Abstract
Reports on the therapeutic efficacy and safety of carbon‐ion radiotherapy (C‐ion RT) for oligometastatic liver disease are limited, with insufficient evidence. This study aimed to evaluate the clinical outcomes of C‐ion RT for oligometastatic liver disease at all Japanese facilities using the nationwide cohort data. We reviewed the medical records to obtain the nationwide cohort registry data on C‐ion RT between May 2016 and June 2020. Patients (1) with oligometastatic liver disease as confirmed by histological or diagnostic imaging, (2) with ≤3 synchronous liver metastases at the time of treatment, (3) without active extrahepatic disease, and (4) who received C‐ion RT for all metastatic regions with curative intent were included in this study. C‐ion RT was performed with 58.0–76.0 Gy (relative biological effectiveness [RBE]) in 1–20 fractions. In total, 102 patients (121 tumors) were enrolled in this study. The median follow‐up duration for all patients was 19.0 months. The median tumor size was 27 mm. The 1‐year/2‐year overall survival, local control, and progression‐free survival rates were 85.1%/72.8%, 90.5%/78.0%, and 48.3%/27.1%, respectively. No patient developed grade 3 or higher acute or late toxicity. C‐ion RT is a safe and effective treatment for oligometastatic liver disease and may be beneficial as a local treatment option in multidisciplinary treatment.
Keywords: carbon‐ion radiotherapy, multicentric study, nationwide cohort data, oligometastatic liver disease, radiotherapy
Carbon‐ion radiotherapy could effectively treat oligometastatic liver disease. Carbon‐ion radiotherapy could be a local treatment option in multidisciplinary treatment.
Abbreviations
- C‐ion RT
carbon‐ion radiotherapy
- CT
computed tomography
- LC
local control
- OS
overall survival
- PFS
progression‐free survival
- RBE
relative biological effectiveness
- SBRT
stereotactic body radiotherapy
1. INTRODUCTION
Oligometastatic tumors are often encountered in clinical practice, and the efficacy of local treatment for oligometastatic tumors has been studied in recent years. Hellman and Weichselbaum reported that oligometastatic tumors are in an intermediate state between localization and widespread dissemination 1 ; therefore, local treatment to control oligometastatic tumors might improve survival, depending on the primary disease. Although surgery is one of the most effective local treatments for oligometastatic tumors, the best treatment option for those not amenable to surgery due to the patient's background, complications, or age remains debatable. SBRT has been reported as a local treatment option for oligometastatic tumors in various cancers. 2 , 3 , 4 , 5 , 6 , 7 The American Society for Radiation Oncology (ASTRO) model policies state that SBRT is a suitable local treatment for oligometastatic tumors. 8 Although SBRT showed favorable results, the 1‐year or 2‐year OS and LC rates were 26%–85% and 32%–95% in oligometastatic liver disease, respectively. 2 , 3 , 4 , 5 , 6 We hypothesize that carbon‐ion radiotherapy (C‐ion RT) could show similar or better results than SBRT.
C‐ion RT has physical and biological advantages with higher dose localization properties due to the sharp lateral penumbra and Bragg‐peak and higher cell‐killing effect due to higher linear energy transfer compared with X‐ray RT. 9 Although these advantages of C‐ion RT are expected to have favorable clinical outcomes compared with SBRT, there have been limited reports on the clinical outcomes of oligometastatic liver disease treated with C‐ion RT. 10 , 11 Since May 2016, all patients treated with C‐ion RT have been registered in a multi‐institutional nationwide cohort study. These data allow the analysis of clinical outcomes in all patients at facilities that provide C‐ion RT in Japan. To build evidence for C‐ion RT for oligometastatic liver disease, we report the clinical results of C‐ion RT for oligometastatic liver disease at all Japanese C‐ion RT facilities using nationwide cohort data.
2. MATERIALS AND METHODS
2.1. Patient eligibility
We reviewed the medical records to obtain the nationwide cohort registry data of C‐ion RT between May 2016 and June 2020. In total, 102 patients met the following criteria:
histological or diagnostic imaging confirmed oligometastatic liver disease;
≤3 synchronous liver metastases at the time of treatment;
absence of recurrence in the primary disease site after primary curative treatment;
absence or control of extrahepatic disease;
not indicated or refused surgery for metastatic disease of the liver;
radiographically measurable tumor;
initial enrollment for C‐ion RT;
delivery of C‐ion RT to all metastatic regions with curative intent.
Before patient registration, medical history, physical examination, routine testing of blood cell counts, chemistry, urine analysis, CT, MRI, and/or fluorodeoxyglucose PET were acquired as a pretreatment evaluation.
The treatment protocol for this study was reviewed and approved by the Institutional Review Board (approval number: 16‐007), and all patients signed an informed consent form before treatment initiation.
2.2. Carbon‐ion radiotherapy
A heavy‐ion accelerator at each C‐ion RT facility was used to generate C‐ion beams. Doses of C‐ion RT were expressed as the RBE weighted dose (Gy [RBE]), which was defined as the physical dose multiplied by the RBE of the C‐ions. 12 Before C‐ion RT, patients were immobilized using tailor‐made fixation cushions and thermoplastic shells to acquire treatment‐planning CT images. Respiratory‐gated and four‐dimensional CT images were then acquired. Images from the expiratory phase were used for treatment planning. Patients received C‐ion RT once daily for 4 days a week. Treatment‐planning CT images were merged with MRI and/or PET images to precisely delineate the target. Gross tumor, clinical target, and planning target volumes were determined. The patient position was verified using digital orthogonal X‐ray and reference images that were digitally reconstructed based on the planning CT for daily patient position matching. C‐ion RT was performed with 58.0–76.0 Gy (RBE) in 1–20 fractions (3.8–58.0 Gy [RBE] per fraction).
2.3. Evaluation during follow‐up
The patients were followed up every 3 months after completion of C‐ion RT. The follow‐up examinations performed included routine testing of blood cell counts, blood chemistry, and diagnostic imaging using CT, MRI, and/or PET. LC was defined as no evidence of tumor regrowth on CT, MRI, and/or PET in the irradiated tumor bed, with or without a continuous elevation of blood levels of tumor markers. PFS was defined as the absence of progression of both local and distant metastases. Acute and late toxicities were graded using the Common Terminology Criteria for Adverse Events, version 4.0 of the National Cancer Institute. 13 Acute and late toxicities were evaluated as the highest grade of toxicity that occurred within 3 months and after 3 months, respectively, from the initiation of C‐ion RT.
2.4. Statistical analysis
All statistical analyses were performed using JMP Pro 12.2.0 software (SAS Institute, Inc.). OS was measured from the date of C‐ion RT initiation to the date of death or most recent follow‐up. LC was measured from the date of C‐ion RT initiation to the date of observation of local failure or most recent follow‐up. PFS was measured from the date of C‐ion RT initiation to the date of observation of tumor progression or death from any cause. The probabilities of OS, LC, and PFS were calculated using the Kaplan–Meier method. Additionally, we analyzed OS and LC rates in patients treated with hypofractionation (≤4 fractions) and nonhypofractionation (>4 fractions). We used the log‐rank test for the univariate analysis and the Cox proportional hazards regression model for multivariate analysis to compare the clinical outcomes based on the patients' characteristics. All factors with a p‐value of <0.20 in the univariate analysis were subjected to multivariate analysis. A p‐value of <0.05 was considered statistically significant. Variable risk was expressed as a hazard ratio with a corresponding 95% confidence interval (CI).
3. RESULTS
3.1. Patient characteristics
In this study, 102 patients with 121 tumors were enrolled. The patient characteristics are summarized in Table 1. The median follow‐up duration in all patients was 19.0 months (range: 0.7–72.0 months). The median age at the time of C‐ion RT initiation was 67 years (range, 38–85 years). The median tumor size was 27 mm (range: 7–90 mm). The numbers of patients and tumors along with their origin were, respectively, 60 and 68 from colorectal cancer, 15 and 21 from cholangiocarcinoma, 11 and 13 from pancreatic cancer, and 16 and 19 from other cancer types (choroid, lung, duodenum, esophagus, stomach, breast, ovary, and uterine cervical cancer). The dose fractions of C‐ion RT are listed in Table 1. The median dose was 60 Gy (RBE; range: 58–76 Gy), and the median fraction was four fractions (range: 1–20 fractions). All patients completed C‐ion RT as scheduled.
TABLE 1.
Patient and tumor characteristics of 102 patients with 121 tumors.
| Characteristic | Value |
|---|---|
| Age, years | |
| Median (range) | 67 (38–85) |
| PS, number of patients | |
| 0 | 81 |
| 1 | 19 |
| 2 | 2 |
| Sex, number of patients | |
| Male | 43 |
| Female | 59 |
| Origin of the cancer, number of patients | |
| Colorectal cancer | 60 |
| Cholangiocarcinoma | 15 |
| Pancreatic cancer | 11 |
| Other cancer | 16 |
| Child–Pugh class, number of patients | |
| A | 95 |
| B | 3 |
| Not applicable | 4 |
| Previous history of liver resection, number of patients | |
| Presence | 34 |
| Absence | 58 |
| Not applicable | 10 |
| Tumor number, number of patients | |
| 1 | 84 |
| 2 | 17 |
| 3 | 1 |
| Surgical indication, number of patients | |
| Not indicated by disease status | 55 |
| Not indicated by age or complication | 18 |
| Refused | 29 |
| Tumor size, mm | |
| Median (range) | 27 (7–90) |
| Dose fraction of C‐ion RT, number of tumors | |
| 58 Gy (RBE) in 1 fr. | 10 |
| 60 Gy (RBE) in 4 fr. | 68 |
| 60 Gy (RBE) in 6 fr. | 1 |
| 60 Gy (RBE) in 8 fr. | 1 |
| 64 Gy (RBE) in 8 fr. | 7 |
| 68 Gy (RBE) in 8 fr. | 2 |
| 60 Gy (RBE) in 12 fr. | 16 |
| 64.8 Gy (RBE) in 12 fr. | 3 |
| 69.6 Gy (RBE) in 12 fr. | 1 |
| 68 Gy (RBE) in 16 fr. | 4 |
| 60 Gy (RBE) in 20 fr. | 1 |
| 76 Gy (RBE) in 20 fr. | 7 |
Abbreviations: C‐ion RT, carbon‐ion radiotherapy; fr., fractions; PS, performance status; RBE, relative biological effectiveness.
3.2. Clinical outcomes
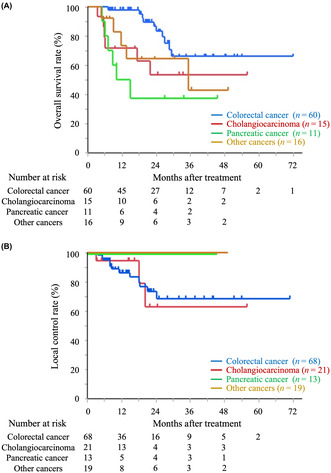
The median survival time was not available at the time of analysis. The 1‐year/2‐year OS rates were 85.1%/72.8% (95% CI: 76.0%–91.2% and 61.5%–81.7%, respectively), the LC rates were 90.5%/78.0% (95% CI: 81.9%–95.2% and 65.3%–86.9%, respectively), and the PFS rates were 48.3%/27.1% (95% CI: 38.3%–58.4% and 18.5%–37.8%, respectively). Figure 1 shows the OS, LC, and PFS curves for all patients. In total, 14 patients with 15 tumors had local recurrence, and 27 died at the time of analysis. None of the patients developed grade 3 or higher acute or late toxicity.
FIGURE 1.
Kaplan–Meier curves of overall survival (A), local control (B), and progression‐free survival (C) for the all‐patient cohort.
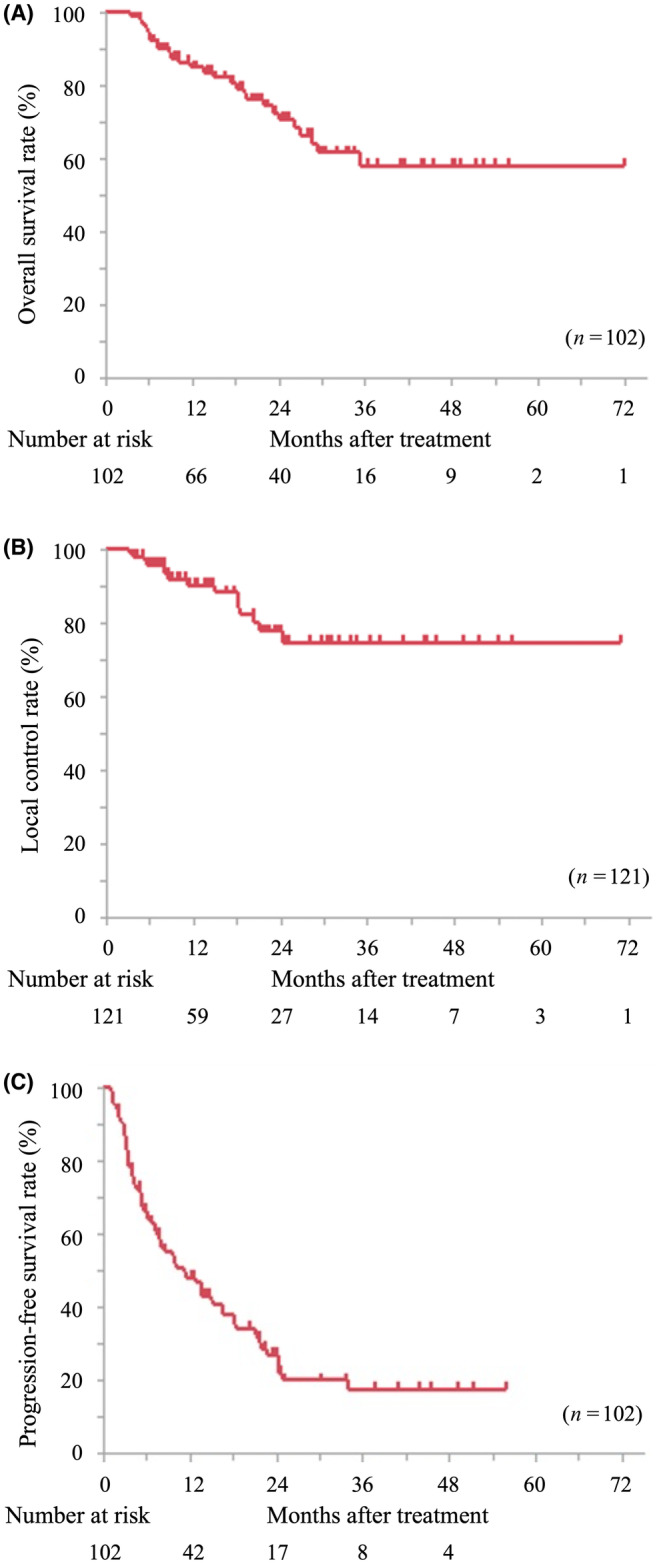
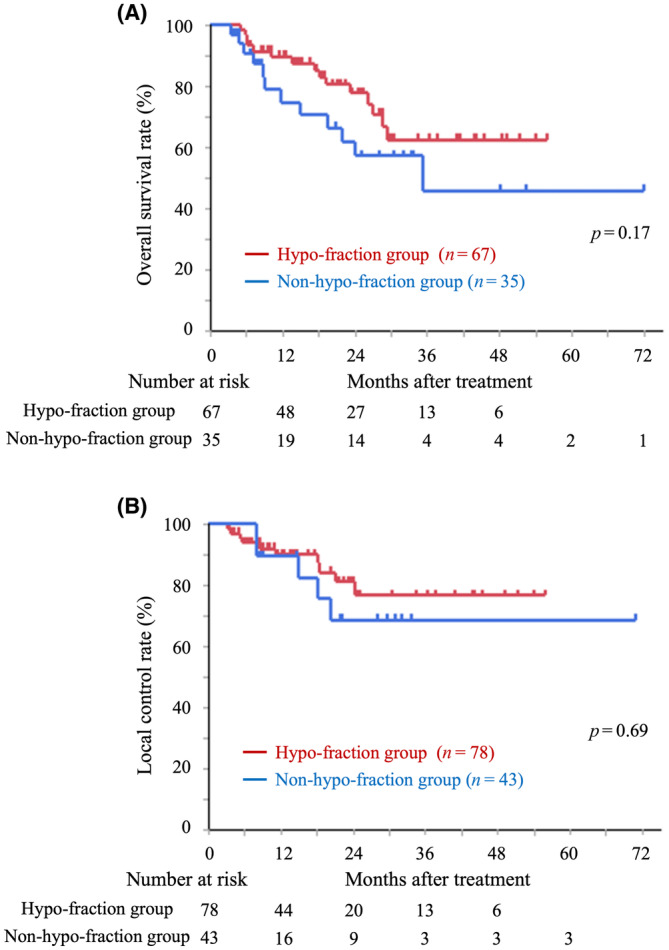
We analyzed OS and LC rates in patients treated with hypofractionation (≤4 fractions) and nonhypofractionation (>4 fractions; Figure 2). The 2‐year OS and LC rates in the hypofractionation group were 78.0% (95% CI: 64.0%–87.6%) and 81.1% (95% CI: 66.5%–90.3%), respectively, and those in the nonhypofractionation group were 62.1% (95% CI: 42.3%–78.6%) and 68.8% (95% CI: 43.0%–86.6%), respectively. There were no significant differences in OS and LC between the hypofractionation and the nonhypofractionation group (p = 0.17 and 0.69, respectively). Additionally, we analyzed OS and LC based on the origin of the cancers (Figure 3 and Table 2). For colorectal cancer, the 1‐year/2‐year OS rates were 98.1%/86.8% (95% CI: 87.6%–99.7% and 71.6%–94.5%, respectively), and the LC rates were 86.5%/73.8% (95% CI: 74.1%–93.5% and 57.6%–85.4%, respectively). Patients with colorectal cancer showed significantly better OS than those with cholangiocarcinoma (p < 0.05) and pancreatic cancer (p < 0.01); in contrast, no significant differences were observed in the LC rate analyzed by cancer origin.
FIGURE 2.
Kaplan–Meier curves of overall survival (A) and local control (B) in the patient treated using hypofractionation (≤4 fractions) and nonhypofractionation (>4 fractions).
FIGURE 3.
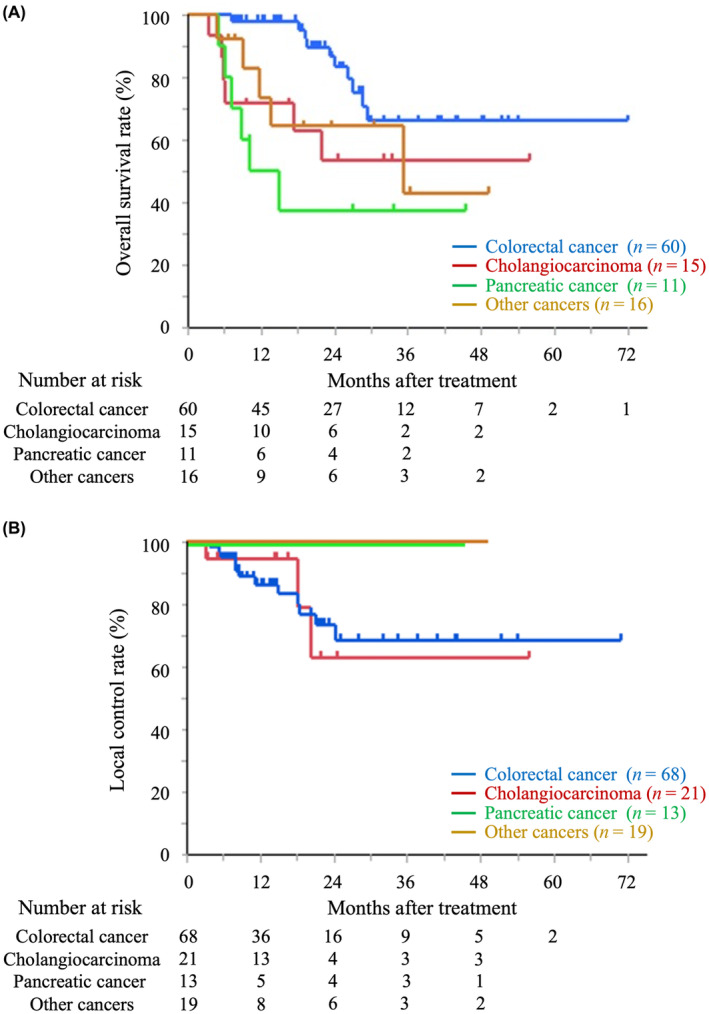
Kaplan–Meier curves of overall survival (A), local control (B), based on the origin of the cancers.
TABLE 2.
Overall survival and local control rates by the origin of the cancers.
| Origin of the cancer | Overall survival rates | Local control rates | ||
|---|---|---|---|---|
| n | 1 y/2 y | n | 1 y/2 y | |
| Colorectal cancer | 60 | 98.1%/86.8% | 68 | 86.5%/73.8% |
| Cholangiocarcinoma | 15 | 71.8%/53.8% | 21 | 94.7%/63.2% |
| Pancreatic cancer | 11 | 50.0%/37.5% | 13 | 100%/100% |
| Other cancer | 16 | 73.8%/64.6% | 19 | 100%/100% |
Abbreviation: y, years.
The results of the univariate and multivariate analyses are shown in Table 3. In multivariate analysis, the origin of the cancer other than colorectal cancer was the only independent poor prognostic factor of OS.
TABLE 3.
Univariate and multivariate analyses of the overall survival and local control rates.
| Univariate analysis | ||||||
|---|---|---|---|---|---|---|
| Variables | Overall survival | Local control | ||||
| n | 2‐y overall survival rate | p‐value | n | 2‐y local control rate | p‐value | |
| Age, years | ||||||
| ≤67 | 52 | 76.9% | 0.34 | 62 | 85.0% | 0.29 |
| >67 | 50 | 67.4% | 59 | 70.1% | ||
| PS | ||||||
| 0 | 81 | 71.3% | 0.81 | 98 | 76.0% | 0.90 |
| 1, 2 | 21 | 77.2% | 23 | 84.3% | ||
| Sex | ||||||
| Male | 59 | 71.4% | 0.59 | 68 | 78.7% | 0.73 |
| Female | 43 | 74.6% | 53 | 77.7% | ||
| Origin of the cancer | ||||||
| Colorectal cancer | 60 | 86.8% | <0.01 | 68 | 73.8% | 0.15 |
| Other than colorectal cancer | 42 | 53.2% | 53 | 85.6% | ||
| Tumor size, mm | ||||||
| ≤40 | 73 | 73.6% | 0.16 | 83 | 83.6% | 0.06 |
| >40 | 29 | 69.0% | 27 | 57.4% | ||
| Surgical indication | ||||||
| Indicated | 29 | 73.8% | 0.68 | 35 | 77.4% | 0.97 |
| Not indicated | 73 | 72.0% | 86 | 78.2% | ||
| Fraction size | ||||||
| ≤4 | 67 | 78.0% | 0.17 | 78 | 81.1% | 0.69 |
| >4 | 35 | 62.1% | 43 | 68.8% | ||
| Multivariate analysis | ||||||
|---|---|---|---|---|---|---|
| Variables | Multivariate for overall survival | Multivariate for local control | ||||
| HR | 95% CI | p‐value | HR | 95% CI | p‐value | |
| Origin of the cancer | ||||||
| Colorectal cancer vs. Other than colorectal cancer | 0.39 | 0.17–0.86 | <0.05 | 2.04 | 0.56–7.41 | 0.28 |
| Tumor size | ||||||
| ≤40 mm vs. >40 mm | 1.79 | 0.77–4.17 | 0.19 | 2.82 | 0.91–8.73 | 0.07 |
| Fraction size | ||||||
| ≤4 vs. >4 | 1.31 | 0.59–2.88 | 0.51 | |||
Abbreviations: CI, confidence interval; C‐ion RT, carbon‐ion radiotherapy; HR, hazard ratio; y, years.
4. DISCUSSION
We showed the clinical outcomes of C‐ion RT for oligometastatic liver disease using nationwide cohort registry data. The 1‐year/2‐year OS rates were 85.1% and 72.8%, respectively. The 1‐year/2‐year LC rates were 90.5% and 78.0%, respectively. Finally, the 1‐year/2‐year PFS rates were 48.3% and 27.1%, respectively. No grade 3 or higher severe toxicities were observed. These results suggest that C‐ion RT for oligometastatic liver disease is safe and effective and could be a local treatment option for oligometastatic liver disease.
Previous reports of SBRT for oligometastatic liver disease showed that the 1‐year or 2‐year OS and LC rates were 26%–85% and 32%–95% (Table 4), respectively, with 0%–9.8% of grade 3 or higher toxicities. ASTRO model policies indicated that SBRT is a suitable local treatment for oligometastatic tumors. 8 Our study showed that the clinical results of C‐ion RT for oligometastatic liver disease are comparable with or better than those of SBRT. Additionally, the frequency of grade 3 or higher toxicities in C‐ion RT was similar to or lower than those in previous SBRT reports, even though our analysis of C‐ion RT included tumors larger than 4 cm (Tables 3 and 4). Furthermore, although it is difficult to compare clinical outcomes between C‐ion RT and SBRT in large tumors because there are no reports on clinical outcomes in SBRT for larger tumors, C‐ion RT showed favorable clinical results with no difference in treatment efficacy of LC and OS in tumors that were larger than 4 cm compared with those that were 4 cm or smaller. We consider that this safe and effective treatment with C‐ion RT for larger tumors in the liver is due to the better dose distribution of C‐ion RT than that of SBRT. 14
TABLE 4.
Comparison of the present study with previous studies on oligometastatic liver disease.
| Reference | Year | Patient and tumor number | Total dose of SBRT | Origin of the cancer | Overall survival rates | Local control rates | Late toxicities ≥grade 3 |
|---|---|---|---|---|---|---|---|
| Yamashita et al. 2 | 2014 | 51 patients with 64 tumors | 30–60 Gy | Various | 2 y: 71.9% | 2 y: 64% | 9.8% |
| Scorsetti et al. 3 | 2018 | 61 patients with 76 tumors | 52.5–75 Gy in 3 fr. | Various | 1 y/3 y/5 y: 85.2%/31.1%/18.0% | 1 y/3 y/5 y: 94%/78%/78% | 1.0% |
| Comito et al. 4 | 2014 | 41 patients with 52 tumors | 75 Gy in 3 fr. | Colorectum | 1 y/2 y/3 y: 78%/61%/44% | 1 y/2 y/3 y: 95%/90%/81% | 0% |
| Scorsetti et al. 5 | 2015 | 42 patients with 65 tumors | 75 Gy in 3 fr. | Colorectum | 2 y: 65% | 1 y/2 y/3 y: 95%/91%/85% | 0% |
| McPartlin et al. 6 | 2017 | 51 patients with 93 tumors | 37.6 Gy in 6 fr. | Colorectum | 1 y/2 y/4 y: 63%/26%/9% | 1 y/2 y/4 y: 50%/32%/26% | 0% |
| Current study | 102 patients with 121 tumors | 58–76 Gy in 1–20 fr. with C‐ion RT | Various | 1 y/2 y: 85.1%/72.8% | 1 y/2 y: 90.5%/78.0% | 0% |
Abbreviations: C‐ion RT, carbon‐ion radiotherapy; fr, fraction; SBRT, stereotactic body radiotherapy; y, years.
Local treatment, including surgery for oligometastatic liver disease of colorectal cancer, has been reported to improve survival, and those studies reported that 2‐year OS rates were 49%–74%. 15 , 16 In our study, the 2‐year OS rate in colorectal cancer was 86.8%, and the clinical results of C‐ion RT were comparable with those of surgery, despite the inclusion of inoperable patients. However, whether C‐ion RT contributes to OS in oligometastatic liver diseases other than colorectal cancer was unclear. A previous report showed that surgery improved OS in oligometastatic liver disease of intrahepatic cholangiocarcinoma, 17 and local treatment, including C‐ion RT, for this condition may contribute to improved OS. In contrast, current guidelines do not recommend local treatment or chemotherapy in oligometastatic liver disease of pancreatic cancer. 18 Regarding indications for oligometastatic liver disease treated with C‐ion RT, it is important to select the origin of the cancer types for which local treatment has been reported to improve survival. Further analysis using a larger sample size and extended follow‐up duration is required, and the origin of the cancers for which local treatment is effective in oligometastatic liver disease should be determined.
The median number of fractions is four, and C‐ion RT can be completed within 1 week; therefore, depending on the chemotherapy regimen, C‐ion RT can be performed between each chemotherapy cycle or during the withdrawal of chemotherapy. Additionally, hypofractionated C‐ion RT showed 2‐year OS and LC rates of 78.0% and 81.1%, respectively, which were similar to, or better than, those of nonhypofractionated C‐ion RT. The availability of local treatment between chemotherapy would be an advantage in multidisciplinary treatment and may contribute to the prolongation of OS. Additionally, if a patient has multiple metastases in which only one lesion is uncontrolled, and the other lesions are controlled by chemotherapy, the chemotherapy effect is considered a progressive disease, and a change in the chemotherapy regimen is considered. However, if an uncontrollable lesion can be controlled by local treatment such as C‐ion RT, it may be possible to consider not changing the chemotherapy regimen. This approach of local treatment using C‐ion RT may be a new treatment option for cancer patients, especially for cancers with fewer chemotherapy regimens. Therefore, as a multidisciplinary treatment in cancer therapy, C‐ion RT may be a treatment option for oligometastatic liver disease.
This study had some limitations. This analysis was performed on patients with heterogeneous backgrounds, especially those with significantly different prognoses regarding the origin of the cancers and the availability of chemotherapy. Second, the registry data do not adequately describe the presence or absence of adjuvant therapy after the administration of C‐ion RT or systemic therapy after recurrence. Therefore, a competing risk analysis of the local effects cannot be performed. In addition, as the management of chemotherapy such as adjuvant chemotherapy differs depending on the origin of the cancers, the impact of chemotherapy before and after C‐ion RT on LC and OS needs to be clarified in the future based on the cancer‐specific prospective studies. Further analysis is needed to increase the number of patients and to analyze the origin of each cancer.
In conclusion, we evaluated the clinical outcomes of C‐ion RT for oligometastatic liver disease using nationwide cohort registry data, suggesting that C‐ion RT for oligometastatic liver disease is a safe and effective treatment. Furthermore, hypofractionated C‐ion RT with chemotherapy would be an advantage of multidisciplinary treatment. Therefore, C‐ion RT is a local treatment option for oligometastatic liver disease and may play a role in multidisciplinary treatment.
CONFLICT OF INTEREST STATEMENT
Tatsuya Ohno received research funding from Hitachi, and Hiroyuki Katoh received research funding from Toshiba Energy Systems and Solutions Corporation. All other authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study was reviewed and approved by the Institutional Review Board (approval number: 16‐007), and this study conforms to the provisions of the Declaration of Helsinki.
Informed Consent: All patients included in this study signed an informed consent form before treatment initiation.
Registry and the registration number of the study/trial: N/A.
Animal Studies: N/A.
DISCLOSURE
None of the authors is an Editor or an Editorial Board Member of Cancer Science.
ACKNOWLEDGMENTS
The authors would like to thank all patients involved in this study, our colleagues in the Japan Carbon‐Ion Radiation Oncology Study Group, and Editage (www.editage.com) for English language editing.
Shiba S, Wakatsuki M, Toyama S, et al. Carbon‐ion radiotherapy for oligometastatic liver disease: A national multicentric study by the Japan Carbon‐Ion Radiation Oncology Study Group (J‐CROS). Cancer Sci. 2023;114:3679‐3686. doi: 10.1111/cas.15871
DATA AVAILABILITY STATEMENT
The datasets generated for this study are available from the corresponding author upon request.
REFERENCES
- 1. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8‐10. doi: 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 2. Yamashita H, Onishi H, Matsumoto Y, et al. Local effect of stereotactic body radiotherapy for primary and metastatic liver tumors in 130 Japanese patients. Radiat Oncol. 2014;9:112. doi: 10.1186/1748-717X-9-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scorsetti M, Comito T, Clerici E, et al. Phase II trial on SBRT for unresectable liver metastases: long‐term outcome and prognostic factors of survival after 5 years of follow‐up. Radiat Oncol. 2018;13:234. doi: 10.1186/s13014-018-1185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Comito T, Cozzi L, Clerici E, et al. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer. 2014;14:619. doi: 10.1186/1471-2407-14-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015;141:543‐553. doi: 10.1007/s00432-014-1833-x [DOI] [PubMed] [Google Scholar]
- 6. McPartlin A, Swaminath A, Wang R, et al. Long‐term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys. 2017;99:388‐395. doi: 10.1016/j.ijrobp.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 7. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long‐term results of the SABR‐COMET phase II randomized trial. J Clin Oncol. 2020;38:2830‐2838. doi: 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ASTRO model policies stereotactic body radiation therapy. ASTROSBRTModelPolicy pdf. Accessed June 23, 2021.
- 9. Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy‐ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201‐210. doi: 10.1016/s0360-3016(98)00544-6 [DOI] [PubMed] [Google Scholar]
- 10. Makishima H, Yasuda S, Isozaki Y, et al. Single fraction carbon ion radiotherapy for colorectal cancer liver metastasis: a dose escalation study. Cancer Sci. 2019;110:303‐309. doi: 10.1111/cas.13872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiba S, Shibuya K, Okamoto M, et al. Carbon‐ion radiotherapy for oligometastatic colorectal cancer in the liver or lung. Anticancer Res. 2021;41:1997‐2005. doi: 10.21873/anticanres.14967 [DOI] [PubMed] [Google Scholar]
- 12. Inaniwa T, Kanematsu N, Matsufuji N, et al. Reformulation of a clinical‐dose system for carbon‐ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys Med Biol. 2015;60:3271‐3286. doi: 10.1088/0031-9155/60/8/3271 [DOI] [PubMed] [Google Scholar]
- 13. NCI common terminology criteria for adverse events (CTCAE). Version 4.0. Data file. ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed February 1, 2022.
- 14. Abe T, Saitoh J, Kobayashi D, et al. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat Oncol. 2015;10:187. doi: 10.1186/s13014-015-0491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elias D, Liberale G, Vernerey D, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12:900‐909. doi: 10.1245/ASO.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 16. Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677‐3683. doi: 10.1200/JCO.2008.20.5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kojima T, Umeda Y, Fuji T, et al. Efficacy of surgical management for recurrent intrahepatic cholangiocarcinoma: a multi‐institutional study by the Okayama study group of HBP surgery. PLoS One. 2020;15:e0238392. doi: 10.1371/journal.pone.0238392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NCCN guidelines for patients, pancreatic cancer . https://www.nccn.org/patients/guidelines/content/PDF/pancreatic‐patient.pdf. Accessed August 22, 2022; 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available from the corresponding author upon request.


