Abstract
Intratumor bacteria modify the tumor immune microenvironment and influence outcomes of various tumors. Periodontal pathogen Fusobacterium nucleatum has been detected in pancreatic cancer tissues and is associated with poor prognosis. However, it remains unclear how F. nucleatum affects pancreatic cancer. Here, we compared clinical features with F. nucleatum colonization in pancreatic cancer tissues. F. nucleatum was detected in 15.5% (13/84) of pancreatic cancer patients. The tumor size was significantly larger in the F. nucleatum‐positive group than in the negative group. To clarify the biological effect of intratumor F. nucleatum on pancreatic cancer progression, we performed migration/invasion assays and cytokine array analysis of cancer cells cocultured with F. nucleatum. F. nucleatum promoted CXCL1 secretion from pancreatic cancer cells, leading to cancer progression through autocrine signaling. Intratumor F. nucleatum suppressed tumor‐infiltrating CD8+ T cells by recruiting myeloid‐derived suppressor cells (MDSCs) to the tumor in an F. nucleatum‐injected subcutaneous pancreatic cancer mouse model, resulting in tumor progression. Furthermore, tumor growth accelerated by F. nucleatum was suppressed by MDSC depletion or cytokine inhibitors. Intratumor F. nucleatum promoted pancreatic cancer progression through autocrine and paracrine mechanisms of the CXCL1‐CXCR2 axis. Blockade of the CXCL1‐CXCR2 axis may be a novel therapeutic approach for patients with intratumor F. nucleatum‐positive pancreatic cancer.
Keywords: CXCL1, CXCR2, Fusobacterium nucleatum, pancreatic cancer, tumor immune microenvironment
Fusobacterium nucleatum, a periodontal pathogen, stimulated pancreatic cancer cells to secrete CXCL1, leading to cancer progression. In an autocrine manner, F. nucleatum promoted the migration of pancreatic cancer cells. In a paracrine manner, F. nucleatum suppressed CD8+ T cells by recruiting myeloid‐derived suppressor cells to the tumor.

Abbreviations
- BHI
brain heart infusion
- CXCL1
C‐X‐C motif chemokine ligand 1
- CXCR2
C‐X‐C motif chemokine receptor 2
- IL‐8
interleukin‐8
- KPC
LSL‐Kras G12D/+ ; LSL‐Trp53 R172H/+ ; Pdx‐1‐Cre
- MDSCs
myeloid‐derived suppressor cells
- TCGA
The Cancer Genome Atlas
1. INTRODUCTION
Pancreatic cancer is among the deadliest cancers worldwide, and the 5‐year survival rate of patients with pancreatic cancer is only 12%. 1 Conventional therapies for pancreatic cancer do not provide an adequate prognosis, and novel treatment approaches are required. 2 The gut microbiota acts as the primary mediator of tumor responses to chemotherapy and immunotherapy in cancer patients. 3 , 4 Dysbiosis, the loss of gut microbial diversity, suppresses antitumor immunity, promotes cancer‐associated inflammation, and increases resistance to cancer therapy. 5 , 6 , 7
In addition to the gut microbiota, intratumor bacteria have been highlighted as a key regulator of tumor behavior. 8 Colonization of intratumor bacteria has been reported not only in gastrointestinal cancers, such as colorectal cancers 9 and esophageal cancers, 10 , 11 but also in nongastrointestinal cancers, including breast cancers 12 , 13 and pancreatic cancers. 14 , 15 , 16 , 17 Pushalkar et al. 15 showed that depletion of intratumor bacteria by antibiotics enhanced the efficacy of immune checkpoint inhibitors by upregulating the expression of programmed cell death protein 1 in a mouse model of pancreatic cancer, suggesting that intratumor bacteria modify the tumor immune microenvironment of pancreatic cancer.
Fusobacterium nucleatum is a gram‐negative anaerobic periodontal pathogen that has been detected in esophageal cancer 10 , 11 and colorectal cancer. 18 , 19 Fusobacterium adhesin A, an outer membrane protein derived from F. nucleatum, attaches to E‐cadherin on cancer cells and promotes colorectal cancer cell proliferation by activating β‐catenin signaling. 16 Fusobacterium species have also been detected in pancreatic cancer tissues by 16S rRNA gene sequencing, and intratumor Fusobacterium species are associated with a poor prognosis of pancreatic cancer. 17 These findings suggest that Fusobacterium species are potential therapeutic targets. However, it remains unclear how F. nucleatum affects pancreatic cancer.
We hypothesized that F. nucleatum is a key molecule promoting pancreatic cancer progression by modifying tumor biology and the tumor immune microenvironment. To clarify the effect of intratumor F. nucleatum on pancreatic cancer progression, we investigated the biological alterations of cancer cells by migration and invasion assays in direct cocultures with F. nucleatum in vitro experiments. Cytokine array analysis of the culture supernatant from cancer cells cocultured with F. nucleatum identified the key molecules that modified pancreatic cancer behavior. Additionally, we revealed that inhibition of F. nucleatum‐specific molecules restored the antitumor microenvironment and suppressed tumor growth in a subcutaneous tumor mouse model injected with F. nucleatum intratumorally.
2. MATERIALS AND METHODS
2.1. Human pancreatic ductal adenocarcinoma tissue samples
We reviewed patients who underwent pancreatic resection for pancreatic ductal adenocarcinoma with curative intent at the Department of Surgery and Oncology, Kyushu University Hospital (Fukuoka, Japan) between 2012 and 2020 with available fresh‐frozen tissue. Patients with positive intraoperative ascites cytology, noncancer death, and acinar cell carcinoma at pathological diagnosis were excluded. Normal pancreatic tissues were obtained from nontumor areas in patients with resected pancreatic neuroendocrine tumors. Informed consent was received from all patients in accordance with the Declaration of Helsinki. Clinical data including histopathological findings were obtained from electronic medical records.
2.2. Bacterial strains and growth conditions
Fusobacterium nucleatum strain ATCC 23726 9 and Porphyromonas gingivalis strain ATCC 33277 20 were purchased from the American Type Culture Collection (ATCC). Detailed procedures are described in Appendix S1.
2.3. Human cell culture
SUIT‐2 cells were purchased from the Japan Health Science Research Resources Bank and maintained as described previously. 21 Passages three to eight were used in assays.
2.4. Mice
LSL‐Kras G12D/+ ; LSL‐Trp53 R172H/+ ; Pdx‐1‐Cre; (KPC) mice have been described previously. 22 , 23 , 24 KPC mice were bred and maintained at Charles River Laboratories Japan, Inc. and the Research Animal Facility at Kyushu University in accordance with Kyushu University guidelines. Four‐week‐old female C57BL/6 mice were purchased from CLEA Inc.
2.5. Murine cells and culture conditions
Murine pancreatic cancer cells were established in our laboratory from primary tumors of KPC mice using the outgrowth method as described previously. 24 Murine pancreatic cancer cells were maintained in Dulbecco's modified Eagle's medium (Sigma Chemical Co.) containing 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin.
2.6. Matrigel invasion and migration assays
Detailed procedures are described in Appendix S1.
2.7. Cell proliferation assay
Detailed procedures are described in Appendix S1.
2.8. DNA extraction
Detailed procedures are described in Appendix S1.
2.9. Quantitative reverse‐transcription polymerase chain reaction (qRT‐PCR)
We examined the expression levels of the following genes using qRT‐PCR. Primer sequences are listed in Table 1. We defined a Cycle threshold value of >40 or nonspecific amplification in the melting curve analysis as not detected. All RT‐PCRs were performed in triplicate. Cases in which F. nucleatum was detected in at least two replicates were defined as positive for F. nucleatum. Detailed procedures are described in Appendix S1.
TABLE 1.
Primers used for quantitative reverse‐transcription polymerase chain reactions.
| Gene | Primer | Sequence 5′‐3′ |
|---|---|---|
| Fusobacterium nucleatum | Forward | CAACCATTACTTTAACTCTACCATGTTCA |
| Reverse | GTTGACTTTACAGAAGGAGATTATGTAAAAATC | |
| FAM probe | GTTGACTTTACAGAAGGAGATTA | |
| SLCO2A1 | Forward | ATCCCCAAAGCACCTGGTTT |
| Reverse | AGAGGCCAAGATAGTCCTGGTAA | |
| VIC probe | CCATCCATGTCCTCATCTC | |
| Human CXCL1 | Forward | GAAAGCTTGCCTCAATCCTG |
| Reverse | CTCCCTTCTGGTCAGTTGGA | |
| Human IL‐8 | Forward | CACTGCGCCAACACAGAAAT |
| Reverse | GCCCTCTTCAAAAACTTCTCCAC | |
| Human GAPDH | Forward | GCACCGTCAAGGCTGAGAAC |
| Reverse | TGGTGAAGACGCCAGTGGA | |
| Mouse Cxcl1 | Forward | CTGGGATTCACCTCAAGAACATC |
| Reverse | CAGGGTCAAGGCAAGCCTC | |
| Mouse Gapdh | Forward | TGTGTCCGTCGTGGATCTGA |
| Reverse | TTGCTGTTGAAGTCGCAGGAG |
2.10. Immunohistochemistry
Detailed procedures are described in Appendix S1.
2.11. Cytokine array
Detailed procedures are described in Appendix S1.
2.12. The Cancer Genome Atlas (TCGA)
Detailed procedures are described in Appendix S1.
2.13. Flow cytometry
Detailed procedures are described in Appendix S1.
2.14. In vivo experiments
KPC cells (KPCF453‐PC; 5 × 105) were suspended in 50 μL Dulbecco's modified Eagle's medium and subcutaneously injected into the abdomen skin of 5‐week‐old female C57/B6 mice. Seven days after implantation, three to six mice were used in each of the bacterium‐injected group and non‐bacterium‐injected group. Bacterium‐injected group, F. nucleatum or P. gingivalis was suspended at a dose of 5 × 107 colony forming unit in 50 μL BHI medium and injected into each mouse tumor every 3 days per week for 2 weeks; control group, mice were injected with 50 μL BHI medium only. The tumor volume was calculated using the following formula: π/6 × (L × W × W), where L is the largest tumor diameter, and W is the smallest tumor diameter.
2.15. In vivo drug treatments
In some experiments, mice were injected with six doses of a C‐X‐C motif chemokine receptor 2 (CXCR2) antagonist (5 mg/kg, i.p.; SB225002, Selleck) or neutralizing anti‐C‐X‐C motif chemokine ligand 1 (CXCL1) mAb (4 mg/kg, intratumor injection; R&D Systems) at 72‐h intervals unless specified otherwise. Control‐group mice were injected with dimethyl sulfoxide as described previously. 25 To deplete myeloid‐derived suppressor cells (MDSCs), six doses of 100 μg/dose of InVivoMAb anti‐mouse Ly6G 26 (clone 1A8; BioXCell, Cat # BP0075‐1) or isotype control antibody (clone 2A3, BioXcell, Cat # BP0089) diluted in InVivoPure pH 7.0 Dilution Buffer (BioXcell, Cat # IP0070) was intraperitoneally injected at 72‐h intervals unless specified otherwise.
2.16. Statistical analysis
The χ 2 test was used to analyze the correlation between intratumor F. nucleatum and clinicopathological characteristics in qRT‐PCR analyses. Survival analyses were performed by the Kaplan–Meier method and compared using the log‐rank test. Results are presented as the mean ± standard deviation (SD). For each result, group comparisons were conducted using Student's t‐test. Statistical significance was defined as p < 0.05. Statistical analyses were performed using GraphPad Prism 8 (v8.4.3; GraphPad Software) or JMP software (v17.0.0; SAS Institute).
3. RESULTS
3.1. Intratumor F. nucleatum affects pancreatic tumor behavior
To confirm bacterial colonization in human pancreatic cancers, we performed immunohistochemistry using antibodies against bacterial lipopolysaccharide, a molecule in the outer membrane of bacteria. 27 Lipopolysaccharide was detected in pancreatic tumor tissue, but not in normal pancreatic tissue (Figure 1A). To assess colonization of F. nucleatum in the human pancreas, we performed qRT‐PCR analysis of F. nucleatum DNA in fresh‐frozen tissues of 84 resected pancreatic tumors and 41 normal pancreatic tissues. 11 F. nucleatum DNA was detected in 13 cases (15.5%) of pancreatic tumors, while only three cases of normal tissues (7.3%) were positive (p < 0.05; Figure 1B). Tumors with F. nucleatum colonization were significantly larger and more frequently showed retroperitoneal invasion and multiple lymph node metastases (more than four) compared with tumors without F. nucleatum colonization (p < 0.01; Figure 1C–E). Patients with F. nucleatum in the tumor had shorter disease‐free survival and overall survival times (Figure 1F). These findings suggest that intratumor F. nucleatum promotes pancreatic tumor growth and its metastasis, leading to a poor prognosis.
FIGURE 1.
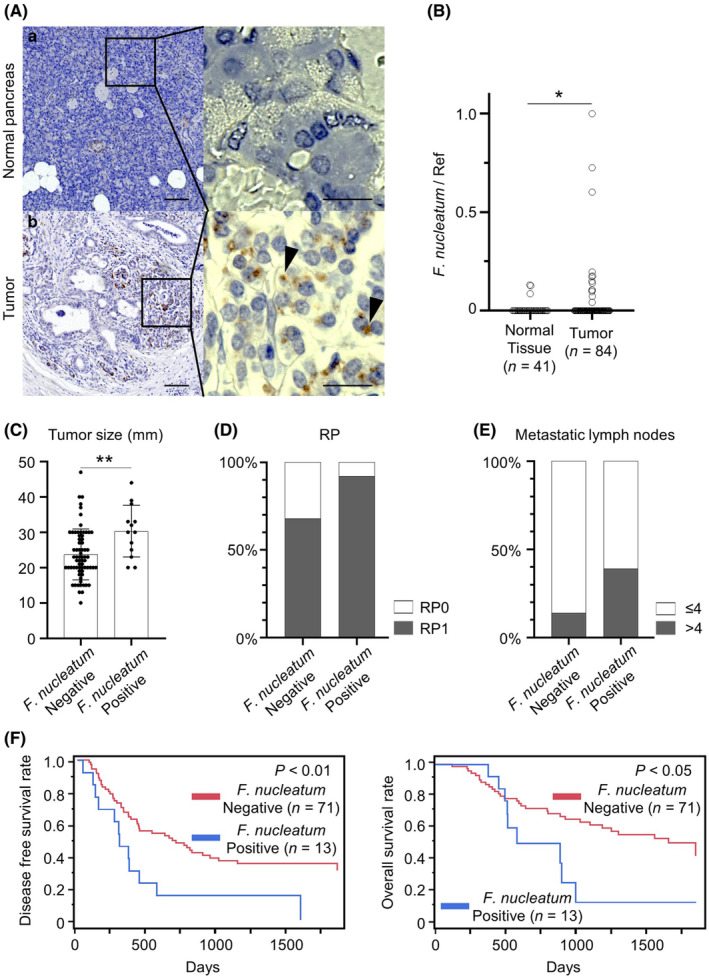
Characterization of intratumor Fusobacterium nucleatum in pancreatic cancer. (A) Immunohistochemistry of bacterial lipopolysaccharide in normal pancreatic tissue (A‐a: upper case) and pancreatic tumor tissue (A‐b: lower case). Arrowheads in the insets indicate positive staining of bacterial lipopolysaccharide in the pancreatic tumor (original magnification: ×40; scale bar 100 μm, insets: ×400; scale bar, 20 μm). (B) Relative abundance of F. nucleatum in normal pancreatic tissues (N = 41) versus pancreatic tumor tissues (N = 84). (C–E) The relationship of the tumor size (C), retroperitoneal invasion (D), and multiple lymph node metastases (more than four) (E) in pancreatic cancer patients with or without intratumor F. nucleatum. (F) Kaplan–Meier curves demonstrating disease‐free survival (left) and overall survival (right) of pancreatic cancer patients with or without intratumor F. nucleatum. *p < 0.05, **p < 0.01.
3.2. Fusobacterium nucleatum stimulates CXCL1 and IL‐8 secretion from pancreatic cancer cells, promoting cell migration in an autocrine manner
To evaluate the effect of F. nucleatum on the biological activities of human pancreatic cancer cells, we performed proliferation, migration, and invasion assays using a coculture system of a pancreatic cancer cell line SUIT‐2 cocultured with F. nucleatum. As a result, we found significant promotion of cell migration and invasion compared with monocultured SUIT‐2 cells (p < 0.05; Figure 2A,B). Another periodontal pathogen, P. gingivalis, did not promote migration or invasion of SUIT‐2 cells. Pancreatic cancer cell proliferation did not change after coculture with F. nucleatum or P. gingivalis (Figure 2C). To assess the molecules that changed the biological activities of cancer cells, we performed cytokine array analysis of the culture supernatant from SUIT‐2 cells cocultured with periodontal pathogens. CXC family cytokines, CXCL1 and interleukin‐8 (IL‐8) were upregulated in the culture supernatants of SUIT‐2 cells cocultured with F. nucleatum, but not with P. gingivalis (Figure 2D). Increased mRNA expression of CXCL1 and IL‐8 in F. nucleatum‐cocultured SUIT‐2 cells was also confirmed by qRT‐PCR (p < 0.01; Figure 2E). CXCL1 and IL‐8 secreted by cancer cells bind to CXCR2 expressed on cancer cells and induce autocrine signaling to promote cancer cell migration. 28 , 29 Therefore, we assessed the effect of CXCL1 and IL‐8 on cancer cell activity by inhibiting CXCR2. CXCR2‐specific inhibitor SB225002 significantly suppressed the migration of F. nucleatum‐cocultured SUIT‐2 cells (p < 0.01; Figure 2F), suggesting that enhanced migration of pancreatic cancer cells by F. nucleatum is mediated through autocrine signaling.
FIGURE 2.

Effects of Fusobacterium nucleatum on migration and invasion of pancreatic cancer cells. (A) Representative photomicrographs of migrating and invading SUIT‐2 cells cocultured with F. nucleatum and P. gingivalis or monocultured. (B) F. nucleatum promoted the migration and invasion of SUIT‐2 cells compared with P. gingivalis‐cocultured and monocultured cells. (C) Proliferation rate of SUIT‐2 cells cocultured with F. nucleatum was similar to that of P. gingivalis‐cocultured and monocultured SUIT‐2 cells. (D) Representative human cytokine array images of conditioned medium derived from SUIT‐2 cells cocultured with F. nucleatum and P. gingivalis or monocultured. Black boxes: positive controls; orange boxes: CXCL1; green boxes: IL‐8. CXCL1 and IL‐8 were upregulated in the conditioned medium of SUIT‐2 cells cocultured with F. nucleatum, but not with P. gingivalis or monocultured. (E) CXCL1 (left) and IL‐8 (right) expression was increased in SUIT‐2 cells cocultured with F. nucleatum, but not with P. gingivalis. (F) Representative photomicrographs of migrating F. nucleatum‐cocultured SUIT‐2 cells with or without the CXCR2 inhibitor (SB225002). The CXCR2 inhibitor significantly suppressed migration of F. nucleatum‐cocultured SUIT‐2 cells. Original magnification: ×40; scale bar 100 μm. *p < 0.05, **p < 0.01.
3.3. CXCL1 and IL‐8 expression in pancreatic cancer patients correlates with a poor prognosis
Because in vitro experiments showed that F. nucleatum upregulated CXCL1 expression in cancer cells, we confirmed the association between F. nucleatum colonization and CXCL1 expression in human pancreatic cancer tissues. Pancreatic cancers colonized with F. nucleatum showed significantly higher CXCL1 expression than those without F. nucleatum colonization (p < 0.05; Figure 3A). Next, we investigated CXCL1 and IL‐8 expressions in human pancreatic tumor samples using publicly available data provided by TCGA and the Genotype‐Tissue Expression project. Patient samples were compared with noncancerous control tissues using GEPIA. 30 CXCL1 and IL‐8 expression levels were higher in tumor samples compared with normal samples (p < 0.01; Figure 3B). High expression of CXCL1 and IL‐8 was significantly associated with poor overall survival in all pancreatic cancer patient data from TCGA (Figure 3C). These findings indicate that high expression of CXCL1 and IL‐8 in pancreatic tumors contributes to the aggressiveness of pancreatic cancer.
FIGURE 3.

Comparison of CXCL1 and IL‐8 expression in tumors and the prognosis of pancreatic cancer patients. (A) CXCL1 expression in human pancreatic cancer tissues in the Fusobacterium nucleatum‐negative group (N = 13) versus the F. nucleatum‐positive group (N = 13). (B) Gene expression profiling interactive analysis of CXCL1 (left) and IL‐8 (right) in normal (gray bars) and tumor (pink bars) samples from pancreatic cancer data of The Cancer Genome Atlas (TCGA) and Genotype‐Tissue Expression databases. (C) Kaplan–Meier analysis of overall survival of TCGA pancreatic cancer patients with high and low expression of CXCL1 (left) and IL‐8 (right). *p < 0.05, **p < 0.01.
3.4. Intratumor F. nucleatum modifies the tumor immune microenvironment in pancreatic cancer
To investigate the effects of intratumor F. nucleatum on the tumor immune microenvironment in vivo experiments, we established subcutaneous pancreatic cancer models with KPC cells in C57BL/6 mice and injected bacteria into the tumors as shown in Figure 4A. Intratumor F. nucleatum increased the tumor volume compared with the control or P. gingivalis (p < 0.05; Figure 4B). Cxcl1 expression was significantly increased in F. nucleatum‐injected tumors compared with the control and P. gingivalis‐injected tumors (p < 0.05; Figure 4C). To investigate the effect of intratumor F. nucleatum on the tumor immune microenvironment, flow cytometric analysis was used to evaluate tumor‐infiltrating immune cells. The composition of tumor‐infiltrating immune cells showed significantly increased infiltration of CD11b+ Gr‐1+ MDSCs into F. nucleatum‐injected tumors (p < 0.05; Figure 4D). Conversely, CD8+ T cells were decreased in F. nucleatum‐injected tumors compared with the control and P. gingivalis‐injected tumors (Figure 4D). Infiltration of dendritic cells and macrophages was not affected by intratumor bacteria (Figure 4D). Immunohistochemistry confirmed that colonization of F. nucleatum in tumors increased the number of CD11b+ cells and reduced the number of CD8a+ cells compared with the control (*p < 0.05, **p < 0.01; Figure 4E). These data suggest that intratumor F. nucleatum promotes in vivo tumor growth and contributes to the establishment of an immunosuppressive tumor microenvironment in pancreatic cancer.
FIGURE 4.

Effects of intratumor Fusobacterium nucleatum on in vivo tumor growth of pancreatic cancer. (A) Experimental schema. KPC cells were subcutaneously implanted into C57/B6 mice. From 1 week after implantation, the mice were intratumorally injected with F. nucleatum or P. gingivalis every 3 days for 11 days. Control mice were intratumorally injected with BHI medium. (B) Representative quantified graph of tumor growth curve. Comparisons of the tumor volume in control, F. nucleatum, and P. gingivalis are shown. (C) Cxcl1 expression was increased in F. nucleatum‐injected tumors compared with the control and P. gingivalis‐injected tumors. (D) Representative flow cytometric analysis of CD11b+Gr‐1+ MDSCs, CD8+ T cells, CD11c+MHCII+F4/80− dendritic cells, and F4/80+ macrophages gated on CD45+ cells. (E) Representative immunohistochemical staining of CD11b+ myeloid cells and CD8a+ T cells. Original magnification, ×100. Scale bar, 50 μm. *p < 0.05, **p < 0.01.
3.5. Intratumor F. nucleatum promotes pancreatic cancer progression through modification of the tumor immune microenvironment via the CXCL1‐CXCR2 axis
To investigate whether intratumor F. nucleatum promotes tumor progression via CXCR2 signaling, we administrated the CXCR2 antagonist in F. nucleatum‐injected subcutaneous pancreatic cancer models (Figure 5A). Tumor growth promoted by F. nucleatum was significantly suppressed by CXCR2 inhibition (*p < 0.05, **p < 0.01; Figure 5B,C). Increased infiltration of MDSCs in F. nucleatum‐injected tumors was also suppressed by CXCR2 inhibition, although a change in CD8+ T cell infiltration was not observed (*p < 0.05, **p < 0.01; Figure 5D). Infiltration of dendritic cells and macrophages did not change after CXCR2 inhibition. In increased two cytokines (CXCL1 and IL‐8) detected in the supernatant of human pancreatic cancer cells treated with F. nucleatum, IL‐8 is not expressed in mice 31 ; therefore, we investigated the role of CXCL1 in modification of the tumor immune microenvironment in F. nucleatum‐injected pancreatic tumors. Intratumor administration of the anti‐CXCL1 Ab into F. nucleatum‐injected tumors suppressed tumor growth in mice (p < 0.05; Figure 5E–G) with suppressed MDSC infiltration and reversal of CD8+ T cell infiltration (p < 0.01; Figure 5H). These data indicate that intratumor F. nucleatum promotes pancreatic cancer progression via the CXCL1‐CXCR2 axis by affecting cancer cells themselves in an autocrine manner and modifying the tumor immune microenvironment in a paracrine manner.
FIGURE 5.

Effects of intratumor Fusobacterium nucleatum on the tumor immune microenvironment via CXCR2. (A) Experimental schema. KPC cells were subcutaneously implanted into C57/B6 mice. From 1 week after implantation, the mice were intratumorally injected F. nucleatum or BHI and treated with or without CXCR2 inhibitor every 3 days for 2 weeks. (B) Representative quantified graph of tumor weight. (C) Representative in situ images of tumors. Tumor growth promoted by F. nucleatum was significantly suppressed by CXCR2 inhibition. (D) Representative flow cytometric analysis of CD11b+Gr‐1+ MDSCs, CD8+ T cells, CD11c+MHCII+F4/80− dendritic cells, and F4/80+ macrophages gated on CD45+ cells. (E) Experimental schema. KPC cells were subcutaneously implanted into C57/B6 mice. From 1 week after implantation, the mice were intratumorally injected with F. nucleatum or BHI and treated with or without an anti‐CXCL1 Ab every 3 days for 2 weeks. (F) Representative quantified graph of tumor volumes. (G) Representative in situ images of tumors. Tumor growth promoted by F. nucleatum was significantly suppressed by the anti‐CXCL1 Ab. (H) Representative immunohistochemical staining of CD11b+ myeloid cells and CD8a+ T cells. Original magnification, ×100. Scale bar, 50 μm. *p < 0.05, **p < 0.01.
3.6. Intratumor F. nucleatum promotes pancreatic cancer progression through recruitment of MDSCs to the tumor immune microenvironment
The results so far indicate that intratumor F. nucleatum induces CXCR2‐mediated recruitment of MDSCs to the tumor immune microenvironment, resulting in tumor progression. Finally, we investigated whether MDSC recruitment induced by intratumor F. nucleatum was critical to suppress antitumor immunity and tumor progression. To abrogate MDSC infiltration in pancreatic tumors, we injected anti‐Ly6G Ab intraperitoneally (Figure 6A). Tumor growth promoted by F. nucleatum was significantly suppressed by the anti‐Ly6G Ab treatment (*p < 0.05, **p < 0.01; Figure 6B,C). Flow cytometry confirmed that anti‐Ly6G Ab administration significantly suppressed the infiltration of MDSCs in F. nucleatum‐injected tumors, but not that of dendritic cells or macrophages (p < 0.05; Figure 6D). F. nucleatum‐induced effects on the infiltration of MDSCs or CD8+ T cells did not observe with anti‐Ly6G Ab administration (Figure 6D), which was confirmed by immunohistochemistry (p < 0.01; Figure 6E). Taken together, our findings suggest that intratumor F. nucleatum facilitates progression by modifying the tumor immune environment, inducing MDSC infiltration and inhibiting CD8+ T cell proliferation.
FIGURE 6.

Effects of intratumor Fusobacterium nucleatum on myeloid‐derived suppressor cell (MDSC) recruitment to the tumor immune microenvironment. (A) Experimental schema. KPC cells were subcutaneously implanted into C57/B6 mice. From 1 week after implantation, the mice were intratumorally injected with F. nucleatum or BHI and treated with an anti‐Ly6G Ab or isotype control Ab every 3 days for 2 weeks. (B) Representative quantified graph of tumor weights. (C) Representative in situ images of tumors. Tumor growth promoted by F. nucleatum was significantly suppressed by the anti‐Ly6G Ab. (D) Representative flow cytometric analysis of CD11b+Gr‐1+ MDSCs, CD8+ T cells, CD11c+MHCII+F4/80− dendritic cells, and F4/80+ macrophages gated on CD45+ cells. (E) Representative immunohistochemical staining of CD8a+ T cells. Original magnification, ×100. Scale bar, 50 μm. *p < 0.05, **p < 0.01.
4. DISCUSSION
In this study, we elucidated the mechanisms by which intratumor F. nucleatum promoted pancreatic cancer progression. Fusobacterium species in tumors have been reported to impair the prognosis of pancreatic cancer patients, but the underlying mechanisms have not been elucidated so far. 17 We found that tumors with F. nucleatum colonization were significantly larger and more frequently showed retroperitoneal invasion compared with tumors without F. nucleatum colonization, suggesting that intratumor F. nucleatum contributes to the aggressiveness of pancreatic cancer. F. nucleatum stimulated pancreatic cancer cells to secrete CXCL1, leading to cancer progression through two pathways. First, in an autocrine manner, F. nucleatum promoted pancreatic cancer cell migration and invasion, which was confirmed by in vitro coculture experiments with F. nucleatum; second, in a paracrine manner, F. nucleatum suppressed tumor‐infiltrating CD8+ T cells by recruiting MDSCs to the tumor, which was confirmed by in vivo experiments (Figure 7). Finally, we revealed that inhibition of the CXCL1‐CXCR2 axis restored antitumor immunity and suppressed tumor growth in F. nucleatum‐injected mice. To the best of our knowledge, we are the first to show that intratumor F. nucleatum promotes the progression of pancreatic cancer by modifying the tumor immune microenvironment.
FIGURE 7.

Model of the mechanism of Fusobacterium nucleatum stimulating CXCL1 secretion from pancreatic cancer cells, leading to cancer progression through two pathways: first, in an autocrine manner, F. nucleatum promoted pancreatic cancer cell migration and invasion, which was confirmed by in vitro coculture experiments with F. nucleatum; second, in a paracrine manner, F. nucleatum suppressed tumor‐infiltrating CD8+ T cells by recruiting MDSCs in vivo experiments.
Bacterial colonization in human pancreatic tumors has been reported in several studies. 14 , 15 , 16 , 17 , 32 Nejman et al. 32 detected bacterial DNA in more than 60% of pancreatic tumors by 16S rRNA gene sequencing. Depletion of intratumor bacteria by antibiotics reshapes the tumor immune microenvironment, by decreasing MDSCs and increasing CD8+ T cell activation in tumors. 15 Riquelme et al. 14 showed that the composition and diversity of intratumor bacteria were associated with improved CD8+ T cell responses and prolonged survival of pancreatic cancer patients. Specifically, enrichment of Sachharopolyspora, Pseudoxanthomas, and Streptomyces in a tumor was positively correlated with tumor‐infiltrating CD8+ T cells. This suggests that pancreatic tumors contain a mixture of functionally different bacterial species that modify the tumor immune microenvironment positively or negatively. Intratumor bacteria that modify antitumor immunity are a therapeutic target, but functional analysis of a specific bacterium has not been reported. This study showed that intratumor F. nucleatum promoted tumor growth by mediating antitumor immunity represented by suppression of tumor‐infiltrating CD8+ T cells. The negative effect of intratumor F. nucleatum on tumors was confirmed by clinicopathological analysis of resected pancreatic cancers with F. nucleatum colonization. Selective antibiotic depletion of specific bacteria promoting pancreatic cancer progression such as F. nucleatum might be a novel approach to immunostimulatory therapy of pancreatic cancer.
CXCL1 is a chemoattractant for immune cells, mediating inflammatory responses at an infectious site. 33 CXCL1 is mainly secreted from cancer cells, specifically binds to its receptor, CXCR2, expressed on MDSCs and cancer cells, and promotes sarcoma tumor growth in a paracrine manner. 34 Binding of CXCL1 to CXCR2 on prostate cancer cells promotes migration and invasion via NF‐κB activation through the Akt pathway in an autocrine manner. 35 It is also known that CXCL1 recruits CXCR2+ MDSCs to tumors, which suppresses CD8+ T cell proliferation at the site and establishes an immunosuppressive microenvironment. 34 Our TCGA analysis showed that CXCL1 was highly expressed in pancreatic tumors and correlated with a poor prognosis of pancreatic cancer patients. Considering the aforementioned findings about CXCL1 in other tumors, the CXCL1‐CXCR2 axis may be a candidate therapeutic target in pancreatic cancer. However, a previous report has demonstrated that depletion of CXCR2 alone does not suppress pancreatic tumors in in vivo experiments. 36 Our study revealed that CXCL1 secretion from cancer cells was enhanced by F. nucleatum. Inhibition of the CXCL1‐CXCR2 axis by the anti‐CXCL1 Ab or CXCR2 inhibitor restored antitumor immunity and suppressed the growth of F. nucleatum‐injected tumors. This effect was not observed in tumors without F. nucleatum injection, suggesting that the contribution of the CXCL1‐CXCR2 axis to tumor growth is limited to F. nucleatum‐colonized tumors. We found that a certain proportion of human pancreatic cancers harbored F. nucleatum, indicating that inhibition of the CXCL1‐CXCR2 axis might be effective only in these cases. The composition of intratumor bacteria would influence the tumor immune microenvironment, and a therapeutic strategy in accordance with the intratumor bacteria might be proposed in the future.
While a large cohort study has demonstrated that periodontitis is a risk factor of pancreatic cancer development, 37 the causal relationship between periodontal pathogens and pancreatic cancer progression has not been revealed. Periodontal pathogens are mainly composed of F. nucleatum, P. gingivalis, Tannerella denticola, and Tannerella forsythia. 38 Among them, F. nucleatum and P. gingivalis are reported to promote the proliferation of pancreatic cancer cells by enhancing Akt signaling and cyclin D1 expression in in vitro experiments. 33 , 39 In this study, we found that intratumor F. nucleatum promoted tumor growth by modifying the tumor immune microenvironment in multiple in vivo experiments. This is the first evidence that periodontal pathogens in pancreatic tumors alter antitumor immunity and contribute to tumor progression. Although the relationship between oral and pancreatic microbiomes has not been revealed yet, it is known that some bacteria in pancreatic tumors originate from the duodenum. 16 It is also shown that bacteria administered to mice by oral gavage migrate to the pancreas and activate Ki67+ cells in tumors. 40 It is reasonable that the oral microbiome partially represents the intratumor microbiome in the pancreas. Considering that the intratumor microbiome affects malignancy, analysis of the oral microbiome might provide useful information about therapeutic options for pancreatic cancer.
We acknowledge there are some limitations in this study. First, we did not observe interactions between intratumor bacteria and immune cells. Intratumor injection of bacteria might directly act on immune cells and modify antitumor immunity. However, F. nucleatum promoted CXCL1 secretion from cancer cells, and depletion of CXCL1 changed the composition of intratumor immune cells. Interactions between cancer cells and intratumor bacteria should affect the progression of pancreatic cancer. The direct effect of intratumor bacteria on immune cells in the tumor will be elucidated in the next study. Second, we did not find that P. gingivalis, which has been reported to be epidemiologically associated with pancreatic cancer, 41 promoted pancreatic cancer progression. P. gingivalis varies in virulence among strains, 42 and the virulent strain W83 has been reported to promote pancreatic cancer progression. 40 We used the less virulent strain ATCC 33277 in this study, which may have led to the different results from the previous report.
In conclusion, F. nucleatum can colonize pancreatic cancer and affect the prognosis of pancreatic cancer patients. Intratumor F. nucleatum promotes CXCL1 secretion from cancer cells, contributing to cancer progression in an autocrine and paracrine manners. The CXCL1‐CXCR2 axis is activated by F. nucleatum to create an immunosuppressive tumor microenvironment, and blockade of this axis suppresses tumor growth. Our results suggest a novel therapeutic approach for intratumor F. nucleatum‐positive pancreatic cancer patients.
AUTHOR CONTRIBUTIONS
M.H. performed most experiments, analyzed data, and wrote the manuscript. N.I. coordinated the project, supervised manuscript writing, and contributed to data interpretation and discussion. K.N. supervised the study and contributed to data interpretation, designed the experimental approach, and critically revised the manuscript. H.L., P.Z., S.D., K.O., N.H., A.K., C.I., and T.A. performed mouse breeding and assisted with animal experiments and assays. N.T., Y.Y., and Y.O. performed immunohistochemistry and contributed resources. K.O. contributed to discussion and supervised manuscript writing. M.N. supervised the study and manuscript writing. All authors approved the final manuscript.
FUNDING INFORMATION
This work was supported by JSPS KAKENHI grant numbers JP22H02922, JP22J11407, and JP22K15560, and grants from GSK Japan Research Grant 2021, The Shin‐Nihon Foundation of Advanced Medical Research, and Pancreas Research Foundation of Japan.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest. Yoshinao Oda, the coauthor for this study, is the Associate Editor of Cancer Science.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The study was approved by the Ethics Committee of Kyushu University (approval numbers: 30–230 and 858–00; Fukuoka, Japan).
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: All mouse experiments were conducted in accordance with the guidelines of the institutional animal committee of Kyushu University (approval number: A21‐383).
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank S. Sadatomi and E. Manabe (Department of Surgery and Oncology, Kyushu University Hospital) and the members of the Research Support Center, Graduate School of Medical Sciences, Kyushu University for their expert technical assistance. Figures were created using BioRender.com. We thank Mitchell Arico from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Hayashi M, Ikenaga N, Nakata K, et al. Intratumor Fusobacterium nucleatum promotes the progression of pancreatic cancer via the CXCL1‐CXCR2 axis. Cancer Sci. 2023;114:3666‐3678. doi: 10.1111/cas.15901
Contributor Information
Naoki Ikenaga, Email: ikenaga.naoki.533@m.kyushu-u.ac.jp.
Masafumi Nakamura, Email: nakamura.masafumi.861@m.kyushu-u.ac.jp.
REFERENCES
- 1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17‐48. doi: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 2. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140‐2141. doi: 10.1056/NEJMc1412266 [DOI] [PubMed] [Google Scholar]
- 3. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science. 2015;350(6264):1084‐1089. doi: 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079‐1084. doi: 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science. 2018;359(6371):97‐103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104‐108. doi: 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science. 2018;359(6371):91‐97. doi: 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 8. Poore GD, Kopylova E, Zhu Q, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579(7800):567‐574. doi: 10.1038/s41586-020-2095-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299‐306. doi: 10.1101/gr.126516.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nomoto D, Baba Y, Liu Y, et al. Fusobacterium nucleatum promotes esophageal squamous cell carcinoma progression via the NOD1/RIPK2/NF‐kappa B pathway. Cancer Lett. 2022;530:59‐67. doi: 10.1016/j.canlet.2022.01.014 [DOI] [PubMed] [Google Scholar]
- 11. Yamamura K, Baba Y, Miyake K, et al. Fusobacterium nucleatum in gastroenterological cancer: evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol Lett. 2017;14(6):6373‐6378. doi: 10.3892/ol.2017.7001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xuan C, Shamonki JM, Chung A, et al. Microbial dysbiosis is associated with human breast cancer. PLoS One. 2014;9(1):e83744. doi: 10.1371/journal.pone.0083744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82(16):5039‐5048. doi: 10.1128/aem.01235-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(4):795‐806.e12. doi: 10.1016/j.cell.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pushalkar S, Hundeyin M, Daley D, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403‐416. doi: 10.1158/2159-8290.Cd-17-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geller LT, Barzily‐Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(6356):1156‐1160. doi: 10.1126/science.aah5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitsuhashi K, Nosho K, Sukawa Y, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6(9):7209‐7220. doi: 10.18632/oncotarget.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E‐cadherin/beta‐catenin signaling via its FadA Adhesin. Cell Host Microbe. 2013;14(2):195‐206. doi: 10.1016/j.chom.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll‐like receptor 4 signaling to nuclear factor‐kappa B, and up‐regulating expression of MicroRNA‐21. Gastroenterology. 2017;152(4):851‐866.e24. doi: 10.1053/j.gastro.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. PORPHYROMONAS‐GINGIVALIS invasion of gingival epithelial‐cells. Infect Immun. 1995;63(10):3878‐3885. doi: 10.1128/iai.63.10.3878-3885.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohuchida K, Mizumoto K, Murakami M, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor‐stromal interactions. Cancer Res. 2004;64(9):3215‐3222. doi: 10.1158/0008-5472.Can-03-2464 [DOI] [PubMed] [Google Scholar]
- 22. Kibe S, Ohuchida K, Ando Y, et al. Cancer‐associated acinar‐to‐ductal metaplasia within the invasive front of pancreatic cancer contributes to local invasion. Cancer Lett. 2019;444:70‐81. doi: 10.1016/j.canlet.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 23. Okumura T, Ohuchida K, Kibe S, et al. Adipose tissue‐derived stromal cells are sources of cancer‐associated fibroblasts and enhance tumor progression by dense collagen matrix. Int J Cancer. 2019;144(6):1401‐1413. doi: 10.1002/ijc.31775 [DOI] [PubMed] [Google Scholar]
- 24. Okumura T, Ohuchida K, Sada M, et al. Extra‐pancreatic invasion induces lipolytic and fibrotic changes in the adipose microenvironment, with released fatty acids enhancing the invasiveness of pancreatic cancer cells. Oncotarget. 2017;8(11):18280‐18295. doi: 10.18632/oncotarget.15430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun X, He X, Zhang Y, et al. Inflammatory cell‐derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast‐hijacked cancer escape mechanism. Gut. 2022;71(1):129‐147. doi: 10.1136/gutjnl-2020-322744 [DOI] [PubMed] [Google Scholar]
- 26. Zhang QF, Ma C, Duan Y, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021;11(5):1248‐1267. doi: 10.1158/2159-8290.Cd-20-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635‐700. doi: 10.1146/annurev.biochem.71.110601.135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyamoto M, Shimizu Y, Okada K, Kashii Y, Higuchi K, Watanabe A. Effect of interleukin‐8 on production of tumor‐associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol Immunother. 1998;47(1):47‐57. doi: 10.1007/s002620050503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth‐related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66(6):3071‐3077. doi: 10.1158/0008-5472.Can-05-2871 [DOI] [PubMed] [Google Scholar]
- 30. Tang ZF, Li CW, Kang BX, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98‐W102. doi: 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asfaha S, Dubeykovskiy AN, Tomita H, et al. Mice that express human Interleukin‐8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144(1):155‐166. doi: 10.1053/j.gastro.2012.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type‐specific intracellular bacteria. Science. 2020;368(6494):973‐980. doi: 10.1126/science.aay9189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Udayasuryan B, Ahmad RN, Nguyen TTD, et al. Fusobacterium nucleatum induces proliferation and migration in pancreatic cancer cells through host autocrine and paracrine signaling. Sci Signal. 2022;15(756):eabn4948. doi: 10.1126/scisignal.abn4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Highfill SL, Cui YZ, Giles AJ, et al. Disruption of CXCR2‐mediated MDSC tumor trafficking enhances anti‐PD1 efficacy. Sci Transl Med. 2014;6(237):237ra67. doi: 10.1126/scitranslmed.3007974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuo P‐L, Shen K‐H, Hung S‐H, Hsu YL. CXCL1/GRO alpha increases cell migration and invasion of prostate cancer by decreasing fibulin‐1 expression through NF‐kappa B/HDAC1 epigenetic regulation. Carcinogenesis. 2012;33(12):2477‐2487. doi: 10.1093/carcin/bgs299 [DOI] [PubMed] [Google Scholar]
- 36. Steele CW, Karim SA, Leach JDG, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832‐845. doi: 10.1016/j.ccell.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent. 2010;38(2):83‐95. doi: 10.1016/j.jdent.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 38. Mohanty R, Asopa SJ, Joseph MD, et al. Red complex: polymicrobial conglomerate in oral flora: a review. J Family Med Primary Care. 2019;8(11):3480‐3486. doi: 10.4103/jfmpc.jfmpc_759_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gnanasekaran J, Gallimidi AB, Saba E, et al. Intracellular Porphyromonas gingivalis promotes the tumorigenic behavior of pancreatic carcinoma cells. Cancers (Basel). 2020;12(8):2331. doi: 10.3390/cancers12082331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan Q, Ma X, Yang B, et al. Periodontitis pathogen Porphyromonas gingivalis promotes pancreatic tumorigenesis via neutrophil elastase from tumor‐associated neutrophils. Gut Microbes. 2022;14(1). doi: 10.1080/19490976.2022.2073785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fan XZ, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population‐based nested case‐control study. Gut. 2018;67(1):120‐127. doi: 10.1136/gutjnl-2016-312580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naito M, Hirakawa H, Yamashita A, et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P‐gingivalis. DNA Res. 2008;15(4):215‐225. doi: 10.1093/dnares/dsn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


