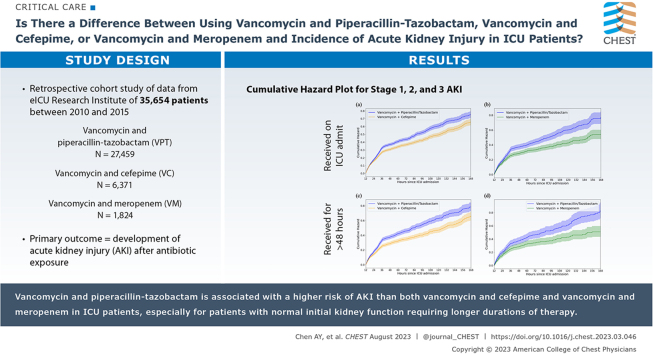
Abstract
Background
Evidence regarding acute kidney injury associated with concomitant administration of vancomycin and piperacillin-tazobactam is conflicting, particularly in patients in the ICU.
Research Question
Does a difference exist in the association between commonly prescribed empiric antibiotics on ICU admission (vancomycin and piperacillin-tazobactam, vancomycin and cefepime, and vancomycin and meropenem) and acute kidney injury?
Study Design and Methods
This was a retrospective cohort study using data from the eICU Research Institute, which contains records for ICU stays between 2010 and 2015 across 335 hospitals. Patients were enrolled if they received vancomycin and piperacillin-tazobactam, vancomycin and cefepime, or vancomycin and meropenem exclusively. Patients initially admitted to the ED were included. Patients with hospital stay duration of < 1 h, receiving dialysis, or with missing data were excluded. Acute kidney injury was defined as Kidney Disease: Improving Global Outcomes stage 2 or 3 based on serum creatinine component. Propensity score matching was used to match patients in the control (vancomycin and meropenem or vancomycin and cefepime) and treatment (vancomycin and piperacillin-tazobactam) groups, and ORs were calculated. Sensitivity analyses were performed to study the effect of longer courses of combination therapy and patients with renal insufficiency on admission.
Results
Thirty-five thousand six hundred fifty-four patients met inclusion criteria (vancomycin and piperacillin-tazobactam, n = 27,459; vancomycin and cefepime, n = 6,371; vancomycin and meropenem, n = 1,824). Vancomycin and piperacillin-tazobactam was associated with a higher risk of acute kidney injury and initiation of dialysis when compared with that of both vancomycin and cefepime (Acute kidney injury: OR, 1.37 [95% CI, 1.25-1.49]; dialysis: OR, 1.28 [95% CI, 1.14-1.45]) and vancomycin and meropenem (Acute kidney injury: OR, 1.27 [95%, 1.06-1.52]; dialysis: OR, 1.56 [95% CI, 1.23-2.00]). The odds of acute kidney injury developing was especially pronounced in patients without renal insufficiency receiving a longer duration of vancomycin and piperacillin-tazobactam therapy compared with vancomycin and meropenem therapy.
Interpretation
VPT is associated with a higher risk of acute kidney injury than both vancomycin and cefepime and vancomycin and meropenem in patients in the ICU, especially for patients with normal initial kidney function requiring longer durations of therapy. Clinicians should consider vancomycin and meropenem or vancomycin and cefepime to reduce the risk of nephrotoxicity for patients in the ICU.
Key Words: acute kidney injury, cefepime, meropenem, piperacillin, tazobactam, vancomycin
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 273
Take-home Points.
Study Question: Does a difference exist in the association between commonly prescribed empiric antibiotics on ICU admission (vancomycin and piperacillin-tazobactam, vancomycin and cefepime, and vancomycin and meropenem) and acute kidney injury?
Results: Greater risk of acute kidney injury exists when using vancomycin and piperacillin-tazobactam compared with vancomycin and cefepime or vancomycin and meropenem, especially in patients with normal kidney function on admission requiring antibiotic treatment for longer than 48 h. In addition, patients receiving vancomycin and piperacillin-tazobactam showed greater odds of initiating dialysis and dialysis or in-hospital mortality compared with those receiving vancomycin and cefepime or vancomycin and meropenem.
Interpretation: When prescribing empiric antibiotic regimens to critically ill patients, clinicians should consider vancomycin and meropenem or vancomycin and cefepime over vancomycin and piperacillin-tazobactam to reduce the risk of nephrotoxicity and adverse clinical outcomes.
Infection is common in the ICU. Some of the most common empiric broad-spectrum antibiotic regimens used include a combination of vancomycin and piperacillin-tazobactam (VPT), vancomycin and cefepime (VC), and vancomycin and meropenem (VM). These regimens are used widely in the initial management of critically ill patients with suspected infection and have methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa coverage.1 Notably, VPT has been linked to the development of acute kidney injury (AKI), although the data supporting this association have been primarily in general medical and mixed population studies.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 When studied exclusively in patients in the ICU, however, the evidence has been inconclusive.9,13, 14, 15, 16, 17, 18, 19
To date, several retrospective studies have examined the incidence of AKI in critically ill patients treated with VPT, VC, and VM.9,14,15,17,18 Of the four smaller studies (n < 500), two demonstrated an association with AKI9,15 and two demonstrated no difference in AKI rates across groups.13,14 Two larger studies recently were published. A large cohort study (n = 789,200) on a mixed population demonstrated that VPT increases AKI risk, but did not focus on the critically ill population.20 More recently, a single-site retrospective study focused on the critically ill population (n = 15,673) reported an increased risk of VPT compared with other regimens with antipseudomonal and anti-methicillin-resistant S aureus coverage.21 Our study adds to this growing body of literature by comparing VPT with two commonly prescribed regimens, VC and VM, in the critically ill population across multiple sites.
In this study, we aimed to address the uncertainty in the literature through a large multicenter retrospective cohort study on nephrotoxicity associated with empiric broad-spectrum antibiotics in patients in the ICU. We examined patients admitted to the ICU who were receiving one of three empiric regimens (VPT, VC, or VM) and were assessed for risk of AKI over the first 7 days of ICU admission. We focused on critically ill patients in the ICU and used a more stringent definition of AKI, defined as Kidney Disease: Improving Global Outcomes (KDIGO) stages 2 and 3. We further performed subanalyses to investigate the effect of treatment duration and baseline renal function on AKI development. These design choices allowed us to present a more comprehensive understanding of how AKI risk varies between VPT regimens and VC and VM regimens in the critically ill.
Study Design and Methods
This large multicenter retrospective cohort study was conducted using patient data from the eICU Research Institute database, which contains records for 3,089,748 unique ICU stays between 2010 and 2015 across 335 distinct hospital units.22,23 We included the first ICU stay for each patient, excluding all admissions shorter than 1 h. Only patients who were admitted directly from the ED to the ICU were included to incorporate ED data before ICU admission in determining baseline creatinine levels. Hospitals that did not have accurate digital medical administration records were excluded. In addition, patients with missing variables necessary for the outcome definition or for propensity score matching, who were receiving chronic dialysis or incident dialysis before the administration of antibiotics, or who were not administered antibiotics or were administered more than one combination therapy were excluded. Patients were enrolled in treatment groups (VPT, VM, or VC) if they exclusively received one of these combination therapies on admission to the ICU to ensure independent comparisons among the three antibiotic regimens.
Baseline serum creatinine (SCr) was estimated using the lowest SCr value recorded within the window of ICU admission. The primary outcome was development of AKI in the first week after antibiotic exposure, or more explicitly (12 h, 7 days) from recorded time of admission to the ICU. AKI was defined via the KDIGO guidelines based on the SCr component alone.24 A stringent definition of AKI was used for robustness of the study and to mitigate the effects of pseudonephrotoxicity and incidental fluctuations in SCr, which is discussed in the Discussion section. For the main analysis, the outcome of AKI was defined as KDIGO stage 2 or 3 disease. Stage 1 AKI was considered a negative outcome. The analyses were repeated with AKI defined as all three KDIGO stages.
The study included two comparison groups (VPT vs VC and VPT vs VM). Patients were matched via propensity score matching as discussed herein. The primary outcome was AKI. Secondary outcomes of dialysis initiation, dialysis or in-hospital mortality, and in-hospital mortality also were included. Two sensitivity analyses were conducted. An additional requirement of at least 48 h of therapy was applied to study the effect of longer antibiotic regimens on AKI development. A threshold of 48 h was chosen because 48 to 72 h is a common time frame used for antibiotic time-outs, where antibiotic appropriateness is reassessed to inform de-escalation or discontinuation.25 Patients were subdivided further based on the estimated glomerular filtration rate (eGFR) to study the effect of initial renal sufficiency on the difference in AKI risk between the treatment groups. An eGFR cutoff of 60 mL/min/1.73 m2 at the time of admission was used to define abnormal initial renal function.
Propensity score matching is a statistical method for causal inference amidst confounding factors.26 We estimated the probability that an individual received an antibiotic treatment using logistic regression with the following features selected a priori based on expert guidance: age, eGFR, immunocompromised state, Acute Physiology and Chronic Health Evaluation (APACHE) IV predicted risk of mortality score, BMI, and use of nephrotoxic agents.26 Nephrotoxic agents were defined as eight common culprits of drug-induced nephrotoxic injury (IV contrast, aminoglycosides, amphotericin B, antiviral agents, calcineurin inhibitors, loop diuretics, nonsteroidal antiinflammatory drugs, and vasopressors). Patients in the VM and VC groups were matched 1:1 with patients in the VPT group, allowing for replacement in the VPT group because this reduces bias in the estimated score.27 We used a caliper width of 0.01 to ensure similar matches. Because considerably more patients were administered VPT exclusively, all patients in the minority VC and VM groups were matched to their closest pair in the VPT group. All unmatched patients in the VPT groups were not included in that specific analysis.
ORs and 95% CIs were calculated to assess the risk of the primary and secondary outcomes for the control group (VC or VM) compared with that of the exposure group (VPT). The Mantel-Haenszel test and the Cochran-Mantel-Haenszel χ2 test were used to calculate the composite OR for each of the matched pairs and to determine statistical significance, respectively. Significance was defined as a P value of < .05. Cumulative hazard censored by ICU discharge or death was calculated for each antibiotic group to assess the probability of AKI as a function of ICU admission length in hours. Inverse probability treatment weighting was used to balance the three antibiotic groups when comparing cumulative hazard over time. The slope of the hazard was calculated between hours 48 and 144, because it takes approximately 48 h for true kidney injury to occur.
The study was exempt from institutional review board approval because of its retrospective design, lack of direct patient intervention, and the security schema, for which the re-identification risk was certified as meeting safe harbor standards by an independent privacy expert (Privacert; Health Insurance Portability and Accountability Act Certification no. 1031219-2). SQL software (BigQuery; Google) and PostgreSQL (PostgreSQL Global Development Group) was used to query data and Python software (Python Software Foundation) was used to perform all analyses. All code for data extraction and analysis associated with the current submission is available online.28 Detailed definitions of concepts are provided in e-Appendix 1.
Results
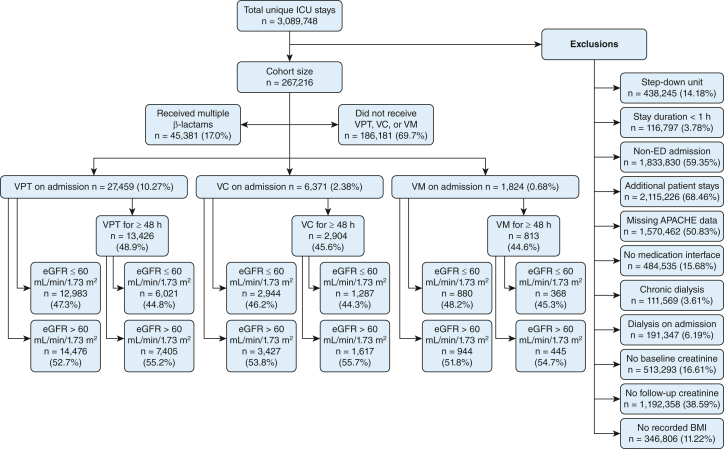
A total of 267,216 patients comprised the cohort after applying the exclusion criteria (Fig 1). Of those, 35,654 patients received an exclusive antibiotic combination regimen on ICU admission (VPT, n = 27,459; VC, n = 6,371; VM, n = 1,824). The percentage of patients with normal kidney function on admission (eGFR > 60 mL/min/1.73 m2) were comparable with that of abnormal initial renal function among all three antibiotic therapy groups. Almost one-half of the treatment groups received longer durations of antibiotic therapy (≥ 48 h).
Figure 1.
Sequential flow chart showing cohort selection procedure. Reported number of exclusions are absolute numbers rather than sequential. One patient stay can meet multiple exclusion criteria. APACHE = Acute Physiology and Chronic Health Evaluation; eGFR = estimated glomerular filtration rate; VC = vancomycin and cefepime; VM = vancomycin and meropenem; VPT = vancomycin and piperacillin-tazobactam.
All patients were matched successfully 1:1 with replacement via propensity score matching (e-Appendix B, e-Table 1). Tables 1 and 2 display the baseline demographics and clinical characteristics of patients receiving VPT with those receiving VC and VM, respectively. A greater percentage of male patients, Hispanic patients, and Native American patients received VPT when compared with both VM and VC for the matched cohorts. Sepsis was the most common diagnosis, comprising 48.4% of all the matched patients. Most patients were exposed to another nephrotoxic agent on admission.
Table 1.
Baseline Characteristics and Demographic Features After Propensity Score Matching: VC Compared With VPT
| Variable | VC (n = 6,371) | VPT (n = 6,371) | Overall (N = 12,742) |
|---|---|---|---|
| Demographics | |||
| Age, y | 66.2 ± 15.7 | 66.3 ± 15.9 | 66.3 ± 15.8 |
| Sex | |||
| Female | 3,064 (48.1) | 2,854 (44.8) | 5,918 (46.4) |
| Male | 3,307 (51.9) | 3,517 (55.2) | 6,824 (53.6) |
| Ethnicity | |||
| Black | 657 (10.3) | 653 (10.3) | 1,310 (10.3) |
| Asian | 67 (1.1) | 84 (1.3) | 151 (1.2) |
| White | 5,161 (81.3) | 4,907 (77.5) | 10,068 (79.4) |
| Hispanic | 226 (3.6) | 401 (6.3) | 627 (4.9) |
| Native American | 36 (0.6) | 63 (1.0) | 99 (0.8) |
| Other/unknown | 203 (3.2) | 226 (3.6) | 429 (3.4) |
| BMI group | |||
| Underweight | 262 (4.1) | 282 (4.4) | 544 (4.3) |
| Normal | 1,943 (30.5) | 1,928 (30.3) | 3,871 (30.4) |
| Overweight | 1,688 (26.5) | 1,707 (26.8) | 3,395 (26.6) |
| Obese | 2,478 (38.9) | 2,454 (38.5) | 4,932 (38.7) |
| ICU admission diagnoses | |||
| Cancer | 63 (1.0) | 53 (0.8) | 116 (0.9) |
| Cardiac | |||
| Hypertension | 11 (0.2) | 11 (0.2) | 22 (0.2) |
| Other | 23 (0.4) | 15 (0.2) | 38 (0.3) |
| Arrhythmia | 87 (1.4) | 109 (1.7) | 196 (1.5) |
| Chest pain | 17 (0.3) | 15 (0.2) | 32 (0.3) |
| Heart failure | 195 (3.1) | 168 (2.6) | 363 (2.8) |
| Myocardial infarction | 212 (3.3) | 251 (3.9) | 463 (3.6) |
| Drug | |||
| Other | 1 (0.0) | 1 (0.0) | 2 (0.0) |
| Overdose | 50 (0.8) | 76 (1.2) | 126 (1.0) |
| Withdrawal | 9 (0.1) | 5 (0.1) | 14 (0.1) |
| Endocrine | 72 (1.1) | 91 (1.4) | 163 (1.3) |
| GI | |||
| Bleed | 70 (1.1) | 114 (1.8) | 184 (1.4) |
| Liver failure | 20 (0.3) | 32 (0.5) | 52 (0.4) |
| Other | 32 (0.5) | 96 (1.5) | 128 (1.0) |
| Hematologic | 94 (1.5) | 72 (1.1) | 166 (1.3) |
| Neurologic | |||
| Infection | 37 (0.6) | 15 (0.2) | 52 (0.4) |
| Other | 230 (3.6) | 293 (4.6) | 523 (4.1) |
| Stroke | 68 (1.1) | 72 (1.1) | 140 (1.1) |
| Obstetric and gynecologic | . . . | 1 (0.0) | 1 (0.0) |
| Other | 260 (4.1) | 377 (5.9) | 637 (5.0) |
| Postoperative | 35 (0.5) | 56 (0.9) | 91 (0.7) |
| Renal | 168 (2.6) | 156 (2.4) | 324 (2.5) |
| Respiratory | |||
| COPD | 188 (3.0) | 136 (2.1) | 324 (2.5) |
| Failure | 197 (3.1) | 185 (2.9) | 382 (3.0) |
| Other | 154 (2.4) | 189 (3.0) | 343 (2.7) |
| Pneumonia | 731 (11.5) | 900 (14.1) | 1,631 (12.8) |
| Rheumatologic | 1 (0.0) | . . . | 1 (0.0) |
| Sepsis | 3,306 (51.9) | 2,860 (44.9) | 6,166 (48.4) |
| Trauma | 40 (0.6) | 22 (0.3) | 62 (0.5) |
| Comorbidities | |||
| APACHE score | 70.2 ± 25.6 | 70.4 ± 26.3 | 70.3 ± 26.0 |
| APACHE comorbidities | |||
| AIDS | 15 (0.2) | 40 (0.6) | 55 (0.4) |
| Hepatic failure | 91 (1.4) | 94 (1.5) | 185 (1.5) |
| Lymphoma | 100 (1.6) | 57 (0.9) | 157 (1.2) |
| Metastatic cancer | 294 (4.6) | 272 (4.3) | 566 (4.4) |
| Leukemia | 185 (2.9) | 79 (1.2) | 264 (2.1) |
| Cirrhosis | 129 (2.0) | 95 (1.5) | 224 (1.8) |
| Surgical admission | 30 (0.5) | 54 (0.8) | 84 (0.7) |
| Immunocompromised | 605 (9.5) | 604 (9.5) | 1209 (9.5) |
| Nephrotoxic exposure | 4,244 (66.6) | 4,217 (66.2) | 8,461 (66.4) |
| Nephrotoxic agent | |||
| Aminoglycosides | 281 (6.6) | 251 (6.0) | 532 (6.3) |
| Amphotericin B | 6 (0.1) | 1 (0.0) | 7 (0.1) |
| Antivirals | 248 (5.8) | 139 (3.3) | 387 (4.6) |
| Calcineurin inhibitors | 32 (0.8) | 29 (0.7) | 61 (0.7) |
| Contrast | 38 (0.9) | 58 (1.4) | 96 (1.1) |
| Loop diuretics | 1,072 (25.3) | 1,003 (23.8) | 2,075 (24.5) |
| NSAIDs | 1,613 (38.0) | 1,544 (36.6) | 3,157 (37.3) |
| Vasopressors | 2,500 (58.9) | 2,525 (59.9 | 5,025 (59.4) |
| Hospital information | |||
| Region | |||
| Midwest | 2,302 (37.5) | 2,455 (41.7) | 4,757 (39.6) |
| Northeast | 1,360 (22.2) | 930 (15.8) | 2,290 (19.1) |
| South | 1,596 (26.0) | 1,690 (28.7) | 3,286 (27.3) |
| West | 874 (14.3) | 813 (13.8) | 1,687 (14.0) |
| Hospital teaching status | 2,172 (35.3) | 1,966 (32.9) | 4,138 (34.1) |
| No. of beds | |||
| < 100 | 161 (2.7) | 197 (3.4) | 358 (3.0) |
| 100-249 | 1,205 (20.1) | 1,616 (27.8) | 2,821 (23.9) |
| 250-500 | 1,450 (24.2) | 1,265 (21.8) | 2,715 (23.0) |
| > 500 | 3,170 (53.0) | 2,737 (47.1) | 5,907 (50.1) |
| Laboratory data | |||
| eGFR, mL/min/1.73 m2 | 66.4 ± 34.2 | 67.1 ± 33.8 | 66.7 ± 34.0 |
| Baseline SCr, mg/dL | 1.4 ± 1.2 | 1.4 ± 1.1 | 1.4 ± 1.2 |
| Baseline BUN, mg/dL | 31.6 ± 24.0 | 30.5 ± 23.3 | 31.1 ± 23.6 |
Data are presented as No. (%) or mean ± SD. APACHE = Acute Physiology and Chronic Health Evaluation; eGFR = estimated glomerular filtration rate; NSAID = nonsteroidal antiinflammatory drug; SCr = serum creatinine; VC = vancomycin and cefepime; VPT = vancomycin and piperacillin-tazobactam.
Table 2.
Baseline Characteristics and Demographic Features After Propensity Score Matching: VM Compared With VPT
| Variable | VM (n = 1,824) | VPT (n = 1,824) | Overall (N = 3,648) |
|---|---|---|---|
| Demographics | |||
| Age, y | 64.3 ± 16.2 | 64.1 ± 17.1 | 64.2 ± 16.6 |
| Sex | |||
| Female | 925 (50.7) | 860 (47.1) | 1,785 (48.9) |
| Male | 898 (49.2) | 964 (52.9) | 1,862 (51.0) |
| Ethnicity | |||
| Black | 263 (14.6) | 204 (11.2) | 467 (12.9) |
| Asian | 33 (1.8) | 28 (1.5) | 61 (1.7) |
| White | 1,313 (72.9) | 1,388 (76.4) | 2,701 (74.7) |
| Hispanic | 71 (3.9) | 110 (6.1) | 181 (5.0) |
| Native American | 5 (0.3) | 14 (0.8) | 19 (0.5) |
| Other/unknown | 116 (6.4) | 72 (4.0) | 188 (5.2) |
| BMI group | |||
| Underweight | 98 (5.4) | 106 (5.8) | 204 (5.6) |
| Normal | 580 (31.8) | 580 (31.8) | 1,160 (31.8) |
| Overweight | 479 (26.3) | 464 (25.4) | 943 (25.8) |
| Obese | 667 (36.6) | 674 (37.0) | 1,341 (36.8) |
| ICU admission diagnoses | |||
| Cancer | 12 (0.7) | 13 (0.7) | 25 (0.7) |
| Cardiac | |||
| Hypertension | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Other | 2 (0.1) | 4 (0.2) | 6 (0.2) |
| Arrhythmia | 30 (1.6) | 28 (1.5) | 58 (1.6) |
| Chest pain | 5 (0.3) | 5 (0.3) | 10 (0.3) |
| Heart failure | 36 (2.0) | 39 (2.1) | 75 (2.1) |
| Myocardial infarction | 79 (4.3) | 67 (3.7) | 146 (4.0) |
| Drug | |||
| Other | |||
| Overdose | 7 (0.4) | 31 (1.7) | 38 (1.0) |
| Withdrawal | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Endocrine | 25 (1.4) | 34 (1.9) | 59 (1.6) |
| GI | |||
| Bleed | 21 (1.2) | 37 (2.0) | 58 (1.6) |
| Liver failure | 8 (0.4) | 7 (0.4) | 15 (0.4) |
| Other | 24 (1.3) | 35 (1.9) | 59 (1.6) |
| Hematologic | 19 (1.0) | 12 (0.7) | 31 (0.8) |
| Neurologic | |||
| Infection | 29 (1.6) | 2 (0.1) | 31 (0.8) |
| Other | 81 (4.4) | 72 (3.9) | 153 (4.2) |
| Stroke | 18 (1.0) | 23 (1.3) | 41 (1.1) |
| Obstetric and gynecologic | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Other | 107 (5.9) | 127 (7.0) | 234 (6.4) |
| Postoperative | 13 (0.7) | 11 (0.6) | 24 (0.7) |
| Renal | 59 (3.2) | 53 (2.9) | 112 (3.1) |
| Respiratory | |||
| COPD | 27 (1.5) | 37 (2.0) | 64 (1.8) |
| Failure | 44 (2.4) | 46 (2.5) | 90 (2.5) |
| Other | 44 (2.4) | 45 (2.5) | 89 (2.4) |
| Pneumonia | 181 (9.9) | 253 (13.9) | 434 (11.9) |
| Rheumatologic | . . . | 1 (0.1) | 1 (0.0) |
| Sepsis | 941 (51.6) | 824 (45.2) | 1,765 (48.4) |
| Trauma | 8 (0.4) | 15 (0.8) | 23 (0.6) |
| Comorbidities | |||
| APACHE score | 71.2 ± 26.2 | 70.1 ± 27.1 | 70.7 ± 26.7 |
| APACHE comorbidities | |||
| AIDS | 8 (0.4) | 9 (0.5) | 17 (0.5) |
| Hepatic failure | 29 (1.6) | 24 (1.3) | 53 (1.5) |
| Lymphoma | 20 (1.1) | 14 (0.8) | 34 (0.9) |
| Metastatic cancer | 59 (3.2) | 55 (3.0) | 114 (3.1) |
| Leukemia | 32 (1.8) | 17 (0.9) | 49 (1.3) |
| Cirrhosis | 28 (1.5) | 35 (1.9) | 63 (1.7) |
| Surgical admission | 12 (0.7) | 10 (0.5) | 22 (0.6) |
| Immunocompromised | 123 (6.7) | 112 (6.1) | 235 (6.4) |
| Nephrotoxic exposure | 1,234 (67.7) | 1,248 (68.4) | 2,482 (68.0) |
| Nephrotoxic agent | |||
| Aminoglycosides | 66 (5.3) | 79 (6.3) | 145 (5.8) |
| Amphotericin B | 3 (0.2) | 3 (0.2) | 6 (0.2) |
| Antivirals | 94 (7.6) | 33 (2.6) | 127 (5.1) |
| Calcineurin inhibitors | 8 (0.6) | 7 (0.6) | 15 (0.6) |
| Contrast | 15 (1.2) | 21 (1.7) | 36 (1.5) |
| Loop diuretics | 253 (20.5) | 274 (22.0) | 527 (21.2) |
| NSAIDs | 410 (33.2) | 447 (35.8) | 857 (34.5) |
| Vasopressors | 825 (66.9) | 784 (62.8 | 1,609 (64.8) |
| Hospital information | |||
| Region | |||
| Midwest | 637 (37.0) | 710 (42.5) | 1,347 (39.7) |
| Northeast | 97 (5.6) | 266 (15.9) | 363 (10.7) |
| South | 660 (38.3) | 467 (28.0) | 1,127 (33.2) |
| West | 328 (19.0) | 226 (13.5) | 554 (16.3) |
| Hospital teaching status | 556 (32.0) | 602 (35.7) | 1,158 (33.8) |
| No. of beds | |||
| < 100 | 78 (4.6) | 41 (2.5) | 119 (3.6) |
| 100-249 | 593 (35.2) | 439 (26.8) | 1,032 (31.1) |
| 250-500 | 339 (20.1) | 346 (21.1) | 685 (20.6) |
| > 500 | 673 (40.0) | 811 (49.5) | 1,484 (44.7) |
| Laboratory data | |||
| eGFR, mL/min/1.73 m2 | 65.0 ± 34.6 | 66.1 ± 33.9 | 65.6 ± 34.3 |
| Baseline SCr, mg/dL | 1.5 ± 1.2 | 1.5 ± 1.1 | 1.5 ± 1.2 |
| Baseline BUN, mg/dL | 32.1 ± 24.2 | 30.9 ± 24.0 | 31.5 ± 24.1 |
Data are presented as No. (%) or mean ± SD. APACHE = Acute Physiology and Chronic Health Evaluation; eGFR = estimated glomerular filtration rate; NSAID = nonsteroidal anti-inflammatory drug; SCr = serum creatinine; VM = vancomycin and meropenem; VPT = vancomycin and piperacillin-tazobactam.
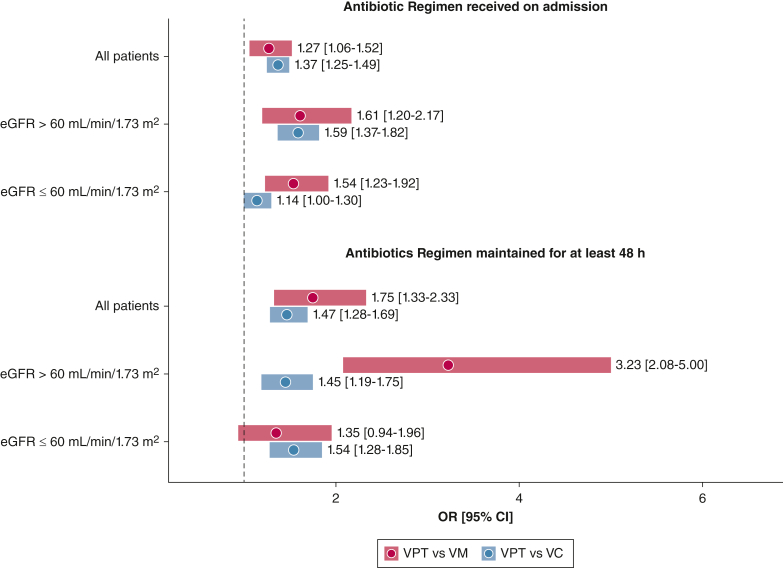
The odds of AKI for patients receiving VPT were statistically significantly higher than those of patients receiving VC or VM on admission (Fig 2). A patient administered VPT in the cohort showed 1.37 times greater odds of AKI compared with a similar patient administered VC and showed a 1.27 times greater odds compared with a similar patient administered VM (VPT vs VC: 95% CI, 1.25-1.49; VPT vs VM: 95% CI, 1.06-1.52). For patients with normal initial renal function, this effect was even more pronounced (VPT vs VC: OR, 1.59 [95% CI, 1.37-1.82]; VPT vs VM: OR, 1.61 [95% CI, 1.20-2.17]). When focusing specifically on the subset of patients who received a longer duration of antibiotic therapy (≥ 48 h), patients receiving VPT showed higher odds of AKI developing across all analyses except longer duration of VM vs VPT, for which no difference was found in patients with abnormal initial renal function. The association of VPT with AKI was greatest for patients with normal initial kidney function receiving continued empiric antibiotic treatment when compared with that of patients receiving prolonged VM treatment (VPT vs VM: OR, 3.23 [95% CI, 2.08-5.00]). A more stringent sensitivity analysis with AKI defined as all three KDIGO stages is described in e-Appendix 3. The corresponding results are shown in e-Figures 1 and 2.
Figure 2.
Forest plot showing ORs and 95% CIs for stage 2 and 3 acute kidney injury across all control (VC, VM) vs exposure (VPT) comparison groups after propensity score matching. Each comparison contains a balanced number of exposure patients and control participants. eGFR = estimated glomerular filtration rate; VC = vancomycin and cefepime; VM = vancomycin and meropenem; VPT = vancomycin and piperacillin-tazobactam.
Tables 3 and 4 display the secondary clinical outcomes. When compared with VC, VPT was associated with greater odds of dialysis initiation (OR, 1.28; 95% CI, 1.14-1.45) and dialysis or in-hospital mortality (OR, 1.14; 95% CI, 1.04-1.23). Similarly, when compared with VM, VPT was associated with greater odds of dialysis initiation (OR, 1.56; 95% CI, 1.23-2.00) and dialysis or in-hospital mortality (OR, 1.28; 95% CI, 1.10-1.52). No significant difference in mortality was found between the VPT group and the VC and VM groups.
Table 3.
Clinical Outcomes: VC Compared With VPT
| Variable | VC (n = 6,371) | VPT (n = 6,371) | OR |
|---|---|---|---|
| KDIGO AKI stage | |||
| 1 | 423 (6.6) | 455 (7.1) | . . . |
| 2 | 206 (3.2) | 322 (5.1) | . . . |
| 3 | 762 (12.0) | 935 (14.7) | . . . |
| 2/3 | 968 (15.2) | 1,257 (19.7) | 1.37 (1.25-1.49) |
| AKI requiring dialysis | 503 (7.9) | 633 (9.9) | 1.28 (1.14-1.45) |
| Dialysis or in-hospital mortality | 1,254 (19.7) | 1,383 (21.7) | 1.14 (1.04-1.23) |
| In-hospital mortality | 894 (14.0) | 936 (14.7) | 1.05 (0.95-1.16) |
| Hospital length of stay, d | 10.9 ± 10.3 | 10.6 ± 7.9 | . . . |
| Hospital discharge location | |||
| Death | 894 (14.0) | 936 (14.7) | . . . |
| Home | 2,663 (41.8) | 2,779 (43.6) | . . . |
| Nursing home | 368 (5.8) | 346 (5.4) | . . . |
| Other | 145 (2.3) | 145 (2.3) | . . . |
| External | 472 (7.4) | 487 (7.6) | . . . |
| Hospital | 272 (4.3) | 307 (4.8) | . . . |
| Skilled nursing facility | 1,557 (24.4) | 1,371 (21.5) | . . . |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. AKI = acute kidney injury; KDIGO = Kidney Disease: Improving Global Outcomes; VC = vancomycin and cefepime; VPT = vancomycin and piperacillin-tazobactam.
Table 4.
Clinical Outcomes: VM Compared With VPT
| Variable | VM (n = 1,824) | VPT (n = 1,824) | OR |
|---|---|---|---|
| KDIGO AKI stage | |||
| 1 | 110 (6.0) | 152 (8.3) | . . . |
| 2 | 65 (3.6) | 79 (4.3) | . . . |
| 3 | 209 (11.5) | 255 (14.0) | . . . |
| 2/3 | 274 (15.0) | 334 (18.3) | 1.27 (1.06-1.52) |
| AKI requiring dialysis | 120 (6.6) | 180 (9.9) | 1.56 (1.23-2.00) |
| Dialysis or in-hospital mortality | 331 (18.1) | 403 (22.1) | 1.28 (1.10-1.52) |
| In-hospital mortality | 245 (13.4) | 270 (14.8) | 1.12 (0.93-1.35) |
| Hospital length of stay, d | 11.1 ± 9.2 | 11.1 ± 9.2 | . . . |
| Hospital discharge location | |||
| Death | 245 (13.4) | 270 (14.8) | . . . |
| Home | 779 (42.7) | 787 (43.1) | . . . |
| Nursing home | 88 (4.8) | 108 (5.9) | . . . |
| Other | 52 (2.9) | 40 (2.2) | . . . |
| External | 123 (6.7) | 137 (7.5) | . . . |
| Hospital | 112 (6.1) | 74 (4.1) | . . . |
| Skilled nursing facility | 425 (23.3) | 408 (22.4) | . . . |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. AKI = acute kidney injury; KDIGO = Kidney Disease: Improving Global Outcomes; VM = vancomycin and meropenem; VPT = vancomycin and piperacillin-tazobactam.
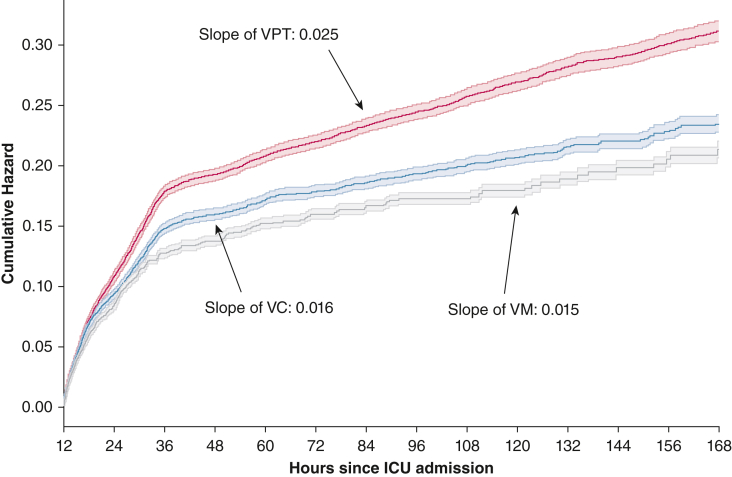
Figure 3 shows the cumulative hazard plot for AKI over time among the three antibiotic regimens. The difference in cumulative hazard becomes pronounced within the first 36 h, possibly suggestive of pseudonephrotoxic effects of VPT. Between hours 48 and 144, the slopes remain relatively constant. VPT showed the greatest slope (0.025) compared with VC (0.016) and VM (0.015), suggesting a greater risk of AKI over time.
Figure 3.
Cumulative hazard plot showing stage 2 and 3 acute kidney injury. The solid line represents the cumulative hazard, and the transparent boundaries represent 95% CIs. The slopes were calculated between hours 48 and 144. VC = vancomycin and cefepime; VM = vancomycin and meropenem; VPT = vancomycin and piperacillin-tazobactam.
Discussion
To our knowledge, this multicenter retrospective cohort study is the largest study to date comparing the risk of AKI for VPT against two other empiric antibiotic regimens, VM and VC, in the critically ill population. Our analyses found that for patients receiving antibiotics on admission to the ICU, VPT was associated with an increased risk of AKI compared with VC or VM. This effect was found to be stronger in patients with a longer duration of antibiotic treatment as well as for those with normal kidney function on admission. In addition, VPT was associated with greater odds of dialysis initiation and dialysis or in-hospital mortality compared with VC and VM.
Prior studies have examined the relative risk of nephrotoxicity among VPT, VC, and VM. Our study adds to the literature by focusing on a multicenter ICU population using a stringent definition of AKI and including subanalyses to study the effect of prolonged antibiotic treatment and baseline kidney function on AKI development. To our knowledge, this study is the largest study to date of critically ill patients (n = 35,654) and comprises data from across 158 distinct hospital units. Our results are consistent with those of Blevins et al17 that VPT poses an increased risk of AKI compared with VC or VM for the critically ill population.19
The decision to prescribe one antibiotic regimen over another always should be made considering patient-specific factors. The risk of AKI found to be associated with VPT will need to be weighed against the specifics of a particular patient’s history and clinical condition, as well as local resistance patterns, colonization with multidrug-resistant organisms, risk of neurotoxicity, risk of Clostridium difficile infection, allergy profiles, and more.
Some of the strengths of this study include the size of the dataset and its composition of critically ill patients from multiple hospitals, allowing for greater generalizability across diverse populations. Additionally, we performed propensity score matching to reduce confounding and to ensure comparability among different combination therapies. Because patient weight is a major consideration when determining drug dosage, BMI group was included to control for potential effects of weight on treatment decisions and outcomes. In addition, to account for comparable nephrotoxic risk, admission eGFR and eight common culprits of drug-induced nephrotoxic injury were considered in the matching as well. Finally, overall patient status was ensured to be comparable among groups by matching on age, immunocompromised status, and APACHE score, which is an indicator of disease severity. Thus, given the large sample size and bias reduction from the propensity score matching, we believe the three treatment groups are comparable for the purposes of our study.
Although we acknowledge that matching will not eliminate confounding by indication, we believe that the clinical indications for VPT, VM, and VC on ICU admission are similar. The detailed breakdown of ICU admission diagnoses are shown in Tables 1 and 2. We recognize that patients with sepsis and with greater disease severity have an increased risk of AKI. Although we match by APACHE score, the limitations of a retrospective analysis can never guarantee comparable patient severity between groups. However, we note that sepsis was more common in the matched VC and VM groups (51.9% and 51.6%) than in the VPT group (44.9% and 45.2%). Thus, it is possible that our results may underestimate the actual nephrotoxic risk of VPT.
Like all retrospective studies, our study has limitations. As with other large retrospective studies of AKI in the literature, we are limited by the inability to analyze accurate urine output.18 Because urine output is dependent on consistent catheter use and variations in urine collection and reporting, this makes urine output difficult to quantify accurately across all hospitals and an unreliable variable in our study; thus, our definition of AKI did not include the urine output of the KDIGO guidelines.29,30 Using sCR alone has limitations, including a delay after insult and variations with fluid, nutrition, and muscle mass.31 However, prior analysis of KDIGO criteria with and without the inclusion of urine output has shown that the inclusion of urine output criteria may double the diagnosis of AKI in critically ill patients.32 This suggests that by excluding urine output criteria in our definition of AKI, our study may underestimate the risk of AKI. Because the true risk may be higher, the findings in this large-scale, multisite study are still valuable despite this discrepancy. Future randomized controlled trials that accurately factor in urine output are still needed to provide the definitive word.
Studies have demonstrated the usefulness of kidney function and stress biomarkers, such as cystatin C, TIMP2, and IGBP7, in the determination of AKI, especially when considering the pseudonephrotoxic phenomenon of VPT.33, 34, 35 Piperacillin has been hypothesized to compete with the tubular secretion of creatinine, leading to a phenomenon referred to as pseudonephrotoxicity, in which the rise in SCr mimics renal damage, but is merely a result of reduced secretion.3 It is possible that the mild increase in SCr from competition with piperacillin may be sufficient to meet the KDIGO criteria for stage 1 AKI. Miano et al34 measured cystatin C, creatinine, and BUN values and found that VPT is associated with increased creatinine without associated changes in the other two biomarkers by day 2, suggesting that the bump in creatinine may not reflect true nephrotoxic injury. Pais et al36 previously showed histopathologic evidence supporting a lack of synergistic nephroxicity with the addition of piperacillin/tazobactam to vancomycin. In contrast, Kane-Gill et al35 reported that critically ill patients at risk of AKI showed higher levels of kidney stress biomarkers in those receiving VPT when compared with those receiving monotherapy, supporting a potential synergistic nephrotoxic effect of VPT.
To help discriminate between true nephrotoxicity and pseudonephrotoxicity, we used a stringent definition of AKI defined as KDIGO stage 2 or 3 AKI. Unfortunately, cystatin C was not available in the eICU database, which was built from records dating between 2010 and 2015. Future multicenter, large-scale studies with kidney functional and stress biomarkers in addition to kidney biopsy results are needed to distinguish better the nephrotoxic potential of VPT compared with VC and VM. In the absence of such objective data, we focused on clinical end points. Patients receiving VPT were associated with greater odds of dialysis initiation when compared with patients receiving VM and VC. Dialysis initiation is a decision that considers the broader clinical context, rather than creatinine thresholds alone, which suggests that a clinically significant difference exists among the groups.
Prior studies have analyzed and reported vancomycin dosage and trough levels, which have been shown to correlate with risk.17 Unfortunately, precise dose timing data was not available in the eICU Research Institute database, thus making inference of peak and trough levels impractical. The cohort included a broad range of ICUs across the country, and as such, information is limited regarding specific hospital-based dosing regimens and protocols for these agents. Although this limits our ability to understand how dosing changes might affect risk of AKI, it illustrates a useful representation of the effects of real-world practice patterns.
Although we have performed a subanalysis to evaluate the effect of longer duration of antibiotic therapy (≥ 48 h) on the development of AKI, we recognize that limitations exist related to defining antibiotic therapy duration retrospectively as well. We classified patients as receiving a longer duration of antibiotic therapy if patients received both an administration at ICU admission and another administration of the same antibiotic regimen of between 48 h and 1 week. Unfortunately, this generalizable definition does not validate that the patient receives a clinically therapeutic dosage or at therapeutic intervals. However, as above, although this limits our ability to understand how consecutive antibiotic therapy may affect AKI risk, it again is a useful representation of the effects of real-world practice patterns.
Our study uses a surrogate value for baseline SCr; this is a common limitation of studies that do not have preadmission data.37, 38, 39 Of the common surrogate options—imputing an eGFR value of 75 mL/min/1.73 m2, using SCr level on admission, and using minimum observed SCr level—the latter has been shown to have the second highest sensitivity (81.7%), the highest specificity (79.8%), and the fewest stage misclassifications.40 Although using minimum SCr level as the baseline creatinine may overestimate AKI incidence and staging, it is one of the most robust methods when preadmission data are not available. Additionally, a key interest of this study was identifying how kidney function changes from ICU admission rather than in comparison with a patient’s normal kidney health. Thus, our use of minimum value allows us to track how kidney function changes effectively from ICU admission.
Finally, our study focused on the subset of patients admitted to the ICU from the ED. Because patients frequently received antibiotics before ICU admission, it was imperative to have reliable data across all hospital sites on antibiotics received on admission. Inpatient data outside of the ICU and ED were not widely available across all hospital sites, so we focused on only the subset of patients admitted from the ED to maximize accuracy of the treatment groups. Although our study is the largest retrospective analysis of critically ill patients (n = 35,654) to our knowledge, many patients were excluded because criteria including non-ED admission (n = 1,833,830 [59.35%]), missing APACHE data (n = 1,570,462 [50.83%]), and exposure to multiple β-lactams (n = 45,381 [17.0%]), among others. These exclusions were necessary to limit confounders in our study, although at the expense of reducing the generalizability of this study. Further analyses should be carried out before generalizing the results of this study to patients who do not meet our exclusion criteria.
Interpretation
In conclusion, this large-scale multicenter cohort retrospective study of critically ill patients suggests that there is greater risk of AKI when using VPT over VC or VM, especially in patients with normal kidney function on admission requiring antibiotic treatment for longer than 48 h. When prescribing empiric antibiotic regimens to critically ill patients, clinicians should consider VM or VC over VPT to reduce risk of nephrotoxicity. Additional prospective research is necessary to evaluate this association.
Funding/Support
L. A. C. is funded by the National Institute of Health through NIBIB R01 EB017205.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: All authors contributed to the study conception and design, data interpretation, and manuscript revision. Data extraction and analysis were performed by A. Y. C., A. E. W. J., C.-Y. D., and M. A. A. d. l. H. A. D. and A. Y. C. contributed to the writing of the manuscript.
Availability of data and material: A subset of the full eICU dataset with > 200,000 patients admitted from 2014 through 2015 is publicly available as the eICU Collaborative Research Database.23 All code for data extraction and analysis associated with the current submission is available at https://doi.org/10.5281/zenodo.3956338. Any updates will also be published on Zenodo.41
Other contributions: This manuscript was a culmination of the work completed in the Harvard/MIT HST.953 course, Collaborative Data Science in Medicine. The authors thank the HST.953 faculty for their support, guidance, and encouragement throughout the course of this project and Dr Eric Gottlieb for his feedback and advice on our analysis.
Additional information: The e-Appendixes, e-Figures, and e-Table are available online under “Supplementary Data.”
Supplementary Data
References
- 1.MacArthur R.D., Miller M., Albertson T., et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis. 2004;38(2):284–288. doi: 10.1086/379825. [DOI] [PubMed] [Google Scholar]
- 2.Rutter W.C., Burgess D.S. Incidence of acute kidney injury among patients treated with piperacillin-tazobactam or meropenem in combination with vancomycin. Antimicrob Agents Chemother. 2018;62(7):e00264–18. doi: 10.1128/AAC.00264-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeon N., Staley B., Klinker K.P., Hincapie Castillo J., Winterstein A.G. Acute kidney injury risk associated with piperacillin/tazobactam compared with cefepime during vancomycin therapy in hospitalised patients: a cohort study stratified by baseline kidney function. Int J Antimicrob Agents. 2017;50(1):63–67. doi: 10.1016/j.ijantimicag.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Chen X.Y., Xu R.X., Zhou X., Liu Y., Hu C.Y., Xie X.F. Acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam administration: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50(11):2019–2026. doi: 10.1007/s11255-018-1870-5. [DOI] [PubMed] [Google Scholar]
- 5.Rutter W.C., Cox J.N., Martin C.A., Burgess D.R., Burgess D.S. Nephrotoxicity during vancomycin therapy in combination with piperacillin-tazobactam or cefepime. Antimicrob Agents Chemother. 2017;61(2):e02089–16. doi: 10.1128/AAC.02089-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balci C., Uzun O., Arici M., Hayran S.A., Yuce D., Unal S. Nephrotoxicity of piperacillin/tazobactam combined with vancomycin: should it be a concern? Int J Antimicrob Agents. 2018;52(2):180–184. doi: 10.1016/j.ijantimicag.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Mullins B.P., Kramer C.J., Bartel B.J., Catlin J.S., Gilder R.E. Comparison of the nephrotoxicity of vancomycin in combination with cefepime, meropenem, or piperacillin/tazobactam: a prospective, multicenter study. Ann Pharmacother. 2018;52(7):639–644. doi: 10.1177/1060028018757497. [DOI] [PubMed] [Google Scholar]
- 8.Navalkele B., Pogue J.M., Karino S., et al. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis. 2017;64(2):116–123. doi: 10.1093/cid/ciw709. [DOI] [PubMed] [Google Scholar]
- 9.Kang S., Park J., Yu Y.M., Park M.S., Han E., Chang M.J. Comparison of acute kidney injury and clinical prognosis of vancomycin monotherapy and combination therapy with beta-lactams in the intensive care unit. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson A.D., Li C., Hammond D.A., Dickey T.A. Incidence of acute kidney injury among patients receiving the combination of vancomycin with piperacillin-tazobactam or meropenem. Pharmacotherapy. 2018;38(12):1184–1193. doi: 10.1002/phar.2179. [DOI] [PubMed] [Google Scholar]
- 11.Hammond D.A., Smith M.N., Li C., Hayes S.M., Lusardi K., Bookstaver P.B. Systematic review and meta-analysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clin Infect Dis. 2017;64(5):666–674. doi: 10.1093/cid/ciw811. [DOI] [PubMed] [Google Scholar]
- 12.Giuliano C.A., Patel C.R., Kale-Pradhan P.B. Is the combination of piperacillin-tazobactam and vancomycin associated with development of acute kidney injury? A meta-analysis. Pharmacotherapy. 2016;36(12):1217–1228. doi: 10.1002/phar.1851. [DOI] [PubMed] [Google Scholar]
- 13.Buckley M.S., Hartsock N.C., Berry A.J., et al. Comparison of acute kidney injury risk associated with vancomycin and concomitant piperacillin/tazobactam or cefepime in the intensive care unit. J Crit Care. 2018;48:32–38. doi: 10.1016/j.jcrc.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Hammond D.A., Smith M.N., Painter J.T., Meena N.K., Lusardi K. Comparative incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam or cefepime: a retrospective cohort study. Pharmacotherapy. 2016;36(5):463–471. doi: 10.1002/phar.1738. [DOI] [PubMed] [Google Scholar]
- 15.Inage S., Nakamura S., Isoe Y., et al. Acute kidney injury in non-intensive care and intensive care patients treated with vancomycin and piperacillin-tazobactam. J Nippon Med Sch. 2020;87(2):66–72. doi: 10.1272/jnms.JNMS.2020_87-203. [DOI] [PubMed] [Google Scholar]
- 16.Arnaud F.C.S., Liborio A.B. Attributable nephrotoxicity of vancomycin in critically ill patients: a marginal structural model study. J Antimicrob Chemother. 2020;75(4):1031–1037. doi: 10.1093/jac/dkz520. [DOI] [PubMed] [Google Scholar]
- 17.Blevins A.M., Lashinsky J.N., McCammon C., Kollef M., Micek S., Juang P. Incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam, cefepime, or meropenem. Antimicrob Agents Chemother. 2019;63(5):e02658–18. doi: 10.1128/AAC.02658-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreier D.J., Kashani K.B., Sakhuja A., et al. Incidence of acute kidney injury among critically ill patients with brief empiric use of antipseudomonal beta-lactams with vancomycin. Clin Infect Dis. 2019;68(9):1456–1462. doi: 10.1093/cid/ciy724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luther M.K., Timbrook T.T., Caffrey A.R., Dosa D., Lodise T.P., LaPlante K.L. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med. 2018;46(1):12–20. doi: 10.1097/CCM.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.D., Heintz B.H., Mosher H.J., Livorsi D.J., Egge J.A., Lund B.C. Risk of acute kidney injury and Clostridioides difficile infection with piperacillin/tazobactam, cefepime, and meropenem with or without vancomycin. Clin Infect Dis. 2021;73(7):e1579–e1586. doi: 10.1093/cid/ciaa1902. [DOI] [PubMed] [Google Scholar]
- 21.Côté J.M., Desjardins M., Cailhier J.F., Murray P.T., Beaubien Souligny W. Risk of acute kidney injury associated with anti-pseudomonal and anti-MRSA antibiotic strategies in critically ill patients. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0264281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McShea M., Holl R., Badawi O., Riker R.R., Silfen E. The eICU research institute—a collaboration between industry, health-care providers, and academia. IEEE Eng Med Biol Mag. 2010;29(2):18–25. doi: 10.1109/MEMB.2009.935720. [DOI] [PubMed] [Google Scholar]
- 23.Pollard T.J., Johnson A.E.W., Raffa J.D., Celi L.A., Mark R.G., Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018;5 doi: 10.1038/sdata.2018.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 25.Vasina L., Dehner M., Wong A., et al. The impact of a pharmacist driven 48-hour antibiotic time out during multidisciplinary rounds on antibiotic utilization in a community non-teaching hospital. Open Forum Infect Dis. 2017;4(suppl 1):S272–S273. [Google Scholar]
- 26.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 27.Austin P.C., Cafri G. Variance estimation when using propensity-score matching with replacement with survival or time-to-event outcomes. Stat Med. 2020;39(11):1623–1640. doi: 10.1002/sim.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen A, Johnson A, Deng C-Y, de la Hoz MA. MIT-LCP/vancomycin-nephrotoxicity: vancomycin nephrotoxicity in eICU, Zonodo. Version 1.0. Updated July 22, 2020. https://zenodo.org/record/3956338
- 29.Macedo E. Urine output assessment as a clinical quality measure. Nephron. 2015;131(4):252–254. doi: 10.1159/000437312. [DOI] [PubMed] [Google Scholar]
- 30.Kramer G.C., Luxon E., Wolf J., Burnett D.R., Nanduri D., Friedman B.C. Inaccuracy of urine output measurements due to urinary retention in catheterized patients in the burn ICU. J Burn Care Res. 2017;38(1):e409–e417. doi: 10.1097/BCR.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiersema R., Jukarainen S., Eck R.J., et al. Different applications of the KDIGO criteria for AKI lead to different incidences in critically ill patients: a post hoc analysis from the prospective observational SICS-II study. Crit Care. 2020;24(1):164. doi: 10.1186/s13054-020-02886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koeze J., Keus F., Dieperink W., van der Horst I.C., Zijlstra J.G., van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017;18(1):70. doi: 10.1186/s12882-017-0487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Côté J.M., Kane-Gill S.L., Murray P.T. A ray of hope in the discord: is adding piperacillin-tazobactam to vancomycin truly more nephrotoxic? Intensive Care Med. 2022;48(9):1208–1210. doi: 10.1007/s00134-022-06861-4. [DOI] [PubMed] [Google Scholar]
- 34.Miano T.A., Hennessy S., Yang W., et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144–1155. doi: 10.1007/s00134-022-06811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane-Gill S.L., Ostermann M., Shi J., Joyce E.L., Kellum J.A. Evaluating renal stress using pharmacokinetic urinary biomarker data in critically ill patients receiving vancomycin and/or piperacillin-tazobactam: a secondary analysis of the Multicenter Sapphire Study. Drug Saf. 2019;42(10):1149–1155. doi: 10.1007/s40264-019-00846-x. [DOI] [PubMed] [Google Scholar]
- 36.Pais G.M., Liu J., Avedissian S.N., et al. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother. 2020;75(5):1228–1236. doi: 10.1093/jac/dkz563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 38.Hsu C.-Y., McCulloch C.E., Fan D., Ordoñez J.D., Chertow G.M., Go A.S. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nash K., Hafeez A., Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 40.Siew E.D., Matheny M.E., Ikizler T.A., et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77(6):536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.