector-based vaccines are the two main types of vaccines that have been authorised for global use.8 The effect of vaccination on cytokines and chemokines is complicated. On the one hand, clinical trials have shown that both types of vaccines generate high concentrations of neutralising antibodies to prevent cytokine dysregulation and reduce severe outcomes, and mRNA vaccines have better efficacy than adenovirus vaccines.9,10 On the other hand, a comparison of cytokine concentrations pre-vaccination and 8–16 days post-vaccination among individuals without COVID-19 showed that mRNA and adenovirus-vector vaccines increased inflammation biomarkers immediately after injection, and adenovirus-vector vaccines induced a larger increase in biomarker concentration.11 Cytokine dysregulation has also been proposed to be a potential cause of myocarditis, a rare and usually transient complication of COVID-19 vaccination.12 Concomitantly, elevated concentrations of cytokines and chemokines (interleukin [IL]-15, interferon [IFN]-γ, and inducible protein [IP]-10) are related to antibody development.13 High concentrations of post-vaccination, pro-inflammatory cytokines are also associated with an increased antibody response to vaccines in blood donors and organ transplant recipients.5,14
Cytokines and chemokines play a key role in the response to infection and vaccination. However, little is known about the short-term or long-term association of COVID-19 vaccination with cytokine and chemokine concentrations and the trajectories among individuals with symptomatic COVID-19. This study evaluated the longitudinal association of previous vaccination against COVID-19 on cytokine and chemokine concentrations among adult outpatients with symptomatic SARS-CoV-2 infection.
Methods
Study design and participants
In this prospective cohort study, blood samples were used from participants enrolled in the Convalescent Plasma to Limit SARS-CoV-2 Associated Complications (CSSC-004) trial, a double-blind, randomised controlled trial that evaluated the efficacy of outpatient convalescent plasma therapy to prevent severe COVID-19 outcomes among individuals recently infected with SARS-CoV-2 and symptomatic with COVID-19.15 The trial was conducted in 23 outpatient sites in the USA. Between June 3, 2020, and October 1, 2021, 1225 participants were enrolled and randomly assigned to study groups; 1181 participants received transfusion with convalescent plasma or pre-pandemic control plasma within 9 days after onset of COVID-19 symptoms. During the enrolment period, the variants of concern were the alpha and delta strains. Exclusion criteria were previous COVID-19-related admission to hospital, planned hospitalisation after enrolment, previous history of adverse reactions to transfusions, inability of compliance to study protocols, and receipt of monoclonal antibody therapies. Five people elected to receive monoclonal antibody therapies after transfusion, and outpatient oral antiviral drugs were not available before the end of trial enrolment. Participants returned for in-person follow-up visits at days 14, 28, and 90 post-transfusion.
This longitudinal study was restricted to participants with COVID-19 before vaccination or with breakthrough infections who had blood samples and symptom data collected at screening (pre-transfusion), day 14, and day 90 visits. Detailed selection of the study population is presented in the appendix (p 2). Day 28 samples were excluded since the cytokine concentrations at day 28 were similar to day 14 samples in a pilot study. Evaluating cytokine concentrations among recipients of convalescent plasma and control plasma was a pre-planned secondary outcome of the trial (NCT04373460) and assessing cytokine concentration by vaccination status (this study) was a post-hoc analysis. All participants provided written informed consent.
Johns Hopkins Institutional Review Board (JHU IRB00247590), Human Research Protection Office of the US Department of Defense (A-21080·2b), Navajo Nation Human Research Review Board, and the Indian Health Service National Institutional Review Board provided ethics approval. The trial was performed in accordance with the Declaration of Helsinki.
Procedures
At each visit, participants provided blood samples. At screening, self-reported data on demographics (age, sex, race, ethnicity) and comorbidities (obesity class, hypertension, diabetes, HIV infection, asthma) were collected. Options were female or male for sex and yes or no for hypertension and diabetes, whereas BMI (restricted cubic spline with 5 knots at 5, 35, 50, 65, and 95 percentiles16) and age were on a continuous scale. Calendar time at transfusion was used as a surrogate for waves of the pandemic to control for SARS-CoV-2 variants and account for differences in virulence. Variants of concern during the study were the alpha and delta variants. Participants who were enrolled and randomly assigned before June 15, 2021, were considered to be infected during the pre-alpha or alpha wave, whereas those who were enrolled and randomly assigned between June 15, and Oct 1, 2021, were considered to be infected during the delta wave.17
COVID-19 vaccine status at each visit was self-reported, including vaccine date, dose, and type of vaccine (adenovirus or mRNA). Participants who did not receive a COVID-19 vaccine before the study visit were classified as unvaccinated. Participants were classified as partly vaccinated if they had received one dose of adenovirus-vector vaccine (Johnson & Johnson, New Brunswick, USA), one dose of monovalent mRNA vaccine (Pfizer & BioNTech, New York, USA or Moderna, Cambridge), or two doses of mRNA vaccine but had received the final dose less than 14 days before the study visit. Participants who had received either one dose of adenovirus-vector vaccine or the second dose of mRNA vaccine at least 14 days before the study visit were considered fully vaccinated. Vaccine status was considered as a time-varying component.
Plasma was separated from collected blood samples by centrifugation within 8 h post-phlebotomy and stored at −80°C. Cytokine concentrations in thawed plasma were quantified using custom multiplexed sandwich immunoassays (MesoScale Discovery, Gaithersburg, MD, USA) according to the manufacturer’s protocols. There were 21 cytokines: IL-1RA, IL-2RA, IL-6, IL-7, IL-8, IL-10, IL-15, IL-16, IL17A, IL-18, IL21, IL-22, IFN-α2a, IL-29 (IFN-λ), IFN-γ, IFN-γ-IP-10, monocyte chemoattractant protein-1 (MCP-1), macrophage-inflammatory protein-1α (MIP-1α), tumour necrosis factors-α (TNF-α), vascular endothelial growth factor-A (VEGF-A), and vascular cell adhesion molecule-1 (VCAM-1) selected on the basis of previous studies of COVID-19.5,14,18 All samples were tested in duplicate on the same plate, and samples with a coefficient of variation greater than 25% and a electrochemiluminescence signal of more than 2000 were re-run for quality control. Detection limits of the assay were the electrochemiluminescence signals back-calculated from the top and bottom calibrator concentrations offset by 2·5 times of signal standard deviation of top or bottom replicates. Fit curve ranges were the signals back-calculated from the top and bottom calibrator concentrations. Each plate and analyte had specific detection limits and fit curve ranges. Values out of detection limits but within fit curve ranges were extrapolated and reported by the assay, whereas values outside of fit curve ranges were reported as missing.
Statistical analysis
Cytokine concentrations outside the fit curve range were imputed using a single random draw from the extrapolated tails of a log10 normal distribution fit to available cytokine data for each cytokine individually. Imputed values were bounded by 0 and the minimal available value for the extrapolated lower tail, and by the maximum available value and ten times that maximum for the extrapolated upper tail. Since each sample was tested twice, the log10 transformed average value was used for the analysis. Outliers (ie, >Q3 + [1·5 · IQR] or <Q1 − [1·5 · IQR], where Q1 is the first quartile and Q3 is the third quartile) for each cytokine at each visit were excluded from the analysis. Four plates with all samples out of the fit curve range were also excluded from the study. Density plots were produced to inspect the normality and homogeneity of the variance of the remaining log10-transformed cytokine concentrations.
Baseline demographic, clinical, and comorbidity characteristics of the participants were summarised overall and by their vaccination status at baseline. Student t tests were used to compare log10-transformed cytokine concentrations between vaccination groups at each study visit (screening and days 14 and 90).
Linear mixed-effect regression models with an interaction term between time-varying vaccine status and days after screening were used to evaluate the longitudinal association of vaccine status with log10 cytokine levels, with separate models used for each cytokine. To account for correlation among repeated measures, random intercepts and slopes for time were included in the model with an unstructured covariance matrix describing the covariance among random effects. The ratio of geometric mean concentrations of cytokines and chemokines comparing vaccination status was obtained by 10x where x is the coefficient. 95% CIs were estimated to measure uncertainty. Potential confounders, including age, sex, BMI, self-reported hypertension, self-reported diabetes, trial group (control, convalescent plasma transfused >5 days since symptom onset, or convalescent plasma transfused ≤5 days since symptom onset), and COVID-19 waves, were included in the regression models as covariates. Specifically, trial groups were not adjusted at screening since participants were transfused after screening. In a sensitivity analysis, individuals who changed vaccination status during follow-up were censored at the time of additional vaccine dose to assess the effect of changing vaccination status. Additional subgroup analyses were conducted, stratified by age (<50 years and ≥50 years) and sex (female and male), to evaluate the effect measure modification of demographic factors on the association between vaccination and inflammation.
A separate post-hoc analysis was done among individuals who were fully vaccinated before screening, using student t tests to evaluate the difference in cytokine concentrations between recipients of adenovirus and mRNA vaccines. Correlations between days since last vaccine dose and cytokine concentrations were also assessed among this subgroup using Spearman rank correlation coefficients.
Available case method was used to handle missing covariate data. Bonferroni corrections were used to account for multiple comparison, and a two-tailed p value of less than 0·0024 (0–05 ÷ 21) was considered significant. All analyses were conducted in R (version 4.2) and STATA (version 15.1).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of 1181 participants who received transfusion in the CSSC-004 trial, 882 were enrolled between June 29, 2020, and Sept 30, 2021, and were included in this study and contributed 2646 person-visits to the analysis (appendix p 2). 688 (78%) of 882 participants were unvaccinated, 55 (6%) were partly vaccinated, and 139 (16%) were fully vaccinated at baseline (screening visit; table 1). 506 (57%) of 882 participants were female and 376 (43%) were male. 252 (37%) of 688 unvaccinated participants were aged 50 years or older, whereas only 29 (21%) of 139 fully vaccinated participants were aged 50 years or older. Hypertension was more frequent in the unvaccinated group than the fully vaccinated group (175 [25%] of 688 vs 16 [12%] of 139). Most of the unvaccinated group were infected during the pre-alpha or alpha wave (642 [93%] of 688), whereas most of the fully vaccinated group were enrolled during the delta wave of the pandemic (123 [88%] of 139). Among the pre-enrolment fully vaccinated group, the first fully vaccinated participant was transfused on March 10, 2021, and the median time since full vaccination was 157 days. Sex, race, ethnicity, obesity, diabetes, HIV infection, asthma, and trial treatment were distributed evenly between unvaccinated, partly, and fully vaccinated participants. During follow-up, 21 (2%) of 882 participants were admitted to hospital, of whom 20 (95%) were unvaccinated and one (5%) was partly vaccinated at screening. The geometric mean of SARS-CoV-2 viral load was significantly different between vaccination groups (p<0·0001), and the unvaccinated group had the highest geometric mean of 8737 copies (95% CI 6922–11028).
Table 1:
Baseline characteristics at screening, stratified by vaccination status
| Overall (n=882) |
Unvaccinated (n=688) |
Partly vaccinated (n=55) |
Fully vaccinated (n=139) |
p value | |
|---|---|---|---|---|---|
| Age, years | .. | .. | .. | .. | 0·012 |
| 18–29 | 263 (30%) | 190 (28%) | 16 (29%) | 57 (41%) | .. |
| 30–49 | 320 (36%) | 246 (36%) | 21 (38%) | 53 (38%) | .. |
| 50–64 | 247 (28%) | 207 (30%) | 16 (29%) | 24 (17%) | .. |
| ≥65 | 52 (6%) | 45 (7%) | 2 (4%) | 5 (4%) | .. |
| Sex | .. | .. | .. | .. | 0·75 |
| Female | 506 (57%) | 390 (57%) | 33 (60%) | 83 (60%) | |
| Male | 376 (43%) | 298 (43%) | 22 (40%) | 56 (40%) | |
| Race | .. | .. | .. | .. | 0·42 |
| Asian | 28 (3%) | 18 (3%) | 4 (7%) | 6 (4%) | .. |
| Black | 116 (13%) | 89 (13%) | 10 (18%) | 17 (12%) | .. |
| Mixed or other | 17 (2%) | 15 (2%) | 0 (0) | 2 (1%) | .. |
| American Indian or Alaska Native | 15 (2%) | 14 (2%) | 0 (0) | 1 (1%) | .. |
| White | 706 (80%) | 552 (80%) | 41 (75%) | 113 (81%) | .. |
| Ethnicity | .. | .. | .. | .. | 0·11 |
| Hispanic or Latino, Latina, or Latinx | 109 (12%) | 90 (13%) | 2 (4%) | 17 (12%) | .. |
| Non-Hispanic or Latino, Latina, or Latinx | 773 (88%) | 598 (87%) | 53 (96%) | 122 (88%) | .. |
| BMI | .. | .. | .. | .. | 0·80 |
| <30·0 | 522 (59%) | 398 (58%) | 34 (62%) | 90 (65%) | .. |
| 30·0–34·9 | 179 (20%) | 141 (20%) | 9 (16%) | 29 (21%) | .. |
| ≥35·0 | 141 (16%) | 110 (16%) | 11 (20%) | 20 (14%) | .. |
| Missing | 40 (5%) | 39 (6%) | 1 (2%) | 0 (0) | .. |
| Hypertension | .. | .. | .. | .. | 0·0005 |
| No | 675 (77%) | 513 (75%) | 39 (71%) | 123 (88%) | .. |
| Yes | 207 (23%) | 175 (25%) | 16 (29%) | 16 (12%) | .. |
| Diabetes | .. | .. | .. | .. | 0·27 |
| No | 810 (92%) | 629 (91%) | 49 (89%) | 132 (95%) | .. |
| Yes | 72 (8%) | 59 (9%) | 6 (11%) | 7 (5%) | .. |
| HIV infection | .. | .. | .. | .. | 0·22 |
| No | 867 (98%) | 674 (98%) | 54 (98%) | 139 (100%) | .. |
| Yes | 15 (2%) | 14 (2%) | 1 (2%) | 0 (0) | .. |
| Asthma | .. | .. | .. | .. | 0·58 |
| No | 784 (89%) | 609 (89%) | 48 (87%) | 127 (91%) | .. |
| Yes | 98 (11%) | 79 (11%) | 7 (13%) | 12 (9%) | .. |
| Treatment group | .. | .. | .. | .. | 0·99 |
| Control | 437 (50%) | 342 (50%) | 28 (51%) | 67 (48%) | .. |
| CCP >5 days since symptom onset | 248 (28%) | 192 (28%) | 16 (29%) | 40 (29%) | .. |
| CCP ≤5 days since symptom onset | 197 (22%) | 154 (22%) | 11 (20%) | 32 (24%) | .. |
| COVID-19 wave during infection | .. | .. | .. | .. | <0·0001 |
| Pre-alpha or alpha | 704 (80%) | 642 (93%) | 46 (84%) | 16 (12%) | .. |
| Delta | 178 (20%) | 46 (7%) | 9 (16%) | 123 (88%) | .. |
| SARS-CoV-2 viral load, copies | 7248 (5909– 8889) | 8737 (6922–11 028) | 1874 (976–3598) | 4933 (2951–8246) | <0·0001 |
Data are n (%) or geometric mean (95% CI). p values were estimated using Fisher’s exact test for categorical variables and Kruskal-Wallis rank sum test for the continuous variable (SARS-CoV-2 viral load) to compare the differences between the three groups. BMI missingness is reported in the table. SARS-CoV-2 viral load had 51 missing data (unvaccinated participants n=39, partly vaccinated participants n=2, fully vaccinated participants n=10). Other variables had no missing values. CCP=convalescent plasma.
Of 2646 person visits, the prevalence of imputed values for observations outside the fit curve was greater than 10% for IL-17A, IL-22, and IFN-α2a. IL-17A and IL-22 also had more than 10% of resulting samples that were considered outliers and were excluded from the analysis (appendix p 3).
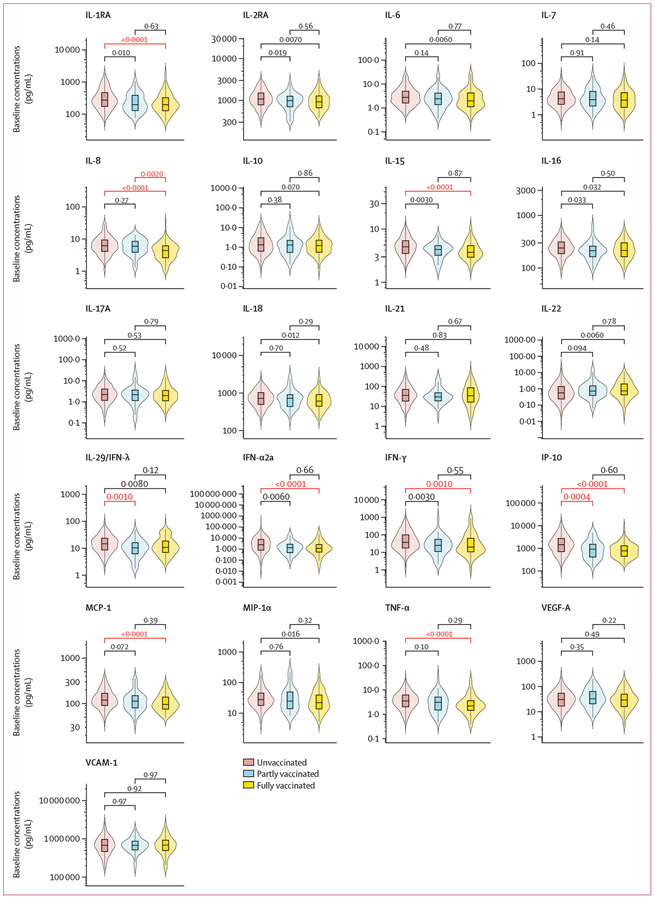
The distribution of log10 concentrations had similar variance between vaccination groups and showed a normal distribution for most analytes, except for IFN-α2a and VCAM-1 (appendix pp 4-6). At screening (1–8 days after infection), the unvaccinated group had the highest mean log10 concentrations, the partly vaccinated group had lower, and the fully vaccinated group had the lowest concentrations of almost all the cytokines evaluated (figure 1). After accounting for multiple comparisons, the mean log10 concentrations of IL-1RA, IL-8, IL-15, IFN-α2a, IFN-γ, IP-10, MCP-1, and TNF-α were significantly lower in the fully vaccinated group than the unvaccinated group. The partly vaccinated group had lower concentrations of IL-29 (IFN-λ) and IP-10 than the unvaccinated group. The mean log10 concentration of IL-8 was significantly lower in the fully vaccinated group than the partly vaccinated group, and all the other cytokine concentrations were not significantly different between the partly and fully vaccinated groups at screening.
Figure 1: Baseline cytokine and chemokine concentrations, stratified by vaccination status at screening.
IFN=interferon. IL=interleukin. IP-10=inducible protein-10. MCP-1=monocyte chemoattractant protein-1. MIP-1α=macrophage-inflammatory protein-1α. TNF-α=tumour necrosis factor-α. VCAM-1=vascular cell adhesion molecule-1. VEGF-A=vascular endothelial growth factor-A. *p values were calculated from student t tests, using log10 transformed cytokine and chemokine concentrations. Concentration differences between groups were considered significant after adjusting for multiple comparison using Bonferroni correction when p<0·0024. Significant p values are marked in red.
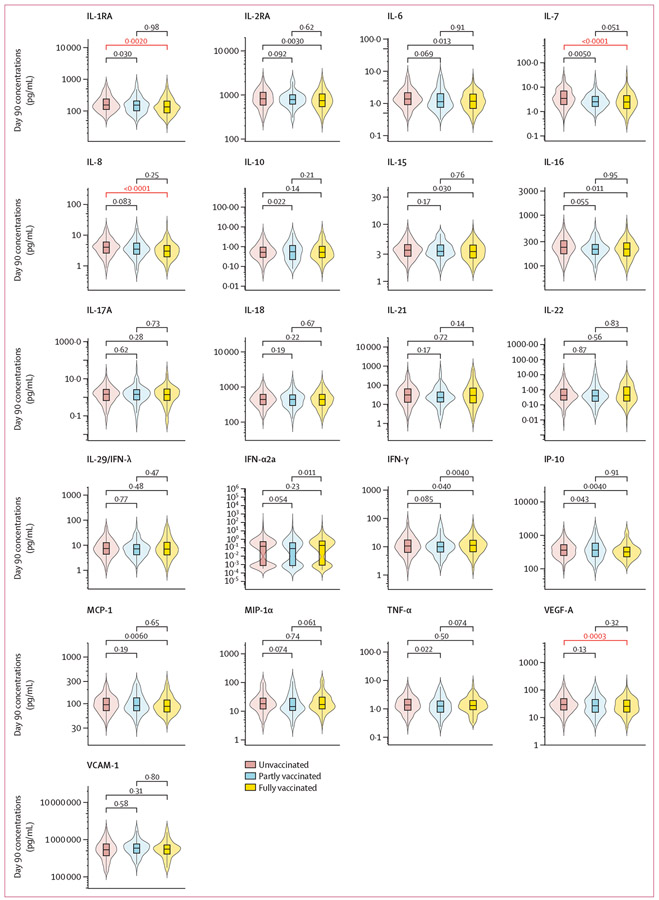
On day 14 of follow-up, fewer differences in cytokine concentrations were found between vaccination groups (appendix p 7). Mean log10 concentrations of IL-1RA, IL-7, IL-8, and VEGF-A were significantly lower in the fully vaccinated group than the unvaccinated group, whereas no significant differences were found between the partly vaccinated group and the fully vaccinated and unvaccinated groups. On day 90, the mean log10 concentrations of IL-1RA, IL-7, IL-8, and VEGF-A remained significantly lower among the fully vaccinated group than the unvaccinated group (figure 2). No other significant differences were observed.
Figure 2: Day 90 cytokine and chemokine concentrations, stratified by day 90 vaccination status.
*p values were calculated from student t tests, using log10 transformed cytokine and chemokine concentrations. Concentration differences between groups were considered significant after adjusting for multiple comparison using Bonferroni correction when p<0·0024. Significant p values are marked in red. IFN=interferon. IL=interleukin. IP-10=inducible protein-10. MCP-1=monocyte chemoattractant protein-1. MIP-1α=macrophage-inflammatory protein-1α TNF-α=tumour necrosis factor-α. VCAM-1=vascular cell adhesion molecule-1. VEGF-A=vascular endothelial growth factor-A.
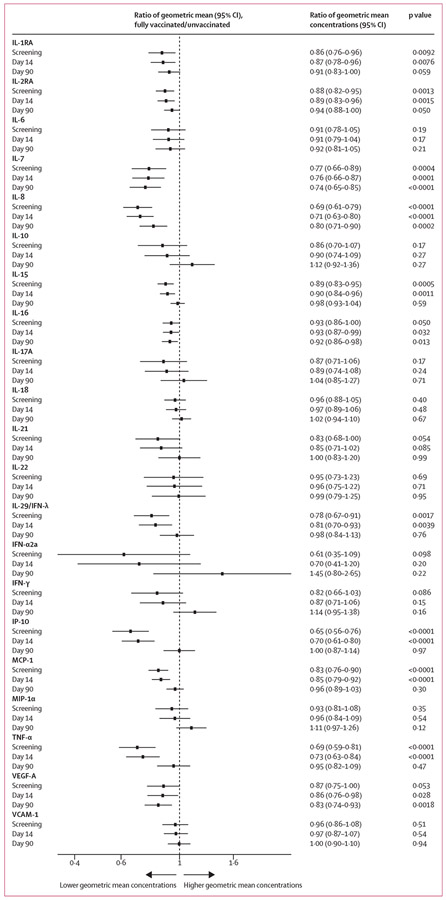
After conducting Bonferroni correction of multiple comparison and adjusting for age, sex, BMI, hypertension, diabetes, convalescent or control plasma treatment, and COVID-19 waves, at screening, the geometric mean values of IL-2RA, IL-7, IL-8, IL-15, IL-29 (IFN-λ), IP-10, MCP-1, and TNF-α were significantly lower among the fully vaccinated group than the unvaccinated group (figure 3). At day 14, the fully vaccinated group had significantly lower geometric mean concentrations of IL-2RA, IL-7, IL-8, IL-15, IP-10, MCP-1, and TNF-α than the unvaccinated group. At day 90, the geometric mean concentrations of IL-7, IL-8, and VEGF-A remained significantly lower in the fully vaccinated group than the unvaccinated group (p<0·0024). The fully vaccinated group at day 90 had 26% lower concentrations of IL-7, 20% lower concentrations of IL-8, and 17% lower concentrations of VEGF-A. The partly vaccinated group had significantly lower geometric mean concentrations of IP-10 and MCP-1 than unvaccinated individuals at screening and significantly lower concentrations of IP-10 at day 14; no differences were found between the two groups at day 90 (appendix p 8). Overall, the fully vaccinated group had lower geometric mean concentrations of cytokine and chemokine than the partly vaccinated group, although the differences were not significant (appendix p 9).
Figure 3: Differences in cytokine and chemokine concentrations between unvaccinated and fully vaccinated participants at screening, day 14, and day 90.
Each analyte had a separate mixed effect model, and all the models were adjusted for age, sex, BMI, hypertension, diabetes, trial groups, and COVID-19 waves. Trial groups were not adjusted at screening, since participants were transfused after screening. Among the covariates, only BMI has missing data, and the prevalence of missingness was 5%. Available case method was used to handle missing data. Significance after adjusting for multiple comparison using Bonferroni correction was defined by a p value cutoff of 0·0024. IFN=interferon. IL=interleukin. IP-10=inducible protein-10. MCP-1=monocyte chemoattractant protein-1. MIP-1α=macrophage-inflammatory protein-1α TNF-α=tumour necrosis factor-α. VCAM-1=vascular cell adhesion molecule-1. VEGF-A=vascular endothelial growth factor-A.
In the sensitivity analysis that censored (ie, excluded) visits following a change in vaccination status, results were similar to those in the main analysis (appendix p 10). In the subgroup analysis stratified by age and sex, we did not detect heterogeneity in the association of vaccination status with cytokine concentrations by age or sex, except for IL-22. Among participants aged 50 years or older, fully vaccinated participants had a reduced geometric mean concentration of IL-22 by about 40% throughout the recovery process from screening to day 90, whereas among participants younger than 50 years, those fully vaccinated had slightly increased concentrations of IL-22 for all timepoints, although these differences were not significant after accounting for multiple comparisons (appendix pp 11-14).
After adjusting for age, sex, BMI, hypertension, diabetes, convalescent or control plasma treatment (not adjusted at screening), and COVID-19 waves, a significant decreasing trajectory between baseline and day 90 of IL-1RA, IL-2RA, IL-6, IL-7, IL-8, IL-10, IL-15, IL-17A, IL-18, IL-22, IL-29 (IFN-λ), IFN-α2a, IFN-γ, IP-10, MCP-1, MIP-1α, TNF-α, and VCAM-1 was observed in the unvaccinated group (table 2). In the fully vaccinated group, a significant decreasing trajectory between baseline and day 90 was seen for IL-1RA, IL-2RA, IL-6, IL-7, IL-18, IFN-α2a, IFN-γ, and IP-10. In the partly vaccinated group, a significant decreasing trajectory between baseline and day 90 was only seen for IL-18. Overall, the log10 concentrations of cytokines and chemokines decreased faster in the unvaccinated group than the other vaccination groups. The mean log10 concentrations of IL-15, IL-29 (IFN-λ), IP-10, MCP-1, and TNF-α in the unvaccinated group decreased significantly faster than the fully vaccinated group (appendix p 15).
Table 2:
Slope of daily changes of cytokine and chemokine levels from screening to day 90
| Unvaccinated participants | p value | Partly vaccinated participants | p value | Fully vaccinated participants | p value | |
|---|---|---|---|---|---|---|
| IL-1RA | −0·0016 (−0·0019 to −0·0014) | <0·0001 | −0·0005 (−0·0012 to 0·0003) | 0·22 | −0·0013 (−0·0018 to −0·0009) | <0·0001 |
| IL-2RA | −0·0012 (−0·0013 to −0·0010) | <0·0001 | −0·0005 (−0·0009 to 0·0000) | 0·032 | −0·0009 (−0·0011 to −0·0006) | <0·0001 |
| IL-6 | −0·0018 (−0·0022 to −0·0015) | <0·0001 | −0·0011 (−0·0021 to 0·0000) | 0·040 | −0·0017 (−0·0024 to −0·0011) | <0·0001 |
| IL-7 | −0·0010 (−0·0013 to −0·0007) | <0·0001 | −0·0010 (−0·0019 to −0·0001) | 0·035 | −0·0012 (−0·0017 to −0·0006) | <0·0001 |
| IL-8 | −0·0010 (−0·0013 to −0·0007) | <0·0001 | −0·0003 (−0·0012 to 0·0005) | 0·43 | −0·0004 (−0·0009 to 0·0001) | 0·16 |
| IL-10 | −0·0026 (−0·0031 to −0·0021) | <0·0001 | −0·0006 (−0·0021 to 0·0008) | 0·40 | −0·0014 (−0·0023 to −0·0005) | 0·0032 |
| IL-15 | −0·0008 (−0·0009 to −0·0006) | <0·0001 | −0·0002 (−0·0006 to 0·0003) | 0·45 | −0·0003 (−0·0005 to 0·0000) | 0·038 |
| IL-16 | −0·0001 (−0·0003 to 0·0000) | 0·090 | 0·0004 (−0·0001 to 0·0008) | 0·095 | −0·0002 (−0·0005 to 0·0001) | 0·15 |
| IL-17A | −0·0016 (−0·0021 to −0·0011) | <0·0001 | −0·0008 (−0·0021 to 0·0006) | 0·29 | −0·0008 (−0·0016 to 0·0001) | 0·076 |
| IL-18 | −0·0014 (−0·0016 to −0·0012) | <0·0001 | −0·0009 (−0·0015 to −0·0004) | 0·0010 | −0·0012 (−0·0015 to −0·0008) | <0·0001 |
| IL-21 | −0·0004 (−0·0008 to 0·0000) | 0·057 | 0·0004 (−0·0008 to 0·0016) | 0·50 | 0·0005 (−0·0002 to 0·0012) | 0·14 |
| IL-22 | −0·0009 (−0·0015 to −0·0004) | 0·0009 | −0·0011 (−0·0027 to 0·0005) | 0·20 | −0·0007 (−0·0017 to 0·0003) | 0·15 |
| IL-29 (IFN-λ) | −0·0014 (−0·0018 to −0·0011) | <0·0001 | −0·0003 (−0·0013 to 0·0007) | 0·58 | −0·0004 (−0·0010 to 0·0002) | 0·24 |
| IFN-α2a | −0·0088 (−0·0103 to −0·0072) | <0·0001 | −0·0058 (−0·0099 to −0·0016) | 0·0066 | −0·0046 (−0·0072 to −0·0020) | 0·0004 |
| IFN-γ | −0·0034 (−0·0040 to −0·0028) | <0·0001 | −0·0017 (−0·0034 to −0·0001) | 0·042 | −0·0018 (−0·0028 to −0·0007) | 0·0008 |
| IP-10 | −0·0037 (−0·0041 to −0·0033) | <0·0001 | −0·0015 (−0·0026 to −0·0003) | 0·012 | −0·0017 (−0·0024 to −0·0009) | <0·0001 |
| MCP-1 | −0·0009 (−0·0011 to −0·0007) | <0·0001 | 0·0001 (−0·0005 to 0·0006) | 0·77 | −0·0002 (−0·0005 to 0·0001) | 0·22 |
| MIP-1α | −0·0013 (−0·0016 to −0·0010) | <0·0001 | −0·0009 (−0·0018 to 0·0000) | 0·044 | −0·0005 (−0·0010 to 0·0001) | 0·079 |
| TNF-α | −0·0024 (−0·0028 to −0·0020) | <0·0001 | −0·0012 (−0·0024 to −0·0001) | 0·027 | −0·0009 (−0·0015 to −0·0002) | 0·012 |
| VEGF-A | −0·0004 (−0·0007 to −0·0001) | 0·0073 | −0·0003 (−0·0012 to 0·0006) | 0·49 | −0·0006 (−0·0011 to −0·0001) | 0·018 |
| VCAM-1 | −0·0007 (−0·0010 to −0·0005) | <0·0001 | −0·0003 (−0·0010 to 0·0004) | 0·39 | −0·0006 (−0·0010 to −0·0002) | 0·0060 |
Data are slope (95% CI) of the daily changes of log10 pg/mL values of each analyte during follow-up. Each analyte had a separate mixed effect model, and all models were adjusted for age, sex, BMI, hypertension, diabetes, treatment groups, and COVID-19 waves. Treatment groups were not adjusted at screening since participants were transfused after screening. Among the covariates, only BMI had missing data, and the prevalence of missingness was 5%. Available case method was used to handle missing data. Significance after adjusting for multiple comparison using Bonferroni correction was defined by a p value cut off of 0·0024. IFN=interferon. IL=interleukin.
IP-10=inducible protein-10. MCP-1=monocyte chemoattractant protein-1. MIP-1 α=macrophage inflammatory protein-1 α. TNF-α=tumour necrosis factor-α. VCAM-1=vascular cell adhesion molecule-1. VEGF-A= vascular endothelial growth factor-A.
For the separate analysis in the fully vaccinated population before trial enrolment, 127 (91%) of 139 participants received mRNA vaccines. The distribution of IL-8 (p=0·028) and VEGF-A (p=0·038) was higher among recipients of the adenovirus vaccine than the mRNA vaccine. However, the difference was not significant after adjusting for multiple comparisons (appendix p 16). No correlation was observed between days since full vaccination and cytokine concentrations at screening (appendix p 17).
Discussion
Our study found that, among individuals recently infected with SARS-CoV-2, those who were fully vaccinated had lower concentrations of almost all cytokines and chemokines than those who were unvaccinated in the short-term and long-term after symptomatic SARS-CoV-2 infection. All vaccination groups had a decreasing trajectory of all cytokine and chemokine concentrations during recovery from symptomatic COVID-19. Although unvaccinated participants had a larger decrease of cytokine and chemokine concentrations over time, their mean concentrations of IL-7, IL-8, and VEFG-A were still higher than those in fully vaccinated participants more than 3 months after COVID-19 diagnosis, due to higher concentrations of inflammation at disease onset. These data suggest that vaccination might play a protective role against inflammation even with breakthrough symptomatic infection.
Our findings are consistent with the literature. A study showed that vaccinated individuals with breakthrough infection had better cellular immune reactions, which reduces inflammation, than unvaccinated individuals.19 Previous studies have also shown that cytokines and chemokines are associated with disease severity related to SARS-CoV-2 infection.3,20 Additionally, up-regulated IL-7 was associated with chronic inflammatory diseases, and increasing IL-8 concentrations were associated with lung hyperinflammation and longer illness among people with severe COVID-19.21-23 VEGF-A was over-expressed in lung tissues of people who died from COVID-19.24
In the context of existing knowledge about the pathogenesis and immunology of SARS-CoV-2 infection, our findings of decreased inflammatory cytokine levels are consistent with and explainable by several facets of antibody-mediated immunity. Administering convalescent plasma containing SARS-CoV-2 antibodies to patients with COVID-19 decreases inflammatory cytokine concentrations, a phenomenon attributed to viral neutralisation and reduced infection.25 SARS-CoV-2 infection in vaccinated individuals with neutralising antibody responses and defined antibody avidity maturation would similarly reduce viral load and the extent of infection, and results in the formation of antigen-antibody complexes that could further modulate the inflammatory response by engaging Fc receptors.26 Disease severity and death in COVID-19 is thought to generally result from an overexuberant inflammatory response that interferes with pulmonary gas exchange.27 Our findings that vaccinated individuals with COVID-19 have reduced inflammatory mediators provide a mechanistic explanation for their reduced symptomatology and mortality when infected with SARS-CoV-2.
Even unvaccinated individuals who recovered from COVID-19 had higher concentrations of IL-7, IL-8, and VEGF-A than fully vaccinated individuals, suggesting that unvaccinated individuals take longer to recover from the inflammation caused by COVID-19. A systematic review of case-control and cohort studies found that vaccination was associated with reduced odds of post-COVID-19 conditions.28 Elevated cytokines among unvaccinated participants long after infection in this study might provide an explanation for a subgroup of individuals for their increased odds of post-COVID-19 conditions.
We did stratified analyses by age and sex, to evaluate whether these factors modified the association between vaccination and inflammation biomarkers. We found that among older participants, fully vaccinated participants had 40% lower concentrations of IL-22 than unvaccinated participants during their recovery, although this finding was not significant after accounting for multiple comparisons. However, among younger participants, vaccination limited impact. A study suggested that IL-22 expression is dependent on age, which could be a possible explanation of the effect.29 No difference was observed for other analytes between age groups. There was no effect modification by sex. Future studies with larger sample sizes in the subgroup are needed to confirm our results, given the reduced sample sizes in stratified analyses. Additionally, future research with sex hormone data could further evaluate the effect of sex.
Recipients of adenovirus and mRNA vaccines had similar cytokine profiles in our study, although mRNA vaccines might reduce concentrations of IL-8 and VEGF-A. A previous study suggested that specific components of adenovirus vaccines might increase inflammation.11 However, information remains scarce regarding vaccination and cytokine profiles among patients with symptomatic COVID-19. Additional research is needed to confirm our findings since the number of individuals who received adenovirus-based vaccine was small.
Our study had several strengths. First, to our knowledge, this is the largest study to date measuring cytokine and chemokine concentrations during COVID-19, providing sufficient power to detect differences between vaccinated and unvaccinated participants. We can generalise our findings to a wider group with the large diverse study population. Second, we collected blood samples from multiple visits, allowing the trajectories of cytokines and chemokines to be assessed after infection and differences in trajectories to be evaluated between vaccinated and unvaccinated participants. Furthermore, the source population of our study is a double-blind, randomised clinical trial and this analysis is among the few COVID-19 cytokine studies that adjusted for potential confounders, which could increase the validity of the findings.5,18
This study also has limitations. First, the timing of vaccination varied among participants in our study, and participants could receive vaccine doses during follow-up. We assumed that the individuals who were vaccinated months earlier had the same cytokine and chemokine profile as those who were vaccinated later. To assess this assumption, the correlation between days since vaccination and cytokine concentrations were evaluated and no differences were found. A sensitivity analysis was also performed to censor the visits that had a change in vaccination status, which also returned similar results. Second, outliers of cytokines at each visit were excluded from the analysis. However, most of them were out of fit-curve range or unstable estimates and were reported missing by the assay. Third, the calendar year is only a surrogate of SARS-CoV-2 variants and it might not fully capture the alpha and delta waves. A potential concern would be whether the alpha variant would be more aggressive or elicit enhanced inflammatory responses than the delta variant. However, a previous study found that alpha and delta variants showed similar disease severity.30 Fourth, our study was also limited by sample size for comparing vaccine types, since only 12 individuals received adenovirus vaccine and were fully vaccinated at screening. Studies with larger sample size might be able to assess differences more definitively in cytokine concentrations between recipients of adenovirus-based and mRNA vaccines. Most of our study population was unvaccinated at screening and the sample size of the partly vaccinated group was small (n=55 at screening). We might have low power to compare the partly vaccinated group with other groups. Furthermore, with the increasing availability of booster vaccine doses, future studies on these doses are need. Finally, although we adjusted for convalescent or control plasma treatment in regression models, it might have more complicated effects on cytokines and chemokines. However, convalescent plasma was not associated with vaccination status, and it likely did not confound the existing analysis.
This study found a lower inflammatory profile among fully vaccinated participants across all stages of COVID-19 recovery, suggesting that vaccination might have a long-lasting protective effect against inflammation induced by COVID-19. These findings suggest that vaccination might have short-term and long-term benefits after symptomatic infection and indicates a mechanistic explanation for the significantly reduced disease severity and mortality among vaccinated cohorts. Thus, it is essential to raise public awareness of the benefits of vaccination and guarantee equitable distribution of vaccines globally.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles published between Jan 1, 2020, and Feb 14, 2023, on COVID-19 vaccination and inflammation, using the terms “vaccin*”, “cytokine OR inflam*”, and “COVID-19”, without language restrictions. It is well documented that cytokine storm is associated with increased disease severity and risk of death following infection with SARS-CoV-2. Previous research also suggests people in early stages of recovery from COVID-19 have higher concentrations of pro-inflammatory cytokines and chemokines than pre-pandemic controls. Persistence of inflammation might also be related to the development of post-COVID-19 conditions. COVID-19 vaccination has been proven to reduce the risk of severe disease and death and might also reduce the risk of post-COVID-19 conditions. However, the effect of COVID-19 vaccination on longitudinal concentrations and trajectories of cytokines and chemokines among people with COVID-19 and throughout various stages of recovery remains poorly characterised.
Added value of this study
This is one of the largest studies to characterise cytokine and chemokine dynamics following infection with SARS-CoV-2, and among the first studies to evaluate the effect of COVID-19 vaccination on longitudinal cytokine and chemokine concentrations and trajectories. In a well characterised prospective cohort of people with symptomatic COVID-19, fully vaccinated individuals had lower concentrations of cytokines and chemokines than unvaccinated individuals throughout recovery (1–8 days since symptom onset to 90 days after enrolment). Cytokine and chemokine concentrations in partly vaccinated individuals were generally higher than those fully vaccinated and lower than unvaccinated individuals. Cytokine and chemokine concentrations decreased faster among unvaccinated individuals than fully and partly vaccinated individuals, but their mean concentrations were still higher than fully vaccinated participants at day 90. Specifically, at day 90, concentrations of interleukin (IL)-7, IL-8 and vascular endothelial growth factor-A were significantly higher in the unvaccinated group than in the fully vaccinated group. Collectively, this longitudinal prospective cohort study found that vaccinated individuals with COVID-19 have reduced inflammatory mediators, which provides a mechanistic explanation for their reduced morbidity and mortality when infected with SARS-CoV-2.
Implications of all the available evidence
COVID-19 vaccination might blunt the elevation of cytokine and chemokine concentrations and shorten the duration of pro-inflammatory responses following SARS-CoV-2 infection. This finding could partly explain how COVID-19 vaccination reduces severe morbidity and mortality. Vaccination could also have long-term benefits of decreasing inflammation after a breakthrough infection, adding to the evidence base for vaccine scale-up to combat COVID-19.
Acknowledgments
We thank the plasma donors and study participants who generously gave their time and biological specimens to the CSSC-001 and CSSC-004 trials and the passionate study personnel who facilitated these studies. This study was funded principally by the US Department of Defense’s Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND), in collaboration with the Defense Health Agency (W911QY2090012), and with additional support from Bloomberg Philanthropies, State of Maryland, Mental Wellness Foundation, Moriah Fund, Octapharma, HealthNetwork Foundation, the Shear Family Foundation and the National Institutes of Health (NIH): National Institute of Allergy and Infectious Diseases (NIAID; 3R01AI152078–01S1 and 3R01AI120938–05S1), National Institute on Drug Abuse (F31DA054849), National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK131926), NIH National Center for Advancing Translational Sciences (U24TR001609), and Division of Intramural Research NIAID NIH.
Footnotes
Declaration of interests
KAG reports consultancy work for the Aspen Institute, Teach for America, serving as a non-paid member of a scientific advisory board for Pfizer, and writing COVID-19 management guidelines for UpToDate. AGA reports consultancy work for Implementation Group, Hirslanden Klinik, and Elsevier. ERC reports receiving unrestricted research grants from Gilead and Merck paid to the Regents of the University of California and participating in an advisory board to Theratechnologies for an unrelated topic. JSC reports consultancy work for Merck and Company in 2021. TJG reports employment by Fenwal, a Fresenius Kabi Company. LLH reports research funding to Johns Hopkins Center of American Indian Health from AstraZeneca, US Centers for Disease Control and Prevention, Merck, NIH, and Pfizer. MAH reports contracts from Gilead Sciences, Insmed, and AN2 Therapeutics to the University of Cincinnati. GSM reports research grant support from Teva, Alk-Abello, Genentech, Novartis, GlaxoSmithKline, and Sanofi-Regeneron, serving as an immediate past president of the American Academy of Allergy Asthma and Immunology, and is co-chair of the Continuous Assessment Program Examination for the American Board of Allergy and Immunology BP reports participating in part of the COVID-19 trials and pulmonary arterial hypertension trials. JHP reports research funding from MindRhythm. JSR is a consultant and advisor with Sanofi Genzyme, and a board of directors member with the American Society for Apheresis. SK reports helping to produce educational materials related to HIV with Integritas Communications and Vindico Medical Education. AC reports serving on the scientific advisory board of SAB Biotherapeutics. EMB reports personal fees and non-financial support from Terumo BCT, Abbott Laboratories, Tegus, and UptoDate, is a member of the US Food and Drug Administration Blood Products Advisory Committee, and served on a convalescent plasma guideline panel. DH reports personal fees from Neurelis, Neurotrope, and medicolegal consulting. DJS is a founder and board member with stock options (macrolide for malaria) for AliquantumRx and reports consulting for Hemex Health and royalties for malaria diagnostic test control standards to Alere. SLH reports serving on the data monitoring committee for Pfizer. All other authors declare no competing interests.
Contributor Information
Xianming Zhu, Department of Pathology, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Kelly A Gebo, Department of Medicine, Division of Infectious Diseases, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Alison G Abraham, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA; Department of Epidemiology, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA.
Feben Habtehyimer, Department of Medicine, Division of Infectious Diseases, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Eshan U Patel, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Oliver Laeyendecker, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Washington, DC, USA.
Thomas J Gniadek, Department of Pathology and Laboratory Medicine, Northshore University Health System, Evanston, IL, USA.
Reinaldo E Fernandez, Department of Medicine, Division of Infectious Diseases, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Owen R Baker, Department of Medicine, Division of Infectious Diseases, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Malathi Ram, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Edward R Cachay, Department of Medicine, Division of Infectious Diseases, University of California, San Diego, San Diego, CA, USA.
Judith S Currier, Department of Medicine, Division of Infectious Diseases, University of California, Los Angeles, CA, USA.
Yuriko Fukuta, Department of Medicine, Section of Infectious Diseases, Baylor College of Medicine, Houston, TX, USA.
Jonathan M Gerber, Department of Medicine, Division of Hematology and Oncology, University of Massachusetts Chan Medical School, Worcester, MA, USA.
Sonya L Heath, Department of Medicine, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, AL, USA.
Barry Meisenberg, Department of Medicine and Research Institute of Luminis Health, Annapolis, MD, USA.
Moises A Huaman, Department of Medicine, Division of Infectious Diseases University of Cincinnati, Cincinnati, OH, USA.
Adam C Levine, Department of Emergency Medicine, Warren Alpert Medical School of Brown University, Providence, RI, USA.
Aarthi Shenoy, Division of Hematology, Medstar DC Hospital, Washington, DC, USA.
Shweta Anjan, Department of Medicine, Division of Infectious Diseases, University of Miami, Miller School of Medicine, Miami, FL, USA.
Janis E Blair, Department of Medicine, Division of Infectious Diseases, Mayo Clinic Hospital, Phoenix, AZ, USA.
Daniel Cruser, Department of Pathology, Nuvance Health Vassar Brothers Medical Center, Poughkeepsie, NY, USA.
Donald N Forthal, Department of Medicine, Division of Infectious Diseases, University of California, Irvine, CA, USA.
Laura L Hammitt, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Seble Kassaye, Division of Infectious Diseases, Georgetown University Medical Center, Washington, DC, USA.
Giselle S Mosnaim, Division of Allergy and Immunology, Department of Medicine, Northshore University Health System, Evanston, IL, USA.
Bela Patel, Department of Medicine, Divisions of Pulmonary and Critical Care Medicine, University of Texas Health Science Center, Houston, TX, USA.
James H Paxton, Department of Emergency Medicine, Wayne State University, Detroit, MI, USA.
Jay S Raval, Department of Pathology, University of New Mexico, Albuquerque, NM, USA.
Catherine G Sutcliffe, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Matthew Abinante, Ascada Research, Los Angeles, CA, USA.
Patrick Broderick, Department of Emergency Medicine, Nuvance Health Danbury Hospital, Danbury, CT, USA.
Valerie Cluzet, Department of Infectious Disease, Nuvance Health Vassar Brothers Medical Center, Poughkeepsie, NY, USA.
Marie Elena Cordisco, Department of Emergency Medicine, Nuvance Health Danbury Hospital, Danbury, CT, USA.
Benjamin Greenblatt, Department of Emergency Medicine, Nuvance Health Norwalk Hospital, Norwark, CT, USA.
Joann Petrini, Department of Emergency Medicine, Nuvance Health Danbury Hospital, Danbury, CT, USA.
William Rausch, Department of Emergency Medicine, Nuvance Health Danbury Hospital, Danbury, CT, USA.
David Shade, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Karen Lane, Department of Neurology, Brain Injury Outcomes Division, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Amy L Gawad, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Sabra L Klein, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Andrew Pekosz, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Shmuel Shoham, Department of Medicine, Division of Infectious Diseases, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Arturo Casadevall, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Evan M Bloch, Department of Pathology, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Daniel Hanley, Department of Neurology, Brain Injury Outcomes Division, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
David J Sullivan, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Aaron A R Tobian, Department of Pathology, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Data sharing
Data are available from DJS or AART on request with a reply expected in 14 days. Deidentified data from clinical trial will be deposited in the Vivli server for public access before the end of 2023.
References
- 1.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science 2022; 375: 1122–27. [DOI] [PubMed] [Google Scholar]
- 2.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020; 383: 2255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi Y, Ge Y, Wu B, et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J Infect Dis 2020; 222: 746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tufa A, Gebremariam TH, Manyazewal T, et al. Inflammatory mediators profile in patients hospitalized with COVID-19: a comparative study. Front Immunol 2022; 13: 964179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonny TS, Patel EU, Zhu X, et al. Cytokine and chemokine levels in coronavirus disease 2019 convalescent plasma. Open Forum Infect Dis 2020; 8: ofaa574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peluso MJ, Lu S, Tang AF, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis 2021; 224: 1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebo KA, Heath SL, Fukuta Y, et al. Early treatment, inflammation and post-COVID conditions. medRxiv 2023; published online Feb 16. https://www.medrxiv.org/content/10.1101/2023.02.13.23285855v1(preprint) [Google Scholar]
- 8.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021; 21: 195–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383: 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 2021; 384: 2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostrowski SR, Søgaard OS, Tolstrup M, et al. Inflammation and platelet activation after COVID-19 vaccines—possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis. Front Immunol 2021; 12: 779453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021; 144: 471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergamaschi C, Terpos E, Rosati M, et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep 2021; 36: 109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaba AH, Zhu X, Benner SE, et al. Higher proinflammatory cytokines are associated with increased antibody titer after a third dose of SARS-CoV-2 Vaccine in solid organ transplant recipients. Transplantation 2022; 106: 835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan DJ, Gebo KA, Shoham S, et al. Early outpatient treatment for COVID-19 with convalescent plasma. N Engl J Med 2022; 386: 1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DH, Keum N, Hu FB, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ 2018; 362: k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huai Luo C, Paul Morris C, Sachithanandham J, et al. Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta variant is associated with higher recovery of infectious virus compared to the alpha variant in both unvaccinated and vaccinated individuals. Clin Infect Dis 2022; 75: e715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noroozi R, Branicki W, Pyrc K, et al. Altered cytokine levels and immune responses in patients with SARS-CoV-2 infection and related conditions. Cytokine 2020; 133: 155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huapaya JA, Higgins J, Kanth S, et al. Vaccination ameliorates cellular inflammatory responses in SARS-CoV-2 breakthrough infections. J Infect Dis 2023; published online Feb 18. 10.1093/infdis/jiad045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Qin L, Zhang P, et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 2020; 5: e139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barata JT, Durum SK, Seddon B. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol 2019; 20: 1584–93. [DOI] [PubMed] [Google Scholar]
- 22.Ma A, Zhang L, Ye X, et al. High levels of circulating IL-8 and soluble IL-2R are associated with prolonged illness in patients with severe COVID-19. Front Immunol 2021; 12: 626235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesta MC, Zippoli M, Marsiglia C, et al. The role of interleukin-8 in lung inflammation and injury: implications for the management of COVID-19 and hyperinflammatory acute respiratory distress syndrome. Front Pharmacol 2022; 12: 808797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 2020; 383: 120–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta-Ampudia Y, Rojas M, Monsalve DM, Rodríguez Y, Ramírez-Santana C, Anaya JM. Nature and dimensions of the systemic hyper-inflammation and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis 2021; 223: 1833–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben Mkaddem S, Benhamou M, Monteiro RC. Understanding Fc receptor involvement in inflammatory diseases: from mechanisms to new therapeutic tools. Front Immunol 2019; 10: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirofski LA, Casadevall A. Pathogenesis of COVID-19 from the perspective of the damage-response framework. MBio 2020; 11: e01175–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Notarte KI, Catahay JA, Velasco JV, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine 2022; 53: 101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cagan E, Tezcan G, Simsek A, et al. The age-dependent role of Th22, Tc22, and Tc17 cells in the severity of pneumonia in COVID-19 immunopathogenesis. Viral Immunol 2022; 35: 318–27. [DOI] [PubMed] [Google Scholar]
- 30.Esper FP, Adhikari TM, Tu ZJ, et al. Alpha to omicron: disease severity and clinical outcomes of major SARS-CoV-2 variants. J Infect Dis 2023; 227: 344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from DJS or AART on request with a reply expected in 14 days. Deidentified data from clinical trial will be deposited in the Vivli server for public access before the end of 2023.