Abstract
Background
The use of a Left Ventricular Assist Device (LVAD) in patients with advanced heart failure refractory to optimal medical management has progressed steadily over the past two decades. Data have demonstrated reduced LVAD efficacy, worse clinical outcome, and higher mortality for patients who experience significant ventricular tachyarrhythmia (VTA). We hypothesize that a novel prophylactic intra‐operative VTA ablation protocol at the time of LVAD implantation may reduce the recurrent VTA and adverse events postimplant.
Methods
We designed a prospective, multicenter, open‐label, randomized‐controlled clinical trial enrolling 100 patients who are LVAD candidates with a history of VTA in the previous 5 years. Enrolled patients will be randomized in a 1:1 fashion to intra‐operative VTA ablation (n = 50) versus conventional medical management (n = 50) with LVAD implant. Arrhythmia outcomes data will be captured by an implantable cardioverter defibrillator (ICD) to monitor VTA events, with a uniform ICD programming protocol. Patients will be followed prospectively over a mean of 18 months (with a minimum of 9 months) after LVAD implantation to evaluate recurrent VTA, adverse events, and procedural outcomes. Secondary endpoints include right heart function/hemodynamics, healthcare utilization, and quality of life.
Conclusion
The primary aim of this first‐ever randomized trial is to assess the efficacy of intra‐operative ablation during LVAD surgery in reducing VTA recurrence and improving clinical outcomes for patients with a history of VTA.
Keywords: ablation, left ventricular assist device, ventricular tachycardia
The use of a Left Ventricular Assist Device (LVAD) in patients with advanced heart failure refractory to optimal medical management has progressed steadily over the past two decades. Data have demonstrated reduced LVAD efficacy, worse clinical outcome, and higher mortality for patients who experience significant ventricular tachyarrhythmia (VTA). We hypothesize that a novel prophylactic intra‐operative VTA ablation protocol at the time of LVAD implantation may reduce the recurrent VTA and adverse events postimplant. We designed a prospective, multicenter, open‐label, randomized‐controlled clinical trial enrolling 100 patients who are LVAD candidates with a history of VTA in the previous 5 years. The primary aim of this first‐ever randomized trial is to assess the efficacy of intra‐operative ablation during LVAD surgery in reducing VTA recurrence and improving clinical outcomes for patients with a history of VTA.
1. INTRODUCTION
The use of left ventricular assist devices (LVAD) has continued to progress for patients with advanced heart failure, driven by improvements in symptoms, morbidity, quality of life, and mortality (Drakos et al., 2011; Mehra et al., 2017; Miller et al., 2007; Slaughter et al., 2009, 2011). The currently approved indications for LVAD implantation include advanced heart failure symptoms (New York Heart Association class IIIB or IV) refractory to optimal medical management in patients who are deemed to be candidates for LVAD, either as bridge therapy while awaiting cardiac transplantation or as destination therapy for those who are not eligible for cardiac transplantation (Yancy et al., 2013). It is estimated that the number of potential recipients for LVAD support in the U.S. ranges from 150,000 to 250,000 (Kirklin et al., 2015). Improvements in device design, hemocompatibility, and technology have resulted in survival rates approaching 60% in the current generation of LVADs (Mehra et al., 2022). Several factors continue to limit the benefit of LVAD support among patients with advanced heart failure. Due to their advanced cardiomyopathies, many LVAD recipients have ventricular tachyarrhythmias (VTAs), specifically ventricular tachycardia (VT) or ventricular fibrillation (VF), with 20% to 50% of patients having recurrent VTA or new VTA after implant (Bedi et al., 2007; Garan et al., 2013; Nakahara et al., 2013; Oswald et al., 2010; Raasch et al., 2012; Refaat et al., 2008; Ziv et al., 2005).
VTA is associated with worse outcomes. Published data from the University of Rochester (URMC) show that 28% of ambulatory LVAD recipient patients experienced post‐implant VTAs. At the end of follow‐up, the observed mortality was 46% for patients who experienced VTAs versus 19% for patients without VTAs (p < .001). This corresponded to a 7.3‐fold increase in all‐cause mortality risk (HR = 7.3, p < .001) based on multivariate analysis (Yoruk et al., 2016). Therefore, it is imperative to identify and treat factors associated with VTA risk in recipients with LVAD. The URMC data show that the most powerful predictor of VTA post‐LVAD implant is a history of VTA at any time prior to the procedure (Brenyo et al., 2012). In multivariate analysis, a history of VTA was associated with a 2.8‐fold increase in the risk of VTA post‐LVAD implant. Similarly, Moss et al. (2017) reported that VT recurrence after catheter ablation was associated with increased mortality after LVAD implantation. Notably, the association between a history of VTA and subsequent arrhythmic risk is consistent for patients who did not experience VTA in the years preceding the implant procedure.
These findings suggest that early intervention that modifies the arrhythmia substrate, such as effective ablation, prior to or at the time of LVAD implantation in candidates with a history of VTA may reduce VTA burden and improve clinical outcomes following LVAD implantation. This is especially important since primary prevention implantable cardioverter defibrillators (ICDs) were not shown to improve outcomes in recipients with LVAD (Garan et al., 2013; Lee et al., 2015) and may also be associated with an increased risk of infection.
As a history of VT is the most powerful predictor of post‐LVAD implant VTA, we hypothesize that a preventive strategy of intraoperative VT ablation at the time of LVAD implant may lead to a significant reduction of post‐implant VTA. At present, there are no prospective data on the role of ablation in the prevention of post‐implant VTA in candidates with high‐risk LVAD. In a study of 36 patients at two centers, high‐density intra‐operative epicardial voltage mapping during LVAD implantation was shown to be safe and efficient, requiring a median of only 12 min to characterize potentially arrhythmogenic substrate. Patients who experienced post‐implant VTA had a significantly higher burden of epicardial low‐voltage areas (Moss et al., 2018). We recently published the URMC experience in 10 patients who underwent prophylactic intra‐operative VT ablation at the time of LVAD implant and their pre‐/post‐procedural outcomes. All patients had cardiomyopathy and an ICD. The mean age was 59.2 ± 11.1 years and 3 were women. INTERMCS profile ≤II was present in 20% of patients. Using negative binominal regression, the number of events per 10 patients per month prior versus post‐LVAD implant was 17 versus 1.6, respectively (p < .001) (Tankut et al., 2022). No acute procedural‐related complications were reported. We believe these preliminary data support the feasibility and possible efficacy of our proposed approach.
In light of the promising preliminary data, plus the increasing utilization of LVAD in patients with end‐stage heart failure, the timing is appropriate for a randomized clinical trial. We have designed and proposed the Prophylactic Intra‐Operative Ventricular Arrhythmia Ablation in High‐Risk LVAD Candidates (PIVATAL) Trial, which will explore the safety of a strategy that incorporates prophylactic intra‐operative VT ablation strategy during LVAD implant for the reduction of post‐implant VTA recurrence and death from any cause from index discharge to 18 months following LVAD implantation.
2. METHODS
2.1. Objectives
The primary objective of the PIVATAL study is to prospectively evaluate the effect of prophylactic intra‐operative VTA at the time of LVAD implantation on post‐implant total recurrent VTA events, after accounting for the competing risk of death. The secondary objectives of this trial are:
To evaluate the effect of a management strategy that incorporates intra‐operative VT ablation at the time of LVAD implantation on adverse events (comprising a composite of hospital readmissions, clinical RV failure, stroke, and death) following LVAD implantation. We will collect comprehensive post‐implant adverse event data over an average follow‐up of 18 months. We hypothesize that the reduction in VTA burden associated with a prophylactic versus a conventional approach will translate into a corresponding reduction in adverse events, including right heart failure, hospital readmissions, stroke, and death.
To collect prospective data on peri‐procedural outcomes associated with the proposed novel approach of prophylactic intra‐operative VT ablation. The study will utilize a prespecified protocol for intra‐operative VT ablation with high‐risk candidates with a history of VTA (without an active arrhythmia such as VTA storm) at the time of LVAD implant. We will collect comprehensive peri‐procedural data and post‐procedural complications. We hypothesize that a strategy that incorporates prophylactic intra‐operative VT ablation at the time of LVAD implantation will be associated with a post‐operative safety profile similar to conventional medical management without prophylactic VT ablation.
In addition, we will also explore mechanisms associated with (a) recurrent VTA post‐LVAD implant with electrophysiology studies. For patients who develop recurrent VTA, and those eligible for electrophysiology study and mapping, we will collect comprehensive data that may elucidate the mechanisms of recurrent VTA; (b) the ramifications of recurrent VTA on right ventricular hemodynamics post‐LVAD implant with echocardiographic ramp studies and right heart catheterization. All the patients will undergo exercise ramp test including an echo at 6 months to assess whether the reduction in VT burden associated with prophylactic VT ablation translates into preservation in RV function and hemodynamics.
2.2. Study design
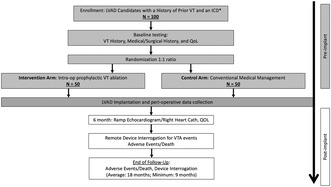
We propose a randomized multi‐center clinical trial that will evaluate the effect of prophylactic intra‐operative VT ablation in high‐risk LVAD candidate patients on VTA recurrence and adverse outcomes. We plan to enroll 100 candidates with LVAD with advanced cardiomyopathy, prior to or intended ICD implant and history of VTA from 15 experienced medical centers, who will be randomized in a 1:1 ratio to prophylactic intra‐operative VT ablation versus conventional medical management (Figure 1). These centers include the University of Pennsylvania, Vanderbilt University, University of Colorado Denver, University of Pittsburgh, Ascension St. Vincent Indianapolis, Henry Ford Hospital, Spectrum/Corewell Health Grand Rapids, Piedmont Atlanta Hospital, University of California San Francisco, Emory University, University of Arizona College of Medicine‐ Phoenix, Tufts Medical Center, Cleveland Clinic, University of California Los Angeles, and University of Rochester. This study protocol has been approved by the URMC ethics committee and conducted in compliance with standard institutional operating procedures. All the patients enrolled in the study will provide written informed consent.
FIGURE 1.
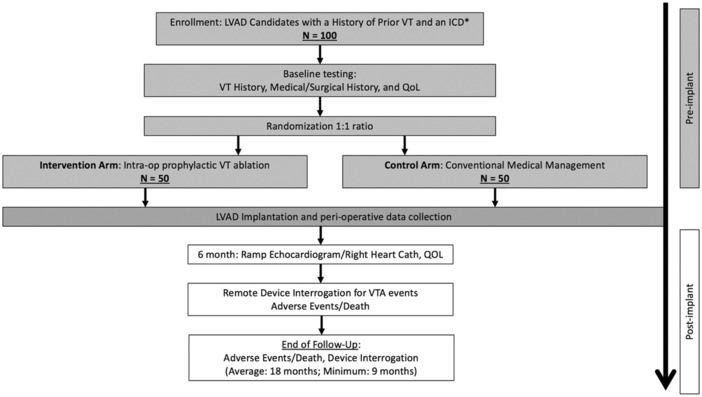
Study design of PIVATAL trial. *If an ICD is not present prior to LVAD implant, it will be implanted prior to hospital discharge (an ICM is an alternative if an ICD cannot be implanted prior to discharge for a clinical reason, such as infection).
2.3. Study population
The PIVATAL trial will enroll 100 candidates with LVAD with the following eligibility.
2.3.1. Inclusion criteria
Age > 18 years.
Presence of advanced cardiomyopathy (of all INTERMACS classification) and eligible for LVAD implant per the decision of the Heart Failure clinical team.
Implanted cardioverter defibrillator (ICD) any time in past with remote monitoring or planned to undergo ICD (or ICM as an alternative, if an ICD cannot be implanted for a clinical reason) implant within the index hospitalization for LVAD implant.
History of treated or monitored sustained (i.e., >30 s in duration) VT or VF episode within the past 5 years.
2.3.2. Exclusion criteria
Past successful VTA ablation without recurrent VTA prior to LVAD implant (Patients who continue to experience VTA post‐ablation and pre‐LVAD implant qualify to be enrolled).
Participation in other clinical trials (observational registries are allowed with approval).
Unable or unwilling to provide informed consent.
2.4. End points
The primary specific aim for PIVATAL is to evaluate the effect of prophylactic intra‐operative VTA ablation at the time of LVAD implantation on post‐implant total recurrent VTA events, defined as an average number of VTA episodes per patient per year, after accounting for the competing risk of death (Table 1). The primary endpoint of recurrent VTA is calculated as the total number of VTA episodes (ICD‐treated or monitored VT episodes >30 s or VF) divided by the total follow‐up time (in years).
TABLE 1.
Endpoints and endpoint definitions.
| Specific aims | Endpoint | Endpoint definition |
|---|---|---|
| Aim 1 (primary endpoint) | Recurrent VTA |
|
| Aim 2 | Adverse events |
|
| Aim 3 | Procedural outcomes |
|
Note: Exploratory outcomes will include: (a) Mechanisms of recurrent VTA post‐LVAD implant with catheter‐based electrophysiology studies. (b) The ramifications of recurrent VTA on right ventricular hemodynamics and function post‐LVAD implant with echocardiographic ramp studies and right heart catheterization.
Defined as an average number of VTA episodes per patient per year: Calculated as the total number of VTA episodes (ICD‐treated or monitored VT episodes > 30 s or VF) divided by the total follow‐up time (in years).
There are two secondary specific aims for the trial (Table 1). First, we will evaluate the effects of a management strategy that incorporates intra‐operative VT ablation at the time of LVAD implantation on adverse clinical outcomes following LVAD implantation. The endpoint for this will comprise a composite of hospital readmissions, clinical right heart failure, stroke, and death. Second, we will collect prospective data on peri‐procedural outcomes associated with the proposed novel approach of prophylactic intra‐operative VT ablation. The endpoints will include the duration of surgery and ablation, rate of peri‐procedural complications and length of stay in the intensive care units.
We will also explore the mechanisms associated with: (a) recurrent VTA post‐LVAD implant with electrophysiology studies and (b) the ramifications of recurrent VTA on right ventricular hemodynamics post‐LVAD implant with echocardiographic ramp studies and right heart catheterization. If a patient receives a heart transplant during the follow‐up phase, this will be regarded as a censuring event, i.e., regarded as the patient will be withdrawn from the study and all data on subsequent outcomes, including arrhythmias will not be collected. Transplants are expected to be rare.
2.5. Study procedures
2.5.1. Recruitment
Each site participating in the study will develop and implement recruitment methods that are appropriate to their institutional policies and guidelines, along with consideration for the rights and welfare of human subject research. The study staff will identify potential subjects who meet inclusion criteria by reviewing patient databases, electronic medical records, inpatient admissions, and daily clinic appointments. The enrolling site investigator and/or study coordinator will then approach the potential subject at the clinical inpatient or outpatient encounter to introduce and gauge interest about participating in the study after confirming that the potential subject has an interest in learning more about the PIVATAL study with the treating physician. The enrolling site study team will then explain the details of study participation and if the potential subject is interested in continuing the informed consent process.
2.5.2. Consent
The study, including the details of the clinical equipoise posed by the study intent and design along with the potential benefits and risks will be discussed with the patient. The patient will be required to sign a consent for participation in PIVATAL.
2.5.3. Randomization
After signing consent, all the subjects will undergo randomization and study‐related procedures participating patients will be randomized from all of the enrolling centers and randomization will be 1:1 to either an intervention arm, consisting of intra‐operative VTA ablation vs. a control arm.
2.6. Allocation
In the PIVATAL study, for each of the 15 potential enrolling sites, treatment/control strings using 10 blocks with 4 subjects per block were created to try and ensure a balanced allocation for enrolling centers with smaller enrollment numbers. SAS' PLAN procedure was utilized for this process and is widely used for randomization designs for factorial experiments including randomized allocation of treatment levels. The treatment strings were incorporated in the TrialMaster software and a treatment assignment specific to each enrolling is delivered to them in an automated fashion when a subject's enrolling information is entered.
2.6.1. Baseline evaluation
Potential PIVATAL patients will be screened for eligibility at the time of evaluation for advanced heart failure therapy. Comprehensive data on arrhythmia history, past medical history and medication history will be collected in all subjects. For those patients with pre‐existing ICD, a full interrogation of the arrhythmia device will be performed and specific VTA events recorded. For patients with a history of VTA within 5 years with existing or planned ICD and a decision to proceed with LVAD implant, they will be approached for enrollment and consent.
All the consented subjects will be eligible for implantation of LVAD using standard‐of‐care procedures and indication and then undergo randomization to intra‐operative VT ablation versus medical management. All LVADs including future upgrades that are utilized in this study will be Food and Drug Administration (FDA) approved and used per the current FDA indications. The clinical research team is experienced in cardiovascular trials and the clinical management of subjects with heart disease/heart failure and will be monitoring for any adverse effects.
2.6.2. ICD therapy programming
To ensure uniformity in the detection and capture of VTA data, the programming of ICD/CRT‐D devices for therapy should be guided by pre‐established protocols. Table 2 specifies the detection zones and therapy programming for randomized subjects, based on with or without prior history of VTA, before and after LVAD implant. As advocated in the recently published AHA Scientific Statement on Arrhythmia Management in LVAD Recipients, a high heart rate for VF detection of 250 bpm will be programmed (Gopinathannair et al., 2019). A zone for VT detection of at least 10 bpm below clinical VT should be mandated in subjects who experience VTA events at any time during the study in order to ensure a uniform surveillance capture of VTA events. Therapy programming of ICD should be left up to the discretion of the clinicians with the caveat that if a patient experiences symptomatic VTA with hypoperfusion, worsened heart failure, or reduction in pump flow and cardiac output, ICD therapy for VT should be turned on. Pacing parameters should be maintained as previously programmed before LVAD implant. Post‐LVAD, pacing in non‐CRT devices should be minimized unless subjects develop bradycardic heart rates <30 bpm consistently. In CRT devices, biventricular pacing will be per clinician discretion. Throughout the study, all changes in ICD programming will be recorded.
TABLE 2.
Prespecified ICD programming protocol. a
| ICD programming post‐LVAD | ||
|---|---|---|
| No further VTA | Further VTA | |
| VT monitor zone | 150 bpm | 150 bpm or per clinician discretion b |
| VT zone rate | 188–220 bpm | 170 bpm or ≥10 bpm below clinical VTA |
| VT zone delay | Maximal delay c | Maximal delay c |
| VF zone rate | >250 bpm | >250 bpm |
| VF delay | Maximal delay c | Maximal delay c |
| ATP therapy | On for all zones (≥1 attempt) | On for all zones (≥1 attempt) |
| Shock energy | Maximum for all shocks | Per clinician discretion |
| Discrimination algorithms | On | On |
In patients with an ICM monitoring will be identical to ICD programming (150 bpm).
If the patient has documented VTA slower than 150 bpm, the provider may choose to program detection rate per individualized clinical needs.
If the patient has a symptomatic or hemodynamic compromise with VTA after LVAD, delays can be programmed per clinician discretion.
2.6.3. Antiarrhythmic medical regimen
Upon randomization, the medical therapy for each subject will be reviewed and documented. For subjects who are already on medical antiarrhythmic therapy (AAD), these should be continued as prescribed pre‐LVAD implant. All efforts should be made to ensure that beta‐blocker use is compliant. Following randomization and the surgical LVAD procedures, subjects will be monitored per protocol.
Intervention arm
The planned surgical VTA ablation at the time of LVAD implant will be based on electrophysiologic and anatomic data on the arrhythmic substrate as described below. We will collect comprehensive peri‐procedural and electroanatomic data along with ablation details.
Surgical VTA ablation at the time of LVAD implant
For surgical intra‐operative ablation, efforts will be made to identify scarred myocardium based on cardiac magnetic resonance imaging, nuclear scans, and/or echocardiogram. Mapping and ablation will be performed at the time of LVAD surgery with the currently approved and updated mapping and ablation systems available at each center. If a prior electrophysiological study and mapping have previously been performed, i.e., any prior catheter mapping and ablation procedure, the details may be incorporated to supplement the intra‐operative mapping data. Ventricular scars will be delineated through electroanatomic mapping (voltage < 1.5 mV). Activation and entrainment mapping may be performed during VT to allow more precise VT circuit and critical isthmus identification. If a pre‐operative electrophysiology study and catheter mapping have been performed, the data will be incorporated. An ablation strategy will be formulated and may be modified based on visualization during surgical exposure to modify and/or circumscribe the scars. Ablation may be carried out by radiofrequency or cryotherapy at the operator's discretion. The specific substrate scar modification method (i.e., late potential/LAVA abolition, de channeling, circumscribing, intersecting lesions, deceleration zone, etc.) chosen for substrate ablation will be performed based on physician discretion. The target locations for ablation, intra‐operative mapping, scar location by imaging or direct visualization during LVAD implant, will be recorded. For surgical ablation, we will evaluate the short‐term success as defined by the recurrence of VTA within 30 days after surgery. The feasibility and safety of this procedure will be assessed through tracking of peri‐operative parameters.
Medical management in the intervention arm: Subjects who undergo intra‐operative VTA ablation and have been on a stable regimen of antiarrhythmic medical therapy, these should be continued. Otherwise, these subjects should remain on beta‐blocker therapy post‐LVAD implant.
Control arm
To ensure uniformity in control arm, a standardized AAD regimen is recommended among subjects randomized to the medical management control arm for VTA recurrences following LVAD implant. There is no stipulation for the use of amiodarone or other antiarrhythmics in the control arm. For subjects who are already on a stable AAD regimen, such as amiodarone, sotalol or dofetilide, these are allowed to be continued.
Intervention arm
Post‐implant VTA recurrence in both arms
In cases of recurrences, we have devised a uniform AAD regimen (Table 3 and Figure 2) to allow escalation of AAD therapy for subjects in both the control and intervention arms. If there may be recurrence of VTA post‐LVAD implant and once the clinical decision is made to initiate medical antiarrhythmics, amiodarone, either intravenous or oral, will be administered. If a subject is not a candidate for amiodarone therapy, sotalol, or quinidine can be used. If subjects are experiencing recurrent VTA despite amiodarone or sotalol regimen, intravenous lidocaine can be added. For VTA refractory to amiodarone or sotalol therapy, mexiletine can be added. If subjects are deemed inappropriate for mexiletine or the other antiarrhythmic combination therapy, ranolazine with its action on inhibition of late inward sodium channel current may be considered as an adjunctive oral therapy. Patients who prove to be refractory to medical antiarrhythmic control should undergo a catheter‐based electrophysiology study and ablation on LVAD support. Throughout the study, all changes including interim changes in antiarrhythmic medical therapy will be recorded during the scheduled visits.
TABLE 3.
Prespecified antiarrhythmic medical regimen.
| Antiarrhythmic medication | ||
| Amiodarone | Initiating regimen | |
| 150 mg IV bolus over 10 min, then IV infusion of 0.5–1 mg/min for 24 h or longer as needed | ||
| For refractory VTA, repeated boluses over 10 min every 10 to 15 min up to 2.2 g in 24 h | ||
| Maintenance Regimen | ||
| Oral 400–1200 mg a day for 7–10 days then maintenance dosing of 200 mg daily | ||
| Sotalol | Regimen | |
| 80 mg twic a day and titrate upwards as needed and tolerated | ||
| Lidocaine | Initiating Regimen | |
| 1 to 1.5 mg/kg loading with 0.5 to 0.75 mg/kg repeated every 5 to 10 min as needed | ||
| Maintenance Regimen | ||
| Continuous IV infusion of 1 to 4 mg/min may be used. | ||
| Mexilitine | Regimen | |
| 150–300 mg every 8–12 h (may load with 400 mg if necessary) | ||
| Ranolazine | Regimen | |
| 500 to 1000 mg every 12 h | ||
FIGURE 2.
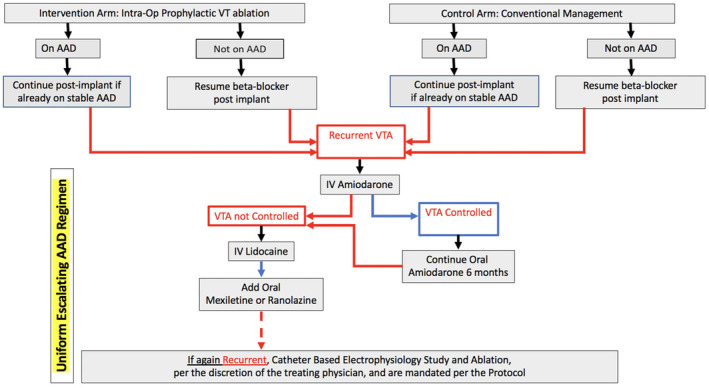
Antiarrhythmic medical therapy flow chart and escalating AAD regimen for VTA recurrences.
Catheter electrophysiology study and VTA ablation for patients with recurrent VTA in both arms
Eligible subjects who develop recurrent VTA after LVAD not responsive to medical antiarrhythmics may undergo catheter‐based electrophysiology study, mapping and ablation in the Cardiac Electrophysiology Laboratory. This will be performed as a further treatment strategy to control recurrent VTA resistant to medical therapy and is not considered as a crossover of treatment arms in the study. Due to technical issues with mapping and ablation, there often is a higher threshold, i.e., multiple recurrent VTA events taking place prior to proceeding with catheter‐based electrophysiology procedures post‐LVAD implant. The suggested criteria for post‐LVAD implant VTA EP study and ablation include: repeated VTA not responsive to medical therapy, VTA episodes associated with hemodynamic compromise, and/or RV dysfunction. The decision to proceed with post‐LVAD electrophysiology study and ablation is per the judgment and discretion of the treating clinician. If performed, voltage mapping of the ventricle(s) to delineate scars will be carried out through electroanatomic mapping. As subjects are supported by LVAD during VTA, activation and entrainment mapping may be performed during VT to establish precise mechanisms (reentry vs. focal), and locations of VT circuits and any critical isthmus. Acute success will be defined as the non‐inducibility of any monomorphic VT with programmed stimulation of a basic drive train and up to three extra stimuli from at least one ventricular site. We believe detailed electrophysiologic data from cardiac mapping will further elucidate the mechanisms of recurrent VTA post‐LVAD implant.
2.6.4. Follow‐up in both arms
Following LVAD implantation office visits will be scheduled as part of standard clinical care every three months, during which data on VTA events, ICD therapy, medication lists, and adverse events will be collected (Table 4). All subjects will have ICD devices with remote interrogation capabilities enabled, which will be interrogated for any potential arrhythmic events, treated and monitored, at 3‐month intervals. Plus, ICD data will be collected with every subject report of ICD therapy, as well as clinic visits, adverse events, emergency room visits, and hospitalization, planned or unplanned. All the ICD data and therapy will be adjudicated by the Adjudication Committee. Enrolled patients will be followed for an average duration of 18 months with a minimum of 9 months.
TABLE 4.
Schedule of activities and data collection.
| Activity | Pre‐LVAD implantation visit | LVAD implant a | 1‐month LVAD clinic visit (±14 days) a | 3‐month, 9‐month, 15‐month LVAD clinic visit (±1 calendar month) a | 6‐month LVAD clinic visit (±1 calendar month) a | 12‐month and following every 6‐month LVAD clinic visit (±1 calendar month) up to 24 months a |
|---|---|---|---|---|---|---|
| Study timefsrame | Following randomization | Count from discharge date after LVAD implant | Count from discharge date after LVAD implant | Count from discharge date after LVAD implant | Count from discharge date after LVAD implant | |
| Eligibility/informed consent/ | ✓ | |||||
| Future use | ✓ | |||||
| Randomization | ✓ | |||||
| Physical exam a | ✓ | ✓ | ✓ | ✓ | ✓ | |
| CV Medications | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Pre‐discharge LVAD parameters a | ✓ | |||||
| Electrocardiogram a | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Echocardiogram a | ✓ | ✓ | ✓ | |||
| Medical history/Cardiac Events | ✓ | ✓ | ✓ | ✓ | ✓ | |
| RAMP study/Echo/Right Heart Cath Study a | ✓ | ✓ | ✓ (month 12 and if available thereafter) | |||
| Laboratory Tests/Pregnancy Test a | ✓ | |||||
| VT Ablation Procedure b | ✓ | |||||
| LVAD parameters a | ✓ | ✓ | ✓ | ✓ | ||
| CIED device status | ✓ | By occ | By occ | By occ | By occ | |
| Remote CIED interrogation | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Quality of Life (KCCQ −12) | ✓ c | ✓ (month 06) | ||||
| Adverse events | By occ | By occ | By occ | By occ | By occ | |
| Protocol deviations | By occ | By occ | By occ | By occ | By occ |
SOC (Standard of Care) procedure if done.
If randomized to ablation SOC procedure.
Prior to LVAD implant.
Ramp testing with an echocardiogram will be performed at 6 months per standard of care at the enrolling centers. At 6 months a follow‐up QoL assessment will also be collected using the Kansas City Cardiopulmonary Questionnaire 12. Data regarding deaths will be reported based on information from enrolling sites obtained by study coordinators at the respective clinics. We will also collect detailed data on post‐LVAD implant VTA ablation procedures whenever applicable.
2.7. Statistical analysis
Our sample size justification is based on having sufficient statistical power to detect an effect on the intensity of recurrent VTA (primary endpoint) between subjects randomized to the intervention arm of undergoing prophylactic intra‐operative VT ablation (n = 50) versus randomized to the control arm of medical management (n = 50). We used Cook's (1995) (Lin et al., 2000) method for designing a study with recurrent events; for study design purposes, competing death events are regarded as censored at the time of death.
The SMASH–VT (Reddy et al., 2007) did not evaluate VTA burden as an endpoint. However, the study did show that VT ablation in subjects with a history of VT is associated with a 65% reduction (hazard ratio in the ablation group, 0.35; 95% confidence interval, 0.15 to 0.78, p = .007) in the occurrence of a first appropriate ICD therapy (anti‐tachycardia pacing or shocks). Accordingly, based on SMASH–VT, we assume a more conservative effect size of 44% reduction (or intensity ratio of 0.56 – our primary hypothesis). Using a two‐sided test with a 5% Type I error rate, we have 90% statistical power to detect an intensity ratio of 0.56, that is, a 44% reduction in VTA intensity from 3 events per year to 1.7 events per year, on average. Note, an intensity ratio of 0.56 is interpreted as a 44% reduction in the intensity of VTAs from 3 events per year to 1.8 events per year, on average, after accounting for a mortality rate of 10% at 18 months and a loss to follow‐up rate of 5% (patients will be censored at loss to follow‐up). For a lower effect size of 40% reduction, we have 85% statistical power to detect an intensity ratio of 0.60 from 3 events per year to 1.7 events per year.
For the weighted composite endpoint, Cook's method can also give us an idea of the power to detect reductions in the weighed composite endpoint. Under a constant weighting scheme, all adverse events are regarded as equal and the weighed composite endpoint reduces to an ordinary recurrent event process. Thus, for this specific choice of weighting scheme, we can expect roughly 90% statistical power to detect a 44% reduction in the composite adverse event process, from 3 AEs per year in the control medical management arm to 1.7 events per year in the prophylactic ablation arm.
3. DISCUSSION
Even though patients with LVAD can often tolerate sustained VTA and even VF without syncope or sudden death, the occurrence of VTA is associated with increased complications and poor outcomes (Bouchez et al., 2016; Javed et al., 2016; Naito et al., 2014; Patel et al., 2011). VTA may be associated with reduced LVAD efficacy, right heart failure, and thromboembolic events (Kadado et al., 2018). Data on the association between VTA and mortality are conflicting, but most observational studies have shown that VTA post‐LVAD is associated with a significantly higher mortality (Clerkin et al., 2017; Greet et al., 2018; Kirklin et al., 2015). A meta‐analysis of nine retrospective observational studies comprising of 1179 patients consistently noted an association of post‐LVAD implant VTA with mortality and demonstrated that pre‐LVAD VTA is also a major risk factor for mortality (Makki et al., 2015).
Despite the clinical importance of these arrhythmias, there are little data to guide the treatment of post‐LVAD implant VTAs. In fact, the recent Scientific Statement from the American Heart Association concluded with no consensus on optimal strategies for medical management or procedural interventions in patients with recurrent VTA (Gopinathannair et al., 2019). Currently, antiarrhythmic medical therapy with amiodarone is widely utilized for patients who develop VTA post‐LVAD implant. However, this may have adverse effects, as retrospective data suggest that long‐term use of amiodarone pre‐heart transplant is associated with an increased mortality (Cooper et al., 2017). Ablation therapy for recurrent VTA following LVAD implant has been reported in several small retrospective studies (Table 5) (Asirvatham et al., 2017; Cantillon et al., 2012; Dandamudi et al., 2007; Garan et al., 2014; Herweg et al., 2012; Hottigoudar et al., 2011; Moss et al., 2017; Sacher et al., 2015). These data were compiled from centers without uniform pre‐ and post‐LVAD arrhythmia surveillance, VTA episode definition, ablation strategies, or post‐ablation medical management. One of the largest single‐center experiences included only 21 patients accumulated over a 20‐year period using different ablation techniques, surgical techniques, and generations of LVAD (Cantillon et al., 2012). A compiled report included 34 patients from 9 international centers utilizing varied approaches and techniques for the ablation (Sacher et al., 2015). These reports also suggest that post‐LVAD implant VTA ablation is associated with relatively high‐acute recurrence rates (Table 5), and the impact of ablation on long‐term outcomes is uncertain.
TABLE 5.
Prior reports of VT ablation in LVAD recipients.
| N | ICM, n (%) | CF–LVAD, n (%) | Follow‐up, mo | Recurrence, n (%) | Epicardial ablation, n | |
|---|---|---|---|---|---|---|
| Dandamudi et al. (2007) | 3 | 2 (66) | 0 (0) | 4–12 | 1 (33) | 0 |
| Hottigoudar et al. (2011) | 3 | 1 (33) | 3 | 2–10 | 2 (66) | 0 |
| Cantillon et al. (2012) | 21 | 12 (57) | NA | 4.4 ± 3.3 | 7 (33) | 0 |
| Herweg et al. (2012) | 6 | 4 (66) | 4 (66) | 7.5 ± 6.9 | 2 (33) | 0 |
| Garan et al. (2014) | 7 | 5 (71) | 7 (100) | 5 ± 3.6 | 6 (86) | 1 |
| Sacher et al. (2015) | 34 | 21 (62) | 34 (100) | 25 ± 15 | 5 (15) | 0 |
| Snipelisky et al. (2017) | 6 | 2 (33) | 6 (100) | 6 | 5 (83) | 1 |
| Moss et al. (2017) | 21 | 14 (66) | 21 (100) | 9 | 7 (33) | 0 |
A lack of prospective, randomized data on the potential benefits of ablation prior to or at the time of LVAD implant in patients with a history of VTA has led to disparate implementation of this approach among clinicians, even though it is well‐recognized that these patients are at high risk of recurrent VTA post‐LVAD implant and poor outcomes, including increased mortality. Some centers advocate ablation more aggressively, whereas others avoid ablation and lean on pharmacologic therapy and the hemodynamic benefit of an unloaded left ventricle to improve post‐LVAD arrhythmic issues. However, selected arrhythmia mapping data suggest that a majority of the VTA that occur post‐LVAD implant are due to arrhythmic substrate arising from the underlying cardiomyopathy and is unlikely to be altered significantly by the implant of LVAD alone (Cantillon et al., 2012; Sacher et al., 2015). The recent Scientific Statement from American Heart Association advocated that LVAD should not be considered as a treatment for VTA and that the majority of VTA results from the underlying substrate, not from heart failure and the associated worsening hemodynamic status (Gopinathannair et al., 2019). At the same time, there are no agreed upon recommendations for VTA management beyond medical therapy due to a lack of well‐conducted trial data. Thereby, there exists an ongoing clinical equipoise regarding the proper management plans for these high‐risk patients. In light of the preliminary data from small case series suggesting prophylactic intra‐operative VTA ablation can be successful in this patient population, plus the increasing utilization of LVAD in patients with end‐stage heart failure, the timing is appropriate for a randomized clinical trial. The proposed study will explore a strategy that incorporates a prophylactic intra‐operative VT ablation strategy during LVAD implant for the reduction of post‐implant VTA recurrence and death from any cause from index discharge to 18 months following LVAD implantation.
PIVATAL is the first randomized trial to test the clinical utility of prophylactic VT ablation therapy in patients with high‐risk LVAD. We will prospectively evaluate acute success rates, safety, and efficacy of a novel management approach of prophylactic VTA ablation at the time of LVAD implantation. The protocol plans to enroll 100 candidates with LVAD with advanced cardiomyopathy, a history of VTA and prior or intended ICD implant from 15 experienced medical centers with clinical expertise in LVAD surgery and VTA ablation. Patients will be randomized in a 1:1 ratio to prophylactic intra‐operative VT ablation versus conventional medical management. We hypothesize that a prophylactic ablation strategy will result in a significant reduction in VTA events compared with conventional management and translate into a reduction in adverse events, including hospital readmissions, clinical RV failure, stroke, and death. PIVATAL has the potential to change clinical practice and lead to a paradigm change in the management of ventricular arrhythmias in the growing population of patients with LVAD.
AUTHOR CONTRIBUTIONS
DTH, IG, KLW, HV, WS, BAJ, SM, VK, WZ, IG: Conceptualization, Methodology, Investigation. DTH, IG, WZ: Funding acquisition DTH, CW, ST, IG: Writing – Original Draft, Visualization. IG, KLW, HV, WS, FM, GS, SZ, PW, RT, WST, JDM, KK, SH, PJP, AMK, CS, GR, MSK, GSC, ML, EJM. ADH, ML, JR, ES, KS BAJ, SM, VK, WZ: Writing – Review and Editing, Investigation.
FUNDING INFORMATION
This study is supported by the National Institutes of Health NIH Grant R01HL159401.
CONFLICT OF INTEREST STATEMENT
Dr. David T. Huang is an Editorial Board member of the Annals of Noninvasive Electrocardiology and a co‐author of this article. To minimize bias, he was excluded from all editorial decision‐making related to the acceptance of this article for publication. Dr. Wojciech Zareba is the Editor‐in‐Chief of the journal and co‐author of this article. He was excluded from the peer‐review process and all editorial decisions related to the acceptance and publication of this article. Peer review was handled independently by Associate Editor, Dr. Mark Haigney to minimize bias. David T. Huang: Research grants from Biosense‐Webster, Medtronic, Biotronik. Fellowship support from Abbott, Boston Scientific, and Medtronic. Wojciech Zareba: Research grants from Boston Scientific, Biotronik, Zoll Inc. Ilan Goldenberg: Research grants from Boston Scientific, Zoll, Medtronic, Biosense‐Webster, Biotronik, Abbott, Astra Zeneca. Igor Gosev: Consultant for Abbott. Himabindu Vidula: Research grant from Abbott; Consultant/speaker for Abbott. William G. Stevenson: Teaching honoraria: Abbott, Biotronik, Boston Scientific, Medtronic, Johnson and Johnson. J. Peter Weiss: Consulting/Honoraria: Abbott, Biotronik, Stereotaxis, Galaxy Medical. Joshua D. Moss: Teaching honoraria from Abbott, Baylis, Biosense Webster. Fellowship support from Abbott, Boston Scientific, and Medtronic. Roderick Tung: Speaking and Consulting honoraria from Abbott, Biosense Webster, Boston Scientific, Biotronik, Atricure, Access Point Technologies, Medtronic. Sunit‐Preet Chaudhry: Consulting honoraria from Edwards Lifesciences and Medtronic. Claudio Schuger: Research grant from Boston Scientific; Honoraria: Boston Scientific and Medtrronic. Ezequiel J Molina: Consultant: Abbott. Michael Lloyd: research and fellowship support: Biosense webster, Medtronic, Boston Scientific. Consultant, Medtronic, Boston Scientific. Edward G. Soltesz: Research: Abiomed; Honoraria: Atricure, Abiomed and Abbott.
FUNDING INFORMATION
This study has been formally reviewed by the institutional IRB at the University of Rochester. Authors listed here within are investigators, steering committee members, and/or collaborators.
ETHICS STATEMENT
All participating patients will grant their informed consent for inclusion prior to participating in the study. The study is being conducted in accordance with the Declaration of Helsinki. The PIVATAL trial was approved by the institutional IRB and the Ethics Committee with full approval to proceed with a randomized trial design.
Supporting information
Appendix S1
Huang, D. T. , Gosev, I. , Wood, K. L. , Vidula, H. , Stevenson, W. , Marchlinski, F. , Supple, G. , Zalawadiya, S. K. , Weiss, J. P. , Tung, R. , Tzou, W. S. , Moss, J. D. , Kancharla, K. , Chaudhry, S.‐P. , Patel, P. J. , Khan, A. M. , Schuger, C. , Rozen, G. , Kiernan, M. S. … Goldenberg, I. (2023). Design and characteristics of the prophylactic intra‐operative ventricular arrhythmia ablation in high‐risk LVAD candidates (PIVATAL) trial. Annals of Noninvasive Electrocardiology, 28, e13073. 10.1111/anec.13073
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Asirvatham, S. J. , Packer, D. L. , Cha, Y. M. , Kapa, S. , Brady, P. A. , Noseworthy, P. A. , Maleszewski, J. J. , & Mulpuru, S. K. (2017). Effect of ventricular arrhythmia ablation in patients with Heart Mate II left ventricular assist devices: An evaluation of ablation therapy. Journal of Cardiovascular Electrophysiology, 28, 68–77. [DOI] [PubMed] [Google Scholar]
- Bedi, M. , Kormos, R. , Winowich, S. , McNamara, D. M. , Mathier, M. A. , & Murali, S. (2007). Ventricular arrhythmias during left ventricular assist device support. The American Journal of Cardiology, 99, 1151–1153. [DOI] [PubMed] [Google Scholar]
- Bouchez, S. , De Somer, F. , Herck, I. , Van Belleghem, Y. , De Pauw, M. , & Stroobandt, R. (2016). Shock‐refractory ventricular fibrillation in a patient implanted with a left ventricular assist device. Resuscitation, 107, e1–e2. [DOI] [PubMed] [Google Scholar]
- Brenyo, A. , Rao, M. , Koneru, S. , Hallinan, W. , Shah, S. , Massey, H. T. , Chen, L. , Polonsky, B. , McNitt, S. , Huang, D. T. , Goldenberg, I. , & Aktas, M. (2012). Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. Journal of Cardiovascular Electrophysiology, 23, 515–520. [DOI] [PubMed] [Google Scholar]
- Cantillon, D. J. , Bianco, C. , Wazni, O. M. , Kanj, M. , Smedira, N. G. , Wilkoff, B. L. , Starling, R. C. , & Saliba, W. I. (2012). Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm, 9, 859–864. [DOI] [PubMed] [Google Scholar]
- Clerkin, K. J. , Topkara, V. K. , Mancini, D. M. , Yuzefpolskaya, M. , Demmer, R. T. , Dizon, J. M. , Takeda, K. , Takayama, H. , Naka, Y. , Colombo, P. C. , & Garan, A. R. (2017). The role of implantable cardioverter defibrillators in patients bridged to transplantation with a continuous‐flow left ventricular assist device: A propensity score matched analysis. The Journal of Heart and Lung Transplantation, 36, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, L. B. , Mentz, R. J. , Edwards, L. B. , Wilk, A. R. , Rogers, J. G. , Patel, C. B. , Milano, C. A. , Hernandez, A. F. , Stehlik, J. , & Lund, L. H. (2017). Amiodarone use in patients listed for heart transplant is associated with increased 1‐year post‐transplant mortality. The Journal of Heart and Lung Transplantation, 36, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandamudi, G. , Ghumman, W. S. , Das, M. K. , & Miller, J. M. (2007). Endocardial catheter ablation of ventricular tachycardia in patients with ventricular assist devices. Heart Rhythm, 4, 1165–1169. [DOI] [PubMed] [Google Scholar]
- Drakos, S. G. , Kfoury, A. G. , Selzman, C. H. , Verma, D. R. , Nanas, J. N. , Li, D. Y. , & Stehlik, J. (2011). Left ventricular assist device unloading effects on myocardial structure and function: Current status of the field and call for action. Current Opinion in Cardiology, 26, 245–255. 10.1097/HCO.0b013e328345af13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garan, A. R. , Iyer, V. , Whang, W. , Mody, K. P. , Yuzefpolskaya, M. , Colombo, P. C. , Te‐Frey, R. , Takayama, H. , Naka, Y. , Garan, H. , Jorde, U. P. , & Uriel, N. (2014). Catheter ablation for ventricular tachyarrhythmias in patients supported by continuous‐flow left ventricular assist devices. ASAIO Journal, 60, 311–316. [DOI] [PubMed] [Google Scholar]
- Garan, A. R. , Yuzefpolskaya, M. , Colombo, P. C. , Morrow, J. P. , Te‐Frey, R. , Dano, D. , Takayama, H. , Naka, Y. , Garan, H. , Jorde, U. P. , & Uriel, N. (2013). Ventricular arrhythmias and implantable cardioverter‐defibrillator therapy in patients with continuous‐flow left ventricular assist devices: Need for primary prevention? Journal of the American College of Cardiology, 61, 2542–2550. [DOI] [PubMed] [Google Scholar]
- Gopinathannair, R. , Cornwell, W. K. , Dukes, J. W. , Ellis, C. R. , Hickey, K. T. , Joglar, J. A. , Pagani, F. D. , Roukoz, H. , Slaughter, M. S. , & Patton, K. K. (2019). Device therapy and arrhythmia management in left ventricular assist device recipients, a scientific Statement from the American Heart Association. Circulation, 139, e967–e989. [DOI] [PubMed] [Google Scholar]
- Greet, B. D. , Pujara, D. , Burkland, D. , Pollet, M. , Sudhakar, D. , Rojas, F. , Costello, B. , Postalian, A. , Hale, Z. , Jenny, B. , Lai, C. , Igbalode, K. , Wadhera, D. , Nair, A. , Ono, M. , Morgan, J. , Simpson, L. , Civitello, A. , Cheng, J. , & Mathuria, N. (2018). Incidence, predictors, and significance of ventricular arrhythmias in patients with continuous‐flow left ventricular assist devices. A 15‐year institutional experience. 2017. JACC: Clinical Electrophysiology, 4, 257–264. [DOI] [PubMed] [Google Scholar]
- Herweg, B. , Ilercil, A. , Kristof‐Kuteyeva, O. , Rinde‐Hoffman, D. , Caldeira, C. , Mangar, D. , Karlnosky, R. , & Barold, S. S. (2012). Clinical observations and outcome of ventricular tachycardia ablation in patients with left ventricular assist devices. Pacing and Clinical Electrophysiology, 35, 1377–1383. [DOI] [PubMed] [Google Scholar]
- Hottigoudar, R. U. , Deam, A. G. , Slaughter, M. S. , Sutton, B. S. , Mccants, K. C. , Birks, E. J. , & Gopinathannair, R. (2011). Ventricular tachycardia ablation in patients with HeartMate II left ventricular assist devices: Rhythm still matters in the bionic age. Journal of Innovations in Cardiac Rhythm Management, 2, 537–547. [Google Scholar]
- Javed, W. , Chaggar, P. S. , Venkateswaran, R. , & Shaw, S. M. (2016). Prolonged asystole in a patient with an isolated left ventricular assist device. Future Cardiology, 12, 533–538. 10.2217/fca-2016-0022 [DOI] [PubMed] [Google Scholar]
- Kadado, A. J. , Akar, J. G. , & Hummel, J. P. (2018). Arrhythmias after left ventricular assist device implantation: Incidence and management. Trends in Cardiovascular Medicine, 28, 41–50. [DOI] [PubMed] [Google Scholar]
- Kirklin, J. K. , Naftel, D. C. , Pagani, F. D. , Kormos, R. L. , Stevenson, L. W. , Blume, E. D. , Myers, S. L. , Miller, M. A. , Baldwin, J. T. , & Young, J. B. (2015). Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of Heart and Lung Transplantation, 34, 1495–1504. [DOI] [PubMed] [Google Scholar]
- Lee, W. , Tay, A. , Subbiah, R. N. , Walker, B. D. , Kuchar, D. L. , Muthiah, K. , Macdonald, P. S. , Keogh, A. M. , Kotlyar, E. , Jabbour, A. , Spratt, P. , Jansz, P. C. , Granger, E. , Dhital, K. , & Hayward, C. S. (2015). Impact of implantable cardioverter defibrillators on survival of patients with centrifugal left ventricular assist devices. Pacing and Clinical Electrophysiology, 38, 925–933. [DOI] [PubMed] [Google Scholar]
- Lin, D. Y. , Wei, L. J. , Yang, I. , & Ying, Z. (2000). Semiparametric regression for the mean and rate functions of recurrent events. Journal of the Royal Statistical Society, Series B (Statistical Methodology), 62, 711–730. [Google Scholar]
- Makki, N. , Mesubi, O. , Steyers, C. , Olshansky, B. , & Abraham, W. T. (2015). Meta‐analysis of the relation of ventricular arrhythmias to all‐cause mortality after implantation of a left ventricular assist device. The American Journal of Cardiology, 116, 1385–1390. [DOI] [PubMed] [Google Scholar]
- Mehra, M. R. , Naka, Y. , Uriel, N. , Goldstein, D. J. , Cleveland, J. C., Jr. , Colombo, P. C. , Walsh, M. N. , Milano, C. A. , Patel, C. B. , Jorde, U. P. , Pagani, F. D. , Aaronson, K. D. , Dean, D. A. , McCants, K. , Itoh, A. , Ewald, G. A. , Horstmanshof, D. , Long, J. W. , Salerno, C. , & MOMENTUM 3 Investigators . (2017). A fully magnetically levitated circulatory pump for advanced heart failure. The New England Journal of Medicine, 376, 440–450. 10.1056/NEJMoa1610426 [DOI] [PubMed] [Google Scholar]
- Mehra, M. R. , Goldstein, D. J. , Cleveland, J. C. , Cowger, J. A. , Hall, S. , Salerno, C. T. , Naka, Y. , Horstmanshof, D. , Chuang, J. , Wang, A. J. , & Uriel, N. (2022). Five‐year outcomes in patients with fully magnetically levitated vs axial‐flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA, 328(12), 1233–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, L. W. , Pagani, F. D. , Russell, S. D. , John, R. , Boyle, A. J. , Aaronson, K. D. , Conte, J. V. , Naka, Y. , Mancini, D. , Delgado, R. M. , MacGillivray, T. E. , Farrar, D. J. , Frazier, O. H. , & HeartMate II Clinical Investigators . (2007). Use of a continuous‐flow device in patients awaiting heart transplantation. The New England Journal of Medicine, 357, 885–896. 10.1056/NEJMoa067758 [DOI] [PubMed] [Google Scholar]
- Moss, J. D. , Flatley, E. E. , Beaser, A. D. , Shin, J. H. , Nayak, H. M. , Upadhyay, G. A. , Burke, M. C. , Jeevanandam, V. , Uriel, N. , & Tung, R. (2017). Characterization of ventricular tachycardia after left ventricular assist device implantation as destination therapy: A single‐center ablation experience. JACC: Clinical Electrophysiology, 3, 1412–1424. [DOI] [PubMed] [Google Scholar]
- Moss, J. D. , Oesterle, A. , Raiman, M. , Flatley, E. E. , Beaser, A. D. , Jeevanandam, V. , Klein, L. , Ota, T. , Wieselthaler, G. , Uriel, N. , & Tung, R. (2018). Feasibility and utility of intraoperative epicardial scar characterization during left ventricular assist device implantation. Journal of Cardiovascular Electrophysiology, 30(2), 183–192. 10.1111/jce.13803 [DOI] [PubMed] [Google Scholar]
- Naito, N. , Kinoshita, O. , & Ono, M. (2014). Prolonged left ventricular assist device support (18 months) in refractory ventricular fibrillation. The Journal of Heart and Lung Transplantation, 33, 772–773. [DOI] [PubMed] [Google Scholar]
- Nakahara, S. , Chien, C. , Gelow, J. , Dalouk, K. , Henrikson, C. A. , Mudd, J. , & Stecker, E. C. (2013). Ventricular arrhythmias after left ventricular assist device. Circulation. Arrhythmia and Electrophysiology, 6, 648–654. [DOI] [PubMed] [Google Scholar]
- Oswald, H. , Schultz‐Wildelau, C. , Gardiwal, A. , Lüsebrink, U. , König, T. , Meyer, A. , Duncker, D. , Pichlmaier, M. A. , Klein, G. , & Strüber, M. (2010). Implantable defibrillator therapy for ventricular tachyarrhythmia in left ventricular assist device patients. European Journal of Heart Failure, 12, 593–599. [DOI] [PubMed] [Google Scholar]
- Patel, P. , Williams, J. G. , & Brice, J. H. (2011). Sustained ventricular fibrillation in an alert patient: Preserved hemodynamics with a left ventricular assist device. Prehospital Emergency Care, 15, 533–536. [DOI] [PubMed] [Google Scholar]
- Raasch, H. , Jensen, B. C. , Chang, P. P. , Mounsey, J. P. , Gehi, A. K. , Chung, E. H. , Sheridan, B. C. , Bowen, A. , & Katz, J. N. (2012). Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous‐flow left ventricular assist device implantation. American Heart Journal, 164, 373–378. [DOI] [PubMed] [Google Scholar]
- Reddy, V. Y. , Reynolds, M. R. , Neuzil, P. , Richardson, A. W. , Taborsky, M. , Jongnarangsin, K. , Kralovec, S. , Sediva, L. , Ruskin, J. N. , & Josephson, M. E. (2007). Prophylactic catheter ablation for the prevention of defibrillator therapy. The New England Journal of Medicine, 357, 2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaat, M. , Chemaly, E. , Lebeche, D. , Gwathmey, J. K. , & Hajjar, R. J. (2008). Ventricular arrhythmias after left ventricular assist device implantation. Pacing and Clinical Electrophysiology, 31, 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher, F. , Reichlin, T. , Zado, E. S. , Field, M. E. , Viles‐Gonzalez, J. F. , Peichl, P. , Ellenbogen, K. A. , Maury, P. , Dukkipati, S. R. , Picard, F. , Kautzner, J. , Barandon, L. , Koneru, J. N. , Ritter, P. , Mahida, S. , Calderon, J. , Derval, N. , Denis, A. , Cochet, H. , … Jais, P. (2015). Characteristics of ventricular tachycardia ablation in patients with continuous flow left ventricular assist devices. Circulation. Arrhythmia and Electrophysiology, 8, 592–597. [DOI] [PubMed] [Google Scholar]
- Slaughter, M. S. , Meyer, A. L. , & Birks, E. J. (2011). Destination therapy with left ventricular assist devices: Patient selection and outcomes. Current Opinion in Cardiology, 26, 232–236. 10.1097/HCO.0b013e328345aff4 [DOI] [PubMed] [Google Scholar]
- Slaughter, M. S. , Rogers, J. G. , Milano, C. A. , Russell, S. D. , Conte, J. V. , Feldman, D. , Sun, B. , Tatooles, A. J. , Delgado, R. M., 3rd , Long, J. W. , Wozniak, T. C. , Ghumman, W. , Farrar, D. J. , Frazier, O. H. , & HeartMate II Investigators . (2009). Advanced heart failure treated with continuous‐flow left ventricular assist device. The New England Journal of Medicine, 361, 2241–2251. 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- Snipelisky, D. , Reddy, Y. N. , Manocha, K. , Patel, A. , Dunlay, S. M. , Friedman, P. A. , Munger, T. M. , Asirvatham, S. J. , Packer, D. L. , Cha, Y. M. , Kapa, S. , Brady, P. A. , Noseworthy, P. A. , Maleszewski, J. J. , & Mulpuru, S. K. (2017). Effect of ventricular arrhythmia ablation in patients with heart mate II left ventricular assist devices: An evaluation of ablation therapy. Journal of Cardiovascular Electrophysiology, 28(1), 68–77. 10.1111/jce.13114 [DOI] [PubMed] [Google Scholar]
- Tankut, S. , Gosev, I. , Yoruk, A. , Younis, A. , McNitt, S. , Bjelic, M. , Vidula, H. , Wu, I. , Aktas, M. K. , Goldenberg, I. , & Huang, D. T. (2022). Intraoperative ventricular tachycardia ablation during left ventricular assist device implantation in high‐risk heart failure patients. Circulation. Arrhythmia and Electrophysiology, 15(6), e010660. [DOI] [PubMed] [Google Scholar]
- Yancy, C. W. , Jessup, M. , Bozkurt, B. , Butler, J. , Casey, D. E., Jr. , Drazner, M. H. , Fonarow, G. C. , Geraci, S. A. , Horwich, T. , Januzzi, J. L. , Johnson, M. R. , Kasper, E. K. , Levy, W. C. , Masoudi, F. A. , McBride, P. E. , McMurray, J. J. , Mitchell, J. E. , Peterson, P. N. , Riegel, B. , … American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . (2013). ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 2013(62), e147–e239. [DOI] [PubMed] [Google Scholar]
- Yoruk, A. , Sherazi, S. , Massey, H. T. , Kutyifa, V. , McNitt, S. , Hallinan, W. , Huang, D. T. , Chen, L. , & Aktas, M. K. (2016). Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm, 13(5), 1052–1056. 10.1016/j.hrthm.2016.01.033 [DOI] [PubMed] [Google Scholar]
- Ziv, O. , Dizon, J. , Thosani, A. , Naka, Y. , Magnano, A. R. , & Garan, H. (2005). Effects of left ventricular assist device therapy on ventricular arrhythmias. Journal of the American College of Cardiology, 45, 1428–1434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


