Abstract
Desmoplastic reaction is a fibrosis reaction that is characterized by a large amount of dense extracellular matrix (ECM) and dense fibrous stroma. Fibrotic stroma around the tumor has several different components, including myofibroblasts, collagen, and other ECM molecules. This stromal reaction is a natural response to the tissue injury process, and fibrosis formation is a key factor in pancreatic cancer development. The fibrotic stroma of pancreatic cancer is associated with tumor progression, metastasis, and poor prognosis. Reportedly, multiple processes are involved in fibrosis, which is largely associated with the upregulation of various cytokines, chemokines, matrix metalloproteinases, and other growth factors that promote tumor growth and metastasis. Fibrosis is also associated with immunosuppressive cell recruitment, such as regulatory T cells (Tregs) with suppressing function to antitumor immunity. Further, dense fibrosis restricts the flow of nutrients and oxygen to the tumor cells, which can contribute to drug resistance. Furthermore, the dense collagen matrix can act as a physical barrier to block the entry of drugs into the tumor, thereby further contributing to drug resistance. Thus, understanding the mechanism of desmoplastic reaction and fibrosis in pancreatic cancer will open an avenue to innovative medicine and improve the prognosis of patients suffering from this disease.
Keywords: cancer, desmoplastic reaction, fibroblasts, fibrosis, pancreas
Desmoplastic reaction is a phenomenon seen in many cancers, including pancreatic cancer. It occurs when the tumor invades the surrounding tissues, causing an inflammatory response that leads to fibrosis.
Abbreviations
- CAFs
cancer‐associated fibroblasts
- CF
cystic fibrosis
- CTGF
connective tissue growth factor
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- HA
hyaluronic acid
- HDAC
histone deacetylase
- HGF
hepatocyte growth factor
- HIF1
hypoxia‐inducible factor 1
- IL
interleukin
- MMPs
matrix metalloproteinases
- NF‐κB
nuclear factor–kappa B
- PDGF
platelet‐derived growth factor
- PD‐L1
programmed death‐ligand 1
- PSC
pancreatic stellate cell
- RNA
ribonucleic acid
- TGFB
transforming growth factor beta
- TIMP‐1
tissue inhibitor of metalloproteinase 1
- TNF
tumor necrosis factor
- Treg
regulatory T cell
1. INTRODUCTION
Desmoplastic reaction is a phenomenon in many cancers, including breast, metastatic colorectal, and pancreatic cancer. 1 , 2 , 3 However, elucidating the mechanisms is one of the currently little‐known areas regarding desmoplastic reaction and its morphologic characteristics, including fibrosis occurrence. For example, the association between the interactions among cancer cells and surrounding fibroblasts and desmoplastic reaction and its relationship to fibrosis remains unclear. Currently, a desmoplastic reaction is known to occur when the tumor invades the surrounding tissue, causing an inflammatory response that results in fibrosis and scar tissue formation. This reaction is thought to be an adaptive response of the living body to the presence of invasive cancers, as it helps to limit the spread of tumors, but it can also limit the efficacy of chemotherapy, 4 as the dense scar tissue will make the drug penetration into tumors difficult. The significant involvement of the pancreas needs to be investigated to overcome this life‐threatening cancer with a very poor prognosis probably due to its immunosuppressive characteristics although fibrosis research is documented in diseases in several organs, such as the lung, heart, and liver. 5 Currently, treatments for fibrosis are limited, with a need to develop new therapies that can slow the disease progression (Figure 1). Here, we update the recent advancement in research and discuss the significance of desmoplastic reaction and cancer fibrosis, especially focusing on pancreatic cancer.
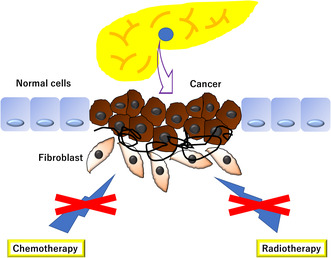
FIGURE 1.
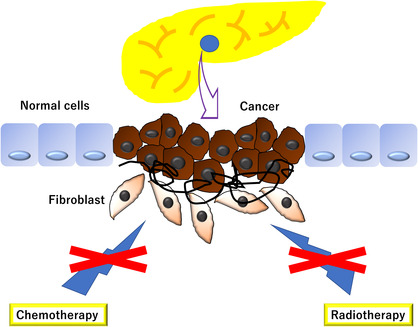
Desmoplastic reaction is a phenomenon seen in many cancers, including pancreatic cancer, and it occurs when the tumor invades the surrounding tissues, inducing an inflammatory response that causes fibrosis and scar tissue formation. This reaction is believed to be responsible for limiting tumor spread, but dense scar tissue can limit the effectiveness of chemotherapy and radiation therapy.
2. DESMOPLASTIC REACTION AND FIBROSIS
Desmoplastic reaction and fibrosis are two distinct, but related, pathological processes that are commonly seen in various cancer types. 6 Desmoplastic reaction is a general term used to describe the fibrous tissue proliferation in the area surrounding a tumor. 6 This tissue, which is composed of collagen and other connective tissue components, is referred to as the desmoplastic stroma and serves to physically separate the tumor from the surrounding healthy tissue. 6 Fibrosis is a term used to describe the progressive accumulation of extracellular matrix (ECM) components, such as hyaluronic acid (HA), in the interstitial space of a tissue that interacts with collagen to affect cancer cell activity. 6 This accumulation of matrix material is thought to be a response to chronic inflammation or injury and causes tissue scarring and stiffness. 7 Desmoplastic reaction is thought to contribute to fibrosis formation, as the desmoplastic stroma can impede the diffusion of nutrients and oxygen to the surrounding tissue, thereby creating an environment conducive to ECM component accumulation and affecting cancer cell activity. 6
3. DESMOPLASTIC REACTION AND PANCREATIC CANCER
Desmoplastic reaction is a host response to pancreatic cancer, whereby the tumor triggers excessive fibroblasts, collagen, and other stromal component production. This causes a rigid desmoplastic stroma that is resistant to chemotherapy and radiotherapy and limits treatment effectiveness. 1 , 8 Single‐cell RNA sequencing of pancreatic cancer revealed that high fibroblast levels were indicative of poor prognosis. 9 Recent evidence suggests that desmoplastic reaction may also provide a physical barrier that helps the tumor resist chemotherapy and radiation. Additionally, desmoplastic reaction decreases the immune response by accumulating type I collagen and prevents the immune system from recognizing and attacking the tumor. 2
4. FIBROSIS AND PANCREATIC CANCER
Pancreatic cancer is related to fibrosis as it is a potentially serious chronic pancreatitis complication, which is a form of pancreatic fibrosis. Chronic pancreatitis is caused by long‐term pancreatic inflammation and gradual endocrine and exocrine compartment loss due to atrophy and replacement with fibrous tissue, 10 thereby developing scar tissue in the organ. This scar tissue can become cancerous over time, leading to pancreatic cancer. 11
5. MOLECULES CONTROLLING DESMOPLASTIC REACTION AND FIBROSIS
The following factors should be considered because multiple factors control the process in desmoplastic reaction (Figure 2):
Inflammation: Inflammation is an important factor in developing fibrosis, as it can stimulate fibroblast proliferation, increase ECM deposition, and mediate inflammatory cell recruitment. 12
Growth factors: Growth factors, such as fibroblast growth factor (FGF), transforming growth factor beta (TGFB), hepatocyte growth factor (HGF), and platelet‐derived growth factor (PDGF), are important fibrosis regulators and are involved in fibroblast recruitment, proliferation, and differentiation. 13
Cytokines: Cytokines, such as interleukin‐1 (IL‐1) and tumor necrosis factor alpha (TNF‐α), are involved in fibrosis initiation and progression, as they promote the inflammatory cell recruitment and activation and modulate growth factor production. 14
Oxidative stress: Oxidative stress can increase inflammatory mediator production, which can cause fibrotic changes. 15
Matrix metalloproteinases (MMPs): MMPs are important proteases that are involved in ECM degradation and can affect fibrosis development by modulating growth factor and cytokine productions. 16
FIGURE 2.
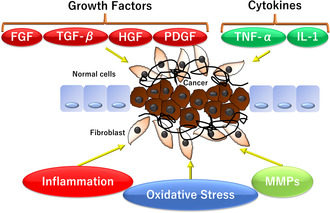
The process of demyelination and fibrosis is governed by multiple factors, including (1) inflammation, (2) growth factors such as fibroblast growth factors (FGFs), transforming growth factor beta (TGFB), hepatocyte growth factor (HGF), and platelet‐derived growth factor (PDGF), (3) cytokines such as tumor necrosis factor alpha (TNF‐α and interleukin‐1 (IL‐1), (4) oxidative stress, and (5) matrix metalloproteinases (MMPs).
5.1. Fibroblast growth factor
Desmoplastic reaction and fibrosis are a type of wound healing response in which the body produces increased amounts of collagen in an attempt to repair the tissue. 17 FGF is a type of growth factor that stimulates fibroblast growth and proliferation, which are cells that produce collagen. 17 , 18 Alternative splicing of FGF genes can generate extracellular variants with distinct specificity to FGF‐family peptide ligands, whereas another splicing gives rise to variations in C‐terminals of FGF receptor proteins, which stimulate signals to tumor cell growth and differentiation. 17 , 18 FGF is believed to play a role in desmoplastic reaction and fibrosis by promoting the formation of new collagen, which can help reduce inflammation and speed up the healing process. 18
Inflammation is thought to be an important factor in desmoplastic reaction and fibrosis development. 19 The presence of inflammatory cells, such as macrophages and neutrophils, can lead to the release of several proinflammatory cytokines, which can further stimulate the release of growth factors and other signaling molecules, resulting in increased fibrotic material deposition. As genetic factors, certain genetic variants associated with desmoplastic reactions and fibrosis have been identified, indicating the role of genetic factors in the development of these conditions. 20
5.2. Transforming growth factor beta (TGFB)
The main factor controlling desmoplastic reaction and fibrosis is TGFB. 21 TGFB is a cytokine that is released in response to injury and inflammation. It plays an important role in the body's healing process by promoting collagen production, which is the major fibrotic tissue component. 22 TGFB also increases ECM protein production, which is important for maintaining tissue structure and integrity. 23 Additionally, TGFB is known to regulate the immune response and inflammatory processes, which can contribute to desmoplastic reaction and fibrosis development. 24
Hepatocyte growth factor The major factors controlling desmoplastic reaction and fibrosis are the growth factors, including HGF, which binds to the c‐met receptor on fibroblasts. 25 HGF induces ECM molecule and collagen production, causing desmoplastic reaction and fibrosis development.
Platelet‐derived growth factor Desmoplastic reaction and fibrosis are both processes that involve ECM component deposition in tissue. PDGF is a type of growth factor that is released from platelets and plays an important role in new blood vessel formation, as well as existing ECM component remodeling. Studies have revealed that PDGF can stimulate fibroblast proliferation and collagen deposition, which are both important components of desmoplastic reaction and have synergistic effects on tumor progression. 26 Additionally, PDGF increases the secretion of ECM components by fibroblasts, which may be beneficial in forming a denser and more organized ECM in the desmoplastic reaction.
5.3. Interleukin‐1
Expression of the cytokine IL‐1 is important in desmoplastic reaction and fibrosis development. 27 In vitro studies have revealed that IL‐1 is capable of inducing fibrosis in various cell types, including fibroblasts, endothelial cells, and mesenchymal cells. 28 Additionally, increased IL‐1 expression has been observed in several types of fibrosis in both animal models and humans. IL‐1 inhibition is effective in reducing the severity of fibrosis in some studies. 29
5.4. Tumor necrosis factor alpha
Desmoplastic reaction is a type of fibrotic response initiated by tissue injury or inflammation. It is characterized by ECM component accumulation, including collagen and fibronectin, and an increase in myofibroblast number and activity. The underlying cause of desmoplastic reaction is not completely understood, but several cytokines and growth factors, such as TNFα, have been implicated in its development. 30 TNFα is a proinflammatory cytokine that is released in response to injury and inflammation and can stimulate ECM component production as well as promote cell proliferation and cell migration. 31 Additionally, TNFα is known to activate fibroblasts and increase their production of collagen and other ECM components.
5.5. Histone deacetylase (HDAC)
Desmoplastic reaction and fibrosis are associated with the HDAC pathway activation. 32 HDAC is an enzyme involved in gene expression regulation. It is known to be involved in regulating ECM protein production and tissue structure remodeling. Thus, HDAC inhibitors have been studied in the context of desmoplastic reaction and fibrosis, as they may reverse the pathological changes caused by an overactive HDAC pathway. 33
5.6. Hypoxia‐inducible factor 1 (HIF1)
HIF1 is a transcription factor that is involved in several gene regulations related to desmoplastic reaction and fibrosis. 34 HIF1 plays an important role in fibrosis development, as it can modulate the production of collagen, ECM proteins, and other key regulators of wound healing. 35 Additionally, HIF1 can regulate the expression of several proinflammatory cytokines, chemokines, and adhesion molecules that play a role in immune cell recruitment and activation and interact with viral oncogenes that induce multiple genomic networks in precancerous states that promote advanced cervical cancer development. 36
5.7. Nuclear factor–kappa B (NF‐κB) subunit 1
The NF‐κB pathway is an important desmoplastic reaction and fibrosis regulator. NF‐κB activation increases inflammation, fibroblast proliferation, and ECM deposition. NF‐κB is involved in multiple inflammatory pathway regulations, including cytokine, chemokine, and growth factor production. 37 Additionally, NF‐κB can regulate the expression of several genes involved in desmoplastic response regulation, such as collagen, fibronectin, and tenascin‐C. 38
5.8. Hyaluronic acid
The gradual accumulation of ECM components, such as HA, in the tissue pore space and its interaction with collagen to affect cancer cell activity results in fibrosis and desmoplastic reaction. 6 Preclinical and clinical data indicate that hyaluronan accumulation in TME is associated with invasive metastatic disease, drug resistance, and poor prognosis, 39 and a randomized phase III trial for hyaluronan‐high metastatic pancreatic ductal adenocarcinoma (PDAC) was attempted. 40
5.9. Pancreatic stellate cells (PSCs)
Oxidative stress, which is thought to be an important factor in desquamation response and fibrosis development, activates several signaling pathways and promotes fibrotic substance deposition, which in turn activates PSCs, which respond to cytokines and growth factors to produce ECM adhesion molecules, and various chemokines are produced. 24 Recent studies have reported that the knockdown of pyruvate kinase isozyme M2 expression inhibits PSC‐induced proliferation, migration, and epithelial–mesenchymal transition. 41 PSC activation has also been tested to create a three‐dimensional artificial vascular model that mimics the PDAC vasculature, allowing the study of vascular compaction in a controlled manner, something that has only been observed in vivo and under clinical conditions. 42 Eventually, we envision that this will be widely applied to evaluate new therapeutic strategies to modulate TME and ultimately help reduce or improve animal testing and design optimal combination therapies (Table 1).
TABLE 1.
Classification of molecules that control desmoplastic reaction and fibrosis and their specific functions.
| Molecules | Function |
|---|---|
| Fibroblast growth factor (FGF) | Stimulates growth and proliferation of fibroblasts that produce collagen, reduces inflammation, and speeds up the healing process 18 |
| Transforming growth factor beta (TGFB) | Controls desmoplastic reaction and fibrosis, 21 promotes the production of collagen, 22 increases the production of extracellular matrix proteins, 23 and regulates immune response and inflammatory processes 24 |
| Hepatocyte growth factor (HGF) | Induces the production of extracellular matrix molecules and collagen, leading to the formation of desmoplastic reaction and fibrosis 25 |
| Platelet‐derived growth factor (PDGF) | Stimulates fibroblast proliferation and collagen deposition and increases the secretion of extracellular matrix components, leading to the formation of denser and more organized extracellular matrix in desmoplastic reaction 26 |
| Interleukin‐1 (IL‐1) | Induces fibrosis in various cell types and is important in the development of desmoplastic reaction and fibrosis 28 |
| Tumor necrosis factor alpha (TNFα) | Promotes the production of extracellular matrix components, cell proliferation, and cell migration, contributing to the development of desmoplastic reaction 31 |
| Histone deacetylase (HDAC) | Involved in the regulation of gene expression, production of extracellular matrix proteins, and tissue structure remodeling. HDAC inhibitors are studied as potential therapy for desmoplastic reaction and fibrosis 32 |
| Hypoxia‐inducible factor 1 (HIF1) | Transcription factor involved in regulation of genes related to desmoplastic reaction and fibrosis. 34 Modulates the production of collagen, extracellular matrix proteins, and other regulators of wound healing. 35 Also regulates proinflammatory cytokines, chemokines, and adhesion molecules and interacts with viral oncogenes that promote the development of advanced cervical cancer 36 |
| Nuclear factor–kappa B (NF‐κB) | Important regulator of desmoplastic reaction and fibrosis. Activation leads to increased inflammation, fibroblast proliferation, and extracellular matrix deposition. Regulates multiple inflammatory pathways including production of cytokines, chemokines, and growth factors. 37 Also regulates expression of genes involved in the regulation of desmoplastic response 38 |
| Hyaluronic acid (HA) | Accumulation in tissue pore space and interaction with collagen contributes to fibrosis and desmoplastic reaction. 6 Associated with invasive metastatic disease, drug resistance, and poor prognosis 39 |
| Pancreatic stellate cells (PSCs) | Respond to cytokines and growth factors to produce extracellular matrix, adhesion molecules, and chemokines. Activation promoted by oxidative stress, which is important in the development of desmoplastic reaction and fibrosis. 24 Knockdown of pyruvate kinase isozyme M2 expression inhibits PSC‐induced proliferation, migration, and epithelial‐mesenchymal transition. 41 PSCs are used to create 3D artificial vascular model to study vascular compaction and evaluate new therapeutic strategies 42 |
6. CELLULAR PROCESSES CONTROLLING DESMOPLASTIC REACTION AND FIBROSIS
Recent studies revealed that several cellular processes are involved in desmoplastic reaction and fibrosis, which can provide key factors to produce efficient diagnostic technologies and innovative therapeutic approaches.
6.1. DNA methylation
Studies have revealed that epigenetic marker alteration, such as DNA methylation and histone modification, can be associated with desmoplastic reaction. 43 DNA methylation is a key epigenetic modification in gene expression regulation, which can alter the chromatin structure and gene expression by adding a methyl group to the 5‐carbon position of the cytosine in the CpG dinucleotide of the gene promoter region. 44 DNA methylation in organ fibrosis is involved in ECM remodeling, profibrotic cytokine expression, and cell proliferation and differentiation regulation. 45 DNA methylation in the heart modulates the expression of profibrotic genes, such as PDGF‐A and TGFB1, and inhibits the expression of antifibrotic genes, such as MMP‐9. 46 DNA methylation in the liver is involved in regulating profibrotic gene expressions, such as TGFB1 and connective tissue growth factor (CTGF), and represses antifibrotic gene expression, such as MMP‐9 and tissue inhibitor of metalloproteinase 1 (TIMP‐1). 47 DNA methylation in the lung is associated with processes, such as alveolar epithelial cell differentiation, fibrosis promotion, and inflammatory response development. 48 It has been shown to play a role in the regulation of inflammatory responses to airway injury and may be important in pulmonary disease development and progression such as asthma and chronic obstructive pulmonary disease. 49 Additionally, DNA methylation has been implicated in lung cancer development and other disease progression, such as idiopathic pulmonary fibrosis. 50
6.2. MicroRNAs (miRNAs)
Recent research has suggested that miRNAs play an important role in regulating desmoplastic reaction. 51 miRNAs are small noncoding RNAs that can bind to mRNAs and inhibit their translation. They can affect gene expressions involved in ECM deposition and fibroblast activation by targeting specific mRNAs. 52 Several studies have demonstrated that miRNAs are involved in modulating desmoplastic reaction, including miR‐21, 53 miR‐31, 54 miR‐210, 55 miR‐335, 56 and miR‐449a. 57 Additionally, miR‐21 has played a role in regulating several factors involved in desmoplastic reaction, including TGFB, CTGF, and collagen. 58 Further, it has increased MMP expression, which causes ECM degradation and remodeling. 59 MiR‐29a and miR‐29b are upregulated in kidney fibrosis and can regulate genes involved in inflammation and fibrosis regulation, such as TGFB. 60 MiR‐29a and miR‐29b have been implicated in other types of organ fibrosis, such as the liver 61 and heart. 62 Finally, miR‐199a‐3p has been reported to play a negative regulatory role in the NF‐κB signaling pathway, and its low expression in patients with CF contributes to chronic pulmonary inflammation. 63
6.3. Epithelial‐mesenchymal transition (EMT)
Desmoplastic reaction is a type of fibrotic response that is characterized by increased ECM component deposition such as collagen, fibronectin, and laminin. 64 This response is often seen in the context of a cancerous tumor, where the tumor cells secrete a variety of cytokines and growth factors that increase fibrosis. This process is known as EMT, which allows the cancer cells to change shape and migrate away from their original location (Figure 3). The increased fibrosis with desmoplastic reaction can increase the risk of metastasis, as the EMT program is activated and maintained by glycolytic enzymes. 3
FIGURE 3.
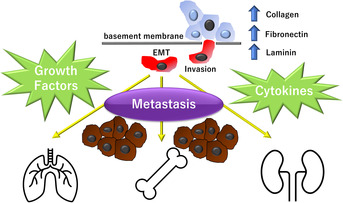
Desmoplastic reaction results in increased deposition of extracellular matrix components such as collagen, fibronectin, and laminin. The process by which tumor cells secrete a variety of cytokines and growth factors, leading to increased fibrosis, is called epithelial‐mesenchymal transition (EMT) and causes an increased risk of metastasis.
6.4. Hypoxia
Desmoplastic reaction is a type of tissue response to cancer, injury, or inflammation. It is characterized by increased collagen and other ECM protein productions that cause the formation of dense, fibrous scar tissue. 17 This fibrous tissue can interfere with the normal functioning of the area, leading to rigid and inflexible structure formation. Hypoxia is a condition in which the body's tissues and organs are deprived of adequate oxygen supply. This can occur due to a variety of reasons, including a decreased availability of oxygen in the air, blockage of the airways, or decreased blood circulation. Hypoxia can cause tissue damage and cell death, resulting in fibrosis, or excessive scarring. 65
7. IMMUNITY
Recent research has shown that Tregs may play a role in fibrosis inhibition in pancreatic cancer. 5 Pancreatic cancer is an aggressive type of cancer that is highly resistant to treatment and has a very poor prognosis. Studies have revealed that fibrosis Tregs can suppress pancreatic cancer cell proliferation, reduce inflammatory cytokine production, and reduce pancreatic cancer‐associated inflammation severity. Additionally, fibrosis Tregs may also play a role in promoting cancer cell destruction and tumor metastasis reduction. 66 Additionally, cancer‐associated fibroblasts (CAFs) are major players in PDAC progression and drug resistance; a unique population of CAFs, also known as antigen‐presenting CAFs (apCAFs), have been shown to activate CD4+ T cells in an antigen‐specific manner and have immunomodulatory capacity. 67 These apCAFs are derived from mesothelial cells and directly ligate naive CD4+ T cells in an antigen‐specific manner to induce regulatory T cells (Tregs). Furthermore, treatment with antibodies targeting mesothelial cell marker mesothelin effectively suppresses mesothelial cell migration to apCAFs and Treg formation by apCAFs. 68 Further research is needed to better understand the role of fibrosis Tregs in pancreatic cancer and to develop potential therapeutic strategies to target these cells to improve patient outcomes (Figure 4).
FIGURE 4.
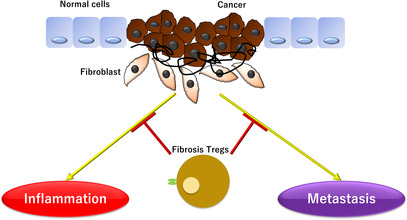
Fibrosis regulatory T cell (Tregs) are involved in suppressing fibrosis in pancreatic cancer and may play a role in reducing inflammation and metastasis of pancreatic cancer cells.
8. CONCLUSION
Desmoplastic reaction is a form of fibrosis that occurs in response to pancreatic cancer. 69 It is characterized by a dense, fibrotic stroma that is composed of myofibroblasts and ECM proteins, such as collagen, fibronectin, and laminin. 64 This stroma serves to protect the cancer cells from the immune system, making them more resistant to chemotherapy and radiotherapy. 70 Additionally, the dense stroma contributes to a protumorigenic environment by providing structural support for the tumor cells, promoting angiogenesis, and inhibiting apoptosis. 71 Furthermore, desmoplastic reaction can cause desmoplastic wall formation around the tumor, which can further inhibit the infiltration and destruction of cancer cells by the immune system. 2 The molecular and cellular mechanisms underlying desmoplastic reaction and fibrosis should be understood to develop new treatments for pancreatic cancer. For example, TNF‐α and TGFB are known as two key cytokines that drive desmoplastic reaction in pancreatic cancer. 72 TNF‐α activates the NF‐κB pathway and generally promotes proinflammatory cytokine, chemokine, and adhesion molecule production, which are involved in inflammatory cell recruitment, such as macrophages, to the tumor microenvironment. 73 In contrast, TGFB suppresses the NF‐κB pathway and is thought to inhibit inflammation. It can also upregulate MMP expression, which is involved in stroma remodeling and cancer cell invasion into surrounding tissues. Additionally, TGFB has been shown to increase the expression of immunosuppressive molecules, such as PD‐L1, which can help cancer cells evade the immune system. 74 Taken together, these two cytokines appear to play opposing roles in desmoplastic reaction, with TNF‐α promoting inflammation and TGFB suppressing it.
Prospects include the need for improved diagnostic methods for desmoplastic reaction and fibrosis. Histological analysis is currently required for diagnosing patients with cancer, and this requires a biopsy, as summarized primarily in Sections 5–7 of this article. However, since biopsy is a highly invasive procedure, there is a need to develop more noninvasive, yet accurate, diagnostic methods, such as biomarkers and imaging tests. 19
AUTHOR CONTRIBUTIONS
H.I. conceptualized the study objectives and obtained the funding. H.S., T.H., S.M., Y.T., Y.A., Y.S., and H.I. contributed to drawing artwork, collecting information, and writing the manuscript. H.S., T.H., K.S., S.K., Y.D., H.E., and H.I. constructed the study design and outlined the content. All authors have read and approved the final manuscript version for publication.
FUNDING INFORMATION
This work was supported in part by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (17cm0106414h0002; JP21lm0203007; 18KK0251; 19 K22658; 20H00541; 21K19526; 22H03146; 22K19559; 16H06279 [PAGS]). Partial support was offered by Mitsubishi Foundation to H.I.
CONFLICT OF INTEREST STATEMENT
Partial institutional endowments were received from Hirotsu Bio Science Inc. (Tokyo, Japan); Kinshu‐kai Medical Corporation (Osaka, Japan); Kyowa‐kai Medical Corporation (Osaka, Japan); IDEA Consultants Inc. (Tokyo, Japan); and Unitech Co. Ltd. (Chiba, Japan). Y.D., H.E., and H.I are associate editors of this journal. Other authors have no conflicts of interest to this journal.
ETHICS STATEMENT
Although this is a review article, every author followed a research protocol that has been approved by a suitably constituted Ethics Committee at Osaka University, Japan and that conforms to the provisions of the Declaration of Helsinki.
Approval of the research protocol by an Institutional Reviewer Board: Not applicable.
Registry and the Registration No. of the study/trial: Not applicable.
Patient Consent for Publication: Not applicable.
Animal Studies: Not applicable.
ACKNOWLEDGMENTS
The authors are thankful to every lab member.
Sato H, Hara T, Meng S, et al. Multifaced roles of desmoplastic reaction and fibrosis in pancreatic cancer progression: Current understanding and future directions. Cancer Sci. 2023;114:3487‐3495. doi: 10.1111/cas.15890
REFERENCES
- 1. Geng X, Chen H, Zhao L, et al. Cancer‐associated fibroblast (CAF) heterogeneity and targeting therapy of CAFs in pancreatic cancer. Front Cell Dev Biol. 2021;9:655152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Y, Kim J, Yang S, et al. Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021;39(4):548‐565e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang J, Ren B, Yang G, et al. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77(2):305‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prieto‐Garcia E, Diaz‐Garcia CV, Agudo‐Lopez A, et al. Tumor‐stromal interactions in a Co‐culture model of human pancreatic adenocarcinoma cells and fibroblasts and their connection with tumor spread. Biomedicine. 2021;9(4):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ozdemir BC, Pentcheva‐Hoang T, Carstens JL, et al. Depletion of carcinoma‐associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu S, Xu H, Wang W, et al. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kenny FN, Drymoussi Z, Delaine‐Smith R, et al. Tissue stiffening promotes keratinocyte proliferation through activation of epidermal growth factor signaling. J Cell Sci. 2018;131(10):jcs215780. [DOI] [PubMed] [Google Scholar]
- 8. Yoon JH, Jung YJ, Moon SH. Immunotherapy for pancreatic cancer. World J Clin Cases. 2021;9(13):2969‐2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chijimatsu R, Kobayashi S, Takeda Y, et al. Establishment of a reference single‐cell RNA sequencing dataset for human pancreatic adenocarcinoma. iScience. 2022;25(8):104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kleeff J, Whitcomb DC, Shimosegawa T, et al. Chronic pancreatitis. Nat Rev Dis Primers. 2017;7(3):17060. [DOI] [PubMed] [Google Scholar]
- 11. Lu YY, Tu HP, Wu CH, et al. Risk of cancer development in patients with keloids. Sci Rep. 2021;11(1):9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke DL, Carruthers AM, Mustelin T, Murray LA. Matrix regulation of idiopathic pulmonary fibrosis: the role of enzymes. Fibrogenesis Tissue Repair. 2013;6(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roth KJ, Copple BL. Role of hypoxia‐inducible factors in the development of liver fibrosis. Cell Mol Gastroenterol Hepatol. 2015;1(6):589‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu N, Wang C, Dai X, et al. Phillygenin inhibits LPS‐induced activation and inflammation of LX2 cells by TLR4/MyD88/NF‐kappaB signaling pathway. J Ethnopharmacol. 2020;248:112361. [DOI] [PubMed] [Google Scholar]
- 15. Otoupalova E, Smith S, Cheng G, Thannickal VJ. Oxidative stress in pulmonary fibrosis. Compr Physiol. 2020;10(2):509‐547. [DOI] [PubMed] [Google Scholar]
- 16. Roeb E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018;68‐69:463‐473. [DOI] [PubMed] [Google Scholar]
- 17. Akimoto N, Vayrynen JP, Zhao M, et al. Desmoplastic reaction, immune cell response, and prognosis in colorectal cancer. Front Immunol. 2022;13:840198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L715‐L731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baues M, Dasgupta A, Ehling J, et al. Fibrosis imaging: current concepts and future directions. Adv Drug Deliv Rev. 2017;121:9‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaps L, Schuppan D. Targeting cancer associated fibroblasts in liver fibrosis and liver cancer using nanocarriers. Cell. 2020;9(9):2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stylianou A, Gkretsi V, Stylianopoulos T. Transforming growth factor‐beta modulates pancreatic cancer associated fibroblasts cell shape, stiffness and invasion. Biochim Biophys Acta Gen Subj. 2018;1862(7):1537‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lodyga M, Hinz B. TGF‐beta1 ‐ a truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol. 2020;101:123‐139. [DOI] [PubMed] [Google Scholar]
- 23. Rosell‐Garcia T, Palomo‐Alvarez O, Rodriguez‐Pascual F. A hierarchical network of hypoxia‐inducible factor and SMAD proteins governs procollagen lysyl hydroxylase 2 induction by hypoxia and transforming growth factor beta1. J Biol Chem. 2019;294(39):14308‐14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shimizu K. Pancreatic stellate cells: molecular mechanism of pancreatic fibrosis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S119‐S121. [DOI] [PubMed] [Google Scholar]
- 25. Corsino P, Davis B, Law M, et al. Tumors initiated by constitutive Cdk2 activation exhibit transforming growth factor beta resistance and acquire paracrine mitogenic stimulation during progression. Cancer Res. 2007;67(7):3135‐3144. [DOI] [PubMed] [Google Scholar]
- 26. Gioni V, Karampinas T, Voutsinas G, et al. Imatinib mesylate inhibits proliferation and exerts an antifibrotic effect in human breast stroma fibroblasts. Mol Cancer Res. 2008;6(5):706‐714. [DOI] [PubMed] [Google Scholar]
- 27. Schellerer VS, Langheinrich M, Hohenberger W, et al. Tumor‐associated fibroblasts isolated from colorectal cancer tissues exhibit increased ICAM‐1 expression and affinity for monocytes. Oncol Rep. 2014;31(1):255‐261. [DOI] [PubMed] [Google Scholar]
- 28. Liubomirski Y, Lerrer S, Meshel T, et al. Tumor‐stroma‐inflammation networks promote pro‐metastatic chemokines and aggressiveness characteristics in triple‐negative breast cancer. Front Immunol. 2019;10:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrasek J, Bala S, Csak T, et al. IL‐1 receptor antagonist ameliorates inflammasome‐dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122(10):3476‐3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE. Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down‐regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator‐activated receptor gamma: mechanism of desmoplastic reaction. Cancer Res. 2001;61(5):2250‐2255. [PubMed] [Google Scholar]
- 31. Zeng S, Cui J, Zhang Y, Zheng Z, Meng J, du J. MicroRNA‐15b‐5p inhibits tumor necrosis factor alpha‐induced proliferation, migration, and extracellular matrix production of airway smooth muscle cells via targeting yes‐associated protein 1. Bioengineered. 2022;13(3):5396‐5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jimenez‐Uribe AP, Gomez‐Sierra T, Aparicio‐Trejo OE, et al. Backstage players of fibrosis: NOX4, mTOR, HDAC, and S1P; companions of TGF‐beta. Cell Signal. 2021;87:110123. [DOI] [PubMed] [Google Scholar]
- 33. Lyu X, Hu M, Peng J, Zhang X, Sanders YY. HDAC inhibitors as antifibrotic drugs in cardiac and pulmonary fibrosis. Ther Adv Chronic Dis. 2019;10:2040622319862697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei X, Hou Y, Long M, Jiang L, du Y. Molecular mechanisms underlying the role of hypoxia‐inducible factor‐1 alpha in metabolic reprogramming in renal fibrosis. Front Endocrinol (Lausanne). 2022;13:927329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keely S, Glover LE, MacManus CF, et al. Selective induction of integrin beta1 by hypoxia‐inducible factor: implications for wound healing. FASEB J. 2009;23(5):1338‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu ZH, Wright JD, Belt B, Cardiff RD, Arbeit JM. Hypoxia‐inducible factor‐1 facilitates cervical cancer progression in human papillomavirus type 16 transgenic mice. Am J Pathol. 2007;171(2):667‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58(2):88‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang YJ, Downey MA, Choi S, Shoup TM, Elmaleh DR. Cromolyn platform suppresses fibrosis and inflammation, promotes microglial phagocytosis and neurite outgrowth. Sci Rep. 2021;11(1):22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X, Shepard HM, Cowell JA, et al. Parallel accumulation of tumor hyaluronan, collagen, and other drivers of tumor progression. Clin Cancer Res. 2018;24(19):4798‐4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Cutsem E, Tempero MA, Sigal D, et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab‐paclitaxel plus gemcitabine for patients with hyaluronan‐high metastatic pancreatic adenocarcinoma. J Clin Oncol. 2020;38(27):3185‐3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masamune A, Hamada S, Yoshida N, Nabeshima T, Shimosegawa T. Pyruvate kinase isozyme M2 plays a critical role in the interactions between pancreatic stellate cells and cancer cells. Dig Dis Sci. 2018;63(7):1868‐1877. [DOI] [PubMed] [Google Scholar]
- 42. Heinrich MA, Uboldi I, Kuninty PR, et al. Microarchitectural mimicking of stroma‐induced vasculature compression in pancreatic tumors using a 3D engineered model. Bioact Mater. 2023;22:18‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geismann C, Arlt A. Coming in the air: hypoxia meets epigenetics in pancreatic cancer. Cell. 2020;9(11):2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bronner C, Alhosin M, Hamiche A, Mousli M. Coordinated dialogue between UHRF1 and DNMT1 to ensure faithful inheritance of methylated DNA patterns. Genes (Basel). 2019;10(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bian EB, Huang C, Wang H, et al. Repression of Smad7 mediated by DNMT1 determines hepatic stellate cell activation and liver fibrosis in rats. Toxicol Lett. 2014;224(2):175‐185. [DOI] [PubMed] [Google Scholar]
- 46. Liu C, Zhao W, Meng W, et al. Platelet‐derived growth factor blockade on cardiac remodeling following infarction. Mol Cell Biochem. 2014;397(1–2):295‐304. [DOI] [PubMed] [Google Scholar]
- 47. Zhou X, Xiong J, Lu S, et al. Inhibitory effect of Corilagin on miR‐21‐regulated hepatic fibrosis signaling pathway. Am J Chin Med. 2019;47(7):1541‐1569. [DOI] [PubMed] [Google Scholar]
- 48. Liu H, Nie H, Lai W, et al. Different exposure modes of PM(2.5) induces bronchial asthma and fibrosis in male rats through macrophage activation and immune imbalance induced by TIPE2 methylation. Ecotoxicol Environ Saf. 2022;247:114200. [DOI] [PubMed] [Google Scholar]
- 49. Chen YC, Chang YP, Huang KT, Hsu PY, Hsiao CC, Lin MC. Unraveling the pathogenesis of asthma and chronic obstructive pulmonary disease overlap: focusing on epigenetic mechanisms. Cell. 2022;11(11):1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Duan J, Zhong B, Fan Z, et al. DNA methylation in pulmonary fibrosis and lung cancer. Expert Rev Respir Med. 2022;16(5):519‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aslan M, Shahbazi R, Ulubayram K, et al. Targeted therapies for pancreatic cancer and hurdles ahead. Anticancer Res. 2018;38(12):6591‐6606. [DOI] [PubMed] [Google Scholar]
- 52. Liu J, Ren L, Li S, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11(9):2783‐2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Uozaki H, Morita S, Kumagai A, et al. Stromal miR‐21 is more important than miR‐21 of tumour cells for the progression of gastric cancer. Histopathology. 2014;65(6):775‐783. [DOI] [PubMed] [Google Scholar]
- 54. Chen Y, Huang C, Duan ZB, Chen YX, Xu CY. LncRNA NEAT1 accelerates renal fibrosis progression via targeting miR‐31 and modulating RhoA/ROCK signal pathway. Am J Physiol Cell Physiol. 2022;324:C292‐C306. [DOI] [PubMed] [Google Scholar]
- 55. Zaccagnini G, Greco S, Longo M, et al. Hypoxia‐induced miR‐210 modulates the inflammatory response and fibrosis upon acute ischemia. Cell Death Dis. 2021;12(5):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang F, Li L, Piontek K, Sakaguchi M, Selaru FM. Exosome miR‐335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67(3):940‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang M, Zhang B, Wang X, et al. LncRNA CFAR promotes cardiac fibrosis via the miR‐449a‐5p/LOXL3/mTOR axis. Sci China. Life Sci. 2022;66:783‐799. [DOI] [PubMed] [Google Scholar]
- 58. Cardozo ER, Foster R, Karmon AE, et al. MicroRNA 21a‐5p overexpression impacts mediators of extracellular matrix formation in uterine leiomyoma. Reprod Biol Endocrinol. 2018;16(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smieszek A, Marcinkowska K, Pielok A, Sikora M, Valihrach L, Marycz K. The role of miR‐21 in osteoblasts‐osteoclasts coupling In vitro. Cell. 2020;9(2):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dalgaard LT, Sorensen AE, Hardikar AA, et al. The microRNA‐29 family: role in metabolism and metabolic disease. Am J Physiol Cell Physiol. 2022;323(2):C367‐C377. [DOI] [PubMed] [Google Scholar]
- 61. Jampoka K, Muangpaisarn P, Khongnomnan K, Treeprasertsuk S, Tangkijvanich P, Payungporn S. Serum miR‐29a and miR‐122 as potential biomarkers for non‐alcoholic fatty liver disease (NAFLD). Microrna. 2018;7(3):215‐222. [DOI] [PubMed] [Google Scholar]
- 62. Zhang Y, Huang XR, Wei LH, Chung ACK, Yu CM, Lan HY. miR‐29b as a therapeutic agent for angiotensin II‐induced cardiac fibrosis by targeting TGF‐beta/Smad3 signaling. Mol Ther. 2014;22(5):974‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bardin P, Marchal‐Duval E, Sonneville F, et al. Small RNA and transcriptome sequencing reveal the role of miR‐199a‐3p in inflammatory processes in cystic fibrosis airways. J Pathol. 2018;245(4):410‐420. [DOI] [PubMed] [Google Scholar]
- 64. Mahadevan D, Von Hoff DD. Tumor‐stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6(4):1186‐1197. [DOI] [PubMed] [Google Scholar]
- 65. Lokmic Z, Musyoka J, Hewitson TD, et al. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol. 2012;296:139‐185. [DOI] [PubMed] [Google Scholar]
- 66. Korbecki J, Bajdak‐Rusinek K, Kupnicka P, et al. The role of CXCL16 in the pathogenesis of cancer and other diseases. Int J Mol Sci. 2021;22(7):3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elyada E, Bolisetty M, Laise P, et al. Cross‐species single‐cell analysis of pancreatic ductal adenocarcinoma reveals antigen‐presenting cancer‐associated fibroblasts. Cancer Discov. 2019;9(8):1102‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang H, Wang Z, Zhang Y, et al. Mesothelial cell‐derived antigen‐presenting cancer‐associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell. 2022;40(6):656‐673 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hartel M, Di Mola FF, Gardini A, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004;28(8):818‐825. [DOI] [PubMed] [Google Scholar]
- 70. Han X, Xu Y, Geranpayehvaghei M, Anderson GJ, Li Y, Nie G. Emerging nanomedicines for anti‐stromal therapy against desmoplastic tumors. Biomaterials. 2020;232:119745. [DOI] [PubMed] [Google Scholar]
- 71. Turaga RC, Sharma M, Mishra F, et al. Modulation of cancer‐associated fibrotic stroma by an integrin alpha(v)beta(3) targeting protein for pancreatic cancer treatment. Cell Mol Gastroenterol Hepatol. 2021;11(1):161‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8(1):10‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ji RC. Macrophages are important mediators of either tumor‐ or inflammation‐induced lymphangiogenesis. Cell Mol Life Sci. 2012;69(6):897‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hussain SM, Kansal RG, Alvarez MA, et al. Role of TGF‐beta in pancreatic ductal adenocarcinoma progression and PD‐L1 expression. Cell Oncol (Dordr). 2021;44(3):673‐687. [DOI] [PubMed] [Google Scholar]


