Abstract
The diagnosis, prognostication, and differentiation of phenotypes of COPD can be facilitated by CT scan imaging of the chest. CT scan imaging of the chest is a prerequisite for lung volume reduction surgery and lung transplantation. Quantitative analysis can be used to evaluate extent of disease progression. Evolving imaging techniques include micro-CT scan, ultra-high-resolution and photon-counting CT scan imaging, and MRI. Potential advantages of these newer techniques include improved resolution, prediction of reversibility, and obviation of radiation exposure. This article discusses important emerging techniques in imaging patients with COPD. The clinical usefulness of these emerging techniques as they stand today are tabulated for the benefit of the practicing pulmonologist.
Key Words: COPD, chest CT scan, CT scan of chest, imaging, quantitative analysis, biomarkers
COPD is an important cause of morbidity, mortality, and disability globally.1 In part 1 of this series,2 the authors discuss the clinical usefulness of imaging in COPD, focusing on imaging techniques, CT scan-definable subtypes, and clinical evaluation and management of patients with this diagnosis. In this article (part 2), the authors discuss novel advanced imaging techniques and emerging concepts in the imaging of patients with COPD to increase awareness among practicing pulmonologists. The role of imaging in various clinical scenarios is considered, with a focus on quantitative assessment. In particular, we describe the role of imaging in the preprocedural evaluation of patients undergoing lung volume reduction and also describe the usefulness of imaging in patients with coexistent central airways abnormalities and COPD.
The evolving imaging techniques described in this article include both advanced imaging methods (eg, micro-CT scan, ultra-high-resolution and photon-counting CT scan imaging, MRI, radionuclide scintigraphy, and PET) as well as advanced analysis tools (eg, parametric response mapping and machine learning). It also should be emphasized that newer imaging methods are being developed and trialed in academic radiology centers, siloed from clinicians who in many instances are unaware of their potential usefulness in clinical practice. Because many of these techniques are not developed fully, detailed descriptions have not been included.
The aim of this article is to provide an update on the potential of modern imaging for diagnosis and management of patients with COPD beyond the traditionally applied spirometry. For example, patients with mild COPD may have normal spirometry findings, yet have significant emphysema on CT scans. Patients with mild emphysema on spirometry may have distinct abnormalities on chest CT scans such as mucus plugging, combined pulmonary fibrosis and emphysema, and clues to pulmonary hypertension. These additional findings may explain why such patients experience frequent exacerbations or are symptomatic beyond the extent of obstruction on spirometry. A recent article by Stolz et al3 indicated that an average of approximately 85 million thoracic CT scans are obtained annually in the United States alone. The authors advocate that these images should be reviewed for COPD, including early abnormalities. They propose that CT scan-detected emphysema, air trapping, and airway remodeling should be considered diagnostic of COPD, even in the absence of airways obstruction on spirometry. Furthermore, the authors of this article recommend that when spirometry is challenging to obtain, CT scan imaging of the chest should be used to diagnose COPD. If these recommendations become widely accepted, lung imaging will have a greater importance in the early detection of COPD and prevention of disease progression.
Methods
Evidence Used in This Review
We conducted a targeted search of PubMed/MEDLINE, Web of Science, and Google Scholar from January 1, 2000, through March 30, 2022, for randomized controlled trials, observational studies, scoping reviews, systematic reviews, meta-analyses, and clinical practice guidelines. We searched for the terms chronic obstructive pulmonary disease, COPD, and imaging. We further enhanced our search by evaluating the reference lists of selected articles and supplemented our search with literature from our own collections. A detailed description of the methodology used for searching articles is included in e-Figure 1.
Imaging in the Evaluation of Interventional and Surgical Techniques in COPD
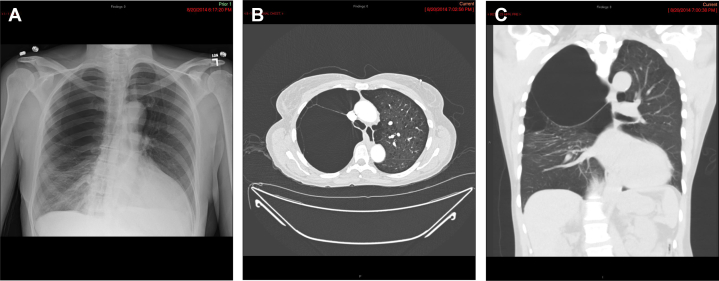
Giant bullae may be present with normal surrounding lung parenchyma or with associated generalized emphysema (Fig 1). A recent study of 41 patients confirmed that surgical outcomes are better in patients with giant bullae with normal surrounding lung parenchyma and that the benefits are maintained over at least 5 years.4 Although bullectomy has been associated with improved outcomes, identifying optimal candidates is sometimes challenging. Parametric response mapping, discussed herein, has identified less functional areas of the lung that potentially may be targeted for bullectomy or bronchoscopic lung volume reduction (BLVR).5
Figure 1.
A-C, Chest radiograph (A), axial CT scan image (B), and coronal CT image (C) in a man with bullous emphysema in the right upper lobe. Chest radiograph shows hyperlucency in the right upper and mid lung zone with compressive atelectasis of the right lower lung zone.
Lung volume reduction surgery is an established method in the management of advanced emphysema. BLVR is an emerging technique that also can improve quality of life, can postpone the need for lung transplantation, and possibly can improve survival in select patients with emphysema who already are optimized with medical management.6, 7, 8, 9 BLVR is being carried out using a variety of devices through a flexible bronchoscope. BLVR also has been found to be effective in patients with lower lobe disease as well as those with homogenous emphysema.
Proper selection of patients is of utmost importance for successful outcomes from the procedure. This is achieved using a combined assessment of symptoms, pulmonary function, and chest imaging. High-resolution CT scan imaging remains the mainstay in initial evaluation of these patients to assess both for potential target lobes and any concurrent disease that may affect candidacy. A thin-slice (1 mm) noncontrast high-resolution CT scan is performed and reconstructed for both visual assessment and quantitative analysis.
Preliminary visual assessment is performed using the combination of axial, coronal, and sagittal views. This can help to evaluate the extent of emphysema and to evaluate the lobe with the maximum emphysema as well as to assess fissure integrity (Fig 2). Although a role exists for visual assessment in terms of screening, its limitations include a wide range of interobserver variability, lack of sensitivity to early disease, and the time-consuming nature of the process.6
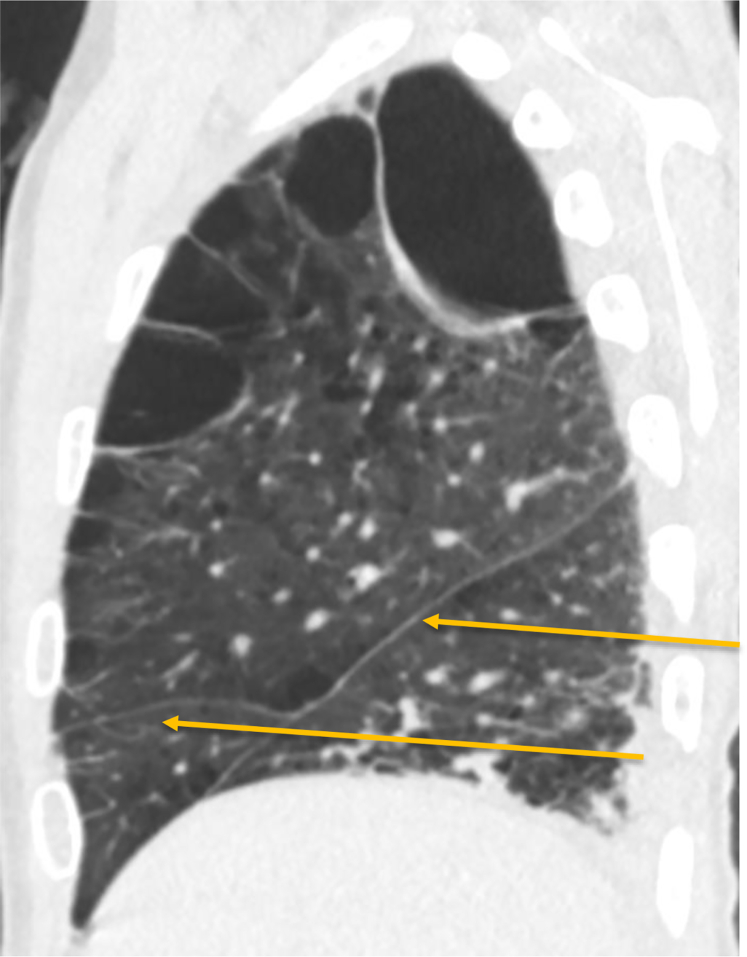
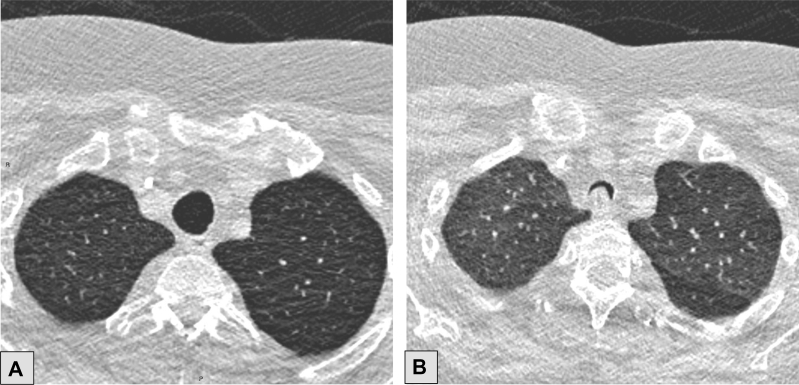
Figure 2.
Sagittal CT scan showing well-visualized complete right-sided fissures (arrow).
Thus, given the limitations, definitive evaluation is performed by means of quantitative analysis CT (QCT) scan imaging using CT scan densitometry (discussed herein). This is used routinely for BLVR and recently was suggested for patients being evaluated for lung volume reduction surgery.7, 8, 9, 10 This provides information about the distribution of disease (homogenous vs heterogenous), degree of emphysema per lobe as well as lobar volumes, and fissure integrity.
In patients undergoing BLVR, a certain degree of emphysematous destruction is essential to occlude the lobe safely without worsening mismatch owing to resultant atelectasis. The goal would be to impair air flow only to portions of the lung that have severe emphysema and not to healthy lung, which would create a worse mismatch than already exists. In addition, lobar volumes also are important in evaluating target lobes, especially the ipsilateral nontarget lobe to ensure that it is capable of occupying the entire hemithorax after treatment. This can be achieved by means of QCT imaging (Fig 3). Additionally, fissure integrity using QCT imaging can serve as a surrogate for the presence or absence of collateral ventilation, which is important for assessing candidacy for BLVR.11 Fissure integrity can be evaluated definitively using the Chartis (Pulmonx, Inc.) measurement,12 but that requires bronchoscopic intervention. The Chartis System is a device used to detect collateral ventilation between the lobes of the lung under consideration for endobronchial volume reduction.13 The device uses a balloon-tipped catheter that is advanced through the channel of a bronchoscope. Inflation of the balloon allows for temporary occlusion of the airway, during which airflow coming from the lobe is assessed for volume, pressure, and resistance and is visualized on the console. Absence of airflow confirms appropriate placement of endobronchial valves.
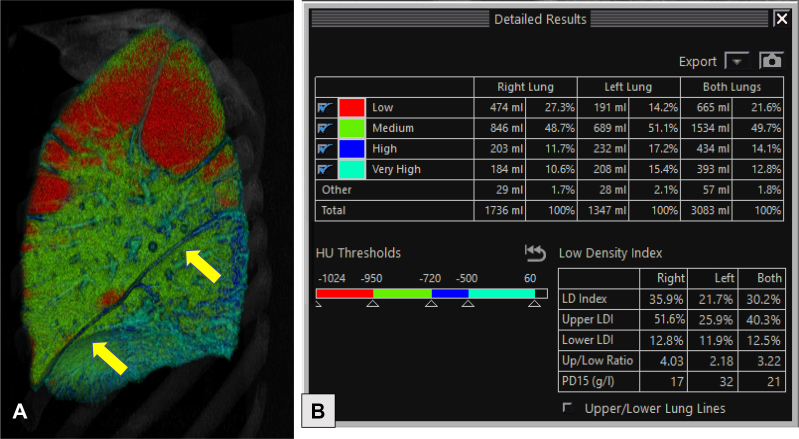
Figure 3.
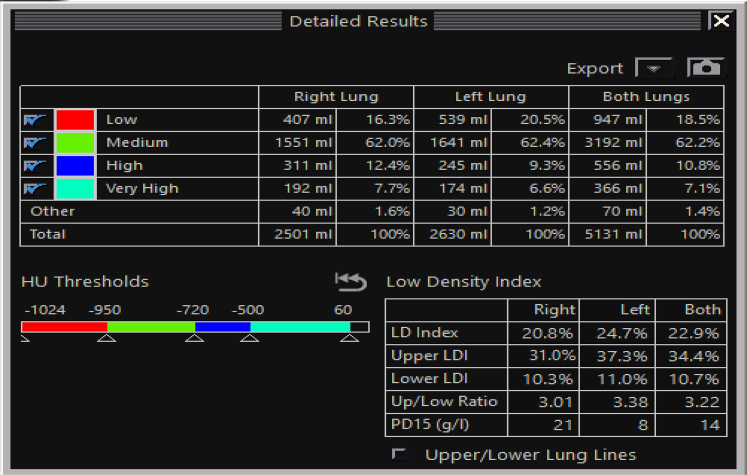
A, Sagittal multiplanar volume reformatted image of the right lung from quantitative CT scan imaging using lung density analysis program demonstrating heterogeneous, bullous emphysema, with the emphysematous portions of the lung shaded red. The integrity of the major fissure is demonstrated (arrows). B, Quantitative data for use in the evaluation of the suitability of the patient for bronchoscopic lung volume reduction. In this example, the LDI, or proportion of the lung with < –950 HU, is higher in the upper lung (upper LDI) than in the lower lung (lower LDI), which is a favorable finding when considering suitability for bronchoscopic lung volume reduction. HU = Hounsfield units; LDI = low-density index.
Recent studies demonstrated that patients with a fissure integrity of < 80% based on QCT imaging can be excluded from Chartis evaluation and BLVR.14, 15 In patients with > 80% complete fissures, Chartis measurements are recommended. Thus, in BLVR, the ideal patient is characterized by the lobar level of emphysema, emphysema heterogeneity, preserved fissure integrity, and preserved ipsilateral lobe volume. This can be achieved with qualitative and quantitative CT scan analysis.
Lung Transplant
More than 1,000 lung transplantations are performed each year for end-stage COPD, including in those with alpha-1 antitrypsin deficiency, worldwide.16 Either single or a bilateral lung transplantation is performed for the indication, leading to unique radiographic findings. In patients who have undergone a single lung transplantation, chest radiography usually reveals a mediastinal shift toward the side of the surgery resulting from hyperinflated native lung.17 Incidentally, the shift worsens after chronic rejection develops in the allograft. This shift often is misinterpreted as progression of emphysema in the native lung. In such cases, the temptation to perform any invasive procedure on the native side should be avoided.18 CT scan of the chest is performed in all patients being considered for lung transplantation, regardless of the indication. Besides defining parenchymal pathologic features, it also allows for study of the mediastinum as well as pleural anatomic features. The CT scan also is used to follow up any nodular abnormalities seen among the patients either with emphysema or interstitial lung disease, because diagnostic procedures are usually contraindicated in these group of patients. Incidentally, lung cancer is found in approximately 2.5% of all explanted lungs, with a slightly higher incidence among patients with interstitial lung disease.19
Imaging in the Evaluation of Central Airway Abnormalities Associated with COPD
In recent years, tracheobronchomalacia and excessive dynamic airways collapse (EDAC) have been recognized with higher frequency mainly because of the increased usefulness of CT scan imaging of the chest and the popularity of interventional pulmonology techniques. Because the standard definition of these conditions is still debated, the exact incidence remains to be determined. In a recent review in Radiographics, the severity of expiratory collapse was defined as mild if on exhalation the airway luminal narrowing was 70%-80%, moderate if 81%-90%, and severe if > 90%. The authors recommended that definitive treatment is typically reserved for patients demonstrating > 90% narrowing.20 EDAC is seen more frequently in patients with COPD and obesity, although it may occur without any known associations. CT scans can identify COPD-associated exaggerated collapse of the central airways during exhalation. This may be owing to three mechanisms.
-
1.
Tracheobronchomalacia refers to collapse of tracheobronchial tree resulting from loss of structural integrity of the cartilages. The involvement could be localized or diffuse and can involve pediatric as well as adult populations.
-
2.
EDAC is characterized by exaggerated (> 50%) dynamic bulging of the posterior membranous wall of the trachea and bronchi leading to luminal narrowing.21 The tracheal and bronchial cartilages are structurally intact. The mechanism is believed to be reduced tone of the myoelastic fibers of the posterior wall resulting from chronic inflammation or increased transpulmonic pressure.22
-
3.
Tracheobronchial smooth muscle atrophy and separation is characterized by separation of the posterior wall of the trachea or the main bronchi from the cartilaginous rings, adding to EDAC. This diagnosis is usually confirmed by a bronchoscopic examination.23
These conditions are suspected in patients with a seal-like barking cough, dyspnea on exertion, lack of response to inhaled bronchodilators and steroids, an inflection point on flow volume loop, and abnormal collapse of the trachea and main bronchi on dynamic CT scan or bronchoscopic examination (Figure 4, Figure 5, Figure 6).
Figure 4.
A-D, Axial CT scan images with lung windows obtained at end inspiration (A) and during dynamic expiration (B) and corresponding virtual bronchoscopic images (C and D), respectively. An abnormal lunate configuration of the trachea is present at end inspiration (A and C), with excessive airway collapse during dynamic expiration (B and D). These findings are consistent with tracheobronchomalacia.
Figure 5.
A, B, Axial CT scan images with lung windows obtained at end inhalation (A) and during dynamic exhalation (B). A, Normal-appearing trachea is seen on an end inhalation CT scan. B, In this patient, excessive dynamic airways collapse, as evidenced by an exaggerated bulging of the posterior membranous wall, can be identified only during exhalation (dynamic expiratory image).
Figure 6.
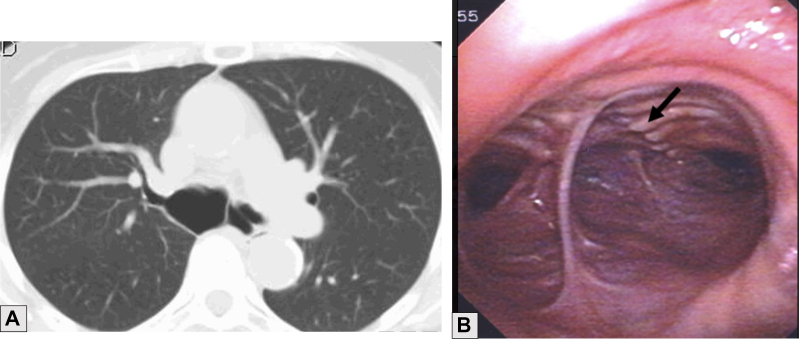
A, B, CT scan showing marked dilatation of the proximal right main bronchus (3 × 2.5 cm) (A) and endoscopic imaging showing separation of the cartilages from the posterior wall evident in the right main bronchus (arrow) (B) in tracheobronchial smooth muscle atrophy.
A full discussion of CT scan imaging techniques is covered in part 1 of this series.2 Recommended scanning protocol includes volumetric full chest scanning in the supine position at full inspiration and routine end-expiratory imaging. Dynamic expiratory imaging, with images obtained during forced expiration, is the most useful technique to assess dynamic narrowing of the airways.24 Virtual bronchoscopy is an optional technique using images generated from the CT scan data that allows a noninvasive intraluminal evaluation of the tracheobronchial tree, simulating what may be seen with fiberoptic bronchoscopy. Although it is a useful noninvasive tool in assessing EDAC, it is not considered the gold standard for making this diagnosis because of inaccuracies in assessing the degree of collapse.25 Treatment of this disorder is based on its severity and may range from pneumatic stenting with positive airway pressure to tracheopexy.
Imaging for Quantitative Assessment of COPD
Postprocessing approaches, such as densitometric analysis and airway quantification, are feasible after segmentation of the lung and airways from the surrounding chest wall and mediastinal structures. Fixed thresholds such as –910 Hounsfield units (HU), –950 HU, or –960 HU then are capable of delineating emphysema-like tissue. The percent or fraction of lung occupied by these low attenuating areas is highly correlated with explanted lung pathologic characteristics and is related directly to degrees of clinical impairment (Fig 7).26, 27, 28 A more recent histogram-based technique for disease quantification that delineates the lowest 15th percentile of the lung histogram (PD15) from the remaining lung tissue can be used to derive the grams of lung tissue per 1 L of lung volume.29 This approach is thought to provide a more sensitive metric of progression in early lung disease than threshold methods generating low attenuating areas and can be statistically adjusted more readily for differing reconstruction algorithms and scanner make and model (Fig 8).30
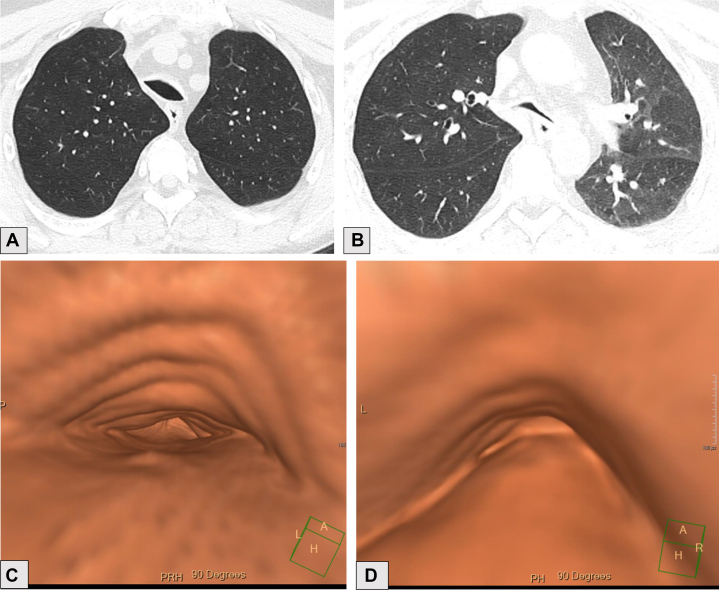
Figure 7.
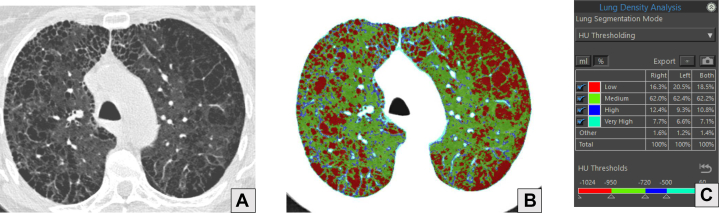
A-C, Axial CT scan image with lung windows (A), axial image from lung density analysis (B), and quantitative analysis of lung density (C) of a 60-year-old male with a history of COPD who uses tobacco. A, Advanced destructive emphysema and mild paraseptal emphysema. B, Red portions of the lungs correspond to the areas of lucency caused by emphysema on CT scan and represent pixels with density measurements of < –950 HU. C, Percentages of each lung with density measurements falling into the defined ranges. In this example, 16.3% of the right lung, 20.5% of the left lung, and 18.5% of both lungs have density measurements in the range of emphysema (red). HU = Hounsfield units.
Figure 8.
Quantitative data for the same patient as Figure 7, with additional data including PD15, as explained in text. HU = Hounsfield units; LD = low density; LDI = low density index; PD15 = lowest 15th percentile of lung histogram.
The quantitative density measurements both are repeatable and may be applied to evaluate disease progression. Importantly, volume-adjusted lung density provides individualized measurements. Efforts are ongoing to have this qualified as a biomarker with the US Food and Drug Administration as an acceptable outcome measure for therapeutic interventions.
Although lung density is used widely and is a well-accepted metric of disease progression in the respiratory and imaging community, it does have limitations. Differences in lung density resulting from the inspiratory level can be corrected by adjusting for lung volume.31 Differences in density resulting from the scanner reconstruction technique can be addressed by normalization techniques.32 For example, some subtypes of emphysema are based on morphologic appearance and distribution within the lung.33,34 Centrilobular, paraseptal, and panlobular emphysema may be equally quantified by densitometry, but they cannot be distinguished by either a simple HU threshold or volume-adjusted lung density. Recognition of this limitation spurred the development of more advanced machine learning and deep learning techniques that leverage local patterns of attenuation, combined with their distribution in the lung (cranial or caudal and central vs subpleural).35, 36, 37, 38 These techniques have been quite successful at providing more granular label maps of emphysema subtype as well as stage.
Quantitative assessment of central airway morphologic features has become a cornerstone in the CT scan-based characterization of COPD. Early investigations demonstrated that measurements of the wall area percent (the percent of total bronchial cross-sectional area occupied by airway wall) were related inversely to lung function and clinical outcomes.39 Subsequent work demonstrated that the clinical correlation of this feature could be enhanced when extracted from higher-generation airways, presumably because airways deeper in the lung provide a better reflection of disease in the distal small airways.40
Direct measurements of airways are challenging because in COPD, the primary area of interest is small airways, which cannot be well observed because of limited spatial resolution with current CT scan imaging technology. Endobronchial ultrasound provides direct airway assessment, but this is also limited to larger airways. Despite challenges in measuring small airways, measurements of larger airways such as airway-wall thickness and the internal perimeter of a hypothetical 10-mm airway have a number of clinically relevant correlations to spirometry and other measurements of lung function.41,42
Biomarker measurement of pulmonary arteries and veins also has been established in the research setting. The most well-established measurements are the pulmonary artery to aorta ratio (Fig 9) and the estimated blood volume contained in blood vessels with cross-sectional area of approximately 5 or 10 mm, which have been associated with multiple clinical measurements.43,44
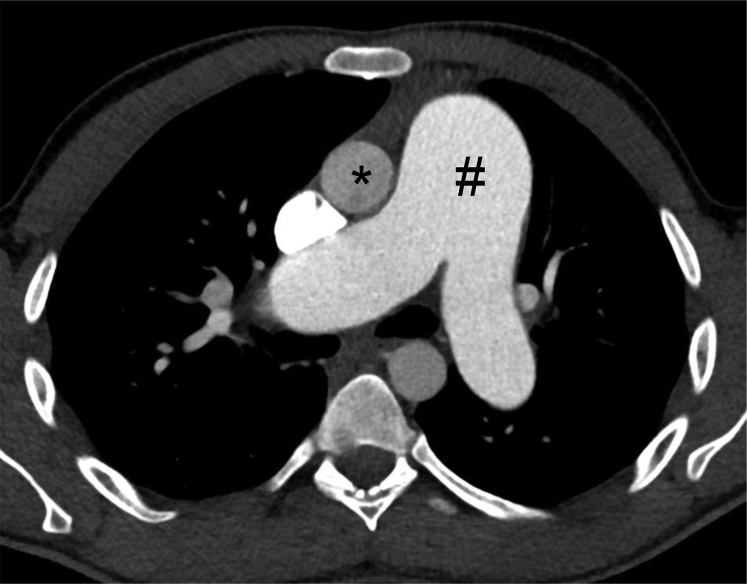
Figure 9.
Axial contrast-enhanced CT scan image obtained through the main pulmonary artery in a patient with pulmonary hypertension demonstrating a dilated main pulmonary artery (number symbol), larger in caliber than the adjacent ascending aorta (asterisk).
A number of other promising measurements and methods are in somewhat earlier stages of development. These include CT scan-based biomarkers of early fibrosis that have been shown to be detectable and associated with clinical measurements in patients with COPD, detailed measurements of pulmonary ventilation using inhaled xenon and dual-energy CT scan imaging, and various modifications of traditional MRI. Other, more complex, biomarkers such as texture-based measurements of local patterns of emphysema, gas trapping, and functional small airways disease are well established in the research setting.35,45 These new and evolving techniques are tabulated (Table 1, Table 2) to add context to their clinical usefulness and applications at present. Table 1 describes advanced imaging methods, such as micro-CT imaging, ultra-high-resolution and photon-counting CT scan imaging, MRI using hyperpolarized helium, and radionuclide scintigraphy including PET. Specific indications for each of these methods that are being studied currently are tabulated. However, we clearly state that most of these evolving techniques do not have defined clinical indications at present. Table 2 defines advanced analysis tools like CT scan densitometry and parametric response mapping. We similarly put into context the indications that these advanced tools have in the clinical arena.
Table 1.
Advanced Imaging Methods
| Advanced Imaging Method | Use | Current Clinical Usefulness |
|---|---|---|
| Micro-CT scan imaging | Higher resolution than typical HRCT, can visualize terminal bronchioles | Research setting: ex vivo or in vivo for small animals |
| Ultra-high-resolution and photon-counting CT scan imaging | Higher spatial resolution, greater image contrast, and less noise that typical HRCT, using less radiation | Clinical value is under evaluation |
| MRI, including hyperpolarized helium | Can quantify and evaluate lung parenchyma | Research setting, but clinical opportunities expanding |
| Radionuclide scintigraphy | Detailed measurements of pulmonary ventilation using inhaled xenon and dual-energy CT scan imaging is in early stage of development; SPECT imaging with Technegas correlates with spirometric lung function | Research setting |
| PET | Widely explored as a biomarker of pulmonary inflammation, can distinguish asthma from COPD and α1-antitrypsin deficiency | Available, but not first-line method for diagnosis of COPD |
HRCT = high-resolution CT; SPECT = single photon emission CT.
Table 2.
Advanced Analysis Tools
| Advanced Analysis Tool | Use | Current Clinical Usefulness |
|---|---|---|
| CT scan imaging densitometry or QCT scan imaging |
|
|
| Parametric response mapping |
|
|
| Machine learning or deep learning |
|
|
BLVR = bronchoscopic lung volume reduction; FDA = US Food and Drug Administration; LAA = low attenuating area; LVRS = lung volume reduction surgery; PD15 = lowest 15th percentile of lung histogram; QCT = quantitative analysis CT.
Evolving Imaging Techniques
Additional Methods
Micro-CT Imaging
Typical high-resolution CT scan imaging is capable of noninvasive imaging with spatial resolution in the range of 600 to 1000 μm. Parenchymal and airway abnormalities related to airflow obstruction in COPD occur in the conducting airways at a similar scale and are difficult to resolve. Ex vivo micro-CT scans with resolution of up to 1 μm can enable visualization of terminal bronchioles, although currently it can only be used on ex vivo lung tissue samples because of the high radiation required (Fig 10).46 Although not widely available, synchrotron micro-CT scan imaging also has been used to produce artifact-free, 3-D ex vivo reconstructions of distal lung anatomic features in patients with emphysema, showing coalescence and overexpansion of acini.47
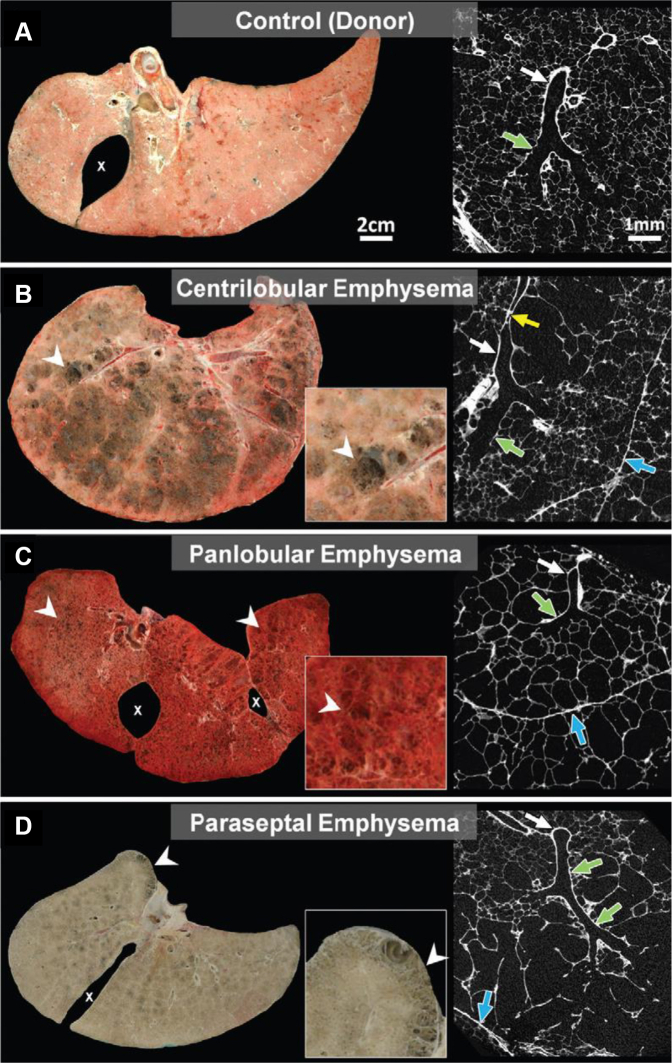
Figure 10.
A-D, Comparison of frozen lung slices and micro-CT scan images. A, Micro-CT image of normal donor lung showing a terminal bronchiole (white arrow) connecting to respiratory bronchiole (green arrow) supplying alveoli of normal size. B, Frozen lung slice showing extensive centrilobular emphysema (arrowheads) and micro-CT scan image showing dilation and destruction of proximal respiratory bronchioles (green arrow), with sparing of alveoli near lobular septa (blue arrow). Terminal bronchiole leading into centrilobular lesion is narrowed (yellow arrow) and then opens up again (white arrow). C, In contrast, frozen lung slice showing panlobular emphysema in α1-antitrypsin deficiency with uniform destruction of alveoli (arrowheads) extending right up to lobular septa (blue arrow) on the micro-CT scan image. Terminal bronchiole (white arrow) and respiratory bronchiole (green arrow) are normal. D, Frozen lung slice showing paraseptal emphysema with typical subpleural lesions (arrowheads) and micro-CT scan image showing that alveoli adjacent to lobular septa are dilated and destroyed, with sparing of center of the lobule. Terminal bronchiole (white arrow) and respiratory bronchiole (green arrow) are normal. (Reprinted with permission from Lynch et al.34)
Ultra-high-Resolution and Photon-Counting CT Scan Imaging
Historically, the most common matrix size for CT scan images has been 512 × 512. Newer systems are capable of reconstructing larger matrix sizes (eg, 1,024 or 2,048), potentially improving spatial resolution to 150 to 500 μm, which may allow improved assessment of emphysema and airways.48 New detector technology is capable of counting individual photons, whereas traditional photodetectors used in CT scan imaging integrate detected energy. This new photon counting CT scan imaging technique is a major advance in radiologic imaging, producing images not only with higher spatial resolution, but also with greater image contrast, less noise, and using less radiation (Fig 11).49
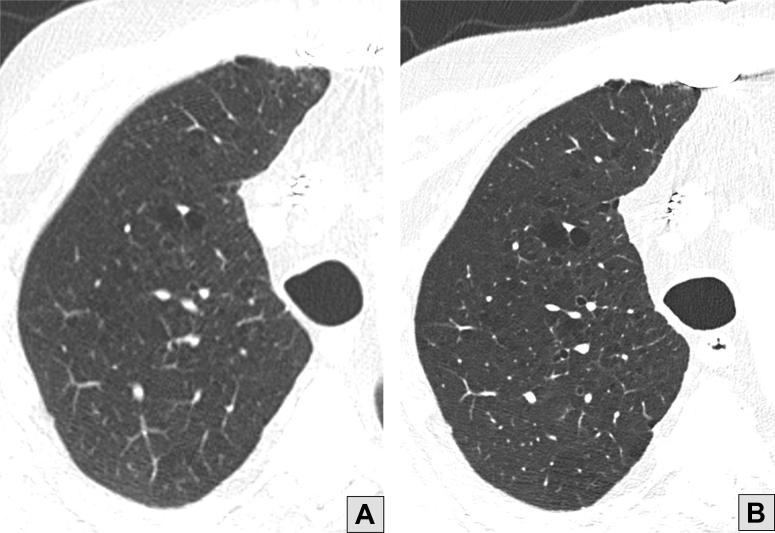
Figure 11.
A, Conventional high-resolution CT scan image of the right lung showing centrilobular emphysema. B, Photon-counting CT scan image obtained with a substantially lower acquisition dose and a sharper reconstruction kernel showing improved conspicuity of emphysema resulting from increased resolution and decreased image noise.
MRI (Including Hyperpolarized Helium)
Because of radiation dose considerations, CT scan imaging typically is not used to perform true dynamic imaging. Major advantages of MRI are the ability to quantify ventilation directly, to identify progression and reversibility with bronchodilators, to measure airspace dimensions directly, and to quantify pulmonary perfusion.50 Inhaled 3He provides a contrast media that maps regional ventilation of the lungs. Apparent diffusion coefficient, 129Xe dissolved phase, and ventilation defect percentage are imaging parameters derived from hyperpolarized MRI that have been used in the study of COPD (Fig 12).51 Hyperpolarized helium MRI apparent diffusion coefficient values could explain ventilation improvements after bronchodilator treatment.52 Moreover, when comparing with CT scan and pulmonary function tests, only hyperpolarized helium MRI enabled the prediction of exacerbations in patients with mild to moderate COPD without previous exacerbations.53 For determining perfusion, pulmonary microvascular blood flow was reduced in patients with mild COPD compared with control participants who used tobacco, independent of small airways disease on CT scan imaging and gas trapping on pulmonary function tests.54 Pulmonary microvascular blood flow would be measured using gadolinium-enhanced MRI, associated with signs of endothelial injury, including elevated endothelial microparticles and reduced circulating endothelial cells.55 Fortunately, recent MRI techniques including ultrashort echo time and zero echo time, as well as multicoil parallel acquisitions and acceleration methods, are expanding clinical opportunities for pulmonary MRI to evaluate lung parenchymal and pulmonary vascular diseases.56
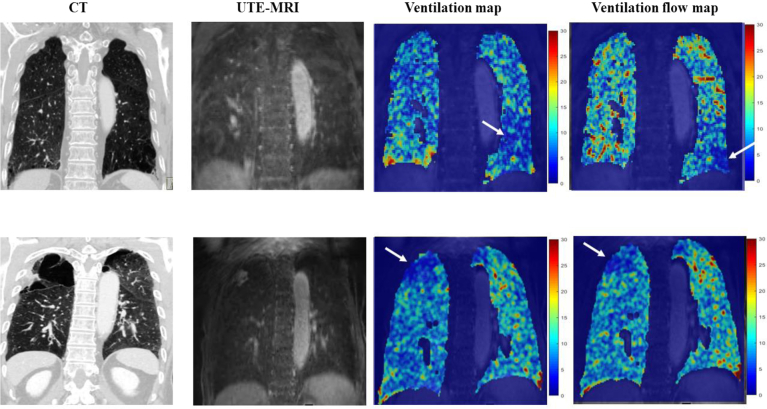
Figure 12.
CT scan images and ultrashort echo time (UTE) MRI scans in two patients with COPD. The ventilation map and the ventilation flow map from the UTE MRI scans present two types of voxel-wise information about ventilation. A ventilation map was obtained by calculating the relative ratio of the signal difference between end-inspiration (Sins) and end expiration (Sexp) with reference to end expiration, which is written as ventilation = (Sexp – Sins) / Sexp. A ventilation flow map can be obtained from 3-D UTE images by calculating the rate of change of ventilation over time between two consecutive respiratory phases. It can provide regional information on airflow in the lung parenchyma or small airways, allowing for further evaluation of pulmonary ventilation function. Ventilation flow can be described more clearly by introducing the concept of fractional ventilation (FV), which is defined as ventilation at a specific respiratory phase relative to the end-expiratory ventilation: FV(m) = (S(mexp) – S(m)) / (S(mexp)), where a positive integer m represents the mth respiratory phase image in the breathing cycle (m = 1, 2, . . ., ms, where ms is the number of all respiratory phases considered) and mexp represents the end-expiration image. Then, assuming the interval t between two consecutive respiratory phases, ventilation flow (VF) can be defined as the change in FV over Δt: VF(n) = (ΔFV(n + 1) – ΔFV(n)) / Δt . Here, ΔFV(n) = FV(n + 1) – FV(n), where FV(n + 1) is assumed to be the same as FV(1) for n = ms. Given this definition of VF, a VF map can be obtained by calculating the voxel-wise difference between the maximum and minimum values of VF(n). Accordingly, the ventilation map indicates the amount of ventilation, whereas the VF map shows the ventilation rate. A, Blue area in the left lower lobe on the ventilation map (arrow) shows an area of decreased ventilation, but the corresponding area on the ventilation rate map (arrow) is smaller. B, In contrast, this patient has a large right upper lobe bulla (arrow), with matching decreases in ventilation and ventilation rate.
Radionuclide Lung Scanning and PET Scanning
Molecular imaging approaches such as PET and single photon emission CT could meet the need for noninvasive biomarkers of lung disease (Fig 13). Because inflammatory cell recruitment leads to increased glucose use in the lungs, 18F-fludeoxyglucose PET has been explored widely as a biomarker of pulmonary inflammation.57 Currently, molecular imaging is not a first-line method for the diagnosis of COPD. However, it can provide information for enhancing diagnosis, advancing individualized disease phenotyping, and assisting with the assessment of responses to treatments.58 For example, fludeoxyglucose in addition to the use of a macrophage-targeted 11C-PK1195 PET radiotracer was shown to be able to discriminate asthma from COPD in patients.59 A study of 30 patients, divided into healthy, α1-antitrypsin deficiency, and COPD groups reflecting different severities of inflammation, showed that fludeoxyglucose PET could differentiate these three study groups.60 Patients with COPD also have been evaluated using single photon emission CT imaging procedures with Technegas (Cyclomedica Ltd.) (99mTc-labelled carbon particles) because it has been demonstrated to correlate positively with spirometric lung function.61 Development of molecular imaging agents specific to matrix metalloproteases secreted by macrophages and other inflammatory cells also would aid in COPD diagnosis.62
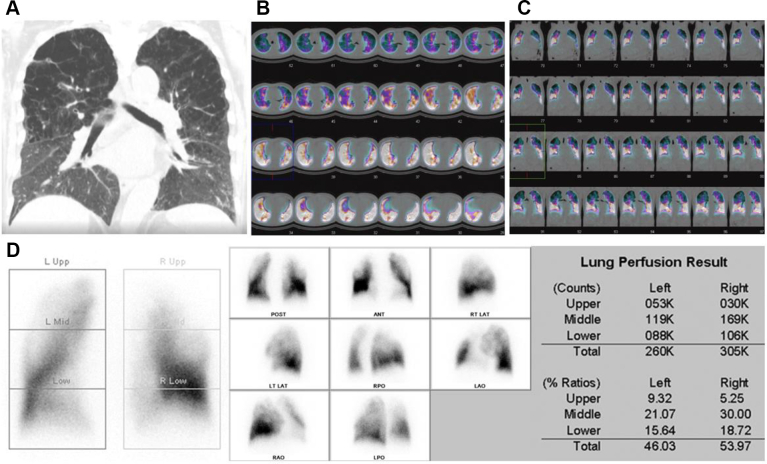
Figure 13.
A-D, Single photon emission CT scan images from a 72-year-old male with COPD. A, High-resolution CT image showing centrilobular emphysema in upper lobes with an extent of 40% of the lung volume. B-D, Transverse (B) and frontal (C) perfusion single photon emission CT images revealing areas with reduced perfusion in all segments of the right upper lobe and apicoposterior and the anterior segments of the left upper lobes. ANT = anterior; LAO = left anterior oblique; LAT = lateral; LT = left; POST = posterior; RPO = right posterior oblique; RT = right; Upp = upper.
Analysis Methods
Parametric Response Mapping
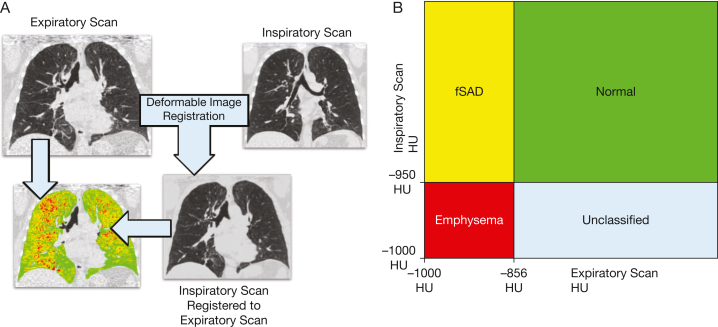
Airways < 2 mm in diameter are a primary site of airflow obstruction in COPD.63 Because they are difficult to measure, one can assess their effect indirectly by quantifying gas trapping on expiratory CT scan imaging by measuring the percent of voxels < –856 HU on expiratory CT scan imaging.64,65 However, this methodology cannot distinguish areas of small airways disease from emphysema. The parametric response mapping methodology overcomes this challenge using image registration to match inspiratory and expiratory lung to examine local changes in density so that healthy lung can be distinguished from functional small airways disease and emphysema (Fig 14).66,67 This methodology has the potential to identify at-risk patients for greater lung function decline.68
Figure 14.
A, B, Parametric response mapping: deformable image registration (A) is used to match voxels on inspiratory and expiratory scans, and voxels are assigned a parametric response mapping category based on their HU values on the expiratory and corresponding registered inspiratory scan (B). For example, a voxel with a value of > –950 HU on the registered inspiratory scan and < –856 HU on the expiratory scans would be classified as fSAD. fSAD = functional small airways disease; HU = Hounsfield unit.
Machine Learning
Machine learning is a subfield of artificial intelligence that focuses on algorithms that adapt to example data, rather than using explicitly programmed rules, to improve accuracy of a prediction task. Deep learning is a type of machine learning using neural networks, which have the benefit of simultaneously optimizing feature extraction and outcome prediction. The last decade has seen great interest in applying machine learning and deep learning in medicine. Deep learning in particular has provided remarkable advances in historically challenging image analysis tasks. Machine learning and deep learning methods for automatic anatomic segmentation have improved the robustness and precision of quantitative CT scan imaging. Separate machine learning and deep learning algorithms for COPD have shown the ability to classify phenotypes and to stratify disease severity on CT scan imaging.36,69,70
Additional Considerations Regarding Health Disparities
Lung health disparities may emerge related in part because of race or ethnicity, sex, age, and country of origin. To provide the best clinical care, attention to all aspects of COPD subgroups and the recognition of the intersectionality of race or ethnicity, sex, and age should be addressed.71
The question of whether race is a useful construct in pulmonary medicine is timely.72,73 Slightly less emphysema seems to occur in Black individuals with a history of tobacco use.74,75 However, using visual scoring of CT scans, Black participants showed greater 5-year progression of emphysema.76 CT scan imaging of bone density in the vertebral column found that Black individuals who used tobacco generally showed higher measured bone mineral density, but with advancing COPD in higher Global Initiative for Chronic Obstructive Lung Disease stages, they still showed lower bone mineral density and more risk of fracture, highlighting the intersectionality of these issues with aging and comorbidities.77
Recognition of aging factors is a key opportunity to address lung health equity. The diagnosis of early COPD is an opportunity globally to improve lung health78; the aging of the global populations portends higher prevalence of COPD in the future. Physical frailty emerges as a major syndrome to evaluate and intervene in COPD.79 Frailty in COPD is prevalent with increasing age. Chest CT scan imaging has a role in identifying age-associated multiple morbidities (eg, osteoporosis, vertebral fractures, degenerative spine disease, and cardiovascular disease).
Although national COPD mortality rates in women have continued to increase,79 research has demonstrated that COPD tends to be underdiagnosed in women and that the use of spirometry mitigates the underdiagnosis.4 Menopause is associated with accelerated lung function decline,5 and women are at increased risk for severe, early-onset COPD (younger than 55 years),6 highlighting the need for sex- and gender-aware approaches to addressing lung health disparities.7 The reason for this apparent increased risk for early disease is unclear and is a topic of investigation. In addition to the imperative of suspecting and diagnosing COPD in all women, it is clear that across all ages, women with COPD manifest more severe symptoms and comorbid conditions when compared with men with COPD.8
In addition to race, age, and sex, international differences may lead to health disparities. COPD is accepted widely to be caused by noxious compounds that lead to lung and airways damage. An estimated 50% of global COPD is caused by non-tobacco use risk factors that vary by geographical region.80 In low-income countries, these risk factors are attributed to environmental pollution, with smoke from biomass fuel being the most statistically significant risk factor.80 In general, patients with COPD who have never used tobacco have relatively mild dyspnea and sputum production, mild airflow limitation, and a normal diffusing capacity. CT scans show less emphysema than individuals with COPD who use tobacco and are more likely to show bronchial wall thickening and mucus plugging. Racial differences in presentation and symptoms, coupled with different health care systems, lead to vastly different approaches to the diagnosis and management of COPD.
Finally, socioeconomic disparities both in the United States and internationally result in different levels of access and use of advanced and emerging imaging methods. Physicians and other health care leaders can improve the health and well-being of patients with COPD by promoting more equitable distribution of advanced imaging for diagnosis and treatment planning.
Summary
Advanced imaging can offer valuable information in the diagnosis, management, and prognostication of COPD. Novel imaging techniques include both advanced imaging methods (eg, micro-CT scan imaging, ultra-high-resolution and photon-counting CT scan imaging, MRI, radionuclide scintigraphy, and PET) as well as advanced analysis tools (eg, parametric response mapping and machine learning). Because these newer imaging methods are being developed and trialed in academic radiology centers, clinicians may be unaware of their potential usefulness in clinical practice. With increased awareness and by promoting equitable use of advanced imaging for diagnosis and treatment planning, practicing physicians can improve the health and well-being of patients with COPD significantly.
Funding/Support
D. D. has received support from NIH. S. H. is supported by the National Heart, Lung, and Blood Institute. D. L. is supported by the National Heart, Lung, and Blood Institute [Grants U01 HL089897 and U01 HL089856].
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: S. B. is a speaker for Merck and Genentech consultant for Boehringer Ingelheim and Bellus. P. C. has received grant support from Bayer and GSK and consulting fees from Novartis. D. D. has received support from Bayer and Novartis. M. K. H. reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, Amgen, UpToDate, Altesa Biopharma, Medscape, NACE, MDBriefcase, Integrity and Medwiz. She has received either in kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, Astra Zeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation and the American Lung Association. She has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. She has received stock options from Meissa Vaccines and Altesa Biopharma. S. H. reports research grants from Boehringer Ingelheim paid to the institution, service contracts from Calyx paid to the institution, and the patent “System and Methods for Classifying the Severity of COPD” pending (unlicensed and assigned to the institution). B. Mina is a medical consultant for Inari Medical. Y. O. reports a research grant from Canon Medical Systems Corporation. E. J. R. v. B. is founder/owner of QCTIS, Ltd.; a consultant for Astra Zeneca, Contextflow, and Lunit; serves on the medical advisory board of Aidence; and has received speakers fees from Roche Diagnostics and Astra Zeneca. None declared (S. R., M. S., A. A., H. A., R. B., S. F., C. S. H., J. H., H. Y. L., K. S. L., D. L., S. Machnicki, A. M., S. Mehta, D. N., J. N., E. R., G. W., B. Make).
Acknowledgments
Other contributions: The authors thank Erin Brown and William Self for advising with the literature searches and Patrice Balistreri for secretarial assistance.
Additional information: The e-Figure is available online under “Supplemental Data.”
Supplementary Data
References
- 1.Adeloye D., Song P., Zhu Y., et al. Global, regional and national prevalence of, and risk factors for, chronic obstructive lung disease (COPD) in 2019: a systemic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raoof S., Shah M., Make B., et al. Lung imaging in COPD part 1: clinical usefulness. Chest. 2023;164(1):69–84. doi: 10.1016/j.chest.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolz D., Mkorombindo T., Schumann D.M., et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400(10356):921–972. doi: 10.1016/S0140-6736(22)01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palla A., Desideri M., Rossi G., et al. Elective surgery for giant bullous emphysema: a 5-year clinical and functional follow-up. Chest. 2005;128(4):2043–2050. doi: 10.1378/chest.128.4.2043. [DOI] [PubMed] [Google Scholar]
- 5.Lor K.L., Chang Y.C., Yu C.J., Wang C.Y., Chen C.M., TCORE Consortium Bullous parametric response map for functional localization of COPD. J Digit Imaging. 2022;35(2):115–126. doi: 10.1007/s10278-021-00561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman A., Martinez F., Naunheim K., et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 7.Criner G.J., Sue R., Wright S., et al. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE) Am J Respir Crit Care Med. 2018;198(9):1151–1164. doi: 10.1164/rccm.201803-0590OC. [DOI] [PubMed] [Google Scholar]
- 8.Criner G.J., Delage A., Voelker K., et al. Improving lung function in severe heterogenous emphysema with the Spiration Valve System (EMPROVE). A multicenter, open-label randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200(11):1354–1362. doi: 10.1164/rccm.201902-0383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klooster K., ten Hacken N.H., Hartman J.E., Kerstjens H.A., van Rikxoort E.M., Slebos D.J. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373(24):2325–2335. doi: 10.1056/NEJMoa1507807. [DOI] [PubMed] [Google Scholar]
- 10.Caviezel C., Froehlich T., Schneiter D., et al. Identification of target zones for lung volume reduction surgery using three-dimensional computed tomography rendering. ERJ Open Res. 2020;6(3) doi: 10.1183/23120541.00305-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koster T.D., Slebos D.J. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis. 2016;11:765–773. doi: 10.2147/COPD.S103807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klooster K., Koster T.D., Ruwwe-Glösenkamp C., et al. An integrative approach of the fissure completeness score and Chartis assessment in endobronchial valve treatment for emphysema. Int J Chron Obstruct Pulmon Dis. 2020;15:1325–1334. doi: 10.2147/COPD.S242210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantri S., Macaraeg C., Shetty S., et al. Technical advances: measurement of collateral flow in the lung with a dedicated endobronchial catheter system. J Bronchology Interv Pulmonol. 2009;16(2):141–144. doi: 10.1097/LBR.0b013e3181a40a3e. [DOI] [PubMed] [Google Scholar]
- 14.Klooster K., Slebos D.J. Endobronchial valves for the treatment of advanced emphysema. Chest. 2021;159(5):1833–1842. doi: 10.1016/j.chest.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wienker J., Karpf-Wissel R., Funke F., et al. Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620932507. 1753466620932507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui F.M., Diamond J.M. Lung transplantation for chronic obstructive pulmonary disease: past, present, and future directions. Curr Opin Pulm Med. 2018;24:199–204. doi: 10.1097/MCP.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejwani V., Panchabhai T.S., Kotloff R., Mehta A.C. Complications of lung transplantation: a roentgenographic perspective. Chest. 2016;149(6):1535–1545. doi: 10.1016/j.chest.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Oey I., Steiner M., Morgan M., Waller D. Patient-directed volume reduction for emphysema: sequential surgical and endobronchial techniques. Ann Thorac Surg. 2021;112:295–301. doi: 10.1016/j.athoracsur.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Panchabhai T.S., Arrossi A.V., Patil P.D., et al. Unexpected neoplasms in lungs explanted from lung transplant recipients: a single-center experience and review of literature. Transplant Proc. 2018;50(1):234–240. doi: 10.1016/j.transproceed.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Aslam A., De Luis Cardenas J., Morrison R.J., et al. Tracheobronchomalacia and excessive dynamic airway collapse: current concepts and future directions. Radiographics. 2022;42(4):1012–1027. doi: 10.1148/rg.210155. [DOI] [PubMed] [Google Scholar]
- 21.Boiselle P.M., Feller-Kopman D., Ashiku S., et al. Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am. 2003;41(3):627–636. doi: 10.1016/s0033-8389(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 22.Jokenen K., Palva T., Sutinen S., Nuutinen J. Acquired tracheobronchomalacia. Ann Clin Res. 1977;9:52–57. [PubMed] [Google Scholar]
- 23.Mehta A.C., Zaki K.S., Banga A., Singh J., Gildea T., Arrossi V. Tracheobronchial smooth muscle atrophy and separation. Respiration. 2015;90(3):256–262. doi: 10.1159/000431381. [DOI] [PubMed] [Google Scholar]
- 24.Lee E., Litmanovich D., Boiselle P. Multidetector CT evaluation of tracheobronchomalacia. Radiol Clin N Am. 2009;47:261–269. doi: 10.1016/j.rcl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 25.De Wever W., Bogaert J., Verschakelen J.A. Virtual bronchoscopy: accuracy and usefulness—an overview. Semin Ultrasound CT MR. 2005;26(5):364–373. doi: 10.1053/j.sult.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Muller N.L., Staples C.A., Miller R.R., Abboud R.T. “Density mask.” An objective method to quantitate emphysema using computed tomography. Chest. 1988;94(4):782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella M., Muller N.L., Abboud R.T., Morrison N.J., DyBuncio A. Quantitation of emphysema by computed tomography using a “density mask” program and correlation with pulmonary function tests. Chest. 1990;97(2):315–321. doi: 10.1378/chest.97.2.315. [DOI] [PubMed] [Google Scholar]
- 28.Gevenois P.A., de Maertelaer V., De Vuyst P., Zanen J., Yernault J.C. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 29.Stolk J., Ng W.H., Bakker M.E., et al. Correlation between annual change in health status and computer tomography derived lung density in subjects with alpha1-antitrypsin deficiency. Thorax. 2003;58(12):1027–1030. doi: 10.1136/thorax.58.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parr D.G., Dirksen A., Piitulainen E., Deng C., Wencker M., Stockley R.A. Exploring the optimum approach to the use of CT densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antitrypsin deficiency. Respir Res. 2009;10:75. doi: 10.1186/1465-9921-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pompe E., Strand M., van Rikxoort E.M., et al. Five-year progression of emphysema and air trapping at CT in smokers with and those without chronic obstructive pulmonary disease: results from the COPDGene Study. Radiology. 2020;295(1):218–226. doi: 10.1148/radiol.2020191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallardo-Estrella L., Lynch D.A., Prokop M., et al. Normalizing computed tomography data reconstructed with different filter kernels: effect on emphysema quantification. Eur Radiol. 2016;26(2):478–486. doi: 10.1007/s00330-015-3824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb W.R. Thin-section CT of the secondary pulmonary lobule: anatomy and the image—the 2004 Fleischner Lecture. Radiology. 2006;239(2):322–338. doi: 10.1148/radiol.2392041968. [DOI] [PubMed] [Google Scholar]
- 34.Lynch D.A., Austin J.H., Hogg J.C., et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015;277(1):192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castaldi P.J., San Jose Estepar R., Mendoza C.S., et al. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188(9):1083–1090. doi: 10.1164/rccm.201305-0873OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humphries S.M., Notary A.M., Centeno J.P., et al. Deep learning enables automatic classification of emphysema pattern at CT. Radiology. 2020;294(2):434–444. doi: 10.1148/radiol.2019191022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorensen L., Shaker S.B., de Bruijne M. Quantitative analysis of pulmonary emphysema using local binary patterns. IEEE Trans Med Imaging. 2010;29(2):559–569. doi: 10.1109/TMI.2009.2038575. [DOI] [PubMed] [Google Scholar]
- 38.Bermejo-Pelaez D., Ash S.Y., Washko G.R., San Jose Estepar R., Ledesma-Carbayo M.J. Classification of interstitial lung abnormality patterns with an ensemble of deep convolutional neural networks. Sci Rep. 2020;10(1):338. doi: 10.1038/s41598-019-56989-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano Y., Muro S., Sakai H., et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa M., Nasuhara Y., Onodera Y., et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(12):1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 41.Martinez C.H., Chen Y.-H., Westgate P.M., et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coxson H.O. Quantitative computed tomography assessment of airway wall dimensions: current status and potential applications for phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(9):940–945. doi: 10.1513/pats.200806-057QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells J.M., Washko G.R., Han M.K., et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estépar R.S.J., Kinney G.L., Black-Shinn J.L., et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uppaluri R., Mitsa T., Sonka M., Hoffman E.A., McLennan G. Quantification of pulmonary emphysema from lung computed tomography images. Am J Respir Crit Care Med. 1997;156(1):248–254. doi: 10.1164/ajrccm.156.1.9606093. [DOI] [PubMed] [Google Scholar]
- 46.Hogg J., Tanabe N., Vasilescu D.M., et al. Pathological comparisons of paraseptal and centrilobular emphysema in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202(6):803–811. doi: 10.1164/rccm.201912-2327OC. [DOI] [PubMed] [Google Scholar]
- 47.Kampschulte M., Schneider C.R., Litzlbauer H.D., et al. Quantitative 3D micro-CT imaging of human lung tissue. Rofo. 2013;185(9):869–876. doi: 10.1055/s-0033-1350105. [DOI] [PubMed] [Google Scholar]
- 48.Hata A., Yanagawa M., Honda O., et al. Effect of matrix size on the image quality of ultra-high-resolution CT of the lung: comparison of 512 × 512, 1024 × 1024, and 2048 × 2048. Acad Radiol. 2018;25(7):869–876. doi: 10.1016/j.acra.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Sotoudeh-Paima S., Segars W.P., Samei E., Abadi E. Photon-counting CT versus conventional CT for COPD quantifications: intra-scanner optimization and inter-scanner assessments using virtual imaging trials. Proc SPIE Int Soc Opt Eng. 2022;12031:120312I. doi: 10.1117/12.2613003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch D.A. Functional imaging of COPD by CT and MRI. Br J Radiol. 2022;95(1132) doi: 10.1259/bjr.20201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiboutot J., Yuan W., Park H.C., et al. Current advances in COPD imaging. Acad Radiol. 2019;26(3):335–343. doi: 10.1016/j.acra.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirby M., Mathew L., Heydarian M., Etemad-Rezai R., McCormack D.G., Parraga G. Chronic obstructive pulmonary disease: quantification of bronchodilator effects by using hyperpolarized ³He MR imaging. Radiology. 2011;261(1):283–292. doi: 10.1148/radiol.11110403. [DOI] [PubMed] [Google Scholar]
- 53.Kirby M., Pike D., Coxson H.O., McCormack D.G., Parraga G. Hyperpolarized (3)He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology. 2014;273(3):887–896. doi: 10.1148/radiol.14140161. [DOI] [PubMed] [Google Scholar]
- 54.Hueper K., Vogel-Claussen J., Parikh M.A., et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. the MESA COPD Study. Am J Respir Crit Care Med. 2015;192(5):570–580. doi: 10.1164/rccm.201411-2120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomashow M.A., Shimbo D., Parikh M.A., et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am J Respir Crit Care Med. 2013;188(1):60–68. doi: 10.1164/rccm.201209-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatabu H., Ohno Y., Gefter W.B., et al. Expanding applications of pulmonary MRI in the clinical evaluation of lung disorders: Fleischner Society position paper. Radiology. 2020;297(2):286–301. doi: 10.1148/radiol.2020201138. [DOI] [PubMed] [Google Scholar]
- 57.Chen D.L., Cheriyan J., Chilvers E.R., et al. Quantification of lung PET images: challenges and opportunities. J Nucl Med. 2017;58(2):201–207. doi: 10.2967/jnumed.116.184796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myc L.A., Shim Y.M., Laubach V.E., Dimastromatteo J. Role of medical and molecular imaging in COPD. Clin Transl Med. 2019;8(1):12. doi: 10.1186/s40169-019-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones H.A., Marino P.S., Shakur B.H., Morrell N.W. In vivo assessment of lung inflammatory cell activity in patients with COPD and asthma. Eur Respir J. 2003;21(4):567–573. doi: 10.1183/09031936.03.00048502. [DOI] [PubMed] [Google Scholar]
- 60.Subramanian D.R., Jenkins L., Edgar R., Quraishi N., Stockley R.A., Parr D.G. Assessment of pulmonary neutrophilic inflammation in emphysema by quantitative positron emission tomography. Am J Respir Crit Care Med. 2012;186(11):1125–1132. doi: 10.1164/rccm.201201-0051OC. [DOI] [PubMed] [Google Scholar]
- 61.Jögi J., Ekberg M., Jonson B., Bozovic G., Bajc M. Ventilation/perfusion SPECT in chronic obstructive pulmonary disease: an evaluation by reference to symptoms, spirometric lung function and emphysema, as assessed with HRCT. Eur J Nucl Med Mol Imaging. 2011;38(7):1344–1352. doi: 10.1007/s00259-011-1757-5. [DOI] [PubMed] [Google Scholar]
- 62.Golestani R., Razavian M., Ye Y., et al. Matrix metalloproteinase-targeted imaging of lung inflammation and remodeling. J Nucl Med. 2017;58(1):138–143. doi: 10.2967/jnumed.116.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogg J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 64.Jain N., Covar R.A., Gleason M.C., Newell J.D., Jr., Gelfand E.W., Spahn J.D. Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol. 2005;40(3):211–218. doi: 10.1002/ppul.20215. [DOI] [PubMed] [Google Scholar]
- 65.Matsuoka S., Kurihara Y., Yagihashi K., Hoshino M., Watanabe N., Nakajima Y. Quantitative assessment of air trapping in chronic obstructive pulmonary disease using inspiratory and expiratory volumetric MDCT. AJR Am J Roentgenol. 2008;190(3):762–769. doi: 10.2214/AJR.07.2820. [DOI] [PubMed] [Google Scholar]
- 66.Galban C.J., Han M.K., Boes J.L., et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B., Christensen G.E., Hoffman E.A., McLennan G., Reinhardt J.M. Pulmonary CT image registration and warping for tracking tissue deformation during the respiratory cycle through 3D consistent image registration. Med Phys. 2008;35(12):5575–5583. doi: 10.1118/1.3005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhatt S.P., Soler X., Wang X., et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(2):178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.González G., Ash S.Y., Vegas-Sánchez-Ferrero G., et al. Disease staging and prognosis in smokers using deep learning in chest computed tomography. Am J Respir Crit Care Med. 2018;197(2):193–203. doi: 10.1164/rccm.201705-0860OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh A.S., Baraghoshi D., Lynch D.A., et al. Emphysema progression at CT by deep learning predicts functional impairment and mortality: results from the COPDGene study. Radiology. 2022;304(3):672–679. doi: 10.1148/radiol.213054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Celedon J.C., Burchard E.G., Schraufnagel D., et al. An American Thoracic Society/National Heart, Lung, and Blood Institute Workshop report: addressing respiratory health equality in the United States. Ann Am Thorac Soc. 2017;14:814–826. doi: 10.1513/AnnalsATS.201702-167WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schluger N.W., Dozor A.J., Jung Y.E.G. Rethinking the race adjustment in pulmonary function testing. Ann Am Thorac Soc. 2022;19:353–356. doi: 10.1513/AnnalsATS.202107-890PS. [DOI] [PubMed] [Google Scholar]
- 73.Braun L. Race correction and spirometry: why history matters. Chest. 2021;159:1670–1675. doi: 10.1016/j.chest.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 74.Hansel N.N., Washko G.R., Foreman M.G., et al. Racial differences in CT phenotypes in COPD. COPD. 2013;10:20–27. doi: 10.3109/15412555.2012.727921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lynch D.A., Moore C.M., Wilson C., et al. CT-based visual classification of emphysema: association with mortality in the COPDGene Study. Radiology. 2018;288:859–866. doi: 10.1148/radiol.2018172294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Kaddouri B., Strand M.J., Baraghoshi D., et al. Fleischner Society visual emphysema CT patterns help predict progression of emphysema in current and former smokers: results from the COPDGene Study. Radiology. 2021;298:441–449. doi: 10.1148/radiol.2020200563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaramillo J.D., Wilson C., Stinson D.S., et al. Reduced bone density and vertebral fractures in smokers. Men and COPD patients at increased risk. Ann Am Thorac Soc. 2015;12:648–656. doi: 10.1513/AnnalsATS.201412-591OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colak Y., Afzal S., Nordestgaard B.G., Lange P., Vestbo J. Importance of early COPD in young adults for development of clinical COPD: findings from the Copenhagen General Population Study. Am J Respir Crit Care Med. 2021;203:1245–1256. doi: 10.1164/rccm.202003-0532OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee S.Y., Nyunt M.S.Z., Gao Q., et al. Co-occurrence of physical frailty and COPD and association with disability and mortality: Singapore Longitudinal Ageing Study. Chest. 2022;161:1225–1238. doi: 10.1016/j.chest.2021.12.633. [DOI] [PubMed] [Google Scholar]
- 80.Yang I.A., Jenkins C.R., Salvi S.S. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022;10:497–511. doi: 10.1016/S2213-2600(21)00506-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.